OVERVIEW
The growing population of cancer survivors often faces adverse effects of treatment, which have a substantial impact on morbidity and mortality. Although certain adverse effects are thought to have a significant heritable component, much work remains to be done to understand the role of germline genetic factors in the development of treatment-related toxicities. In this article, we review current understanding of genetic susceptibility to a range of adverse outcomes among cancer survivors (e.g., fibrosis, urinary and rectal toxicities, ototoxicity, chemotherapy-induced peripheral neuropathy, subsequent malignancies). Most previous research has been narrowly focused, investigating variation in candidate genes and pathways such as drug metabolism, DNA damage and repair, and inflammation. Few of the findings from these earlier candidate gene studies have been replicated in independent populations. Advances in understanding of the genome, improvements in technology, and reduction in laboratory costs have led to recent genome-wide studies, which agnostically interrogate common and/or rare variants across the entire genome. Larger cohorts of patients with homogeneous treatment exposures and systematic ascertainment of well-defined outcomes as well as replication in independent study populations are essential aspects of the study design and are increasingly leading to the discovery of variants associated with each of the adverse outcomes considered in this review. In the long-term, validated germline genetic associations hold tremendous promise for more precisely identifying patients at highest risk for developing adverse treatment effects, with implications for frontline therapy decision-making, personalization of long-term follow-up guidelines, and potential identification of targets for prevention or treatment of the toxicity.
The population of cancer survivors has increased dramatically in many countries over the last several decades as a result of major advances in treatment, improvements in early detection, and population growth and aging. In the United States, the number of cancer survivors has grown from fewer than 3 million in 1975 to over 15 million today and is expected to reach 20 million by 2026.1
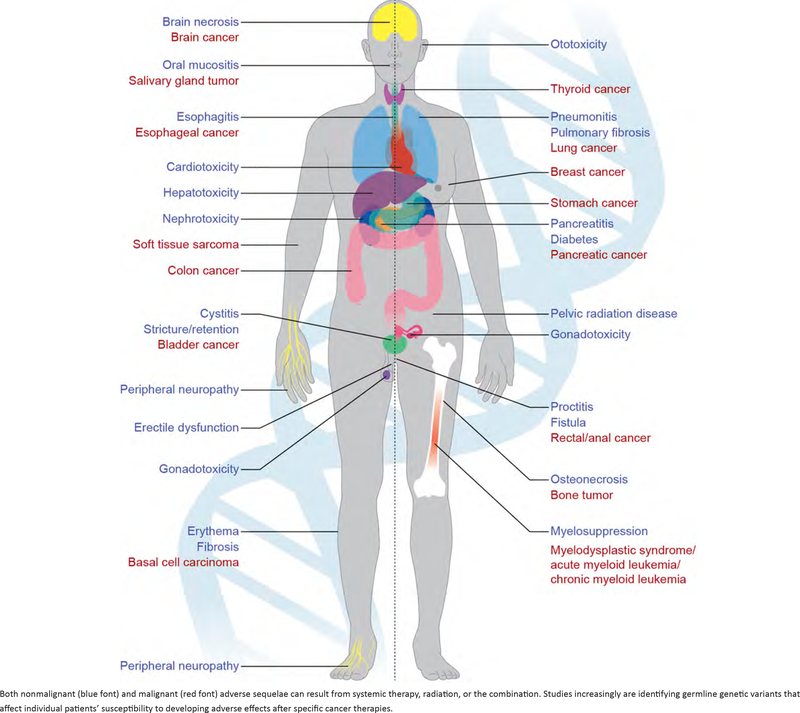
As a result of the substantial growth in the cancer survivor population, understanding factors that influence both short- and long-term health of survivors has gained importance from the public health as well as clinical perspective. Although many general population health guidelines for screening and prevention can reasonably be applied to cancer survivors, the morbidity and mortality associated with treatment-related adverse effects—that can impact nearly any organ system (Fig. 1)—strikingly differentiate survivors from others in the general population. Severe toxicities are dose limiting for both chemotherapy and radiotherapy.2 Despite such constraints, a substantial percentage of cancer survivors develop mild and moderate adverse effects that may cause substantial morbidity and even mortality; incur costs for diagnosis, monitoring, medication, and other interventions; and negatively impact quality of life. For example, progressive, and permanent bilateral hearing loss occurs in as many as 56% of children3 and 80% of adults4 treated with cisplatin; the lifetime costs/patient associated with hearing loss is $300,000 for adults and over $1,000,000 for a child.5 A recent systematic review found that concerns about the discomfort of treatment and fear of side effects are important factors for declining cancer treatment among older patients with cancer.6
FIGURE 1.
Examples of Radiotherapy and Systemic Therapy-Related Adverse Effects on a Range of Organ Systems
Rapid advances in genomics hold tremendous promise for identifying inherited genetic factors that may influence risk for treatment-related adverse effects. Discovery of such factors provides insight into the biologic processes leading to the development of adverse effects. Additionally, such discoveries have potential for clinical application by more precisely quantifying risks and benefits of different therapeutic options at the treatment planning stage, tailoring long-term follow-up guidelines for individual survivors, and identifying potential targets for prevention or treatment of the adverse event. In this article, we review the current landscape of germline genomics research in relation to nonmalignant and malignant adverse effects of therapy. We then provide a roadmap for future research that is needed to realize the promise of employing germline genetics to bring precision medicine into survivorship.
PERSPECTIVES ON GENOMICS RESEARCH AND HUMAN HEALTH
Inherited predisposition to cancer has been recognized for well over a century based on the identification of families with strikingly elevated risk for breast cancer. Over the last several decades, specific genes that underlie many rare inherited disease predisposition syndromes have been systematically identified.7,8 More recently, studies increasingly have attempted to identify common genetic variants that could be associated with cancer risk in the general population9 as well as variants that could be associated with response to cancer treatment.10 Those studies generally investigated known variants in candidate genes, selected based on hypotheses of the key genes and pathways relevant for the outcome of interest.
Major advances in genotyping and sequencing technology and large initiatives, such as the International HapMap,11,12 ENCODE (ENCyclopedia Of DNA Elements),13,14 and 1000 Genomes15 projects, have resulted in major changes in research on germline genomics and cancer in recent years. Critically, a reduction in costs of genotyping and sequencing has enabled a dramatic expansion in the sample sizes for genomics studies. These expansions have led to the unfortunate realization that very few of the results from candidate gene studies are consistently replicated in independent study populations.9 Additionally, advances in understanding of the genome and technological advances have enabled agnostic, genome-wide study designs, including genome-wide association studies (GWAS), which leverage linkage disequilibrium to assess common genetic variation across the genome through direct genotyping and imputation of millions of single nucleotide polymorphisms (SNPs), and or whole-genome sequencing, which can identify rare genetic variants. A major strength of genome-wide approaches is that they do not require a priori assumptions about the genes or pathways involved in the outcome, and so they are a powerful tool for uncovering novel biologic mechanisms. Indeed, the shift to genome-wide study designs has demonstrated that the early candidate gene studies were too narrowly focused and instead discovered novel genes not previously suspected to be involved in many diseases.
Research on genomics of treatment-related adverse effects generally has followed the path described above, albeit at a slower pace, with fewer studies completed thus far. As with other complex diseases, much of the initial research has focused on candidate genes, the results of which typically either have not been investigated or have failed to replicate in independent populations. Only more recently has the field begun to include agnostic, genome-wide study designs (Table 1). The good news is that pharmacogenomic and radiogenomic studies tend to have larger effect sizes than complex disease susceptibility studies primarily because the relevant environmental factors (drug and radiation exposure, respectively) are known.16 The challenge, however, is implementing large, well-powered studies with homogeneous treatment exposures and consistent measures of adverse effects, with replication of results in independent populations.
TABLE 1.
List of Genome-Wide Association Studies of Selected Treatment-Related Adverse Effects
| Treatment, Study Reference | Adverse Effect | Study Population, by Ancestry (ndiscovery){nby population}[nreplication]* |
|---|---|---|
| Nonmalignant Outcomes | ||
| Radiotherapy17 | Erectile dysfunction | African American (79) |
| Radiotherapy18 | Rectal incontinence | European (579)[516] |
| Radiotherapy19 | Overall late toxicity | European and European American (741)[633 and 368] |
| Radiotherapy20 | Increased urinary frequency | European and European American/Canadian (1,564; meta-analysis of four studies: 597, 527, 290, 151) |
| Radiotherapy20 | Decreased urinary stream | European and European American/Canadian (1,564; meta-analysis of four studies: 597, 527, 290, 151) |
| Paclitaxel21 | Neuropathy | European (144) |
| Paclitaxel22 | Neuropathy | European American (855)[154 European American; 117 African American] |
| Paclitaxel/docetaxel**23 | Neuropathy | Diverse (1,570){1,357 European American; 213 African American}[789 European American; 90 African Amercan; 56 other] |
| Docetaxel24 | Neuropathy | European American (623) |
| Vincristine25 | Neuropathy | Diverse (321){209 European American; 43 African American; 2 Asian; 44 Hispanic; 23 other} |
| Platinating (combination)26 | Neuropathy | Korean (96)[247] |
| Bortezomib27 | Neuropathy | European (469)[114] |
| Bortezomib28 | Neuropathy | European (646) |
| Cisplatin29 | Neuropathy | European American (680) |
| Cisplatin30 | Ototoxicity | European American (511) |
| Cisplatin31 | Ototoxicity | Diverse (238){European American, African American, and other}[68] |
| Subsequent Neoplasms | ||
| Radiotherapy32 | Any subsequent neoplasm | European American (178)[227] |
| Radiotherapy33 | Breast cancer | European American (2378)[603] |
| Radiotherapy, chemotherapy34 | MDS/AML | European American (230)[165] |
Replication refers to any study that performs a second association study in another cohort within the same publication.
Received additional chemotherapeutic drugs; however, study was intended to evaluate taxane-induced neuropathy.
Abbreviation: MDS/AML, myelodysplastic syndrome/acute myeloid leukemia.
NONMALIGNANT ADVERSE EFFECTS OF CANCER TREATMENT
Radiotherapy
Toxicities following radiotherapy vary depending on the tumor site and surrounding normal tissues exposed. For example, common toxicities following radiotherapy for head and neck tumors include oral mucositis, dysphasia, and xerostomia resulting from damage to the oral epithelium and salivary glands, development of fibrosis in the pharynx, as well as inflammation.35–37 Pelvic radiotherapy used for treatment of prostate, cervical, bladder, and rectal cancers can cause intestinal, bowel, and bladder damage that can result in adverse gastrointestinal and urinary effects including bleeding, pain, frequency, and urgency.38,39 Local radiation damage can also lead to subsequent systemic effects. For example, damage to the oral cavity can lead to poor dental hygiene, increased susceptibility to oral infections, oral pain, and difficulty chewing and swallowing that can in turn result in sleep disturbances, nutritional deficiencies, and overall decrease in quality of life. Similarly, damage to the gastrointestinal tract can lead to chronic dysfunction resulting in altered intestinal transit and nutritional malabsorption.40
It has long been recognized that substantial variation exists among patients in the incidence and severity of normal tissue reactions to radiotherapy. Individual variation of normal tissue response for a given radiation dose was first described in the scientific literature in 1936, with the publication of a sigmoid dose response curve for the development of skin telangiectasia.41,42 The hypothesis that radiation sensitivity may be heritable is supported by the existence of rare genetic syndromes associated with hypersensitivity to radiation, where rare mutations in genes involved in DNA double-strand break repair, such as ATM (ataxia telangiectasia mutated), NBS1 (Nijmegen breakage syndrome), MRE11 (ataxia telangiectasia-like disorder), and LIG4 (DNA ligase IV deficiency), result in syndromes characterized by extreme radiosensitivity and increased risk for developing cancer.43–45 The variable responses to radiotherapy observed in patients who are treated with protocols involving similar dosimetric characteristics but are not affected by one of these rare syndromes suggest the importance of common genetic factors. In vitro studies of apoptotic response or chromosome damage following irradiation have estimated the heritability of radiosensitivity to range from 58% to 82%.46–50 Similar estimates have been made for related clinical outcomes, such as skin telangiectasia.51
The biologic mechanisms underlying development of radiotherapy adverse effects involve general processes common to multiple normal tissues, such as fibrosis, necrosis, inflammation, and vascular damage. However, the relative importance of specific genes and pathways may vary depending on the specific normal tissues involved and the endpoints of interest.52 For example, pathways involved in repair of muscle damage may be more important in the context of bladder function following pelvic radiotherapy, whereas pathways regulating the development of collagen deposition and fibrosis may be more important in development of lung damage. There are also differences in the biologic pathways underlying early versus late effects of radiation for most adverse effects. Thus, studies aiming to identify genetic risk factors must take tissue specificity and endpoint specificity into consideration at the design stage.
Early studies of SNP-toxicity associations focused on candidate genes known to be important in cellular radiation response from in vitro radiobiologic studies. These include genes involved in DNA damage response, cellular survival, free radical metabolism, wound healing, and inflammation. While those early studies were limited by high genotyping costs, incomplete understanding of the genetic architecture of the human genome, and lack of attention to the confounding effects of ancestry, some associations were successfully replicated. For example, the missense SNP rs1139793 in TXNRD2 was significantly associated with radiation-induced fibrosis after breast cancer in a study of candidate genes involved in reactive oxygen species metabolism.53 Furthermore, rs1139793 was significantly associated with TXNRD2 mRNA expression in blood, suggesting a functional impact of the SNP. In another candidate gene study, rs1800629 in the inflammatory cytokine TNF showed a replicated association with skin toxicity in breast cancer survivors.54 A study of patients with non-small cell lung cancer found that the functional promoter variant rs2868371 in HSPB1 was significantly associated with pneumonitis following chemoradiation,55 and this same variant was subsequently shown to be associated with radiotherapy-induced esophagitis, suggesting it may play a broad role in radiosensitivity across different tissue types.56 Although early studies of the common SNP rs1801516 in ATM showed inconclusive results, a recent meta-analysis of individual patient data provides convincing evidence that the minor allele of this SNP is associated with an increased risk of overall radiotherapy-induced acute and late toxicity,57 confirming that both common and rare ATM variants contribute to general radiosensitivity. In contrast, a replicated association has been reported between rs1800469 in TGFB1 and esophagitis,58,59 whereas a large meta-analysis of individual patient data (2,782 patients, 11 independent studies) reported no association of this SNP with radiotherapy-induced fibrosis,60 indicating that some SNPs show tissue specificity.
GWAS have begun to identify additional, novel radiosensitivity loci within genes not previously known to be involved in cellular or tissue response to radiation. The first radiogenomics GWAS, though underpowered, was able to detect a risk locus within the FSHR gene associated with erectile dysfunction following radiotherapy for prostate cancer based on 79 cases.17 A much larger (> 1,500 patients) three-stage GWAS of overall late toxicity including urinary and rectal effects in patients with prostate cancer identified a locus in TANC1,19 which is expressed in myoblasts and plays a central role in regeneration of damaged muscle. A recent individual patient data meta-analysis of four GWAS of late toxicity in prostate cancer radiotherapy patients identified two more risk SNPs: rs17599026 in KDM3B associated with increased urinary frequency and rs7720298 in DNAH5 associated with decreased urinary stream.20 Notably, these SNPs lie within genes that are expressed in tissues likely underlying these symptoms, including the bladder, which is exposed to radiation during treatment of prostate cancer. Indeed, the genes identified via GWAS of radiotherapy toxicity have not previously been implicated in cellular radiation response, but initial laboratory data suggest they may be involved in key radio-response pathways such as muscle cell regeneration after radiation induced damage (TANC1) and DNA double-strand break repair following irradiation (KDM3B; unpublished data). Ongoing functional studies are underway to further characterize these genes. Expanded efforts currently underway through the Radiogenomics Consortium61 and the REQUITE study62 are expected to uncover additional risk SNPs and will allow for investigation of gene-environment interaction, investigation of effect modifiers, and validation of prior SNP-toxicity associations.
Systemic Therapy
Toxicities related to systemic therapy, such as peripheral neuropathy, myelosuppression, hepatotoxicity, ototoxicity, pancreatitis, cardiotoxicity, and osteonecrosis, could be lifelong and often have debilitating effects on a survivor’s physical and psychological well-being. Below, we focus on chemotherapy-induced peripheral neuropathy and ototoxicity to exemplify current understanding of the genomics of nonmalignant adverse effects of systemic therapy.
Chemotherapy-induced peripheral neuropathy.
Chemotherapy-induced peripheral neuropathy is one of the most common adverse effects of chemotherapy63,64 and may arise as a result of mechanistically different chemotherapeutics.65 In part because of a paucity of genetically diverse human models of chemotherapy-induced peripheral neuropathy, there are no preventive measures or effective treatments for this devastating adverse drug effect.66 Patient demographics (i.e., race and history of neuropathy) and treatment (i.e., cumulative dose and drug exposure) factors have been associated with chemotherapy-induced peripheral neuropathy.67–69 Race was also a major predictor of paclitaxel induced neuropathy, with patients of African descent experiencing increased risk of grade 2 to 4 as well as grade 3 to 4 peripheral neuropathy compared with others.23 In addition, peripheral neuropathy resulting from cisplatin treatment has been shown to be negatively associated with self-reported health and physical activity level and positively correlated with weight gain after treatment, suggesting a less active lifestyle due to complications of neuropathy.29
Early studies exploring the genetic contribution to chemotherapeutic toxicities relied heavily upon candidate gene approaches, associating SNPs in genes encoding known drug metabolizing enzymes, DNA repair pathways, receptors, and transporters. For example, SNPs in GSTP1,70,71 ABCG272 XPC72 and ERCC173 were associated with cisplatin-induced neuropathy when evaluated singly, but the findings did not replicate in a subsequent GWAS.29 SNPs in candidate genes also were reported to be associated with paclitaxel-74–78 and docetaxel-79 induced neuropathy.
There have been a number of GWAS of chemotherapy-induced peripheral neuropathy associated with vincristine,25 paclitaxel,21–23 docetaxel,24 bortezomib,27,28 oxaliplatin,80 and cisplatin.29 GWAS of paclitaxel-induced peripheral neuropathy in a large cooperative trial identified a signal in EPHA5 (rs7349683)22 that was replicated by others,21,81 but interestingly also met replication significance (p < .05) in a study of cisplatin-induced neuropathy.29 A common polymorphism in FGD4 (rs10771973), a congenital peripheral neuropathy gene, also was associated with paclitaxel-induced neuropathy and was replicated in an African American cohort.22 More recent work has identified a panel of SNPs associated with increased risk of grade 3 to 4 paclitaxel-induced peripheral neuropathy in patients of European descent,23 but they were not in agreement with a previous GWAS of paclitaxel-induced neuropathy.22 However, both studies implicated the importance of the Wnt pathway (Wntless [M/S]23 and frizzled [FZD3]22) in paclitaxel-induced peripheral neuropathy. The most compelling study focused on vincristine-induced peripheral neuropathy in a pediatric population, identifying a genome-wide significant SNP in CEP72, with accompanying functional studies showing that knockdown of the gene resulted in greater sensitivity to vincristine25 and subsequent replication in adult patients with acute lymphoblastic leukemia.82
A recent large-scale GWAS of testicular cancer survivors estimated that cisplatin-induced peripheral neuropathy was significantly heritable (h2 = 0.74; p = .03).29 A transcriptome-wide association study implicated lower (genetically determined) expression of RPRD1B in cisplatin-induced peripheral neuropathy, which was replicated in an independent cohort of patients who developed drug-induced polyneuropathy.29 Importantly, RPRD1B functions in DNA repair, transcription, and cell cycle control and may be a target for drug development.83
Ototoxicity.
Ototoxicity (including both hearing loss and tinnitus) is another notable side effect of systemic therapy that can create functional limitations, ranging from impairment of speech development and academic achievement in children to detrimental effects on quality of life, socialization, and cognition in adults.84 In contrast to chemotherapy-induced peripheral neuropathy, ototoxicity results primarily from the use of platinating agents, specifically cisplatin.85,86 Cisplatin is used in the treatment of many adult-onset (cervical, endometrial, head/neck, lung, ovarian, and testicular) and pediatric (germ cell tumors, medulloblastoma, neuroblastoma, osteosarcoma, and retinoblastoma) malignancies, making it one of the most commonly applied chemotherapeutic agents worldwide. The incidence of hearing loss following cisplatin treatment is high and dependent on the cumulative dose and regimen. For example, in testicular cancer survivors receiving cisplatin, 80% had some degree of hearing loss and 18% had severe to profound hearing loss as measured by audiometry.4
Until recently, genetic studies of cisplatin-associated ototoxicity have been almost exclusively conducted in small pediatric cohorts (130–254 patients) that predominantly involved candidate gene investigations87–90 with conflicting results.91,92 In adults, previous candidate gene studies were confounded by agents known to induce ototoxicity, including vincristine93–95 and cranial radiotherapy.96–99
The first GWAS of cisplatin-induced ototoxicity identified an association with a genetic variant (rs1872328) in ACYP2 in pediatric patients with brain tumors, with replication of results in a second cohort of pediatric patients31 as well as three additional studies.100–102 Another GWAS of cisplatin-induced ototoxicity was performed in testicular cancer survivors and identified a significant SNP (rs62283056) in the first intron of Mendelian deafness gene WFS1 (wolframin ER transmembrane glycoprotein) associated with cisplatin-induced hearing loss30 that was replicated in an independent population of patients with testicular cancer when evaluating the same phenotype (geometric mean).102 That SNP is an expression quantitative trait locus (eQTL) for the WFS1 gene, with the risk (and minor) allele being associated with lower expression of the gene. Deleterious mutations in WFS1 cause Wolfram syndrome, a Mendelian disorder characterized by deafness and other neurodevelopmental conditions. The shared genetic architecture between cisplatin-induced ototoxicity with Mendelian forms of deafness could potentially impact those who live with disabling deafness. Notably, both variants identified through GWAS are extremely rare in the East Asian population (0.011 for rs1872328 in ACYP2 and 0.003 for rs62283056 in WFS1) pointing to the importance of inclusion of diverse cohorts in future pharmacogenomics studies to ensure that the benefits of genomic medicine are realized for all.103
MALIGNANT ADVERSE EFFECTS OF RADIOTHERAPY AND SYSTEMIC THERAPY
Radiotherapy
The development of a subsequent malignancy substantially impacts morbidity and mortality and is thus one of the most serious treatment-related adverse effects. Detailed studies of cancer survivors and other populations exposed to ionizing radiation demonstrate increased risk for a wide range of malignancy types.104–108 The highest risks (> fivefold) have been reported for malignancies of the skin (basal cell carcinoma), soft tissue, central nervous system, bone, thyroid, and breast, whereas more modest but still significantly elevated risks have been reported for malignancies of the lung, gastrointestinal tract, pancreas, bladder, and salivary gland. Risks generally increase linearly with increasing radiation dose, with the exception of thyroid cancer, for which a downturn in risk is evident above doses of approximately 20 Gy. Most radiation-associated subsequent malignancies do not appear for at least 5 to 10 years following exposure, and the elevated risks persist for decades. Several factors have been identified to modify radiotherapy-related risks for subsequent malignancies, including certain systemic therapies as well as age at exposure, with generally higher risks for younger ages at exposure.
Candidate gene studies of radiotherapy-related subsequent malignancies generally have focused on genetic variants in DNA damage detection and repair mechanisms, as reviewed in 2015.109 For example, the Women’s Environmental Cancer and Radiation Epidemiology (WECARE) Study is a multicenter U.S.-based case-control study of contralateral breast cancer among breast cancer survivors.110 In that study, sequencing of ATM for 708 cases and 1,397 controls revealed a stronger radiation dose-response relation among women who carried deleterious missense variants (excess relative risk / Gy = 2.6; 95% CI, 0.0–10.6) than among those without deleterious (i.e., “tolerated”) missense ATM variants (ERR/Gy = 0.8; 95% CI, 0.1–3.6) or without any missense ATM variants (ERR/Gy = 0.0; 95% CI, < 0–0.3).111 Intriguingly, the findings were more pronounced among women diagnosed with breast cancer at a younger age or whose contralateral breast cancer occurred 5 years or more after first primary breast cancer. In contrast to those results, a similar pattern was not found for women who carry deleterious BRCA1/2 mutations.112 In an expanded study (1,459 contralateral breast cancer cases, 2,126 unilateral breast cancer controls), common genetic variants known to be associated with breast cancer also were associated with risk of contralateral breast cancer, but the risk patterns did not differ significantly by prior treatment exposures.113 A candidate gene study of central nervous system tumors also has been conducted among childhood cancer survivors, including 82 cases and 228 matched controls in the initial study set and an additional 25 cases and 54 controls in a replication set.114 That study found marginal associations with a number of SNPs known to be associated with central nervous system tumors in adults, but, similar to the WECARE study, did not clearly demonstrate differences by prior treatment exposures. Overall, the candidate gene studies generally have not had sufficient sample size to conduct analyses stratified by homogenous treatment exposures with specific phenotypes and/or have not replicated results in independent populations.
More recently, several GWAS of subsequent malignancies after radiotherapy have been conducted. A two-stage GWAS investigated risk of any subsequent malignancy after childhood Hodgkin lymphoma, using a discovery set of 96 cases (61% breast cancer) and 82 controls (followed for ≥ 27 years with no subsequent malignancy reported) from the Childhood Cancer Survivor Study and a replication set of 119 cases (89% breast cancer) and 108 controls from high-risk cancer predisposition clinics.32 That study found a significant association with several variants at chromosome 6q21 that were correlated with expression of PRDM1, a zinc finger transcriptional repressor, and radiation-induced MYC repression. Notably, in the replication set, the association was restricted to younger cases and controls.
Another GWAS investigated risk of breast cancer among survivors of any childhood malignancy,33 leveraging data from two large-scale cohort studies of childhood cancer survivors that both have available DNA, detailed treatment data, and long-term, systematic follow-up: the Childhood Cancer Survivor Study115 and the St. Jude Lifetime Cohort.116 Comparing 207 female survivors of European descent who developed breast cancer with 2,774 survivors who had not developed any subsequent malignancy as of the date of last follow-up, the GWAS identified a relatively common locus on 1q41 as well as a rare variant at 11q23 that both appeared to be associated with breast cancer risk, but only among survivors who had received at least 10 Gy radiation exposure to the breast (1q41: rs4342822, nearest gene PROX1, risk allele frequency in controls = 0.46; HR 1.92; 95% CI, 1.49–2.44; p = 7.09 × 10−9; 11q23: rs74949440, TAGLN, risk allele frequency in controls = 0.02; HR 2.59; 95% CI, 1.62–4.16; p = 5.84 × 10−8).33 Because genotyping was conducted in the full cohorts for both the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort, additional analyses of other specific subsequent malignancies are expected to be published in coming years.
Systemic Therapy
Certain cytotoxic agents, particularly alkylating agents, platinum-based compounds, and topoisomerase II inhibitors, are well-established very strong risk factors for chemotherapy-related myelodysplastic syndrome/acute myeloid leukemia (MDS/AML).117 Numerous studies have investigated the genomics of chemotherapy-related MDS/AML, but most have been candidate gene studies related to drug metabolism, DNA damage detection, and DNA repair, as reviewed recently.109,118 Unfortunately, as with other candidate gene studies, few of these results have been investigated or replicated in independent populations.
A single GWAS of chemotherapy-related MDS/AML used a two-stage design (discovery: 80 cases, 150 cancer-free controls; replication: 70 cases, 95 controls).34 Three SNPs were identified as top associations, including rs1394384, which is intronic to ACCN1, encoding an amiloride-sensitive sodium channel; rs1199098, which is in linkage disequilibrium with IPMK, in the inositol phosphokinase family; and rs1381392, which is not near any known genes. Intriguingly, the results were stronger when the cases were restricted to those with chromosome 5 and/or 7 abnormalities, which are typically associated with prior exposure to alkylating agents, but detailed chemotherapy exposure data were not available.
Systemic therapy is also increasingly understood to play a role in risk for solid tumors.106 Elevated risks have been reported for certain alkylating agent-containing regimens with lung cancer and gastrointestinal tract malignancies, for example, whereas gonadotoxic chemotherapy has been associated with decreased risk of developing subsequent breast cancer. In the WECARE study, SNPs in genes associated with metabolism of breast cancer chemotherapeutic agents did not appear to alter the protective effect of chemotherapy on contralateral breast cancer risk.119 Beyond that study, the genomics of chemotherapy-related risks of solid tumors has not been investigated.
FUTURE DIRECTIONS
A major goal in cancer survivorship research is to improve the understanding of factors that contribute to treatment-related toxicities. The establishment of long-term cancer survivorship studies in children and young adults is especially important because patients are often cured and thus remain at lifelong risk for the emergence of either the late effects of cancer therapy or the long-term persistence of acute-onset toxicity.120 The focus of precision cancer medicine in recent years has been on identifying somatic alterations in individual patient tumors and attempting to match those mutations with specific therapeutic strategies.121 To bring precision medicine into survivorship, a better understanding of the impact of germline genetic variation on incidence of treatment-related adverse effects is critical. However, much work remains to realize the promise of precision medicine in cancer survivorship. Above, we reviewed the current state of knowledge surrounding the genomics of both nonmalignant and malignant treatment-related adverse effects. In general, advances in our understanding have been hampered by a number of key methodologic limitations that must be addressed in future studies, and large-scale collaborative efforts are essential.
The difficulty and expense associated with large, prospective pharmacogenomics and radiogenomics studies is the primary challenge facing studies of the genomics of treatment-related adverse effects, which require the collection of DNA, detailed treatment data, and long-term systematic follow-up for well-defined outcomes in large numbers of patients. Increasingly, investigators are therefore leveraging clinic-based cohorts and clinical trials to conduct such studies. For example, the International Radiogenomics Consortium formed with the goal of fostering collaborative efforts to pool data from existing cohorts and clinical trials to increase sample sizes for genetic studies of radiotherapy adverse effects.61 Although these populations may not be wholly representative of all cancer survivors in the general population, they have tremendous potential for advancing the field. In studies of late adverse effects, particularly subsequent malignancies, the need for very long-term follow-up is an added challenge particularly in prospectively designed studies. A number of ongoing cohort studies of cancer survivors, such as the Childhood Cancer Survivor Study, have both detailed treatment data and systematic long-term follow-up. Recently collected DNA make these studies an invaluable contribution to the genomics of survivorship, although biases in sample collection due to either nonresponse or survival bias must be considered in the design of studies and interpretation of results.
A further challenge in the field derives from potential heterogeneity in treatment effects and phenotypes. Studies of the genomics of survivorship would optimally be designed with very well-defined outcomes ascertained in patient populations that are homogeneous with respect to treatment exposures and other key factors that may modify risk for adverse effects, such as age at exposure. An example of this is the Platinum Study, an international consortium of cancer centers that studies cisplatin-treated testicular cancer survivors. The study includes detailed collection of dose information for all drugs, systematic evaluation of human hearing using state of the art audiometric methods, and collection of extensive health information related to other toxicities.122 The REQUITE study aims to achieve a similar goal in radiogenomics, by using standardized data collection and patient reported outcome forms to prospectively assess late radiation toxicity in patients with breast, lung, and prostate cancer.62 With the exception of some cancers, the last several decades have seen dramatic changes in treatment approaches, including changes in doses or intensity of therapy, development of novel radiotherapy techniques, and introduction of new systemic therapies, but the impacts of these changes on risk for adverse effects is often poorly understood, and the joint effects with genetic variants are unknown. For example, advances in radiation delivery technology and treatment planning have allowed clinicians to optimize radiotherapy, but newer delivery methods can result in more variable dose distribution in surrounding normal tissues, with larger volumes receiving low doses.123 The effect of genetic factors on toxicity risk may differ depending on the radiation dose and volume of tissue exposed. Radiation and systemic cancer therapies can interact and synergistically impact adverse effects, and this interplay is becoming increasingly complex with the introduction of targeted biologics and immunotherapies. The studies described above for chemotherapy-induced peripheral neuropathy and ototoxicity emphasize the importance of conducting studies in patients with homogeneous treatment exposures, since different variants have been identified for the same chemotherapeutic-induced toxicity. Additionally, differences in the variants identified for specific phenotypes (e.g., specific types of subsequent malignancies) highlights the importance of studying well-defined outcomes.
The lack of available data and potential heterogeneity in treatment effect impact the likelihood of discovering genetic factors that influence treatment-related adverse effects. An important determinant of statistical power is the estimated effect size. Currently, complex human traits and diseases are generally thought to be polygenic, with heritable components arising from a combination of rare variants with relatively strong effects and many common variants, each of which has a weak effect.124 The extent to which this paradigm is applicable to the genomics of treatment-related adverse effects is as yet unknown but has important implications for statistical power because it is difficult to imagine large-scale survivorship studies of more than 10,000 patients with a specific adverse effect. However, to date, some of the GWAS of treatment-related adverse effects have identified common variants with relatively high risk estimates (> twofold risk per allele, compared with 1.1- to 1.2-fold for most adult sporadic diseases) consistent with studies demonstrating pharmacogenomic studies tend to have larger effect sizes, as described above.16
Once genetic variants associated with specific adverse events have been identified, risk prediction models constructed in independent patient populations are needed, combining genetic information with current models based primarily on treatment exposures. Evidence is beginning to emerge from modeling and experimental approaches that supports the hypothesis that incorporation of genetic or other biologic data into toxicity risk prediction models can result in improvements in sensitivity and specificity. For example, a data simulation study showed that incorporation of SNPs can improve the area under the receiver operating characteristic curve of a radiation dose-based model, and the same concept would apply to a model of systemic therapy.125 As expected, the magnitude of improvement increased with increasing number of SNPs and was affected by assumptions about minor allele frequency, effect size, and toxicity prevalence. If the majority of risk SNPs has very modest effects sizes (< 1.2), hundreds of SNPs are needed to substantially improve models; in contrast, if some risk SNPs have larger risk estimates (1.2–2.0), fewer than 100 may be sufficient to improve models to an extent that is clinically meaningful. With large-scale collaboration for variant discovery, validation, and translation into risk prediction models, a substantial number of patients have the potential to benefit from precision medicine not only in selecting the optimal therapeutic strategy to shrink or eradicate their tumor, but also to estimate their acute and long-term treatment-related risks.
PRACTICAL APPLICATIONS.
Certain adverse effects of cancer therapy are thought to have a significant heritable component.
Most previous research on germline genetic susceptibility to adverse effects has narrowly focused on variation in candidate genes and pathways, and few of the findings have been replicated in independent populations.
Advances in understanding of the genome, improvements in technology, and reduction in laboratory costs have led to recent genome-wide studies, which agnostically interrogate common and/or rare variants across the entire genome, enabling identification of novel genes and pathways that may impact the development of adverse effects.
Large-scale collaborative eff orts are essential for replicating results from genomics studies of adverse treatment effects to translate the findings into clinical practice.
In the long-term, validated germline genetic associations hold tremendous promise for more precisely identifying patients at highest risk for developing adverse treatment effects, with implications for frontline therapy decision-making, personalization of long-term follow-up guidelines, and potential identification of targets for prevention or treatment of the toxicity.
Footnotes
Disclosures of potential conflicts of interest provided by the authors are available with the online article at asco.org/edbook.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 2.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010; 76:S10–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight KR, Chen L, Freyer D, et al. Group-wide, prospective study of ototoxicity assessment in children receiving cisplatin chemotherapy (ACCL05C1): a report from the Children’s Oncology Group. J Clin Oncol. 2017;35:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisina RD, Wheeler HE, Fossa SD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol. 2016;34:2712–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohr PE, Feldman JJ, Dunbar JL. The societal costs of severe to profound hearing loss in the United States. Policy Anal Brief H Ser. 2000;2:1–4. [PubMed] [Google Scholar]
- 6.Puts MT, Tapscott B, Fitch M, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41:197–215. [DOI] [PubMed] [Google Scholar]
- 7.Rahman N Realizing the promise of cancer predisposition genes. Nature. 2014;505:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung CC, Chanock SJ. Current status of genome-wide association studies in cancer. Hum Genet. 2011;130:59–78. [DOI] [PubMed] [Google Scholar]
- 10.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frazer KA, Ballinger DG, Cox DR, et al. ; International HapMap Consortium. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consortium EP; ENCODE Project Consortium. The ENCODE (ENCyclopedia Of DNA Elements) Project. Science. 2004;306:636–640. [DOI] [PubMed] [Google Scholar]
- 14.Consortium EP; ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012; 489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abecasis GR, Auton A, Brooks LD, et al. ; 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012:491:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maranville JC, Cox NJ. Pharmacogenomic variants have larger effect sizes than genetic variants associated with other dichotomous complex traits. Pharmacogenomics J. 2016;16:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerns SL, Ostrer H, Stock R, et al. Genome-wide association study to identify single nucleotide polymorphisms (SNPs) associated with the development of erectile dysfunction in African-American men after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett GC, Thompson D, Fachal L, et al. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother Oncol. 2014;111:178–185. [DOI] [PubMed] [Google Scholar]
- 19.Fachal L, Gómez-Caamaño A, Barnett GC, et al. A three-stage genome-wide association study identifies a susceptibility locus for late radiotherapy toxicity at 2q24.1. Nat Genet. 2014;46:891–894. [DOI] [PubMed] [Google Scholar]
- 20.Kerns SL, Dorling L, Fachal L, et al. ; Radiogenomics Consortium. Meta-analysis of genome wide association studies identifies genetic markers of late toxicity following radiotherapy for prostate cancer. EBioMedicine. 2016;10:150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leandro-García LJ, Inglada-Pérez L, Pita G, et al. Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J Med Genet. 2013; 50:599–605. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin RM, Owzar K, Zembutsu H, et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res. 2012;18:5099–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider BP, Li L, Radovich M, et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21:5082–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hertz DL, Owzar K, Lessans S, et al. Pharmacogenetic discovery in CALGB (Alliance) 90401 and mechanistic calidation of a VAC14 polymorphism that increases risk of docetaxel-induced neuropathy. Clin Cancer Res. 2016;22:4890–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Won HH, Lee J, Park JO, et al. Polymorphic markers associated with severe oxaliplatin-induced, chronic peripheral neuropathy in colon cancer patients. Cancer. 2012;118:2828–2836. [DOI] [PubMed] [Google Scholar]
- 27.Magrangeas F, Kuiper R, Avet-Loiseau H, et al. A genome-wide association study identifies a novel locus for bortezomib-induced peripheral neuropathy in European patients with multiple myeloma. Clin Cancer Res. 2016;22:4350–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campo C, da Silva Filho MI, Weinhold N, et al. Bortezomib-induced peripheral neuropathy: A genome-wide association study on multiple myeloma patients. Hematol Oncol. 2018:236:232–237. [DOI] [PubMed] [Google Scholar]
- 29.Dolan ME, El Charif O, Wheeler HE, et al. ; Platinum Study Group. Clinical and genome-wide analysis of cisplatin-induced peripheral neuropathy in survivors of adult-onset cancer. Clin Cancer Res. 2017;23:5757–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler HE, Gamazon ER, Frisina RD, et al. Variants in WFS1 and other Mendelian deafness genes are associated with cisplatin-associated ototoxicity. Clin Cancer Res. 2017;23:3325–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Robinson GW, Huang J, et al. Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nat Genet. 2015;47:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Best T, Li D, Skol AD, et al. Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med. 2011;17:941–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morton LM, Sampson JN, Armstrong GT, et al. Genome-wide association study to identify susceptibility loci that modify radiation-related risk for breast cancer after childhood cancer. J Natl Cancer Inst. 2017;109:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight JA, Skol AD, Shinde A, et al. Genome-wide association study to identify novel loci associated with therapy-related myeloid leukemia susceptibility. Blood. 2009;113:5575–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010; 76:S58–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Troffi A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. [DOI] [PubMed] [Google Scholar]
- 37.Cooper JS, Fu K, Marks J, et al. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31:1141–1164. [DOI] [PubMed] [Google Scholar]
- 38.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010; 76:S101–S107. [DOI] [PubMed] [Google Scholar]
- 39.Marks LB, Carroll PR, Dugan TC, et al. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257–1280. [DOI] [PubMed] [Google Scholar]
- 40.Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnet NG, Johansen J, Turesson I, et al. Describing patients’ normal tissue reactions: concerning the possibility of individualising radiotherapy dose prescriptions based on potential predictive assays of normal tissue radiosensitivity. Int J Cancer. 1998;79:606–613. [DOI] [PubMed] [Google Scholar]
- 42.Holthusen H Erfahrungen uber die Vertraglichkeitsgrenze fur Rontgen-strahlen and deren Nutzanwendung zur Verhutung von Schaden. Strahlentherapie. 1936;57:254–269. [Google Scholar]
- 43.Gaffi RA, Berkel I, Boder E, et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22–23. Nature. 1988;336:577–580. [DOI] [PubMed] [Google Scholar]
- 44.Gaffi RA. The inherited basis of human radiosensitivity. Acta Oncol. 2001;40:702–711. [DOI] [PubMed] [Google Scholar]
- 45.Pollard JM, Gaffi RA. Clinical radiation sensitivity with DNA repair disorders: an overview. Int J Radiat Oncol Biol Phys. 2009;74:1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curwen GB, Winther JF, Tawn EJ, et al. G(2) chromosomal radiosensitivity in Danish survivors of childhood and adolescent cancer and their offspring. Br J Cancer. 2005;93:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curwen GB, Cadwell KK, Winther JF, et al. The heritability of G2 chromosomal radiosensitivity and its association with cancer in Danish cancer survivors and their offspring. Int J Radiat Biol. 2010;86:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finnon P, Robertson N, Dziwura S, et al. Evidence for significant heritability of apoptotic and cell cycle responses to ionising radiation. Hum Genet. 2008;123:485–493. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz A, Bayer J, Dechamps N, et al. Heritability of susceptibility to ionizing radiation-induced apoptosis of human lymphocyte subpopulations. Int J Radiat Oncol Biol Phys. 2007;68:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surowy H, Rinckleb A, Luedeke M, et al. Heritability of baseline and induced micronucleus frequencies. Mutagenesis. 2011;26:111–117. [DOI] [PubMed] [Google Scholar]
- 51.Safwat A, Bentzen SM, Turesson I, et al. Deterministic rather than stochastic factors explain most of the variation in the expression of skin telangiectasia after radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:198–204. [DOI] [PubMed] [Google Scholar]
- 52.Andreassen CN, Alsner J, Overgaard J. Does variability in normal tissue reactions after radiotherapy have a genetic basis--where and how to look for it? Radiother Oncol. 2002;64:131–140. [DOI] [PubMed] [Google Scholar]
- 53.Edvardsen H, Landmark-H0yvik H, Reinertsen KV, et al. SNP in TXNRD2 associated with radiation-induced fibrosis: a study of genetic variation in reactive oxygen species metabolism and signaling. Int J Radiat Oncol Biol Phys. 2013;86:791–799. [DOI] [PubMed] [Google Scholar]
- 54.Talbot CJ, Tanteles GA, Barnett GC, et al. A replicated association between polymorphisms near TNFa and risk for adverse reactions to radiotherapy. Br J Cancer. 2012;107:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang Q, Wei Q, Xu T, et al. Functional promoter variant rs2868371 of HSPB1 is associated with risk of radiation pneumonitis after chemoradiation for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1332–1339. [DOI] [PubMed] [Google Scholar]
- 56.Lopez Guerra JL, Wei Q, Yuan X, et al. Functional promoter rs2868371 variant of HSPB1 associates with radiation-induced esophageal toxicity in patients with non-small-cell lung cancer treated with radio(chemo) therapy. Radiother Oncol. 2011;101:271–277. [DOI] [PubMed] [Google Scholar]
- 57.Andreassen CN, Rosenstein BS, Kerns SL, et al. ; International Radiogenomics Consortium (RgC). Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother Oncol. 2016;121:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guerra JL, Gomez D, Wei Q, et al. Association between single nucleotide polymorphisms of the transforming growth factor β1 gene and the risk of severe radiation esophagitis in patients with lung cancer. Radiother Oncol. 2012;105:299–304. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Yang M, Bi N, et al. Association of TGF-ß1 and XPD polymorphisms with severe acute radiation-induced esophageal toxicity in locally advanced lung cancer patients treated with radiotherapy. Radiother Oncol. 2010;97:19–25. [DOI] [PubMed] [Google Scholar]
- 60.Barnett GC, Elliott RM, Alsner J, et al. Individual patient data meta-analysis shows no association between the SNP rs1800469 in TGFB and late radiotherapy toxicity. Radiother Oncol. 2012;105:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.West C, Rosenstein BS, Alsner J, et al. ; EQUAL-ESTRO. Establishment of a radiogenomics consortium. Int J Radiat Oncol Biol Phys. 2010;76: 1295–1296. [DOI] [PubMed] [Google Scholar]
- 62.West C, Azria D, Chang-Claude J, et al. The REQUITE project: validating predictive models and biomarkers of radiotherapy toxicity to reduce side-effects and improve quality of life in cancer survivors. Clin Oncol (R Coll Radiol). 2014;26:739–742. [DOI] [PubMed] [Google Scholar]
- 63.Argyriou AA, Bruna J, Marmiroli P, et al. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. [DOI] [PubMed] [Google Scholar]
- 64.Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013; 63:419–437. [DOI] [PubMed] [Google Scholar]
- 65.Brewer JR, Morrison G, Dolan ME, et al. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hershman DL, Laccheffi C, Dworkin RH, et al. ; American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. [DOI] [PubMed] [Google Scholar]
- 67.van Gerven JM, Moll JW, van den Bent MJ, et al. Paclitaxel (taxol) induces cumulative mild neurotoxicity. Eur J Cancer. 1994;30:1074–1077. [DOI] [PubMed] [Google Scholar]
- 68.Mielke S, Sparreboom A, Steinberg SM, et al. Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin Cancer Res. 2005;11:4843–4850. [DOI] [PubMed] [Google Scholar]
- 69.Schneider BP, Zhao F, Wang M, et al. Neuropathy is not associated with clinical outcomes in patients receiving adjuvant taxane-containing therapy for operable breast cancer. J Clin Oncol. 2012;30:3051–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oldenburg J, Kraggerud SM, Bryd0y M, et al. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goekkurt E, Al-Batran SE, Hartmann JT, et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol. 2009;27:2863–2873. [DOI] [PubMed] [Google Scholar]
- 72.Lamba JK, Fridley BL, Ghosh TM, et al. Genetic variation in platinating agent and taxane pathway genes as predictors of outcome and toxicity in advanced non-small-cell lung cancer. Pharmacogenomics. 2014;15:1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim HS, Kim MK, Chung HH, et al. Genetic polymorphisms affecting clinical outcomes in epithelial ovarian cancer patients treated with taxanes and platinum compounds: a Korean population-based study. Gynecol Oncol. 2009;113:264–269. [DOI] [PubMed] [Google Scholar]
- 74.Sissung TM, Mross K, Steinberg SM, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42:2893–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leskelä S, Jara C, Leandro-García LJ, et al. Polymorphisms in cytochromes P450 2C8 and 3A5 are associated with paclitaxel neurotoxicity. Pharmacogenomics J. 2011;11:121–129. [DOI] [PubMed] [Google Scholar]
- 76.Leandro-García LJ, Leskelä S, Jara C, et al. Regulatory polymorphisms in β-tubulin Ila are associated with paclitaxel-induced peripheral neuropathy. Clin Cancer Res. 2012;18:4441–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sucheston LE, Zhao H, Yao S, et al. Genetic predictors of taxane-induced neurotoxicity in a SWOG phase III intergroup adjuvant breast cancer treatment trial (S0221). Breast Cancer Res Treat. 2011;130:993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hertz DL, Roy S, Motsinger-Reif AA, et al. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann Oncol. 2013;24:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sissung TM, Baum CE, Deeken J, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14:4543–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terrazzino S, Argyriou AA, Cargnin S, et al. Genetic determinants of chronic oxaliplatin-induced peripheral neurotoxicity: a genome-wide study replication and meta-analysis. J Peripher Nerv Syst. 2015;20: 15–23. [DOI] [PubMed] [Google Scholar]
- 81.Boora GK, Kanwar R, Kulkarni AA, et al. Testing of candidate single nucleotide variants associated with paclitaxel neuropathy in the trial NCCTG N08C1 (Alliance). Cancer Med. 2016;5:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stock W, Diouf B, Crews KR, et al. An inherited genetic variant in CEP72 promoter predisposes to vincristine-induced peripheral neuropathy in adults with acute lymphoblastic leukemia. Clin Pharmacol Ther. 2017;101:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patidar PL, Motea EA, Fattah FJ, et al. The Kub5-Hera/RPRD1B interactome: a novel role in preserving genetic stability by regulating DNA mismatch repair. Nucleic Acids Res. 2016;44:1718–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grewal S, Merchant T, Reymond R, et al. Auditory late effects of childhood cancer therapy: a report from the Children’s Oncology Group. Pediatrics. 2010;125:e938–e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. [DOI] [PubMed] [Google Scholar]
- 86.Ruggiero A, Trombatore G, Triarico S, et al. Platinum compounds in children with cancer: toxicity and clinical management. Anticancer Drugs. 2013;24:1007–1019. [DOI] [PubMed] [Google Scholar]
- 87.Ross CJ, Katzov-Eckert H, Dubé MP, et al. ; CPNDS Consortium. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–1349. [DOI] [PubMed] [Google Scholar]
- 88.Lanvers-Kaminsky C, Sprowl JA, Malath I, et al. Human OCT2 variant c.808G>T confers protection effect against cisplatin-induced ototoxicity. Pharmacogenomics. 2015;16:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang JJ, Lim JY, Huang J, et al. The role of inherited TPMT and COMT genetic variation in cisplatin-induced ototoxicity in children with cancer. Clin Pharmacol Ther. 2013;94:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pussegoda K, Ross CJ, Visscher H, et al. ; CPNDS Consortium. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin Pharmacol Ther. 2013;94:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ratain MJ, Cox NJ, Henderson TO. Challenges in interpreting the evidence for genetic predictors of ototoxicity. Clin Pharmacol Ther. 2013;94:631–635. [DOI] [PubMed] [Google Scholar]
- 92.Carleton BC, Ross CJ, Bhavsar AP, et al. Role of TPMT and COMT genetic variation in cisplatin-induced ototoxicity. Clin Pharmacol Ther. 2014;95:253. [DOI] [PubMed] [Google Scholar]
- 93.Glendenning JL, Barbachano Y, Norman AR, et al. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116:2322–2331. [DOI] [PubMed] [Google Scholar]
- 94.Brydøy M, Oldenburg J, Klepp O, et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 2009;101: 1682–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schell MJ, McHaney VA, Green AA, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol. 1989;7:754–760. [DOI] [PubMed] [Google Scholar]
- 97.Theunissen EA, Zuur CL, Józwiak K, et al. Prediction of hearing loss due to cisplatin chemoradiotherapy. JAMA Otolaryngol Head Neck Surg. 2015;141:810–815. [DOI] [PubMed] [Google Scholar]
- 98.Zuur CL, Simis YJ, Lansdaal PE, et al. Ototoxicity in a randomized phase Ill trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol. 2007;25:3759–3765. [DOI] [PubMed] [Google Scholar]
- 99.Chen WC, Jackson A, Budnick AS, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106:820–829. [DOI] [PubMed] [Google Scholar]
- 100.Vos HI, Guchelaar HJ, Gelderblom H, et al. Replication of a genetic variant in ACYP2 associated with cisplatin-induced hearing loss in patients with osteosarcoma. Pharmacogenet Genomics. 2016;26:243–247. [DOI] [PubMed] [Google Scholar]
- 101.Thiesen S, Yin P, Jorgensen AL, et al. TPMT, COMT and ACYP2 genetic variants in paediatric cancer patients with cisplatin-induced ototoxicity. Pharmacogenet Genomics. 2017;27:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drögemöller BI, Brooks B, Critchley C, et al. Further investigation of the role of ACYP2 and WFS1 pharmacogenomic variants in the development of cisplatin-induced ototoxicity in testicular cancer patients. Clin Cancer Res. Epub 2018. January 22. [DOI] [PubMed] [Google Scholar]
- 103.Mapes B, El Charif O, Al-Sawwaf S, et al. Genome-wide association studies of chemotherapeutic toxicities: genomics of inequality. Clin Cancer Res. 2017;23:4010–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Inskip PD, Sigurdson AJ, Veiga L, et al. Radiation-related new primary solid cancers in the Childhood Cancer Survivor Study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys. 2016;94:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morton LM, Swerdlow AJ, Schaapveld M, et al. Current knowledge and future research directions in treatment-related second primary malignancies. EJCSuppl. 2014;12:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Travis LB, Demark Wahnefried W, Allan JM, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10:289–301. [DOI] [PubMed] [Google Scholar]
- 108.Wood ME, Vogel V, Ng A, et al. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30:3734–3745. [DOI] [PubMed] [Google Scholar]
- 109.Bhatia S Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer. 2015;121:648–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bernstein JL, Langholz B, Haile RW, et al. ; WECARE study. Study design: evaluating gene-environment interactions in the etiology of breast cancer - the WECARE study. Breast Cancer Res. 2004;6:R199–R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernstein JL, Haile RW, Stovall M, et al. ; WECARE Study Collaborative Group. Radiation exposure, the ATM Gene, and contralateral breast cancer in the women’s environmental cancer and radiation epidemiology study. J Natl Cancer Inst. 2010;102:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bernstein JL, Thomas DC, Shore RE, et al. ; WECARE Study Collaborative Group. Contralateral breast cancer after radiotherapy among BRCA1 and BRCA2 mutation carriers: a WECARE study report. Eur J Cancer. 2013;49:2979–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Robson ME, Reiner AS, Brooks JD, et al. ; WECARE Study Collaborative Group. Association of common genetic variants with contralateral breast cancer risk in the WECARE Study. J Natl Cancer Inst. 2017;109:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X, Sun CL, Hageman L, et al. Clinical and genetic risk prediction of subsequent CNS tumors in survivors of childhood cancer: A report from the COG ALTE03N1 study. J Clin Oncol. 2017;35:3688–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol. 2007;137:513–529. [DOI] [PubMed] [Google Scholar]
- 119.Brooks JD, Teraoka SN, Bernstein L, et al. ; WECARE Study Collaborative Group. Common variants in genes coding for chemotherapy metabolizing enzymes, transporters, and targets: a case-control study of contralateral breast cancer risk in the WECARE Study. Cancer Causes Control. 2013;24:1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Letai A Functional precision cancer medicine-moving beyond pure genomics. Nat Med. 2017;23:1028–1035. [DOI] [PubMed] [Google Scholar]
- 122.Travis LB, Beard C, Allan JM, et al. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010; 102:1114–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kerns SL, Kundu S, Oh JH, et al. The prediction of radiotherapy toxicity using single nucleotide polymorphism-based models: a step toward prevention. Semin Radiat Oncol. 2015;25:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]