Abstract
While both pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine and TA-HPV recombinant vaccinia viral vector-based vaccines have elicited HPV-specific CD8+ T cell responses in HPV16/E7-expressing tumor models, and been used as prime-boost regimen to enhance HPV-specific immune responses in humans (NCT00788164), the optimal route of administration for TA-HPV remains unclear. In a preclinical model, we examined the immunogenicity of priming with intramuscular pNGVL4a-Sig/E7(detox)/HSP70 followed by TA-HPV boost through different administration routes. We observed that priming twice with a pNGVL4a-Sig/E7(detox)/HSP70 followed by a single TA-HPV immunization boost through skin scarification generated the strongest antigen-specific CD8+ T cell response in C57BL/6 mice. These data translate to tumor control and prolonged survival of treated mice. Our results provide rationale for future clinical testing of intramuscular pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine prime, TA-HPV vaccine skin scarification boost immunization regimen for the control of HPV-associated diseases.
Keywords: Human papillomavirus, Therapeutic HPV vaccine, pNGVL4a-Sig/E7(detox)/HSP70, TA-HPV, Pre-clinical model
1. Introduction
The prevalence of human papillomavirus (HPV) infections and HPV-associated diseases has remained a global burden, even in an age where preventative HPV vaccines are proven effective (Gupta et al., 2017; Yang et al., 2016a). Not only is HPV a known biologic carcinogen for several cancers including penile, vaginal, anal, vulva, and oropharyngeal (Forman et al., 2012; Maxwell et al., 2016; Mehanna et al., 2013; Wakeham and Kavanagh, 2014), it is also recognized as an etiological factor for many other diseases (Forman et al., 2012; Wakeham and Kavanagh, 2014). Notably, HPV is responsible for causing nearly all cervical cancer cases worldwide, which remains the fourth deadliest female cancer (Wakeham and Kavanagh, 2014). Due to the adverse health effects associated with HPV infections, the need for treatment options for patients with established HPV infections and HPV-associated diseases is imperative.
HPV is a circular, double-stranded DNA virus belonging to the Papillomaviridae family (Lee et al., 2016). Uncleared HPV infection can progress into persistent infection, which may further develop into precancerous lesions or cancer, or regress at any stage of the transformation process (Ghittoni et al., 2015; Ostor, 1993). HPV oncoproteins E6 and E7 are believed to assist in the carcinogenesis of HPV-associated lesions by inhibiting the function of tumor suppressive proteins p53 and pRb (Doorbar, 2016; zur Hausen, 2002) and are therefore, required for the initiation and upkeep of HPV-associated malignancies (Doorbar, 2016). E6/E7 can circumvent immune tolerance against self-antigens because they are foreign proteins constitutively-expressed in transformed cells (Ma et al., 2012). For these reasons, E6/E7 have risen to the forefront of therapeutic HPV treatment strategies as ideal therapeutic HPV vaccine targets.
One promising treatment method for existing HPV infections and HPV-associated diseases are therapeutic HPV DNA vaccines (for review see Yang et al., 2016a). They have been widely studied in preclinical and clinical trials for the treatment of HPV-associated diseases (Bagarazzi et al., 2012; Kim et al., 2014; Maldonado et al., 2014). Therapeutic HPV DNA vaccines encode E6 and/or E7 antigens into the plasmid DNA, which are then introduced into the host cells upon vaccination. DNA vaccines do not lead to neutralizing antibodies against the DNA plasmids and can therefore be repeatedly administered (Ma et al., 2012); however, they have low intrinsic immunogenicity. To overcome this and enhance the therapeutic efficacy of DNA vaccines, strategies have been developed to strengthen the immune responses generated by DNA vaccination, including boosting the immune system with a heterologous therapeutic HPV vaccine (for review see Yang et al., 2016b).
Vaccinia virus is a viral vector commonly used to deliver E6/E7 antigens for therapeutic HPV vaccination (Hsieh et al., 2004) because it is extremely infectious and has a low probability of irregular DNA integration into the host’s genome (Borysiewicz et al., 1996). TA-HPV is a live recombinant vaccinia virus expressing HPV16/18 E6/E7 proteins. TA-HPV was first used in a 2006 clinical trial in eight patients with late stage cervical cancer (Borysiewicz et al., 1996). TA-HPV has since been frequently used alone or in a prime boost regimen to enhance immune responses of several heterologous vaccines, including TA-CIN (Davidson et al., 2004; Smyth et al., 2004) and pNGVL4a-Sig/E7(detox)/HSP70 (Maldonado et al., 2014).
Both DNA vaccines and TA-HPV are often administered via intramuscular (IM) injection. Although this is a common administration route, it is still unclear what route of administration produces the most robust immune response. Previous data have shown that administration of vaccinia virus-based vaccines through skin scarification (SS) can induce potent immune responses (Liu et al., 2010; Rice et al., 2014). In this study, we examined the therapeutic efficacy of heterologous pNGVL4a-Sig/E7(detox)/HSP70 DNA prime, TA-HPV vaccinia virus boost vaccination regimen in preclinical TC-1 tumor model. We also explored the optimal route of vaccinia virus vaccination to elicit a desired potent antitumor immune response.
2. Materials and methods
2.1. Mice
5–8-week old female C57BL/6 mice were purchased from Charles River Laboratories (Frederick, MD). All mice were maintained at the Johns Hopkins University School of Medicine Oncology Animal Facility (Baltimore, MD) under specific-pathogen free conditions. All procedures were performed according to Johns Hopkins Institutional Animal Care and Use Committee and in accordance with recommendations for proper use and care of laboratory animals.
2.2. Peptides, antibodies and reagents
HPV16/E7aa49–57 peptide, RAHYNIVTF and HPV18/E6aa67–75 peptide, KCIDFYSRI, were synthesized by GenScript (Piscataway, NJ) at a purity of ≥ 80%. FITC and PE-conjugated anti-mouse CD8a (clone 53.6.7), and FITC-conjugated anti-mouse IFN-γ (clone XMG1.2) antibodies, purified anti-mouse CD16/32 (Fc Block™), and 7-Amino-Actinomycin D (7-AAD) were purchased from BD Pharmingen (BD Pharmingen, San Diego, CA). Purified anti-HPV16/E7 monoclonal antibody (clone 8C9) was purchased from Thermo Scientific (Rockford, IL). PE-conjugated, HPV16/E7aa49–57 peptide loaded H2-Db tetramers were obtained from the National Institute of Allergy and Infectious Diseases Tetramer Facility (Atlanta, GA). G 418 disulfate salt was purchased from Sigma-Aldrich (St. Louis, MO). Purified HPV16 E7 protein was purchased from Protein X Lab (San Diego, CA). Bifurcated needles were purchased from Precision Medical Products, INC (Denver, PA).
2.3. Cell line
TC-1/luc cells expressing the HPV16-E6/E7 proteins and firefly luciferase were developed in our laboratory and have been described previously (Huang et al., 2007). The cells were maintained in RPMI medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, 400 μg/mL of G418, and 10% fetal bovine serum (FBS).
2.4. Vaccine and vaccination
The generation of pNGVL4a-Sig/E7(detox)/HSP70 (Trimble et al., 2003), pcDNA3-CRT, and pcDNA3-CRT/HPV16/E7 (Cheng et al., 2001a) DNA vaccines have been described previously. To generate pcDNA3-CRT/HPV18/E6 DNA vaccine, HPV18/E6 DNA was synthesized by GenScript and cloned into EcoRI/HindIII of pcDNA3-CRT. A recombinant vaccinia virus expressing HPV16/18-E6/E7, TA-HPV, has been described previously (Borysiewicz et al., 1996). The generation of vaccinia virus expressing luciferase (Lister strain, rVV4) (Chang et al., 2009) and wild-type vaccinia virus (WR strain) (Wu et al., 1995) has also been described previously. For DNA vaccination, 25 μg of designated DNA was prepared in 50 μL using endotoxin-free kit (Qiagen) and injected intramuscularly into biceps femoris muscle. For TA-HPV intramuscular vaccination, mice were injected with TA-HPV virus (50 μL) intramuscularly (biceps femoris muscle). For virus skin scarification, mice were anesthetized and 5 μL of virus at designated dose was applied to tail skin 1 cm from the base of the tail or on the ear. The skin area was then gently scratched 15 times with a bifurcated needle.
2.5. Tetramer staining
For tetramer staining, mice PBMCs were stained with purified anti-mouse CD16/32 first, and then stained with anti-mouse CD8-FITC, and PE-conjugated H-2Db tetramer loaded with HPV16/E7aa49–57 peptide at 4 °C. After washing, the cells were stained with 7-AAD before flow cytometry analysis to exclude dead cells. The cells were acquired with FACSCalibur flow cytometer and analyzed with CellQuest Pro software.
2.6. Intracellular cytokine staining and flow cytometry analysis
To detect HPV16/E7- and HPV18/E6-specific CD8+ T cell responses by IFN-γ intracellular staining, splenocytes were stimulated with either HPV16/E7aa49–57 or HPV18/E6aa67–75 peptide (1 μg/mL) in the presence of GolgiPlug (BD Pharmingen, San Diego, CA) at 37 °C overnight. The stimulated splenocytes were then washed once with PBS containing 0.5% BSA and stained with PE-conjugated anti-mouse CD8 antibody. Cells were permeabilized and fixed with Cytofix/Cytoperm kit according to the manufacturer’s instruction (BD Pharmingen, San Diego, CA). Intracellular IFN-γ was stained with FITC-conjugated rat antimouse IFN-γ. Flow cytometry analysis was performed using FACSCalibur flow cytometer with CellQuest Pro software (BD biosciences, Mountain View, CA).
2.7. ELISA
HPV16 E7-specific antibody response was detected through enzyme-linked immunoabsorbent assays (ELISA) as described previously (Cheng et al., 2001b). Optical density (OD) value was read with xMark Microplate Spectrophotometer (BioRad, Hercules, CA) ELISA reader at 405 nm.
2.8. In vivo bioluminescence imaging of luciferase expression by either vaccinia virus or TC-1/luciferase tumor cells
The expression of luciferase by inoculated vaccinia virus or TC-1/luciferase tumor cells was monitored by bioluminescence using a Xenogen imaging system. Mice were given D-Luciferin by intraperitoneally (i.p.) injection (200 μL/mouse, 75 mg/kg) and anesthetized with isoflurane. In vivo bioluminescence imaging for luciferase expression was conducted on a cryogenically cooled IVIS system using Living Image acquisition and analysis software (Xenogen). Mice were placed onto the warmed stage inside the light-tight camera box with continuous exposure to 1–2% isoflurane. Images were acquired 10 min after D-luciferin administration and imaged for appropriate time. The levels of light from the bioluminescent cells were detected by the IVIS imager, integrated, and digitized. The region of interest from displayed images was designated and quantified as total photon counts using Living Image 2.50 software (Xenogen).
2.9. In vivo tumor protection experiment
For the in vivo tumor protection experiment, female C57BL/6 mice (five/group) were vaccinated with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 DNA (50 μL) through intramuscular injection. The mice were boosted with the same regimen once with 2-week intervals. Two weeks later, one group of pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccinated and one group of naïve mice were vaccinated with 5 × 105 pfu of TA-HPV vaccinia virus through skin scarification on the tail. Two weeks (4 weeks after the last DNA vaccination) after TA-HPV vaccination, mice were injected with 1 × 105 of TC-1/luciferase tumor cells intravaginally. The growth of the tumor was monitored by the expression of luciferase with bioluminescence using a Xenogen imaging system.
2.10. In vivo tumor treatment experiment
For the in vivo tumor treatment experiment, female C57BL/6 mice (five/group) were injected with 2 × 104 of TC-1/luciferase tumor cells intravaginally. One day after tumor cell injection, one group of TC-1/luciferase tumor cell challenged mice was vaccinated with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 DNA (50 μL) through intramuscular injection at hind leg muscle and boosted twice with the same regimen with 4-day interval. Another group of TC-1/luciferase tumor cell challenged mice was vaccinated with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 DNA (50 μL) through intramuscular injection in hind leg muscle and boosted once with the same regimen and further boosted with 5 × 105 pfu of TA-HPV vaccinia virus through skin scarification on the tail at 4-day intervals. Another group of TC-1/luciferase tumor cell challenged mice was vaccinated with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 DNA (50 μL) through intramuscular injection in front leg muscle and boosted once with the same regimen and further boosted with 5 × 105 pfu of TA-HPV vaccinia virus through skin scarification on the ear at 4-day intervals. One group of TC-1/luciferase tumor cell challenged mice was vaccinated with 5 × 105 pfu of TA-HPV vaccinia virus through skin scarification on the tail 9 days after tumor cell injection. The growth of the tumor was monitored by the expression of luciferase with bioluminescence using a Xenogen imaging system. Tumor-bearing mice survival was recorded.
2.11. Statistical analyses
All experiments were repeated three times. All data were expressed as means ± standard deviations (SD). Comparisons between individual data points were analyzed by two-tailed Student’s t-test. The non-parametric Mann-Whitney test was used for comparing two different groups. Survival distributions for mice in different groups were compared by the Kaplan-Meier curves and by use of log-rank tests. A p-value < 0.05 was considered significant.
3. Results
3.1. Administration of TA-HPV vaccinia virus through skin scarification enhanced HPV16/E7-specific CD8+ T cell responses in pNGVL4a-Sig/E7(detox)/HSP70 vaccinated mice
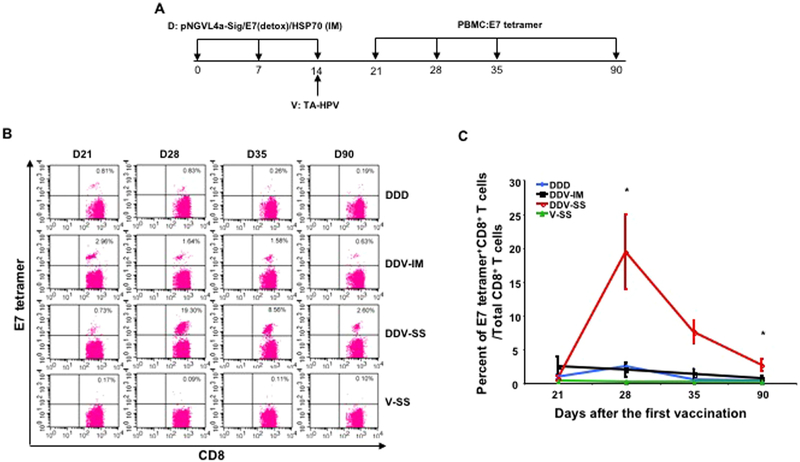
To evaluate the immune response elicited by different vaccine regimens and routes of administration, 5–8-week old C57BL/6 mice received one of four vaccination regimens: 1) IM injection of pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine in the hind leg on day 0, then boosted with the same vaccination on day 7 and day 14 (DDD); 2) IM injection of pNGVL4a-Sig/E7(detox)/HSP70 in the hind leg on day 0, boosted with the same vaccination on day 7, and vaccinated with TAHPV vaccinia virus via IM injection on day 14 (DDV-IM); 3) IM injection of pNGVL4a-Sig/E7(detox)/HSP70 in the hind leg on day 0, boosted with the same vaccination on day 7, and TA-HPV via SS on the tail on day 14 (DDV-SS); or 4) As a control, a group of naïve mice received IM PBS treatment in the hind leg on day 0 and day 7, and TAHPV via SS on the tail on day 14 (V-SS). PBMCs were then prepared on day 21, 28, 35, and 90, and stained with anti-mouse CD8 and HPV16/E7 tetramer and analyzed using flow cytometry (Fig. 1A). Mice that received DDV-SS vaccination showed a long-lasting HPV16 E7-specific CD8+ T cell response, which peaked on day 28, but continued to remain higher than the background even 90 days after the first vaccination. Mice vaccinated with all other regimens showed similar, weak HPV16/E7-specific CD8+ T cell responses (Fig. 1B and C).
Fig. 1. TA-HPV administration through skin scarification boosted pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine-induced HPV16 E7-specific CD8+ T cells resulting in a robust and long-lasting response.
A. Schematic illustration of the experiment. Briefly, 5–8-week old female C57BL/6 mice (5 mice/group) were vaccinated with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine in 50 μL via IM injection (hind leg muscle). The mice were boosted with the same regimen 7 days later. One week after the last vaccination, one group of mice was further boosted with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine in 50 μL via IM injection (leg muscle). Another group of mice was injected with 5 × 105 pfu of TA-HPV vaccinia virus (50 μL) via IM injection (leg muscle). A group of mice was vaccinated with 5 × 105 pfu of TA-HPV vaccinia virus (5 μL) on the tail through SS. Lastly, a group of naïve mice was vaccinated with 5 × 105 pfu of TA-HPV vaccinia virus (5 μL) on the tail through SS. 7, 14, 21 and 76 days after the last vaccination, PBMCs were prepared and stained with anti-mouse CD8 and HPV16/E7 tetramer. The data were acquired with FACSCalibur flow cytometer and analyzed with CellQuest. B. Representative flow cytometry data from each group. C. Summary of the flow cytometry data.
We then sought to determine whether the boosting effect observed from TA-HPV SS was mediated by antigen-specific or non-specific immunity. In an additional study, mice received two DNA vaccinations and a boost of either: DNA vaccine (DDD), TA-HPV (DDV-TAHPV) or wild type vaccinia virus (DDV-wtVV) via SS. As shown in Supplemental Fig. 1, significant E7-specific CD8+ T cell responses were observed in mice boosted with TA-HPV (DDV-TAHPV); however, mice that received wild type vaccinia virus boost (DDV-wtVV) had a weaker immune response than mice that received DDD regimen.
Next, we showed that a biologically active, live TA-HPV vaccinia virus is required to effectively boost the antigen-specific CD8+ T cell responses elicited by the priming with pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine. Mice that received live TA-HPV boost via SS (DDV-SS-Live) had a significant E7-specific CD8+ T cell response, which was strongest on day 28. Mice that received heat inactivated (HI) TA-HPV boost via SS (DDV-SS-HI) had comparable levels of E7-specific CD8+ T cells to mice that received DDD and DDV-IM regimens (Supplemental Fig. 2).
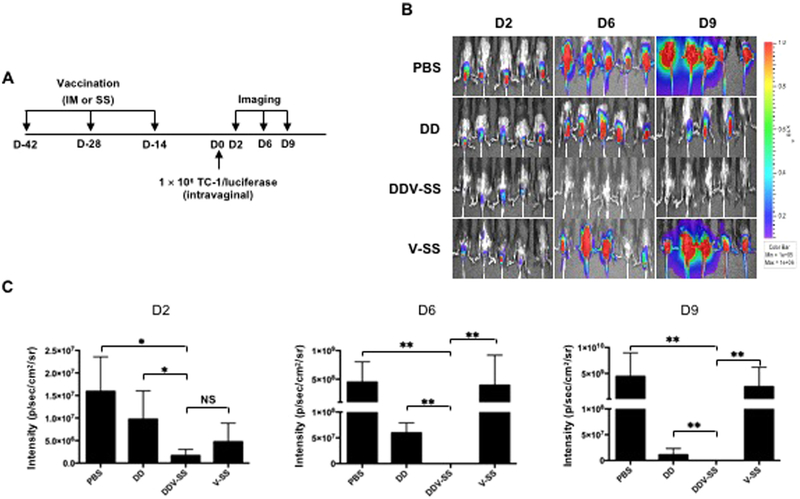
3.2. Administration of vaccinia virus through skin scarification generated greater luciferase intensity in mice compared to vaccination through intramuscular injection
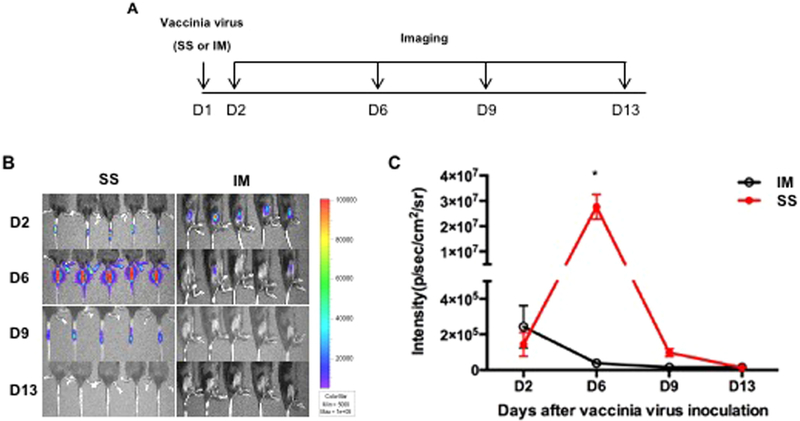
As we observed, mice primed with pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccination elicited stronger immune responses when boosted with TA-HPV vaccine via SS compared to IM injection (Fig. 1). To examine the potential mechanism that contributes to this observation, vaccinia virus encoding luciferase was administered to 5–8-week old C57BL/6 mice via IM injection or SS on the tail. Luciferase expression was then monitored using luminescence imaging at indicated intervals as illustrated in Fig. 2A. As shown in Fig. 2B–C, the luciferase signal in mice that received vaccinia virus through SS was detectable on days 2, 6 and 9; however, it was strongest on day 6. Mice that received vaccinia virus via IM injection showed a weak luminescence signal after injection, which was not detectable on day 9 and 13. The increase in luminescence intensity in mice translates to an increase in the abundance of luciferase-expressing cells around the administration site. Thus, our data suggests that vaccinia virus experienced greater viral replication when administered via SS, amplifying the expression of encoded protein antigens, resulting in a more potent immune response.
Fig. 2. Comparison of luciferase expression by vaccinia virus administered through either skin scarification or intramuscular injection.
A. Schematic illustration of the experiment. Briefly, one group of 5–8-week old female C57BL/6 mice (5 mice/group) was injected with 5 × 105 pfu of vaccinia virus expressing luciferase (50 μL) via IM injection (hind leg muscle). Another group of mice was vaccinated with 5 × 105 pfu of vaccinia virus expressing luciferase (5 μL) on the tail through SS. Expression of luciferase was monitored with luminescence imaging at indicated time points. B. Representative luminescence imaging data from each group. C. Summary of the luminescence imaging data.
3.3. Skin scarification of Luciferase-expressing vaccinia virus at tested doses resulted in similar protein expression kinetics
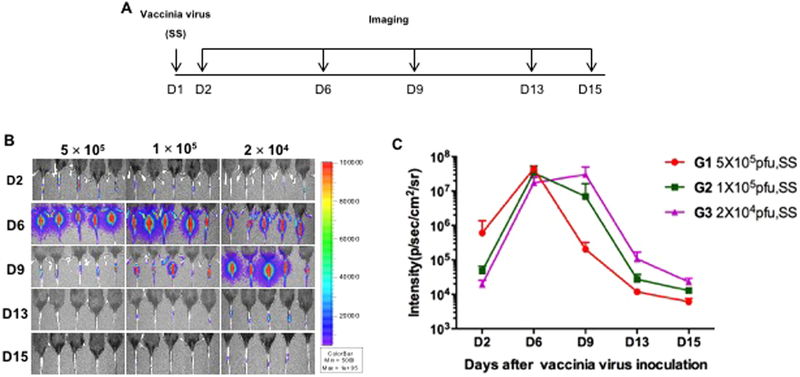
After observing that vaccinia virus administration via SS resulted in elevated expression of virus-encoded protein compared to IM administration, we sought to determine whether changes in administration dosage would influence the protein expression kinetics. 5–8-week old mice were administered with varying doses of vaccinia virus (5 × 105 pfu (G1), 1 × 105 pfu (G2), or 2 × 104 pfu (G3)) via SS on the tail. Luciferase expression was then monitored for 15 days using luminescence imaging at indicated intervals (Fig. 3A). As shown in Fig. 3B–C, SS of luciferase encoded vaccinia virus at the three tested doses showed similar luminescence kinetics, all reaching their peak luminescence intensity 6–9 days after administration, and decreased rapidly to almost background level by day 15. Importantly, no significant differences in luminescence signals were observed between the groups with high (5 × 105 pfu) or low (2 × 104 pfu) dose of vaccinia virus, suggesting protein expression was not dose dependent. This further implies that only a small dose of vaccinia virus administered through SS would be capable of mounting a substantial immune response.
Fig. 3. Dose response of luciferase expression by vaccinia virus administered through skin scarification.
A. Schematic illustration of the experiment. Briefly, groups of 5–8-week old female C57BL/6 mice (5 mice/group) were injected with either 5 × 105, 1 × 105, or 2 × 104 pfu of vaccinia virus expressing luciferase (5 μL) on the tail through SS. Expression of luciferase was monitored with luminescence imaging at indicated time points. B. Representative luminescence imaging data from each group. C. Summary of the luminescence imaging data.
3.4. Boosting with skin scarification of TA-HPV at tested doses generated comparable magnitude of HPV16 E7-specific CD8+ T cell response following priming with pNGVL4a-Sig/E7(detox)/HSP70N
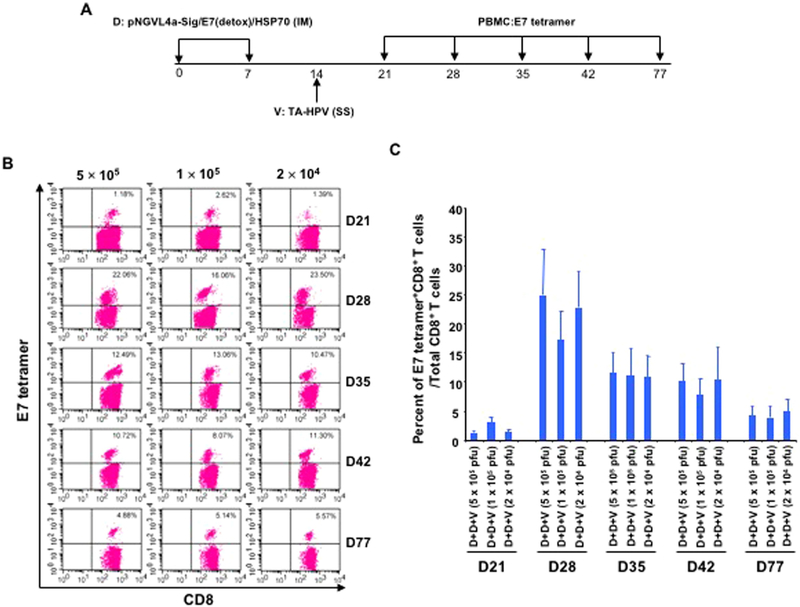
Since we demonstrated that SS of vaccinia virus at different doses resulted in a comparable amount of expression of encoded proteins, we hypothesize that the observed phenomenon would translate into the generation of similar levels of antigen-specific immune responses. To test this hypothesis, 5–8-week old C57BL/6 mice received IM injection of pNGVL4a-Sig/E7(detox)/HSP70 DNA on days 0 and 7, followed by a boost with different doses of TA-HPV (5 × 105 pfu, 1 × 105 pfu, or 2 × 104 pfu) via SS on day 14. PBMCs were then prepared on days 21, 28, 35, 42, and 77, and stained with anti-mouse CD8 and HPV16-E7 tetramer and analyzed through flow cytometry (Fig. 4A). As shown in Fig. 4B–C, the resulted E7-specific CD8+ T cell responses elicited by TA-HPV booster vaccination were comparable among the three tested doses of TA-HPV vaccine. This data, together with the findings observed in Fig. 3, suggest that the pNGVL4a-Sig/E7(detox)/HSP70 DNA IM injection prime, TA-HPV vaccinia virus SS boost vaccination regimen is capable of eliciting a potent HPV16/E7-specific CD8+ T cell response even at lower tested doses.
Fig. 4. Response of HPV16 E7-specific CD8+ T cells to different doses of TA-HPV administered through skin scarification.
A. Schematic illustration of the experiment. Briefly, groups of 5–8-week old female C57BL/6 mice (5 mice/group) were vaccinated with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine in 50 μL via IM injection (hind leg muscle). The mice were boosted with the same regimen 7 days later. One week after the last vaccination, the mice were further boosted with either 5 × 105, 1 × 105, or 2 × 104 pfu of TA-HPV (5 μL) on the tail through SS. 7, 14, 21, 28 and 63 days after the last vaccination, PBMCs were prepared and stained with anti-mouse CD8 and HPV16/E7 tetramer. The data were acquired with FACSCalibur flow cytometer and analyzed with CellQuest. B. Representative flow cytometry data from each group. C. Summary of the flow cytometry data.
3.5. TA-HPV skin scarification can boost the antigen-specific CD8+ T cells responses induced by DNA vaccine against multiple antigens
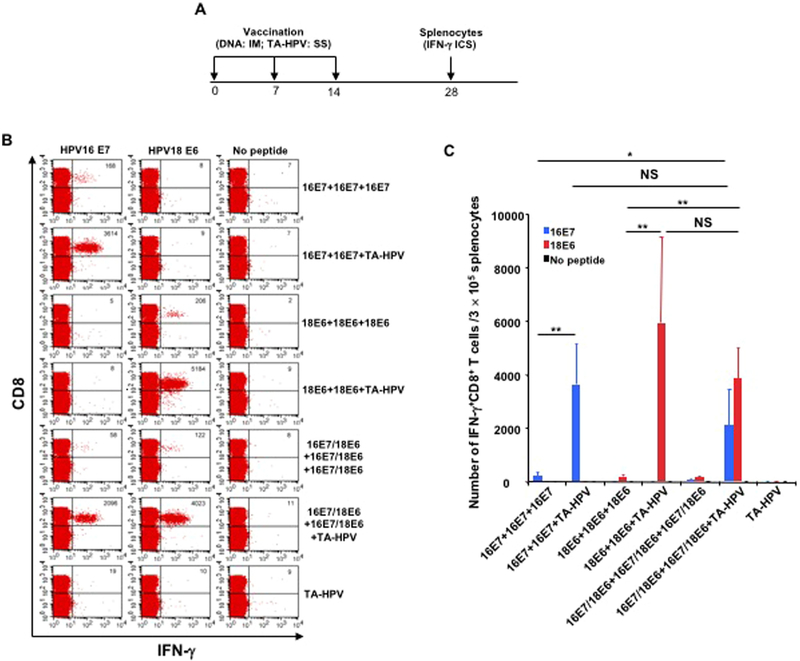
We have demonstrated that SS of TA-HPV can significantly boost the antigen-specific immune responses generated by IM DNA priming vaccination. Since TA-HPV encodes four antigens (HPV16-E6/E7 and HPV18-E6/E7) it can potentially enhance the immune responses against multiple antigens simultaneously. To evaluate the ability of TAHPV to boost the immune response against multiple HPV antigens, mice were vaccinated with DNA encoding HPV16-E7 and/or HPV18-E6 via IM injection on days 0 and 7, followed by the administration of TA-HPV through SS on day 14 (Fig. 5A). On day 28, splenocytes were harvested from different groups of mice, stimulated with either HPV16-E7 peptide or HPV18-E6 peptide, and analyzed using flow cytometry. As shown in Fig. 5B–C, TA-HPV SS significantly enhanced the immune responses elicited by the priming of DNA encoding a single antigen (either HPV16-E7 or HPV18-E6) in vaccinated mice. Furthermore, mice that received the combinational DNA vaccination containing both HPV16-E7 and HPV18-E6 antigens followed by boosting with TA-HPV SS induced both enhanced HPV16-E7 and HPV18-E6 specific CD8+ T cell responses. These results demonstrate that TA-HPV SS is able to enhance the antigen-specific CD8+ T cell immune responses primed by DNA vaccination against multiple antigens simultaneously.
Fig. 5. TA-HPV administration through skin scarification is able to boost multiple DNA vaccine induced CD8+ T cell responses either individually or simultaneously.
A. Schematic illustration of the experiment. Briefly, groups of 5–8-week old female C57BL/6 mice (5 mice/group) were vaccinated as indicated in the figure with one-week interval. One group of mice received three 25 μg vaccinations of pcDNA3-CRT/HPV16-E7 DNA vaccine via IM injection, the next group received two 25 μg vaccinations of pcDNA3-CRT/HPV16-E7 DNA vaccine via IM injection and 5 μL TA-HPV via SS, the third group received three 25 μg vaccinations of pcDNA3-CRT/HPV18-E6 DNA vaccine via IM injection, the fourth group received two 25 μg vaccinations of pcDNA3-CRT/HPV18-E6 DNA vaccine via IM injection and 5 μL TA-HPV via SS, the next group received three combined vaccinations of 25 μg pcDNA3-CRT/HPV16-E7 DNA vaccine and pcDNA3-CRT/HPV18-E6 DNA vaccine via IM injection, the sixth group received two combined vaccinations of 25 μg pcDNA3-CRT/HPV16-E7 DNA vaccine and pcDNA3-CRT/HPV18-E6 DNA vaccine via IM injection and 5 μL TA-HPV via SS, the last group received one administration of 5 μL TA-HPV via SS. Two weeks after the last vaccination, splenocytes were prepared from the mice and stimulated with either 1 μg/mL of HPV16-E7aa49–57 peptide, or HPV18-E6aa67–75 peptide at the presence of GolgiPlug (1 μL/mL) overnight at 37 °C. The cells were then stained with anti-mouse CD8 followed by intracellular IFN-γ The data were acquired with FACSCalibur flow cytometer and analyzed with CellQuest. B. Representative flow cytometry data from each group. C. Summary of the flow cytometry data.
3.6. TA-HPV skin scarification boost leads to enhanced anti-tumor immunity following priming with pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine
Since we observed a significant antigen-specific CD8+ T cell immune response generated by IM pNGVL4a-Sig/E7(detox)/HSP70 DNA prime, TA-HPV vaccinia virus SS boost vaccination regimen, we sought to examine whether the immunogenicity of the vaccine regimen translates into potent antitumor effects against HPV+ cervical tumors. We first tested the antitumor efficacy of the vaccination regimen in a tumor protection model. C57BL/6 mice were vaccinated with various regimens: 1) PBS control (PBS); 2) two IM pNGVL4a-Sig/E7(detox)/HSP70 DNA injections (DD); 3) two IM pNGVL4a-Sig/E7(detox)/HSP70 injection followed by one TA-HPV SS (DDV-SS); or 4) one TA-HPV SS (V-SS) (Fig. 6A). Two weeks after vaccination, mice were challenged with 1 × 106 TC-1/luciferase tumor cells intravaginally, and the subsequent luminescence intensity was measured as a representation of tumor growth. As shown in Fig. 6B–C, mice treated with PBS or V-SS did not generate protective immunity against TC-1 tumor challenge, as the luciferase expression increased during the examined time points. Furthermore, mice treated with DD only demonstrated some degree of protective immunity against TC-1 tumor challenge, while mice that received DDV-SS generated the most potent protective immune response against tumor challenge.
Fig. 6. TA-HPV boosted pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine-induced HPV16 E7-specific CD8+ T cell responses through skin scarification is able to completely protect mouse from a lethal cervicovaginal challenge of HPV16 E7-expressing tumor cells.
A. Schematic illustration of the experiment. Briefly, groups of 5–8-week old female C57BL/6 mice (5 mice/group) were vaccinated as indicated in the figure at two-week intervals. Two or four (pNGVL4a-Sig/E7(detox)/HSP70 only group) weeks after the last vaccination, the mice were challenged with 1 × 106 of TC-1/luciferase cells intravaginally. Expression of luciferase was monitored with luminescence imaging at indicated time points. B. Representative luminescence imaging data from each group at indicated time point. C. Summary of the luminescence imaging data. * = p < 0.05, * * = p < 0.01, NS = not significant.
Next we sought to study the HPV16 E7 antibody response in vaccinated mice. Mice received DD, DDV-IM, DDV-SS, or V-SS vaccination combination as described in Fig. 6A at two-week intervals. Two weeks after the last vaccination, the sera from mice were harvested and the antibody titer for antibody against HPV16-E7 proteins was examined using ELISA. As shown in Supplemental Fig. 4, mice from all treatment groups showed a similar levels of E7-specific antibody titer, suggesting the vaccination regimen predominantly elicited T-cell mediated immunity and not B-cell mediated humoral immunity. Overall, these data suggest that TA-HPV, administered through SS, is able to boost the HPV16/E7-specific CD8+ T cell responses induced by the priming with IM pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine.
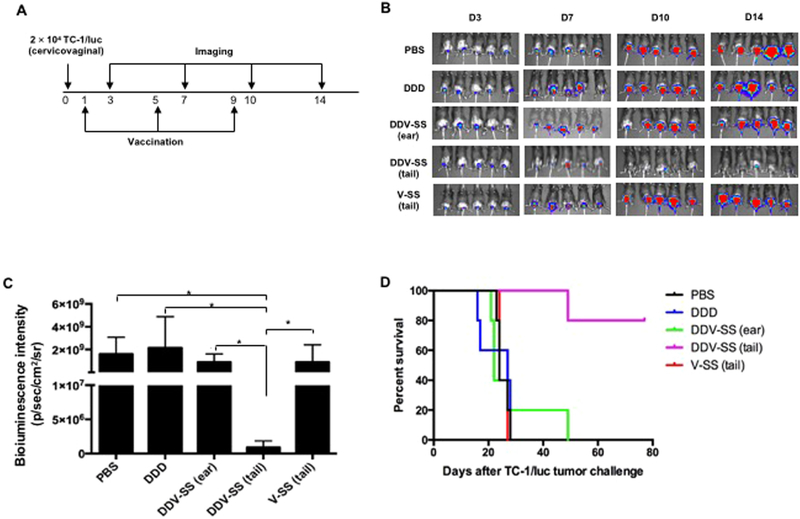
3.7. TA-HPV skin scarification boost in the tail generated the strongest antitumor immunity against established HPV16 E7-expressing cervicovaginal tumors
To evaluate the therapeutic efficacy of the proposed prime boost vaccination regimen against pre-existing tumors, C57BL/6 mice were challenged with 2 × 104 TC-1/luciferase tumor cells intravaginally, followed by one of the following treatments one day after tumor challenge: 1) PBS control (PBS), 2) IM pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccination three times in 4-day intervals (DDD), 3) IM pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccination twice in 4-day intervals followed by TA-HPV SS on the ear 4 days after last DNA vaccination (DDV-SS (ear)), 4) two times IM pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccination in 4 day interval followed by TA-HPV SS on the tail 4 days after last DNA vaccination (DDV-SS (tail)), and 5) TA-HPV SS on the tail 9 days after tumor challenge (V-SS (tail)) (Fig. 7A). Luminescence imaging was performed starting 3 days after tumor challenge and continued in 4-day intervals. As shown in Fig. 7B, at 14 days after tumor challenge, the mice treated with DDV-SS (tail) regimen showed a significantly lower luminescence intensity compared to all other treatment groups, suggesting DDV-SS (tail) resulted in better tumor control. In addition, PBMCs from tumor-bearing mice were harvested 14 days after tumor challenge, stained with E7 tetramer and anti-CD8 antibody and analyzed using flow cytometry, which showed the generation of significant amounts of E7-specific CD8+ T cells in mice that received DDV-SS (tail) and DDV-SS (ear) vaccination regimen (Supplementary Fig. 3). Furthermore, 80% of mice that received DDV-SS (tail) treatment survived more than 80 days after TC-1/luc tumor challenge, while mice that received all other regimens died within 50 days of tumor challenge. These results indicate that DDV-SS (tail) is able to elicit antitumor immunity against established HPV16/E7-expressing cervicovaginal tumors in mice.
Fig. 7. TA-HPV boosted pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine-induced HPV16 E7-specific CD8+ T cell responses through skin scarification generated the strongest antitumor immunity against established HPV16 E7-expressing cervicovaginal tumors.
A. Schematic illustration of the experiment. Briefly, groups of 5–8-week old female C57BL/6 mice (5 mice/group) were challenged with 2 × 104 of TC-1/luciferase cells intravaginally to establish cervicovaginal tumor. One day later, the mice were vaccinated with 25 μg/mouse of pNGVL4a-Sig/E7(detox)/HSP70 via intramuscular injection (hind leg muscle for DDD, DDV-SS tail, and front leg for DDV-SS ear). The mice were boosted as indicated in the figure at 4-day intervals. Expression of luciferase by TC-1 tumor cells was monitored with luminescence imaging at indicated time points. B. Luminescence imaging data from each group 14 days after tumor challenge. C. Kaplan-Meier survival analysis of TC-1/luciferase tumor cell challenged mice treated with the different regimens. * = p < 0.05.
4. Discussion
In this study, we evaluated the heterologous therapeutic HPV vaccination regimen consisting of IM pNGVL4a-Sig/E7(detox)/HSP70 DNA priming followed by TA-HPV vaccinia virus boost. We tested different routes of TA-HPV administration either via IM injection or through SS. We showed that boosting with TA-HPV through SS effectively generated a significant level of E7-specific CD8+ T cell responses following with IM pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine priming and is much more potent compared to boosting with TA-HPV through IM injection. When examining the properties of TA-HPV that contribute to the generation of enhanced antigen-specific immune responses, we demonstrated that live TA-HPV administered through SS resulted in significantly more viral expansion and antigen expression as compared to IM injection, and that the antigen expression kinetics, as well as the ability to elicit antigen-specific CD8+ T cell responses, is comparable across various TA-HPV doses administered through SS. Lastly, we showed that the ability of TA-HPV SS to generate a strong antigen-specific CD8+ T cell immune response translates into a potent anti-tumor effect against HPV16-E6/E7-expressing TC-1 tumor model, controlling tumor growth, and prolonging tumor-bearing mice survival in both tumor protection and tumor treatment experiments. We believe that these insights will help improve therapeutic HPV vaccines in treating HPV infection and HPV-associated diseases.
The mode of delivery for vaccine administration is very important for inducing strong immune responses. Vaccination delivery routes include intramuscular (IM), subcutaneous (SC), intravenous (IV) and skin scarification (SS). Vaccine administration via SS has been shown to generate a more potent immune response in comparison to other routes of vaccination. In previous studies that administered recombinant vaccinia virus via SS, skin resident dendritic cells (DC) were found to be critical for the generation of a robust CD8+ T cell immune response (Seneschal et al., 2014). DCs are professional antigen presenting cells required for naïve T cell priming and activation (Seneschal et al., 2014). After vaccination via SS, the epidermis and follicular epithelium are infected and express the viral genome. Following scarification, antigen-specific T cells are imprinted with skin-homing markers in draining lymph nodes (tdLN) and migrate to the site of injection (Liu et al., 2010). Recent studies have also shown that epithelial infection produces skin resident memory T cells (TRM), which have been shown to prevent the appearance of lesions (Hersperger et al., 2014). Our data further supports this claim, showing a more robust HPV16/E7-specific CD8+ T cell response and stronger antitumor effect in mice that received TA-HPV through SS following priming with pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine (DDV-SS). Through these data, we can infer that skin resident DCs may play an important role in inducing a strong TRM response. As further supported by our data, and discussed in previous studies, the process of scarification is a major contributor to immunoprotection (Rice et al., 2014) and should be critically considered when developing vaccination protocols for other kinds of diseases and infectious agents.
In the current study, we show that SS on the tail was able to induce a larger population of HPV16/E7-specific CD8+ T cells against intravaginal TC-1 tumor than SS on the ear. One possible explanation for this observation may be the site of vaccination and its proximity to the tumor location and tumor-draining lymph nodes (tdLN). Previous studies have reported that vaccination location plays an important role in eliciting potent antitumor immune responses (Jeanbart et al., 2014). In particular, vaccinating near the tdLNs, proximal to the tumor location has been shown to induce a stronger CD8+ T cell responses (Jeanbart et al., 2014; van Poelgeest et al., 2016). It is likely that cervicovaginal tumor-bearing mice receiving TA-HPV via SS on the tail had better immunotherapeutic response and survival rates because the vaccination site was closer to the tumor and respective tdLNs.
When investigating protein expression kinetics and varying vaccinia virus dosages we found that SS of different doses of vaccinia virus encoding luciferase resulted in similar luciferase expression levels and intensity. Furthermore, we demonstrated that varying doses of TA-HPV boost via SS resulted in similar E7-specific immune responses. These data indicated that the magnitude of the immune response elicited by TA-HPV via SS is reflected by the increase in luciferase bioluminescence antigen expression as an indicator for T cell response, as we saw similar E7-specific CD8+ T cell responses across the range of administered doses. We believe that these data warrant further testing to identify the minimal dose of TA-HPV capable of generating a potent antigen-specific immune response in vivo.
In previous studies, heat-inactivated vaccinia virus through SS failed to elicit a significant immune response, while administration of live vaccinia virus via SS resulted in potent immune stimulation (Liu et al., 2010). Our data further supports these findings, showing that live TAHPV administered through SS was able to induce a greater HPV16/E7-specific CD8+ T cell response than heat inactivated TA-HPV. A previous study also determined that SS with recombinant vaccinia virus expressing tumor antigen was able to provide protection against tumor challenge (Liu et al., 2010). We demonstrated that the HPV16/E7-specific CD8+ T cell immunity boosted by TA-HPV SS was able to translate into effective protection against TC-1 tumor challenge. Furthermore, mice that received DDV-SS generated the strongest, therapeutic antitumor response against established HPV16/E7-expressing cervicovaginal TC-1 tumors. Although there are abundant amounts of evidence to support the notion that vaccine administration through SS can induce a robust immune response, the mechanisms by which SS provides this robust immune response are not completely understood (Liu et al., 2010).
5. Conclusions
In this study, we provide data to suggest that the ability of TA-HPV SS to generate potent antigen-specific immune responses is potentially due to the vaccinia virus’ ability to replicate more efficiently when administered via SS compared to IM injection; however, further investigation is warranted in order to fully understand the mechanics behind the immune response triggered by SS immunization. Therapeutic HPV DNA vaccines and TA-HPV have been successfully utilized as a combinational therapeutic treatment strategy in preclinical and clinical studies; however, the administration of TA-HPV via SS in DNA prime, TA-HPV boost regimen has not been previously explored. In the current study, we show that administration of TA-HPV through SS is able to produce a more potent HPV16/E7-specific CD8+ T cell response than IM injection. The results of this study warrant future clinical translation of heterologous pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine prime, TA-HPV SS boost vaccination regimen as a method to treat HPV infection and HPV-associated diseases.
Supplementary Material
Funding
This work was supported by the National Institutes of Health (NIH) Cervical Cancer Specialized Program of Research Excellence (SPORE) [P50CA098252]; (U.S.A.); NIH R01 grant [R01CA114425] (U.S.A.); and NIH R21 grants [R21CA194896] and [R21AI109259]. This work was also supported by Papivax Biotech Inc.
Footnotes
Conflict of interest
Yung-Nien Chang is the Chief Scientific Officer of Papivax Biotech Inc. and owns stock options in Papivax Biotech Inc. T.-C. Wu and Richard B.S. Roden are co-founders of and have equity ownership interests in Papivax LLC. They also own Papivax Biotech Inc. stock options and are members of Papivax Biotech Inc.’s Scientific Advisory Board. Additionally, under a licensing agreement between Papivax Biotech Inc. and Johns Hopkins University, Dr. Wu, Dr. Hung, and Dr. Roden are entitled to royalties on an invention described in this article. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Other coauthors have declared no conflicts of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi.org/10.1016/j.virol.2018.09.019.
References
- Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, Knott C, Lin F, Boyer JD, Draghia-Akli R, White CJ, Kim JJ, Weiner DB, Sardesai NY, 2012. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med 4, 155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, Evans AS, Adams M, Stacey SN, Boursnell ME, Rutherford E, Hickling JK, Inglis SC, 1996. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 347, 1523–1527. [DOI] [PubMed] [Google Scholar]
- Chang CL, Ma B, Pang X, Wu TC, Hung CF, 2009. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol. Ther 17, 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, Wu TC, 2001a. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J. Clin. Investig 108, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Ling M, Slater LA, Roden RB, Wu TC, 2001b. Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments. Hum. Gene Ther 12, 235–252. [DOI] [PubMed] [Google Scholar]
- Davidson EJ, Faulkner RL, Sehr P, Pawlita M, Smyth LJ, Burt DJ, Tomlinson AE, Hickling J, Kitchener HC, Stern PL, 2004. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7). Vaccine 22, 2722–2729. [DOI] [PubMed] [Google Scholar]
- Doorbar J, 2016. Model systems of human papillomavirus-associated disease. J. Pathol 238, 166–179. [DOI] [PubMed] [Google Scholar]
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, Franceschi S, 2012. Global burden of human papillomavirus and related diseases. Vaccine 30 (Suppl. 5), SF12–SF23. [DOI] [PubMed] [Google Scholar]
- Ghittoni R, Accardi R, Chiocca S, Tommasino M, 2015. Role of human papilloma-viruses in carcinogenesis. Ecancermedicalscience 9, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Glueck R, Patel PR, 2017. HPV vaccines: global perspectives. Hum. Vaccin. Immunother 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Siciliano NA, DeHaven BC, Snook AE, Eisenlohr LC, 2014. Epithelial immunization induces polyfunctional CD8+ T cells and optimal mousepox protection. J. Virol 88, 9472–9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CJ, Kim TW, Hung CF, Juang J, Moniz M, Boyd DA, He L, Chen PJ, Chen CH, Wu TC, 2004. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine 22, 3993–4001. [DOI] [PubMed] [Google Scholar]
- Huang B, Mao CP, Peng S, He L, Hung CF, Wu TC, 2007. Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine 25, 7824–7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanbart L, Ballester M, de Titta A, Corthesy P, Romero P, Hubbell JA, Swartz MA, 2014. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res 2, 436–447. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, Lee CW, Kim S, Woo JW, Park KS, Hwang YY, Park J, Lee IH, Lim KT, Lee KH, Jeong MS, Surh CD, Suh YS, Park JS, Sung YC, 2014. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun 5, 5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Yang A, Wu TC, Hung CF, 2016. Immunotherapy for human papilloma-virus-associated disease and cervical cancer: review of clinical and translational research. J. Gynecol. Oncol 27, e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS, 2010. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat. Med 16, 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Maraj B, Tran NP, Knoff J, Chen A, Alvarez RD, Hung CF, Wu TC, 2012. Emerging human papillomavirus vaccines. Expert Opin. Emerg. Drugs 17, 469–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, Clark RA, Trimble CL, 2014. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci. Transl. Med 6 (221ra213). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JH, Grandis JR, Ferris RL, 2016. HPV-associated head and neck cancer: unique features of epidemiology and clinical management. Annu. Rev. Med 67, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S, 2013. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer – systematic review and meta-analysis of trends by time and region. Head Neck 35, 747–755. [DOI] [PubMed] [Google Scholar]
- Ostor AG, 1993. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol 12, 186–192. [PubMed] [Google Scholar]
- Rice AD, Adams MM, Lindsey SF, Swetnam DM, Manning BR, Smith AJ, Burrage AM, Wallace G, MacNeill AL, Moyer RW, 2014. Protective properties of vaccinia virus-based vaccines: skin scarification promotes a nonspecific immune response that protects against orthopoxvirus disease. J. Virol 88, 7753–7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneschal J, Jiang X, Kupper TS, 2014. Langerin+ dermal DC, but not Langerhans cells, are required for effective CD8-mediated immune responses after skin scar-ification with vaccinia virus. J. Investig. Dermatol 134, 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, Pawlita M, Man S, Hickling JK, Fiander AN, Tristram A, Kitchener HC, Offringa R, Stern PL, Van Der Burg SH, 2004. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin. Cancer Res 10, 2954–2961. [DOI] [PubMed] [Google Scholar]
- Trimble C, Lin CT, Hung CF, Pai S, Juang J, He L, Gillison M, Pardoll D, Wu L, Wu TC, 2003. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine 21, 4036–4042. [DOI] [PubMed] [Google Scholar]
- van Poelgeest MI, Visconti VV, Aghai Z, van Ham VJ, Heusinkveld M, Zandvliet ML, Valentijn AR, Goedemans R, van der Minne CE, Verdegaal EM, Trimbos JB, van der Burg SH, Welters MJ, 2016. Potential use of lymph node-derived HPV-specific T cells for adoptive cell therapy of cervical cancer. Cancer Immunol. Immunother 65, 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeham K, Kavanagh K, 2014. The burden of HPV-associated anogenital cancers. Curr. Oncol. Rep 16, 402. [DOI] [PubMed] [Google Scholar]
- Wu TC, Guarnieri FG, Staveley-O’Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, Cho KR, August JT, Pardoll DM, 1995. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc. Natl. Acad. Sci. USA 92, 11671–11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Farmer E, Wu TC, Hung CF, 2016a. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci 23, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, Hung CF, 2016b. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev. Vaccin 15, 989–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H, 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2, 342–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.