Abstract
Purpose of Review
This article will review the current techniques in cardiac magnetic resonance imaging (CMR) for diagnosing and assessing primary valvular heart disease.
Recent Findings
The recent advancements in CMR have led to an increased role of this modality for qualifying and quantifying various native valve diseases. Phase-contrast velocity encoded imaging is a well-established technique that can be used to quantify aortic and pulmonic flow. This technique, combined with the improved ability for CMR to obtain accurate left and right ventricular volumetrics, has allowed for increased accuracy and reproducibility in assessing valvular dysfunction. Advancements in CMR technology also allows for improved spatial and temporal resolution imaging of various valves and their regurgitant or stenotic jets. Therefore, CMR can be a powerful tool in evaluation of native valvular heart disease.
Summary
The role of CMR in assessing valvular heart disease is growing and being recognized in recent guidelines. CMR has the ability to assess valve morphology along with qualifying and quantifying valvular disease. In addition, the ability to obtain accurate volumetric measurements may improve more precise management strategies and may lead to improvements in mortality and morbidity.
Keywords: Cardiac magnetic resonance imaging, Cardiac valve disease, Mitral regurgitation, Phase-contrast imaging, Velocity encoded imaging, Aortic regurgitation
Introduction
With the aging population and greater survival of patients with heart failure, more patients are suffering from complications related to valvular heart disease. Up to 11.7% of people 75 years or older in the USA are afflicted by moderate to severe valvular heart disease [1]. Cardiac magnetic resonance imaging (CMR) provides a comprehensive evaluation of cardiac anatomy, function, and myocardial tissue characterization and is increasingly being used to assess valvular heart disease. The utility of CMR for the evaluation of valvular regurgitation has recently been recognized as part of the joint American Society of Echocardiography (ASE) and the Society of Cardiovascular Magnetic Resonance (SCMR) recommendations for the noninvasive evaluation of native valvular regurgitation [2••]. In previous guidelines, CMR was only advocated in valvular disease in situations where echocardiographic evaluation was insufficient or inconclusive [3]. However, with excellent spatial and temporal resolution, CMR may provide added benefits for valvular evaluation, including visualization of valve structure, direct measurements of flow, assessment of ventricular volumes, and quantification of regurgitant volumes and fractions [4]. In this article, we will comprehensively review the use of CMR for the evaluation of the structure and function of native heart valves.
MRI Techniques for Valvular Assessment and Flow Quantification
Assessment of Valve Structure
One of the primary advantages to CMR for valvular assessment is the ability to visualize the morphology and motion of each valve from any desired image orientation. Balanced steady-state free-precession (SSFP) sequences are “bright blood” cine imaging techniques (Fig. 1) which offer high signal-to-noise ratio and excellent contrast between the blood pool and myocardium and have become widely used for the evaluation of valvular structures in motion [4–8].
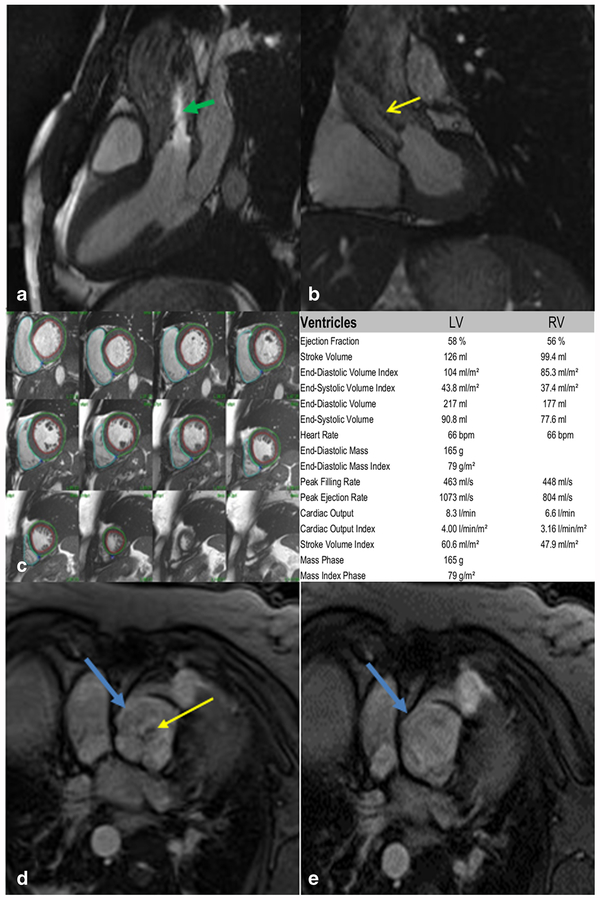
Fig. 1.
a Three-chamber steady-state free precession (SSFP) cine imaging of a bicuspid aortic valve. Note the bright signal (green arrow) indicating a turbulent jet through the stenotic valve. b LVOT SSFP imaging for the same stenotic bicuspid aortic valve. Low-signal void and dephasing is indicated by the yellow arrow. c Short-axis SSFP cine stacks with regions of interest drawn in the LV endocardium, LVepicardium, and RVendocardium. Post-processing analysis software can allow for calculation of ejection fraction and volumes. d Gradient echo (GRE) of an incompetent quadricuspid aortic valve (blue arrow) in the diastolic phase with a regurgitant jet. e GRE of the same quadricuspid valve in the systolic phase
Cine imaging requires either prospective or retrospective electrocardiographic (ECG) gated data acquisition over multiple cardiac cycles during a respiratory breath-hold [9, 10]. These images can be obtained in a series of short-axis or long-axis slices with a spatial resolution of 1.2–1.5 mm per pixel and a temporal resolution of 20–40 ms per cardiac phase [2••, 11]. However, cine imaging is limited by relatively thick slices of 5 to 8 mm, which can lead to partial volume effects and hinders the visualization of thin structures or small vegetations [7, 12]. Therefore, precise placement of the imaging slice perpendicular to the valve plane with a slice thickness of 4 to 6 mm is of critical importance to minimize partial volume effects. Another limitation of cine SSFP imaging is sensitivity to arrhythmias, as ECG-gating is used to combine data over multiple successive heartbeats. There are also older sequences that can be used to assess valve anatomy, such as “black blood” turbo-spin-echo (TSE) techniques [5, 13]. T1-weighted and T2-weighted TSE imaging techniques have been used for the assessment of valvular masses or vegetations [14–16].
Assessment of Ventricular Volumes and Function
The decision to intervene in valvular disease is determined by both the severity of valvular heart disease, and assessment of ventricular dimensions, ventricular volumes, and ejection fraction. As such, quantification of LV size and volumes should be an integral part of a comprehensive valvular assessment by CMR [3]. SSFP techniques have been well validated for the quantification of ventricular volumes, function, and assessment of myocardial masses [17, 18]. Ventricular volumes are determined from a short-axis stack of 6–8 mm thick slices with an interslice gap of −4 mm [11]. The short-axis stacks are analyzed using post-processing software which allows the user to draw endocardial and epicardial borders of the left ventricle and endocardial border of the right ventricle as seen in Fig. 1c. The software then uses the Simpson method to calculate ventricular volumes, ejection fractions, and myocar-dial mass. Axial stacks with a slice thickness of 4–8 mm and interslice gap of 0 to 20% may be more accurate and reproducible for the assessment of right ventricle (RV) volumes [19, 20].
Flow Visualization
Another advantage of CMR cine sequences is the ability to visualize post stenotic and regurgitant blood flow. Qualitative analysis is based on the visualization of flow voids, which result from intravoxel spin dephasing due to turbulent flow [21]. Magnetic spins, moving along the direction of a magnetic gradient, acquire phase shifts depending on the velocity of the spin. In the case of turbulent flow, there are spins with different phase shifts leading to dephasing of the net magnetization and signal loss that is more easily seen in gradient echo imaging (GRE) [5, 21]. Cine GRE is an older CMR “bright blood” cine imaging technique which has reduced myocardial-blood contrast, lower signal-to-noise ratio, and longer acquisition times compared to SSFP (Fig. 1d, e) [22]. The major advantage of GRE imaging compared to SSFP for qualitative valvular assessment is the increased spin dephasing effect which can improve sensitivity for detecting flow abnormalities [23, 24]. In valvular stenosis, a vena contracta can be measured as a high-signal central jet of laminar flow surrounded by low signal flow voids of turbulent flow; however, this technique is not commonly used or recommended [25]. Regurgitant jets can be visualized as dark flow voids originating from the malfunctioning valve [26]. Semi-quantitative assessment of regurgitation by visual assessment of flow voids on CMR imaging has excellent correlation with Doppler echocardiography and cardiac catheterization with > 90% sensitivity and specificity for the evaluation of aortic valve and mitral valve regurgitation [15, 23, 27, 28]. It is critical to examine flow voids in multiple planes as to evaluate the extent of regurgitant or stenotic flow, as sometimes, the eccentric jets can lead to incomplete characterization and inaccurate semi-quantitative assessments [26, 29].
Flow Quantification
Flow quantification can be performed using specific CMR sequences and post-processing software. Phase-contrast velocity encoding is a technique that utilizes velocity-encoding (VENC) gradients to generate a phase shift in the magnetic resonance imaging signal, which is proportional to the velocity of the moving protons. Phase velocity images display the velocity of spins (positive and negative) in the direction of the applied velocity encoding gradients for each voxel. The VENC is an operator-controlled parameter which defines the maximum velocity that can be encoded without aliasing (similar to the aliasing velocity in pulse-wave color Doppler echocardiography). The phase-velocity images are typically displayed such that zero velocity is gray, and the maximum velocities in either direction and encoded as black or white respectively (Fig. 2) [30, 31]. These two-dimensional phase-velocity images can have velocity encoded in the “in-plane” or “through-plane direction.” The in-plane sequence allows for visualization of the stenotic or regurgitant jet and allow for planning of the “through-plane” phase-contrast image to quantify blood velocity and flow [7]. These images can be processed using specialized software to calculate the velocity, volume, and direction of flow, thus allowing for quantification of stenosis or regurgitation [32]. There are some limitations to phase-contrast velocity imaging such as a limited temporal resolution of 20–25 ms, which is much lower than continuous wave Doppler echocardiography (2 m). However, the temporal resolution is usually sufficient for most velocity and flow measurements [33]. Another limitation is that the dephasing due to turbulent motion described above results in a loss of signal resulting from high-velocity turbulent flows. This limits the accuracy for velocities greater than 3.5 m/s. This may be problematic when assessing severity of aortic stenosis, or peak mitral regurgitation velocity [34, 35, 36]. For quantification of peak velocities, it is important to insure that the VENC direction is collinear with the direction of flow, or peak velocities may be underestimated. An advantage of quantifying flow with VENC imaging over Doppler-based flow measurements is that it does not require any geometric assumptions, as the velocity is known at each pixel location, and the number of pixels within a region of interest (ROI) is known. As such, CMR flow measurements have less angular dependence than Doppler techniques for quantifying flow. One other caveat for quantifying flow is that the slice location is fixed in space, and the valve moves relative to the slice, meaning that the velocities are not measured at the same anatomic location throughout the cardiac cycle. This may lead to some challenges when directly quantifying tricuspid or mitral flow but is usually not much of an issue for quantifying flow in the proximal aorta and pulmonary artery. Despite these potential issues and caveats, CMR flow quantification compares favorably with invasive hemodynamic measurements [37, 38].
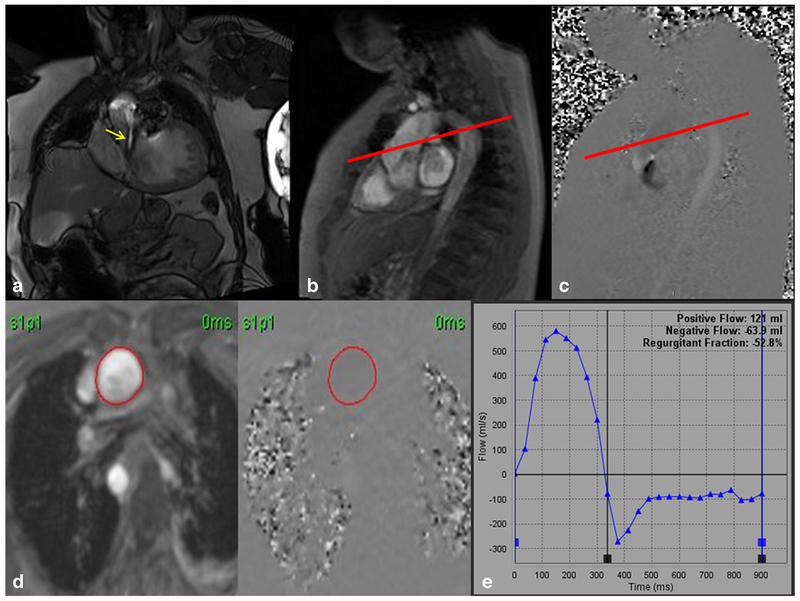
Fig. 2.
a Steady-state free precession (SSFP) left ventricular outflow tract (LVOT) cine imaging of a quadricuspid aortic valve with severe aortic regurgitation (yellow arrow). b, c “In-plane” phase-contrast velocity encoded imaging. The in-plane image allows for perpendicular planning (red line) to obtain through-plane images above the aortic valve leaflets. d Phase-contrast velocity encoded through plane images with a region of interest drawn in the aorta. e Plotted graph of forward flow and regurgitant flow with calculated regurgitant fraction of 52.8% and regurgitant volume of 63.9 mL indicating severe aortic regurgitation
Aortic Valve
Aortic Regurgitation
CMR offers a comprehensive evaluation of the aorta, aortic valve, and left ventricle (LV) to compliment echocardiography in aortic regurgitation (AR) evaluation. This includes quantification of LV volume and function, assessment for evidence of LV remodeling, assessment of aortic valve (AV) morphology to help determine mechanism of AR, quantification of AR by determining the aortic regurgitant volume (RVol), and assessment of the aorta for potential aortopathy. Recent guidelines have suggested that CMR is indicated for AR assessment when (1) echo images are suboptimal, (2) discordance exists between echo parameters, (3) discordance exists between clinical assessment and severity of AR by echocardiography, (4) patients with moderate/severe AR and suboptimal echocardiographic assessment of LV volumes and systolic function, and (5) patients with bicuspid aortic valves and inadequate aorta assessment by echocardiography [2••].
Cine Imaging for Valve Morphology and LV Volumes
SSFP and GRE cine images can be used for visual assessment of the AV, aortic root anatomy, and quantification of AR. CMR is the gold standard for LV chamber quantification. Two-dimensional (2D) and three-dimensional (3D) echocardiography tends to underestimate LV volumes as compared to CMR [2••, 39]. An LV end diastolic volume > 246 mL by CMR was shown in a multicenter observational study to predict patients that would need future AV replacement during a mean follow-up of 2.6 years [40].
CMR Quantification of AR Severity
The preferred method to quantify AR severity by CMR is to quantify the RVol which can be assessed even in the presence of coexisting valvular lesions. Flow through the aortic valve can be measured with phase-contrast velocity mapping in a plane perpendicular to the aorta. The imaging slice should be halfway between aortic annulus and sinotubular junction, near the leaflet tips in the sinuses orthogonal to aortic flow in two imaging planes [41, 42, 43]. Chatzimavroudis et al. previously demonstrated that the ascending aorta slice location with the most accurate measurement of AR Rvol is between the AV annulus and coronary ostia [41]. Above this location, coronary flow and aortic compliance negatively affects accuracy of measurements [44]. In the absence of obvious stenosis, a maximum VENC of 150 or 200 cm/s is typically sufficient. If aliasing occurs, then the VENC can be increased by 50 cm/s until aliasing does not occur. This allows for a more accurate assessment of peak velocity in the aorta. Some software packages determine peak velocity in one pixel in the ROI and others take the peak velocity of the average of a few adjacent pixels in the ROI; thus, this must be taken into consideration when quantifying peak velocities [45]. Aortic Rvol is calculated from the area under the retrograde diastolic flow curve as shown in Fig. 2. In the absence of pulmonic regurgitation, aortic RVol can also be calculated by subtracting the pulmonic forward flow, as assessed by phase-contrast velocity imaging of the pulmonary artery, from the aortic forward flow [2••, 46]. Regurgitant fraction (RF) is calculated with the following equation: RF = (regurgitant volume/forward volume) × 100%. Repeating the phase-contrast sequences of the aorta multiple times allows for assessment of consistent results and identification of artifacts or erroneous measurements, which can occur in the presence of cardiac arrhythmias.
Gelfand et al. previously found that CMR-RF thresholds for AR that had maximal agreement with echo were mild ≤ 15%, moderate 16–27%, and severe > 27% [47]. Myerson et al. found that an RF threshold of > 33% and an RVol threshold > 42 ml were strong predictors of patients with asymptomatic chronic AR who would develop indications for surgical replacement during a mean follow-up of 2.6 years [40]. These studies suggest that clinical trials are needed to better define CMR-specific thresholds of RF and RVol used to define severe AR by CMR, as these may be different than the values that are measured with echocardiography [2••]. As per the 2014 ACC/AHA guidelines, current values for echoc ardiographic-defined severe AR are RF > 50% and RVol > 60, which are the values commonly used today for CMR quantification [3].
Mitral Valve
Mitral Regurgitation
Current guidelines recommend the use of CMR for assessment of mitral regurgitation (MR) severity when echocardio-graphic assessment is felt to be unsatisfactory or there is discrepancy between MR severity and clinical findings. In addition, CMR can provide further information about the mechanism of MR and assess cardiac remodeling and viability, which may influence decisions regarding the appropriateness of surgery [2••]. Cine imaging can be performed through the different scallops of the mitral valve (MV) leaflets to help assess the mechanism of MR. Assessment of late gadolinium enhancement of the inferior wall and papillary muscles can also provide clues to the presence of ischemic MR. Secondary MR can be identified by assessing LV functional impairment and LV enlargement.
The jet of MR can be visualized by either SSFP or GRE cine images; however, qualitative assessment is problematic as the appearance of signal void on cine images may be dependent on pulse sequence parameters [29]. Thus, evaluation of regurgitant severity based on jet area or length by CMR is not recommended. The measurement of ventricular volumes, rather than measuring cavity diameter, is recommended as the diameter-volume relationship is nonlinear [2••]. Furthermore, using indexed volumes allows for better assessment of ventricular dilation for different body sizes. Dilation of cardiac chambers is a supportive sign of chronic severe valvular regurgitation.
Primary mitral regurgitation from mitral valve prolapse (MVP) is an excellent example where CMR can help confirm severity of mitral regurgitation. Typically in MVP, the MR occurs in mid-late systole when leaflet malcoaptation is greatest, and the regurgitant jet may be eccentric. Measurement of the estimated regurgitant orifice area (EROA) by flow convergence methods, or assessment of the vena contracta on color-flow images, which are measured at the peak of MR, can overestimate the severity of mitral regurgitation [2••, 48]. Thus, volumetric methods such as quantification of Rvol by echocardiography or CMR are more accurate for assessment of MR severity [48].
CMR Quantification of MR Severity
In addition to visual assessment of MR based on spin dephasing in the left atrium, measurement of vena contracta area or EROA from short-axis cine of phase-contrast images or quantification of RVol and RF has been studied. Current guidelines recommend RVol and RF as the best method for quantification of MR severity [2••]. Measuring mitral regurgitation with phase-contrast imaging of the mitral valve is difficult due to the movement of the mitral valve annulus during ventricular systole, and due to the high velocity and turbulence of the regurgitant jet [29]. Quantification of MR should be performed using SSFP imaging to quantify LV stroke volume (SV) and phase-contrast imaging to quantify forward flow volume across the aortic (or pulmonic) valves to quantify MR RVol and RF. The currently preferred method is to calculate the total LV SV and subtract this from the aortic forward flow volume as shown in Fig. 3 [49••]. Phase velocity imaging has been shown to precisely quantify blood flow in the absence of significant aortic regurgitation or cardiac shunts [44]. This technique is reproducible, correlates well with invasive angiography, and can quantify MR in patients irrespective of regurgitant jet morphology (i.e., multiple and eccentric jets) [49••, 50]. Alternative techniques include using pulmonic artery flow in place of aortic flow, or calculating the difference in LV and RV SV quantified by SSFP imaging, or by measuring the difference between mitral inflow SV and aortic SV by phase-contrast images. RF can be calculated by dividing RVol by LV SV in the volume in the first two methods and by mitral inflow SV for the third method [2••].
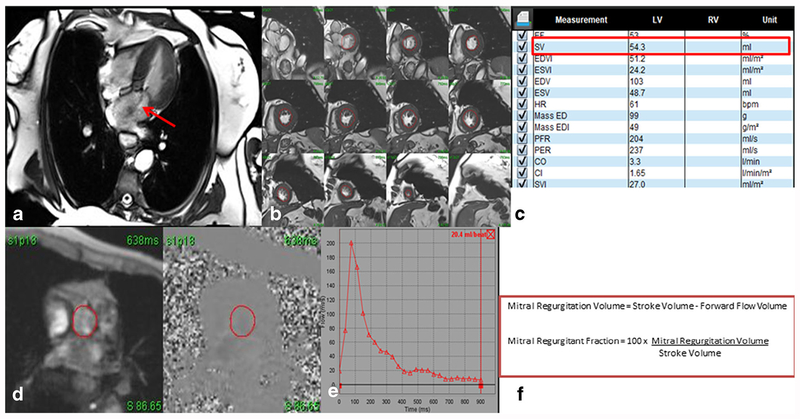
Fig. 3.
Four-chamber steady-state free precession (SSFP) cine image showing mitral regurgitation (red arrow) (a). Quantification was performed using volumetric analysis by obtaining short axis cine stacks (b) to obtain a stroke volume (c). Velocity encoded imaging of the aorta (d) above the level of the valve is obtained for forward-flow volume measurement (e). The regurgitant fraction can be obtained with a simple mathematical formula using the stroke volume and the forward flow volume (f). In this case, the regurgitant fraction of 62% indicates severe mitral regurgitation
Similar to echocardiography, quantification of RF by CMR can be influenced by technical factors (artifacts) and physiologic factors such as blood pressure and heart rate.[2••]. Peak velocities can also be underestimated by limited temporal resolution or if imaging slice is not oriented perpendicular to flow of blood [29]. CMR studies ordered for a primary indication of MR quantification should include quantification of RVand LV volumes as well as aortic and pulmonary flow to provide an internal quality check and detect possible errors in acquisition or analysis. Internal quality validation should be employed such as calculating the difference between LV SV and aortic total forward flow and the difference between LV SV (or RV SV) and pulmonary artery total forward flow as these values should be similar in isolated MR. Also, similar to AR quantification as above, multiple phase-contrast velocity sequences of the aorta and pulmonary artery should be obtained for internal quality validation [49••].
Currently, there are no CMR-specific RVol/RF thresholds for MR severity but guidelines suggest using the same as those established for echocardiography: mild < 30 mL, moderate 30–59 mL, and severe ≥ 60 mL [2••]. Gelfand et al. previously found that CMR-RF thresholds for MR with maximal agreement with echo were mild ≤ 15%, moderate 16–24%, moderate-severe 25–42%, severe > 42% [47]. More recently, Myerson et al. showed that a Rvol threshold of > 55 mL and RF > 40% accurately identified patients who progressed to symptomatic MR or other indications for mitral valve surgery [51]. These studies suggest that CMR-specific thresholds need to be better defined. Furthermore, randomized controlled trials using echo and CMR parameters to grade severity of MR and predict benefit of mitral valve surgery are warranted. The utility of fibrosis measurement by late gadolinium enhancement and T1 mapping in determining surgical intervention also need to be better studied.
Comparison of CMR and TTE Assessment of MR
Echocardiography uses several semi-quantitative and quantitative parameters to assess MR, and standard cutoffs for degree of severity were based primarily on comparison to left ventriculography and retrospective studies [49••, 52].
Comparing severity of MR by CMR and echocardiogram has had modest concordance for qualitative and quantitate evaluation of MR severity; however, CMR has consistently showed higher reproducibility [42, 53, 54]. The low agreement (26 to 66%) [49••] in patients diagnosed with severe MR is concerning, since these patients are often referred for surgery. Cawley et al. demonstrated that in patients with either chronic AR or MR with no more than mild regurgitation of a second valve, quantification of RVol by CMR has lower intra-observer and inter-observer variability as compared to TTE. This was true for a variety of TEE measurements including transvalvular antegrade stroke volumes at the LVoutflow tract and mitral annulus, and the proximal isovelocity hemispheric surface area (PISA) method [42].
In a small prospective multicenter trial, severe MR determined by CMR had a better correlation with improved post-surgical LV remodeling (r = 0.85) as compared to echocardiography (r = 0.32). Rvol quantified by echocardiography using the 2D PISA method were consistently higher than those quantified using CMR [54]. Of patients determined to have severe MR by TTE, only 22% had severe MR by CMR. Another study used volumetric pulsed Doppler flow quantification and found a modest correlation between RVol and RF determined using echo and CMR without consistent overestimation by either method [53]. Higher prevalence of discrepancy was seen in functional MR. A recent paper assessed chronic moderate and severe organic MR defined by echo and found discordant severity in 24% of the patients; however, the majority occurred in cases of late systolic or multiple jets. There was good agreement in cases with holosystolic, central, and single jet characteristics present (K statistic = 0.90; 95% CI, 0.92–0.9) [55•]. This study also found that a lower Rvol cut-off (≥ 50 mL) by
CMR was more sensitive than the traditional 60 mL used in guidelines and had a higher predictive accuracy for severe MR than echo-derived RV [2••, 55•]. The majority of patients that had a poor clinical outcome with a Rvol between 45 and 60 mL had a dilated LV (end-systolic volume index > 35 mL/m2) suggesting that combining Rvol and LV volumes can enhance specificity when using a lower cut off for Rvol.
3D echocardiography (3DE) MR assessment could have potential advantages for quantifying MR due to fewer geometric assumptions; however, 3DE PISA-derived Rvol has shown mixed results in terms of correlations with CMR [56, 57].
Right-Sided Valve Assessment
Pulmonary Valve
CMR has distinct advantages over echocardiography for the evaluation of the right-sided valves. In particular, the pulmonic valve, which is not visualized well on either transthoracic or transesophageal echocardiography, can clearly be visualized in both en-face and right ventricular outflow tract (RVOT) views on CMR. It is also advantageous to use CMR for right-sided valve evaluation in congenital disease where distortion of the anatomy or other associated cardiac defects can be missed with echocardiography [58]. Furthermore, RV volumetric assessment using either SSFP short axis or axial stacked imaging can provide more accurate assessment as compared to 2D or 3D echo [19, 59]. Therefore, CMR is largely considered the “gold standard” for evaluation of the pulmonary valve and RVOT and for the assessment of RV volumes and function [7].
Multiple views beyond the standard views may be needed to visualize the pulmonary valve leaflets. Flow jets can be visualized on SSFP or GRE RVOT cine views allowing for qualitative assessment of regurgitation or stenosis as demonstrated in Fig. 4. Using the RVOT cine view, a perpendicular slice through the pulmonic valve tips can be defined and used for pulmonic valve orifice planimetry, which is the preferred method for pulmonic valve area calculation. In addition, along with standard 2-chamber, 3-chamber, and 4-chamber cine imaging, RV inflow view should be performed to assess for secondary tricuspid regurgitation and for qualitative assessment of RV size. RV volumes and RVEF should also be routinely assessed in pulmonic valve disease. Other techniques such as non-contrast “dark blood” TSE imaging may be useful for improved anatomic visualization of the outflow tract. ECG-gated contrast-enhanced magnetic resonance angiography can delineate the valve leaflets and the pulmonary arteries [60].
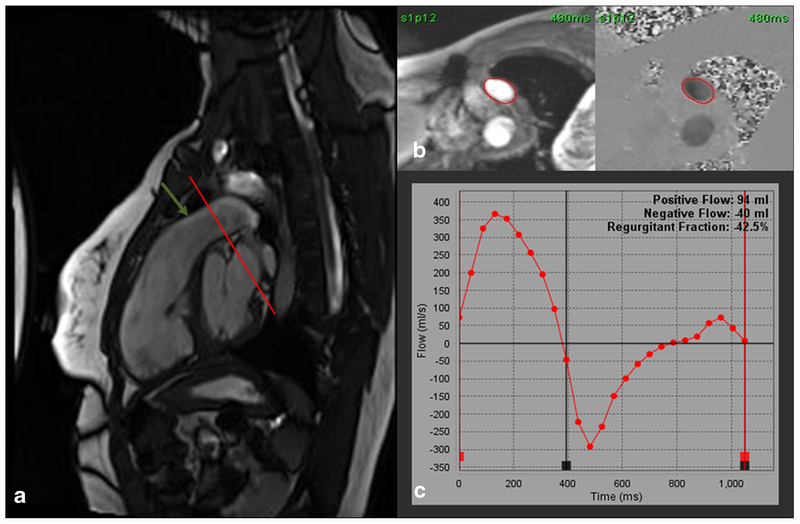
Fig. 4.
a Steady-state free precession (SSFP) right ventricular outflow tract (RVOT) cine image showing severe pulmonic regurgitation. The regurgitation is so severe that there is no turbulent flow to cause dephasing and a low signal flow void. A through-plane phase-contrast velocity-encoded image (VENC) can be obtained at the cross-sectional plane (red line) of the pulmonary artery (green arrow). b Through-plane VENC image with a region of interest drawn in the pulmonary artery. c Graphic plot of forward and regurgitant flow through the pulmonary artery showing a regurgitant fraction of 42.5% indicating severe pulmonic regurgitation
Qualitative assessment of pulmonic stenosis (PS) can be performed with “through-plane” phase-contrast imaging at the level of maximal stenosis at valvular, supravalvular, or subvalvular positions. Using post-processing software, a peak velocity can be obtained through the stenotic region. Pulmonic regurgitation (PR) can be quantified by assessing the regurgitant fraction using a through-plane phase-contrast image acquired at the level of the pulmonary artery just above the pulmonic valve. This method has been validated against echocardiographic measurements and stroke volume comparisons between the right and left ventricle in patients with Tetralogy of Fallot (TOF) [61, 62]. Currently, a regurgitant fraction of ≥ 40% is considered severe [7, 62]. CMR is now being widely used in patients with pulmonic regurgitation after TOF repair to determine optimal timing of surgery [63, 64]. Specifically for TOF repair, CMR-derived RV end systolic and diastolic volume index prior to pulmonic valve replacement (PVR) best predict improvement in post-PVR RV volumes regardless of the severity of PR [65, 66]. There are certain limitations in the assessment of PS and PR. In the case of pulmonic stenosis, high peak velocities can be difficult to measure due to the limitations of phase-contrast imaging of high-velocity turbulent jets. PR jets may be difficult to identify on SSFP images, as they tend to have less turbulence and may not produce a significant flow voids [7].
Tricuspid Valve
CMR poses similar advantages and disadvantages for evaluation of the tricuspid valve as described above for evaluation of mitral regurgitation. When evaluating the tricuspid valve, RV volumes and function should be assessed as described above for evaluation of the pulmonic valve. The tricuspid valve can be seen on cine imaging of the RV inflow, short axis at the aortic valve level, and 4-chamber views. Qualitative assessment is similar to echocardiography. Dephasing allows for visualization of a vena contracta and > 7 mm infers severe regurgitation [2••, 67]. The vena-contracta can also be measured by planimetry at the level of the tricuspid valve tips [2••, 67]. Quantification of flow across the tricuspid valve by phase velocity imaging is difficult due to the transannular motion of the valve [67]. Indirect quantification of Rvol can be obtained by subtracting forward pulmonary artery flow from RV stroke volume [68]. However, this method is limited in the setting of concomitant pulmonic disease and therefore only qualitative assessment can usually be performed in those situations. Tricuspid stenosis is a rare disease, and CMR use in that setting has not been well studied.
Future Directions
CMR research in valvular heart disease is focused on a number of areas such as free breathing sequences [69], fibrosis evaluation [70, 71], CMR-derived right ventricular strain evaluation [72], and microvascular disease assessment [73]. These advances in CMR technology will provide new information for assessing the heart in patients with valvular heart disease, which may yield new diagnostic and prognostic information. In addition, there has been a significant focus on improving flow visualization and quantification. Current, phase-contrast techniques typically use 2D slices with assessment of flow in a single direction. In the 1990s, 3D phase-contrast techniques were developed which could provide time-resolved velocity data in three dimensions with volumetric coverage of the entire heart but were limited by long acquisition times [74, 75]. With improvements in image reconstruction techniques, temporally resolved 3D phase velocity imaging with encoding in multiple directions, which is known as 4D flow imaging, is becoming clinically feasible. Blood motion can be tracked in the 3D images to derive volumetric streamline images which can provide new insights into the visualization of stenotic or regurgitant jets [76, 77]. This is particularly useful in the identification and qualitative assessment in the case of eccentric regurgitant jets or difficult-to-visualize flows. With the use of post-processing software, quantification of flow from 4D flow data can be performed in regurgitant valves and major vessels [78–80]. Therefore, 4D flow may be of significant use in the setting of stenosis or complex flow patterns as compared to 2D-derived phase-contrast imaging [80].
Conclusion
While in the past the role of CMR was isolated to situations where echocardiographic assessment was inadequate, CMR is playing an increasingly important role in the assessment of valvular heart disease. The high spatial resolution and ability to image the valves from multiple slice orientations enables a comprehensive visual analysis of valvular morphology and function. CMR cine imaging remains the gold standard for measuring left and right ventricular volumes and function providing essential information regarding ventricular remodeling in response to valvular heart disease. Phase-contrast velocity imaging provides the ability to quantify peak velocities and flow across the aortic and pulmonic valves. The combination of volumetrics from cine imaging and flow quantified from phase-contrast velocity imaging provides an accurate and reproducible assessment of aortic and mitral regurgitation providing important diagnostic and prognostic information. This has been highlighted in the comprehensive guide-lines published by the American Society of Echocardiography. New techniques such as 4D-Flow promise to provide new insights into valvular heart disease, and techniques such as T1 mapping and LGE imaging can provide unique information regarding ventricular remodeling. CMR provides complementary information to echocardiography and is increasingly becoming an essential modality that can reproducibly quantify the severity of valvular heart disease.
Funding Information
Authors receive salary support from the following grants R01 HL131919–01A1 and 5T32EB003841.
Michael Salerno declares he has no relevant conflicts of interest related to this publication. He does note grants from AstraZeneca and NIH not related to this work. In addition, he receives non-financial support from Seimens Healthineers, also unrelated to this publication.
Footnotes
This article is part of the Topical Collection on Valvular Heart Disease
Conflict of Interest Roshin C. Mathew and Adrián I. Löffler declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.••.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation a report from the American Society of Echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. JASE. 2017;30(4):303–71. 10.1016/j.echo.2017.01.007.Defines the current recommendations for the qualitative and quantitative assessment of for native valve regurgitation by CMR and echo.
- 3.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148:e1–132. 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55: 2614–62. 10.1016/j.jacc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommer G, Bremerich J, Lund G. Magnetic resonance imaging in Valvular heart disease: clinical application and current role for patient management. J Magn Reson Imaging. 2012;35:1241–52. 10.1002/jmri.23544. [DOI] [PubMed] [Google Scholar]
- 6.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J Nittka M, Jellus V, Wang J, Kiefer B, Haase A Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47:1202–1210. DOI: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 7.Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:7 10.1186/1532-429X-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieri O, Scheffler K. Fundamental of balanced steady state free precession MRI. J Magn Reson Imaging. 2013;38:2–11. 10.1002/jmri.24163. [DOI] [PubMed] [Google Scholar]
- 9.Van Dijk P ECG-triggered NMR imaging of the heart. Diagn Imaging Clin Med. 1984;53:29–37. [PubMed] [Google Scholar]
- 10.Lenz GW, Haacke EM, White RD. Retrospective cardiac gating: a review of technical aspects and future directions. Magn Reson Imaging. 1989;7:445–55. [DOI] [PubMed] [Google Scholar]
- 11.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. The Society for Cardiovascular Magnetic Resonance Board of Trustees task force on standardized protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longmore DB, Klipstein RH, Underwood SR, Firmin DN, Hounsfield GN, Watanabe M, et al. Dimensional accuracy of magnetic resonance in studies of the heart. Lancet. 1985;1:1360–2. [DOI] [PubMed] [Google Scholar]
- 13.Dursun M, Yılmaz S, Yılmaz E, Yılmaz R, Onur İ, Oflaz H, et al. The utility of cardiac MRI in diagnosis of infective endocarditis: preliminary results. Diagn Interv Radiol. 2015;21(1):28–33. 10.5152/dir.2014.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caduff JH, Hernandez RJ, Ludomirsky A. MR visualization of aortic valve vegetations. J Comput Assist Tomogr. 1996;20:613–5. [DOI] [PubMed] [Google Scholar]
- 15.Wagner S, Auffermann W, Buser P, Lim TH, Kircher B, Pflugfelder P, et al. Diagnostic accuracy and estimation of the severity of valvular regurgitation from the signal void on cine magnetic resonance images. Am Heart J. 1989;118:760–7. [DOI] [PubMed] [Google Scholar]
- 16.Sievers B, Brandts B, Franken U, Trappe HJ. Cardiovascular magnetic resonance imaging demonstrates mitral valve endocarditis. Am J Med. 2003;115:681–2. [DOI] [PubMed] [Google Scholar]
- 17.Sechtem U, Pflugfelder PW, Gould RG, Cassidy MM, Higgins CB. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology. 1987;163:697–702. 10.1148/radiology.163.3.3575717. [DOI] [PubMed] [Google Scholar]
- 18.Katz J, Whang J, Boxt LM, Barst RJ. Estimation of right ventricular mass in normal subjects and in patients with pulmonary hypertension by magnetic resonance imaging. J Am Coll Cardiol. 1993;21: 1475–81. [DOI] [PubMed] [Google Scholar]
- 19.Clarke CJ, Gurka MJ, Norton PT, Kramer CM, Hoyer AW. Assessment of the accuracy and reproducibility of RV volume measurements by CMR in congenital heart disease. J Am Coll Cardiol Img. 2012;5:28–37. 10.1016/j.jcmg.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Alfakih K, Plein S, Bloomer T, Jones T, Ridgeway J, Sivananthan M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging. 2003;18:25–32. 10.1002/jmri.10329. [DOI] [PubMed] [Google Scholar]
- 21.Evans AJ, Blinder RA, Herfkens RJ, Spritzer CE, Kuethe DO, Fram EK, et al. Effects of turbulence on signal intensity in gradient echo images. Investig Radiol. 1988;23:512–8. [DOI] [PubMed] [Google Scholar]
- 22.Malayeri AA, Johnson WC, Macedo R, Bathon J, Lima JA, Bluemke DA. Cardiac cine MRI: quantification of the relationship between fast gradient echo and steady-state free precession for determination of myocardial mass and volumes. J Magn Reson Imaging. 2008;28:60–6. 10.1002/jmri.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aurigemma G, Reichek N, Schiebler M, Axel L. Evaluation of aortic regurgitation by cardiac cine magnetic resonance imaging: planar analysis and comparison to Doppler echocardiography. Cardiology. 1991;78(4):340–7. 10.1159/000174815. [DOI] [PubMed] [Google Scholar]
- 24.Ozdogan O, Yuksel A, Gurgun C, Kayikcioglu M, Yavuzgil O, Cinar CS. Evaluation of the severity of mitral regurgitation by the use of signal void in magnetic resonance imaging. Echocardiography. 2009;26(10):1127–35. 10.1111/j.1540-8175.2009.00968.x. [DOI] [PubMed] [Google Scholar]
- 25.Spielmann RP, Schneider O, Thiele F, Heller M, Bucheler E. Appearance of poststenotic jets in MRI: dependence on flow velocity and on imaging parameters. Magn Reson Imaging. 1991;9: 67–72. [DOI] [PubMed] [Google Scholar]
- 26.Sondergaard L, Stahlberg F, Thomsen C. Magnetic resonance imaging of valvular heart disease. J Magn Reson Imaging. 1999;10: 627–38. [DOI] [PubMed] [Google Scholar]
- 27.Utz JA, Herfkens RJ, Heinsimer JA, Shimakawa A, Glover G, Pelc N. Valvular regurgitation: dynamic MR imaging. Radiology. 1988;168:91–4. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell L, Jenkins JPR, Watson Y, Rowlands DJ, Isherwood I. Diagnosis and assessment of mitral and aortic valve disease by cine-flow magnetic resonance imaging. Magn Reson Med. 1989;12:181–97. [DOI] [PubMed] [Google Scholar]
- 29.Cawley PJ, Maki JH, Otton CM. Cardiovascular magnetic resonance imaging for Valvular heart disease technique and validation. Circulation. 2009;119:468–78. 10.1161/CIRCULATIONAHA.107.742486. [DOI] [PubMed] [Google Scholar]
- 30.Underwood SR, Firmin DN, Rees RS, Longmore DB. Magnetic resonance velocity mapping. Clin Phys Physiol Meas. 1990;11(Suppl A):37–43. [DOI] [PubMed] [Google Scholar]
- 31.Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging. Magn Reson Q. 1991;7:229–54. [PubMed] [Google Scholar]
- 32.Kilner PJ, Gatehouse PD, Firmin DN. Flow measurement by magnetic resonance: a unique asset worth optimising. J Cardiovasc Magn Reson. 2007;9:723–8. 10.1080/10976640701465090. [DOI] [PubMed] [Google Scholar]
- 33.Gatehouse PD, Keegan J, Crowe LA, Masood S, Mohiaddin RH, Kreitner KF, et al. Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol. 2005;15:2172–84. 10.1007/s00330-005-2829-3. [DOI] [PubMed] [Google Scholar]
- 34.Firmin DN, Nayler GL, Kilner PJ, Longmore DB. The application of phase shifts in NMR for flow measurement. Magn Reson Med. 1990;14:230–41. [DOI] [PubMed] [Google Scholar]
- 35.Gatehouse PD, Rolf MP, Graves MJ, Hofman MB, Totman J, Werner B, et al. Flow measurement by cardiovascular magnetic resonance: a multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J Cardiovasc MagnReson. 2010;12:5 10.1186/1532-429X-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien KR, Cowan BR, Jain M, Stewart RA, Kerr AJ, Young AA. MRI phase contrast velocity and flow errors in turbulent stenotic jets. J Magn Reson Imaging. 2008;28:210–8. 10.1002/jmri.21395. [DOI] [PubMed] [Google Scholar]
- 37.Hundley WG, Li HF, Hillis LD, Meshack BM, Lange RA, Willard JE, et al. Quantitation of cardiac output with velocity-encoded, phase-difference magnetic resonance imaging. Am J Cardiol. 1995;75:1250–5. [DOI] [PubMed] [Google Scholar]
- 38.Sondergaard L, Thomsen C, Stahlberg F, Gymoese E, Lindvig K, Hildebrandt P, et al. Mitral and aortic valvular flow: quantification with MR phase mapping. J Magn Reson Imaging. 1992;2:295–302. [DOI] [PubMed] [Google Scholar]
- 39.Mor-Avi V, Jenkins C, Kuhl HP, Nesser HJ, Marwick T, Franke A, et al. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes Multicenter Study for Validation With Magnetic Resonance Imaging and Investigation of Sources of Error. JACC Img. 2008;1(4):413–23. 10.1016/j.jcmg.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Myerson SG, d’Arcy J, Mohiaddin R, Greenwood JP, Karamitsos TD, Francis JM, et al. Aortic regurgitation quantification using cardiovascular magnetic resonance association with clinical outcome. Circulation. 2012;126:1452–60. 10.1161/CIRCULATIONAHA.111.083600. [DOI] [PubMed] [Google Scholar]
- 41.Chatzimavroudis GP, Walter PG, Oshinski JN, Franch RH, Pettigrew RI, Yoganathan AP. Slice location dependence of aortic regurgitation measurements with MR phase velocity mapping. Mag Reson Med. 1997;37:545–51. [DOI] [PubMed] [Google Scholar]
- 42.Cawley PJ, Hamilton-Crai C, Owens DS, Krieger EV, Strugnell WE, Mitsumori L, et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging. 2013;6: 48–57. 10.1161/CIRCIMAGING.112.975623. [DOI] [PubMed] [Google Scholar]
- 43.Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013. June 13;15:51 10.1186/1532-429X-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatzimavroudis GP, Oshinski JN, Franch RH, Walker PG, Yoganathan AP, Pettigrew RI. Evaluation of the precision of magnetic resonance phase velocity mapping for blood flow measurements. J Cardio Mag Reson. 2001;3(1):11–9. [DOI] [PubMed] [Google Scholar]
- 45.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwamoto Y, Inage A, Tomlinson G, Lee KJ, Grosse-Wortmann L, Seed M, et al. Direct measurement of aortic regurgitation with phase-contrast magnetic resonance is inaccurate: proposal of an alternative method of quantification. Pediatr Radiol. 2014;44(11): 1358–69. 10.1007/s00247-014-3017-x. [DOI] [PubMed] [Google Scholar]
- 47.Gelfand EV, Hughes S, Hauser TH, Yeon SB, Goepfert L, Kissinger KV. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson. 2006;8(3): 503–7. [DOI] [PubMed] [Google Scholar]
- 48.Topilsky Y, Michelena H, Bichara V, Maalouf J, Mahoney DW, Enriquez-Sarano M, et al. Mitral valve prolapse with mid-late systolic mitral regurgitation: pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation. 2012;125(13):1643–51. 10.1161/CIRCULATIONAHA.111.055111. [DOI] [PubMed] [Google Scholar]
- 49.••.Uretsky S, Argulian E, Narula J, Wolff SD. Use of cardiac magnetic resonance imaging in assessing mitral regurgitation: current evidence. J Am Coll Cardiol. 2018;71(5):547–63. 10.1016/j.jacc.2017.12.009.One of the most recent review articles published describing the utility of CMR in the evaluation of mitral regurgitation, including a proposed clinical pathway and guidelines.
- 50.Hundley WG, Li HF, Willard JE, Landau C, Lange RA, Meshack BM, et al. Magnetic resonance imaging assessment of the severity of mitral regurgitation. Comparison with invasive techniques. Circulation. 1995;92(5):1151–8. [DOI] [PubMed] [Google Scholar]
- 51.Myerson SG, d’Arcy J, Christiansen JP, Dobson LE, Mohiaddin R, Francis JM, et al. Determination of clinical outcome in mitral regurgitation with cardiovascular magnetic resonance quantification. Circulation. 2016;133(23):2287–96. 10.1161/CIRCULATIONAHA.115.017888. [DOI] [PubMed] [Google Scholar]
- 52.Dujardin KS, Enriquez-Sarano M, Bailey KR, Nishimura RA, Seward JB, Tajik AJ. Grading of mitral regurgitation by quantitative Doppler echocardiography: calibration by left ventricular angiography in routine clinical practice. Circulation. 1997;96(10): 3409–15. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Mattei JC, Ibrahim H, Shaikh KA, Little SH, Shah DJ, Maragiannis D, et al. Comparative assessment of mitral regurgitation severity by transthoracic echocardiography and cardiac magnetic resonance using an integrative and quantitative approach. Am J Cardiol. 2016;117(2):264–70. 10.1016/j.amjcard.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 54.Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65(11):1078–88. 10.1016/j.jacc.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 55.•.Penicka M, Vecera J, Mirica DC, Kotrc M, Kockova R, Van Camp G. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with organic mitral regurgitation: comparison with doppler echocardiography-derived integrative approach. Circulation. 2018;137(13):1349–60. 10.1161/CIRCULATIONAHA.117.029332.One of the few studies detailing the prognostic implications of CMR use in evaluating MR in asymptomatic individuals.
- 56.Choi J, Heo R, Hong GR, Chang HJ, Sung JM, Shin SH, et al. Differential impact of three-dimensional color Doppler echocardiography for the quantification of mitral regurgitation according to the severity and characteristics. Circ Cardiovasc Imaging. 2014;7(3):535–44. 10.1161/CIRCIMAGING.113.001457. [DOI] [PubMed] [Google Scholar]
- 57.Thavendiranathan P, Liu S, Datta S, Rajagopalan S, Ryan T, Igo SR, et al. Quantification of chronic functional mitral regurgitation by automated 3-dimensional peak and integrated proximal isovelocity surface area and stroke volume techniques using real-time 3-dimensional volume color Doppler echocardiography: in vitro and clinical validation. Circ Cardiovasc Imaging. 2013;6(1):125–33. 10.1161/CIRCIMAGING.112.980383. [DOI] [PubMed] [Google Scholar]
- 58.Mercer-Rosa L, Yang W, Rychik J, Fogel M, Goldmuntz E. Quantifying pulmonary regurgitation and right ventricular function in surgically repaired tetralogy of Fallot a comparative analysis of echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:637–43. 10.1161/CIRCIMAGING.112.972588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medvedofsky D, Mor-Avi V, Kruse E, Guile B, Ciszek B, Weinert L, et al. Quantification of right ventricular size and function from contrast-enhanced three-dimensional echocardiographic images. J Am Soc Echocardiogry. 2017;30(12):1193–202. 10.1016/j.echo.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Saremi F, Gera A, Ho SY, Hijazi ZM, Sánchez-Quintana D. CT and MR imaging of the pulmonary valve. Radiographics. 2014. Jan-Feb;34(1):51–71. 10.1148/rg.341135026. [DOI] [PubMed] [Google Scholar]
- 61.Li W, Davlouros PA, Kilner PJ, Pennell DJ, Gibson D, Henein MY, et al. Doppler-echocardiographic assessment of pulmonary regurgitation in adults with repaired tetralogy of Fallot: comparison with cardiovascular magnetic resonance imaging. Am Heart J. 2004;147:165–72. [DOI] [PubMed] [Google Scholar]
- 62.Rebergen SA, Chin JG, Ottenkamp J, van der Wall EE, de Roos A. Pulmonary regurgitation in the late postoperative follow-up of tetralogy of Fallot. Volumetric quantitation by nuclear magnetic resonance velocity mapping. Circulation. 1993;88:2257–66. [DOI] [PubMed] [Google Scholar]
- 63.Vliegen HW, van Straten A, de Roos A, Roest AA, Schoof PH, Zwinderman AH, et al. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of fallot. Circulation. 2002;106:1703–7. [DOI] [PubMed] [Google Scholar]
- 64.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–82. [DOI] [PubMed] [Google Scholar]
- 65.Henkens IR, van Straten A, Schalij MJ, Hazekamp MG, Roos d, van der Wall EE, et al. Predicting outcome of pulmonary valve replacement in adult tetralogy of Fallot patients. Ann Thorac Surg. 2007;83:907–11. [DOI] [PubMed] [Google Scholar]
- 66.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007. July 31;116(5):545–51. 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 67.Jun H, Park EA, Bahn YE, Lee W, Kim HK, Chung JW. Quantification of tricuspid regurgitation using two-dimensional velocity encoding cine: optimal plane and reproducibility. Int J Cardiovasc Imaging. 2015;31(2):233–40. 10.1007/s10554-015-0715-x. [DOI] [PubMed] [Google Scholar]
- 68.Westenberg JJ, Roes SD, Ajmone Marsan N, Binnendijk NM, Doornbos J, Bax JJ, et al. Mitral valve and tricuspid valve blood flow: accurate quantification with 3D velocity-encoded MR imaging with retrospective valve tracking. Radiology. 2008;249(3):792–800. 10.1148/radiol.2492080146. [DOI] [PubMed] [Google Scholar]
- 69.Moghari MH, Komarlu R, Annese D, Geva T, Powell AJ. Free-breathing steady-state free precession cine cardiac magnetic resonance with respiratory navigator gating. Magn Reson Med. 2015;73(4):1555–61. 10.1002/mrm.25275. [DOI] [PubMed] [Google Scholar]
- 70.Geiger J, Rahsepar AA, Suwa K, Powell A, Ghasemiesfe A, Barker AJ, et al. 4D flow MRI, cardiac function, and T1-mapping: association of valve-mediated changes in aortic hemodynamics with left ventricular remodeling. J Magn Reson Imaging J Magn Reson Imaging. 2018;48(1):121–31. 10.1002/jmri.25916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Podlesnikar T, Delgado V, Bax JJ. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int J Cardiovasc Imaging. 2018. January;34(1):97–112. 10.1007/s10554-017-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Auger DA, Zhong X, Epstein FH, Spottiswoode BS. Mapping right ventricular myocardial mechanics using 3D cine DENSE cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14: 4 10.1186/1532-429X-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zorach B, Shaw PW, Bourque J, Kuruvilla S, Balfour PC Jr, Yang Y, et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J Cardiovasc Magn Reson. 2018;20(1):14 10.1186/s12968-018-0435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wigstrom L, Sjoqvist L, Wranne B. Temporally resolved 3D phase-contrast imaging. Magn Reson Med. 1996;36:800–3. [DOI] [PubMed] [Google Scholar]
- 75.Pelc NJ, Bernstein MA, Shimakawa A, Glover GH. Encoding strategies for three-direction phase-contrast MR imaging of flow. J Magn Reson Imaging. 1991;1:405–13. [DOI] [PubMed] [Google Scholar]
- 76.Napel S, Lee DH, Frayne R, Rutt BK. Visualizing three-dimensional flow with simulated streamlines and three-dimensional phase-contrast MR imaging. J Magn Reson Imaging. 1992;2:143–53. [DOI] [PubMed] [Google Scholar]
- 77.Kozerke S, Hasenkam JM, Pedersen EM, Boesiger P. Visualization of flow patterns distal to aortic valve prostheses in humans using a fast approach for cine 3D velocity mapping. J Magn Reson Imaging. 2001;13(5):690–8. [DOI] [PubMed] [Google Scholar]
- 78.Brix L, Ringgaard S, Rasmusson A, Sørensen TS, Kim WY. Three dimensional three component whole heart cardiovascular magnetic resonance velocity mapping: comparison of flow measurements from 3D and 2D acquisitions. J Cardiovasc Magn Reson. 2009;11:3 10.1186/1532-429X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nordmeyer S, Riesenkampff E, Crelier G, Khasheei A, Schnackenburg B, Berger F, et al. Flow-sensitive four-dimensional cine magnetic resonance imaging for offline blood flow quantification in multiple vessels: a validation study. J Magn Reson Imaging. 2010;32:677–83. 10.1002/jmri.22280. [DOI] [PubMed] [Google Scholar]
- 80.Nordmeyer S, Riesenkampff E, Messroghli D, Kropf S, Nordmeyer J, Berger F, et al. Four-dimensional velocity-encoded magnetic resonance imaging improves blood flow quantification in patients with complex accelerated flow. J Magn Reson Imaging. 2013;37(1): 208–16. 10.1002/jmri.23793. [DOI] [PubMed] [Google Scholar]