Abstract
Peripheral artery disease (PAD) in the lower extremities often leads to intermittent claudication. In the present study, we proposed a low-dose DCE MRI protocol for quantifying calf muscle perfusion stimulated with plantar flexion and multiple new metrics for interpreting perfusion maps, including the ratio of gastrocnemius over soleus perfusion (G/S; for assessing the vascular redistribution between the two muscles) and muscle perfusion normalized by whole body perfusion (for quantifying the muscle’s active hyperemia). Twenty-eight human subjects participated in this Institutional Review Board-approved study, with 10 healthy subjects (group A) for assessing interday reproducibility and 8 healthy subjects (group B) for exploring the relationship between plantar-flexion load and induced muscle perfusion. In a pilot group of five elderly healthy subjects and five patients with PAD (group C), we proposed a protocol that measured perfusion for a low-intensity exercise and for an exhaustion exercise in a single MRI session. In group A, perfusion estimates for calf muscles were highly reproducible, with correlation coefficients of 0.90–0.93. In group B, gastrocnemius perfusion increased linearly with the exercise workload (P < 0.05). With the low-intensity exercise, patients with PAD in group C showed substantially lower gastrocnemius perfusion compared with elderly healthy subjects [43.4 (SD 23.5) vs. 106.7 (SD 73.2) ml·min−1·100 g−1]. With exhaustion exercise, G/S [1.0 (SD 0.4)] for patients with PAD was lower than both its low-intensity level [1.9 (SD 1.3)] and the level in elderly healthy subjects [2.7 (SD 2.1)]. In conclusion, the proposed MRI protocol and the new metrics are feasible for quantifying exercise-induced muscle hyperemia, a promising functional test of PAD.
NEW & NOTEWORTHY To quantitatively map exercise-induced hyperemia in calf muscles, we proposed a high-resolution MRI method shown to be highly reproducible and sensitive to exercise load. With the use of low contrast, it is feasible to measure calf muscle hyperemia for both low-intensity and exhaustion exercises in a single MRI session. The newly proposed metrics for interpreting perfusion maps are promising for quantifying intermuscle vascular redistribution or a muscle’s active hyperemia.
Keywords: calf muscles, cardiac output, magnetic resonance imaging, peripheral artery disease, plantar flexion
INTRODUCTION
With lower extremity peripheral artery disease (PAD), patients often develop intermittent claudication during walking activities, thereby decreasing their mobility (57). PAD is caused by atherosclerosis that results in arterial narrowing in one or more segments of the peripheral vasculature. The severity of PAD is currently diagnosed based on the ankle-brachial index (ABI), the ratio of blood pressures measured at the ankle and arm. Before surgical intervention, an imaging exam such as computed topography or magnetic resonance (MR) angiography is applied to map the spatial distribution of the artery stenosis along the arterial tree (32, 34). However, no clinical method provides quantitative information on the impact of arterial stenosis to the downstream calf muscles, particularly in the presence of collateral vessels. As a research tool, venous occlusion plethysmography can be used to estimate arterial blood flow into a limb by occluding the venous outflow (35, 39); despite its simplicity and robustness, the method does not provide regional perfusion for individual muscle groups. Accurate mapping of exercise-stimulated muscle perfusion could improve the diagnosis of PAD and serve as a functional biomarker for guiding therapeutic management.
For measuring muscle perfusion, an imaging approach has multiple advantageous features. First, most imaging techniques are noninasive and can potentially be performed multiple times, e.g., at different exercise intensities, in a single visit. Second, cross-sectional imaging can reveal perfusion of different muscle groups within a same field of view and even reveal intramuscle heterogeneity of blood flow. Dynamic contrast-enhanced (DCE) MR imaging (MRI) has been widely adopted as a clinical tool for assessing tissue perfusion in many organs (27, 36, 41, 53) and in cancers (26, 31, 51). In skeletal muscle (2, 13, 15, 29, 30), several groups have explored potential value of DCE MRI in assessing PAD. For example, Luo et al. (29) detected a decrease in the DCE MRI-measured muscle perfusion index (PI) after the induction of femoral artery ligation in a rat model. To date, published studies have used a relatively high dosage of contrast agent (0.1~0.2 mmol/kg), often leading to arterial signal saturation (59). To avoid this artifact, Isbell et al. (15) proposed to acquire muscle and arterial signals with different sequence settings. Nevertheless, high dosages of contrast agent have precluded repeated perfusion studies and the performance of same-day contrast-enhanced MR angiography.
DCE MRI using a low dosage of gadolinium chelates (~0.05 mmol/kg or less) has been established for measuring tissue perfusion with high precision and spatial resolution (1, 59, 61, 63). This technique has been mostly applied in renal imaging (25, 55, 62). In the present study, we adapted the low-dose DCE MRI protocol for mapping exercise-stimulated muscle perfusion and proposed multiple new metrics for interpreting exercise-stimulated muscle perfusion. We tested the hypotheses that the perfusion metrics are reproducible and are sensitive to exercise intensity. We also demonstrated in a pilot study the feasibility of a dual-intensity protocol in assessing the performance of calf muscles in elderly subjects and patients with PAD.
MATERIALS AND METHODS
Subjects and exercise protocols.
This prospective study was approved by the Institutional Research Board and was Health Insurance Portability and Accountability Act of 1996 compliant. Written informed consent was obtained from all participants before the experimental procedures. A total of 28 subjects participated in this study (April 2015 to November 2017). The characteristics of the subjects are shown in Table 1. In group C, the five elderly healthy subjects were selected based on the absence of intermittent claudication, any leg pain, and history of any atherosclerosis. None of the subjects had type 2 diabetes; one subject had hypertension, and another subject smoked. Of the five patients with PAD in group C, three patients had computed topography angiographic data showing the presence of stenosis in the iliac and proximal popliteal arteries, superficial femoral artery, and iliac and posterior tibial arteries, respectively. The other two patients with PAD were diagnosed based on their low ABI values and symptom of intermittent claudication. Of the five patients with PAD, two patients had type 2 diabetes, all five patients had hypertension, and four patients smoked or had a history of smoking.
Table 1.
Characteristics of the subjects enrolled in the present study
| Aim | Subjects | Protocol | |
|---|---|---|---|
| Group A | To test the reproducibility of measured muscle perfusion | 10 healthy volunteers, 4 men and 6 women, 29.4 (SD 6.4) yr old | Repeated 8 lb on 2 separate days |
| Group B | To investigate the relationship between exercise intensity and muscle perfusion | 8 healthy volunteers, 6 men and 2 women, 26.0 (SD 4.6) yr old | Exercise with loads of 4, 8, and 16 lb |
| Group C | A pilot study to test the feasibility of a dual-intensity protocol for assessing PAD | 5 elderly healthy volunteers, 4 men and 1 woman, 62.6 (SD 4.3) yr old, BMI 28.4 (SD 3.5); | 4 lb and exercise to exhaustion on a same day |
| 5 patients with PAD, 4 men and 1 woman, 61.2 (SD 4.8) yr old, ABI 0.73 (SD 0.10), BMI 25.2 (SD 5.5) |
Plantar flexion with a load of 4 lb and frequency of 1 Hz in our experiments corresponds to a work output of ~2.7 W. PAD, peripheral artery disease; BMI, body mass index; ABI, ankle-brachial index.
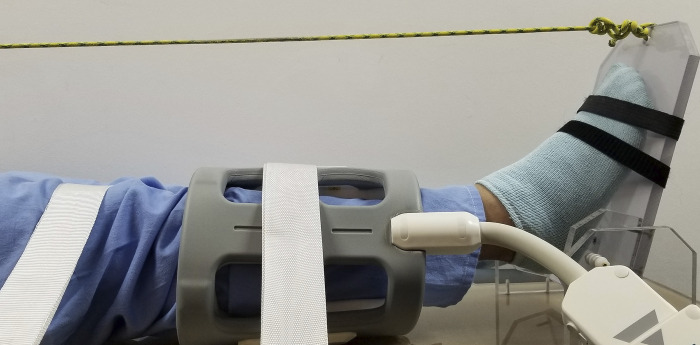
All experiments were performed in a thermoneutral environment with participants in an overnight fasted state and having refrained from any intensive physical activity for 24 h before the experimental protocol. With the subject in a supine feet-first position in the MR scanner, a strap was used to fasten the imaged leg to the scanner table, so that the leg kept straight and stationary in exercise and imaging. Single-leg dynamic plantar flexion was performed with an MRI-compatible apparatus that allowed for adjustable load (Fig. 1). The right leg was scanned in all healthy subjects, whereas the more symptomatic leg was scanned in patients with PAD. Immediately after exercise stopped, DCE MRI was initiated to capture exercise-stimulated hyperemia. From the recruited subjects (Table 1), we collected three groups of data. For group A, which consisted of 10 healthy subjects, we repeated the MRI measurement on 2 separate days [37 (SD 21] days apart, ranging from 13 to 82 days] for each subject. The same exercise protocol was used in the MRI measurement: plantar flexion of 3 min, a frequency of 1 Hz, and load of 8 lb. For group B, each of the eight young healthy subjects underwent three MRI perfusion measurements for plantar flexion at different workloads; each exercise lasted for 3 min at a flexion frequency of 1 Hz and was loaded with constant weight of 4, 8, and 16 lb, respectively. The three DCE MRI scans were finished in 2 days. For group C, each of the five elderly healthy subjects and five patients with PAD underwent MRI after two different exercises (“dual-intensity protocol”). For the low-intensity measurement, subjects performed plantar flexion with a low and constant load (4 lb) at 1 Hz for 3 min. For the exhaustion measurement, the plantar flexion exercise started with a load at 2 lb and increasing by 2 lb every minute until exhaustion (22, 23). A period of 15 min was put between the two measurements for the subject to recover. The low-intensity exercise was aimed to stimulate measurable calf muscle perfusion in light activities, such as walking. None of the recruited subjects, including the patients with PAD, developed any symptoms during the low-intensity exercise. The exhaustion exercise was aimed to stimulate the maximum perfusion in the calf muscles during plantar flexion.
Fig. 1.
Our experimental setup of plantar-flexion exercise inside the MRI scanner. A flexible surface radiofrequency coil is wrapped around the calf. The imaged leg is fastened to the scanner table by the white straps to make sure that straight-leg plantar flexion is performed. The other end of the rope is attached to adjustable load. For better demonstration effect, this picture was taken outside of the MRI scanner.
DCE MRI to measure exercise-stimulated perfusion.
Each subject was positioned supine feet-first in a 3-T clinical MRI scanner (TimTrio, Siemens, Erlangen, Germany). Two four-channel flex coils were wrapped around the leg, one around the calf and the other around the knee of the exercising leg. Before exercise, 10 images were acquired to establish a baseline (see sequence details below). The subject was then instructed to perform the plantar flexion exercise protocol. Five seconds before the end of exercise, 0.05 mmol/kg gadoteridol (ProHance, Bracco Diagnostics) was injected intravenously followed by injection of 20 ml saline, both at a rate of 4 ml/s. Immediately after exercise cessation, with the patient’s ankle immobile in its neutral position, dynamic imaging started and continued for 4 min. Both the baseline and dynamic imaging used a saturation-recovery prepared fast low angle shot (FLASH) sequence with the following parameters (59): delay time (TD), 307 ms; repetition time (TR), 527 ms; echo time, 1.42 ms; flip angle, 15°; pixel bandwidth, 1,002 Hz; slice thickness, 10 mm; number of averages, 1; image matrix, 128 × 128; and field of view, 180 × 180 mm2. Dynamic signals of two axial slices, one at the level of maximum diameter of the calf and the other at the level of knee, were acquired at 1 s/frame. In some patients with PAD, the arteries on the calf level may be too narrow for sampling arterial input, so we chose to image the knee-level slice as well to cover the popliteal artery as an alternative option. Immediately after the 4-min dynamic imaging, we performed three additional acquisitions with the above sequence but with a long TR of 4 s to estimate proton density (PD). PD images were used to quantify contrast concentration from the dynamic saturation-recovery signals (55).
Quantify muscle perfusion with tracer kinetic modeling.
All acquired images were transferred to a personal computer for postprocessing using Matlab-based in-house programs. We first converted each dynamic image (S) to a map of spin-lattice relaxation time (T1) based on the following saturation-recovery formula: S = PD × [1 − exp(−TD/T1)]. Postcontrast T1 maps were converted to maps of contrast concentration (M) based on the following relaxivity formula:
| (1) |
where is the baseline T1 value before contrast enhancement and r1 is T1 relaxivity. Subtraction of the baseline T1 relaxation rate in Eq. 1 also eliminates the effect of potential contrast residue in our dual-injection acquisition protocol. For each voxel in the field of view, the temporal course of contrast concentration was analyzed using an established tracer kinetic model to estimate tissue perfusion. The model expresses tissue contrast concentration (per voxel) in terms of perfusion (F) and the convolution of the arterial input function (AIF) and the impulse retention function (R) (7, 46, 61), as follows:
| (2) |
AIF is the contrast concentration converted from arterial signals, which in this application were manually sampled from the peroneal artery, anterior tibial artery, and/or posterior tibial artery in the calf slice. As muscle perfusion decreases after exercise stops, we included only the dynamic data of the first 40 s for quantifying exercise-induced perfusion. For perfusion quantification, dynamic images acquired during contrast uptake (typically the initial 30–40 s) would be adequate. We acquired the dynamic images for 4 min to study contrast excretion in exercising muscles for a separate study. With the use of a numerical optimization technique, F in Eq. 2 can be optimized so that the right side of the equation fits the concentration data [M(t)]. To evaluate the goodness of fit, we computed root mean square error between M(t) and its model fit and expressed it as a percentage of the averaged magnitude of M(t). As the result of the voxel-wise model fitting, we obtained a perfusion map with an in-plane resolution of 1.4 × 1.4 mm2. Under the supervision of a musculoskeletal radiologist (10 yr of experience), regions of interest were manually drawn for the medial and lateral gastrocnemius (MG and LG, respectively), soleus (S), peroneal (PE), tibilalis posterior (TP), tibialis anterior (TA), and extensor digitorum longus (EDL) to compute average perfusion for these muscle groups.
New quantitative metrics of skeletal muscle perfusion.
In addition to computing average perfusion for the muscle groups, we also propose here new quantitative metrics for normalizing muscle perfusion to overall whole body perfusion and for comparing response to exercise across the different plantar-flexion muscles in the calf. The first new parameter, termed “G/WBP,” is the ratio of gastrocnemius perfusion (G) to average whole body perfusion (WBP). WBP (in ml·min−1·100 g−1) is estimated by normalizing cardiac output (CO) by body weight. In the present study, we estimated CO for each subject from the measured AIF using an established method (60). Briefly, the Stewart-Hamilton equation [D = CO × AUC] enables the calculation of CO, where D is the dosage of injected contrast (in mmol) and AUC is the area under the first pass of AIF (in mmol·min−1·ml−1). The equation is valid for both the aorta and peripheral arteries, even those with arterial stenosis. As the AIF was measured immediately after exercise cessation, the above computed CO reflects exercise-stimulated CO, the same as the muscle perfusion estimates. If the increase in G were solely caused by the higher heart rate and stroke volume (reflected in the increased CO), like all the other organs, then the parameter G/WBP would be constant at value of 1; active hyperemia of gastrocnemius would be reflected as G/WBP >1. The same ratio parameter can be computed for S to obtain S/WBP.
A second new parameter, termed as “G/S,” is the ratio of averaged gastrocnemius perfusion over averaged soleus perfusion. Both the gastrocnemius and soleus contribute to support plantar flexion in walking but with different roles (33). The literature also suggested that with aging or PAD the two muscles deteriorate by different rates (9, 19, 56). For example, with aging, the gastrocnemius tends to deteriorate earlier than the soleus. G/S from noninvasive imaging would reveal the real-time performance of these two muscles in plantar flexion of any specified intensity.
Statistical analysis.
For group A, consisting of 10 healthy subjects, we compared the perfusion of the MG, LG, and S measured from two visits using the Pearson correlation coefficient. The intervisit agreement of the perfusion values was also assessed by computing the relative difference, i.e., the difference divided by the average of each pair of perfusion values.
Each of the eight healthy subjects in group B performed plantar flexion at three different intensities: 4, 8, and 16 lb. For each parameter, the values from two adjacent exercise intensities (4 vs. 8 lb and 8 vs. 16 lb) were first compared using paired t-tests to detect perfusion difference between the adjacent intensities. To further assess the association between each parameter (G, S, G/WBP, S/WBP, and G/S) and exercise intensity, we used a linear mixed effect model to estimate the change in parameter values across the three exercise intensities and reported the point estimate and 95% confidence intervals (CIs) of the slope (change of the parameter for 1-lb increase in exercise load). For this group of data, we also computed the correlation matrix, with each element being the Pearson’s correlation coefficient between perfusion estimates of two different muscle groups. The involved muscle groups included S, MG, LG, PE, TP, TA, and EDL. Each muscle group had 24 perfusion estimates (8 subjects, each with 3 exercise intensities). With this correlation matrix, we can identify which muscle groups would get simultaneous hyperemia in plantar flexion.
Group C included five elderly healthy subjects and five patients with PAD. The parameters G, S, G/WBP, S/WBP, and G/S were computed for each subject and each exercise protocol (low-intensity or exhaustion). As this was a pilot study with just five subjects in each group, we aimed only to identify any promising metric that could potentially differentiate the two groups. Between the healthy subjects and patients with PAD, each of the parameters was compared with a two-sample t-test. The comparisons for the exhaustion-exercise estimates were adjusted for their respective maximal exercise load.
All statistical tests were two sided. P < 0.05 indicates statistically significant results. Statistical Packages R version 3.1 (https://www.r-project.org/) and Stata version 13 (College Station, TX) were used for the statistical analyses.
RESULTS
Reproducibility of the proposed method (group A).
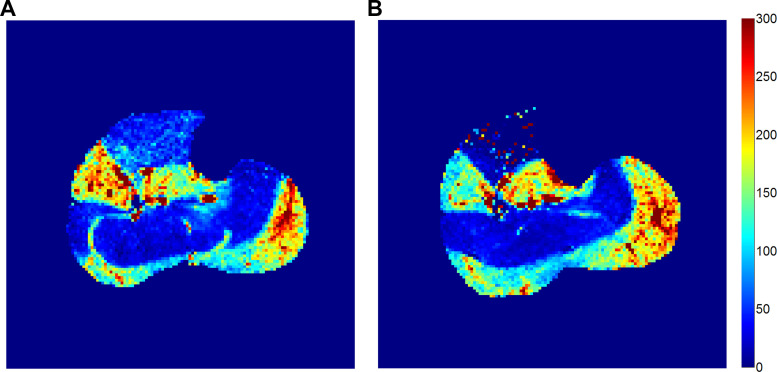
For each of the 10 subjects, three muscle groups (MG, LG, and S) were included in this analysis. The 30 perfusion estimates were averaged at 113.4 (SD 80.0) ml·min−1·100 g−1 for visit 1 and 111.8 (SD 71.5) ml·min−1·100 g−1 for visit 2, with relative differences of 5.1% (SD 31.6%). Perfusion estimates from the two visits correlated with each other significantly, with coefficient of 0.93, 0.92, and 0.90 for the MG, LG, and S, respectively. Figure 2 shows perfusion maps quantified from two MRI visits of a same subject for demonstration.
Fig. 2.
Calf-muscle perfusion maps measured on 2 separate days for the same healthy subject (woman, 27 yr old, from group A). A and B: visit 1 (A) and visit 2 (B) for this subject were 28 days apart.
The relationship between the exercise intensity and the stimulated muscle perfusion (group B).
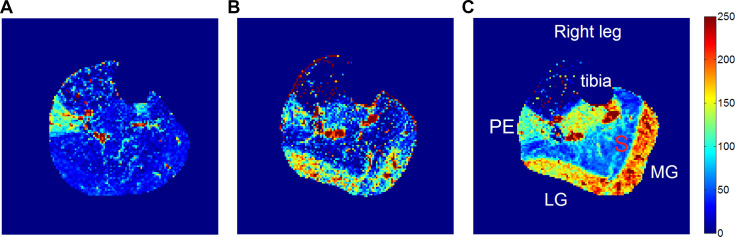
Figure 3 shows perfusion maps stimulated by exercise of different intensities in one subject, where perfusion of both the medial and lateral heads of gastrocnemius increased with exercise load. In some subjects, high perfusion can also be observed in other muscles that could assist plantar flexion, such as PE and TP. In more rare cases, the tibialis-anterior muscle was sometimes used to dorsiflex to reposition the load to its baseline position when the load was too light for the gravity to do so on its own.
Fig. 3.
Calf muscle perfusion maps at different loads of plantar flexion for a healthy subject (from group B). A: 4 lb; B: 8 lb; C: 16 lb. For this case, perfusion increased progressively with load in the lateral gastrocnemius (LG) and medial gastrocnemius (MG). PE, peroneal. Units for the perfusion are ml·min−1·100 g−1.
Table 2 shows the perfusion values of the posterior muscle groups, including the soleus and gastrocnemius, which are predominantly involved during plantar flexion exercise. Gastrocnemius perfusion increased significantly when the exercise load increased from 4 to 8 lb (P = 0.048) and then significantly from 8 to 16 lb (P = 0.040). Compared with the gastrocnemius, soleus perfusion was much lower, 33.6 (SD 9.8) (P = 0.017) and 39.6 (SD 21.2) (P = 0.002) at exercise loads of 4 and 8 lb, respectively, and at 16 lb increased significantly to 86.5 (SD 50.8)) ml·min−1·100 g−1 (P = 0.008).
Table 2.
Calf muscle perfusion measurements with increasing plantar-flexion load in healthy subjects (group B)
| 4 lb | 8 lb (SD, P1) | 16 lb (SD, P2) | Slope (P3) | |
|---|---|---|---|---|
| G, ml·min−1·100 g−1 | 100.1 (71.1) | 141.4 (56.8, 0.048) | 184.4 (39.3, 0.040) | 6.7 (0.001) |
| S, ml·min−1·100 g−1 | 33.6 (9.8) | 39.6 (21.2, 0.140) | 86.5 (50.8, 0.008) | 4.6 (0.001) |
| G/WBP | 3.7 (2.6) | 4.6 (2.7, 0.048) | 6.7 (1.8, 0.038) | 0.25 (0.001) |
| S/WBP | 1.3 (2.5) | 1.2 (2.7, 0.416) | 2.9 (11.2, 0.001) | 0.16 (0.001) |
| G/S | 3.1 (2.2) | 4.3 (2.0, 0.048) | 2.6 (1.2, 0.035) | −0.07 (0.342) |
Values are means (SD). “Slope” from the linear mixed effect model analysis quantifies how much a parameter changes per pound of exercise load. G, gastrocnemius perfusion; S, soleus perfusion; WBP, whole body perfusion (equal to cardiac output divided by body weight); P1, P value (paired t-test) compared with the 4-lb value; P2, P value (paired t-test) compared with the 8-lb value; P3, P value for the slope parameter based on the linear mixed effect model analysis.
G/WBP increased linearly with exercise load, from 3.7 (SD 2.6) at 4 lb to 4.6 (SD 2.7) at 8 lb and increased to 6.7 (SD 1.8) at 16 lb, with statistically significant differences (P = 0.048 and 0.038, respectively). G/WBP values were much greater than 1 at all three exercise loads. In contrast, S/WBP was not significantly different from 1 at 4 lb [1.3 (SD 2.5)] and at 8 lb [1.2 (SD 2.7)] but at 16 lb increased to 2.9 (SD 11.2).
In this cohort of healthy subjects, G/S increased from 3.1 (SD 2.2) at 4 lb to 4.3 (SD 2.0) at 8 lb and then decreased to 2.6 (SD 1.2) at 16 lb. There were statistically significant differences in G/S values between exercise loads of 4 and 8 lb (P = 0.048) as well as between 4 and 16 lb (P = 0.035).
Linear mixed effect model analysis showed statistically significant increases in both G [averaged slope of 6.7 per lb, 95% CI (3.3, 10.1)] and G/WBP [averaged slope of 0.25 per lb, 95% CI (0.12, 0.38)] with increased workload. For the soleus, average slopes were lower than those of the gastrocnemius, but the associations were still significant (Table 2). For these young healthy subjects, no statistically significant linear association was detected between G/S and exercise intensities (P = 0.342).
Table 3 shows the correlation coefficients between perfusion estimates of the different muscle groups. The pairs with correlation coefficients higher than 0.60 included the MG and LG (0.65), MG/LG, and PE (0.81 and 0.66), TA, and EDL (0.95). The high correlation between TA and EDL indicates that these two muscles were often activated simultaneously, and their negative correlation with the gastrocnemius was due to the frequent activation of TA and EDL in low-load plantar flexion to pull the foot back to the neutral position (i.e., dorsiflexion). Correlation coefficients between the soleus and all other muscles were low (<0.3).
Table 3.
Correlation coefficients between perfusion estimates of the different calf muscle groups during plantar flexion
| MG | LG | S | PE | TP | EDL | TA | |
|---|---|---|---|---|---|---|---|
| MG | 1.00 | ||||||
| LG | 0.65* | 1.00 | |||||
| S | 0.29 | 0.22 | 1.00 | ||||
| PE | 0.81* | 0.66* | 0.11 | 1.00 | |||
| TP | 0.58 | 0.46 | 0.17 | 0.56 | 1.00 | ||
| EDL | −0.38 | −0.30 | −0.06 | −0.36 | −0.27 | 1.00 | |
| TA | −0.52 | −0.34 | −0.12 | −0.50 | −0.41 | 0.95* | 1.00 |
The data were from 8 young healthy subjects (group B), each with an exercise load of 4, 8, and 16 lb. MG, medial gastrocnemius; LG, lateral gastrocnemius; S, soleus; PE, peroneal, TP, tibilalis posterior; TA, tibialis anterior; EDL, extensor digitorum longus.
Coefficients > 0.60.
A pilot test of a dual-intensity protocol (group C).
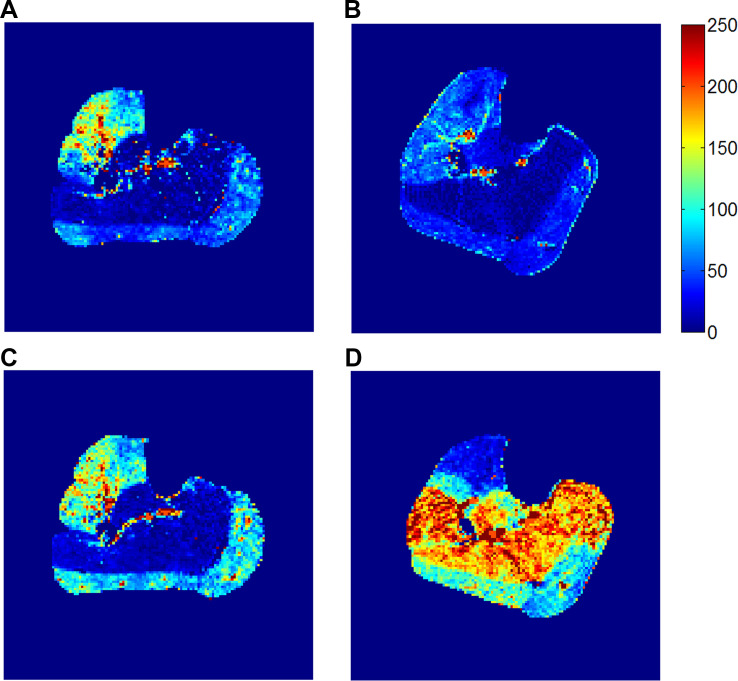
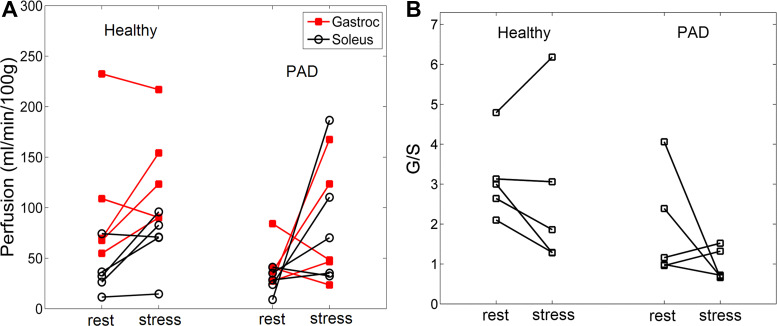
This protocol was implemented in 10 human subjects: 5 elderly healthy subjects and 5 patients with PAD. Even though the protocol required subjects to exercise to maximal tolerance, the exhaustion-exercise perfusion was measured at comparable maximal loads for the two groups: 14.0 (SD 3.5) and 14.2 (SD 5.5) lb, respectively. Representative examples of perfusion maps after the low-intensity and exhaustion exercises are shown in Fig. 4. The perfusion values of the gastrocnemius and soleus for all subjects are shown in Fig. 5. Means (SD) of the perfusion values and the derived metrics are shown in Table 4.
Fig. 4.
Calf muscle perfusion maps measured with the dual-intensity protocol (group C). A and B: perfusion map after the low-intensity exercise (4-lb load for 3 min) for a healthy elderly subject (man, 64 yr old; A) and for a patient with peripheral artery disease (PAD; man, 61 yr old, right-leg ankle-brachial index: 0.84; B). C and D: perfusion map after the exhaustion exercise for the healthy subject (exercised for 6 min to 12 lb; C) and for the patient with PAD (exercised for 9 min to 18 lb; D). In contrast to the healthy subject, the patient with PAD had a much higher perfusion increase in the soleus than in the gastrocnemius from the low-intensity to the exhaustion exercise. Units for the perfusion are ml·min−1·100 g−1.
Fig. 5.
Calf perfusion measured for the dual-intensity protocol in 5 healthy subjects and 5 patients with peripheral artery disease (PAD). A: perfusion values for the gastrocnemius and soleus. B: gastrocnemius over soleus perfusion ratio (G/S). Compared with elderly healthy subjects, patients with PAD showed lower gastrocnemius perfusion at low-intensity exercise and a higher perfusion increase in the soleus at exhaustion.
Table 4.
Perfusion parameters from the dual-intensity protocol
| Elderly Healthy Subjects | Patients With PAD | |||
|---|---|---|---|---|
| “Low intensity” | “Exhaustion” 14.0 (3.5) lb | “Low intensity” | “Exhaustion” 14.2 (5.5) lb | |
| G, ml·min−1·100 g−1 | 106.7 (73.2) | 135.0 (52.9) | 43.4 (23.5) | 81.8 (60.9) |
| S, ml·min−1·100 g−1 | 36.1 (23.3) | 66.9 (31.0) | 27.6 (12.2) | 87.0 (64.0) |
| G/WBP | 4.0 (2.6) | 5.0 (1.6) | 2.3 (2.2) | 3.5 (3.1) |
| S/WBP | 1.4 (1.0) | 2.6 (1.5) | 1.2 (0.8) | 3.6 (2.6) |
| G/S | 3.1 (1.0) | 2.7 (2.1) | 1.9 (1.3) | 1.0 (0.4) |
Values are means (SD); n = 5. G, gastrocnemius perfusion; S, soleus perfusion; WBP, whole body perfusion (equal to cardiac output divided by body weight); PAD, peripheral artery disease.
The differences of the perfusion estimates between the elderly healthy subjects and patients with PAD did not reach statistical significance. However, there were a few notable trends. Compared with elderly healthy subjects, patients with PAD had lower perfusion in the gastrocnemius after both exercises. From the low-intensity to exhaustion exercise, patients with PAD showed perfusion increases in both the gastrocnemius and soleus but the higher increase in the soleus resulted in decreased G/S values. One notable feature for the patients with PAD was the comparable perfusion for both the gastrocnemius and soleus at exhaustion, leading to an average G/S value of 1.0 (SD 0.4). In contrast, the G/S values for the elderly healthy subjects at exhaustion averaged 2.7 (SD 2.1), much higher than 1.
Other technical considerations.
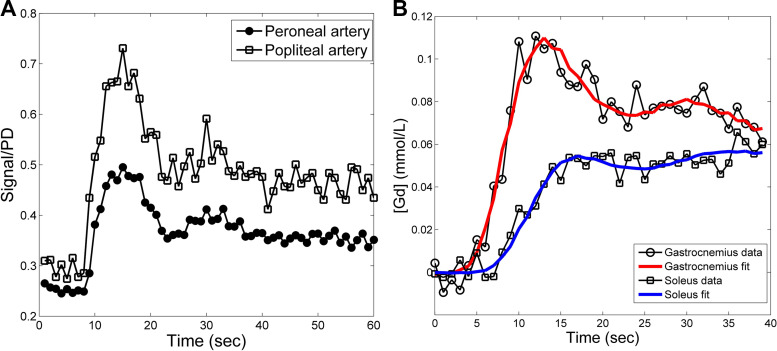
From the 28 subjects that participated, overall 64 exercise-stimulated perfusion data were acquired. No visible motion was detected in any dynamic data, so image registration was not needed. For all the cases, arterial signals were manually sampled from the lower calf slice, i.e., the peroneal artery and anterior and/or posterior tibial artery, and to avoid partial volume artifacts, only the voxels in the center of the arterial regions (combined size of 2~4 voxels) were selected. Due to the use of low contrast dosage, the sampled arterial signals even at their peak were substantially lower than their respective proton density level, indicating the absence of signal saturation artifacts. It was also noted that the sampled arterial signals were lower than the signals sampled from the popliteal artery in the upper knee slice (Fig. 6A). Compared with the upper knee slice, the lower calf slice was farther away from the upper boundary of the saturation volume, so its arterial signals suffered less from inflow artifacts. The contrast-to-noise ratio of contrast enhancement curves from individual muscle voxels was high enough for reliable curve fitting. Figure 6B shows representative curve examples from a voxel of the gastrocnemius and a voxel of the soleus. For all cases, the percent root mean square error in fitting contrast enhancement of individual voxels was typically around 10~30%, reflecting the noise level of the acquired single voxel signals.
Fig. 6.
Examples of arterial and muscle contrast enhancement curves. A: arterial signals sampled from the lower calf slice (peroneal artery for this example) suffered less from inflow artifacts than signals from the popliteal artery (the upper knee slice). For comparison, the two signals were normalized by their respective proton density (PD). B: contrast enhancement curves in a single voxel of the gastrocnemius and soleus muscle overlaid with fitted curves (red and blue lines, respectively) to estimate muscle perfusion. For this example, gastrocnemius perfusion was 85.1 ml·min−1·100 g−1 and soleus perfusion was 29.5 ml·min−1·100 g−1.
DISCUSSION
The present study tested the feasibility and reproducibility of mapping exercise-stimulated perfusion in the calf muscles using a clinical MRI scanner. In young healthy subjects, gastrocnemius perfusion increased linearly with intensity of plantar flexion, even when normalized by CO. In a pilot test with the dual-intensity protocol, gastrocnemius perfusion stimulated by the light-intensity exercise was lower in the symptomatic limb of patients with PAD than in healthy elderly subjects. More intensive stimulation with the exhaustion exercise did not substantially increase the gastrocnemius perfusion in patients with PAD, unlike their healthy counterparts, but instead elicited a greater increase in soleus perfusion.
To our knowledge, this is the first report demonstrating that calf muscle perfusion increases linearly with increasing workloads of dynamic plantar flexion exercise in healthy subjects. Most previous studies (2, 30, 38, 50, 58) stimulated hyperemia in calf muscles of human subjects with a cuffing-release procedure at rest, which may not reflect the real exercise physiology. Isbell et al. (15) used DCE MRI to quantify calf muscle perfusion stimulated with plantar flexion exercise until exhaustion (similar to our exhaustion exercise). Unfortunately, a direct comparison with the present findings is not feasible, because the prior study computed the ratio of the muscle signal enhancement slope over arterial slope (termed as PI), and, probably due to a relatively low signal-to-noise ratio, the calculation was done for regions of interest as opposed to each individual voxel. Nevertheless, the study did find that perfusion was higher in healthy control subjects compared with patients with PAD (median PI: 0.48 vs. 0.29) for the same exercise intensity, and an increase in exercise intensity led to a further increased PI of 0.69 in healthy control subjects. These trends agree with the findings in the present study. Using arterial spin labeling MRI to estimate perfusion during peak exercise, Pollack et al. (37) found higher perfusion in age-matched healthy control subjects than in patients with PAD [80 (SD 23) vs. 49 (SD 16) ml·min−1·100 g−1, respectively]. While this pattern is similar to our result shown in Table 4, their reported values were sampled from the highest perfused muscle voxels but not measured across a specific muscle group. Contrast enhanced ultrasound has also been actively explored for assessing muscle perfusion (8, 20, 44, 50), with relatively high accuracy and precision. However, the relatively low spatial resolution of the method (20) prevents reliable assessment of perfusion heterogeneity across muscle groups. With the proposed reproducible and high-resolution mapping of muscle perfusion, a future direction of research is to analyze the spatial heterogeneity and pattern of muscle perfusion to gain more understanding on capillary recruitment in skeletal muscles (12, 17, 54).
With exercise, a greater fraction of CO is delivered to the activated skeletal muscle (3, 16, 47). In the present study, we estimated CO and muscle perfusion from a same set of postexercise MRI data to assess whether with exercise hyperemia in the activated muscles exceeded the overall average increase in systemic perfusion (11). In our experiments with low-intensity exercise, S/WBP estimates of all subjects were close to 1, suggesting that hyperemia in the soleus with exercise was no greater than the average increase in perfusion with low-intensity plantar flexion. For young healthy subjects, G/WBP increased progressively with exercise load, suggesting that the gastrocnemius muscle was preferentially hyperperfused at heavier loads. With exhaustion exercise, G/WBP was lower in patients with PAD than in elderly healthy subjects [3.5 (SD 3.1) vs. 5.0 (SD 1.6)], possibly because vasodilatory capacity of the gastrocnemius may have been compromised in the patients. The proposed ratio of muscle perfusion over WBP, particularly measured at the exhaustion exercise, may be useful in assessing the potential benefit of exercise therapy in improving muscle vasodilation for patients with PAD. As obesity is often associated with PAD, estimation of WBP for the patients could be improved by eliminating the impact of body fat, i.e., using “lean” body weight instead to normalize CO. The weight of body fat can be readily estimated by a whole body MRI scan (28).
For assessing the relative contribution of the gastrocnemius versus soleus in plantar flexion, we computed G/S as the ratio of averaged gastrocnemius to soleus perfusion. In young healthy subjects, G/S maintained at a high level, around 2.6~4.3, and at the maximal load of 16 lb dropped to a lower level. The more activated gastrocnemius relative to soleus in our experiment agrees with the previous finding that, with straight leg (or fully extended knee), the gastrocnemius is most efficient and thus used in supporting plantar flexion (21). With exercise load increasing from 8 to 16 lb, gastrocnemius perfusion increased by ~30% only, and, as compensation, the soleus perfusion doubled, which explains the drop in G/S at the heaviest load. Elderly healthy subjects showed comparable perfusion results as the young healthy subjects, and, even at exhaustion, were able to maintain a high averaged G/S level of 2.7 (SD 2.1). The relatively high variability of G/S among the elderly healthy subjects was possibly due to their different status of physical fitness, which will be further investigated with a larger group of elderly subjects in future study. Now we look at the results of the patients with PAD. Due to the low gastrocnemius perfusion, G/S was low at the low-intensity exercise and decreased further to less than one at the exhaustion exercise. One possible cause for these findings with patients with PAD is the myofiber oxidative damage that is selective for type II (or “fast-twitch”) muscle fibers by lower extremity PAD (19, 56). As the gastrocnemius contains much type II fibers and the soleus does not, in patients with PAD the gastrocnemius would be impaired more severely than the soleus (10). Therefore, in exhaustion exercise, the impaired gastrocnemius would get fatigued easily, whereas the soleus, with mostly type-I (or “slow-twitch”) fibers, would be more sustainable (4). Lower extremity PAD could also affect capillary density in calf muscles. A previous study with muscle biopsy found reduced capillary density in the gastrocnemius of patients with PAD (40), and this was confirmed by the relatively low gastrocnemius perfusion measured by our proposed method. With imaging, we had opportunity of measuring perfusion for the soleus, which is not quite accessible for muscle biopsy, and observed relatively high soleus perfusion, suggesting less or no damage in the capillaries of the soleus by PAD. Hence, with perfusion measurements by our dual-intensity protocol, patients with PAD showed a promising characteristic feature: blood redistribution from the gastrocnemius to the soleus (or lower G/S) in the exhaustion exercise compared with the low-intensity exercise. This blood redistribution is different from the conventional “vascular steal” phenomenon (5), in that the latter is caused by stenosis in large artery branches. Future studies with more patients with PAD are needed to validate this feature of blood redistribution for assessing calf muscle functions in patients with PAD.
With current clinical standards, PAD is diagnosed by a low ABI of the affected leg and can be further characterized by computed topography or MR angiography to determine the location and the extent of arterial stenosis. Ultrasound has also been used in some clinical centers to assess blood pressure or flow in leg arteries (14, 49). With these measurements, intervention can be performed percutaneously with angioplasty and possible stenting or surgically with a vascular bypass (48). However, ~30% of treated patients do not experience significant improvement in symptoms after surgical interventions (18). Such failure could be due to diminished capillary density in the affected muscles (40), which would not be detected by either ABI or angiography nor be reversed by surgery. Conversely, as suggested by numerous studies (6, 24, 42, 52), muscle hypoperfusion due to arterial stenosis may be compensated by newly grown collateral arteries in some patients with PAD; for these patients, invasive surgical treatment should not be applied (42, 64). With the proposed method, we can assess the effects of vascular disease on exercise-stimulated muscle perfusion. To properly interpret muscle perfusion distribution as revealed by the proposed protocol, for patients with PAD it is recommended to determine the exact location and significance of the peripheral artery stenoses using angiography. A comprehensive protocol can be achieved by first performing noncontrast MR angiography (32) and then the proposed dual-intensity perfusion scans in a same visit. The contrast dosage used in our dual-intensity perfusion protocol (overall 0.1 mmol/kg gadoteridol) has been shown to be safe for patients with an estimated glomerular filtration rate of 30–59 or <30 ml·min−1·1.73 m−2 (45). Such a clinical protocol has the potential to improve the management of patients with PAD by quantitatively evaluating the effect of therapeutic interventions, such as medication, exercise, or surgery, on regional muscle perfusion.
This study has multiple limitations. First, sample sizes of patients with PAD and elderly healthy subjects were small, and without angiographic scans, the elderly healthy subjects could have asymptomatic PAD. While these preliminary data do demonstrate the feasibility and promise of the proposed method, validation of the method in larger groups of patients with PAD and elderly healthy subjects is necessary in future work. Second, in comparing patients with PAD and elderly healthy subjects in group C, we did not consider the potential impact from confounding factors such as diabetes, hypertension, and smoking. These factors could affect blood flow to the calf muscles and should be considered in subject recruitment in future studies. Fourth, we measure perfusion during exercise recovery but not during exercise, so as to avoid artifacts that would impair our measurements, such as motion artifacts and possible muscle contraction effects (43) from exercise. To best capture exercise hyperemia, we used only the images of the first 40 s for perfusion quantification. The large differences between perfusion after the different loads (Table 2) suggest that our measured perfusion reflects the effects of exercise. Finally, in this study, images were acquired for only one axial slice of the calf. In future studies, we will cover multiple slices or even a volume of the calf so as to reduce repositioning variation across different visits.
In conclusion, we have proposed a method that measures exercise-induced calf muscle perfusion and can be readily implemented on clinical MRI scanner. The pulse sequences used are conventional and universally available, and low doses of Gd contrast agent are generally considered safe. The measured calf muscle perfusion was reproducible and increased linearly with plantar-flexion intensity in young healthy subjects. The proposed metrics (G/WBP and G/S) for quantifying perfusion maps reveal promising insights into the impact of PAD on exercise tolerance and perfusion of calf muscles.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-092439 (to V. S. Lee) and R01-HL-135242 (to J. L. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.Z., C.C.C., and N.H. analyzed data; J.L.Z., G.L., C.H., C.R.H., L.K., M.T.M., and V.S.L. interpreted results of experiments; J.L.Z. prepared figures; J.L.Z. drafted manuscript; J.L.Z., G.L., C.H., C.C.C., N.H., L.K., and V.S.L. edited and revised manuscript; J.L.Z., G.L., C.H., C.C.C., C.R.H., N.H., L.K., M.T.M., and V.S.L. approved final version of manuscript; G.L., C.C.C., and C.R.H. performed experiments.
REFERENCES
- 1.Bokacheva L, Rusinek H, Chen Q, Oesingmann N, Prince C, Kaur M, Kramer E, Lee VS. Quantitative determination of Gd-DTPA concentration in T1-weighted MR renography studies. Magn Reson Med 57: 1012–1018, 2007. doi: 10.1002/mrm.21169. [DOI] [PubMed] [Google Scholar]
- 2.Brunner G, Bismuth J, Nambi V, Ballantyne CM, Taylor AA, Lumsden AB, Morrisett JD, Shah DJ. Calf muscle perfusion as measured with magnetic resonance imaging to assess peripheral arterial disease. Med Biol Eng Comput 54: 1667–1681, 2016. doi: 10.1007/s11517-016-1457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carù B, Colombo E, Santoro F, Laporta A, Maslowsky F. Regional flow responses to exercise. Chest 101, Suppl: 223S–225S, 1992. doi: 10.1378/chest.101.5_Supplement.223S. [DOI] [PubMed] [Google Scholar]
- 4.Cronin NJ, Avela J, Finni T, Peltonen J. Differences in contractile behaviour between the soleus and medial gastrocnemius muscles during human walking. J Exp Biol 216: 909–914, 2013. doi: 10.1242/jeb.078196. [DOI] [PubMed] [Google Scholar]
- 5.Crottogini AJ, Guth BD, Barra JG, Willshaw P, Lascano EC, Pichel RH. Interventricular coronary steal induced by stenosis of left anterior descending coronary artery in exercising pigs. Circulation 83: 1361–1370, 1991. doi: 10.1161/01.CIR.83.4.1361. [DOI] [PubMed] [Google Scholar]
- 6.de Lussanet QG, van Golde JC, Beets-Tan RG, de Haan MW, Zaar DV, Post MJ, Huijberts MS, Schaper NC, van Engelshoven JM, Backes WH. Magnetic resonance angiography of collateral vessel growth in a rabbit femoral artery ligation model. NMR Biomed 19: 77–83, 2006. doi: 10.1002/nbm.1003. [DOI] [PubMed] [Google Scholar]
- 7.Dennis Cheong LH, Markus Tan CK, Koh TS, Tchoyoson Lim CC, Bisdas S. Functional imaging: dynamic contrast-enhanced ct using a distributed-parameter physiologic model for accessing stroke and intracranial tumor. Conf Proc IEEE Eng Med Biol Soc 1: 294–297, 2005. doi: 10.1109/IEMBS.2005.1616402. [DOI] [PubMed] [Google Scholar]
- 8.Duerschmied D, Zhou Q, Rink E, Harder D, Freund G, Olschewski M, Bode C, Hehrlein C. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis 202: 505–512, 2009. doi: 10.1016/j.atherosclerosis.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara K, Asai H, Toyama H, Kunita K, Yaguchi C, Kiyota N, Tomita H, Jacobs JV. Changes in muscle thickness of gastrocnemius and soleus associated with age and sex. Aging Clin Exp Res 22: 24–30, 2010. doi: 10.1007/BF03324811. [DOI] [PubMed] [Google Scholar]
- 10.Gollnick PD, Sjödin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflügers Arch 348: 247–255, 1974. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- 11.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol 85: 609–618, 1998. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 12.Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol 299: R72–R79, 2010. doi: 10.1152/ajpregu.00056.2010. [DOI] [PubMed] [Google Scholar]
- 13.Hindel S, Sauerbrey A, Maaß M, Maderwald S, Schlamann M, Lüdemann L. Validation of perfusion quantification with 3d gradient echo dynamic contrast-enhanced magnetic resonance imaging using a blood pool contrast agent in skeletal swine muscle. PLoS One 10: e0128060, 2015. doi: 10.1371/journal.pone.0128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hur KY, Jun JE, Choi YJ, Lee YH, Kim DJ, Park SW, Huh BW, Lee EJ, Jee SH, Huh KB, Choi SH. Color Doppler ultrasonography is a useful tool for diagnosis of peripheral artery disease in type 2 diabetes mellitus patients with ankle-brachial index 0.91 to 1.40. Diabetes Metab J 42: 63–73, 2018. doi: 10.4093/dmj.2018.42.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isbell DC, Epstein FH, Zhong X, DiMaria JM, Berr SS, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 25: 1013–1020, 2007. doi: 10.1002/jmri.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalliokoski KK, Kuusela TA, Laaksonen MS, Knuuti J, Nuutila P. Muscle fractal vascular branching pattern and microvascular perfusion heterogeneity in endurance-trained and untrained men. J Physiol 546: 529–535, 2003. doi: 10.1113/jphysiol.2002.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasapis C, Gurm HS. Current approach to the diagnosis and treatment of femoral-popliteal arterial disease. A systematic review. Curr Cardiol Rev 5: 296–311, 2009. doi: 10.2174/157340309789317823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutakis P, Weiss DJ, Miserlis D, Shostrom VK, Papoutsi E, Ha DM, Carpenter LA, McComb RD, Casale GP, Pipinos II. Oxidative damage in the gastrocnemius of patients with peripheral artery disease is myofiber type selective. Redox Biol 2: 921–928, 2014. doi: 10.1016/j.redox.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krix M, Krakowski-Roosen H, Kauczor HU, Delorme S, Weber MA. Real-time contrast-enhanced ultrasound for the assessment of perfusion dynamics in skeletal muscle. Ultrasound Med Biol 35: 1587–1595, 2009. doi: 10.1016/j.ultrasmedbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Landin D, Thompson M, Reid M. Knee and ankle joint angles influence the plantarflexion torque of the gastrocnemius. J Clin Med Res 7: 602–606, 2015. doi: 10.14740/jocmr2107w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layec G, Hart CR, Trinity JD, Kwon OS, Rossman MJ, Broxterman RM, Le Fur Y, Jeong EK, Richardson RS. Oxygen delivery and the restoration of the muscle energetic balance following exercise: implications for delayed muscle recovery in patients with COPD. Am J Physiol Endocrinol Metab 313: E94–E104, 2017. doi: 10.1152/ajpendo.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layec G, Haseler LJ, Richardson RS. The effect of higher ATP cost of contraction on the metabolic response to graded exercise in patients with chronic obstructive pulmonary disease. J Appl Physiol 112: 1041–1048, 2012. doi: 10.1152/japplphysiol.00986.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CE, Ng HB, Yip CW, Lim CC. Imaging collateral circulation: magnetic resonance angiography and perfusion magnetic resonance imaging at 3 T. Arch Neurol 62: 492–493, 2005. doi: 10.1001/archneur.62.3.492. [DOI] [PubMed] [Google Scholar]
- 25.Lee VS, Rusinek H, Bokacheva L, Huang AJ, Oesingmann N, Chen Q, Kaur M, Prince K, Song T, Kramer EL, Leonard EF. Renal function measurements from MR renography and a simplified multicompartmental model. Am J Physiol Renal Physiol 292: F1548–F1559, 2007. doi: 10.1152/ajprenal.00347.2006. [DOI] [PubMed] [Google Scholar]
- 26.Little RA, Barjat H, Hare JI, Jenner M, Watson Y, Cheung S, Holliday K, Zhang W, O’Connor JPB, Barry ST, Puri S, Parker GJM, Waterton JC. Evaluation of dynamic contrast-enhanced MRI biomarkers for stratified cancer medicine: How do permeability and perfusion vary between human tumours? Magn Reson Imaging 46: 98–105, 2018. doi: 10.1016/j.mri.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Lüdemann L, Warmuth C, Plotkin M, Förschler A, Gutberlet M, Wust P, Amthauer H. Brain tumor perfusion: comparison of dynamic contrast enhanced magnetic resonance imaging using T1, T2, and T2* contrast, pulsed arterial spin labeling, and H2(15)O positron emission tomography. Eur J Radiol 70: 465–474, 2009. doi: 10.1016/j.ejrad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig UA, Klausmann F, Baumann S, Honal M, Hövener JB, König D, Deibert P, Büchert M. Whole-body MRI-based fat quantification: a comparison to air displacement plethysmography. J Magn Reson Imaging 40: 1437–1444, 2014. doi: 10.1002/jmri.24509. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Mohning KM, Hradil VP, Wessale JL, Segreti JA, Nuss ME, Wegner CD, Burke SE, Cox BF. Evaluation of tissue perfusion in a rat model of hind-limb muscle ischemia using dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 16: 277–283, 2002. doi: 10.1002/jmri.10169. [DOI] [PubMed] [Google Scholar]
- 30.Lutz AM, Weishaupt D, Amann-Vesti BR, Pfammatter T, Goepfert K, Marincek B, Nanz D. Assessment of skeletal muscle perfusion by contrast medium first-pass magnetic resonance imaging: technical feasibility and preliminary experience in healthy volunteers. J Magn Reson Imaging 20: 111–121, 2004. doi: 10.1002/jmri.20092. [DOI] [PubMed] [Google Scholar]
- 31.Martens MH, Subhani S, Heijnen LA, Lambregts DM, Buijsen J, Maas M, Riedl RG, Jeukens CR, Beets GL, Kluza E, Beets-Tan RG. Can perfusion MRI predict response to preoperative treatment in rectal cancer? Radiother Oncol 114: 218–223, 2015. doi: 10.1016/j.radonc.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki M, Lee VS. Nonenhanced MR angiography. Radiology 248: 20–43, 2008. doi: 10.1148/radiol.2481071497. [DOI] [PubMed] [Google Scholar]
- 33.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech 34: 1387–1398, 2001. doi: 10.1016/S0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 34.Ouzounian M, Liu PP. Review: contrast-enhanced MRA is more sensitive and specific than CT angiography or ultrasonography for detection of lower-limb PAD. ACP J Club 147: 77, 2007. [PubMed] [Google Scholar]
- 35.Patterson SD, Ferguson RA. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur J Appl Physiol 108: 1025–1033, 2010. doi: 10.1007/s00421-009-1309-x. [DOI] [PubMed] [Google Scholar]
- 36.Perkiö J, Soinne L, Østergaard L, Helenius J, Kangasmäki A, Martinkauppi S, Salonen O, Savolainen S, Kaste M, Tatlisumak T, Aronen HJ. Abnormal intravoxel cerebral blood flow heterogeneity in human ischemic stroke determined by dynamic susceptibility contrast magnetic resonance imaging. Stroke 36: 44–49, 2005. doi: 10.1161/01.STR.0000150495.96471.95. [DOI] [PubMed] [Google Scholar]
- 37.Pollak AW, Meyer CH, Epstein FH, Jiji RS, Hunter JR, Dimaria JM, Christopher JM, Kramer CM. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging 5: 1224–1230, 2012. doi: 10.1016/j.jcmg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raynaud JS, Duteil S, Vaughan JT, Hennel F, Wary C, Leroy-Willig A, Carlier PG. Determination of skeletal muscle perfusion using arterial spin labeling NMRI: validation by comparison with venous occlusion plethysmography. Magn Reson Med 46: 305–311, 2001. doi: 10.1002/mrm.1192. [DOI] [PubMed] [Google Scholar]
- 39.Reilly H, Lane LM, Egaña M. Lack of age-specific influence on leg blood flow during incremental calf plantar-flexion exercise in men and women. Eur J Appl Physiol 118: 989–1001, 2018. doi: 10.1007/s00421-018-3833-z. [DOI] [PubMed] [Google Scholar]
- 40.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol 111: 81–86, 2011. doi: 10.1152/japplphysiol.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schalkx HJ, van Stralen M, Coenegrachts K, van den Bosch MA, van Kessel CS, van Hillegersberg R, van Erpecum KJ, Verkooijen HM, Pluim JP, Veldhuis WB, van Leeuwen MS. Liver perfusion in dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI): comparison of enhancement in Gd-BT-DO3A and Gd-EOB-DTPA in normal liver parenchyma. Eur Radiol 24: 2146–2156, 2014. doi: 10.1007/s00330-014-3275-x. [DOI] [PubMed] [Google Scholar]
- 42.Schirmer SH, van Royen N. Stimulation of collateral artery growth: a potential treatment for peripheral artery disease. Expert Rev Cardiovasc Ther 2: 581–588, 2004. doi: 10.1586/14779072.2.4.581. [DOI] [PubMed] [Google Scholar]
- 43.Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol 56: 287–295, 1984. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- 44.Sellei RM, Waehling A, Weber CD, Jeromin S, Zimmermann F, McCann PA, Hildebrand F, Pape HC. Contrast enhanced ultrasound (CEUS) reliably detects critical perfusion changes in compartmental muscle: a model in healthy volunteers. Eur J Trauma Emerg Surg 40: 535–539, 2014. doi: 10.1007/s00068-014-0443-2. [DOI] [PubMed] [Google Scholar]
- 45.Soulez G, Bloomgarden DC, Rofsky NM, Smith MP, Abujudeh HH, Morgan DE, Lichtenstein RJ, Schiebler ML, Wippold FJ 2nd, Russo C, Kuhn MJ, Mennitt KW, Maki JH, Stolpen A, Liou J, Semelka RC, Kirchin MA, Shen N, Pirovano G, Spinazzi A. Prospective cohort study of nephrogenic systemic fibrosis in patients with stage 3-5 chronic kidney disease undergoing MRI with injected gadobenate dimeglumine or gadoteridol. AJR Am J Roentgenol 205: 469–478, 2015. doi: 10.2214/AJR.14.14268. [DOI] [PubMed] [Google Scholar]
- 46.St Lawrence KS, Lee TY. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: I. Theoretical derivation. J Cereb Blood Flow Metab 18: 1365–1377, 1998. doi: 10.1097/00004647-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Stevens ED. The effect of moderate exercise on the regional distribution of blood flow in the rat. Can J Physiol Pharmacol 47: 771–780, 1969. doi: 10.1139/y69-130. [DOI] [PubMed] [Google Scholar]
- 48.Stoner MC, Calligaro KD, Chaer RA, Dietzek AM, Farber A, Guzman RJ, Hamdan AD, Landry GJ, Yamaguchi DJ; Society for Vascular Surgery . Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg 64: e1–e21, 2016. doi: 10.1016/j.jvs.2016.03.420. [DOI] [PubMed] [Google Scholar]
- 49.Styczynski G, Szmigielski C, Leszczynski J, Abramczyk P, Kuch-Wocial A, Szulc M. Descending aortic Doppler flow pattern in patients with proximal peripheral artery disease. Am J Cardiol 103: 1774–1776, 2009. doi: 10.1016/j.amjcard.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 50.Thomas KN, Cotter JD, Lucas SJ, Hill BG, van Rij AM. Reliability of contrast-enhanced ultrasound for the assessment of muscle perfusion in health and peripheral arterial disease. Ultrasound Med Biol 41: 26–34, 2015. doi: 10.1016/j.ultrasmedbio.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Totman JJ, O’gorman RL, Kane PA, Karani JB. Comparison of the hepatic perfusion index measured with gadolinium-enhanced volumetric MRI in controls and in patients with colorectal cancer. Br J Radiol 78: 105–109, 2005. doi: 10.1259/bjr/13525061. [DOI] [PubMed] [Google Scholar]
- 52.Traupe T, Ortmann J, Stoller M, Baumgartner I, de Marchi SF, Seiler C. Direct quantitative assessment of the peripheral artery collateral circulation in patients undergoing angiography. Circulation 128: 737–744, 2013. doi: 10.1161/CIRCULATIONAHA.112.000516. [DOI] [PubMed] [Google Scholar]
- 53.Tsushima Y, Niemi P, Komu M, Dean PB, Haapanen A, Kormano M. Dynamic contrast-enhanced magnetic resonance imaging of the kidney: comparison of T1-weighted and T2*-weighted sequences. Acad Radiol 3, Suppl 2: S176–S178, 1996. doi: 10.1016/S1076-6332(96)80526-5. [DOI] [PubMed] [Google Scholar]
- 54.Tyml K. Capillary recruitment and heterogeneity of microvascular flow in skeletal muscle before and after contraction. Microvasc Res 32: 84–98, 1986. doi: 10.1016/0026-2862(86)90045-2. [DOI] [PubMed] [Google Scholar]
- 55.Vivier PH, Storey P, Rusinek H, Zhang JL, Yamamoto A, Tantillo K, Khan U, Lim RP, Babb JS, John D, Teperman LW, Chandarana H, Friedman K, Benstein JA, Skolnik EY, Lee VS. Kidney function: glomerular filtration rate measurement with MR renography in patients with cirrhosis. Radiology 259: 462–470, 2011. doi: 10.1148/radiol.11101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss DJ, Casale GP, Koutakis P, Nella AA, Swanson SA, Zhu Z, Miserlis D, Johanning JM, Pipinos II. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J Transl Med 11: 230, 2013. doi: 10.1186/1479-5876-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss T, Fujita Y, Kreimeier U, Messmer K. Effect of intensive walking exercise on skeletal muscle blood flow in intermittent claudication. Angiology 43: 63–71, 1992. doi: 10.1177/000331979204300108. [DOI] [PubMed] [Google Scholar]
- 58.Wu WC, Wang J, Detre JA, Ratcliffe SJ, Floyd TF. Transit delay and flow quantification in muscle with continuous arterial spin labeling perfusion-MRI. J Magn Reson Imaging 28: 445–452, 2008. doi: 10.1002/jmri.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang JL, Conlin CC, Carlston K, Xie L, Kim D, Morrell G, Morton K, Lee VS. Optimization of saturation-recovery dynamic contrast-enhanced MRI acquisition protocol: monte carlo simulation approach demonstrated with gadolinium MR renography. NMR Biomed 29: 969–977, 2016. doi: 10.1002/nbm.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang JL, Rusinek H, Bokacheva L, Chen Q, Storey P, Lee VS. Use of cardiac output to improve measurement of input function in quantitative dynamic contrast-enhanced MRI. J Magn Reson Imaging 30: 656–665, 2009. doi: 10.1002/jmri.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang JL, Rusinek H, Bokacheva L, Lerman LO, Chen Q, Prince C, Oesingmann N, Song T, Lee VS. Functional assessment of the kidney from magnetic resonance and computed tomography renography: impulse retention approach to a multicompartment model. Magn Reson Med 59: 278–288, 2008. doi: 10.1002/mrm.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang JL, Rusinek H, Bokacheva L, Lim RP, Chen Q, Storey P, Prince K, Hecht EM, Kim DC, Lee VS. Angiotensin-converting enzyme inhibitor-enhanced MR renography: repeated measures of GFR and RPF in hypertensive patients. Am J Physiol Renal Physiol 296: F884–F891, 2009. doi: 10.1152/ajprenal.90648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang JL, Rusinek H, Conlin C, Lee VS. Feasibility of regional renal blood flow and vascular volume fraction measurement with cardiac-output corrected MR renography. In: Proceedings of the 20th Annual Meeting of ISMRM 2012. Melbourne, VIC, Australia: ISMRM, 2012. https://cds.ismrm.org/protected/12MProceedings/files/4060.pdf. [Google Scholar]
- 64.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, Akingba AG, Sturek M, Dalsing MC, Unthank JL. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation 17: 3–20, 2010. doi: 10.1111/j.1549-8719.2010.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]