Abstract
We previously found relationships between body condition and physiological function affecting health and welfare of female tourist camp elephants in Thailand, and used that approach to conduct a similar study of bull elephants in the same camps (n = 13). A body condition score (BCS) was done every other month, and fecal glucocorticoid metabolite (FGM) concentrations were measured twice monthly for 1 year. Effects of season, camp management and tourist activity on lipid profiles [total cholesterol (TC), low density lipoproteins (LDL), high density lipoproteins (HDL), triglycerides (TG)] and metabolic factors [insulin, glucose, fructosamine, glucose to insulin ratio (G:I)] were determined and correlated to measures of body condition, testosterone and FGM. Positive correlations were found between BCS and TG, between FGM and TG, HDL and glucose, and between testosterone and HDL, whereas BCS and testosterone were negatively associated with the G:I. There was a significant positive relationship between FGM and testosterone. Elevated FGM concentrations were associated with altered lipid and metabolic profiles and were higher in winter compared to summer and rainy seasons. Insulin and glucose levels were higher, while the G:I was lowest in the winter season. Strong positive associations were found between TC and HDL, LDL and HDL and glucose, and glucose and insulin. By contrast, negative relationships were found between the G:I and HDL and glucose, and between insulin and G:I. Differences also were found between High and Low tourist season months for FGM, insulin, and G:I. Last, there was notable variation among the camps in measured parameters, which together with tourist season effects suggests camp management may affect physiological function and welfare; some negatively like feeding high calorie treats, others positively, like exercise. Last, compared to females, bull elephants appear to be in better physical health based on normal BCSs, lower insulin levels and higher G:I ratios.
Introduction
The Asian elephant (Elephas maximus) has been listed as endangered in Appendix 1 of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES 2018) since 1973, with wild populations declining in several range countries. Some captive elephant populations in Asia also are not sustaining, due in part to low reproductive and high mortality rates. As elephants are a long-lived species that produce only a few calves in their lifetime, it is important to better understand factors affecting health and reproduction to prevent further population declines. Considerable information now is available about the basic biology of elephants, especially the reproductive physiology of females, while bulls have received comparatively less attention (e.g., [1]).
In recent years, studies have focused on associations between health and reproduction in captive elephants, particularly females, with problems linked at least in part to obesity because of too little exercise, diets that are too high in calories, or both [2–4]. There are numerous documented links between obesity, metabolic and lipid problems in other species, including humans, companion and domestic animals [5–7]. Abnormally high blood glucose, triglycerides (TG), and cholesterol have been linked to a number of health issues, collectively called metabolic syndrome, such as hypertension, hyperlipidemia, insulin resistance, and type 2 diabetes [8, 9]. In zoo Asian and African elephant females, negative associations have been found between body condition scores (BCSs) and the glucose to insulin ratio (G:I) [4], suggesting that, as is the case in women, low G:I reflects an unhealthy state [10]. Recently, Norkaew et al. [11] found high BCSs also were associated with a low G:I in captive female Asian elephants in Thailand, as well as higher total cholesterol (TC) and low density lipoprotein (LDL). To our knowledge, only one group has measured glucose and insulin in bull Asian elephants, and found a significant sex difference; mean G:I was 143 units lower in females than males, which corresponded to higher BCSs [12]. Taken together, evidence suggest that being overweight may lead to potentially negative health consequences, at least in females, leading to questions about whether bull elephants are prone to similar obesity-related risks.
In addition to body condition, stress can affect metabolic health and lipid parameters. Glucocorticoids (GCs) are endogenous stress hormones that affect nearly every organ and tissue in the body, modulating various physiological processes including energy homeostasis (metabolism), immunology, behavior, reproductive function, cell proliferation, and survival [13]. While important for maintaining metabolic equilibrium, excessive GC exposure for prolonged periods can have devastating effects on health [14]. Working elephants in Thailand interact with the public in a variety of ways, including performing in shows, trekking, bathing, and painting. Often these activities are not closely monitored or regulated, and could be sources of stress to individual animals. Tourist activities have been shown to compromise welfare and negatively affect behavior and physiology in other species, resulting in increased hiding behaviors [15, 16], heightened vigilance [17, 18], stereotypies [19, 20], poor body condition [21], and elevated cortisol [22, 23]. In a recent study, stress levels based on fecal GC metabolite (FGM) measures, and several lipid and metabolic factors were higher in female elephants during the high tourist season, suggesting some tourist activities may have a negative impact on health and well-being (Norkaew et al., [24]. However, there appeared to be a protective effect of exercise, with hours of daily exercise being associated with better body condition and lipid and metabolic profiles, so elephant trekking per se may not be as bad for welfare as some animal activist organizations claim [25].
Therefore, the aims of this study were to examine: 1) relationships between BCS and FGM and metabolic function (insulin, glucose, fructosamine, G:I) and lipid profiles (TC, TG, HDL, LDL) in male Asian elephants in Thailand; 2) the effect of age on FGM, metabolic function and lipid profiles; and 3) how camp management and the tourist season affects adrenal, lipid and metabolic function in working bull elephants. In addition, because obesity has been shown to be related to declining circulating testosterone levels [26], and reductions in sex hormone binding globulin (SHBG) associated with hyperinsulinemia in obese men [27], we also examined lipid and metabolic relationships with serum testosterone levels. Understanding how management affects health could aid in developing science-based strategies to create sustainable populations of elephants that take into consideration both physical and psychological welfare needs.
Materials and methods
Environmental data
Weather in Thailand is hot and humid, with three official seasons: summer (16 February–15 May), rainy (16 May–15 October) and winter (16 October–15 February). Information on daily temperature (°C), amount of rainfall (mm/day), and humidity (%), averaged by month, was obtained from The Northern Meteorological Center, Meteorological Department, Ministry of Information and Communication Technology, Chiang Mai, Thailand [28]. A thermal–humidity index (THI) was calculated based on air temperature and relative humidity using the following formula: THI = (1.8×Tdb+32) − (0.55−0.0055×RH) × (1.8×Tdb−26), where the Tdb is the temperature of air measured by a thermometer freely exposed to the air, but shielded from radiation and moisture, and RH is the relative humidity (%) [29]. High (November–February) and Low (March–October) tourist seasons were defined by the Tourism Authority of Thailand.
Animals
This study was approved by the Faculty of Veterinary Medicine, Chiang Mai University, Animal Care and Use Committee (FVM-ACUC; permit number S39/2559). Table 1 describes the elephants and tourist camp activities in this study, which are a subset of camps described in Norkeaw et al. [11]. Thirteen adult male Asian elephants (age range, 16–50; mean±SEM, 35.1±2.9 years) were housed at three tourist camps within 43–72 km of the Faculty of Veterinary Medicine, Chiang Mai University (latitude 18°47'N, longitude 98°59'E, altitude 330 m). Tourists interacted with elephants through riding programs (bareback or with a saddle) and feeding of supplementary foods. Elephants were fed primarily corn stalk, napier grass (Pennisetum purpureum) and bana grass (Pennisetum purpureum X, P. americanum hybrid) with unlimited access to fresh water. Animals were given an annual physical examination by staff veterinarians, and were in good health during the study.
Table 1. Description of elephant camps in the study.
Information includes number of years the camp has been in operation (camp age), total number of elephants in each camp, number of elephants participating in the study, participating elephant mean (±SEM) and range age and work time, the general type of work conducted with tourists, and estimated amounts of primary and supplemental food items provided.
| Variable | Camp A | Camp B | Camp E |
|---|---|---|---|
| Camp age (years) | 9 | 27 | 40 |
| Total elephant number | 46 | 66 | 76 |
| Participating elephant number | 4 | 4 | 5 |
| Elephant age (years) | 29.25±7.50 | 41.50±1.94 | 32.67±3.15 |
| (16–50) | (38–47) | (22–40) | |
| Type of work | Bareback riding | Saddle riding | Saddle riding |
| Work time (hours/day) | 1.11±0.03 | 3.12±0.14 | 3.92±0.12 |
| Primary diet | Napier grass, cornstalk | Napier grass, cornstalk | Napier grass |
| Amount per day (kg/day) | 210±0.00 | 186±2.94 | 290±9.79 |
| Supplementary diet | Bamboo, sugarcane, banana | Banana, sugarcane | Bamboo, sugarcane, banana |
| Amount per day (kg/day) | 30 | 10 | 5 |
Body condition scoring
Once every 2 months, rear and side view photographs were taken of each elephant to permit a visual evaluation of the backbone, rib bone and pelvic bone areas, and scored 1–5 (1 = thinnest; 5 = fattest) in 0.5-point increments as described by Norkaew et al. [11]. All photos were evaluated by three experienced elephant veterinarians, and the scores averaged. Intra-class correlations determined the inter-assessor reliability was 0.85.
Blood collection
Blood samples (10 ml) were collected from each elephant from an ear vein by elephant camp staff or Chiang Mai University veterinarians in the morning (between 1000–1200 hours) twice monthly for 1 year. All elephants were conditioned to the blood sampling procedure; however, for safety reasons, blood collection was not attempted on any bulls exhibiting signs of musth. Three bulls (Camp B = 1 elephant, 4 samples were not collected; Camp E = 2 elephants, 3 and 5 samples were not collected) came into musth during October and December, for 2–3 months each. Blood was centrifuged at 1,500 x g for 10 minutes within a few hours of collection, and the serum stored at -20°C until processing and analysis.
Metabolic and lipid marker analysis
Metabolic and lipid markers were analyzed as described by Norkaew et al. [11]. Serum glucose was measured using an automated glucose analyzer (Glucinet T01-149, Bayer, Barcelona, Spain), serum fructosamine was measured by a colorimetric method in a Biosystems BA400 clinical chemistry analyzer (Biosystems S.A., Barcelona, Spain), and a validated solid-phase, two-site bovine insulin enzyme immunoassay (EIA; Cat. No. 10-1113-01; Mercodia, Uppsala, Sweden) was used to measure serum insulin concentrations. All samples were analyzed in duplicate; intra- and inter-assay CVs were <10% and <15%, respectively.
Serum lipids were quantified using a Mindray BS Series analyzer (Mindray BS-380, Shenzhen Mindray Bio-Medical Electronics Co., Ltd.), total cholesterol was measured by a cholesterol oxidase-peroxidase (CHOD-POD) method, and triglycerides were measured by a glycerokinase peroxidase-peroxidase (GPO-POD) method.
Fecal extraction and GC metabolite analysis
Fecal samples were collected immediately after defecation, and about 50 g of well-mixed sub aliquots were placed in plastic ziplock bags, and stored on ice (1–2 hours) until transported to freezers at the Chiang Mai University. The fecal extraction technique is described in Norkaew et al. [11]. Briefly, samples were dried in a conventional oven at 60°C for ~24–48 hours and stored at -20°C until extraction. Frozen dried fecal samples were thawed at room temperature (RT), mixed well and 0.1 g (± 0.01) of dry powdered feces extracted twice in 90% ethanol in distilled water by boiling in a water bath (96°C) for 20 minutes and adding 100% ethanol as needed to keep from boiling dry. Samples were centrifuged at 1,500 x g for 20 min, and the combined supernatants dried under air in a 50°C water bath. Dried extracts were reconstituted in 1 ml methanol (Cat. No. X065, Arbor Assays, Arbor, MI, USA) and stored at –20°C until enzyme immunoassay (EIA) analysis.
Concentrations of FGM were determined using a double-antibody EIA with a polyclonal rabbit anti-corticosterone antibody (CJM006) validated for Asian elephants [30] and described by Norkaew et al. [11]. The absorbance was measured at 405 nm by a microplate reader (TECAN, Sunrise microplate reader, Salzburg, Austria). Assay sensitivity (based on 90% binding) was 0.14 ng/ml (0.014 ng/g). Samples were diluted 1:3 in assay buffer for analysis. The inter-assay CV for high (30% binding) and low (70% binding) control samples was <15%. Samples were reanalyzed if the duplicate CV was >10%; thus, intra-assay CVs were <10%. Fecal data are expressed as ng/g dried feces.
Concentrations of serum testosterone were quantified by a double-antibody EIA validated for Asian elephants [31] utilizing an anti-testosterone antibody (R156/7 50 μl, 1:110,000 dilution) and testosterone-horseradish peroxidase (HRP, 25 μl, 1:10,000 dilution) label. Serum samples were diluted 1:1 with assay buffer for analysis in duplicate (50 μl), and absorbance was measured at 405 nm. Assay sensitivity (based on 90% binding) was 0.08 ng/ml (0.04 ng/ml) and the intra- and inter-assay CVs were <10% and <15%, respectively.
Statistical analysis
Descriptive data were reported as the mean ± standard error of the mean (SEM) and camp management variables were presented as a range or frequency, depending on the type of data. Statistical analyses were performed using R version 3.4.0 [32]. Repeated measures data were analyzed using Generalized Estimating Equations (GEE) to determine: 1) the effects of BCS, FGM and testosterone on metabolic and lipid panel results; 2) seasonal and climate factor effects on metabolic and lipid function; and 3) relationships among metabolic and lipid panel measures. Differences in mean metabolic and lipid profiles between age groups and seasons were further analyzed by Tukey’s post-hoc tests after GEE analyses. Correlations between individual FGM and metabolic hormones or lipid measures in each elephant were analyzed using Pearson's tests for aggregated data. Differences in mean FGM, metabolic, lipid profiles and work type between High and Low tourist seasons were analyzed by Tukey’s post-hoc tests. Note that not all months in the seasonal analysis (summer, rainy, winter) were included in the tourist season analysis (high, low) (see Environmental data). The significance level was set at α = 0.05.
Results
Descriptive BCS, testosterone, FGM, metabolic marker, and lipid profile measures are presented in Table 2, highlighting the variability in mean and range values across individuals. Relationships between BCS, FGM and testosterone on metabolic markers and lipid profiles are presented in Table 3. There was a significant positive relationship between BCS and TG. FGM concentrations also were positively related to TG, HDL and glucose. Testosterone concentrations were significantly positively associated with HDL, while both BCS and testosterone were negatively correlated to the G:I (Table 3). There was a positive relationship between FGM and testosterone concentrations (p<0.01), but no relationships between BCS and FGM or BCS and testosterone.
Table 2. Descriptive statistics.
Mean (±SEM) and range values for body condition score (BCS), fecal glucocorticoid metabolite (FGM) and serum testosterone concentrations, lipid panel measures and metabolic factors in captive Asian elephant bulls (n = 13) in Thailand tourist camps.
| Factors | Mean | Min–Max | Mean range |
|---|---|---|---|
| BCS | 2.80±0.04 | 2.00–4.50 | 2.38–3.70 |
| FGM (ng/g) | 61.60±2.19 | 1.40–330.00 | 37.40–98.30 |
| Testosterone (ng/mL) | 2.10±0.18 | 0.04–29.39 | 0.51–5.11 |
| TC (mg/dL) | 39.90±0.54 | 19.00–101.00 | 28.80–46.30 |
| TG (mg/dL) | 26.20±1.11 | 6.00–145.00 | 18.20–36.70 |
| HDL (mg/dL) | 12.00±0.22 | 2.00–29.00 | 7.64–17.50 |
| LDL (mg/dL) | 29.20±0.45 | 11.00–98.00 | 20.70–35.90 |
| Glucose (mg/dL) | 86.10±1.27 | 37.00–162.00 | 69.60–109.00 |
| Fructosamine (mM) | 0.58±0.01 | 0.38–0.82 | 0.54–0.62 |
| Insulin (ng/mL) | 0.50±0.05 | 0.03–6.82 | 0.05–1.59 |
| G:I | 681.00±34.50 | 19.50–778.61 | 82.50–701.00 |
TC = total cholesterol; TG = triglycerides; HDL = high density lipoproteins; LDL = low density lipoproteins; G:I = glucose to insulin ratio.
Table 3. General estimation equation analyses.
Relationships between health factors and body condition, FGM, and testosterone in captive Asian elephant bulls (n = 13) in Thailand tourist camps.
| Factors | BCS | FGM | Testosterone | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | Beta | P value | Intercept | beta | P value | Intercept | beta | P value | |
| TC (mg/dL) | 33.47 | 1.610 | 0.270 | 38.88 | 0.013 | 0.110 | 39.66 | 0.146 | 0.350 |
| TG (mg/dL) | 1.87 | 9.280 | 0.009 | 29.09 | 0.101 | 0.009 | 26.93 | -0.155 | 0.570 |
| HDL (mg/dL) | 9.97 | 0.863 | 0.379 | 9.97 | 0.033 | <0.001 | 11.21 | 0.311 | 0.002 |
| LDL (mg/dL) | 24.63 | 0.961 | 0.500 | 29.68 | -0.011 | 0.750 | 29.38 | 0.021 | 0.870 |
| Glucose (mg/dL) | 13.36 | 8.040 | 0.090 | 79.01 | 0.114 | 0.005 | 84.23 | 0.517 | 0.360 |
| Fructosamine (mM) | 0.60 | -0.008 | 0.390 | 0.58 | -2.69-e5 | 0.680 | 0.577 | 0.001 | 0.270 |
| Insulin (ng/mL) | 0.01 | 0.314 | 0.300 | 0.28 | 0.004 | 0.200 | 0.37 | 0.024 | 0.320 |
| G:I | 776.00 | -189.000 | 0.015 | 348.33 | -1.177 | 0.130 | 307.42 | -13.090 | 0.016 |
BCS = body condition score; FGM = fecal glucocorticoid metabolites; TC = total cholesterol; TG = triglycerides; HDL = high density lipoproteins; LDL = low density lipoproteins; G:I = glucose to insulin ratio.
Differences in FGM, testosterone, metabolic marker and lipid profile measures related to age are shown in Table 4. Because of limited numbers, elephants were grouped into two age classes: ≤30 years and >30 years for further analysis. Higher levels of TC, TG, LDL, glucose, fructosamine and insulin were found in younger elephants, especially insulin levels, which were three times higher than in older elephants. The G:I was lower in young compared to older elephants. By contrast, testosterone and HDL concentrations were similar across the age groups (Table 4).
Table 4. Effect of age on physiological parameters in bull elephants.
Mean (± SEM) and range values depicting the effect of age on adrenal and testicular steroid hormones, and health factors in captive Asian elephant bulls (n = 13) in Thailand tourist camps.
| Age | FGM (ng/g) |
Testosterone (ng/mL) |
TC (mg/dL) |
TG (mg/dL) |
HDL (mg/dL) |
LDL (mg/dL) |
Glucose (mg/dL) |
Fructosamine (mM) |
Insulin (ng/mL) |
G:I |
|---|---|---|---|---|---|---|---|---|---|---|
| ≤30 years | 60.2±3.44a | 2.18±0.24a | 42.10±0.84b | 30.80±1.84b | 12.40±0.36a | 31.30±0.87b | 98.70±2.14b | 0.58±0.005b | 0.84±0.15b | 204.00±23.30a |
| (n = 5) | 14.10–177.00 | 0.05–11.40 | 19.00–101.00 | 6.00–96.00 | 2.00–23.00 | 17.00–98.00 | 47.00–162.00 | 0.38–0.78 | 0.04–6.82 | 19.50–748.00 |
| >30 years | 70.70±3.12b | 2.05±0.29a | 38.4±0.73a | 23.00±1.44a | 11.80±0.30a | 27.80±0.55a | 77.60±1.36a | 0.57±0.003a | 0.27±0.05a | 336.00±33.20b |
| (n = 8) | 14.60–330.00 | 0.04–29.40 | 22.00–101.00 | 7.00–145.00 | 6.00–29.00 | 11.00–55.00 | 37.00–158.00 | 0.49–0.82 | 0.03–2.82 | 51.10–779.00 |
a,bColumn values are significantly different between the two age groups (p<0.05).
FGM = fecal glucocorticoid metabolites; TC = total cholesterol; TG = triglycerides; HDL = high density lipoproteins; LDL = low density lipoproteins; G:I = glucose to insulin ratio.
Seasonal effects on measured parameters are summarized in Table 5. All but BCS and TG were significantly affected by season. FGM was highest in elephants during the winter months, as was insulin. By contrast, the G:I ratio was lowest during the winter season compared to rainy and summer season months. Glucose also was higher in the winter and summer compared to the rainy season. Serum testosterone was highest in the winter, lowest in the summer, and intermediate in the rainy season. Relationships between environmental factors and BCS, FGM and testosterone concentrations are presented in Table 6, with significant correlations noted between BCS and temperature and humidity. There were no significant negative effects of monthly temperature, rainfall, humidity and THI on FGM or testosterone measures, although an effect of humidity on testosterone approached significance (p = 0.052).
Table 5. Seasonal effects on physiological parameters in bull elephants.
Mean (±SEM) and range values in body condition scores (BCS), fecal glucocorticoid metabolite (FGM) and serum testosterone concentrations, lipid panel measures and metabolic factors across the summer, rainy and winter seasons in captive Asian elephant bulls (n = 13) in Thailand tourist camps.
| Factors | Summer | Rainy | Winter |
|---|---|---|---|
| BCS | 2.62±0.06a | 2.75±0.05a | 2.94±0.07a |
| (2.00–3.00) | (2.00–4.00) | (2.00–4.00) | |
| FGM (ng/g) | 54.70±3.64a | 59.50±2.81a | 72.80±4.55b |
| (14.90–179.00) | (14.10–197.00) | (14.60–166.00) | |
| Testosterone (ng/mL) | 1.57±0.25a | 1.96±0.03ab | 2.84±0.45b |
| (0.07–11.40) | (0.04–29.40) | (0.18–24.00) | |
| TG (mg/dL) | 24.40±2.43a | 25.70±1.45a | 28.50±2.41a |
| (6.00–97.00) | (9.00–90.00) | (8.00–145.00) | |
| HDL (mg/dL) | 13.70±0.54b | 11.10±0.27a | 11.90±0.13a |
| (6.00–29.00) | (5.00–19.00) | (2.00–21.00) | |
| LDL (mg/dL) | 27.50±0.72a | 28.80±0.68ab | 31.20±1.09b |
| (11.00–45.00) | (16.00–59.00) | (15.00–98.00) | |
| Glucose (mg/dL) | 88.40±1.80b | 81.00±1.86a | 91.50±3.06b |
| (63.00–153.00) | (37.00–143.00) | (47.00–162.00) | |
| Fructosamine (mM) | 0.61±0.006c | 0.56±0.004a | 0.58±0.004b |
| (0.48–0.78) | (0.38–0.82) | (0.49–0.68) | |
| Insulin (ng/mL) | 0.37±0.08a | 0.33±0.06a | 0.89±0.21b |
| (0.04–2.89) | (0.03–2.47) | (0.03–6.82) | |
| G:I | 290.00±41.60ab | 320.00±33.80b | 186.00±30.80a |
| (34.20–779.00) | (40.80–702.00) | (19.50–648.00) |
a,b,cRow values for each Factor differ significantly across the Summer, Rainy and Winter seasons (p<0.05).
TC = total cholesterol; TG = triglycerides; HDL = high density lipoproteins; LDL = low density lipoproteins; G:I = glucose to insulin ratio. Summer: 16 February–15 May, rainy: 16 May–15 October, winter: 16 October–15 February
Table 6. General Estimation Equation analysis of seasonal relationships.
Relationships between body condition scores (BCS), fecal glucocorticoid metabolite (FGM) and serum testosterone concentrations, and environmental factors in captive Asian elephant bulls (n = 13) in Thailand tourist camps.
| Factors | BCS | FGM | Testosterone | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | Beta | P value | Intercept | Beta | P value | Intercept | Beta | P value | |
| Temperature (°C) | 4.410 | -0.057 | 0.048 | 114.300 | -1.810 | 0.093 | 3.895 | -0.065 | 0.248 |
| Rainfall (mm) | 2.832 | 0.007 | 0.900 | 65.714 | -0.450 | 0.620 | 2.098 | 0.001 | 0.980 |
| Humidity (%) | 1.761 | 0.007 | 0.030 | 47.205 | 0.240 | 0.175 | 0.197 | 0.027 | 0.052 |
| THI | 5.144 | -0.030 | 0.175 | 165.570 | 0.864 | 0.132 | 2.790 | -0.009 | 0.840 |
THI = temperature-humidity index
There were significant differences across camps in adrenal activity, metabolic marker and lipid profiles, with FGM, testosterone, BCS, TC, TG, HDL, insulin and glucose being among the highest, and G:I being the lowest in Camp A, the facility with least amount work activities for elephants and the one where tourists fed the highest amounts of treats (e.g., bananas, sugar cane) (Table 7). During the High tourist season, elephants exhibited higher FGM and insulin concentrations than during the Low season (Table 8). In particular, insulin levels were three times higher during the High compared to the Low season, whereas glucose concentrations were unchanged, resulting in a G:I that was 40% lower during the High season.
Table 7. Camp differences in body condition, adrenal activity and health markers in elephants.
Mean (±SEM) and range values (min–max) for body condition score (BCS), fecal glucocorticoid metabolite (FGM) and serum testosterone concentrations, lipid panel measures and metabolic factors in captive Asian elephant bulls (n = 13) in three Thailand tourist camps.
| Factors | Camp A | Camp B | Camp E |
|---|---|---|---|
| BCS | 3.27±0.12b | 2.52±0.10a | 2.62±0.14a |
| (2.00–4.50) | (2.00–3.00) | (2.00–4.00) | |
| FGM (ng/g) | 82.40±3.90c | 58.80±3.40b | 46.50±2.43a |
| (21.90–179.00) | (14.90–197.00) | (14.10–161.00) | |
| Testosterone (ng/mL) | 3.06±0.33b | 2.34±0.53ab | 1.13±0.11a |
| (0.05–16.83) | (0.07–29.39) | (0.04–6.38) | |
| TC (mg/dL) | 43.40±0.75c | 36.20±0.94a | 39.70±1.03b |
| (19.00–67.00) | (22.00–77.00) | (27.00–101.00) | |
| TG (mg/dL) | 31.30±1.96b | 21.90±2.11a | 25.10±1.84ab |
| (6.00–96.00) | (7.00–145.00) | (8.00–97.00) | |
| HDL (mg/dL) | 15.10±0.35c | 11.00±0.43b | 10.20±0.23a |
| (7.00–23.00) | (6.00–29.00) | (2.00–18.00) | |
| LDL (mg/dL) | 29.80±0.65b | 26.90±0.95a | 30.50±0.87b |
| (17.00–53.00) | (11.00–55.00) | (20.00–98.00) | |
| Glucose (mg/dL) | 101.20±2.44c | 73.90±1.54a | 82.00±1.81b |
| (47.00–162.00) | (45.00–107.00) | (37.00–158.00) | |
| Fructosamine (mM) | 0.57±0.005a | 0.57±0.004a | 0.59±0.006b |
| (0.41–0.71) | (0.49–0.69) | (0.38–0.82) | |
| Insulin (ng/mL) | 1.01±0.18b | 0.20±0.05a | 0.20±0.09a |
| (0.04–6.82) | (0.03–1.59) | (0.04–1.53) | |
| G:I | 179.00±26.30a | 305.00±52.30ab | 349.00±33.00b |
| (19.50–748.00) | (65.50–701.00) | (69.00–779.00) |
a,b,cRow values for each Factor differ significantly across the three Camps (p<0.05).
TC = total cholesterol; TG = triglycerides; HDL = high density lipoproteins; LDL = low density lipoproteins; G:I = glucose to insulin ratio
Table 8. Tourist season effects on physiological parameters in bull elephants.
Mean (±SEM) and range values for captive Asian elephant bulls in lipid profiles and metabolic factors between High and Low tourist seasons in captive bull Asian elephants (n = 13) in Thailand tourist camps.
| Factors | High season | Low season |
|---|---|---|
| BCS | 2.98±0.07a | 2.70±0.04a |
| (2.00–4.50) | (2.00–4.00) | |
| FGM (ng/g) | 73.50±5.07b | 60.40±2.33a |
| (14.60–330.00) | (14.10–197.00) | |
| TC (mg/dL) | 41.80±1.14a | 39.10±0.59a |
| (22.00–109.00) | (10.00–85.00) | |
| TG (mg/dL) | 26.30±2.18a | 26.10±1.27a |
| (7.00–145.00) | (6.00–97.00) | |
| HDL (mg/dL) | 12.20±0.38a | 12.00±0.27a |
| (2.00–22.00) | (5.00–29.00) | |
| LDL (mg/dL) | 30.30±1.01a | 28.70±0.46a |
| (11.00–98.00) | (12.00–59.00) | |
| Glucose (mg/dL) | 89.30±2.59a | 84.70±1.41a |
| (47.00–162.00) | (37.00–153.00) | |
| Fructosamine (mM) | 0.58±0.01a | 0.58±0.01a |
| (0.48–0.78) | (0.38–0.82) | |
| Insulin (ng/mL) | 0.90±0.13b | 0.35±0.03a |
| (0.03–6.82) | (0.03–2.89) | |
| G:I | 186.00±15.70a | 307.00±13.80b |
| (19.50–648.00) | (34.20–779.00) |
a,b,cRow values for each Factor differ significantly across the High and Low tourist seasons (p<0.05).
BCS = body condition score; FGM = fecal glucocorticoid metabolites; TC = total cholesterol; TG = triglycerides; HDL = high density lipoproteins; LDL = low density lipoproteins; G:I = glucose to insulin ratio. High: November–February, Low: March–October
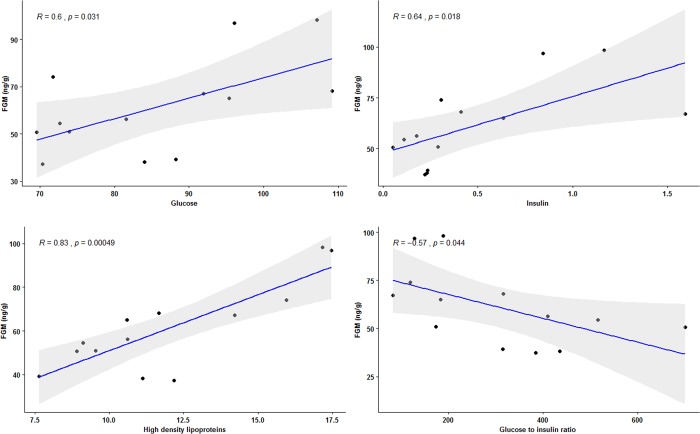
Several correlations were noted amongst the metabolic and lipid factors as shown in Table 9. Strong positive associations (p<0.001) were found between TC and HDL, LDL and HDL and glucose, and glucose and insulin. By contrast, negative relationships (p<0.001) were found between the G:I and HDL and glucose, and between insulin and G:I. In separate Pearson's correlation analyses of individual means (n = 13), FGM levels were similarly correlated to HDL, glucose and insulin, and negatively correlated to G:I (p<0.05) (Fig 1).
Table 9. Relationships among physiological factors in bull elephants.
Correlation matrix presenting relationships between testosterone and lipid panel measures and metabolic factors in captive Asian elephant bulls (n = 13) in Thailand tourist camps.
| Test | |||||||||
| TC | TC | ||||||||
| TG | TG | ||||||||
| HDL | HDL | ||||||||
| LDL | LDL | ||||||||
| GLU | GLU | ||||||||
| FRUC | FRUC | ||||||||
| INS | INS | ||||||||
| G:I | G:I |
***p<0.001 (dark color)
**p<0.01 (medium color)
*p<0.05 (light color)
Blue = positive correlation. Red = negative correlation. Test = Testosterone; TC = total cholesterol; TG = triglycerides; HDL = high density lipoproteins; LDL = low density lipoproteins; GLU = glucose; FRUC = fructosamine; INS = insulin; G:I = glucose to insulin ratio.
Fig 1. Significant relationships between adrenal steroids and lipid and metabolic factors.
Pearson's correlations between FGM concentrations and lipid panel measures and metabolic factors in captive Asian elephant bulls (n = 13) in Thailand tourist camps. (R = correlation coefficient).
Discussion
This was the first study to assess lipid profiles, and evaluate relationships between BCS, FGMs and metabolic factors in male Asian elephants in Thailand. The mean BCS was 2.8, which is within a normal/ideal distribution of body fat for elephants [33]. In general, the working bulls in our study had better body condition than western zoo bull elephants; 69% of Thai bulls had a BCS = 3, compared to only 33% in the U.S. Furthermore, 48% of bulls in the U.S. had a BCS = 4, whereas no bulls in Thailand had an average BCS of 4 or 5 over the study period, although a few scored up to 4.5 in individual months. This could be due to higher amounts of exercise, with tourist elephants engaged in many activities, including trekking, bathing, shows or walking with tourists [34], so inactivity is less of a concern. This result is consistent with a study of female Asian elephants in Thailand where most (~60%) had a BCS = 3 [11], although compared to males, females had on average higher BCSs than males (3.50±0.02 vs 2.80±0.04; GEE, p<0.001). In the U.S., elephants that walked more than 14 hours/week had a decreased risk of BCS = 4 or 5 [35]. Thus, increased exercise associated with tourist activities likely helps elephants maintain better body condition. From a previous study, female elephants that worked more hours per day in the form of saddle or bareback riding were found to have better BCSs and metabolic health [11].
Insulin plays a central role in the regulation of blood glucose and energy homeostasis; however, high levels are associated with hypertension, obesity, dyslipidemia, and glucose intolerance in humans [36, 37]. Glucose and insulin are generally measured after a patient has fasted, but that was not possible in our elephants because they had access to forage overnight. Instead, we evaluated the G:I [38], which has been used to detect insulin sensitivity in women [10]. One popular activity for tourists is feeding the elephants, particularly with bananas and sugar cane, which possess high concentrations of sucrose and other soluble sugars that could contribute to weight problems. Blood glucose values agreed with our previous study of female elephants (male: 86.10±1.27 vs female: 88.90±0.75 mg/dL) that found elephants with a BCS of 4.5–5.0 had higher glucose and insulin levels, and a lower G:I [11]. Compared to females at the same camps, bulls exhibited a higher, and thus potentially healthier, G:I (male: 681.00±34.50 vs female: 196.00±6.72; GEE, p<0.001). Last, the G:I of bulls in Thailand was higher than that of bulls in the U.S. (253±18) [4], commenserate with the higher BCSs observed in the latter population. These results agree with insulin measures, which were lower in male than female elephants (0.50±0.05 vs 0.75±0.03 ng/mL; GEE, p = 0.003), respectively [11]. Thus, overall, it appears the bull elephants in our study population are healthier than their female cohorts, both physically and physiologically, perhaps because feeding of bulls is more limited. They also differ somewhat in metabolic profiles compared to bulls in the U.S., which deserves further study.
Serum TG concentrations were within the range reported for female Asian elephants [2, 11] and correlated positively with BCS, consistent with other findings [11, 35]. From work in other species, serum TGs are useful indicators of overall adiposity [39–42]. In humans, a number of metabolic changes are associated with obesity, high BMI or poor eating habits, including elevated TC, TG and LDL levels. Similarly, in dogs, increased plasma TC and TG concentrations are observed in association with obesity [3], and in horses, TG concentration is correlated with body condition [43, 44]. Hyperlipidemia could pose problems for elephants, although incidence of cardiovascular disease appears to be relatively low [45], and none of the bulls in our study were obese (BCS = 5).
There were positive associations between FGM and TG, HDL and glucose, indicating relationships between adrenal activity and metabolic and lipid function, as has been demonstrated in other species [46, 47], including female elephants [11]. Elevated and sustained cortisol secretion during chronic stress can lead to central obesity, hypertension, glucose intolerance, and dyslipidemia in humans [48]. Studies to understand how management factors affect stress responses in captive animals are key to improving welfare, and are beginning to be applied to elephants in westerns zoos [49, 50], and to working tourist elephants in Asia [11].
In exploring age effects, older bull elephants (>30 years) exhibited comparatively lower metabolic and lipid function than those under 30 years, in agreement with studies in other species that show basal metabolic rate decreases almost linearly with age [51–53]. By contrast, in humans, diabetes is a progressive disease, and glucose levels tend to increase with age. The incidence of type-2 diabetes increases in older individuals primarily due to age-related declines in beta cell function and impaired insulin secretion, rather than to insulin resistance [54]. The aging process can also be associated with pancreatic islet cell dysfunction as a contributing factor to abnormal glucose metabolism [55]. We are aware of only one study of diabetes in an older Asian elephant bull [56], so this condition may not be common. Nevertheless, these results highlight a number of physiological changes associated with age in this species.
There was a significant effect of season on FGM, with higher concentrations during the winter when temperatures and rainfall are lower. Salivary cortisol and FGM in female Asian elephants in Spain and Thailand, respectively, were highest during the winter [11, 57]. In goral (Naemorhedus griseus), another indigenous ungulate species in Thailand, FGMs also were higher in winter [58, 59]. The need for more energy to maintain optimum body temperature and ensure survival in cooler temperatures could be related to this finding. Elevated circulating GC levels as a response to cold stress have been documented in red deer (Cervus elephas) [60] and in farm animals [61]. Although High season months overlapped with the winter season, not all months in the seasonal analysis (October–February) were included in the tourist season analysis (November–February), so more work is needed to identify the primary drivers of FGM production in working elephants.
Significant differences were identified across camps in stress hormone levels, metabolic status and lipid profiles, which may be related to management and tourist activities. Elephants at Camp A exhibited overall lipid and metabolic results that were higher on average than the other camps. BCS at Camp A also was the highest, while G:I was the lowest, indicative of metabolic derangements [4, 11]. These effects appear to be related, in part, to the feeding of greater amounts of supplementary foods (30 vs 5–10 kg/day) and lower levels of activity (1.1 vs 3–4 hours/day). Female elephants at Camp A also exhibited higher BCS, FGM concentrations, and metabolic and lipid measures, suggesting management changes may be needed to provide elephants with better diets or increased exercise opportunities. Some of these camp effects may be related to tourists, given that there was a significant tourist season effect on health status, with levels of FGMs and several metabolic markers being higher during the High tourist season. Higher numbers of tourists likely are associated with increases in amounts of food treats offered to elephants, given that feeding is one of the most popular activities. The food given to elephants in this study consisted of items with a high sugar content and glycemic index, including bananas, sugarcane, watermelon, and pumpkin. High glycemic index foods induce an exaggerated insulin response, which can increase body fat and weight, and lead to insulin resistance, and eventual exhaustion of endocrine pancreatic function and insulin release [62, 63]. There is growing recognition and concern that obesity and metabolic conditions are negatively impacting the health of many species, including humans, companion and domestic animals. A similar health concern exists for zoo-held species, including elephants, that often are fed diets high in calories and given inadequate exercise [2, 4, 33]. However, for the bulls in this study, body condition, adrenal activity, metabolic and lipid status all appeared to indicate a comparatively normal health status.
By contrast, overall FGM concentrations in bulls were higher than those in females [24] (Camp A, 82.40±3.90 vs 60.40±2.43; Camp B, 58.80±3.40 vs 49.60±2.40; Camp E, 46.50±2.43 vs 39.60±2.06 ng/g, GEE, p<0.001), respectively. One explanation could be that bulls are controlled more vigilantly (i.e., using an ankus) around tourists, and are often kept on shorter chains because of their more aggressive nature [64], which could lead to higher stress levels. A big challenge in captive elephant management is how to care for bulls in a way that meets their welfare needs; a problem that often is ignored because of limited capacity to safely and humanely manage them. However, in mice, males in general excreted higher amounts of corticosterone metabolites via the feces than females [65]. Therefore, the higher level of FGM in males may not be because males experience more stress, but may be due to sex differences in GC metabolism.
Testosterone serves a number of biological functions, including development of male reproductive tissues, promoting secondary sexual characteristics, and supporting breeding behaviors. However, low testosterone in men and animals has been associated with a metabolic syndrome characterized by obesity, diabetes, hypertension, and dyslipidemia [66–69], and clinical trials have demonstrated that testosterone replacement therapy moderates problems with insulin resistance and aids in glycemic control [70]. Although none of the bulls in our study were overweight or obese (mean BCS = 4 or 5), there was a positive correlation between testosterone and HDL (good cholesterol), in agreement with previous studies in humans [71, 72], and a negative relationship with G:I, suggesting thinner bulls may experience better health parameters, not unlike our studies in female elephants. We did note a seasonal effect on serum testosterone. Three of the bulls came into musth between October and December, for 2–3 months each, which is the general pattern for elephants in Thailand [73, 74]. Although blood samples were not collected during musth due to safety concerns, average testosterone concentrations were still higher in winter as compared to summer and rainy season months, presumably reflecting changes in baseline concentrations. A study of bulls in Thailand by Thongtip et al. [74] also found higher serum testosterone during the winter, despite not collecting samples during musth. Overall, there was no difference in testosterone concentrations in bulls >30 years, which differs from that of Brown et al. [75], who found an overall increase with respect to age (r = 0.69) in Asian bulls in the U.S., at least up to the age of 42 years. That study did include samples from musth bulls, which could account in part for the study differences.
Compared to the females in Norkeaw et al. [11], there were similar positive relationships between TC and HDL and LDL, HDL and glucose, glucose and insulin, and negative relationships between HDL and G:I, glucose and G:I, and insulin and G:I. By contrast, males differed from females by exhibiting a negative correlation between TC and G:I, and a lack of positive relationships between glucose and fructosamine, and fructosamine and insulin. In the cat, fructosamine concentrations are tightly correlated with blood glucose levels in obese animals [76], but there was no such relationship in bull elephants, likely because we saw no incidence of obesity in this population.
Conclusion
Used as outcome variables in regression models, greater BCS and FGM measures were predictors of higher metabolic and lipid levels in male Asian elephants, although not to the extent observed in female elephants in these same camps. There also was a relationship between higher BCSs and adrenal steroid hormone measures in bulls during the winter, when there are more tourists. Thus, elephant health and well-being could be promoted by limiting tourist interactions with individual elephants, especially during the high tourist season. However, it may be significant that overall, bull elephants in Thailand appear to be in better physical and physiological health compared to females at the same camps. This difference could be related to how bulls are managed, keeping them at a lower body condition to lessen musth symptoms and perhaps to fewer feeding opportunities with tourists.
Acknowledgments
We are grateful to the elephant owners and mahouts for participating in this study and allowing us to work with the elephants. We would like to thank our colleagues, Dr. Muyao Li, Ms. Patcharapa Towiboon, Mr. Pallop Tankaew, Dr. Khajohnpat Boonprasert, Dr. Patiparn Toin, Dr. Tithipong Plangsangmas, Dr. Channarong Saisaard, Dr. Panida Muanghong and Dr. Siripat Khammesi for help with collecting blood samples and for laboratory assistance.
Data Availability
Data cannot be shared publicly because of the sensitive nature of the results (e.g., subject to use by animal rights groups). Data are available from the Chiang Mai University Institutional Data Access / Ethics Committee (Assoc. Prof. Dr. Nattawooti Sthitmatee, e-mail: nattawooti.s@cmu.ac.th, drneaw@gmail.com) for researchers who meet the criteria for access to confidential data.
Funding Statement
Faculty of Veterinary Medicine, Chiang Mai University (grant number R000017077) www.vet.cmu.ac.th, Center of Excellence in Elephant and Wildlife Research, Chiang Mai University (grant number 002/2559) www.asianelephantresearch.com.
References
- 1.Brown JL. Comparative reproductive biology of elephants. Adv Exp Med Biol. 2014;53:135–69. 10.1007/978-1-4939-0820-2_8 [DOI] [PubMed] [Google Scholar]
- 2.Clubb R, Rowcliffe M, Lee P, Mar K, Moss C, Mason G. Fecundity and population viability in female zoo elephants: Problems and possible solutions. Anim Welf. 2009; 18:237–247. [Google Scholar]
- 3.Morfeld KA, Brown JL. Ovarian acyclicity in zoo African elephants (Loxodonta africana) is associated with high body condition scores and elevated serum insulin and leptin. Reprod Fertil Dev. 2016;28(5):640–7. Epub 2014/11/07. 10.1071/RD14140 [DOI] [PubMed] [Google Scholar]
- 4.Morfeld KA, Brown JL. Metabolic health assessment of zoo elephants: Management factors predicting leptin levels and the glucose-to-insulin ratio and their associations with health parameters. PLOS ONE. 2017;12(11):e0188701 Epub 2017/12/01. 10.1371/journal.pone.0188701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeusette IC, Lhoest ET, Istasse LP, Diez MO. Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res. 2005;66(1):81–6. [DOI] [PubMed] [Google Scholar]
- 6.Jung HS, Chang Y, Eun Yun K, Kim CW, Choi ES, Kwon MJ, et al. Impact of body mass index, metabolic health and weight change on incident diabetes in a Korean population. Obesity (Silver Spring, Md). 2014;22(8):1880–7. 10.1002/oby.20751 [DOI] [PubMed] [Google Scholar]
- 7.Watson TD, Packard CJ, Shepherd J, Fowler JN. An investigation of the relationships between body condition and plasma lipid and lipoprotein concentrations in 24 donkeys. Vet Rec. 1990;127(20):498–500. [PubMed] [Google Scholar]
- 8.Lanktree MB, Joy TR, Hegele RA. Chapter 83—The metabolic syndrome In: Ginsburg GS, Willard HF, editors. Genomic and Personalized Medicine (Second Edition): Academic Press; 2013. p. 1006–1. [Google Scholar]
- 9.Lee L, Sanders RA. Metabolic syndrome. Pediatr Rev. 2012;33(10):459–68. PMC4109314 10.1542/pir.33-10-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83(8):2694–8. 10.1210/jcem.83.8.5054 [DOI] [PubMed] [Google Scholar]
- 11.Norkaew T, Brown JL, Bansiddhi P, Somgird C, Thitaram C, Punyapornwithaya V, et al. Body condition and adrenal glucocorticoid activity affects metabolic marker and lipid profiles in captive female elephants in Thailand. PLOS ONE. 2018;13(10):e0204965 10.1371/journal.pone.0204965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morfeld KA, Brown JL. Metabolic health assessment of zoo elephants: Management factors predicting leptin levels and the glucose-to-insulin ratio and their associations with health parameters. PLOS ONE. 2017;12(11):e0188701 10.1371/journal.pone.0188701 ; PMCID: PMCPMC5706714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel R, Williams-Dautovich J, Cummins CL. Minireview: new molecular mediators of glucocorticoid receptor activity in metabolic tissues. Mol Endocrinol. 2014;28(7):999–1011. 10.1210/me.2014-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabasa C, Dickson SL. Impact of stress on metabolism and energy balance. Curr Opin Behav Sci. 2016;9:71–7. [Google Scholar]
- 15.Birke L. Effects of browse, human visitors and noise on the behaviour of captive orang utans. 2002;11(2):189–202. [Google Scholar]
- 16.Stevens J, Thyssen A, Laevens H, Vervaecke H. The influence of zoo visitor numbers on the behaviour of Harbour seals (Phoca vitulina). J Zoo Aquar Res. 2013;1(1):31–4. [Google Scholar]
- 17.Clark FE, Fitzpatrick M, Hartley A, King AJ, Lee T, Routh A, et al. Relationship between behavior, adrenal activity, and environment in zoo-housed western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. 2012;31(3):306–21. 10.1002/zoo.20396 [DOI] [PubMed] [Google Scholar]
- 18.Larsen MJ, Sherwen SL, Rault J-L. Number of nearby visitors and noise level affect vigilance in captive koalas. Appl Anim Behav Sci. 2014;154:76–82. 10.1016/j.applanim.2014.02.005 [DOI] [Google Scholar]
- 19.Mallapur A, Chellam R. Environmental influences on stereotypy and the activity budget of Indian leopards (Panthera pardus) in four zoos in Southern India. Zoo Biol. 2002;21(6):585–95. 10.1002/zoo.10063 [DOI] [Google Scholar]
- 20.Wells DL. A note on the influence of visitors on the behaviour and welfare of zoo-housed gorillas. Appl Anim Behav Sci. 2005;93(1):13–7. 10.1016/j.applanim.2005.06.019 [DOI] [Google Scholar]
- 21.Maréchal L, Semple S, Majolo B, MacLarnon A. Assessing the effects of tourist provisioning on the health of wild Barbary Macaques in Morocco. PLOS ONE. 2016;11(5):e0155920 10.1371/journal.pone.0155920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis N, Schaffner CM, Smith TE. Evidence that zoo visitors influence HPA activity in spider monkeys (Ateles geoffroyii rufiventris). Appl Anim Behav Sci. 2005;90(2):131–41. 10.1016/j.applanim.2004.08.020 [DOI] [Google Scholar]
- 23.Pifarré M, Valdez R, González-Rebeles C, Vázquez C, Romano M, Galindo F. The effect of zoo visitors on the behaviour and faecal cortisol of the Mexican wolf (Canis lupus baileyi). Appl Anim Behav Sci. 2012;136(1):57–62. 10.1016/j.applanim.2011.11.015 [DOI] [Google Scholar]
- 24.Norkaew T. Evaluation of relationships between glucocorticoid hormones and physiological function in Asian elephants (Elephas maximus). Ph.D. Thesis, Chiang Mai University. 2018.
- 25.Schmidt-Burbach J, Ronfot D, Srisangiam R. Asian elephant (Elephas maximus), pig-tailed Macaque (Macaca nemestrina) and tiger (Panthera tigris) populations at tourism venues in Thailand and aspects of their welfare. PLOS ONE. 2015;10(9):e0139092 10.1371/journal.pone.0139092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz-Arjonilla M, Schwarcz M, Swerdloff RS, Wang C. Obesity, low testosterone levels and erectile dysfunction. Int J Impot Res. 2009;21: 89–98. 10.1038/ijir.2008.42 [DOI] [PubMed] [Google Scholar]
- 27.Fui MNT, Dupuis P, Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl. 2014;16(2):223–31. 10.4103/1008-682X.122365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thai Meteorological Department—Chiang Mai Weather (2016). Available: http://www.tmd.go.th. Accessed 2017 January 9.
- 29.Herbut P, Angrecka S. Forming of temperature-humidity index (THI) and milk production of cows in the free-stall barn during the period of summer heat. Anim Sci Pap Rep. 2012;30(4):363–72. [Google Scholar]
- 30.Watson R, Munro C, Edwards KL, Norton V, Brown JL, Walker SL. Development of a versatile enzyme immunoassay for non-invasive assessment of glucocorticoid metabolites in a diversity of taxonomic species. Gen Comp Endocrinol. 2013;186:16–24. 10.1016/j.ygcen.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 31.Kumar V, Palugulla Reddy V, Kokkiligadda A, Shivaji S, Umapathy G. Non-invasive assessment of reproductive status and stress in captive Asian elephants in three south Indian zoos. Gen Comp Endocrinol. 2014;201:37–44. 10.1016/j.ygcen.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 32.R Development Core Team (2017) R: A language and environment for statistical computing Vienna: R Foundation for Statistical Computing; Available: http://www.R-project.org/. Accessed 2017 July 8. [Google Scholar]
- 33.Morfeld KA, Lehnhardt J, Alligood C, Bolling J, Brown JL. Development of a body condition scoring index for female African elephants validated by ultrasound measurements of subcutaneous fat. PLOS ONE. 2014;9(4):e93802 10.1371/journal.pone.0093802 ; PMCID: PMCPMC3981750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malikhao P, Servaes L. Elephants in tourism. sustainable and practical approaches to captive elephant welfare and conservation in Thailand In: Malikhao P, editor. Culture and Communication in Thailand. Singapore: Springer Singapore; 2017. p. 127–38. [Google Scholar]
- 35.Morfeld KA, Meehan CL, Hogan JN, Brown JL. Assessment of body condition in African (Loxodonta africana) and Asian (Elephas maximus) elephants in North American Zoos and management practices associated with high body condition scores. PLOS ONE. 2016;11(7):e0155146 Epub 2016/07/16. 10.1371/journal.pone.0155146 ; PMCID: PMCPMC4944958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boden G. Chapter 8—Insulin resistance and inflammation: Links between obesity and cardiovascular disease In: Dokken BB, editor. Glucose Intake and utilization in pre-diabetes and diabetes. Boston: Academic Press; 2015. p. 95–101. [Google Scholar]
- 37.Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985;75(3):809–17. PMC423608 10.1172/JCI111776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralston SL. Insulin and glucose regulation. Vet Clin North Am Equine Pract. 2002;18(2):295–304. [DOI] [PubMed] [Google Scholar]
- 39.García AI, Niño-Silva LA, González-Ruíz K, Ramírez-Vélez R. Body adiposity index as marker of obesity and cardiovascular risk in adults from Bogotá, Colombia. Endocrinol Nutr. 2015;62(3):130–7. 10.1016/j.endonu.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 40.PeÑA C, Suarez L, Bautista-CastaÑO I, Juste MC, CarretÓN E, Montoya-Alonso JA. Effects of low-fat high-fibre diet and mitratapide on body weight reduction, blood pressure and metabolic parameters in obese dogs. J Vet Med Sci. 2014;76(9):1305–8. 10.1292/jvms.13-0475 PMC4197164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamka RM, Friesen KG, Frantz NZ. Identification of canine markers related to obesity and the effects of weight loss on the markers of interest. Int J Appl Res Vet Med. 2006;4(4):282. [Google Scholar]
- 42.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 43.Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine veterinary journal. 2017;49(5):570–6. 10.1111/evj.12703 [DOI] [PubMed] [Google Scholar]
- 44.de Laat MA, Hampson BA, Sillence MN, Pollitt CC. Sustained, low‐intensity exercise achieved by a dynamic feeding system decreases body fat in ponies. J Vet Intern Med. 2016;30(5):1732–8. 10.1111/jvim.14577 PMC5032883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fowler M, Mikota SK. Biology, medicine, and surgery of elephants: Wiley; 2008. [Google Scholar]
- 46.Mudron P, Rehage J, Sallmann HP, HoÈ ltershinken M, Scholz H. Stress response in dairy cows related to blood glucose. Acta Vet Brno. 2005; 74:37–42. [Google Scholar]
- 47.Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr Metab. 2005;2:3 10.1186/1743-7075-2-3 PMC548667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang M. The role of glucocorticoid action in the pathophysiology of the metabolic syndrome. Nutr Metab (Lond). 2005;2(1):3 10.1186/1743-7075-2-3 ; PMCID: PMCPMC548667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris DM, Sherwin DC, Harris PS. The welfare, housing and husbandry of elephants in UK Zoos. Report to DEFRA. 2008.
- 50.Proctor C, Brown J. Influence of handling hethod on adrenal activity in zoo African and Asian elephants. J Zoo Aquar Res. 2015;3:(1). [Google Scholar]
- 51.Goh VH, Tong TY, Mok HP, Said B. Differential impact of aging and gender on lipid and lipoprotein profiles in a cohort of healthy Chinese Singaporeans. Asian J Androl. 2007;9(6):787–94. 10.1111/j.1745-7262.2007.00294.x [DOI] [PubMed] [Google Scholar]
- 52.St-Onge M-P, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26(2):152–5. 10.1016/j.nut.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weerasinghe D, Elsom J. Investigating the effects of age and exercise on HbA1c and lipid profiles in healthy individuals. Atherosclerosis. 2017;263:e267 10.1016/j.atherosclerosis.2017.06.862 [DOI] [Google Scholar]
- 54.Selvin E, Parrinello CM. Age-related differences in glycaemic control in diabetes. Diabetologia. 2013;56(12):2549–51. 10.1007/s00125-013-3078-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am. 2013;42(2):333–47. 10.1016/j.ecl.2013.02.010 ; PMCID: PMCPMC3664017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Kolk JH, Hoyer MJ, Verstappen FA, Wolters MS, Treskes M, Grinwis GC, et al. Diabetes mellitus in a 50-year-old captive Asian elephant (Elaphas maximus) bull. Vet Q. 2011;31(2):99–101. 10.1080/01652176.2011.585793 [DOI] [PubMed] [Google Scholar]
- 57.Menargues Marcilla A, Urios V, Liminana R. Seasonal rhythms of salivary cortisol secretion in captive Asian elephants (Elephas maximus). Gen Comp Endocrinol. 2012;176(2):259–64. Epub 2012/03/01. 10.1016/j.ygcen.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 58.Khonmee J, Brown JL, Rojanasthien S, Aunsusin A, Thumasanukul D, Kongphoemphun A, et al. Gender, season and management affect fecal glucocorticoid metabolite concentrations in captive goral (Naemorhedus griseus) in Thailand. PLOS ONE. 2014;9(3):e91633 10.1371/journal.pone.0091633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khonmee J, Brown JL, Rojanasthien S, Thumasanukul D, Kongphoemphun A, Siriaroonrat B, et al. Seasonality of fecal androgen and glucocorticoid metabolite excretion in male goral (Naemorhedus griseus) in Thailand. Anim Reprod Sci. 2014;146(1–2):70–8. 10.1016/j.anireprosci.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 60.Huber S, Palme R, Arnold W. Effects of season, sex, and sample collection on concentrations of fecal cortisol metabolites in red deer (Cervus elaphus). Gen Comp Endocrinol. 2003;130: 48–54. 10.1016/S0016-6480(02)00535-X [DOI] [PubMed] [Google Scholar]
- 61.Dantzer R, Mormede P. Stress in farm animals: a need for reevaluation. J Anim Sci. 1983;57(1):6–18. [DOI] [PubMed] [Google Scholar]
- 62.Ludwig DS. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23. 10.1001/jama.287.18.2414 [DOI] [PubMed] [Google Scholar]
- 63.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. The Lancet. 2004;364(9436):778–85. 10.1016/S0140-6736(04)16937-7 [DOI] [PubMed] [Google Scholar]
- 64.Bansiddhi P, Brown JL, Thitaram C, Punyapornwithaya V, Somgird C, Edwards KL, et al. Changing trends in elephant camp management in northern Thailand and implications for welfare. PeerJ. 2018;6: e5996 10.7717/peerj.5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touma C, Sachser N, Möstl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol. 2003;130(3):267–78. 10.1016/S0016-6480(02)00620-2 [DOI] [PubMed] [Google Scholar]
- 66.Akishita M, Fukai S, Hashimoto M, Kameyama Y, Nomura K, Nakamura T, et al. Association of low testosterone with metabolic syndrome and its components in middle-aged Japanese men. Hypertension research: Official journal of the Japanese Society of Hypertension. 2010;33(6):587–91. 10.1038/hr.2010.43 [DOI] [PubMed] [Google Scholar]
- 67.Donner DG, Elliott GE, Beck BR, Bulmer AC, Du Toit EF. Impact of diet-induced obesity and testosterone deficiency on the cardiovascular system: A novel rodent model representative of males with testosterone-deficient metabolic syndrome (TDMetS). PLOS ONE. 2015;10(9):e0138019 10.1371/journal.pone.0138019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haring R, Baumeister SE, Volzke H, Dorr M, Felix SB, Kroemer HK, et al. Prospective association of low total testosterone concentrations with an adverse lipid profile and increased incident dyslipidemia. Eur J Cardiovasc Prev Rehabil. 2011;18(1):86–96. 10.1097/HJR.0b013e32833c1a8d [DOI] [PubMed] [Google Scholar]
- 69.Tong PCY, Ho C-S, Yeung VTF, Ng MCY, So W-Y, Ozaki R, et al. Association of testosterone, insulin-like growth factor-I, and C-reactive protein with metabolic syndrome in Chinese middle-aged men with a family history of type 2 diabetes. J Clin Endocrinol Metab. 2005;90(12):6418–23. 10.1210/jc.2005-0228 [DOI] [PubMed] [Google Scholar]
- 70.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45. 10.1530/JOE-12-0455 [DOI] [PubMed] [Google Scholar]
- 71.Haffner SM, Mykkanen L, Valdez RA, Katz MS. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab. 1993;77(6):1610–5. 10.1210/jcem.77.6.8263149 [DOI] [PubMed] [Google Scholar]
- 72.Van Pottelbergh I, Braeckman L, De Bacquer D, De Backer G, Kaufman JM. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166(1):95–102. [DOI] [PubMed] [Google Scholar]
- 73.Somgird C, Sripiboon S, Mahasawangkul S, Boonprasert K, Brown JL, Stout TAE, et al. Differential testosterone response to GnRH-induced LH release before and after musth in adult Asian elephant (Elephas maximus) bulls. Theriogenology. 2016;85(7):1225–32. 10.1016/j.theriogenology.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 74.Thongtip N, Saikhun J, Mahasawangkul S, Kornkaewrat K, Pongsopavijitr P, Songsasen N, et al. Potential factors affecting semen quality in the Asian elephant (Elephas maximus). Reprod Biol Endocrinol: RB&E. 2008;6:9 10.1186/1477-7827-6-9 ; PMCID: PMCPMC2276508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown JL, Somerville M, Riddle HS, Keele M, Duer CK, Freeman EW. Comparative endocrinology of testicular, adrenal and thyroid function in captive Asian and African elephant bulls. Gen Comp Endocrinol. 2007;151(2):153–62. 10.1016/j.ygcen.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 76.Woo J, Cockram C, Lau E, Chan A, Swaminathan R. Influence of obesity on plasma fructosamine concentration. Clin Chem. 1992;38(11):2190–2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because of the sensitive nature of the results (e.g., subject to use by animal rights groups). Data are available from the Chiang Mai University Institutional Data Access / Ethics Committee (Assoc. Prof. Dr. Nattawooti Sthitmatee, e-mail: nattawooti.s@cmu.ac.th, drneaw@gmail.com) for researchers who meet the criteria for access to confidential data.