Abstract
Biomaterial-mediated inflammation and fibrosis remain a prominent challenge in designing materials to support tissue repair and regeneration. Despite the many biomaterial technologies that have been designed to evade or suppress inflammation (i.e. delivery of anti-inflammatory drugs, hydrophobic coatings, etc.) many materials are still subject to a foreign body response, resulting encapsulation of dense, scar-like extracellular matrix. The primary cells involved in biomaterial-mediated fibrosis are macrophages, which modulate inflammation, and fibroblasts, which primarily lay down new extracellular matrix. While macrophages and fibroblasts are implicated in driving biomaterial-mediated fibrosis, the signaling pathways and spatiotemporal crosstalk between these cell types remain loosely defined. In this review we set out to decipher the role of M1 and M2 macrophages (and soluble cues) involved in the fibrous encapsulation of biomaterials in vivo. Additionally we also focused this review on fibroblast and macrophage crosstalk in vitro, along with in vitro models to study the foreign body response. Lastly, we highlight several strategies that have been used to specifically modulate macrophages and fibroblast behavior in vitro and in vivo to control biomaterial-mediated fibrosis.
Keywords: macrophage, fibroblast, foreign body response, fibrosis, biomaterial
1. Introduction
Each year in the U.S., tens of thousands of biomaterial implants and medical devices are implanted into patients to repair, replace, or regenerate damaged tissues throughout the body [1, 2]. As the size of the biomaterials-based medical devices market and number of Food and Drug Administration (FDA)-cleared and -approved products continues to grow [3, 4], failure to achieve clinical or patient satisfaction remains a significant challenge for biomaterials engineers. The clinical and research-based incidences of such failures are difficult to quantify due to the diversity of biomaterial therapies, applications in which they are used, and reasons for biomaterial removal or revision. However, it is known that all materials are subject to the inflammatory response upon implantation. The host response to an implanted biomaterial remains a continuing challenge for the future of regenerative biomaterials for applications in tissue repair and regeneration [5].
When diseased or damaged tissue becomes non-functional (i.e. from trauma, degenerative processes, etc.), biomaterials are employed to aid in repair and regeneration. When biomaterials require surgical implantation, this additional injury may magnify the biological response to the biomaterial. Following biomaterial implantation, a series of well-established biological events is initiated [6], starting with protein adsorption to the biomaterial surface. Through damaged blood vessels, platelets migrate to the site, where complement activation is initiated to form a transient provisional matrix on and around the biomaterial. The extravasation of blood also initiates the recruitment of innate immune cells, including neutrophils, monocytes, and macrophages to the biomaterial implant and surrounding tissue. The provisional matrix and influx of innate immune cells present myriad microenvironmental signals including structural and cellular components along with cytokines, growth factors, chemoattractants, and other bioactive signals to initiate the inflammatory response by macrophages. Macrophages function to clear debris, bacteria, apoptotic cells, and foreign material via phagocytosis; secrete enzymes that remodel the blood-based provisional matrix; and produce signals that recruit additional support cells, such as fibroblasts. Fibroblasts migrate, proliferate, and produce new extracellular matrix (ECM) to repair and replace damaged tissue in the vicinity of the implanted biomaterial. The newly formed ECM surrounding the biomaterial either (1) integrates with the biomaterial and facilitates tissue repair or regeneration of new tissue over time, or (2) develops into a pathological positive feedback loop where more and more ECM is deposited. This process, known as the foreign body response, isolates the biomaterial from the rest of the body in a thick collagenous scar-like tissue capsule, rendering the biomaterial nonfunctional (for a review of the foreign body response, see Ref. [6]). During the foreign body response, macrophages fuse into multinucleated foreign body giant cells (FBGCs) that secrete enzymes that degrade the foreign material if possible [7, 8]. With macrophages being the driver of inflammation and fibroblasts being critically involved with laying down new ECM, it is clear that these cells are tightly regulated in vivo to facilitate healthy tissue repair [9, 10]. Although macrophages and fibroblasts have each been shown to play independent roles in tissue repair and the foreign body response, it remains unclear how these cells communicate with one another in these processes to promote healthy tissue regeneration.
This review highlights recent work that has investigated macrophage and fibroblast behavior within the context of biomaterial-mediated fibrosis, macrophage-fibroblast crosstalk, and various biomaterial and drug delivery strategies that modulate macrophage and fibroblast behavior to promote tissue regeneration. Lastly, we provide perspective on remaining questions and future directions within the scope of macrophages and fibroblasts in biomaterial-mediated fibrosis.
1.1. Macrophages
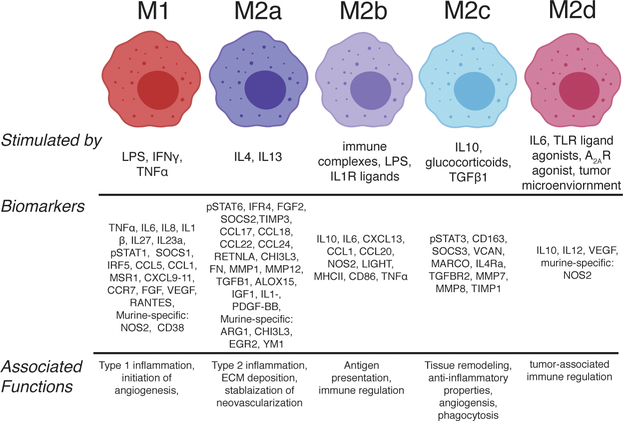
It has long been established that macrophages are the primary cell at the biomaterial-tissue interface [11]. Several studies from the 1950’s and 1960’s described how sutures derived from various materials elicited different responses in vivo. For example, materials like multifilament silk sutures implanted in heart tissue elicited early (24 hours to one week) qualitative inflammation, thrombus, and suppuration formation, which were associated with a high number of macrophages, FBGCs, and edema; three to six months later, the same silk sutures were associated with sterile abscesses and granuloma formation, suggesting that the early immune response to biomaterials was critical to the long term function and outcome [12, 13]. What we now appreciate is that macrophages are highly plastic cells that exhibit a spectrum of phenotypes in response to local cues in vitro and in vivo [14]. Although macrophages are most commonly referred to as either classically activated/pro-inflammatory “M1” or alternatively activated/anti-inflammatory “M2”, aligning with the helper T cell nomenclature [15], many other phenotypes exist. Specific phenotypes that have been identified and characterized in the context of tissue repair include M1, stimulated with pro-inflammatory signals like lipopolysaccharide (LPS), interferon-gamma (IFNγ), or tumor necrosis factor alpha (TNFα); M2a, stimulated with Th2 cytokines such as interleukin-4 (IL4) with or without interleukin-13 (IL13); M2b, stimulated with immune complex (IC) and LPS; M2c, stimulated with the anti-inflammatory cytokine interleukin-10 (IL10), glucocorticoids, or transforming growth factor beta (TGFβ1) [16, 17]; M2d, simulated with Toll-like receptor ligands and adenosine A2A receptor agonists [18]; and M2f, stimulated via uptake of apoptotic cells [19, 20] (Figure 1). It is worth noting that the nomenclature of macrophage phenotype is somewhat controversial [17]. In this review we will use the M1/M2 nomenclature, as opposed to “classically/alternatively activated,” or others, because it is agnostic about macrophage function, which is context-dependent, as discussed in Ref [21]. Macrophage fusion into FBGCs might also be considered a distinct macrophage phenotype; while the M2a-promoting cytokine IL4 has been used to promote macrophage fusion in vitro [22, 23] and has been associated with increased scar formation and fibrous encapsulation of biomaterials [24, 25], FBGCs appear to exhibit characteristics that are associated with M1 and M2 subtypes [26–28]. It is also important to note that macrophage fusion in vivo requires significant signaling from various lymphocytes, including natural killer cells, T and B cells [29], along with activation of chemotactic pathways [30]. Indeed, a recent study used a series of macrophage, neutrophil and lymphocyte knockout models to identify the macrophage-specific gene, colony stimulating factor 1 receptor (CSF1R) as being key to the foreign body response and associated with FBGC formation [31]. Doloff et al., found that inhibiting CSF1R resulted in no fibrous encapsulation formation and protected normal macrophage functions important for normal tissue regeneration, including secretion of VEGF, reactive oxygen species (ROS) production, and phagocytosis [31]. While FBGC formation has typically been associated with fibrous encapsulation of biomaterials, one study showed that FBGCs aid in phagocytosis of larger debris including fibrotic tissue deposits [32], suggesting that FBGC formation is not always a detrimental process. Relatedly, biomaterial-mediated fibrosis in general is not always detrimental; for example, fibrotic ingrowth into surgical meshes is an important means by which internal wounds are sealed [33].
Figure 1.
Outline of macrophage phenotypes, including polarizing stimuli, biomarkers, and associated functions. Figure created with ©BioRender.io.
In addition to the stimuli described, macrophage behavior is affected by microenvironmental features, including biomaterial properties such as shape and geometry [34], biochemical surface or composition [35–41], mechanical stiffness [42], topography [43, 44], porosity [45, 46], and release of proteins or drugs [37, 47, 48]. Furthermore, tissue-resident and monocyte-derived macrophages also display distinct phenotypes and functions when cultured in vitro, and are indistinguishable from each other in vivo [49]. Lastly, macrophages often exhibit hybrid phenotypes, displaying characteristics of M1 and M2 phenotypes simultaneously, especially in the complex environment surrounding implanted biomaterials [40, 47]; the role and function of macrophages displaying a hybrid phenotype, which could be an entirely distinct phenotype, remain unclear in biomaterial-mediated fibrosis. These reports illustrate that macrophages are intimately involved in the body’s natural response to biomaterials (and soluble cues), and can ultimately exhibit a vast array of possible phenotypes and functions.
1.2. Fibroblasts
All connective tissues in the body are comprised of highly dynamic interstitial fibroblasts that are incorporated in an extracellular matrix comprised of proteins such as collagens, elastin, laminin, and proteoglycans [50]. Fibroblasts are ECM-producing mesenchymal cells derived from embryonic mesoderm [50], although their progenitor source is somewhat controversial, with evidence suggesting they derive from circulating cells from bone marrow, epithelial, endothelial, and hematopoietic origin (for reviews, see Refs. [51–53]). More recently, it has become appreciated that fibroblasts are particularly heterogenous and highly plastic in their phenotypes across tissues and even within the same tissue, such as the skin [10]. Despite the challenges in defining fibroblast phenotype, it is well established that fibroblasts play a significant role beyond that of ECM deposition, including indirect roles in inflammation [54], angiogenesis [55], cancer progression [56], others. Additionally, fibroblasts can be activated or transformed into distinct phenotypes with unique behaviors and functions, including proto-myofibroblasts, myofibroblasts, and fibrocytes (extensively reviewed by Reilkoff et al. [57]). Following tissue injury, fibroblasts are activated by a variety of chemokines and cytokines, along with mechanical and physical microenvironmental changes. One of the first steps following injury, besides complement and the inflammatory cascade, is fibroblast migration and activation into ‘proto-myofibroblasts’ in response to changes in the ECM and the various signaling factors from immune cells and platelets. These provisional cells generate cell-ECM contacts via weak focal adhesions, and later undergo mature differentiation into myofibroblasts [58]. Myofibroblasts are hallmarked by their excessive ECM deposition, high contractile stress fibers incorporated with alpha-smooth muscle actin (αSMA) [59], with differentiation occurring via chemical (i.e. TGFβ1 and reactive oxygen species (ROS)) [60, 61] and/or mechanical (i.e. tensile forces) [62] mechanisms. As normal healing occurs, myofibroblasts undergo programmed apoptosis, mediated by the lack of chemical signals (i.e. decreased TFGβ1) [63] and/or when the newly formed ECM provides independent mechanical support, facilitating mechanical release of weak focal adhesions [64], which collectively facilitate ECM remodeling [65]. Similar to macrophages, fibroblast behavior and differentiation is context- and time-dependent. The controlled and transient presence of myofibroblasts is critical for normal tissue repair [66, 67]. On the other hand, chronic inflammation, disease, cancer, and the foreign body response are associated with a myofibroblast positive feedback loop causing proliferation and migration, ultimately leading to excessive ECM deposition, contracture, and fibrosis [68–70].
Due to the highly dynamic nature of macrophages and fibroblasts, assessing and tracking their behavior in vivo can be particularly challenging; surface markers, genes, and protein secretion often overlap between phenotypes, and depending on the progenitor source, the same cell might express a completely different set of markers and have completely different functional behaviors. Several review articles have highlighted the particularly heterogenous nature of fibroblasts [10, 71, 72], as they exist in nearly every tissue and organ; for example, fibroblasts within the skin are derived from two distinct lineages, which are functionally and phenotypically different within the context of tissue repair [73]. A concomitant challenge is that a specific marker to distinguish fibroblasts and MSCs has not been identified [74]. In addition to macrophages and fibroblasts, other innate immune cells and lymphocytes are involved in the cellular response to implanted biomaterials [75]. However, within the scope of this review, we will primarily focus on the role of macrophages and their crosstalk with fibroblasts.
2. The role of macrophages in the foreign body response and fibrosis in vivo
2.1. Overview
Primary research on macrophage behavior typically falls under the M1-M2 paradigm. Investigating macrophage behavior within this context has been particularly useful, albeit oversimplified, for establishing that macrophage phenotype is spatiotemporally regulated and unique to various disease states and pathologies. For example, it is known that macrophages exhibit temporal shifts from an M1 phenotype (immediately to three days-post injury) to an M2-like phenotype that may be most closely related to M2a (four to seven days-post injury) during normal wound healing and tissue regeneration in vivo [47, 76, 77]. M1 macrophages are known to secrete elevated levels of a potent angiogenic factor, vascular endothelial growth factor (VEGF), and pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNFα) and interleukin-1-beta (IL1β), while M2a macrophages have been shown to secrete high levels of a powerful mitogen, platelet-derived growth factor-BB (PDGF-BB) [41] along with pro-fibrotic chemokine (C-C motif) ligand 17 (CCL17) and CCL18 [78–80]. Failure of macrophages to naturally and sequentially transition over time from M1 to M2a has been associated with dysregulated functions including the non-healing nature of chronic wounds [81] and inhibited wound re-epithelialization [82, 83], while the elimination of M1 macrophage behavior and/or premature promotion of the M2a phenotype have been associated with delayed wound healing [84], excessive ECM deposition [85], poorly formed neo-vascularization [86], and pathological fibrosis [87, 88].
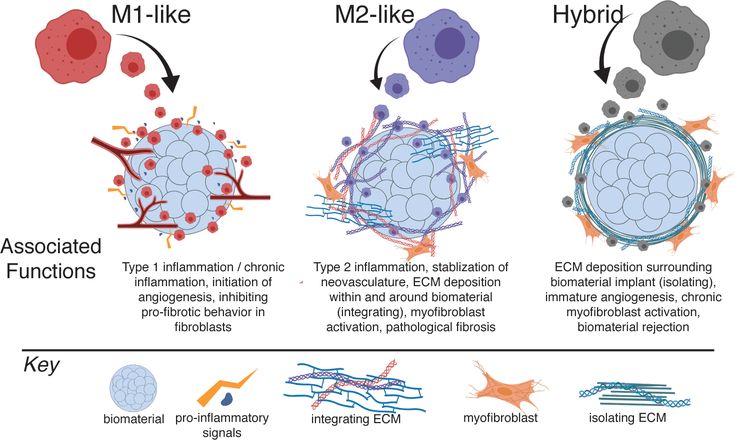
Despite research illustrating the dynamic behavior of macrophages, a common misconception remains that biomaterials that promote the M2 phenotype are “good” because of anti-inflammatory properties, while materials that promote the M1 phenotype are “bad” due to the risk for chronic inflammation. To illustrate that a delicate and temporally controlled balance in these two phenotypic extremes is required to facilitate optimal tissue repair and regeneration, in this section we break down and review studies that have examined the role of specific macrophage phenotypes within the context of the foreign body response in vivo (Figure 2).
Figure 2.
Outline of M1- and M2-like, and hybrid macrophage phenotypes in biomaterial-mediated fibrosis. Figure created with ©BioRender.io.
2.2. Role of M1 macrophages in fibrosis
Traditional investigations of the foreign body response have been performed using histological and immunohistochemical techniques; recently, Li and Liu completed a thorough review examining various visualization techniques that have been developed and used to evaluate cell behavior in vivo, including more advanced techniques such as second and third harmonic generation, which facilitates visualization of cells and structures in vivo in real time without fluorescent labeling [89]. In a recent study, second and third harmonic generation combined with immunohistochemistry were used to interrogate macrophage phenotype and fibrous capsule surrounding a 3D electrospun poly(caprolactone) (PCL) scaffold implanted subcutaneously within a dorsal window chamber in C57BL/6-Tg(UBC-GFP) mice, in which cells of hematopoietic origin are fluorescent [90]. Dondossola et al. found that VEGF was produced by cells surrounding the PCL scaffold fibers that were positive for CD68+, a general macrophage marker, as well as interferon regulatory factor 5 (IRF5) [90], which is a validated M1 marker in murine and human macrophages [91]. Cells surrounding the PCL scaffold were negative for CD163, which is expressed specifically by human M2c macrophages but is co-expressed by murine M2a and M2c macrophages [90, 92, 93]. These M1-like and VEGF-producing macrophages were associated with an immature neovascular network and a dense fibrous capsule after 2–4 weeks [90]. Administration of clodronate liposomes, which kill macrophages by inducing apoptosis following phagocytosis, resulted in significantly reduced neovascularization and fibrous capsule thickness. Equivalent reductions in the fibrous capsule thickness and the numbers of blood vessels and FBGCs were observed even four weeks after the initial treatment with clodronate liposomes, as well as after blocking angiogenesis by neutralizing VEGF via a chemical inhibitor. Together, these results suggest that M1-derived VEGF may directly contribute to the fibrous capsule formation [90]. Additionally, low-fouling poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogels implanted subcutaneously in C57BL/6 mice resulted in significantly elevated collagen density, decreased blood vessel count, and a higher proportion of M1 macrophages (iNOS, IL1R1, TNFα, CCR7, IL12) in the surrounding tissue relative to ultra-low fouling, zwitterionic poly(carboxybetatine methyacrylate) (CBMA) hydrogels [94]. Moreover, CBMA hydrogels exhibited a higher proportion of M2 macrophages (MRC1, Arg1, IL10, scavenger receptor B I/II (SRBI/II), FIZZ1) with significantly lower collagen density in the surrounding tissue [94]. These findings may suggest that materials that promote a more M1-like response (relative to M2) may lead to fibrous capsule formation and subsequently implicate M1 macrophages in the process. On the other hand contradictory findings concerning the role of M1 macrophages in fibrous capsule formation were found in another study in which hexamethylenediisocyanate-crosslinked dermal sheep collagen (HDSC) discs were subcutaneously implanted into macrophage Fas-induced apoptosis (MaFIA) mice [95]. MaFIA mice are derived from the C57BL/6 background and facilitate user-controlled macrophage depletion via administration of the small molecule AP20187 [95]. In contrast to the previously described study, macrophage depletion resulted in significantly increased fibrous capsule thickness and increase in number of fibroblast-like cells around the HDSC disc [95]. Moreover, the tissue surrounding the HDSC discs in macrophage-depleted mice exhibited decreased expression of M1 markers (IL1β, TNFα, CCL2 (a.k.a. macrophage inflammatory protein-1 alpha (MIP-1α)), and MMP13 [96–98]), along with increased expression of collagens, TIMPs, fibroblast-stimulating growth factors, and MMP2 [95]. These results suggest that the HDSC scaffolds also promoted an early M1-like phenotype, similar to the electrospun PCL fibers in the aforementioned study by Dondossola et al. It is possible that experimental differences, such as macrophage depletion methods in MaFIA mice compared to clodronate liposomes [26, 95], biomaterial architecture (porosity, thickness), and surface chemistry may have caused the disparate effects of M1-like macrophages in fibrous capsule thickness. Nonetheless, these studies support the fact that M1-like macrophages play a critical regulatory role in biomaterial-mediated ECM deposition. These studies and other seminal works regarding macrophage phenotype in the foreign body response are summarized in Table 1.
Table 1.
Studies implicating macrophage phenotype in biomaterial-mediated fibrosis.
| Model | Biomaterial | Fibrosis-associated Outcome | Biomarkers | Ref | |
|---|---|---|---|---|---|
| M1-associated studies | murine subcutaneous, dorsal window chamber (C57BL/6) | 3D-electrospun poly(caprolactone) (PCL) scaffold | immature neovascular network, dense fibrous encapsulation after 2–4 weeks | ↑ expression of VEGF, CD68, IRF5 | [90] |
| murine subcutaneous (C57BL/6) | hexamethylenediisocyanate-crosslinked dermal sheep collagen (HDSC) discs | decreased fibrous capsule thickness and decrease in number of fibroblast-like cells surrounding discs. | ↑ expression of IL1β, TNFα, CCL2, MIP1α, MMP13. ↓ collagens, TIMPs, FGFs, MMP2 | [95] | |
| murine subcutaneous (C57BL/6) | poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogel | more M1 macrophage than M2 macrophages, increased fibrous encapsulation, decreased blood vessel growth in tissue surrounding biomaterial | M1: iNOS, IL1R1, TNFα, CCR7, IL12; M2: MRC1, Arg1, IL10, SRBI/II, FIZZ1 | [94] | |
| Mixed M1 and M2-associated studies | murine intraperitoneal (IP) space | cube of boiled chicken egg white | increased macrophage infiltration, fibrous encapsulation; possible macrophage-myofibroblast transition. Later stages (>14 days) of response were associated with both pro- and anti-inflammatory genes, MSC-associated genes, and ECM-related molecules via microarray. | EGFP signal from MacGreen mice), F4/80, Ly6C, along with aSMA. 85% of aSMA+ cells were co-expressed for EGFP. Gene lists provided in Ref. 107 | [26, 133] |
| intraperitoneal (IP) space non-human primates (cynomolgus macaque) | alginate hydrogels | cylinders and smaller spheres (0.5mm) ⟹ ↑ cellular deposition and fibrous encapsulation vs. larger spheres (1.5mm) | Masson’s Trichrome stain (collagen) | [34] | |
| intraperitoneal (IP) space mice (C57BL/6) | alginate hydrogels | smaller spheres (0.5mm) ↑ macrophage deposition of M1, M2a and M2c phenotypes, whicle larger spheres (1.5mm), ↑ macrophage deposition of M1 and M2a | M1: CXCL10, TNFa, IL1, IRF5; M2a: Arg1, YM1, CCL22, Stab1, Dcir; M2c: Sphk1, Light, IL10 | [34] | |
| intraperitoneal (IP) space rats (albino oxford) | HDSC discs | fibrous encapsulation, ↑ deposition of M2a macrophages, no M1 macrophages over 21 days | Macrophage: CD68; M1: iNOS; M2a: CD206 | [134] | |
| murine subcutaneous (C57BL/6) | glutaraldehyde-crosslinked gelatin hydrogels | fibrous enapsulation, consistent M1 macrophage deposition over time, increasing M2a macrophage deposition over time. | M1: iNOS, M2: Arg1 and CD163. aSMA present near firbous capsule edge | [40] | |
| murine intraperitoneal (IP) space (C57BL/6) | murine intraperitoneal (IP) space (C57BL/6) | fibrous encapsulation, ↑ deposition M1 and M2 macrophages | M1: TNFα, IL1β, IL6, iNOS; M2: IL10, Arg1, CD36, Ym1 | [135] |
M1-related signals have also been implicated in promoting fibrosis in the absence of biomaterials. Macrophages from patients with idiopathic pulmonary fibrosis (IPF) express elevated levels of IL1β, which in turn promoted increased expression of collagen (type I and III) and fibronectin [99]. IL1β appears to serve an important role in the initiation of pulmonary fibrosis, based on the ability of intratracheal delivery of IL1β to recapitulate bleomycin-induced pulmonary fibrosis [100]. Moreover, activation and cleavage of IL1β and IL18, which are also found at elevated levels in patients with cardiac fibrosis [101] in addition to idiopathic pulmonary fibrosis, have been associated with inflammasome activation [102]. Inflammasome activation, which is upregulated in M1 macrophages [103] and inhibited in M2 macrophages [104], involves the formation of intracellular multimeric protein complexes that primarily control the response to pathogen-associated molecular patterns (PAMPs) and endogenous cell damage signals. There are several canonical pathways for inflammasome activation; chief among them is NOD-like receptor-family Pyrin domain containing 3 (NLRP3) [105]. Activation of the NLRP3 pathway is regulated by caspase 1 and apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), which are specifically upstream of IL1β and IL18. Inflammasome activation occurs in both macrophages and fibroblasts, but investigations into its role in biomaterials and the foreign body response have so far been limited to macrophages [106]. Another canonical inflammasome pathway, AIM2, along with ASC has been shown to modulate fibrotic encapsulation and inflammatory cell infiltration in response to polymethyl methacrylate (PMMA) beads in vivo, which are particularly useful for local antimicrobial and antibiotic drug delivery in orthopedic applications [107]. In AIM2 and ASC-deficient mice, PMMA bead delivery promoted significantly less inflammatory cell infiltration and fibrous encapsulation [108]. Further work was done using PMMA beads to explore the relationship between inflammasomes and the foreign body response, demonstrating that caspase 1, in addition to ASC, mediate fibrous encapsulation and inflammation [109]. Interestingly, a recent study designed a biomaterial to inhibit inflammasome activation in order to mitigate the foreign body response, further supporting a critical role of M1 macrophages in this process. Yao et al. developed a novel surface coating peptide for lanthanide-based nanocrystal nanoparticles to effectively reduce NAPDH oxide-generated ROS, which subsequently inhibited NLRP3 inflammasome activation in macrophages [110]. These data identify and provide proof of concept that targeting the inflammasome may be a unique strategy in biomaterial design to mitigate chronic inflammation.
Another pro-inflammatory cytokine, IL6, which both activates and is secreted by M1 macrophages [111], has been associated with initiation of fibrosis, as IL6-deficient mice have been reported to undergo less severe angiotensin II-induced cardiac fibrosis as evidenced by reduced αSMA, TGFβ1, and collagen expression [112]. The role of IL6 in fibrosis was further implicated in vitro when authors showed that a 1:1 fibroblast and macrophage co-culture caused fibroblasts to produce significantly more IL6 along with αSMA and collagen I [112]. When in vitro co-cultures were treated with an IL6-neutralizing antibody, significant decreases were observed in TGFβ1 expression and phosphorylation of SMAD3, a downstream signal in the TGFβ pathway [112]. Ma et al. collectively concluded that macrophages may stimulate cardiac fibroblasts to secrete IL6 and in turn drive fibrosis via the TGFβ pathway [112]. In addition to affecting the TGFβ pathway, IL6 has been shown to induce STAT3 signaling in macrophages [113]. STAT3 signaling has a controversial role in tissue fibrosis, with reports suggesting it may play a role in both anti- and pro-fibrotic behavior [114]. Fibroblast-specific knockout of STAT3 or blocking via pharmacologic inhibitors resulted in significantly reduced models of systemic sclerosis, a fibrotic disease characterized by the hardening of connective tissue [115], suggesting that STAT3 signaling in fibroblasts is pro-fibrotic. However, STAT3-deficient macrophages isolated from bleomycin-induced fibrotic lesions displayed decreased expression of suppressor of cytokine signaling 3 (SOCS3), IL10, and decorin, as well as increased expression of TGFβ1 compared to control macrophages [114, 116], which suggests that STAT3 signaling may also be anti-fibrotic. Interestingly, when IL10 is used to induce STAT3 signaling in macrophages, it appears to have anti-fibrotic effects on fibroblasts via regulation of TGFβ1 signaling [114]. Collectively, these studies of fibrosis both in the presence and absence of biomaterials suggest that pro-inflammatory signals from M1 macrophages may be important and early regulators of fibrosis (Table 2). The contradictory nature of the findings motivate the need for future research to understand the optimal level of M1 macrophage behavior that is advantageous to inhibit excessive ECM deposition, while limiting the risk for chronic inflammation.
Table 2.
Studies implicating macrophage phenotype in non-biomaterial-mediated fibrosis
| Fibrosis Model | Species | Macrophage-associated Experimental Condition | Outcome | Reference | |
|---|---|---|---|---|---|
| M1-associated studies | idopathic pulmonary fibrosis | human | - | ↑ expression of IL1β, collagen type I and III, fibronectin | [99] |
| cardiac fibrosis | murine | IL6 knockout | ↓ expression of αSMA, TGFβ1, collagen | [112] | |
| bleomycin-induced fibrotic legions | murine | STAT3 knockout | ↓ expression of SOCS3, IL10, and decorin. ↑ expression of TGFβ1 | [115] | |
| M2-associated studies | scenescent muscle | murine | - | ↑ collagen, tissue stiffness, ↓ nNOS | [118] |
| scenescent muscle | murine | muscle-specific nNOS transgene | ↓ M2 macrophages and muscle fibrosis in older muscle tissue | [87] | |
| endometriosis | murine (Balb/C) | - | ↓ M2 macrophages (CD163), ↑ collagen, αSMA, SMAD3, and CD41 | [120] | |
| endometriosis | murine (C57BL/6) | macrophage ablation and adoptive transfer of M1, M2a, or M2c macrophages | adoptive transfer of M2a macrophages (MRC1) ⟹ ↑ collagen I, TGFβ1, αSMA compared to M1 (iNOS) or M2c (VTCN1) | [120] | |
| tissue fibrosis | murine | IL4Rα knockout | ↓ M2 markers (CD163, MRC1, PDGFB, IL10) and unchanged M1 markers (IL1β, TNFα, IL) vs. controls. M2a-derived RELMα ⟹ ↑ LH2 in fibroblasts | [124] | |
| fibrotic chronic pancreatitis | murine and human | IL4Rα knockout, macrophage depletion, and IL4/IL13 blocking antibodies | IL4/IL13 blocking ⟹ ↓ M2a macrophages and decrease in fibrosis | [125] | |
| bleomycin-induced lung fibrosis | murine | IL13Rα2 blocking antibody | ↓ TGFβ1 and collagen deposition | [126] | |
| heart-allograft fibrosis | murine | IL13Rα2 blocking via siRNA | fibrosis was prevented | [127] | |
| failing renal allograft | human | - | CD68, aSMA, MRC1 | [129] | |
| renal fibrosis | murine | SMAD3 knockout | macrophage-myofibroblast transition | [130] |
2.3. Role of M2 macrophages in fibrosis
For as many studies that have implicated M1 macrophages and pro-inflammatory signals in fibrosis, equal numbers have implicated M2 macrophages. For example, M2 macrophages (Arg1+CD206+) macrophages were associated with the fibrous capsule surrounding porous collagen scaffolds implanted subcutaneously in mice, while vascularized collagen scaffolds were characterized by fewer Arg1+ cells and more cells that were positive for M1 markers[41]. Other studies have demonstrated increasing expression of the M2 marker Arg1 over time as the fibrous capsule thickened surrounding subcutaneously implanted hydrogels [40, 117], further implicating M2 macrophages in biomaterial-mediated fibrosis, although M1 macrophages were also present in the fibrous capsule in these studies.
With respect to fibrosis in the absence of biomaterials, previous research has shown that senescent murine muscle is characterized by increased collagen content and tissue stiffness with reduced expression of neuronal nitric oxide synthase (nNOS) compared to young mice, which have been specifically linked with an upstream arginase-mediated mechanism governed by M2 macrophages [118]. Wang et al. went on to show that the prevention of age-related decreases in muscle nNOS expression, via a murine model that expresses a muscle-specific transgene of nNOS, was associated with significantly fewer M2 macrophages and a subsequent reduction in muscle fibrosis in older murine muscle tissue [87]. Another series of studies implicated macrophages and fibrosis in endometriosis, which have been described as wounds that repeatedly undergo reinjury and repair [119]. To elucidate the role of macrophages (and identify the specific phenotype of those was involved), Duan et al. initiated a three-pronged study; first, endometriosis was induced in Balb/C mice via intraperitoneal (IP) injection of endometrial fragments, followed by histological and immunohistochemical analysis of markers related to macrophage phenotype, epithelial-mesenchymal transition (EMT), fibroblast-to-myofibroblast transdifferentiation (FMT), smooth muscle metaplasia (abnormal smooth muscle formation) (SMM), and fibrosis [120]. Follow up experiments utilized C57BL/6 transgenic mice expressing the human diphtheria toxin receptor under the CD11b promotor (DTR-CD11b), which allows for macrophage depletion upon administration of diphtheria toxin [121]. Authors examined the effects of macrophage ablation and adoptive transfer of M1, M2a, and M2c macrophages after induction of endometriosis for an additional three weeks [120]. The number of M2 macrophages (CD163+ cells) significantly increased in mice with induced endometriosis over time, along with increased markers of fibrosis including total collagen (via Masson’s Trichrome staining), and expression of αSMA (myofibroblast marker), phosphorylated SMAD3 (epithelial marker), and CD41 (platelet aggregation) [120]. Interestingly, following induction of endometriosis and macrophage ablation, administration of M2a macrophages (cells positive for mannose receptor C-type 1 (MRC1), a.k.a CD206) promoted significantly increased expression of fibrosis markers, collagen I, TGFβ1, and αSMA compared to administration of M1 (positive for induced nitric oxide synthase (iNOS)) or M2c (positive for V-set domain-containing T-cell activation inhibitor (VTCN1), a.k.a. B7-H4)) macrophages [120].
Further elucidating the mechanisms behind the role of M2 macrophages in fibrosis, others have investigated M2-associated signaling pathways. IL4 and IL13, which are frequently used to promote the M2a phenotype in vitro, are secreted by Th2-polarized T cells, granulocytes, monocytes, and macrophages. IL4 signaling is initiated via two heterodimeric transmembrane receptor complexes: the type I receptor, found on most hematopoietic cells, includes IL4Rα and γc subunits, and the type II receptor, found on many non-hematopoietic cell types which include IL4Rα and IL13Rα1 [122]. Importantly, IL13 signaling is primarily initiated through the type II receptor, but also through a functional cell surface IL13Rα2 subunit [123]. Macrophages express both type I and type II receptors and subsequently, the M2a macrophage phenotype is initiated through the binding of IL4 to the type I receptor or the type II receptor, or both, in combination with IL13 binding to the type II receptor [122]. Knockout studies have demonstrated the importance of IL4 and IL13 signaling in tissue fibrosis; macrophages in cutaneous wounds of IL4Rα−/− mice exhibited a disrupted M1-to-M2 transition, as evidenced by significantly decreased expression of M2 markers, including CD163, MRC1, PDGFB, and IL10, and unchanged expression of M1 markers, IL1β, TNF, and IL6 over time, relative to controls [124]. Additionally, Knipper et al. also showed that secretion of Retnla resistin-like alpha (RELMα) by M2a macrophages directly promoted expression of the collagen-modifying enzyme LH2 in fibroblasts [124]. This study suggested that IL4 signaling was critical to the crosstalk between fibroblasts and macrophages by promoting M2 polarization among macrophages, which in turn mediated collagen production in fibroblasts during skin repair [124]. Indeed, other studies employed a series of knockout models to demonstrate that mice with induced fibrotic chronic pancreatitis that lacked IL4Rα, macrophage-specific IL4Rα, or IL4 and IL13 resulted in diminished evidence of fibrosis [125]. Moreover, when IL4 and IL13 were blocked in murine or human ex vivo models with chronic pancreatitis, the number of M2a macrophages were significantly reduced along with a subsequent decrease in fibrosis [125].
Macrophages stimulated with both IL13 and the pro-inflammatory stimulus TNFα produced significantly higher levels of TGFβ1 relative to macrophages stimulated with IL4, IL13, or IL4 in combination with TNFα [126]. Relatedly, when the IL13Rα2 pathway was blocked, there was a significant downregulation of TGFβ1 production and collagen deposition in a murine model of bleomycin-induced lung fibrosis [126]. Moreover, in a follow-up study where the IL13/TGFβ1 interaction was blocked by IL13Rα2 silencing RNA (siRNA), heart allograft fibrosis was prevented [127].
Another new and still developing area of research in fibrosis is macrophage-to-myofibroblast transition (MMT). Recent work has shown that in renal fibrosis using macrophage lineage tracing in mice, macrophages appear to transdifferentiate into myofibroblasts with elevated αSMA and collagen 1 expression [128]. Biopsies of fibrotic lesions from human patients with a failing renal allograft revealed CD68+ macrophages that were also αSMA+ and CD206+, implicating M2 macrophages in this process [128]. Using murine knockout models, authors found that SMAD3 signaling in M2 macrophages may be a key driver of the macrophage-myofibroblast transition [129, 130]. Interestingly, MMT has also been implicated in response to biomaterial implantation; sterile cubes of boiled chicken egg white that were implanted into MacGreen transgenic mice (csf1r-EGFP), which labels macrophage, trophoblast, and granulocyte lineages [131], resulted in elevated levels of EGFP, F4/80, Ly6C, along with αSMA [26]. Flow cytometric analysis of fibrous capsule tissue after 14 days demonstrated that 85% of αSMA+ cells co-expressed EGFP. These results suggest that macrophages transdifferentiated into myofibroblasts, although in theory, they could also suggest that macrophages fused with or phagocytosed αSMA+ fibroblasts. However, another study that employed a model of renal fibrosis in Lyz2-Cre/Rosa26-Tomato lineage tracing mouse demonstrated that approximately half of all αSMA+ myofibroblasts were of bone marrow-derived macrophage origin [130]. Flow cytometry demonstrated that cell fusion was not occurring among the myofibroblasts from the lineage tracing mice undergoing renal fibrosis. These data shown were also collected on day 28, a time point by which the contents of the phagolysosome would have been destroyed, indicating αSMA+ positive signal is unlikely to be a result of phagocytosis [130]. Additional work by Sinha et al. demonstrated through a series of knockout models, cross-reference immunostaining, transcriptomic analysis, and ex vivo culture models, that over two-thirds of fibroblasts found in wound granulation tissue were confirmed myeloid-origin [132]. Based on these observations, it appears likely that macrophage to (myo)fibroblast transition does occur.
These studies demonstrate a strong connection of M2-like macrophages and M2-associated signaling (i.e. IL4, IL13, TGFβ1) with fibrotic conditions ranging from the foreign body response to endometriosis, renal- and heart-associated allograft fibrosis, and pulmonary fibrosis.
2.4. Mixed M1 and M2 macrophages in biomaterial-mediated fibrosis in vivo
While several studies have identified important roles in fibrosis for either M1 or M2 macrophages or their signaling pathways, other studies have demonstrated a mixture of both M1 and M2 macrophages in fibrotic regions. It stands to reason that the conflicting information regarding the role of M1 and M2 macrophages in fibrosis may result from the presence of both phenotypes cooperating to promote fibrosis, or that there is a hybrid M1/M2 phenotype driving fibrosis.
Mooney et al. implanted cubes of boiled chicken egg white, as a model biomaterial, into the intraperitoneal (IP) space of MacGreen mice; EGFP+ cells were sorted from the fibrous capsule surrounding the foreign material at time points ranging from 2 days to 28 days post-implantation, and subsequently processed for gene expression analysis by microarray followed by network analysis [133]. Cells present at later stages (>14 days) of the foreign body response expressed a mixture of M1 and M2-associated genes in addition to MSC-associated genes [133]. The authors discussed similarities in the macrophages found in foreign body response with fibrocytes, which are hypothesized to play a combined role in inflammation and ECM deposition / remodeling (for review, see Ref. [57]). The transcriptional analysis demonstrated that macrophages within biomaterial-mediated fibrosis, especially at later time points (>14 days), do not exbibit discrete M1 or M2 phenotypes, but rather express markers of both phenotypes and share similarities with fibroblasts and fibrocytes [133].
In a separate series of studies, the primary cells involved in the foreign body response to various biomaterials of different sizes and shapes (spherical or cylindrical) implanted into the IP space of mice, rats, and non-human primates were characterized [34]. Biomaterial shape, rather than material origin or stiffness, was the significant driver of the foreign body response [34]. Alginate hydrogel cylinders and 0.5mm-diameter spheres implanted subcutaneously in non-human primates for two weeks resulted in significantly more macrophage deposition and fibrous encapsulation compared to 1.5mm-diameter spheres [34]. Further analysis was conducted in C57BL/6 mice to facilitate kinetic profiling of the host response to alginate spheres via flow cytometry, intravital imaging, and gene expression analysis to elucidate macrophage phenotype. The results revealed that the fibrosis-inducing 0.5mm-diameter spheres elicited significantly elevated numbers of macrophages at all time points analyzed (1, 4, 7, 14, and 28 days) relative to the 1.5mm-diameter spheres via flow cytometry and intravital imaging. Gene expression analysis showed that 0.5mm-diameter spheres also promoted increased levels of markers associated with M1, M2a, and M2c phenotypes, while 1.5mm-diameter spheres promoted increased levels of M1 and M2a macrophages over seven days [34]. These findings may implicate M2c macrophages in driving fibrous capsule formation and/or that a hybrid M1/M2a phenotype is responsible for promoting fibrous encapsulation. Indeed, colocalization of M1 and M2 macrophages has also been observed surrounding HDSC scaffolds subcutaneously implanted in Albino Oxford rats [134], glutaraldehyde-crosslinked gelatin hydrogels subcutaneously implanted in C57BL/6 mice over three weeks [40], and mixed cellulose filters or polydimethylsiloxane (PDMS) disks implanted into intraperitoneally in C57BL/6 mice for one week [135]. Moore et al. investigated the foreign body response in mice deficient for MCP1 (aka CCL2) [135], which has been associated with the M1 macrophage phenotype as well as macrophage fusion into FBGCs [136]. In wild-type mice, macrophages surrounding the PDMS disks upregulated the M1-associated TNFα and NFκB, as well as increased expression and secretion of TGFβ1, which is typically associated with the M2 phenotype [135]. These processes, along with a diminished fibrotic response, were inhibited in MCP1-knockout mice, suggesting a possible role of MCP1 in reducing inflammation and fibrotic behavior [135]. Moreover, Moore et al. also found that M1 (TNFα, IL1β, IL6, iNOS) and M2 (IL10, Arg1, CD36, Ym1) macrophages were both present in the fibrous encapsulation, but iNOS and Arg1 were not co-localized via immunohistochemical staining, which may suggest both M1 and M2 macrophages were present individually (as opposed to a hybrid phenotype) [135].
Collectively, these studies illustrate that macrophages exhibiting characteristics of both M1 and M2 macrophages are associated with the fibrous encapsulation of biomaterials.
3. Macrophage-fibroblast crosstalk in vitro
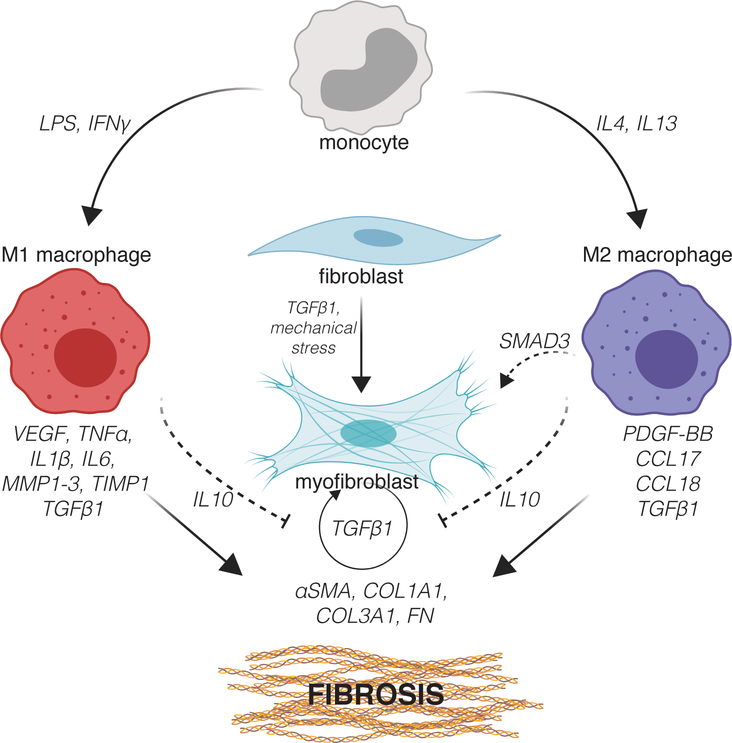
Due to the challenges in tracking macrophages and fibroblasts in vivo, studies often employ in vitro models to elucidate the how these distinct cell populations communicate to facilitate tissue repair. Despite being oversimplified relative to the in vivo environment, in vitro studies have been critical to understanding the behavior of these cells and how they directly and indirectly affect each other’s behavior within the context of fibrosis (Figure 3).
Figure 3.
Macrophage-fibroblast crosstalk in fibrosis. Figure created with ©BioRender.io.
In the in vivo environment, macrophages and fibroblasts communicate via juxtracrine and paracrine signaling, which have been modeled in vitro by culturing macrophages with fibroblasts in direct contact or separated via transwell membranes. Utilizing murine primary and cell-line derived cells, Holt et al. found that conditioned media from unactived macrophages of the cell line RAW 264.7 as well as bone marrow-derived origin promoted an increase in NIH 3T3 fibroblast secretion of pro-inflammatory cytokines (IL-6, TNFα, MCP1, macrophage inflammatory proteins 1β (MIP1β) and 1α (MIP1α)) primarily during the first three days of culture, an effect that tended to diminish over time [137]. In contrast, NIH 3T3 fibroblast-derived conditioned media caused reduced pro-inflammatory cytokine production in macrophages, suggesting that macrophages and fibroblasts may function in a feedback loop to limit excessive pro-inflammatory cytokine generation [137]. Additionally, fibroblasts and macrophages cultured under endotoxin challenge (LPS) resulted in significant upregulation of all pro-inflammatory markers in both cell types and led to macrophage fusion into FBGCs.
A more recent study was designed to delineate the effects of myofibroblast-derived signals on M2a macrophages; primary murine bone marrow derived macrophages were pre-treated with IL4 and IL13 to generate M2a macrophages and subsequently cultured in primary murine dermal myofibroblast conditioned media [138]. The authors found that myofibroblast-conditioned media promoted increased gene expression of M2 markers arginase 1 (ARG1) and chitinase-3-like 3 (YM1), along with spontaneous secretion of IL10 and suppressed nitric oxide (NO) production. To understand the downstream effects of this behavioral shift in the macrophages, myofibroblasts were then cultured in conditioned media derived from the M2a macrophages that had been cultured with myofibroblast-derived signals, which resulted in reduced migration, expression of COX2, and production of PGE2 and PGD2 [138]. These results suggest that M2a macrophages cultured with myofibroblast conditioned media may in turn be able to inhibit downstream myofibroblast behavior in another feedback loop [138]. Similar results were observed in another study when primary murine cardiac fibroblasts were pre-treated with TGFβ1, simulating a myofibroblast-like phenotype and subsequently co-cultured with monocytes; after 72 hour incubation, monocytes adopted an anti-inflammatory phenotype, secreting increased levels of IL10 and decreased levels of pro-inflammatory IL12 [139, 140]. Taken together, these results illustrate that M2a macrophage-myofibroblast-signaling may bi-directionally regulate aberrant cell behavior [138].
In another study, conditioned media generated from different macrophage phenotypes was employed to examine the effect of macrophage signals on fibroblast behavior. Conditioned media from M1 macrophages promoted pro-inflammatory, but anti-fibrotic behavior in fibroblasts, demonstrated by decreased collagen production and increased production of pro-inflammatory factors (CCL2, IL6, CCL7, MMP-1, −2, and −3) [141]. The M2a macrophage-conditioned media did not have a major effect on fibroblasts; however, when fibroblasts were initially exposed to M1 signals and subsequently changed to M2a macrophage-conditioned media, to mimic the natural sequential behavior of the M1-to-M2a macrophage transition that is observed in wound healing in vivo, fibroblasts downregulated pro-inflammatory and enzymatic genes CCL2, IL6, and MMP1, and significantly upregulated COL1A1, after 72 and 144 hours [141]. In contrast, another study showed that M2a macrophage signals did have a significant effect on fibroblast behavior, in that THP1-derived M2a macrophage conditioned media promoted myofibroblast differentiation (evidenced by increased levels of αSMA) of human dermal fibroblasts as well as increased collagen production and expression of COL1A1, TGFβ1 and TIMP1 [142].
Collectively, these results suggest that the M2 macrophage-derived signals directly influence fibroblasts to express genes and secrete proteins to promote ECM deposition. Interestingly, these findings appear to be in agreement with previously described results that suggested a direct link between M2-like macrophages, MMT and fibrosis.
3.1.1. Models to examine biomaterial-mediated fibrosis
Several models and systems have been developed to specifically characterize macrophage and fibroblast behavior in vitro in 2D and 3D. These studies often use model biomaterials with modifiable properties to demonstrate the versatility of the model in studying or predicting changes in cell behavior.
Several studies have described 2D monoculture models to examine the behavior of macrophages (thoroughly reviewed in Ref. [143]) or fibroblasts (reviewed in Ref. [144]) in response to changing biomaterial properties. Other in vitro models have strived to add complexity to the culture systems by adding signals from conditioned media generated from other monocultures, or by introducing the other cell type separated by a transwell insert. In one model, 54 different polymer surfaces were developed by manufacturing three different polymer rods made of polycaprolactone (PCL) and two poly(ethylene oxide terephthalate)/poly(butylene terephthalate)-based co-polymers PA300 and PA1000, which were each subjected to changes in topography, roughness, hydrophobicity/hydrophilicity via solvent surface etching with sodium hydroxide, physical surface etching with chloroform or dichloromethane, or gas plasma treatment with argon, oxygen, or trifluoroethane [145]. Macrophages or fibroblasts were then cultured on the polymer rods placed on top of an agarose mold to prevent cell attachment to the cell culture wells [145]. Cell attachment and proliferation, pro-inflammatory cytokine (IL1β and IL6) and anti-inflammatory cytokine (TGFβ1 and IL10) secretion, and collagen and elastin synthesis were measured. Physically etched polymers via chloroform resulted in decreased pro-inflammatory and increased anti-inflammatory cytokine secretion in both fibroblasts and macrophages, along with the highest levels of collagen and elastin production by fibroblasts. To add an additional element of complexity, another model was developed to combine self-assembled monolayer biomaterials with various changes in surface wettability and charge with cell culture fence chambers with internal and external compartments that facilitated co-cultures of THP1-derived macrophages and fibroblasts [146]. Hydrophobic surfaces prepared with methyl (CH3)-terminated groups yielded the most significant pro-inflammatory response in macrophage-fibroblast co-cultures, while hydrophilic/anionic surfaces prepared with carboxylic acid (COOH)-terminated groups resulted in significantly diminished inflammatory response [146]. Interestingly, macrophages in this system were significantly more motile in the presence of fibroblasts compared to macrophages in monoculture. Notably, the fence chamber design in combination with novel biomaterials were particularly advantageous for assessing and simulating paracrine, autocrine, and juxtracrine signaling [146, 147].
Recent studies have demonstrated the utility of 3D in vitro systems and biomaterial platforms to aid in studying the complex mechanotransduction, phenotypic changes, and signaling between macrophages and fibroblasts. Jannasch et al. embedded fibroblasts within a 3D collagen hydrogel incubated in media derived from macrophages cultured on various biomaterial surfaces (glass, titanium, and polytetrafluoroethylene (PTFE)) [148]. Live cell imaging combined with computational modeling allowed the authors to determine the chemotactic potential of the fibroblasts and long-term behavior of fibroblasts within the 3D collagen matrix environment [148]. Fibroblasts were significantly more motile within the hydrogel when cultured in conditioned media derived from macrophages cultured on titanium compared to conditioned media derived from macrophages cultured on glass or PTFE. On the other hand, fibroblasts cultured in conditioned media derived from macrophages cultured on PTFE demonstrated significantly greater remodeling capacity, evidenced by increased collagen fibril deposition and organization within the hydrogel, compared to the other conditions [148]. Lastly, Caires et al. demonstrated the utility of a 3D co-culture system to examine the interdependent effects of macrophages, fibroblasts and MSCs on cell migration in response to poly(lactic acid) scaffolds or chitosan scaffolds [149]. This model showed that macrophages cultured with chitosan scaffolds induced the greatest recruitment of fibroblasts (whether cocultured with MSCs or not) compared to natural killer cells, monocytes, or peripheral blood mononuclear cells cultured in the same fashion. Interestingly, if MSCs and macrophages were cocultured together, macrophages no longer recruited fibroblasts [149], suggesting that the arrival and signaling of MSCs may limit excessive fibroblast recruitment associated with fibrosis.
In addition to 2D and 3D in vitro models, other studies have examined changes in macrophage and/or fibroblast behavior in vitro and in vivo in response to various biomaterials to assess for a potential foreign body response. Indeed, we have investigated and characterized macrophage behavior in response to several different types of biomaterials including commercially-available wound matrices [38, 39], drug-eluting nanoparticles [37] and microparticles [48], ceramic scaffolds [36, 41], collagen scaffolds [47], and gelatin scaffolds [40], which have illustrated that various material properties modulate macrophage phenotype, often promoting hybrid phenotypes, which are important considerations for the future of immunomodulatory biomaterials.
4. Strategies for modulating macrophage and fibroblast behavior to control biomaterial-mediated fibrosis
The design of biomaterials to specifically modulate macrophage behavior has emerged as a promising strategy in regenerative medicine and tissue engineering (See Refs. [21, 150–154]), including utilization of controlled release systems [155] or microRNA-focused techniques [156]. Here we highlight key studies that have modified biomaterial surfaces and structures to control both macrophage and fibroblast behavior along with combination strategies that include delivery of soluble factors through the use of coatings, films, electrospinning, liposomes, and polymeric particles.
4.1. Biomaterial surface / structure modification-focused approaches
Non-degradable biomaterials such as silicone or bulk metallic glasses (BMGs) can provide long term mechanical stability in the body but can elicit a strong foreign body response. Many have investigated engineering the surfaces of such materials using nano- and micro-patterning techniques to control the behavior of surrounding cells, especially macrophages and fibroblasts, to reduce adhesion and cellular activation. Padmanabhan et al. showed that nanopatterning of highly tunable BMGs with topographical features ranging in size from 55nm to 200nm resulted in changes in fibroblast, macrophage, and endothelial cell attachment [157]. More specifically, fibroblasts adhered to nanopatterns of all sizes, while macrophages and endothelial cells did not adhere to nanopatterns smaller than 150nm and 100nm, respectively [157]. A follow-up study showed that BMGs with nanopatterns that were 55nm in size (BMG-55) seeded with either M1 (stimulated with LPS/IFNγ) or M2 (stimulated with IL4) murine bone marrow-derived macrophages resulted in a significant reduction in pro-inflammatory protein secretion (TNFα, IL1α, IL12, CCL2, and CXCL1), increased phagocytosis and decreased cell area, after 24 hours in culture relative to unpatterned BMG [158]. Histological analysis following a two-week subcutaneous study in mice showed that BMG-55 implants promoted an increase in the ratio of cells positive for Arg1 (an M2 marker) to iNOS (an M1 marker), as well as in the number and size of blood vessels, with decreased macrophage fusion and fibrous capsule thickness, compared to unpatterned BMG implants [158]. Similar strategies to use surface patterning to modulate cell behavior were also applied to silicone and hydrogel implants; Robotti et al. showed that a micron-range symmetrical array of hexagonal pits interfered with human dermal fibroblast focal adhesion and maturation into myofibroblasts [159]. Moreover, culture of fibroblasts or THP1-derived macrophages on the array for over 72 hours resulted in significantly reduced cell adhesion, proliferation, and pro-fibrotic CCL17 expression, suggesting that the biomaterial surface might limit fibrosis in vivo, although this was not directly tested [159].
In another approach, Majd et al. designed a biomaterial surface that would facilitate macrophage and fibroblast adhesion while eliminating a pro-fibrotic response by controlling the size of focal adhesions via surface micropattern size and coating [160]. Fibronectin, collagen or poly N-acetyl glucosamine (sNAG) were patterned via micro-stenciling onto silicone substrates, creating evenly spaced points of controlled geometry (a.k.a. islets), and compared to coatings with no pattern. After seeding TGFβ1-activated subcutaneous rat fibroblasts onto the materials, an islet size of 4×2μm regardless of coating material yielded fibroblast attachment and proliferation, while simultaneously suppressed αSMA organization into contractile fibers compared to uncoated, 2×2μm, 6×2μm, 8×2μm, 10×2μm, or 20×2μm micropatterns. More macrophages adhered to fibronectin-coated and uncoated surfaces compared to the 4×2μm collagen-micropatterned materials. Histological analysis following a 30-day subcutaneous implantation in rats revealed the collagen-coated 4×2μm micropatterned material had significantly less macrophages (with no FBGCs) and myofibroblasts, along with a significantly thinner layer of αSMA+ cells and fibrous capsule formation compared to collagen-coated or uncoated materials. These results suggest that biomaterial surface features can be optimized to control focal adhesions and attenuate fibrous capsule formation.
4.2. Drug delivery systems to modulate macrophage and fibroblast behavior
To provide an additional level of control over cell behavior, several have designed drug delivery systems for bolus, controlled, and/or sequential release of various soluble factors to modulate macrophage and/or fibroblast behavior.
Despite silicone being a particularly popular biomaterial in aesthetic surgery [161], silicone biomaterials have been shown to elicit a classic foreign body response [162]. Controlled release of tranilast (a TGFβ1 inhibitor) from a PLGA spray coating on silicone implants implanted subcutaneously in Sprague-Dawley rats resulted in significant reduction in fibrous capsule thickness and collagen density surrounding the implant compared to controls, including a tranilast-adsorbed silicone implant, over 15 days [163]. These results were correlated with significantly reduced numbers of macrophages and fibroblasts by 12 weeks, suggesting a role for TGFβ1 in recruiting these cells or otherwise mediating their interactions with biomaterials [163]. In another study, Riabov et al, incorporated an M2-stimulating cocktail of soluble factors (IL4, IL10, and TGFβ1) into thin films of gelatin-hyaluronic acid-tyramine via spin coating [164]. In an in vitro co-culture of primary human macrophages and GFP-labeled human lung fibroblasts (MRC-5 cell line), the M2-stimulating cocktail promoted fibroblast migration and increased MMP7 secretion in a scratch assay [164]. In a monoculture of macrophages, sustained release of the M2-stimulating cocktail promoted decreased secretion of pro-inflammatory cytokines TNFα, IL6, and IL1β, and increased secretion of the M2 marker (and pro-fibrotic cytokine) CCL18 after 6 days in vitro [164].
Electrospinning provides a tunable platform to alter cellular function based on both surface topography as well as controlled release of biochemical cues. One strategy first employed a core-shell electrospun fiber, yielding two different components that each contained different drugs; core-shell fibers were electrospun into a mat and then electrosprayed, creating a third component [165]. Capitalizing on previous work that nuclear factor-κB (NFκB) signaling was required for macrophages to fuse into FBGC, Morris et al. designed their multicomponent system to release Bay 11–7082, a nuclear factor-κB (NFκB) inhibitor from the shell, along with an antibiotic, ampicillin from the core and an anti-fibrotic pirfenidone from electrosprayed coating [165]. The strategy behind this design was to inhibit FBGC formation, reduce encapsulation of the implant, and prevent bacterial infection [165]. In vitro benchtop release studies illustrated drug release on different timescales, which was also reflected in vivo, where macrophage fusion was successfully mitigated after one week, TGFβ1 secretion was suppressed, and fibrotic encapsulation was decreased in a dose-dependent manner over four weeks [165].
Long term use of glucocorticoids has been shown to inhibit healthy cell function within the context of wound healing [166]. However, Gauthier et al., sought to promote a “hyper-local” delivery of dexamethasone (DEX), a known anti-inflammatory drug, by releasing it directly and preferentially to macrophages [167]. DEX-loaded liposomes were fabricated with 10% of the surface modified to present phosphatidylserine (PS) [167], which is an apoptotic cell surface marker that is critical in the phagocytic clearing of dying cells by macrophages (a.k.a. efferocytosis) [168]. PS-modified liposomes cultured with macrophages resulted in decreased M1 macrophage cytokine release (IL6 and TNFα), increased thrombospondin 1 and efferocytosis activity with elevated uptake and potency relative to control liposomes that were modified with polyethylene glycol (PEG). Additionally, PS-modified liposomes were preferentially targeted and phagocytosed by macrophages even when cultured with fibroblasts and keratinocytes, or a 3D ex vivo skin model [167]. In another study, macrophage-fibroblast interactions were controlled to promote tissue deposition to enhance healing of diabetic wounds. Following the discovery that the small non-coding RNA miR-21 was more abundant in healing human diabetic wounds compared to non-healing wounds, and to mediate the transition of macrophages into fibroblasts in murine skin wounds, Sinha et al. administered miR-21-containing lipid nanoparticles to a murine model of delayed diabetic wound healing [132]. MiR-21 delivery caused macrophages to transition into fibroblast-like cells, leading to increased collagen deposition and normal skin stiffness.
Generally, it is appreciated that during normal wound healing and tissue repair macrophages exhibit an initial M1-like phenotype, which transitions into an M2-like phenotype over time [76]. In fact, studies have shown that controlled release of the M2a-promoting cytokine IL4 from either a nanometer-thick polyelectrolyte coating of polypropylene meshes implanted in the IP space [169] or collagen scaffolds containing IL4-loaded microparticles implanted subcutaneously [170] resulted in significantly elevated levels of local M2-like macrophages and decreased levels of M1 macrophages during early stages (7 days) of the foreign body response. The early macrophage response to the IL4-coated polypropylene meshes was correlated with significantly decreased fibrous capsule thickness and improved tissue integration after 90 days [169]. Others have also investigated macrophage and fibroblast behavior in response to other M2-promoting biomaterials. Truong and Jones hypothesized that biomaterial release of capsaicin, an anti-inflammatory capsinoid derived from chili peppers, would promote M2-like behavior to reduce the foreign body response [171]. To test this hypothesis, capsaicin-laden PLGA films were developed for in vitro studies and capsaicin-laden PLGA discs for subcutaneous implantation in C57BL/6 mice. Macrophages of the RAW 264.7 cell line cultured on capsaicin-PLGA films showed a marked upregulation in M2 makers (Arg1 and IL10) and downregulation of M1 markers (iNOS and IL12) compared to PLGA films alone. In vitro results were corroborated by in vivo subcutaneous implantation of capsaicin-PLGA discs, which showed reduced collagen deposition within and surrounding the discs by 40% and induced a significant increase in IL10 secretion relative to PLGA discs alone. Collectively, these studies and models establish that biomaterials can be designed to modulate macrophage and fibroblast behavior, in order to control inflammation and ECM deposition.
5. Conclusions
We have provided an extensive review of 1) the role of macrophage phenotypes in fibrosis, 2) in vitro models to study the foreign body response, 3) macrophage and fibroblast crosstalk in vitro, and 4) biomaterial strategies to modulate macrophage and fibroblast behavior for tissue regeneration. Collective information from in vitro and in vivo studies indicate that both M1 and M2 macrophages are present within biomaterial-mediated fibrosis. The presence of both phenotypes may suggest unique functions within the fibrous capsule or implicate the formation of a new hybrid macrophage phenotype. It is now clear that strategies to promote either the M1 or M2 phenotypes of macrophages will not necessarily be successful because of the complexities of the involvement of both of these phenotypes in fibrosis. Moreover, various clinical pathologies or diseases may require yet-to-be-defined macrophage and fibroblast phenotypes to facilitate optimal tissue remodeling. Additional basic research will be necessary to understand the optimal temporal and spatial presentation of macrophages and fibroblasts (and their phenotypes) in order to control fibrosis, which will allow the design of better, more targeted biomaterials. Because of the importance of fibrosis in many diseases, better understanding of the relationship between fibroblasts and macrophages and how to control it is of great importance across many clinical applications.
Acknowledgements
The authors would like to gratefully acknowledge NHLBI R01 HL130037 to KLS.
Biography

Dr. Kara Spiller is an Associate Professor in Drexel University’s School of Biomedical Engineering, Science, and Health Systems. Her research interests include the role of immune cells in tissue regeneration, the design of immunomodulatory biomaterials, and international engineering education. Her research is funded by the NIH, the NSF, and private foundations. Her awards include the NSF CAREER award, the United States nominee for the ASPIRE prize, and a Fulbright fellowship (Portugal).

Claire Witherel is a PhD Candidate in Biomedical Engineering at Drexel University. Her current research, under the advisement of Dr. Kara Spiller, is focused on investigating macrophage and fibroblast interactions with biomaterials in the context of wound healing and the foreign body response. She is interested immunomodulatory biomaterial design to support tissue regeneration. She graduated with a BS/MS in Biomedical Engineering from Drexel University in 2012 and was awarded a Whitaker Fellowship (UK) in 2013.
Dr. Tom Barker is a Professor in the Department of Biomedical Engineering at the University of Virginia and the Director of the UVA Fibrosis Initiative. Dr. Barker’s research activities center cell-extracellular matrix biology. His research integrates engineering and basic cell and molecular biology approaches to understand the fundamental roles of cell mechanotransduction and mechanical forces in regulating the biochemical activity of proteins in the extracellular matrix toward wound repair, regeneration, and fibrosis. His research is funded by the NIH and DOD. His awards include both the Young Investigator Award and Iozzo Award from the American Society for Matrix Biology and the NIH Director’s Transformative Research Award.
Contributor Information
Claire E. Witherel, Drexel University, School of Biomedical Engineering, Science and Health Systems, 3141 Chestnut Street, Philadelphia, Pennsylvania 19104, USA
Dr. Daniel Abebayehu, University of Virginia, Department of Biomedical Engineering, School of Engineering & School of Medicine, 415 Lane Road, Charlottesville, Virginia 22904, USA
Prof. Thomas H. Barker, University of Virginia, Department of Biomedical Engineering, School of Engineering & School of Medicine, 415 Lane Road, Charlottesville, Virginia 22904, USA
Prof. Kara L. Spiller, Drexel University, School of Biomedical Engineering, Science and Health Systems, 3141 Chestnut Street, Philadelphia, Pennsylvania 19104, USA, spiller@drexel.edu
References
- [1].Bhat S, Kumar A, Biomaterials and bioengineering tomorrow’s healthcare, Biomatter, 3 (2013) e24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].I.o. Medicine, “Front Matter” Medical Devices and the Public’s Health: The FDA 510(k) Clearance Process at 35 Years, The National Academies Press; (2011). [Google Scholar]
- [3].Biomaterials Market by Type of Materials (Metallic, Ceramic, Polymers, Natural) & Application (Cardiovascular, Orthopedic, Dental, Plastic Surgery, Wound Healing, Neurology, Tissue Engineering, Ophthalmology) - Global Forecast to 2021, Markets and Markets, BT1556 (2016). [Google Scholar]
- [4].Quarterly Meeting on MDUFA III (FY 2013–2017) Performance, Food and Drug Administration, (2015). [Google Scholar]
- [5].Williams DF, Biocompatibility Pathways: Biomaterials-Induced Sterile Inflammation, Mechanotransduction, and Principles of Biocompatibility Control, ACS Biomaterials Science & Engineering, 3 (2017) 2–35. [DOI] [PubMed] [Google Scholar]
- [6].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin Immunol, 20 (2008) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM, Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells, Journal of Biomedical Materials Research Part A, 83A (2007) 585–596. [DOI] [PubMed] [Google Scholar]
- [8].MacLauchlan S, Skokos EA, Meznarich N, Zhu DH, Raoof S, Shipley JM, Senior RM, Bornstein P, Kyriakides TR, Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9, Journal of Leukocyte Biology, 85 (2009) 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wynn TA, Barron L, Macrophages: Master Regulators of Inflammation and Fibrosis, Seminars in liver disease, 30 (2010) 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hannan RT, Peirce SM, Barker TH, Fibroblasts: Diverse Cells Critical to Biomaterials Integration, ACS Biomaterials Science & Engineering, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Coleman DL, King RN, Andrade JD, The foreign body reaction: a chronic inflammatory response, J Biomed Mater Res, 8 (1974) 199–211. [DOI] [PubMed] [Google Scholar]
- [12].Harrison J, Swanson DS, Lincoln AF, A comparison of the tissue reactions to plastic materials: Dacron, ivalon sponge, nylon, orlon, and teflon, A.M.A. Archives of Surgery, 74 (1957) 139–144. [DOI] [PubMed] [Google Scholar]
- [13].Britt CI, Miller EM, Felder ME, Sirak HD, Comparative Reaction of Mersilene and Silk Sutures Implanted Within the Heart, Annals of surgery, 153 (1961) 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nat Rev Immunol, 8 (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL, Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins, The Journal of Immunology, 136 (1986) 2348. [PubMed] [Google Scholar]
- [16].Martinez FO, Gordon S, The M1 and M2 paradigm of macrophage activation: time for reassessment, F1000Prime Reports, 6 (2014) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murray Peter J., Allen Judith E., Biswas Subhra K., Fisher Edward A., Gilroy Derek W., Goerdt S, Gordon S, Hamilton John A., Ivashkiv Lionel B., Lawrence T, Locati M, Mantovani A, Martinez Fernando O., Mege J-L, Mosser David M., Natoli G, Saeij Jeroen P., Schultze Joachim L., Shirey Kari A., Sica A, Suttles J, Udalova I, van Ginderachter Jo A., Vogel Stefanie N., Wynn Thomas A., Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines, Immunity, 41 (2014) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ, The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of Interleukin-4 receptor alpha (IL4Rα) signaling, Inflammation, 36 (2013) 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM, Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF, The Journal of Clinical Investigation, 101 (1998) 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brecht K, Weigert A, Hu J, Popp R, Fisslthaler B, Korff T, Fleming I, Geisslinger G, Brüne B, Macrophages programmed by apoptotic cells promote angiogenesis via prostaglandin E2, The FASEB Journal, 25 (2011) 2408–2417. [DOI] [PubMed] [Google Scholar]
- [21].Spiller KL, Koh TJ, Macrophage-based therapeutic strategies in regenerative medicine, Advanced Drug Delivery Reviews, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McNally AK, Anderson JM, Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: dependence on material surface properties, J Biomed Mater Res A, 103 (2015) 1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McNally AK, Anderson JM, Foreign body-type multinucleated giant cells induced by interleukin-4 express select lymphocyte co-stimulatory molecules and are phenotypically distinct from osteoclasts and dendritic cells, Experimental and Molecular Pathology, 91 (2011) 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Helming L, Gordon S, Molecular mediators of macrophage fusion, Trends in Cell Biology, 19 (2009) 514–522. [DOI] [PubMed] [Google Scholar]
- [25].Sheikh Z, Brooks JP, Barzilay O, Fine N, Glogauer M, Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials, Materials, 8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mooney JE, Rolfe BE, Osborne GW, Sester DP, van Rooijen N, Campbell GR, Hume DA, Campbell JH, Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response, Am J Pathol, 176 (2010) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Prokop S, Heppner FL, Goebel HH, Stenzel W, M2 polarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis, The American journal of pathology, 178 (2011) 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF, Macrophage polarization in response to ECM coated polypropylene mesh, Biomaterials, 35 (2014) 6838–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brodbeck WG, MacEwan M, Colton E, Meyerson H, Anderson JM, Lymphocytes and the foreign body response: Lymphocyte enhancement of macrophage adhesion and fusion, Journal of Biomedical Materials Research Part A, 74A (2005) 222–229. [DOI] [PubMed] [Google Scholar]
- [30].Khan UA, Hashimi SM, Bakr MM, Forwood MR, Morrison NA, CCL2 and CCR2 are Essential for the Formation of Osteoclasts and Foreign Body Giant Cells, Journal of Cellular Biochemistry, 117 (2015) 382–389. [DOI] [PubMed] [Google Scholar]
- [31].Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, Aresta-Dasilva S, Griffin M, Jhunjhunwala S, Webber M, Siebert S, Tang K, Chen M, Langan E, Dholokia N, Thakrar R, Qi M, Oberholzer J, Greiner DL, Langer R, Anderson DG, Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates, Nat Mater, 16 (2017) 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Milde R, Ritter J, Tennent Glenys A., Loesch A, Martinez Fernando O., Gordon S, Pepys Mark B., Verschoor A, Helming L, Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction, Cell Reports, 13 (2015) 1937–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baylón K, Rodríguez-Camarillo P, Elías-Zúñiga A, Díaz-Elizondo JA, Gilkerson R, Lozano K, Past, Present and Future of Surgical Meshes: A Review, Membranes, 7 (2017) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacik I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG, Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates, Nat Mater, 14 (2015) 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Thevenot P, Hu W, Tang L, Surface Chemistry Influence Implant Biocompatibility, Current topics in medicinal chemistry, 8 (2008) 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Graney PL, Roohani-Esfahani S-I, Zreiqat H, Spiller KL, In vitro response of macrophages to ceramic scaffolds used for bone regeneration, Journal of The Royal Society Interface, 13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pentecost AE, Witherel CE, Gogotsi Y, Spiller KL, Anti-inflammatory effects of octadecylamine-functionalized nanodiamond on primary human macrophages, Biomaterials Science, 5 (2017) 2131–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Witherel CE, Graney PL, Freytes DO, Weingarten MS, Spiller KL, Response of human macrophages to wound matrices in vitro, Wound Repair Regen, 24 (2016) 514–524. [DOI] [PubMed] [Google Scholar]
- [39].Witherel CE, Yu T, Concannon M, Dampier W, Spiller KL, Immunomodulatory Effects of Human Cryopreserved Viable Amniotic Membrane in a Pro-Inflammatory Environment In Vitro, Cellular and Molecular Bioengineering, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yu T, Wang W, Nassiri S, Kwan T, Dang C, Liu W, Spiller KL, Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels, Journal of Biomaterials Science, Polymer Edition, 27 (2016) 721–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G, The role of macrophage phenotype in vascularization of tissue engineering scaffolds, Biomaterials, 35 (2014) 4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Féréol S, Fodil R, Labat B, Galiacy S, Laurent VM, Louis B, Isabey D, Planus E, Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties, Cell Motility and the Cytoskeleton, 63 (2006) 321–340. [DOI] [PubMed] [Google Scholar]
- [43].Tous E, Weber HM, Lee MH, Koomalsingh KJ, Shuto T, Kondo N, Gorman JH Iii, Lee D, Gorman RC, Burdick JA, Tunable hydrogel-microsphere composites that modulate local inflammation and collagen bulking, Acta Biomaterialia, 8 (2012) 3218–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Luu TU, Gott SC, Woo BW, Rao MP, Liu WF, Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype, ACS Appl Mater Interfaces, 7 (2015) 28665–28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD, Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction, Annals of Biomedical Engineering, 42 (2014) 1508–1516. [DOI] [PubMed] [Google Scholar]
- [46].Garg K, Pullen NA, Oskeritzian CA, Ryan JJ, Bowlin GL, Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds, Biomaterials, 34 (2013) 4439–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds, Biomaterials, 37 (2015) 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Witherel CE, Gurevich D, Collin JD, Martin P, Spiller KL, Host–Biomaterial Interactions in Zebrafish, ACS Biomaterials Science & Engineering, (2017). [DOI] [PubMed] [Google Scholar]
- [49].Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, Wiens KE, Tang MS, Basu-Roy U, Mansukhani A, Allen JE, Loke P, Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct, Blood, 123 (2014) e110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kendall RT, Feghali-Bostwick CA, Fibroblasts in fibrosis: novel roles and mediators, Frontiers in Pharmacology, 5 (2014) 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kramann R, DiRocco DP, Humphreys BD, Understanding the origin, activation and regulation of matrix‐producing myofibroblasts for treatment of fibrotic disease, The Journal of Pathology, 231 (2013) 273–289. [DOI] [PubMed] [Google Scholar]
- [52].Iwata M, Sandstrom RS, Delrow JJ, Stamatoyannopoulos JA, Torok-Storb B, Functionally and Phenotypically Distinct Subpopulations of Marrow Stromal Cells Are Fibroblast in Origin and Induce Different Fates in Peripheral Blood Monocytes, Stem Cells and Development, 23 (2014) 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mack M, Yanagita M, Origin of myofibroblasts and cellular events triggering fibrosis, Kidney Int, 87 (2015) 297–307. [DOI] [PubMed] [Google Scholar]
- [54].Jordana M, Sarnstrand B, Sime PJ, Ramis I, Immune-inflammatory functions of fibroblasts, European Respiratory Journal, 7 (1994) 2212. [DOI] [PubMed] [Google Scholar]
- [55].Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CCW, The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation, Molecular Biology of the Cell, 22 (2011) 3791–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tripathi M, Billet S, Bhowmick NA, Understanding the role of stromal fibroblasts in cancer progression, Cell Adh Migr, 6 (2012) 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Reilkoff RA, Bucala R, Herzog EL, Fibrocytes: emerging effector cells in chronic inflammation, Nat Rev Immunol, 11 (2011) 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hinz B, The role of myofibroblasts in wound healing, Current Research in Translational Medicine, 64 (2016) 171–177. [DOI] [PubMed] [Google Scholar]
- [59].Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G, Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts, The Journal of Cell Biology, 122 (1993) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bocchino M, Agnese S, Fagone E, Svegliati S, Grieco D, Vancheri C, Gabrielli A, Sanduzzi A, Avvedimento EV, Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis, PLoS One, 5 (2010) e14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Alili L, Sack M, Puschmann K, Brenneisen P, Fibroblast-to-myofibroblast switch is mediated by NAD(P)H oxidase generated reactive oxygen species, Biosci Rep, 34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kollmannsberger P, Bidan CM, Dunlop JWC, Fratzl P, Vogel V, Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts, Science Advances, 4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang H-Y, Phan SH, Inhibition of Myofibroblast Apoptosis by Transforming Growth Factor β1, American Journal of Respiratory Cell and Molecular Biology, 21 (1999) 658–665. [DOI] [PubMed] [Google Scholar]
- [64].Carlson MA, Longaker MT, Thompson JS, Wound splinting regulates granulation tissue survival, Journal of Surgical Research, 110 (2003) 304–309. [DOI] [PubMed] [Google Scholar]
- [65].Desmoulière A, Redard M, Darby I, Gabbiani G, Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar, The American Journal of Pathology, 146 (1995) 56–66. [PMC free article] [PubMed] [Google Scholar]
- [66].Darby I, Skalli O, Gabbiani G, Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing, Lab Invest, 63 (1990) 21–29. [PubMed] [Google Scholar]
- [67].Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, Kubota T, Takeshita A, Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction, Cardiovasc Res, 64 (2004) 526–535. [DOI] [PubMed] [Google Scholar]
- [68].Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A, Autocrine TGF- and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts, Proceedings of the National Academy of Sciences, 107 (2010) 20009–20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB, Fibrotic extracellular matrix activates a profibrotic positive feedback loop, The Journal of Clinical Investigation, 124 (2014) 1622–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].DiEgidio P, Friedman HI, Gourdie RG, Riley AE, Yost MJ, Goodwin RL, Biomedical implant capsule formation: lessons learned and the road ahead, Ann Plast Surg, 73 (2014) 451–460. [DOI] [PubMed] [Google Scholar]
- [71].Lynch MD, Watt FM, Fibroblast heterogeneity: implications for human disease, Journal of Clinical Investigation, 128 (2018) 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Costa-Almeida R, Soares R, Granja PL, Fibroblasts as maestros orchestrating tissue regeneration, J Tissue Eng Regen Med, 12 (2018) 240–251. [DOI] [PubMed] [Google Scholar]
- [73].Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM, Distinct fibroblast lineages determine dermal architecture in skin development and repair, Nature, 504 (2013) 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Denu RA, Nemcek S, Bloom DD, Goodrich AD, Kim J, Mosher DF, Hematti P, Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable, Acta Haematol, 136 (2016) 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gardner AB, Lee SKC, Woods EC, Acharya AP, Biomaterials-based modulation of the immune system, BioMed research international, 2013 (2013) 732182–732182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B, Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis, J Exp Med, 204 (2007) 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mirza RE, Fang MM, Ennis WJ, Koh TJ, Blocking Interleukin-1β Induces a Healing-Associated Wound Macrophage Phenotype and Improves Healing in Type 2 Diabetes, Diabetes, 62 (2013) 2579–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E, Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages, Am J Respir Cell Mol Biol, 53 (2015) 676–688. [DOI] [PubMed] [Google Scholar]
- [79].Lewis C, Zhu M, Lieu M, Moodley S, Wang Y, McConaghy T, Larner B, Widdop R, Sobey C, Drummond G, Kemp-Harper B, CCL18 as a Mediator of the Pro-Fibrotic Actions of M2 Macrophages in the Vessel Wall during Hypertension, The FASEB Journal, 31 (2017) 825.822–825.822. [Google Scholar]
- [80].Belperio JA, Dy M, Murray L, Burdick MD, Xue YY, Strieter RM, Keane MP, The Role of the Th2 CC Chemokine Ligand CCL17 in Pulmonary Fibrosis, The Journal of Immunology, 173 (2004) 4692–4698. [DOI] [PubMed] [Google Scholar]
- [81].Nassiri S, Zakeri I, Weingarten MS, Spiller KL, Relative Expression of Proinflammatory and Antiinflammatory Genes Reveals Differences between Healing and Nonhealing Human Chronic Diabetic Foot Ulcers, Journal of Investigative Dermatology, 135 (2015) 1700–1703. [DOI] [PubMed] [Google Scholar]
- [82].Mirza R, Koh TJ, Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice, Cytokine, 56 (2011) 256–264. [DOI] [PubMed] [Google Scholar]
- [83].Kimball AS, Joshi AD, Boniakowski AE, Schaller M, Chung J, Allen R, Bermick J, Carson WF, Henke PK, Maillard I, Kunkel SL, Gallagher KA, Notch Regulates Macrophage-Mediated Inflammation in Diabetic Wound Healing, Frontiers in Immunology, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jetten N, Roumans N, Gijbels MJ, Romano A, Post MJ, de Winther MPJ, van der Hulst RRWJ, Xanthoulea S, Wound Administration of M2-Polarized Macrophages Does Not Improve Murine Cutaneous Healing Responses, PLOS ONE, 9 (2014) e102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Maione AG, Smith A, Kashpur O, Yanez V, Knight E, Mooney DJ, Veves A, Tomic-Canic M, Garlick JA, Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair, Wound Repair and Regeneration, 24 (2016) 630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA, Decreased Macrophage Number and Activation Lead to Reduced Lymphatic Vessel Formation and Contribute to Impaired Diabetic Wound Healing, The American Journal of Pathology, 170 (2007) 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang Y, Wehling-Henricks M, Samengo G, Tidball JG, Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide, Aging Cell, 14 (2015) 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Weng S-Y, Wang X, Vijayan S, Tang Y, Kim YO, Padberg K, Regen T, Molokanova O, Chen T, Bopp T, Schild H, Brombacher F, Crosby JR, McCaleb ML, Waisman A, Bockamp E, Schuppan D, IL-4 Receptor Alpha Signaling through Macrophages Differentially Regulates Liver Fibrosis Progression and Reversal, EBioMedicine, 29 (2018) 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Li Y, Liu T-M, Discovering Macrophage Functions Using In Vivo Optical Imaging Techniques, Frontiers in Immunology, 9 (2018) 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW, Friedl P, Examination of the foreign body response to biomaterials by nonlinear intravital microscopy, Nature Biomedical Engineering, 1 (2016) 0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Weiss M, Blazek K, Byrne AJ, Perocheau DP, Udalova IA, IRF5 is a specific marker of inflammatory macrophages in vivo, Mediators Inflamm, 2013 (2013) 245804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, Panicker LM, Feldman RA, Urbanska AM, Santambrogio L, Vunjak-Novakovic G, Freytes DO, Differential gene expression in human, murine, and cell line-derived macrophages upon polarization, Exp Cell Res, 347 (2016) 1–13. [DOI] [PubMed] [Google Scholar]
- [93].Lurier EB, Dalton D, Dampier W, Raman P, Nassiri S, Ferraro NM, Rajagopalan R, Sarmady M, Spiller KL, Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing, Immunobiology, 222 (2017) 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irvin C, Ratner BD, Jiang S, Zwitterionic hydrogels implanted in mice resist the foreign-body reaction, Nat Biotechnol, 31 (2013) 553–556. [DOI] [PubMed] [Google Scholar]
- [95].Bank RA, Zandstra J, Room H, Petersen AH, van Putten SM, Biomaterial Encapsulation Is Enhanced in the Early Stages of the Foreign Body Reaction During Conditional Macrophage Depletion in Transgenic Macrophage Fas-Induced Apoptosis Mice, Tissue Engineering Part A, (2017). [DOI] [PubMed] [Google Scholar]
- [96].Hayes E, Tsaousi A, Di Gregoli K, Jenkinson S, Bond A, Johnson J, Bevan L, Thomas A, Newby A, Classical and alternative activation and metalloproteinase expression occurs in foam cell macrophages in male and female ApoE null mice in the absence of T- and B-lymphocytes, Frontiers in Immunology, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Newby AC, Metalloproteinase production from macrophages - a perfect storm leading to atherosclerotic plaque rupture and myocardial infarction, Exp Physiol, 101 (2016) 1327–1337. [DOI] [PubMed] [Google Scholar]
- [98].Craig VJ, Zhang L, Hagood JS, Owen CA, Matrix Metalloproteinases as Therapeutic Targets for Idiopathic Pulmonary Fibrosis, American Journal of Respiratory Cell and Molecular Biology, 53 (2015) 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN, Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure, The Journal of Immunology, 150 (1993) 4188. [PubMed] [Google Scholar]
- [100].Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA, Bleomycin and IL-1β–mediated pulmonary fibrosis is IL-17A dependent, The Journal of Experimental Medicine, 207 (2010) 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Xiao H, Li H, Wang J-J, Zhang J-S, Shen J, An X-B, Zhang C-C, Wu J-M, Song Y, Wang X-Y, Yu H-Y, Deng X-N, Li Z-J, Xu M, Lu Z-Z, Du J, Gao W, Zhang A-H, Feng Y, Zhang Y-Y, IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult, European Heart Journal, 39 (2018) 60–69. [DOI] [PubMed] [Google Scholar]
- [102].Sharma D, Kanneganti TD, The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation, J Cell Biol, 213 (2016) 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Awad F, Assrawi E, Jumeau C, Georgin-Lavialle S, Cobret L, Duquesnoy P, Piterboth W, Thomas L, Stankovic-Stojanovic K, Louvrier C, Giurgea I, Grateau G, Amselem S, Karabina SA, Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation, PLoS One, 12 (2017) e0175336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Czimmerer Z, Daniel B, Horvath A, Rückerl D, Nagy G, Kiss M, Peloquin M, Budai MM, Cuaranta-Monroy I, Simandi Z, Steiner L, Nagy B Jr., Poliska S, Banko C, Bacso Z, Schulman IG, Sauer S, Deleuze J-F, Allen JE, Benko S, Nagy L, The Transcription Factor STAT6 Mediates Direct Repression of Inflammatory Enhancers and Limits Activation of Alternatively Polarized Macrophages, Immunity, 48 (2018) 75–90. e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhou R, Yazdi AS, Menu P, Tschopp J, A role for mitochondria in NLRP3 inflammasome activation, Nature, 469 (2010) 221. [DOI] [PubMed] [Google Scholar]
- [106].Vasconcelos DP, Águas AP, Barbosa MA, Pelegrín P, Barbosa JN, The inflammasome in host response to biomaterials: Bridging inflammation and tissue regeneration, Acta Biomaterialia, (2018). [DOI] [PubMed] [Google Scholar]
- [107].Hake ME, Young H, Hak DJ, Stahel PF, Hammerberg EM, Mauffrey C, Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature, Injury, 46 (2015) 1447–1456. [DOI] [PubMed] [Google Scholar]
- [108].Christo S, Bachhuka A, Diener KR, Vasilev K, Hayball JD, The contribution of inflammasome components on macrophage response to surface nanotopography and chemistry, Scientific Reports, 6 (2016) 26207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, Shi Y, Kyriakides TR, Mehal WZ, Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response, Proceedings of the National Academy of Sciences, 108 (2011) 20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Yao H, Zhang Y, Liu L, Xu Y, Liu X, Lin J, Zhou W, Wei P, Jin P, Wen L-P, Inhibition of lanthanide nanocrystal-induced inflammasome activation in macrophages by a surface coating peptide through abrogation of ROS production and TRPM2-mediated Ca2+ influx, Biomaterials, 108 (2016) 143–156. [DOI] [PubMed] [Google Scholar]
- [111].Kechagia JZ, Ezra DG, Burton MJ, Bailly M, Fibroblasts profiling in scarring trachoma identifies IL-6 as a functional component of a fibroblast-macrophage pro-fibrotic and pro-inflammatory feedback loop, Sci Rep, 6 (2016) 28261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, Qi Y, Du J, Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II, PLoS One, 7 (2012) e35144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH, The Transcription Factor STAT3 Is Required for T Helper 2 Cell Development, Immunity, 34 (2011) 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Do NN, Willenborg S, Eckes B, Jungst C, Sengle G, Zaucke F, Eming SA, Myeloid Cell-Restricted STAT3 Signaling Controls a Cell-Autonomous Antifibrotic Repair Program, J Immunol, 201 (2018) 663–674. [DOI] [PubMed] [Google Scholar]
- [115].Chakraborty D, Šumová B, Mallano T, Chen C-W, Distler A, Bergmann C, Ludolph I, Horch RE, Gelse K, Ramming A, Distler O, Schett G, Šenolt L, Distler JHW, Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis, Nature Communications, 8 (2017) 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Carow B, Rottenberg ME, SOCS3, a Major Regulator of Infection and Inflammation, Frontiers in immunology, 5 (2014) 58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lynn AD, Blakney AK, Kyriakides TR, Bryant SJ, Temporal progression of the host response to implanted poly(ethylene glycol)-based hydrogels, Journal of Biomedical Materials Research Part A, 96A (2011) 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wehling-Henricks M, Jordan MC, Gotoh T, Grody WW, Roos KP, Tidball JG, Arginine Metabolism by Macrophages Promotes Cardiac and Muscle Fibrosis in mdx Muscular Dystrophy, PLOS ONE, 5 (2010) e10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Leyendecker G, Wildt L, Mall G, The pathophysiology of endometriosis and adenomyosis: tissue injury and repair, Archives of gynecology and obstetrics, 280 (2009) 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Duan J, Liu X, Wang H, Guo SW, The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice, Reprod Biomed Online, 37 (2018) 254–268. [DOI] [PubMed] [Google Scholar]
- [121].Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M, Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques, Circulation research, 100 (2007) 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Van Dyken SJ, Locksley RM, Interleukin-4- and Interleukin-13-Mediated Alternatively Activated Macrophages: Roles in Homeostasis and Disease, Annual Review of Immunology, 31 (2013) 317–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].McCormick SM, Heller NM, Commentary: IL-4 and IL-13 receptors and signaling, Cytokine, 75 (2015) 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Knipper Johanna A., Willenborg S, Brinckmann J, Bloch W, Maaß T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg Marc E., Niehoff A, Richardson R, Hammerschmidt M, Allen Judith E., Eming Sabine A., Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair, Immunity, 43 (2015) 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A, Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis, Nature communications, 6 (2015) 7158–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A, IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis, Nat Med, 12 (2006) 99–106. [DOI] [PubMed] [Google Scholar]
- [127].Brunner SM, Schiechl G, Kesselring R, Martin M, Balam S, Schlitt HJ, Geissler EK, Fichtner-Feigl S, IL-13 signaling via IL-13Rα2 triggers TGF-β1-dependent allograft fibrosis, Transplantation Research, 2 (2013) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Meng XM, Wang S, Huang XR, Yang C, Xiao J, Zhang Y, To KF, Nikolic-Paterson DJ, Lan HY, Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis, Cell Death Dis, 7 (2016) e2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wang YY, Jiang H, Pan J, Huang XR, Wang YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY, Chen JH, Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury, J Am Soc Nephrol, 28 (2017) 2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang S, Meng X-M, Ng Y-Y, Ma FY, Zhou S, Zhang Y, Yang C, Huang X-R, Xiao J, Wang Y-Y, Ka S-M, Tang Y-J, Chung ACK, To K-F, Nikolic-Paterson DJ, Lan H-Y, TGF-β/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis, Oncotarget, 7 (2016) 8809–8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA, A macrophage colony-stimulating factor receptor–green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse, Blood, 101 (2003) 1155. [DOI] [PubMed] [Google Scholar]
- [132].Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, Blackstone B, Powell HM, Khanna S, Roy S, Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue, Nature Communications, 9 (2018) 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Mooney JE, Summers KM, Gongora M, Grimmond SM, Campbell JH, Hume DA, Rolfe BE, Transcriptional switching in macrophages associated with the peritoneal foreign body response, Immunol Cell Biol, 92 (2014) 518–526. [DOI] [PubMed] [Google Scholar]
- [134].van Putten SM, Ploeger DTA, Popa ER, Bank RA, Macrophage phenotypes in the collagen-induced foreign body reaction in rats, Acta Biomaterialia, 9 (2013) 6502–6510. [DOI] [PubMed] [Google Scholar]
- [135].Moore LB, Sawyer AJ, Charokopos A, Skokos EA, Kyriakides TR, Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFkappaB activation in the foreign body response, Acta Biomater, 11 (2015) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Skokos EA, Charokopos A, Khan K, Wanjala J, Kyriakides TR, Lack of TNF-alpha-induced MMP-9 production and abnormal E-cadherin redistribution associated with compromised fusion in MCP-1-null macrophages, Am J Pathol, 178 (2011) 2311–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Holt DJ, Chamberlain LM, Grainger DW, Cell-cell signaling in co-cultures of macrophages and fibroblasts, Biomaterials, 31 (2010) 9382–9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Fernando MR, Giembycz MA, McKay DM, Bidirectional crosstalk via IL-6, PGE2 and PGD2 between murine myofibroblasts and alternatively activated macrophages enhances anti-inflammatory phenotype in both cells, Br J Pharmacol, 173 (2016) 899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Humeres C, Vivar R, Boza P, Munoz C, Bolivar S, Anfossi R, Osorio JM, Olivares-Silva F, Garcia L, Diaz-Araya G, Cardiac fibroblast cytokine profiles induced by proinflammatory or profibrotic stimuli promote monocyte recruitment and modulate macrophage M1/M2 balance in vitro, J Mol Cell Cardiol, (2016). [DOI] [PubMed] [Google Scholar]
- [140].Trinchieri G, Interleukin-12: A Proinflammatory Cytokine with Immunoregulatory Functions that Bridge Innate Resistance and Antigen-Specific Adaptive Immunity, Annual Review of Immunology, 13 (1995) 251–276. [DOI] [PubMed] [Google Scholar]
- [141].Ploeger DTA, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA, Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts, Cell Communication and Signaling : CCS, 11 (2013) 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE, Alternatively activated macrophages derived from THP-1 cells promote the fibrogenic activities of human dermal fibroblasts, Wound Repair Regen, 25 (2017) 377–388. [DOI] [PubMed] [Google Scholar]
- [143].Boersema GS, Grotenhuis N, Bayon Y, Lange JF, Bastiaansen-Jenniskens YM, The Effect of Biomaterials Used for Tissue Regeneration Purposes on Polarization of Macrophages, Biores Open Access, 5 (2016) 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Smithmyer ME, Sawicki LA, Kloxin AM, Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease, Biomater Sci, 2 (2014) 634–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Damanik FF, Rothuizen TC, van Blitterswijk C, Rotmans JI, Moroni L, Towards an in vitro model mimicking the foreign body response: tailoring the surface properties of biomaterials to modulate extracellular matrix, Sci Rep, 4 (2014) 6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zhou G, Loppnow H, Groth T, A macrophage/fibroblast co-culture system using a cell migration chamber to study inflammatory effects of biomaterials, Acta Biomaterialia, 26 (2015) 54–63. [DOI] [PubMed] [Google Scholar]
- [147].Zhou G, Liedmann A, Chatterjee C, Groth T, In vitro study of the host responses to model biomaterials via a fibroblast/macrophage co-culture system, Biomaterials Science, 5 (2017) 141–152. [DOI] [PubMed] [Google Scholar]
- [148].Jannasch M, Gaetzner S, Weigel T, Walles H, Schmitz T, Hansmann J, A comparative multi-parametric in vitro model identifies the power of test conditions to predict the fibrotic tendency of a biomaterial, Scientific Reports, 7 (2017) 1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Caires HR, Barros da Silva P, Barbosa MA, Almeida CR, A co-culture system with three different primary human cell populations reveals that biomaterials and MSC modulate macrophage-driven fibroblast recruitment, Journal of Tissue Engineering and Regenerative Medicine, 12 (2017) e1433–e1440. [DOI] [PubMed] [Google Scholar]
- [150].Smith TD, Nagalla RR, Chen EY, Liu WF, Harnessing macrophage plasticity for tissue regeneration, Advanced Drug Delivery Reviews, 114 (2017) 193–205. [DOI] [PubMed] [Google Scholar]
- [151].Graney PL, Lurier EB, Spiller KL, Biomaterials and Bioactive Factor Delivery Systems for the Control of Macrophage Activation in Regenerative Medicine, ACS Biomaterials Science & Engineering, (2017). [DOI] [PubMed] [Google Scholar]
- [152].Garash R, Bajpai A, Marcinkiewicz BM, Spiller KL, Drug delivery strategies to control macrophages for tissue repair and regeneration, Experimental Biology and Medicine, 241 (2016) 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Alvarez MM, Liu JC, Santiago G. Trujillo-de, Cha B-H, Vishwakarma A, Ghaemmaghami AM, Khademhosseini A, Delivery strategies to control inflammatory response: Modulating M1–M2 polarization in tissue engineering applications, Journal of Controlled Release, 240 (2016) 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ, Biomaterial based modulation of macrophage polarization: a review and suggested design principles, Materials Today, 18 (2015) 313–325. [Google Scholar]
- [155].Dumont CM, Park J, Shea LD, Controlled release strategies for modulating immune responses to promote tissue regeneration, Journal of Controlled Release, 219 (2015) 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ong S-M, Biswas SK, Wong S-C, MicroRNA-mediated immune modulation as a therapeutic strategy in host-implant integration, Advanced Drug Delivery Reviews, 88 (2015) 92–107. [DOI] [PubMed] [Google Scholar]
- [157].Padmanabhan J, Kinser ER, Stalter MA, Duncan-Lewis C, Balestrini JL, Sawyer AJ, Schroers J, Kyriakides TR, Engineering Cellular Response Using Nanopatterned Bulk Metallic Glass, ACS Nano, 8 (2014) 4366–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Shayan M, Padmanabhan J, Morris AH, Cheung B, Smith R, Schroers J, Kyriakides TR, Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization, Acta Biomater, 75 (2018) 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Robotti F, Bottan S, Fraschetti F, Mallone A, Pellegrini G, Lindenblatt N, Starck C, Falk V, Poulikakos D, Ferrari A, A micron-scale surface topography design reducing cell adhesion to implanted materials, Scientific Reports, 8 (2018) 10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Majd H, Scherer SS, Boo S, Ramondetti S, Cambridge E, Raffoul W, Friedrich M, Pittet B, Pioletti D, Hinz B, Pietramaggiori G, Novel micropatterns mechanically control fibrotic reactions at the surface of silicone implants, Biomaterials, 54 (2015) 136–147. [DOI] [PubMed] [Google Scholar]
- [161].Curtis J, Colas A, Chapter II.5.18 - Medical Applications of Silicones, in: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (Eds.) Biomaterials Science (Third Edition), Academic Press; 2013, pp. 1106–1116. [Google Scholar]
- [162].O’Malley JT, Burgess BJ, Galler D, Nadol JB Jr., Foreign Body Response to Silicone in Cochlear Implant Electrodes in the Human, Otology & Neurotology, 38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Park S, Park M, Kim BH, Lee JE, Park HJ, Lee SH, Park CG, Kim MH, Kim R, Kim EH, Heo CY, Choy YB, Acute suppression of TGF-b with local, sustained release of tranilast against the formation of fibrous capsules around silicone implants, J Control Release, 200 (2015) 125–137. [DOI] [PubMed] [Google Scholar]
- [164].Riabov V, Salazar F, Htwe SS, Gudima A, Schmuttermaier C, Barthes J, Knopf-Marques H, Kluter H, Ghaemmaghami AM, Vrana NE, Kzhyshkowska J, Generation of anti-inflammatory macrophages for implants and regenerative medicine using self-standing release systems with a phenotype-fixing cytokine cocktail formulation, Acta Biomater, 53 (2017) 389–398. [DOI] [PubMed] [Google Scholar]
- [165].Morris AH, Mahal RS, Udell J, Wu M, Kyriakides TR, Multicompartment Drug Release System for Dynamic Modulation of Tissue Responses, Advanced Healthcare Materials, 6 (2017) 1700370. [DOI] [PubMed] [Google Scholar]
- [166].Beer HD, Fassler R, Werner S, Glucocorticoid-regulated gene expression during cutaneous wound repair, Vitam Horm, 59 (2000) 217–239. [DOI] [PubMed] [Google Scholar]
- [167].Gauthier A, Fisch A, Seuwen K, Baumgarten B, Ruffner H, Aebi A, Rausch M, Kiessling F, Bartneck M, Weiskirchen R, Tacke F, Storm G, Lammers T, Ludwig MG, Glucocorticoid-loaded liposomes induce a pro-resolution phenotype in human primary macrophages to support chronic wound healing, Biomaterials, 178 (2018) 481–495. [DOI] [PubMed] [Google Scholar]
- [168].Elliott MR, Koster KM, Murphy PS, Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses, Journal of immunology (Baltimore, Md. : 1950), 198 (2017) 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Hachim D, LoPresti ST, Yates CC, Brown BN, Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration, Biomaterials, 112 (2017) 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Minardi S, Corradetti B, Taraballi F, Byun JH, Cabrera F, Liu X, Ferrari M, Weiner BK, Tasciotti E, IL-4 Release from a Biomimetic Scaffold for the Temporally Controlled Modulation of Macrophage Response, Annals of Biomedical Engineering, 44 (2016) 2008–2019. [DOI] [PubMed] [Google Scholar]
- [171].Truong T, Jones KS, Capsaicin reduces PLGA-induced fibrosis by promoting M2 macrophages and suppressing overall inflammatory Response, Journal of Biomedical Materials Research Part A, 106 (2018) 2424–2432. [DOI] [PubMed] [Google Scholar]