Abstract
Background.
Persistent/recurrent primary hyperparathyroidism (pHPT) occurs frequently in multiple endocrine neoplasia type 1 (MEN1). We assessed the usefulness of intraoperative PTH (IOPTH) and preoperative localizing studies based on the outcome of patients with MEN1-associated pHPT undergoing reoperative surgery.
Methods.
A retrospective analysis identified MEN1 patients with persistent/recurrent pHPT. Patient outcome was defined as postoperative serum calcium and PTH levels (cured, persistent or recurrent) at last follow-up. Positive predictive value (PPV) was calculated for imaging studies and IOPTH.
Results.
Thirty patients with MEN1-associated recurrent/persistent pHPT underwent 69 reoperative parathyroidectomies. Median follow-up time was 33 months. Persistent pHPT occurred in four (13 %) patients. IOPTH had a 92 % PPV for postoperative eucalcemia. Ultrasound and Tc99msestamibi had sensitivities of 100 and 85 % for localizing an enlarged parathyroid gland. However, five (17 %) patients had additional enlarged glands, not visualized preoperatively that were removed after IOPTH did not drop appropriately. Bone mineral density scores did not improve after reoperation (p = 0.60), but the rate of postoperative nephrocalcinosis did (p = 0.046). Patients with pancreatic neuroendocrine tumors had significantly higher rates of persistent/recurrent pHPT compared with those without (40 vs. 0 %, p = 0.021). Intraoperative and delayed parathyroid autotransplantation was performed in nine (30 %) and four (14 %) patients, respectively.
Conclusions.
Although preoperative localizing studies are helpful for guiding reoperative strategy in MEN1 with persistent/recurrent pHPT, additional enlarged glands may be missed by conventional imaging. IOPTH should therefore be employed routinely in this setting. Routine cryopreservation should be considered in all patients. Pancreatic manifestation may be associated with earlier recurrence or persistent disease.
Primary hyperparathyroidism (pHPT) is the most common presentation in patients with multiple endocrine neoplasia type 1 (MEN1), occurring in more than 90 % of patients.1–4 MEN1 is an autosomal dominant syndrome caused by loss-of-function mutations in the MEN1 tumor suppressor gene. pHPT in MEN1 patients typically results from multiple, enlarged parathyroid glands, but it is not uncommon to find fewer than four enlarged glands during initial surgical exploration.5,6 Inadequate recognition of more than one affected gland or an unrecognized diagnosis of MEN1 during initial operation and thus incomplete resection of all affected parathyroid glands in these patients is associated with postoperative persistent or recurrent pHPT.5,6 Presence of ectopic or supernumerary glands and regrowth of remnant and/or autografted parathyroid tissue are all possible sites of recurrent or persistent pHPT in patients with MEN1. Early age at diagnosis (third and fourth decade) and parathyroidectomy is associated with high rates of persistent/recurrent disease and reoperative surgery is often required during their lifetime.7–12 Reoperation in the neck is associated with higher rates of complications, such as recurrent laryngeal nerve injury and hypoparathyroidism and therefore should be planned carefully.13,14 Although a few studies have examined the role of preoperative localizing studies, outcome after reoperative parathyroidectomy, and genotype/recurrence association in MEN1-associated pHPT, no study exists to date incorporating all these variables in the same cohort of patients.15–18
In this study, we aimed to assess the usefulness of intraoperative PTH (IOPTH) and preoperative localizing studies to identify the remaining culprit gland(s) in patients with MEN1-associated pHPT undergoing reoperative surgery as well as to assess the outcome of these patients and factors associated with persistent or recurrent disease.
METHODS
Thirty patients with recurrent/persistent pHPT and MEN1 undergoing reoperation were identified in a prospectively maintained database at the National Institutes of Health (NIH). Incomplete follow-up data was supplemented by telephone interview. Information on previous surgery was obtained from prior pathology and operative reports. The diagnosis of MEN1 was established based on family history and/or evidence of two or more endocrinopathies associated with the syndrome (27 %) and genetic testing (73 %) results for the MEN1 gene of at least one affected family member. Of the eight patients with negative or no mutation testing, all eight had pituitary adenoma and pHPT. Four of eight also had a pancreatic neuroendocrine tumor and two had adrenocortical adenomas. The 22 patients who had positive genetic testing had insertion/deletions or missense mutations in exon 2 (n = 8), exon 3 (n = 1), exon 4 (n = 1), exon 7 (n = 4), exon 9 (n = 1), and exon 10 (n = 7). pHPT was diagnosed in all patients through elevated serum and/or ionized calcium (Ca) levels as well as elevated parathyroid hormone (PTH) levels. All patients had a screening biochemical workup for pancreatic neuroendocrine tumors, including fasting plasma insulin, gastrin, somatostatin, glucagon, chromogranin, pancreatic polypeptide, and urine 5-HIAA levels, as well as anatomical imaging, including abdominal CT scan and/or MRI at presentation to NIH. All but two patients had documented metabolic complications in the form of bone mineral density (BMD) loss (femur and/or spine) and/or nephrocalcinosis before reoperation. Preoperative localizing studies were used in all patients and included either ultrasound of the neck (n = 24), CT scan of the neck/mediastinum (n = 18), MRI of the neck (n = 17), Tc99m-sestamibi scan (MIBI) (n = 28), or invasive testing consisting of intraarterial hypocalcemic infusion and venous sampling, selective venous sampling, selective arteriography or direct transcutaneous aspiration, and PTH measurement (n = 4). All but one patient had at least two preoperative localizing studies. Extent of reoperation was based on review of previous operative and pathology reports, as well as localizing studies. Cryopreservation was performed in all patients according to a previously described procedure. Parathyroid tissue was sectioned into 1–2 mm pieces placed in a solution of the patient’s serum and DMSO (1:1 by volume) and cryopreserved.19
Intraoperative parathyroid hormone assay (IOPTH) was performed as previously described in all but one patient.20 More information on definitions of variables used in this analysis can be found in Supplemental Table 1.
Statistical Analysis
Cumulative disease-free survival was calculated using the Kaplan–Meier method. Differences in recurrent/persistent pHPT among variables were determined by using the log-rank test (Mantel–Cox). Group comparisons of categorical variables were performed using Pearson’s χ2 testing, and the comparison of continuous variables was performed with a two-tailed t test or Wilcoxon rank test. All p values were considered statistically significant if p < 0.05. The Statistical Package for Social Sciences (SPSS v.16 IBM Corporation, Armonk, NY) was used for statistical analysis.
RESULTS
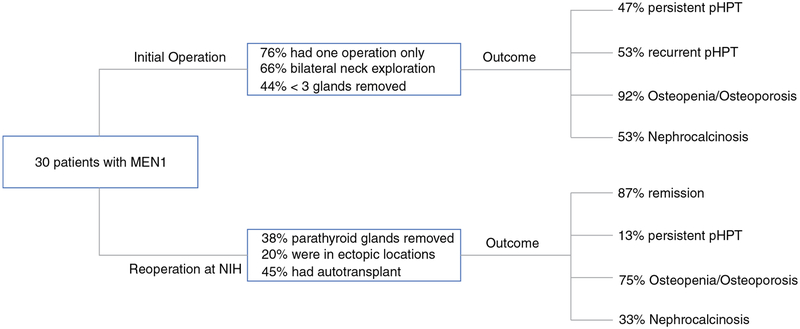
Patient demographics are summarized in Table 1. Thirty consecutive patients with MEN1 and recurrent/persistent pHPT undergoing reoperation at NIH were included from 1998 to 2014. Median follow-up time was 33 (range 0.5–180) months. Surgical and metabolic variables for initial and reoperation can be found in Table 2 and Fig. 1. Fourteen patients had a known diagnosis of MEN1 at their initial operation, 11 did not, and it was unknown in 5 patients.
TABLE 1.
Patient demographics and clinical characteristics at presentation to NIH
| Variable | Number | % |
|---|---|---|
| Sex | ||
| Female | 19 | 63 |
| Male | 11 | 37 |
| MEN1 diagnosis first surgery | ||
| Yes | 14 | 56 |
| No | 11 | |
| Unknown | 5 | 44 |
| MEN1 manifestations | ||
| Pancreatic neuroendocrine tumor | 20 | 67 |
| Nonfunctioning | 6 | |
| Gastrinoma | 5 | |
| Insulinoma | 11 | |
| Adrenocortical adenoma | 7 | 23 |
| Pituitary adenoma | 19 | 63 |
| Type of surgery | ||
| Neck exploration | 28 | 93 |
| Autograft removal | 2 | 7 |
| Total number of surgeries | ||
| 2 | 23 | 76 |
| >2 | 7 | 24 |
| Recurrence after first surgery | 16 | 53 |
| Persistence after first surgery | 14 | 47 |
| Number of surgery before reoperation at NIH | Mean (range) | 1.3 (1–4) |
| Follow-up (mo) | Median (range) | 33 (0.5–180) |
TABLE 2.
Surgical and metabolic variables at initial and reoperative surgery
| Initial surgery | ||
|---|---|---|
| Variable | Number | % |
| First surgery at NIH | 4 | 13 |
| Bilateral neck exploration outside | 20 | 66 |
| Glands resected in previous operations | ||
| 1 | 8 | 27 |
| 2–2.5 | 5 | 17 |
| 3–3.5 | 14 | 56 |
| 4–4.5 | 3 | 10 |
| Bone density | ||
| Normal | 1 | 8 |
| Osteopenia | 8 | 67 |
| Osteoporosis | 3 | 25 |
| Nephrocalcinosis | ||
| Yes | 16 | 53 |
| No | 11 | 37 |
| Age at first surgery (year) | (Median) | 31 (15–59) |
| Time to recurrence (year) | (Median) | 10 (2–28) |
| Time between first surgery and reoperation (year) | (Median) | 10 (0.5–36) |
| Reoperation | ||
| Variable | Number | % |
| Elevated Gastrin preop | ||
| Yes | 8 | 47 |
| No | 9 | 53 |
| Thymectomy | 7 | 23 |
| Ectopic gland | 6 | 20 |
| Autograft transplantation | ||
| None | 16 | 55 |
| Immediate | 9 | 31 |
| Delayed | 5 | 17 |
| Outcome | ||
| Remission (last follow-up) | 22 | 74 |
| Persistent pHPT <6 months) | 4 | 13 |
| Recurrent pHPT(>6 months) | 4 | 13 |
| Hypoparathyroidism | ||
| None | 16 | 53 |
| Transient <6 months) | 10 | 33 |
| Permanent >6 months) | 4 | 13 |
| Bone density | ||
| Normal | 3 | 25 |
| Osteopenia | 4 | 33 |
| Osteoporosis | 5 | 42 |
| Nephrocalcinosis | ||
| Yes | 7 | 23 |
| No | 12 | 40 |
| Age reoperation (year) | (Median) | 46 (21–71) |
FIG. 1.
Surgical and outcome variables for initial and reoperation in a cohort of 30 patients with MEN1 and recurrent/persistent pHPT
Reoperation at NIH
Performance of Preoperative Localizing Studies and Intraoperative Findings
All patients underwent at least one preoperative localizing study; 70 % (21/30) of patients had three or more preoperative localizing studies. Twenty-four patients had US, 28 patients MIBI, 19 CT scan, 18 MRI, and 4 patients had invasive preoperative localizing studies. Sensitivities and positive predictive values (PPV) for localizing an enlarged parathyroid gland for each test are listed in Table 3. US and MIBI were discordant in 4 cases and CT or MRI correctly identified the correct gland in two of these four cases. Invasive studies were only obtained in four patients because of discordant noninvasive imaging results or at the discretion of the attending physician and were found to be helpful to identify an enlarged parathyroid gland in three patients. Sensitivity of these tests was not significantly different if three or more versus less than three glands were removed before reoperation. Seventeen percent (5/30) of patients had supernumerary glands identified during reoperation that were not found on preoperative localizing studies, which decreased sensitivity of US and MIBI to detect all enlarged glands to 88 and 72 %, respectively. Of these five patients, three had one additional enlarged gland identified on the same side and two patients had one additional gland identified on the opposite side; three patients had additional thymectomy during that reoperation. Of the four patients who experienced persistent pHPT, three had a positive preoperative localizing study and none had venous sampling. One had two, one had three, and two had 4.5 glands removed after all operative procedures. Thymectomy was performed in one of four patients who experienced persistent pHPT during reoperation at our institution.
TABLE 3.
Performance of preoperative localizing tests for localizing an enlarged parathyroid gland in patients with MEN1 and pHPT undergoing reoperation
| Sensitivity (%) | PPV (%)a | |
|---|---|---|
| Ultrasound (n = 24) | 100 | 92 |
| Sestamibi (n = 28) | 85 | 96 |
| CT neck (n = 18) | 75 | 86 |
| MRI neck (n = 17) | 63 | 91 |
| Venous sampling (n = 4) | 100 | 75 |
Positive predictive value
Performance of Intraoperative Parathyroid Hormone Measurement
All but one patient had IOPTH measured during the procedure. Sensitivity and PPV for IOPTH were 92 and 92 % for postoperative eucalcemia, respectively. Of the five patients with supernumerary glands identified intraoperatively and not preoperatively, all had <50 % decrease in IOPTH 10 min postexcision of the initial parathyroid gland, which prompted further exploration and identification of another enlarged gland in all patients. One patient developed recurrent pHPT at 1 year postoperatively. The remaining four patients were in remission at last follow-up. An IOPTH level below the lower limit of normal after reoperation was not associated with postoperative hypoparathyroidism (p = 0.1).
Immediate vs. Delayed Parathyroid Autotransplantation
Of the ten patients with transient hypoparathyroidism, three patients underwent immediate and one patient required delayed autotransplant. All other cases resolved with oral calcium and 1,25-dihydroxycholecalciferol suppleme ntation. Nine patients (30 %) underwent immediate autotransplantation during reoperation, because it was thought to be their last parathyroid gland and/or because the IOPTH dropped <20 pg/mL; seven had ≥3.5 glands removed initially, and two patients had 2.5 glands removed. One patient had permanent hypoparathyroidism and three patients had transient hypoparathyroidism. Two patients underwent a second delayed autotransplant of cryopreserved parathyroid tissue 8 weeks after the initial one. Seven of nine patients with immediate autotransplant had normal serum calcium levels at last follow-up. Long-term data on two patients was missing. Five patients (17 %) underwent delayed autotransplant for persistent hypoparathyroidism; four of those patients had normal serum calcium levels at last follow-up. None of the autotransplant grafts had to be removed. There were no additional comorbidities encoun tered after reoperation.
Metabolic Outcome and Genotype/Phenotype Associations
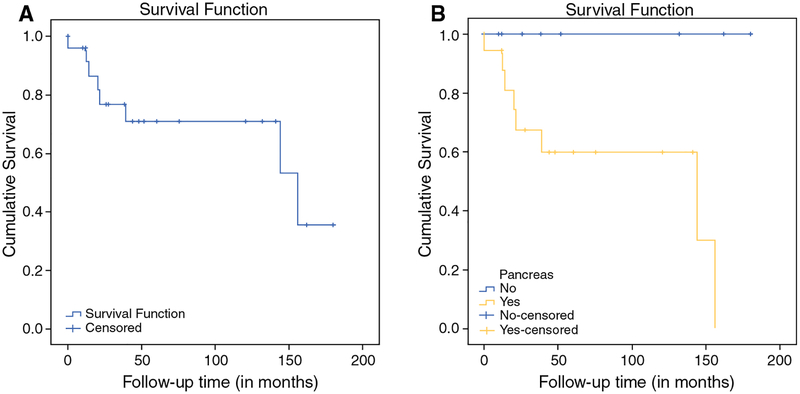
Pre- and postoperative data on bone mineral density t-scores (BMD) and nephrocalcinosis (assessed by US or CT) was available in 12 and 18 patients, respectively. BMD did not change significantly after reoperation (p = 0.6); however, the number of patients who had nephrocalcinosis significantly decreased (p = 0.046; Supplemental Table 2). When comparing patients with recurrent/persistent pHPT versus patients with biochemical cure, the former group had a significantly higher fasting gastrin elevation (p = 0.03) and were more likely to have pancreatic tumors (p = 0.02). Estimated disease-free survival curves using the Kaplan–Meier method showed that the presence of pancreatic tumors was associated with a significant higher rate of recurrent/persistent pHPT (p = 0.021; Fig. 2b). There was no association between MEN1 genotype (group 1 vs. group 2) and recurrent/persistent pHPT.
FIG. 2.
a Estimated cumulative disease-free survival of patients with MEN1 undergoing reoperation for recurrent/persistent pHPT using the Kaplan–Meier method. b Estimated disease-free survival according to pancreatic phenotype (log rank test: p = 0.021)
DISCUSSION
pHPT occurs in almost all patients with MEN1. Initial surgical approaches comprise subtotal (3–3.5 gland) parathyroidectomy (SPTX) and thymectomy or total parathyroidectomy (TPTX), thymectomy and reimplantation of parathyroid tissue in the forearm. Recurrence rates for SPTX range from 12 to 67 % and for TPTX 4–55 % at long-term follow-up (8 years or more). These numbers emphasize that a vast majority of these patients will recur, if one watches them long enough.15 Therefore, one can probably not refer to “cure rate” but rather “remission rate” when referring to long-term follow-up in these patients. Additionally, the diagnosis of MEN1 is not always evident at initial exploration which can lead to a high rate of persistent disease if less than SPTX is performed during the initial operation; and ectopic or supernumerary glands have been documented in up to 30 % of cases.15 In this study, the rate of unrecognized MEN1 diagnosis at initial operation was 37 % and the rate of persistent disease after initial operation was 47 %.
A previous report from our institution comprising 75 patients yielded a biochemical cure/remission rate of 91 and a 33 % recurrence rate after reoperative surgery.15 In our study, a similar surgical cure/remission rate of 87 % was noted, but our recurrence rate was lower at 13 %. This may be due to the shorter median follow-up time (33 vs. 58 months) or perhaps due to the usage of preoperative localizing studies combined with IOPTH, which may have allowed a better pre- and intraoperative assessment of the remaining parathyroid tissue, and therefore improved our remission rates in this patient population.
One study examining the role of sestamibi scan in reoperative surgery of MEN1 patients with recurrent pHPT showed 100 % sensitivity in 13 patients to identify enlarged parathyroid gland(s) preoperatively. However, seven supernumerary glands in four patients and a parathyroid remnant in one patient were not localized preoperatively with sestamibi scan, therefore yielding a sensitivity of 61 % in localizing all the enlarged glands.18 In our study, using all four routinely available preoperative localizing studies in most patients, we found that US and sestamibi had a sensitivity of 100 and 85 % at detecting an enlarge parathyroid gland, respectively. This number, however, decreases to 88 and 72 % when analyzing the detection rate of these modalities for all enlarged glands. Of the four patients with discordant US and MIBI, CT, or MRI correctly identified a parathyroid tumor in 50 % of cases, which suggests that a combination of US and sestamibi should be used as routine preoperative localizing tools before reexploration and that CT and MRI should be considered as useful adjuncts when these studies are discordant or nonlocalizing. Only four patients had invasive localizing studies; therefore, we cannot draw conclusions in terms of the performance of this modality. However, invasive localizing studies should be used when discordant or negative results are obtained preoperatively from other noninvasive imaging modalities.
In this study, IOPTH was particularly useful to identify patients with more than one enlarged gland not detected on preoperative localizing studies. Indeed, IOPTH did not drop appropriately in 5 patients, which prompted additional exploration and yielded additional enlarged glands that were not visualized by preoperative localizing studies. Since routine bilateral neck exploration for reoperative surgery in patients with pHPT and MEN1 is not usually recommended due to higher risk of recurrent laryngeal nerve injury, we recommend the routine use of IOPTH to help guide the extent of exploration, even when preoperative localizing studies appear to identify an enlarged parathyroid gland.
Controversial data exists on whether recurrence in patients with pHPT and MEN1 is associated with a specific genotype.16 A recent report by the DutchMEN1 Study Group, found that genotype did affect the chance of recurrence after surgical exploration in 73 patients with pHPT and MEN1. However, the decreased incidence of recurrence was only found in patients with mutations in exons 2, 7, and 9 who had less than three parathyroid glands resected during initial operation.17 In our study, we could not identify a genotype that was associated with higher recurrence rates after reoperative surgery in these patients. However, it is striking that no patients without pancreatic manifestations of MEN1 had persistent/recurrent pHPT after reoperation at last follow-up. We believe that this may be important information for clinicians as it could help them adjust patient expectations and surveil-lance strategy. A specific correlation of gastrin levels and recurrence could not be found, although the preoperative gastrin levels were significantly higher in the group of patients with recurrent/persistent disease. Because we did not have postoperative gastrin levels available in most patients, it was not possible to assess improvement of those levels after reoperation, as previously described.21
Even though success rates vary widely for autotrans-plantation of parathyroid glands after cryopreservation, it should be performed in all patients undergoing reoperative for MEN1-associated pHPT. This is because delayed autotransplantation was required in five patients in our study at >6 months postoperative and in two patients 8 weeks after failed immediate autotransplant (total of 23 % of all patients), which is similar to previously reported rates (17–43 %).15,19,22
Management of recurrent pHPT in MEN1 after initial exploration is complex. A key aspect of the disease management is the decision as to when one should reoperate for pHPT in MEN1 patients, as re-do neck operations have been associated with increased morbidity.13,14 In this study all patients undergoing reoperation where symptomatic and/or had metabolic complications (decreased BMD scores). Even though BMD scores did not significantly improve after reoperation at last follow-up, presence of nephrocalcinosis was significantly lower at last follow-up.
There are several limitations to this study. For one, the retrospective nature of this study and small sample size limit the ability to apply these concepts to all patients. Moreover, the relatively short follow-up time may have skewed some results, especially those related to genotype/phenotype correlation as well as BMD, as re-mineralization of bone mass can take several years. Lastly, BMD was measured at different testing locations and included only information on femur/hip t scores.
CONCLUSIONS
Our current strategy for reoperation in MEN1 patients with pHPT include: (1) review of past operative and pathology reports to gain maximal information about current anatomy and the remaining parathyroid glands; (2) obtain preoperative non-invasive localizing studies to get strong prediction of the dominant tumor(s); (3) obtain invasive studies if further information is warranted; (4) use IOPTH to guide decisions about when to finish the neck exploration; (5) routine cryopreservation and immediate autotransplantation when the last parathyroid gland is resected or delayed autotransplantation for persistent hypoparathyroidism.
Although preoperative localizing studies can be helpful for guiding reoperative strategy in MEN1 with persistent/recurrent pHPT, a significant percentage of additional enlarged glands may be missed by conventional preoperative imaging. IOPTH should be employed routinely, because it helps to identify additional glands missed by preoperative imaging. Routine cryopreservation should be considered in all patients. Additionally, patients with pancreatic neuroendocrine tumor phenotype may be more likely to recur earlier or persist than those without. Further larger cohort studies are needed to confirm these findings.
Supplementary Material
Footnotes
Accepted for Poster Presentation at the American Association of Endocrine Surgery (AAES) Annual Meeting, Baltimore, MD, April 10–12, 2016.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-016-5467-x) contains supplementary material, which is available to authorized users.
DISCLOSURE The authors declare no conflict of interest.
REFERENCES
- 1.Goudet P, Murat A, Binquet C, Cardot-Bauters C, Costa A, Ruszniewski P, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34(2):249–55. [DOI] [PubMed] [Google Scholar]
- 2.Lourenco DM Jr., Toledo RA, Coutinho FL, Margarido LC, Siqueira SA, dos Santos MA, et al. The impact of clinical and genetic screenings on the management of the multiple endocrine neoplasia type 1. Clinics. 2007;62(4):465–76. [PubMed] [Google Scholar]
- 3.Pieterman CR, Schreinemakers JM, Koppeschaar HP, Vriens MR, Rinkes IH, Zonnenberg BA, et al. Multiple endocrine neoplasia type 1 (MEN1): its manifestations and effect of genetic screening on clinical outcome. Clin Endocrinol. 2009;70(4):575–81. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann J, Fendrich V, Habbe N, Bartsch DK, Slater EP, Kann PH, et al. Screening of patients with multiple endocrine neoplasia type 1 (MEN-1): a critical analysis of its value. World J Sur. 2009;33(6):1208–18. [DOI] [PubMed] [Google Scholar]
- 5.Kraimps JL, Duh QY, Demeure M, Clark OH. Hyperparathyroidism in multiple endocrine neoplasia syndrome. Surgery. 1992;112(6):1080–6; discussion 6-8. [PubMed] [Google Scholar]
- 6.Marx SJ, Menczel J, Campbell G, Aurbach GD, Spiegel AM, Norton JA. Heterogeneous size of the parathyroid glands in familial multiple endocrine neoplasia type 1. Clin Endocrinol. 1991;35(6):521–6. [DOI] [PubMed] [Google Scholar]
- 7.Elaraj DM, Skarulis MC, Libutti SK, Norton JA, Bartlett DL, Pingpank JF, et al. Results of initial operation for hyper-parathyroidism in patients with multiple endocrine neoplasia type 1. Surgery. 2003;134(6):858–64; discussion 64-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee CH, Tseng LM, Chen JY, Hsiao HY, Yang AH. Primary hyperparathyroidism in multiple endocrine neoplasia type 1: individualized management with low recurrence rates. Ann Surg Oncol. 2006;13(1):103–9. [DOI] [PubMed] [Google Scholar]
- 9.Dotzenrath C, Cupisti K, Goretzki PE, Yang Q, Simon D, Ohmann C, et al. Long-term biochemical results after operative treatment of primary hyperparathyroidism associated with multiple endocrine neoplasia types I and IIa: is a more or less extended operation essential? Eur J Surg. 2001;167(3):173–8. [DOI] [PubMed] [Google Scholar]
- 10.Hellman P, Skogseid B, Oberg K, Juhlin C, Akerstrom G, Rastad J. Primary and reoperative parathyroid operations in hyperparathyroidism of multiple endocrine neoplasia type 1. Surgery. 1998;124(6):993–9. [PubMed] [Google Scholar]
- 11.O’Riordain DS, O’Brien T, Grant CS, Weaver A, Gharib H, van Heerden JA. Surgical management of primary hyperparathyroidism in multiple endocrine neoplasia types 1 and 2. Surgery. 1993;114(6):1031–7; discussion 7-9. [PubMed] [Google Scholar]
- 12.Rizzoli R, Green J, 3rd, Marx SJ. Primary hyperparathyroidism in familial multiple endocrine neoplasia type I. Long-term follow-up of serum calcium levels after parathyroidectomy. Am J Med. 1985;78(3):467–74. [DOI] [PubMed] [Google Scholar]
- 13.Carling T, Udelsman R. Parathyroid surgery in familial hyperparathyroid disorders. J Intern Med. 2005;257(1):27–37. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard JG, Sebag F, Maweja S, Henry JF. Primary hyper-parathyroidism in MEN 1: how radical should surgery be? Langenbeck’s Arch Surg. 2002;386(8):553–7. [DOI] [PubMed] [Google Scholar]
- 15.Kivlen MH, Bartlett DL, Libutti SK, Skarulis MC, Marx SJ, Simonds WF, et al. Reoperation for hyperparathyroidism in multiple endocrine neoplasia type 1. Surgery. 2001;130(6):991–8. [DOI] [PubMed] [Google Scholar]
- 16.Langer P, Wild A, Schilling T, Nies C, Rothmund M, Bartsch DK. [Multiple endocrine neoplasia type 1. Surgical therapy of primary hyperparathyroidism]. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2004;75(9):900–6. [DOI] [PubMed] [Google Scholar]
- 17.Pieterman CR, van Hulsteijn LT, den Heijer M, van der Luijt RB, Bonenkamp JJ, Hermus AR, et al. Primary hyperparathyroidism in MEN1 patients: a cohort study with longterm follow-up on preferred surgical procedure and the relation with geno-type. Ann Surg. 2012;255(6):1171–8. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd JJ, Burgess JR, Greenaway TM, Ware R. Preoperative sestamibi scanning and surgical findings at bilateral, unilateral, or minimal reoperation for recurrent hyperparathyroidism after subtotal parathyroidectomy in patients with multiple endocrine neoplasia type 1. Arch Surg. 2000;135(7):844–8. [DOI] [PubMed] [Google Scholar]
- 19.Feldman AL, Sharaf RN, Skarulis MC, Bartlett DL, Libutti SK, Weinstein LS, et al. Results of heterotopic parathyroid autotrans-plantation: a 13-year experience. Surgery. 1999;126(6):1042–8. [DOI] [PubMed] [Google Scholar]
- 20.Libutti SK, Alexander HR, Bartlett DL, Sampson ML, Ruddel ME, Skarulis M, et al. Kinetic analysis of the rapid intraoperative parathyroid hormone assay in patients during operation for hyperparathyroidism. Surgery. 1999;126(6):1145–50; discussion 50-1. [DOI] [PubMed] [Google Scholar]
- 21.Norton JA, Cornelius MJ, Doppman JL, Maton PN, Gardner JD, Jensen RT. Effect of parathyroidectomy in patients with hyperparathyroidism, Zollinger–Ellison syndrome, and multiple endocrine neoplasia type I: a prospective study. Surgery. 1987;102(6):958–66. [PubMed] [Google Scholar]
- 22.Borot S, Lapierre V, Carnaille B, Goudet P, Penfornis A. Results of cryopreserved parathyroid autografts: a retrospective multicenter study. Surgery. 2010;147(4):529–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.