Abstract
Necrotizing enterocolitis (NEC) is associated with low plasma arginine and vascular dysfunction. It is not clear whether low intestinal citrulline production, the precursor for arginine synthesis, occurs before and thus predisposes to NEC or if it results from tissue damage. This study was designed to test the hypothesis that whole body rates of citrulline, arginine, and nitric oxide synthesis are low in premature pigs and that they precede NEC. Piglets delivered by cesarean section at 103 days [preterm (PT)], 110 days [near-term (NT)], or 114 days [full-term (FT)] of gestation were given total parenteral nutrition and after 2 days orogastrically fed infant formula for 42 h to induce NEC. Citrulline and arginine fluxes were determined before and during the feeding protocol. Gross macroscopic and histological NEC scores and plasma fatty acid binding protein (iFABP) concentration were determined as indicators of NEC. Intestinal gene expression for enzymes of the arginine pathway were quantitated. A lower (P < 0.05) survival rate was observed for PT (8/27) than for NT (9/9) and FT pigs (11/11). PT pigs had higher macroscopic gross (P < 0.05) and histological NEC (P < 0.05) scores and iFABP concentration (P < 0.05) than pigs of more advanced gestational age. PT pigs had lower citrulline production and arginine fluxes (P < 0.05) throughout and a reduced gene expression in genes of the citrulline-arginine pathway. In summary, intestinal enzyme expression and whole body citrulline and arginine fluxes were reduced in PT pigs compared with animals of more advance gestational age and preceded the development of NEC.
NEW & NOTEWORTHY Arginine supplementation prevents necrotizing enterocolitis (NEC), the most common gastrointestinal emergency of prematurity. Citrulline (precursor for arginine) production is reduced during NEC, and this is believed to be a consequence of intestinal damage. In a swine model of NEC, we show that intestinal gene expression of the enzymes for citrulline production and whole body citrulline and arginine fluxes are reduced and precede the onset of NEC in premature pigs. Reduced citrulline production during prematurity may be a predisposition to NEC.
Keywords: arginine, citrulline, necrotizing enterocolitis, nitric oxide, prematurity
INTRODUCTION
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in premature infants. NEC affects 1–3 live births per 1,000 in North America, and mortality reaches ~50% in the most severe cases (36, 45). Prematurity, microbial dysbiosis, and formula feeding are prominent risk factors for NEC that lead to intestinal edema, hemorrhage, leukocyte infiltration, transmural necrosis, and gaseous build-up (pneumatosis intestinalis) (31, 32, 46). NEC pathophysiology is not thoroughly described and is likely multifactorial. Feeding infant formula versus human milk and the colonization by a dysbiotic microbiota seem to be necessary elements in the pathogenesis of NEC (23, 35, 38). A major factor in the premature host intestine that has been linked to NEC susceptibility is innate immunity, especially the expression and activity of Toll-like receptor signaling (11, 30, 32). Modeling the elements of prematurity in animals can be challenging, but the preterm piglet replicates these key elements of human NEC, including spontaneous development with infant formula feeding (39, 43).
There is a strong indication that inflammation that occurs with NEC is preceded by intestinal hypoxia (7, 17, 33, 34); indeed, early observations showed that hypoxia in piglets reproduced NEC-like lesions (48). Furthermore, hypoxia is necessary for the development of most rodent models of NEC (43). More recently, using noninvasive, continuous abdominal near-infrared spectroscopy, it was shown in preterm piglets that low intestinal oxygenation precedes the onset of NEC (18, 52). This potential link between hypoxia and NEC pathogenesis has implicated nitric oxide (given its role in endothelial relaxation), inhibition of platelet aggregation, and modulation of inflammation (5, 10, 21, 25). Nitric oxide is produced from arginine by three different nitric oxide synthase (NOS) isoenzymes that are associated with inflammation and NEC in human infants (16, 51). Importantly, arginine supplementation has been shown to successfully reduce NEC incidence in clinical trials (1, 14, 37, 41) and animal studies (13).
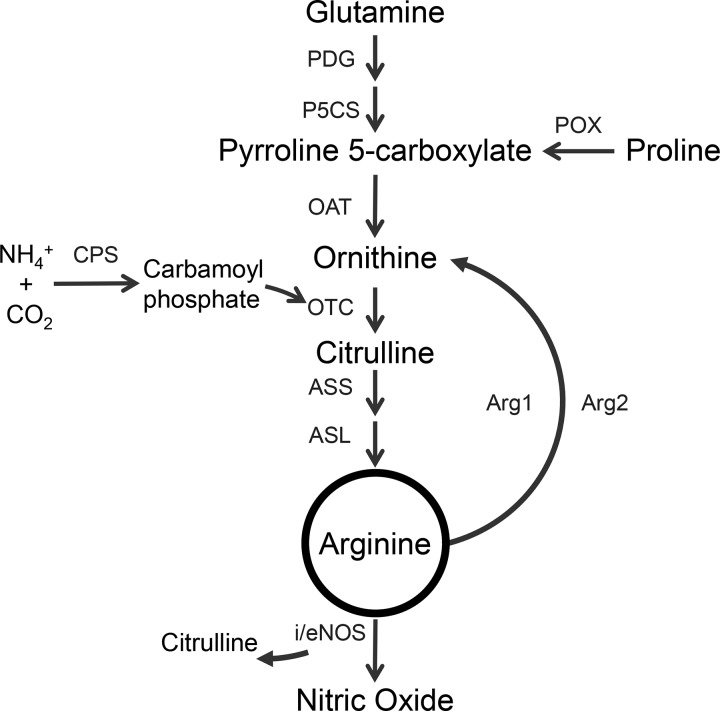
Arginine is a conditionally indispensable amino acid synthesized from glutamine and proline (Fig. 1) that may be especially important for preterm and low birth weight infants (50). Furthermore, low plasma arginine concentration has been reported in infants with NEC (3, 42). Arginine is synthesized de novo from citrulline, a nonprotein amino acid made primarily by enterocytes that has been used as a biomarker of intestinal health (12). Gut-derived citrulline circulates to the kidney where it is converted to arginine by argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL; Fig. 1) (29). Arginine is largely catabolized to ornithine (and urea) by two arginase (Arg1/2) isoenzymes in liver and immune cells (Arg1), as well as kidney and gut (Arg2). Although a small fraction of arginine is utilized for the production of nitric oxide, an adequate arginine availability is needed to sustain the production of this molecule.
Fig. 1.
Enzymes involved in the production and catabolism of arginine. Arg1, arginase 1; Arg2, arginase 2; ASL, argininosuccinate lyase; ASS, argininosuccinate synthase; CPS, carbamoyl phosphate synthetase; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; OAT, ornithine aminotransferase; OTC, ornithine transcarbamylase; P5CS, pyrroline 5-carboxylate synthase; PDG, phosphate-dependent glutaminase; POX, proline oxidase.
The aim of this study was to investigate whether arginine metabolism is regulated by gestational age during the perinatal period and whether this is associated with the development of NEC in the neonatal piglet model. The underlying hypothesis tested was that whole body rates of citrulline, arginine, and nitric oxide synthesis are low in premature pigs and that they precede NEC.
MATERIALS AND METHODS
Animals and treatments.
Six commercial crossbred sows were transported to the Children’s Nutrition Research Center 6–8 days before delivery by cesarean section. Piglets were delivered at 103 days gestation [preterm (PT)], 110 days gestation [near-term (NT)], or 114 days gestation [full-term (FT)], followed by insertion of catheters into the jugular vein and the umbilical artery. During surgery, 0.3 ml of blood were sampled into a K2 EDTA tube for a complete blood count analysis. Piglets were also fitted with an orogastric feeding tube (6-Fr, Portex, Smith Medical, Dublin, OH) and placed into heated acrylic incubators at 31–32°C for the remainder of the study, as described elsewhere (19, 44, 52). To establish an enteral morphological and gene expression baseline, intestinal tissues were collected from additional PT (n = 6), NT (n = 5), and FT (n = 6) pigs immediately after birth. These newborn pigs were delivered by cesarean section, resuscitated, and euthanized within 3–4 h of birth. All animal procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine.
NEC protocol.
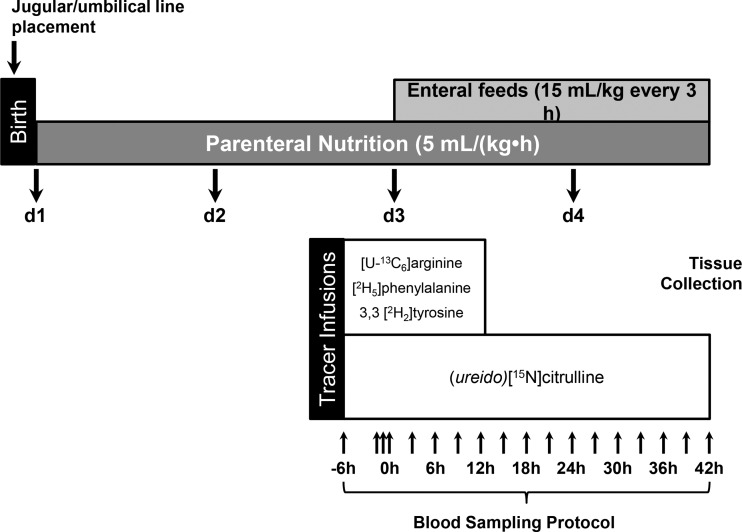
The NEC protocol, shown schematically in Fig. 2, has been described in more detail elsewhere (19, 39, 52). Briefly, total parenteral nutrition (TPN) was initiated immediately after line placement at 5 ml/(kg·h) on day 1. Parenteral nutrition provided (kg/day) 410 kJ energy, 12.5 g dextrose, 6.5 g amino acids, and 2.5 g fat (Intralipid 20%; Fresenius Kabi, Bad Homburg, Germany) (44). Maternal sow plasma was provided intravenously at 4, 5, and 7 ml/kg at 6, 12, and 22 h after birth, respectively, to provide passive immunity. Parenteral nutrition was maintained at this rate throughout the study, and on day 3, enteral feeding commenced at 15 ml/kg administered every 3 h for 42 h (Fig. 2). Enteral infant formula (Pepdite Junior, Nutricia, Gaithersburg, MD) plus additional whey protein isolate and medium chain triacylglyceride oil was administered at room temperature via orogastric tube. Enteral formula provided (kg/d) 487 kJ energy, 7.6 g protein (mainly whey protein isolate), 5.9 g carbohydrate (corn syrup solids), 7.3 g fat, and minerals and vitamins (22, 39). Rectal temperatures and signs of distress (i.e., emesis, blood oxygen saturation, bloody diarrhea, distension) were monitored closely throughout the study. Piglets were euthanized at the end of the 42-h enteral feeding protocol or when there was strong clinical suspicion that a piglet developed NEC (e.g., abdominal distension, bloody diarrhea, fever, emesis, low oxygen saturation, etc.) (19, 39, 52). Samples of proximal jejunum, distal ileum, kidney, and colon were frozen and fixed in 10% formalin.
Fig. 2.
A schematic diagram detailing the study protocol. After cesarean section delivery, piglets were provided total parenteral nutrition (TPN) until day 3 when additional enteral feeding was initiated. Tracers were infused with TPN starting 6 h before the initiation of enteral feeding. Blood was sampled regularly to generate time-course kinetic profiles of these piglets. Piglets were euthanized after they were diagnosed with necrotizing enterocolitis (NEC) or on the morning of day 5, and tissues were scored for NEC and collected for subsequent analysis.
NEC scoring.
Anatomical NEC severity was assessed at necropsy, and a histological NEC evaluation was done later on formalin-fixed intestinal sections (n = 19/27 for PT sections because of poor integrity during processing). A gross NEC score was assigned by visually scoring different gastrointestinal segments between 1 and 6; a macroscopic lesion score of ≥3 in any segment was considered NEC. Gross scoring was based on the presence of inflammation, necrosis, and pneumatosis intestinalis (4, 19). Gross NEC score was used to stratify animals as fulminant NEC (positive NEC score throughout the bowel), colonic NEC (NEC lesions localized to the distal bowel), and NEC resistant (for piglets without lesions). Histological NEC score was performed on hematoxylin-eosin-stained sections of proximal jejunum, distal ileum, and colon (4, 8). Sections were assigned a histological NEC severity score (0–4) based on the degree of epithelial and/or mucosal damage; a tissue score of ≥3 indicated necrosis and/or pneumatosis. Histological scoring was assessed by a trained observer who was blinded to animal identifiers. A histological NEC severity score of ≥2 was considered NEC. In addition, a biochemical marker for enterocyte damage, intestinal fatty acid binding protein (iFABP), was measured in the last plasma sample before necropsy.
Isotope infusions and blood sampling.
Isotope infusions commenced 6 h before the onset of enteral feeds (0 h) and were delivered with the TPN solution at a rate of 1 ml/(kg·h) (Fig. 2). The primed continuous infusion of (ureido)[15N]citrulline (0.6 µmol/kg and 0.6 µmol/kg·h, respectively) was given between −6 and 42 h. Additionally, piglets received primed continuous infusions of [U-13C6]arginine (prime: 14.5 µmol/kg; continuous infusion: 14.5 µmol/kg·h), (ring)[2H5]phenylalanine (prime: 13.5 µmol/kg; continuous infusion: 13.5 µmol/kg·h), and 3,3 [2H2]tyrosine (prime: 1.5 µmol/kg; continuous infusion: 1.5 µmol/kg·h) from −6 to 12 h (Fig. 2). Blood was collected into K2EDTA tubes during the tracer infusion just before each oral feed bolus, immediately centrifuged at 4°C for 10 min at 5,000 g, and plasma was frozen and stored at −80°C.
Enteroid culture.
Intestinal crypts were isolated from the proximal jejunum of newborn cesarean-derived, 103-day gestational age, PT (n = 2), and from 10-day-old term born, vaginally-delivered, sow-reared pigs (n = 3). Crypt isolation and growth parameters were based on methods described by others (40). Briefly, 1–2-cm sections of jejunum were flushed and rinsed with phosphate buffered saline (PBS) containing gentamicin, penicillin, and streptomycin. Crypt units were dispersed by shaking for 90 min in EDTA-chelating buffer at 4°C. Crypt units were dispersed in PBS fetal bovine serum and centrifuged at 150 g for 5 min at 4°C. The cell pellet was suspended in 150 µl Matrigel (Corning Biosciences, Tewksbury, MA), and 30 µl drops were plated. Matrigel was solidified by incubating the 24-well plate in a 37°C incubator (5% CO2) for 10 min. Complete media with growth factor (CMGF+) was added to each well, and plates remained in the 37°C incubator. Media was obtained from the Digestive Disease Center (Texas Medical Center, Houston, TX) and was composed by DMEM/12-containing key growth factors, including Wnt3a-conditioned media (mouse cell line obtained from ATCC, Manassas, VA), Rspo1-Fc-conditioned media (the Rspo1-Fc-expressing mouse cell line was a kind gift from Dr. Calvin Kuo, Stanford University), Noggin-conditioned media (mouse cell line was a kind gift from Dr. V. Van den Brink, Tytgat Institute), human recombinant EGF (R&D Systems, Minneapolis, MN), and human Gastrin I (Sigma-Aldrich, St. Louis, MO). Enteroid cultures were monitored daily and passaged when the enteroids began to bud prolifically, usually 7 days after plating. Intestinal crypts were maintained in proliferation media (CMGF+) for 6 days to promote the development of undifferentiated enteroid structures. On day 6, enteroid cultures were either maintained in proliferation media (CMGF+) or differentiation media (CMGF−, lacking Wnt3a) for 3 days (10 wells/group). Citrulline production was measured in fresh media over 24 h. The DNA content per well was assessed with Hoechst 33258 dye and measured by fluorescence spectrophotometry (excitation: 350 nm, emission: 473 nm). Citrulline concentration was measured in culture media.
Sample analysis.
Complete blood cell count was measured at birth (day 1) and at the end of the study. Plasma amino acid isotopic enrichments were determined as their dansyl derivatives by LC-MS/MS utilizing a TSQ Quantum Ultra System (Thermo Finnigan, San Jose, CA) as previously described (28). Citrulline concentration in culture media was quantified by isotope dilution relative to [5-13C, 4,4,5,5 2H4]citrulline internal standard. Amino acid concentrations in plasma and feed were measured using phenyl isothiocyanate derivatives relative to a methionine sulfone internal standard by HPLC (6). iFABP was measured using a human iFABP Quantikine ELISA Kit (R&D Systems).
RNA was extracted from proximal jejunum, distal ileum, and kidney with TRIzol (Thermo Fisher Scientific, Waltham, MA). Gene expression (Ct) was measured using SYBRGreen chemistry (Applied Biosystems, Branchburg, NJ) and calculated relative to β-actin expression by ΔΔCt (26). Primer sequences are presented in Table 1. Intestinal tissue was fixed in 10% buffered formalin and hematoxylin-eosin-stained, and villus height and crypt depth were measured as previously described (2) using Scion Image Software (Scion Corporation 2000–2001, version Alpha 4.0.3.2, MD).
Table 1.
Primers sequences and accession codes used in quantitative PCR analysis
| Gene Name | Accession | Primer Sequence (5′-3′) | Product Size, bp | Tm, °C | Efficiency |
|---|---|---|---|---|---|
| NAGS | NM_001097520.1 | F: CAGGCTGTCTTTCCTTCTGG R: CACAGGATGGGAATGCTACC |
226 | 60 | 99.0 |
| PDG* | AF490841.1 | F: AAGGCACAGACATGGTTGGT R: AAGCAAACTGCCCTGAGAAA |
213 | 60 | 97.4 |
| OTC* | NM_001164002.2 | F: GCCTTTGTGAACAAGGTGGT R: TGAGATGCATGACAAGCACA |
135 | 60 | 99.3 |
| OAT* | NM_001185141 | F: CCAGTTACGACGGGTTTGGA R: GAACGCAGCAACGTTTGGAT |
107 | 60 | 97.8 |
| ARG2* | XM_001928679.3 | F: TACGTCCTGCCCTTCGTATC R: CAAGCCAGCTTCCCTTACAG |
182 | 60 | 95.7 |
| ASS* | XM_005660523.1 | F: GCTGGTGTACACGGGTTTCT R: CCAGCTCCTCGTTGTAGAGG |
167 | 60 | |
| CPS1* | XM_005672159.1 | F: CTGAGGCCCAGACAAGGGAA R: TGCAGCCAGTGTATCAATCTGT |
77 | 61 | 102.0 |
| P5CS* | XM_005671336.1 | F: CGGAACCTCAATGGGACACT R: CCTTAACACTAATAACCCCCTGGA |
127 | 60 | 95.3 |
| POX/PRODH | AK231562.1 | F: CCGATCGCCAGGTGTACTTT R: CTCCATCACGGGGCCATAAG |
115 | 60 | 93.9 |
| NOS3* | NM_214215.1 | F: CAGCCCTGATGGAGATGTCG R: GGTCTGAGCAGGAAACGCTA |
99 | 60 | 99.5 |
| ASL* | XM_003124443.2 | F: AGTTCCTGTTCTGGGCTTCG R: GCTTCCAGTGCTGTAGGCAT |
122 | 60 | 105.3 |
| ARG1* | NM_214048.2 | F: GGCTGGTCTGCTTGAGAAAC R: AGCCAGCTGTTGATTTGCTT |
144 | 60 | 105.3 |
F, forward; NAGS, N-acetylglutamate synthase; POX, proline oxidase; PRODH, proline dehydrogenase 1; R, reverse; Tm, temperature.
Gene definition is provided in the manuscript.
For immunohistochemistry, enteroids were grown in chamber slides and formalin-fixed for 20 min at room temperature. Enteroids were permeabilized with 0.5% Triton (in PBS) for 10 min at 4°C and blocked with buffer and serum. Primary antibodies obtained from Abcam (Cambridge, MA) [ASS, dilution 1:4,000, ab109753; carbamoyl phosphate synthetase 1, dilution 1:1,000, ab45956; and ornithine aminotransferase (OAT), dilution 1:1,000, ab137679] and Sigma-Aldrich [ornithine transcarbamylase (OTC), dilution 1:400, AV41766] were applied to the enteroids overnight at 4°C. After washing with 1X PBST for 3 × 10 min, secondary antibodies were applied and incubated at room temperature for 1 h (Alexa Fluor 488 donkey anti-goat, A-11055 and Alexa Fluor 555 donkey anti-rabbit, A-31572; Thermo Fisher Scientific). Signal was detected by deconvolution microscope (DVLive fluorescence microscope, GE Healthcare, Pittsburgh, PA).
Calculations.
Whole body citrulline, arginine, tyrosine and phenylalanine fluxes were estimated based on the dilution of the respective infused tracers in plasma (27). Whole body conversion of arginine to citrulline (i.e., nitric oxide production) and phenylalanine to tyrosine (phenylalanine hydroxylation) were based on the transfer of the infused tracer label to the corresponding product (27). Nonhydroxylative phenylalanine disposal, an indication of protein synthesis, was calculated during TPN by subtracting tyrosine hydroxylation from phenylalanine flux as described by others (47). The endogenous production of arginine was calculated by subtracting dietary (TPN and enteral) arginine from the flux (citrulline was not present in the diet).
Statistics.
PT, NT, and FT piglets were compared using parametric and nonparametric analysis using Prism 5.0. One-way ANOVA was performed on data sets that passed the D’Agostino and Pearson omnibus normality test. Between-group differences were identified by Tukey’s post hoc test. Nonparametric data sets (clinical and histological NEC score, iFABP, amino acids, and intestinal gene expression) were compared by the Kruskal-Wallis test with Dunn’s multiple comparison test. Data are presented as mean ± SE or 95% confidence interval. P < 0.05 was considered significant in all cases.
RESULTS
Clinical characteristics and NEC assessment.
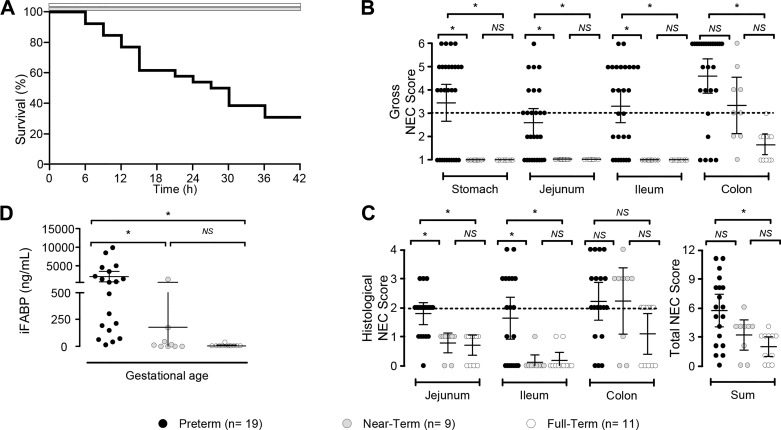
PT piglets had lower (P < 0.05) birth and final body weights than NT and FT piglets, but fractional growth rates were not different (P = 0.9) among groups (Table 2). Approximately 30% (8/27) of PT piglets survived the NEC protocol versus 100% of NT (9/9) and FT (11/11) piglets (P < 0.05). PT pig losses started as early as 6 h after the initiation of enteral feeding and occurred throughout the 42-h protocol (Fig. 3A).
Table 2.
Body weight, growth rate, and intestinal morphology of PT, NT, and FT piglets
| Growth Parameters* | PT | NT | FT | P Value |
|---|---|---|---|---|
| Birth weight*, g | 956 ± 43b | 1212 ± 89a | 1430 ± 96a | <0.001 |
| Final weight*, g | 1163 ± 51b | 1583 ± 108a | 1859 ± 132a | <0.001 |
| Fractional growth rate*, g·kg−1·day−1 | 62 ± 5 | 67 ± 5 | 65 ± 4 | 0.9 |
| Villus height§, µm | ||||
| Proximal jejunum | ||||
| At birth† | 322 ± 18 | 372 ± 19 | 372 ± 41 | 0.3 |
| Final‡ | 227 ± 32b | 323 ± 27a,b | 464 ± 23a | <0.001 |
| Distal ileum | ||||
| At birth† | 383 ± 41 | 255 ± 31 | 352 ± 26 | 0.06 |
| Final‡ | 240 ± 39b | 508 ± 42a | 503 ± 31a | <0.001 |
| Crypt depth, µm | ||||
| Proximal jejunum | ||||
| At birth† | 51 ± 3b | 70 ± 4a | 70 ± 7a | 0.03 |
| Final‡ | 69 ± 3 | 79 ± 4 | 77 ± 4 | 0.2 |
| Distal ileum | ||||
| At birth† | 64 ± 11 | 51 ± 2 | 53 ± 2 | 0.45 |
| Final‡ | 58 ± 4b | 76 ± 5a | 83 ± 5a | <0.001 |
| Colon | ||||
| At birth† | 209 ± 21 | 175 ± 8 | 209 ± 18 | 0.18 |
| Final‡ | 176 ± 11 | 183 ± 10 | 210 ± 10 | 0.15 |
Values are means ± SE; n = no. of pigs. Data analyzed by one-way ANOVA and Tukey’s post hoc test, except for villus height data. FT, full term; NT, near term; PT, preterm.
,
Means within a row with different superscripted letter differ (P < 0.05);
n = 27 PT pigs, n = 9 NT pigs, and n = 11 FT pigs;
n = 7 PT pigs, n = 5 NT pigs, and n = 5 FT pigs;
n = 19 PT pigs, n = 9 NT pigs, and n = 11 FT pigs;
Villus height data analyzed by Kruskal-Wallis test and Dunn’s multiple comparison test.
Fig. 3.
Multiple end-point measurements of necrotizing enterocolitis (NEC) incidence and severity in piglets. A: Kaplan-Meir graph detailing piglet survival. B: gross NEC score in piglets of advancing gestational age exposed to the NEC protocol. A threshold of 3 in any one segment was considered positive for NEC (dashed line). C: histological NEC score was determined in hematoxylin-eosin-stained tissue sections, and a threshold of ≥2 in any tissue section was considered positive for NEC (dashed line). D: plasma intestinal fatty-acid binding protein (iFABP) concentration in the final plasma sample for piglet groups in response to the NEC protocol. B–D: *P < 0.05, significant differences between groups, and error bars show 95% confidence interval. Data analyzed by Kruskal-Wallis test and Dunn’s multiple comparison test. NS, not significant.
There was no effect of gestational age on intestinal crypt and villus morphological parameters tissues at birth, except slightly shallower crypts in the proximal jejunum of PT pigs (P = 0.03; Table 2). However, villus height was greater in NT and FT pigs than in PT piglets at necropsy after the NEC protocol (Table 2); reduced villus height in the small bowel of PT piglets was likely due to NEC. There was an effect (P < 0.001) of gestational age on crypt depth in the ileum, but no effect (P > 0.15) was found for the proximal jejunum and colon.
PT pigs had significantly greater (P < 0.05) gross NEC scores in the stomach, proximal jejunum, and distal ileum versus NT and FT groups (Fig. 3B). NT piglets also had higher (P < 0.05) gross NEC scores in the colon compared with FT piglets. NEC incidence based on the gross NEC score was 94% (25/27), 66% (6/9), and 6% (1/11) in PT, NT, and FT piglets, respectively. Although NT and FT piglets were not entirely NEC resistant, the injury was exclusive to the colon in NT and FT piglets.
PT piglets scored positive for histological NEC in 74% (14/19) of cases, and the incidence of histological NEC was 66% (6/9) in NT piglets and 55% (6/11) in FT piglets (Fig. 3C). Consistent with the gross NEC score, NT and FT piglets only showed histological damage in the colon; PT piglets had greater (P < 0.05) histological NEC scores in the proximal jejunum and distal ileum versus the other groups. Summed histological NEC values from each section of the bowel were greater (P < 0.05) in PT piglets versus FT piglets (Fig. 3C). Plasma iFABP concentrations were markedly greater (P < 0.05) in PT piglets than in the NT and FT groups (Fig. 3D).
Complete blood count.
PT and NT pigs had lower (15.8 ± 1.0% and 18.2 ± 1.3%, P < 0.05) hematocrit at the end of the study compared with FT pigs (22.4 ± 1.0%), and PT pigs had a lower (2.0 ± 0.1 106/µl, P < 0.05) red blood cell count at the end of the study versus the NT and FT piglets (3.0 ± 0.3 and 3.3 ± 0.1 106/µl, respectively). Hemoglobin concentration was lower (4.6 ± 0.3 g/dl, P < 0.05) in PT pigs than FT piglets throughout the study (5.1 ± 0.3 g/dl and 6.3 ± 0.3 g/dl, respectively), whereas mean corpuscular volume was elevated (77 ± 2 fl, P < 0.01) in the PT piglets relative to the other groups (65.2 ± 1.2 fl). Initial platelet count among groups was similar initially, but final platelet count was lower (168 ± 30 103/µl, P < 0.01) in PT piglets relative to other groups. However, mean platelet volume was greater in the PT (23.7 ± 8.6 fl, P < 0.01) versus NT and FT piglets throughout the study (12.8 ± 2.6 fl and 16.8 ± 1.9 fl, respectively).
Tissue gene expression.
In proximal jejunum, arginine-producing OAT, ASS, and ASL gene expression at birth was lower (P < 0.05) in PT versus NT and FT piglets (data not shown). Furthermore, the jejunal inducible NOS (iNOS) gene expression was lower (P < 0.05) in newborn PT piglets versus newborn NT and newborn FT piglets. However, phosphate-dependent glutaminase (PDG) gene expression was greater (P < 0.05) in the PT pigs than in the other gestational age groups. In distal ileum, the gene expression of pyrroline 5-carboxylate synthase (P5CS), proline oxidase, and ASS was lower at birth (P < 0.05) in PT piglets relative to NT and FT animals (data not shown).
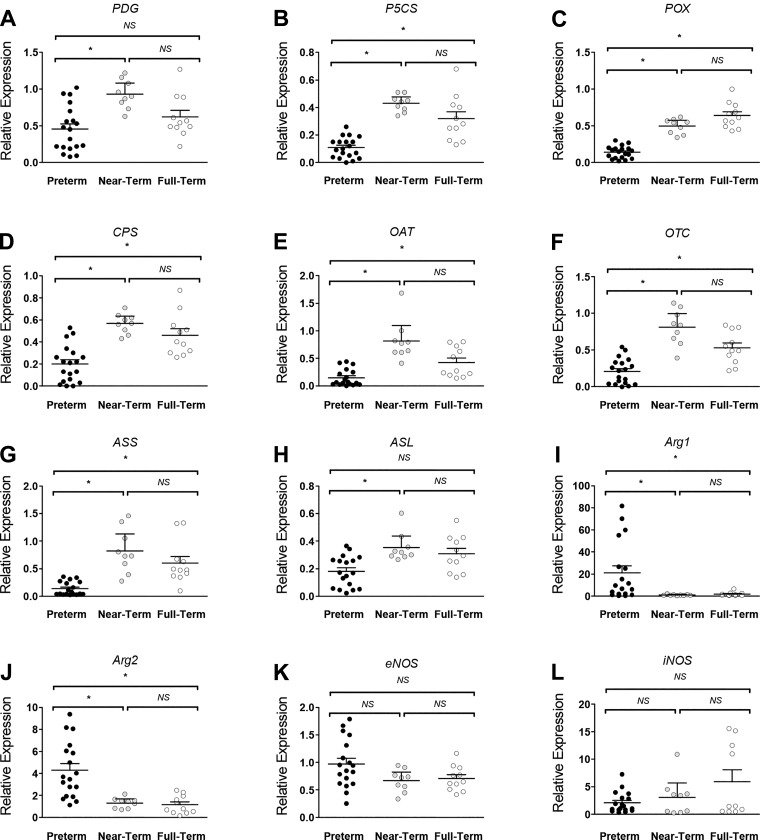
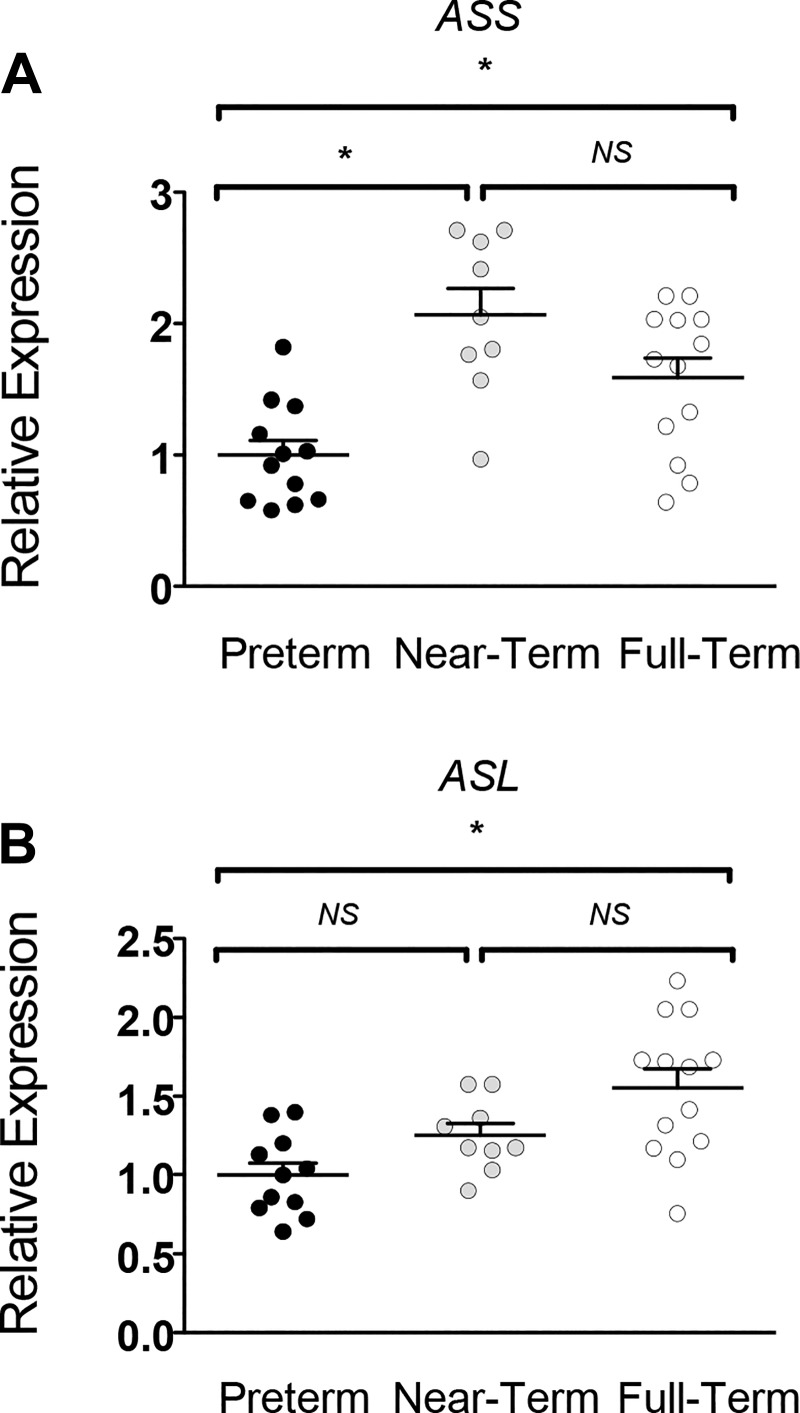
At the end of the NEC protocol, in the distal ileum, the gene expression of P5CS, proline oxidase, carbamoyl phosphate synthetase (CPS), OAT, and OTC was ~50% lower (P < 0.05) in PT piglets compared with NT and FT piglets (Fig. 4). Furthermore, PDG and ASL expression were greater in NT versus PT piglets only (P < 0.05). In contrast, gene expression of the arginine catabolizing enzymes Arg1 and Arg2 was greater (P < 0.05) in PT piglets relative to the other groups. Similar trends were observed in the proximal jejunum, except that PT piglet tissue had greater (P < 0.05) gene expression of endothelial NOS (eNOS) and PDG and lower (P < 0.05) iNOS expression versus FT piglets (data not shown). In the kidney, the expression of ASS was greater (P < 0.05) in NT and FT pigs versus the PT piglets (Fig. 5). However, ASL gene expression was only greater (P < 0.05) in FT pigs.
Fig. 4.
A–L: gene expression profile of genes of arginine metabolism in the distal ileum of piglets after exposure to the necrotizing enterocolitis (NEC) protocol. The relative expression of genes are grouped by gestational age and presented as mean ± 95% confidence interval. *Statistical difference between groups (P < 0.05). A: phosphate-dependent glutaminase (PDG). B: pyrroline 5-carboxylate synthase (P5CS). C: proline oxidase (POX). D: carbamoyl phosphate synthetase (CPS). E: ornithine aminotransferase (OAT). F: ornithine transcarbamylase (OTC). G: argininosuccinate synthase (ASS). H: argininosuccinate lyase (ASL). I: arginase (Arg) 1. J: Arg2. K: endothelial nitric oxide synthase (eNOS). L: inducible NOS (iNOS). Data analyzed by Kruskal-Wallis test and Dunn’s multiple comparison test. NS, not significant.
Fig. 5.
Expression of argininosuccinate synthase (ASS) (A) and argininosuccinate lyase (ASL) (B) in the kidney of piglets of advancing gestational age after the necrotizing enterocolitis (NEC) protocol. Relative expression is plotted as mean ± SE. *P < 0.05, significant difference between groups. Data analyzed by one-way ANOVA and Tukey’s post hoc test. NS, not significant.
Gene expression was further examined in PT piglets stratified as fulminant NEC, colonic NEC, and NEC resistant. In the proximal jejunum, P5CS expression was greater (P < 0.05) in colonic NEC piglets versus fulminant NEC (data not shown). In the distal ileum of pigs with colonic NEC and pigs resistant to NEC, the expression of OAT, CPS, OTC, and ASL was greater (P < 0.05) than in piglets with fulminant NEC (data not shown); however, Arg1 gene expression was lower in these animals (P < 0.05).
Plasma amino acids.
There was an effect (P < 0.05) of gestational age on plasma arginine, ornithine, glutamine, and proline concentrations with lower values for the PT pigs during TPN feeding (Table 3). However, no differences (P > 0.7) were found for proline and arginine for the different groups during enteral feeding. Plasma citrulline concentrations were not different (P = 0.2) among the three gestational age groups, irrespective of feeding modality (Table 3).
Table 3.
Concentrations of plasma amino acids relevant to arginine metabolism in PT, NT, and FT piglets during an NEC-inducing protocol
| Plasma Concentration (µmol/l) |
||||
|---|---|---|---|---|
| PT | NT | FT | P < | |
| Arginine | ||||
| TPN (day 3) | 90.8 (74.3–107.1)b | 98.8 (65.2–132.3)b | 195.2 (123.9–266.5)a | 0.002 |
| Final | 62.8 (35.9–89.7) | 32.1 (23.3–40.9) | 38.0 (25.9–50.2) | 0.9 |
| Citrulline | ||||
| TPN (day 3) | 81.2 (72.9–89.5) | 65.2 (39.5–90.9) | 86.2 (67.5–104.9) | 0.3 |
| Final | 73.3 (62.8–83.8) | 113.3 (78.7–147.9) | 72.9 (53.3–92.5) | 0.2 |
| Glutamine | ||||
| TPN (day 3) | 301.2 (272.4–330.0)c | 438.1 (381.8–464.4)b | 583.6 (487.7–679.4)a | <0.0001 |
| Final | 611.3 (502.1–720.5)b | 719.3 (607.5–831.0)ab | 910.4 (782.9–1038.0)a | 0.005 |
| Ornithine | ||||
| TPN (day 3) | 31.1 (26.8–35.4)b | 34.1 (20.3–48.0)ab | 51.9 (37.43–66.3)a | 0.02 |
| Final | 28.7 (20.5–36.8)b | 46.2 (35.1–57.2)ab | 72.8 (57.0–88.7)a | <0.0001 |
| Proline | ||||
| TPN (day 3) | 378.0 (298.9–457.0)b | 393.2 (345.5–440.9)b | 642.4 (555.6–729.1)a | 0.001 |
| Final | 562.3 (434.9–689.6) | 532.9 (467.7–598.2) | 586.2 (613.6–658.7) | 0.7 |
Values are means and 95% confidence interval; n = 27 PT pigs, n = 9 NT pigs, and n = 11 FT pigs. Data analyzed by Kruskal-Wallis test and Dunn’s multiple comparison test. FT, full term; NEC, necrotizing enterocolitis; NT, near term; PT, preterm; TPN, total parenteral nutrition.
Means with different superscript differ (P < 0.05).
Protein synthesis.
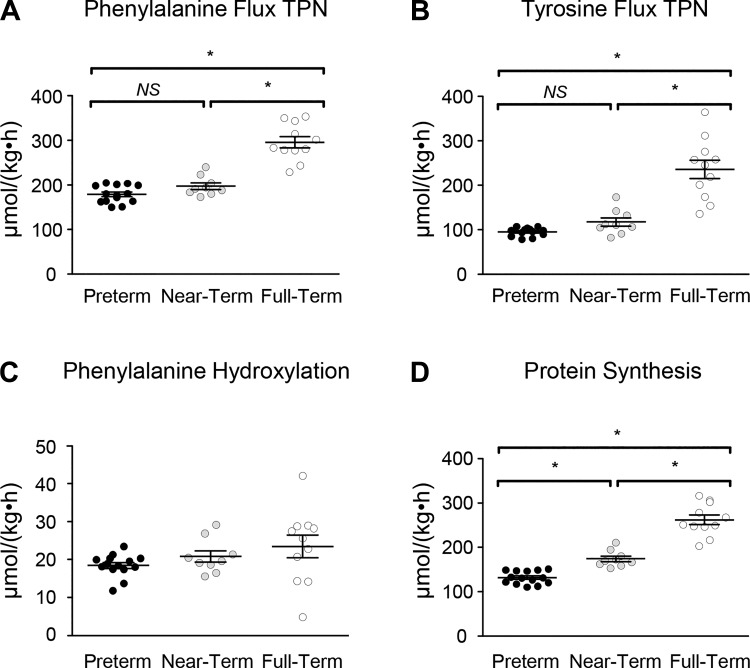
FT pigs had greater (P < 0.05) phenylalanine (a measurement of whole body protein degradation) and tyrosine fluxes than PT and NT pigs during parenteral nutrition (Fig. 6, A and B). Phenylalanine hydroxylation was not different among groups (Fig. 6C). However, the nonhydroxylative disposal of phenylalanine, which reflects whole body protein synthesis, increased (P < 0.05) with gestational age (Fig. 6D). NS, not significant.
Fig. 6.
Phenylalanine and tyrosine kinetics were monitored during total parenteral nutrition (TPN) in piglets of advancing gestational age. The flux rate of phenylalanine (A) and tyrosine (B) was greatest in full-term piglets (*P < 0.05). Phenylalanine hydroxylation to tyrosine was not affected by gestational age (C). Nonhydroxylative disposal, a measure of protein synthesis (D), was associated with gestational age (*P < 0.05). Data analyzed by one-way ANOVA and Tukey’s post hoc test. NS, not significant.
Arginine, citrulline, and nitric oxide kinetics.
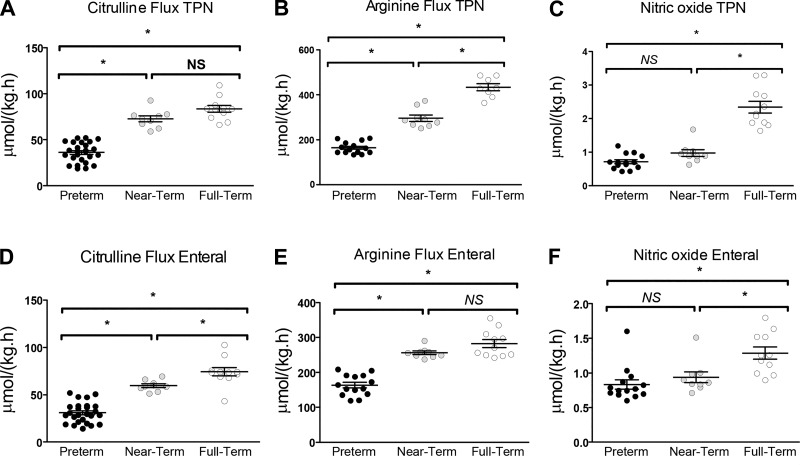
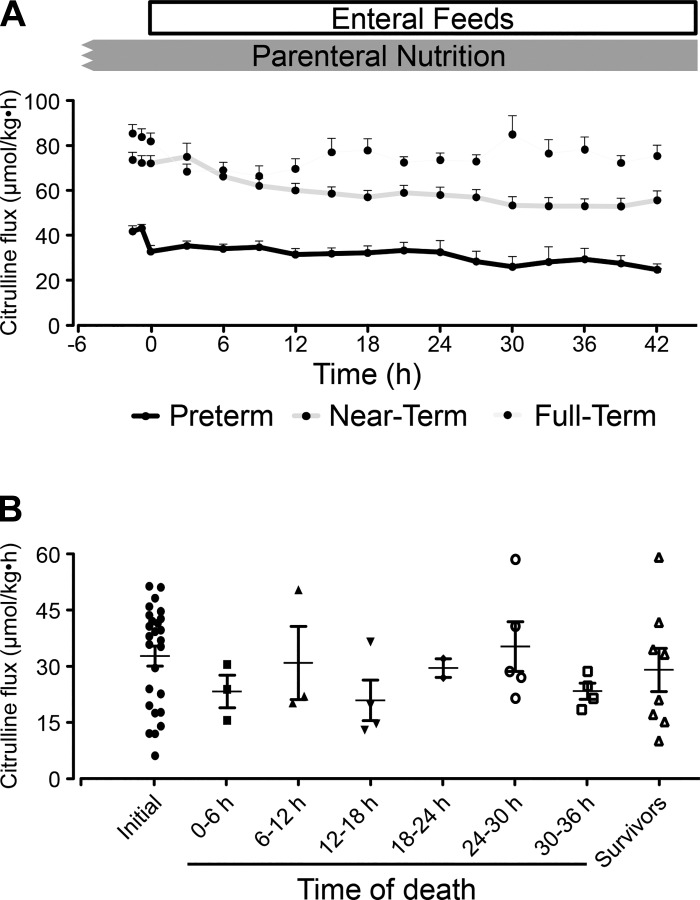
During TPN (Fig. 7A) and enteral feeding (Fig. 7D), citrulline flux was ~50% lower (P < 0.05) in PT piglets than in the NT and FT groups. However, time course citrulline flux remained rather constant throughout the NEC protocol (Fig. 8A), and in vivo citrulline flux did not change in response to suspected intestinal necrosis (Fig. 8B).
Fig. 7.
Profile of in vivo arginine metabolism in piglets of different gestational age. The flux of citrulline (A), arginine (B), and nitric oxide (C) was quantified during total parenteral nutrition (TPN) feeding as well during the necrotizing enterocolitis protocol (D, E, and F for citrulline, arginine, and nitric oxide, respectively). Bars are means ± SE. *P < 0.05. Data analyzed by one-way ANOVA and Tukey’s post hoc test. NS, not significant.
Fig. 8.
A: time course of citrulline flux throughout the necrotizing enterocolitis (NEC) protocol in piglets of different gestational ages. B: initial citrulline flux of preterm piglets followed by the final citrulline flux of piglets that had NEC or survived the protocol. Data analyzed by one-way ANOVA and Tukey’s post hoc test.
Whole body arginine and nitric oxide fluxes were monitored during TPN and 12 h after initiation of the enteral feeding protocol (Fig. 7, B, C, E, and F). During TPN, arginine flux in PT piglets was ~45% and ~60% lower (P < 0.05) than NT and FT piglets, respectively (Fig. 7B). Arginine flux was ~45% lower (P < 0.05) in PT piglets versus NT and FT groups during oral feeds (Fig. 7E). Whole body nitric oxide flux was 60%–70% lower (P < 0.05) in PT and NT piglets versus FT animals during TPN (Fig. 7C). Furthermore, low nitric oxide flux persisted (P < 0.05) in PT and NT piglets upon initiating oral feeds (Fig. 7F).
Enteroid citrulline production.
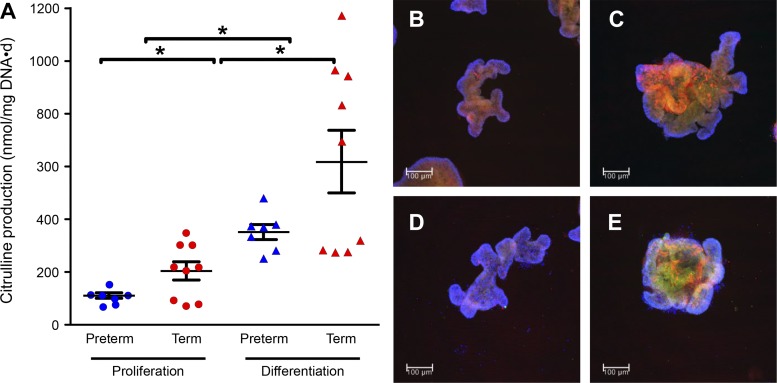
Citrulline production was measured in proliferating and differentiated enteroids. Enteroids originating from PT piglets produced less (P < 0.05) citrulline than enteroids from FT pigs (Fig. 9A). Furthermore, terminally differentiated Wnt3a-restricted enteroids produced significantly more (P < 0.05) citrulline than proliferating enteroids. Proliferating and differentiated enteroids derived from PT and FT pigs expressed OTC, ASS (Fig. 9, B–E), CPS, and OAT (not shown).
Fig. 9.
Citrulline production (A) and ornithine transcarbamylase localization (OTC) (B–E) in enteroids derived from the proximal jejunum of preterm and term piglets. Bars are means ± SE; *P < 0.05. Proliferating enteroids derived from preterm and term piglets (B and C, respectively). Differentiated enteroids derived from preterm and term piglets (D and E, respectively). OTC (green) is costained with argininosuccinate synthase (ASS) in red and DAPI in blue. Colocalization of ASS with OTC appears in yellow. Scale bar = 100 μm. The experiment was conducted twice with similar results. Data analyzed by one-way ANOVA and Tukey’s post hoc test.
DISCUSSION
Arginine is considered an indispensable amino acid in newborns (50), and lower plasma arginine concentrations have been observed in PT infants with NEC than in healthy infants (3). For several decades, arginine has been implicated in the pathogenesis of NEC (42), and the supplementation with this amino acid has been shown to reduce NEC incidence in PT infants (1, 37, 42). However, the underlying biological mechanism that explains how NEC depresses blood arginine and how dietary arginine supplementation prevents NEC is poorly understood. In the current study, newborn piglets were used as a model of human perinatal infants to test the hypothesis that whole body rates of citrulline, arginine, and nitric oxide synthesis are low in premature pigs and that they precede NEC.
Effect of gestational age on NEC incidence and presentation.
Our results confirm that the incidence and severity of NEC was related to gestational age, with the highest incidence and mortality in premature pigs. Although all of the NT pigs survived the 42-h protocol, some animals showed evidence of NEC localized mainly in colonic, but not in small intestinal, tissue. Similar to these macroscopic findings, PT pigs had shorter villi in both the jejunum and ileum compared with NT and FT pigs. As a consequence of NEC, there was a release of iFABP by the damaged epithelial cells that entered the circulation; this marker of small intestinal injury was negligible in NT and FT pigs. Thus, anatomical, histological, and plasma biomarkers indicated that incidence and severity of NEC was reduced with advancing gestational age.
Effect of gestational age on gene expression of enzymes involved in citrulline and arginine synthetic pathways.
In newborn pigs at birth, the intestinal expression of genes for the enzymes that synthesize citrulline and arginine were lower in PT than in NT and FT pigs; this was especially true for P5CS, OAT, ASS, and ASL. In contrast, the intestinal gene expression of enzymes that catabolize arginine was not different among the three gestational age groups. By the end of the feeding protocol, these differences in the expression of genes involved in the metabolism of citrulline and arginine synthesis became larger in PT versus the NT and FT groups. Furthermore, renal gene expression of the enzymes of arginine production, ASS, and ASL, was elevated in piglets of more advanced gestational age (15, 20). Reduced intestinal gene expression of citrulline- and arginine-synthesizing enzymes has also been described in infants with NEC (24). Thus, while it is possible that the lower gene expression of enzymes involved in the synthesis of citrulline and arginine in PT pigs was due to NEC-related intestinal injury and reduced villus height compared with NT and FT pigs, in vivo citrulline kinetics suggest that the relationship is associated with gestational age or intestinal maturation.
Effect of gestational age on citrulline and arginine fluxes and nitric oxide production.
Plasma citrulline concentration has been used as a marker for gut mass and health (12), and it has been hypothesized that whole body citrulline production rate would be a sensitive marker for in vivo intestinal injury. Contrary to this hypothesis, in vivo citrulline fluxes were relatively constant during the course of the TPN and enteral feeding protocol, despite marked differences in NEC-associated acute intestinal injury in PT piglets, colonic injury in NT pigs, and no injury in FT pigs. This strongly suggests that the differences in citrulline fluxes between PT, NT, and FT groups were a function of gestational age (or intestinal maturation) and were not due to intestinal injury. This was supported by the lower citrulline production of PT intestinal enteroids compared with those derived from older term pigs, further suggesting that reduced citrulline production is an intrinsic feature of the premature intestine. However, the term enteroids were derived from older pigs that were born naturally, and this may have also affected the production of citrulline.
The gestational age differences in citrulline production translated in lower arginine fluxes, which were also consistent with intestinal gene expression. In vivo arginine flux was significantly higher with advancing gestational age during both the TPN and enteral feeding. It is important to note that dietary arginine intake from TPN and enteral feeds were identical among piglet groups. This low arginine availability during prematurity is likely the reason for the reduction in NEC incidence in PT infants supplemented with arginine (1, 37, 42). Increasing arginine availability not only restores nitric oxide production but it may also mitigate tissue necrosis by modulating hypoxia (5), inhibit platelet activation and leukocyte adherence, and increase by free radical scavenging (9). Reduced nitric oxide production in PT piglets, but not in NT animals, coincided with thrombocytopenia and may have created an environment that favors platelet activation and coagulopathy. Implicit in NEC pathophysiology is that the activation of toll-like receptor 4 and NF-κB signaling and increased iNOS (49) and inhibition of eNOS mediated nitric oxide synthesis (51). In contrast to reports in rodent NEC models, we observed no evidence of changes in the expression of either iNOS or eNOS because of gestational age or NEC phenotype.
In summary, our results show that in neonatal pigs, the expression of intestinal enzymes and whole body citrulline and arginine fluxes were greater in animals of more advanced gestational age, which was associated with a reduction in the incidence and severity of NEC. The reduced citrulline production seems to be intrinsic of the premature intestine since it preceded the initiation of the NEC protocol and was replicated by the ex vivo enteroid system. The implication of our findings is that the low citrulline production, together with a lower arginine availability and NO production in PT pigs, is an underlying metabolic deficiency that results in poor intestinal perfusion and hypoxia, which are central to the pathogenesis of NEC. Citrulline and arginine supplementation have the potential to overcome this deficiency of prematurity and reduce the incidence of NEC.
GRANTS
This work was supported by federal funds from the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement No. 58-3092- 5-001 and California State University Agricultural Research Institute Grant 58982.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.G.B. and J.C.M. conceived and designed research; J.L.R., V.A.S., B.S., U.A., M.H.P., P.L., S.M.C., R.M., O.O., and J.C.M. performed experiments; J.L.R. and J.C.M. analyzed data; J.L.R., D.G.B., and J.C.M. interpreted results of experiments; J.L.R. and J.C.M. prepared figures; J.L.R. and J.C.M. drafted manuscript; J.L.R., D.G.B., and J.C.M. edited and revised manuscript; J.L.R., V.A.S., B.S., U.A., M.H.P., P.L., S.M.C., R.M., O.O., D.G.B., and J.C.M. approved final version of manuscript.
REFERENCES
- 1.Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr 140: 425–431, 2002. doi: 10.1067/mpd.2002.123289. [DOI] [PubMed] [Google Scholar]
- 2.Bauchart-Thevret C, Stoll B, Chacko S, Burrin DG. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 296: E1239–E1250, 2009. doi: 10.1152/ajpendo.91021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J Pediatr 137: 785–793, 2000. doi: 10.1067/mpd.2000.109145. [DOI] [PubMed] [Google Scholar]
- 4.Benight NM, Stoll B, Olutoye OO, Holst JJ, Burrin DG. GLP-2 delays but does not prevent the onset of necrotizing enterocolitis in preterm pigs. J Pediatr Gastroenterol Nutr 56: 623–630, 2013. doi: 10.1097/MPG.0b013e318286891e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berchner-Pfannschmidt U, Yamac H, Trinidad B, Fandrey J. Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J Biol Chem 282: 1788–1796, 2007. doi: 10.1074/jbc.M607065200. [DOI] [PubMed] [Google Scholar]
- 6.Bidlingmeyer BA, Cohen SA, Tarvin TL. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr A 336: 93–104, 1984. doi: 10.1016/S0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- 7.Caplan MS, Fanaroff A. Necrotizing: a historical perspective. Semin Perinatol 41: 2–6, 2017. doi: 10.1053/j.semperi.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol 14: 1017–1028, 1994. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 9.Caplan MS, Hedlund E, Hill N, MacKendrick W. The role of endogenous nitric oxide and platelet-activating factor in hypoxia-induced intestinal injury in rats. Gastroenterology 106: 346–, 1994. doi: 10.1016/0016-5085(94)90591-6. [DOI] [PubMed] [Google Scholar]
- 10.Chokshi NK, Guner YS, Hunter CJ, Upperman JS, Grishin A, Ford HR. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin Perinatol 32: 92–99, 2008. doi: 10.1053/j.semperi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J 15: 1398–1403, 2001. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 12.Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 124: 1210–1219, 2003. doi: 10.1016/S0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 13.Di Lorenzo M, Bass J, Krantis A. Use of l-arginine in the treatment of experimental necrotizing enterocolitis. J Pediatr Surg 30: 235–241, 1995. doi: 10.1016/0022-3468(95)90567-7. [DOI] [PubMed] [Google Scholar]
- 14.El-Shimi MS, Awad HA, Abdelwahed MA, Mohamed MH, Khafagy SM, Saleh G. Enteral L-arginine and glutamine supplementation for prevention of NEC in preterm neonates. Int J Pediatr 2015: 856091, 2015. doi: 10.1155/2015/856091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erez A, Nagamani SCS, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK, Black JO, Zeng H, Tang Y, Reddy AK, Summar M, O’Brien WE, Harrison DG, Mitch WE, Marini JC, Aschner JL, Bryan NS, Lee B. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 17: 1619–1626, 2011. doi: 10.1038/nm.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg 32: 275–282, 1997. doi: 10.1016/S0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 17.Gamsu HR, Kempley ST. Enteral hypoxia/ischaemia and necrotizing enterocolitis. Semin Neonatol 2: 245–254, 1997. doi: 10.1016/S1084-2756(97)80031-0. [DOI] [Google Scholar]
- 18.Gay AN, Lazar DA, Stoll B, Naik-Mathuria B, Mushin OP, Rodriguez MA, Burrin DG, Olutoye OO. Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J Pediatr Surg 46: 1034–1040, 2011. doi: 10.1016/j.jpedsurg.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghoneim N, Bauchart-Thevret C, Oosterloo B, Stoll B, Kulkarni M, de Pipaon MS, Zamora IJ, Olutoye OO, Berg B, Wittke A, Burrin DG. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS One 9: e106888, 2014. doi: 10.1371/journal.pone.0106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin BL, Solomonson LP, Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem 279: 18353–18360, 2004. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- 21.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen AR, Elnif J, Burrin DG, Sangild PT. Development of intestinal immunoglobulin absorption and enzyme activities in neonatal pigs is diet dependent. J Nutr 131: 3259–3265, 2001. doi: 10.1093/jn/131.12.3259. [DOI] [PubMed] [Google Scholar]
- 23.Kantorowska A, Wei JC, Cohen RS, Lawrence RA, Gould JB, Lee HC. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rates. Pediatrics 137: e20153123, 2016. doi: 10.1542/peds.2015-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung KT, Chan KY, Ma TPY, Yu JWS, Tong JH, Tam YH, Cheung HM, To KF, Lam HS, Lee KH, Li K, Ng PC. Dysregulated expression of arginine metabolic enzymes in human intestinal tissues of necrotizing enterocolitis and response of CaCO2 cells to bacterial components. J Nutr Biochem 29: 64–72, 2016. doi: 10.1016/j.jnutbio.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, Keggi C, Helms CC, Lee AN, Belanger AM, Diz DI, Laurienti PJ, Caudell DL, Wang J, Gladwin MT, Kim-Shapiro DB. Mechanisms of human erythrocytic bioactivation of nitrite. J Biol Chem 290: 1281–1294, 2015. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 142: 572–580, 2012. doi: 10.3945/jn.111.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 25: 1291–1296, 2011. doi: 10.1002/rcm.5007. [DOI] [PubMed] [Google Scholar]
- 29.Marini JC, Agarwal U, Robinson JL, Yuan Y, Didelija IC, Stoll B, Burrin DG. The intestinal-renal axis for arginine synthesis is present and functional in the neonatal pig. Am J Physiol Endocrinol Metab 313: E233–E242, 2017. doi: 10.1152/ajpendo.00055.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA 97: 6043–6048, 2000. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 13: 590–600, 2016. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowicki PT. Ischemia and necrotizing enterocolitis: where, when, and how. Semin Pediatr Surg 14: 152–158, 2005. doi: 10.1053/j.sempedsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Nowicki PT, Dunaway DJ, Nankervis CA, Giannnone PJ, Reber KM, Hammond SB, Besner GE, Caniano DA. Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr 146: 805–810, 2005. doi: 10.1016/j.jpeds.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 35.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, Engstrand L, Lilja HE, Hollister EB, Versalovic J, Neu J. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5: 31, 2017. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papillon S, Castle SL, Gayer C, Ford HR. Necrotizing enterocolitis: Contemporary management and outcomes. Adv Pediatr 60: 263–279, 2013. doi: 10.1016/j.yapd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Polycarpou E, Zachaki S, Tsolia M, Papaevangelou V, Polycarpou N, Briana DD, Gavrili S, Kostalos C, Kafetzis D. Enteral L-arginine supplementation for prevention of necrotizing enterocolitis in very low birth weight neonates: a double-blind randomized pilot study of efficacy and safety. JPEN J Parenter Enteral Nutr 37: 617–622, 2013. doi: 10.1177/0148607112471561. [DOI] [PubMed] [Google Scholar]
- 38.Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 6: CD002971, 2018. doi: 10.1002/14651858.CD002971.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangild PT, Siggers RH, Schmidt M, Elnif J, Bjornvad CR, Thymann T, Grondahl ML, Hansen AK, Jensen SK, Boye M, Moelbak L, Buddington RK, Weström BR, Holst JJ, Burrin DG. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130: 1776–1792, 2006. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 41.Shah P, Shah V. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev: CD004339, 2007. doi: 10.1002/14651858.CD004339.pub3. [DOI] [PubMed] [Google Scholar]
- 42.Shah PS, Shah VS, Kelly LE. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev 4: CD004339, 2017. doi: 10.1002/14651858.CD004339.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech 1: 94–98, 2008. doi: 10.1242/dmm.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoll B, Puiman PJ, Cui L, Chang X, Benight NM, Bauchart-Thevret C, Hartmann B, Holst JJ, Burrin DG. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. JPEN J Parenter Enteral Nutr 36: 538–550, 2012. doi: 10.1177/0148607112444756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP, Wyckoff M, Das A, Hale EC, Ball MB, Newman NS, Schibler K, Poindexter BB, Kennedy KA, Cotten CM, Watterberg KL, D’Angio CT, DeMauro SB, Truog WE, Devaskar U, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314: 1039–1051, 2015. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanner SM, Berryhill TF, Ellenburg JL, Jilling T, Cleveland DS, Lorenz RG, Martin CA. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol 185: 4–16, 2015. doi: 10.1016/j.ajpath.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tessari P, Kiwanuka E, Vettore M, Barazzoni R, Zanetti M, Cecchet D, Orlando R. Phenylalanine and tyrosine kinetics in compensated liver cirrhosis: effects of meal ingestion. Am J Physiol Gastrointest Liver Physiol 295: G598–G604, 2008. doi: 10.1152/ajpgi.00355.2007. [DOI] [PubMed] [Google Scholar]
- 48.Touloukian RJ, Posch JN, Spencer R. The pathogenesis of ischemic gastroenterocolitis of the neonate: selective gut mucosal ischemia in asphyxiated neonatal piglets. J Pediatr Surg 7: 194–205, 1972. doi: 10.1016/0022-3468(72)90496-4. [DOI] [PubMed] [Google Scholar]
- 49.Tun X, Yasukawa K, Yamada K. Involvement of nitric oxide with activation of Toll-like receptor 4 signaling in mice with dextran sodium sulfate-induced colitis. Free Radic Biol Med 74: 108–117, 2014. doi: 10.1016/j.freeradbiomed.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Wu G, Jaeger LA, Bazer FW, Rhoads JM. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem 15: 442–451, 2004. doi: 10.1016/j.jnutbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, Branca MF, Prindle T, Richardson WM, Ozolek J, Billiar TR, Binion DG, Gladwin MT, Hackam DJ. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci USA 110: 9451–9456, 2013. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zamora IJ, Stoll B, Ethun CG, Sheikh F, Yu L, Burrin DG, Brandt ML, Olutoye OO. Low abdominal NIRS values and elevated plasma intestinal fatty acid-binding protein in a premature piglet model of necrotizing enterocolitis. PLoS One 10: e0125437, 2015. doi: 10.1371/journal.pone.0125437. [DOI] [PMC free article] [PubMed] [Google Scholar]