Abstract
This review discusses sexual dimorphism in arterial stiffening, disease pathology interactions, and the influence of sex on mechanisms and pathways. Arterial stiffness predicts cardiovascular mortality independent of blood pressure. Patients with increased arterial stiffness have a 48% higher risk for developing cardiovascular disease. Like other cardiovascular pathologies, arterial stiffness is sexually dimorphic. Young women have lower stiffness than aged-matched men, but this sex difference reverses during normal aging. Estrogen therapy does not attenuate progressive stiffening in postmenopausal women, indicating that currently prescribed drugs do not confer protection. Although remodeling of large arteries is a protective adaptation to higher wall stress, arterial stiffening increases afterload to the left ventricle and transmits higher pulsatile pressure to smaller arteries and target organs. Moreover, an increase in aortic stiffness may precede or exacerbate hypertension, particularly during aging. Additional studies are needed to elucidate the mechanisms by which females are protected from arterial stiffness to provide insight into its mechanisms and, ultimately, therapeutic targets for treating this pathology.
Keywords: arterial stiffness, estrogen, hypertension, pulse wave velocity, sex
INTRODUCTION
Arterial stiffening is defined as resistance to deformation or a loss of elastic compliance due to changes in the geometry and microstructure of the vascular wall (11, 157). Stiffening alters the mechanical behavior and properties of the vasculature, which can be described as a decrease in distensibility (a change in diameter with pressure) or compliance (the ability to store volume) (155). Furthermore, stiffening encompasses a decrease in elasticity, minimizing the ability to store energy during deformation, which is integral for performing work on the blood during recoil of the artery (60). Compared with smaller muscular arteries, the increased elastin content in large arteries forms a reservoir for storing energy during the cardiac cycle (64). These characteristics of large blood vessels allow them to maintain peripheral perfusion during diastole (Fig. 1). Passive material properties and active cellular processes contribute to the stiffness of the vessel wall (11). Arterial stiffening also increases cardiac afterload and transmits pressure oscillations to end organs, both of which promote cardiovascular morbidity and mortality (113).
Fig. 1.
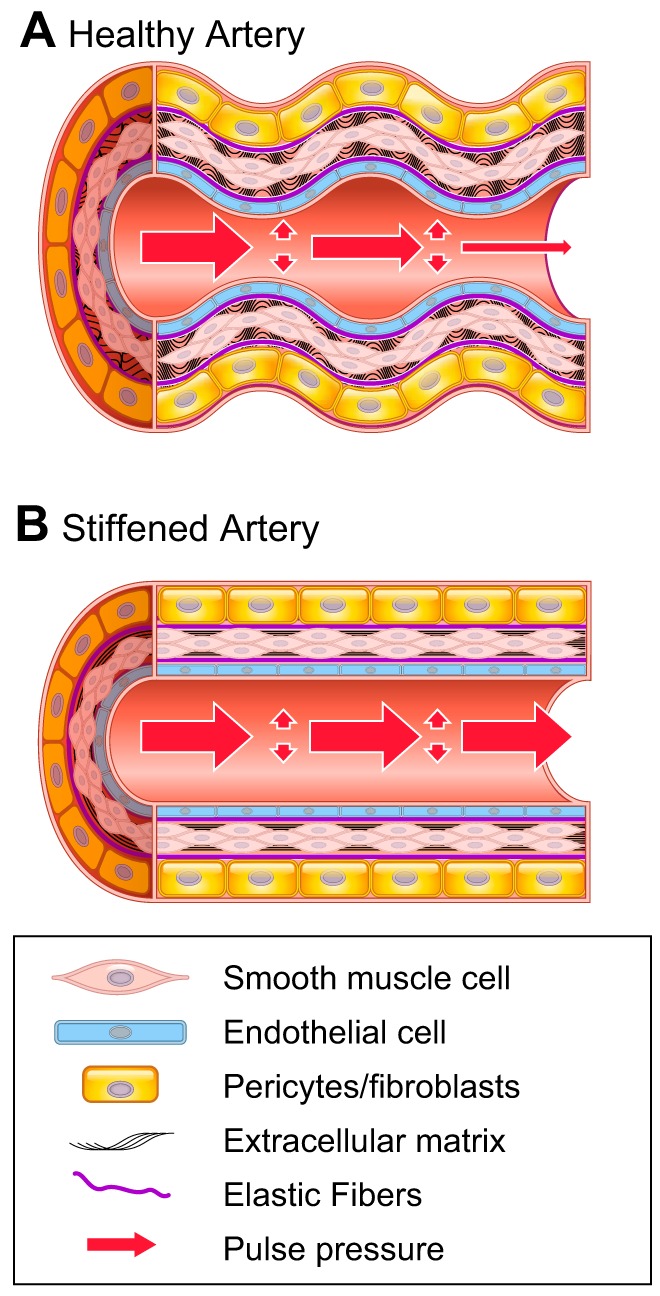
A: healthy artery. Energy generated by distension of the arterial wall during systole is used during diastole to maintain blood flow and decrease pulse pressure downstream. B: stiffened artery. Poor energy absorption results in higher pulse pressure, reduced flow during diastole, increased cardiac afterload, and end organ damage.
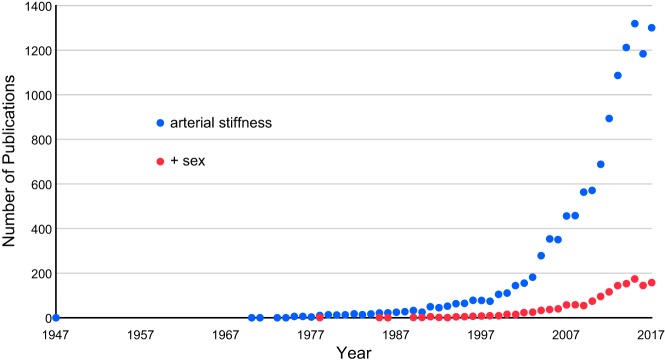
While the elastic properties of arteries have been studied since the late 1800s (175), the first publication containing the term “arterial stiffness” was published 70 yr ago in 1947 (12) and the next did not appear until 1970 (228) (Fig. 2). Interest in this topic steadily rose in the 1990s, and the number of annual publications broke the 100 mark in 1999. A rapid surge in publications started in the 2000s, perhaps in response to the 2003 European guidelines for the management of arterial hypertension, which acknowledged the need for further research on the potential predictive power of large artery compliance (55). Recognition of arterial stiffness as a clinically important variable has lagged in the United States. The National Heart, Lung, and Blood Institute identified this knowledge gap in 2009 and requested applications for grants to study arterial stiffness, resulting in a significant increase in funding on this topic (157). In 2015, the American Heart Association recommended more research on arterial stiffening (208), but still no clinical guidelines exist in the United States (100).
Fig. 2.
Graphical representation of the number of peer-reviewed articles containing the search term “arterial stiffness” alone or with the additional search term “sex” through 2017.
Inclusion of arterial stiffness with standard, well-established risk factors improves the prediction of cardiovascular events. In a 1999, in a study of patients with hypertension, arterial stiffness was found to be an even stronger predictor of cardiovascular mortality than blood pressure (18). The Rotterdam Study found that, in healthy, normotensive patients, arterial stiffness independently predicts stroke and coronary heart disease (135). The Framingham Heart Study supported these results, finding that elevated stiffness correlates with an increased probability for a major cardiovascular event (139). Together, these studies have provided strong clinical evidence that arterial stiffening precedes or accompanies detrimental cardiovascular events, and treatment of this pathology in otherwise healthy patients may provide protection.
Despite the impressive increase in research on “arterial stiffness” in the past decade, addition of the term “sex” to a PubMed search reveals a dire lag in the consideration of sex as an important biological variable in arterial stiffness (Fig. 2). Carotid artery compliance is similar in children but is significantly greater in adult women than men (130). While increased arterial stiffness is lower in young women than men, older women have increased arterial stiffness, particularly with aging (34). In patients with cardiovascular comorbidities, the sexual dimorphism is also reversed, with greater arterial stiffness in women than men. For example, in patients with diastolic heart failure, measures of arterial stiffness are greater in women than men (33). This greater arterial stiffness in women with cardiovascular comorbidities holds true even after adjustment for age, body mass index, mean arterial pressure, and heart rate (177). Given that sex differences play an important role in cardiovascular physiology, inclusion of arterial stiffness as an additional variable during diagnosis may serve as a biomarker for monitoring cardiovascular health.
MEASUREMENTS OF ARTERIAL STIFFNESS
Pulse Pressure
Blood pressure is the conventional method for assessing cardiovascular risk because of its accessibility and high predictive value (100). While systolic pressure increases continuously with aging, diastolic pressure increases until midlife before declining (69). The divergence of systolic and diastolic pressures causes a plateau in mean arterial pressure but an amplification of pulse pressure. Moreover, the increase in pulse pressure is deemed a product of aging-induced arterial stiffening, since it occurs in normotensive and hypertensive patients. Hence, pulse pressure is a better predictor of cardiovascular risk in older patients (75) than in midlife adults (46). Moreover, tracking the longitudinal change in pulse pressure provides an even stronger indication of cardiovascular disease (166). Despite the potential for this biomarker, some meta-analyses failed to find added benefit for including pulse pressure; hence, it has been excluded from current clinical guidelines (100, 117). Pulse pressure should be interpreted with caution, because increased aortic stiffness propagates transmission of energy to distal vessels and does not necessarily increase pulse pressure (82, 191).
Pulse wave velocity (PWV), a measure of the distance traveled by the pulse wave over time, is the gold standard for assessing arterial stiffness (18). The two arterial sites most commonly used are carotid and femoral, although slight variations do exist. Clinical devices used to measure PWV include Complior, SphygmoCor, PulsePen, ultrasound, and magnetic resonance imaging (22, 113). Drawbacks of carotid-femoral PWV include averaging over heterogeneous vascular structures and accurately measuring the distance traveled (212). To circumvent these issues, mathematical models have been developed to estimate PWV using only one arterial site (218) or local PWV of a single arterial segment (162). European guidelines suggest that patients with PWV > 10 m/s should be considered at higher risk for cardiovascular events (212). Average adult values are 7.4 m/s for women and 8.2 m/s for men, and this sex difference is maintained even after correction for age and blood pressure (170). Reference values start at 6.1 (4.6–7.5) m/s for young, healthy individuals but increase with aging and hypertension.
The augmentation index (AIx) is a measurement of arterial stiffness derived from the pressure waveform using applanation tonometry by applying slight pressure on the radial artery. Values are calculated as the difference between the height of the reflected wave and the systolic peak divided by the pulse pressure (113). Radial artery applanation tonometry avoids amplification of peripheral pressure and enables analysis of the central waveform (149). As arteries stiffen, the reflected wave travels faster, resulting in an increased height and, hence, a higher AIx (154). However, clinical studies have shown that decreasing arterial stiffness does not always reduce AIx (116, 140), which may be related to the influence of heart rate on this measurement (222, 223). AIx may have better predictive value when pressure and flow measurements are obtained to separate forward and backward waves (219).
The β-stiffness index [β = ln(SBP/DBP) × D/∆D, where SBP is systolic blood pressure, DBP is diastolic blood pressure, and D is artery diameter (155)] and the cardioankle vascular index (CAVI) are closely related and were developed to be independent of blood pressure. Normal values obtained from the carotid artery are ~10.3 in normotensive men and women (118), but values can be much higher, depending on the artery that is measured (84). Unlike PWV, the β-stiffness index takes into account the blood pressure and is a local measurement that can be used to assess stiffening in both large and small vessels. Because PWV is influenced by blood pressure and the β-stiffness index measures local vascular properties, CAVI overcomes the individual limitations of each by combining the two approaches (186): CAVI involves recording the distance from the brachial artery to the ankle as well as the time difference between the closing of the aortic valve and the arrival of the arterial pressure wave. Not surprisingly, CAVI highly correlates with the β-stiffness index, increases with aging, and is lower in women than men before 70 yr of age (147, 205). Reference values in healthy patients range from 6.6 in young women to 9.4 in older men (147). While this technique is promising, it has been subjected to limited clinical testing and, therefore, has yet to be proven as a universally applicable predictor of cardiovascular outcomes (178).
PATHOLOGIES ASSOCIATED WITH ARTERIAL STIFFENING
Aging
Although aging is not a disease, it correlates more highly with arterial stiffness than any other variable (53). The Baltimore Longitudinal Study of Aging found that PWV increases with each decade of life starting at 40 yr of age (5). Vascular aging occurs across species and in otherwise healthy individuals, but cardiovascular diseases such as hypertension and metabolic syndrome exacerbate these changes (146). Interventional studies have shown that lifestyle modifications, such as caloric restriction, weight loss, and reduced Na+ intake, can partially attenuate the impact of aging on arterial stiffening (153). This benefit is found in individuals with cardiovascular risk factors as well as in healthy subjects.
Women have lower arterial stiffness than age-matched men from puberty (3) to menopause (220). After the surge in sex hormones during puberty, stiffness increases in men, while arterial elasticity is maintained or improved in women. Adults of both sexes experience an increase in arterial stiffening with aging, but the increase is steeper in men than women (5). The SardiNIA Study showed that the rate of stiffening parallels increases in blood pressure in women but becomes dissociated in men over time (182). Since changes in stiffness accompany adolescence, it is not surprising that the sexual dimorphism in arterial stiffening disappears after menopause (7). The largest change in female vascular compliance occurs around the menopausal transition (150). In healthy premenopausal women, arterial stiffening measurements, such as pulse pressure and AIx, remain constant over the menstrual cycle (160). However, postmenopausal women have stiffer arteries than aged-matched men, even though mean arterial pressure is similar (32, 151, 215).
Hypertension
Increased systolic pressure is associated with arterial stiffening due to vascular wall degeneration, progressive remodeling, and loss of stored energy (198). However, the temporal relationship between these two pathologies is still under debate. Longitudinal analysis of data from the Genetic Epidemiology Network of Arteriopathy Study shows that higher PWV and AIx are associated with greater increases in blood pressure over time (33). Another longitudinal study of Framingham offspring found that arterial stiffening measured by PWV predicts future hypertensive status (102). In contrast, evidence from young patients in the Bogalusa Heart Study indicates that hypertension precedes changes in arterial stiffening (27). These disparate findings may indicate the importance of age, with hypertension preceding stiffening in the young population but after stiffening in aging patients.
Hypertension is a confounding factor that clouds most clinical studies of the impact of sex on arterial stiffening. However, a few studies have provided evidence that sex impacts the relationship between blood pressure and arterial stiffening. For example, the Australian National Blood Pressure Study found that, despite similar blood pressures, the β-stiffness index and AIx are greater in aging women with hypertension than age-matched men (17). In a longitudinal multiethnic cohort, arterial stiffening is associated with aging and blood pressure in both sexes (200). Race, diabetes, and cholesterol are associated with stiffness in men, but only educational level is a significant predictor in women. Women born to parents with hypertension are at higher risk for increased arterial stiffness than men, suggesting sex differences in genetic factors (21). These data indicate that pathological or environmental factors may be important, depending on sex.
Metabolic Syndrome
After adjustment for age, PWV is higher in patients with metabolic syndrome (143). The elevation is most striking in older patients with multiple risk factors (53). Obesity, hyperglycemia, and hypertension independently contribute to this increase (179). Insulin resistance also plays a part in the development of metabolic syndrome and is associated with lower distensibility in young obese patients (211). Trajectories show that greater body fat and blood pressure in adolescence predict stiffer arteries in adulthood, indicating a cumulative effect of multiple risk factors on arterial elasticity (58).
Type 2 diabetes is associated with an increase in PWV in women but not in men (37), and the arterial stiffness that accompanies metabolic syndrome is more pronounced in women than men (220). Obesity is a more important risk factor for women, since body mass index correlates positively with arterial stiffening in women, but, surprisingly, a negative relationship is found in men (152). The mechanisms that underlie these sex differences in arterial stiffness are complex but may provide insights into potential pathways for promoting healthy vascular aging.
Immune Disorders
Increased PWV and AIx are observed in patients with autoimmune disorders, including Hashimoto’s thyroiditis (197), inflammatory bowel disease, rheumatoid arthritis, and systemic lupus erythematosus (50, 185). In patients with rheumatoid arthritis without clinical signs of cardiovascular disease, 8 wk of treatment with an antibody against TNF-α significantly attenuated vascular inflammatory markers as well as PWV (125). Inflammation also provides a link between the cardiovascular conditions mentioned above and their impact on stiffening. In stroke patients with metabolic syndrome, higher levels of inflammatory markers, such as C-reactive protein, are associated with stiffer arteries (210). Hypertension is also linked to cytokine production and immune cell infiltration (137) and involves accumulation of inflammatory cells in the kidneys and vasculature that induce oxidative stress and end organ damage.
The Gutenberg Health Study found that arterial stiffness correlates with inflammatory markers, including C-reactive protein and interleukins, in both men and women (8). Female subjects of multiple species have more robust immune responses and quicker pathogen clearance than male subjects, but this heightened response may lead to a higher incidence of autoimmune disorders (105). This female phenotype includes greater antibody response, higher B cell populations, and more circulating immunoglobulins, regardless of age (207). Circulating inflammatory markers in female subjects are increased even at baseline, and cell populations differ by sex, with natural killer CD8+ cells predominating in male subjects and CD4+ cells predominating in female subjects (1). While research on the interactions between inflammation and arterial stiffness is only at the beginning stages, since sex influences the role of immune cells in the development of hypertension (232), it most likely plays a role in arterial stiffness as well.
MECHANISMS AND PATHWAYS IN ARTERIAL STIFFENING
Arterial stiffening is a multifaceted disorder that involves the interaction of several molecular mechanisms, including sex hormones, extracellular matrix (ECM) alterations, endothelial and smooth muscle cell pathophysiology, oxidative stress, and inflammation. Each of these factors will be discussed in regard to sex differences.
Male Sex Hormones
Testosterone belongs to a family of androgens, including androstenedione and dihydrotestosterone, which bind to the androgen receptor to induce physiological actions. Multiple studies have indicated that low serum testosterone is associated with increasing mortality in men (81). Furthermore, population-based studies show that low total serum testosterone impairs endothelial function in men (54), while administration of testosterone increases endothelium-derived relaxation factor and improves flow-mediated vasodilation in hypogonadal men (16, 68, 187). Androgens also play a role in vascular remodeling, since decreased circulating testosterone levels correlate with increased arterial stiffness (45, 93, 111). Testosterone deficiency also increases intima-media thickness in the common carotid artery in men with metabolic disorders (39). In aging men, hypogonadism is associated with increased arterial stiffening in central and peripheral vessels, while testosterone replacement reduces PWV (226). Conversely, blockade of the production of testosterone as a treatment for prostate cancer increases arterial stiffening (190). These results indicate a protective role for testosterone in men.
Female Sex Hormones
Estrogen binds to estrogen receptor (ER)α and ERβ to initiate genomic responses, while G protein-coupled ER (GPER) induces rapid signaling (171). Which receptor is responsible for the positive vascular effects of estrogen has not been clearly delineated. Genetic deletion of endothelial ERα reduces high-fat diet (HFD)-induced arterial stiffness in both male (126) and female (128) subjects, suggesting that this genomic receptor has adverse effects on vascular remodeling. Conversely, the mesenteric arteries of female mice lacking ERβ are significantly less distensible than those of wild-type control mice, indicating a protective role (49). The ratio of ERs may also be important, since increasing GPER expression or decreasing ERα attenuates the vascular remodeling induced by carotid artery injury (78). Previous results from our laboratory have shown that, during salt-sensitive hypertension, membrane-initiated signaling via GPER has no impact on collagen or elastin but decreases oxidative stress and glycosaminoglycan (GAG) deposition in the medial layer of the aorta (123). Therefore, there is still much to understand about ER signaling and how these multiple receptor pathways collaborate to impact arterial stiffening.
Collagen, elastin, and glycoproteins make up the ECM and provide physical scaffolding for blood vessels as well as a platform for de novo biochemical reactions (180). Collagen contributes to the strength and stability of the arterial wall. Fibrillar collagen types I and III are major components of the intima, media, and adventitia, whereas collagen types I, III, IV, and V are present in the basement membrane. Collagen fibers are continuously deposited and removed from the arterial wall throughout life (112, 199). While elastic fibers form during development and are stretched during somatic growth, collagen fibers develop an undulated pattern, which contributes to their mechanical function. During significant levels of strain, such as an acute increase in blood pressure, collagen fibers straighten to limit overdistension and rupture of vessels. Arterial stiffening is frequently associated with alterations in collagen turnover and may include increased collagen production (189), altered ratio of collagen fiber types (52), and enhanced collagen cross-linking (13).
Elastic fibers provide compliance and resilience to enable storage of energy in the arterial wall during systole to maintain flow during diastole. Elastic fibers are susceptible to proteolysis (92) and mechanical fatigue (119) because they do not regenerate during adulthood (36). Acquired or congenital disruption of elastic fiber integrity induces mechanical consequences, such as loss of resilience, decrease in elastic recoil, and diminished distensibility (61). Moreover, elastic fiber disruption promotes changes in smooth muscle cell phenotype (103), inflammatory status (2), and the cell cycle (138). Increased structural stiffness is frequently reported after loss of elastic fiber integrity (56, 131, 216), but recent biaxial assessments have found that intrinsic material stiffness is maintained while elastic energy storage is decreased (59, 60). Therefore, despite the ability to maintain mechanical homeostasis, physiological vascular function is still compromised with loss of elastic fibers.
GAGs are unbranched polysaccharides that covalently bind to proteins to form proteoglycans. These highly charged hydrophilic macromolecules are secreted into the extracellular space to form a porous hydrated gel. GAGs reinforce collagen and elastic fibers and play an important role in arterial stiffening and mechanohomeostasis (196). The luminal glycocalyx is the first line of defense for the vascular wall, and changes in these proteoglycans impact the mechanics of the underlying endothelium (57). A high-salt diet causes damage to the glycocalyx, allowing Na+ to permeate the vascular wall (156). As a result, nitric oxide (NO) production is suppressed, leading to endothelial dysfunction and vascular stiffening (110). In the early stages of vascular injury before changes in collagen are detected, GAGs are upregulated and promote alterations in smooth muscle cell phenotype (221). Elevated GAGs are detected in aortas of spontaneously hypertensive rats before the onset of hypertension and compromise distensibility and mechanosensing in the aorta (172, 173).
Sex Differences in the ECM
Few studies have directly compared the arterial ECM composition of men with that of women. However, theoretical investigation indicates that aging has a greater effect on ECM stiffness in men (10). Estrogen deficiency promotes ECM remodeling that is associated with arterial stiffening, including an increase in collagen synthesis and a decrease in degradation (65, 193, 230), while estrogen administration to ovariectomized rats decreases the total amount of both collagen and elastin (63). Femoral arteries from female mice lacking endothelial ERα show no alterations in collagen or the internal elastic lamina (128). Male mesenteric arteries with endothelial-specific deletion of ERα have more elastin and collagen as well as decreased activity of matrix metalloproteinases, which break down the ECM (126). On the other hand, mesenteric arteries from female mice lacking ERβ have more elastin than wild-type control mice (49). Estrogen decreases GAGs in the aorta of ovariectomized rabbits and attenuates carotid injury by reducing proteoglycan deposition (4, 161). The mechanism for these estrogenic effects may be transcriptional, as estrogen reduces gene expression for proteoglycans as well as the enzyme involved in their synthesis (70, 136). Alternatively, since proteoglycan synthesis is inhibited by antioxidants in vascular smooth muscle, estrogen may alter GAGs indirectly by reducing oxidative stress (26). Along these lines, we found that in vivo GPER activation attenuates salt-induced increases in aortic GAGs in hypertensive mRen2 female rats and highly correlates with tissue oxidant levels (123). The male sex hormone testosterone inhibits collagen synthesis in cardiac fibroblasts (225) but increases GAGs in vascular smooth muscle cells (83). In cultured aortic smooth muscle cells, estrogen, progesterone, and testosterone decrease collagen, while only the female sex hormones increase elastin (148). The significance of this latter finding is unclear, since functional elastic fibers are not synthesized in adulthood (36). These data indicate mixed results with regard to the impact of sex hormones on ECM factors and are most likely complicated by the presence of estrogens and androgens in both male and female subjects as well as the presence of multiple receptor signaling pathways.
Vascular Smooth Muscle Cells
Intrinsic changes in the structural properties and phenotype of vascular smooth muscle cells contribute to arterial stiffening, yet there has been limited work assessing this cell type. Vascular smooth muscle cells play a critical role in vascular function by regulating tone, pressure, and flow. Moreover, aging, oxidative stress, and hypertension directly impact vascular smooth muscle cells by increasing apoptosis (231) and inducing cytoskeletal stiffening (169, 184). Mechanical homeostasis between smooth muscle and the ECM impacts cell phenotype, as well as the structure and content of the ECM, during remodeling (94). Additional investigation is needed to fully understand this relationship and the molecular mechanisms that contribute to arterial stiffening.
Transcriptional profiling of aortas from young and old male and female monkeys reveals higher expression of genes regulating the ECM as well as genes that play a role in switching smooth muscle cells from the contractile to the synthetic phenotype in aging male monkeys (167). In addition, the observation that aging-induced alterations in stiffness were absent after actin depolymerization (169) emphasized the importance of smooth muscle cell phenotype. Sex differences also impact the proliferation of vascular smooth muscle cells. While both male and female rat aortic smooth muscle cells express ERs, the impact of genetic deletion of these receptors has, in some cases, opposite effects on cell proliferation (91). Sexual dimorphism in this cell type is also found in response to induced apoptosis: more resistant cells and increased organization of actin fibers in female subjects (201). Therefore, in smooth muscle cells, genomic as well as hormonal programming most likely contributes to sex differences in function.
Endothelial Dysfunction and NO
Arterial stiffening and endothelial dysfunction are frequently observed simultaneously (188) and are key components of vascular aging (183). Endothelial dysfunction is characterized by impaired NO release and may be due to decreased availability of substrate and/or cofactors necessary for enzymatic action by NO synthases (23). This “uncoupling” event also results in oxidative stress (44), both of which promote vascular remodeling. Systemic inhibition of NO synthase increases the carotid β-stiffness index in men and postmenopausal women within 15 min (203). Similarly, inhibition of NO synthase for 3 wk increases PWV in spontaneously hypertensive rats (98). In the reverse direction, structural changes in the vascular wall increase endothelial permeability, allowing infiltration of inflammatory molecules and exacerbating stiffness (96). Arterial stiffening is attenuated when either the NO substrate l-arginine or the NO precursor nitrite is increased (188). The endothelial dysfunction observed in autoimmune disorders most likely contributes to the increased prevalence of stiffness in this patient population (204).
While arterial stiffening is associated with lower NO bioavailability, it is well established that estrogen increases NO in the vasculature (25). Estrogen increases NO bioavailability and decreases vascular tone (24) and negates arterial stiffening in postmenopausal women (72, 181) and ovariectomized rats (206). Although estrogen improves endothelial function, cross-sectional studies on early-menopausal women have indicated that it does not decrease carotid intima-media thickness (14). In postmenopausal women, administration of estrogen or the NO synthase cofactor tetrahydrobiopterin improves carotid artery compliance (142). Interestingly, tetrahydrobiopterin provides no additional benefit when administered with estrogen, suggesting that these treatments share a similar mechanism. Estrogen increases endothelial NO through membrane-initiated signaling (85) via ERα (28) and/or GPER (120) as well as transcriptionally by upregulating mRNA for endothelial NO synthase (106). Estrogen regulates inducible NO synthase in a bidirectional manner, with ERα repressing and ERβ increasing its transcription (209). Acute signaling by GPER also increases NO production in brain microvascular endothelial cells (6). Interestingly, the increased NO was associated with increased cell stiffness, as measured by atomic force microscopy. The antiaging effects of resveratrol are also associated with increased NO bioavailability, which may be, at least in part, mediated by ERα (107). These studies have suggested a close relationship between ER signaling and NO bioavailability, which may at times be difficult to separate.
Oxidative Stress
Increases in reactive oxygen species (ROS), including superoxide and hydrogen peroxide, are detrimental to vascular function and remodeling (71). Sources of ROS include NADPH oxidases in endothelial and smooth muscle cells as well as mitochondrial production. In addition to the intimal and medial layers of the vasculature, perivascular adipose tissue is another source of ROS that may become particularly important during aging (67). ROS directly induce vascular damage and decrease NO bioavailability by combining with NO to form peroxynitrite (80). Reductions in superoxide levels (67) and inhibition of mitochondrial ROS (73) attenuate aortic PWV in aging mice.
While ROS contribute to vascular remodeling, estrogen protects against oxidative damage (224). Infusion of the antioxidant ascorbic acid improves carotid artery compliance in postmenopausal women, indicating that menopause-induced oxidative damage promotes arterial stiffening (89). Mechanisms for estrogenic protection from oxidative stress include decreased NADPH oxidase activity, increased superoxide dismutase activity, stimulation of NO production to quench ROS, and neutralization of excess ROS by the phenolic hydroxyl group of estrogen (109). Selective activation of the GPER reduces oxidative stress in endothelial cells (20) and pancreatic β-cells (108), and our work in the salt-sensitive mRen2 female rat provides evidence that GPER reduces ROS and arterial remodeling in vivo (121, 123).
Inflammation
Changes in the inflammatory profile of the vasculature may promote arterial stiffening. Expression of proinflammatory cytokines is increased in isolated coronary arteries from 25-mo-old Fischer-344 rats (35), whereas IL-6, TNF-α, and monocyte chemoattractant protein-1 are increased in vascular endothelial cells from aging humans (47). Androgen receptors are present in the thymus, and testosterone has suppressive effects on the immune system by decreasing production of both B and T cells (158). ERs are expressed in the thymus, peripheral T cells, and bone marrow and normalize immune function by increasing T helper cells and lowering B cell production in ovariectomized animal models (158). Estrogen exhibits protective anti-inflammatory properties, such as its ability to reduce production of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α (164, 227). Production of TNFα and IL-1β increases during the naturally occurring drops in estrogen during the ovarian cycle, and ovariectomy increases production of TNF-α as well as IL-17, a key modulator of IL-1, IL-6, and TNF-α (38). In addition to the impact of estrogen, there is evidence for a suppressive effect of androgens on male macrophage activity that may contribute to sex differences in the inflammatory response (74, 163).
ANIMAL MODELS OF ARTERIAL STIFFNESS
Disease modeling in animals is important for understanding cardiovascular pathophysiology and advancing translational research. However, designing a “pure” model of arterial stiffness is complex, because arterial stiffening develops gradually over time. Instead, arterial stiffening is usually induced by dietary or genetic manipulations, aging, or hypertension.
Few studies have addressed the impact of estrogen on arterial stiffening in female subjects in the absence of other pathologies. In ovariectomized Sprague-Dawley rats, low-dose estrogen increases the distensibility of coronary but not mesenteric arteries (229). Estrogen was also found to attenuate aging-induced increases in mesenteric artery wall thickness and restore vascular matrix metalloproteinase activity (230). While studies in ER global knockout mice have not been performed, genetic deletion of steroid receptor coactivator-1, which associates with and promotes the actions of the nuclear ERs, results in increased blood pressure and aortic stiffness (90). These studies have indicated that estrogen and, in particular, the transcriptional actions of estrogen may significantly protect from arterial stiffening. Many questions remain, however, regarding the receptors and signaling pathways that mediate the actions of this hormone.
Consumption of a HFD promotes arterial stiffening in male Wistar rats (165), young and old male B6D2F1 mice (87), and male C57BL/6J mice (15). Although a side-by-side comparison of both sexes was not performed, female C57BL/6J mice are susceptible to HFD-induced arterial stiffness, and either mineralocorticoid receptor blockade (41) or genetic deletion of the endothelial mineralocorticoid receptor (101) ameliorates the damage. Additional studies using this same model have shown benefits for exercise (159), inhibition of dipeptidyl peptidase-4 (127), and amiloride treatment (132). Contrary to the well-known benefits of vascular ER signaling, deletion of ERα from endothelial cells attenuates vascular stiffening in female (128) and male (126) mice. In contrast to studies in healthy animals, these results indicate that genomic estrogen signaling in endothelial cells may promote, rather than attenuate, arterial stiffening. Since the protective effect of female sex in rats with metabolic syndrome is absent during feeding of a HFD (95), these dietary models may not be optimal for studying sex differences in arterial stiffening.
Multiple genetic mouse models show the significant impact of ECM factors on arterial stiffening. Few studies have examined the impact of sex, and many have found a lack of sex differences. Large arteries from mice heterozygous for the elastin gene (Eln+/−) have increased blood pressure and more smooth muscle cell layers with smaller luminal diameters, which normalize wall stress in the face of decreased elastin (56). Analysis of sex differences in this mouse model found no significant differences in blood pressure, pulse pressure, length of the ascending aorta, and axial stretch (114). The impact of genetic deletion of other proteins that are critical for the formation of mature elastic fibers, such as fibulin-4 (115) and fibulin-5 (217), has also been characterized; fibulin-5 shows a phenotype of structural stiffness and decreased energy storage that is independent of sex (60). The aortas of male and female fibrillin-1 hypomorphic mice are similarly stiffer, with smaller internal diameters and disorganized elastic fibers (131). In addition to a lack of sex differences at baseline, exercise training of fibrillin-1 hypomorphic mice induces no sex differences in vascular structure (133). Although genetic deletion of these ECM components affects arterial stiffening to the same degree in male and female animals, the relatively few studies indicate the need for additional work, including studies of other ECM components, addressing the potential impact on sexual dimorphism in arterial stiffening.
Aging is a nonmodifiable factor that contributes to arterial stiffening. Remarkably, the longest-lived rodent, the naked mole rat, has no age-related cardiovascular diseases and no detectable increase in PWV (77). In contrast, PWV is increased in <26-mo-old male C57BL/6 mice and associated with alterations in NO (188). Arterial stiffening also manifests in macaque monkeys during aging, along with alterations in collagen and elastin (167). This nonhuman primate study provides strong evidence of sex differences, with female monkeys having less stiffness and maintaining elastin density, despite equal pulse pressures. Analysis of gene expression from these aortas found that while >600 genes were impacted by aging, <5% of these altered genes were the same in male and female monkeys (168). Another nonrodent animal model that displays sex differences in PWV is the domestic fowl (176). Interestingly, while young female chicks have less stiffness than age-matched male chicks, this sex difference is absent in the prepubertal age group but returns in sexually mature chickens. Therefore, aging-induced mechanisms, as well as sex differences in the clinical measurements, may be necessary to diagnose vascular changes.
Multiple models of hypertension show increases in arterial stiffness, including animals infused with angiotensin II (51) or N-nitro-l-arginine methyl ester (66) and genetic models such as the spontaneously hypertensive rat (31). Unfortunately, the majority of these studies have not included female animals, and even fewer studies have directly compared the two sexes. In the Dahl salt-sensitive rat model, male and female rats display a similar increase in PWV from 3 to 6 mo of age (88), but the quantitative trait loci associated with arterial stiffening are sex-specific (40). In studies focused on arterial stiffening in female subjects, estrogen was found to be protective. For example, when SU-5416 and chronic hypoxia were used to induce pulmonary hypertension, compliance decreased in ovariectomized female mice but not in mice supplemented with estrogen (122). While animal models provide data on key mechanisms involved in arterial stiffening, major gaps remain, because relatively few models have been used to study sex differences.
INTERVENTIONS FOR ARTERIAL STIFFENING
While there are no drugs indicated for counteracting arterial stiffening, many antihypertensive drugs simultaneously improve elasticity. While many studies have included women, few were powered to assess whether sex is a factor. In a cohort containing men and women, the angiotensin receptor blocker eprosartan had greater benefits in terms of arterial stiffening than the β-blocker atenolol, despite similar blood pressures (42). Similarly, in men and women with resistant hypertension, addition of the angiotensin receptor blocker valsartan to a regimen containing an angiotensin-converting enzyme inhibitor enhanced the reduction in both blood pressure and arterial stiffening (124). In contrast to these studies showing the importance of renin-angiotensin system blockade, a study of only postmenopausal women found that valsartan and the Ca2+ channel blocker amlodipine equally reduce arterial stiffening (86). Therefore, additional studies are needed to determine whether certain antihypertensive medications are more effective in attenuating arterial stiffening in women than men.
The impact of menopausal hormone therapy on large artery remodeling is varied. Arterial stiffness is decreased with estrogen treatment in combination with the synthetic progestins drospirenone (214) and dydrogesterone (134). The combination of estrogen and progestin has a positive impact on the carotid intima-to-media ratio in healthy women (144) and women with mild hypercholesterolemia (76), but a reduction in PWV does not accompany the improvements in carotid measurements. Similarly, treatment with estrogen alone does not improve PWV after 12 wk (9) or 4 mo (43). In contrast, administration of the phytoestrogen resveratrol for 12 wk significantly reduces CAVI in patients with type 2 diabetes (97) but not in healthy individuals (192). These mixed results indicate the need for knowledge of the estrogenic signaling pathways that attenuate arterial stiffening, so that more selective approaches can be tested.
In contrast to the controversy surrounding estrogen therapy, clear evidence indicates that testosterone improves arterial elasticity and does not increase overall cardiovascular risk (30). However, based on a relatively small number of studies that show increased cardiovascular events in patients taking this therapy, the United States Food and Drug Administration requires a warning for increased cardiovascular risk on testosterone prescriptions (213). While most studies that have assessed arterial stiffening in male and female subjects still fail to measure and report hormonal status, one recent analysis from the Multi-Ethnic Study of Atherosclerosis found that higher levels of testosterone in women and, conversely, estradiol in men are associated with greater aortic stiffness (202). More adequate assessment of the impact of aging-induced decreases in sex hormones on arterial stiffness is needed in future studies.
Exercise improves multiple measures of arterial health in humans (99) and animal models (79). Sex differences emerge when the type and intensity of exercise and its impact on arterial stiffening are considered. While cardiorespiratory fitness and physical activity are associated with better arterial elasticity in both sexes, leisure activities have adverse effects on stiffening only in men, an effect most likely resulting from the predominance of sedentary leisure in men compared with active leisure in women (19). PWV after acute exercise is higher in men than women (29, 48), but chronic aerobic exercise in men reduces arterial stiffening to a greater extent than resistance training (141, 145). In fact, resistance training acutely increases PWV to a similar extent in young men and women (104). High-intensity resistance training for 8 wk, on the other hand, improves endothelial function but does not impact PWV in young and older women (174). A combination of resistance and aerobic exercise significantly decreased arterial stiffness in postmenopausal women (62, 195), and this regimen also reduced stiffness, along with blood pressure and insulin resistance, in adolescent girls (194). Together, these studies have emphasized that even the type of exercise may need to be tailored according to sex to obtain the optimal reduction in arterial stiffness.
Perspectives
While reducing blood pressure improves health outcomes, inclusion of other vascular measurements may provide more information about the underlying vascular pathology. Additional research is needed to understand the cellular mechanisms that precede and follow changes in arterial stiffening. Integration of measurements of arterial stiffening into clinical practice will provide more opportunities to improve methodology and a platform for additional clinical studies. Moreover, the impact of sex and sex hormones on arterial stiffening should be acknowledged, with separate acceptable ranges defined for each stage of life. Since arterial stiffening arises from several etiologies (Fig. 3), assessment of familial genetic inheritance and environmental exposure should provide further insights into potential targets for interventions aimed at reversing arterial stiffening. Unresolved issues, such as the significance of assessing local PWV on specific vessels (e.g., carotid artery vs. carotid-femoral PWV), can improve risk stratification on cardiovascular disease.
Fig. 3.
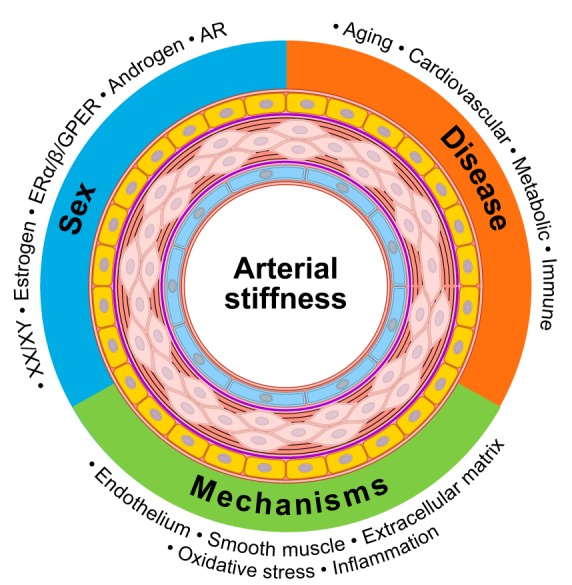
Graphical abstract denoting interactions of sex, disease, and mechanisms in arterial stiffness. ER, estrogen receptor; GPER, G protein-coupled ER; AR, androgen receptor.
GRANTS
This work was supported by National Institutes of Health Grant 5-R01-HL-133619 (to S. H. Lindsey).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.O.O., M.A.Z., and S.H.L. interpreted results of experiments; B.O.O. and S.H.L. prepared figures; B.O.O., M.A.Z., G.L.C., C.M.A., K.M.G., K.S.M., and S.H.L. drafted manuscript; B.O.O., M.A.Z., G.L.C., C.M.A., K.M.G., K.S.M., and S.H.L. edited and revised manuscript; B.O.O., M.A.Z., K.S.M., and S.H.L. approved final version of manuscript.
REFERENCES
- 1.Abdullah M, Chai PS, Chong MY, Tohit ER, Ramasamy R, Pei CP, Vidyadaran S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol 272: 214–219, 2012. doi: 10.1016/j.cellimm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol 40: 1101–1110, 2008. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and postpuberty. J Clin Endocrinol Metab 88: 5375–5380, 2003. doi: 10.1210/jc.2003-030722. [DOI] [PubMed] [Google Scholar]
- 4.Aikawa J, Munakata H, Isemura M, Ototani N, Yosizawa Z. Hormonal effects on glycosaminoglycans in thoracic aortas of rabbits. Tohoku J Exp Med 143: 113–116, 1984. doi: 10.1620/tjem.143.113. [DOI] [PubMed] [Google Scholar]
- 5.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 62: 934–941, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altmann JB, Yan G, Meeks JF, Abood ME, Brailoiu E, Brailoiu GC. G protein-coupled estrogen receptor-mediated effects on cytosolic calcium and nanomechanics in brain microvascular endothelial cells. J Neurochem 133: 629–639, 2015. doi: 10.1111/jnc.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics−2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold N, Gori T, Schnabel RB, Schulz A, Prochaska JH, Zeller T, Binder H, Pfeiffer N, Beutel M, Espinola-Klein C, Lackner KJ, Blankenberg S, Münzel T, Wild PS. Relation between arterial stiffness and markers of inflammation and hemostasis−data from the population-based Gutenberg Health Study. Sci Rep 7: 6346, 2017. doi: 10.1038/s41598-017-06175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arruda CG, Aldrighi JM, Bortolotto LA, Alecrin IN, Ramires JA. Effects of estradiol alone and combined with norethisterone acetate on pulse-wave velocity in hypertensive postmenopausal women. Gynecol Endocrinol 22: 557–563, 2006. doi: 10.1080/09513590601005342. [DOI] [PubMed] [Google Scholar]
- 10.Astrand H, Stalhand J, Karlsson J, Karlsson M, Sonesson B, Länne T. In vivo estimation of the contribution of elastin and collagen to the mechanical properties in the human abdominal aorta: effect of age and sex. J Appl Physiol 110: 176–187, 2011. doi: 10.1152/japplphysiol.00579.2010. [DOI] [PubMed] [Google Scholar]
- 11.Avolio A. Arterial stiffness. Pulse (Basel) 1: 14–28, 2013. doi: 10.1159/000348620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahrs AM, Wulzen R. Effect of deficiency in anti-stiffness factor on relative arterial occlusion pressure of guinea pigs. Fed Proc 6: 71, 1947. [PubMed] [Google Scholar]
- 13.Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens 17: 23S–30S, 2004. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Bechlioulis A, Kalantaridou SN, Naka KK, Chatzikyriakidou A, Calis KA, Makrigiannakis A, Papanikolaou O, Kaponis A, Katsouras C, Georgiou I, Chrousos GP, Michalis LK. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab 95: 1199–1206, 2010. doi: 10.1210/jc.2009-2262. [DOI] [PubMed] [Google Scholar]
- 15.Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Heart Circ Physiol 309: H574–H582, 2015. doi: 10.1152/ajpheart.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernini G, Versari D, Moretti A, Virdis A, Ghiadoni L, Bardini M, Taurino C, Canale D, Taddei S, Salvetti A. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab 91: 1691–1697, 2006. doi: 10.1210/jc.2005-1398. [DOI] [PubMed] [Google Scholar]
- 17.Berry KL, Cameron JD, Dart AM, Dewar EM, Gatzka CD, Jennings GL, Liang YL, Reid CM, Kingwell BA. Large-artery stiffness contributes to the greater prevalence of systolic hypertension in elderly women. J Am Geriatr Soc 52: 368–373, 2004. doi: 10.1111/j.1532-5415.2004.52107.x. [DOI] [PubMed] [Google Scholar]
- 18.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 33: 1111–1117, 1999. doi: 10.1161/01.HYP.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 19.Boreham CA, Ferreira I, Twisk JW, Gallagher AM, Savage MJ, Murray LJ. Cardiorespiratory fitness, physical activity, and arterial stiffness: the Northern Ireland Young Hearts Project. Hypertension 44: 721–726, 2004. doi: 10.1161/01.HYP.0000144293.40699.9a. [DOI] [PubMed] [Google Scholar]
- 20.Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol 298: H1055–H1061, 2010. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- 21.Buus NH, Carlsen RK, Khatir DS, Eiskjær H, Mulvany MJ, Skov K. Arterial stiffness and peripheral vascular resistance in offspring of hypertensive parents: influence of sex and other confounders. J Hypertens 36: 815–823, 2018. doi: 10.1097/HJH.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 22.Calabia J, Torguet P, Garcia M, Garcia I, Martin N, Guasch B, Faur D, Vallés M. Doppler ultrasound in the measurement of pulse wave velocity: agreement with the Complior method. Cardiovasc Ultrasound 9: 13, 2011. doi: 10.1186/1476-7120-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation 88: 2541–2547, 1993. doi: 10.1161/01.CIR.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti S, Morton JS, Davidge ST. Mechanisms of estrogen effects on the endothelium: an overview. Can J Cardiol 30: 705–712, 2014. doi: 10.1016/j.cjca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23: 665–686, 2002. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 26.Chang MY, Han CY, Wight TN, Chait A. Antioxidants inhibit the ability of lysophosphatidylcholine to regulate proteoglycan synthesis. Arterioscler Thromb Vasc Biol 26: 494–500, 2006. doi: 10.1161/01.ATV.0000200135.61362.27. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Li S, Fernandez C, Sun D, Lai C-C, Zhang T, Bazzano L, Urbina EM, Deng H-W. Temporal relationship between elevated blood pressure and arterial stiffening among middle-aged black and white adults: the Bogalusa Heart Study. Am J Epidemiol 183: 599–608, 2016. doi: 10.1093/aje/kwv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor-α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103: 401–406, 1999. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collier SR, Frechette V, Sandberg K, Schafer P, Ji H, Smulyan H, Fernhall B. Sex differences in resting hemodynamics and arterial stiffness following 4 weeks of resistance versus aerobic exercise training in individuals with pre-hypertension to stage 1 hypertension. Biol Sex Differ 2: 9, 2011. doi: 10.1186/2042-6410-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corona G, Rastrelli G, Maseroli E, Sforza A, Maggi M. Corrigendum: Testosterone replacement therapy and cardiovascular risk: a review. World J Mens Health 34: 154, 2016. doi: 10.5534/wjmh.2016.34.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosson E, Herisse M, Laude D, Thomas F, Valensi P, Attali JR, Safar ME, Dabire H. Aortic stiffness and pulse pressure amplification in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 292: H2506–H2512, 2007. doi: 10.1152/ajpheart.00732.2006. [DOI] [PubMed] [Google Scholar]
- 32.Costa-Hong VA, Muela HCS, Macedo TA, Sales ARK, Bortolotto LA. Gender differences of aortic wave reflection and influence of menopause on central blood pressure in patients with arterial hypertension. BMC Cardiovasc Disord 18: 123, 2018. doi: 10.1186/s12872-018-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coutinho T, Bailey KR, Turner ST, Kullo IJ. Arterial stiffness is associated with increase in blood pressure over time in treated hypertensives. J Am Soc Hypertens 8: 414–421, 2014. doi: 10.1016/j.jash.2014.03.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 61: 96–103, 2013. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J 17: 1183–1185, 2003. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 36.Davis EC. Elastic lamina growth in the developing mouse aorta. J Histochem Cytochem 43: 1115–1123, 1995. doi: 10.1177/43.11.7560894. [DOI] [PubMed] [Google Scholar]
- 37.De Angelis L, Millasseau SC, Smith A, Viberti G, Jones RH, Ritter JM, Chowienczyk PJ. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension 44: 67–71, 2004. doi: 10.1161/01.HYP.0000130482.81883.fd. [DOI] [PubMed] [Google Scholar]
- 38.De M, Sanford TR, Wood GW. Interleukin-1, interleukin-6, and tumor necrosis factor-α are produced in the mouse uterus during the estrous cycle and are induced by estrogen and progesterone. Dev Biol 151: 297–305, 1992. doi: 10.1016/0012-1606(92)90234-8. [DOI] [PubMed] [Google Scholar]
- 39.De Pergola G, Pannacciulli N, Ciccone M, Tartagni M, Rizzon P, Giorgino R. Free testosterone plasma levels are negatively associated with the intima-media thickness of the common carotid artery in overweight and obese glucose-tolerant young adult men. Int J Obes Relat Metab Disord 27: 803–807, 2003. doi: 10.1038/sj.ijo.0802292. [DOI] [PubMed] [Google Scholar]
- 40.Decano JL, Pasion KA, Black N, Giordano NJ, Herrera VL, Ruiz-Opazo N. Sex-specific genetic determinants for arterial stiffness in Dahl salt-sensitive hypertensive rats. BMC Genet 17: 19, 2016. doi: 10.1186/s12863-015-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents Western diet-induced arterial stiffening in female mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhakam Z, McEniery CM, Yasmin, Cockcroft JR, Brown MJ, Wilkinson IB. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens 19: 214–219, 2006. doi: 10.1016/j.amjhyper.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Dias AR Jr, de Mello NR, Eluf Gebara OC, Nussbacher A, Wajngarten M, Petti DA. Conjugated equine estrogen, raloxifene and arterial stiffness in postmenopausal women. Climacteric 11: 390–396, 2008. doi: 10.1080/13697130802325635. [DOI] [PubMed] [Google Scholar]
- 44.Dikalova AE, Góngora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dockery F, Bulpitt CJ, Donaldson M, Fernandez S, Rajkumar C. The relationship between androgens and arterial stiffness in older men. J Am Geriatr Soc 51: 1627–1632, 2003. doi: 10.1046/j.1532-5415.2003.51515.x. [DOI] [PubMed] [Google Scholar]
- 46.Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, Grimm R, Cohen J, Stamler J, Group MR; MRFIT Research Group . Pulse pressure and cardiovascular disease-related mortality: follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 287: 2677–2683, 2002. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 47.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doonan RJ, Mutter A, Egiziano G, Gomez YH, Daskalopoulou SS. Differences in arterial stiffness at rest and after acute exercise between young men and women. Hypertens Res 36: 226–231, 2013. doi: 10.1038/hr.2012.158. [DOI] [PubMed] [Google Scholar]
- 49.Douglas G, Cruz MN, Poston L, Gustafsson JA, Kublickiene K. Functional characterization and sex differences in small mesenteric arteries of the estrogen receptor-β knockout mouse. Am J Physiol Regul Integr Comp Physiol 294: R112–R120, 2008. doi: 10.1152/ajpregu.00421.2007. [DOI] [PubMed] [Google Scholar]
- 50.Dregan A. Arterial stiffness association with chronic inflammatory disorders in the UK Biobank Study. Heart 104: 1257–1262, 2018. doi: 10.1136/heartjnl-2017-312610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberson LS, Sanchez PA, Majeed BA, Tawinwung S, Secomb TW, Larson DF. Effect of lysyl oxidase inhibition on angiotensin II-induced arterial hypertension, remodeling, and stiffness. PLoS One 10: e0124013, 2015. doi: 10.1371/journal.pone.0124013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eberth JF, Gresham VC, Reddy AK, Popovic N, Wilson E, Humphrey JD. Importance of pulsatility in hypertensive carotid artery growth and remodeling. J Hypertens 27: 2010–2021, 2009. doi: 10.1097/HJH.0b013e32832e8dc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El Feghali R, Topouchian J, Pannier B, Asmar R. Ageing and blood pressure modulate the relationship between metabolic syndrome and aortic stiffness in never-treated essential hypertensive patients. A comparative study. Diabetes Metab 33: 183–188, 2007. doi: 10.1016/j.diabet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Empen K, Lorbeer R, Dörr M, Haring R, Nauck M, Gläser S, Krebs A, Reffelmann T, Ewert R, Völzke H, Wallaschofski H, Felix SB. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol 32: 481–486, 2012. doi: 10.1161/ATVBAHA.111.232876. [DOI] [PubMed] [Google Scholar]
- 55.European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 21: 1011–1053, 2003. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112: 1419–1428, 2003. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fels J, Jeggle P, Liashkovich I, Peters W, Oberleithner H. Nanomechanics of vascular endothelium. Cell Tissue Res 355: 727–737, 2014. doi: 10.1007/s00441-014-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferreira I, van de Laar RJ, Prins MH, Twisk JW, Stehouwer CD. Carotid stiffness in young adults: a life-course analysis of its early determinants: the Amsterdam Growth and Health Longitudinal Study. Hypertension 59: 54–61, 2012. doi: 10.1161/HYPERTENSIONAHA.110.156109. [DOI] [PubMed] [Google Scholar]
- 59.Ferruzzi J, Bersi MR, Mecham RP, Ramirez F, Yanagisawa H, Tellides G, Humphrey JD. Loss of elastic fiber integrity compromises common carotid artery function: implications for vascular aging. Artery Res 14: 41–52, 2016. doi: 10.1016/j.artres.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferruzzi J, Bersi MR, Uman S, Yanagisawa H, Humphrey JD. Decreased elastic energy storage, not increased material stiffness, characterizes central artery dysfunction in fibulin-5 deficiency independent of sex. J Biomech Eng 137: 137, 2015. doi: 10.1115/1.4029431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferruzzi J, Collins MJ, Yeh AT, Humphrey JD. Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: implications for Marfan syndrome. Cardiovasc Res 92: 287–295, 2011. doi: 10.1093/cvr/cvr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 18: 980–984, 2011. doi: 10.1097/gme.0b013e3182135442. [DOI] [PubMed] [Google Scholar]
- 63.Fischer GM. In vivo effects of estradiol on collagen and elastin dynamics in rat aorta. Endocrinology 91: 1227–1232, 1972. doi: 10.1210/endo-91-5-1227. [DOI] [PubMed] [Google Scholar]
- 64.Fischer GM, Llaurado JG. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res 19: 394–399, 1966. doi: 10.1161/01.RES.19.2.394. [DOI] [PubMed] [Google Scholar]
- 65.Fischer GM, Swain ML. Effects of estradiol and progesterone on the increased synthesis of collagen in atherosclerotic rabbit aortas. Atherosclerosis 54: 177–185, 1985. doi: 10.1016/0021-9150(85)90177-7. [DOI] [PubMed] [Google Scholar]
- 66.Fitch RM, Vergona R, Sullivan ME, Wang YX. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res 51: 351–358, 2001. doi: 10.1016/S0008-6363(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 67.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13: 576–578, 2014. doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francomano D, Fattorini G, Gianfrilli D, Paoli D, Sgrò P, Radicioni A, Romanelli F, Di Luigi L, Gandini L, Lenzi A, Aversa A. Acute endothelial response to testosterone gel administration in men with severe hypogonadism and its relationship to androgen receptor polymorphism: a pilot study. J Endocrinol Invest 39: 265–271, 2016. doi: 10.1007/s40618-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franklin SS, Gustin W I4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96: 308–315, 1997. doi: 10.1161/01.CIR.96.1.308. [DOI] [PubMed] [Google Scholar]
- 70.Freudenberger T, Röck K, Dai G, Dorn S, Mayer P, Heim HK, Fischer JW. Estradiol inhibits hyaluronic acid synthase 1 expression in human vascular smooth muscle cells. Basic Res Cardiol 106: 1099–1109, 2011. doi: 10.1007/s00395-011-0217-5. [DOI] [PubMed] [Google Scholar]
- 71.García-Redondo AB, Aguado A, Briones AM, Salaices M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol Res 114: 110–120, 2016. doi: 10.1016/j.phrs.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 72.Gavin KM, Jankowski C, Kohrt WM, Stauffer BL, Seals DR, Moreau KL. Hysterectomy is associated with large artery stiffening in estrogen-deficient postmenopausal women. Menopause 19: 1000–1007, 2012. doi: 10.1097/gme.0b013e31825040f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol 124: 1194–1202, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Girón-González JA, Moral FJ, Elvira J, García-Gil D, Guerrero F, Gavilán I, Escobar L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol 143: 31–36, 2000. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 75.Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch Intern Med 160: 2765–2772, 2000. doi: 10.1001/archinte.160.18.2765. [DOI] [PubMed] [Google Scholar]
- 76.Gompel A, Boutouyrie P, Joannides R, Christin-Maitre S, Kearny-Schwartz A, Kunz K, Laurent S, Boivin JM, Pannier B, Pornel B, Struijker-Boudier HA, Thuillez C, Van Bortel L, Zannad F, Pithois-Merli I, Jaillon P, Simon T. Association of menopause and hormone replacement therapy with large artery remodeling. Fertil Steril 96: 1445–1450, 2011. doi: 10.1016/j.fertnstert.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Grimes KM, Reddy AK, Lindsey ML, Buffenstein R. And the beat goes on: maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. Am J Physiol Heart Circ Physiol 307: H284–H291, 2014. doi: 10.1152/ajpheart.00305.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gros R, Hussain Y, Chorazyczewski J, Pickering JG, Ding Q, Feldman RD. Extent of vascular remodeling is dependent on the balance between estrogen receptor α and G-protein-coupled estrogen receptor. Hypertension 68: 1225–1235, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07859. [DOI] [PubMed] [Google Scholar]
- 79.Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Contribution of receptor for advanced glycation end products to vasculature-protecting effects of exercise training in aged rats. Eur J Pharmacol 741: 186–194, 2014. doi: 10.1016/j.ejphar.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 80.Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 39: 1088–1094, 2002. doi: 10.1161/01.HYP.0000018041.48432.B5. [DOI] [PubMed] [Google Scholar]
- 81.Haring R, Völzke H, Steveling A, Krebs A, Felix SB, Schöfl C, Dörr M, Nauck M, Wallaschofski H. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 31: 1494–1501, 2010. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 82.Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58: 839–846, 2011. doi: 10.1161/HYPERTENSIONAHA.111.177469. [DOI] [PubMed] [Google Scholar]
- 83.Hashimura K, Sudhir K, Nigro J, Ling S, Williams MR, Komesaroff PA, Little PJ. Androgens stimulate human vascular smooth muscle cell proteoglycan biosynthesis and increase lipoprotein binding. Endocrinology 146: 2085–2090, 2005. doi: 10.1210/en.2004-1242. [DOI] [PubMed] [Google Scholar]
- 84.Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens 33: 1742–1757, 2015. doi: 10.1097/HJH.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 85.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87: 677–682, 2000. doi: 10.1161/01.RES.87.8.677. [DOI] [PubMed] [Google Scholar]
- 86.Hayoz D, Zappe DH, Meyer MA, Baek I, Kandra A, Joly MP, Mazzolai L, Haesler E, Periard D. Changes in aortic pulse wave velocity in hypertensive postmenopausal women: comparison between a calcium channel blocker vs. angiotensin receptor blocker regimen. J Clin Hypertens (Greenwich) 14: 773–778, 2012. doi: 10.1111/jch.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henson GD, Walker AE, Reihl KD, Donato AJ, Lesniewski LA. Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice. Physiol Rep 2: e00268, 2014. doi: 10.1002/phy2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herrera VL, Decano JL, Giordano N, Moran AM, Ruiz-Opazo N. Aortic and carotid arterial stiffness and epigenetic regulator gene expression changes precede blood pressure rise in stroke-prone Dahl salt-sensitive hypertensive rats. PLoS One 9: e107888, 2014. doi: 10.1371/journal.pone.0107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause 21: 624–632, 2014. doi: 10.1097/GME.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hinton AO Jr, Yang Y, Quick AP, Xu P, Reddy CL, Yan X, Reynolds CL, Tong Q, Zhu L, Xu J, Wehrens XHT, Xu Y, Reddy AK. SRC-1 regulates blood pressure and aortic stiffness in female mice. PLoS One 11: e0168644, 2016. doi: 10.1371/journal.pone.0168644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hogg ME, Vavra AK, Banerjee MN, Martinez J, Jiang Q, Keefer LK, Chambon P, Kibbe MR. The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res 173: e1–e10, 2012. doi: 10.1016/j.jss.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hornebeck W, Emonard H. The cell-elastin-elastase(s) interacting triade directs elastolysis. Front Biosci 16: 707–722, 2011. doi: 10.2741/3714. [DOI] [PubMed] [Google Scholar]
- 93.Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, Blackman MR, Metter EJ. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab 290: E234–E242, 2006. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 94.Humphrey JD. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50: 53–78, 2008. doi: 10.1007/s12013-007-9002-3. [DOI] [PubMed] [Google Scholar]
- 95.Hunter I, Soler A, Joseph G, Hutcheson B, Bradford C, Zhang FF, Potter B, Proctor S, Rocic P. Cardiovascular function in male and female JCR:LA-cp rats: effect of high-fat/high-sucrose diet. Am J Physiol Heart Circ Physiol 312: H742–H751, 2017. doi: 10.1152/ajpheart.00535.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res 116: 895–908, 2015. doi: 10.1161/CIRCRESAHA.116.305720. [DOI] [PubMed] [Google Scholar]
- 97.Imamura H, Yamaguchi T, Nagayama D, Saiki A, Shirai K, Tatsuno I. Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with type 2 diabetes mellitus. Int Heart J 58: 577–583, 2017. doi: 10.1536/ihj.16-373. [DOI] [PubMed] [Google Scholar]
- 98.Isabelle M, Simonet S, Ragonnet C, Sansilvestri-Morel P, Clavreul N, Vayssettes-Courchay C, Verbeuren TJ. Chronic reduction of nitric oxide level in adult spontaneously hypertensive rats induces aortic stiffness similar to old spontaneously hypertensive rats. J Vasc Res 49: 309–318, 2012. doi: 10.1159/000337470. [DOI] [PubMed] [Google Scholar]
- 99.Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor κB signalling. J Hypertens 33: 2477–2482, 2015. doi: 10.1097/HJH.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 101.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 118: 935–943, 2016. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130: 411–423, 2003. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 104.Kingsley JD, Tai YL, Mayo X, Glasgow A, Marshall E. Free-weight resistance exercise on pulse wave reflection and arterial stiffness between sexes in young, resistance-trained adults. Eur J Sport Sci 17: 1056–1064, 2017. doi: 10.1080/17461391.2017.1342275. [DOI] [PubMed] [Google Scholar]
- 105.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 16: 626–638, 2016. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 106.Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, Förstermann U. Estrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involved. Hypertension 31: 582–588, 1998. doi: 10.1161/01.HYP.31.2.582. [DOI] [PubMed] [Google Scholar]
- 107.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor-α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22: 2185–2197, 2008. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 108.Kooptiwut S, Mahawong P, Hanchang W, Semprasert N, Kaewin S, Limjindaporn T, Yenchitsomanus PT. Estrogen reduces endoplasmic reticulum stress to protect against glucotoxicity induced-pancreatic β-cell death. J Steroid Biochem Mol Biol 139: 25–32, 2014. doi: 10.1016/j.jsbmb.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 109.Kumar S, Lata K, Mukhopadhyay S, Mukherjee TK. Role of estrogen receptors in pro-oxidative and anti-oxidative actions of estrogens: a perspective. Biochim Biophys Acta 1800: 1127–1135, 2010. doi: 10.1016/j.bbagen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 110.Kusche-Vihrog K, Schmitz B, Brand E. Salt controls endothelial and vascular phenotype. Pflugers Arch 467: 499–512, 2015. doi: 10.1007/s00424-014-1657-1. [DOI] [PubMed] [Google Scholar]
- 111.Kyriazis J, Tzanakis I, Stylianou K, Katsipi I, Moisiadis D, Papadaki A, Mavroeidi V, Kagia S, Karkavitsas N, Daphnis E. Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol Dial Transplant 26: 2971–2977, 2011. doi: 10.1093/ndt/gfq847. [DOI] [PubMed] [Google Scholar]
- 112.Langille BL. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol 21, Suppl 1: S11–S17, 1993. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- 113.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 114.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol 301: H221–H229, 2011. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le VP, Yamashiro Y, Yanagisawa H, Wagenseil JE. Measuring, reversing, and modeling the mechanical changes due to the absence of fibulin-4 in mouse arteries. Biomech Model Mechanobiol 13: 1081–1095, 2014. doi: 10.1007/s10237-014-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lemogoum D, Flores G, Van den Abeele W, Ciarka A, Leeman M, Degaute JP, van de Borne P, Van Bortel L. Validity of pulse pressure and augmentation index as surrogate measures of arterial stiffness during β-adrenergic stimulation. J Hypertens 22: 511–517, 2004. doi: 10.1097/00004872-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 117.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Collaboration PS; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 118.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension 34: 201–206, 1999. doi: 10.1161/01.HYP.34.2.201. [DOI] [PubMed] [Google Scholar]
- 119.Lillie MA, Gosline JM. Limits to the durability of arterial elastic tissue. Biomaterials 28: 2021–2031, 2007. doi: 10.1016/j.biomaterials.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 120.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 81: 99–102, 2014. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58: 665–671, 2011. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu A, Tian L, Golob M, Eickhoff JC, Boston M, Chesler NC. 17β-Estradiol attenuates conduit pulmonary artery mechanical property changes with pulmonary arterial hypertension. Hypertension 66: 1082–1088, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu L, Kashyap S, Murphy B, Hutson DD, Budish RA, Trimmer EH, Zimmerman MA, Trask AJ, Miller KS, Chappell MC, Lindsey SH. GPER activation ameliorates aortic remodeling induced by salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 310: H953–H961, 2016. doi: 10.1152/ajpheart.00631.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahmud A, Feely J. Favourable effects on arterial wave reflection and pulse pressure amplification of adding angiotensin II receptor blockade in resistant hypertension. J Hum Hypertens 14: 541–546, 2000. doi: 10.1038/sj.jhh.1001053. [DOI] [PubMed] [Google Scholar]
- 125.Mäki-Petäjä KM, Elkhawad M, Cheriyan J, Joshi FR, Ostör AJ, Hall FC, Rudd JH, Wilkinson IB. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126: 2473–2480, 2012. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 126.Manrique-Acevedo C, Ramirez-Perez FI, Padilla J, Vieira-Potter VJ, Aroor AR, Barron BJ, Chen D, Haertling D, Declue C, Sowers JR, Martinez-Lemus LA. Absence of endothelial ERα results in arterial remodeling and decreased stiffness in Western diet-fed male mice. Endocrinology 158: 1875–1885, 2017. doi: 10.1210/en.2016-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manrique C, Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Martinez-Lemus LA, Ramirez-Perez FI, Klein T, Meininger GA, DeMarco VG. Dipeptidyl peptidase-4 inhibition with linagliptin prevents Western diet-induced vascular abnormalities in female mice. Cardiovasc Diabetol 15: 94, 2016. doi: 10.1186/s12933-016-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manrique C, Lastra G, Ramirez-Perez FI, Haertling D, DeMarco VG, Aroor AR, Jia G, Chen D, Barron BJ, Garro M, Padilla J, Martinez-Lemus LA, Sowers JR. Endothelial estrogen receptor-α does not protect against vascular stiffness induced by Western diet in female mice. Endocrinology 157: 1590–1600, 2016. doi: 10.1210/en.2015-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marlatt KL, Kelly AS, Steinberger J, Dengel DR. The influence of gender on carotid artery compliance and distensibility in children and adults. J Clin Ultrasound 41: 340–346, 2013. doi: 10.1002/jcu.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marque V, Kieffer P, Gayraud B, Lartaud-Idjouadiene I, Ramirez F, Atkinson J. Aortic wall mechanics and composition in a transgenic mouse model of Marfan syndrome. Arterioscler Thromb Vasc Biol 21: 1184–1189, 2001. doi: 10.1161/hq0701.092136. [DOI] [PubMed] [Google Scholar]
- 132.Martinez-Lemus LA, Aroor AR, Ramirez-Perez FI, Jia G, Habibi J, DeMarco VG, Barron B, Whaley-Connell A, Nistala R, Sowers JR. Amiloride improves endothelial function and reduces vascular stiffness in female mice fed a Western diet. Front Physiol 8: 456, 2017. doi: 10.3389/fphys.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mas-Stachurska A, Siegert AM, Batlle M, Gorbenko Del Blanco D, Meirelles T, Rubies C, Bonorino F, Serra-Peinado C, Bijnens B, Baudin J, Sitges M, Mont L, Guasch E, Egea G. Cardiovascular benefits of moderate exercise training in Marfan syndrome: insights from an animal model. J Am Heart Assoc 6: e006438, 2017. doi: 10.1161/JAHA.117.006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matsui S, Yasui T, Tani A, Kato T, Uemura H, Kuwahara A, Matsuzaki T, Arisawa K, Irahara M. Effect of ultra-low-dose estradiol and dydrogesterone on arterial stiffness in postmenopausal women. Climacteric 17: 191–196, 2014. doi: 10.3109/13697137.2013.856399. [DOI] [PubMed] [Google Scholar]
- 135.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113: 657–663, 2006. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 136.McDaniel JS, Akula Suresh Babu R, Navarro MM, LeBaron RG. Transcriptional regulation of proteoglycan 4 by 17β-estradiol in immortalized baboon temporomandibular joint disc cells. Eur J Oral Sci 122: 100–108, 2014. doi: 10.1111/eos.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Michel JB. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol 23: 2146–2154, 2003. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- 139.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mitchell GF, Lacourcière Y, Arnold JM, Dunlap ME, Conlin PR, Izzo JL Jr. Changes in aortic stiffness and augmentation index after acute converting enzyme or vasopeptidase inhibition. Hypertension 46: 1111–1117, 2005. doi: 10.1161/01.HYP.0000186331.47557.ae. [DOI] [PubMed] [Google Scholar]
- 141.Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med 47: 393–396, 2013. doi: 10.1136/bjsports-2012-090488. [DOI] [PubMed] [Google Scholar]
- 142.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mulè G, Cottone S, Mongiovì R, Cusimano P, Mezzatesta G, Seddio G, Volpe V, Nardi E, Andronico G, Piazza G, Cerasola G. Influence of the metabolic syndrome on aortic stiffness in never treated hypertensive patients. Nutr Metab Cardiovasc Dis 16: 54–59, 2006. doi: 10.1016/j.numecd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 144.Muscat Baron Y, Brincat M, Galea R. Carotid artery wall thickness in women treated with hormone replacement therapy. Maturitas 27: 47–53, 1997. doi: 10.1016/S0378-5122(97)01115-8. [DOI] [PubMed] [Google Scholar]
- 145.Mutter AF, Cooke AB, Saleh O, Gomez YH, Daskalopoulou SS. A systematic review on the effect of acute aerobic exercise on arterial stiffness reveals a differential response in the upper and lower arterial segments. Hypertens Res 40: 146–172, 2017. doi: 10.1038/hr.2016.111. [DOI] [PubMed] [Google Scholar]
- 146.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 147.Namekata T, Suzuki K, Ishizuka N, Shirai K. Establishing baseline criteria of cardio-ankle vascular index as a new indicator of arteriosclerosis: a cross-sectional study. BMC Cardiovasc Disord 11: 51, 2011. doi: 10.1186/1471-2261-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, Kingwell BA. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 46: 1129–1134, 2005. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- 149.Nelson MR, Stepanek J, Cevette M, Covalciuc M, Hurst RT, Tajik AJ. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clin Proc 85: 460–472, 2010. doi: 10.4065/mcp.2009.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nethononda RM, Lewandowski AJ, Stewart R, Kylinterias I, Whitworth P, Francis J, Leeson P, Watkins H, Neubauer S, Rider OJ. Gender specific patterns of age-related decline in aortic stiffness: a cardiovascular magnetic resonance study including normal ranges. J Cardiovasc Magn Reson 17: 20, 2015. doi: 10.1186/s12968-015-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noon JP, Trischuk TC, Gaucher SA, Galante S, Scott RL. The effect of age and gender on arterial stiffness in healthy Caucasian Canadians. J Clin Nurs 17: 2311–2317, 2008. doi: 10.1111/j.1365-2702.2007.02155.x. [DOI] [PubMed] [Google Scholar]
- 152.Nordstrand N, Gjevestad E, Dinh KN, Hofsø D, Røislien J, Saltvedt E, Os I, Hjelmesæth J. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord 11: 7, 2011. doi: 10.1186/1471-2261-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for achieving healthy vascular aging. Hypertension 71: 389–402, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 45: 652–658, 2005. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 155.O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 15: 426–444, 2002. doi: 10.1016/S0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 156.Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, Oberleithner K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch 462: 519–528, 2011. doi: 10.1007/s00424-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oh YS, Berkowitz DE, Cohen RA, Figueroa CA, Harrison DG, Humphrey JD, Larson DF, Leopold JA, Mecham RP, Ruiz-Opazo N, Santhanam L, Seta F, Shyy JYJ, Sun Z, Tsao PS, Wagenseil JE, Galis ZS. A special report on the NHLBI initiative to study cellular and molecular mechanisms of arterial stiffness and its association with hypertension. Circ Res 121: 1216–1218, 2017. doi: 10.1161/CIRCRESAHA.117.311703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev 17: 369–384, 1996. [DOI] [PubMed] [Google Scholar]
- 159.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA, Sowers JR. Regular exercise reduces endothelial cortical stiffness in Western diet-fed female mice. Hypertension 68: 1236–1244, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Papaioannou TG, Stamatelopoulos KS, Georgiopoulos G, Vlachopoulos C, Georgiou S, Lykka M, Lambrinoudaki I, Papamichael CM, Stefanadis CI. Arterial wave reflections during the menstrual cycle of healthy women: a reproducibility study. Hypertension 54: 1021–1027, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137703. [DOI] [PubMed] [Google Scholar]
- 161.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res 90: 1087–1092, 2002. doi: 10.1161/01.RES.0000021114.92282.FA. [DOI] [PubMed] [Google Scholar]
- 162.Pereira T, Correia C, Cardoso J. Novel methods for pulse wave velocity measurement. J Med Biol Eng 35: 555–565, 2015. doi: 10.1007/s40846-015-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, Northoff H, Samuelsson B, Rådmark O, Sautebin L, Werz O. ERK-mediated regulation of leukotriene biosynthesis by androgens: a molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci USA 105: 19881–19886, 2008. doi: 10.1073/pnas.0809120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pernis AB. Estrogen and CD4+ T cells. Curr Opin Rheumatol 19: 414–420, 2007. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- 165.Poudyal H, Panchal SK, Ward LC, Waanders J, Brown L. Chronic high-carbohydrate, high-fat feeding in rats induces reversible metabolic, cardiovascular, and liver changes. Am J Physiol Endocrinol Metab 302: E1472–E1482, 2012. doi: 10.1152/ajpendo.00102.2012. [DOI] [PubMed] [Google Scholar]
- 166.Protogerou AD, Vlachopoulos C, Thomas F, Zhang Y, Pannier B, Blacher J, Safar ME. Longitudinal changes in mean and pulse pressure, and all-cause mortality: data from 71,629 untreated normotensive individuals. Am J Hypertens 30: 1093–1099, 2017. doi: 10.1093/ajh/hpx110. [DOI] [PubMed] [Google Scholar]
- 167.Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen YT, Vatner DE, Vatner SF. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation 116: 669–676, 2007. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- 168.Qiu H, Tian B, Resuello RG, Natividad FF, Peppas A, Shen Y-T, Vatner DE, Vatner SF, Depre C. Sex-specific regulation of gene expression in the aging monkey aorta. Physiol Genomics 29: 169–180, 2007. doi: 10.1152/physiolgenomics.00229.2006. [DOI] [PubMed] [Google Scholar]
- 169.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RRG, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107: 615–619, 2010. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reference Values for Arterial Stiffness’ Collaboration Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J 31: 2338–2350, 2010. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 172.Reynertson RH, Parmley RT, Rodén L, Oparil S. Proteoglycans and hypertension. I. A biochemical and ultrastructural study of aorta glycosaminoglycans in spontaneously hypertensive rats. Coll Relat Res 6: 77–101, 1986. doi: 10.1016/S0174-173X(86)80033-4. [DOI] [PubMed] [Google Scholar]
- 173.Roccabianca S, Bellini C, Humphrey JD. Computational modelling suggests good, bad and ugly roles of glycosaminoglycans in arterial wall mechanics and mechanobiology. J R Soc Interface 11: 20140397, 2014. doi: 10.1098/rsif.2014.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rossow LM, Fahs CA, Thiebaud RS, Loenneke JP, Kim D, Mouser JG, Shore EA, Beck TW, Bemben DA, Bemben MG. Arterial stiffness and blood flow adaptations following eight weeks of resistance exercise training in young and older women. Exp Gerontol 53: 48–56, 2014. doi: 10.1016/j.exger.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 175.Roy CS. The elastic properties of the arterial wall. J Physiol 3: 125–159, 1881. doi: 10.1113/jphysiol.1881.sp000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ruiz-Feria CA, Yang Y, Thomason DB, White J, Su G, Nishimura H. Pulse wave velocity and age- and gender-dependent aortic wall hardening in fowl. Comp Biochem Physiol A Mol Integr Physiol 154: 429–436, 2009. doi: 10.1016/j.cbpa.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension 60: 362–368, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Nagayama D, Ohira M, Endo K, Tatsuno I. The role of a novel arterial stiffness parameter, cardio-ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb 23: 155–168, 2016. doi: 10.5551/jat.32797. [DOI] [PubMed] [Google Scholar]
- 179.Schillaci G, Pirro M, Vaudo G, Mannarino MR, Savarese G, Pucci G, Franklin SS, Mannarino E. Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension 45: 1078–1082, 2005. doi: 10.1161/01.HYP.0000165313.84007.7d. [DOI] [PubMed] [Google Scholar]
- 180.Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res 339: 83–92, 2010. doi: 10.1007/s00441-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Scuteri A, Lakatta EG, Bos AJ, Fleg JL. Effect of estrogen and progestin replacement on arterial stiffness indices in postmenopausal women. Aging (Milano) 13: 122–130, 2001. [DOI] [PubMed] [Google Scholar]
- 182.Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, Ferreli LA, Loi F, Pilia MG, Delitala A, Spurgeon H, Najjar SS, AlGhatrif M, Lakatta EG. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 64: 1219–1227, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41: 501–507, 2006. doi: 10.1016/j.exger.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 184.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension 65: 370–377, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shang Q, Tam LS, Li EK, Yip GW, Yu CM. Increased arterial stiffness correlated with disease activity in systemic lupus erythematosus. Lupus 17: 1096–1102, 2008. doi: 10.1177/0961203308092160. [DOI] [PubMed] [Google Scholar]
- 186.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 13: 101–107, 2006. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 187.Shoskes DA, Tucky B, Polackwich AS. Improvement of endothelial function following initiation of testosterone replacement therapy. Transl Androl Urol 5: 819–823, 2016. doi: 10.21037/tau.2016.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Skalska A, Gasowski J, Cwynar M, Grodzicki T. The relationship between pulse wave velocity and indexes of collagen synthesis in hypertensive patients, according to the level of systolic blood pressure. J Hum Hypertens 19: 731–735, 2005. doi: 10.1038/sj.jhh.1001892. [DOI] [PubMed] [Google Scholar]
- 190.Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, Cockcroft JR, Scanlon MF, Davies JS. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86: 4261–4267, 2001. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 191.Smulyan H, Mookherjee S, Safar ME. The two faces of hypertension: role of aortic stiffness. J Am Soc Hypertens 10: 175–183, 2016. doi: 10.1016/j.jash.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 192.Soare A, Weiss EP, Holloszy JO, Fontana L. Multiple dietary supplements do not affect metabolic and cardio-vascular health. Aging (Albany NY) 6: 149–157, 2014. doi: 10.18632/aging.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sokolis DP, Dimitriou CA, Lelovas P, Kostomitsopoulos NG, Dontas IA. Effect of ovariectomy and Sideritis euboea extract administration on large artery mechanics, morphology, and structure in middle-aged rats. Biorheology 54: 1–23, 2017. doi: 10.3233/BIR-16113. [DOI] [PubMed] [Google Scholar]
- 194.Son WM, Sung KD, Bharath LP, Choi KJ, Park SY. Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens 39: 546–552, 2017. doi: 10.1080/10641963.2017.1288742. [DOI] [PubMed] [Google Scholar]
- 195.Son WM, Sung KD, Cho JM, Park SY. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause 24: 262–268, 2017. doi: 10.1097/GME.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 196.Sorrentino TA, Fourman L, Ferruzzi J, Miller KS, Humphrey JD, Roccabianca S. Local versus global mechanical effects of intramural swelling in carotid arteries. J Biomech Eng 137: 041008, 2015. doi: 10.1115/1.4029303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stamatelopoulos KS, Kyrkou K, Chrysochoou E, Karga H, Chatzidou S, Georgiopoulos G, Georgiou S, Xiromeritis K, Papamichael CM, Alevizaki M. Arterial stiffness but not intima-media thickness is increased in euthyroid patients with Hashimoto’s thyroiditis: the effect of menopausal status. Thyroid 19: 857–862, 2009. doi: 10.1089/thy.2008.0326. [DOI] [PubMed] [Google Scholar]
- 198.Stefanadis C, Dernellis J, Vlachopoulos C, Tsioufis C, Tsiamis E, Toutouzas K, Pitsavos C, Toutouzas P. Aortic function in arterial hypertension determined by pressure-diameter relation: effects of diltiazem. Circulation 96: 1853–1858, 1997. doi: 10.1161/01.CIR.96.6.1853. [DOI] [PubMed] [Google Scholar]
- 199.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 59: 89–144, 1997. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 200.Stern R, Tattersall MC, Gepner AD, Korcarz CE, Kaufman J, Colangelo LA, Liu K, Stein JH. Sex differences in predictors of longitudinal changes in carotid artery stiffness: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 35: 478–484, 2015. doi: 10.1161/ATVBAHA.114.304870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Straface E, Vona R, Gambardella L, Ascione B, Marino M, Bulzomi P, Canu S, Coinu R, Rosano G, Malorni W, Franconi F. Cell sex determines anoikis resistance in vascular smooth muscle cells. FEBS Lett 583: 3448–3454, 2009. doi: 10.1016/j.febslet.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 202.Subramanya V, Ambale-Venkatesh B, Ohyama Y, Zhao D, Nwabuo CC, Post WS, Guallar E, Ouyang P, Shah SJ, Allison MA, Ndumele CE, Vaidya D, Bluemke DA, Lima JA, Michos ED. Relation of sex hormone levels with prevalent and 10-year change in aortic distensibility assessed by MRI: the Multi-Ethnic Study of Atherosclerosis. Am J Hypertens 31: 774–783, 2018. doi: 10.1093/ajh/hpy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Yokoi T, Otsuki T, Shimojo N, Miyauchi T, Maeda S, Tanaka H. Effect of systemic nitric oxide synthase inhibition on arterial stiffness in humans. Hypertens Res 30: 411–415, 2007. doi: 10.1291/hypres.30.411. [DOI] [PubMed] [Google Scholar]
- 204.Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, Ferrannini E, Salvetti A, Monzani F. Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto’s thyroiditis. J Clin Endocrinol Metab 91: 5076–5082, 2006. doi: 10.1210/jc.2006-1075. [DOI] [PubMed] [Google Scholar]
- 205.Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Matsuda T, Hiratsuka A, Matsuzaki M. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J 71: 1710–1714, 2007. doi: 10.1253/circj.71.1710. [DOI] [PubMed] [Google Scholar]
- 206.Tatchum-Talom R, Martel C, Marette A. Influence of estrogen on aortic stiffness and endothelial function in female rats. Am J Physiol Heart Circ Physiol 282: H491–H498, 2002. doi: 10.1152/ajpheart.00589.2001. [DOI] [PubMed] [Google Scholar]
- 207.Teixeira D, Longo-Maugeri IM, Santos JL, Duarte YA, Lebrão ML, Bueno V. Evaluation of lymphocyte levels in a random sample of 218 elderly individuals from São Paulo city. Rev Bras Hematol Hemoter 33: 367–371, 2011. doi: 10.5581/1516-8484.20110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tsutsumi S, Zhang X, Takata K, Takahashi K, Karas RH, Kurachi H, Mendelsohn ME. Differential regulation of the inducible nitric oxide synthase gene by estrogen receptors 1 and 2. J Endocrinol 199: 267–273, 2008. doi: 10.1677/JOE-07-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tuttolomondo A, Pecoraro R, Di Raimondo D, Di Sciacca R, Canino B, Arnao V, Buttà C, Della Corte V, Maida C, Licata G, Pinto A. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke with and without metabolic syndrome. Diabetol Metab Syndr 6: 28, 2014. doi: 10.1186/1758-5996-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia 55: 625–631, 2012. doi: 10.1007/s00125-011-2412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T, Society A; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries . Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30: 445–448, 2012. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 213.Vigen R, O’Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310: 1829–1836, 2013. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 214.Vitale C, Mammi C, Gambacciani M, Russo N, Spoletini I, Fini M, Volterrani M, Rosano GMC. Effect of hormone replacement therapy with the anti-mineralocorticoid progestin drospirenone compared to tibolone on endothelial function and central haemodynamics in post-menopausal women. Int J Cardiol 227: 217–221, 2017. doi: 10.1016/j.ijcard.2016.11.149. [DOI] [PubMed] [Google Scholar]
- 215.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens 19: 2205–2212, 2001. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 216.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289: H1209–H1217, 2005. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 217.Wan W, Yanagisawa H, Gleason RL Jr. Biomechanical and microstructural properties of common carotid arteries from fibulin-5 null mice. Ann Biomed Eng 38: 3605–3617, 2010. doi: 10.1007/s10439-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Weber T, Wassertheurer S, Hametner B, Parragh S, Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J Hypertens 33: 1023–1031, 2015. doi: 10.1097/HJH.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 219.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension 60: 534–541, 2012. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
- 220.Weng C, Yuan H, Yang K, Tang X, Huang Z, Huang L, Chen W, Chen F, Chen Z, Yang P. Gender-specific association between the metabolic syndrome and arterial stiffness in 8,300 subjects. Am J Med Sci 346: 289–294, 2013. doi: 10.1097/MAJ.0b013e3182732e97. [DOI] [PubMed] [Google Scholar]
- 221.Wight TN. Arterial remodeling in vascular disease: a key role for hyaluronan and versican. Front Biosci 13: 4933–4937, 2008. doi: 10.2741/3052. [DOI] [PubMed] [Google Scholar]
- 222.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 525: 263–270, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xiao H, Tan I, Butlin M, Li D, Avolio AP. Mechanism underlying heart rate dependency of wave reflection in the aorta: a numerical simulation. Am J Physiol Heart Circ Physiol 314: H443–H451, 2018. [DOI] [PubMed] [Google Scholar]
- 224.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol 29: 289–295, 2009. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang X, Wang Y, Yan S, Sun L, Yang G, Li Y, Yu C. Effect of testosterone on the proliferation and collagen synthesis of cardiac fibroblasts induced by angiotensin II in neonatal rat. Bioengineered 8: 14–20, 2017. doi: 10.1080/21655979.2016.1227141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yaron M, Greenman Y, Rosenfeld JB, Izkhakov E, Limor R, Osher E, Shenkerman G, Tordjman K, Stern N. Effect of testosterone replacement therapy on arterial stiffness in older hypogonadal men. Eur J Endocrinol 160: 839–846, 2009. doi: 10.1530/EJE-09-0052. [DOI] [PubMed] [Google Scholar]
- 227.Youssef H, Stashenko P. Interleukin-1 and estrogen protect against disseminating dentoalveolar infections. Int J Oral Sci 9: 16–23, 2017. doi: 10.1038/ijos.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zelis R, Mason DT. Diminished forearm arteriolar dilator capacity produced by mineralocorticoid-induced salt retention in man. Implications concerning congestive heart failure and vascular stiffness. Circulation 41: 589–592, 1970. doi: 10.1161/01.CIR.41.4.589. [DOI] [PubMed] [Google Scholar]
- 229.Zhang Y, Davidge ST. Estrogen replacement increases coronary artery distensibility in ovariectomized rats. Can J Physiol Pharmacol 77: 75–78, 1999. doi: 10.1139/y98-145. [DOI] [PubMed] [Google Scholar]
- 230.Zhang Y, Stewart KG, Davidge ST. Estrogen replacement reduces age-associated remodeling in rat mesenteric arteries. Hypertension 36: 970–974, 2000. doi: 10.1161/01.HYP.36.6.970. [DOI] [PubMed] [Google Scholar]
- 231.Zhou RH, Vendrov AE, Tchivilev I, Niu XL, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR, Runge MS. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arterioscler Thromb Vasc Biol 32: 745–755, 2012. doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zimmerman MA, Baban B, Tipton AJ, O’Connor PM, Sullivan JC. Chronic ANG II infusion induces sex-specific increases in renal T cells in Sprague-Dawley rats. Am J Physiol Renal Physiol 308: F706–F712, 2015. doi: 10.1152/ajprenal.00446.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]