Abstract
Advanced nanomaterials such as carbon nano-tubes (CNTs) display unprecedented properties such as strength, electrical conductance, thermal stability, and intriguing optical properties. These properties of CNT allow construction of small microfluidic devices leading to miniaturization of analyses previously conducted on a laboratory bench. With dimensions of only millimeters to a few square centimeters, these devices are called lab-on-a-chip (LOC). A LOC device requires a multidisciplinary contribution from different fields and offers automation, portability, and high-throughput screening along with a significant reduction in reagent consumption. Today, CNT can play a vital role in many parts of a LOC such as membrane channels, sensors and channel walls. This review paper provides an overview of recent trends in the use of CNT in LOC devices and covers challenges and recent advances in the field. CNTs are also reviewed in terms of synthesis, integration techniques, functionalization and superhydrophobicity. In addition, the toxicity of these nanomaterials is reviewed as a major challenge and recent approaches addressing this issue are discussed.
Keywords: Lab-on-a-chip, Single-walled carbon nanotubes, Microfluidics, Superhydrophobicity, Electrochemical signal transduction, Nanotoxicity
1. Introduction
There has been an unstoppable trend toward miniaturization in all aspects of modern technology. This trend has been powered by remarkable advances in materials science, including nanotechnology. These advanced materials can be integrated into the new field of microfluidics to produce lab-on-a-chip (LOC) devices (Haeberle and Zengerle 2007). These devices enable several operations and procedures formerly carried out on the laboratory bench to now be carried out in a small chip (Fair 2007; Ríos et al. 2012). Microfluidic technologies have provided researchers with the potential to make groundbreaking advances. Microfluidics allows for the manipulation of very small volumes of fluids, and for the miniaturization of complex procedures onto small chips, called microfluidic platforms (Volpatti and Yetisen 2014; Chin et al. 2012). These platforms also known as “micro total analysis systems” (μTAS) or LOC, makes possible the incorporation of a variety of laboratory functions in a small micro/nanofluidic chip (Neuži et al. 2012). This technology relies on the complimentary use of microfluidics and bio-sensing devices that can handle fluid volumes as low as a few picoliters to femtoliters inside tiny narrow channels (Alfi et al. 2016). These devices have advantages consisting of systemic miniaturization, portability, automation, low cost per analysis, reduced consumption of reagents and samples, rapid turnaround time, and precise microenvironment control (Daw and Finkelstein 2006; Chin et al. 2007; Wang and Zhe 2011). The field of LOC and its associated microsystem equivalent technologies (microfluidics, MEMS/NEMS, μTAS, etc.) have now come to be seen as a real multidiscipli-nary field, necessitating contributions from a variety of disciplines including biology, chemistry, software development, physics and materials science (Daw and Finkelstein 2006; Guo et al. 2015a; Mohammed et al. 2015). LOC devices contain microfluidic elements and added components for fluid control, processing and detection capability, with various applications ranging from point-of-care diagnostics, drug discovery, environmental monitoring, and other industrial applications (Daw and Finkelstein 2006).
Nanomaterials (NMs) are widely used in different parts of LOC devices (Medina-Sánchez et al. 2012). Nanostructures can be considered the building blocks needed for novel biosensing systems detect DNA, proteins, cells, etc. Moreover, nanomaterials make vital contributions to precise fluid control and are increasingly being used in LOC platforms (Medina-Sánchez et al. 2012). Carbon nanotubes (CNTs) enjoy many unique properties (which in some cases are also tunable) namely superhydrophobicity, high aspect ratio, outstanding mechanical strength, chemical stability, electrical and thermal conductivity, that have made nanostructures one of the important components used in microfluidic devices (Mogensen et al. 2011). Due to their superhydrophobic property, CNTs minimize the contact surface between the microfluidic device channels and, leading to new levels of precision in fluid control (Joseph et al. 2006; Ma and Hill 2006). CNTs can be modified via chemical functionalization through creation of covalent or non-covalent bonds to either the sides or the ends of CNTs. Functionalized CNT can have applications in molecular and cellular biology and also point-of-care diagnostic tools such as immunosensors (Karimi et al. 2015a, b). Nonetheless, there still remain many challenges concerning their practical use in micro-fluidic devices that need to be overcome. These challenges include their practical integration into useful biomedical devices, undesirable surface chemistry and insolubility in biological environments (Heister et al. 2010; Bhattacharya et al. 2016; Li et al. 2012a). All those aside, there remain many additional safety fears regarding CNTs, which have a structure and shape (long and thin) similar to asbestos fibers, which are considered to be extremely hazardous to human health, including proven carcinogenicity (Donaldson et al. 2013; Firme and Bandaru 2010; Poland et al. 2008).
The current publication presents an overview of the recent studies concerning the development of CNTs and different approaches and challenges regarding their integration into various parts of a lab-on-a-chip microfluidic device. Although a comprehensive review article on the same theme was published in Choon et al. (2008) (due to the great number of papers published since), another review was needed to cover the more recent literature. In the first part of this review, a general overview of the lab-on-a-chip devices and carbon nanotubes is presented. The next parts focus on the reports concerning the synthesis and integration techniques for microfluidic lab-on-a-chip applications.
Afterward, studies on CNT sensors and membranes are summarized. The investigations on the toxicity of these nanomaterials are then presented in the final part. In addition, future challenges and possible approaches addressing them are also examined.
2. Carbon nanotubes: structure and properties
Carbon is on route to becoming the wonder element of the “Nano-Age” due to the small size of atoms and the number of electrons they can share, which together leads to the ability to form multiple bonding patterns, and thence to the existence of various allotropes. Actually, each carbon atom contains six electrons with the following electron configu-ration: 1s2 2s2 2p2. The extremely small energy difference between the 2s- and the 2p-state makes it possible to transfer one electron from the 2s-state into the 2p-state (Peschel 2011).
Fullerene is a member of a large class of carbon allotropes with a geometric closed cage-like structure. The structure was firstly proposed at Rice University by Richard Smalley et al. in 1985 (Niemeyer 2001). During the same year, C60 or “bucky-ball,” an icosahedral-shaped ball with 60 carbon atoms, was the first fullerene isolated by Kroto et al. (1985). A few years later, in 1991, cylindrical fullerenes, called carbon nanotubes (CNTs) or bucky-tubes, consisting of “rolled-up” cylindrical graphitic sheets, were discovered by Iijima as a minor by-product in a fullerene synthesis. Such nanotubes, grown during arc-discharge evaporation of carbon, contained more than one graphitic sheet (2–50 concentric sheets) in the cylinder with an approximate inner diameter of 4 nm (Ebbesen and Ajayan 1992; Iijima 1991; Harris 2009).
CNTs are classified based on the number of concentric tubes of graphene. For instance, one of the most common types of CTNs are called multi-walled carbon nanotubes (MWCNTs) which usually range from 1 to 50 μm in length and from 2 to 100 nm in diameter (Popov 2004). In general, the aspect ratio (L/D ratio) of CNTs is about 1/1000 (Popov 2004). In 1993, Iijima, Toshinar, and Bethune et al., independently synthesized single-walled carbon nanotubes (SWCNTs) (Harris 2009). These individual tubes with a length range from 20 to 1000 nm typically have very small diameters of about only 1 nm. Moreover, their structure tends to be curved rather than straight which provides remarkable properties in comparison with MWCNTs (Harris 2009; Popov 2004). Double-walled carbon nanotubes (DWNTs) are unique nanotubes containing precisely two concentric SWCNTs, which has been explored less than SWCNTs and MWCNTs (Tanaka and Iijima 2014).
In a single graphene sheet, each carbon atom is covalently bonded to three neighboring sp2 hybridized carbon atoms by sigma(ơ) bonds (Tanaka and Iijima 2014). The distance between two graphene sheets in a single crystal graphite is 0.335 nm, while in MWCNTs (due to the presence of weak Van der Waals interactions between cylinders) this distance is 0.340 nm (Popov 2004). The walls of CNTs are made from carbon hexagonal lattice similar to that seen in the graphite atomic planes. The ends of walls are covered with a hemisphere of a fullerene-like molecule (Gogotsi and Presser 2013). In practice, however, CNTs do not possess perfect structures, and their structures generally contain defects that are introduced into them during synthesis. These defects are vacancies, di-vacancies, adatoms, and a frequently encountered defect called Stone–Wales defect (SWD). When Stone–Wales transformation occurs, hexagonal carbon network transforms into pentagon–heptagon pairs (Harris 2009; Lu et al. 2012; Farooq et al. 2015).
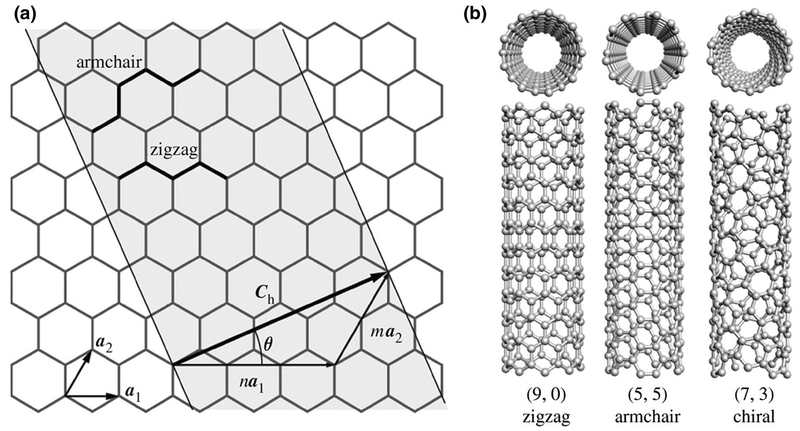
As previously mentioned, a single CNT is a cylinder that can be visualized as rolled-up graphene sheet. Accordingly, a rolling vector consisting of n and m indices in the honeycomb crystal lattice of graphene can characterize the structure of CNT. As shown in Fig. 1, the magnitude of unit chiral vectors can be defined by these indices (n, m) in two directions. For instance, if the n and m indices are equal (n = m), CNTs would be called “armchair,” if m = 0 they would be called “zigzag,” and called “chiral” for the remaining combinations of (n, m) indices (Tanaka and Iijima 2014) (Fig. 1).
Fig. 1.
An illustration of the structures of a unrolled sheet of a SWCNT, b zigzag, armchair, and chiral SWCNTs (reproduced from Kis and Zettl 1870. Copyright 2008 with permission from The Royal Society)
The properties of CNTs depend on parameters such as their size, structure, number of walls, and presence of defects. For instance, the chirality of CNTs has a considerable impact on the electronic properties of CNTs based on the zone-folding approach. Therefore, a nanotube can be either metallic or semiconducting (Tanaka and Iijima 2014; Baughman et al. 2002).
As far as mechanical and physical properties are concerned, CNTs are extremely mechanically strong, stiff, lightweight materials with excellent electrical conductivity, low thermal expansion, and high chemical stability. In order to illustrate these outstanding properties, two of their mechanical properties are given in comparison with other materials. The Young’s modulus of defect-free SWCNTs and MWCNTs is ~1 and 1.11 TPa, respectively, which is comparable to that of diamond. Moreover, the ultimate tensile strength of SWCNTs is 56 and 1.7 times that of steel wire and silicon carbide nanorods, respectively (Harris 2009; Tanaka and Iijima 2014; Gogotsi 2006; Wong et al. 1997; Chhowalla and Unalan 2011).
3. Carbon nanotubes: synthesis and integration techniques into microfluidic LOC devices
The synthesis of CNTs can be accomplished by three different methods, namely high temperature processing, laser ablation, and chemical vapor deposition (CVD) performed at somewhat lower temperatures. These methods have been widely covered in our previously reviewed articles (Karimi et al. 2015c, d). Each of the above-mentioned methods were briefly summarized and their advantages and disadvantages compared and contrasted. Transition-metals nanoparticles (NPs) such as Ni, Co, Fe, or their alloys like Fe–W, and Ni–Y are needed to act as catalysts in CVD techniques. The catalyst which is in contact with a hydrocarbon precursor or carbon source (e.g. acetylene, ethylene, methane) hosts the deposited carbon atoms on its surface and allows the controlled growth of nanotubes to gradually build up (Karimi et al. 2015c; Mogensen and Kutter 2012; Justino et al. 2013).
Arc discharge and laser ablation have roughly the same mechanism, i.e., both an arc discharge and a laser are applied to carbon materials within a chamber under reduced pressure, causing an abrupt rise in temperature and consequent sublimation of carbon atoms. However, in the CVD method, the catalytic growth of CNT results from hydrocarbons decomposition. The quality and properties of CNTs, which are produced by this process, depend on the operating parameters, namely: reaction temperature, gas pressure, type of gas, diameter of catalyst particles, etc. (Karimi et al. 2015c). Each process has its own advantages and disadvantages, which are summarized in Table 1. Among the above-mentioned techniques for synthesizing CNTs, there are considerably more reports of CNTs produced using the CVD and arc discharge methods compared to the laser ablation method in microfluidic LOC applications (Vilela et al. 2012a; Song 2012; Ali et al. 2013, 2015). Various methods can be used for depositing or growing CNTs on different surfaces, and these need to be individually designed for the integration of CNTs into different parts of microfluidic LOC devices including the channel walls, membranes, and different kinds of nanosensors (Mogensen and Kutter 2012; Gencoglu and Minerick 2014; Merkoçi and Kutter 2012; Choong et al. 2008).
Table 1.
Advantages and disadvantages of CNTs synthesis methods including arc discharge, laser ablation, and CVD techniques
| Synthesis techniques | Advantages | Disadvantages |
|---|---|---|
| Arc discharge | High rate of CNT production (Justino et al. 2013) Easy availability of raw materials (Rafique and Iqbal 2011) Ease of process control (Rafique and Iqbal 2011) | Relatively low-yield process (Harris 2009) Non-continuous process (Harris 2009; Justino et al. 2013) Lack of control over the impurities in CNTs (Karimi et al. 2015c) High energy consumption (high temperature process) (Karimi et al. 2015c) |
| Laser ablation | Production of high purity CNTs (Maser et al. 2001) | Difficult purification process (Maser et al. 2001) Labor-intensive and expensive process (Harris 2009; Justino et al. 2013) High energy consumption (high temperature process) (Karimi et al. 2015c)) |
| Chemical vapor deposition (CVD) | High yield process (Karimi et al. 2015c) Tunable growth direction of CNTs (Justino et al. 2013) | Production of CNTs with poorer mechanical properties (less perfect structure in CNTs with higher density of defects) (Harris 2009) |
3.1. Integrating CNTs into microfluidic LOCs through growth
A successful method for the integration of CNT into LOC needs a synthesis method in which the type of CNTs and their growth orientation can be controlled. Typically, CNTs are grown on the surfaces of LOC components via CVD techniques. Reviewing the literature shows that the main mechanism in the integration of CNTs into LOCs through growth requires heating a specific location within the microsystem. By so doing, the growth is limited to only the selected location. In other words, the temperature of remaining areas is much lower, and CNT growth will not occur.
Chen et al. (2012) introduced nonporous vertically aligned multi-walled CNT (VACNT) arrays inside microfluidic channels by growing the arrays on silicon wafers using the CVD technique for particle interception in a gas flow. They showed that the ultra-high porosity arrays resulting from this CVD technique gave enhanced particle interception and subsequently allowed microfluidic cell separation (equivalent to cell sorting). In order to fabricate SWCNT electrodes that could be used to detect dopamine in a flow stream, Sansuk et al. (2012) successfully formed a two-dimensional sparse network of SWCNTs on an insulating Si/ SiO2 substrate via a catalyzed CVD technique. It was shown that this detector effectively improved dopamine detection. Another promising CVD technique used to fabricate VACNTs is called plasma-enhanced CVD (PECVD), in which a gas plasma is used to dissociate hydrocarbon molecules at lower temperatures compared to laser ablation and arc discharge (Saghafi et al. 2014).
The growth of CNTs on the surfaces of LOC components via the CVD technique is considered to be a low-cost process, which makes it possible to easily control the wafer level. Kim et al. (2013) introduced a low-cost integrated vertically aligned microfluidic sensor based on MWCNTs. In this system, MWCNTs were grown on Ni dots, as a catalyst, by DC-biased PECVD. PECVD was used along with a set of other processes, in order to produce embedded MWCNT in a working electrode for electrochemical bio-sensing of nucleic acids. Abdolahad et al. (2012) also used DC-PECVD to grow VACNTs for an electrical cell impedance sensing biosensor. This biosensor was prepared using a photolithography process on Ni/SiO2/Si layers following the growth of CNTs in the desired locations. It was concluded that the VACNTs obtained using this process, exhibited good adhesion along with good conductivity which resulted in a considerable better sensing ability in comparison with other impedance-based cell biosensors of the same type. Seo et al. (2012) introduced a new type of microfluidic system to capture streptavidin which consisted of Si pillars with 3D interconnected CNT networks between them. Firstly, using a solution of Fe–Mo catalyst, a uniform density of the Fe–Mo catalyst was achieved over the entire surface of the Si pillar array and then a horizontal quartz tube reactor was used in order to synthesize CNTs by means of a low pressure thermal CVD method. Finally, CNTs were coated with Al2O3 to hinder aggregation of CNTs. This 3D network of CNTs was effective in selective filtering of nanoparticles and recognition and capturing of streptavidin by biotin affinity. Other methods such as pyrolysis have also been reported to allow growth of CNTs on sensing devices. Rajavel et al. (2015) reported the use of a pyrolysis method for growing a random network of CNTs to function as electrodes for oxygen sensing purposes. In this study, mixtures of ferrocene and xylene were placed in a quartz tube reactor, which was flushed with a flow of Ar gas, and the growth of CNTs followed at various temperatures.
3.2. Integration of CNTs into microfluidic LOCs by other techniques
One of the simplest ways for the deposition of CNTs onto surfaces in various components of LOCs is through simple drop-wise addition, or casting a solution, suspension or a gel-like network containing CNTs (Vilela et al. 2012a; Song 2012; Merkoçi and Kutter 2012; Shim and Ahn 2012).
For instance, Shim and Ahn (2012) fabricated an optical immunosensing platform employing an individual CNT-assembled electrode. For this purpose, an array of SWCNTs was first sonicated in a solution of distilled water and Tween20 and then was separated from metal impurities utilizing magnetic force. The resulting solution was pipetted onto a Ni-self aligned electrode. Subsequently, microchannels were fabricated using soft lithography and detached from the SiO2 substrate. The dispersed SWCNTs formed flow-through microchannels with Fe catalyst at their ends as a result of the CVD technique. By applying an external magnetic field, the ends of the SWCNTs were absorbed onto the Ni pattern edge and were aligned to be parallel with the flow direction. In another attempt, Song (2012) produced a passive valve in a microfluidic channel by casting a hydro-gel composite reinforced with CNT. The hydrogel composite showed enhanced mechanical properties owing to the presence of CNTs. Besides, the composite showed suitable swelling properties in distinct liquids, which made it suitable for passive valves in microfluidic devices. Vilela et al. (2012a) also modified the electrode of an electrochemical sensor for sensing dopamine and/or catechol by casting a dispersant solution containing SWCNTs, and subsequently drying.
A novel approach by Vilela et al. (2012b) that did not require a classical casting process was press-transferring CNTs onto a substrate. For instance, Vilela et al. (2012b) press-transferred CNTs onto a poly-(methyl methacrylate) (PMMA) substrate, to act as a transducer for electrochemical sensing. In this scheme, first they dispersed SWCNTs by tip sonicating in 1,2-dichloroethane and then filtered the dispersion using a Teflon filter and dried under vacuum. The SWCNT films that collected on the filter were then cut and press-transferred under pressure onto the PMMA substrate. After cleaning and drying the PMMA, the filter was removed, and a homogeneous CNT film remained.
Some of the advantages of this approach are as follows:
It facilitates direct detection of target analytes on the CNT surface by direct coupling of the material into a microchip.
The assembly of electrochemical sensors would be simplified since there is no need for other electrode substrates in the process.
This approach requires only a small amount of SWCNTs with high surface sensitivity, with no need for other transducer materials.
Pressing a paste of CNT-containing material into a cavity is another approach which could be used to prepare electrodes for electrochemical sensors (Choong et al. 2008). Li et al. (2012b) mixed and hand ground graphite powder mixed with mineral oil and purified MWCNTs in a mortar. Then, the obtained MWCNT/graphite paste was pressed into a silica capillary. The resulting electrode was polished and subsequently dried at room temperature.
Although in recent years, many scientists have focused on the integration of CNTs onto polymeric substrates, there are still many challenges in this method of integration. For example, the low temperatures of the glass transition (Tg) in polymers result in limitations in the direct synthesize of CNTs upon them. However, Murray et al. (2013) used CNT electrodes to prevent premature dielectric breakdown in electro-adaptive microfluidics consisting of polymer actuators. In this system, SWCNTs would absorb the heat caused by a short circuit (dielectric breakdown at a defect) and thereby protected the dielectric-elastomer actuator from destruction. The flexible SWCNT electrodes were deposited onto the pre-strained elastomer via spray coating with a SWCNT/chloroform solution, thereby illustrating another approach to integration of CNTs into LOCs. Ali et al. (2015) reported the fabrication of a CNT–nickel oxide (CNT–NiO) nanocomposite electrode in a CNT-based smart LOC via a dip-coating technique. In this scheme, a thick gel-like solution of CNT–Ni(OH)2 was deposited onto transparent conductive indium tin oxide (ITO) electrodes, dried at 110 °C for 1 h, and annealed at 450 °C for about 2 h in order to form a CNT–NiO composite matrix on the ITO surface.
In addition, scientists have developed other methods for the integration of CNTs onto a polymeric substrate in LOC applications such as solution-based processing and soft lithography. Hot embossing is also another method to integrate CNTs onto a polymeric substrate, which has the ability to transfer CNTs onto polymers with critical dimensions smaller than 10 μm.
Another approach implemented to deposit CNTs onto microfluidic devices is dielectrophoresis (DEP). For this purpose, CNTs should be provided in the form of a powder, since DEP is a solution-based technology by which the particles or powders in the solution move under the influence of an electric field (Pethig 2010). Li et al. (2012) diluted acid-treated SWCNTs with DI water. A minute amount of SWCNT dispersion was deposited onto a probe location connected to external electronics by micropositioner-controlled metal connections. SWCNTs were attracted to the sensors and assembled across the electrodes of the device under the influence of the electric field. Electrical characterization and microscopic images confirmed the efficiency of this DEP process to assemble SWCNTs at desired locations. Zhao et al. (2012) fabricated a SWCNT-based nanosensor for glycerol detection via assembly of SWCNTs between electrodes using DEP technology.
CNTs also can be coupled into microfluidic devices by preparing “CNT-ink.” Novell et al. (2014) integrated CNTs into paper sensors designed to monitor the level of Li+ in blood. In this work, CNT-inks were prepared by suspension of SWCNTs in a SDBS solution, and sonicating them in a tip sonicator. The paper was printed with the prepared CNT-ink by means of a conventional paint brush. After the evaporation of the ink solvent, the paper was rinsed with water. The results showed that owing to the presence of CNT, the paper became conductive and showed enhanced sensitivity.
Using an inkjet printer to integrate CNTs into microfluidic devices is another novel approach in LOC fabrication. Venkatanarayanan et al. (2012) prepared a electrochemiluminescence sensor, for detection of immunoglobulin G (IgG), by printing acid-functionalized CNTs dispersed in DMF (dimethylformamide) onto ITO. This process resulted in the formation of a vertically aligned “SWCNT forest” on ITO to for the sensor.
Digital microfluidics is a technology in which liquid droplets can be controlled on an open array of electrodes. In these systems, chemical processes can occur in each droplet (Choi et al. 2012). The inkjet printing technique is a time-saving and cost-effective technique that can also be utilized in digital microfluidic technology. Ko et al. (2014) reported fabrication of paper chips with inkjet-printed patterned CNT electrodes for active digital microfluidics. In this scheme, the patterns of electrodes were first designed by Adobe Flash graphic software. CNT-ink was prepared by mixing 1 g of MWCNTs in 200 mL of DI water and 30 g of a dispersant, for 5 h at 80 °C (Kwon et al. 2013). In order to homogenize the CNT-ink, it was ball-milled in the presence of a nonionic surfactant containing a naphthyl group. Finally, the resulting ink was printed according to the desired pattern.
According to the above-mentioned studies, various methods have been utilized to assemble CNTs into microfluidic LOCs. These techniques can be chosen according to the cost, ease, and location of the CNTs within the LOC assembly, and also the availability of facilities. According to these parameters, inkjet printing technology seems to be a novel, and cost-effective technique which we suggest should be further investigated.
4. Functionalization of CNTs for biomedical applications
One of the main drawbacks of CNTs to be used for biomedical applications is that as-prepared CNTs are virtually insoluble and are extremely difficult to disperse conventional water-based solvents, which is critical for biological applications. Hence, it is essential to improve the dispersion of CNT in biological environments by manipulating their surface chemistry via chemical functionalization (Vardharajula et al. 2012). The functionalization of CNTs is a process, in which molecules are attached via covalent or non-covalent bond formation to the sides, or the ends of the CNTs.
In non-covalent functionalization, the attachment takes place via physical adsorption mediated by various forces including Van der Waals and electrostatic forces, hydrogen bonds, and π stacking interactions. Non-covalent functionalization possesses many advantages including being a nondestructive, cheap, and easy method. However, in covalent functionalization, the attachment occurs via formation of covalent linkages between the attached functional groups and the carbon atoms of the CNTs. Covalent functionalization has two subcategories: direct covalent functionalization and indirect covalent functionalization mediated by carboxylic groups that exist on the surface of CNTs. In comparison with non-covalent functionalization, covalent functionalization introduces distortion to the pristine CNT framework, and is a difficult, and an expensive method (Karimi et al. 2015c). Functionalized CNTs have been reported in micro-fluidic LOC devices. In these studies, the high surface area of CNTs has been covalently or non-covalently functionalized with biomolecules such as antibodies (Venkatanarayanan et al. 2012; Kadimisetty et al. 2015; Rasooly et al. 2013), enzymes (Ali et al. 2013), proteins (Ali et al. 2015), aptamers (He et al. 2013), and single-stranded DNA molecules (Moraes et al. 2012), or alternatively with non-biological polymers (Ge et al. 2013), and transition-metal hexacyanoferrates (TMHCFs) such as cobalt-hexacyanoferrate (Li et al. 2012b). As examples of covalent and non-covalent functionalization, one report concerning covalent and non-covalent functionalization is described in detail.
Ali et al. (2015) reported the preparation of proteins [antiapolipoprotein B (AAB) and bovine serum albumin (BSA)] immobilized onto COOH-functionalized CNT–NiO composite electrodes [using carboxylic (−COOH) groups on the CNT–NiO surface]. In this study, the CNTs were synthesized by the CVD technique using ferrocene and toluene and then functionalized with acid to produce carboxylic groups. After CNT preparation, AAB solution was spread on the CNT–NiO surface and subsequently attached via EDC (N-ethyl-N0-(3-(dimethylamino)propylcarbodiimide)-NHS(N-hydroxysuccinimide) coupling chemistry and also allowed to react for 4 h in a humid chamber at 4 °C. The −COOH groups on the CNT–NiO surface reacted with −NH2 groups of the AAB protein and formed a strong covalent amide bond. It was shown that this system exhibited an enhanced detection sensitivity for LDL (low-density lipoprotein), owing to the covalent functionalization of BSA–AAB with CNT–NiO composite matrix on the transducer surface.
Rassoly et al. (2013) fabricated an enzyme-linked immunosorbent assay (ELISA) using CNTs to increase antibody absorption, and improve ELISA sensitivity. In this scheme, to functionalize the CNTs with an antibody, a positively charged linker molecule, polydiallyldimethylammonium chloride (PDDA), was used to aid the functionalization. The positively charged molecule was electrostatically (non-covalently) adsorbed onto negatively charged SEB (Staphylococcal enterotoxin B) antibody. Through dispersing CNTs at room temperature in rabbit anti-SEB IgG in phosphate buffer solution for an hour, the CNTs were successfully attached. The polycationic PDDA was then adsorbed by dispersing CNTs in a solution containing PDDA and in NaCl solution for half an hour, then centrifuging for 15 min, and finally washing with water.
5. Superhydrophobic surfaces
The “lotus effect” refers to self-cleaning properties that arise as a result of ultrahydrophobicity as exhibited by the leaves of Nelumbo or “lotus flower.” Scientists realized that the rough surfaces were capable of air-trapping which leads to superhydrophobicity, which minimizes the droplet adhesion to that surface and causes the droplet to be suspended on the surface, and thus readily roll off the surface at a small tilt angle (Ensikat et al. 2011; Liu et al. 2007; Feng et al. 2002). The emergence of microfluidics and consequently the need to achieve precise fluid control on both the macro-scale and micro-scale, has led scientists to actively revisit superhydrophobic materials during recent years (Joseph et al. 2006). Besides the low adhesiveness of these materials that enables efficient liquid transport, the possibility of minimizing the surface interaction between the fluid and microfluidic channel walls could provide considerable analytical advantages when it comes to avoiding fouling or non-specific surface adsorption (Zhu et al. 2014; Wang et al. 2014; Rodzi 2013). Superhydrophobic surfaces can be characterized via measurement of the contact angle, Ѳc, produced between the surface and a water droplet in contact with each other (Miwa et al. 2000). Generally, surfaces can be classified according to their degree of wettability using water, into the following groups: hydrophobic (Ѳc > 90°), hydrophilic (Ѳc < 90°), superhydrophobic (Ѳc > 150°), and superhydrophilic (Ѳc < 5°) (Miwa et al. 2000; Yu et al. 2015; Wang and Jiang 2007). Accordingly, surfaces are commonly called superhydrophobic when the water contact angle is greater than 150°. Two separate models suggested by Wenzel and Cassie-Baxter link the surface roughness with the apparent contact angle (Marmur 2004). As illustrated in Fig. 2b, Wenzel suggested that part of the liquid would fill up the rough surface valleys. Then anchored by these small water droplets a high-contact mode would be created and subsequently a high contact angle hysteresis would be observed (Yu et al. 2015). The interaction between the water and the surface would be relatively strong.
Fig. 2.
a Surface tension of a water drop on a smooth surface; b Wenzel state: penetration of liquid droplet into surface irregularities; c Cassie–Baxter state: trapped air makes liquid drop suspend on the rough surface spikes) (reproduced from Yu et al. 2015. Copyright 2015 with permission from Royal Society of Chemistry)
On the other hand, the Cassie–Baxter model assumes that when the liquid is simply in contact with the top of the peaks of the solid (not the troughs), the air pockets trapped under the liquid form an interface between solid and air which has a very weak interaction as shown in Fig. 2c (Yu et al. 2015).
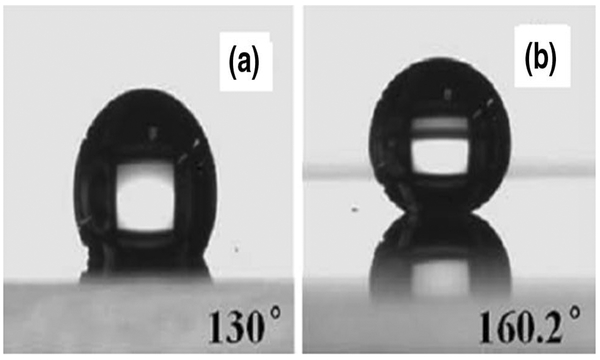
“CNT forests,” which can be synthesized on a nanoscale by growing a forest of parallel nanotube pillars, can mimic nature’s design and create a superhydrophobic effect through their rough surface property (Lau et al. 2003). The chemical stability and ability of CNTs to form microstructures and nanostructures has made them good candidates for micro-fluidic devices. However, most as-grown CNTs have a CA of 136° with water droplets and hence further treatment is needed to increase the contact angle of fluids on CNT surfaces (Journet et al. 2005; Ramos et al. 2010). Besides, as-grown CNTs are susceptible to oil fouling as a result of their high surface energy, and also the mechanically fragile nature of the grown CNTs causes them to collapse upon light friction or penetration of water and to lose their superhydrophobic effect (Zhu et al. 2014; Li et al. 2010). A method that has been suggested by many researchers to increase the superhydrophobicity of CNTs is to modify the as-pre-pared surface with low surface energy materials (Babu 2014). In this regard, Meng and Park (2010), carried out surface modifications of MWCNTs through dispersal in a fluoropolymer (FP) solution thus forming FP-grafted MWCNTs. The obtained coating showed a higher contact angle of 160.2° (compared to 130°) (Fig. 3), and the results showed that the 3D network of FP-grafted MWCNTs played a significant role in the superhydrophobicity of the film. Addressing the same issue, a simpler and faster method was suggested by Wang et al. (2011), who fabricated a CNTs/polytetrafluoroethylene composite film with a hierarchically structured superhydrophobic surface using a spray coating method. They dispersed CNTs in an aqueous solution containing perfluoroalkoxy resin. The film obtained in their work had a sliding angle about 5°, which was stable even in high-stress conditions, and a contact angle of 153.1° ± 2°. Zhu et al. (2014) also fabricated a superhydrophobic coating by spraying MWCNTs followed by surface fluorination. The obtained coating showed an acceptable superhydrophobicity which in cases of mechanical damage, could be repaired easily. Furthermore, if the MWCNT-coated layer should be contaminated with oil, the superhydrophobicity of the coating could be regenerated by thermally removing the oil contamination in air.
Fig. 3.
Contact angle of a water drop on a Pristine MWCNT-coated films; b MWCNT–FP coated films (MWCNT/FP volume ratio = 10:3, coated seven times) (reproduced from Meng and Park 2014. Copyright 2014)
Besides these surface modification methods, another approach is to fabricate a composite coating to enhance CNT superhydrophobicity and improve their properties. In this regard, Mokarian et al. (2016) fabricated a superhydrophobic coating made of randomly laid down MWCNTs on a silicone rubber substrate, which was inert, non-toxic, and biocompatible. Their results indicated that a superhydrophobic surface with ultra-high water repellency and a contact angle of near 159° for deionized water droplets could be obtained using this composite coating. In addition, the composite coating surface showed good mechanical properties and high corrosion resistance in both acidic and alkaline medias, and its anti-icing ability remained constant during exposure to UV radiation and immersion in acidic media, aqueous solution containing 5 wt% NaCl, and boiling water. Another inexpensive and simple CNT composite coating was suggested by Wang (2016), who fabricated a layer of expanded graphite (EG)/CNT/polymer composite as the surface. The fabricated layer, for a 6 μL water droplet, showed a high water contact angle of 162° and a sliding angel of 6°. In addition, since the contact angle was resistant to both heating and exposure to organic solvents, the coating had good environmental stability.
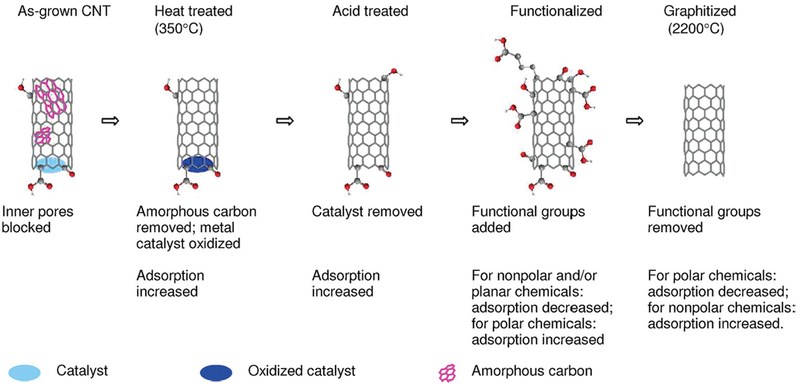
More recent investigations suggested that surfaces with a tunable superhydrophobic effect could better manipulate fluids in terms of acceleration, deceleration, merging, and control of the fluid flow (Shiu et al. 2005; Krupenkin et al. 2007). Researchers have tried to achieve a reversible and reliable localized switching between superhydrophobicity and superhydrophilicity on the same surface with potential for better-controlled fluid manipulation. For instance, a surface with a gradient in wettability could cause the water droplet to predictably roll on the surface and be transported to the desired area. CNT wettability may be changed through chemical modification methods such as oxidative treatment of the CNT surface or treating the surface with acids. Modifying the CNT surfaces leads to a significant rise in the wettability of the surface for polar liquids including water and also to more reactive surfaces (Shiu et al. 2005; Krupenkin et al. 2007; Draper et al. 2013). The presence of functional groups not only increases diffusional resistance, but it also changes the adsorption properties of the CNTs. Figure 4 illustrates the general changes of adsorption properties of CNTs after different treatments (Pan and Xing 2008). For instance, the functionalization of CNTs by increasing the oxygen content decreases the surface area in a significant manner. As a result, the adsorption of nonpolar hydrocarbons will be reduced mainly because of reduced hydrophobicity (Pan and Xing 2008).
Fig. 4.
Effects of functional groups on the adsorption properties of CNTs (reproduced from Pan and Xing 2008. Copyright 2008 with permission from ACS)
In an interesting study with the purpose of constructing a superhydrophobicity gradient on the surface, Lobo et al. (2012) used the oxygen plasma treatment technique to modify vertically aligned MWCNTs. The VACNTs etched with pure oxygen plasma developed a superhydrophilic effect on their surface. Following the functionalization, the VACNT tips showed more polar properties (due to attachment of polar groups onto their surfaces) which encourage surface interactions with polar liquids. Also, another study by the same team showed that irradiation of the obtained super-hydrophilic surface with a CO2 laser led to evaporation of the surface of the VACNT containing oxygen polar groups and thus, the superhydrophobic effect of the surface could be restored (Lobo et al. 2010). Another study with the same purpose was carried out by Babu (2014), who fabricated superhydrophobic 3D-aligned CNT arrays, possessing a wettability gradient, without any coating or micropatterning of the CNT surfaces, and only using “water-assisted chemical vapor deposition” (WACVD). The required surface roughness was achieved by a CVD regrowth technique and then the surface was passivated by polydimethylsiloxane functionalization. Using a DC corona discharge, the wettability gradient was induced, and the resulting surface became capable of transporting water droplets via the wettability gradients. Besides, the wettability of these fabricated surfaces was reversible which enables the surfaces to be reversed to their initial superhydrophobic state. In the future, these efforts may allow the fabrication of “reprogrammable surfaces,” which could possibly find a variety of applications in microfluidics.
6. Lab-on-a-chip
Lab-on-a-chip devices are miniaturized controllers and sensors for small volumes of fluids flowing in channels which have been designed for desired purpose such as drug screening, cell growth, promoting interactions between different cell types and biological substrates, etc. (Karimi et al. 2016). The essential components of these systems can be summarized as follows: actuators (mechanisms for controlling volume, access of materials, varying pressure); sensors (in order to monitor the changes in various parameters such as color, fluorescence, ion concentration, pH changes, etc.); and reservoirs for efflux and waste. Here, we take a look at different applications of CNTs in these LOC components.
6.1. Channel walls and membranes
In nanoscale capillaries, the flow behavior is mostly affected by the interaction of the fluid with capillary walls rather than its bulk properties (Whitby and Quirke 2007). Thus, with respect to the wall-induced effects, one of the main challenges in nanopores and nanochannels is whether the selected type of nanotube can be filled with water (Hummer et al. 2001; Waghe et al. 2002). Furthermore, the structure of walls at the molecular level is very important in controlling surface friction. Graphite surfaces, due to their high surface density of atoms, decrease surface corrugation to a very low extent (Whitby and Quirke 2007). CNTs can serve as the fundamental component of channels in nanofluidic devices due to their controllable geometry, simple chemistry and their hollow structure (Guo et al. 2015b). The CNTs diameter can be controlled during synthesis to be range between the size of a small molecule (<1 nm) to tens of nanometers, while their length can be up to few millimeters. The inner surface of CNTs is hydrophobic and molecularly smooth (Karimi et al. 2015c). CNTs with diameters less than 10 nm have been reported to display these features: (1) spontaneous wettability of interior CNT surfaces in contact with aqueous solutions; (2) ability to transport ions and molecules selectively when the openings or ends of CNTs are functionalized; and (3) ultra-fast penetration of gas, water and protons (Guo et al. 2015b). Solid-state CNT platforms were first introduced as vertical channels made of a single MWCNT with the important advantage of reproducibility and the ability to make hundreds of identical pores for mass transport. They were prepared with diameters greater than 50 nm (Sun and Crooks 2000). Another platform, first introduced by Lindsay, was made from SWCNT with diameters between 0.9–4.2 nm and 2 μm in length. In their platform, a poly(methacrylate) (PMMA) layer was coated onto SWCNT and then used to study ionic and molecular translocation triggered by an external electric field (Liu et al. 2010). Moreover, the selective permeability of CNTs makes them a suitable choice to be used as semi-permeable membranes in LOC configurations. For instance, membrane-spanning SWCNTs was evaluated using transportation of various ions such as potassium, calcium and chloride. The study concluded that the overall selectivity was mostly affected by the net charge of the CNT filters (García-Fandiño and Sansom 2012). Although this endeavor did not applied in a LOC setup, this study, besides many others, shows the great potential of CNTs in filtration at molecular level. Introducing samples into microfluidic devices often needs a filtration process which is necessary to prevent contamination of the system. CNTs, due to their large surface-to-volume ratio, are good candidates for separation and concentration steps (Bakajin 2003). Membranes of mammalian cells have ion channels or pores, which selectively allow the trafficking of specific kinds of molecules through the membrane. Such control relies on membrane potential (voltage-gated channels) can be triggered by a specific ligand (ligand-gated channels) and can affected by movement (mechanically gated channels) (Cohen 2002; Davis et al. 2001; Webster et al. 2004). Addressing this issue, one group studied the mechanism of phosphorylation and dephosphorylation by using aligned CNTs impregnated in a polystyrene matrix. The openings of the core CNTs (inner diameter: 7 nm) were functionalized with the plasma-oxidization technique to form carboxylate groups. Additionally, a polypeptide of [GRTGRNSI-NH2] which is a substrate of protein kinase A was attached at the tip of the CNTs to make a ligand-gated protein channel (Nednoor et al. 2007).
Matching the size of pore to the targeted molecule is necessary for the optimization of nanoporous membranes, especially when the size range is in the order of 1–10 nm (Hinds et al. 2004). The reports show that aligned MWCNTs have been produced with well-controlled inner diameters of 4.3 ± 2.3 nm (Andrews et al. 1999). In one of the early attempts, the primary aim of a study was to prepare a MWCNT membrane with a structure that could allow molecular transport through the CNT core. The process of oxidative trimming was used to introduce carboxylate groups at the openings of the CNT inner core. The results showed permeability to nitrogen gas in accordance with the Knudsen diffusion rate (Hinds et al. 2004). However, it demonstrated that gas permeation rates in CNT membranes, produced by gas-phase catalytic CVD followed by an epoxy encapsulation step, were higher than predicted by Knudsen permeability, while the permeation of hydrogen was in the Knudsen range. Thus, this observation could be considered a proof of existence of a non-Knudsen transport process (Ge et al. 2012). Having flows in rates higher than Knudsen predictions can be attributed to the smooth surface of CNTs and a positive resume for these NTs to further investigate their potential in microfluidic walls and channels.
Holt et al. (2006) also reported that the gas flow of a membrane constructed with aligned CNTs with diameters less than 2 nm, also surpassed the Knudsen prediction. Moreover, Falk et al. (2010) investigated the interfacial friction of water in graphitic surfaces including CNTs. Their results demonstrated that decreasing the CNT radius would result in reducing the friction experienced by water inside, but also the friction was increased for water outside. Carboxylate groups can act as gatekeepers to regulate ionic transport through CNTs. In this regard, Majumder et al. (2011) proposed that the specificity of bulk diffusion was similar to the CNT pore transport of various ions with defined charges and sizes. Based on this conclusion, the estimation of open pores number and the amount of membrane porosity would be possible. A recent study claimed that sub-10 nm channels do not always show the same electrokinetic behavior of ion transportation. For example, the magnitude and trend of conductivity in CNT membranes with pore diameters ranging from 2 to 10 nm increases in relation to the channel height if measured in 1 mM KCl, while for smaller channel sizes, the study showed that the conductivity was less significantly increased in channels with diameters less than 2 nm (Cheng et al. 2016).
6.2. Sensors
Detection methodologies play a vital role in LOC devices considering the tiny amount of solution available, nanoto pico-liter volumes. Therefore, precise techniques are necessary to report such small amounts of analyte. These methodologies can be divided into fluorescence, electro-chemical and electronic detection. Due to their inherent electroactivity, portability, sensitivity and low-power requirements, CNTs are a good candidate for detection in microfluidic devices (Pumera 2011). Generally, CNT-based biosensors consist of two parts which are a biologically specific detection site (for instance functionalized with cell receptors, enzymes, antibodies, polynucleotides and microorganisms) and a signal transducer. The transducer converts the presence of recognized analytes to detectable signals such as currents, absorbance, fluorescence, mass or acoustic variables (Yang et al. 2015). In addition to CNTs alone, composites made of CNTs and polymers have shown superior features compared to thin film of CNTs for detection purposes due to their robust mechanically stability, lower background currents and less signal noise (Pumera 2011).
6.2.1. Flow sensors
The field of fluid dynamics changed significantly when microfabrication was developed and nanostructures and nanochannels were used to develop nanoprobes that could perform as flow sensors. Flow sensors can detect both liquid and gas flows.
Electrokinetic phenomena, such as electro-osmosis or electrophoresis, rely on the interaction between ions in an aqueous solution and charged surfaces. That is, these interactions are affected by fluid dynamic properties, and by evaluating changes in electrostatic potential or charge distribution which occurs during the process of detection (Bourlon et al. 2007).
Two main mechanisms have been proposed for enabling the flow sensing behavior of CNTs. First mechanism suggests momentum transfer from the molecules of a flowing liquid to the acoustic phonons in CNTs, then this quasi momentum of the phonons attracts the non-charged carriers into the CNTs (Park et al. 2004). The second mechanism proposes that scattering of free carriers is the main reason for fluctuations within CNTs (Xu et al. 2007). The second mechanism (compared to the first one) requires a current which is five times smaller in magnitude (Ghosh et al. 2003).
In this regard, a report showed that voltage generation occurred due to flow of a polar liquid over a SWCNT. The results confirmed the induced voltage became saturated at a flow velocity even as small as 10−5 m s−1 (Ghosh et al. 2003). This experiment also showed the logarithmic nature of the voltage dependency on flow velocity which is in a contrast with a previous study by Král and Shapiro (2001). A few patents have been filed on flow sensing using SWCNTs such as the patent registered by Ghosh et al. (2003). The measured voltage was dependent on the flowing liquid ionic strength, and this report was the first attempt to create a flow sensor based on SWCNT which when they encounter a fluid flow, produce an electrical signal (Ghosh et al. 2003). Furthermore, it was reported that the current induced by the flow on the surface of MWCNT thin films, significantly depended on the flow velocity and temperature (Liao et al. 2003). Although the previously mentioned endeavors did not result in an actual LOC device, Qu et al. utilized aligned MWCNTs for sensing the aqueous flow in a microfluidic system made from PDMS. Figure 5 shows a schematic illustration of how the flow sensor was designed. The study results showed a linear relation between aqueous flow rate of deionized water and alterations in electrical resistance in CNT-based sensors. The sensor can function at low-level powers as small as 1 μW with considerable sensitivity (Qu 2007). In order to enhance the robustness of CNTs in a flow sensor, Cao et al. used SWCNTs wrapped around by polymer chains of PDMS which were fixed into a matrix of PDMS in order to prepare a composite material, which prevented washing of SWCNTs by the liquid flow. When the thin film of the composite was bathed in solutions having different flow rates, it showed the induced voltage dependency on the alterations of the ionic concentration and flow velocity (Cao et al. 2010).
Fig. 5.
Fabrication process for CNT-based flow sensor for aqueous solutions in PDMS microfluidic systems (reproduced from Qu 2007. Copyright 2007)
6.2.2. Optical sensors
The optical features of CNTs allow them to be used as solution-phase sensors which respond to analyte adsorption by regulating fluorescence emission (Barone et al. 2005). It has been demonstrated that SWCNTs display a bright, structured photoluminescence in the near-infrared (NIR) region (O’connell et al. 2002). Additionally, spectrofluorimetric and resonance Raman scattering measurements of SWCNTs can be used to analyze the detailed bulk composition of CNT samples according to their optical properties. Based on optical evaluation, CNTs can be divided into separate groups (Bachilo et al. 2002). NIR light ranging from 0.9 to 1.3 eV (1370–950 nm) (Barone et al. 2005) has been shown to have good penetration in human tissues (Kim et al. 2004; Frangioni 2003) and biological fluids (Wray et al. 1988) as well as a reduced autofluorescence background. SWCNTs can absorb light at their surface (Barone et al. 2005) and are resistant to photobleaching (Heller et al. 2005). One report took advantage of the photobleaching resistant feature of CNTs (photostability) for detection of DNA hybridization by measuring the NIR fluorescence signal from a DNA-SWCNT complex (Jeng et al. 2006). During DNA hybridization, the CNT emission underwent solvatochromic shifts that could be used as optical biosensors for detection of single nucleotide polymorphisms (SNP), which is a DNA mutation where a single nucleotide base is replaced by a different one (Wang et al. 1998).
Recently a group took a combinational approach to detect E. coli bacteria in a point-of-a-care approach. They used a multilayer of MWCNT to make a LOC biosensor in a loop-mediated isothermal amplification configuration in order to capture the bacteria with high sensitivity as low as 1 CFU mL−1 (Li et al. 2017).
6.2.3. Electrochemical sensors
The most prevalent types of biosensors are electrochemical (EC) biosensors, and carbon structures, which includes various types such as amorphous carbon, graphite, carbon fibers, and CNTs, are one of the most commonly used materials to make EC electrodes (Choong et al. 2008). The selection of carbon materials for electrodes must overcome such challenges like heterogeneous electron transfer and the potential passive state of electrodes; however, the use of CNTs can address these issues (Yang et al. 2015). For instance, Pumera et al. (2007) compared SWCNTs and MWCNTs with graphite powder films on different substrates such as glassy carbon and surfaces of electrodes made of gold, platinum, and glassy carbon. The results showed that MWCNT on a glassy carbon surface had the best electrocatalytic effect for oxidation of dopamine and catechol when compared to a bare glassy carbon electrode. Moreover, in comparison with a gold electrode, the MWCNT film and SWCNT film showed a shift of 0.08 in E1/2 and E1/2 shift of 0.10 V for catechol. SWCNTs have electrical properties which can be directly affected by molecular adsorption (Smalley et al. 2003).
There are four types of EC biosensors comprising those that are amperometric-based, potentiometric-based, conductometric-based and impedimetric-based.
Enzymatic reactions can take place on the surface of an electrode coated with an enzyme when it is immersed in the sample solution, and the analytes will penetrate via diffusion to its surface, so that amperometric-based biosensors convert the enzymatic chemical reactions into electrical signals (Yang et al. 2015). It is worth mentioning that in order to improve selectivity and sensitivity, immobilized enzymes can be coated onto CNT modified electrodes, so that CNT act as transducers to produce the signal from the enzyme reaction (Choong et al. 2008). The carboxyl groups usually present on the surface of CNTs have been used to immobilize enzyme molecules (Pavlidis et al. 2010). For instance, melatonin and its precursor tryptophan have been detected with SWCNT press-transferred electrodes in a microchip electrophoresis process. SWCNTs filtered on a non-conductive PMMA substrate acted as transducers. This system resulted in rapid detection of melatonin, tryptophan and serotonin in periods shorted than 150 s (Gomez et al. 2015). Furthermore, it was found that an amperometric sensor could be made of CNTs and ferrocene (as a redox-responsive reporter) that was applied in order to detect pathogenic viral DNA from hepatitis C and genomic DNA from Myocobacterium tuberculosis with high sensitivity (Zribi et al. 2016).
In impedimetric biosensors, impedance changes that arise either due to the conformational alterations or to DNA damage, and in some cases the formation of a blocking layer is detectable (Bahadır and Sezgintürk 2016). Considering interaction between an antigen and antibody as an immuno-sensing mechanism, one study showed that the deposition of Au NPs onto the aligned SWCNT on the SiO2/Si substrate could significantly improve selectivity to detect interleukin-6. The biosensor could detect IL6 as low as 0.01 fg mL−1 (Yang et al. 2013).
Moreover, in another non-enzymatic electrocatalytic method, SWCNTs that were sandwiched in Ni–Ag hybrid NPs were utilized to prepare an electrode surface for detection of glucose in blood samples. The use of SWCNTs significantly improved the electron transfer ability and gave better hydrophilicity. This sensor provided good sensitivity, selectivity, strong resistance against interference, and good stability (Li et al. 2016). This sensor was not used in a specific LOC setup, but another group employed a layer-by-layer approach to fabricate bilayers of poly (allylamine hydrochloride)/poly (styrene sulfonate) on each individual CNT by repeated deposition from an aqueous solution in microfluidic channels. Their work provided a promising platform for tailoring and grading of nanoporous scaffolds for LOC applications. Additionally, detection of prostate-specific antigen was demonstrated which used this platform (Yost et al. 2015).
The accumulation of charge potential at the sample electrodes compared to the reference electrode when the current flow is zero (equilibrium conditions) can be measured by potentiometric biosensors. In this regard, the Nernst equation or field-effect transistor devices have usually been used (Miserere and Merkoçi 2015). As an example, Wang et al. used a potentiometric approach to measure the human serum albumin (HSA) adsorption on MWCNT. When the HSA was adsorbed on the surface of the MWCNT an electrical potential was produced which was linearly related to the zero current potential ranging from 2.8 × 10−8 to 3.4 × 10−7 M with a detection limit of 2 × 10−8 M (Wang et al. 2015a). In another study, potentiometric sensing using a hybrid structure of transduction capabilities of SWCNTs and a protein-specific RNA Aptamer were used to detect protein at concentrations as low as 4 fM for real-time detection (Zelada-Guillén et al. 2013).
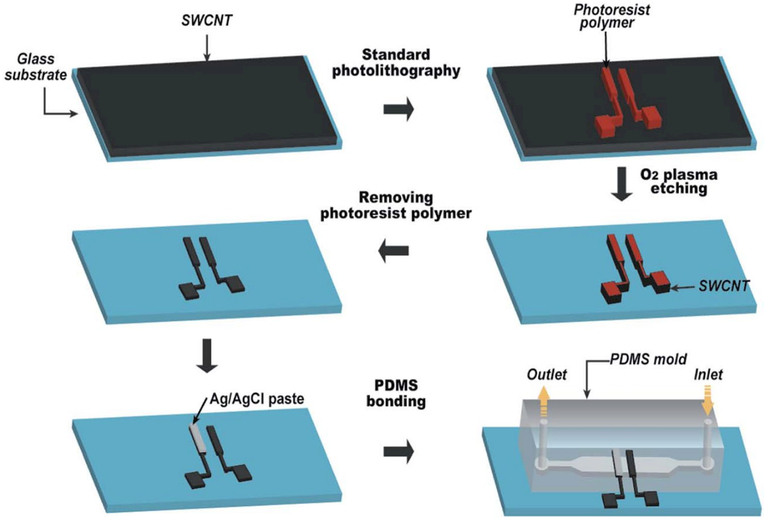
A pH-sensing chip made of SWCNT thin films was developed which simultaneously acted as an electrode and a pH-sensing membrane. The changes in electronic structure in response to varying pH values were measured with semiconductor SWCNTs (Li et al. 2014). The schematic steps of fabrication of such a pH-sensing chip are shown in Fig. 6.
Fig. 6.
Schematic fabrication process of the microfluidic pH-sensing chip (reproduced from Li et al. 2014. Copyright 2014 with permission from Royal Society of Chemistry)
Conductimetric biosensors can compare the ability of a material to conduct electrical current between two electrodes (Miserere and Merkoçi 2015). For instance, a MWCNT/TiO2
composite biosensor prepared via a hydrothermal technique then was blended with cellulose to give a hybrid composite of TiO2/MWCNT/cellulose in order to measure pH. The results showed that the nanocomposite displayed two linear regions for its conductance ranging between pH 1–6 (highly sensitive region) and pH 7–12 (less sensitive region) (Chen et al. 2013). More information about recent reports of EC LOC biosensors is given in Table 2.
Table 2.
Recent researches on EC biosensors based on CNTs
| Target | Transducer | Considerations | References |
|---|---|---|---|
| Fe2+ and Fe3+ions | Electrode | CNT electrodes modified with bismuth acetate and magnetic nano-particles | Jothimuthu et al. (2016) |
| DNA | Electrode | A SWCNT membrane assembled on a chromium/gold electrode using the dielectrophoresis technique with a sensitivity limit of 50 nM and advantages of achieving a low-noise frequency output along with real-time detection based on solution, on-chip integration capability | Wang et al. (2015) |
| Aptamer | Nanoparticles/electrode | Catalytic activity of Ag NPs toward the reduction of onitrophenol to oaminophenol by NaBH4. Comparison of the electric signals from the electrodeposition product of o-aminophenol and MWCNT on a screen-printed electrode | Song et al. (2016) |
| Enzyme | Field-effect transistor (FET) | Electrolyte-gated CNT-FETs have been used as an ion- and pH-sensing platform with detection limit of 100 uM | Melzer et al. (2016) |
| Protein | FET | A conducting copolymer functionalized with SWCNTs-based biosensor was used with a detection limit of 24.2 pg mL”1 to detect cardiac myoglobin | Puri et al. (2014) |
| Protein | Electrode | SWCNT deposited on two Au/ITO (indium tin oxide) electrodes combined with monoclonal antibodies specific for osteopontin (a protein which is seen during cancer) immobilized covalently on SWCNTs. The detection limit reported was 0.3 pg mL”1 | Sharma et al. (2015) |
| Glucose | Nanowires | Both CNTs and reduced graphene oxide nanosheets were utilized in combination with Au nanowires to make a glucose sensor with a detection limit of 20 uM | Qinetal. (2016) |
| Cancer cell | Electrode | SWCNT arrays coated with anti-epithelial cell adhesion molecule antibodies to detect the breast cancer cell line MCF7 with 91% sensitivity, 82% specificity and 86% accuracy in the blind classification test | Khosravi et al. (2016) |
We should mention some of the challenges that may prevent making CNTs a practical choice for LOC biosensing. First of all, different CNT platforms may lead to different results, so further investigations are needed to make it clear whether the design step in the platforms has any important role in discrepancies in the results (Guo et al. 2015b). According to some reports, controlling the helicity of CNTs during manufacturing is difficult, therefore producing the optimal CNT-based biosensor could be complicated. Additionally, while the production of high purity CNTs (99.99%) is essential for high accuracy, selectivity and sensitivity, nevertheless higher purity means higher costs. So, most of the techniques are not yet cost-effective for mass production of CNTs. Additionally, due to the difficulties that could occur during processes for immobilizing enzymes on the surface of CNTs, leading to damage occurring to enzymatic molecules, and reductions in bio-activity, biocompatibility and chemical stability (Yang et al. 2015). Another limiting factor for wider use of CNTs could be their toxicity, which is discussed in detail in the next section.
7. Toxicity of carbon nanotubes
As the worldwide applications of CNTs increase due to their unique properties, there is an urgent need for more toxicological studies of CNTs (Karimi et al. 2015e). To date, our knowledge concerning the toxicity of such important materials and their impact on humans and the environment is inadequate. It is realized that CNTs have a morphological structure similar to that of needle-like asbestos fibers which are well known for their harmful health effects such as causing lung damage and carcinogenicity (Donaldson et al. 2013; Poland et al. 2008).
As a general rule, the reduction of size typical of nano-materials, endows them with new biomedical properties, attributed to an exponential increase in surface area relative to volume, which makes their surface more reactive toward its immediate environment. This may apply even when the nanoparticles are made of inert elements like gold (Medina-Sánchez et al. 2012; Beg et al. 2011). Another problem is the biological accumulation of nano-sized materials, since their small size increases their uptake into cells and tissues, altering the critical biological function of cells (Beg et al. 2011). Deposition of CNTs into the respiratory system and lungs is thought to cause health hazards in a similar manner to that observed following exposure to asbestos (Teeguarden et al. 2011). After CNTs are inhaled, they may become trapped in the lungs and eventually can accumulate, causing lung scarring or fibrosis, and also inducing formation of granulomas in the lungs and increasing pulmonary inflammation. These effects might be equal to or even greater, than those caused by asbestos (Poland et al. 2008; Guo et al. 2012; Ravichandran et al. 2011; Snyder-Talkington et al. 2015). There have been several reviews looking at potential toxicity of CNT (Dong and Ma 2015; Ema et al. 2016; Bussy et al. 2013). However, it must be admitted that the majority of toxicology studies have looked either at inhalation and potential lung damage or lung carcinogenesis, or else at potential toxicity toward blood cells. Neither of these administration routes has high relevance to the dangers posed by microfluidic devices, where the CNTs would likely be borne along by liquid effluent and oral absorption would be the most likely route of entry into the human body.
It can be concluded from the researches concerning toxicity of CNTs, various parameters including their thickness, length, and state of agglomeration, affect their toxicity, and although the issue has been discussed widely, more studies are still needed for a comprehensive understanding of their toxicity and the mechanisms.
8. Conclusion and future trends
Microfluidic devices such as LOCs offer many advantages including lowered consumption of fluid and reagent per each analysis, faster response times, and possibility for high-throughput analysis. LOC enables better control over the process, and automation, consequently opening the doors toward new powerful diagnostic instruments. Carbon nano-tubes have been widely employed in LOC devices due to their unique properties. CNTs are able to provide us with tunable superhydrophobic surfaces with extremely high aspect ratios (length/diameter), thus permitting a new level of fluid manipulation which was not achievable before.
In the present review, some recent studies and progress in the utilization of these materials in lab-on-a-chip devices and relevant challenges have been presented and discussed. Some of the main challenges of the field were highlighted including: the search for practical methods to integrate CNTs into LOC; the necessity for dealing with the mechanically fragile nature of the as-grown CNTs; handling the oil-fouling problem that can occur with CNTs; increasing and tuning the hydrophobicity properties of these materials, etc. Various approaches addressing these issues were presented and compared.
Future progress in CNT-based LOC may allow undreamt medical applications. Implantable sensors may allow continuous vital monitoring of critical biomolecules. Glucose monitoring is the most often discussed of these ambulatory applications. By definition, implantable biosensors must be ultra-miniaturized, have excellent signal transduction capability, and have the capability to be wirelessly powered. The advantages of CNT discussed above suggest they may have an important role to play in this regard. Implantable sensors for oxygen, sodium, and pharmaceuticals or their metabolites will likely have extensive uses. It could even be imagined that an implantable sensor for illegal drugs and banned performance enhancing substances might be developed. The other area of future development is likely to be based on low-cost analytical instruments for the less developed world. There has been a trend for smart-phone-based biochemical and biological assays, and it is likely that CNT-based LOC will play a role in this future development. Advances in production methods and automation for microfluidics devices will be required before this futuristic dream becomes a reality. Moreover, concerns about the nanotoxicity of CNT still linger and these will need to be assuaged.
Acknowledgements
Michael R. Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700.
References
- Abdolahad M et al. (2012) A vertically aligned carbon nanotube-based impedance sensing biosensor for rapid and high sensitive detection of cancer cells. Lab Chip 12(6):1183–1190 [DOI] [PubMed] [Google Scholar]
- Alfi M, Nasrabadi H, Banerjee D (2016) Experimental investigation of confinement effect on phase behavior of hexane, heptane and octane using lab-on-a-chip technology. Fluid Phase Equilib 423:25–33 [Google Scholar]
- Ali MA et al. (2013) Highly efficient bienzyme functionalized nano-composite-based microfluidics biosensor platform for biomedical application. Sci Rep 3:2661–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MA et al. (2015) Protein functionalized carbon nanotubes-based smart lab-on-a-chip. ACS Appl Mater Interfaces 7(10):5837–5846 [DOI] [PubMed] [Google Scholar]
- Andrews R et al. (1999) Continuous production of aligned carbon nano-tubes: a step closer to commercial realization. Chem Phys Lett 303(5):467–474 [Google Scholar]
- Babu DJ et al. (2014) Inscribing wettability gradients onto superhydrophobic carbon nanotube surfaces. Adv Mater Interfaces 1(2) [Google Scholar]
- Bachilo SM et al. (2002) Structure-assigned optical spectra of single-walled carbon nanotubes. Science 298(5602):2361–2366 [DOI] [PubMed] [Google Scholar]
- Bahadır EB, Sezgintürk MK (2016) A review on impedimetric biosensors. Artif Cells Nanomed Biotechnol 44(1):248–262 [DOI] [PubMed] [Google Scholar]
- Bakajin O et al. (2003) Carbon nanotube based microfluidic elements for filtration and concentration. In: 7th International conference on miniaturized chemical and biochemical analysts systems [Google Scholar]
- Barone PW et al. (2005) Near-infrared optical sensors based on single-walled carbon nanotubes. Nat Mater 4(1):86–92 [DOI] [PubMed] [Google Scholar]
- Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes— the route toward applications. Science 297(5582):787–792 [DOI] [PubMed] [Google Scholar]
- Beg S et al. (2011) Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J Pharm Pharmacol 63(2):141–163 [DOI] [PubMed] [Google Scholar]
- Bhattacharya K et al. (2016) Biological interactions of carbon-based nanomaterials: from coronation to degradation. Nanomed Nanotechnol Biol Med 12(2):333–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlon B et al. (2007) A nanoscale probe for fluidic and ionic transport. Nat Nanotechnol 2(2):104–107 [DOI] [PubMed] [Google Scholar]
- Bussy C, Methven L, Kostarelos K (2013) Hemotoxicity of carbon nanotubes. Adv Drug Deliv Rev 65(15):2127–2134 [DOI] [PubMed] [Google Scholar]
- Cao H et al. (2010) Single-walled carbon nanotube network/poly composite thin film for flow sensor. Microsyst Technol 16(6):955–959 [Google Scholar]
- Chen GD et al. (2012) Nanoporous micro-element arrays for particle interception in microfluidic cell separation. Lab Chip 12(17):3159–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mun SC, Kim J (2013) A wide range conductometric pH sensor made with titanium dioxide/multiwall carbon nanotube/ cellulose hybrid nanocomposite. IEEE Sens J 13(11):4157–4162 [Google Scholar]
- Cheng C et al. (2016) Ion transport in complex layered graphene-based membranes with tuneable interlayer spacing. Sci Adv 2(2):e1501272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhowalla M, Unalan HE (2011) Cathodic arc discharge for synthesis of carbon nanoparticles. Plasma processing of nanomaterials. Taylor & Francis, New York, p 147 [Google Scholar]
- Chin CD, Linder V, Sia SK (2007) Lab-on-a-chip devices for global health: past studies and future opportunities. Lab Chip 7(1):41–57 [DOI] [PubMed] [Google Scholar]
- Chin CD, Linder V, Sia SK (2012) Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 12(12):2118–2134 [DOI] [PubMed] [Google Scholar]
- Choi K et al. (2012) Digital microfluidics. Annu Rev Anal Chem 5:413–440 [DOI] [PubMed] [Google Scholar]
- Choong C-L, Milne WI, Teo KB (2008) Review: carbon nanotube for microfluidic lab-on-a-chip application. IntJ Mater Form 1(2):117–125 [Google Scholar]
- Cohen P (2002) The origins of protein phosphorylation. Nat Cell Biol 4(5):E127–E130 [DOI] [PubMed] [Google Scholar]
- Davis MJ et al. (2001) Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 281(5):H1835–H1862 [DOI] [PubMed] [Google Scholar]
- Daw R, Finkelstein J (2006) Lab on a chip. Nature 442(7101):367 Donaldson K et al. (2013) Pulmonary toxicity of carbon nanotubes and asbestos—similarities and differences. Adv Drug Deliv Rev 65(15):2078–2086 [DOI] [PubMed] [Google Scholar]
- Dong J, Ma Q (2015) Advances in mechanisms and signaling pathways of carbon nanotube toxicity. Nanotoxicology 9(5):658–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper MC et al. (2013) Superhydrophobic surfaces as an on-chip microfluidic toolkit for total droplet control. Anal Chem 85(11):5405–5410 [DOI] [PubMed] [Google Scholar]
- Ebbesen T, Ajayan P (1992) Large-scale synthesis of carbon nanotubes. Nature 358(6383):220–222 [Google Scholar]
- Ema M, Gamo M, Honda K (2016) A review of toxicity studies of single-walled carbon nanotubes in laboratory animals. Regul Toxicol Pharmacol 74:42–63 [DOI] [PubMed] [Google Scholar]
- Ensikat HJ et al. (2011) Superhydrophobicity in perfection: the outstanding properties of the lotus leaf. Beilstein J Nanotechnol 2(1):152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair RB (2007) Digital microfluidics: is a true lab-on-a-chip possible? Microfluid Nanofluid 3(3):245–281 [Google Scholar]
- Falk K et al. (2010) Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett 10(10):4067–4073 [DOI] [PubMed] [Google Scholar]
- Farooq MU, Hashmi A, Hong J (2015) Anisotropic bias dependent transport property of defective phosphorene layer. Sci Rep 5:12482–1–12482–11. doi: 10.1038/srep12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L et al. (2002) Super, Äêhydrophobic surfaces: from natural to artificial. Adv Mater 14(24):1857–1860 [Google Scholar]
- Firme CP, Bandaru PR (2010) Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed Nanotechnol Biol Med 6(2):245–256 [DOI] [PubMed] [Google Scholar]
- Frangioni JV (2003) In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7(5):626–634 [DOI] [PubMed] [Google Scholar]
- García-Fandiño R, Sansom MS (2012) Designing biomimetic pores based on carbon nanotubes. Proc Natl Acad Sci 109(18):6939–6944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L et al. (2012) Vertically-aligned carbon nanotube membranes for hydrogen separation. RSC Adv 2(12):5329–5336 [Google Scholar]
- Ge L et al. (2013) Photoelectrochemical lab-on-paper device based on an integrated paper supercapacitor and internal light source. Anal Chem 85(8):3961–3970 [DOI] [PubMed] [Google Scholar]
- Gencoglu A, Minerick AR (2014) Electrochemical detection techniques in micro-and nanofluidic devices. Microfluid Nanofluid 17(5):781–807 [Google Scholar]
- Ghosh S, Sood A, Kumar N (2003) Carbon nanotube flow sensors. Science 299(5609):1042–1044 [DOI] [PubMed] [Google Scholar]
- Gogotsi Y (2006) Nanotubes and nanofibers. CRC Press, Boca Raton [Google Scholar]
- Gogotsi Y, Presser V(2013) Carbon nanomaterials. CRC Press, Boca Raton [Google Scholar]
- Gomez FJV et al. (2015) Microchip electrophoresis-single wall carbon nanotube press-transferred electrodes for fast and reliable electro-chemical sensing of melatonin and its precursors. Electrophoresis 36(16):1880–1885 [DOI] [PubMed] [Google Scholar]
- Guo NL et al. (2012) Multiwalled carbon nanotube-induced gene signatures in the mouse lung: potential predictive value for human lung cancer risk and prognosis. J Toxicol Environ Health A 75(18):1129–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L et al. (2015a) Application of microfluidic “lab-on-a-chip” for the detection of mycotoxins in foods. Trends Food Sci Technol 46(2):252–263 [Google Scholar]
- Guo S et al. (2015b) Nanofluidic transport through isolated carbon nanotube channels: Advances, controversies, and challenges. Adv Mater 27(38):5726–5737 [DOI] [PubMed] [Google Scholar]
- Haeberle S, Zengerle R (2007) Microfluidic platforms for lab-on-a-chip applications. Lab Chip 7(9):1094–1110 [DOI] [PubMed] [Google Scholar]
- Harris PJF (2009) Carbon nanotube science: synthesis, properties and applications. Cambridge University Press, Cambridge [Google Scholar]
- He P et al. (2013) Label-free electrochemical monitoring of vasopressin in aptamer-based microfluidic biosensors. Anal Chim Acta 759:74–80 [DOI] [PubMed] [Google Scholar]
- Heister E et al. (2010) Higher dispersion efficacy of functionalized carbon nanotubes in chemical and biological environments. ACS Nano 4(5):2615–2626 [DOI] [PubMed] [Google Scholar]
- Heller DA et al. (2005) Single-walled carbon nanotube spectroscopy in live cells: towards long-term labels and optical sensors. Adv Mater 17(23):2793–2799 [Google Scholar]
- Hinds BJ et al. (2004) Aligned multiwalled carbon nanotube membranes. Science 303(5654):62–65 [DOI] [PubMed] [Google Scholar]
- Holt JK et al. (2006) Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312(5776):1034–1037 [DOI] [PubMed] [Google Scholar]
- Hummer G, Rasaiah JC, Noworyta JP (2001) Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414(6860):188–190 [DOI] [PubMed] [Google Scholar]
- Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56–58 [Google Scholar]
- Jeng ES et al. (2006) Detection of DNA hybridization using the near-infrared band-gap fluorescence of single-walled carbon nano-tubes. Nano Lett 6(3):371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph P et al. (2006) Slippage of water past superhydrophobic carbon nanotube forests in microchannels. Phys Rev Lett 97(15):156104. [DOI] [PubMed] [Google Scholar]
- Jothimuthu P et al. (2016) Enhanced electrochemical sensing with carbon nanotubes modified with bismuth and magnetic nanoparticles in a lab-on-a-chip. ChemNanoMat 2(9):904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet C et al. (2005) Contact angle measurements on superhydrophobic carbon nanotube forests: effect of fluid pressure. Europhys Lett EPL 71(1):104 [Google Scholar]
- Justino CI, Rocha-Santos TA, Duarte AC (2013) Advances in point-of-care technologies with biosensors based on carbon nanotubes. TrAC Trends Anal Chem 45:24–36 [Google Scholar]
- Kadimisetty K et al. (2015) Automated multiplexed ECL immunoar-rays for cancer biomarker proteins. Anal Chem 87(8):4472–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M et al. (2015a) Temperature-sensitive nanocarriers Morgan & Claypool Publishers, San Rafael [Google Scholar]
- Karimi M et al. (2015b) pH-sensitive micro/nanocarriers, in smart internal stimulus-responsive nanocarriers for drug and gene delivery. Morgan & Claypool Publishers, San Rafael [Google Scholar]
- Karimi M et al. (2015c) Carbon nanotubes part I: preparation of a novel and versatile drug-delivery vehicle. Expert Opin Drug Deliv 12(7):1071–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M et al. (2015d) Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert Opin Drug Deliv 12(7):1089–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M et al. (2015e) Nanotoxicology and future scope for smart nanoparticles, in smart external stimulus-responsive nanocarriers for drug and gene delivery. Morgan & Claypool Publishers, San Rafael [Google Scholar]
- Karimi M et al. (2016) Microfluidic systems for stem cell-based neural tissue engineering. Lab Chip 16(14):2551–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi F et al. (2016) Label-free capture of breast cancer cells spiked in buffy coats using carbon nanotube antibody micro-arrays. Nanotechnology 27(13):13LT02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S et al. (2004) Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol 22(1):93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J et al. (2013) Microfluidic integrated multi-walled carbon nano-tube (MWCNT) sensor for electrochemical nucleic acid concentration measurement. Sens Actuators B Chem 185:370–376 [Google Scholar]
- Kis A, Zettl A (1870) Nanomechanics of carbon nanotubes. Philos Trans R Soc Lond A Math Phys Eng Sci 2008(366):1591–1611 [DOI] [PubMed] [Google Scholar]
- Ko H et al. (2014) Active digital microfluidic paper chips with inkjet-printed patterned electrodes. Adv Mater 26(15):2335–2340 [DOI] [PubMed] [Google Scholar]
- Král P, Shapiro M (2001) Nanotube electron drag in flowing liquids. Phys Rev Lett 86(1):131. [DOI] [PubMed] [Google Scholar]
- Kroto HW et al. (1985) C 60: buckminsterfullerene. Nature 318(6042):162–163 [Google Scholar]
- Krupenkin TN et al. (2007) Reversible wetting–dewetting transitions on electrically tunable superhydrophobic nanostructured surfaces. Langmuir 23(18):9128–9133 [DOI] [PubMed] [Google Scholar]
- Kwon O-S et al. (2013) Fabrication and characterization of inkjet-printed carbon nanotube electrode patterns on paper. Carbon 58:116–127 [Google Scholar]
- Lau KK et al. (2003) Superhydrophobic carbon nanotube forests. Nano Lett 3(12):1701–1705 [Google Scholar]
- Li J, Wang L, Jiang W (2010) Super-hydrophobic surface of bulk carbon nanotubes compacted by spark plasma sintering followed by modification with polytetrofluorethylene. Carbon 48(9):2668–2671 [Google Scholar]
- Li X et al. (2012a) Biocompatibility and toxicity of nanoparticles and nanotubes. J Nanomater 2012:6 [Google Scholar]
- Li X et al. (2012b) Cobalt hexacyanoferrate modified multi-walled carbon nanotubes/graphite composite electrode as electrochemical sensor on microfluidic chip. Anal Chim Acta 710:118–124 [DOI] [PubMed] [Google Scholar]
- Li P et al. (2012c) Integration of nanosensors into a sealed microchannel in a hybrid lab-on-a-chip device. Sens Actuators B Chem 166:870–877 [Google Scholar]
- Li CA et al. (2014) A single-walled carbon nanotube thin film-based pH-sensing microfluidic chip. Analyst 139(8):2011–2015 [DOI] [PubMed] [Google Scholar]
- Li W et al. (2016) Single walled carbon nanotube sandwiched Ni–Ag hybrid nanoparticle layers for the extraordinary electrocatalysis toward glucose oxidation. Electrochim Acta 188:197–209 [Google Scholar]
- Li T et al. (2017) Selective capture and rapid identification of E. coli O157: H7 by carbon nanotube multilayer biosensors and micro-fluidic chip-based LAMP. RSC Adv 7(48):30446–30452 [Google Scholar]
- Liao K-J et al. (2003) Experimental studies on flow velocity sensors based on multiwalled carbon nanotubes. Microfabr Technol 4:57–59 [Google Scholar]
- Liu Y et al. (2007) Artificial lotus leaf structures from assembling carbon nanotubes and their applications in hydrophobic textiles. J Mater Chem 17(11):1071–1078 [Google Scholar]
- Liu H et al. (2010) Translocation of single-stranded DNA through single-walled carbon nanotubes. Science 327(5961):64–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo AO et al. (2010) Fast preparation of hydroxyapatite/superhydrophilic vertically aligned multiwalled carbon nanotube composites for bioactive application. Langmuir 26(23):18308–18314 [DOI] [PubMed] [Google Scholar]
- Lobo AO et al. (2012) Fast functionalization of vertically aligned multiwalled carbon nanotubes using oxygen plasma. Mater Lett 70:89–93 [Google Scholar]
- Lu W et al. (2012) State of the art of carbon nanotube fibers: opportunities and challenges. Adv Mater 24(14):1805–1833 [DOI] [PubMed] [Google Scholar]
- Ma M, Hill RM (2006) Superhydrophobic surfaces. Curr Opin Colloid Interface Sci 11(4):193–202 [Google Scholar]
- Majumder M, Chopra N, Hinds BJ (2011) Mass transport through carbon nanotube membranes in three different regimes: ionic diffusion and gas and liquid flow. ACS Nano 5(5):3867–3877 [DOI] [PubMed] [Google Scholar]
- Marmur A (2004) The lotus effect: superhydrophobicity and metastability. Langmuir 20(9):3517–3519 [DOI] [PubMed] [Google Scholar]
- Maser WK et al. (2001) Production of carbon nanotubes by CO2-laser evaporation of various carbonaceous feedstock materials. Nano-technology 12(2):147 [Google Scholar]
- Medina-Sánchez M, Miserere S, Merkoçi A (2012) Nanomaterials and lab-on-a-chip technologies. Lab Chip 12(11):1932–1943 [DOI] [PubMed] [Google Scholar]
- Melzer K et al. (2016) Enzyme assays using sensor arrays based on ion-selective carbon nanotube field-effect transistors. Biosens Bioelectron 84:7–14 [DOI] [PubMed] [Google Scholar]
- Meng L-Y, Park S-J (2010) Effect of fluorination of carbon nanotubes on superhydrophobic properties of fluoro-based films. J Colloid Interface Sci 342(2):559–563 [DOI] [PubMed] [Google Scholar]
- Meng L-Y, Park S-J (2014) Superhydrophobic carbon-based materials: a review of synthesis, structure, and applications. Carbon Lett 15(2):89–104 [Google Scholar]
- Merkoçi A, Kutter JP (2012) Analytical miniaturization and nanotechnologies. Lab Chip 12(11):1915–1916 [DOI] [PubMed] [Google Scholar]
- Miserere S, Merkoçi A (2015) Microfluidic electrochemical biosensors: fabrication and applications In: Lab-on-a-chip devices and micro-total analysis systems. Springer, pp 141–160 [Google Scholar]
- Miwa M et al. (2000) Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 16(13):5754–5760 [Google Scholar]
- Mogensen KB, Kutter JP (2012) Carbon nanotube based stationary phases for microchip chromatography. Lab Chip 12(11):1951–1958 [DOI] [PubMed] [Google Scholar]
- Mogensen KB et al. (2011) Carbon nanotube based separation columns for high electrical field strengths in microchip electrochromatography. Lab Chip 11(12):2116–2118 [DOI] [PubMed] [Google Scholar]
- Mohammed MI, Haswell S, Gibson I (2015) Lab-on-a-chip or chip-ina-lab: challenges of commercialization lost in translation. Proc Technol 20:54–59 [Google Scholar]
- Mokarian Z, Rasuli R, Abedini Y (2016) Facile synthesis of stable superhydrophobic nanocomposite based on multi-walled carbon nanotubes. Appl Surf Sci 369:567–575 [Google Scholar]
- Moraes FC et al. (2012) Glass/PDMS hybrid microfluidic device integrating vertically aligned SWCNTs to ultrasensitive electrochemical determinations. Lab Chip 12(11):1959–1962 [DOI] [PubMed] [Google Scholar]
- Murray C et al. (2013) Electro-adaptive microfluidics for active tuning of channel geometry using polymer actuators. Microfluid Nanofluid 14(1–2):345–358 [Google Scholar]
- Nednoor P et al. (2007) Carbon nanotube based biomimetic membranes: mimicking protein channels regulated by phosphorylation. J Mater Chem 17(18):1755–1757 [Google Scholar]
- Neuži P et al. (2012) Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discovery 11(8):620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer CM (2001) Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angew Chem Int Ed 40(22):4128–4158 [DOI] [PubMed] [Google Scholar]
- Novell M et al. (2014) A paper-based potentiometric cell for decentralized monitoring of Li levels in whole blood. Lab Chip 14(7):1308–1314 [DOI] [PubMed] [Google Scholar]
- O’connell MJ et al. (2002) Band gap fluorescence from individual single-walled carbon nanotubes. Science 297(5581):593–596 [DOI] [PubMed] [Google Scholar]
- Pan B, Xing B (2008) Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol 42(24):9005–9013 [DOI] [PubMed] [Google Scholar]
- Park J-Y et al. (2004) Electron–phonon scattering in metallic single-walled carbon nanotubes. Nano Lett 4(3):517–520 [Google Scholar]
- Pavlidis I et al. (2010) Functionalized multi-wall carbon nanotubes for lipase immobilization. Adv Eng Mater 12(5):B179–B183 [Google Scholar]
- Peschel G (2011) Carbon–carbon bonds: hybridization. http://www.physik.fu-berlin.de/einrichtungen/ag/ag-reich/lehre/Archiv/ss2011/docs/Gina_Peschel-Handout.pdf. Published on 2011. 5(5) [Google Scholar]
- Pethig R (2010) Review article—dielectrophoresis: status of the theory, technology, and applications. Biomicrofluidics 4(2):022811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland CA et al. (2008) Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3(7):423–428 [DOI] [PubMed] [Google Scholar]
- Popov VN (2004) Carbon nanotubes: properties and application. Mater Sci Eng R Rep 43(3):61–102 [Google Scholar]
- Pumera M (2011) Nanomaterials meet microfluidics. Chem Commun 47(20):5671–5680 [DOI] [PubMed] [Google Scholar]
- Pumera M, Merkoçi A, Alegret S (2007) Carbon nanotube detectors for microchip CE: comparative study of single-wall and multiwall carbon nanotube, and graphite powder films on glassy carbon, gold, and platinum electrode surfaces. Electrophoresis 28(8):1274–1280 [DOI] [PubMed] [Google Scholar]
- Puri N et al. (2014) Conducting polymer functionalized single-walled carbon nanotube based chemiresistive biosensor for the detection of human cardiac myoglobin. Appl Phys Lett 105(15):153701 [Google Scholar]
- Qin Y et al. (2016) Integration of microfluidic injection analysis with carbon nanomaterials/gold nanowire arrays-based biosensors for glucose detection. Sci Bull 61(6):473–480 [Google Scholar]
- Qu Y et al. (2007) CNTs as Ultra-Low-Powered Aqueous Flow Sensors in PDMS Microfluidic Systems. In: 1st Annual IEEE international conference on nano/molecular medicine and engineering (IEEE-NANOMED) [Google Scholar]
- Rafique MMA, Iqbal J (2011) Production of carbon nanotubes by different routes—a review. J Encapsul Adsorpt Sci 1(02):29 [Google Scholar]
- Rajavel K et al. (2015) Multiwalled carbon nanotube oxygen sensor: enhanced oxygen sensitivity at room temperature and mechanism of sensing. ACS Appl Mater Interfaces 7(43):23857–23865 [DOI] [PubMed] [Google Scholar]
- Ramos S et al. (2010) CO2 laser treatment for stabilization of the superhydrophobicity of carbon nanotube surfaces. J Vac Sci Technol B Microelectron Nanometer Struct 28(6):1153 [Google Scholar]
- Rasooly A, Bruck HA, Kostov Y (2013) An ELISA lab-on-a-chip (ELISA-LOC). Microfluid Diagn Methods Protoc 949:451–471 [DOI] [PubMed] [Google Scholar]
- Ravichandran P et al. (2011) Pulmonary biocompatibility assessment of inhaled single-wall and multiwall carbon nanotubes in BALB/c mice. J Biol Chem 286(34):29725–29733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos Á, Zougagh M, Avila M (2012) Miniaturization through lab-ona-chip: Utopia or reality for routine laboratories? A review. Anal Chim Acta 740:1–11 [DOI] [PubMed] [Google Scholar]
- Rodzi N et al. (2013) Hydrophobicity studies of polymer thin films with varied CNT concentration : SPIE micro + nano materials, devices, and applications. International Society for Optics and Photonics [Google Scholar]
- Saghafi M et al. (2014) Preparation of vertically aligned carbon nano-tubes and their electrochemical performance in supercapacitors. Synth Met 195:252–259 [Google Scholar]
- Sansuk S et al. (2012) Ultrasensitive detection of dopamine using a carbon nanotube network microfluidic flow electrode. Anal Chem 85(1):163–169 [DOI] [PubMed] [Google Scholar]
- Seo J et al. (2012) Hierarchical and multifunctional three-dimensional network of carbon nanotubes for microfluidic applications. Adv Mater 24(15):1975–1979 [DOI] [PubMed] [Google Scholar]
- Sharma A et al. (2015) Single-walled carbon nanotube based transparent immunosensor for detection of a prostate cancer biomarker osteopontin. Anal Chim Acta 869:68–73 [DOI] [PubMed] [Google Scholar]
- Shim JS, Ahn CH (2012) Optical immunosensor using carbon nano-tubes coated with a photovoltaic polymer. Biosens Bioelectron 34(1):208–214 [DOI] [PubMed] [Google Scholar]
- Shiu J-Y, Kuo C-W, Chen P (2005) Fabrication of tunable superhydrophobic surfaces : Proceedings of SPIE—the international society for optical engineering [Google Scholar]
- Smalley RE et al. (2003) Carbon nanotubes: synthesis structure properties and applications, vol 80 Springer, Berlin [Google Scholar]
- Snyder-Talkington BN et al. (2015) Multi-walled carbon nanotube-induced gene expression in vitro: concordance with in vivo studies. Toxicology 328:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YS (2012) A passive microfluidic valve fabricated from a hydro-gel filled with carbon nanotubes. Carbon 50(3):1417–1421 [Google Scholar]
- Song W et al. (2016) Enzyme-free electrochemical aptasensor by using silver nanoparticles aggregates coupling with carbon nanotube inducing signal amplification through electrodeposition. J Electroanal Chem 781:62–69 [Google Scholar]
- Sun L, Crooks RM (2000) Single carbon nanotube membranes: a well-defined model for studying mass transport through nanoporous materials. J Am Chem Soc 122(49):12340–12345 [Google Scholar]
- Tanaka K, Iijima S (2014) Carbon nanotubes and graphene. Newnes, Oxford [Google Scholar]
- Teeguarden JG et al. (2011) Comparative proteomics and pulmonary toxicity of instilled single-walled carbon nanotubes, crocidolite asbestos, and ultrafine carbon black in mice. Toxicol Sci 120(1):123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardharajula S et al. (2012) Functionalized carbon nanotubes: biomedical applications. Int J Nanomed 7:5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatanarayanan A et al. (2012) High sensitivity carbon nanotube based electrochemiluminescence sensor array. Biosens Bioelectron 31(1):233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela D et al. (2012a) High NIR-purity index single-walled carbon nanotubes for electrochemical sensing in microfluidic chips. Lab Chip 12(11):2006–2014 [DOI] [PubMed] [Google Scholar]
- Vilela D et al. (2012b) Carbon nanotubes press-transferred on PMMA substrates as exclusive transducers for electrochemical microfluidic sensing. Anal Chem 84(24):10838–10844 [DOI] [PubMed] [Google Scholar]
- Volpatti LR, Yetisen AK (2014) Commercialization of microfluidic devices. Trends Biotechnol 32(7):347–350 [DOI] [PubMed] [Google Scholar]
- Waghe A, Rasaiah JC, Hummer G (2002) Filling and emptying kinetics of carbon nanotubes in water. J Chem Phys 117(23):10789–10795 [Google Scholar]
- Wang C-F et al. (2016) Preparation and characterization of biomimetic superhydrophobic expanded graphite/carbon nanotube/polymer composites. In: 2016 International conference on electronics packaging (ICEP) The Japan Institute of Electronics Packaging [Google Scholar]
- Wang S, Jiang L (2007) Definition of superhydrophobic states. Adv Mater 19(21):3423–3424 [Google Scholar]
- Wang Z, Zhe J (2011) Recent advances in particle and droplet manipulation for lab-on-a-chip devices based on surface acoustic waves. Lab Chip 11(7):1280–1285 [DOI] [PubMed] [Google Scholar]
- Wang DG et al. (1998) Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science 280(5366):1077–1082 [DOI] [PubMed] [Google Scholar]
- Wang K et al. (2011) Stable superhydrophobic composite coatings made from an aqueous dispersion of carbon nanotubes and a fluoro-polymer. Carbon 49(5):1769–1774 [Google Scholar]
- Wang C-F et al. (2014) Combining hierarchical surface roughness with fluorinated surface chemistry to preserve superhydrophobicity after organic contamination. Appl Surf Sci 320:658–663 [Google Scholar]
- Wang H, Wu Y, Song J-F (2015a) Interface potential sensing from adsorption of human serum albumin (HSA) on carbon nanotube (CNT) monitored by zero current potentiometry for HSA determination. Biosens Bioelectron 72:225–229 [DOI] [PubMed] [Google Scholar]
- Wang T et al. (2015b) Electrical sensing of DNA-hybridization using two-port network based on suspended carbon nanotube membrane. Biomed Microdevice 17(6):1–7 [DOI] [PubMed] [Google Scholar]
- Webster SM et al. (2004) Intracellular gate opening in Shaker K+ channels defined by high-affinity metal bridges. Nature 428(6985):864–868 [DOI] [PubMed] [Google Scholar]
- Whitby M, Quirke N (2007) Fluid flow in carbon nanotubes and nano-pipes. Nat Nanotechnol 2(2):87–94 [DOI] [PubMed] [Google Scholar]
- Wong EW, Sheehan PE, Lieber CM (1997) Nanobeam mechanics: elasticity, strength, and toughness of nanorods and nanotubes. Science 277(5334):1971–1975 [Google Scholar]
- Wray S et al. (1988) Characterization of the near infrared absorption spectra of cytochrome aa3 and haemoglobin for the non-invasive monitoring of cerebral oxygenation. Biochim Biophys Acta Bioenerget 933(1):184–192 [DOI] [PubMed] [Google Scholar]
- Xu Z, Zheng Q-S, Chen G (2007) Thermally driven large-amplitude fluctuations in carbon-nanotube-based devices: molecular dynamics simulations. Phys Rev B 75(19):195445 [Google Scholar]
- Yang T et al. (2013) An electrochemical impedance sensor for the label-free ultrasensitive detection of interleukin-6 antigen. Sens Actuators B Chem 178:310–315 [Google Scholar]
- Yang N et al. (2015) Carbon nanotube based biosensors. Sens Actuators B Chem 207:690–715 [Google Scholar]
- Yost AL et al. (2015) Layer-by-layer functionalized nanotube arrays: a versatile microfluidic platform for biodetection. Microsyst Nanoeng 1:15037 [Google Scholar]
- Yu S, Guo Z, Liu W (2015) Biomimetic transparent and superhydrophobic coatings: from nature and beyond nature. Chem Commun 51(10):1775–1794 [DOI] [PubMed] [Google Scholar]
- Zelada-Guillén GA et al. (2013) Ultrasensitive and real-time detection of proteins in blood using a potentiometric carbon-nanotube aptasensor. Biosens Bioelectron 41:366–371 [DOI] [PubMed] [Google Scholar]
- Zhao J et al. (2012) A compact lab-on-a-chip nanosensor for glycerol detection. Appl Phys Lett 100(24):243109 [Google Scholar]
- Zhu X et al. (2014) Fabrication of a superhydrophobic carbon nanotube coating with good reusability and easy repairability. Colloids Surf A 444:252–256 [Google Scholar]
- Zribi B et al. (2016) A microfluidic electrochemical biosensor based on multiwall carbon nanotube/ferrocene for genomic DNA detection of Mycobacterium tuberculosis in clinical isolates. Biomicrofluidics 10(1):014115. [DOI] [PMC free article] [PubMed] [Google Scholar]