Abstract
Abnormal pulmonary vascular development is a critical factor in the pathogenesis of bronchopulmonary dysplasia (BPD). Despite the well-established sex-specific differences in the incidence of BPD, the molecular mechanism(s) behind these are not completely understood. Exposure to a high concentration of oxygen (hyperoxia) contributes to BPD and creates a profibrotic environment in the lung. Our objective was to elucidate the sex-specific differences in neonatal human pulmonary microvascular endothelial cells (HPMECs) in normoxic and hyperoxic conditions, including the propensity for endothelial-to-mesenchymal transition. HPMECs (18- to 24-wk gestation donors, 6 male donors and 5 female donors) were subjected to hyperoxia (95% O2 and 5% CO2) or normoxia (air and 5% CO2) up to 72 h. We assessed cell migration and angiogenesis at baseline. Cell proliferation, viability, and expression of endothelial (CD31) and fibroblast markers (α-smooth muscle actin) were measured upon exposure to hyperoxia. Female HPMECs had significantly higher cell migration when assessed by the wound healing assay (40.99 ± 4.4%) compared with male HPMECs (14.76 ± 3.7%) and showed greater sprouting (1710 ± 962 μm in female cells vs. 789 ± 324 in male cells) compared with male endothelial cells in normoxia. Hyperoxia exposure decreased cell viability (by 9.8% at 48 h and 11.7% at 72 h) and proliferation (by 26.7% at 72 h) markedly in male HPMECs, whereas viability was sustained in female endothelial cells. There was greater expression of α-smooth muscle actin (2.5-fold) and decreased expression (5-fold) of CD31 in male HPMECs upon exposure to hyperoxia. The results indicate that cellular sex affects response in HPMECs in normoxia and hyperoxia.
NEW & NOTEWORTHY Cellular sex affects response in human neonatal pulmonary microvascular endothelial cells in normoxia and hyperoxia. Under normoxic conditions, female human neonatal pulmonary microvascular endothelial cells display greater migration and angiogenic sprouting compared with male endothelial cells. Compared with female endothelial cells, hyperoxia exposure decreased cell viability and proliferation and increased α-smooth muscle actin and decreased CD31 expression in male endothelial cells, indicating an increased endothelial-mesenchymal transition.
Keywords: bronchopulmonary dysplasia, endothelial-mesenchymal transition, human pulmonary microvascular endothelial cells, hyperoxia, sex dimorphism
INTRODUCTION
Arrest in alveolarization and abnormal pulmonary vascular development are hallmarks of bronchopulmonary dysplasia (BPD). The etiology of this disease is multifactorial, and exposure to high concentrations of oxygen (hyperoxia) contributes to its development (28). Pulmonary hypertension develops in as many as 50% of infants with severe BPD (3, 5). This is associated with significant morbidity, with studies reporting 33–48% mortality at 2 yr (11, 15).
The incidence of BPD is higher among premature boys after adjusting for other confounders (6, 23, 37). The biological mechanisms responsible for these sex differences are not yet fully understood. Sex-specific phenotypic (proliferation, migration, and autophagy) and gene expression differences in endothelial cells at baseline and under various pathological stressors (serum starvation and shear stress) have been reported (2, 20, 24). Pulmonary endothelial cells are among the most sensitive to exposure to high concentrations of O2 compared with other cell populations in the lung (22). Neonatal male mice exposed to hyperoxia show greater arrest in lung alveolarization and angiogenesis compared with females (10, 19). Enomoto et al. (12) reported that male neonatal rat pups had greater pulmonary arterial muscle contraction upon exposure to hyperoxia. The severity of BPD is associated with male sex (27, 37), and pulmonary hypertension is more common in infants with severe BPD.
The endothelial-mesenchymal transition is a complex biological process in which endothelial cells lose their surface expression of endothelium-specific markers (e.g., CD31 and vonWillebrand factor) and acquire a mesenchymal phenotype and express markers like α-smooth muscle actin (SMA) (25). Exposure to hyperoxia leads to a profibrotic phenotype leading to an increase in myofibroblasts (30). The role of the endothelial-mesenchymal transition is known in lung diseases such as idiopathic pulmonary fibrosis (significant contribution to fibrosis and vascular regression) and pulmonary arterial hypertension (causing pulmonary vascular remodeling and endothelial dysfunction) (13, 14, 18). However, the role for the endothelial-mesenchymal transition in BPD has not been determined. We hypothesized that human pulmonary microvascular endothelial cells (HPMECs) will exhibit sexual dimorphism under normoxic and hyperoxic conditions. For this purpose, we analyzed migratory and angiogenic properties (under normoxia) as well as cellular proliferation, viability, and propensity for the endothelial-mesenchymal transition (under hyperoxia).
METHODS
Cell culture.
HPMECs (male and female) from 18- to 24-wk-old donors (canalicular stage of human lung development) were obtained from ScienCell laboratories (catalog no. 3000, Carlsbad, CA) and maintained in endothelial cell medium (catalog no. 1001, ScienceCell) at 37°C in 5% CO2. Characteristics of these cells are shown in Table 1. Male and female HPMECs were used from passages 3−6. Per ScienCell, the tissue was obtained from nonprofit tissue providers who strictly adhere to the guidelines for tissue collection and distribution according to established protocols, in compliance with local, state, and federal laws and regulations governing the procurement and distribution of human tissue after informed consent. For each result, three independent experiments were performed.
Table 1.
Gestational age, sex, and lot numbers of human pulmonary microvascular endothelial cells
| Lot No. | Sex | Gestational Age, wk |
|---|---|---|
| 5016 | Female | 20 |
| 10885 | Male | 22 |
| 10160 | Female | 24 |
| 11367 | Male | 23 |
| 10899 | Male | 22 |
| 10169 | Female | 24 |
| 11422 | Male | 19 |
| 17799 | Female | 19 |
| 11816 | Male | 19 |
| 16021 | Male | 18 |
| 15900 | Female | 18 |
Wound healing assay.
Two-dimensional wound healing assays were performed using ibidi culture inserts. Briefly, confluent monolayers of HPMECs were established on the ibidi Culture-Insert 2 well in a 35-mm μ-Dish. Images were captured immediately after wounding and 8 h later. The percentage of wound closure was measured with the National Institutes of Health ImageJ software package.
Angiogenesis assay.
Angiogenesis potential was measured based on the maximum length of sprouts protruding from HPMEC-coated cytodex-3 microcarrier beads suspended in fibrin gel as previously described (21). Coated beads were then incubated in media overnight to ensure confluence before resuspension in 2 mg/ml fibrin gels supplemented with 0.15 U/ml aprotinin at a concentration of 250 beads/ml. Gels were maintained in an extracellular matrix with media changes on alternating days. After culture, gels were fixed in 4% paraformaldehyde supplemented with 0.1% Triton X-100 for 2 h at 4°C, washed three times with 1× PBS, and stained overnight with CF594-conjugated phalloidin (Cell Signaling Technologies, Danvers, MA). Gels were again washed three times with 1× PBS. Sprout length was determined with ImageJ by generating a circle (centered on the bead center) whose radius intersected the longest angiogenic sprout. Maximum sprout distance was calculated by subtracting the radius of the bead from the radius of the overlaid circle.
Vasculogenesis assay.
The ability of cells to form a three-dimensional self-assembled vascular network, known as a plexus, was evaluated within collagen gels cultured over 7 days as previously described (16). Confocal z-stacks were taken of four random regions of each gel, and each was converted into a maximum projection image. The resulting images were binarized in ImageJ, and the percentage of fluorescent pixels was identified based on the resulting signal histogram.
Exposure of cells to hyperoxia.
Before hyperoxia treatment, 1 × 105 male or female HPMECs were seeded in a 6-mm dish. Later (2 h), these cells were incubated at 37°C in normoxia (21% O2-5% CO2) or in hyperoxia (95% O2-5% CO2) as previously described for up to 72 h (32).
Trypan blue staining.
Ten microliters of the cell suspension after exposure to room air or hyperoxia were combined with 10 μl trypan blue (0.4%). The mixture was gently pipetted up and down 10 times to mix the cells, and dye and quantitation were performed using the TC20 Automated Cell Counter (Bio-Rad, Hercules, CA) as previously described (29).
Cell proliferation assay.
Cell proliferation was assayed by measuring the incorporation of bromodeoxyuridine (BrdU) into newly synthesized DNA using the BrdU cell proliferation ELISA kit (catalog no. ab126556, Abcam, Cambridge, MA) as per the manufacturer's recommendations. After 24 h of preincubation at 37°C in 5% CO2, cells were incubated in room air or hyperoxia (95% O2-5% CO2). BrdU stock solution was added to each well in a 96-well plate in the last 2 h of incubation, after which the cells were placed back in normoxia/hyperoxia. At 0, 24, 48, and 72 h after hyperoxia incubation, absorbance was measured at dual wavelength of 450/550 nm using a microplate reader (Molecular Devices, Sunnyvale, CA).
Western immunoblot analysis.
Total protein of HPMECs (baseline and after 72 h either in normoxia or after exposure to hyperoxia) was isolated using RIPA buffer containing protease inhibitors, and protein concentration was measured. Total protein (20–30 μg) was resolved by 4–12% SDS-PAGE and then transferred to a PVDF membrane. The primary antibodies used were as follows: rabbit anti-CD31 (1:1,000, Cell Signaling, Beverly, MA), rabbit anti-α-SMA (1:1,000, Cell Signaling), and rabbit anti-β-actin (1:5,000, Cell Signaling). Goat anti-rabbit secondary antibody (1:2,000, Abcam) was used. Protein bands were normalized against β-actin (used as a loading control) on the same membrane. Relative quantitation was performed using Bio-Rad image-analysis software.
Statistical analysis.
Data are presented as means ± SE. Student’s t-tests and two- or three-way ANOVA following the Bonferroni posttest were performed for statistical evaluation. In all analyses, significance level was set at P < 0.05. Main effects of hyperoxia, sex, time point, and interaction between the independent variables were also assessed.
RESULTS
Wound healing assay revealed increased migration in female HPMECs.
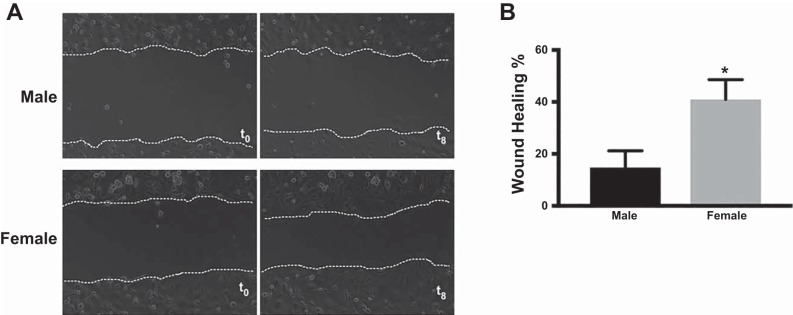
Representative phase-contrast images from the two-dimensional wound healing assay at baseline and after 8 h are shown in Fig. 1A. After 8 h of incubation, the percentage wound healing was greater (P = 0.01; Fig. 1B) in female HPMECs (40.99 ± 4.4%) compared with male HPMECs (14.76 ± 3.7%).
Fig. 1.
Two-dimensional wound healing assay reveals increased migration in female human pulmonary microvascular endothelial cells (HPMECs). A: representative images of male and female HPMECs taken at baseline [time 0 (t0)] and after 8 h incubation. B: percentage of wound closure at 8 h. Values are means ± SE of 3 independent biological replicates (n = 3/group). Significant differences between male and female HPMECs are indicated by *P < 0.05.
Female HPMECs exhibited greater sprouting angiogenesis.
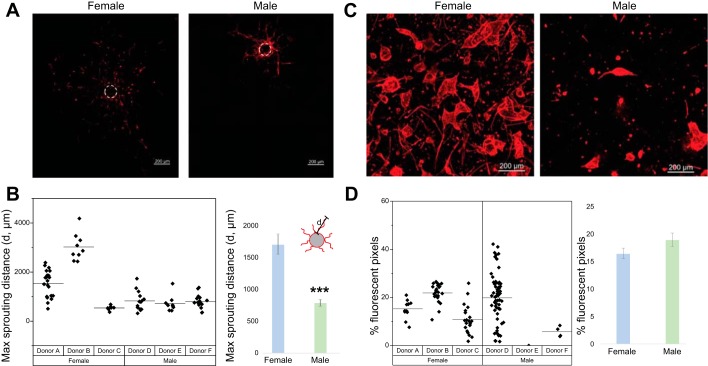
Representative images of sprouting angiogenesis assay are shown in Fig. 2A. Measurements from individual donors as well as from male and female donors are shown in Fig. 2B. Female HPMECs produced sprouts with a maximum sprout distance of 1,710 ± 962 μm, whereas male HPMECs had a maximum sprout distance of 789 ± 324 μm. Although female cells showed statistically significant population-based differences from cells of male origin, there was large variability between individual donors.
Fig. 2.
Female human pulmonary microvascular endothelial cells (HPMECs) exhibit greater sprouting angiogenesis. Angiogenic potential was quantified based on the maximum length of sprouts protruding from HPMEC-coated cytodex-3 microcarrier beads suspended in a fibrin gel. A: representative images from male and female HPMECs subjected to a sprouting angiogenesis assay. B: maximum sprouting distance in male and female HPMECs. The ability of cells to form a three-dimensional self-assembled vascular network, known as a plexus, was evaluated within collagen gels cultured over 7 days. Each point represents one coated bead. C: representative images from male and female HPMECs subjected to a vasculogenesis assay. D: percentage of fluorescent pixels (fluorescent pixels/total pixels). Each point represents one collagen gel. Values are means ± SE of 3 independent biological replicates (n = 3/group). Significant differences between male and female HPMECs are indicated by ***P < 0.001.
No sex-specific differences were noted in the vasculogenesis assay.
The differences between male and female HPMECs were not statistically significant in the vasculogenesis assay (Fig. 2, C and D). Marked interdonor variability was seen among male endothelial cells, with an inability of a plexus to self-assemble in one of the male donors (donor E).
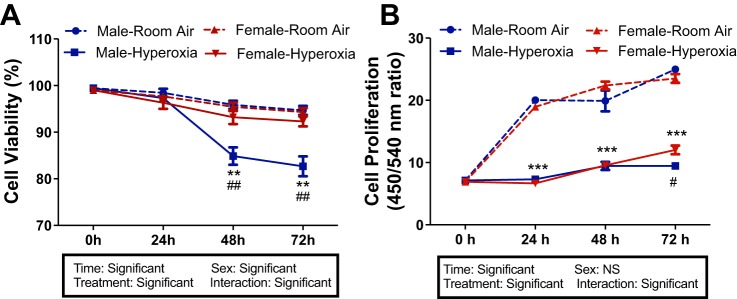
Female HPMECs have preserved cell viability upon exposure to hyperoxia compared with male HPMECs.
Hyperoxia significantly decreased cell viability (Fig. 3A) and proliferation (Fig. 3B) in male HPMECs compared with controls incubated in room air. Cell viability in male cells was decreased significantly after 48 h (9.8%, P < 0.001) and 72 h (11.7%, P < 0.001) of hyperoxia exposure compared with female cells and compared with male control cells in normoxia (P < 0.001) at 48 h (13.1%) and 72 h (14.5%). All of the independent variables (time, treatment, and sex) and the interaction term were significant in the statistical analysis. There was greater decline (P = 0.02) in cell proliferation in male cells (26.7%) at 72 h compared with female cells. Although sex was not significant among the independent variables, the interaction term was significant at 72 h.
Fig. 3.
Female human pulmonary microvascular endothelial cells (HPMECs) have preserved cell viability upon exposure to hyperoxia. Male and female HPMECs exposed to room air (RA) (RA-5% CO2) and 24, 48, or 72 h of hyperoxia (95% O2-5% CO2) were subjected to Trypan blue exclusion (A) or a bromodeoxyuridine (BrdU) incorporation assay (B) as described in materials and methods. Values are means ± SE of 3 independent biological replicates (n = 3/group). Significant differences between baseline and subsequent time points within the same sex are indicated by **P < 0.01 and ***P < 0.001; significant differences between male and female HPMECs at each time point are indicated by #P < 0.5 and ##P < 0.01. NS, not significant.
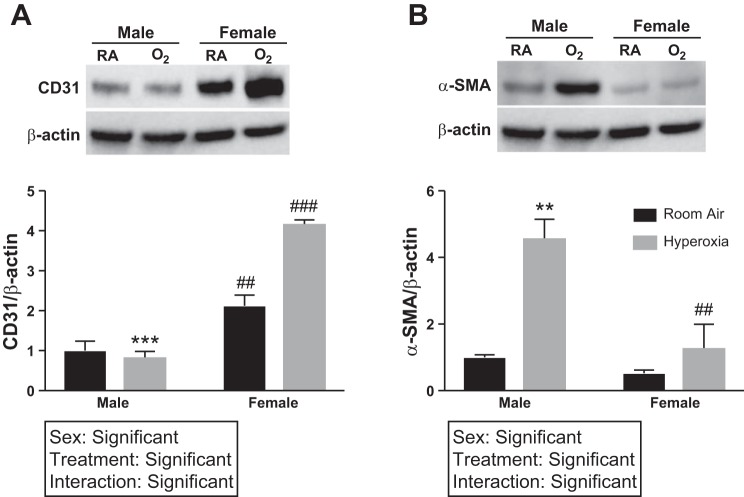
Male HPMECs have greater expression of α-SMA and decreased expression of CD31 upon exposure to hyperoxia.
Under normoxic and hyperoxic conditions, there was greater (5-fold) expression of CD31 in female HPMECs (Fig. 4A). Upon exposure to hyperoxia, male HPMECs showed increased α-SMA expression (Fig. 4B) compared with female HPMECs (2.5-fold, P = 0.006) and compared with normoxic control HPMECs (4.6-fold, P = 0.003). Two-way ANOVA showed significance of both the independent variables (treatment and hyperoxia) and the interaction term for the results. This indicates an increased endothelial-mesenchymal transition in male HPMECs compared with female HPMECs.
Fig. 4.
CD31 and α-smooth muscle actin (SMA) expression in male and female human pulmonary microvascular endothelial cells (HPMECs) upon exposure to hyperoxia. CD31 and α-SMA protein expression in male and female HPMECs exposed to room air (RA) (RA-5% CO2) and 72 h of hyperoxia (HO) (95% O2-5% CO2) are shown. Representative Western blot images and densitometry analyses are shown. Values are means ± SE of 3 independent biological replicates (n = 3/group). Significant differences between baseline and subsequent time points within the same sex are indicated by **P < 0.01 or ***P < 0.001; significant differences between male and female HPMECs at each time point are indicated by ##P < 0.01 or ###P < 0.001.
DISCUSSION
We show in this study that neonatal HPMECs show distinct responses based on cellular sex both under normoxic and hyperoxic conditions. Female HPMECs exhibit greater cellular migration and sprouting angiogenesis under normoxic conditions. When subjected to hyperoxia, female HPMECs have preserved cellular viability and proliferation compared with similarly exposed male cells. Male HPMECs show a greater acquisition of the mesenchymal marker α-SMA and a lower expression of the endothelial marker CD31, thus showing a greater propensity toward endothelial-mesenchymal transition. These results are similar to previous studies with human umbilical vein endothelial cells (HUVECs) (34) and can explain the differences in pulmonary vascular development in vivo in the murine BPD model (19).
Premature male neonates are at a higher risk for developing BPD, even in the postsurfactant era (6, 23, 37). Zysman-Coleman et al. (37) reported that the severity of BPD is associated with male sex with an adjusted odds ratio of 1.51 [95% confidence interval: 1.14–2.0]. Another study (27) reported an odds ratio of 3.01 (95% confidence interval: 1.3–7.5) for the same association. Male sex also predicts poorer long-term lung function in babies with BPD (17, 31, 36).
Abnormal or dysmorphic pulmonary vasculature is a key finding in human patients and animal models of BPD (1). Based on our findings, female sex may confer advantage to the developing lung endothelium exposed to postnatal hyperoxia and preserve angiogenesis and secondarily alveolarization as well. Differences between male and female endothelial cells have been shown in previous studies both at baseline and in response to various stressors (2, 20, 24). HUVECs, typically extracted from term gestation umbilical cords, have been used in many studies to study endothelial cell function and biology. Cattaneo et al. (9) reported that female HUVECs express greater endothelial nitric oxide synthase expression and that angiogenesis in female HUVECs was endothelial nitric oxide synthase dependent. Lorenz et al. (20) reported enrichment of immune-related genes in female HUVECs at baseline and differential gene expression upon exposure to shear stress. Female HUVECs also displayed preserved cell viability and tube formation capacity after serum starvation (20). In a previous study (34), we showed that male HUVECs have decreased survival, greater oxidative stress, and impairment in angiogenesis upon exposure to hyperoxia. Sex-specific differences can be attributed to differential sex hormone production and responsiveness or to sex chromosome-mediated cell intrinsic differences in male and female subjects. Cells from mouse embyros as early as embryonic day 10.5 before gonadal differentiation still show sexually dimorphic responses (24). Future studies that dissect the role of sex chromosomes versus sex hormones behind these differences will delineate the contribution of each behind these findings.
The endothelial-to-mesenchymal transition could be an additional source of fibroblasts in pathological lung conditions, including idiopathic pulmonary fibrosis and pulmonary arterial hypertension (13, 14, 18). In our study, neonatal male HPMECs show a higher propensity toward endothelial-to-mesenchymal transition, suggesting a greater pathological contribution of this process in premature neonatal male lungs exposed to hyperoxia.
X chromosome-linked genes, which may have a higher expression in female subjects because of incomplete X inactivation, could possibly explain some of the above findings (8). X chromosome-linked inhibitor of apoptosis increases antioxidant gene expression and prevents apoptosis under oxidative stress (26, 35). Another X-linked gene that has proangiogenic effects is angiomotin (7), which has been shown to increase endothelial cell motility and tube formation (33).
Our study has some limitations. Although the sex and gestational age of the endothelial cells used in this study are known, we do not have information on donor tissue health. Also, the media used in the experiments contained hormones. We only used a single hyperoxia concentration (95% fraction of inspired O2) for our experiments. A dose-dependent effect on cell viability and proliferation and sex-specific differences were not studied. Current evidence advocates for the avoidance of hyperoxia or hypoxia in the neonatal intensive care unit (4). The sex-specific effects of different saturation ranges in the premature newborn population have not been studied.
In conclusion, we show that cellular sex plays a major role in dictating the response in HPMECs in normoxia and hyperoxia. This could be one of the reasons for differences in the incidence of bronchopulmonary dysplasia in premature neonates.
GRANTS
This work was supported by National Institutes of Health Grants K08-HL-127103 and R03-HL-141572 (to K. Lingappan) and R01-HL-133163, R21-ES-027962, and S10-OD-016361 (to J. P. Gleghorn), American Lung Association Grant RG-418067 (to K. Lingappan), National Science Foundation Grant 1537256 (to J. P. Gleghorn), IGERT Traineeship 1144726 (to J. Shirazi), and March of Dimes Basil O’Connor Award 5-FY16-33 (to J. P. Gleghorn).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Z., X.D., J.S., and K.L. performed experiments; Y.Z., J.S., J.P.G., and K.L. analyzed data; Y.Z., X.D., J.S., and K.L. prepared figures; Y.Z., J.S., J.P.G., and K.L. edited and revised manuscript; Y.Z., J.S., J.P.G., and K.L. approved final version of manuscript; X.D., J.P.G., and K.L. drafted manuscript; J.S., J.P.G., and K.L. interpreted results of experiments; K.L. conceived and designed research.
ACKNOWLEDGMENTS
We acknowledge Dr. Peter Westenskow for help with the two-dimensional wound healing assay.
REFERENCES
- 1.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med 164: 1755–1756, 2001. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 2.Addis R, Campesi I, Fois M, Capobianco G, Dessole S, Fenu G, Montella A, Cattaneo MG, Vicentini LM, Franconi F. Human umbilical endothelial cells (HUVECs) have a sex: characterisation of the phenotype of male and female cells. Biol Sex Differ 5: 18, 2014. doi: 10.1186/s13293-014-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Mourani P. Pulmonary hypertension in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100: 240–246, 2014. doi: 10.1002/bdra.23241. [DOI] [PubMed] [Google Scholar]
- 4.Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, Davis PG, Carlo WA, Brocklehurst P, Davies LC, Das A, Rich W, Gantz MG, Roberts RS, Whyte RK, Costantini L, Poets C, Asztalos E, Battin M, Halliday HL, Marlow N, Tin W, King A, Juszczak E, Morley CJ, Doyle LW, Gebski V, Hunter KE, Simes RJ; Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration . Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA 319: 2190–2201, 2018. doi: 10.1001/jama.2018.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129: e682–e689, 2012. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binet M-E, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B; Canadian Neonatal Network . Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol 29: 159–166, 2012. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- 7.Bratt A, Birot O, Sinha I, Veitonmäki N, Aase K, Ernkvist M, Holmgren L. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem 280: 34859–34869, 2005. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- 8.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434: 400–404, 2005. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo MG, Vanetti C, Decimo I, Di Chio M, Martano G, Garrone G, Bifari F, Vicentini LM. Sex-specific eNOS activity and function in human endothelial cells. Sci Rep 7: 9612, 2017. doi: 10.1038/s41598-017-10139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coarfa C, Zhang Y, Maity S, Perera D, Jiang W, Wang L, Couroucli X, Moorthy B, Lingappan K. Sexual dimorphism of the pulmonary transcriptome in neonatal hyperoxic lung injury: identification of angiogenesis as a key pathway. Am J Physiol Lung Cell Mol Physiol 313: L991–L1005, 2017. doi: 10.1152/ajplung.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Cerro MJ, Sabaté Rotés A, Cartón A, Deiros L, Bret M, Cordeiro M, Verdú C, Barrios MI, Albajara L, Gutierrez-Larraya F. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol 49: 49–59, 2014. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto M, Gosal K, Cubells E, Escobar J, Vento M, Jankov RP, Belik J. Sex-dependent changes in the pulmonary vasoconstriction potential of newborn rats following short-term oxygen exposure. Pediatr Res 72: 468–478, 2012. doi: 10.1038/pr.2012.120. [DOI] [PubMed] [Google Scholar]
- 13.Good RB, Gilbane AJ, Trinder SL, Denton CP, Coghlan G, Abraham DJ, Holmes AM. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 185: 1850–1858, 2015. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 43: 161–172, 2010. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 120: 1260–1269, 2007. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 16.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol 443: 83–101, 2008. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- 17.Kotecha SJ, Lowe J, Kotecha S. Does the sex of the preterm baby affect respiratory outcomes? Breathe (Sheff) 14: 100–107, 2018. doi: 10.1183/20734735.017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Lui KO, Zhou B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat Rev Cardiol 15: 445–456, 2018. doi: 10.1038/s41569-018-0023-y. [DOI] [PubMed] [Google Scholar]
- 19.Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 311: L481–L493, 2016. doi: 10.1152/ajplung.00047.2016. 27343189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenz M, Koschate J, Kaufmann K, Kreye C, Mertens M, Kuebler WM, Baumann G, Gossing G, Marki A, Zakrzewicz A, Miéville C, Benn A, Horbelt D, Wratil PR, Stangl K, Stangl V. Does cellular sex matter? Dimorphic transcriptional differences between female and male endothelial cells. Atherosclerosis 240: 61–72, 2015. doi: 10.1016/j.atherosclerosis.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsu MN, Davis J, Hughes CCW. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp 3: 186, 2007. doi: 10.3791/186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Reilly MA. DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol 281: L291–L305, 2001. doi: 10.1152/ajplung.2001.281.2.L291. [DOI] [PubMed] [Google Scholar]
- 23.O’Shea JE, Davis PG, Doyle LW; Victorian Infant Collaborative Study Group . Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res 71: 210–214, 2012. doi: 10.1038/pr.2011.27. [DOI] [PubMed] [Google Scholar]
- 24.Penaloza C, Estevez B, Orlanski S, Sikorska M, Walker R, Smith C, Smith B, Lockshin RA, Zakeri Z. Sex of the cell dictates its response: differential gene expression and sensitivity to cell death inducing stress in male and female cells. FASEB J 23: 1869–1879, 2009. doi: 10.1096/fj.08-119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piera-Velazquez S, Mendoza FA, Jimenez SA. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med 5: 45, 2016. doi: 10.3390/jcm5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resch U, Schichl YM, Sattler S, de Martin R. XIAP regulates intracellular ROS by enhancing antioxidant gene expression. Biochem Biophys Res Commun 375: 156–161, 2008. doi: 10.1016/j.bbrc.2008.07.142. [DOI] [PubMed] [Google Scholar]
- 27.Rutkowska M, Hożejowski R, Helwich E, Borszewska-Kornacka MK, Gadzinowski J. Severe bronchopulmonary dysplasia−incidence and predictive factors in a prospective, multicenter study in very preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med 58: 1–7, 2018. doi: 10.1080/14767058.2017.1422711. [DOI] [PubMed] [Google Scholar]
- 28.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39–49, 2003. doi: 10.1016/S1084-2756(02)00194-X. [DOI] [PubMed] [Google Scholar]
- 29.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3: Appendix 3B, 2001. doi: 10.1002/0471142735.ima03bs21. [DOI] [PubMed] [Google Scholar]
- 30.Sucre JMS, Vijayaraj P, Aros CJ, Wilkinson D, Paul M, Dunn B, Guttentag SH, Gomperts BN. Posttranslational modification of β-catenin is associated with pathogenic fibroblastic changes in bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 312: L186–L195, 2017. doi: 10.1152/ajplung.00477.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas MR, Marston L, Rafferty GF, Calvert S, Marlow N, Peacock JL, Greenough A. Respiratory function of very prematurely born infants at follow up: influence of sex. Arch Dis Child Fetal Neonatal Ed 91: F197–F201, 2006. doi: 10.1136/adc.2005.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari KK, Moorthy B, Lingappan K. Role of GDF15 (growth and differentiation factor 15) in pulmonary oxygen toxicity. Toxicol In Vitro 29: 1369–1376, 2015. doi: 10.1016/j.tiv.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troyanovsky B, Levchenko T, Månsson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol 152: 1247–1254, 2001. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Lingappan K. Differential sex-specific effects of oxygen toxicity in human umbilical vein endothelial cells. Biochem Biophys Res Commun 486: 431–437, 2017. doi: 10.1016/j.bbrc.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu C, Xu F, Fukuda A, Wang X, Fukuda H, Korhonen L, Hagberg H, Lannering B, Nilsson M, Eriksson PS, Northington FJ, Björk-Eriksson T, Lindholm D, Blomgren K. X chromosome-linked inhibitor of apoptosis protein reduces oxidative stress after cerebral irradiation or hypoxia-ischemia through up-regulation of mitochondrial antioxidants. Eur J Neurosci 26: 3402–3410, 2007. doi: 10.1111/j.1460-9568.2007.05948.x. [DOI] [PubMed] [Google Scholar]
- 36.Zisk JL, Genen LH, Kirkby S, Webb D, Greenspan J, Dysart K. Do premature female infants really do better than their male counterparts? Am J Perinatol 28: 241–246, 2011. doi: 10.1055/s-0030-1268239. [DOI] [PubMed] [Google Scholar]
- 37.Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatr Child Health 18: 86–90, 2013. doi: 10.1093/pch/18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]