Abstract
One significant drawback of current probiotic therapy for the prevention of necrotizing enterocolitis (NEC) is the need for at least daily administration because of poor probiotic persistence after enteral administration, increasing the risk of the probiotic bacteria causing bacteremia or sepsis if the intestines are already compromised. We previously showed that the effectiveness of Lactobacillus reuteri (Lr) in preventing NEC is enhanced when Lr is grown as a biofilm on the surface of dextranomer microspheres (DM). Here we sought to test the efficacy of Lr administration by manipulating the Lr biofilm state with the addition of biofilm-promoting substances (sucrose and maltose) to DM or by mutating the Lr gtfW gene (encoding an enzyme central to biofilm production). Using an animal model of NEC, we determined that Lr adhered to sucrose- or maltose-loaded DM significantly reduced histologic injury, improved host survival, decreased intestinal permeability, reduced intestinal inflammation, and altered the gut microbiome compared with Lr adhered to unloaded DM. These effects were abolished when DM or GtfW were absent from the Lr inoculum. This demonstrates that a single dose of Lr in its biofilm state decreases NEC incidence. Importantly, preloading DM with sucrose or maltose further enhances Lr protection against NEC in a GtfW-dependent fashion, demonstrating the tunability of the approach and the potential to use other cargos to enhance future probiotic formulations.
NEW & NOTEWORTHY Previous clinical trials of probiotics to prevent necrotizing enterocolitis have had variable results. In these studies, probiotics were delivered in their planktonic, free-living form. We have developed a novel probiotic delivery system in which Lactobacillus reuteri (Lr) is delivered in its biofilm state. In a model of experimental necrotizing enterocolitis, this formulation significantly reduces intestinal inflammation and permeability, improves survival, and preserves the natural gut microflora compared with the administration of Lr in its free-living form.
Keywords: biofilm, Lactobacillus reuteri, necrotizing enterocolitis, probiotics
INTRODUCTION
Necrotizing enterocolitis (NEC) remains an overwhelming source of morbidity and mortality for premature infants. Ten percent of infants born under 1,500 g will develop NEC (24), manifested by excessive intestinal inflammation that often progresses to tissue destruction, bacterial translocation, sepsis, and even death. Despite decades of research and an estimated $500 million to $1 billion spent annually on care for infants with NEC, mortality remains as high as 30%. The pathogenesis of NEC is undoubtedly multifactorial, but strong evidence indicates that gut dysbiosis plays a prominent role in development of the disease (40, 42, 66).
Bacterial colonization of the intestine is essential to healthy gut development (20, 23). A neonate’s commensal microbial community, dictated largely by ingestion of maternal vaginal and colonic microorganisms during passage through the birth canal and oral feedings (69), strengthens the intestinal mucosal barrier and primes the immune system to react appropriately to bacteria that will subsequently be encountered (69). Although significant variability exists among individuals, full-term breast-fed infants have a high number of beneficial bacteria including Lactobacillus and Bifidobacterim species (21, 32, 69). In contrast, premature infants tend to have reduced microbiome diversity and stability and increased levels of pathogenic Gammaproteobacteria, which ultimately lead to delayed transition to adult microbial patterns (9, 19). These trends are particularly evident in infants that develop NEC (11, 18, 48, 64, 66). Such findings contribute to the mounting evidence that dysbiosis is key to susceptibility to and eventual development of NEC.
To counteract the altered intestinal microbiome associated with NEC, probiotics [“live microorganisms that confer a health benefit on the host” (50)] were introduced for the prevention of NEC as early as 1999 (26). Probiotics are generally orally administered to infants in conjunction with enteral feeds. There have been abundant trials evaluating the efficacy of probiotics for the prevention of NEC, with some trials demonstrating beneficial effects (3, 63). Animal models of experimental NEC indicate that many probiotic species inhibit countereffective inflammation (17, 59), reduce apoptosis (68), inhibit TLR4 activation (5, 17), and protect against breakdown of the intestinal mucosal barrier (8). However, there are a number of limitations to current probiotic administration, including the fact that they must be given repeatedly for efficacy. Following enteral administration, probiotic bacteria are quickly rendered ineffective because of the acidic pH of the stomach, interaction with bile salts, pressure from the host immune system, and competition with host commensal bacteria (16). These factors likely contribute to the inability of probiotics to adhere to and colonize the gut (2). When probiotic administration is halted, their beneficial effects are lost (39). Importantly, repeated probiotic administrations, either daily or multiple times per day, create a double-edged sword: they are necessary for even modest probiotic efficacy but could themselves induce bacteremia or sepsis if gut barrier function has already been compromised (13, 33, 34, 53).
To address these limitations, we developed a novel probiotic formulation strategy that delivers probiotics in a more stable biofilm state rather than the traditional administration of planktonic, free-living bacteria (44, 47). To create a probiotic biofilm, cultures of the probiotic Lactobacillus reuteri (Lr) are introduced to porous, biocompatible, biodegradable, ~50-µm diameter dextranomer microspheres (DM). During a brief incubation period, DM provide a surface for rapid Lr adherence and biofilm formation (Fig. 1). Probiotics in their biofilm state have increased resistance to gastric pH and laminar/turbulent fluid forces compared with planktonic bacteria (52). In addition to providing a surface for Lr biofilm formation, DM are particularly useful because they can be preloaded (because of their porosity) with substances that contribute to probiotic growth and further promote biofilm formation. Disaccharides that would normally be rapidly diluted, metabolized, and absorbed in the duodenum remain undiluted in the confines of DM, where they slowly diffuse out and are delivered directly, selectively, and in high concentration to probiotic bacteria adhered to the DM surface, whereas nonadhered bacteria receive no benefit.
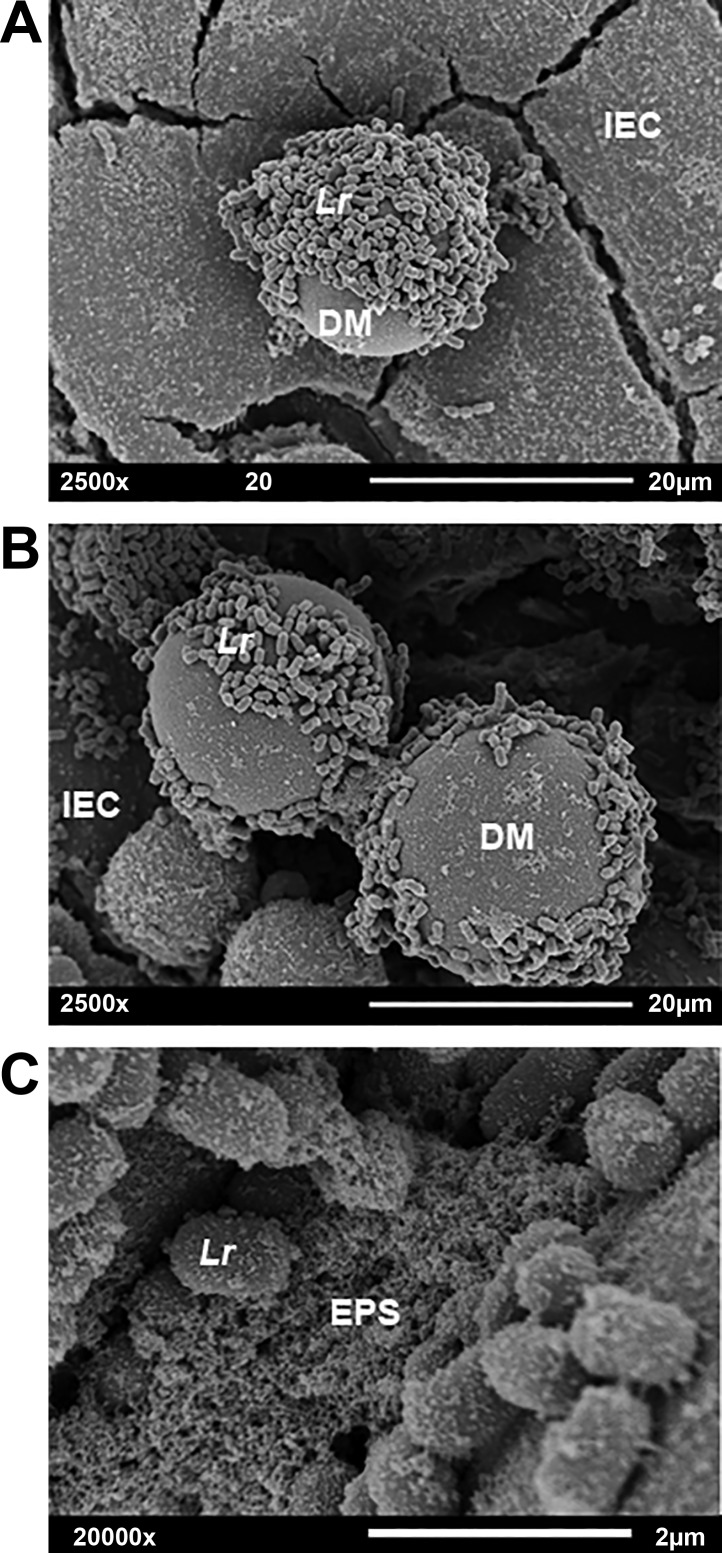
Fig. 1.
Scanning electron microscopy of Lr + DM. Samples of Lr + DM were incubated on the surface of DLD-1 human colonic epithelial cells for 1 h. All nonadhered bacteria were removed by washing, and the remaining samples were fixed, stained, dehydrated, sputter coated with gold and palladium, and imaged with scanning electron microscopy. A: Lr adhered to DM, 2500× magnification. B: Lr-produced EPS is seen joining two microspheres. C: image B at 20,000× magnification, more clearly demonstrating the EPS. DM, dextranomer microsphere; EPS, extracellular polymeric substance; IEC, intestinal epithelial cell; Lr, Lactobacillus reuteri.
Our initial results showed that in contrast to a single dose of free-living Lr, a single dose of Lr administered in its biofilm state (Lr + DM) decreased the incidence of experimental NEC by 50% in a neonatal rat model of the disease (47). These results were in marked contrast to other studies, in which the beneficial effects of planktonic Lr are maintained only by repeated Lr administration (35, 37). Given these findings, we sought to improve Lr + DM efficacy further by investigating potential substances that could be preloaded into DM before incubation with Lr.
In the current study, we sought to determine whether enhanced formulations could further protect the intestines from NEC and improve pup survival. We also evaluated intestinal mucosal barrier function and markers of intestinal inflammation to begin to determine how Lr protects the intestines from NEC. Additionally, we sought to assess the roles of DM and the glucosyltransferase enzyme GtfW. GtfW is a strain-specific, cell-associated extracellular enzyme involved in Lr biofilm formation. GtfW has two domains: a catalytic domain that transfers a glucose moiety from its sole substrate, maltose, to a polysaccharide made of glucose monomers (glucan), and a glucan-binding domain that binds to the aforementioned growing glucan chain. Moreover, the glucan-binding domain of GtfW also enables the bacterial cell to adhere to other chemically similar glucans, such as the cross-linked dextran found in DM. Furthermore, the gtfW gene is upregulated in the presence of sucrose (44). Given the critical role of GtfW for Lr adherence to DM, we hypothesized that the absence of GtfW would abrogate the protective effects of Lr in vivo.
Preparatory in vitro studies demonstrated that preloading DM with sucrose (DM-Sucr) or maltose (DM-Malt) enhanced Lr biofilm formation, significantly increased Lr adherence to human intestinal epithelial cells, and prolonged Lr survival in acidic pH (44). These improved results were specific for the disaccharides sucrose or maltose and not observed with their monosaccharide subunits, glucose and fructose. Such in vitro results suggested that Lr + DM-Sucr and Lr + DM-Malt may represent superior formulations compared with the original Lr + DM treatments tested in our previous studies (47).
Here we set out to determine whether strains of Lr or Lr adhered to DM with tunable cargos could effectively prevent NEC and to what degree biofilm formation played a role. In addition, we determined if these same treatments would alter the endogenous microbiome during a NEC insult, with a particular focus on whether Lr + DM-Malt would limit the expansion potential of Proteobacteria commonly observed during NEC.
METHODS
Neonatal rat model of experimental NEC.
All animal studies were conducted in compliance with protocol #AR15–00012 approved by the Institutional Animal Care and Use Committee of The Research Institute at Nationwide Children’s Hospital. Sprague-Dawley rat pups at 20.5 days gestational age were delivered from timed-pregnant dams (Envigo, Indianapolis, IN) via cesarean section under CO2 anesthesia. Immediately after delivery, pups were randomized into experimental groups that received a single enteral dose of Lr or control treatment via gastric gavage. Pups were then subjected to our well-established model of experimental NEC (47, 49), which is a modification of a stress protocol used to induce NEC that was first introduced by Barlow et al. in 1974 (6, 7). In short, pups were subjected to repeated episodes of the following: 1) hypertonic, hypercaloric formula feeds via orogastric gavage 5 times daily with 15 g Similac 60/40 (Abbott Nutrition, Columbus, OH) in 75 mL of Esbilac (Pet-Ag, New Hampshire, IL), providing a combined 836.8 kJ·kg−1·day−1; 2) 3 episodes of hypoxia and hypothermia each day (placement in a chamber of N2 gas calibrated to fraction of inspired oxygen <1.5% for 90 s directly followed by placement in a 4°C environment for 10 min); and 3) gastric gavage of 2 mg/kg lipopolysaccharide (Sigma-Aldrich, St. Louis, MO) on the first day of life. Between each of these episodes, pups were housed in an incubator at 35°C. Breast-fed control pups were placed with a surrogate dam immediately after cesarean delivery and were not exposed to experimental stress.
Lr biofilm preparation and administration.
Human feces-derived Lr 23272 (also commonly cited as DSM 20016) was purchased from American Type Culture Collection (ATCC, Manassas, VA) and grown in de Man, Rogosa, and Sharpe (MRS) broth (Fisher Scientific, Pittsburg, PA) (14) overnight at 37°C under 5% CO2. For planktonic Lr administration, Lr was pelleted and resuspended in sterile 0.9% saline; 100 µl of the treatment was administered to neonatal pups via gastric gavage for a final dose of 2 × 108 colony forming units (CFU)/pup (a dose consistent with other published studies) (70). For Lr administered in its biofilm state, Lr was introduced to DM before administration as described previously (44). In short, sterile, dry DM (Sephadex G-25 Superfine, GE Healthcare Bio-Sciences, Pittsburgh, PA) were hydrated in water at 50 mg/mL and then autoclaved for 20 min. For treatment groups that contained sucrose or maltose, DM were removed from solution and collected into 1 mL of a sterile 1 M solution of the sugar. The solution was vortexed and incubated at room temperature for 24 h. DM were then removed from the solution using a vacuum filter and aseptically scraped with a sterile loop into a tube containing resuspended bacteria. Lr was allowed to incubate with DM for 30 min at room temperature to facilitate binding and biofilm formation. Pups were then gavaged with 100 µl of the bacteria-DM solution, resulting in a final dose of 2 × 108 CFU/pup and 0.5 mg DM/pup.
Incidence and severity of experimental NEC.
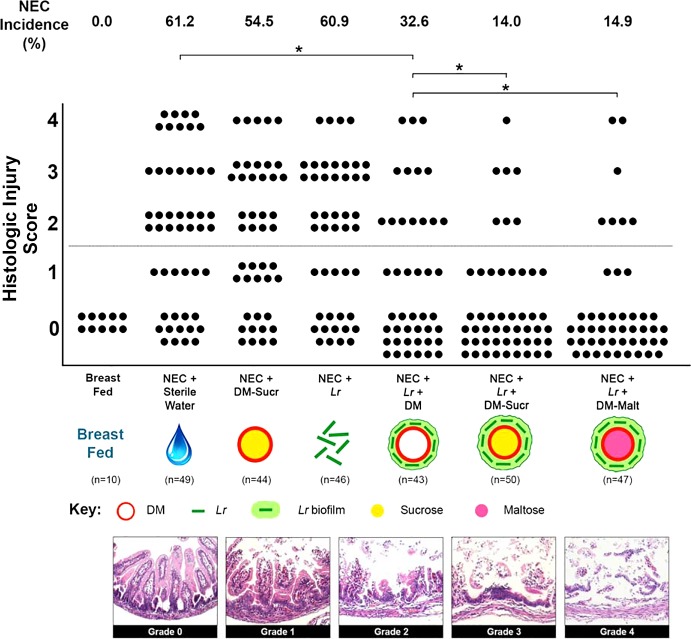
Immediately after delivery, pups were randomly divided into 1 of 7 experimental groups that received one of the following treatments via gastric gavage: 1) 100 µl sterile water (vehicle control; n = 49); 2) 0.5 mg DM-Sucr (n = 44); 3) 2 × 108 CFU Lr (n = 46); 4) 2 × 108 CFU Lr + 0.5 mg DM (n = 43); 5) 2 × 108 CFU Lr + 0.5 mg DM-Sucr (n = 50); or 6) 2 × 108 CFU Lr + 0.5 mg DM-Malt (n = 47). An additional group of pups was returned to surrogate dams and served as unstressed breast-fed controls. After receiving their single treatment dose, all pups (with the exception of the unstressed breast-fed controls) were subjected to the experimental NEC protocol. When signs of NEC developed (i.e., bloody stools, severe abdominal distention, lethargy, respiratory distress, cyanosis), pups were euthanized. All remaining pups were euthanized 96 h after delivery. Upon euthanasia, intestinal tissue was harvested and fixed in 10% formalin for 24 h. Fixed tissue was paraffin embedded, and then hematoxylin and eosin-stained transverse sections were prepared. Two independent observers graded each section in a blinded fashion using an established histologic injury grading scale initially established by Caplan et al. (10, 47, 49). Histologic injury was classified as follows: grade 0, no visible histological villus damage; grade 1, distal villus enterocyte detachment; grade 2, sloughing of enterocytes to the midvillus level; grade 3, loss of entire villus with preservation of the crypts; and grade 4, transmural necrosis (Fig. 2). Experimental NEC was defined as an injury score of grade 2 or higher; severe NEC was defined as an injury score of grade 3 or 4.
Fig. 2.
Incidence and severity of NEC. Rat pups were delivered prematurely, subjected to the experimental NEC protocol, and euthanized when signs of clinical NEC developed or after 96 h. Each dot represents a single rat pup with their histologic injury score depicted. H&E stained intestinal tissue sections demonstrate the following grades of histologic injury: grade 0, no visible histological villus damage; grade 1, distal villus enterocyte detachment; grade 2, sloughing of enterocytes to the midvillus level; grade 3, loss of the entire villus with preservation of the crypts; and grade 4, transmural necrosis. Grade 2 injury and above are consistent with histologic NEC. NEC incidence for each experimental group of pups is indicated. All images are 20× magnification. *P < 0.05, Kruskal-Wallis test followed by Dunn’s multiple comparisons test. DM, dextranomer microspheres; DM-Malt, DM with maltose; DM-Sucr, DM with sucrose; H&E, hematoxylin-eosin; Lr, Lactobacillus reuteri; NEC, necrotizing enterocolitis.
Intestinal permeability.
Immediately after delivery, pups were randomized to receive one of the following: 1) 100 µl sterile water (vehicle control; n = 20); 2) 2 × 108 CFU Lr (n = 20); 3) 2 × 108 CFU Lr + 0.5 mg DM-Sucr (18); or 4) 2 × 108 CFU Lr + 0.5 mg DM-Malt (n = 15). An additional group of pups was returned to surrogate dams and served as unstressed breast-fed controls. All pups (with the exception of the unstressed breast-fed controls) were then subjected to the experimental NEC protocol for 48 h, at which time each received 1,500 mg/kg of fluorescein isothiocyanate-labeled dextran (FD70, molecular weight 70,000; Sigma-Aldrich) suspended in sterile PBS via orogastric gavage. Pups were euthanized 4 h later, and serum was collected into BD Microtainer SST tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). Serum was extracted and fluorescence measured with a fluorescent plate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA) using a 492/518 nm filter set. The plasma concentration of FD70 for each pup was then extrapolated using a standard curve generated from a 1:2 serial dilution of a known FD70 concentration.
DNA isolation and 16S rRNA gene sequencing.
Immediately after delivery, pups were randomly divided into 1 of 6 experimental groups that received one of the following treatments via gastric gavage: 1) 100 µl sterile water (vehicle control); 2) 0.5 mg DM-Malt; 3) 2 × 108 CFU Lr; or 4) 2 × 108 CFU Lr + 0.5 mg DM-Malt. Additional groups included the following: 5) pups that were returned to surrogate dams and served as unstressed breast-fed controls and 6) pups that were unstressed but formula fed. After receiving their single treatment dose, all pups (with the exception of groups 5 and 6) were subjected to the experimental NEC protocol (n = 5–8/group, total n = 32). Fecal samples were collected on days 2, 3, and 4 of the 4-day NEC protocol, with a minimum of three samples per group per day analyzed. DNA was isolated using the QIAamp DNA stool kit (Qiagen, Hilden, Germany) according to manufacturer recommendations. Prior to library submission, DNA was checked for quality through gel electrophoresis. Next, a DNA library was constructed using the Fluidigm Access Array System in the Molecular and Cellular Imagine Center at The Ohio State University. After library construction, 250 × 250 paired end reads from the V4-V5 hypervariable region of the 16S rRNA gene (515F FGAGTGCCAGCMGCCGCGGTAA and 806R ACGGACTACHVGGGTWTCTAAT) were sequenced using a MiSeq2000 (Illumina, San Diego, CA).
Sequencing and downstream microbiome analysis.
High-quality sequencing data (>25) from forward and reverse reads were analyzed using the IM-TORNADO40 pipeline. Briefly, IM-TORNADO merges trimmed paired end reads into a single multiple alignment, obtains taxa calls, and clusters sequences into operational taxonomic units at 97% similarity from the Greengenes database. After taxa file generation, downstream diversity and taxonomic analysis was performed through QIIME 1.9.1 and R statistical software. β-diversity (unweighted UniFrac) analysis (averaged across rat pups from day 2 to day 4 of life) were computed at an even sampling depth of 4,500 sequences/sample based on α-diversity rarefaction plots and compared for group differences through permutational analysis of variance. Genera representation as percentage of total 16S rRNA gene (relative abundance) was used for all taxa level analyses.
Scanning electron microscopy.
Scanning electron microscopy was performed using a Hitachi S-4800 field emission microscope (Hitachi, Tokyo, Japan). Samples were prepared as previously described (44). Briefly, samples of Lr + DM were incubated on the surface of DLD-1 human colonic epithelial cells (ATCC CCL-221) grown on 15-mm diameter thermanox coverslips (Electron Microscopy Sciences, Hatfield, PA) for 1 h, and nonadhered bacteria were removed by washing. The remaining bacteria, DM, and DLD-1 cells were then fixed, washed, and stained twice with a 1% solution of tetroxide (Sigma-Aldrich). Samples were dehydrated using a graded series of ethanol at 25, 50, 70, 95, and 100% ethanol, followed by final immersion in 100% hexamethyldisilazane (Sigma-Aldrich), and air-dried overnight. Dehydrated sample coverslips were then mounted onto 15-mm diameter metal SE specimen stubs (Electron Microscopy Sciences) using colloidal silver (Electron Microscopy Sciences) and sputter coated with gold and palladium for 2 min at 25 mA using an Emitech K550X sputter coater (Quorum Technologies, Laughton, UK).
RNA isolation and quantitative real-time PCR for inflammatory gene expression.
Neonatal rat pups were delivered via cesarean section and subjected to experimental NEC. When signs of NEC developed, pups were euthanized, and intestinal tissue was harvested and formalin fixed as described above. Total RNA was isolated from paraffin-embedded tissue sections using RNeasy FFPE kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. cDNA was generated from 2 μg RNA using the SuperScript VILO cDNA synthesis kit (ThermoFisher, Waltham, MA). Quantitative (q) real-time PCR was performed in duplicate with primers for rat IL-6, IL-1β, CCL2, CXCL-1, and IL-10 (Qiagen) and SYBR Green PCR Master mix (ThermoFisher) on an ABI PRIZM 7900 machine (ThermoFisher). Target gene expression was normalized to GAPDH endogenous control and expressed as relative copy number using the ΔΔCT method.
qPCR of Lr.
Lr presence was quantified from fecal samples of Lr and Lr + DM-Malt treated pups throughout NEC treatment (n = 5 pups per group per day). Standards were developed from pure culture of the experimental Lr ATCC 23272 (108 CFU) and diluted in a 10-fold series dilution. qPCR primers (F: 5′ CCACTTGCTAAGGAGGTTGC 3′; R: 5′ GGCAGCCATTAAGGGTGTAA 3′) were developed against the click beetle luciferase probe inserted into ATCC 23272. Primers were tested against local alignment search tool (BLAST) and fulfilled criteria required for specificity. qPCR analysis was carried out on a Applied Biosystems Quant Studio 3 PCR machine with Power SYBR Green master mix in a 30 μL reaction with 250 nM of each primer and 2.5 μL of extracted DNA. qPCR conditions were as follows: 95°C for 10 min followed 40 cycles of 95°C for 15 s and 59°C (annealing) for 1 min. Samples and standards were run in duplicate. Amplicon specificity was again verified by melt curve analysis, and efficiency of amplification was verified through monoculture Lr ATCC 23272 spiked samples. Lr ATCC 23272 abundance was quantified in amplicons per gram of contents.
ΔGtfW strain of Lr.
A GtfW-deficient strain of Lr was generated as previously described (44). In short, a gtfW knockout plasmid was constructed and introduced into Lr ATCC 23272 by electroporation. Immediately after electroporation, cells were resuspended in MRS, incubated, and then serially diluted onto MRS agar containing 5 µg/mL chloramphenicol. The mutant strain was then selected using previously published methods (44). To estimate gtfW promoter (PgtfW) transcription, the PgfW-CBluc reporter plasmid was constructed and transformed into Lr 23272 to create the reporter strain LMW501.
Statistics.
Unless otherwise noted, all data are expressed as mean ± SE. NEC severity between groups was analyzed using the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. NEC incidence was compared by chi-square analysis. For animal survival, a log-rank test was performed. Intestinal permeability and RNA expression were assessed by one-way analysis of variance followed by Tukey’s multiple comparison test. For RNA expression, the interquartile range method was used to identify outliners. Microbiome composition was compared across NEC scores using stepwise linear regression analysis. Microbiome principle components determined from unweighted UniFrac [(PC1-PC3 (62.23% of total variance explained)] were used as independent variables in regression model. Lactobacillus spp. and Lr ATCC 23272 + click beetle luciferase abundance across NEC treatments was analyzed by a 2 × 2 (time × treatment) analysis of variance comparing Lr and Lr + DM-Malt groups. Enterobacter spp. presence was analyzed across NEC treatments by a similar 2 × 2 (time × treatment) analysis of variance comparing Lr and Lr + DM-Malt groups to other controls. Tukey’s honest significant difference post-hoc was used to compare individual group differences. Pearson correlation analysis was used to compare Lactobacillus spp. abundance to NEC injury grade at the time of euthanasia. Statistical analyses were performed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA) and SAS 9.4 software (SAS Institute, Cary, NC). Statistical significance was defined as P ≤ 0.05.
RESULTS
Lr biofilm formulation decreases NEC incidence and severity.
Nearly two thirds of pups subjected to the experimental NEC protocol and treated with sterile water only developed NEC (Fig. 2). The severity of NEC for stressed pups that received DM-Sucr alone or planktonic Lr alone was not significantly different from stressed pups that received sterile water only (P > 0.999 and P > 0.999, respectively). However, compared with pups that received sterile water alone, pups that received Lr + DM experienced lower NEC injury (P = 0.021), whereas pups that received a single dose of Lr + DM-Sucr or Lr-DM-Malt experienced the lowest NEC incidence [14% (P < 0.001) and 15% (P < 0.001), respectively]. Importantly, both Lr + DM-Sucr and Lr + DM-Malt resulted in an even lower NEC incidence compared with Lr + DM (P = 0.033 and P = 0.048, respectively). No unstressed breast-fed pups developed NEC.
Lr biofilm formulation improves rat pup survival.
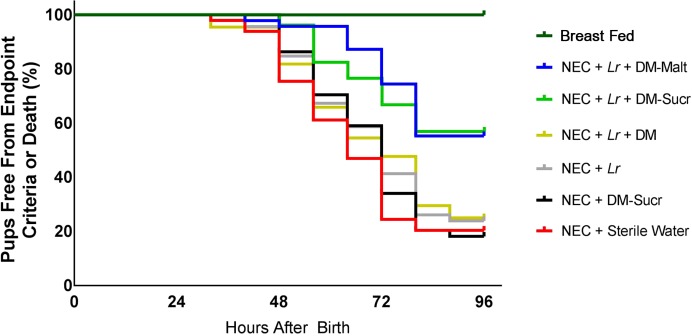
All unstressed breast-fed pups survived the entire 96 h protocol (Fig. 3). In contrast, only 20.4% of untreated stressed pups subjected to the protocol were alive and free from end-point criteria (lethargy, bloody stools, agonal breathing, cyanosis) after 96 h. Stressed pups treated with DM-Sucr (18.2%), Lr (23.9%), or Lr + DM (25.6%) displayed no difference in survival compared with untreated stressed pups. However, pups that were treated with a single dose of Lr + DM-Sucr or Lr + DM-Malt exhibited superior survival compared with untreated pups. Pups treated with Lr + DM-Sucr displayed 58.0% survival (hazard ratio of 2.62 with 95% confidence interval 1.57–4.37), whereas pups treated with Lr + DM-Malt exhibited 55.3% survival (hazard ratio of 2.88 with 95% confidence interval of 1.72–4.84).
Fig. 3.
Rat pup survival. The number of pups alive and free from end-point criteria (lethargy, bloody stools, agonal breathing, cyanosis) are depicted for each experimental group in 8 h intervals over the course of the 96 h experimental NEC protocol. Statistical analysis via log-rank test. DM, dextranomer microspheres; DM-Malt, DM with maltose; DM-Sucr, DM with sucrose; Lr, Lactobacillus reuteri; NEC, necrotizing enterocolitis.
Lr biofilm formulation decreases intestinal mucosal permeability.
After 48 h of the experimental NEC protocol, untreated pups experienced higher intestinal permeability compared with breast-fed control pups, as demonstrated by elevated serum levels of FD70 4 h after enteral FD70 administration (31.99 ± 6.5 µg/mL vs. 2.22 ± 0.3 µg/mL; P = 0.001) (Fig. 4). Although pups treated with planktonic Lr alone displayed numerically lower serum FD70 levels (17.32 ± 5.0 µg/mL; P = 0.117), pups treated with a single dose of Lr + DM-Sucr or Lr + DM-Malt had significantly lower levels of serum FD70 (10.83 ± 1.2 µg/mL; P = 0.009 and 8.98 ± 3.2 µg/mL; P = 0.006).
Fig. 4.
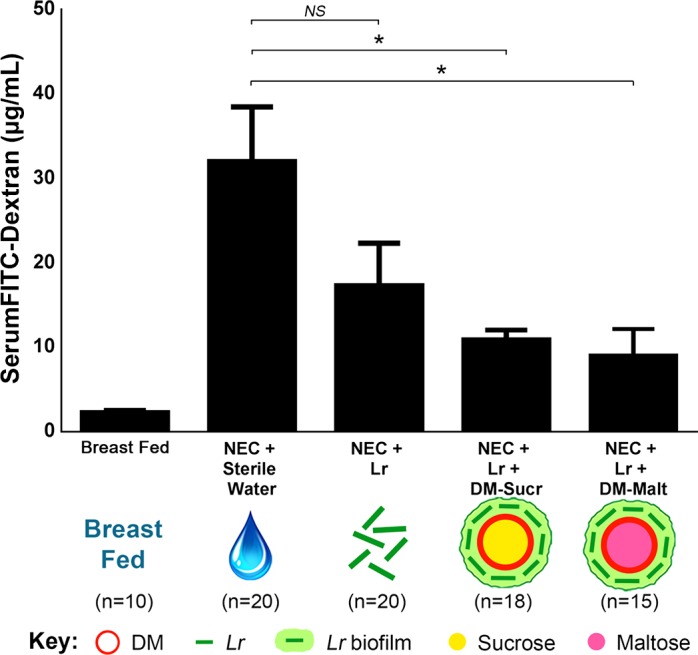
Intestinal permeability of rat pups subjected to experimental NEC. Intestinal permeability was determined by measuring serum levels of FITC dextran 4 h after gastric administration, with greater levels of serum FITC dextran indicating increased intestinal permeability. *P < 0.05, one-way ANOVA followed by Tukey’s multiple comparison test. DM, dextranomer microspheres; DM-Malt, DM with maltose; DM-Sucr, DM with sucrose; FITC, fluorescein isothiocyanate; Lr, Lactobacillus reuteri; NEC, necrotizing enterocolitis; NS, no significance.
Lr biofilm formulation decreases small intestine inflammatory cytokine production.
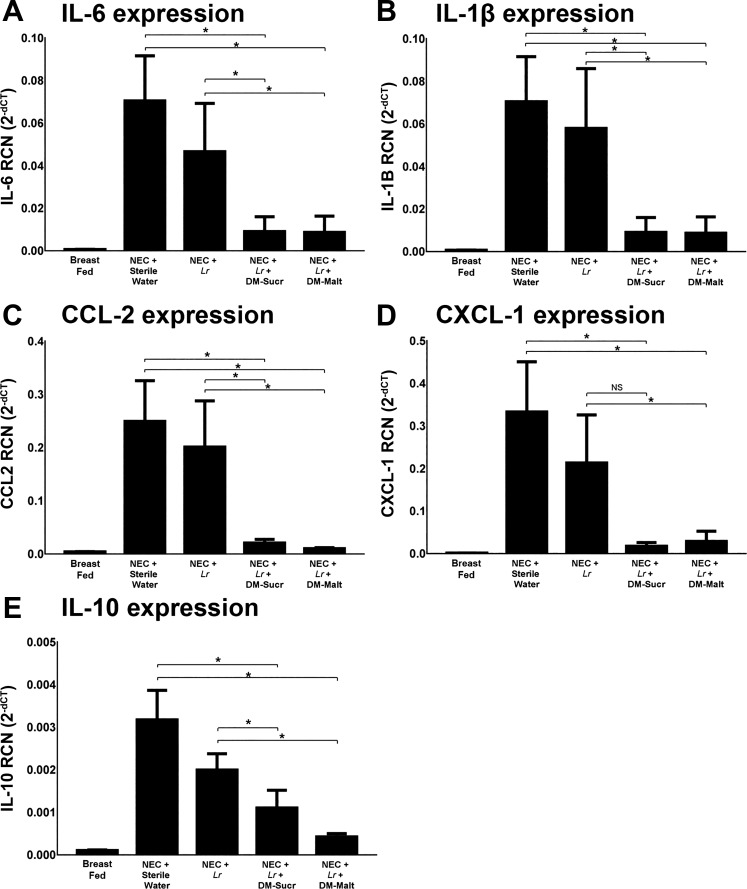
Untreated pups subjected to experimental NEC experienced significant elevation of IL-6 (P < 0.002), IL-1β (P < 0.002), CCL-2 (P < 0.001), CXCL-1 (P < 0.001), and IL-10 (P < 0.002) expression within small intestinal tissue compared with breast-fed unstressed pups (Fig. 5). However, compared with untreated pups, expression of IL-6, IL-1β, CCL-2, CXCL-1, and IL-10 were substantially lower when pups were treated with a single dose of Lr + DM-Sucr (P = 0.010, P = 0.010, P = 0.001, P = 0.012, and P = 0.035, respectively) or Lr + DM-Malt (P = 0.009, P = 0.009, P = 0.001, P = 0.008, and P = 0.010, respectively). Compared with pups treated with planktonic Lr, the administration of Lr + DM-Sucr led to lower expression of IL-6 (P = 0.006), IL-1β (P = 0.005), CCL-2 (P = 0.043), and IL-10 (P = 0.028); CXCL-1 expression was not significantly lower (P = 0.083). Compared with pups treated with planktonic Lr, the administration of Lr + DM-Malt led to lower expression of IL-6 (P = 0.001), IL-1β (P = 0.002), CCL-2 (P = 0.003), CXCL-1 (P = 0.017), and IL-10 (P < 0.001).
Fig. 5.
Small intestine inflammatory gene expression. RNA was isolated and analyzed with real-time quantitative PCR for the expression of IL-6 (A), IL-1β (B), CCL-2 (C), CXCL-1 (D), and IL-10 (E). Results represent the mean ± SE of 7–10 different rat pups, performed in duplicate. *P < 0.05, one-way ANOVA followed by Tukey’s multiple comparison test. DM, dextranomer microspheres; DM-Malt, DM with maltose; DM-Sucr, DM with sucrose; Lr, Lactobacillus reuteri; NEC, necrotizing enterocolitis; NS, no significance; RCN, relative copy number.
Lr biofilm formulation preserves the natural intestinal microbiome.
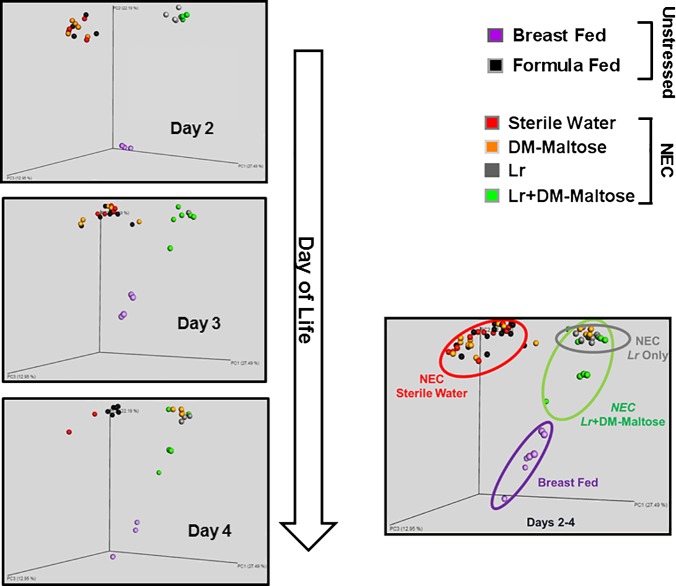
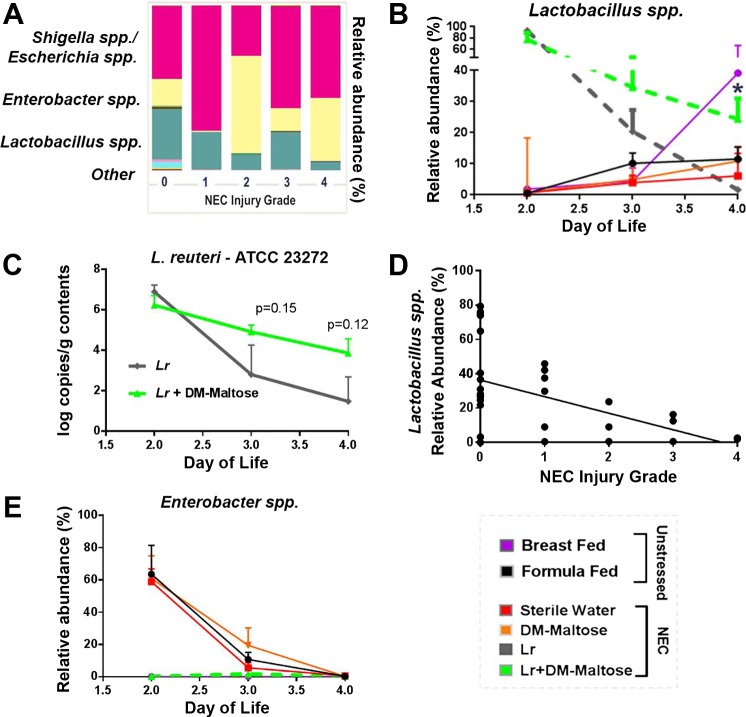
16S rRNA gene sequencing analysis revealed that gut microbiota community structure and taxa composition were differentially affected by Lr and Lr + DM-Malt administration. Unweighted UniFrac analysis (average of all samples over days 2–4 of life) revealed distinct clustering of stressed pups treated with Lr (gray circles) or stressed pups receiving Lr + DM-Malt (green circles) away from unstressed formula-fed pups (black circles), stressed pups that received sterile water only (red circles), or stressed pups that received DM-Malt (orange circles) (Fig. 6; permutational analysis of variance P < 0.05, respectively). Additionally, the microbiota community arrangement of Lr + DM-Malt pups (green circles) clustered more closely with that of vaginally delivered breast-fed controls (purple circles) compared with pups treated with planktonic Lr (Fig. 6). Taxa-level analysis revealed that the microbiota of both unstressed and stressed pups were dominated by three bacterial genera (Lactobacillus spp., Escherichia/Shigella spp., and Enterobacter spp.) at each NEC injury grade (Fig. 7A). Microbial community composition was significantly different based on NEC injury grade [microbiome principle components (PC1-PC3) vs. NEC injury grade, β = −0.560; P < 0.001]. When comparing changes in these taxa throughout NEC treatment among groups, Lactobacillus spp. levels were highly represented at day 2 of life in both Lr and Lr + DM-Malt groups (>80%, respectively, vs. <5% in all other groups), which then declined during day 3 and day 4 of life (Fig. 7B). However, Lactobacillus spp. abundance was maintained more effectively in Lr + DM-Malt treated pups compared with Lr treated pups, the former of which contained Lactobacillus spp. levels close to that of unstressed breast-fed controls (30–40%) at day 4 of life (Fig. 7B; time × Lr treatment P < 0.05). Importantly, Lactobacillus spp. abundance was negatively associated with NEC injury grade at the time of euthanasia (Fig. 7D; Pearson r = −0.480, P = 0.010). qPCR was also used to investigate whether the experimental Lr strain persisted longer in Lr + DM-Malt fed pups. Here, Lr ATCC 23272 was numerically (but not significantly) higher in the feces of pups fed Lr + DM-Malt compared with pups fed Lr only, particularly at day 3 and day 4 of life (Fig. 7C; Lr group main effect P = 0.11). Lastly, despite the differences in Lr and Lr + DM-Malt in maintaining Lactobacillus levels during NEC, both treatments were effective at limiting the abundance of the potential enteric pathogen Enterobacter spp. (averaged across days 2–4 of treatment) compared with unstressed formula-fed control pups or with stressed pups that received sterile water only or DM-Malt (Fig. 7E; Tukey honest significant difference, P < 0.05).
Fig. 6.
Microbiome β-diversity throughout NEC. Principle coordinates analysis (PCoA) based on the unweighted UniFrac phylogenetic distance metric. Left: circles represent microbiota community structure of individual rat pups throughout day 2–day 4 of life (day 2, n = 3–7/group; day 3, n = 3–6/group; day 4, n = 3–7/group). Right: combined PCoA represents all samples collected in rat pups combined across time points (days 2–4). Colored ovals are placed as visual indicators depicting clustering of Lr + DM-Malt with unstressed breast-fed controls. Two × 2 (time × treatment) ANOVA. DM, dextranomer microspheres; DM-Malt, DM with maltose; Lr, Lactobacillus reuteri; NEC, necrotizing enterocolitis.
Fig. 7.
Microbiome composition throughout NEC treatment. A: fecal genera representation expressed as relative abundance (% of total 16S rRNA gene reads) compared across NEC injury grades at day of euthanasia. B: Lactobacillus spp. abundance is maintained more effectively in Lr + DM-Malt treated pups compared with planktonic Lr treated pups throughout the NEC experimental protocol. (n = 5–8 per group per day). *ANOVA time × Lr group interaction at P < 0.05 comparing Lr and Lr + DM-Malt groups only. C: Lr (ATCC 23272 + CBluc) concentration in feces 2–4 days after probiotic inoculation in Lr and Lr + DM-Malt fed pups exposed to experimental NEC as measured by quantitative PCR (n = 5 per group per day). Lr group × time F2,28 = 1.67, P = 0.28; time main effect: F2,28 = 11.78, P < 0.01; Lr group: F1,28 = 2.71, P = 0.11. Tukey honest significant difference was used to compare Lr ATCC 23272 concentrations among groups within each day of life. P values are designated above day 3 (P = 0.15) and day 4 (P = 0.12) of life on figure. D: Lactobacillus spp. abundance at day of rat pup euthanasia is negatively associated with NEC injury grade (Spearman’s Rho = −0.480; P = 0.01). E: Enterobacter spp. presence (n = 5–8 per group per day) across NEC treatment as measured by 16S rRNA gene sequencing [relative abundance (% of total bacteria)]. Lr and Lr + DM-Malt groups exhibit low Enterobacter spp. presence across days 2 and 3 of NEC treatment compared with controls [sterile water (NEC), DM-Malt (NEC)] as well as formula fed unstressed pups. Lr group main effect *P < 0.05. ATCC, American Type Culture Collection; DM, dextranomer microspheres; DM-Malt, DM with maltose; Lr, Lactobacillus reuteri; NEC, necrotizing enterocolitis.
Biofilm-deficient Lr have decreased ability to prevent NEC.
In this experiment, stressed pups that received water alone had an NEC incidence of 65% (Fig. 8). In contrast, stressed pups that received either Lr + DM-Sucr or Lr + DM-Malt had a significantly lower NEC incidence of 21% (P = 0.001) and 22% (P = 0.004), respectively. However, the protective effects of Lr were not seen with administration of Lr-Sucr or Lr-Malt alone (DM-deficient treatments), which had NEC incidences of 52% (P > 0.999) and 51% (P > 0.999), respectively, or with administration of ΔGtfW + DM-Sucr or ΔGtfW + DM-Malt (GtfW-deficient treatments), which had NEC incidences of 50% (P > 0.999) and 41% (P = 0.666), respectively (Fig. 8). Compared with Lr + DM-Sucr, the incidence of severe NEC (injury grades 3 or 4) was significantly higher for Lr + Sucr (P = 0.004) or ΔGtfW + DM-Sucr (P = 0.016). Similarly, compared with Lr + DM-Malt, the incidence of severe NEC was higher for Lr + Malt (P = 0.003) or ΔGtfW + DM-Malt (P = 0.035). No unstressed breast-fed pups developed NEC.
Fig. 8.
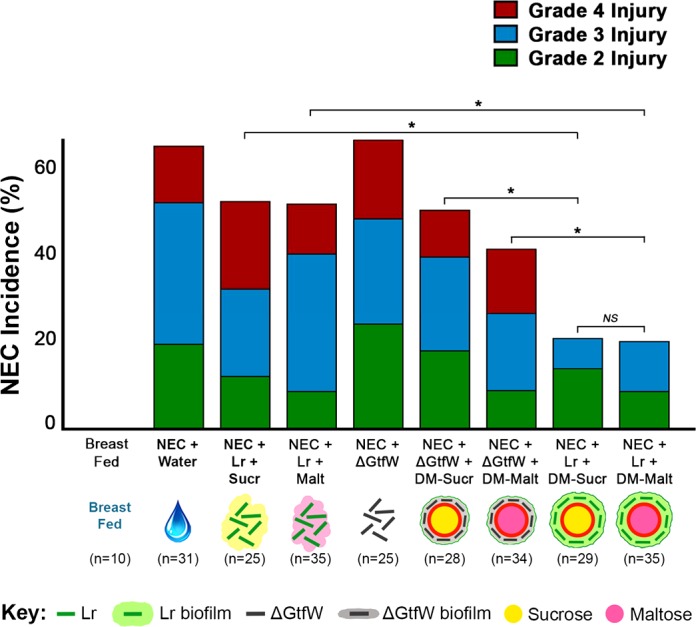
Incidence and severity of NEC with Lr GtfW mutation. Rat pups were delivered prematurely, given a single enteral treatment as indicated, and then subjected to the experimental NEC protocol. Pups were euthanized when signs of clinical NEC developed or after 96 h, intestinal tissue was harvested, and H&E sections were graded to determine the extent of intestinal damage. The incidence of NEC for each experimental group of pups is shown. For each treatment group, the percentage of pups with grade 2, grade 3, and grade 4 injury are depicted. *P < 0.05, Kruskal-Wallis test followed by Dunn’s multiple comparisons test. DM, dextranomer microspheres; DM-Malt, DM with maltose; DM-Sucr, DM with sucrose; H&E, hematoxylin-eosin; Lr, Lactobacillus reuteri; NEC, necrotizing enterocolitis.
DISCUSSION
In the current study, we have shown that administration of a single dose of Lr adhered to DM in a biofilm state can be significantly enhanced with the addition of either sucrose or maltose as diffusible cargo to the DM lumen. In addition to reducing NEC incidence, Lr + DM-Sucr and Lr + DM-Malt improved animal survival, reduced intestinal mucosal barrier breakdown, and limited intestinal inflammation. Furthermore, Lr + DM-Malt augmented Lactobacillus spp. persistence in the intestinal tract and shifted the composition of the gut microbial community to be more similar to the breast-fed community.
Lr was originally isolated from human breast milk (60) and is present in healthy human intestine (51, 61). The strain of Lr used for our current studies was clade II Lr ATCC 23272 (also known as DSM 20016), and it possesses both anti-inflammatory and antimicrobial capabilities (56). Lr ATCC 23272 downregulates both cytokine and chemokine production by colonic epithelial cells stimulated with Citrobacter rodentium (38, 39). Lr has also been shown to reduce intestinal inflammation in both juvenile and adult animals (36, 55). Additionally, Lr produces antimicrobial compounds such as reuterin (31), which is derived from the substrate glycerol. Reuterin inhibits the growth of numerous pathogenic microorganisms, such as Gram-positive bacteria, Gram-negative bacteria, fungi, and protozoa (58). Furthermore, Lr readily forms biofilms (community architectures of bacteria adhered to a surface) in which the bacteria are encased in a self-produced matrix of extracellular polymeric substance. In addition, Lr has great affinity for the cross-linked dextran of DM (dependent on the GtfW enzyme), which results in excellent binding and subsequent biofilm formation (44). For these reasons, along with the accumulating evidence that Lr is beneficial in human diseases such as colic (54), diarrhea (22), IgE-mediated eczema (1), and NEC (27), Lr was chosen for use in the current experiments.
The beneficial effects of Lr adhered to microspheres may be linked to alterations in the endogenous gut microbiota. Administering Lr + DM-Malt maintained Lactobacillus spp. relative abundances throughout NEC treatment. We hypothesize that these effects stem directly from enhanced biofilm production, which gives Lr a greater ability to withstand acidic environments and adhere to intestinal epithelial cells (44), potentially allowing Lr to integrate more fully into the host microbial community.
Given the dysbiosis observed in preterm infants who go on to develop NEC (66), an ability to effectively alter and maintain the host microbiome in a beneficial way would hold great promise in the prevention of NEC. Changes in the gut microbial community, such as the increasing prevalence of Proteobacteria (which includes many commonly observed Gram-negative pathogens) (40), have been reported in infants before the onset of NEC (12). One large observational, prospective study showed an increased proportion of Gammaproteobacteria and decreased proportion of Negativicutes in infants that went on to develop NEC compared with control infants (66). A separate systematic review provided similar findings, demonstrating an increase in Proteobacteria and a decrease in Firmicutes and Bacteroidetes preceding NEC in preterm infants, providing further evidence that dysbiosis is central to the development of NEC (48).
In the current study, microbiota characteristics in NEC-stressed rats paralleled what has been observed previously in humans. For instance, Enterobacter spp. and Shigella/Escherichia spp. (class Gammaproteobacteria, family Enterobacteriaceae) represented over 50% of the genera in NEC-stressed rats and together were associated with higher NEC injury grade. Importantly, these genera have also been strongly linked with gastrointestinal inflammation and, in some cases, directly implicated as causative agents for NEC in humans (42, 65). For example, a metagenomic study in 144 preterm and 22 term infants identified numerous microbial genes associated with uropathogenic Escherichia coli that contributed to NEC pathogenesis and mortality. Moreover, the pathogen Enterobacter sakazakii has been definitively linked to NEC outbreaks through contamination of powdered milk formula (28, 43). In the present study, both Lr and Lr + DM-Malt treatments limited Enterobacter spp. expansion, providing evidence that Lr treatment attenuates NEC-induced microbiota profiles that parallel dysbiosis in humans with NEC. Moreover, a higher Lactobacillus spp. abundance (more effectively maintained in the Lr + DM-Malt treated pups) was negatively associated with NEC injury grade, indicating a role of this taxa in preventing disease pathogenesis. Future studies are warranted to determine which specific Lactobacillus species and strains (including both endogenous and delivered) are maintained by the Lr + DM-Malt treatment.
Lr adherence to DM is central to its improved protective effects seen in this study. Lr is able to adhere to DM through the expression of glucan-binding proteins. Previous results from our laboratory demonstrate that Lr lacking GtfW (which contains a glucan-binding domain) have diminished capacity to adhere to DM, whereas in another study, inhibiting a similar enzyme (GtfA) in another Lr strain reduced its ability to aggregate, form biofilms, and colonize the GI tract (44, 62). Here, we expand on these findings to show that the absence of GtfW abolishes the ability of the Lr + DM treatment to attenuate NEC severity, highlighting a key role of this biofilm-forming enzyme in adherence to DM and its subsequent effects in vivo. Of importance, few bacteria make glucan homopolymers that facilitate binding to DM. In our previous studies, the pathogens Escherichia coli, Salmonella typhimurium, and Clostridium difficile did not detectably bind to DM (44), making it unlikely that administration of DM would provide a scaffold on which pathogenic bacteria can create an active biofilm.
One commonly cited drawback to any type of probiotic administration is the potential for bacteremia. Several case reports exist of patients who received prophylactic probiotic treatments and subsequently developed bacteremia (though not necessarily symptomatic) (30, 45, 71). Bacteremia is presumably a result of administering bacteria, albeit probiotic, each day to an injured intestine with an immature immune system, a problem that runs counter to the intent of our treatment. We are optimistic that our novel Lr administration strategy will reduce the ability of probiotic bacteria to translocate into the host bloodstream. First, our therapeutic is given with just a single dose, predictably in advance of intestine damage; daily dosing of probiotics run the risk of administration at a time when there are already breaches in mucosal integrity. Second, we have shown that intestinal barrier breakdown is prevented with administration of our Lr biofilm formulation. This is consistent with others that have shown that Lr can help maintain a functional mucosal barrier even with severe intestinal inflammation (15). Third, we hypothesize that prophylactic Lr administration allows the probiotic to establish itself in the gut, averting some of the damage to the gut seen with the development of NEC. Furthermore, Lr administered in this delivery method are in their stable biofilm state. Once Lr adherence and biofilm expression has occurred, over 500 × g centrifugal force is required to dissociate Lr from DM in vitro (unpublished data). The tight binding of Lr to DM potentially makes Lr less likely to translocate through a weakened intestinal mucosal barrier. Determining more specific answers to some of these questions regarding bacterial translocation will be the focus of future investigations.
Importantly, the DM used in this study was also chosen for its ability to be safely used in a weakened gut. DM are biodegradable, nonimmunogenic, nonmutagenic, nonallergenic, and Generally Recognized As Safe by the FDA. They have been used in numerous FDA-approved medical products to date, including Solesta, a bulking gel injected submucosally in the anal canal for treatment of fecal incontinence (25); Debrisan, a cicatrizant wound dressing (29); and Deflux, a bulking gel used to treat vesicoureteral reflux (57). These long-standing uses of DM provide evidence for safety in human administration. Furthermore, the DM lumen can be filled with compounds useful to Lr but otherwise limited in vivo, which diffuse over time directly to Lr adhered to DM (Lr + DM) as they transit the GI tract after enteral administration.
The use of biofilm-promoting DM loaded with utilizable substrate represents an exciting development in the improvement of probiotic administration. The information provided from this current study, namely that biofilm-promoting probiotic formulations may be superior to planktonic probiotic administration and that such formulations can be “tuned” with apropos luminal cargo to enhance specific probiotic effects, provide great insight. As we continue to learn more about the human microbiome and its interaction with health and disease, probiotic administration will likely play an increasingly prominent role in disease prevention and management. This novel treatment strategy may not only contribute to improved prevention of clinical NEC in the future but may be applicable to other areas of emerging interest for probiotic administration including C. difficile colitis (46), inflammatory bowel disease (67), obesity (41), and colorectal cancer (4). Management of these and multiple other disease states stands to benefit greatly from superior probiotic biofilm administration strategies.
GRANTS
This work was supported by National Institutes of Health Grants R01-GM-123482 (to G. Besner, S. Goodman, and M. Bailey) and R41-GM-122130 (to G. Besner and S. Goodman) and American College of Surgeons Grant 82048615 (to J. Olson).
DISCLOSURES
G. Besner, S. Goodman, and M. Bailey are Scientific Founders and hold stock options in Scioto Biosciences, Inc. They are also inventors on US2017/0209504 A1 (Probiotic Formulations and Methods for Use) and US provisional application 15/649,352 (Prebiotic Formulations). None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.K.O., J.B.N., J.M.A., V.A.V., M.T.B., S.D.G., and G.E.B. conceived and designed research; J.K.O., J.B.N., J.M.A., C.J.M., Y.W., and V.A.V. performed experiments; J.K.O., J.B.N., J.M.A., L.M.-W., V.A.V., M.T.B., S.D.G., and G.E.B. analyzed data; J.K.O., J.B.N., J.M.A., C.J.M., L.M.-W., Y.W., V.A.V., M.T.B., S.D.G., and G.E.B. interpreted results of experiments; J.K.O., J.B.N., J.M.A., Y.W., and G.E.B. prepared figures; J.K.O., J.B.N., J.M.A., L.M.-W., M.T.B., S.D.G., and G.E.B. drafted manuscript; J.K.O., J.B.N., J.M.A., C.J.M., L.M.-W., Y.W., V.A.V., M.T.B., S.D.G., and G.E.B. edited and revised manuscript; J.K.O., J.B.N., J.M.A., C.J.M., L.M.-W., Y.W., V.A.V., M.T.B., S.D.G., and G.E.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Melissa Moore-Clingenpeel from the Biostatistics Core of the Research Institute at Nationwide Children’s Hospital for assistance with statistical analysis.
REFERENCES
- 1.Abrahamsson TR, Jakobsson T, Böttcher MF, Fredrikson M, Jenmalm MC, Björkstén B, Oldaeus G. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 119: 1174–1180, 2007. doi: 10.1016/j.jaci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol 65: 351–354, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev (4): CD005496, 2014. doi: 10.1002/14651858.CD005496.pub4. [DOI] [PubMed] [Google Scholar]
- 4.Ambalam P, Raman M, Purama RK, Doble M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol 30: 119–131, 2016. doi: 10.1016/j.bpg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Arciero J, Bard Ermentrout G, Siggers R, Afrazi A, Hackam D, Vodovotz Y, Rubin J. Modeling the interactions of bacteria and Toll-like receptor-mediated inflammation in necrotizing enterocolitis. J Theor Biol 321: 83–99, 2013. doi: 10.1016/j.jtbi.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery 77: 687–690, 1975. [PubMed] [Google Scholar]
- 7.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis–the importance of breast milk. J Pediatr Surg 9: 587–595, 1974. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol 182: 1595–1606, 2013. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berrington JE, Stewart CJ, Embleton ND, Cummings SP. Gut microbiota in preterm infants: assessment and relevance to health and disease. Arch Dis Child Fetal Neonatal Ed 98: F286–F290, 2013. doi: 10.1136/archdischild-2012-302134. [DOI] [PubMed] [Google Scholar]
- 10.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol 14: 1017–1028, 1994. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 11.Cassir N, Simeoni U, La Scola B. Gut microbiota and the pathogenesis of necrotizing enterocolitis in preterm neonates. Future Microbiol 11: 273–292, 2016. doi: 10.2217/fmb.15.136. [DOI] [PubMed] [Google Scholar]
- 12.Claud EC, Keegan KP, Brulc JM, Lu L, Bartels D, Glass E, Chang EB, Meyer F, Antonopoulos DA. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 1: 20, 2013. doi: 10.1186/2049-2618-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr Infect Dis J 24: 278–280, 2005. doi: 10.1097/01.inf.0000154588.79356.e6. [DOI] [PubMed] [Google Scholar]
- 14.de Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of Lactobacilli. J Appl Microbiol 23: 130–135, 1960. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- 15.Dicksved J, Schreiber O, Willing B, Petersson J, Rang S, Phillipson M, Holm L, Roos S. Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. PLoS One 7: e46399, 2012. doi: 10.1371/journal.pone.0046399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding WK, Shah NP. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J Food Sci 72: M446–M450, 2007. doi: 10.1111/j.1750-3841.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 17.Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, Vikram A, Bibby K, Morowitz MJ, Firek B, Lu P, Hackam DJ. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 306: G1021–G1032, 2014. doi: 10.1152/ajpgi.00452.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grishin A, Papillon S, Bell B, Wang J, Ford HR. The role of the intestinal microbiota in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg 22: 69–75, 2013. doi: 10.1053/j.sempedsurg.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome 2: 38, 2014. doi: 10.1186/2049-2618-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr 28: 19–25, 1999. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, Krabshuis J, Lemair T, Kaufmann P, de Paula JA, Fedorak R, Shanahan F, Sanders ME, Szajewska H, Ramakrishna BS, Karakan T, Kim N; World Gastroenterology Organization . World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol 46: 468–481, 2012. doi: 10.1097/MCG.0b013e3182549092. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez-Castrellon P, Lopez-Velazquez G, Diaz-Garcia L, Jimenez-Gutierrez C, Mancilla-Ramirez J, Estevez-Jimenez J, Parra M. Diarrhea in preschool children and Lactobacillus reuteri: a randomized controlled trial. Pediatrics 133: e904–e909, 2014. doi: 10.1542/peds.2013-0652. [DOI] [PubMed] [Google Scholar]
- 23.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 30: 61–67, 2000. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med 60: 111–124, 2009. doi: 10.1146/annurev.med.60.050207.092824. [DOI] [PubMed] [Google Scholar]
- 25.Hoy SM. Dextranomer in stabilized sodium hyaluronate (Solesta®): in adults with faecal incontinence. Drugs 72: 1671–1678, 2012. doi: 10.2165/11209030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis 3: 197–202, 1999. doi: 10.1016/S1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 27.Hunter C, Dimaguila MA, Gal P, Wimmer JE Jr, Ransom JL, Carlos RQ, Smith M, Davanzo CC. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight < 1000 grams: a sequential analysis. BMC Pediatr 12: 142, 2012. doi: 10.1186/1471-2431-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter CJ, Petrosyan M, Ford HR, Prasadarao NV. Enterobacter sakazakii: an emerging pathogen in infants and neonates. Surg Infect (Larchmt) 9: 533–539, 2008. doi: 10.1089/sur.2008.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsson S, Jonsson L, Rank F, Rothman U. Studies on healing of Debrisan-treated wounds. Scand J Plast Reconstr Surg 10: 97–101, 1976. doi: 10.3109/02844317609105196. [DOI] [PubMed] [Google Scholar]
- 30.Jenke A, Ruf EM, Hoppe T, Heldmann M, Wirth S. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch Dis Child Fetal Neonatal Ed 97: F217–F218, 2012. doi: 10.1136/archdischild-2011-300838. [DOI] [PubMed] [Google Scholar]
- 31.Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9: 35, 2009. doi: 10.1186/1471-2180-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleessen B, Bunke H, Tovar K, Noack J, Sawatzki G. Influence of two infant formulas and human milk on the development of the faecal flora in newborn infants. Acta Paediatr 84: 1347–1356, 1995. doi: 10.1111/j.1651-2227.1995.tb13567.x. [DOI] [PubMed] [Google Scholar]
- 33.Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr 38: 457–458, 2004. doi: 10.1097/00005176-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115: 178–181, 2005. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Fatheree NY, Dingle BM, Tran DQ, Rhoads JM. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One 8: e56547, 2013. doi: 10.1371/journal.pone.0056547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 299: G1087–G1096, 2010. doi: 10.1152/ajpgi.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol 302: G608–G617, 2012. doi: 10.1152/ajpgi.00266.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackos AR, Eubank TD, Parry NM, Bailey MT. Probiotic Lactobacillus reuteri attenuates the stressor-enhanced severity of Citrobacter rodentium infection. Infect Immun 81: 3253–3263, 2013. doi: 10.1128/IAI.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackos AR, Galley JD, Eubank TD, Easterling RS, Parry NM, Fox JG, Lyte M, Bailey MT. Social stress-enhanced severity of Citrobacter rodentium-induced colitis is CCL2-dependent and attenuated by probiotic Lactobacillus reuteri. Mucosal Immunol 9: 515–526, 2016. doi: 10.1038/mi.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 6: e20647, 2011. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molinaro F, Paschetta E, Cassader M, Gambino R, Musso G. Probiotics, prebiotics, energy balance, and obesity: mechanistic insights and therapeutic implications. Gastroenterol Clin North Am 41: 843–854, 2012. doi: 10.1016/j.gtc.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, Altaye M, Wagner M, Gevers D, Ward DV, Kennedy MA, Huttenhower C, Newburg DS. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 1: 13, 2013. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mshana SE, Gerwing L, Minde M, Hain T, Domann E, Lyamuya E, Chakraborty T, Imirzalioglu C. Outbreak of a novel Enterobacter sp. carrying blaCTX-M-15 in a neonatal unit of a tertiary care hospital in Tanzania. Int J Antimicrob Agents 38: 265–269, 2011. doi: 10.1016/j.ijantimicag.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Navarro JB, Mashburn-Warren L, Bakaletz LO, Bailey MT, Goodman SD. Enhanced probiotic potential of Lactobacillus reuteri when delivered as a biofilm on dextranomer microspheres that contain beneficial cargo. Front Microbiol 8: 489, 2017. doi: 10.3389/fmicb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohishi A, Takahashi S, Ito Y, Ohishi Y, Tsukamoto K, Nanba Y, Ito N, Kakiuchi S, Saitoh A, Morotomi M, Nakamura T. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J Pediatr 156: 679–681, 2010. doi: 10.1016/j.jpeds.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 46.Ollech JE, Shen NT, Crawford CV, Ringel Y. Use of probiotics in prevention and treatment of patients with Clostridium difficile infection. Best Pract Res Clin Gastroenterol 30: 111–118, 2016. doi: 10.1016/j.bpg.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Olson JK, Rager TM, Navarro JB, Mashburn-Warren L, Goodman SD, Besner GE. Harvesting the benefits of biofilms: a novel probiotic delivery system for the prevention of necrotizing enterocolitis. J Pediatr Surg 51: 936–941, 2016. doi: 10.1016/j.jpedsurg.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 48.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, Engstrand L, Lilja HE, Hollister EB, Versalovic J, Neu J. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5: 31, 2017. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg 51: 942–947, 2016. doi: 10.1016/j.jpedsurg.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16: 658–672, 2003. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol 2: 43–53, 2001. [PubMed] [Google Scholar]
- 52.Salas-Jara MJ, Ilabaca A, Vega M, García A. Biofilm forming Lactobacillus: new challenges for the development of probiotics. Microorganisms 4: E35, 2016. doi: 10.3390/microorganisms4030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salminen MK, Tynkkynen S, Rautelin H, Saxelin M, Vaara M, Ruutu P, Sarna S, Valtonen V, Järvinen A. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis 35: 1155–1160, 2002. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]
- 54.Schreck Bird A, Gregory PJ, Jalloh MA, Risoldi Cochrane Z, Hein DJ. Probiotics for the treatment of infantile colic: a systematic review. J Pharm Pract, 30: 366–374, 2017. doi: 10.1177/0897190016634516. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber O, Petersson J, Phillipson M, Perry M, Roos S, Holm L. Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. Am J Physiol Gastrointest Liver Physiol 296: G534–G542, 2009. doi: 10.1152/ajpgi.90470.2008. [DOI] [PubMed] [Google Scholar]
- 56.Spinler JK, Sontakke A, Hollister EB, Venable SF, Oh PL, Balderas MA, Saulnier DM, Mistretta TA, Devaraj S, Walter J, Versalovic J, Highlander SK. From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol 6: 1772–1789, 2014. doi: 10.1093/gbe/evu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stenberg A, Läckgren G. A new bioimplant for the endoscopic treatment of vesicoureteral reflux: experimental and short-term clinical results. J Urol 154: 800–803, 1995. doi: 10.1016/S0022-5347(01)67168-4. [DOI] [PubMed] [Google Scholar]
- 58.Talarico TL, Dobrogosz WJ. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob Agents Chemother 33: 674–679, 1989. doi: 10.1128/AAC.33.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Underwood MA, Arriola J, Gerber CW, Kaveti A, Kalanetra KM, Kananurak A, Bevins CL, Mills DA, Dvorak B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr Res 76: 326–333, 2014. doi: 10.1038/pr.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urbańska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr 173: 1327–1337, 2014. doi: 10.1007/s00431-014-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 70: 1176–1181, 2004. doi: 10.1128/AEM.70.2.1176-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walter J, Schwab C, Loach DM, Gänzle MG, Tannock GW. Glucosyltransferase A (GtfA) and inulosucrase (Inu) of Lactobacillus reuteri TMW1.106 contribute to cell aggregation, in vitro biofilm formation, and colonization of the mouse gastrointestinal tract. Microbiology 154: 72–80, 2008. doi: 10.1099/mic.0.2007/010637-0. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 47: 241–248, 2012. doi: 10.1016/j.jpedsurg.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944–954, 2009. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward DV, Scholz M, Zolfo M, Taft DH, Schibler KR, Tett A, Segata N, Morrow AL. Metagenomic sequencing with strain-level resolution implicates uropathogenic E. coli in necrotizing enterocolitis and mortality in preterm infants. Cell Reports 14: 2912–2924, 2016. doi: 10.1016/j.celrep.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, Shaikh N, Hoffmann JA, Linneman LA, Hamvas A, Khanna G, Rouggly-Nickless LC, Ndao IM, Shands BA, Escobedo M, Sullivan JE, Radmacher PG, Shannon WD, Tarr PI. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387: 1928–1936, 2016. doi: 10.1016/S0140-6736(16)00081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wasilewski A, Zielińska M, Storr M, Fichna J. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm Bowel Dis 21: 1674–1682, 2015. doi: 10.1097/MIB.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 68.Weng M, Ganguli K, Zhu W, Shi HN, Walker WA. Conditioned medium from Bifidobacteria infantis protects against Cronobacter sakazakii-induced intestinal inflammation in newborn mice. Am J Physiol Gastrointest Liver Physiol 306: G779–G787, 2014. doi: 10.1152/ajpgi.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weng M, Walker WA. The role of gut microbiota in programming the immune phenotype. J Dev Orig Health Dis 4: 203–214, 2013. doi: 10.1017/S2040174412000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu SF, Chiu HY, Chen AC, Lin HY, Lin HC, Caplan M. Efficacy of different probiotic combinations on death and necrotizing enterocolitis in a premature rat model. J Pediatr Gastroenterol Nutr 57: 23–28, 2013. doi: 10.1097/MPG.0b013e3182929210. [DOI] [PubMed] [Google Scholar]
- 71.Zbinden A, Zbinden R, Berger C, Arlettaz R. Case series of Bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology 107: 56–59, 2015. doi: 10.1159/000367985. [DOI] [PubMed] [Google Scholar]