Abstract
In the current study, we explored exaggerated physiological startle responses in posttraumatic stress disorder (PTSD) and examined startle reactivity as a biomarker of PTSD in a large veteran sample. We assessed heart rate (HR), skin conductance (SC), and electromyographic (EMG) startle responses to acoustic stimuli under low-, ambiguous-, and high-threat conditions in Gulf War veterans with current (n = 48), past (n = 42), and no history of PTSD (control group; n = 152). We evaluated PTSD status using the Clinician-Administered PTSD Scale and trauma exposure using the Trauma History Questionnaire. Participants with current PTSD had higher HR, ds = 0.28−0.53; SC, d = 0.37; and startle responses than those with past or no history of PTSD. The HR startle response under ambiguous threat best differentiated current PTSD; however, sensitivity and specificity analyses revealed it to be an imprecise indicator of PTSD status, ROC AUC = .66. Participants with high levels of trauma exposure only showed elevated HR and SC startle reactivity if they had current PTSD. Results indicate that startle is particularly elevated in PTSD when safety signals are available but a possibility of danger remains and when trauma exposure is high. However, startle reactivity alone is unlikely to be a sufficient biomarker of PTSD.
Posttraumatic stress disorder (PTSD) is a chronic and disabling mental health condition affecting 9−10% of the population. The diagnosis of PTSD relies entirely on patient symptom accounts, which are obtained through self-report scales or semistructured interviews. Such approaches fail to account for complex factors that underlie and influence psychopathology, including neural structure and function, endocrine and hormonal systems, the autonomic nervous system, attention, memory, decision-making, sociocultural context, stress, early life experience, genetics, and temperament (Craske, 2017). Incorporation of additional markers of risk and resilience in PTSD beyond self-report could improve reliability of the diagnosis and increase efficiency of treatment. One leading candidate biomarker of PTSD is the acoustic startle response, or the physiologic response to sudden loud stimuli that varies between people and is highly conserved across species.
Prior studies have demonstrated elevated physiological reactivity to startling tones among people with PTSD (Butler et al., 1990; Orr, Lasko, Shalev, & Pitman, 1995; Orr, Solomon, Peri, Pitman, & Shalev, 1997; Shalev, Orr, Peri, Schreiber, & Pitman, 1992). Researchers have aimed to determine the precise conditions under which physiological startle responses are elevated by (a) examining multiple physiological measures (Orr et al., 1995; Orr et al., 1997; Shalev et al., 1992), (b) manipulating contextual threat during the startle paradigm (Grillon, Morgan, Davis, & Southwick, 1998a, 1998b; Morgan, Grillon, Southwick, Davis, & Charney, 1995; Pole et al., 2009; Pole, Neylan, Best, Orr, & Marmar, 2003), (c) examining whether exaggerated startle is a risk factor or an acquired symptom of PTSD (Blanchard, Hickling, Galovski, & Veazey, 2002; Bryant, Harvey, Guthrie, & Moulds, 2000; Carson et al., 2007; Griffin, 2008; Macklin et al., 2012; Metzger et al., 1999; Pole et al., 2003; Shalev et al., 1998, 2000), and (d) examining the association between startle reactivity and trauma exposure independent of PTSD (Bryant et al., 2000; Jovanovic et al., 2009; Pole et al., 2007). Yet, prior studies have produced conflicting results, as described in the following paragraphs. In addition, prior studies have used small samples and have not tested these factors simultaneously in one sample, limiting our understanding of how they interact. Finally, to our knowledge, no prior study has examined startle reactivity as a biomarker of PTSD status. Thus, the goals of the current study were to test how PTSD status (presence of current, past, or no history of PTSD), contextual factors (low, ambiguous, and high threat), and lifetime trauma exposure impact the startle response across multiple physiological measures in a sample of 242 veterans of the Gulf War. We also examined startle response as a diagnostic indicator of PTSD status.
Eye-blink electromyogram (EMG) of the startle response is thought to be a direct measure of neurobiological threat sensitivity and a primary indicator of the startle response (Davis, 1984). However, whereas the authors of many published studies have found greater EMG reactivity to startle probes in participants with PTSD compared to controls (Butler et al., 1990; Grillon et al., 1998a; Morgan et al., 1995; Morgan, Grillon, Southwick, Davis, & Charney, 1996; Orr et al., 1995), others have failed to detect an effect (Carson et al., 2007; Metzger et al., 1999; Orr et al., 1997; Shalev et al., 1992). Researchers have also examined autonomic responses, including heart rate (HR) and skin conductance (SC), to startling sounds. Neural control of SC is exerted by the sympathetic branch of the autonomic nervous system, whereas HR receives projections from both sympathetic and parasympathetic branches (Hugdahl, 1995). People with PTSD reliably show greater startle reactivity in terms of HR compared to controls, whereas findings for SC are more variable (Carson et al., 2007; Metzger et al., 1999; Orr et al., 1995; Orr et al., 1997; Shalev et al., 1992). A meta-analysis by Pole (2007) indicated a moderate effect of PTSD on startle, with effect sizes of d = 0.41, 0.43, and 0.63 for SC, EMG, and HR responses, respectively.
Researchers have also modulated the level of contextual threat during the startle paradigm to examine fear-potentiated startle, or the tendency to have greater startle responses in fearful states (e.g., Pole et al., 2003; Pole et al., 2009). One study comparing individuals with and without PTSD (Grillon et al., 1998b) showed elevated startle reactivity in terms of EMG in PTSD when participants were placed in a threatening context (e.g., shock electrodes attached to skin) both with and without a safety signal indicating that no shock would be delivered. Thus, startle response may be elevated in individuals with PTSD during both high threat and ambiguous threat where a safety signal is available but the context is potentially dangerous. To our knowledge, no prior study has compared autonomic responses to differing threat conditions in individuals with and without PTSD. Although Pole and colleagues (2003) found a positive association between skin conductance responses and PTSD symptoms in police officers who were under low and medium, but not high, threat, the authors did not directly compare autonomic startle responses between the threat conditions, nor did they compare police officers with and without PTSD, due to low incidence of diagnosable PTSD in the police officers.
Other questions include whether exaggerated startle is a stable trait among people who develop PTSD (i.e., present in those with current PTSD as well as those with PTSD in remission) and whether trauma exposure is independently associated with startle reactivity regardless of PTSD status. To examine the stability of elevated startle in individuals with PTSD, studies have compared those with current to those with past PTSD with inconsistent findings (Carson et al., 2007; Metzger et al., 1999). Other studies have examined whether startle response predicts later onset of PTSD, again with mixed results (Blanchard et al., 2002; Bryant et al., 2000; Griffin, 2008; Macklin et al., 2012; Pole et al., 2009; Shalev et al., 1998; Shalev et al., 2000), and one study that examined monozygotic twins with and without PTSD found elevated startle response only in those with PTSD (Orr et al., 2003). Thus, further research is needed to determine the stability of elevated startle response among people with a lifetime history of PTSD. Only a handful of studies have examined how trauma exposure is related to startle reactivity or have attempted to parse the effects of trauma exposure and PTSD on startle response. Childhood trauma appears to increase startle reactivity in adulthood over and above PTSD status (Jovanovic et al., 2009; Pole et al., 2007), whereas trauma exposure that occurs in adulthood does not seem to have a measureable effect on startle responses (Bryant et al., 2000). To our knowledge, no prior study has examined whether the effect of trauma exposure on startle response differs depending on PTSD symptom severity or the cumulative effects of trauma exposure over the lifespan on startle.
How might measures of physiology be useful in clinical practice? One possibility is that physiological reactivity could serve as a biomarker for PTSD. Discriminant function analysis is one approach to assessing the utility of a purported biomarker. This approach identifies, based on a diagnostic clinical interview, how accurately a biological measure classifies individuals who meet criteria for PTSD (sensitivity) and those who do not (specificity). Prior studies have examined physiological reactivity to trauma-related imagery and cues as a potential biomarker of PTSD, but to our knowledge, no prior study has examined sensitivity and specificity of startle reactivity to an acoustic startle probe. Based on a meta-analysis of studies examining physiological reactivity to standardized trauma cues, sensitivity was 0.77 and specificity was 0.91; for studies examining reactivity to idiographic trauma cues, sensitivity was 0.65 and specificity was 0.83 (Pole, 2007). These findings suggest that measures of physiological reactivity to trauma-related cues can more accurately identify individuals who do not meet PTSD criteria than those who do, meaning that many people who do meet criteria for PTSD based on a clinician-administered interview do not show elevated physiological reactivity to trauma-related cues. Given that exaggerated startle response falls under the hyperarousal and reactivity symptom cluster of the PTSD diagnosis, whereas physiological reactivity to trauma reminders is categorized under the intrusion symptom cluster, it would be useful to test sensitivity and specificity for these different categories of physiological reactivity as they may capture different facets of PTSD.
In the current study, we tested the effects of threat context and trauma exposure on startle reactivity in PTSD using a large sample of Gulf War veterans with current, past, and no history of PTSD. We conducted analyses for three measures of the physiological startle response: EMG, HR, and SC. First, we tested the magnitude of the difference in startle responses between participants with current PTSD and those with past or no history of PTSD and examined whether those group differences were augmented by experimentally increasing contextual threat under which startle was administered. We hypothesized that individuals with current PTSD would show greater startle reactivity than those with past or no history of PTSD and that those differences would be particularly pronounced in the high- and ambiguous-threat conditions. We also conducted sensitivity (proportion of positive results correctly identified) and specificity (proportion of negative results correctly identified) analyses testing startle reactivity as a biomarker of PTSD status. Second, we examined whether trauma exposure moderated the effect of PTSD on startle reactivity. We hypothesized that exposure to more traumatic events over the lifespan would predict greater startle reactivity but had no a priori hypotheses about the interaction between traumatic events and PTSD group.
Method
Participants
The current analysis utilized data from a larger cross-sectional study that examined the impact of Gulf War Illness on the brain among male and female veterans of the first Persian Gulf War (n = 316 Weiner et al., 2011). For the current analysis, 242 participants were examined (current PTSD, n = 48; past PTSD, n = 42; no PTSD, n = 152). Demographic features are reported in Table 1 and types of trauma exposure, by group, are reported in Table 2. Participants were recruited via convenience sampling through contacts with physicians at Veterans Affairs (VA) clinics and hospitals and from a list of veterans furnished by the U.S. Department of Defense. The sole inclusion criterion was status as a U.S. veteran of the First Persian Gulf War. The exclusion criteria were severe physical impairment, medical illnesses accounting for symptoms of Gulf War Illness, current or lifetime history of psychotic disorder, suicidal or homicidal ideation, use of antipsychotic medications in the past 6 weeks, history of neurologic or systemic illness affecting central nervous system function, history of head injury with prolonged loss of consciousness, contraindication for magnetic resonance imaging (due to imaging component of parent study), current substance other than alcohol abuse, or being pregnant or nursing. The VA Medical Center and the University of California, San Francisco Committees on Human Research, and the Department of Defense Human Subjects Research Review Board approved the protocol (protocol number 10–04027).
Table 1.
Demographic Data by Posttraumatic Stress Symptom (PTSD) Status
| Control |
Past PTSD |
Current PTSD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | M | SD | n | % | M | SD | N | % | M | SD | Statistical Test |
| Sample size | 152 | 62.6 | 42 | 17.3 | 48 | 19.8 | |||||||
| Age, years | 45.1 | 9.78 | 44.9 | 10.23 | 41.2 | 8.30 | F(2, 240) = 3.19* | ||||||
| Years of education | 14.8 | 2.15 | 14.0 | 1.51 | 14.1 | 1.82 | F(2, 230) = 4.14* | ||||||
| Male gender | 137 | 89.5 | 37 | 88.1 | 40 | 83.3 | χ2(2, N = 243) = 1.34 | ||||||
| Ethnicity | χ2(6, N = 230) = 16.71* | ||||||||||||
| Caucasian | 99 | 69.2 | 21 | 52.5 | 27 | 57.5 | |||||||
| Black | 15 | 10.5 | 8 | 20.0 | 15 | 31.9 | |||||||
| Latino | 13 | 9.1 | 7 | 17.5 | 3 | 6.4 | |||||||
| Other | 16 | 11.2 | 4 | 10.0 | 2 | 4.3 | |||||||
| Lifetime traumaa | 70.2 | 127.4 | 122.9 | 163.7 | 143.0 | 185.2 | χ2(2, N = 242) = 14.43*** | ||||||
| Lifetime CAPSb | 7.4 | 12.30 | 61.7 | 18.46 | 80.8 | 24.28 | F(2, 240) = 452.30*** | ||||||
| Current CAPSb | 3.2 | 7.31 | 19.6 | 11.91 | 61.5 | 15.69 | F(2, 240) = 584.75*** | ||||||
Note. CAPS = clinician administered PTSD scale.
Because the number of trauma events is a count variable, we tested the effect of PTSD group on trauma events using negative binomial regression, and the test statistic is chi-square.
CAPS scores can be interpreted as follows: 0–19 for asymptomatic or few symptoms, 20–39 for mild or subthreshold PTSD, 40–59 for moderate or threshold-level PTSD, 60–79 for severe PTSD symptomatology, and ≥ 80 for extreme PTSD symptomatology (Weathers, Keane, & Davidson, 2001).
p < .05.
p < .01.
p < .001.
Table 2.
Frequency of Trauma Type by Posttraumatic Stress Disorder (PTSD) Status
| Control |
Past PTSD |
Current PTSD |
|||||
|---|---|---|---|---|---|---|---|
| Trauma Type | n | % | n | % | n | % | χ2a |
| Crime events | 82 | 54.0 | 25 | 59.5 | 25 | 52.1 | 0.56 |
| Accident | 73 | 48.0 | 22 | 52.4 | 36 | 75.0 | 10.75** |
| Natural or manmade disaster | 60 | 39.5 | 22 | 52.4 | 35 | 72.9 | 16.67*** |
| Chemical exposure | 62 | 40.8 | 27 | 64.3 | 3 | 70.8 | 16.86*** |
| Terrorist attack | 8 | 5.3 | 5 | 11.9 | 10 | 20.8 | 10.62** |
| War combat | 131 | 86.2 | 41 | 97.6 | 47 | 97.9 | 8.84* |
| Injured self/other or killed other | 119 | 78.3 | 39 | 92.9 | 46 | 95.8 | 11.30** |
| Death of someone close | 96 | 63.2 | 37 | 88.1 | 35 | 72.9 | 9.98** |
| Life-threatening illness | 24 | 15.8 | 8 | 19.1 | 13 | 27.1 | 3.08 |
| Sexual trauma/abuse | 20 | 13.2 | 7 | 16.7 | 13 | 27.1 | 5.13 |
| Physical attack/abuse | 45 | 29.6 | 18 | 42.9 | 23 | 47.9 | 6.53* |
| Other | 20 | 13.2 | 9 | 21.4 | 16 | 33.3 | 10.08** |
Note. df = 2, N = 242.
p < .05.
p < .01.
p < .001.
Procedure
In preparation for the startle protocol, participants were asked to abstain from activities and substances that might impact results, including exercise, cigarettes, and coffee, on the day of testing and from eating within 1 hr of testing. Adherence was assessed by self-report.
Startle responses were assessed under low, ambiguous, and high threat of shock following acoustic startle probes separated by intertrial intervals of 30–50 s. At the beginning of the protocol, participants were told that they would receive shocks later in the study but that they could not occur until participants were fitted with the shock device. Participants first underwent 10 startle trials in the low-threat condition with no finger shock device and were told that they would not be shocked. In the low-threat condition, the first 5 startle trials were habituation trials, and only the last 5 were used in analyses. Following low threat, participants had the finger shock device fitted and were told that when shocks were possible, up to three shocks at any point in time would be administered. Participants then underwent 10 startle trials (5 per condition) under two additional threat levels: ambiguous threat and high threat. Under ambiguous threat, participants were fitted with the finger shock device but were given a cue via text on the computer monitor that they would not be shocked (i.e., “NO SHOCK”). In the high-threat condition, participants were fitted with the finger shock device and signaled with a message that indicated that shocks were possible (i.e., “SHOCK POSSIBLE”). The threat cue message remained on the computer screen throughout the startle protocol for the corresponding condition. Each condition lasted approximately 4 min, with 1 min between conditions. The order of the ambiguous- and high-threat conditions was counterbalanced across participants, and order was included as a covariate in all statistical models. One shock was delivered following the last startle probe in the high-threat condition.
Measures
PTSD diagnosis and severity.
We used the Clinician Administered PTSD Scale-IV (CAPS; Blake et al., 1995) to assess current and lifetime PTSD status and severity as defined by the Diagnostic and Statistical Manual for Mental Disorders (4th ed., text rev.; DSM-IV; American Psychiatric Association [APA], 2000). Symptoms were rated on frequency and intensity on a scale of 0 to 4; symptoms with a frequency score of 1 or greater and an intensity score of 2 or greater were classified as “endorsed symptoms.” The diagnosis was made based on the DSM-IV criteria for each PTSD symptom cluster (APA, 2000). Severity scores were calculated by summing the frequency and intensity scores. To administer the CAPS, one supervisor was responsible for the training and supervision of all assessors and verbally reviewed each CAPS to arrive at a consensus on PTSD diagnosis and severity. Interviewers were graduate students completing clinical placements at the hospital in which these data were collected. Our research group has established an intraclass correlation coefficient (ICC; established via independent review of recorded CAPS interviews) of .984 for CAPS severity scores.
Number of traumatic events.
The number of traumatic events each participant had experienced was measured using the 24-item Trauma History Questionnaire (THQ; Hooper, Stockton, Krupnick, & Green, 2011). The participant indicates whether or not he or she experienced specific types of trauma and how many discrete instances of that type of trauma were experienced. The total number of traumatic events was calculated by summing the number of instances reported for each event assessed.
Physiological and startle responses.
Stimuli consisted of 106 db(A) white noise bursts of 40 ms duration (Coulbourn Instruments Audio Source Module V85–05; Holliston, MA) delivered by headphones. A Coulbourn Instruments Transcutaneous Aversive Finger Stimulator E13–22 worn on the second and third digits of the dominant hand was used to administer 2.5 mA shocks.
Startle was measured using three indicators of physiological reactivity: HR, SC, and EMG. Measures were sampled at 1000 Hz for a 5-s duration starting 1 s before each startle stimulus. Signals were collected and digitized by a Coulbourn Instruments LabLinc V system and stored for offline analysis. We used Human Startle Software by Coulbourn Instruments to generate mean values for each measure in the 1 s prior to startle onset for EMG and SC and 5 s prior to startle onset for HR, as well as the maximum value in the 1–4 s poststartle for SC and HR, and 21–200 ms poststartle for EMG. We then calculated the change in each physiological measure by subtracting the value prior to startle onset from the value following startle administration. For each participant, change values were averaged across trials within each of the three threat conditions to obtain one measure of HR, EMG, and SC per threat condition.
Heart rate.
We measured HR in beats per minute using 3-Dot-model 3M Corporation (Maplewood, MN) electrocardiogram electrodes attached to each participant’s left and right arms as well as a ground electrode placed behind the participant’s left ear. The signal was filtered for 8–13 Hz activity and amplified by 10,000. Interbeat intervals were calculated from the EKG signal and converted to HR measurements.
Skin conductance response.
We used a Coulbourn V71–23 Isolated Skin Conductance Coupler to deliver a constant 0.5 V direct current through 8 mm sensor diameter In Vivo Metric (Healdsburg, CA) Ag/AgCl electrodes. The electrodes were attached to participants’ medial phalanges of the left middle and index fingers. We analyzed SC amplitude; average values across trials were calculated using only trials that included a response greater than or equal to .02 μS.
Electromyogram.
Participants’ EMG was measured in microvolts (μV) using three 4-mm sensor diameter In Vivo Metric Ag/AgCl surface electrodes. Two electrodes were placed on participants’ left orbicularis oculi muscles, and a ground electrode was placed behind each participant’s left ear (ground for both EMG and EKG signals). The signal was amplified by 10,000, rectified, and filtered to keep the range between 10 and 500 Hz. Impedance levels were maintained below 10 kΩ and notch-filtered at 60 Hz by a Coulbourn Instruments V75–04 Isolated Bioamplifier. The signal was digitized at 1000 Hz for 5 s beginning 1 s before stimulus onset, smoothed using a 5-ms time constant by a Coulbourn Instruments V76–23A Contour Following Integrator, and stored.
Data Analysis
Using scatterplots, we examined data by trial for all trials included in analyses (15 trials per participant). For HR, 71 (1.4%) trials were replaced with missing values because HR fluctuations were not detected or the increase in HR pre- to poststartle probe administration was greater than 4 standard deviations from the mean. We chose 4 standard deviations (as opposed to the more conventional 3 standard deviations) as an outlier cutoff for HR because scatterplots showed clusters of outliers falling above 4, but not 3, standard deviations from the mean. For EMG, 292 (6.0%) of trials were replaced with missing values when EMG was recorded as less than 0 or greater than 300 μV or when the change in EMG pre- to poststartle was greater than 3 standard deviations from the mean. For SC, 21 (0.4%) trials were replaced with missing values because SC was recorded as greater than 40 μS. In addition, 62 participants (no PTSD, 25.0%; past PTSD, = 26.2%; current PTSD, 23.4%) were nonresponders who had limited variability in the SC measurement across trials or never showed an SC response (increase of 0.02 μS) to the startle probe. These 62 participants were excluded from SC analyses (approximately 10% of individuals, or more in clinical samples, do not show a reliable GSR response; Braithwaite, Watson, Jones, & Rowe, 2013). For consistency with some prior studies, analyses were conducted with and without outliers for EMG and SC, and the results were unchanged. Thus, we report findings for analyses excluding outliers. For the number of trauma events, outlier values greater than 3 standard deviations from the mean (more than 542 events) were replaced with the next highest value based on the Winsor method (Guttman, 1973).
Analyses were conducted in Stata (Version 14). Both EMG and SC were square-root transformed to reduce skew. We used multilevel modeling (MLM), which accounts for within- and between-participant variance and effectively handles missing data by including all participants in the model regardless of missing data points. Condition was modeled at Level 1 and group at Level 2. Models used maximum likelihood and included only intercept random effects for parsimony, as inclusion of additional parameters did not measurably alter the values of the fixed effects. The ICCs were .45, .59, and .74 for HR, SC, and EMG, respectively.
We first examined the group (no PTSD, past PTSD, current PTSD) by condition (low, ambiguous, and high threat) interaction on each outcome (HR, EMG, and SC) separately. Main effects of group and condition were also included in the model. Regardless of statistical significance of the interaction, we tested pairwise group effects within each condition because of our a priori research questions regarding the magnitude of between-group differences at different levels of contextual threat. Achieved power to detect an effect size of Cohen’s d = 0.5 between past and no PTSD and current and no PTSD was 81% and 85%, respectively. Further, we tested main effects of threat condition followed by pairwise comparisons as a manipulation check. To examine the interaction between trauma exposure and PTSD group on startle response, we tested the Group × Condition × Number of Trauma Events interaction (all lower order interaction and main effects were included in the model) and examined pairwise interactions within each condition. For significant pairwise interactions, we used the Potthoff extension to the Johnson-Neyman technique (1964) to examine the significance of the predicted group difference in startle response at 1 standard deviation above the mean and the minimum (because 1 standard deviation below the mean fell below zero) on trauma events. Confidence intervals (CI) are 95%, and reported effect sizes are Cohen’s d. Reported means were predicted from the MLM. Effects interpreted as statistically significant were those with 95% CIs that did not include zero.
Results
Descriptive Statistics
Table 1 presents descriptive values for the sample by PTSD status. Mean values for age, years of education, and race/ethnicity differed among PTSD groups. We examined the correlation between these demographic variables and startle response averaged across threat conditions and found that more years of education was correlated with greater EMG response, r = .21, 95% CI [.082, .33]; older age was correlated with lower SC, r = −.17,95% CI [−.31, −.028], and HR, r = −.21, 95% CI [−.32, −.081], responses. Consistent with prior studies (Kredlow et al., 2017), race was also correlated with startle such that White participants had greater SC compared to other ethnic groups, r = .24, 95% CI [.097, .38]. Thus, age was included as a covariate in analyses that included SC and HR, education in analyses of EMG, and race in analyses of SC. Due to missing data on age, education, and race/ethnicity, we used statistical significance (α = .05) of the association between each covariate and the physiological measure as a threshold for inclusion to limit the number of covariates in each model to maintain the largest possible sample size.
Effect of PTSD and Condition on Startle Reactivity
Heart rate.
For heart rate startle reactivity (Table 3), the Group × Condition interaction was not significant, χ2(4, N = 237) = 2.02, p = .732. Under low and ambiguous threat, individuals with current PTSD (low, M = 2.81, SD = 3.32; ambiguous, M = 4.77, SD = 3.01) had larger HR responses compared to those with no PTSD (low, M = 2.19, SD = 2.74; ambiguous, M = 2.86, SD = 2.55) and past PTSD (low, M = 2.46, SD = 3.50; ambiguous, M = 2.75, SD = 3.01). Under high threat, participants with current PTSD (M = 4.32, SD = 4.42) had larger HR responses compared to those with no PTSD (M = 3.17, SD = 3.56). No other significant pairwise comparisons emerged. Averaging across groups, startle responses significantly differed by condition, χ2(2, N = 237) = 13.92, p = .001, such that participants had larger HR responses under high (M = 3.40, SD = 3.74) versus low (M = 2.55, SD = 3.04) threat, B = 0.85, 95% CI [0.38, 1.32], d = 0.22, and under ambiguous (M = 3.21, SD = 3.18) versus low threat, B = 0.66, 95% CI [0.19, 1.13], d = 0.20, but not under high versus ambiguous threat, B = 0.19, 95% CI [−0.28, 0.66], d = 0.05.
Table 3.
Posttraumatic Stress Disorder (PTSD) Group Comparisons Within Low, Ambiguous, and High Threat Conditions for Heart Rate, Skin Conductance, and Electromyogram
| Heart Rate |
Skin Conductance |
Electromyogram |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference | 95% CI | d | Difference | 95% CI | d | Difference | 95% CI | d | |
| Low threat | |||||||||
| Past vs. none | 0.27 | [−0.86, 1.40] | 0.09 | 0.08 | [−0.02, 0.17] | 0.31 | 0.44 | [−0.45, 1.32] | 0.18 |
| Current vs. none | 1.62 | [.53, 2.72] | 0.53 | 0.07 | [−.02, .16] | 0.30 | 0.12 | [−.72, .95] | 0.05 |
| Current vs. past | 1.35 | [−.04, 2.74] | 0.40 | 0.01 | [−0.12, 0.11] | −0.03 | −0.32 | [−1.39, .75] | −0.13 |
| Ambiguous threat | |||||||||
| Past vs. none | −0.11 | [−1.24, 1.02] | −0.04 | 0.05 | [−0.05, 0.14] | 0.24 | 0.49 | [−0.40, 1.37] | 0.19 |
| Current vs. none | 1.90 | [0.81, 3.00] | 0.51 | 0.09 | [0.00, 0.18] | 0.37 | 0.08 | [−0.75, 0.92] | 0.03 |
| Current vs. past | 2.01 | [0.63, 3.39] | 0.52 | 0.04 | [−0.07, 0.16] | 0.19 | −0.40 | [−1.47, 0.66] | −0.17 |
| High threat | |||||||||
| Past vs. none | 0.05 | [−1.08, 1.17] | 0.01 | 0.08 | [−0.01, 0.17] | 0.35 | 0.79 | [−0.10, 1.68] | 0.31 |
| Current vs. none | 1.16 | [0.07, 2.24] | 0.29 | 0.07 | [−0.02, 0.15] | 0.28 | −0.01 | [−0.85, 0.82] | 0.00 |
| Current vs. past | 1.11 | [−0.27, 2.49] | 0.28 | 0.00 | [−0.13, 0.10] | −0.07 | −0.80 | [−1.87, 0.26] | −0.31 |
Skin conductance response.
For SC (Table 3), the Group × Condition interaction was not significant, χ2(4, N = 167) = 1.95, p = .745. Under ambiguous threat, participants with current PTSD (M = 0.62, SD = 0.28) had larger SC responses compared to those with no PTSD (M = 0.52, SD = 0.22). No other significant pairwise comparisons emerged. Averaging across groups, startle responses significantly differed by condition, χ2(2, N = 167) = 50.15, p < .001. Tests of simple effects revealed that SC response was larger following startle under high (M = 0.61, SD = 0.23) versus low (M = 0.51, SD = 0.23) threat, B = 0.10, 95% CI [0.072, 0.13], d = 0.53; under ambiguous (M = 0.55, SD = 0.23) versus low threat, B = 0.0044, 95% CI [0.016, 0.073], d = 0.24; and under high versus ambiguous threat, B = 0.056, 95% CI [0.028, 0.084], d = 0.39.
Electromyogram.
For EMG (Table 3), the Group x Condition interaction was not significant, χ2(4, N = 223) = 5.40, p = .248. No significant pairwise differences were found in any condition. Averaging across groups, startle responses differed by condition, χ2(2, N = 223) = 80.80, p < .001. Tests of simple effects revealed that participants had larger EMG responses under high (M = 3.44; SD = 2.50) versus low (M = 2.79; SD = 2.33) threat, B = 0.66, 95% CI [0.51, 0.80] d = 0.54; under ambiguous (M = 3.12; SD = 2.44) versus low threat, B = 0.33; 95% CI [0.19, 0.48, d = 0.30; and under high versus ambiguous threat, B = 0.32, 95% CI [0.18, 0.47], d = 0.35.
Startle as a Biomarker of PTSD Status
Given that HR reactivity to startle in the ambiguous threat condition differentiated individuals with current PTSD from those with past and no history of PTSD with the largest effect sizes, we conducted sensitivity and specificity analyses (see Supplementary Materials for analyses of additional startle indices). We conducted a logistic regression with current (coded as 1) versus past or no PTSD (coded as 0) as the outcome and HR increase following startle under ambiguous threat as the predictor. We found that HR was a significant predictor of current PTSD status, χ2 (1, N = 235) = 11.77, p < .001. We graphed the receiver operating characteristic (ROC) curve (see Figure 1), and the area under the curve (AUC) was .66, indicating that HR startle reactivity is a poor indicator of PTSD status (AUC values below .8 are not useful for making diagnostic determinations; Zhu, Zeng, & Wang, 2010). The AUC was also .66 when the past PTSD group was not included in the analysis, and the addition of EMG and SC indices did not markedly improve the model fit (AUC increased to .67). Using the model that included only HR under ambiguous threat as the predictor and current PTSD status as the outcome, to determine the optimal cutoff, we identified the maximum value of the Youden Index (Youden, 1950). The optimal cutoff was 3.8 for HR reactivity. Sensitivity for this cutoff was 64% and specificity was 69%. The positive predictive value was 33%.
Figure 1.
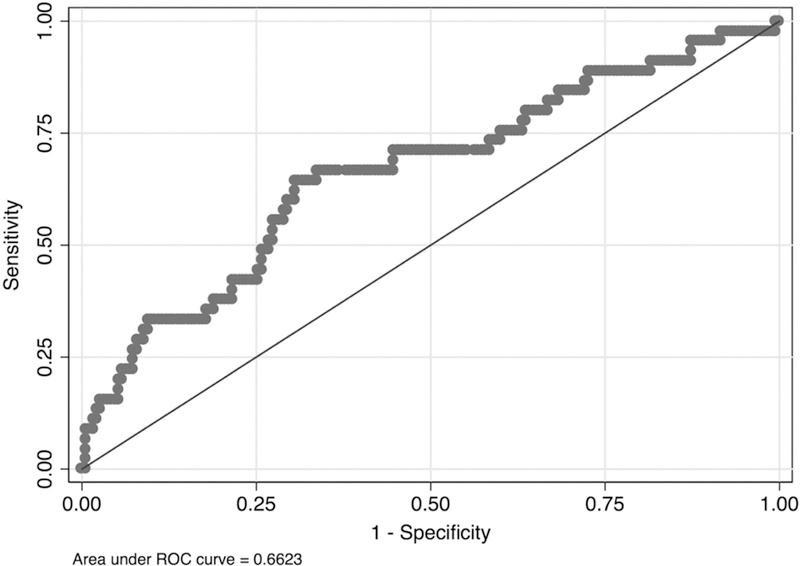
Receiver operating characteristic (ROC) curve showing performance of heart rate as a biomarker of posttraumatic stress disorder status.
Effect of Trauma Exposure on Startle Reactivity
Traumatic events.
Total number of traumatic events was the number of discrete traumatic events the participants had experienced as reported on the THQ (M = 93.64, SD = 150.07, range: 2–993). Means and standard deviations by group are displayed in Table 1. Ranges of traumatic events for participants with no history of PTSD, past PTSD, and current PTSD were 2–993, 4–609, and 7–808, respectively.
Heart rate.
The HR results are displayed in Figure 2A. In the ambiguous threat condition, the association between trauma events and HR startle response was significantly greater in the current PTSD group, r = .25, 95% CI [–.050, .51], than it was in the no PTSD group, r = –.070, 95% CI [–.23, .094]; B = 0.0083, 95% CI [0.000075, 0.017], p = .048. Within the ambiguous threat condition, for traumatic events equal to two (sample minimum), the current PTSD group had significantly larger HR startle responses compared to the past PTSD group, difference = 1.84, 95% CI [0.044, 3.63], d = 0.48, but not the no PTSD group, p = .079. For traumatic events equal to 216 (one standard deviation above the mean), the current PTSD group had larger HR startle responses compared to the no PTSD, difference = 3.05, 95% CI [1.61, 4.48], d = 0.82, and past PTSD groups, difference = 2.57, 95% CI [0.96, 4.18], d = 0.66. No other interaction effects had p values less than .05.
Figure 2.
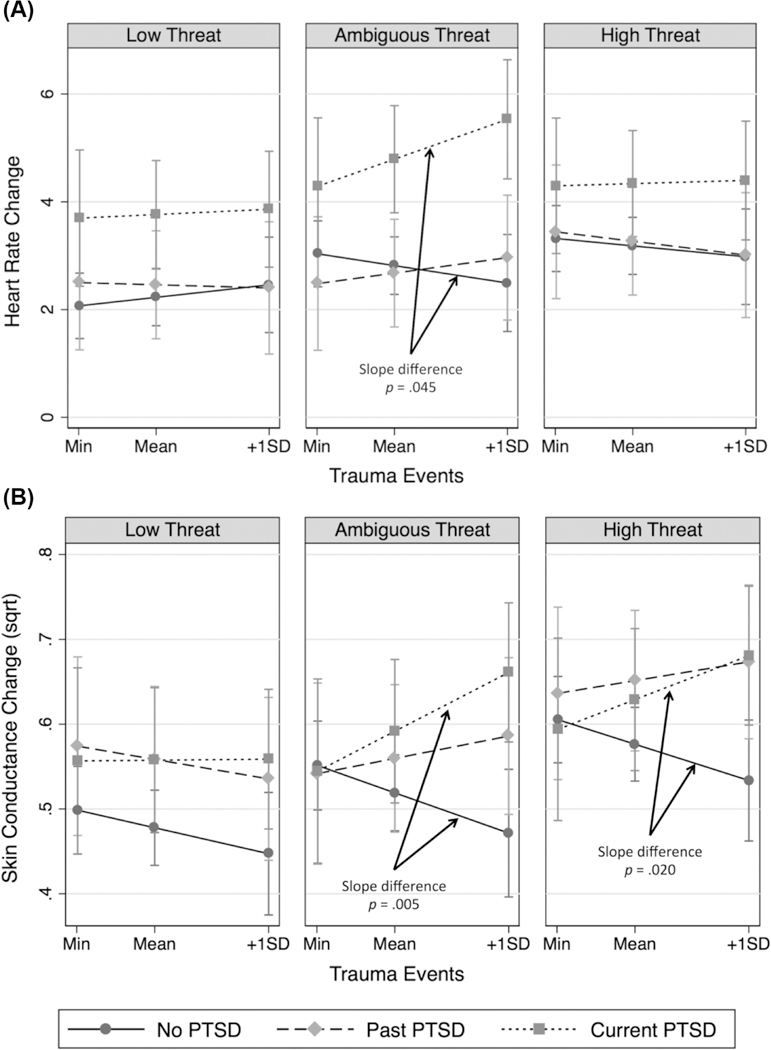
Effect of trauma exposure on startle reactivity by posttraumatic stress disorder (PTSD) group and condition for heart rate (Panel A) and skin conductance rate (Panel B).
Skin conductance response.
The SC response results are displayed in Figure 2B. In the ambiguous threat condition, the association between traumatic events and SC was greater in the current PTSD group, r = .38, 95% CI [.083, .62], than in the no PTSD group, r = –.080,95% CI [–.25, .10]; B = 0.00092,95% CI [0.00028, 0.0016], p = .005. Within the ambiguous threat condition, at traumatic events equal to two (sample minimum), groups did not differ, ps = .877–.970. However, for traumatic events equal to 216 (one standard deviation above the mean), the current PTSD group had a larger SC response compared to the no PTSD group, difference = 0.19, CI [0.077, 0.30], d = 0.77. In the high threat condition, the association between traumatic events and SC response was greater in the current PTSD group, r = .28,95% CI [–.036, .54], than in the no PTSD group, r = –.10 95% CI [–.27, .068]; B = 0.00075, 95% CI [0.00012, 0.0014], p = .020. Within the high threat condition, at traumatic events equal to two (sample minimum), groups did not significantly differ, ps = .572–.851. However, for traumatic events equal to 216 (one standard deviation above the mean), the current PTSD group had a larger SC response compared to the no PTSD group, difference = 0.15, 95% CI [0.038, 0.26], d = 0.65, and the past PTSD group had a larger SC response compared to the no PTSD group, difference = 0.14, 95% CI [0.026, 0.26], d = 0.61. No other interaction effects had p values less than .05.
Electromyogram.
For EMG, no simple interaction effects with p values less than .05 emerged. Thus, trauma exposure did not significantly moderate the effect of PTSD group on EMG startle reactivity.
Discussion
In the current study, we tested EMG, HR, and SC reactivity to startle among veterans with current, past, and no history of PTSD under low, ambiguous, and high threat of shock. Regardless of PTSD group, startle responses increased with increasing threat level for all three physiological measures, with the largest effect sizes for SC and EMG and a small effect size for HR. Participants with current PTSD showed greater startle reactivity than those with no history of PTSD, ds = 0.29–0.53, and past PTSD, d = 0.52, and we found the largest effect sizes for HR. Interestingly, we found the largest differences in HR change following startle between participants with and without current PTSD in the ambiguous and low threat conditions; however, it is important to note that the group by condition interaction was not statistically significant. For SC, the only statistically significant group difference we found was between participants with current PTSD and those with no history of PTSD in the ambiguous threat condition, d = 0.37. Participants with a past history of PTSD had SC similar to those with current PTSD or that fell between those with current and no history of PTSD (depending on threat condition). We found no significant group differences for EMG. Despite its reliability across studies and moderate effect size, HR response to startle was a poor diagnostic indicator of PTSD diagnosis based on a clinical interview.
Consistent with prior research (Carson et al., 2007; Metzger et al., 1999; Orr et al., 1995, 1997; Shalev et al., 1992), we found evidence for elevated HR response and SC to startling sounds among individuals with PTSD. Although electrodermal responses are a more pure measure of sympathetic nervous system activation than HR, which receives input from both sympathetic and parasympathetic branches, it is notable that HR consistently differentiates individuals with current PTSD from healthy individuals. Although fear responding is generally attributed to sympathetic responding, it is possible that hypoactivation of the parasympathetic nervous system may play a key role. Indeed, findings from several meta-analyses have shown elevated basal cardiovascular activity in people with PTSD compared to trauma-exposed individuals without PTSD and non-trauma-exposed individuals (Blanchard, Jones-Alexander, Buckley, & Forneris, 1996; Buckley & Kaloupek, 2001; Pole, 2007). Further, evidence suggests that the parasympathetic nervous system plays a greater role in determining HR compared to the sympathetic nervous system (Katona, McLean, Dighton, & Guz, 1982), and that the parasympathetic system in particular may be responsible for elevated HR in PTSD (Hopper, Spinazzola, Simpson, & van der Kolk, 2006). Another possibility is that HR can be measured with greater precision and ease than both SC and EMG responses. Although all three of the measures can be imprecise due to movement artifacts, a substantial minority of subjects do not show measurable SC responses, and SC appears to differ by ethnicity (Kredlow et al., 2017); additionally, the procedures necessary to obtain an accurate EMG signal are labor-intensive. On the other hand, HR is robust, results in fewer missing data points, and is frequently assessed in clinical settings by a variety of health professionals. Thus, HR may be a more viable clinical biomarker of PTSD than other measures of physiological reactivity.
Partially consistent with our hypotheses, group differences were greatest (albeit not significantly greater) for HR responses in the ambiguous (shock electrodes attached and safety signal present) and in the low threat (no shock electrodes attached) conditions, with the ambiguous condition showing the largest effect size difference between participants with current PTSD and those with past or no history of PTSD. These findings compliment those from studies that have used safety signal conditioning procedures and shown greater EMG responses to safety signals in participants with PTSD compared to those without PTSD (Jovanovic et al., 2010; Jovanovic, Kazama, Bachevalier, & Davis, 2012; Peri, Ben-Shakhar, Orr, & Shalev, 2000; Pole et al., 2003). Thus, evidence for elevated HR startle reactivity under ambiguous threat adds to growing evidence of impaired safety signal processing in PTSD and shows that HR responses in particular may clearly differentiate patients from controls.
Consistent with one study that showed elevated startle in current compared to past PTSD (Carson et al., 2007), but inconsistent with another that showed no difference in startle (Metzger et al., 1999), we found that individuals with past PTSD did not show significantly greater startle responses than controls. However, it should be noted that when trauma exposure was included as a moderator, for very high levels of trauma exposure, participants with past PTSD did show greater reactivity in terms of SC compared to those without PTSD and did not differ from those with current PTSD. Thus, startle reactivity among people with past PTSD may depend on their level of trauma exposure and which physiological system is being measured (i.e., purely sympathetic vs. sympathetic and parasympathetic). For HR, individuals with past PTSD showed significantly less startle reactivity compared to those with current PTSD. These findings support the notion that elevated HR startle responding is not an enduring trait among individuals who develop PTSD, but may wax and wane alongside other PTSD symptoms. The importance of current PTSD symptoms is also evident given that high levels of trauma exposure only predicted elevated startle for individuals who met criteria for current PTSD. Further, this suggests that elevated startle may be most characteristic of individuals with both PTSD and high levels of lifetime trauma exposure, and it highlights the importance of examining the interaction between PTSD and cumulative trauma exposure in future research. Interestingly, participants with high trauma exposure who did not meet PTSD criteria showed startle reactivity similar to those with low trauma exposure, suggesting that this group may be particularly resilient to the negative psychological and physiological effects of trauma exposure.
Sensitivity and specificity analyses used to determine whether HR startle reactivity under ambiguous threat was a viable biomarker of PTSD produced an ROC AUC value of .66, indicating that HR startle reactivity alone is not sufficient as an index of PTSD status; however, it is important to note that a diagnostic interview itself is an imperfect measure. An AUC value of .50, which would be depicted by a straight diagonal line in the ROC curve, indicates that the test performs no better than chance, and values less than .80 have been deemed inadequate for use as a diagnostic test. Results for sensitivity (64%) were similar to those found in previous studies (65–77%) that examined receiver operating characteristics for physiological reactivity to trauma-related reminders as a marker of PTSD status (Pole, 2007). Specificity in the current study (69%), however, was lower than in previous studies (83–91%). One possible explanation for this difference is that startle reactivity to an acoustic burst is more normative and more likely to be demonstrated in someone who does not meet PTSD criteria compared to physiological reactivity to a trauma-related cue. These findings suggest that, although HR reactivity to startle produces the largest difference in effect size between individuals with and without PTSD (compared to EMG and SC), it will likely need to be a component of a multivariate index that incorporates other markers (e.g., physiological reactivity to trauma reminders, neural activity, markers of immune functioning, psychometric data) to serve as a useful biomarker of disease. These findings are disappointing given that, based on a review by Schmidt, Kaltwasser, and Wotjak (2013), elevated startle response is one of the most robust biological features of PTSD. However, biomarkers may not be particularly useful as diagnostic indicators given that the definition of a psychiatric disorder is based on distress and impairment, which is determined clinically. Instead, a biomarker may help clarify diagnostic subgroups, provide information about disease course, or predict differential treatment response. For example, individuals with PTSD who had higher systolic blood pressure have shown a greater response to the α1-adrenergic receptor antagonist, prazosin, compared to those with lower systolic blood pressure (Raskind et al., 2016). Thus, in the case of PTSD, one possibility is that physiological reactivity to startle indexes a subtype of PTSD that is not currently captured in the diagnostic criteria (e.g., physiologically reactive subtype). Future research could examine whether startle reactivity predicts treatment outcome or if it could be used as a prescriptive factor for treatment assignment.
Despite many strengths, the current study has a number of limitations. For the analysis examining trauma exposure as a moderator of the association between PTSD and startle reactivity, it should be noted that trauma exposure significantly differed by PTSD group. This suggests that PTSD may serve as a mediator between trauma exposure and startle reactivity. Because we did not have longitudinal data, we were unable to conduct a rigorous assessment of PTSD as an explanatory variable in the association between trauma exposure and startle reactivity. Future studies that utilize longitudinal data should consider testing a meditational model. Further, the current study was based on a largely male sample of Gulf War veterans with PTSD, meaning that our results may be specific to this population. That said, with the exception of EMG, the effect sizes found in this study are generally similar to those found in a meta-analysis (Pole, 2007), suggesting that this population resembles other PTSD groups in terms of autonomic responding. It should be noted that all startle responses in the current study were measured under anticipation of future shock, which may explain why some results (e.g., null findings for EMG) differ from prior startle studies that did not include anticipatory threat of shock. Finally, diagnostic interviews were conducted by graduate students in training, and recordings of the interviews were not independently reviewed. Thus, diagnoses may have been subject to “groupthink,” and it is possible that misdiagnosis accounts for inconsistencies with prior literature.
In sum, our study demonstrated physiological startle reactivity in Gulf War veterans with current PTSD—a veteran sample in which startle reactivity had not yet been examined. Our findings are consistent with those from prior work showing HR startle reactivity to be the most reliable physiological indicator of greater startle responding in PTSD and are novel in that we showed the greatest startle reactivity (in terms of autonomic responses) for ambiguous threat and among participants with the highest self-reported trauma exposure. These findings add to a growing body of literature indicating that PTSD is characterized by elevated startle when safety signals are available but there remains a possibility of danger. Further, our findings indicate that startle reactivity may be most pronounced for people with a history of extensive trauma exposure. Finally, we showed that HR startle reactivity alone is unlikely to be a sufficient biomarker of PTSD. However, this does not preclude HR startle reactivity from serving as a useful marker in conjunction with other measures or possibly in identifying a subtype of individuals with PTSD.
Supplementary Material
Acknowledgments
This study was supported by Department of Defense Grant DAMD17–011-0764, entitled “Magnetic Resonance and Spectroscopy of the Human Brain in Gulf War Illness,” which was awarded to the Northern California Institute for Research and Education from the Department of Defense Gulf War Illnesses Research Program, U.S. Army Medical Research and Materiel Command. This study was also supported by the Mental Illness Research, Education, and Clinical Center of the Department of Veterans Affairs. Aoife O’Donovan was supported by a National Institute of Mental Health Career Development Award (K01-MH109871) and a Society in Science-Branco Weiss Fellowship. Resources and the use of facilities were provided by the Veterans Administration Medical Center, San Francisco, California.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text revision). Washington, DC: American Psychiatric Association. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician- administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Galovski T, & Veazey C (2002). Emergency room vital signs and PTSD in a treatment seeking sample of motor vehicle accident survivors. Journal of Traumatic Stress, 15(3), 199–204. 10.1023/A:1015299126858 [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, & Forneris CA (1996). Psychometric properties of the PTSD checklist (PCL). Behaviour Research and Therapy, 34(8), 669–673. 10.1016/0005-7967(96)00033-2 [DOI] [PubMed] [Google Scholar]
- Braithwaite JJ, Watson DG, Jones R, & Rowe M (2013). A guide for analysing electrodermal activity (EDA) and skin conductance responses (SCRs) for psychophysiological experiments via the MP36R and AcqKnowledge software (No. 1). University of Birmingham, UK: Selective Attention & Awareness Laboratory (SAAL), Behavioral Brain Sciences Centre, School of Psychology. [Google Scholar]
- Bryant RA, Harvey AG, Guthrie RM, & Moulds ML (2000). A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. Journal of Abnormal Psychology, 109(2), 341–344. [PubMed] [Google Scholar]
- Buckley TC, & Kaloupek DG (2001). A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine, 63(4), 585–594. [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, & Geyer MA (1990). Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. The American Journal of Psychiatry, 147(10), 1308–1312. 10.1176/ajp.147.10.1308 [DOI] [PubMed] [Google Scholar]
- Carson MA, Metzger LJ, Lasko NB, Paulus LA, Morse AE, Pitman RK, & Orr SP (2007). Physiologic reactivity to startling tones in female Vietnam nurse veterans with PTSD. Journal of Traumatic Stress, 20(5), 657–666. 10.1002/jts.20218 [DOI] [PubMed] [Google Scholar]
- Craske MG (2017). Honoring the past, envisioning the future: ABCT’s 50th anniversary presidential address. Behavior Therapy. https://doi.org/10.1016Zj.beth.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Davis M (1984). The mammalian startle response In Eaton RC (Ed.), Neural mechanisms of startle (pp. 287–351). New York: Plenum. [Google Scholar]
- Griffin MG (2008). A prospective assessment of auditory startle alterations in rape and physical assault survivors. Journal of Traumatic Stress, 21(1), 91–99. 10.1002/jts.20300 [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, & Southwick SM (1998a). Effect of darkness on acoustic startle in Vietnam veterans with PTSD. The American Journal of Psychiatry, 155(6), 812–817. 10.1176/ajp.155.6.812 [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, & Southwick SM (1998b). Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry, 44(10), 1027–1036. 10.1016/S0006-3223(98)00034-1 [DOI] [PubMed] [Google Scholar]
- Guttman I (1973). Care and handling of univariate or multivariate outliers in detecting spuriosity: A bayesian approach. Technometrics, 723–738. [Google Scholar]
- Hopper JW, Spinazzola J, Simpson WB, & van der Kolk BA (2006). Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. Journal of Psychosomatic Research, 60(1), 83–90. 10.1016/j.jpsychores.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Hooper LM, Stockton P, Krupnick JL, & Green BL (2011). Development, use, and psychometric properties of the trauma history questionnaire. Journal of Loss and Trauma, 16(3), 258–283. 10.1080/15325024.2011.572035 [DOI] [Google Scholar]
- Hugdahl K (1995). Psychophysiology: The mind-body perspective. Boston, MA: Harvard University Press. [Google Scholar]
- Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, & Ressler KJ (2009). Childhood abuse is associated with increased startle reactivity in adulthood. Depression and Anxiety, 26(11), 1018–1026. 10.1002/da.20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, & Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, & Ressler KJ (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety, 27(3), 244–251. 10.1002/da.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona PG, McLean M, Dighton DH, & Guz A (1982). Sympathetic and parasympathetic cardiac control in athletes and nonathletes at rest. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology, 52(6), 1652–1657. [DOI] [PubMed] [Google Scholar]
- Kredlow A, Pineles SL, Inslicht SS, Marin M-F, Milad MR, Otto MW, & Orr SP (2017). Assessment of skin conductance in African American and non-African American participants in studies of conditioned fear. Psychophysiology, 54(11), 1741–1754. 10.1111/psyp.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin ML, Pineles SL, Orr SP, Lasko NB, Chang Y, & Pitman RK (2012). Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Retrieved from https://dash.harvard.edu/handle/1/10436295 [DOI] [PMC free article] [PubMed]
- Metzger LJ, Orr SP, Berry NJ, Ahern CE, Lasko NB, & Pitman RK (1999). Physiologic reactivity to startling tones in women with posttraumatic stress disorder. Journal of Abnormal Psychology, 108(2), 347–352. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Southwick SM, Davis M, & Charney DS (1995). Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry, 38(6), 378–385. 10.1016/0006-3223(94)00321-S [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Southwick SM, Davis M, & Charney DS (1996). Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. The American Journal of Psychiatry, 153(1), 64–68. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, & Pitman RK (1995). Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. Journal of Abnormal Psychology, 104(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, & Pitman RK (2003). Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: Association with posttraumatic stress disorder. Archives of General Psychiatry, 60(3), 283–288. [DOI] [PubMed] [Google Scholar]
- Orr SP, Solomon Z, Peri T, Pitman RK, & Shalev AY (1997). Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur War. Biological Psychiatry, 41(3), 319–326. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, & Shalev AY (2000). Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry, 47(6), 512–519. [DOI] [PubMed] [Google Scholar]
- Pole N (2007). The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin, 133(5), 725–746. http://doi.org.ucsf.idm.oclc.org/10.1037/0033-2909.133.5.725 [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Best SR, Orr SP, & Marmar CR (2003). Fear-potentiated startle and posttraumatic stress symptoms in urban police officers. Journal of Traumatic Stress, 16(5), 471–479. 10.1023/A:1025758411370 [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, & Marmar CR (2009). Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biological Psychiatry, 65(3), 235–240. 10.1016/j.biopsych.2008.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Metzler TJ, Best SR, Henn-Haase C, & Marmar CR (2007). Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: A study of police cadets. Journal of Abnormal Psychology, 116(2), 352. [DOI] [PubMed] [Google Scholar]
- Potthoff RF (1964). On the Johnson-Neyman technique and some extensions thereof. Psychometrika, 29(3), 241–256. 10.1007/BF02289721 [DOI] [Google Scholar]
- Raskind MA, Millard SP, Petrie EC, Peterson K, Williams T, Hoff DJ, ... Peskind ER(2016). Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with Prazosin. Biological Psychiatry, 80(10), 736–742. 10.1016/j.biopsych.2016.03.2108 [DOI] [PubMed] [Google Scholar]
- Schmidt U, Kaltwasser SF, & Wotjak CT (2013). Biomarkers in post-traumatic stress disorder: Overview and implications for future research. Disease Markers, 35(1), 43–54. 10.1155/2013/835876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Peri T, Schreiber S, & Pitman RK (1992). Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Archives of General Psychiatry, 49(11), 870–875. 10.1001/archpsyc.1992.01820110034005 [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, & Pitman RK (2000). Auditory startle response in trauma survivors with posttraumatic stress disorder: A prospective study. The American Journal of Psychiatry, 157(2), 255–261. 10.1176/appi.ajp.157.2.255 [DOI] [PubMed] [Google Scholar]
- Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, ... Pitman RK (1998). A prospective study of heart rate response following trauma and the subsequent development of post-traumatic stress disorder. Archives of General Psychiatry, 55(6), 553–559. 10.1001/archpsyc.55.6.553 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, & Davidson JRT (2001). Clinician-administered PTSD scale: A review of the first ten years of research. Depression and Anxiety, 13(3), 132–156. 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
- Weiner MW, Meyerhoff DJ, Neylan TC, Hlavin J, Ramage ER, McCoy D, ... McCarthy C (2011). The relationship between Gulf War illness, brain N-acetylaspartate, and post-traumatic stress disorder. Military Medicine, 176(8), 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youden WJ (1950). Index for rating diagnostic tests. Cancer, 3(1), 32–35. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zeng N, & Wang N (2010). Sensitivity, specificity, accuracy, associated confidence interval and ROC analysis with practical SAS implementations. NESUG Health Care and Life Sciences. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


