Abstract
A growing body of literature has demonstrated the potential for ketamine in the treatment of major depression. Sub-anesthetic doses produce rapid and sustained changes in depressive behavior, both in patients and rodent models, associated with reorganization of glutamatergic synapses in the prefrontal cortex (PFC). While ketamine is known to regulate N-methyl-D-aspartate (NMDA) -type glutamate receptors (NMDARs), the full complement of downstream cellular consequences for ketamine administration are not well understood. Here, we combine electrophysiology with 2-photon imaging and glutamate uncaging in acute slices of mouse PFC to further examine how ketamine alters glutamatergic synaptic transmission. We find that four hours after ketamine treatment, glutamatergic synapses themselves are not significantly affected. However, levels of the neuromodulatory Regulator of G-protein Signaling (RGS4) are dramatically reduced. This loss of RGS4 activity is associated with disruption of the normal compartmentalization of synaptic neuromodulation. Thus, under control conditions, α2 adrenergic receptors and type B γ-aminobutyric acid (GABAB) receptors selectively inhibit α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) -type glutamate receptors (AMPARs) and NMDARs, respectively. After ketamine administration and reduction in RGS4 activity, this selectivity is lost, with both modulatory systems broadly inhibiting glutamatergic transmission. These results suggest a novel mechanism by which ketamine may influence synaptic signaling and provide new avenues for the exploration of therapeutics directed at treating neuropsychiatric disorders, such as depression.
Introduction
Major depression, with a lifetime prevalence of 17%, presents a significant psychological and economical burden for both individuals and society [1, 2]. Despite enormous efforts to develop effective treatments, available therapeutic interventions have considerable limitations. For example, most antidepressant medications take several weeks to achieve maximal benefit and a significant fraction of patients remain refractory to treatment [3, 4]. However, recent studies in both clinical and basic science fields have demonstrated promising results using low doses of the drug ketamine. Indeed, sub-anesthetic doses of ketamine produce rapid antidepressant actions within a few hours [5–7], even in otherwise refractory patients [8]. Thus, the potential benefits of this new pharmacological intervention provide great promise for the treatment of major depression.
Surprisingly, the neurological mechanisms underlying the antidepressant actions of ketamine remain poorly understood. Recent efforts have focused on the regulation of glutamatergic synapses in the prefrontal cortex (PFC) as a potential process by which ketamine modulates behavior. Ketamine itself is an antagonist of N-methyl-D-aspartate (NMDA) -type glutamate receptors (NMDARs) [9], though it may have other actions as well [10]. Acute administration of ketamine produces mild dissociative effects that subside within two hours after administration [11], while the antidepressant actions may persist for up to a week [5, 6]. In animal models, ketamine stimulates a signaling cascade that produces long-term enhancement of glutamatergic transmission in the PFC, including increased synaptic protein synthesis and increased density of dendritic spines, the structural sites of individual excitatory inputs [12–15]. Moreover, these synaptic changes persist for several days after administration [13].
The cellular mechanisms underlying these alterations in synaptic structure and function are unclear, and a variety of signaling pathways have been implicated in linking NMDAR blockade to long-term alteration of glutamatergic signaling, including the mammalian target of rapamycin (mTOR) and brain-derived neurotrophic factor (BDNF) [13, 16, 17]. Both these processes have been shown to regulate the growth and stability of glutamatergic synapses [18, 19]. However, ketamine has also been linked to alterations in inhibitory γ-aminobutyric acid (GABA) -ergic signaling, both directly and indirectly by disrupting activity in inhibitory interneurons [20–22].
A recent study suggested that acute administration of ketamine might also impact the function of the protein regulator of G-protein signaling type-4 (RGS4) [23]. RGS4 is a GTPase activating protein that accelerates the hydrolysis of guanosine-5’-triphosphate (GTP) to guanosine-5’-diphosphate (GDP) following the activation of G proteins by a variety of ligand-receptor interactions [24, 25]. Behavioral studies in mice lacking RGS4 demonstrated that this enzyme can act as a key negative modulator of ketamine-mediated antidepressant actions [23], and ketamine itself is capable of reducing levels of RGS4 in the PFC. Previous studies found that RGS4 plays a crucial role in regulating the neuromodulation of glutamatergic synapses in the PFC [26]. These data showed that the specificity of adrenergic and GABAergic control over α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)- and NMDA-type glutamate receptors, respectively, is abolished following pharmacological blockade of RGS4 function.
As neuromodulation may play a key role in normal prefrontal function, we investigated whether ketamine might disrupt the regulation of glutamate receptors in the PFC. We used whole-cell patch clamp electrophysiology and 2-photon calcium imaging in acute slices from the mouse PFC to measure postsynaptic responses evoked by focal 2-photon glutamate uncaging. We found that a single dose of ketamine did not alter basal function of postsynaptic glutamate receptors. However, ketamine did produce a substantial reduction in prefrontal RGS4 levels in the PFC. We also found that 4 hours after ketamine treatment adrenergic and GABAergic neuromodulators no longer displayed selectivity in the regulation of AMPA- and NMDA- type glutamate receptors. Thus, our work suggests a novel mechanism by which acute ketamine can influence glutamatergic signaling and potentially contribute to its antidepressant actions.
Materials and methods
Animals and drug treatment
All animal handling was performed in accordance with guidelines approved by the Yale and UC Irvine Institutional Animal Care and Use Committee and federal guidelines. All experiments were approved by the above committees. Wild-type C57/Bl6 mice (postnatal day 22–42) of either sex were injected i.p. with either saline vehicle or ketamine (15 mg/kg) 4 hours prior to experiments.
Slice preparation
For glutamate uncaging experiments, we prepared acute prefrontal cortical (PFC) slices as previously described [26]. Briefly, mice were anesthetized with isoflurane and decapitated, and coronal slices (300 μm) were cut in ice-cold solution containing (in mM): 110 choline, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 7 MgCl2, 0.5 CaCl2, 10 glucose, 11.6 sodium ascorbate and 3.1 sodium pyruvate, bubbled with 95% O2 and 5% CO2. Slices containing the prelimbic-infralimbic regions of the PFC were then transferred to artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 3 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 0.4 sodium ascorbate, 2 sodium pyruvate and 3 myo-inositol, bubbled with 95% O2 and 5% CO2. After an incubation period of 15 min at 34°C, the slices were maintained at 22–24°C for at least 20 min before use.
Electrophysiology and imaging
All experiments were conducted at near physiological temperature (32–34°C) in a submersion-type recording chamber. Whole-cell recordings in voltage clamp mode were obtained from layer 5 pyramidal cells (400–500 μm from the pial surface) identified with infrared differential interference contrast. Glass electrodes (1.8–3.0 MΩ) were filled with internal solution containing (in mM): 135 CsMeSO3, 10 HEPES, 4 MgCl2, 4 Na2ATP, 0.4 NaGTP, 10 sodium creatine phosphate and 0.2% Neurobiotin (Vector Laboratories) adjusted to pH 7.3 with CsOH. Red-fluorescent Alexa Fluor-594 (10 μM, Invitrogen) and the green-fluorescent calcium (Ca2+)-sensitive Fluo-5F (300 μM, Invitrogen) were included in the pipette solution. Neurons were filled via the patch electrode for 10 min before imaging. Series resistance was 10–22 MΩ and uncompensated. Electrophysiological recordings were made using a Multiclamp 700B amplifier (Molecular Devices), filtered at 4 kHz, and digitized at 10 kHz. Typically, 7–10 trials were averaged into each response.
2-photon imaging was accomplished with a custom-modified Olympus BX51-WI microscope (Olympus, Japan), including components manufactured by Mike’s Machine Company [27]. Fluorophores were excited using 840 nm light from a pulsed titanium-sapphire laser (Ultra2, Coherent). Emitted green and red photons were separated with appropriate optics (Chroma, Semrock) and collected by photomultiplier tubes (Hamamatsu).
For Ca2+ imaging, signals were collected during 500 Hz line scans across a spine. Ca2+ signals were first quantified as increases in green fluorescence from baseline normalized to the average red fluorescence (ΔG/R). We then expressed fluorescence changes as the fraction of the G/R ratio measured in saturating Ca2+ (ΔG/Gsat)(Lur and Higley 2015).
2-Photon glutamate uncaging
For focal stimulation of single dendritic spines, we used 2-photon laser uncaging of glutamate (2PLU). To photorelease glutamate, a second Ti-Sapphire laser tuned to 720 nm was introduced into the light path using polarization optics. Laser power was calibrated for each spine by directing the uncaging spot to the middle of the spine head. We adjusted uncaging power to achieve 50% photobleaching of the Alexa 594 dye filling the spine [26]. The power used for 2PLU ranged from 8 to 25 mW, pulse duration was 0.5 ms. For synaptic stimulation, we typically uncaged glutamate at 3–4 separate locations around a single spine head to find a “hot spot”, the place of the largest response. Our previous measurements indicate that this stimulus resulted in uncaging evoked excitatory post-synaptic currents (uEPSCs) similar in size and kinetics to spontaneous miniature excitatory post-synaptic currents (uEPSCs) [26].
Data acquisition and analysis
Imaging and physiology data were acquired using National Instruments data acquisition boards and custom software written in MATLAB (Mathworks, [28]). Off-line analysis was performed using custom routines written in MATLAB and IgorPro (Wavemetrics). AMPAR-mediated EPSC amplitudes were calculated by finding the peak of the current traces and averaging the values within a 0.3 ms window. NMDAR-mediated currents were measured in a 3 ms window around the peak. 2PLU-evoked ΔCa2+ was calculated as the average ΔG/Gsat over a 100 ms window, starting 5 ms after the uncaging event. Statistical comparisons were conducted in GraphPad Prism 5. All data were analyzed using one-way analysis of variance (ANOVA)-tests corrected for multiple comparisons (Tukey).
Pharmacology and reagents
2PLU experiments were performed in normal ACSF supplemented with MNI-glutamate (2.5 mM) and D-serine (10 μM). To isolate AMPAR-mediated currents in voltage clamp experiments, we added tetrodotoxin (TTX) (1 μM) to block sodium channels, picrotoxin (50 μM) to block GABAA receptors, CGP55845 (3 μM) to block GABAB receptors, and 3-(2-Carboxypiperazin-4-yl) propyl-1-phosphonic acid (CPP) (10 μM) to block NMDA-type glutamate receptors to the ACSF. To isolate NMDAR-mediated currents, we modified our original ACSF to contain 0 mM Mg and 3 mM Ca2+ and included TTX (1 μM), picrotoxin (50 μM), CGP55845 (3 μM), and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) (10 μM) to block AMPA-type glutamate receptors. To activate α2 adrenergic receptors we used 40 μM guanfacine (Tocris). The selective GABAB receptor agonist baclofen (Tocris), a drug primarily used as a muscle relaxant, approved by the US Food and Drug Administration, was applied at 5 μM. Both G-protein coupled receptor (GPCR) agonists were applied 5–7 minutes prior to data collection and remained in the bath for the duration of the experiment, but typically no longer than 20 minutes to avoid receptor desensitization. All compounds and salts were from Tocris and Sigma, respectively.
Western blot analysis
For RGS4 western blot analysis, we prepared 300 μm thick brain slices containing the PFC from C57/bl6 mice as described above. Following the recovery period, the prelimbic region of the prefrontal cortex was dissected out of the slices on ice. Tissue samples were homogenized and sonicated in ice cold lysis buffer containing 20 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA) and 1x Halt protease and phosphatase inhibitor cocktail (Thermo Scientific) and 0.5% SDS, pH 8.0. After a 10-minute centrifugation at 14000 rpm, the supernatant was collected, and protein content was determined using Pierce BCA Protein Assay (Thermo Scientific). Samples containing equal amounts of protein were separated on a 6% poly-acrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking for 1h at room temperature with 3% non-fat milk and 0.02% Na-azide in Tris buffered salt solution with 0.05% Tween 20 (TBST), membranes were immunoreacted with a primary antibody against RGS4 (Millipore, RBT17) [29] in 1% milk and 0.02% Na-azide in TBST, 1:1000, overnight. After washing off excess primary antibody and incubation with the appropriate horseradish peroxidase (HRP) conjugated secondary antibody (GE Healthcare, UK) for 2 hours at room temperature in TBST, bands were visualized using HyGlo Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific Inc.) and exposed onto autoradiography film (Denville Scientific Inc.). Membranes were then stripped from antibodies using Restore Plus Western Blot Stripping Buffer (15 minutes at room temperature, Thermo Scientific), re-blocked and immunoreacted with anti-β-tubulin (SIGMA) primary antibody followed by the appropriate HRP-secondary antibody to establish total amount β-tubulin in the samples. Autoradiography films were developed in a Kodak automatic developer, then scanned and analyzed with ImageJ. RGS4 level was quantified as RGS4 / β-tubulin.
Behavioral analysis
To perform the forced swim test (FST) mice were individually placed in a transparent glass cylinder (40 cm high, 20 cm diameter) containing 2000 ml of clear water at 24–26° C for 6 min. Mice were not able to reach the top of the beaker or touch the bottom with their tail. A mouse was judged to be immobile when it remained floating passively in the water. Using a video recording, immobility time during the 4 last minutes of the test was measured post-hoc by two independent investigators who were blinded to the animal’s condition. A decrease in immobility time indicates an antidepressant-like response.
For the light / dark box test (LDB) a standard mouse cage was split into two regions at the 2:1 ratio with a barrier that had a 5 x 5 cm gate to allow free movement between the chambers. The smaller chamber (one third) was darkened. The apparatus was set up un a brightly lit testing room. Animals were transferred to the dark side of an apparatus and their activity recorded for 6 minutes. Two independent investigators, blinded to the animal’s condition, measured the number of entries and the time spent in the brightly chamber post-hoc.
Behavioral experiments were conducted on 8 mice. On day 1 animals were injected with saline vehicle at 10 am and tested in LDB and FST at 2–4 pm. After a day of rest, on day 3 mice were injected with 15 mg/kg ketamine in saline at 10 am and re-tested in LDB and FST at 2–4 pm. It has been shown numerous times [30, 31] that 2 repeats of these test do not alter the animal’s performance. This design allowed us to perform paired statistics on the data while minimizing animal sacrifice as per IACUC guidelines.
Results
Ketamine exerts no effect on NMDA receptors 4 hours post treatment
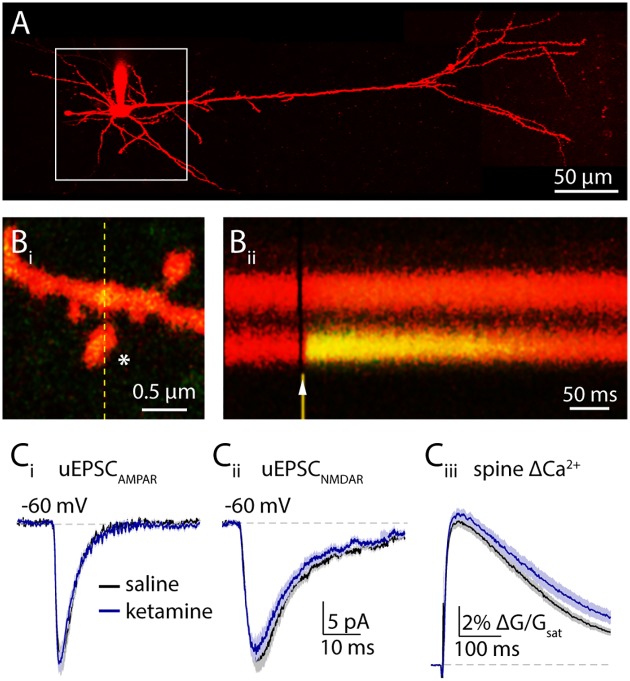
Previous works investigating the rapid onset antidepressant actions of ketamine in rodents have placed the effective dose of ketamine between 5 and 50 mg/kg [13, 23, 32, 33]. To investigate the consequences of ketamine for glutamatergic signaling, we injected mice with either a single dose of ketamine (15 mg/kg) or saline vehicle. At four hours post-injection, we prepared acute brain slices from the medial prefrontal cortex (PFC) of the injected animals and made whole cell voltage clamp recordings from layer 5 pyramidal neurons in the prelimbic region (Fig 1A). We measured excitatory postsynaptic currents (EPSCs) evoked by 2-photon laser uncaging (2PLU) of glutamate onto spines along the basal dendrites, while simultaneously monitoring intra-spine Ca2+ transients using 2-photon laser scanning microscopy (2PLSM)(Fig 1B). We found no difference in AMPAR-mediated EPSCs between vehicle (20.9 ± 1.9 pA, n = 31 spines) and ketamine (21.0 ± 1.4 pA, n = 33 spines, p = 0.96, t-test, Figs 1C and 4A) groups. Notably, we also failed to observe alterations in NMDAR-mediated currents (vehicle: 22.9 ± 2 pA, n = 31 spines, ketamine: 21.4 ± 1.8 pA, n = 31 spines, p = 0.59, t-test, Figs 1C and 4B) or Ca2+ transients (vehicle: 0.52 ± 0.02 ΔG/Gsat, ketamine: 0.54 ± 0.02 ΔG/Gsat, p = 0.31, t-test, Figs 1C and 4B), indicating the absence of persistent NMDAR blockade. Overall, our results suggest that 4 hours post treatment, ketamine produces no change in postsynaptic glutamate receptor responsiveness.
Fig 1. Ketamine administration does not affect postsynaptic glutamate receptor activation 4 hours post treatment.
(A) A 2-photon image of a layer 5 pyramidal neuron visualized by Alexa 594 fluorescence. White box highlights the extent of the basal dendritic arbor searched for spines. (Bi) Image of a dendritic spine. White asterisk shows the uncaging location. Fluorescence intensity was measured in a line scan highlighted by the yellow dashed line. (Bii) Chronogram showing the change in green fluorescence indicating a transient increase of intracellular Ca2+ concentration in the spine head in response to glutamate uncaging (arrowhead). (Ci) Mean AMPAR-mediated uEPSCs in vehicle (black) and in ketamine (blue) treated animals ± SEM (shaded areas). (Cii) 2PLU-evoked NMDAR currents and (Ciii) Ca2+ transients in vehicle (black) and in ketamine treated animals (blue), mean (solid lines) ± SEM (shaded areas). *: p<0.05, unpaired t-test.
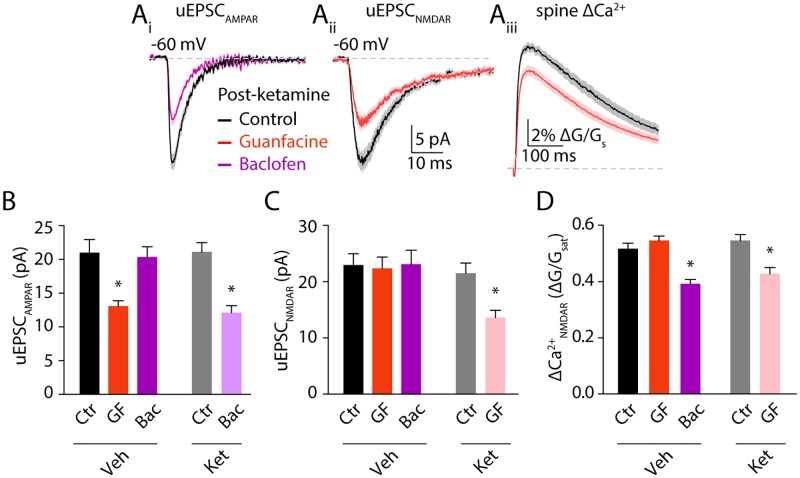
Fig 4. Ketamine treatment eliminates compartmentalized neuromodulation.
(Ai) Mean AMPAR-mediated uEPSCs in control (black) and baclofen (magenta) ± SEM (shaded areas). (Aii) 2PLU-evoked NMDAR currents and (Aiii) Ca2+ transients in control (black) and guanfacine (salmon), mean (solid lines) ± SEM (shaded areas). (B) Bars represent mean amplitude ± SEM of AMPAR currents, (C) NMDAR-mediated currents and (D) NMDAR-mediated Ca2+ transients in control (gray), in guanfacine (orange) or in baclofen (magenta) from vehicle- versus ketamine-treated mice. Ctr: control, GF: guanfacine treatment, Bac: baclofen treatment, Veh: vehicle injected, Ket: ketamine injected. *: p<0.05, Tukey’s multiple comparison test.
Ketamine significantly reduces RGS4 expression in the PFC
To test whether ketamine administration results in reduced depression-like behaviors 4 hours post-administration, we performed forced swim tests (FST). Ketamine treatment significantly reduced immobility times compared to vehicle injection (p = 0.0034, n = 8, Paired t-test, Fig 2A). To further characterize the effect of ketamine we also measured the animal’s anxiety-like behaviors using the light/dark box test (LDB). We found that vehicle and ketamine treated mice spent the same amount of time in the brightly lit compartment (p = 0.64, n = 8, Paired t-test Fig 2B) but ketamine injected mice achieved this in fewer gate crossings (p = 0.032, n = 8, Paired t-test, Fig 2B), resulting in a significantly higher ratio between time spent in the light compartment and the number of entries (p = 0.0013, n = 8, paired t-test, Fig 2B). These data are in strong agreement with previous literature [13, 23, 32, 33], suggesting that 4 hours of ketamine treatment results in reduced depression- and anxiety-like behaviors in mice.
Fig 2. Acute ketamine treatment decreases depression-like behavior and reduces prefrontal RGS4 levels.
(A) Bars show mean immobility times in forced swim test (FST). (B) Bar graph showing results of light / dark box test. Left: mean time spent in the brightly lit compartment, middle: number of entries to the bright compartment, right: ration of time spent in light and the number of entries. *: p < 0.05, paired t-test. (C) Example western blot of RGS4 and β-tubulin in vehicle- versus ketamine- treated mice. (D) Quantification of RGS4 levels in vehicle (black) and ketamine (blue) treated mice. Bars show mean ± SEM, *: p<0.05, unpaired t-test.
Endogenous control of glutamatergic transmission by G-protein coupled receptors (GPCRs) plays a central role in prefrontal function. Previous studies showed that both noradrenergic and GABAergic modulation of glutamatergic synapses is influenced by the activity of the small GTPase RGS4 [26], whose protein level is thought to be regulated by antidepressants including ketamine [23]. To examine the consequences of acute ketamine on RGS4 function in the prefrontal cortex, we first prepared tissue samples from the prelimbic region four hours after animals were injected with either ketamine or saline. Note that these samples include both Layer 5 pyramidal neurons as well as additional excitatory and inhibitory cells across all layers. Western-blot analysis confirmed a 50 ± 0.1% reduction of RGS4 protein level (p = 0.0016, n = 5 animals, unpaired t-test, Fig 2C and 2D), suggesting that ketamine may disrupt neuromodulation at glutamatergic synapses.
Reduced specificity of postsynaptic neuromodulation after ketamine exposure
Activation of alpha2 adrenergic receptors and GABAB receptors negatively modulate AMPARs and NMDARs, respectively via downregulation of protein kinase A (PKA) activity [26]. Moreover, this selective coupling of GPCRs to distinct synaptic proteins requires RGS4 and is lost following small molecule antagonism of RGS4 activity [26]. We therefore asked whether ketamine produces similar dysregulation of synaptic modulation. First, we confirmed that, in saline-injected mice, application of the alpha2 adrenergic agonist guanfacine significantly reduced 2PLU-evoked AMPAR-mediated currents (to 13.0 ± 0.8 pA, n = 25 spines, one-way ANOVA (F = 9.93, p<0.0001), Tukey’s multiple comparison test p<0.01, Figs 3Ai and 4A) but did not alter 2PLU-evoked NMDAR-mediated currents (22.3 ± 2 pA, n = 33 spines, one-way ANOVA (F = 6.53, p = 0.0004), Tukey’s multiple comparison test p>0.05, Figs 3Aii and 4B) or ΔCa2+ (0.54 ± 0.02 ΔG/Gsat, one-way ANOVA (F = 8.1, p<0.0001), Tukey’s multiple comparison test p>0.05, Figs 3Aiii and 4). Conversely, application of the GABAB agonist baclofen did not alter 2PLU-evoked AMPAR-mediated currents (20.3 ± 1.5 pA, n = 32 spines, one-way ANOVA (F = 10.3, p<0.0001), Tukey’s multiple comparison test p>0.05, Figs 3Bi and 4A) or NMDAR currents (23.0 ± 2.5 pA, n = 25 spines, one-way ANOVA (F = 5.01, p = 0.0008), Tukey’s multiple comparison test p>0.05, Figs 3Bii and 4B) but reduced NMDAR-dependent ΔCa2+ (to 0.39 ± 0.01 ΔG/Gsat, one-way ANOVA (F = 12.11, p<0.0001), Tukey’s multiple comparison test p<0.001, Figs 3Biii and 4C).
Fig 3. Differential control of excitatory transmission by α2- and GABAb receptors.
(Ai) Mean AMPAR-mediated uEPSCs in control (black) and guanfacine (orange) ± SEM (shaded areas). (Aii) 2PLU-evoked NMDAR currents and (Aiii) Ca2+ transients in control (black) and guanfacine (orange), mean (solid lines) ± SEM (shaded areas). (Bi) Mean AMPAR-mediated uEPSCs in control (black) and baclofen (magenta) ± SEM (shaded areas). (Bii) NMDAR-mediated uEPSCs and (Biii) Ca2+ transients in control (black) and baclofen (magenta) ± SEM (shaded areas). *: p<0.05, unpaired t-test.
We then performed similar experiments in mice injected four hours prior to slice preparation with ketamine. In contrast to saline-treated animals, AMPAR-mediated currents were significantly reduced by baclofen (to 12.1 ± 1 pA, n = 33 spines, one-way ANOVA (F = 9.93, p<0.0001), Tukey’s multiple comparison test p<0.001, Fig 4Ai and 4B) while guanfacine significantly reduced both NMDAR currents (to 13.6 ± 1.3 pA, n = 37 spines, one-way ANOVA (F = 5.0, p = 0.0008), Tukey’s multiple comparison test p<0.05, Fig 4Aii and 4C) and ΔCa2+ (to 0.42 ± 0.02 ΔG/Gsat, one-way ANOVA (F = 12.1, p<0.0001), Tukey’s multiple comparison test p<0.001, Fig 4Aiii and 4D). Previous studies showed that blocking RGS4 function does not alter the effect of guanfacine on AMPARs or the baclofen induced reduction of NMDAR dependent Ca2+ influx. This earlier work also showed that multiple methods of RGS4 inhibition introduced baclofen induced reduction of NMDAR currents (Lur et al, 2015). Our current results match these previous observations. Overall, these findings show disrupted neuromodulation of glutamatergic signaling in basal dendrites of layer 5 pyramidal neurons 4 hours after ketamine administration but no direct actions on synaptic potency.
Conclusions
In our present study, we demonstrate significant dysregulation of neuromodulatory control over glutamatergic signaling in the mouse prefrontal cortex following the administration of a single, sub-anesthetic dose of ketamine. Specifically, following ketamine administration, adrenergic and GABAergic receptor activation inhibits NMDARs and AMPARs, respectively, a phenomenon that does not occur in untreated animals. Our results suggest this process may be mediated by acute reduction in levels of the small GTPase RGS4, which were previously shown to prevent neuromodulatory cross-talk in dendritic spines [26]. Our findings thus extend our knowledge of targets for ketamine that may contribute to or interact with its antidepressant actions in vivo.
G-protein coupled receptors (GPCRs) provide a ubiquitous mechanism for regulating synaptic transmission via neuromodulators like norepinephrine, GABA, serotonin, dopamine and adenosine. Despite their vast capacity to distinguish extracellular ligands, GPCR activation may engage only a handful of intracellular signaling cascades, many of which rely on soluble, small molecule second messenger systems. In theory, the paucity of unique intracellular response pathways and the high mobility of second messengers could severely limit the system’s capacity for selective regulation. This would be increasingly true for small volume cellular compartments like the dendritic spine. Our previous work showed that within a single synapse, distinct glutamate receptor subtypes (AMPA and NMDA receptors) are selectively regulated by α2 adrenoreceptors and GABAB receptors, respectively, despite being coupled to identical second messenger pathways. We showed that this segregation was possible due to the establishment of synaptic microdomains through the actions of RGS4. In general, this mechanism allows the close coexistence and nuanced function of neuromodulatory systems.
RGS4 is a small GTPase that limits signaling through G protein-coupled receptor pathways by accelerating the hydrolysis of GTP to GDP [24, 34, 35]. Under control conditions, this activity produces microdomains within single dendritic spines that restricts neuromodulatory cascades. For example, adrenergic α2 receptors and GABAB receptors are both coupled to Gi proteins that down-regulate cAMP production and PKA activity. Surprisingly, we found that both receptors are present in single spines but are selectively able to negatively modulate AMPARs and NMDARs, respectively [26]. However, when RGS4 is blocked pharmacologically, this microdomain organization breaks down, leading to cross talk between the neuromodulatory signaling cascades [26, 35]. Remarkably, a single dose of ketamine appears to produce substantial loss of RGS4 within a few hours [23], a finding confirmed in our present study, suggesting this protein is rapidly turned over in cortical neurons. Consistent with our previous results using small molecule inhibitors of RGS4, ketamine-induced down-regulation of RGS4 is associated with aberrant cross-talk between modulatory signals and glutamate receptors. While these results are strongly suggestive, it will be necessary in future studies to experimentally restore RGS4 to control levels to directly confirm causal links between ketamine, RGS4 signaling, and synaptic modulation.
Previous studies looking at the effects of ketamine administration 24 hours post-exposure showed increased frequency for pharmacologically evoked EPSCs in layer 5 PNs. This result was linked to an increase in both spine volume and the density of mature spines and attributed to changes in postsynaptic gene expression [13]. Others have found increased NMDAR EPSC amplitudes at the 24-hour time point [36]. In contrast, our results indicate that four hours after ketamine administration basal postsynaptic glutamatergic signaling through either AMPARs or NMDARs is unaltered. This difference may be due to a longer time window required for altered synaptic gene expression to manifest. Additionally, changes in pharmacologically evoked EPSCs are difficult to interpret, as pre- or postsynaptic alterations cannot be distinguished. Importantly, we demonstrate behavioral effects of ketamine commensurable with previous findings [13, 23, 32, 33, 37].
Acute doses of ketamine have been shown to produce rapid synaptic reorganization in the prefrontal cortex that coincides with antidepressant actions in rodent models [21, 38]. Interestingly, these effects are thought to be mediated through inhibition of NMDARs by ketamine [38]. This hypothesis is supported by evidence that other NMDAR blockers can produce similar synaptic and behavioral effects [17, 38]. Loss of NMDAR signaling may activate both mTOR and BDNF signaling pathways that may provide molecular mechanisms for synaptic changes following ketamine [13, 16–19, 33]. To ensure compatibility with these previous results we also directed our recordings to the medial PFC. We focused our efforts on layer 5 pyramidal neurons because this cell population generates the majority of the synaptic output of the neocortex. Our findings suggest the intriguing possibility that ketamine may also suppress NMDAR activity by broadening the consequences of adrenergic signaling, even after the NMDAR antagonistic effect of ketamine faded. That is, following ketamine, α2 receptors may further suppress these glutamate receptors. Thus, activation of adrenergic signaling could be an adjunct approach to boost the effects of ketamine. Indeed, guanfacine alone exhibits antidepressant activity in rodents [39, 40].
In conclusion, our current findings provide novel evidence that acute ketamine can influence glutamatergic transmission in the mouse prefrontal cortex, potentially via down-regulation of RGS4 and dysregulation of neuromodulatory signaling. These results expand our view of the possible downstream actions of ketamine and suggest that inhibition of NMDARs by α2 adrenergic signaling may provide benefit in models of depression when delivered alongside sub-anesthetic doses of ketamine. Pending further experimental confirmation, our results may advance the clinical application of ketamine in the treatment of depression and anxiety disorders.
Supporting information
(XLSX)
Acknowledgments
The authors wish to thank Dr. Jessica A. Cardin and members of the Higley laboratory for helpful discussions during the preparation of this manuscript.
Data Availability
All relevant data are available within the paper and its Supporting Information file.
Funding Statement
These studies were funded by grants from the Brain and Behavior Research Foundation (NARSAD Young Investigator Award, GL), the Smith Family Foundation (MJH), and the NIH (R01 MH099045, MJH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105. 10.1001/jama.289.23.3095 . [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–27. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–51. 10.1176/appi.ajp.2007.06111868 . [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–52. 10.1056/NEJMoa052964 . [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. . [DOI] [PubMed] [Google Scholar]
- 6.Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. 10.1001/archpsyc.63.8.856 . [DOI] [PubMed] [Google Scholar]
- 7.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73(12):1133–41. 10.1016/j.biopsych.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, et al. Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs. 2012;26(3):189–204. 10.2165/11599770-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987;58(2):251–66. 10.1152/jn.1987.58.2.251 . [DOI] [PubMed] [Google Scholar]
- 10.Sleigh J, Harvey M, Voss L, Denny B. Ketamine–More mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care. 2014;4(2–3):76–81. 10.1016/j.tacc.2014.03.002 [DOI] [Google Scholar]
- 11.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. . [DOI] [PubMed] [Google Scholar]
- 12.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–58. 10.1038/nrn2699 . [DOI] [PubMed] [Google Scholar]
- 13.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64. 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. 10.1126/science.1222939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996–1005. 10.1016/j.biopsych.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–5. 10.1038/nature10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29(27):8688–97. 10.1523/JNEUROSCI.6078-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24(44):9760–9. 10.1523/JNEUROSCI.1427-04.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–500. 10.1523/JNEUROSCI.2213-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169(11):1150–6. 10.1176/appi.ajp.2012.12040531 . [DOI] [PubMed] [Google Scholar]
- 22.Wohleb ES, Gerhard D, Thomas A, Duman RS. Molecular and Cellular Mechanisms of Rapid-Acting Antidepressants Ketamine and Scopolamine. Curr Neuropharmacol. 2017;15(1):11–20. 10.2174/1570159X14666160309114549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratinaki M, Varidaki A, Mitsi V, Ghose S, Magida J, Dias C, et al. Regulator of G protein signaling 4 [corrected] is a crucial modulator of antidepressant drug action in depression and neuropathic pain models. Proc Natl Acad Sci U S A. 2013;110(20):8254–9. 10.1073/pnas.1214696110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116(3):473–95. 10.1016/j.pharmthera.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature. 1996;383(6596):172–5. 10.1038/383172a0 . [DOI] [PubMed] [Google Scholar]
- 26.Lur G, Higley MJ. Glutamate Receptor Modulation Is Restricted to Synaptic Microdomains. Cell Rep. 2015;12(2):326–34. 10.1016/j.celrep.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13(8):958–66. 10.1038/nn.2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13 10.1186/1475-925X-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumins AM, Barker SA, Huang C, Sunahara RK, Yu K, Wilkie TM, et al. Differentially regulated expression of endogenous RGS4 and RGS7. J Biol Chem. 2004;279(4):2593–9. 10.1074/jbc.M311600200 . [DOI] [PubMed] [Google Scholar]
- 30.Kazavchinsky L, Dafna A, Einat H. Individual variability in female and male mice in a test-retest protocol of the forced swim test. J Pharmacol Toxicol Methods. 2019;95:12–5. 10.1016/j.vascn.2018.11.007 . [DOI] [PubMed] [Google Scholar]
- 31.Pozzi L, Pollak Dorocic I, Wang X, Carlen M, Meletis K. Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS One. 2014;9(1):e83879 10.1371/journal.pone.0083879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, et al. Antidepressant Potential of (R)-Ketamine in Rodent Models: Comparison with (S)-Ketamine. J Pharmacol Exp Ther. 2017;361(1):9–16. 10.1124/jpet.116.239228 . [DOI] [PubMed] [Google Scholar]
- 33.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18(1). 10.1093/ijnp/pyu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arshavsky VY, Pugh EN Jr. Lifetime regulation of G protein-effector complex: emerging importance of RGS proteins. Neuron. 1998;20(1):11–4. . [DOI] [PubMed] [Google Scholar]
- 35.Zhong H, Wade SM, Woolf PJ, Linderman JJ, Traynor JR, Neubig RR. A spatial focusing model for G protein signals. Regulator of G protein signaling (RGS) protien-mediated kinetic scaffolding. J Biol Chem. 2003;278(9):7278–84. 10.1074/jbc.M208819200 . [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Ju W, Zhang H, Sun L. Effect of Ketamine on LTP and NMDAR EPSC in Hippocampus of the Chronic Social Defeat Stress Mice Model of Depression. Front Behav Neurosci. 2018;12:229 10.3389/fnbeh.2018.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salat K, Siwek A, Starowicz G, Librowski T, Nowak G, Drabik U, et al. Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor. Neuropharmacology. 2015;99:301–7. 10.1016/j.neuropharm.2015.07.037 . [DOI] [PubMed] [Google Scholar]
- 38.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–61. 10.1016/j.biopsych.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113(3):523–36. 10.1016/j.pharmthera.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mineur YS, Bentham MP, Zhou WL, Plantenga ME, McKee SA, Picciotto MR. Antidepressant-like effects of guanfacine and sex-specific differences in effects on c-fos immunoreactivity and paired-pulse ratio in male and female mice. Psychopharmacology (Berl). 2015;232(19):3539–49. 10.1007/s00213-015-4001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are available within the paper and its Supporting Information file.