Abstract
Purpose
Angiogenesis actively contributes to tumor growth and metastasis. MACC1 was reported to be associated with tumor progression. In the present study, we aimed to investigate the expression and role of MACC1 in cholangiocarcinoma (CCA) and its correlation with angiogenesis.
Patients and methods
We investigated the expression and correlation of MACC1 and VEGFA in The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) datasets and in 7 paired frozen CCA and matched paracarcinoma tissues. Immunohistochemistry (IHC) was used to examine MACC1 and VEGFA expression as well as microvessel density (MVD) in 122 paraffin-embedded CCA samples. Western blotting, real-time qPCR and ELISA were performed to investigate the effect of MACC1 knockdown on VEGFA expression and secretion in CCA cells. Subsequently, we collected conditioned medium from cells with MACC1 knockdown and used it in angiogenesis assays.
Results
The expression levels of both MACC1 and VEGFA were significantly upregulated in the TCGA and GEO datasets and in the 7 paired frozen CCA tissues compared to the matched paracarcinoma tissues, and MACC1 was significantly correlated with VEGFA. IHC showed that high expression of MACC1 and VEGFA was significantly correlated with lymph node metastasis (P<0.05 and P<0.01) and worse survival (P<0.01, P<0.05) in patients with CCA. We further verified that MACC1 was significantly correlated with VEGFA (P<0.01) and MVD (P<0.01) in clinical samples. Western blotting, real-time qPCR and ELISA results showed that MACC1 knockdown in CCA cells significantly decreased the protein and mRNA expression of VEGFA and reduced the VEGFA concentration in conditioned medium. Moreover, angiogenesis assays showed that conditioned medium from CCA cells with MACC1 knockdown decreased the number of tubes formed.
Conclusion
Our results indicate that MACC1 and VEGFA expression are upregulated in CCA. Moreover, MACC1 is an independent predictor of overall survival and facilitates angiogenesis in CCA by upregulating of VEGFA.
Keywords: microvessel density, TCGA, GEO, prognosis, carcinoma
Introduction
Cholangiocarcinoma (CCA) is an epithelial cell malignancy originating from the intrahepatic and extrahepatic bile duct epithelia and has a dismal prognosis.1,2 Aside from surgical resection, the current therapeutic options for CCA are very limited, and most patients have advanced disease at diagnosis.2 The treatment outcomes of adjuvant radiochemotherapy are still not satisfactory.3 Although antiangiogenic drugs have been used to treat CCA, more side effects and unsatisfactory efficacy have been reported.4,5 Therefore, it is necessary to further understand the biological behavior of CCA to provide new treatment modalities.
Angiogenesis is the biological process that leads to the formation of new vessels from preexisting vasculature, which is a critical event in many solid tumors because rapidly growing tumor cells require extra blood vessels to supply nutrients and to induce distant metastasis.6 Vascular endothelial growth factor A (VEGFA) is the most relevant proangiogenic factor and plays a key role in angiogenesis. The signaling mediated by VEGFA promotes endothelial cell proliferation and migration and results in the formation of new blood vessels.7 High microvessel density (MVD) is correlated with a poor prognosis in pancreatic cancer, breast cancer, and intrahepatic CCA.8–10
Metastasis-associated in colon cancer-1 (MACC1) was first identified in 2009 through a genome-wide search for differentially expressed genes in human colon cancer tissues and metastatic tissues.11 It has been reported that MACC1 mRNA expression might be an independent prognostic indicator of recurrence and disease-free survival in colorectal carcinoma,12 lung adenocarcinoma,13 pancreatic cancer,14 and hilar cholangiocarcinoma.15 MACC1 promotes the proliferation, migration, and invasiveness of cancer cells via the hepatocyte growth factor (HGF)/c-Met/MAPK signaling pathway.16 Previous studies have also indicated that MACC1 participates in angiogenesis in gastric cancer17 and cervical cancer.18 However, the correlation between MACC1 and angiogenesis in CCA has not yet been investigated.
In this study, we found that MACC1 and VEGFA were significantly upregulated in CCA according to The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) datasets, as well as in human paraffin-embedded CCA samples. Moreover, MACC1 and VEGFA expression levels were positively correlated in CCA tissues. MACC1 was also an independent predictor of overall survival. We further confirmed that MACC1 regulated the expression and secretion of VEGFA and promoted angiogenesis in CCA cells.
Patients and methods
TCGA and GEO databases
Data from the TCGA19 (https://cancergenome.nih.gov/) and GEO (https://www.ncbi.nlm.nih.gov/geo/), accession numbers: GSE76297,20 GSE8974921 databases are publicly available.
Patients and tissue samples
We obtained tumor specimens and 31 paracarcinoma specimens from 122 patients with CCA who underwent surgery between 2010 and 2016 at the Department of Hepatobiliary Surgery, Southwest Hospital. Seven paired CCA and matched paracarcinoma tissues were obtained during surgery in 2018 and were immediately stored in liquid nitrogen. The clinical information of the 122 CCA patients is summarized in Table 1. None of the patients either received (neo) adjuvant chemotherapy or underwent liver transplantation. This study was approved by the Ethics Committee of Southwest Hospital at Army Medical University, Chongqing, China. The participants provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Clinical characteristics of MACC1 and VEGFA expressions in 122 cholangiocarcinoma patients
| Characteristics | MACC1 expression | P-valuea | VEGFA expression | P-valuea | ||
|---|---|---|---|---|---|---|
| Low (N=57) | High (N=65) | Low (N=55) | High (N=67) | |||
| Age | 0.483 | 0.251 | ||||
| <60 years | 36 | 37 | 36 | 37 | ||
| ≥60 years | 21 | 28 | 19 | 30 | ||
| Gender | 0.741 | 0.973 | ||||
| Male | 35 | 38 | 33 | 40 | ||
| Female | 22 | 27 | 22 | 27 | ||
| Location | 0.665 | 0.059 | ||||
| Intrahepatic | 16 | 16 | 19 | 13 | ||
| Extrahepatic | 41 | 49 | 36 | 54 | ||
| Histological | 0.980 | 0.325 | ||||
| G1 | 5 | 6 | 5 | 6 | ||
| G2 | 43 | 48 | 38 | 53 | ||
| G3 | 9 | 11 | 12 | 8 | ||
| T classificationb | 0.194 | 0.771 | ||||
| T1 or T2 | 27 | 40 | 31 | 36 | ||
| T3 or T4 | 30 | 25 | 24 | 31 | ||
| Lymph node metastasis | 0.018 | 0.006 | ||||
| Negative | 35 | 26 | 35 | 26 | ||
| Positive | 22 | 39 | 20 | 41 | ||
Notes:
Data in bold indicates a P-value <0.05 and was considered to be statistically significant.
According to the seventh UICC-TNM staging. Statistical analysis was performed by a χ2 or Fisher’s exact test.
Abbreviations: UICC, Union for International Cancer Control; TNM, Tumor Node Metastasis.
Immunohistochemistry (IHC) staining
Paraffin-embedded tissue sections were deparaffinized in xylene, rehydrated in a graded series of ethanol solutions and then incubated for 30 minutes in 3% H2O2 at 37°C to quench endogenous peroxidase activity. Next, antigen retrieval was performed by heating the sections in citrate buffer. Nonspecific binding was blocked by incubating the sections with 10% goat serum for 30 minutes at room temperature. The slides were then incubated overnight at 4°C with a rabbit anti-MACC1 primary antibody (1:100, ab106579, Abcam, Cambridge, UK) or a rabbit anti-VEGFA primary antibody (1:100, ab46154, Abcam). An appropriate secondary antibody was added, and the slides were incubated for 30 minutes at 37°C; antibody binding was visualized with DAB. The staining was scored independently by two observers who were blinded to the clinical data. The percentages of positively stained carcinoma cells were graded as follows: 0= negative; 1=1%–50%; 2=51%–74%; and 3≥75%, and the staining intensity was graded as follows: 0= no staining; 1= weak; 2= moderate; and 3= strong. The two values were multiplied to obtain a final score: negative =0; low expression =1–3; high expression =4–6.
Microvessel density (MVD)
The MVD was determined as described by Weidner et al22 The MVD of the tumor sections was assessed according to IHC using an anti-CD34 antibody (1:100, ab81289, Abcam) to stain the tumor vessels. To quantify MVD, three areas within the tumor with the highest vascular density (vascular hot spots) were identified at low magnification (100×), and the number of vessels was counted microscopically under 200× magnification. All counts were independently reviewed by three observers blinded to the clinical data. According to the average values, the MVD was classified as either high (≥24.1 mm2) or low (<24.1 mm2).
Cell culture and transfection
The human CCA cell line QBC939 was established from an extrahepatic CCA lesion and maintained at the Hepatobiliary Surgery Institute, Southwest Hospital, Army Medical University. RBE cells were purchased from the Japanese Collection of Research Bioresources (Osaka, Japan).23 Two human CCA cell lines were cultured in RPMI 1640 medium with 10% FBS (Zeta, Japan) and were routinely cultured in a humidified incubator at 37°C with 5% CO2. Human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC), Manassas, VA, USA and cultured in DMEM with 10% FBS (Zeta, Japan). The target MACC1 sequence for the short hairpin RNA plasmid against MACC1 was purchased from GenePharma Co. Ltd (Shanghai, China). The shRNA sequences for MACC1 are as follows: forward, 5′-GAGTTAGTCGCACGTCTCA and reverse, 5′-TGAGACGTGCGACTAACTC. Two cell lines were transfected with the plasmid using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The cells were then cultured for 48–72 hours after transfection.
Immunofluorescence (IF) confocal microscopy
CCA cells were seeded on chamber slides overnight, after which they were fixed with 4% formaldehyde for 15 minutes at room temperature, permeabilized with 0.3% Triton X-100 (Sigma-Aldrich Co., St Louis, MO, USA) for 10 minutes and incubated with 1% BAS for 30 minutes at room temperature to block nonspecific binding of the antibodies. The slides were incubated overnight at 4°C with the following primary antibodies: rabbit anti-MACC1 primary antibody (1:100, ab106579, Abcam) and mouse anti-VEGFA primary antibody (1:100, bsm-4572M; Biosynthesis Biotechnology Inc., Beijing, China). The slides were washed three times and incubated with secondary antibody (SA00009-2 and SA00013-5, Beyotime, China) for 1 hour in the dark. DAPI (Beyotime Biotechnology, Shanghai, China) was added to the cells for 5 minutes to stain the nuclei. Finally, the slides were mounted on a coverslip and stored in the dark at 4°C.
Western blotting
Total proteins from cells, human CCA tissues and paracarcinoma tissues were extracted with RIPA lysis buffer (Sigma-Aldrich Co., St Louis, MO, USA) supplemented with protease inhibitor cocktail tablets (Hoffman-La Roche Ltd., Basel, Switzerland). The resulting protein lysates were separated through an SDS-polyacrylamide gel and electro-transferred to polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA). Fat-free milk (5%) was used to block the membranes for 2 hours at room temperature. After blocking, rabbit primary antibodies, including anti-MACC1 (1:2,000, Abcam), anti-VEGFA (1:2,000, Abcam), and anti-GAPDH (1:10,000; Proteintech Group Inc., Wuhan, China), were incubated with the membranes overnight at 4°C. The next day, the membranes were washed with PBST and incubated with horseradish peroxidase-conjugated secondary antibody for 2 hours at room temperature. The immunocomplexes were then visualized using chemiluminescence (Merck Millipore) according to the manufacturer’s protocol.
RNA extraction and real-time qPCR
Total RNA was extracted using an Eastep Super Total RNA extraction kit (Promega Corporation, Fitchburg, WI, USA) according to the manufacturer’s protocols. cDNA synthesis was also performed according to the manufacturer’s instructions (PrimeScript™ RT reagent kit, RR037A; Takara Bio Inc., Shiga, Japan). Quantitative PCR was performed with SYBR Premix Ex TaqTM II (RR820A; Takara Bio Inc.) using a CFX96 real-time system. The data were analyzed, and the expression levels were calculated according to the sample threshold cycle (Ct) value from three independent experiments. The primer sequences are as follows: MACC1 (NCBI Reference Sequence: NM_182762.3), forward primer 5′-TTCTTTTGATTCCTCCGGTGA-3′, reverse primer 5′-ACTCTGATGGGCATGTGCTG-3′; GAPDH (NCBI Reference Sequence: NM_002046.7) forward primer 5′-AGAAGGCTGGGGCTCATTTG-3′, reverse primer 5′-AGGGGCCATCCACAGTCTTC-3′; and VEGFA (NCBI Reference Sequence: NM_001025366.2) forward primer 5′-GGGCAGAATCATCACGAAGT-3′, reverse primer 5′-TGGTGATGTTGGACTCCTCA-3′. The expression levels were normalized to those of GAPDH.
Enzyme-linked immunosorbent assay
CCA cells were seeded in 6-well plates and incubated in serum-free medium for 24 hours. The conditioned medium was collected, and the concentration of VEGFA was quantified using VEGFA ELISA kits (Wuhan Abebio Science Co., Ltd, Wuhan City, China) according to the manufacturer’s instructions. The results represent the mean values from three separate experiments.
In vitro Matrigel-based angiogenesis assays
HUVECs were seeded at a density of 1×105 cells per well in 500 µL of conditioned medium in a 24-well plate that was precoated with Matrigel (Corning Incorporated, Corning, NY, USA); the cells were allowed to grow for 8 hours at 37°C. Images were obtained using an inverted bright-field microscope (Nikon Corporation, Tokyo, Japan). Three randomly selected fields per sample were photographed at 100× magnification. The numbers of connected tubes were determined by ImageJ software (National Institutes of Health, Bethesda, MD, USA) and then compared between the different groups.
Statistical methods
Statistical analysis was performed using the SPSS 22.0 software package (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA). The relationship between MACC1 and VEGFA in the TCGA and GEO datasets was also evaluated by linear regression analysis. Survival analysis was performed using the date of surgery to the date of death according to the Kaplan–Meier method. The statistical significance of the differences in cumulative survival curves was compared using the log-rank test. Multivariate analysis was performed using the Cox regression model. To compare the statistical significance of differential results between two groups, a two-tailed t-test analysis was performed. The quantitative data were expressed as the mean ± SEM (standard error of mean) of three independent experiments. P-values less than 0.05 were defined as statistically significant.
Results
MACC1 and VEGFA expression is significantly upregulated in CCA tissues
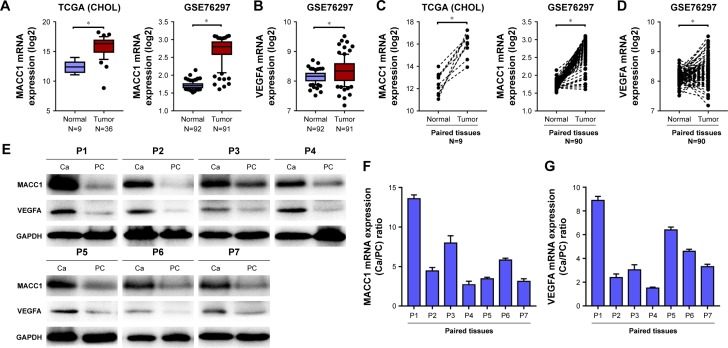
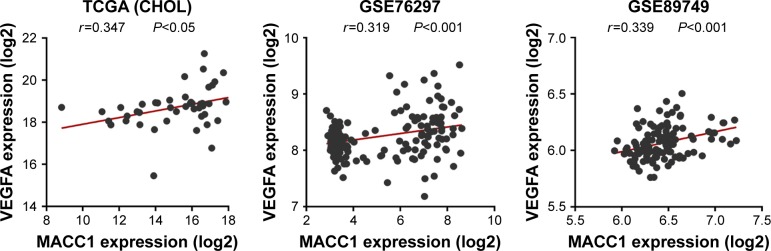
To explore the role of MACC1 and VEGFA in CCA, we analyzed MACC1 and VEGFA expression in publicly available human CCA datasets (TCGA and GEO). Both MACC1 and VEGFA were significantly upregulated in CCA tissues from the datasets and in paired CCA tissues compared with the levels in normal control tissues (Figure 1A–D). Subsequently, we verified in 7 frozen CCA tissues that the protein and mRNA levels of MACC1 and VEGFA were increased compared with those in the matched paracarcinoma tissues (Figure 1E–G). We further analyzed the correlation of MACC1 and VEGFA and found that MACC1 was significantly correlated with VEGFA in the TCGA, GSE76297 and GSE89749 datasets (Figure 2).
Figure 1.
Expression of MACC1 and VEGFA in the TCGA and GEO datasets and in 7 paired frozen CCA and matched paracarcinoma tissues.
Notes: (A) MACC1 mRNA expression in normal and tumor tissues in the TCGA (CHOL) and GSE76297 datasets. (B) VEGFA mRNA expression in normal and tumor tissues in the GSE76297 dataset. (C) MACC1 mRNA expression in paired tissues in the TCGA (CHOL) and GSE76297 datasets. (D) VEGFA mRNA expression in paired tissues in the GSE76297 dataset. (E) Western blotting analysis of MACC1 and VEGFA protein expression in paired frozen CCA tissues and matched paracarcinoma tissues (N=7). Real-time qPCR analysis of MACC1 (F) and VEGFA (G) mRNA expression in paired frozen CCA tissues and matched paracarcinoma tissues (N=7). *P<0.001.
Abbreviations: Ca, carcinoma; CCA, cholangiocarcinoma; GEO, Gene Expression Omnibus; PC, paracarcinoma; TCGA (CHOL), The Cancer Genome Atlas (cholangiocarcinoma).
Figure 2.
Correlation of MACC1 and VEGFA expression in the TCGA (CHOL) and GEO datasets.
Abbreviations: GEO, Gene Expression Omnibus; TCGA (CHOL), The Cancer Genome Atlas (cholangiocarcinoma).
Immunohistochemical analysis of MACC1 expression, VEGFA expression and MVD in paraffin-embedded CCA samples
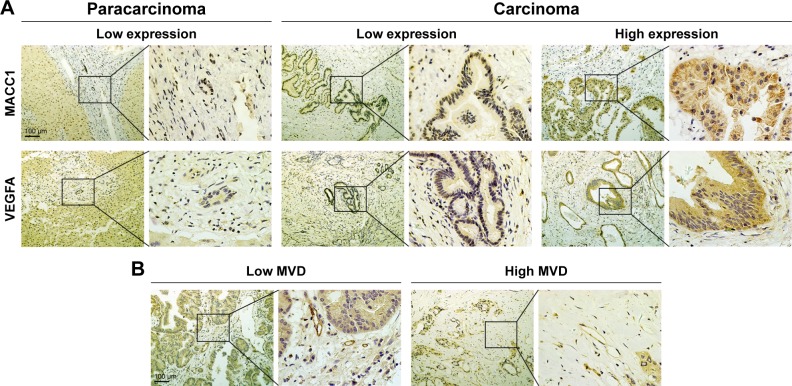
To analyze further the expression of MACC1 and VEGFA, we performed immunohistochemical staining for MACC1 and VEGFA in 122 paraffin-embedded CCA samples and 31 paracarcinoma samples. Representative staining of MACC1 and VEGFA in CCA and paracarcinoma tissues is shown in Figure 3A. MACC1 and VEGFA were primarily expressed in the cytoplasm. MACC1-high and VEGFA-high expression was observed in 53.3% (65/122 cases) and 54.9% (67/122 cases) of CCA cases, respectively. Combined MACC1-high/VEGFA-high expression was observed in 43 cases, and MACC1-low/VEGFA-low expression was observed in 33 cases. However, all 31 paracarcinoma samples showed low expression of MACC1 and VEGFA.
Figure 3.
Immunohistochemical analysis of MACC1 expression, VEGFA expression and MVD in human paraffin-embedded CCA samples.
Notes: (A) Representative images of MACC1 and VEGFA staining in CCA tissues. Paracarcinoma tissues showed low expression, while carcinoma tissues showed either low expression or high expression. (B) CD34 staining indicating high and low MVD in CCA.
Abbreviations: CCA, cholangiocarcinoma; MVD, microvessel density.
Tumor angiogenesis was assessed by MVD. We defined values higher than the cut-off of the MVD (median value; 24.1/mm2) as high MVD and values lower than the cut-off as low MVD. Among the 122 CCA patients, 56 (45.9%) had high MVD, while the remaining 66 (54.1%) were considered to have low MVD. Representative images of high and low MVD are indicated in Figure 3B.
High expression of MACC1 and VEGFA is associated with lymph node metastasis and worse survival in patients with CCA
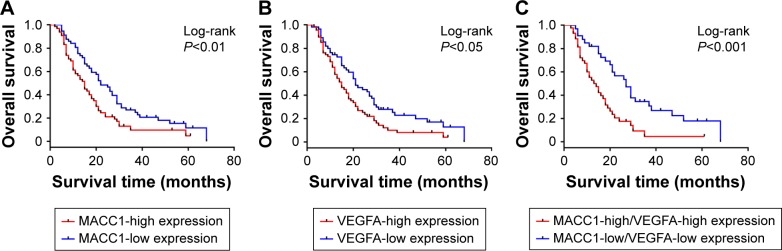
The analysis of the correlation of MACC1 and VEGFA with clinicopathological parameters revealed that MACC1 and VEGFA are associated with lymph node metastasis (P<0.05 and P<0.01, Table 1) but were not significantly associated with age, gender, location, histological type or T grade. To analyze the impact of MACC1 and VEGFA expression on the prognosis of patients with CCA, we performed a survival analysis. The results of the Kaplan–Meier analysis revealed that high expression of MACC1 and VEGFA was significantly associated with reduced overall survival (log-rank P<0.01 and P<0.05, Figure 4A and B). Then, we analyzed the co-expression of MACC1 and VEGFA as a prognostic indicator and found that individuals with MACC1-high/VEGFA-high expression also had a worse overall survival than those with MACC1-low/VEGFA-low expression (log-rank P<0.001, Figure 4C). Cox regression analysis showed that MACC1 and lymph node metastasis were independent predictors of overall survival in CCA patients (Table 2). These results indicated that high expression of MACC1 and VEGFA is significantly correlated with lymph node metastasis and worse survival in patients with CCA.
Figure 4.
Survival analysis of MACC1 and VEGFA in CCA.
Notes: (A) Kaplan–Meier analysis for the overall survival of CCA patients with MACC1-high expression (N=65) and MACC1-low expression (N=57). (B) Kaplan–Meier curves for the overall survival of CCA patients with VEGFA-high expression (N=67) and VEGFA-low expression (N=55). (C) Kaplan–Meier curves for the overall survival of CCA patients with MACC1-high/VEGFA-high expression (N=43) and MACC1-low/VEGFA-low expression (N=33).
Abbreviation: CCA, cholangiocarcinoma.
Table 2.
Cox regression analysis for overall survival of CCA following surgical resection
| Variables | HR (95% CI) | P-valueb |
|---|---|---|
| Gender (male vs female) | 0.679 (0.431–1.071) | 0.096 |
| Age (<60 years vs ≥60 years) | 0.759 (0.439–1.168) | 0.209 |
| Location (intrahepatic vs extrahepatic) | 1.024 (0.603–1.737) | 0.930 |
| Lymph node metastasis (negative vs positive) | 1.820 (1.137–2.912) | 0.013 |
| T classificationa (T1–T2 vs T3–T4) | 1.051 (0.675–1.638) | 0.825 |
| Histological (G1 vs G2 vs G3) | 0.910 (0.607–1.365) | 0.650 |
| MACC1 (low vs high) | 1.544 (1.024–2.328) | 0.038 |
| VEGFA (low vs high) | 1.454 (0.933–2.265) | 0.098 |
Notes:
According to the seventh UICC-TNM staging.
Data in bold indicates a P-value <0.05 and was considered to be statistically significant.
Abbreviation: CCA, cholangiocarcinoma.
MACC1 expression is correlated with VEGFA expression and MVD
We found a significant positive correlation between MACC1 and VEGFA in the TCGA and GEO datasets (Figure 2). A similar significant correlation was observed between MACC1 and VEGFA expression in 122 paraffin-embedded CCA tissues: 66.2% (43/65) of CCA tissues with high MACC1 expression showed high VEGFA expression (P<0.01, Table 3), and 60% (39/65) of CCA tissues with high MACC1 expression showed a high MVD (P<0.01, Table 4). The results revealed that MACC1 expression is significantly correlated with VEGFA expression and MVD.
Table 3.
Correlation between MACC1 and VEGFA expression in 122 CCA tissues
| MACC1 expression | P-value | ||
|---|---|---|---|
| Low | High | ||
| VEGFA expression | 0.008a | ||
| High | 24 (42.1%) | 43 (66.2%) | |
| Low | 33 (57.9%) | 22 (33.8%) | |
Note:
P<0.01, statistical significance by chi-squared test.
Abbreviation: CCA, cholangiocarcinoma.
Table 4.
Correlation between MACC1 expression and MVD in 122 CCA tissues
| MACC1 expression | P-value | ||
|---|---|---|---|
| Low | High | ||
| MVD | 0.001a | ||
| High | 17 (29.8%) | 39 (60.0%) | |
| Low | 40 (70.2%) | 26 (40.0%) | |
Note:
P<0.01, statistical significance by chi-squared test.
Abbreviations: CCA, cholangiocarcinoma; MVD, microvessel density.
MACC1 knockdown reduces VEGFA expression
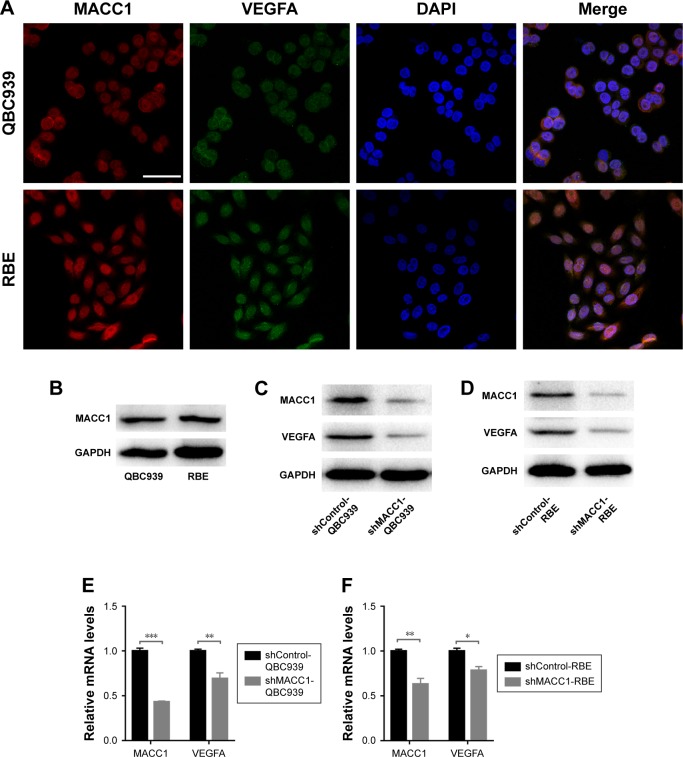
Based on the positive correlation between MACC1 and VEGFA observed in CCA tissues, we speculated that MACC1 regulates VEGFA. We first evaluated the localization of the two proteins by confocal laser scanning microscopy. In both CCA cell lines, MACC1 and VEGFA were expressed in the cytoplasm and nucleus (Figure 5A). Then, we assessed the protein levels of MACC1 in QBC939 and RBE cells. Both cell lines exhibited the same MACC1 protein levels (Figure 5B). We knocked down MACC1 in both types of CCA cells via plasmid transfection. Western blotting and real-time qPCR verified that knocking down MACC1 significantly downregulated the protein and mRNA expression of VEGFA in both CCA cell lines (Figure 5C–F). These results confirmed that MACC1 knockdown significantly reduced VEGFA expression at both the protein and mRNA levels.
Figure 5.
Localization of MACC1 and VEGFA in human CCA cell lines and downregulation of VEGFA expression by MACC1 knockdown in CCA cells.
Notes: (A) Confocal laser scanning microscopy was used to determine the localizations of MACC1 (red) and VEGFA (green) in QBC939 cells (up) and RBE cells (down). Scale bars =50 µm. (B) MACC1 protein levels in QBC939 and RBE cells. (C, D) Impact of MACC1 knockdown on VEGFA levels in CCA cells assessed by Western blotting analysis. GAPDH was used as an internal control. (E, F) Impact of MACC1 knockdown on VEGFA levels in CCA cells assessed by real-time qPCR analysis. The assay was performed in three independent experiments. The expression levels were normalized to those of GAPDH. *P<0.05, **P<0.01, ***P<0.001.
Abbreviation: CCA, cholangiocarcinoma.
MACC1 knockdown reduces VEGFA secretion and angiogenesis
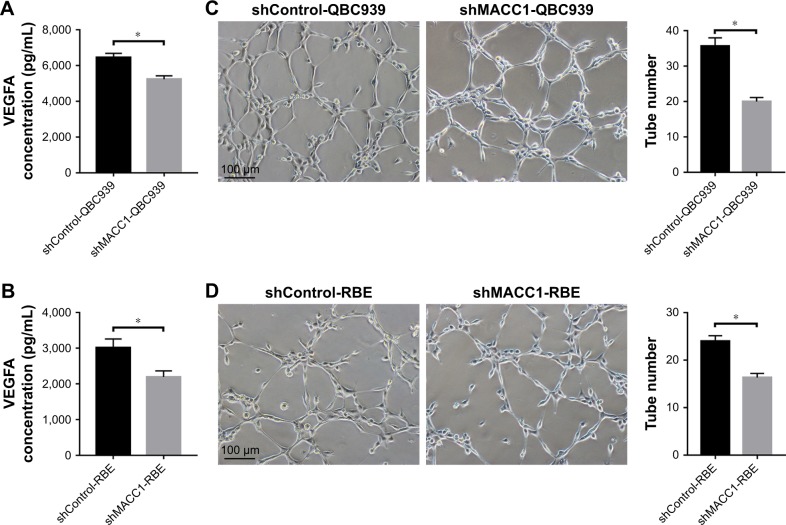
To determine the effect of MACC1 on VEGFA secretion and angiogenesis in CCA, we first used ELISA to measure the levels of secreted VEGFA in conditioned medium of cells with MACC1 knockdown. We found that MACC1 knockdown significantly decreased the level of secreted VEGFA compared with that in the shControl group in both QBC939 and RBE cells (P<0.01 and P<0.01, Figure 6A and B). Then, we performed a tube formation assay in Matrigel using HUVECs cultured in conditioned medium from MACC1-knockdown CCA cells. The number of tubes formed after exposure to medium from the MACC1 knockdown group was significantly lower than that after exposure to medium from the shControl group in both QBC939 and RBE cells (P<0.01 and P<0.01, Figure 6C and D). Taken together, these results suggest that MACC1 knockdown significantly reduces VEGFA secretion and angiogenesis in CCA.
Figure 6.
MACC1 knockdown decreases VEGFA secretion and angiogenesis in CCA cells.
Notes: (A, B) MACC1 knockdown in QBC939 and RBE cells reduced the VEGFA protein concentration in conditioned medium, as detected by ELISA. (C, D) MACC1 knockdown in QBC939 and RBE cells suppressed HUVECs tube formation. Representative images of tube-like structures were obtained, and the mean number of tubes in the entire field was calculated (right). The assay was performed in three independent experiments. Bar graphs show ± SEM. *P<0.01.
Abbreviations: CCA, cholangiocarcinoma; HUVECs, human umbilical vein endothelial cells; SEM, standard error of mean; shControl, short hairpin Control; shMACC1, short hairpin metastasis-associated in colon cancer-1.
Discussion
The early diagnosis rate of CCA is low, and patients have a poor prognosis. Most cases are clinically diagnosed at advanced stages or when distant metastasis has already occurred. MACC1, an oncogene that regulates colon cancer metastasis, has been reported to be highly expressed in several types of cancer cells. High levels of MACC1 are associated with lymph node metastasis and TNM stage in gastric carcinoma as well as with a lower survival in some cancers, including non-small-cell lung cancer, hepatocellular carcinoma, and colorectal cancer.16,24–26 These studies of MACC1 in cancer highlight the importance of MACC1 in predicting patient prognosis. In our study, we found that MACC1 is expressed at a significantly higher level in carcinoma tissues than in paracarcinoma tissues according to the TCGA and GEO datasets. Subsequently, we verified the results in paraffin-embedded and paired frozen CCA and paracarcinoma samples. Moreover, high expression of MACC1 is associated with lymph node metastasis and poor survival and was also an independent predictor for overall survival. These results implied that MACC1 might accelerate the progression of CCA and may serve as a new parameter for the prognostic prediction of CCA.
The sustained growth and metastasis of CCA depends on sufficient blood supply and angiogenesis. VEGFA, the most well-known regulator of angiogenesis, promotes tumor and endothelial cell proliferation and survival through autocrine or paracrine pathways.27 Increased VEGFA expression is associated with the development of multiple tumors and malignancies, including breast,28 colorectal,29 and lung cancer.30 In our study, we found that VEGFA expression is higher in paraffin-embedded and frozen CCA tissues than in paracarcinoma tissues. Moreover, we found a positive correlation between MACC1 and VEGFA in the TCGA and GEO datasets. Subsequently, we further confirmed the positive correlation among MACC1 expression, VEGFA expression, and MVD in CCA tissue samples. Using immunohistochemical analysis of human gastric cancer, previous studies have shown that MACC1 is positively associated with MVD.17 Some researchers have also reported that ectopic expression of MACC1 enhanced cell angiogenesis in cervical cancer cells.18 We first evaluated the correlation of MACC1 with angiogenesis in CCA. In vitro, alterations in MACC1 expression affected VEGFA expression and secretion as well as angiogenesis.
MACC1 transcriptionally activates Met to induce tumor cell invasion, migration and proliferation through the HGF/Met signaling pathway11,31,32 and promotes angiogenesis through the TWIST1/VEGFA signaling pathway.17,33 However, whether MACC1 regulates VEGFA in CCA through direct transcription or through other signaling pathways requires further investigation.
Conclusion
In conclusion, we found that MACC1 and VEGFA are upregulated in CCA and that high expression of MACC1 and VEGFA predicts poor survival. Moreover, MACC1 is an independent predictor of overall survival and facilitates CCA angiogenesis by upregulating VEGFA.
Ethical approval and informed consent
This study was approved by the Ethics Committee of Southwest Hospital, at Army Medical University, Chongqing, China. The participants provided written informed consent and this study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81572456) and the Natural Science Foundation of Southwest Hospital (grant number Swh2016yscxzd-02).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the study of cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13(5):261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut Liver. 2017;11(1):13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell M, Valle JW. The role of adjuvant chemotherapy and radiotherapy for cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):333–343. doi: 10.1016/j.bpg.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Pan TT, Wang W, Jia WD, Xu GL. A single-center experience of sorafenib monotherapy in patients with advanced intrahepatic cholangiocarcinoma. Oncol Lett. 2017;13(5):2957–2964. doi: 10.3892/ol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30(4):1646–1651. doi: 10.1007/s10637-011-9719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 7.Tang D, Nagano H, Yamamoto H, et al. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol Rep. 2006;15(3):525–532. [PubMed] [Google Scholar]
- 8.Benckert C, Thelen A, Cramer T, et al. Impact of microvessel density on lymph node metastasis and survival after curative resection of pancreatic cancer. Surg Today. 2012;42(2):169–176. doi: 10.1007/s00595-011-0045-0. [DOI] [PubMed] [Google Scholar]
- 9.Tynninen O, Sjöström J, von Boguslawski K, et al. Tumour microvessel density as predictor of chemotherapy response in breast cancer patients. Br J Cancer. 2002;86(12):1905–1908. doi: 10.1038/sj.bjc.6600325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thelen A, Scholz A, Weichert W, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105(5):1123–1132. doi: 10.1038/ajg.2009.674. [DOI] [PubMed] [Google Scholar]
- 11.Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15(1):59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 12.Sattler M, Salgia R. C-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr Oncol Rep. 2007;9(2):102–108. doi: 10.1007/s11912-007-0005-4. [DOI] [PubMed] [Google Scholar]
- 13.Shimokawa H, Uramoto H, Onitsuka T, et al. Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg. 2011;141(4):895–898. doi: 10.1016/j.jtcvs.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Kang MX, Lu WJ, Chen Y, Zhang B, Wu YL. MACC1: a potential molecule associated with pancreatic cancer metastasis and chemoresistance. Oncol Lett. 2012;4(4):783–791. doi: 10.3892/ol.2012.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederer A, Herrmann P, Seehofer D, et al. Metastasis-associated in colon cancer 1 is an independent prognostic biomarker for survival in Klatskin tumor patients. Hepatology. 2015;62(3):841–850. doi: 10.1002/hep.27885. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Wu Y, Lin L, et al. Metastasis-associated in colon cancer-1 upregulation predicts a poor prognosis of gastric cancer, and promotes tumor cell proliferation and invasion. Int J Cancer. 2013;133(6):1419–1430. doi: 10.1002/ijc.28140. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zhou R, Zhao Y, et al. MACC-1 promotes endothelium-dependent angiogenesis in gastric cancer by activating TWIST1/VEGF-A signal pathway. PLoS One. 2016;11(6):e0157137. doi: 10.1371/journal.pone.0157137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Xu CJ, Wang JX, et al. Metastasis-associated in colon cancer-1 associates with poor prognosis and promotes cell invasion and angiogenesis in human cervical cancer. Int J Gynecol Cancer. 2015;25(8):1353–1363. doi: 10.1097/IGC.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farshidfar F, Zheng S, Gingras MC, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18(11):2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaisaingmongkol J, Budhu A, Dang H, et al. Common molecular subtypes among Asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell. 2017;32(1):e53, 57–70. doi: 10.1016/j.ccell.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Li D, Li X, et al. 67 laminin receptor promotes the malignant potential of tumour cells up-regulating lysyl oxidase-like 2 expression in cholangiocarcinoma. Dig Liver Dis. 2014;46(8):750–757. doi: 10.1016/j.dld.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Li Z, Wu C, et al. MACC1 overexpression predicts a poor prognosis for non-small cell lung cancer. Med Oncol. 2014;31(1):790. doi: 10.1007/s12032-013-0790-6. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Lu Z, Liang Z, et al. Metastasis-associated in colon cancer-1 is associated with poor prognosis in hepatocellular carcinoma, partly by promoting proliferation through enhanced glucose metabolism. Mol Med Rep. 2015;12(1):426–434. doi: 10.3892/mmr.2015.3416. [DOI] [PubMed] [Google Scholar]
- 26.Koelzer VH, Herrmann P, Zlobec I, Karamitopoulou E, Lugli A, Stein U. Heterogeneity analysis of metastasis associated in colon cancer 1 (MACC1) for survival prognosis of colorectal cancer patients: a retrospective cohort study. BMC Cancer. 2015;15:160–171. doi: 10.1186/s12885-015-1150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 28.Santos LV, Cruz MR, Lopes Gde L, Lima JP. VEGF-A levels in bevacizumab-treated breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2015;151(3):481–489. doi: 10.1007/s10549-015-3410-7. [DOI] [PubMed] [Google Scholar]
- 29.Slattery ML, Lundgreen A, Wolff RK. VEGFA, FLT1, KDR and colorectal cancer: assessment of disease risk, tumor molecular phenotype, and survival. Mol Carcinog. 2014;53(Suppl 1):E140–E150. doi: 10.1002/mc.22058. [DOI] [PubMed] [Google Scholar]
- 30.Frezzetti D, Gallo M, Maiello MR, et al. VEGF as a potential target in lung cancer. Expert Opin Ther Targets. 2017;21(10):959–966. doi: 10.1080/14728222.2017.1371137. [DOI] [PubMed] [Google Scholar]
- 31.Stein U, Smith J, Walther W, Arlt F. MACC1 controls Met: what a difference an Sp1 site makes. Cell Cycle. 2009;8(15):2467–2469. doi: 10.4161/cc.8.15.9018. [DOI] [PubMed] [Google Scholar]
- 32.Sheng XJ, Li Z, Sun M, et al. MACC1 induces metastasis in ovarian carcinoma by upregulating hepatocyte growth factor receptor c-MET. Oncol Lett. 2014;8(2):891–897. doi: 10.3892/ol.2014.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu ZZ, Chen LS, Zhou R, Bin JP, Liao YL, Liao WJ. Metastasis-associated in colon cancer-1 in gastric cancer: beyond metastasis. World J Gastroenterol. 2016;22(29):6629–6637. doi: 10.3748/wjg.v22.i29.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]