Abstract
There are multiple challenges for neuropharmacology in the future. Undoubtedly, one of the greatest challenges is the development of strategies for pharmacological targeting of specific brain regions for treatment of diseases. GABA is the main inhibitory neurotransmitter in the central nervous system, and dysfunction of GABAergic mechanisms is associated with different neurological conditions. Liposomes are lipid vesicles that are able to encapsulate chemical compounds and are used for chronic drug delivery. This short review reports our experience with the development of liposomes for encapsulation and chronic delivery of GABA to sites within the brain. Directions for future research regarding the efficacy and practical use of GABA-containing liposomes for extended periods of time as well as understanding and targeting neurological conditions are discussed.
Keywords: liposomes, GABA, nervous system, neurological diseases, neuroscience
1. Introduction
Nanotechnology involves technology on the molecular scale and research involving the use of nanoparticles in biology and medicine is increasingly becoming of great importance for understanding basic underlying mechanisms and therapeutic efficacy. Nanotechnology offers a wide variety of possibilities for applications [1], for example, nanoparticles are promising for efficient distribution of the drug molecules only to the target region, preserving surrounding tissues and other healthy organs. Nanoparticles are made from different materials, including, polymers, metals and lipids [2–4].
The enormous advances in neuroscience have been able to determine specific brain sites, populations of neurons, local mechanisms and biomolecules that are involved in different neurological conditions. On the other hand, pharmacological targeting of specific sites in the brain for treating a determined neurological disease with minimum side effects is still a huge challenge. In this scenario, nanotechnology seems to offer an important alternative for treating central nervous system diseases and the use of nanoparticles is an option for drug delivery into the brain. The ideal prototype should fulfill the minimum essential requirements; i) the desired drug must be efficiently incorporated into a nanoparticle, ii) the nanoparticle must efficiently cross the blood-brain barrier (BBB) and, iii) the nanoparticle must perform the appropriate release of the drug at the desired site of action. This combination of factors is perhaps the great frontier for nanotechnology as applied in the field of neuropharmacology. Finding an ideal “Trojan horse” for crossing the BBB is not an easy task and requires extensive investigation [5]. On the other hand, the characterization of nanoparticles which are able to encapsulate and produce a controlled release of drugs has advanced with considerable success [6,7].
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system (CNS). It exerts its inhibitory effects by acting on ionotropic GABAA receptors and metabotropic GABAB receptors. Activation of post-synaptic GABAA receptors favors the influx of chloride, hyperpolarizing the neuron [8] and rendering it inhibited from firing. GABAA receptors are widely distributed throughout the mammalian CNS exhibiting specific functions within various specific sites [9]. In this regard, dysfunction of GABAergic mechanisms is associated with several common neurological conditions, including, Huntington’s chorea, Alzheimer’s disease, anxiety, panic disorder and epilepsy [10,11]. The spectrum of actions is so broad that altered GABAergic signaling is also associated with cardiovascular abnormalities such as essential hypertension [12] and heart failure [13] as well as the development of neuroglioma [14]. GABA is a low-molecular, zwitterionic and water-soluble molecule showing reduced permeation through the BBB [15]. On the other hand, the physicochemical properties of GABA make it particularly suitable for encapsulation in vesicular nanoparticles exhibiting high loading capacicity of hydrophilic drug, such as liposomes, polymersomes [16] and multiple emulsions [17]. Indeed, GABA cannot be encapsulated on the basis of electrostatic interactions, like nucleic acids in polyplex, lipoplex or peptide shell [18,19].
Although not yet investigated for the delivery of GABA, polymersomes represent a novel promising alternative to liposomes, because of their expected greater stability, easier industrial production and potentially lower membrane permeability [20].
Recently, GABA-loaded polymeric nanoparticles were prepared by means of inverse emulsion polymerization technique. These positively-charged nanoparticles showed anticonvulsant effect in epileptic rats [21]. However, the rate of drug release from these nanoparticles was quite fast with 47% of GABA released in 6 h.
Another strategy investigated for deliverying GABA to the brain is based on the design of membrane-permeable derivative. A recent study suggests that GABA conjugated to a cationic peptide can deliver GABA to the brain, following parenteral administration [15]. However, potential disadvantages when compared to the nanoparticle strategy may be the lack of tissue specificity, possible immunogenicity and peripheral side effects and limited duration of action. An interesting possibility would be to associate such a derivative to anionic nanoparticles targeted to the brain.
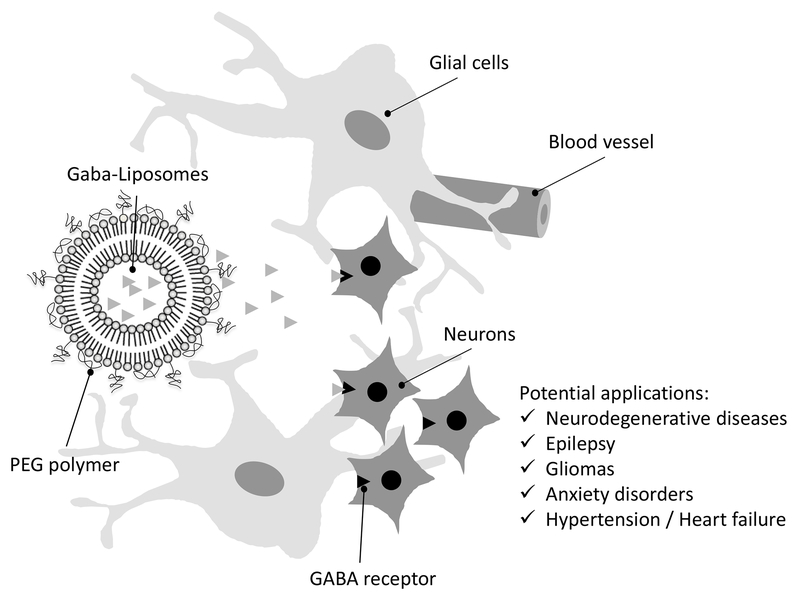
Giving the enormous importance of GABA in several physiological and physiopathological conditions it is surprising that so few nanoparticles have been investigated as potential carriers of GABA. This review focuses on the pros and cons of nano-scale vectors for GABA delivery into the brain. We discuss the applicability of the liposomes for chronic GABA delivery in specific brain regions of interest. These GABA delivery systems could then be used in neuroscience studies aiming potential translational aspects for treating neurological conditions. In this regard, the review also offers insights about possible applications of GABA-containing liposomes with a specific focus on diseases where GABAergic mechanisms are directly involved (Figure 1).
Figure 1 –
Drawing depicting the PEGylated lipid vesicle (PEG liposomes) releasing GABA in the nervous tissue. Given the relevance of GABA as neurotransmitter in the mammalian central nervous system, GABA-liposomes offer a wide range of potential applications to be explored (see text for details).
2. Properties of liposomes: general aspects
Liposomes are microscopic spherical vesicles, consisting of one or more concentric lipid bilayers, which isolate one or more internal aqueous compartments from external medium (Figure 1) [3,22]. They may encapsulate hydrophilic and/or lipophilic substances in the inner aqueous compartment and in the membrane respectively. A great advantage in the use of liposomes, relative to other drug delivery systems, is their high biocompatibility [22], low toxicity and side effect [23]. Besides that, these systems are highly versatile. The size, lamellarity, surface characteristics, lipid composition, volume and composition of the internal aqueous medium can be manipulated depending on pharmaceutical and pharmacological requirements [22].
The manipulation of liposome size and surface is critical to control the pharmacokinetics and biodistribution of this nanosystem. Conventional liposomes, typically made from phosphatidylcholine and cholesterol, undergo opsonization in the plasma and are rapidly cleared from the blood circulation by macrophages of the mononuclear phagocyte system (MPS). Thus, those mainly accumulate in the liver and spleen. As a first means to prolong the circulation time of liposomes and improve their distribution into organs other than MPS, liposome size can be decreased, for instance using vesicles with diameter less than 100 nm [24]. Another more effective means to reduce the rate of liposome clearance in vivo consists in the incorporation into vesicle membrane of a phospholipid conjugated to ethylene glycol polymer (PEG) [25]. The PEG polymer forms a hydrophilic protective layer over the surface of vesicles, diminishes the opsonization process and subsequent clearance of liposomes. Even though the PEGylation of liposomes prolongs their circulation time in the body, it is necessary, but not sufficient, to allow liposomes to cross the BBB. Therefore, their functionalization with ligands, such as peptides, antibodies, aptamers, and others, which specifically bind to receptors at the surface of the brain endothelial cells, can facilitate their transport across the BBB.
Two distinct mechanisms have been shown to contribute to the in vivo release of the encapsulated drug from liposomes: a passive diffusion mechanism consisting of the simple diffusion of the substance across the lipid bilayer, also influenced by the interaction of the liposome membrane with plasma components; a cell-mediated mechanism consisting of the endocytosis of liposomes by cells, their degradation by lysosomal phospholipases and the subsequent release of the drug from cells by exocytosis [22]. Whether one or the other mechanism predominates depends on the characteristics of both drug and liposomes. The diffusion-mediated mechanism prevails for drugs that exhibit low-molecular weight and low-polarity and thus high permeability across the liposome membrane. On the contrary, the cell-mediated mechanism predominates in the case of impermeable drugs. Importantly, the membrane fluidity and surface of liposomes can be manipulated to control the drug release from liposomes. For instance, more rigid membranes usually result in lower rates of drug release [3]. When the cell-mediated drug release mechanism predominates, PEGylated liposomes also promote slower drug release. Interestingly, additional properties can be added to liposomal systems to enhance the delivery of drugs at the targeted site in response to specific stimuli, such as variations in temperature, magnetic field, ultrasound intensity, or changes in pH [26].
Liposomes were first described in the 1960s when Bangham et al., observed that phospholipids in aqueous solution could form a closed bilayer structure [27]. They were first used as cell membrane model because of the similarity with it. It was only in the 1970s that its ability to store drugs [28,29] and its use as a drug carrier were explored [30]. Since then, a great advance in the development and the techniques for the preparation of the liposomes has been achieved, with the approval of several drugs, products and technologies based on their use and efficacy [31].
3. GABA-containing liposomes: characteristics
Loeb et al., were the first to report the use of liposome-encapsulated GABA system [32,33]. These authors reported a reduction in epileptic activity after intravenous administration (lasting for 104 min) and hypothesized that the liposomal carrier might enhance the penetration of GABA across the BBB. The formulation employed was developed only with natural phosphatidylserine [32,33]. Natural phosphatidylserine is expected to form less stable and more leaky vesicles [3] and to favor the capture of liposomes by macrophages [34]. Furthermore, important characteristics such as size and kinetic of GABA release were not determined [32,33]. Therefore, we recently tested the efficiency of another formulation of liposomes for GABA release [35]. These were prepared from L-α-distearoyl-phosphatidylcholine (DSPC), cholesterol (CHOL) and distearoyl-phosphatidyle-thanolamine-polyethylene glycol 2000 (DSPE-PEG2000). The encapsulation of GABA (0.3 M in 0.9% NaCl) was carried out within frozen and thawed multilamellar vesicles (FATMLVs; lipid concentration of 99 g/L). After all formulation steps, we observed that GABA encapsulation rate reached an average efficiency of about 30% [35]. The liposomes prepared had membrane made from high-phase transition temperature phosphatidylcholine (DSPC) and cholesterol, to achieve very low membrane permeability [36]. Accordingly, in vitro results indicate a very slow release of GABA from liposomes, with only 60% of GABA release after 5 days of incubation at 37°C. Other important characteristics of these liposomes were their mean diameter of 200 nm and the incorporation of a pegylated lipid, to slow down their capture by cells, by endocytosis/phagocytosis and the cell-mediated release of encapsulated drug [3].
We have recently demonstrated in an in vitro model that neurons exposed to GABA-containing liposomes for a 24 h period present two important molecular changes. First, a marked increase in GABAA receptors (by 50%). Second, a marked increase in nitric oxide (NO), an important mediator of intracellular signaling in the central nervous system. The increase in nitric oxide possibly results from a reduction in PIN, the endogenous protein inhibitor of neuronal nitric oxide synthase (nNOS) [37]. PIN binding leads to destabilization of active nNOS dimers resulting in the formation of functionally impeded catalytically inactive monomers and hence decrease in NO formation [38]. The observed increase in NO in neurons exposed to GABA-containing liposomes might be due to stabilization of nNOS dimers. Recent functional findings of our GABA liposomes formulation [35,37] are mentioned in some specific topics below.
4. Translational Perspectives
Overcoming the Blood Brain Barrier and Microglial reaction
As any nanotechnology-based drug delivery system for targeting the brain, the perspective of use of GABA-containing liposomes for targeting central nervous system diseases must consider two preponderant challenges; crossing the BBB and microglia reaction.
The BBB protects the brain against harmful blood-borne pathogens but limits the delivery of a large number of drugs for treating neurological diseases [39]. At present, the search for versatile BBB delivery methods is undoubtedly a critical step toward the future of nanotechnology applied to the nervous system [2,6].
Long-circulating nanoparticles can passively cross the BBB following intravenous administration when treating diseases in which the BBB integrity is compromised. BBB integrity is often reduced in brain tumors and it can also be a consequence of other brain diseases [40,41]. Temporary opening of the BBB by osmotic (mannitol) shock or focused ultra-sound has also been used to enhance drug delivery through liposomes [42]. Magnetic nanoparticles were also combined with a magnetic field in the brain to enhance the delivery.
When evaluated for their ability to cross intact BBB, the most effective liposomes were cationic vesicles and liposomes with surface modified by targeting ligands, which specifically bind to receptors or transporter that are expressed on the membrane of the brain endothelial cells [26,42,43]. Deposition of liposomes into the brain using intraarterial injection was found to be more effective from cationic vesicles than from anionic or neutral ones, , possibly due to the electrostatic interactions between the cationic liposomes and the negatively charged cell membranes, enhancing nanoparticle uptake by adsorptive-mediated endocytosis [44]. But the use of cationic nanocarriers for brain delivery is limited by nonspecific uptake by peripheral tissues and their binding to serum proteins that attenuates their surface charge. Thus, large amounts of these nanocarriers will be required to reach therapeutic efficacy, and those carriers are potentially cytotoxic [45].
One of the most effective mechanisms to go through the BBB is receptor-mediated transcytosis, taking advantage of different transporters and receptors present at the BBB. This is the case of the low-density lipoprotein receptor-related protein [46]. Other molecular targets that have been successfully used are the GLUT1 glucose transporter [47], the transferrin receptor and the insulin receptor [43].
Non-invasive routes of administration (ocular or mucosal) have been largely explored for bypassing the BBB. For example, intranasal administration provides a practical approach to deliver drugs to the brain and cationic liposomes were found to be especially effective by this route [48,49].
Invasive techniques can also be an alternative for application of liposomal preparations into specific brain regions. In humans, overcoming the BBB via invasive techniques using intraparenchymal or intracerebroventricular direct applications is considered far from ideal given the need for hospitalization, scarring of brain tissue and risk of infection [2,6]. However, direct application offers the possibility to introduce the therapeutic agent locally, reducing the systemic toxicity and preserving the surrounding healthy tissue [7,50]. Direct application is a real possibility and must be viewed as an alternative for treating some neurological conditions [7,50,51]. In this regard, direct application of dopamine-containing liposomes into the striatum elevated local extracellular levels of dopamine for 25 days reducing the deficits associated with a rodent model of Parkinson’s disease [52]. Direct application of liposomes into the brain via convection-enhanced delivery was tested in a rodent brain tumor model. Results showed that liposomes achieved efficient distribution to the tumor tissue providing a basis for clinical applications to specific site of interest [53,54]. In conclusion, direct application of liposomes into the CNS is a realistic strategy for therapeutic targeting of specific brain regions.
Once inside the central nervous system the question then becomes how microglia reacts against liposomes. Microglia are the principal immune cells of the nervous system, the defense against entry to the central nervous system by microorganisms from the blood or traumatic injury of the nervous tissue. Microglial processes converge towards the injured site, establishing a barrier between the healthy and injured tissue. Migration to the site of injury releases inflammatory cytokines [55]. This chemotactic response seems to be regulated by ATP released from the damaged tissue [56]. Microglial reactivity results in secretion of a large number of substances involved in inflammatory and repair processes including cytokines, lipid mediators and free radicals [57]. Interestingly, evidence indicates that the inflammatory process resulting from microglial activation can be attenuated by a liposomal constituent. Microglia selectively binds liposomes enriched with phosphatidylserine [58]. Phosphatidylserine, acting via specific phosphatidylserine receptors, prevents the classical pro-inflammatory activation of macrophages [59]. For this reason, liposomes containing phosphatidylserine have been shown to reduce the release of pro-inflammatory cytokines from microglia [60] and to inhibit activation of the mitogen-activated protein kinase p38, therefore suppressing pro-inflammatory activities in microglial cells [61]. Moreover, Hashioka et al., showed that liposomes comprising the phospholipids phosphatidylserine and phosphatidylcholine have both neuroprotective and antioxidative properties through the inhibition of microglial activation [62,63]. These findings indicate that the liposomal composition itself can modulate microglial inflammatory reactivity, a positive aspect when considering the usage of liposomes in neurodegenerative diseases (see below) or in other central nervous system conditions.
Neurodegenerative diseases
The hallmark of Alzheimer´s disease is the disruption of basal forebrain cholinergic pathways, however, accumulated evidence indicates that the GABAergic system is also altered. In general, GABA levels are reduced and GABAA receptors are downregulated. Several regions are affected including the prefrontal cortex and hippocampus. Studies have shown that stimulation of GABAergic signaling protects neurons against the neurotoxicity of amyloid β-protein and therefore this strategy can be considered a potential therapy for treatment of Alzheimer’s disease [11]. Reduction of GABA inhibitory input in the basal ganglia circuits also produces the the symptoms of Huntington´s disease [64], and enhancement of GABAergic neurotransmission have been explored as a therapeutic approach [65]. Both Alzheimer´s and Huntington´s diseases, share common features such as the deposition of distinct protein aggregates that alters synaptic function [66]. The deposition of amyloid β-protein in Alzheimer´s disease leads to microglia-mediated inflammatory response and neurotoxicity. Part of the mechanisms by which microglia can enhance the neurotoxicity of amyloid β-protein is via the production of reactive oxygen species [67,68]. Considering our current data showing that GABA-containing liposomes can improve nitric oxide availability in neuronal populations [37], the influence of GABA-containing liposomes on amyloid β-protein toxicity on neurons remains to be examined.
Epilepsy
Evidence from a number of experimental and clinical studies provides strong support for a role of the GABAergic system in the mechanism and treatment of epilepsy. Seizure foci are characterized by a pathological reduction of GABAergic terminals and an increase in glutamatergic, excitatory terminals [69]. The focus of seizure can be determined, for example, in the hippocampus or temporal lobe. In some cases, surgical treatment may be necessary [70]. There are different rat models of epilepsy mimicking different aspects of human epilepsy [71], therefore, GABA-containing liposomes can be very useful for understanding epileptic mechanisms. As mentioned above, Loeb et al.,[32,33] reported a reduction in the epileptic activity after intravenous administration of GABA liposomes and hypothesized that the liposomal carrier may enhance the penetration of GABA across the BBB and enhance the efficacy of treatment. The effects of site-specific administration of liposomes into the brain regions directly involved in epileptic seizures remain to be determined.
Gliomas
Gliomas are commonly associated with epileptic seizures, suggesting interrelated mechanisms between the development of the two pathologies. As elegantly reviewed by Huberfeld and Vecht, GABA signaling is implicated in tumor proliferation as well as in the tumor-associated epilepsy [14]. GABA levels are higher in tissue around gliomas and GABA-mediated inhibition is impaired. Evidence indicates that GABA receptor activation decreases tumor proliferation [14]. Liposomes have been explored as drug carriers for targeting brain tumors for a long time [72] and different animal models of brain tumors are available [54,73]. Therefore, the possibility of GABA-containing liposomes to show an antiproliferative effect on tumoral cells is attractive and remains to be investigated.
Anxiety disorders
The GABA system plays a critical role in the pathophysiology of anxiety disorders. Clinical and animal studies demonstrate that positive modulators of GABA receptors promote anxiety reduction and antidepressant effects, while negative modulators results in anxiogenic effects and depression [74]. The GABAergic circuits and structures involved in anxiety disorders have also been determined, they include the amygdala, dorsomedial hypothalamus, hippocampus and periaqueductal gray amongst others. For example, altered GABAergic input to the amygdala is implicated in the putative fear learning abnormalities and sensitization is also reported in posttraumatic stress disorder [75]. A reduced GABAergic tone to the dorsomedial hypothalamus is implicated in panic disorder [76]. Therefore, the GABAergic systems at the level of amygdala and dorsomedial hypothalamus may be ideal potential targets for anxiety disorder therapeutics. Emotional stress also plays a major contributory role in the development of anxiety disorders. In this regard, we have recently reported that ICV administration of liposome-entrapped GABA reduced the tachycardia evoked by acute stress and promoted a reduction in anxiety in rats. These effects were observed at least 48 hrs after administration and are consistent with the sustained release properties of the liposomal formulation [35]. Given the characteristic of chronic release, GABA-containing liposomes can be a helpful tool for investigating the central mechanisms involved in anxiety disorders.
Hypertension and Heart Failure
Cardiovascular diseases are a major public health epidemic and their prevalence has increased in developing countries. Studies indicate a key role for the GABAergic transmission in the central nervous system for the development of cardiovascular disease. Increased sympathetic tone is present in experimental models of animal hypertension [77] and congestive heart failure [78] and the paraventricular nucleus of the hypothalamus (PVN) is an important region involved in the maintenance of sympathetic output [79,80]. The responses in MAP, HR and sympathetic activity evoked by microinjection of the GABAA antagonist (bicuculline methiodide) or the GABAA receptor agonist (muscimol) into the PVN of hypertensive rats are attenuated when compared to normotensive controls [77]. This finding suggests an attenuation of GABAA receptor function in the PVN of hypertensive rats. Additionally, animals with heart failure presented a decrease in GABAA receptor [13] and in GABA neurotransmitter [81] in the PVN. Recently, we demonstrated that the previous intracerebroventricular microinjection of liposome-entrapped GABA attenuated the increases in renal sympathetic nerve activity evoked by microinjection of bicuculline methiodide and presented blunted tachycardia in stress trials [35].
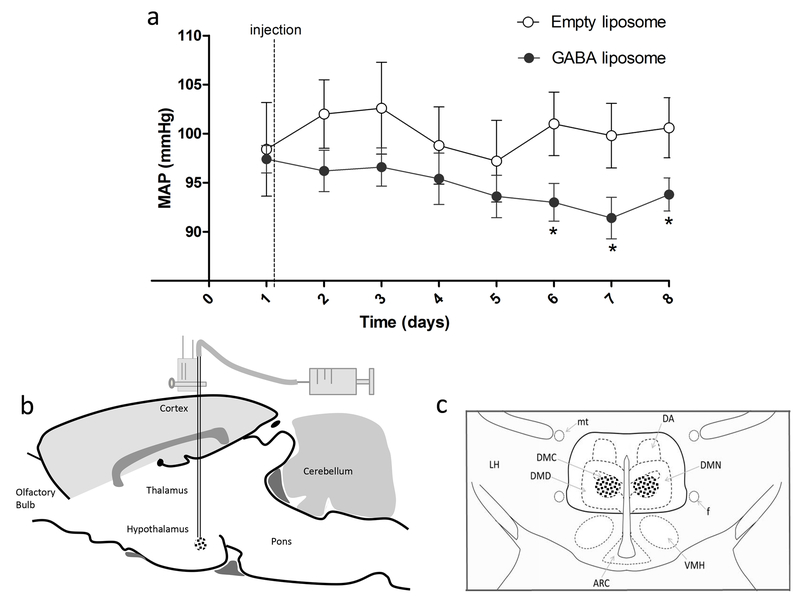
Considering that liposomes can release GABA slowly in the central nervous system as we have previously described [35], the studies of its effects in key regions of cardiovascular control could contribute to provide insights into the mechanisms involved in the onset and or maintenance of hypertension and heart failure complications. In this regard, preliminary experiments from our laboratory indicates that GABA liposomes injected into the dorsomedial hypothalamic region (DMH) resulted in significant long-lasting fall in blood pressure for up to 8 days (Figure 2). The DMH is a key region controlling the sympathetic activity to the heart and vessels [82,83] and it has been implicated as part of the central mechanisms involved in hypertension development [84].
Figure 2 –
(a) Time course of changes (means ± SE) in mean arterial pressure (MAP; measured by telemetry) of Wistar rats after bilateral nanoinjection of GABA liposomes (200 nl, n=5) into the dorsomedial hypothalamic region. (b) Sagittal view of the rat brain illustrating the stereotaxic nanoinjection procedure reaching the target region. (c) Frontal view of the dorsomedial hypothalamic region; dots represent approximate injection sites; the target injection site is delimited by bold line as defined by Fontes et al., 2011. GABA liposomes formulation and microinjection procedures performed according to Vaz et al., 2015. *p<0.05 vs. injection of empty liposome (control, n=5), Two-Way ANOVA followed by Bonferroni. ARC, arcuate hypothalamic nucleus; DA, dorsal hypothalamic area; DMC, compact portion of dorsomedial hypothalamic nucleus; DMD, diffuse portion of the dorsomedial hypothalamic nucleus; DMN, dorsomedial hypothalamic nucleus; f, fornix; mt, mamillothalamic tract; LH, lateral hypothalamus; VMH, ventromedial hypothalamic nucleus; III, third ventricle. All procedures conform to the regulation set by the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 and were approved by our local committee for ethics in animal experimentation (protocol number: 150-2010).
6. Concluding remarks
Since GABA system is implicated in several brain functional networks, an approach to chronic release GABA may represent an important tool for application in neuroscience offering potential future translational perspective. Our recent studies confirm previous findings showing that liposomes can easily incorporate and release GABA chronically. GABA-containing liposomes can improve nitric oxide availability in neuronal populations and this property can be relevant for targeting neurodegenerative diseases. Furthermore, the possibility of using liposomes for site-specific chronic release of GABA can offer a new alternative to understand specific regional and functional aspects of GABAergic mechanisms involved in anxiety, stress, epilepsy and several other physiological and physiopathological conditions such as hypertension and heart failure.
Acknowledgements:
We gratefully acknowledge the financial support from Brazilian agencies Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG CBB-APQ-01463-15; RED-00007-14), Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brazil, CNPq (PQ30600/2013-0; PQ303227/2013-3), PRONEX, INCT-Nanobiofar (CNPq/FAPEMIG). This work was supported by American Heart Association (14SDG19980007) and NIH grants R56 HL124104, P01 HL62222, & RO1 DK114663.
References
- 1.Vo-Dinh T Nanotechnology in biology and medicine: methods, devices, and applications. CRC Press. [Google Scholar]
- 2.Kanwar JR, Sun X, Punj V, et al. Nanoparticles in the treatment and diagnosis of neurological disorders: untamed dragon with fire power to heal. Nanomedicine Nanotechnol. Biol. Med 8(4), 399–414 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Frézard F, dos Santos RA, Fontes MA. Liposome-encapsulated neuropeptides for site-specific microinjection. Neuropept. Methods Protoc , 343–355 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Formoso P, Muzzalupo R, Tavano L, De Filpo G, Pasquale Nicoletta F. Nanotechnology for the environment and medicine. Mini Rev. Med. Chem 16(8), 668–675 (2016). [PubMed] [Google Scholar]
- 5.Pardridge WM. Molecular Trojan horses for blood–brain barrier drug delivery. Curr. Opin. Pharmacol 6(5), 494–500 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Pehlivan SB. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm. Res 30(10), 2499–2511 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Stockwell J, Abdi N, Lu X, Maheshwari O, Taghibiglou C. Novel central nervous system drug delivery systems. Chem. Biol. Drug Des 83(5), 507–520 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA A receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol. Rev 91(3), 1009–1022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA-A receptor subtypes. Curr. Top. Med. Chem 2(8), 795–816 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Ting Wong CG, Bottiglieri T, Snead OC. GABA, γ-hydroxybutyric acid, and neurological disease. Ann. Neurol 54(S6), S3–S12 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Solas M, Puerta E, J Ramirez M. Treatment Options in Alzheimer’ s Disease: The GABA Story. Curr. Pharm. Des 21(34), 4960–4971 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus–a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets 12(6), 717–727 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y-F, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol. 177(1), 17–26 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Huberfeld G, Vecht CJ. Seizures and gliomas–towards a single therapeutic approach. Nat. Rev. Neurol 12(4), 204 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Wang S, Xiao W, Huang C, Li H, Sun M. BBB: Permeable Conjugate of Exogenic GABA. ACS Omega. 2(8), 4108–4111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Discher BM, Won Y-Y, Ege DS, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. 284(5417), 1143–1146 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Yaqoob Khan A, Talegaonkar S, Iqbal Z, Jalees Ahmed F, Krishan Khar R. Multiple emulsions: an overview. Curr. Drug Deliv 3(4), 429–443 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Noble JE, De Santis E, Ravi J, et al. A de novo virus-like topology for synthetic virions. J Am Chem Soc. 138(37), 12202–12210 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Martínez FC, Guerra J, Posadas I, Ceña V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm. Res 28(8), 1843–1858 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Purra M, Ramos V, Petrenko VA, Torchilin VP, Borros S. Double-targeted polymersomes and liposomes for multiple barrier crossing. Int. J. Pharm 511(2), 946–956 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Yurtdaş Kırımlıoğlu G, Menceloğlu Y, Erol K, Yazan Y. In vitro/in vivo evaluation of gamma-aminobutyric acid-loaded N, N-dimethylacrylamide-based pegylated polymeric nanoparticles for brain delivery to treat epilepsy. J. Microencapsul 33(7), 625–635 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Frézard F, Schettini DA, Rocha OG, Demicheli C. Lipossomas: propriedades físico-químicas e farmacológicas, aplicações na quimioterapia à base de antimônio. Quim Nova. 28(3), 511–518 (2005). [Google Scholar]
- 23.Ulrich AS. Biophysical aspects of using liposomes as delivery vehicles. Biosci. Rep 22(2), 129–150 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Senior J, Crawley JC, Gregoriadis G. Tissue distribution of liposomes exhibiting long half-lives in the circulation after intravenous injection. Biochim. Biophys. Acta BBA-Gen. Subj 839(1), 1–8 (1985). [DOI] [PubMed] [Google Scholar]
- 25.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly (ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta BBA-Biomembr 1066(1), 29–36 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Vieira DB, Gamarra LF. Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomedicine. 11, 5381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangham AD, Standish MM, Weissmann G. The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J. Mol. Biol 13(1), 253IN28–259 (1965). [DOI] [PubMed] [Google Scholar]
- 28.Gregoriadis G, Ryman BE. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem. J 124(5), 58P (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregoriadis G, Ryman BE. Fate of Protein-Containing Liposomes Injected into Rats. FEBS J 24(3), 485–491 (1972). [DOI] [PubMed] [Google Scholar]
- 30.Gregoriadis G The carrier potential of liposomes in biology and medicine. N. Engl. J. Med. 295(14), 765–770 (1976). [DOI] [PubMed] [Google Scholar]
- 31.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4(2), 145–160 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Loeb C, Benassi E, Besio G, Maffini M, Tanganelli P. Liposome-entrapped GABA modifies behavioral and electrographic changes of penicillin-induced epileptic activity. Neurology. 32(11), 1237–1237 (1982). [DOI] [PubMed] [Google Scholar]
- 33.Loeb C, Besio G, Mainardi P, et al. Liposome-Entrapped γ-Aminobutyric Acid Inhibits Isoniazid-Induced Epileptogenic Activity in Rats. Epilepsia. 27(2), 98–102 (1986). [DOI] [PubMed] [Google Scholar]
- 34.Tempone AG, Perez D, Rath S, Vilarinho AL, Mortara RA, de Andrade HF Jr. Targeting Leishmania (L.) chagasi amastigotes through macrophage scavenger receptors: the use of drugs entrapped in liposomes containing phosphatidylserine. J. Antimicrob. Chemother 54(1), 60–68 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Vaz GC, Bahia A, De Figueiredo Müller-Ribeiro FC, et al. Cardiovascular and behavioral effects produced by administration of liposome-entrapped GABA into the rat central nervous system. Neuroscience. 285, 60–69 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Silva-Barcellos NM, Frézard F, Caligiorne S, Santos RA. Long-lasting cardiovascular effects of liposome-entrapped angiotensin-(1-7) at the rostral ventrolateral medulla. Hypertension. 38(6), 1266–1271 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Vaz GC, Sharma NM, Zheng H, et al. Liposome-entrapped GABA modulates the expression of nNOS in NG108–15 cells. J. Neurosci. Methods. 273, 55–63 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma NM, Llewellyn TL, Zheng H, Patel KP. Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: role for PIN. Am. J. Physiol.-Heart Circ. Physiol 305(6), H843–H855 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones AR, Shusta EV. Blood–brain barrier transport of therapeutics via receptor-mediation. Pharm. Res 24(9), 1759–1771 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata S, Ochi A, Mori K. Liposomes as Carriers of Cisplatin into the Central Nervous System. Neurol. Med. Chir. (Tokyo). 30(4), 242–245 (1990). [DOI] [PubMed] [Google Scholar]
- 41.Koukourakis MI, Koukouraki S, Fezoulidis I, et al. High intratumoural accumulation of stealth® liposomal doxorubicin (Caelyx®) in glioblastomas and in metastatic brain tumours. Br. J. Cancer 83(10), 1281–1286 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rip J Liposome technologies and drug delivery to the CNS. Drug Discov. Today Technol 20, 53–58 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Spuch C, Navarro C. Liposomes for Targeted Delivery of Active Agents against Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). J. Drug Deliv. 2011, 1–12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi S, Singh-Moon R, Wang M, et al. Cationic surface charge enhances early regional deposition of liposomes after intracarotid injection. J. Neurooncol. 120(3), 489–497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tagalakis AD, Kenny GD, Bienemann AS, et al. PEGylation improves the receptor-mediated transfection efficiency of peptide-targeted, self-assembling, anionic nanocomplexes. J. Controlled Release. 174, 177–187 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Gabathuler R Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. 37(1), 48–57 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Ying X, Wen HE, Lu W-L, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Controlled Release. 141(2), 183–192 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm 379(1), 146–157 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J. Pharm. Sci. 99(4), 1745–1761 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Lewis O, Woolley M, Johnson D, et al. Chronic, intermittent convection-enhanced delivery devices. J. Neurosci. Methods 259, 47–56 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Sampson JH, Brady M, Raghavan R, et al. Colocalization of gadolinium-diethylene triamine pentaacetic acid with high-molecular-weight molecules after intracerebral convection-enhanced delivery in humans. Neurosurgery. 69(3), 668–676 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.During MJ, Freese A, Deutch AY, et al. Biochemical and behavioral recovery in a rodent model of Parkinson’s disease following stereotactic implantation of dopamine-containing liposomes. Exp. Neurol 115(2), 193–199 (1992). [DOI] [PubMed] [Google Scholar]
- 53.Mamot C, Nguyen JB, Pourdehnad M, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J. Neurooncol 68(1), 1–9 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Saito R, Bringas JR, McKnight TR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 64(7), 2572–2579 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 22(1), 219–240 (1999). [DOI] [PubMed] [Google Scholar]
- 56.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8(6), 752 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog. Neurobiol 54(1), 99–125 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Witting A, Müller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic Clearance of Apoptotic Neurons by Microglia/Brain Macrophages In Vitro. J. Neurochem 75(3), 1060–1070 (2000). [DOI] [PubMed] [Google Scholar]
- 59.De Simone R, Ajmone-Cat MA, Minghetti L. Atypical antiinflammatory activation of microglia induced by apoptotic neurons. Mol. Neurobiol 29(2), 197 (2004). [DOI] [PubMed] [Google Scholar]
- 60.De Simone R, Ajmone-Cat MA, Nicolini A, Minghetti L. Expression of phosphatidylserine receptor and down-regulation of pro-inflammatory molecule production by its natural ligand in rat microglial cultures. J. Neuropathol. Exp. Neurol 61(3), 237–244 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Ajmone-Cat MA, De Simone R, Nicolini A, Minghetti L. Effects of phosphatidylserine on p38 mitogen activated protein kinase, cyclic AMP responding element binding protein and nuclear factor-κB activation in resting and activated microglial cells. J. Neurochem 84(2), 413–416 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Hashioka S, Han Y-H, Fujii S, et al. Phosphatidylserine and phosphatidylcholine-containing liposomes inhibit amyloid β and interferon-γ-induced microglial activation. Free Radic. Biol. Med 42(7), 945–954 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Hashioka S, Han Y-H, Fujii S, et al. Phospholipids modulate superoxide and nitric oxide production by lipopolysaccharide and phorbol 12-myristate-13-acetate-activated microglia. Neurochem. Int 50(3), 499–506 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12(10), 366–375 (1989). [DOI] [PubMed] [Google Scholar]
- 65.Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr. Pharm. Des 12(21), 2701–2720 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol 8(2), 101–112 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 77(6), 817–827 (1994). [DOI] [PubMed] [Google Scholar]
- 68.Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong J-S. Microglia enhance β-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J. Neurochem 83(4), 973–983 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Lowenstein DH. Recent advances related to basic mechanisms of epileptogenesis. Epilepsy Res. Suppl. 11, 45–60 (1995). [PubMed] [Google Scholar]
- 70.WIEBE S Effectiveness and efficiency of surgery for Temporal Lobe Epilepsy Study Group : a randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345, 311–318 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Löscher W Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 50(1), 105–123 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Béduneau A, Saulnier P, Benoit J-P. Active targeting of brain tumors using nanocarriers. Biomaterials. 28(33), 4947–4967 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin. Cancer Res. 12(18), 5288–5297 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress. Anxiety 24(7), 495–517 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Steckler T, Risbrough V. Pharmacological treatment of PTSD–established and new approaches. Neuropharmacology. 62(2), 617–627 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural Pathways Underlying Lactate-Induced Panic. Neuropsychopharmacology. 33(9), 2093–2107 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li D-P, Pan H-L. Role of γ-aminobutyric acid (GABA) A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J. Pharmacol. Exp. Ther 320(2), 615–626 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Xu B, Zheng H, Patel KP. Enhanced activation of RVLM-projecting PVN neurons in rats with chronic heart failure. Am. J. Physiol.-Heart Circ. Physiol 302(8), H1700–H1711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 39(2), 275–280 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Sharma NM, Zheng H, Mehta PP, Li Y-F, Patel KP. Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II. Cardiovasc. Res 92(2), 348–357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang R-J, Zeng Q-H, Wang W-Z, Wang W. GABA(A) and GABA(B) receptor-mediated inhibition of sympathetic outflow in the paraventricular nucleus is blunted in chronic heart failure. Clin. Exp. Pharmacol. Physiol 36(5-6), 516–522 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Fontes MAP, Tagawa T, Polson JW, Cavanagh S-J, Dampney RAL. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am. J. Physiol.-Heart Circ. Physiol 280(6), H2891–H2901 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Dampney RAL, Coleman MJ, Fontes MAP, et al. Central mechanisms underlying short-and long-term regulation of the cardiovascular system. Clin. Exp. Pharmacol. Physiol 29(4), 261–268 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Simonds SE, Pryor JT, Ravussin E, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 159(6), 1404–1416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]