Abstract
The present study assessed whether tefillin use (tight, nonocclusive, wrapping of the arm) elicits a remote ischemic preconditioning (RIPC)-like effect in subjects with both acute and chronic use. RIPC, created by short bursts of ischemia-reperfusion, has not been successfully taken to the bedside. Several large population studies have found that Orthodox Jewish men (who wear tefillin almost daily) have decreased cardiovascular mortality compared with non-Orthodox counterparts. We hypothesized that tefillin use is a relevant component in triggering a preconditioning effect. Jewish men (n = 20) were enrolled; 9 men were daily tefillin users (conditioned) and 11 men were nonusers of tefillin as controls (naïve). Subjects were evaluated for adherence to traditional Jewish practice, had vital signs measured, blood drawn for analysis of circulating cytokines and monocyte function, and underwent brachial flow-mediated dilation to evaluate vascular reactivity at baseline (basal) and after 30 min of using tefillin (acute treatment). Under basal conditions, both groups had similar peak systolic velocity (SV), diameter, and flow volume, although the conditioned group had higher SV at 120 s postdeflation (P = 0.05). Acute tefillin use augmented artery diameter and flow volume in both groups, with conditioned subjects experiencing higher SV than control subjects at 90 and 120 s postdeflation (P = 0.03 and P = 0.02, respectively). Conditioned subjects had decreased inflammation, monocyte migration and adhesion, and endothelial activation compared with control subjects at baseline. Acute use of tefillin did not significantly alter monocyte function in either group. In this pilot study, acute tefillin use improves vascular function, whereas chronic tefillin use is associated with an anti-inflammatory RIPC-like phenotype.
NEW & NOTEWORTHY We hypothesized that tefillin use among Orthodox Jewish men (who practice a nonocclusive leather banding of their nondominant arm) will induce a remote ischemic preconditioning phenotype. Chronic use of tefillin in Orthodox Jewish men was associated with increased systolic velocity and attenuated inflammation and monocyte chemotaxis and adhesion versus Jewish men who do not wear tefillin. Acute use of tefillin in both populations augmented brachial artery diameter and blood flow but not inflammatory profiles compared with baseline.
Keywords: chemokines, cytokines, flow-mediated dilatation, monocyte activity, remote ischemic preconditioning
INTRODUCTION
Remote ischemic preconditioning (RIPC) induces cardioprotection against prolonged ischemic injuries via short bursts of vascular obstruction to a distant organ or limb. It has been induced by blood occlusion to the hindlimbs (3), brain (49), kidney (39), and mesentery (37) of various species, including mice (49), rats (37), and rabbits (12, 30), and has been found to provide cardiac protection to subsequent ischemic events. These studies have elucidated the overlapping mechanisms behind RIPC, which include changes to the vascular, inflammatory, neuronal, and humoral systems (for reviews, see Refs. 3 and 7). Specifically, RIPC has been found to decrease the adhesion and functional response of neutrophils in humans (44), whereas vascular reactivity, flow-mediated vascular dilation, and endothelial function are all improved when subjects (including humans) were exposed to RIPC before ischemia-reperfusion (I/R) injury (23). Inflammatory changes that occur during RIPC include regulation of gene expression, neutrophil activation, and secretion of inflammatory markers (26, 27). Despite these studies, RIPC has not yet been incorporated into clinical practice (15a).
Clinical trials using RIPC have attempted to examine the protection of cardiac (14), cerebral (52), and kidney function (6), although few have successfully demonstrated a clinically significant effect (5a, 24). To date, RIPC in ischemic heart disease is limited to a class IIb recommendation (i.e., the weakest benefit-to-risk outcome) for patients undergoing coronary artery bypass surgery per the American Council for Capital Formation/American Heart Association guidelines (1, 16, 54). Several issues have potentially limited the clinical benefit of RIPC in clinical trials, such as 1) the preconditioning stimuli (mostly an inflated and deflated blood pressure cuff), 2) the inherent difficulty predicting future ischemic events in patients, and 3) most clinical trials enroll patients with ongoing or known ischemic processes. As such, an ideal RIPC stimulus would cause transient vascular obstruction without overt tissue damage and would be used regularly before an ischemic event in subjects without known ischemic disease.
Observant Orthodox Jewish adults, traditionally men, tightly bind a nonocclusive leather strap, known as phylacteries or tefillin, onto the nondominant arm and forearm during morning prayers (50). This is performed without causing complete occlusion of the blood flow on a near-daily basis (excluding the Sabbath and certain holidays) after the age of 13 yr for ~30−45 min. While the effects of this precise stimulus have not been scientifically studied, prior population-based studies have suggested that Orthodox Jewish men (not women) have a lower cardiovascular risk even after adjusting for traditional risk factors (11, 21). We assessed whether wearing tefillin both acutely and chronically provided an improved vascular and anti-inflammatory RIPC-like phenotype.
We recruited 20 healthy Jewish men who identify either as Orthodox (wear tefillin daily) or secular/traditional (controls) and examined their vascular reactivity, monocyte function, and inflammatory markers. We further examined the effect of a single acute use of tefillin by having the subjects wear tefillin in the laboratory for 30 min, repeating the previously stated analyses. Acute tefillin usage significantly altered vascular reactivity in both groups. Furthermore, Orthodox subjects were statistically different from control subjects at baseline with regard to vascular reactivity and inflammatory mediators. Our results suggest that tefillin use may result in a protective RIPC-like phenotype.
MATERIALS AND METHODS
Participants
We recruited 20 healthy, self-identified Jewish men 18−40 yr of age from the greater Cincinnati area; specifically, they were recruited from the University of Cincinnati campus, affiliated religious organizations, and the local religious seminary. Nine adults wore tefillin (conditioned) on a daily basis, whereas eleven men did not (naïve). All study procedures were approved by the University of Cincinnati Institutional Review Board (no. 2013-8174).
Study Design
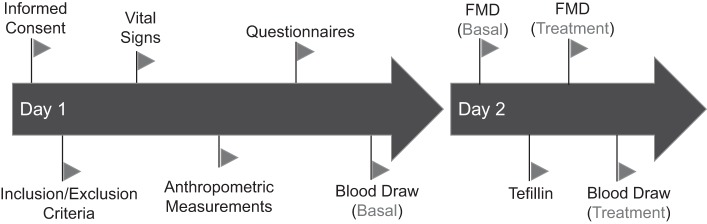
All subjects gave written informed consent and completed a questionnaire ascertaining health and religious practices. Inclusion criteria was defined as healthy Jewish men between the ages of 18 and 40 yr who were able to adhere to study procedures. Major exclusion criteria were nondominant arm trauma, nerve or vascular damage, any significant past medical history, and prescription medications. Subjects underwent a 2-day study protocol investigating the effect of daily tefillin (Fig. 1). On day 1, vital signs and anthropometric measurements were obtained, the subject completed questionnaires regarding their adherence to Jewish culture, diet, and exercise, and blood was drawn upon the completion of the visit. Subjects returned the following day to undergo a basal brachial artery flow-mediated dilatation (BFMD) via ultrasound at Cincinnati Children’s Hospital Medical Center to measure vascular reactivity, endothelial function, reactive hyperemia, and volume flow changes. All subjects then wore tefillin in a traditional manner that did not fully occlude the blood flow (those that were unsure of how to wear tefillin were assisted) for a total of 30 min in a lit, quiet room where they were asked to remain relatively still until the BFMD, which was performed again followed by a final blood draw.
Fig. 1.
Study design of naïve and chronic tefillin users. All subjects underwent the same 2-day protocol to assess the effects of chronic tefillin on vascular reactivity, leukocyte activity (migration and adhesion), and systemic inflammation. Before any study procedures were performed, informed consent was obtained and inclusion/exclusion criteria were then verified. Once enrolled, vital signs and anthropometric measurements were obtained, and subjects answered questions pertaining to their degree of adherence to Jewish Orthodox traditions and cardiovascular health. At the end of day 1, blood was collected for inflammatory and in vitro experiments. On day 2, a brachial flow-mediated dilation (FMD) study was performed to assess vascular reactivity.
Experimental Measures
Brachial artery ultrasound assessment.
Assessment of BFMD using a GE Vivid 7-V7916 Ultrasound System (Horten, Norway) was performed as outlined in guidelines written by the International Brachial Artery Reactivity Task Force (7). Subjects were asked to refrain from ingesting caffeine, using tobacco, or exercising for 8 h and were then asked to lay supine on a standard hospital bed for 10 min of rest in a temperature-controlled room.
Pulse wave velocities and two-dimensional (2-D) diameters were measured with a multifrequency, 11- to 14-MHz, linear array transducer with the subject in the supine position and the dominant arm extending 80−90° from the body. A 5 × 41-cm tourniquet pneumatic cuff was applied to the widest part of the forearm below the antecubital fossa. Images were optimized using ultrasound system settings to display a brachial artery segment perpendicular to the imaging screen and with anterior and posterior intimal interfaces between the lumen and vessel wall and two parallel lines representing echoes that arise from the blood intima and media adventitia boundaries on the near and far walls. Anatomic landmarks (arterial branches, veins, or fascial planes) were noted for reproducible transducer placement. 2-D images and pulse wave Doppler (PWD), 60° and parallel to the vessel wall, were acquired at baseline, preinflation, 2–15 cm above the elbow. The cuff was inflated to 250 mmHg for all subjects. After 5 min, the cuff was deflated. PWD was acquired immediately after cuff deflation and at 60, 90, and 120 s after cuff deflation. Brachial artery 2-D images were acquired at 60, 90, and 120 s after deflation. 2-D diameters for BFMD were acquired at three points along <1-cm length of the brachial artery at end diastole (peak of the R wave) using Digisonics DigiView (Houston, TX) from the trailing edge of intima/blood interface to the leading edge of the blood/intima interface. PWD peak and mean systolic velocities (in cm/s) for reactive hyperemia were measured for three cycles at baseline, the fourth, fifth, and sixth beats after cuff deflation, and 60, 90, and 120 s after cuff deflation using Digisonics DigiView. The time velocity interval (in cm) and flow interval (in ms) were measured immediately after cuff deflation from baseline to baseline at the fourth, fifth, and sixth beats after cuff deflation in triplicate using Digisonic DigiView for the area under the curve. Brachial artery flow volumes (in ml/min) were measured once each at baseline, immediately after cuff deflation, and 60, 90, and 120 s after cuff deflation on the cart from ultrasound manufacturer’s software.
Blood collection and processing.
Blood was collected the day before BFMD examination into 5-ml 3.2% sodium citrated vacutainers (BD glass Vacutainers, Becton Dickinson, Franklin Lakes, NJ). Blood was then pooled into sterile 50-ml conical tubes and spun at 200 g for 10 min. The plasma was removed and spun at 1,500 g for 20 min, and supernatant frozen at −80°C until analysis. The plasma was removed from the original blood and was replaced with an equivalent volume of 10 mM EDTA in HBSS (GIBCO, Rockville, MD). Blood was then layered onto sterile endotoxin 5-free ficoll-paque plus (GE Healthcare, Piscataway, NJ) and spun at 400 g for 30 min with no brake. Peripheral blood mononuclear cells (PBMCs) were collected in the buffy coat layer and washed three times in 1× PBS with repeated spin down at 500 g for 10 min. The final PBMC pellet was stored in Recovery cell culture freezing medium (GIBCO), slowly frozen at −80°C, and transferred to the vapor layer of a liquid nitrogen storage unit until usage.
Plasma cytokine analysis.
We examined plasma and PBMC protein chemokines and cytokines using commercially available ELISAs for the following proteins: chemokine (C-C motif) receptor 2 (CCR2; MyBioSource, San Diego, CA), chemokine (C-X3-C motif) ligand 1 (CX3CL1; R&D Systems, Minneapolis, MN), chemokine (C-X3-C motif) receptor 1 (CX3CR1; Lsbio, LifeSpan BioSciences, Seattle, WA), monocyte chemoattractant protein (MCP)-1 (R&D Systems), and VCAM-1 (Life Technologies, Frederick, MD). Furthermore, each sample was run in duplicate for quality control.
PBMC migration assay.
PBMC migration through a 5-µm membrane was assessed via a 96-well chemotaxis assay (Cell Biolabs, San Diego, CA), as specified by the manufacturer. Briefly, MCP-1 (250 ng/ml, R&D Systems) was placed in the bottom chamber of the well, and 500,000 PBMCs were placed in the upper chamber (triplicate for each subject). After 24 h, PBMC chemotaxis through the membrane was assessed using manufacturer protocols. Cell numbers were assessed using a standard curve of serially diluted PBMCs with known starting numbers.
PBMC adhesion assay.
Adhesion of subject PBMCs was performed as previously described (44). Briefly, human coronary artery endothelial cells (Lonza, Cologne, Germany) were seeded onto 24-well plates and grown to confluence, as per Lonza's instructions. Cells were then washed and treated with recombinant TNF-α (10 ng/ml; R&D Systems) for 24 h. PBMCs were thawed, washed with RPMI medium, and labeled with calcein-red-orange (Life Technologies, Carlsbad, CA) at a final concentration of 10 μM. After three washes, PBMCs were diluted in culture media, and 50,000 cells were added to each well of human coronary artery endothelial cells for 30 min. Unbound PBMCs were washed away (three separate washes), and the monolayer was fixed with 1% glutaraldehyde. Attached fluorescent monocytes were imaged using a Cytation 5 plate reader. Twelve randomly selected portions of each well were then selected at ×20 magnification and counted using thresholding (ImageJ, National Institutes of Health, Bethesda, MD). Representative samples are the average of all 12 sections/subject. All subject PBMCs were replicated in triplicate.
Protein assay.
Where applicable, protein was isolated from PBMCs via incubation with 1× cell lysis buffer (Cell Signaling, Danvers, MA) on ice for 60 min with intermittent vortexing. Protein was quantified using the DC protein assay (Bio-Rad, Hercules, CA) in the 96-well plate assay according to the product manual.
RT-PCR.
RNA was isolated from PBMCs using a Macherey-Nagel NucleoSpin RNA kit, and cDNA was synthesized using a BioScript All-in-One cDNA Synthesis SuperMix (Biotool, Houston, TX). Samples were run on Stratagene Mx3005P (Agilent Technologies) using SYBR Green quantitative PCR Master Mix (Biotool) to assess levels of GAPDH and human antigen R (HuR). Results were analyzed using the ΔΔCt method (46). The following primers were used: GAPDH, forward 5′-ACCACAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTGCTGTA-3′; and HuR, forward 5′-ATTAACCCTCAAAGTTCTCTTCATAACTGC-3′ and reverse 5′-GAGCAAAACAAAATCAAATTTA ATGGTCTT-3′.
Statistics
All statistical analysis was performed using SigmaStat (version 13, SPSS, Chicago, IL) or SAS (version 9.4, SAS Institute, Cary, NC). Data are presented as means ± SE or SE where specified. All data were assessed for normality and equal variance. For multiple group comparisons of nonparametric data, we used ANOVA on ranks with Dunn’s or Tukey’s post hoc analysis where appropriate. BFMD was analyzed via longitudinal mixed modeling, accounting for repeated measures, with group, time, and group × time interaction terms. Subject religious characteristic data were compared using χ2-analysis. Values of P ≤ 0.05 were considered statistically significant. All plots were created using SigmaPlot (version 13, SPSS).
RESULTS
Descriptions of the study population, including demographic information, vital signs, and religious characteristics, are shown in Table 1 and Table 2. Control and tefillin users were similar in heart rate and blood pressure, but tefillin users were somewhat younger and had lower average height and weight. However, body mass index and waist circumference did not differ between groups. As anticipated, tefillin users were more likely to follow Orthodox Jewish practices.
Table 1.
Demographic and vital statistics for the subjects
| Subject Characteristics | Control (Naïve) | Tefillin (Conditioned) | Total Subjects | P Value |
|---|---|---|---|---|
| n, % | 11 (55) | 9 (45) | 20 (100) | |
| Age, yr | 28.3 (7.0) | 21.8 (3.0) | 25.4 (6.4) | 0.02 |
| Waist circumference, cm | 93.2 (10.3) | 85.5 (9.2) | 89.7 (10.3) | 0.10 |
| Height, in. | 69.6 (2.2) | 66.8 (1.8) | 68.4 (2.4) | 0.01 |
| Weight, lbs | 178.5 (26.3) | 154.8 (20.6) | 167.2 (26.3) | 0.04 |
| Body mass index, kg/m2 | 25.8 (2.9) | 24.5 (3.6) | 25.2 (3.2) | 0.38 |
| Systolic blood pressure, mmHg | 127.8 (9.2) | 123.1 (8.1) | 125.7 (8.8) | 0.25 |
| Diastolic blood pressure, mmHg | 81.5 (5.6) | 78.7 (7.6) | 80.3 (6.7) | 0.35 |
| Heart rate, beats/min | 75.0 (11.3) | 71.2 (12.9) | 73.3 (11.9) | 0.49 |
All values are means (SD) except the number of subjects (n), which is in percent. Statistical analyses were performed between naïve and conditioned groups using Student’s t-test or a Mann-Whitney rank U-test where appropriate.
Table 2.
Religious characteristics of the subjects
| Subject Characteristics | Control (Naïve) | Tefillin (Conditioned) | Total Subjects | P Value |
|---|---|---|---|---|
| Faith | 0.002 | |||
| Nonbelievers | 0 (0) | 0 (0) | 0 (0) | |
| Secular | 3 (27) | 0 (0) | 3 (15) | |
| Traditional | 6 (55) | 0 (0) | 6 (30) | |
| Modern Orthodox | 2 (18) | 1 (11) | 3 (15) | |
| Orthodox | 0 (0) | 8 (89) | 8 (40) | |
| Tefillin frequency | 0.001 | |||
| Daily | 0 (0) | 9 (100) | 9 (45) | |
| 4–5 days/wk | 0 (0) | 0 (0) | 0 (0) | |
| 1–3 days/wk | 0 (0) | 0 (0) | 0 (0) | |
| <1/wk | 5 (45) | 0 (0) | 5 (25) | |
| Never | 6 (55) | 0 (0) | 6 (30) | |
| Duration of tefillin | 0.052 | |||
| ≥30 min | 2 (18) | 8 (89) | 10 (50) | |
| 10–29 min | 3 (27) | 1 (11) | 4 (20) | |
| <10 min | 0 (0) | 0 (0) | 0 (0) | |
| Arm marking | 0.915 | |||
| ≥30 min | 2 (18) | 5 (56) | 7 (35) | |
| 10–29 min | 1 (9) | 1 (11) | 2 (10) | |
| <10 min | 1 (9) | 2 (22) | 3 (15) | |
| No marks | 1 (9) | 1 (11) | 2 (10) | |
| Activities on Shabbat | 0.047 | |||
| Traditional Orthodox rules | 3 (27) | 7 (78) | 10 (50) | |
| Some changes | 4 (36) | 2 (22) | 6 (30) | |
| No change | 4 (36) | 0 (0) | 4 (20) | |
| Diet | 0.014 | |||
| Traditional Orthodox rules | 3 (27) | 8 (89) | 11 (55) | |
| Orthodox-style food | 2 (18) | 1 (11) | 3 (15) | |
| No consideration | 6 (55) | 0 (0) | 6 (30) | |
| Alcohol consumption | 0.017 | |||
| Daily | 0 (0) | 0 (0) | 0 (0) | |
| Weekly | 5 (45) | 9 (100) | 14 (70) | |
| Monthly | 6 (55) | 0 (0) | 6 (30) | |
| Not at all | 0 (0) | 0 (0) | 0 (0) |
All values are presented as numbers of subjects (n) with percentages in parenthese. Statistical analyses were performed between control and tefillin groups using χ2-tests.
Chronic Tefillin Use Increases Arterial Blood Flow
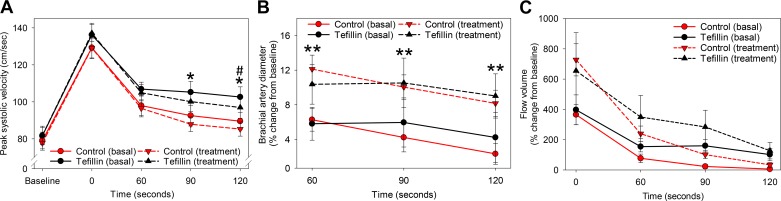
To test whether blood flow and endothelial function were altered with tefillin use, we performed BFMD in naïve subjects without daily tefillin use (naïve, n = 11) and in tefillin-wearing subjects (conditioned, n = 9; Fig. 1). Under resting conditions, the diameter of the contralateral brachial artery on both groups was not significantly different (0.35 ± 0.01 vs. 0.36 ± 0.02 cm, P = 0.31, conditioned vs. naïve). Similarly, peak systolic velocity (SV) (81.70 ± 4.5 vs. 79.09 ± 4.5 cm/s, P = 0.77) and flow volume (63.36 ± 12.42 vs.93.10 ± 17.80 ml/min, P = 0.81) were not significantly different between the conditioned and naïve groups at baseline.
Upon analysis of BFMD, both groups had similarly augmented peak SV immediately after cuff deflation (Fig. 2A, solid lines), with slightly increased peak SV in conditioned versus naïve subjects by 120 s postdeflation (0 s, P = 0.28; 60 s, P = 0.20; 90 s, P = 0.07; and 120 s, P = 0.05).
Fig. 2.
Tefillin use increases arterial flow and vascular diameter. After 10 min of rest in a temperature-controlled room and 8 h of fasting, naïve (control Jewish men, n = 11) and conditioned (tefillin-using Jewish men, n = 9) subjects underwent assessment of brachial artery size and function under basal conditions (solid lines) and immediately after 30 min of tefillin use (treatment, dashed lines). Peak systolic velocity (A), percent change in brachial artery diameter from preinflation baseline (B), and percent change in total blood flow volume from time 0 (C) were measured or calculated immediately after deflation (time 0) and 60, 90, and 120 s later. Circles show means and SEs for each group/time point. #P < 0.05 for comparison of conditioned vs. naïve groups at each time point under basal conditions; *P < 0.05 for comparison of conditioned vs. naïve groups at each time point after treatment, from mixed modeling accounting for multiple measurements per person; **P < 0.05 for comparison before and after treatment within both groups.
Both groups had a similar increase in the brachial artery diameter after deflation of the cuff at 60, 90, and 120 s (all P ≥ 0.48 for difference between groups; Fig. 2B, solid lines). Flow volume was increased in both groups immediately after cuff deflation (P = 0.52 for difference between groups) with no significant differences between groups in flow volume at 60 s (P = 0.21), 90 s (P = 0.15), and 120 s (P = 0.16) compared with naïve subjects (Fig. 2C, solid lines).
Acute Tefillin Use Improves Blood Flow for Both Conditioned and Naïve Groups
Acute tefillin use resulted in a similar pattern in SV to basal conditions after cuff deflation (Fig. 2A, dashed lines). However, with acute tefillin use, the conditioned group experienced a nonstatistically significant trend toward higher SV at cuff removal (P = 0.16) and at 60 s (P = 0.17), with statistically higher SV noted at the 90- and 120-s time points (P = 0.02 and 0.03, respectively).
Interestingly, acute tefillin use resulted in approximately twofold higher increase in brachial artery diameter versus the basal condition after cuff deflation in both conditioned and naïve groups (e.g., at 60 s: 10.5 ± 2.0% vs. 5.8 ± 2.0% increase in conditioned group and 12.1 ± 1.8% vs. 6.3 ± 1.8% increase in the naïve group under acute tefillin and basal conditions, respectively, P < 0.05 between conditions for all time points; Fig. 2B, dashed lines). However, there were no significant differences between conditioned and naïve groups after acute tefillin use for any time point.
Finally, flow volume also increased approximately twofold in both groups immediately after acute tefillin use and remained higher at all time points compared with testing under basal conditions (Fig. 2C, dashed lines). There were no statistically significant differences between the conditioned and naïve groups after acute tefillin use, although the preconditioned group demonstrated nonsignificant but slightly higher flow volume at all time points.
In summary, acute tefillin use resulted in increased brachial artery diameter and flow volume similarly in both groups and improved SV in the conditioned group.
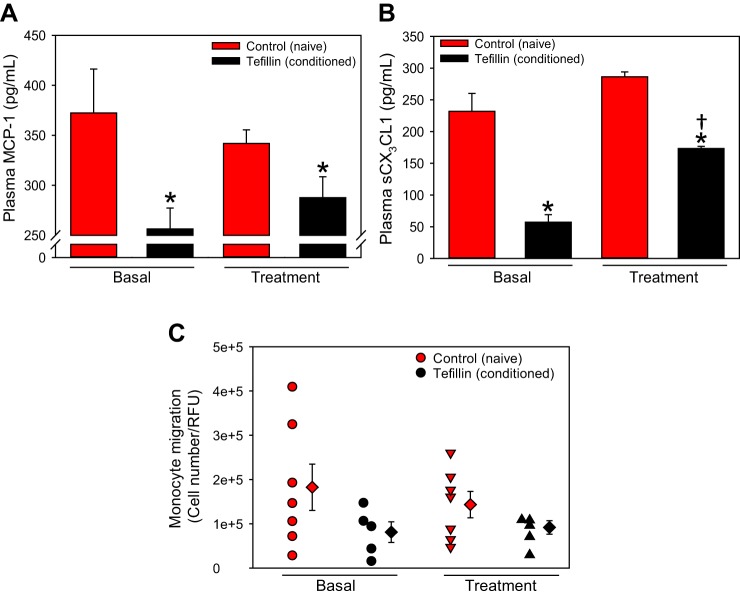
Chronic, But Not Acute, Tefillin Use Is Associated With Decreased Plasma Chemokines and Leukocyte Migration
A previous study (26) has suggested that RIPC suppresses proinflammatory activity in human leukocytes. To determine if tefillin use affected inflammatory markers, we examined the levels of circulating plasma chemokines in naïve and conditioned subject plasma. MCP-1 [also called chemokine (C-C motif) ligand 2; Fig. 3A] and soluble CX3CL1 (also called fractalkine; Fig. 3B) were significantly attenuated in the plasma of conditioned versus naïve control subjects at baseline and after acute tefillin treatment (P < 0.001). Interestingly, acute tefillin increased soluble CX3CL1 in tefillin users versus basal levels (P < 0.01).
Fig. 3.
Chronic use of tefillin attenuates plasma chemokines and leukocyte migration. Plasma monocyte chemoattractant protein 1 (MCP-1; A) and soluble chemokine (C-X3-C motif) ligand 1 (sCX3CL1; B) were assessed in naïve (n = 11) and conditioned (n = 9) subjects basally and after acute use of tefillin. Histobars present means ± SE. Peripheral blood mononuclear cells (PBMCs) were isolated from control (n = 7) or tefillin (n = 5) subjects, and a chemotactic response to MCP-1 (250 ng/ml) was performed using a modified Boyden chamber (C). Circles represent individual subjects; diamonds represent total category means ± SE. *P < 0.05 vs. all control groups, by ANOVA on ranks with Dunn’s post hoc analysis; †P < 0.05 vs. basal tefillin, by ANOVA on ranks with Tukey’s post hoc analysis. RFU, relative fluorescent units.
As circulating chemokines were decreased, we next determined whether the circulating leukocytes of conditioned subjects had altered chemotactic responses. After 24 h, 56% and 36% fewer conditioned leukocytes migrated through the membrane toward MCP-1 versus naïve PBMCs at baseline and after acute tefillin, respectively (Fig. 3C, P = 0.075 and P = 0.079). These results indicate that chronic, but not acute, tefillin use attenuates chemokine secretion and leukocyte chemotaxis.
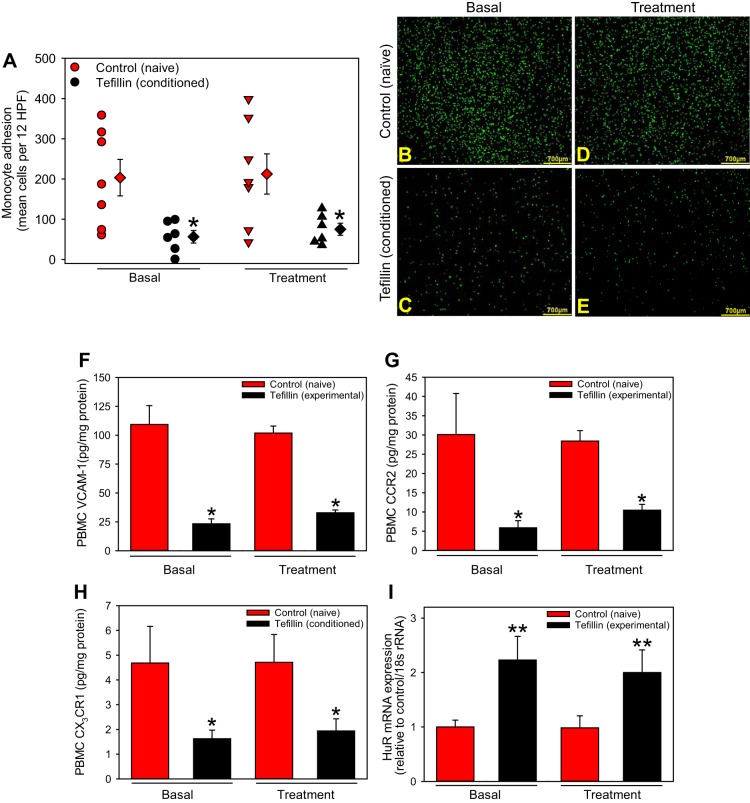
Chronic, But Not Acute, Tefillin Use Is Associated With Reduced Leukocyte Adhesion and Proinflammatory Proteins
Based on the above observation, we speculated that leukocytes from conditioned subjects may also attenuate adhesion to activated endothelial cells. Leukocyte adhesion was significantly diminished in conditioned versus naïve leukocytes at baseline and was unaltered after acute use of tefillin (P < 0.01; Fig. 4, A–E).
Fig. 4.
Chronic tefillin application reduces leukocyte adhesion and adhesion proteins. Peripheral blood mononuclear cells (PBMCs) isolated from control (n = 7) or tefillin (n = 6) subjects basally and after acute use of tefillin were labeled and incubated with TNF-α-treated human coronary artery endothelial cells for 24 h. Monocyte adhesion (A) was assessed by taking the mean of 12 random images/subject. Circles represent individual subjects; diamonds represent total category means ± SE. A representative high-power field is shown for control (B) and tefillin (C) subjects at baseline and for control (D) and tefillin (E) subjects after a single use (bars = 700 µm). PBMC protein concentrations of VCAM-1 (F), chemokine (C-C motif) receptor 2 (CCR2; G), and chemokine (C-X3-C motif) receptor 1 (CX3CR1; H) were measured in both groups at both time points via ELISA. Expression of the RNA-binding protein human antigen R (HuR; I) was assessed by quantitative PCR and extrapolated to GAPDH. Histobars present means ± SE. *P < 0.01 vs. all control groups. by ANOVA on ranks with Dunn’s post hoc analysis; **P < 0.05 vs. controls, by ANOVA on Ranks with Tukey’s post hoc analysis.
To determine if these differences were due to reduced expression of adhesion and chemokine receptors, and a key regulator of inflammatory gene transcription, HuR, leukocyte protein, and mRNA was analyzed. We demonstrate that leukocytes from conditioned subjects had significantly attenuated expression of the adhesion molecule VCAM-1, the MCP-1 receptor CCR2, and the CX3CL1 receptor CX3CR1 versus leukocytes from naïve subjects at baseline, which were not affected after acute use of tefillin (P < 0.001; Fig. 4, F–H). Interestingly, HuR mRNA was similarly augmented in conditioned versus naïve PBMCs at both baseline and after acute tefillin (Fig. 4I). These results suggest chronic, but not acute, tefillin usage reduces the expression of adhesion molecules and chemokine receptors associated with leukocyte adhesion to endothelial cells.
DISCUSSION
RIPC via full vascular occlusion induces a cardioprotective phenotype and reduces the severity of I/R injury and inflammation in both animal models and humans. The data from our study demonstrate that chronic nonocclusive tefillin use is associated with somewhat increased blood velocity and arterial diameter with decreases in inflammatory mediators in the plasma and on circulating PBMCs. Furthermore, acute use of tefillin augmented blood velocity, flow volume, and brachial artery diameter in both naïve and conditioned subjects. Our results suggest that tefillin use may act as an RIPC-like nonocclusive stimulus in a similar fashion to the fully occlusive techniques commonly used in prior animal and human studies.
Vascular Reactivity
Our conditioned subjects had augmented and sustained blood flow, as measured by BFMD, despite no significant differences at baseline compared with naïve subjects. Peak SV increases observed are consistent with Loukogeorgakis and colleagues (31), who demonstrated that three 5-min cycles of ischemia (blood pressure cuff inflated to 200 mmHg) of the contralateral arm blunted endothelial injury induced by arm ischemia in healthy human subjects at both early (20 min) and late (24 and 48 h) time points. Moreover, RIPC induced in a different study by the same group via blood pressure cuff application to the upper part of the contralateral arm or leg was also able to attenuate ischemia-induced reductions in BFMD (32). Our data are consistent with these studies and further demonstrate that both chronic and acute use of tefillin induce measurable vascular effects with chronic use, whereas acute use dramatically increases brachial artery diameter and immediate flow volume after cuff deflation independent of chronic use as well as improved SV among those with chronic use.
In contrast with these acute studies, few studies have focused on long-term preconditioning. Jones et al. (19) demonstrated that 7 days of preconditioning (4 cycles of 5 min of cuff inflation to 220 mmHg) were sufficient to improve BFMD in both preconditioned and contralateral arms. Therefore, the stimulus of wearing tefillin chronically, even though it is nonocclusive in nature, induced a similar vasoreactive effect as in other studies that caused full extremity ischemia.
Inflammatory Pathways
Immediately after the onset of ischemia, endothelial cells upregulate adhesion molecules that, in conjunction with released chemokines, trigger the migration, adhesion, and extravasation of leukocytes, including neutrophils and monocytes (47). MCP-1 and CX3CL1 are two unique chemokines upregulated during injury and inflammation that bind to and recruit leukocytes via engagement of CCR2 and CX3CR1, respectively. Importantly, MCP-1 and CX3CL1 are both acutely upregulated after myocardial infarction and after I/R (4, 15, 28, 29, 56). Considered an early marker of endothelial activation, endothelial and soluble VCAM-1 are also upregulated after reperfusion injury associated with acute injury, resulting in both the binding and extravasation of leukocytes (20, 35, 51). Here, we demonstrate that chronic tefillin users have decreased plasma MCP-1 and soluble CX3CL1 levels along with reduced protein concentrations of CCR2 and CX3CR1 on circulating PBMCs compared with naïve nonusers. Furthermore, soluble VCAM-1 is likewise decreased in chronic tefillin users, which is associated with attenuated leukocyte adhesion and chemotaxis. These data are consistent with chronic tefillin use, inducing an RIPC-like phenotype similar to Konstantinov and colleagues (26), demonstrating RIPC-induced attenuation of genes specific to leukocyte chemotaxis and adhesion and migration. As CCR2hi/CX3CR1low monocytes (referred to as inflammatory LY6Chi monocytes) and CX3CR1high/CCR2low subsets (referred to as LY6Clow monocytes) are recruited to the heart after ischemic injury and are critical for producing high levels of inflammatory cytokines and vascular damage, future studies will ascertain the role of tefillin-induced RIPC-like changes on these monocyte subsets (43).
Several studies have examined the effect of short-term RIPC on inflammation. Shimizu and colleagues (45) examined neutrophils in five healthy adult volunteers who underwent three 3- to 5-min periods of 200-mmHg cuff occlusion separated by 5 min of reperfusion. Blood was examined on days 0, 1, and 10 post-RIPC, resulting in significantly decreased neutrophil adhesion after days 1 and 10, reduced neutrophil apoptosis on days 1 and 10, and decreased phagocytosis activity on day 10. Interestingly, there were no changes in neutrophil-induced cytokine secretion on day 1. It is speculated that cytokines may only contribute to late-phase preconditioning, which occurs up to 24–72 h after the initial RIPC stimulus (53). Indeed, Shimizu and colleagues (45) found that IL-1β and IL-10 were unaltered after day 1 but significantly increased after day 10 of RIPC. Furthermore, Kono and colleagues (25) demonstrated that 10 patients with chronic heart failure had decreased TNFα, IL-6, troponin T, and brain natriuretic peptide after 7 days of RIPC stimulation compared with basal levels. Our results demonstrate acute tefillin did not alter circulating chemokines and leukocyte cell surface receptors (vs. basal levels). This result is not unexpected, as protein synthesis requires several hours poststimulation. Interestingly, acute tefillin use did augment plasma levels of soluble CX3CL1 only in conditioned subjects with acute tefillin treatment versus basal. As CX3CL1 is cleaved by cathepsin S, matrix metalloproteinases, and a disintegrin and metalloproteinase (ADAM)10 and ADAM17 (also called TNF-α-converting enzyme) to become soluble and ADAM17 is upregulated shortly after ischemic preconditioning, we speculate the increased soluble CX3CL1 is due to an activated TNF-α-converting enzyme (5, 9). However, this needs to be explored in future studies.
These results suggest that a nonocclusive RIPC-like stimulus may have clinical relevance to cardiovascular disease. For example, CX3CL1 is a proinflammatory chemokine that is not only linked to several clinical pathologies, such as atherosclerosis and cerebral ischemia, but also rheumatoid arthritis, human immunodeficiency virus infection, cancer, and multiple autoimmune disorders (18). There are currently no available pharmaceutical compounds that target CX3CL1 or its receptor CX3CR1. However, our experiments demonstrated that a chronic nonocclusive RIPC-like stimulus is associated with decreased plasma concentrations of CX3CL1 and monocyte expression of CX3CR1 in healthy subjects. While these results need to be examined in patients with known coronary disease, our results suggest it may be possible to prevent the expression of a key proinflammatory chemokine and its receptor via a noninvasive method without the use of pharmaceuticals, which might also be applied to several other disease processes.
The efficacy of the immune response and subsequent cytokine/chemokine release relies on the production and posttranscriptional regulation of mRNAs encoding effector proteins. One of the archetype proteins of this process is embryonic lethal abnormal vision-like 1 (Elav1)/HuR, which is a ubiquitously expressed protein binding to AU-rich RNA motifs proposed to either enhance or antagonize the stability of its target mRNAs, depending on concentration and localization (8, 36). While HuR has been associated with the stabilization and production of several proinflammatory molecules that play a role in ischemic cardiac injury, overexpression of HuR in mouse macrophages inhibits the translation of several inflammatory mediators, such as IL-1β and TNF-α (22). Moreover, upregulation of HuR in myeloid cells induces posttranscriptional silencing and reduced inflammatory profiles (57). Yiakouvaki and colleagues (57) have suggested a potential HuR/MCP-1/CCR2 axis with respect to monocyte chemotaxis and infiltration in experimental models of colitis and cancer. Interestingly, our results suggest a tefillin-induced RIPC-like nonocclusive stimulus causes a decrease in plasma MCP-1 and PBMC CCR2, with increases in leukocyte HuR resulting in decreased leukocyte adhesion and a trend toward decreased migration. These data are suggestive that HuR-mediated expression of these cytokines may underline the observed decreases in PBMC adhesion and migration phenotype elicited by a tefillin-induced RIPC-like nonocclusive stimulus. The precise mechanisms by which these changes occur and whether HuR plays a role in its regulation can be explored in future studies.
Prior Population-Based Studies
Our findings are limited to our population and require further large-scale studies, although population-based studies from Israel provide interesting supportive data. Specifically, Goldbourt and colleagues (11) examined over 10,000 Israeli male civil servants for 23 yr for common cardiovascular risk factors while also evaluating the effect of country of origin and religious observance in their population. Regardless of religious affiliation or country of origin, Israeli men had similar mortality across all disease states except for death from myocardial infarction, which was significantly lower in Jewish Orthodox men. After adjusting for all variables, the authors stated there was a “statistically significant advantage of at least 20% reduced fatal coronary heart disease risk (that) persisted for the 2,103 ‘most Orthodox’…as compared to the other groups” and suggested that “additional research is suggested to examine other associated life habits and other possible environmental sources of variability.” Thus, in the context of our study, it is plausible that the use of tefillin in this population may in fact be one of the previously unobserved “environmental sources of variability.”
However, the Goldbourt et al. study only included male civil servants. Thus, Kark and colleagues (21) subsequently compared mortality rates in Israeli men and women. Specifically, they conducted a 16-yr historical prospective study to determine the mortality rates in collective farms from 1970 to 1985 and pair matched them to obtain 11 religious and 11 secular farms for analysis. They demonstrate a distinctly lower mortality rate in religious farms than in secular farms across all ages and sexes, although as it pertained to coronary heart disease, the death rate in women was not different between the religious and secular farms (rate ratio: 1.31, confidence interval: 0.54–3.18) but was significantly higher in men in secular farms compared with men in religious farms (rate ratio: 2.4, confidence interval: 1.3–4.4). While a broad spectrum of biases and environmental conditions could have accounted for the overall findings, they did not identify sex-specific activities to account for these findings beyond that Orthodox men who pray together up to three times daily may have a higher “relaxation response” than women who traditionally attend prayers only on the Sabbath and holidays.
Crucially, in neither of the studies was the concept of tefillin discussed as a possible mechanism to explain the findings, but in light of our current data (and the fact that Orthodox women were not protected from cardiovascular mortality), it is suggestive that the act of wearing tefillin may have contributed to the observed protection. Future studies including women and non-Jewish populations are currently being investigated to further elucidate this potential mechanism.
Clinical implications
The clinical implications of these findings, if confirmed in larger studies, are potentially significant as the translation of an RIPC (or RIPC-like) stimuli to the bedside has been hampered by conflicting results from many clinical studies (38, 40, 55). While the data do not support or refute any claims regarding the religious nature of our findings, the potential for a noninvasive and inexpensive method for inducing such a phenotype may lead to relatively quick adoption by the general population with minimal risk is intriguing.
However, substantial experimentation needs to be performed to clarify these findings, including the testing of a nonreligious but otherwise identical device in other populations (women, non-Jews, subjects at risk for coronary artery disease, or subjects with prior coronary artery disease). Furthermore, the testing of a definitive end point (morbidity or mortality in coronary artery disease) would require a much larger study with regard to the number of subjects and length of followup, although the aforementioned population-based studies provide interesting supporting data to this hypothesis.
Study Limitations
The finding of improved vascular function and reactivity associated with both acute and chronic use of tefillin is interesting, but it should be noted that no longitudinal studies in humans have yet proven that young subjects with endothelial dysfunction will go on to develop advanced atherosclerosis or that improving vascular function at an early stage would lead to improved outcomes, as such studies would take decades to complete (41). Despite this, endothelial dysfunction is spatially and temporally linked to atherosclerosis occurring first at coronary branch points where plaques tend to develop and preceding occlusive arterial disease (33, 34, 41). Thus, there is a link between arterial endothelial dysfunction and later advanced atherosclerotic disease, and the use of BFMD as a surrogate end point in clinical trials appears justified. Furthermore, BFMD offers an assessment of endothelial function in conduit arteries, which are the vessels prone to the development of atherosclerosis (10).
Relative disadvantages of the ultrasound technique that we used include that it is technically challenging to perform, requiring a skilled sonographer and an appropriate training period, and that the “error” of the method probably precludes serial study of endothelial function for individuals (41). Future developments are likely to enhance the image quality during peripheral arterial scanning, to automate and improve analytical methods for measuring arterial diameter, and to link abnormalities observed using this method with cardiovascular event rates (41). Finally, as it pertains to the vascular studies, there are concerns regarding the repeatability of BFMD, although in this specific study we minimized this by using a single, highly trained sonographer that analyzed all data points in a blinded manner.
A potential limitation of the trial design was the need for a repeat BFMD study the same day that the tefillin was applied. The current guidelines suggest that repeated studies can be performed within a 10- to 15-min interval and suggest that this may be the ideal time to obtain the highest reproducibility (7). Importantly, Barton and colleagues (2) found there was no significant difference in BFMD among healthy subjects with at least 5 min between examinations. However, a subsequent study (17) demonstrated 15 min between BFMD was inadequate and therefore suggested 30−60 min was a more appropriate interval. Thus, our study was consistent with the current literature as there was at least 30 min between BFMD on all subjects, although it is conceivable that the testing of BFMD before acute tefillin use may have potentially altered the subsequent BFMD.
Other important limitations that will be subject to future studies include the relatively small number of subjects, their recruitment from a single area, and the lack of an appropriate positive control, such as a blood pressure cuff as has been used in other RIPC studies. The study was likely underpowered for many of the comparisons, as BFMD measurement variability was greater than that seen in previous studies of similar size (31, 32). Furthermore, we could not control for the fact that the chronic users may derive a benefit from daily prayer or observance of the Sabbath (among other factors) that may contribute to the anti-inflammatory effect. Thus, future studies will attempt to control for these factors by including women that share almost all other social characteristics except for daily use of tefillin.
Finally, while every attempt was made to ensure that baseline values were identical between the two groups, there was a small but statistically significant difference between the two groups as it pertained to age. However, using post hoc longitudinal mixed modeling adjustments, it was determined that the changes in age, height, and weight did not influence the significance of our results.
Conclusions
We propose that nonocclusive binding of an arm (using tefillin) induces an RIPC-like phenotype with favorable changes in arterial diameter and blood velocity and flow as well as decreased inflammation. Further studies will elucidate the clinical implications of these findings in a broad spectrum of patients, including women and those with vascular disease.
GRANTS
This work was supported by internal funding to J. Rubinstein from the University of Cincinnati.
DISCLOSURES
M. E. Rothenberg is a consultant for Pulm One, Spoon Guru, ClostraBio, Celgene, Shire, Astra Zeneca, GlaxoSmithKline, Allakos, Adare, Regeneron, and Novartis and has an equity interest in the first four listed and Immune Pharmaceuticals and royalties from reslizumab (Teva Pharmaceuticals) and UpToDate. M. E. Rothenberg is an inventor of patents, owned by Cincinnati Children’s. The other authors have no actual or potential perceived conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
A.P.O., E.M.U., M.T., and J.R. conceived and designed research; A.P.O., N.R., K.S., S.M.J., A.K., C.M., and S.S. performed experiments; A.P.O., N.R., K.S., S.M.J., J.G.W., C.M., S.S., and J.R. analyzed data; A.P.O., N.R., K.S., C.M., E.M.U., M.T., and J.R. interpreted results of experiments; A.P.O., N.R., K.S., M.T., and J.R. prepared figures; A.P.O., N.R., and J.R. drafted manuscript; A.P.O., N.R., K.S., S.M.J., A.K., J.G.W., C.M., S.S., M.E.R., E.M.U., M.T., and J.R. edited and revised manuscript; A.P.O., N.R., K.S., S.M.J., A.K., J.G.W., C.M., S.S., M.E.R., E.M.U., M.T., and J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the helpful suggestions and discussions provided by Dr. Sheryl E. Koch (University of Cincinnati), Dr. Evangelina Kranias (University of Cincinnati), and Dr. Michael Brusilovsky (Cincinnati Children’s Hospital Medical Center).
REFERENCES
- 1.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/acc guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 64: e139–e228, 2014. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Barton M, Turner AT, Newens KJ, Williams CM, Thompson AK. Minimum recovery time between reactive hyperemia stimulus in the repeated measurement of brachial flow-mediated dilatation. Ultrasound Med Biol 37: 879–883, 2011. doi: 10.1016/j.ultrasmedbio.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit Circulation 96: 1641–1646, 1997. doi: 10.1161/01.CIR.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 4.Boag SE, Das R, Shmeleva EV, Bagnall A, Egred M, Howard N, Bennaceur K, Zaman A, Keavney B, Spyridopoulos I. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest 125: 3063–3076, 2015. doi: 10.1172/JCI80055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cárdenas A, Moro MA, Leza JC, O’Shea E, Dávalos A, Castillo J, Lorenzo P, Lizasoain I. Upregulation of TACE/ADAM17 after ischemic preconditioning is involved in brain tolerance. J Cereb Blood Flow Metab 22: 1297–1302, 2002. doi: 10.1097/01.WCB.0000033968.83623.D0. [DOI] [PubMed] [Google Scholar]
- 5a.Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery first clinical application in humans. J Am Coll Cardiol 47: 2277–2282, 2006. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 6.Choi YS, Shim JK, Kim JC, Kang K-S, Seo YH, Ahn K-R, Kwak YL. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: a randomized controlled trial. J Thorac Cardiovasc Surg 142: 148–154, 2011. doi: 10.1016/j.jtcvs.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 8.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal 20: 2165–2173, 2008. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Ferretti E, Pistoia V, Corcione A. Role of fractalkine/CX3CL1 and its receptor in the pathogenesis of inflammatory and malignant diseases with emphasis on B cell malignancies. Mediators Inflamm 2014: 480941, 2014. doi: 10.1155/2014/480941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation 126: 753–767, 2012. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldbourt U, Yaari S, Medalie JH. Factors predictive of long-term coronary heart disease mortality among 10,059 male Israeli civil servants and municipal employees. A 23-year mortality follow-up in the Israeli Ischemic Heart Disease Study. Cardiology 82: 100–121, 1993. doi: 10.1159/000175862. [DOI] [PubMed] [Google Scholar]
- 12.Gritsopoulos G, Iliodromitis EK, Zoga A, Farmakis D, Demerouti E, Papalois A, Paraskevaidis IA, Kremastinos DT. Remote postconditioning is more potent than classic postconditioning in reducing the infarct size in anesthetized rabbits. Cardiovasc Drugs Ther 23: 193–198, 2009. doi: 10.1007/s10557-009-6168-5. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; ERICCA Trial Investigators . Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 373: 1408–1417, 2015. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 15.Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 108: 2134–2140, 2003. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- 15a.Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, Coffey JC, Dunne C, Walsh SR, Sadat U, Gaunt ME, Chen S, Tehrani S, Hausenloy DJ, Yellon DM, Kramer RS, Zimmerman RF, Lomivorotov VV, Shmyrev VA, Ponomarev DN, Rahman IA, Mascaro JG, Bonser RS, Jeon Y, Hong DM, Wagner R, Thielmann M, Heusch G, Zacharowski K, Meybohm P, Bein B, Tang TY; Remote Preconditioning Trialists’ Group . Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol 176: 20–31, 2014. doi: 10.1016/j.ijcard.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124: e652–e735, 2011. doi: 10.1016/j.jtcvs.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Inaba H, Takeshita K, Uchida Y, Hayashi M, Okumura T, Hirashiki A, Yoshikawa D, Ishii H, Yamamoto K, Nakayama T, Hirayama M, Matsumoto H, Matsushita T, Murohara T. Recovery of flow-mediated vasodilatation after repetitive measurements is involved in early vascular impairment: comparison with indices of vascular tone. PLoS One 9: e83977, 2014. doi: 10.1371/journal.pone.0083977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv 10: 263–270, 2010. doi: 10.1124/mi.10.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DH. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens 27: 918–925, 2014. doi: 10.1093/ajh/hpu004. [DOI] [PubMed] [Google Scholar]
- 20.Kalawski R, Bugajski P, Smielecki J, Wysocki H, Olszewski R, More R, Sheridan DJ, Siminiak T. Soluble adhesion molecules in reperfusion during coronary bypass grafting. Eur J Cardiothorac Surg 14: 290–295, 1998. doi: 10.1016/S1010-7940(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 21.Kark JD, Shemi G, Friedlander Y, Martin O, Manor O, Blondheim SH. Does religious observance promote health? mortality in secular vs religious kibbutzim in Israel. Am J Public Health 86: 341–346, 1996. doi: 10.2105/AJPH.86.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, Kollias G, Kontoyiannis DL. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell 19: 777–789, 2005. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet 374: 1557–1565, 2009. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbongard P, Peters J, Jakob H, Heusch G, Thielmann M. Persistent survival benefit from remote ischemic pre-conditioning in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 71: 252–254, 2018. doi: 10.1016/j.jacc.2017.10.083. [DOI] [PubMed] [Google Scholar]
- 25.Kono Y, Fukuda S, Hanatani A, Nakanishi K, Otsuka K, Taguchi H, Shimada K. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Devel Ther 8: 1175–1181, 2014. doi: 10.2147/DDDT.S68715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19: 143–150, 2004. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 27.Konstantinov IE, Arab S, Li J, Coles JG, Boscarino C, Mori A, Cukerman E, Dawood F, Cheung MMH, Shimizu M, Liu PP, Redington AN. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg 130: 1326–1332, 2005. doi: 10.1016/j.jtcvs.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 28.Kumar AG, Ballantyne CM, Michael LH, Kukielka GL, Youker KA, Lindsey ML, Hawkins HK, Birdsall HH, MacKay CR, LaRosa GJ, Rossen RD, Smith CW, Entman ML. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation 95: 693–700, 1997. doi: 10.1161/01.CIR.95.3.693. [DOI] [PubMed] [Google Scholar]
- 29.Lakshminarayanan V, Lewallen M, Frangogiannis NG, Evans AJ, Wedin KE, Michael LH, Entman ML. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol 159: 1301–1311, 2001. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung CH, Wang L, Nielsen JM, Tropak MB, Fu YY, Kato H, Callahan J, Redington AN, Caldarone CA. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther 28: 7–17, 2014. doi: 10.1007/s10557-013-6489-2. [DOI] [PubMed] [Google Scholar]
- 31.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system J Am Coll Cardiol 46: 450–456, 2005. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 32.Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation 116: 1386–1395, 2007. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 33.McLenachan JM, Vita J, Fish DR, Treasure CB, Cox DA, Ganz P, Selwyn AP. Early evidence of endothelial vasodilator dysfunction at coronary branch points. Circulation 82: 1169–1173, 1990. doi: 10.1161/01.CIR.82.4.1169. [DOI] [PubMed] [Google Scholar]
- 34.McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation 84: 1273–1278, 1991. doi: 10.1161/01.CIR.84.3.1273. [DOI] [PubMed] [Google Scholar]
- 35.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 18: 228–232, 2008. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myer VE, Fan XC, Steitz JA. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J 16: 2130–2139, 1997. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol 34: 1317–1323, 2002. doi: 10.1006/jmcc.2002.2072. [DOI] [PubMed] [Google Scholar]
- 38.Payne RE, Aldwinckle J, Storrow J, Kong RS, Lewis ME. RIPC remains a promising technique for protection of the myocardium during open cardiac surgery: a meta-analysis and systematic review. Heart Surg Forum 18: E074–E080, 2015. doi: 10.1532/hsf.1251. [DOI] [PubMed] [Google Scholar]
- 39.Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 275: H1542–H1547, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Pilcher JM, Young P, Weatherall M, Rahman I, Bonser RS, Beasley RW. A systematic review and meta-analysis of the cardioprotective effects of remote ischaemic preconditioning in open cardiac surgery. J R Soc Med 105: 436–445, 2012. doi: 10.1258/jrsm.2012.120049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raitakari OT, Celermajer DS. Flow-mediated dilatation. Br J Clin Pharmacol 50: 397–404, 2000. doi: 10.1046/j.1365-2125.2000.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sager HB, Kessler T, Schunkert H. Monocytes and macrophages in cardiac injury and repair. J Thorac Dis 9, Suppl 1: S30–S35, 2017. doi: 10.21037/jtd.2016.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saum K, Campos B, Celdran-Bonafonte D, Nayak L, Sangwung P, Thakar C, Roy-Chaudhury P, Owens AP. Uremic advanced glycation end products and protein-bound solutes induce endothelial dysfunction through suppression of Krüppel-like factor 2. J Am Heart Assoc 7: e007566, 2018. doi: 10.1161/JAHA.117.007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, Zhangdong H, Schmidt M, Downey GP, Redington AN. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res 158: 155–161, 2010. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Slone S, Anthony SR, Wu X, Benoit JB, Aube J, Xu L, Tranter M. Activation of HuR downstream of p38 MAPK promotes cardiomyocyte hypertrophy. Cell Signal 28: 1735–1741, 2016. doi: 10.1016/j.cellsig.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339: 161–166, 2013. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokuno S, Hinokiyama K, Tokuno K, Löwbeer C, Hansson L-O, Valen G. Spontaneous ischemic events in the brain and heart adapt the hearts of severely atherosclerotic mice to ischemia. Arterioscler Thromb Vasc Biol 22: 995–1001, 2002. doi: 10.1161/01.ATV.0000017703.87741.12. [DOI] [PubMed] [Google Scholar]
- 50.Trattner A, David M. Tefillin dermatitis. J Am Acad Dermatol 52: 831–833, 2005. doi: 10.1016/j.jaad.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 51.Videm V, Albrigtsen M. Soluble ICAM-1 and VCAM-1 as markers of endothelial activation. Scand J Immunol 67: 523–531, 2008. doi: 10.1111/j.1365-3083.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 52.Walsh SR, Nouraei SA, Tang TY, Sadat U, Carpenter RH, Gaunt ME. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. 44: 434–439, 2010. doi: 10.1177/1538574410369709. [DOI] [PubMed] [Google Scholar]
- 53.Wang YY, Yin BL. Pro-inflammatory cytokines may induce late preconditioning in unstable angina patients. Med Hypotheses 67: 1121–1124, 2006. doi: 10.1016/j.mehy.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 54.Windecker S, Kolh P, Alfonso F, Collet J-P, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann F-J, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A; Authors/Task Force members . 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 35: 2541–2619, 2014. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 55.Xie J, Zhang X, Xu J, Zhang Z, Klingensmith NJ, Liu S, Pan C, Yang Y, Qiu H. Effect of remote ischemic preconditioning on outcomes in adult cardiac surgery: a systematic review and meta-analysis of randomized controlled studies. Anesth Analg 127: 30–38, 2018. doi: 10.1213/ANE.0000000000002674. [DOI] [PubMed] [Google Scholar]
- 56.Xuan W, Liao Y, Chen B, Huang Q, Xu D, Liu Y, Bin J, Kitakaze M. Detrimental effect of fractalkine on myocardial ischaemia and heart failure. Cardiovasc Res 92: 385–393, 2011. doi: 10.1093/cvr/cvr221. [DOI] [PubMed] [Google Scholar]
- 57.Yiakouvaki A, Dimitriou M, Karakasiliotis I, Eftychi C, Theocharis S, Kontoyiannis DL. Myeloid cell expression of the RNA-binding protein HuR protects mice from pathologic inflammation and colorectal carcinogenesis. J Clin Invest 122: 48–61, 2012. doi: 10.1172/JCI45021. [DOI] [PMC free article] [PubMed] [Google Scholar]