Abstract
Efforts to identify the mechanisms for the initiation and maintenance of human atrial fibrillation (AF) often focus on changes in specific elements of the atrial “substrate,” i.e., its electrophysiological properties and/or structural components. We used experimentally validated mathematical models of the human atrial myocyte action potential (AP), both at baseline in sinus rhythm (SR) and in the setting of chronic AF, to identify significant contributions of the Ca2+-independent transient outward K+ current (Ito) to electrophysiological instability and arrhythmia initiation. First, we explored whether changes in the recovery or restitution of the AP duration (APD) and/or its dynamic stability (alternans) can be modulated by Ito. Recent reports have identified disease-dependent spatial differences in expression levels of the specific K+ channel α-subunits that underlie Ito in the left atrium. Therefore, we studied the functional consequences of this by deletion of 50% of native Ito (Kv4.3) and its replacement with Kv1.4. Interestingly, significant changes in the short-term stability of the human atrial AP waveform were revealed. Specifically, this K+ channel isoform switch produced discontinuities in the initial slope of the APD restitution curve and appearance of APD alternans. This pattern of in silico results resembles some of the changes observed in high-resolution clinical electrophysiological recordings. Important insights into mechanisms for these changes emerged from known biophysical properties (reactivation kinetics) of Kv1.4 versus those of Kv4.3. These results suggest new approaches for pharmacological management of AF, based on molecular properties of specific K+ isoforms and their changed expression during progressive disease.
NEW & NOTEWORTHY Clinical studies identify oscillations (alternans) in action potential (AP) duration as a predictor for atrial fibrillation (AF). The abbreviated AP in AF also involves changes in K+ currents and early repolarization of the AP. Our simulations illustrate how substitution of Kv1.4 for the native current, Kv4.3, alters the AP waveform and enhances alternans. Knowledge of this “isoform switch” and related dynamics in the AF substrate may guide new approaches for detection and management of AF.
Keywords: action potential alternans, action potential restitution, atrial fibrillation, human atrial myocyte, K+ channel isoform switch, mathematical modeling, transient outward K+ currents
INTRODUCTION
In the human atrium, short- and long-term rhythm disturbances [flutter or atrial fibrillation (AF)] are often initiated or dependent on changes (remodeling) in components of the “substrate.” This remodeling can significantly alter the electrophysiological or structural elements or both (10, 11, 18, 48, 67, 70, 79, 91, 97). In practice, electrophysiological remodeling can include alterations in 1) the ion channels and transporters that are responsible for the action potential (AP) and resting potential (18, 44, 91), 2) Ca2+ homeostasis mechanisms (3, 13, 26, 38, 54, 92), 3) the functional balance of autonomic innervation (5, 45, 66, 67), and 4) connexin-mediated intercellular coupling (51, 61, 110). Additional significant changes in the electrophysiological characteristics of atrial myocytes develop as a consequence of both static (diastolic) and phasic (stretch) and have been associated with an increased incidence and duration of human atrial rhythm disturbances (28, 49, 107), particularly in the settings of progressive heart failure (52, 80, 107, 108), hypertension (116), or stroke (131). Structural remodeling is typically described in terms of the extent of fibrosis, as judged by the patterns and extent of collagen deposition (44, 53), as well as atrial enlargement and alterations in T-tubule density and microanatomy (27).
The initiation and progression to AF is often preceded by repetitive cyclic variations in AP duration (APD) (19, 24, 33, 34, 77–79). This phenomenon is denoted as APD alternans (14, 19, 91, 121). Extensive experimental and theoretical (mathematical modeling) work on single myocytes, isolated trabeculae, and Langendorff-perfused mammalian heart preparations has provided firm evidence for the involvement of cyclic changes in intracellular Ca2+ concentration ([Ca2+]i) in the initiation and maintenance of ventricular APD alternans (13, 38, 54, 92, 107). Somewhat similar findings derived from both basic and clinical studies describing the initiation of atrial rhythm disturbances at specific ectopic foci in the atria have been published (56, 78, 92, 106, 117).
These findings, however, need to be considered in the context of earlier studies that revealed a very sensitive and strong relationship between changes in [Ca2+]i and even very small alterations in the repolarization waveform of the AP (7, 17, 58, 89, 101). Such small APD changes (often reported as being most prominent during the early repolarization phase of the AP) also exhibit cyclic changes or alternans (57, 62, 92, 114, 117). For these reasons, we examined whether specific changes in the complement of K+ currents that are responsible for early repolarization in the human atrium can initiate or contribute to the maintenance of APD alternans (71, 80, 85, 95, 99, 100, 117, 122, 125, 126, 130).
We have used two closely related mathematical models of the human atrial myocyte AP in this study. Both of these models (18, 83) have been developed and validated either from data obtained in control conditions [sinus rhythm (SR)] or from results obtained from myocytes exhibiting chronic AF. Our main focus was to assess the effects of changes in specific patterns of K+ channel isoform expression that underlie early repolarization of the atrial AP. Accordingly, our study evaluated the main functional consequences of selective changes in the predominant α-subunits of the Ca2+-independent transient outward K+ current (Ito). A primary impetus for this computational work was the report of significant changes in K+ current ion channel isoforms in well-defined regions of the left atrium (the posterior wall) after chronic AF (46, 97, 100). Specifically, we noted that this pattern of K+ channel expression includes an isoform switch (from Kv4.3 to Kv1.4) that has been previously reported in important pathophysiological settings in human ventricles. These settings include hypertrophy, hypothyroidism, and type 2 diabetes (22, 84, 89, 105). This type of isoform switch is also known to occur during embryonic/fetal development and also during the pathophysiological process known as “return to a fetal/neonatal electrophysiological phenotype” in the mammalian myocardium (23, 93, 97, 120). With respect to regulation of the plateau height and APD in the atrium, it is known that marked differences in the reactivation time course of the adult (mainly Kv4.3) versus neonatal (Kv1.4) isoforms of K+ channel α-subunits are strong contributing factors (30, 31, 120, 121). This regulation is clearly manifested when repolarization dynamics and the underlying currents in rabbit and human atria are compared.
Based on this background, our in silico study was designed to evaluate the hypothesis that a specific change (substitution of 50% of the Kv4.3 conductance with Kv1.4) in the molecular and biophysical properties of Ito in the human atrium can 1) significantly alter the dynamics of the APD and its restitution and 2) introduce APD alternans and perhaps lead to an enhanced likelihood of AF initiation/maintenance.
METHODS
Project design and goals.
Our first objective was to explore and illustrate the relative contributions of K+ currents in the selected models of the AP waveform under SR and AF conditions. The second goal was to determine whether functionally important effects can result from the chosen alterations in the molecular composition and biophysical properties of these K+ current densities. Accordingly, we evaluated the electrophysiological consequences of partial substitution of the K+ channel isoforms that are responsible for Ito. This current strongly modulates early repolarization (89, 101, 113, 119). After the changes in the AP waveform that occur in each of these single myocyte membrane AP models under baseline conditions were confirmed, our computations explored the consequences of a 50% substitution of Kv1.4 for Kv4.3. An important rationale for this maneuver is based on the recent finding that a region in the left atrium of the mammalian heart that is one important site for the initiation of AF consistently exhibits significant expression of Kv1.4 (73, 81, 99, 100, 123). It is also noteworthy that in most healthy mammalian hearts this Kv channel isoform is prominent during early development (23, 81) and is also expressed in healthy Purkinje fibers (2, 98, 102, 128). Interestingly, Kv1.4 expression increases in cardiac tissue that is progressively challenged in a variety of pathophysiological settings. This pattern of change is often referred to as an “isoform switch” to a predominant fetal or neonatal phenotype (73, 81, 93, 98, 119). An apparent exception to this pattern is the molecular identity of Ito in the adult rabbit heart. In the healthy mature rabbit, expression of Kv1.4 makes a significant contribution to Ito even under baseline conditions (31, 65, 121; cf. 2).
Computational details.
This study was made possible by the previous publication of well-documented mathematical models of the human atrial myocyte AP (18, 83; see also 40) that are based on experimental data from human atria obtained under control or SR conditions and also following the electrophysiological remodeling that occurs as a consequence of chronic AF. In this study, we simulated the human atrial AP using a recent mathematical model of human atrial electrophysiology (83). This model was closely based on the well-validated models of Colman et al. (18). In the present work, we incorporated a small, slowly inactivating component (INaL) of Na+ current (6) in the models of both SR and AF conditions. INaL was described as follows:
where GNaL is the maximal conductance of INaL. In all of our simulations, GNaL was assigned to be 0.2% of the maximal conductance for INa (GNa) in both SR and AF settings. mNaL and hNaL are the activation and inactivation variables, V is the transmembrane potential, and ENa is the reversal potential for INaL. Both mNaL and hNaL were formulated identical to those for peak Na+ current (INa). The time constant that captures the slow inactivation of INaL () is as follows:
It was derived from data in Maltsev et al. (72) and also adjusted (= 2.83) for the assumed temperature (37°C). In this study, AF conditions were simulated using the same AF-induced remodeling effects at the cellular level described by Ni et al. (83).
Mathematical formulations of Ito.
V1/2 values for steady-state variables of Ito (Kv4.3) formulations in the original paper by Ni et al. (83) were less positive than the reported values from previous experimental studies (36, 37, 108). Based on this, the present model of Ito (Kv4.3) was updated by shifting the V1/2 of steady-state activation and inactivation variables to the right by 16 and 10 mV, respectively. The maximum conductance of Ito was tuned so that the resulting AP was not changed after this update.
We modeled Kv1.4 current based on published experimental data (16, 31, 84, 120). In previous studies, reactivation kinetics were formulated as two separate exponential components. This was done to account for the very slow recovery of Kv1.4 consistently observed in double-pulse recovery protocols. Accordingly, we described the current as follows:
where Gto is the maximum conductance of this K+ current, which was kept identical to that of Ito, s is the activation gating variable, rfast and rslow are the fast and slow components, respectively, of inactivation variables, and EK is the reversal potential of this K+ current. The kinetics of the gating variables are given as follows:
and
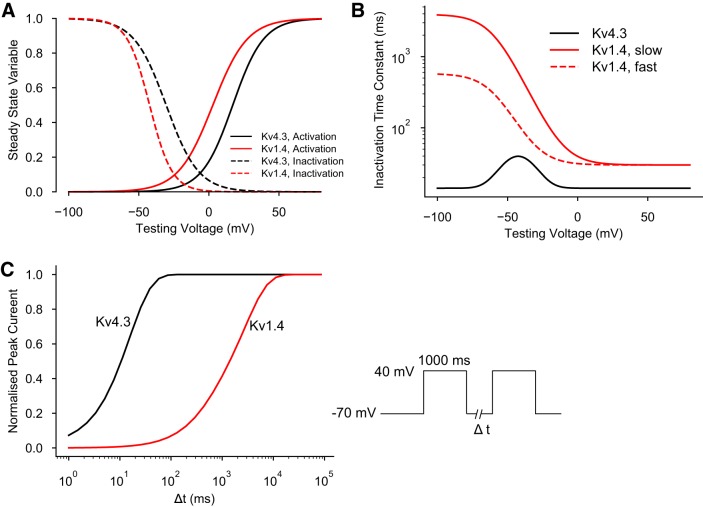
The time constants for inactivation were determined by matching the values recorded at a holding potential of −80 mV (16). The biophysical parameters that govern the electrophysiological behavior of Kv4.3 and Kv1.4 are shown in Fig. 1, A and B. In addition, the two sigmoid traces shown in Fig. 1C show the respective time courses for reactivation of Kv4.3 (black) versus Kv1.4 (red) at a membrane potential of −70 mV. This was selected to closely approximate the resting potential of a human atrial myocyte.
Fig. 1.
Biophysical parameters that regulate the K+ current transcripts Kv4.3 and Kv1.4. These are two of the α-subunits that are known to be involved in generating Ca2+-independent transient outward K+ current (Ito) in the human atrium. A: steady-state activation (solid lines) and inactivation parameters (dashed lines) for these two K+ current transcripts. Black, parameters for Kv4.3; red, parameters for Kv1.4. B: time constants for inactivation of Kv4.3 (black) and Kv1.4 (red). C: time courses for reactivation of Kv4.3 (black) versus Kv1.4 (red) at a membrane potential of −70 mV. Note that these reactivation time courses are plotted on a log scale.
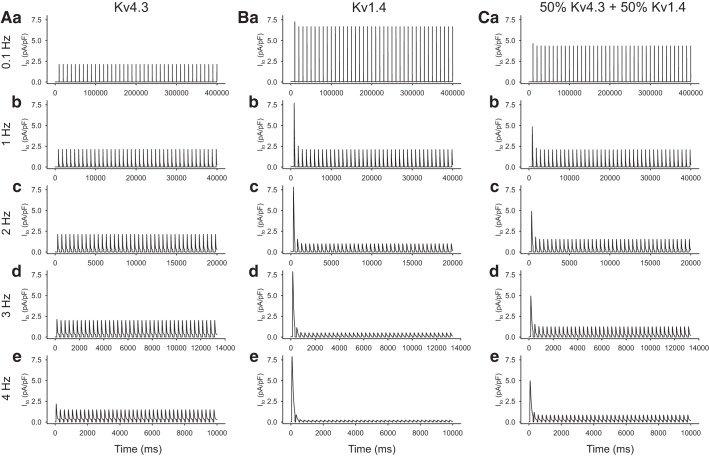
The main consequence of introducing Kv1.4 as a significant contributor to Ito is shown in Fig. 2. The marked difference in the ability of Kv1.4 to respond to repetitive stimulation is noteworthy. This is due to the much slower reactivation kinetics of Kv1.4 compared with those for Kv4.3; specifically, this results in a marked slowing of the reactivation kinetics of the hybrid current (Fig. 2, right).
Fig. 2.
Summary of the contrasting effects of selected rates of stimulation/voltage clamp-induced activation on Ca2+-independent transient outward K+ current (Ito) in the human atrium models in which Ito is generated by only Kv4.3 (A), only Kv1.4 (B), and a 50:50 mixture of Kv4.3 and Kv1.4 (C) while ensuring that Ito conductance remained constant. In all simulations, the holding potential was −60 mV, and each Ito record was elicited by a 200-ms rectangular voltage clamp step to 0 mV.
Simulation protocols.
In silico experiments included voltage-clamp (Figs. 1 and 2) or current-clamp (Figs. 3–7) protocols that were carried out at relevant, fixed heart rates (1 and 4 Hz). Most experiments also included timed applications of a single extra stimulus (S1-S2) to assess APD restitution. Standard S1-S2 stimulus protocols (43) were also used to generate the data (“extra” APs) that form the basis for constructing the APD restitution curves shown in Figs. 6 and 7. These restitution relationships were constructed/derived at both 20% and 90% of full repolarization (APD20 and APD90, respectively). In this analysis, responses to long-lasting trains of stimuli were also used in an attempt to reveal any APD variability (alternans) and gain information about the duration of this instability over a large range of pacing cycle parameters.
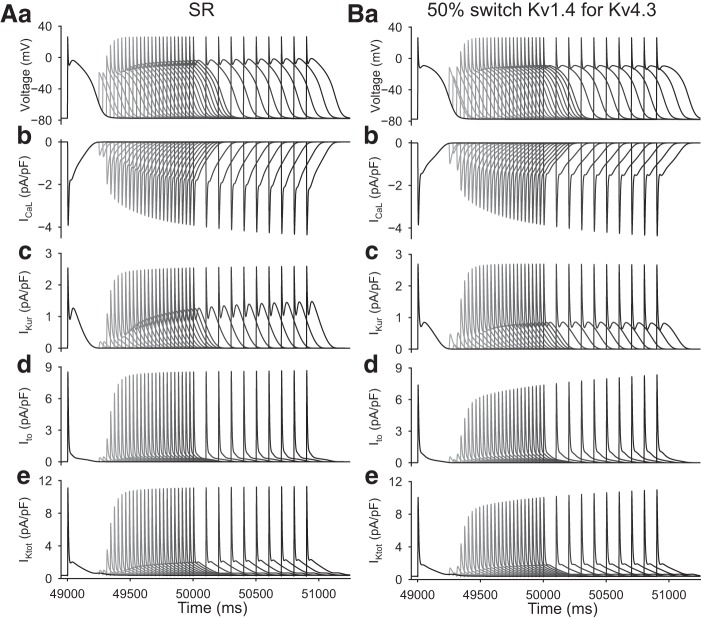
Fig. 3.
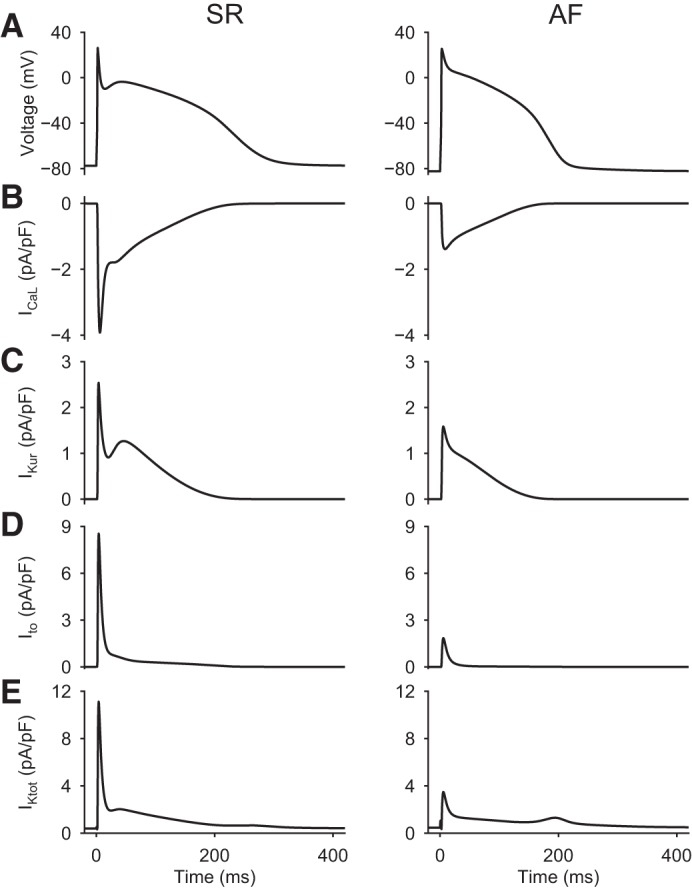
Simulated membrane action potentials (AP) and selected underlying ionic currents generated under sinus rhythm (SR; left) and atrial fibrillation (AF; right) conditions, both paced at 1 Hz. A: AP. B: L-type Ca2+ current (ICaL). C: ultrarapid delayed rectifier K+ current (IKur). D: Ca2+-independent transient outward K+ current (Ito). E: total outward K+ current (IKtot).
Fig. 7.
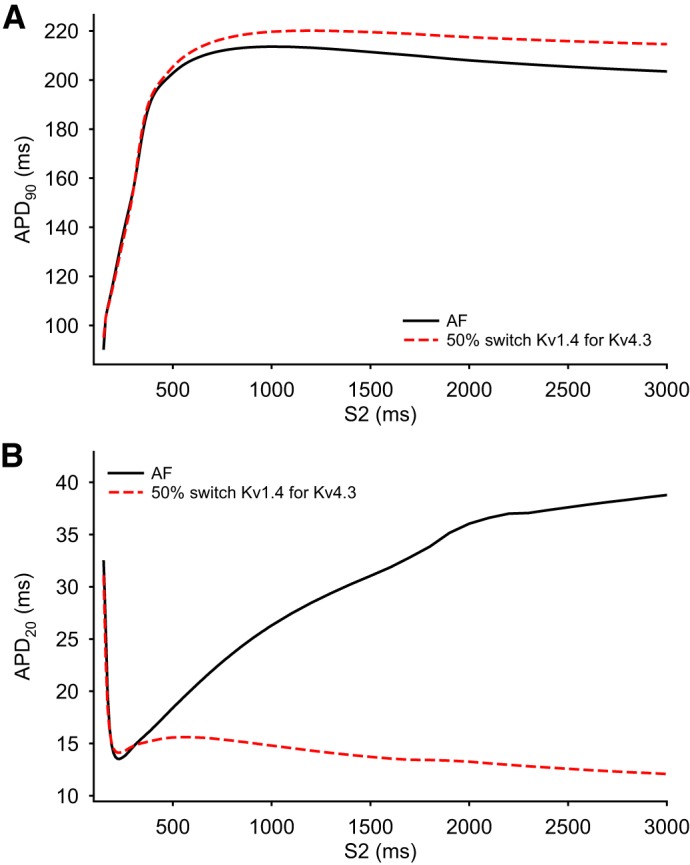
Analysis of the dynamic changes in the action potential (AP) waveform in the human atrium in response to premature stimulation under atrial fibrillation (AF) conditions. The two superimposed AP duration (APD) restitution curves in both A and B compare the simulated effects of a single extra stimulus, S2, applied at selected intervals after a 4-Hz train of stimuli using both the baseline AF model (solid black curved lines) and a variant of this model in which 50% of Kv4.3 was substituted with Kv1.4 (dashed red curved line). Note that in A the restitution curves were constructed using APD at 90% repolarization (APD90) measurements to reflect final repolarization of the AP, whereas in B APD at 20% repolarization (APD20) measurements were made to assess changes in the early repolarization phase.
Fig. 6.
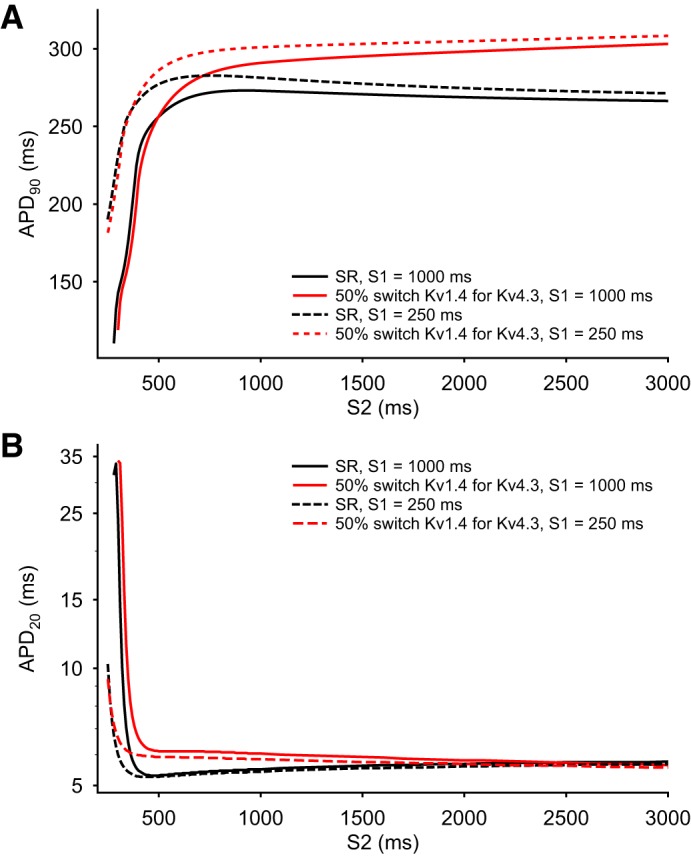
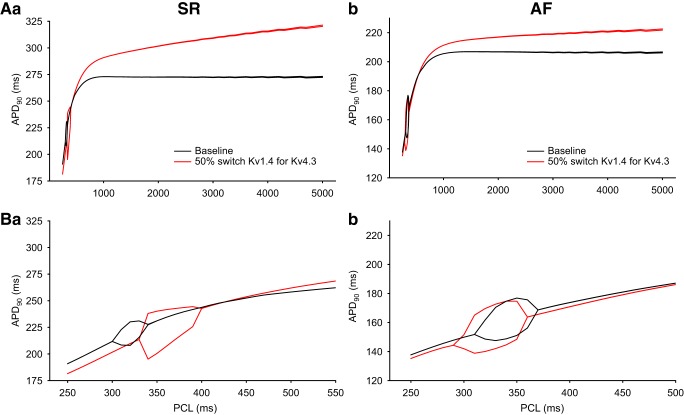
Analysis of the dynamic changes in the action potential (AP) duration (APD) in response to premature stimulation under sinus rhythm (SR) conditions. The four superimposed APD restitution curves in each Panel compare the simulated effects of maintained S1 stimulation rates at either 1 or 4 Hz under SR conditions using the baseline SR model and after 50% switch of Kv1.4 for Kv4.3 was introduced. A: each curved line illustrates the time course and extent of APD restitution based on APD at 90% repolarization (APD90) measurements. B: the four analogous restitution curves were constructed from APD at 20% repolarization (APD20) measurements. The dramatic differences between the patterns of results in A versus B reflect the collapse of the AP plateau that is captured by APD20 measurements. This is in distinction to the progressive changes that are the basis for the APD90 restitution curves.
RESULTS
Illustration of time- and voltage-dependent Ca2+ and K+ currents that underlie the human atrial AP.
The electrophysiological principles that form the starting point for this modeling study are shown in Fig. 3. In each of these two separate sets of simulations, trains of membrane APs were generated at a fixed pacing frequency of 1 Hz using either the SR or AF model. AP waveforms and the selected underlying transmembrane ionic currents were sampled at a fixed time point 50 s into this stimulus train to ensure steady-state conditions. The two columns of data shown in Fig. 3 show control or SR data on the left and AF data on the right. Transmembrane L-type Ca2+ current (ICaL), ultrarapid delayed rectifier K+ current (IKur), and Ito are shown in Fig. 3, B, C, and D, respectively. Figure 3E shows the total outward K+ current (IKtot) that is activated during a single AP in each model. IKtot consists of background inward rectifier K+ current (IK1) as well as Ito, IKur, and very small contributions from the HERG K+ current and so-called slow delayed rectifier K+ current (IKs).
It is apparent from these simulations that after the initial rapid depolarization (not shown), the most prominent transmembrane current changes that underlie/produce the AP are ICaL, Ito, and IKur or Kv1.5. In the previously published AF model (18), each of these currents is significantly (50–70%) reduced with respect to their SR or baseline values. This pattern has been documented in previous publications based on experimental data from myocytes isolated from chronic AF tissue (cf. 10, 11, 18, 40, and 107). One aspect of the IKtot record in AF conditions (Fig. 3E) deserves detailed consideration. Note that in the setting of AF, IKtot exhibits a secondary outward “hump” at ∼200 ms, i.e., during the late repolarization phase of the AP. This is due mainly to IK1, which is known to be significantly increased (10, 11, 40, 42, 125) in the setting of AF (see discussion). This important electrophysiological difference stabilizes the resting potential and augments the final repolarization phase of the AP by enhancing the repolarization reserve.
The data shown in Figs. 4A and 5A reveal the major rate- or frequency-dependent changes in the selected ionic currents in the SR model (Fig. 4) compared with the AF model (Fig. 5) when Ito is generated entirely by Kv4.3. In both sets of computations, these composite data were generated using a standard S1-S2 pacing protocol: the S1 rate was 1,000 ms in Fig. 4 and 250 ms in Fig. 5. These were chosen to correspond to the slow and fast pacing rates characteristic of the SR and AF conditions, respectively. To assess AP waveform dynamics, a single S2 stimulus was applied after each 50-s train of S1 stimuli. The chosen protocol scanned S2 intervals between 250 and 5,000 ms in SR conditions (Fig. 4). In AF conditions (Fig. 5), the chosen range of S2 durations was between 150 and 5,000 ms. The main time- and frequency-dependent changes in transmembrane currents that occur in the SR model in response to S2 stimuli are progressive reactivation of ICaL and a similar but oppositely directed progressive reactivation of Ito as well as residual activation of IKur. These separate and distinct time- and voltage-dependent recovery or reactivation dynamics are also captured in the IKtot record shown in Figs. 4Ae and 5Ae. Note that, due mainly to its larger size (current density), the reactivation of Ito is apparently the major contributor to the total net outward current during the early repolarization phases of the AP. Specifically, under SR conditions in this model (as has been well documented in experimental studies of the human atrium), the current density of Ito is two to three times that of IKur (1, 12, 18, 40, 83). Analogous data generated using the AF model are shown in Fig. 5. Here, however, the details of the time-dependent changes in net current are quite different. In the AF substrate, ICaL, Ito, and IKur were all very small, and in this setting IK1 was dominant.
Fig. 4.
Evaluation of the effects of premature stimulation on the human atrial action potential (AP) waveforms in sinus rhythm (SR) conditions. In the SR baseline model, Ca2+-independent transient outward K+ current (Ito) is generated by Kv4.3 (A); results are also shown after a 50% switch of Kv1.4 for Kv4.3 (B). In both A and B, b–e show the same Ca2+ and K+ currents that are shown in Fig. 3. See results for further information and explanation.
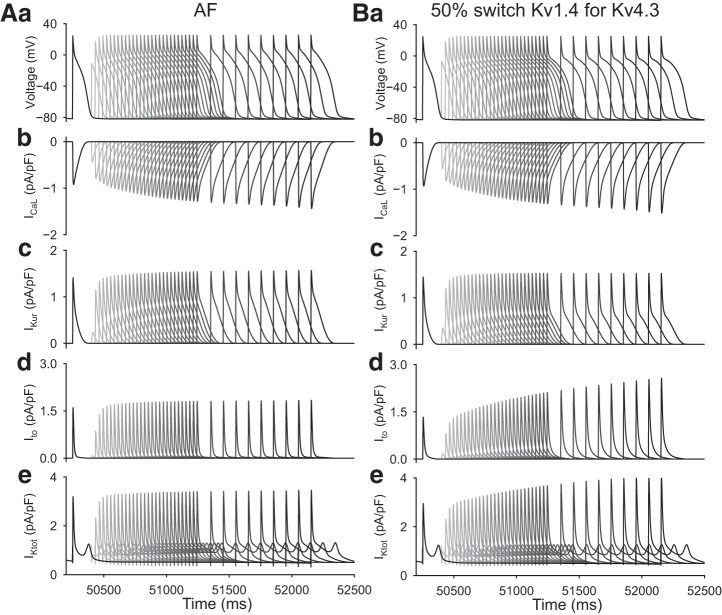
Fig. 5.
Evaluation of the effects of premature stimulation on action potential waveforms generated by the baseline atrial fibrillation (AF) model (A) compared with AF action potential waveforms generated after a switch of 50% of the Kv4.3 conductance for Kv1.4 (B). The layout of this figure is analogous to the sinus rhythm (SR) data shown in Fig. 4 to allow direct comparisons.
Careful inspection of the envelopes of AP recovery data under SR conditions (Fig. 4Aa) shows that the AP plateau becomes more positive and that its profile shows progressively more notching during early repolarization in the approximate diastolic interval range of 5–500 ms. The corresponding pattern of AP waveform changes under AF conditions (Fig. 5Aa) is quite different. Note that the much shorter APD and significantly reduced expression levels or current densities for ICaL and Ito combined to yield no significant AP waveform dynamics in the diastolic interval range that was examined (5–500 ms).
Effects of K+channel isoform “switches” on Ito.
To provide a direct, side-by-side comparison of the baseline data with results obtained after partial substitution of Kv1.4 for Kv4.3, the same stimulus protocols were applied to the altered substrates. The two data sets that illustrate these changes are shown in Figs. 4B and 5B. Before these computations were run, 50% of the baseline or Kv4.3 K+ channel isoform conductance was substituted with Kv1.4. We also ensured that total Ito conductance was unchanged, as described in detail in methods.
Inspection of the superimposed APs shown in Figs. 4Ba and 5Ba reveals that when this change is made using either of the modified SR and AF models, only very small alterations in the recovery kinetics or frequency dependence of Ito are apparent. However, under SR conditions, there is a smaller “recovery envelope” in the action plateau data (see Fig. 4Aa vs. Fig. 4Ba). Moreover, the AP plateau recovers with a more convex waveform and with little evidence of the notched early repolarization phase that is characteristic of the baseline data shown in Fig. 4Aa. Although the reasons for this need to be explored in more detail, when contrasting Fig. 4Bd with Fig. 5Bd, it is apparent that after this partial (50%) replacement of Kv4.3 with Kv1.4, the resulting Ito recovers or reactivates with a time course that is very similar to the reactivation of ICaL (see discussion). In the AF substrate, this partial isoform switch produces changes that closely resemble those in the baseline model (Fig. 4Aa vs. Fig. 5Ba). We note that in the modified AF setting the AP waveform is strongly regulated by the relatively large size and important voltage-dependent contribution of the outward current “hump” that arises from the quasi instantaneous and highly nonlinear behavior of IK1. Importantly, the changed AP waveform and related alterations in impedance profile during final repolarization can also alter the reactivation of ICaL (Fig. 5Bb) and IKur (Fig. 5Bc). As expected from the results shown in Fig. 2, the overall reactivation time course of Ito (Fig. 5Bd) is slowed markedly after the introduction of isoform switch under AF conditions. This change in Ito is sufficiently large that it is also clearly observed in IKtot records (Fig. 5Be).
Restitution of the APD.
To integrate these findings and attempt to relate them to previous experimental and mathematical modeling data sets, the rate-dependent changes in the simulated AP waveforms in Figs. 4 and 5 were analyzed and expressed in terms of conventional APD recovery or restitution curves (9, 13, 19, 35, 43, 69, 78), which are shown in Fig. 6 (SR model) and Fig. 7 (AF model), respectively. In Fig. 6, A and B, four superimposed APD restitution curves are shown in each panel. The data points that are the basis for the smooth curves shown in Fig. 6A were generated using the SR model driven at two different stimulus frequencies for S1: 1 Hz (solid line) and 4 Hz (dashed line). The 4-Hz data set from the SR model was generated in an attempt to assess its behavior under conditions that were comparable to firing rates with the data obtained from the AF model (Fig. 7). In both Figs. 6 and 7, measurements of APD were made at either APD90 (as shown in Figs. 6A and 7A) or APD20 (as shown in Figs. 6B and 7B).
All of these relationships were derived from measurements of dynamic restitution (32, 34) in response to trains of stimuli. Under these circumstances, the K+ channel isoform switch that is the focus of this study resulted in very little change in APD restitution measured at APD90. In contrast, when restitution was measured at APD20, marked differences were observed. In fact, at low rates of stimulation in the mammalian atrium, the AP plateau often essentially “collapses” (see discussion). These restitution relationships illustrate the intrinsic dynamic properties of APD regulation in both SR and AF models.
APD alternans revealed.
We next sought to obtain a detailed understanding of the changes in both the AP waveform and stability of the AP restitution relationship as a consequence of selected changes in the α-subunits for the K+ channels that are responsible for Ito. That is, we wanted to determine the following: 1) does this isoform switch result in enhanced APD90 alternans and 2) if so, does this APD instability relate to or complement previous clinical electrophysiological results? This was determined by testing the transient and maintained responses (changes in APD) of the models to rapid pacing trains at selected, fixed cycle lengths. Important new results are shown in Fig. 8. Note that in all four data sets shown in Fig. 8 there are discontinuities on the initial portion of each APD90 restitution curve. These are due to the presence of substantial beat-to-beat variations in APD90 under these pacing conditions. As shown in more detail in the two graphs in Fig. 8B, these transient changes in APD90 developed under both baseline conditions (SR model, left) and in the setting of AF when the 50% switch of Kv1.4 for Kv4.3 was made. Interestingly, however, this instability was somewhat enhanced (occurs over a broader range of S1 intervals) following the Kv4.3 for Kv1.4 isoform switch, particularly in the AF setting. Initial assessment of these changes in APD90 suggest that they are too small to be of functional importance. We note, however, that based on data obtained from high-resolution monophasic AP recordings as part of clinical electrophysiological assessments, this stimulus-induced APD90 “jitter” is consistently observed. Importantly, the monophasic AP data show that APD90 variability is only in the range of 10–15 ms (62, 78). Rigorous evaluation of atrial rhythm disturbances in an adult sheep model (50) has revealed a similar extent of APD variability.
Fig. 8.
Detection and initial analysis of action potential duration (APD) alternans in single myocyte human atrial action potential models. The rate dependence of APD at 90% repolarization (APD90) changes are illustrated as restitution curves. Each curve was constructed from in silico data obtained after the partial substitution of Kv1.4 for Kv4.3 in a human atrial myocyte model stimulated by rapid pacing in a selected range of pacing cycle lengths (PCLs). A, a and b: APD90 data for two consecutive beats using models that replicate sinus rhythm (SR; a) or atrial fibrillation (AF; b) conditions, respectively. B: the same data plotted on an expanded timescale to more clearly reveal the APD90 alternans behavior.
Figure 9 was included to provide detailed examples of this rate-dependent APD variability. Figure 9 also demonstrates that the pattern of alternans is significantly different when the AF variant of our human atrial AP model is used.
Fig. 9.
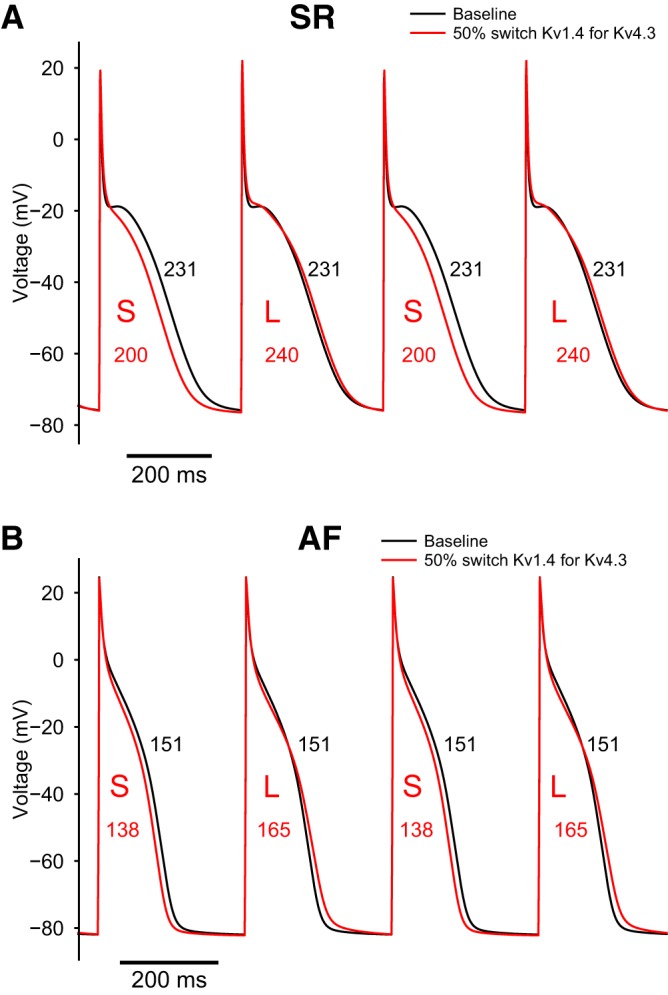
Detailed comparison of individual action potential (AP) waveforms generated by either the baseline model or the model that includes the 50% switch of Kv1.4 for Kv4.3. The superimposed AP records in A were computed under sinus rhythm (SR) conditions, whereas those in B were computed using the atrial fibrillation (AF) model. In both A and B, the numbers within the AP waveforms denote APD values and illustrate the alternans behavior. Note that the isoform switch (red traces) significantly enhances the tendency for the appearance of APD at 90% repolarization (APD90) alternans. L, longer APs; S, shorter APs. The pacing cycle lengths were 350 ms (A) and 310 ms (B).
In summary, this Kv1.4 for Kv4.3 substitution can result in significant changes in the stimulus frequency range (or window) within which APD90 instabilities are observed.
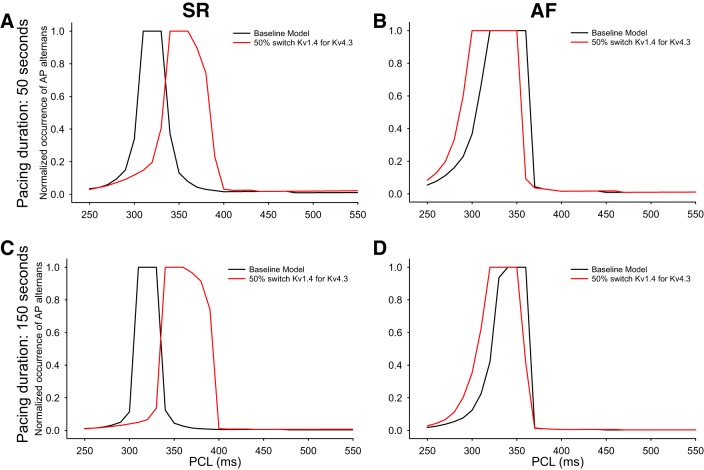
To begin to analyze this propensity for APD alternans, we have expressed our results in terms of the duration or length of the AP train that exhibits alternans and also computed the fraction of APs that show beat-to-beat variation. This analysis, shown in Fig. 10, revealed that the relationship between the fraction of APD alternans and pacing cycle length is sensitive to the K+ channel isoform switch that is the focus of this article but also indicated that the patterns of responses differ significantly when SR and AF data sets are compared.
Fig. 10.
Summary and illustration of the occurrence of short runs of action potential (APD90) alternans in the human atrium at selected pacing cycle lengths. A and B: normalized values for the occurrence of APD90 alternans when human atrial myocyte models were paced for 50 s under sinus rhythm (SR; A) and atrial fibrillation (AF; B) conditions. Results are shown under baseline SR and AF conditions (black traces) and after introduction of the 50% switch of Kv1.4 for Kv4.3 (red traces). C and D: initial evaluation of the duration of the pacemaker train on occurrence of APD90 alternans in the same myocytes that were paced for 150 as opposed to 50 s. To construct the individual graphs, the normalized occurrence of APD90 alternans was calculated as a ratio. For each chosen condition, this ratio consisted of the number of action potentials that exhibited alternans divided by the total number of action potentials that were evoked for each selected pacing cycle length and train duration.
APD90 alternans is reduced by combined block of Ito and IKur.
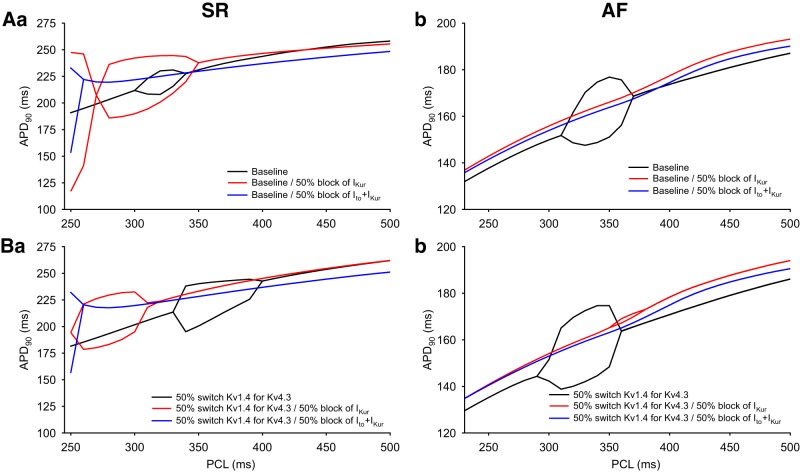
To further explore the role of Ito in the initiation or modulation of rhythm disturbances in human atrial myocytes, we examined the effects of blocking two K+ currents on APD restitution. Our final computations included making the same sets of changes under both SR and AF conditions that were previously done using both the baseline model and also the model that included the partial switch of Kv1.4 for Kv4.3. To extend this analysis to the setting of multiple ion channel block as an emerging approach for antiarrhythmic therapy for AF, we evaluated the combination of a partial block of both Ito and IKur. IKur or Kv1.5 is an independent but significant component of the repolarization reserve in the human atrium (1, 18, 29, 36, 40, 71). These sets of results are shown in Fig. 11. Although the patterns of changes are quite complex (and not fully understood), it is apparent that, in the SR condition when using the baseline model, even a partial (50%) block of IKur enhanced APD90 alternans. This is illustrated by the broader window of cycle lengths in which this alternans can be observed (Fig. 11Aa). After the partial switch of Kv1.4 for Kv4.3 was introduced into the baseline model, the window of cycle lengths in which alternans was induced was shifted to the left in response to the same block of IKur (Fig. 11Ba). When both Ito and IKur were partially blocked, the range of cycle lengths that produced alternans was also shifted substantially to the left and showed reduced susceptibility to induction of APD90 alternans as judged by the smaller envelope of APD variability. This may suggest that this type of regimen or “double-hit approach” for antiarrhythmic drugs in the human atrium has some promise. Under AF conditions, after partial block of only Ito, or partial block of Ito and IKur, neither model exhibited any detectable “fractional” alternans. This pattern of results adds further support to the possibility that double-hit antiarrhythmic pharmacology may be of value (Fig. 11, Ab and Bb; also see discussion).
Fig. 11.
Illustration of the effects of partial block of one or two K+ channels resulting in “double-hit” pharmacological inhibition. The rate dependence of the action potential (AP) duration at 90% repolarization (APD90) after a simulated 50% block of ultrarapid delayed rectifier K+ current (IKur; red) or both Ca2+-independent transient outward K+ current (Ito) and IKur (blue) in baseline model conditions and in the model including partial substitution of Kv1.4 for Kv4.3 was assessed. A: simulated rate dependence of APD90 in response to partial block of both Ito and IKur using the baseline model under sinus rhythm (SR; Aa) and atrial fibrillation (AF; Ab) conditions. B: simulated rate dependence of APD90 after partial (50%) block of both Ito and IKur in the modified human atrial AP model that includes partial substitution of Kv1.4 for Kv4.3. Results are shown for both SR (Ba) and AF (Bb) conditions.
DISCUSSION
Main findings.
Our computations reveal that partial (50%) substitution of Kv4.3 for Kv1.4 can markedly alter the dynamics (reactivation) of Ito and, hence, can significantly change rate-dependent features of the human atrial AP waveform and its stability (24, 62, 77, 78). It is clear from a comparison of the patterns of results in Figs. 4 and 5 that these changes occur and are more pronounced under baseline conditions (SR) than in the setting of chronic AF in these human atrial myocyte models. Some of these effects can be “captured” and related to previous experimental and clinical data obtained from human atrial properties by constructing classical APD restitution curves or relationships (9, 32, 33, 35, 43, 56, 68, 69, 79, 127). By convention, and as shown in Figs. 6 and 7, one way of doing this is to plot APD at a chosen fractional repolarization level (e.g., 90%) against the S1-S2 interval (9, 32–34, 43, 106, 127). Importantly, under some conditions, this K+ channel α-subunit isoform switch can produce a somewhat notched or nonmonotonic initial slope of the restitution waveform (Fig. 8). In these conditions, when even one extra stimulus (S2) is introduced (particularly at relatively low rates of stimulation), the pattern of S1-S2 interval-dependent changes in the AP waveform is revealed to be particularly sensitive to this K+ channel isoform switch. Moreover, under SR conditions, when a stimulus train is applied to the Kv1.4/Kv4.3 hybrid model, the incidence and duration of APD90 alternans can be enhanced (Figs. 8 and 10). In the setting of AF, this isoform switch has much smaller effects. Our final set of in silico studies showed that the susceptibility to alternans was reduced in response to in silico maneuvers that correspond to the combined partial block of both Ito and IKur and that this pattern of results was observed under both SR and AF conditions (Fig. 11).
Relationship to previous work.
Our computations were motivated in part by recent experimental work (46) and reviews (97, 109) on electrophysiological mechanisms of AF in the mouse heart from Kirchhof and colleagues. An interesting finding from this work (46) was that in a murine model of atrial ectopy and arrhythmia there is a significant alteration in the K+ channel α-subunits that are responsible for Ito. These changes were identified in a localized region (the posterior wall) of the adult mouse left atrium that is known to be a site at which rhythm disturbances are initiated. A related paper has shown that under experimental conditions leading to hypertrophy there is a marked (3- to 4-fold) increase in the expression of Kv1.4 (100). In mammalian hearts, it is known that a robust electrophysiological response to mechanical or endocrinological stress causes the myocardium to return to a fetal phenotype. This change includes replacement of much of the Kv4.3 transcript that normally is responsible for Ito with Kv1.4 (73, 84, 89, 99). Accordingly, our simulations were based on one important implication of these patterns of results, i.e., the presence of a spatially localized partial replacement of Kv4.3 with Kv1.4 and resulting changes in Ito in the atrium. We hypothesized that this K+ channel isoform switch would change the ability of the substrate to react to premature stimuli and, therefore, contribute to the initiation of APD alternans.
We also recognized that similar changes in Ito and, therefore, in the AP waveforms (24, 34, 42, 48, 56, 62, 126) may partially explain recent clinical electrophysiological results from mammalian atrial preparations. In particular, several of our studies revealed that even minor perturbations (a single extra stimulus applied soon after repolarization at relatively low heart rates) can trigger atrial flutter/AF (77–79) in human atria. These and other monophasic AP recordings also showed that just before the onset of atrial arrhythmia, APD alternans is often observed (56, 60, 62, 76–79, 117). Furthermore, careful inspection of this clinical electrophysiological data revealed that although the overall change in AP dynamics during alternans can perhaps be captured by standard measurements of APD90, both the early repolarization phase and plateau of these APs also change significantly (Refs. 24, 30, 56, and 60; cf. Ref. 113). We therefore quantified and summarized one aspect of this pattern of waveform changes in the APD restitution plots that are based on APD20 measurements, as shown in Figs. 6 and 7.
A mechanistic working hypothesis.
This isoform or “substrate-dependent” change in APD restitution can be accounted for by considering the marked differences in the reactivation kinetics of Kv1.4 versus Kv4.3, as shown in Fig. 1 (2, 31, 65, 81, 84, 89, 99, 120). Although the reactivation time courses of both of these K+ currents can be quite complex, the main difference is that Kv1.4 recovers or reactivates much more slowly than Kv4.3 at membrane potentials (16, 18, 31, 84, 85, 120, 123) that are close to the resting potential of the human atrial myocyte. This well-established biophysical difference contributes significantly to the observed changes in the characteristics of APD restitution curves. In the atrium, as in other tissues, the initial slope of the restitution curve is strongly modulated by the net current during early and midrepolarization (43, 106). This net current equates to the difference between the inward current generated by L-type Ca2+ channels and the outward current due to Ito (18, 31, 47, 51, 65, 74, 113). At short interstimulus intervals, the time-dependent interaction between the inward current, ICaL, and the outward current, Ito, is such that the ICaL predominates, and this yields a net inward current and produces a normal plateau phase of the atrial AP. In distinction, at slightly longer diastolic intervals, i.e., in the range of interstimulus intervals at which ICaL has inactivated almost completely (14, 37, 113), changes in Ito can dominate the net current. In summary, the independent and significantly different time course of reactivation of Ito and the fact that these two currents (ICaL and Ito) are of similar magnitude can result in a net outward current (18, 65, 91, 113) or repolarization reserve (82). It is well known that even small net outward currents can cause the plateau of the atrial AP to be depressed and produce associated changes in the early repolarization phase of the AP waveform (23, 24, 30, 81, 113, 117, 119, 126). Indeed, the mammalian atrial AP does exhibit a pronounced early repolarization phase followed by a plateau at a membrane potential that is approximately 20 mV negative to the control value (18, 23, 30, 40, 44, 62, 71, 120).
Under some circumstances, the mismatch in the two reactivation time courses of ICaL and Ito can even produce a nonmonotonic APD restitution relationship (32, 35). This “notched” type of APD restitution relationship has been reported previously in studies of the epicardium of the human ventricle, and a nonmonotonic or notched relationship is also characteristic of restitution data derived from Purkinje fibers in mammalian hearts (35, 98, 102). Interestingly, there is evidence that the K+ channel α-subunit that is responsible for a significant fraction of Ito in Purkinje fiber myocytes is Kv1.4 (128), as may also be the case in human atrial myocytes from very young donors (23, 30, 120, cf. 81).
Electrophysiological principles that regulate AP repolarization.
The ability of Ito to modulate the shape of mammalian atrial APs is also the basis for the very significant role for this K+ current in the regulation of the [Ca2+]i transient and excitation-contraction coupling (7, 17, 58, 89, 101). It is well known that even very small changes in the AP height or the contour of early repolarization of the AP can markedly alter the associated [Ca2+]i and phasic contractions (7, 17, 58, 89).
A second, quite closely related phenomenon, sometimes referred to as “cardiac memory,” also arises (at least in part) from the intrinsic biophysical properties of Ito (25, 109). As mentioned above, the reactivation kinetics of Ito can strongly modulate the amplitude of this current in response to either an appropriately timed single extra stimulus or a train of stimuli applied at a frequency that is within the rapidly changing phase of the Ito reactivation time course. Moreover, if this type of K+ current does not turn off or deactivate completely, its presence can also produce quite marked changes in the time course of the response of an atrial myocyte to an applied stimulus and/or alter the short-term firing rate of an ectopic focus (51, 60, 62, 79, 126).
The expression level or size and the detailed biophysical properties of Ito can also strongly modulate both intercellular electrotonic communication and active conduction in atrial syncytia (47, 51, 61, 110). Perhaps the primary reason for this is that when Ito predominates, the contour of the AP plateau is no longer convex but instead “collapses” to approximately −20 mV. Accordingly, the driving force for the transmembrane current that is responsible for intercellular communication is reduced markedly. In addition, the extent to which the final phase of inactivation of Ito is complete represents an important physiological variable. This was first pointed out by Connor and Stevens (20, 21) in their classical studies of the role of this type of K+ current in ectopic pacing in neurons. Later studies from Khaliq and Bean (55) extended this principle and placed it in a new and important context by showing that in mammalian neurons this same type of K+ current can markedly alter latency to firing and change the resting potential and that these effects often are nonlinear. In cardiac syncytia, Huelsing et al. (47), Spitzer et al. (110), and Joyner (51) have shown that the interaction between Ca2+ current and Ito during the plateau phase of the AP can markedly alter intercellular coupling, sometimes resulting in conduction block. The significance of changes in early repolarization in both physiological and pathophysiological settings is now quite well known (23, 24, 108, 113, 119).
Alterations in ion channel isoform ratios/K+ channel isoform switches.
Previous experimental work, mainly in the rat ventricle, has clearly demonstrated that alterations in thyroid hormone levels or induction of diabetes can significantly alter repolarization of the AP (84, 89, 105, 123). Examination of the underlying causes for these changes has often revealed a pathology-related slowing of reactivation of Ito, which is due at least in part to changes in the relative expression levels from Kv4.2/4.3 to Kv1.4 (84, 89, 99, 121). Somewhat similarly, in some experimental models of heart failure (64, 108) or hypertrophy, Ito expressed by epicardial myocytes may exhibit a somewhat similar isoform switch; this is predominantly Kv4.3 to Kv1.4 (73, 99).
It is also interesting to note that in the fetal and early stages of neonatal development of the heart, Ito can be generated mainly by Kv1.4 (cf. 81). As a result, under the wide variety of conditions in which the stressed or injured mammalian myocardium “returns to the fetal phenotype” (93), the AP waveform dynamics would be expected to be strongly influenced by the expression levels of Kv1.4 and its intrinsic biophysical and pharmacological properties. We have previously noted that even under baseline conditions in the mammalian Purkinje fiber, Kv1.4 expression makes a significant contribution to Ito (128). It is also well known that in myocytes from both the atrium and ventricle of the adult rabbit heart, Ito is generated mainly by Kv1.4 (31, 65, 85, 121, 126).
Limitations.
Our computational work has revealed novel and functionally important patterns of changes in human atrial APD waveforms that are strongly dependent on a biophysical property (reactivation or recovery) of Ito. The dynamics of reactivation of Ito are conferred in large part by the α-subunit composition of the K+ channels that underlie this K+ current. Ito strongly regulates the early repolarization phase of the AP (113, 119, 126). When 50% of the Kv4.3 channels that underlie Ito in normal healthy atria are substituted for Kv1.4 channels, the AP waveform and APD change significantly and do so in a rate-dependent manner. These changes can contribute to the initiation of atrial arrhythmias, localized ectopic foci, or associated APD alternans.
We recognize, however, that by design our study is limited in scope. Before our findings can be related meaningfully to the overall multifactorial challenge of understanding the pathogenesis of AF (28, 44, 48, 62, 71, 80, 97, 107), additional work is needed. This will include but not be limited to the following.
Our computational work has been done entirely in a single human atrial myocyte model. Partly for that reason we have not accounted for the known heterogeneity of the AP profiles that are present both at baseline or in SR conditions and in the setting of AF (44, 86, 96, 104). In a single myocyte, axial conduction of the AP is not a major consideration. That is, the conditions are those of a “membrane AP” (cf. Ref. 113). However, it is clear that most settings of pathophysiological interest involve propagated cell-to-cell communication mediated by cell-to-cell intercellular conduction in the atrial or ventricular syncytia (18, 48, 74, 77–79, 114, 126). The K+ channel isoform switch that we have studied needs to be further evaluated under conditions in which there is either two- or three-dimensional conduction (18, 61, 91) using both SR and AF models.
This study focused on only one of the disease-dependent changes in K+ current that have been reported in the setting of AF. Our findings are revealing, but much more extensive study of these changes in conjunction with spatially heterogeneous alterations in vagus nerve discharge (45, 46, 66) and/or contributions from altered adrenergic tone (5, 111) will need to be carried out.
In chronic AF, it is now recognized that significant inflammation is often a cofactor (41). Our study does not address this, although it is known that prominent components of the inflammatory milieu, e.g., hydrogen peroxide production (15), can alter both Ito and INaL (6, 72). It is partly for this reason that the original SR and AF models of the human atrial AP that are the basis for this study have been modified to include a small but significant INaL component (83). Moreover, when the substrate concludes a change that involves a maintained influx of Na+, the contribution of the resulting enhancement of electrogenic Na+/K+ pump current needs to be considered as a factor in the maintenance of AF (103, 125).
Recent studies have drawn attention to the fact that the rapid, disorganized electrophysiological and contractile activity of the atria during AF episodes is likely to result in affected atrial tissue being underperfused (90) and, therefore, perhaps somewhat ischemic (28, 44, 124). Our study does not address any aspect of this important pathophysiological change, e.g., altered extracellular K+ levels (4, 59, 87, 112) or changes in shear stress due to either alterations in localized atrial stretch or related changes in tissue perfusion (8, 28, 49).
Many clinical studies concerning the mechanisms or pharmacological treatments for AF are necessarily carried out in settings where the patients are already receiving other cardiovascular or diuretic therapies. One quite striking example is the recent demonstration that a number of different statin compounds can selectively block Ito in the human atrium (115). If so, the return to the fetal electrophysiological phenotype that occurs in localized atrial tissue areas as a function of progressive AF may need to be taken into account.
Concluding perspectives.
More complete knowledge of the electrophysiological heterogeneity in mammalian atria, including circumstances under which the atrial myocardium significantly changes its intrinsic electrophysiological (substrate) properties, is likely to provide a basis for novel approaches for improved detection or management of AF (44, 107, 117, 129). For example, additional studies may reveal why only certain drugs or prodrugs would be expected to be effective as atria-selective antiarrhythmic agents (42, 80, 83, 124). This insight may be based on emerging atrium-specific properties of Na+ current and associated details of atrial refractoriness (18, 48, 88, 125) or on whether the K+ channels that underlie Ito are predominantly Kv1.4 or Kv4.3 (cf. 85, 121). In addition, attempts to stabilize the electrophysiological substrate in the setting of chronic AF may take place in settings of altered plasma pH or K+ levels (4, 59, 87, 112, 118). If so, it is important to recognize that when Kv1.4 is the predominant drug “target,” it can confer new electrophysiological properties to the atrial substrate (2, 10, 23, 48, 63, 71, 80, 85). Moreover, the spatial heterogeneity of the electrophysiological properties in mammalian atria (right vs. left atrium, right atrium vs. atrial septum) will need to be accounted for (36, 39, 86) since it is known that any significant differences in spatial localized APD within the atrial syncytium can influence arrhythmogenesis (88, 96, 114).
If the AP repolarization contour or a specific selected level of APD is to be targeted in efforts to detect and reduce transient atrial rhythm disturbances or curtail AF, the fact that Ito is only one of the significant K+ currents responsible for repolarization needs to be fully taken into account. In particular, previous work has shown that the so-called ultrarapidly activating delayed rectifier Kv1.5 or IKur can strongly modulate repolarization (1, 29, 40, 63, 71). Important principles of the rate dependence of this current and use-dependent properties of antiarrhythmic drug actions need to be more completely understood and accounted for (1, 29, 42, 83, 94, 115).
Translational work aimed at antiarrhythmic strategies must also take full account of available knowledge concerning the significantly different molecular mechanisms for inactivation when Kv1.4 expression is significant. It is now well known that K+ channels in the Kv1.X family exhibit what has been termed C-type inactivation (75, 94). That is, these channels inactivate or turn off based on molecular rearrangements that involve both the highly charged gating substituents of the K+ channel as well as a change in the diameter of the ion conducting channel pore. C-type inactivation is strongly modulated by a number of factors that would be expected to progressively accumulate in the hypokinetic and perhaps somewhat hypoxic atrial myocardium. Both may occur in the setting of AF. Our simulations (Fig. 11) based on combined partial block of Ito and IKur yielded results that suggest that AF treatment paradigms based on a double-hit approach may be interesting to consider, particularly when combined with high-resolution mapping. If so, more information is needed regarding disease-induced (AF) progressive changes in atrial myocyte microanatomy. One example is the quite recent, convincing demonstration of a functional transverse tubule system in mammalian atria (27). It is known that the transverse tubule system expresses ion channels that are important for AP initiation and repolarization (80; cf. 97, 113).
GRANTS
The main support for this work was derived from National Institutes of Health (NIH) Grants HL-70529 and HL-83359 (to S. M. Narayan). In addition, work at Manchester University was funded by a British Heart Foundation grant to H. Zhang. E. Grandi is supported by NIH Grants R01-HL-131517, R01-HL-41214, and 1OT2-OD-023848-01 and American Heart Association Scientist Development Grant 15SDG24910015. W. R. Giles received grant support from the Canadian Institutes for Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.N., H.Z., E.G., S.M.N., and W.G. conceived and designed research; H.N., E.G., S.M.N., and W.G. analyzed data; H.N., S.M.N., and W.G. interpreted results of simulations; H.N. prepared figures; H.N., H.Z., E.G., S.M.N., and W.G. drafted, edited and revised manuscript; H.N., H.Z., E.G., S.M.N., and W.G. approved final version of manuscript.
REFERENCES
- 1.Aguilar M, Feng J, Vigmond E, Comtois P, Nattel S. Rate-dependent role of IKur in human atrial repolarization and atrial fibrillation maintenance. Biophys J 112: 1997–2010, 2017. doi: 10.1016/j.bpj.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V III, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol 286: H602–H609, 2004. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- 3.Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, Wasserstrom JA. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res 99: e65–e73, 2006. doi: 10.1161/01.RES.0000244087.36230.bf. [DOI] [PubMed] [Google Scholar]
- 4.Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron 15: 951–960, 1995. doi: 10.1016/0896-6273(95)90185-X. [DOI] [PubMed] [Google Scholar]
- 5.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 105: 2753–2759, 2002. doi: 10.1161/01.CIR.0000018443.44005.D8. [DOI] [PubMed] [Google Scholar]
- 6.Bonatti R, Silva AF, Batatinha JA, Sobrado LF, Machado AD, Varone BB, Nearing BD, Belardinelli L, Verrier RL. Selective late sodium current blockade with GS-458967 markedly reduces ischemia-induced atrial and ventricular repolarization alternans and ECG heterogeneity. Heart Rhythm 11: 1827–1835, 2014. doi: 10.1016/j.hrthm.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard RA, Clark RB, Giles WR. Effects of action potential duration on excitation-contraction coupling in rat ventricular myocytes. Action potential voltage-clamp measurements. Circ Res 76: 790–801, 1995. doi: 10.1161/01.RES.76.5.790. [DOI] [PubMed] [Google Scholar]
- 8.Boycott HE, Barbier CSM, Eichel CA, Costa KD, Martins RP, Louault F, Dilanian G, Coulombe A, Hatem SN, Balse E. Shear stress triggers insertion of voltage-gated potassium channels from intracellular compartments in atrial myocytes. Proc Natl Acad Sci USA 110: E3955–E3964, 2013. doi: 10.1073/pnas.1309896110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyett MR, Jewell BR. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol 36: 1–52, 1981. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- 10.Brundel BJJM, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, Grandjean JG, Van Gilst WH, Crijns HJ. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation 103: 684–690, 2001. doi: 10.1161/01.CIR.103.5.684. [DOI] [PubMed] [Google Scholar]
- 11.Brundel BJJM, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, Wilde AAM, Van Gilst WH, Crijns HJGM. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol 37: 926–932, 2001. doi: 10.1016/S0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 12.Burashnikov A, Mannava S, Antzelevitch C. Transmembrane action potential heterogeneity in the canine isolated arterially perfused right atrium: effect of IKr and IKur/Ito block. Am J Physiol Heart Circ Physiol 286: H2393–H2400, 2004. doi: 10.1152/ajpheart.01242.2003. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet E. Intracellular Ca2+ concentration and rate adaptation of the cardiac action potential. Cell Calcium 35: 557–573, 2004. doi: 10.1016/j.ceca.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet E. Electrical alternans: membrane-limited and subcellular components. J Cardiovasc Electrophysiol 17: 94–96, 2006. doi: 10.1111/j.1540-8167.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 15.Carnes CA, Janssen PML, Ruehr ML, Nakayama H, Nakayama T, Haase H, Bauer JA, Chung MK, Fearon IM, Gillinov AM, Hamlin RL, Van Wagoner DR. Atrial glutathione content, calcium current, and contractility. J Biol Chem 282: 28063–28073, 2007. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- 16.Cheng H, Cannell MB, Hancox JC. Differential responses of rabbit ventricular and atrial transient outward current (Ito) to the Ito modulator NS5806. Physiol Rep 5: e13172, 2017. doi: 10.14814/phy2.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark RB, Bouchard RA, Giles WR. Action potential duration modulates calcium influx, Na+-Ca2+ exchange, and intracellular calcium release in rat ventricular myocytes. Ann N Y Acad Sci 779: 417–429, 1996. doi: 10.1111/j.1749-6632.1996.tb44817.x. [DOI] [PubMed] [Google Scholar]
- 18.Colman MA, Aslanidi OV, Kharche S, Boyett MR, Garratt C, Hancox JC, Zhang H. Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodelling: insights from the three-dimensional virtual human atria. J Physiol 591: 4249–4272, 2013. doi: 10.1113/jphysiol.2013.254987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comtois P, Nattel S. Atrial repolarization alternans as a path to atrial fibrillation. J Cardiovasc Electrophysiol 23: 1013–1015, 2012. doi: 10.1111/j.1540-8167.2012.02391.x. [DOI] [PubMed] [Google Scholar]
- 20.Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol 213: 21–30, 1971b. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol 213: 31–53, 1971c. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox EJ, Marsh SA. A systematic review of fetal genes as biomarkers of cardiac hypertrophy in rodent models of diabetes. PLoS One 9: e92903, 2014. doi: 10.1371/journal.pone.0092903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crumb WJ Jr, Pigott JD, Clarkson CW. Comparison of Ito in young and adult human atrial myocytes: evidence for developmental changes. Am J Physiol Heart Circ Physiol 268: H1335–H1342, 1995. doi: 10.1152/ajpheart.1995.268.3.H1335. [DOI] [PubMed] [Google Scholar]
- 24.Dawodu AA, Monti F, Iwashiro K, Schiariti M, Chiavarelli R, Puddu PE. The shape of human atrial action potential accounts for different frequency-related changes in vitro. Int J Cardiol 54: 237–249, 1996. doi: 10.1016/0167-5273(96)02605-8. [DOI] [PubMed] [Google Scholar]
- 25.Delord B, Baraduc P, Costalat R, Burnod Y, Guigon E. A model study of cellular short-term memory produced by slowly inactivating potassium conductances. J Comput Neurosci 8: 251–273, 2000. doi: 10.1023/A:1008902110844. [DOI] [PubMed] [Google Scholar]
- 26.Díaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res 94: 650–656, 2004. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 27.Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail 2: 482–489, 2009. doi: 10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- 28.Eckstein J, Verheule S, de Groot NM, Allessie M, Schotten U. Mechanisms of perpetuation of atrial fibrillation in chronically dilated atria. Prog Biophys Mol Biol 97: 435–451, 2008. [Erratum in Prog Biophys Mol Biol 99: 51, 2009.] doi: 10.1016/j.pbiomolbio.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Ellinwood N, Dobrev D, Morotti S, Grandi E. Erratum: “Revealing kinetics and state-dependent binding properties of IKur-targeting drugs that maximize atrial fibrillation selectivity” [Chaos 27, 093918 (2017)]. Chaos 27: 109902, 2017. doi: 10.1063/1.5007051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escande D, Loisance D, Planche C, Coraboeuf E. Age-related changes of action potential plateau shape in isolated human atrial fibers. Am J Physiol Heart Circ Physiol 249: H843–H850, 1985. doi: 10.1152/ajpheart.1985.249.4.H843. [DOI] [PubMed] [Google Scholar]
- 31.Fermini B, Wang Z, Duan D, Nattel S. Differences in rate dependence of transient outward current in rabbit and human atrium. Am J Physiol Heart Circ Physiol 263: H1747–H1754, 1992. doi: 10.1152/ajpheart.1992.263.6.H1747. [DOI] [PubMed] [Google Scholar]
- 32.Franz MR. The electrical restitution curve revisited: steep or flat slope–which is better? J Cardiovasc Electrophysiol 14, Suppl: S140–S147, 2003. doi: 10.1046/j.1540.8167.90303.x. [DOI] [PubMed] [Google Scholar]
- 33.Franz MR, Jamal SM, Narayan SM. The role of action potential alternans in the initiation of atrial fibrillation in humans: a review and future directions. Europace 14, Suppl 5: v58–v64, 2012. doi: 10.1093/europace/eus273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franz MR, Karasik PL, Li C, Moubarak J, Chavez M. Electrical remodeling of the human atrium: similar effects in patients with chronic atrial fibrillation and atrial flutter. J Am Coll Cardiol 30: 1785–1792, 1997. doi: 10.1016/S0735-1097(97)00385-9. [DOI] [PubMed] [Google Scholar]
- 35.Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest 82: 972–979, 1988. doi: 10.1172/JCI113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol 582: 675–693, 2007. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Z, Sun H, Chiu S-W, Lau CP, Li G-R. Effects of diltiazem and nifedipine on transient outward and ultra-rapid delayed rectifier potassium currents in human atrial myocytes. Br J Pharmacol 144: 595–604, 2005. doi: 10.1038/sj.bjp.0706113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldhaber JI, Xie L-H, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ Res 96: 459–466, 2005. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 39.Gong D, Zhang Y, Cai B, Meng Q, Jiang S, Li X, Shan L, Liu Y, Qiao G, Lu Y, Yang B. Characterization and comparison of Na+, K+ and Ca2+ currents between myocytes from human atrial right appendage and atrial septum. Cell Physiol Biochem 21: 385–394, 2008. doi: 10.1159/000129631. [DOI] [PubMed] [Google Scholar]
- 40.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 109: 1055–1066, 2011. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 60: 2263–2270, 2012. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 42.Hancox JC, James AF, Marrion NV, Zhang H, Thomas D. Novel ion channel targets in atrial fibrillation. Expert Opin Ther Targets 20: 947–958, 2016. doi: 10.1517/14728222.2016.1159300. [DOI] [PubMed] [Google Scholar]
- 43.Hauswirth O, Noble D, Tsien RW. The dependence of plateau currents in cardiac Purkinje fibres on the interval between action potentials. J Physiol 222: 27–49, 1972. doi: 10.1113/jphysiol.1972.sp009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heijman J, Guichard J-B, Dobrev D, Nattel S. Translational challenges in atrial fibrillation. Circ Res 122: 752–773, 2018. doi: 10.1161/CIRCRESAHA.117.311081. [DOI] [PubMed] [Google Scholar]
- 45.Hirose M, Carlson MD, Laurita KR. Cellular mechanisms of vagally mediated atrial tachyarrhythmia in isolated arterially perfused canine right atria. J Cardiovasc Electrophysiol 13: 918–926, 2002. doi: 10.1046/j.1540-8167.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 46.Holmes AP, Yu TY, Tull S, Syeda F, Kuhlmann SM, O’Brien S-M, Patel P, Brain KL, Pavlovic D, Brown NA, Fabritz L, Kirchhof P. A regional reduction in Ito and IKACh in the murine posterior left atrial myocardium is associated with action potential prolongation and increased ectopic activity. PLoS One 11: e0154077, 2016. doi: 10.1371/journal.pone.0154077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huelsing DJ, Pollard AE, Spitzer KW. Transient outward current modulates discontinuous conduction in rabbit ventricular cell pairs. Cardiovasc Res 49: 779–789, 2001. doi: 10.1016/S0008-6363(00)00300-X. [DOI] [PubMed] [Google Scholar]
- 48.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation 124: 2264–2274, 2011. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 49.John B, Stiles MK, Kuklik P, Brooks AG, Chandy ST, Kalman JM, Sanders P. Reverse remodeling of the atria after treatment of chronic stretch in humans: implications for the atrial fibrillation substrate. J Am Coll Cardiol 55: 1217–1226, 2010. doi: 10.1016/j.jacc.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 50.Jousset F, Tenkorang J, Vesin J-M, Pascale P, Ruchat P, Rollin AG, Fromer M, Narayan SM, Pruvot E. Kinetics of atrial repolarization alternans in a free-behaving ovine model. J Cardiovasc Electrophysiol 23: 1003–1012, 2012. doi: 10.1111/j.1540-8167.2012.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joyner RW. Modulation of repolarization by electrotonic interactions. Jpn Heart J 27, Suppl 1: 167–183, 1986. [PubMed] [Google Scholar]
- 52.Kääb S, Dixon J, Duc J, Ashen D, Näbauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 98: 1383–1393, 1998. doi: 10.1161/01.CIR.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 53.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res 106: 47–57, 2010. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanaporis G, Blatter LA. The mechanisms of calcium cycling and action potential dynamics in cardiac alternans. Circ Res 116: 846–856, 2015. doi: 10.1161/CIRCRESAHA.116.305404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khaliq ZM, Bean BP. Dynamic, nonlinear feedback regulation of slow pacemaking by A-type potassium current in ventral tegmental area neurons. J Neurosci 28: 10905–10917, 2008. doi: 10.1523/JNEUROSCI.2237-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim B-S, Kim Y-H, Hwang G-S, Pak H-N, Lee SC, Shim WJ, Oh DJ, Ro YM. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol 39: 1329–1336, 2002. doi: 10.1016/S0735-1097(02)01760-6. [DOI] [PubMed] [Google Scholar]
- 57.Koller ML, Riccio ML, Gilmour RF Jr. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol Heart Circ Physiol 275: H1635–H1642, 1998. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 58.Kondo RP, Dederko DA, Teutsch C, Chrast J, Catalucci D, Chien KR, Giles WR. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol 571: 131–146, 2006. doi: 10.1113/jphysiol.2005.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krijthe BP, Heeringa J, Kors JA, Hofman A, Franco OH, Witteman JCM, Stricker BH. Serum potassium levels and the risk of atrial fibrillation: the Rotterdam Study. Int J Cardiol 168: 5411–5415, 2013. doi: 10.1016/j.ijcard.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 60.Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, Trayanova NA, Narayan SM. Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol 5: 1149–1159, 2012. doi: 10.1161/CIRCEP.111.969022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kucera JP, Rohr S, Kleber AG. Microstructure, cell-to-cell coupling and ion currents as determinants of electrical propagation and arrhythmogenesis. Circ Arrhythm Electrophysiol 10: e004665, 2017. doi: 10.1161/CIRCEP.117.004665. [DOI] [PubMed] [Google Scholar]
- 62.Lalani GG, Schricker AA, Clopton P, Krummen DE, Narayan SM. Frequency analysis of atrial action potential alternans: a sensitive clinical index of individual propensity to atrial fibrillation. Circ Arrhythm Electrophysiol 6: 859–867, 2013. doi: 10.1161/CIRCEP.113.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levitan ES, Takimoto K. Dynamic regulation of K+ channel gene expression in differentiated cells. J Neurobiol 37: 60–68, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 64.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 100: 87–95, 1999. doi: 10.1161/01.CIR.100.1.87. [DOI] [PubMed] [Google Scholar]
- 65.Lindblad DS, Murphey CR, Clark JW, Giles WR. A model of the action potential and underlying membrane currents in a rabbit atrial cell. Am J Physiol Heart Circ Physiol 271: H1666–H1696, 1996. doi: 10.1152/ajpheart.1996.271.4.H1666. [DOI] [PubMed] [Google Scholar]
- 66.Lu Z, Cui B, He B, Hu X, Wu W, Huang C, Jiang H. Effects of autonomic interventions on atrial restitution properties. J Cardiovasc Electrophysiol 22: 84–90, 2011. doi: 10.1111/j.1540-8167.2010.01828.x. [DOI] [PubMed] [Google Scholar]
- 67.Lu Z, Scherlag BJ, Lin J, Niu G, Fung K-M, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol 1: 184–192, 2008. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu Z, Cui B, He B, Hu X, Wu W, Wu L, Huang C, Po SS, Jiang H. Distinct restitution properties in vagally mediated atrial fibrillation and six-hour rapid pacing-induced atrial fibrillation. Cardiovasc Res 89: 834–842, 2011. doi: 10.1093/cvr/cvq334. [DOI] [PubMed] [Google Scholar]
- 69.Lux RL, Ershler PR. Cycle length sequence dependent repolarization dynamics. J Electrocardiol 36, Suppl: 205–208, 2003. doi: 10.1016/j.jelectrocard.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 70.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation 124: 1982–1993, 2011. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am J Physiol Heart Circ Physiol 297: H1398–H1410, 2009. doi: 10.1152/ajpheart.00411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maltsev VA, Sabbah HN, Higgins RSD, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 98: 2545–2552, 1998. doi: 10.1161/01.CIR.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 73.Matsubara H, Suzuki J, Inada M. Shaker-related potassium channel, Kv1.4, mRNA regulation in cultured rat heart myocytes and differential expression of Kv1.4 and Kv1.5 genes in myocardial development and hypertrophy. J Clin Invest 92: 1659–1666, 1993. doi: 10.1172/JCI116751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matthews GDK, Guzadhur L, Grace A, Huang CLH. Nonlinearity between action potential alternans and restitution, which both predict ventricular arrhythmic properties in Scn5a+/− and wild-type murine hearts. J Appl Physiol 112: 1847–1863, 2012. doi: 10.1152/japplphysiol.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer R, Heinemann SH. Temperature and pressure dependence of Shaker K+ channel N- and C-type inactivation. Eur Biophys J 26: 433–445, 1997. doi: 10.1007/s002490050098. [DOI] [PubMed] [Google Scholar]
- 76.Monigatti-Tenkorang J, Jousset F, Pascale P, Vesin J-M, Ruchat P, Fromer M, Narayan SM, Pruvot E. Intermittent atrial tachycardia promotes repolarization alternans and conduction slowing during rapid rates, and increases susceptibility to atrial fibrillation in a free-behaving sheep model. J Cardiovasc Electrophysiol 25: 418–427, 2014. doi: 10.1111/jce.12353. [DOI] [PubMed] [Google Scholar]
- 77.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation 106: 1968–1973, 2002. doi: 10.1161/01.CIR.0000037062.35762.B4. [DOI] [PubMed] [Google Scholar]
- 78.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation 123: 2922–2930, 2011. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol 52: 1222–1230, 2008. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nattel S, Maguy A, Le Bouter S, Yeh Y-H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87: 425–456, 2007. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 81.Nerbonne JM. Regulation of voltage-gated K+ channel expression in the developing mammalian myocardium. J Neurobiol 37: 37–59, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen TP, Singh N, Xie Y, Qu Z, Weiss JN. Repolarization reserve evolves dynamically during the cardiac action potential: effects of transient outward currents on early afterdepolarizations. Circ Arrhythm Electrophysiol 8: 694–702, 2015. doi: 10.1161/CIRCEP.114.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ni H, Whittaker DG, Wang W, Giles WR, Narayan SM, Zhang H. Synergistic anti-arrhythmic effects in human atria with combined use of sodium blockers and acacetin. Front Physiol 8: 946, 2017. doi: 10.3389/fphys.2017.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishiyama A, Ishii DN, Backx PH, Pulford BE, Birks BR, Tamkun MM. Altered K+ channel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. Am J Physiol Heart Circ Physiol 281: H1800–H1807, 2001. doi: 10.1152/ajpheart.2001.281.4.H1800. [DOI] [PubMed] [Google Scholar]
- 85.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J Mol Cell Cardiol 48: 12–25, 2010. doi: 10.1016/j.yjmcc.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nygren A, Lomax AE, Giles WR. Heterogeneity of action potential durations in isolated mouse left and right atria recorded using voltage-sensitive dye mapping. Am J Physiol Heart Circ Physiol 287: H2634–H2643, 2004. doi: 10.1152/ajpheart.00380.2004. [DOI] [PubMed] [Google Scholar]
- 87.Nygren A, Giles WR. Mathematical simulation of slowing of cardiac conduction velocity by elevated extracellular [K+] in a human atrial strand. Ann Biomed Eng 28: 951–957, 2000. doi: 10.1114/1.1308489. [DOI] [PubMed] [Google Scholar]
- 88.Osadchii OE. Arrhythmogenic drugs can amplify spatial heterogeneities in the electrical restitution in perfused guinea-pig heart: An evidence from assessments of monophasic action potential durations and JT intervals. PLoS One 13: e0191514, 2018. doi: 10.1371/journal.pone.0191514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol 33: 851–872, 2001. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- 90.Pacchia CF, Dosdall DJ, Ranjan R, DiBella E. Alterations in atrial perfusion during atrial fibrillation. Exp Physiol 99: 1267–1272, 2014. doi: 10.1113/expphysiol.2014.080242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J 88: 3806–3821, 2005. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 94: 1083–1090, 2004. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 93.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev 12: 331–343, 2007. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 94.Rasmusson RL, Morales MJ, Wang S, Liu S, Campbell DL, Brahmajothi MV, Strauss HC. Inactivation of voltage-gated cardiac K+ channels. Circ Res 82: 739–750, 1998. doi: 10.1161/01.RES.82.7.739. [DOI] [PubMed] [Google Scholar]
- 95.Ravi KC, Krummen DE, Tran AJ, Bullinga JR, Narayan SM. Electrocardiographic measurements of regional atrial fibrillation cycle length. Pacing Clin Electrophysiol 32, Suppl 1: S66–S71, 2009. doi: 10.1111/j.1540-8159.2008.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ridler M-E, Lee M, McQueen D, Peskin C, Vigmond E. Arrhythmogenic consequences of action potential duration gradients in the atria. Can J Cardiol 27: 112–119, 2011. doi: 10.1016/j.cjca.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Riley G, Syeda F, Kirchhof P, Fabritz L. An introduction to murine models of atrial fibrillation. Front Physiol 3: 296, 2012. doi: 10.3389/fphys.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robinson RB, Boyden PA, Hoffman BF, Hewett KW. Electrical restitution process in dispersed canine cardiac Purkinje and ventricular cells. Am J Physiol Heart Circ Physiol 253: H1018–H1025, 1987. doi: 10.1152/ajpheart.1987.253.5.H1018. [DOI] [PubMed] [Google Scholar]
- 99.Rosati B, McKinnon D. Regulation of ion channel expression. Circ Res 94: 874–883, 2004. doi: 10.1161/01.RES.0000124921.81025.1F. [DOI] [PubMed] [Google Scholar]
- 100.Rosenberg MA, Das S, Pinzon PQ, Knight AC, Sosnovik DE, Ellinor PT, Rosenzweig A. A novel transgenic mouse model of cardiac hypertrophy and atrial fibrillation.J Atr Fibrillation 4: 415, 2012. doi: 10.4022/jafib.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito). J Physiol 546: 5–18, 2003. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saitoh H, Bailey JC, Surawicz B. Action potential duration alternans in dog Purkinje and ventricular muscle fibers. Further evidence in support of two different mechanisms. Circulation 80: 1421–1431, 1989. doi: 10.1161/01.CIR.80.5.1421. [DOI] [PubMed] [Google Scholar]
- 103.Sánchez C, Corrias A, Bueno-Orovio A, Davies M, Swinton J, Jacobson I, Laguna P, Pueyo E, Rodríguez B. The Na+/K+ pump is an important modulator of refractoriness and rotor dynamics in human atrial tissue. Am J Physiol Heart Circ Physiol 302: H1146–H1159, 2012. doi: 10.1152/ajpheart.00668.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sarkar AX, Christini DJ, Sobie EA. Exploiting mathematical models to illuminate electrophysiological variability between individuals. J Physiol 590: 2555–2567, 2012. doi: 10.1113/jphysiol.2011.223313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sato T, Kobayashi T, Kuno A, Miki T, Tanno M, Kouzu H, Itoh T, Ishikawa S, Kojima T, Miura T, Tohse N. Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am J Physiol Heart Circ Physiol 306: H1054–H1065, 2014. doi: 10.1152/ajpheart.00414.2013. [DOI] [PubMed] [Google Scholar]
- 106.Shattock MJ, Park KC, Yang HY, Lee AWC, Niederer S, MacLeod KT, Winter J. Restitution slope is principally determined by steady-state action potential duration. Cardiovasc Res 113: 817–828, 2017. doi: 10.1093/cvr/cvx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91: 265–325, 2011. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 108.Schreieck J, Wang Y, Overbeck M, Schömig A, Schmitt C. Altered transient outward current in human atrial myocytes of patients with reduced left ventricular function. J Cardiovasc Electrophysiol 11: 180–192, 2000. doi: 10.1111/j.1540-8167.2000.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 109.Shivkumar K, Weiss JN. Atrial fibrillation: from cells to computers. Cardiovasc Res 52: 171–173, 2001. doi: 10.1016/S0008-6363(01)00454-0. [DOI] [PubMed] [Google Scholar]
- 110.Spitzer KW, Pollard AE, Yang L, Zaniboni M, Cordeiro JM, Huelsing DJ. Cell-to-cell electrical interactions during early and late repolarization. J Cardiovasc Electrophysiol 17, Suppl 1: S8–S14, 2006. doi: 10.1111/j.1540-8167.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 111.Taggart P, Sutton P, Chalabi Z, Boyett MR, Simon R, Elliott D, Gill JS. Effect of adrenergic stimulation on action potential duration restitution in humans. Circulation 107: 285–289, 2003. doi: 10.1161/01.CIR.0000044941.13346.74. [DOI] [PubMed] [Google Scholar]
- 112.Trenor B, Cardona K, Romero L, Gomez JF, Saiz J, Rajamani S, Belardinelli L, Giles W. Pro-arrhythmic effects of low plasma [K+] in human ventricle: an illustrated review. Trends Cardiovasc Med 28: 233–242, 2018. doi: 10.1016/j.tcm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Trenor B, Cardona K, Saiz J, Noble D, Giles W. Cardiac action potential repolarization revisited: early repolarization shows all-or-none behaviour. J Physiol 595: 6599–6612, 2017. doi: 10.1113/JP273651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uzelac I, Ji YC, Hornung D, Schröder-Scheteling J, Luther S, Gray RA, Cherry EM, Fenton FH. Simultaneous quantification of spatially discordant alternans in voltage and intracellular calcium in Langendorff-perfused rabbit hearts and inconsistencies with models of cardiac action potentials and Ca transients. Front Physiol 8: 819, 2017. doi: 10.3389/fphys.2017.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vaquero M, Caballero R, Gómez R, Núñez L, Tamargo J, Delpón E. Effects of atorvastatin and simvastatin on atrial plateau currents. J Mol Cell Cardiol 42: 931–945, 2007. doi: 10.1016/j.yjmcc.2007.03.807. [DOI] [PubMed] [Google Scholar]
- 116.Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res 122: 352–368, 2018. doi: 10.1161/CIRCRESAHA.117.311402. [DOI] [PubMed] [Google Scholar]
- 117.Verrier RL, Fuller H, Justo F, Nearing BD, Rajamani S, Belardinelli L. Unmasking atrial repolarization to assess alternans, spatiotemporal heterogeneity, and susceptibility to atrial fibrillation. Heart Rhythm 13: 953–961, 2016. doi: 10.1016/j.hrthm.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 118.Wagner MB, Golod D, Wilders R, Verheijck EE, Joyner RW, Kumar R, Jongsma HJ, Van Ginneken AC, Goolsby WN. Modulation of propagation from an ectopic focus by electrical load and by extracellular potassium. Am J Physiol Heart Circ Physiol 272: H1759–H1769, 1997. doi: 10.1152/ajpheart.1997.272.4.H1759. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA. Remodeling of early-phase repolarization: a mechanism of abnormal impulse conduction in heart failure. Circulation 113: 1849–1856, 2006. doi: 10.1161/CIRCULATIONAHA.106.615682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, Xu H, Kumar R, Tipparaju SM, Wagner MB, Joyner RW. Differences in transient outward current properties between neonatal and adult human atrial myocytes. J Mol Cell Cardiol 35: 1083–1092, 2003. doi: 10.1016/S0022-2828(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 121.Wang Z, Feng J, Shi H, Pond A, Nerbonne JM, Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ Res 84: 551–561, 1999. doi: 10.1161/01.RES.84.5.551. [DOI] [PubMed] [Google Scholar]
- 122.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res 98: 1244–1253, 2006. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 123.Wickenden AD, Jegla TJ, Kaprielian R, Backx PH. Regional contributions of Kv1.4, Kv4.2, and Kv4.3 to transient outward K+ current in rat ventricle. Am J Physiol Heart Circ Physiol 276: H1599–H1607, 1999. [DOI] [PubMed] [Google Scholar]
- 124.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92: 1954–1968, 1995. doi: 10.1161/01.CIR.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 125.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res 52: 226–235, 2001. doi: 10.1016/S0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 126.Workman AJ, Marshall GE, Rankin AC, Smith GL, Dempster J. Transient outward K+ current reduction prolongs action potentials and promotes afterdepolarisations: a dynamic-clamp study in human and rabbit cardiac atrial myocytes. J Physiol 590: 4289–4305, 2012. doi: 10.1113/jphysiol.2012.235986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu R, Patwardhan A. Mechanism of repolarization alternans has restitution of action potential duration dependent and independent components. J Cardiovasc Electrophysiol 17: 87–93, 2006. doi: 10.1111/j.1540-8167.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 128.Xiao L, Koopmann TT, Ördög B, Postema PG, Verkerk AO, Iyer V, Sampson KJ, Boink GJJ, Mamarbachi MA, Varro A, Jordaens L, Res J, Kass RS, Wilde AA, Bezzina CR, Nattel S. Unique cardiac Purkinje fiber transient outward current β-subunit composition: a potential molecular link to idiopathic ventricular fibrillation. Circ Res 112: 1310–1322, 2013. doi: 10.1161/CIRCRESAHA.112.300227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xiao L, Xiao J, Luo X, Lin H, Wang Z, Nattel S. Feedback remodeling of cardiac potassium current expression: a novel potential mechanism for control of repolarization reserve. Circulation 118: 983–992, 2008. doi: 10.1161/CIRCULATIONAHA.107.758672. [DOI] [PubMed] [Google Scholar]
- 130.Zhou X, Bueno-Orovio A, Orini M, Hanson B, Hayward M, Taggart P, Lambiase PD, Burrage K, Rodriguez B. In vivo and in silico investigation into mechanisms of frequency dependence of repolarization alternans in human ventricular cardiomyocytes. Circ Res 118: 266–278, 2016. doi: 10.1161/CIRCRESAHA.115.307836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ziegler PD, Rogers JD, Ferreira SW, Nichols AJ, Richards M, Koehler JL, Sarkar S. Long-term detection of atrial fibrillation with insertable cardiac monitors in a real-world cryptogenic stroke population. Int J Cardiol 244: 175–179, 2017. doi: 10.1016/j.ijcard.2017.06.039. [DOI] [PubMed] [Google Scholar]