Abstract
microRNAs (miRNAs) are non-coding RNAs highly relevant as biomarkers for disease. A seminal study that explored the role of miRNAs in obstructive sleep apnea syndrome (OSA) demonstrated their usefulness in clinical management. Nevertheless, the miRNAs that may act as endogenous controls (ECs) have not yet been established. The identification of ECs would contribute to the standardization of these biomarkers in OSA. The objective of the study is to identify miRNAs that can be used as ECs in OSA. We evaluated 100 patients divided into two different cohorts: a learning cohort of 10 non-OSA and 30 OSA patients, and a validation cohort (20 non-OSA and 40 OSA patients). In the learning cohort, a profile of 188 miRNAs was determined in plasma by TaqMan Low Density Array. The best EC candidates were identified by mean center+SD normalization and concordance correlation restricted normalization. The results were validated using NormFinder and geNorm to assess the stability of those ECs. Eight miRNAs were identified as EC candidates. The combination miRNA-106a/miRNA-186 was identified as the most stable among all candidates. We identified a set of ECs to be used in the determination of circulating miRNA in OSA that may contribute to the homogeneity of results.
Introduction
Obstructive sleep apnea (OSA) is a prevalent disease that affects approximately 10% of adults [1–3]. It is caused by intermittent collapse of the upper airway during sleep, which leads to oxygen desaturation, arousals and intrathoracic pressure changes. OSA is associated with hypertension, cardiovascular and cerebrovascular diseases, diurnal somnolence and decreased quality of life [4].
Understanding the causes and consequences of OSA and identifying actions to perform personalized medicine are research priorities in the field [5,6]. One of the greatest challenges is identifying accurate and feasible biomarkers useful in diagnosis, prognosis and response to treatment. This challenge has stimulated an interest in novel biomarkers to improve OSA diagnosis and comprehensive management [7], through which micro-ribonucleic acids (miRNAs) have emerged as a new opportunity [8].
microRNAs (miRNAs) are small non-coding RNA molecules that represent an important class of regulatory epigenetic mechanisms [9]. miRNAs influence important cellular functions, such as development and differentiation, and play a pivotal role in many biological processes related to health and disease [10–12]. miRNAs can be rapidly released from tissue into circulation in several pathologies and exist in a high-stability, cell-free form in plasma [13]. Therefore, circulating miRNAs have great potential as non-invasive biomarkers for molecular diagnostics and prognostics and as therapeutic targets [14].
The role of miRNAs in OSA has been little studied. A seminal study demonstrated the utility of these molecules as biomarkers of adequate response to the treatment of OSA with continuous positive airway pressure (CPAP) [8]. However, prior to any analysis of circulating miRNAs, normalization is crucial due to the variability that occurs in plasma RNA isolation and analysis [14,15].
Normalization is a process aimed at differentiating biological variation from experimental artefacts. Normalization reduces the variability due to technical error and contributes to standardization and homogeneity in the analysis of miRNAs [16]. Currently, mean-center normalization is the most accurate method to normalize the results when a high number of miRNAs are being analysed [17]. However, a small number of miRNAs are often analysed, in which case mean-center normalization is not a valid method; instead, endogenous control (EC) normalization is the best option [14,17]. Therefore, suitable ECs (i.e., those that are disease- and tissue-specific, highly detected in all samples, and stable both within and between groups) are needed. The use of unsuitable normalizers can lead to misleading results [18–20].
To the best of our knowledge, there is no current consensus on EC miRNAs for quantitative polymerase chain reaction (qPCR) analysis of plasma miRNAs in OSA. Therefore, the aim of this study was to identify suitable ECs for normalization of circulating miRNAs in OSA studies with the objective of standardizing the analysis of these biomarkers in OSA.
Methods
Study cohort and sample collection
One hundred patients aged between 18 and 60 years and referred because of suspected OSA were enrolled at the Sleep Unit of Santa Maria Hospital (Lleida, Spain). OSA was diagnosed via a conventional polysomnographic sleep study. The results from all sleep studies were analysed by trained personnel, using standard criteria. Apnea was defined as an interruption or reduction of oronasal airflow ≥ 90% for at least 10 seconds. Hypopnea was defined as a 30–90% reduction in oronasal airflow for at least 10 seconds associated with an oxygen desaturation of at least 3% or an arousal on the electroencephalogram. The apnea-hypopnea index (AHI) was calculated based on the average number of apnea plus hypopnea episodes per hour of sleep. The cohort was split into two cohorts: i) TaqMan Low Density Array (TLDA) cohort: an initial sample of 40 male patients (10 patients without OSA and 30 patients with OSA) matched by age and body mass index (BMI). A general miRNA profile was performed. ii) Validation cohort: the remaining 60 patients (20 patients without OSA and 40 with OSA) were used to validate the candidate ECs (Fig 1). The initial exclusion criteria discarded patients with previous use of CPAP and any condition that, in the opinion of the responsible physician investigator, made the person unsuitable for the study (e.g., pregnancy, drug and alcohol consumption or less than one year of life expectancy). TLDA cohort patients were selected who had no other comorbidities apart from those typically associated with OSA (e.g., hypertension, dyslipidaemia, cardiovascular events). All recruited patients signed an informed consent form in accordance with the Helsinki Declaration of 1964 and the ethics committee of the centre (Clinical Research Ethics Committee (CEIC) of the Arnau de Vilanova University Hospital) approved the study. All methods were performed in accordance with current clinical practice guidelines and regulations. A venous fasting blood sample was obtained from each patient between 08.00 and 09.00 a.m. Plasma was obtained by standard venepuncture and centrifugation in EDTA-coated tubes (Vacuette, Greiner Bio-One, Kremsmünster, Austria). Plasma was separated via centrifugation at 1500 g for 10 min at 4°C. All specimens were immediately aliquoted, frozen, and stored in a dedicated -80°C freezer. No freeze-thaw cycles were performed during the experiment.
Fig 1. Flowchart of the study.
Patients who were referred because of suspected OSA were divided into the TLDA cohort (general profile of miRNAs) and the validation cohort and further divided for study on the basis of non-OSA and OSA. Ten non-OSA and 30 OSA patients were used as the TLDA cohort. miRNAs that do not follow the characteristics of a good EC were excluded. Two different strategies (mean-center+SD and CCR) were used in order to select candidate ECs. Stability analysis by GeNorm and NorFinder was performed in the TLDA cohort and in an independent validation cohort (20 non-OSA and 40 OSA patients).
Circulating RNA extraction and purification
RNA extraction was performed from 300 μL of plasma using a mirVana PARIS isolation kit (Applied Biosystems, Vilnius, Lithuania) according to the manufacturer’s instructions. Non-human cel-miR-39 was spiked into the plasma immediately before extraction. RNA concentration and integrity were examined by RT-qPCR quantification of cel-miR-39. RNU6 was selected as the plasma quality indicator because it is highly expressed in different cell types but not well detected in plasma. High detection of RNU6 in plasma indicates cellular contamination [19].
TaqMan low density array
A general profile with 188 miRNAs reported in previous experiments as the most prevalent in plasma were selected for profiling [21–23]. qRT-PCR was performed on the RNA of 40 patients using TaqMan Low Density Array (TLDA) human microRNA custom TLDAs (Life Technologies, Foster City, CA, USA). Briefly, a fixed volume of 3 μL of RNA solution from the 40 μL of RNA isolation eluate was used as the input for retrotranscription using a TaqMan MicroRNA Reverse Transcription Kit and the TaqMan MicroRNA Multiplex RT Assays, which are customised to run the TLDAs. Preamplification was performed using TaqMan PreAmp Master Mix and Megaplex PreAmp Primers for our custom selection. RT-PCR was carried out by means of an Applied Biosystems 7900HT thermocycler.
Candidate EC selection
ECs are miRNAs that are highly represented in all study samples and show similar abundances between groups. Two different approaches were used to choose the best candidates in OSA: i) the miRNA with the values most similar to the global mean expression [24], which was determined by normalizing the data set with the global mean to select the miRNA with the smallest standard deviation (mean-center+SD); and ii) concordance correlation restricted (CCR) normalization procedures, which use a concordance correlation coefficient to select miRNAs that are concordant with the global mean of the fully detected miRNAs [17]. Numerous strategies have been proposed to select the best EC from miRNA arrays [25]. The latest proposals indicate that the similarity between the values of an EC and the global mean (gold standard) is one of the best approximations. Based on these studies, we decided to select the five miRNAs with the lowest variability after normalization by the global mean [23] (mean center+SD) and the five with the highest concordance correlation coefficient of agreement with the global mean of the miRNAs detected in all samples [16] (CCR) as candidates for EC.
RT-PCR validation
Two-step validation was performed. First, the miRNAs selected as candidate ECs were validated internally to check the variability of determination when changing from an RT-PCR miRNA array to individual RT-PCR. Then, external validation of the EC candidates was performed with an external cohort using individual miRNA TaqMan hydrolysis probes.
Candidate EC stability analysis
To determine the miRNAs with the best properties to be ECs, we estimated the stability of the candidate ECs. Stability of each candidate EC was performed using the geNorm [26] and NormFinder [27] algorithms. The ranking resulting from each method and its concordance were studied. Furthermore, we evaluated the best combination with the NormFinder algorithm based on the pair that reduced variability the most.
All the analyses were performed using R-project version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
The 100 patients were middle-aged (less than 60 years old), overweight-obese and mainly males. The clinical and demographic characteristics of the patients are shown in Table 1.
Table 1. Baseline characteristics of the patients.
| TLDA cohort | Validation cohort | ||||||
|---|---|---|---|---|---|---|---|
| Non-OSA (AHI<15 events/h) | OSA (AHI≥15 events/h) | p-value | Non-OSA (AHI<15 events/h) | OSA (AHI≥15 events/h) | p-value | ||
| N = 10 | N = 30 | N = 20 | N = 40 | ||||
| Gender: Male n (%) | 10 (100%) | 30 (100%) | 12 (60.0%) | 30 (75.0%) | 0.37 | ||
| Age (years), median [IQR] | 48.5 [41.8;55.5] | 52.0 [44.2;55.8] | 0.743 | 46.0 [41.5;50.2] | 51.0 [43.8;55.2] | 0.033 | |
| BMI (kg/m2), median [IQR] | 26.1 [23.3;26.9] | 27.1 [25.9;30.0] | 0.089 | 29.8 [26.2;36.3] | 33.1 [28.7;36.0] | 0.279 | |
| Hip perimeter (cm), mean (SD) | 94.6 (10.8) | 101 (8.44) | 0.104 | 106 (15.5) | 109 (13.1) | 0.391 | |
| Waist perimeter (cm), median [IQR] | 102 [98.0;104] | 104 [100;108] | 0.301 | 111 [102;118] | 111 [106;118] | 0.849 | |
| Physical activity n (%) | 0.385 | 0.356 | |||||
| Sedentary | 4 (40%) | 15 (50%) | 9 (45.0%) | 17 (43.6%) | |||
| Moderate | 3 (30%) | 12 (40%) | 6 (30.0%) | 17 (43.6%) | |||
| Active | 3 (30%) | 3 (10%) | 5 (25.0%) | 5 (12.8%) | |||
| AHI (events/h), median [IQR] | 8.45 [6.18;10.8] | 32.3 [27.7;49.6] | <0.001 | 7.52 [4.99;9.01] | 38.9 [24.8;70.0] | <0.001 | |
| TSat90 (%), median [IQR] | 0.08 [0.00;0.14] | 2.34 [0.31;6.90] | <0.001 | 0.12 [0.00;0.32] | 4.23 [1.73;18.8] | <0.001 | |
| TSat90 (%), median [IQR] | 0.08 [0.00;0.14] | 2.34 [0.31;6.90] | <0.001 | 0.12 [0.00;0.32] | 4.23 [1.73;18.8] | <0.001 | |
IQR: interquartile range; SD: standard deviation; BMI: body mass index; AHI: apnea-hypopnea index (number of events·h-1); TSat90: percentage of time spent with oxygen saturation less than 90%.
Selection of the most suitable EC candidates by RT-PCR miRNA array
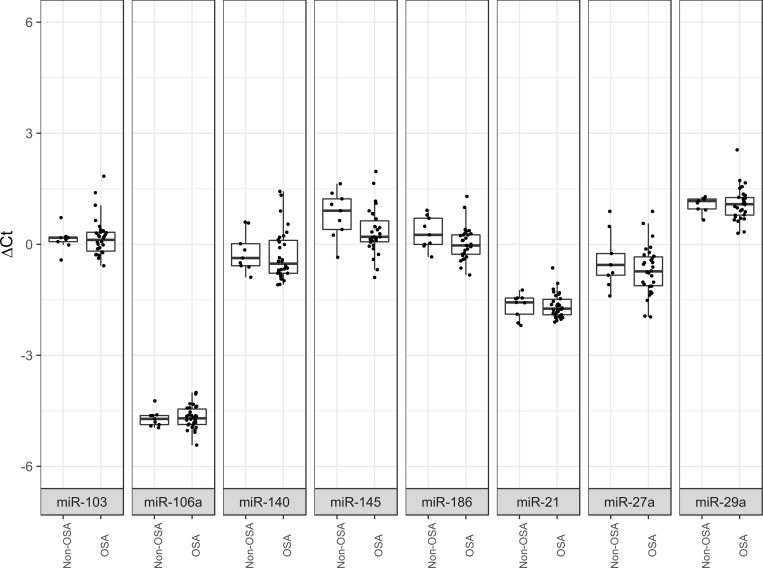
Among the general profiles, 8 candidate ECs were selected based on the two different methods (Table 2). The eight EC candidates exhibited the desirable characteristics of a good normalizer in that they were highly detected in all samples and were not significantly different between the OSA and non-OSA patients (Fig 2).
Table 2. Candidate ECs.
| MiRNA Name | Molecule type | Accesion Number* | Mature Sequence | Selection method |
|---|---|---|---|---|
| hsa-miR-103a-3p | miRNA | MIMAT0000101 | AGCAGCAUUGUACAGGGCUAUGA | CCR and MC+SD |
| hsa-miR-106a-5p | miRNA | MIMAT0000103 | AAAAGUGCUUACAGUGCAGGUAG | MC+SD |
| hsa-miR-140-3p | miRNA | MIMAT0004597 | UACCACAGGGUAGAACCACGG | CCR |
| hsa-miR-145-5p | miRNA | MIMAT0000437 | GUCCAGUUUUCCCAGGAAUCCCU | CCR |
| hsa-miR-186-5p | miRNA | MIMAT0000456 | CAAAGAAUUCUCCUUUUGGGCU | CCR and MC+SD |
| hsa-miR-21-5p | miRNA | MIMAT0000076 | UAGCUUAUCAGACUGAUGUUGA | MC+SD |
| hsa-miR-27a-3p | miRNA | MIMAT0000084 | UUCACAGUGGCUAAGUUCCGC | CRR |
| hsa-miR-29a-3p | miRNA | MIMAT0000086 | UAGCACCAUCUGAAAUCGGUUA | MC+SD |
| cel-miR-39-3p | miRNA | MIMAT0000010 | UCACCGGGUGUAAAUCAGCUUG | Spike-in control |
| RNU6 | snoRNA | NR_004394** | GTGCTCGCTTCGGCAGCACATATACTAAAATTGGAACGATACAGAGAAGATTAGCATGGCCCCTGCGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTT | Plasma quality indicator |
*mirBase database accession number
**NCBI Gene ID
Fig 2. Boxplot comparing OSA and control Ct values.
The eight miRNAs were highly detected, and non-significant differences were found between groups.
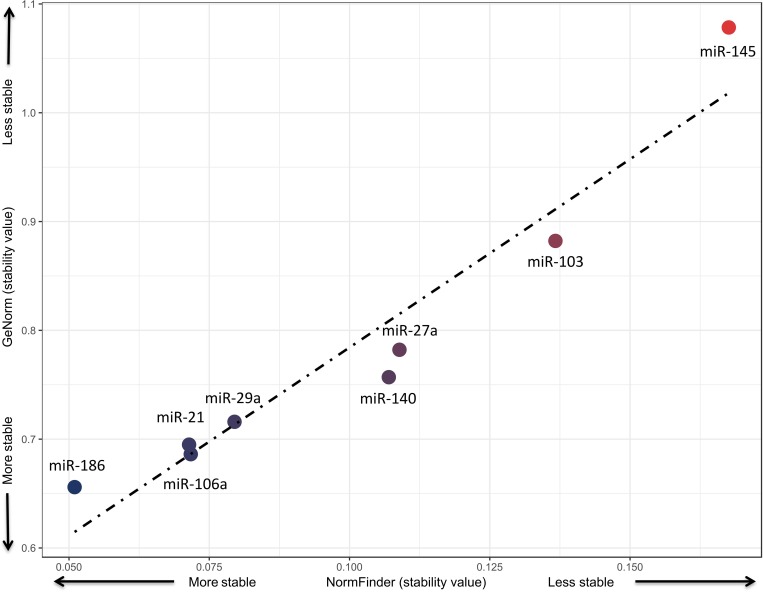
To identify the most stable candidate ECs, the GeNorm and NormFinder algorithms were applied. Both methods reported miR-106a and miR-29a as the most stable. In contrast, miR-27a and miR-145 were the most variable (Table 3). Moreover, NormFinder identified miR-106a and miR-186 as the combination that best reduced the variability among profiles. To verify the ECs’ stability in other OSA classifications, patients were also divided into non-OSA, moderate OSA and severe OSA groups. A similar analysis was performed, and miR-106a/miR-186 remained the most stable pair, followed by miR-29a and miR21 (data not shown).
Table 3. Stability ranking of ECs.
| TaqMan Low Density Array | External validation | |||||||
|---|---|---|---|---|---|---|---|---|
| GeNorm | NormFinder | GeNorm | NormFinder | |||||
| Ranking | Stability value* | Ranking | Stability value | Ranking | Stability value | Ranking | Stability value | |
| 1 | miR-106a | 0.500 | miR-106a | 0.108 | miR-186 | 0.656 | miR-186 | 0.051 |
| 2 | miR-29a | 0.590 | miR-29a | 0.121 | miR-106a | 0.686 | miR-21 | 0.071 |
| 3 | miR-103 | 0.600 | miR-186 | 0.126 | miR-21 | 0.695 | miR-106a | 0.072 |
| 4 | miR-186 | 0.602 | miR-21 | 0.127 | miR-29a | 0.716 | miR-29a | 0.080 |
| 5 | miR-21 | 0.611 | miR-140 | 0.139 | miR-140 | 0.757 | miR-140 | 0.107 |
| 6 | miR-140 | 0.704 | miR-103 | 0.144 | miR-27a | 0.782 | miR-27a | 0.109 |
| 7 | miR-27a | 0.734 | miR-145 | 0.180 | miR-103 | 0.882 | miR-103 | 0.137 |
| 8 | miR-145 | 0.751 | miR-27a | 0.187 | miR-145 | 1.078 | miR-145 | 0.168 |
| Best combination | miR-106a and miR-186 | miR-106a and miR-186 | ||||||
*Stability values are not comparable between methods.
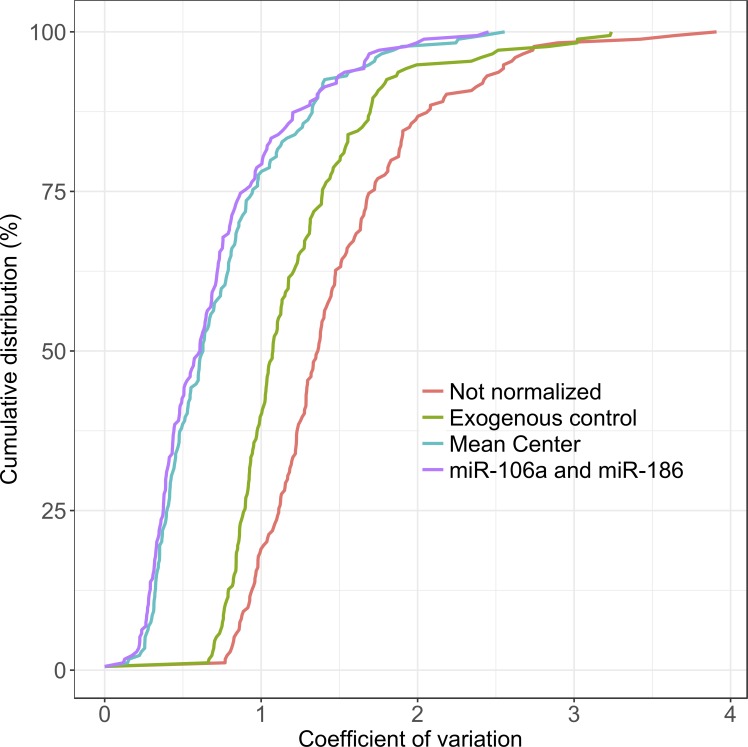
Different strategies of normalization (mean-center, exogenous control and EC selection) were evaluated by comparing their reduction of variability (Fig 3). The variability reduction obtained with mean center (gold standard) was also achieved with EC selection. Exogenous control showed less capacity to reduce variability. A cumulative distribution plot of the coefficient of variation of all miRNA Ct values was examined before and after normalization, confirming the suitability of the ECs as normalizers.
Fig 3. Cumulative distribution plot of the different normalization strategies.
Internal validation by individual probes
The variability between miRNA arrays and individual probes was evaluated by internal validation in the TLDA cohort. Then, the GeNorm and NormFinder algorithms were used to rank the EC candidates. Similar results were obtained from the TLDA and individual probes (data not shown).
Confirmation of ECs by individual probes in an external cohort
Low Ct values of EC candidates were observed in all patients. Furthermore, when comparing differences between groups, none of the miRNAs showed significant differences. The GeNorm and NormFinder algorithms reported miR-186, miR-106a, miR-21 and miR-29a as the miRNAs with the highest stability (see Table 3 and Fig 4). In contrast, miR-145, miR-103 and miR-27a showed the lowest stability (see Table 3 and Fig 4). Both methods showed high concordance with respect to their stability values. The results showed that miR-186 is the best EC, followed by miR-106a, miR-21 and miR-29, which have similar values of stability for both methods (see Fig 4).
Fig 4. Correlation plot of stability values from NormFinder and GeNorm.
Discussion
miRNAs are new, reliable, non-invasive biomarkers. Because they play a major role in the development and prognosis of different diseases, their use could go beyond being a simple biomarker. Their utility has been demonstrated in the management of several diseases [28] as well as in the personalized management of OSA [8]. Plasma miRNAs could help in the identification of new OSA phenotypes for personalized management. In the present study, we identified a set of miRNAs that act as ECs for the use of these biomarkers in sleep apnea.
The use of circulating miRNAs represents an advance in the field of minimally invasive biomarkers [29]. Due to their simple and rapid quantification, hundreds of miRNAs can be evaluated simultaneously in several patients, making them one of the most important tools in precision medicine [30].
There are several methods for quantifying miRNAs [31]. Among them, RT-PCR is currently the most commonly available and reliable method. In a couple of hours, this technique allows the levels of miRNAs to be evaluated to perform relative quantification analysis [31,32]. The main problem that currently exists in the relative quantification of miRNAs is technical variability. Currently, there is not a universal method that eliminates such variability. Different techniques have been used, such as spike-in normalization, gene normalization, and small nucleolar RNA normalization, leading to non-replicable results [14,33]. The gold standard—normalization—is based on mean-center methods and is very useful when high-throughput miRNA approximations are being analysed, but it is not an option for the analysis of a few miRNAs. In such cases, the use of ECs is the best option [17,24]. ECs are specific and should be identified for each disease and tissue. The identification and validation of specific suitable ECs is perhaps the most critical step in the analysis of miRNAs to avoid inaccurate interpretation of the data that may lead to biased results. An ideal EC gene will be detected at a constant level across all samples, exhibit relatively stable levels between samples and groups and have no known association with the disease under investigation [16,34]. To the best of our knowledge, this is the first study that aims to identify and validate suitable ECs in OSA.
In the present study, we performed different bioinformatics analyses to determine the most reliable ECs among OSA patients. We evaluated 188 miRNAs and selected eight EC candidates (miR-106a, miR-186, miR-29a, miR-21, miR-103, miR-27a, miR-140 and miR-145), one spike-in synthetic miRNA (cel-miR-39) and one commonly use normalizer (RNU6). Although RNU6 and cel-miR-39 are commonly used in the normalization of miRNAs [14], we used them only as quality indicators. RNU6 is not highly detected in plasma; low Ct values are obtained when haemolyses occurs. Cel-miR-39 has been used only to assess the efficiency of the process, which requires repeating all samples with low efficiency.
The lack of a comprehensive analysis of normalizers for miRNAs in OSA patients could compromise miRNA results. For this reason, we performed miRNA array screening including 188 miRNAs as potential ECs. The data were analysed using mean-center normalization methods to identify the best candidates to serve as ECs. The best normalization strategy for miRNA analysis in OSA patients was identified as the combined use of miR-186 and miR-106a to normalize RT-PCR results. The combined use of 2 or more miRNAs leads to more reliable results than using either one alone. miR-29a and miR-21 are also suitable as ECs for miRNA analysis in OSA.
Different studies previously used miR-186 [35], miR-106a [14,36,37], miR-29a [38] and miR-21 [39] as ECs in other diseases.
The present study has several limitations that deserve comment. First, only patients between 18 and 60 years old have been studied, and larger studies should be performed to determine the validity of these ECs in patients of other ages. Although a large quantity (188) of miRNAs have been studied, not all of them have been analysed; it is possible that other miRNAs would be better ECs, but this does not invalidate the results, because the stability (when compared with other diseases) is sufficiently high. No other comorbidities apart from those typically associated with OSA (e.g., cardiovascular disease, diabetes, and obesity) have been included in the analysis. However, the use of TLDA did reduce the technical variability of the process, in turn providing reliable results. Despite a limited number of non-OSA patients being included in the learning phase (10 non-OSA subjects in the TLDA cohort), the results were validated in a large patient cohort, and the stability values were assessed using two different methods.
Conclusions
The present study represents the first step in the standardization of the analysis of miRNAs as biomarkers in OSA. In the era of precision medicine, miRNA analysis could open a new field in the search for biomarkers of clinical utility in OSA. These biomarkers could offer insight into the physiopathology of the disease and could have diagnostic and prognostic utility in OSA. The results of this study identified a set of miRNAs that could be used as ECs for standard normalization of miRNA profiling in OSA.
Supporting information
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
CI: Confidence Interval; IQR: Interquartile Range.
(DOC)
Acknowledgments
We thank all the investigators of Sleep Unit of Santa Maria Hospital.
Data Availability
All relevant data are within the manuscript and Supporting Information files.
Funding Statement
The project is supported by PI 14/01266 and PI 18/00449 from the Instituto de Salud Carlos III (ISCIII), Fondo Europeo de Desarrollo Regional (FEDER), Sociedad Española de Neumología y Cirugía Torácica (SEPAR) and Societat Catalana de Pneumologia (SOCAP). The work is supported by IRBLleida Biobank (B.0000682) and PLATAFORMA BIOBANCOS PT17/0015/0027”. We thank all the investigators of Sleep Unit of Santa Maria Hospital. F.S.-M. is the recipient of a predoctoral fellowship from “AGAUR-University of Lleida” and Convocatòria d’Ajuts 2018 de Promoció a la Recerca en Salut. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163: 685–9. 10.1164/ajrccm.163.3.2005065 [DOI] [PubMed] [Google Scholar]
- 2.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3: 310–318. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am J Epidemiol. 2013;177: 1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1: 61–72. 10.1016/S2213-2600(12)70051-6 [DOI] [PubMed] [Google Scholar]
- 5.Pack AI. Application of Personalized, Predictive, Preventative, and Participatory (P4) Medicine to Obstructive Sleep Apnea. A Roadmap for Improving Care? Ann Am Thorac Soc. 2016;13: 1456–1467. 10.1513/AnnalsATS.201604-235PS [DOI] [PubMed] [Google Scholar]
- 6.Torres G, Sánchez-de-la-Torre M, Barbé F. Medicina de precisión: un viaje a Ítaca. Arch Bronconeumol. 2017;52: 455–456. 10.1016/j.arbres.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-de-la-Torre M, Gozal D. Obstructive sleep apnea: in search of precision. Expert Rev Precis Med Drug Dev. Taylor & Francis; 2017;2: 217–228. 10.1080/23808993.2017.1361319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-de-la-Torre M, Khalyfa A, Sánchez-de-la-Torre A, Martinez-Alonso M, Martinez-García MÁ, Barceló A, et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea. J Am Coll Cardiol. 2015;66: 1023–1032. 10.1016/j.jacc.2015.06.1315 [DOI] [PubMed] [Google Scholar]
- 9.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117: 2369–76. 10.1172/JCI33099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008;79: 562–570. 10.1093/cvr/cvn137 [DOI] [PubMed] [Google Scholar]
- 11.Zhang C. MicroRNAs: role in cardiovascular biology and disease. Clin Sci. 2008;114: 699–706. 10.1042/CS20070211 [DOI] [PubMed] [Google Scholar]
- 12.Latronico MVG, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6: 418–429. 10.1038/nrcardio.2009.56 [DOI] [PubMed] [Google Scholar]
- 13.Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem Sci. Elsevier Ltd; 2012;37: 460–465. 10.1016/j.tibs.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 14.Schwarzenbach H, Da Silva AM, Calin G, Pantel K. Data normalization strategies for microRNA quantification. Clin Chem. 2015;61: 1333–1342. 10.1373/clinchem.2015.239459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morata-Tarifa C, Picon-Ruiz M, Griñan-Lison C, Boulaiz H, Perán M, Garcia MA, et al. Validation of suitable normalizers for miR expression patterns analysis covering tumour heterogeneity. Sci Rep. 2017;7: 39782 10.1038/srep39782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott AM, Kerin MJ, Miller N. Identification and Validation of miRNAs as Endogenous Controls for RQ-PCR in Blood Specimens for Breast Cancer Studies. Samant R, editor. PLoS One. 2013;8: e83718 10.1371/journal.pone.0083718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wylie D, Shelton J, Choudhary A, Adai AT. A novel mean-centering method for normalizing microRNA expression from high-throughput RT-qPCR data. BMC Res Notes. 2011;4: 555 10.1186/1756-0500-4-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Tang N, Hui T, Wang S, Zeng X, Li H, et al. Identification of endogenous reference genes for RT-qPCR analysis of plasma microRNAs levels in rats with acetaminophen-induced hepatotoxicity. J Appl Toxicol. 2013;33: 1330–1336. 10.1002/jat.2864 [DOI] [PubMed] [Google Scholar]
- 19.Solayman MHM, Langaee T, Patel A, El-Wakeel L, El-Hamamsy M, Badary O, et al. Identification of Suitable Endogenous Normalizers for qRT-PCR Analysis of Plasma microRNA Expression in Essential Hypertension. Mol Biotechnol. 2016;58: 179–187. 10.1007/s12033-015-9912-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres A, Torres K, Wdowiak P, Paszkowski T, Maciejewski R. Selection and validation of endogenous controls for microRNA expression studies in endometrioid endometrial cancer tissues. Gynecol Oncol. 2013;130: 588–594. 10.1016/j.ygyno.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 21.Ortega FJ, Mercader JM, Catalan V, Moreno-Navarrete JM, Pueyo N, Sabater M, et al. Targeting the Circulating MicroRNA Signature of Obesity. Clin Chem. 2013;59: 781–792. 10.1373/clinchem.2012.195776 [DOI] [PubMed] [Google Scholar]
- 22.Ortega FJ, Mercader JM, Moreno-Navarrete JM, Nonell L, Puigdecanet E, Rodriquez-Hermosa JI, et al. Surgery-Induced Weight Loss Is Associated With the Downregulation of Genes Targeted by MicroRNAs in Adipose Tissue. J Clin Endocrinol Metab. 2015;100: E1467–E1476. 10.1210/jc.2015-2357 [DOI] [PubMed] [Google Scholar]
- 23.Latorre J, Moreno-Navarrete JM, Mercader JM, Sabater M, Rovira Ò, Gironès J, et al. Decreased lipid metabolism but increased FA biosynthesis are coupled with changes in liver microRNAs in obese subjects with NAFLD. Int J Obes. 2017;41: 620–630. 10.1038/ijo.2017.21 [DOI] [PubMed] [Google Scholar]
- 24.Mestdagh P, Van Vlierberghe P, De Weer A, Muth D, Westermann F, Speleman F, et al. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10: R64 10.1186/gb-2009-10-6-r64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandesompele Jo; Kubista M; Pfaffl MW. Reference gene validation software for improved normalization In: Logan Julie; Edwards Kirstin; Saunders N, editor. Real-Time PCR: Current Technology and Applications. Caister Academic Press, U.K; 2009. pp. 1–27. [Google Scholar]
- 26.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64: 5245–50. 10.1158/0008-5472.CAN-04-0496 [DOI] [PubMed] [Google Scholar]
- 28.Anfossi S, Babayan A, Pantel K, Calin GA. Clinical utility of circulating non-coding RNAs—an update. Nat Rev Clin Oncol. 2018; 10.1038/s41571-018-0035-x [DOI] [PubMed] [Google Scholar]
- 29.Brase JC, Wuttig D, Kuner R, Sültmann H. Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer. 2010;9: 1–9. 10.1186/1476-4598-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18: 997–1006. 10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 31.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13: 358–369. 10.1038/nrg3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 2010;50: 298–301. 10.1016/j.ymeth.2010.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin Chem. 2009;55: 3605–3613. [DOI] [PubMed] [Google Scholar]
- 34.Qi R, Weiland M, Gao X-H, Zhou L, Mi Q-S. Identification of endogenous normalizers for serum MicroRNAs by microarray profiling: U6 small nuclear RNA is not a reliable normalizer. Hepatology. 2012;55: 1640–1640. 10.1002/hep.24533 [DOI] [PubMed] [Google Scholar]
- 35.Holm A, Bang-Berthelsen CH, Knudsen S, Kornum BR, Modvig S, Jennum P, et al. miRNA Profiles in Plasma from Patients with Sleep Disorders Reveal Dysregulation of miRNAs in Narcolepsy and Other Central Hypersomnias. Sleep. American Academy of Sleep Medicine, Darien, IL; 2014;37: 1525–1533. 10.5665/sleep.4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders I, Holdenrieder S, Walgenbach-Brünagel G, von Ruecker A, Kristiansen G, Müller SC, et al. Evaluation of reference genes for the analysis of serum miRNA in patients with prostate cancer, bladder cancer and renal cell carcinoma. Int J Urol. 2012;19: 1017–1025. 10.1111/j.1442-2042.2012.03082.x [DOI] [PubMed] [Google Scholar]
- 37.Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, et al. Profiling of Circulating MicroRNAs Reveals Common MicroRNAs Linked to Type 2 Diabetes That Change With Insulin Sensitization. Diabetes Care. 2014;37: 1375–1383. 10.2337/dc13-1847 [DOI] [PubMed] [Google Scholar]
- 38.Suárez-Arriaga M-C, Torres J, Camorlinga-Ponce M, Gómez-Delgado A, Piña-Sánchez P, Valdez-Salazar H-A, et al. A proposed method for the relative quantification of levels of circulating microRNAs in the plasma of gastric cancer patients. Oncol Lett. 2017;13: 3109–3117. 10.3892/ol.2017.5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solayman MHM, Langaee T, Patel A, El-Wakeel L, El-Hamamsy M, Badary O, et al. Identification of Suitable Endogenous Normalizers for qRT-PCR Analysis of Plasma microRNA Expression in Essential Hypertension. Mol Biotechnol. Springer US; 2016;58: 179–187. 10.1007/s12033-015-9912-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
CI: Confidence Interval; IQR: Interquartile Range.
(DOC)
Data Availability Statement
All relevant data are within the manuscript and Supporting Information files.