Abstract
Endothelial to mesenchymal transition (EndMT) was first reported in the embryogenesis. Recent studies show that EndMT also occurs in the disease progression of atherosclerosis, cardiac and pulmonary fibrosis, pulmonary hypertension, diabetic nephropathy, and cancer. Although transforming growth factor-β (TGFβ) is crucial for EndMT, it is not clear which isoform elicits a predominant effect. The current study aims to directly compare the dose-dependent effects of TGFβ1, TGFβ2, and TGFβ3 on EndMT and characterize the underlying mechanisms. In our results, all the three TGFβ isoforms induced EndMT in human microvascular endothelial cells (HMECs) after 72 hours, as evidenced by the increased expression of mesenchymal markers N-cadherin and alpha-smooth muscle actin (αSMA) as well as the decreased expression of endothelial nitric oxide synthase (eNOS). Interestingly, the effect of TGFβ2 was the most pronounced. At 1 ng/ml, only TGFβ2 treatment resulted in significantly increased phosphorylation (activation) of Smad2/3 and p38-MAPK and increased expression of mesenchymal transcription factors Snail and FoxC2. Intriguingly, we observed that treatment with 1 ng/ml TGFβ1 and TGFβ3, but not TGFβ2 resulted in increased expression of TGFβ2 thus indicating that EndMT with TGFβ1 and TGFβ3 treatments was due to the secondary effects through TGFβ2 secretion. Furthermore, silencing TGFβ2 using siRNA blunted the expression of EndMT markers in TGFβ1 and TGFβ3 treated cells. Together, our results indicate that TGFβ2 is the most potent inducer of EndMT and that TGFβ1- and TGFβ3-induced EndMT necessitates a paracrine loop involving TGFβ2.
Keywords: TGFβ1, TGFβ2, TGFβ3, EndMT, Snail, FoxC2, N-cadherin
1. INTRODUCTION
Endothelial to mesenchymal transition (EndMT) is a phenomenon in which endothelial cells (ECs) lose their characteristic features and acquire mesenchymal properties (Azhar et al., 2009; Boyer et al., 1999). EndMT is not only an essential mechanism implicated in the embryonic cardiac development (Azhar et al., 2009) but also in the progression of diseases such as atherosclerosis, pulmonary hypertension, diabetic nephropathy, cardiac and pulmonary fibrosis, and many types of cancers (Arciniegas et al., 2007; Kizu et al., 2009; Lee and Kay, 2006; Long et al., 2009). Aberrant EndMT results in the uncontrolled conversion of ECs into mesenchymal cells (Medici et al., 2010), which further switch their phenotype to myofibroblasts (Zeisberg et al., 2008). Myofibroblast is a diverse mesenchymal cell type greatly implicated in wound healing (Gabbiani et al., 1971; Stone et al., 2016) and organ fibrosis (Gerarduzzi and Di Battista, 2016; Liu, 2006; Zeisberg et al., 2000). Upon activation by biochemical and mechanical signals, myofibroblasts secrete and organize extracellular matrix (ECM), develop specialized matrix adhesions (Hinz et al., 2003), and exhibit cytoskeletal organization characterized by contractile actin filaments (Gabbiani et al., 1971). This allows the re-establishment of mechanical integrity and stability to the damaged tissue thus assisting in both the wound closure and resolution, which can lead to pathological remodeling when aberrantly stimulated and goes unconstrained (Hinz and Gabbiani, 2010; Hinz et al., 2012).
EndMT is characterized by the loss of cell-cell adhesions and changes in cell polarity-inducing a spindle-shaped morphology (Manetti et al., 2011). These changes are accompanied by reduced expression of one or more of the endothelial markers such as VE-cadherin, eNOS, and CD31, and increased expression of mesenchymal markers like fibroblast-specific protein-1 (FSP-1), alpha-smooth muscle actin (αSMA), N-cadherin, and fibronectin (Potenta et al., 2008). Loss of cell-cell adhesion is mediated by transcription factors such as Snail, Slug, ZEB-1, Twist, and FoxC2 that suppress transcription of genes encoding proteins involved in the formation of adherens junctions and tight junctions (Liebner et al., 2004; Medici et al., 2008) that are integral to an intact endothelium. Transforming growth factor-β1 (TGFβ1) is a potent inducer of epithelial to mesenchymal transition (EMT) (Akhurst and Derynck, 2001), a phenomenon very similar in biology to that of EndMT. Whereas TGFβ2 is a more potent inducer of fibrosis than TGFβ1 in amphibians (Rosa et al., 1988), both TGFβ1 (Wermuth et al., 2016) and TGFβ2 (Kokudo et al., 2008; Liebner et al., 2004; Romano and Runyan, 2000) are implicated greatly in mediating myofibroblast activation, EMT, and EndMT in vertebrates leading to organ fibrosis. Although both TGFβ1 and TGFβ2 promote EndMT, only TGFβ2 gene ablation in mice prevented EndMT-mediated cardiac development, and while TGFβ1 or TGFβ3 knockout mice had normal heart development (Azhar et al., 2009). Interestingly, although TGFβ3 is implicated in EMT in cancer (Jalali et al., 2012), there are no reports on the effects of TGFβ3 on EndMT. Thus, it is not clear from the literature which isoform of TGFβ is the predominant inducer of EndMT.
In the current study, we directly compared the isoform-specific effects of TGFβ1, TGFβ2 and TGFβ3 in inducing EndMT in human microvascular ECs (HMECs) in vitro and their effect on the expression of EC markers, mesenchymal markers, transcription factors regulating mesenchymal gene expression and the activity status of TGFβ-mediated canonical and non-canonical pathways. Our results demonstrated that TGFβ2 is the predominant mediator of EndMT in HMECs and that TGFβ1- and TGFβ3-induced EndMT needs EC-mediated paracrine loop through increased TGFβ2 secretion.
2. MATERIALS AND METHODS
2.1 Cell culture
Human dermal (Telomerase-immortalized) microvascular ECs (HMEC) (CRL-4025; ATCC, Manassas, VA) were maintained in EC Basal Medium-2 with a Growth Medium-2 Bullet Kit (Lonza; Walkersville, MD). All cultures were maintained in a humidified 5% CO2 incubator at 37 °C and routinely passaged when 80–90% confluent. TGFβ1, TGFβ2, and TGFβ3 were obtained from R&D Systems (Minneapolis, MN) and were reconstituted according to the manufacturer’s protocol. HMECs monolayers were treated with 1, 2.5 and 5 ng/ml doses of TGFβ1, TGFβ2 and TGFβ3 in 5% serum-containing medium for 72 hours. The growth factors were replenished every 24 hours.
2.2 Western blot analysis
Cell lysates were prepared using complete lysis buffer (EMD Millipore, San Diego, CA) with protease and phosphatase inhibitor cocktails (Roche Diagnostics, Indianapolis, IN). Protein quantification was performed using DC protein assay from Bio-Rad (Hercules, and CA). Western blot analysis was performed as described previously (Abdalla et al., 2013; Al-Azayzih et al., 2015). Antibodies used include N-cadherin (4061), VE-cadherin (2158S), phosphorylated p-38 MAPK (9211S), total p38-MAPK (9212S), phosphorylated Smad2/3 (8828S), total Smad2/3 (8685S), FoxC2 (12974S), Snail (3879S), and GAPDH (2118L) from Cell Signaling Technology (Danvers, MA), αSMA (A2547) and β-actin (A5441) from Sigma (St. Louis, MO), eNOS (610297) from BD Pharmingen (San Diego, CA), and TGFβ2 (MAB612) from R&D (Minneapolis, MN). Band densitometry was done using NIH Image J software.
2.3 SiRNA-mediated TGFβ2 knockdown
HMECs were transfected with TGFβ2 SiRNA (50 nM) (Sigma, St. Louis, MO) using Lipofectamine 2000 (Thermo Scientific, Grand Island, NY) when 70–80% confluent according to the manufacturer’s protocol. After transfection cells were incubated for 5 hours at 37° C in serum-free media (Thermo Scientific, Grand Island, NY). The medium was then discarded and cells were further cultured in the EBM-2 medium. After 12 hours of transfection, cells were treated with TGFβ1 or TGFβ3 (1 ng/ml) for 72 hours. Scrambled SiRNA was used as a control.
2.4 Cell scattering assay
HMECs were seeded at a low density and were allowed to grow to form small colonies. After the formation of small scattered colonies, the EBM-2 medium was replaced with fresh medium containing 5 % FBS and cells were treated with vehicle or 1 ng/ml TGFβ1, 2 and 3. This treatment was done daily for 3 days. Cell scattering images were taken using phase contrast microscope and the images were qualitatively analyzed for consistency in the observations.
2.5 Statistical Analysis
All the data are presented as Mean ± SD and were calculated from multiple independent experiments performed in quadruplicates. For normalized data analysis, data was confirmed that normality assumption was satisfied and analyzed using paired sample t-test (dependent t-test) and/or further confirmed with non-parametric test Wilcoxon signed rank test. For all other analysis, Student’s two-tailed t-test or ANOVA test were used to determine significant differences between treatment and control values using the GraphPad Prism 4.03 and SPSS 17.0 software.
3. RESULTS
3.1 TGFβ1, 2 and 3- induced EndMT in vitro is a long-term process
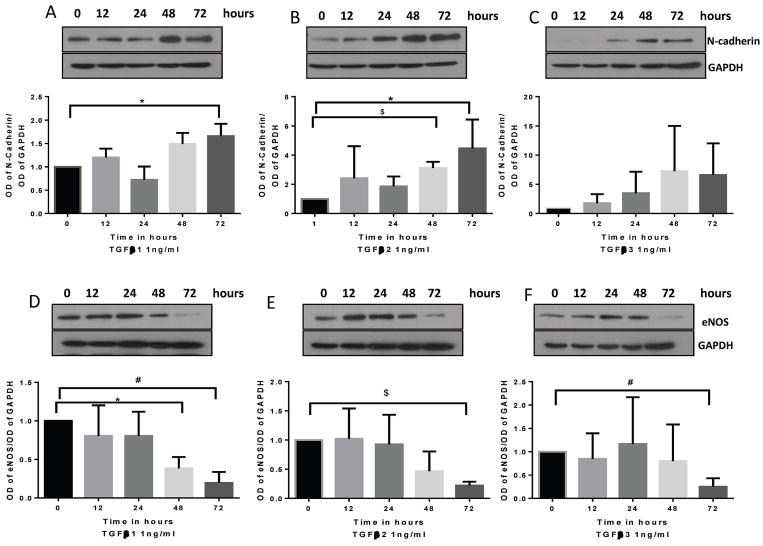
To investigate the time response effects of TGFβ1, 2 and 3 on inducing EndMT, HMEC monolayers were treated with 1 ng/ml dose of the three TGFβ isoforms for 0, 12, 24, 48 and 72 hours and the cell lysates were subjected to Western blot analysis. We observed that although stimulation of HMECs with TGFβ isoforms results in a gradual increase in the expression of mesenchymal marker N-Cadherin (Figure 1A–C) and decrease in the expression of endothelial marker eNOS (Figure 1D–F) we observed that the earliest time point at which any of the isoforms significantly induce EndMT is 48 hours. By 72 hours all the three isoforms induced the expression of N-Cadherin (except for TGFβ3) and loss of eNOS promoting EndMT. These results indicate that TGFβ- induced EndMT in vitro is a long-term process.
FIGURE 1. TGFβ- induced EndMT in HMECs is a long-term process.
(A–C) Representative Western blot images and the corresponding bar graph of band densitometry showing a gradual increase in the expression of mesenchymal marker N-Cadherin in HMECs treated with 1 ng/ml of TGFβ1, 2 and 3 for 0, 12, 24, 48 and 72 hours. (D–E) Representative Western blot images and the corresponding bar graph of band densitometry showing a gradual decrease in the expression of endothelial marker eNOS in HMECs treated with 1 ng/ml of TGFβ1, 2 and 3 for 0, 12, 24, 48 and 72 hours. Data are represented as mean ± SD. (n=3–5), *p<0.05; #p<0.0;, $p<0.001.
3.2 TGFβ2 is the most potent inducer of mesenchymal markers in vitro
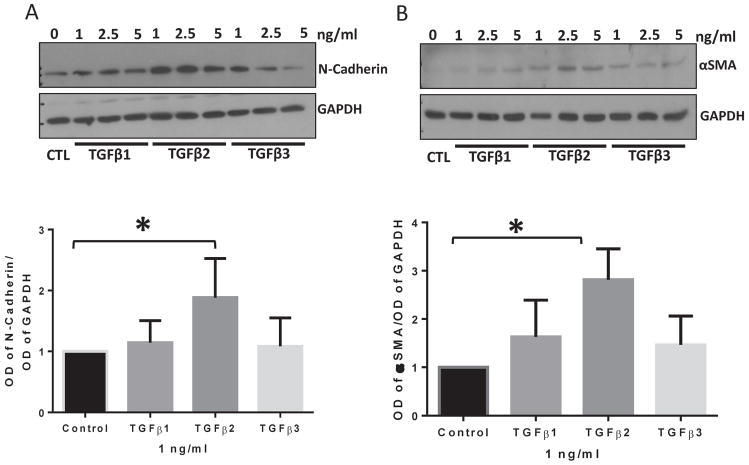
In order to determine which of the three TGFβ isoforms is more potent in inducing the expression of mesenchymal markers and hence EndMT, HMEC monolayers were treated with 1, 2.5, and 5 ng/ml doses of TGFβ1, TGFβ2, and TGFβ3 for 72 hours and the cell lysates were subjected to Western blot analysis. Our results showed that stimulation of HMEC by TGFβ2 at the lowest dose of 1 ng/ml results in a significantly higher expression of mesenchymal markers N-cadherin and αSMA as compared to control, TGFβ1 or TGFβ3 (Figure 2A–B, S1, and S2). The replicate blots are shown in Figures S3 and S4. These results indicate that TGFβ2 is more potent compared to that of TGFβ1 and TGFβ3 in inducing mesenchymal markers thus promoting EndMT.
FIGURE 2. TGFβ2 is a more potent inducer of mesenchymal markers in HMECs compared to TGFβ1 and TGFβ3.
(A) Representative Western blot images and the corresponding bar graph of band densitometry showing increased expression of mesenchymal marker N-cadherin in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. (B) Representative Western blot images and the corresponding bar graph of band densitometry showing increased expression of the mesenchymal marker αSMA in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. Data are represented as mean ± SD. (n=3–5), *p<0.05.
3.3 TGFβ2 is the most potent suppressor of endothelial marker expression in vitro
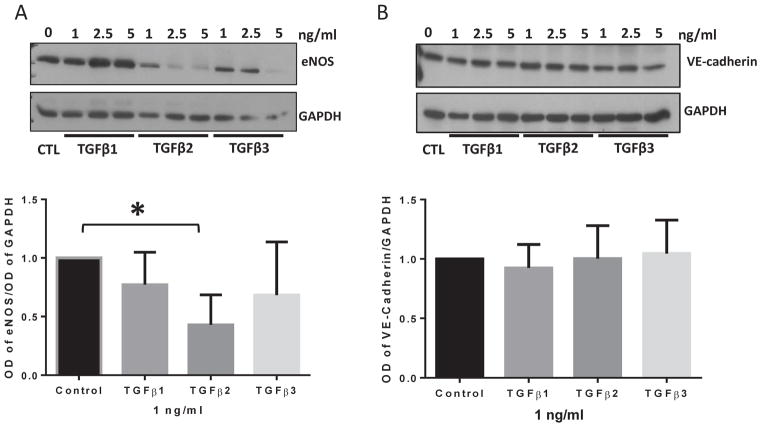
Next, we determined the effects of 1, 2.5, and 5 ng/ml doses of TGFβ1, TGFβ2, and TGFβ3 on the expression of endothelial markers. Analysis of cell lysates after 72 hours of treatment revealed that TGFβ2 treatment resulted in a reduced expression of endothelial marker eNOS and the effect was much higher than similar doses of TGFβ1 and TGFβ3 (Figure 3A–B). However, there was no change in the expression of another endothelial marker VE-cadherin as known previously that epithelial adherens junction protein E-Cadherin expression is not affected by TGFβ (Morin et al., 2011). The replicate blots are shown in Figures S3 and S4. These results indicate that 1 ng/ml dose of TGFβ2 is more potent in downregulating the endothelial marker than that of TGFβ1 or TGFβ3 at a similar dose (Figure 3A–B).
FIGURE 3. TGFβ2 is a more potent suppressor of the endothelial marker expression in HMECs compared to TGFβ1 and TGFβ3.
(A) Representative Western blot images and the corresponding bar graph of band densitometry showing reduced expression of endothelial marker eNOS in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. (B) Representative Western blot images and the corresponding bar graph of band densitometry showing no significant change in the expression of endothelial receptor VE-cadherin in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. Data are represented as mean ± SD. (n=3–5), *p<0.05.
3.4 TGFβ2 is the most potent activator of canonical and non-canonical TGFβ-mediated pathways in HMECs
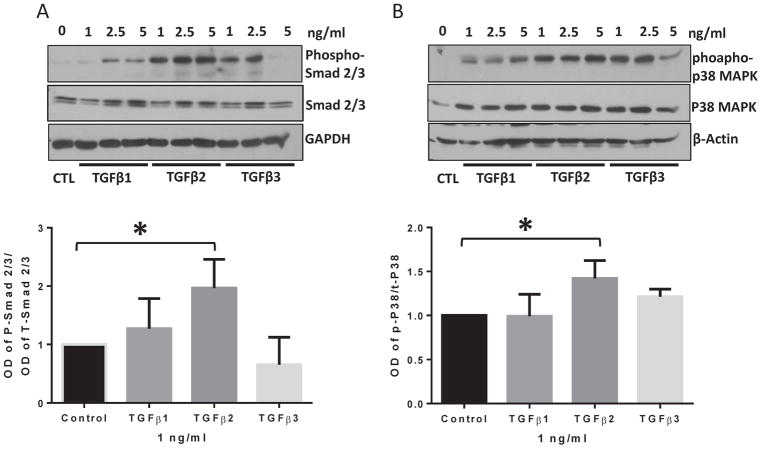
TGFβ superfamily ligands exert their downstream signaling effects via either canonical or non-canonical signaling pathways (Gauldie et al., 2007; Hanahan and Folkman, 1996; Kavsak et al., 2000; Santibanez et al., 2011; Suzuki et al., 2002). In order to investigate whether one or both these pathways are activated by TGFβ isoforms in EndMT, HMEC monolayers were treated with 1, 2.5, and 5 ng/ml doses of TGFβ1, 2, and 3 isoforms for 72 hours and the cell lysates were subjected to Western blot analysis. Our results showed that the lowest dose of 1 ng/ml of TGFβ2 promoted activation (phosphorylation) of receptor-regulated Smad2/3 (Figure 4A), the canonical effector of TGFβ signaling and p38-MAPK (Figure 4B, S1 and S2), the non-canonical effector of TGFβ signaling with higher efficiency as compared to the vehicle, TGFβ1 or TGFβ3 treated cells at the same dose suggesting the involvement of both canonical and non-canonical TGFβ signaling in promoting EndMT. These results indicate that TGFβ2 is the predominant inducer of EndMT in HMECs.
FIGURE 4. TGFβ2 exhibits higher potency in activating both canonical and non-canonical pathways in HMECs compared to TGFβ1 and TGFβ3.
(A) Representative Western blot images and the corresponding bar graph of band densitometry showing increased phosphorylation and total expression of canonical transcription factor Smad2/3 in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. (B) Representative Western blot images and the corresponding bar graph of band densitometry showing increased phosphorylation of non-canonical, stress-induced p38 MAPK in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. Data are represented as mean ± SD. (n=3–5), *p<0.05.
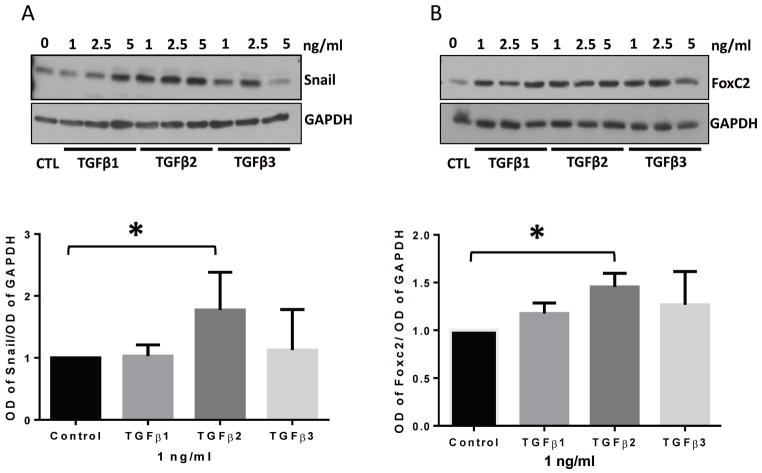
3.5 TGFβ2, but not TGFβ1 or TGFβ3, is the predominant regulator of expression of mesenchymal transcription factors Snail and FoxC2 in HMECs
We wanted to further examine the efficiency of different TGFβ isoforms in inducing EndMT promoting transcription factors Snail and FoxC2. Once again, HMEC monolayers were treated with 1, 2.5, and 5 ng/ml doses of TGFβ1, 2, and 3 isoforms for 72 hours and the cell lysates were subjected to Western blot analysis. We observed that 1 ng/ml dose of TGFβ2 is more potent in upregulating the mesenchymal transcription factors Snail and FoxC2 in HMECs than a similar dose of TGFβ1 or TGFβ3 (Figure 5A–B). The replicate blots are shown in Figures S3 and S4. These results indicate that TGFβ2 is the predominant inducer of mesenchymal transcription factors and hence EndMT.
FIGURE 5. TGFβ2 is a more potent stimulator of mesenchymal transcription factor expression in HMECs compared to TGFβ1 and TGFβ3.
(A) Representative Western blot images and the corresponding bar graph of band densitometry showing increased expression of mesenchymal transcription factor Snail in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. (B) Representative Western blot images and the corresponding bar graph of band densitometry showing increased expression of mesenchymal transcription factor FoxC2 in HMECs in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. Data are represented as mean ± SD. (n=3–5), *p<0.05.
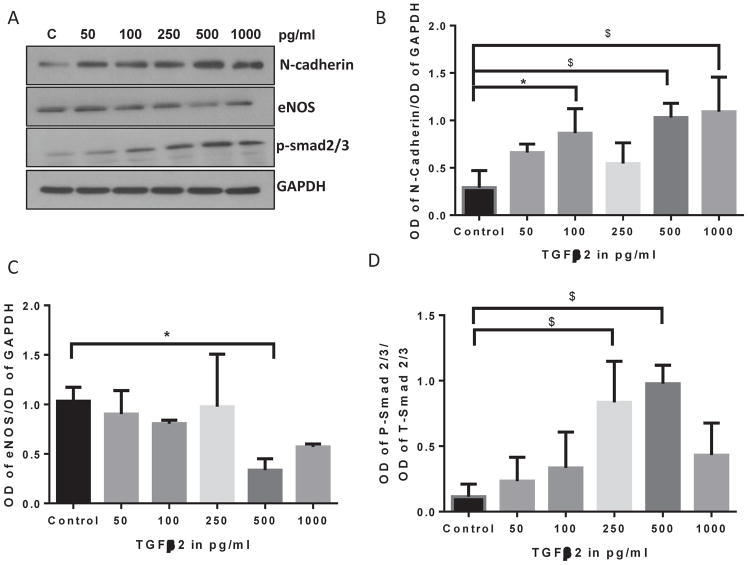
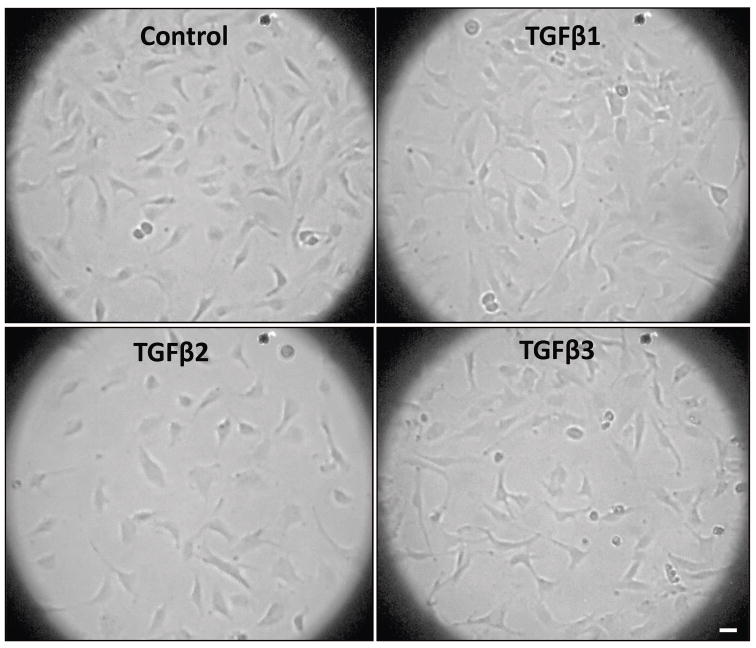
3.6 TGFβ2 induces EndMT and cell scattering at lower doses compared to TGFβ1 or TGFβ3
Whereas both TGFβ1 and TGFβ3 showed dose-dependent effects at a range of 1, 2.5 and 5 ng/ml doses, TGFβ2 effects were almost the same between these doses suggesting that TGFβ2 may still induce EndMT on much lower levels compared to TGFβ1 and TGFβ3. To further investigate that 1 ng/ml dose of TGFβ2 is the right dose for the cell scattering assay, we determined the dose-dependent effect of TGFβ2 on a lower range of 50 pg/ml to 1 ng/ml. Our analysis indicated that the effect of TGFβ2 on mesenchymal marker N-cadherin expression peaks at 0.5 and 1.0 ng/ml doses (Figure 6A–D), thus confirming it as the most appropriate dose to compare the isoform specific-effects. Furthermore, cell scattering analysis, a method that is often used to qualitatively determine mesenchymal phenotype, indicated a predominant effect of 1 ng/ml TGFβ2 on cell scattering, compared to the similar doses of TGFβ1 or TGFβ2 (Figure 7).
FIGURE 6. TGFβ2 induces EndMT at lower doses.
(A) Representative Western blot images showing a gradual increase in the expression of mesenchymal marker N-Cadherin associated with increased p-smad2/3 and decreased eNOS levels in HMECs treated with TGFβ1 for 72 hours with 0, 50, 100, 250, 500 and 1000 pg/ml doses. (B–D) Corresponding bar graph of band densitometry showing a gradual decrease a gradual increase in the expression of mesenchymal marker N-Cadherin associated with increased p-smad2/3 and decreased eNOS levels in HMECs treated with TGFβ1 for 72 hours with 0, 50, 100, 250, 500 and 1000 pg/ml doses. Data are represented as mean ± SD. (n=3–5), *p<0.05; $p<0.001.
FIGURE 7. TGFβ2 predominantly induce endothelial cell scattering compared to other isoforms.
Representative images showing the predominant effect of TGFβ2 on cell scattering, a feature of the invasive mesenchymal cells compared to control, TGFβ1 and TGFβ2 treated cells after 72 hours of treatment. Scale bar: 20 μM.
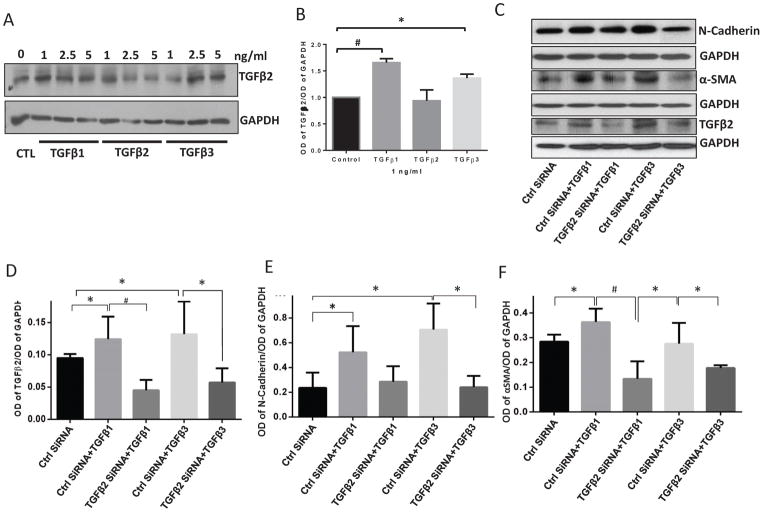
3.7 TGFβ1- and TGFβ3-induced EndMT involve an endothelial TGFβ2-mediated paracrine loop
In order to investigate how TGFβ1 or TGFβ3 were able to induce EndMT in HMEC, we investigated if TGFβ1 or TGFβ3 could increase the production of TGFβ2 by HMEC. Our Western blot analysis indicated that treatment with both TGFβ1 and TGFβ3, but not TGFβ2 itself, promoted synthesis of TGFβ2 by the HMEC (Figure 8A–B). Next, we employed SiRNA-mediated TGFβ2 knockdown in HMECs to determine whether TGFβ2 deficient cells will be resistant to TGFβ1 and TGFβ3-induced EndMT. Our data indicated that HMECs transfected with TGFβ2 SiRNA resulted in >60% knockdown in TGFβ2 (Figure 8C–D) expression blunted the effects of TGFβ1 and TGFβ3 on N-cadherin (Figure 8E) and αSMA (Figure 8F) expression. These results further confirmed that TGFβ2 is the most potent inducer of EndMT and that TGFβ1 and TGFβ3 isoforms initiate a TGFβ2 paracrine loop to indirectly promote EndMT in HMECs (Figure 9).
FIGURE 8. TGFβ1- and TGFβ3-induced EndMT needs activation of a paracrine loop in HMECs involving TGFβ2.
(A) Representative Western blot images showing increased expression of the most potent EndMT stimulating TGFβ isoform, TGFβ2, in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. (B) Bar graph of Western blot band densitometry analysis showing increased expression of TGFβ2 in response to 1, 2.5 and 5 ng/ml of TGFβ1, TGFβ2, and TGFβ3 for 72 hours. (C) Representative Western blot images showing increased expression of N-Cadherin, αSMA, and TGFβ2 by both TGFβ1 and TGFβ3, both of which were blunted upon SiRNA-mediated knockdown of TGFβ2. (D–E) Bar graph of Western blot band densitometry analysis showing increased expression of N-Cadherin and αSMA by both TGFβ1 and TGFβ3, which were blunted upon SiRNA-mediated knockdown of TGFβ2. Data are represented as mean ± SD. (n=3–5), *p<0.05; #p<0.01.
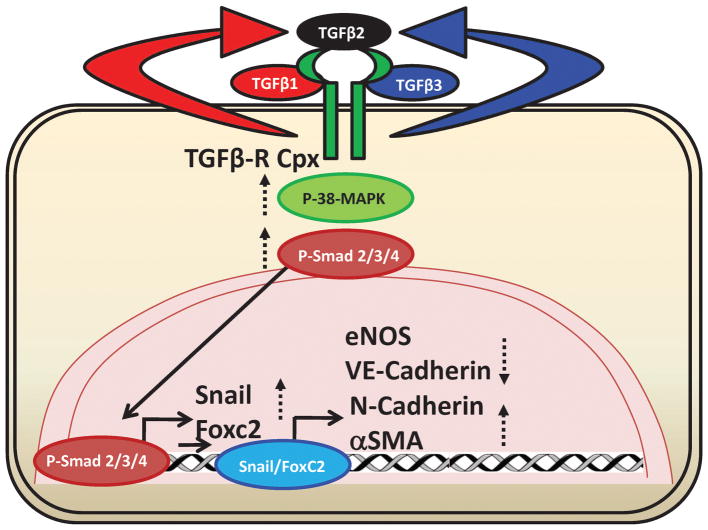
FIGURE 9. Diagrammatic sketch of the working hypothesis.
Both TGFβ1 and TGFβ3, but not TGFβ2 itself, induces the generation of TGFβ2 in HMECs, which in turn, promote EndMT pathways leading to Snail and FoxC2-induced transcriptional activation of mesenchymal markers and repression of endothelial markers.
4. DISCUSSION
Myofibroblasts or activated mesenchymal cells play a crucial role in tissue repair and contribute to the pathogenesis of various fibrotic and vascular diseases including but not limited to interstitial pulmonary fibrosis, systemic sclerosis, and liver or cardiac fibrosis (Hinz and Gabbiani, 2010; Hinz et al., 2012; Neilson, 2006). However, the source of these myofibroblasts remains fairly controversial and is gaining more attention recently due to the emergence of a new type of cellular transdifferentiation, a phenomenon known as EndMT (Abraham et al., 2007; Hinz et al., 2007). EndMT is a biological process in which ECs lose one or more of their specific markers such as VE-cadherin, eNOS, and CD31 and acquire mesenchymal markers such as N-cadherin, αSMA, FSP1 and collagen VI (Arciniegas et al., 2005; Arciniegas et al., 1992). EndMT attributes proliferative properties to the otherwise quiescent and adherent ECs, transforming them into myofibroblasts (Li and Bertram, 2010; Piera-Velazquez et al., 2011). In the recent past, quite a few studies reported the occurrence of EndMT in various fibrotic disorders like cardiac (Zeisberg et al., 2007), pulmonary (Hashimoto et al., 2010; Pinto et al., 2016), corneal (Li et al., 2013), and renal fibrosis (Li and Bertram, 2010). Both TGFβ1 and TGFβ3 have been implicated in the cardiac cushion formation in avian embryo (Nakajima et al., 1997a; Nakajima et al., 1997b; Nakajima et al., 2000; Yamagishi et al., 1999). Although EndMT is implicated in many diseases, the stimuli that trigger the initiation of this cellular differentiation and the mechanisms through which the transformation occurs remain elusive. Several signalling pathways are reported in EndMT, while the most important being TGFβ binding (Doerr et al., 2016; Montorfano et al., 2014; Nakajima et al., 2000; Zeisberg et al., 2008; Zeisberg et al., 2007). Given the popularity of TGFβ as a potent cell differentiation cytokine and the extensive involvement of its signaling in the pathogenesis of fibrotic diseases (Rosenbloom et al., 2010; Wynn, 2008), several groups have investigated its role in the generation of myofibroblasts via EndMT (Hinz et al., 2007; Hinz et al., 2012). Although it is widely accepted that TGFβ1 is a potent inducer of fibrosis via generation of myofibroblasts in various fibrotic models, several emerging reports advocate the involvement of TGFβ2 in promoting EndMT (Chrobak et al., 2013; Medici et al., 2011; Nie et al., 2014; Shi et al., 2016). Whereas TGFβ and its downstream effectors as EndMT inducers are being extensively studied by several groups in various fibrotic and vascular diseases, appropriate knowledge on the contributions of different TGFβ isoforms, TGFβ1, 2, and 3 remains unknown.
In order to identify the most potent inducer of EndMT, we directly compared the dose-dependent effects of TGFβ1, TGFβ2, and TGFβ3 on EndMT in vitro. We examined changes in the expression of endothelial and mesenchymal markers, transcription factors that promote mesenchymal transition, and the activation of TGFβ-induced canonical and non-canonical pathways by treating HMECs with various doses of these TGFβ isoforms for 0–72 hours. Stimulation of HMECs with 1 ng/ml dose of the three isoforms TGFβ1, 2 and 3 for 72 hours revealed that TGFβ is involved in EndMT which was evident from the upregulation of mesenchymal markers N-cadherin and αSMA and a decrease in expression of endothelial marker eNOS. We noticed that the earliest time point at which the EndMT changes start to occur is 48 hours and that at 72 hours most if not all the isoforms induce EndMT. Together these results indicate that TGFβ induced EndMT in vitro is a long-term process.
Among the different receptor-regulated Smads involved in TGFβ signaling, Smad3 was reported as the pro-fibrotic member of the Smad family as its activation (phosphorylation) promotes the progression of fibrosis (Darland et al., 2003; Hirschi et al., 1998). On a similar note, we observed that TGFβ2 increases the phosphorylation (activation) of Smad2/3 and p38 MAPK greater than that of TGFβ1 and TGFβ3 suggesting the predominance of TGFβ2 in inducing mesenchymal transition of ECs and the involvement of both canonical and non-canonical TGFβ signaling pathways in promoting EndMT. We also observed that TGFβ2 increases the expression of EndMT promoting transcription factors Snail and FoxC2 with significantly higher efficiency than the other two TGFβ isoforms further confirming our observation and in agreement with the recent finding reporting a TGFβ2-mediated activation of the ALK5-Smad2/3-Snail pathway (Zeng et al., 2013) leading to EndMT. Interestingly, two most recent studies indicate the unique role of Smad1/5 pathway in EndMT (Ramachandran et al., 2018; Sniegon et al., 2017), in addition to the observed role of Smad2/3-Snail pathway.
Biochemical changes in the expression of EndMT markers must also be accompanied by changes in its morphology and/or functional behavior. Cancer cells that invade and metastasize to distant organs exhibit the ability of cell scattering, invasion and transendothelial migration in vitro (Al-Azayzih et al., 2015; Gao et al., 2015). Unlike cancer cells, motility and adhesion of endothelial cells is a measure of endothelial barrier function and angiogenesis (Chen et al., 2005; Somanath et al., 2007). Although a scattering of endothelial cells in a monolayer is not as robust as the colony forming cancer cells, for a 72-hour long effect of TGFβ isoforms on EndMT, cell scattering is still the best possible assay to be performed in vitro. Our qualitative analysis indicated that 72-hour treatment of endothelial cells with 1 ng/ml of TGFβ2 induced cell scattering more effectively than the comparable doses of TGFβ1 or TGFβ3. Although endothelial cells appeared to be scattered in TGFβ3 wells, this, however, was also contributed by increased cell death with TGFβ3 treatment compared to TGFβ1 or TGFβ2, thus demonstrating that TGFβ2 is a more robust inducer of EndMT that TGFβ1 or TGFβ3.
Another important question that we wanted to address was how TGFβ1 and TGFβ3 were able to induce EndMT, albeit at higher doses. Are the functions of these three isoforms redundant? To address this, we determined the expression changes in TGFβ2 post 72-hour treatment with TGFβ1, TGFβ2, and TGFβ3. The most intriguing and prominent finding of our study that came from this experiment was that both TGFβ1 and TGFβ3, but not TGFβ2, stimulated the expression of TGFβ2 by the HMECs. This indicated the existence of a positive feedback loop between different TGFβ isoforms via paracrine effects involving TGFβ2 synthesis in inducing EndMT. Together, our results demonstrate that although all the three isoforms of TGFβ moderately induce EndMT, TGFβ2 is the most potent inducer. This is in agreement with observations from several reports that suggested the predominance of TGFβ2 in inducing EndMT of mouse embryonic stem cell-derived ECs (Kokudo et al., 2008) and that TGFβ2 is a more potent fibrotic inducer than TGFβ1 in amphibians (Hsuan, 1989; Rosa et al., 1988). To our knowledge, this is the first study to examine and directly compare the dose-dependent effects of three different TGFβ isoforms- TGFβ1, TGFβ2, and TGFβ3 on EndMT demonstrating a paracrine TGFβ2-mediated EndMT loop in HMECs stimulated by TGFβ1 and TGFβ3. Here we indicate the predominance of TGFβ2 in inducing EndMT thus paving a way to direct future investigations on this pathway in EndMT to yield a better understanding of the mechanisms involved.
Supplementary Material
FIGURE S1: TGFβ2 exhibits predominance in inducing EndMT. (A) Representative Western blot images and the corresponding bar graph of band densitometry showing a trend in the increased expression of the mesenchymal marker N-Cadherin in HMECs at 72 hours treated with TGFβ2 compared to that of control and TGFβ1 and TGFβ3. (B) Representative Western blot images and the corresponding bar graph of band densitometry showing decreased expression of the endothelial marker eNOS in HMECs at 72 hours treated with TGFβ2 compared to that of control and TGFβ1 and TGFβ3. Data are represented as mean ± SD. (n=3–5), *p<0.05.
FIGURE S2: TGFβ2 exhibits predominance in promoting expression of mesenchymal markers. Bar graphs of band densitometry showing a trend in the increased expression of the mesenchymal marker αSMA, Snail, P-P38 MAPK, P-Smad2/3 and TGFβ2 in HMECs at 72 hours treated with 5 ng/ml dose of TGFβ2 compared to that of control and 5 ng/ml dose of TGFβ1 and TGFβ3.
FIGURE S3: Replicate Western blot images indicating the predominant, dose-dependent effect of TGFβ2 on the expression of N-Cadherin, αSMA, and p-p38MAPK and decreased expression of eNOS compared to control, TGFβ1 and TGFβ3 treated cells after 72 hours of treatment.
FIGURE S4: Replicate Western blot images indicating the predominant, dose-dependent effect of TGFβ2 on the expression of p-Smad1/2, and Snail1 with no changes in the expression of VE-Cadherin compared to control, TGFβ1 and TGFβ3 treated cells after 72 hours of treatment. Replicate images of dose-dependent effect of TGFβ1 and TGFβ3 on increasing the level of TGFβ2 is also shown.
Acknowledgments
Funds were provided by the National Institutes of Health grant (R01HL103952) to PRS. This work has been accomplished using the resources and facilities at the Charlie Norwood Medical Center in Augusta, GA. The funders had no role in the study design, data collection, analysis, and decision to publish the data. The contents of the manuscript do not represent the views of Department of Veteran Affairs or the United States Government.
Footnotes
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists.
References
- Abdalla M, Goc A, Segar L, Somanath PR. Akt1 mediates alpha-smooth muscle actin expression and myofibroblast differentiation via myocardin and serum response factor. The Journal of biological chemistry. 2013;288(46):33483–33493. doi: 10.1074/jbc.M113.504290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9(2):136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends in cell biology. 2001;11(11):S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- Al-Azayzih A, Gao F, Somanath PR. P21 activated kinase-1 mediates transforming growth factor beta1-induced prostate cancer cell epithelial to mesenchymal transition. Biochimica et biophysica acta. 2015;1853(5):1229–1239. doi: 10.1016/j.bbamcr.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2007;293(1):L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- Arciniegas E, Neves CY, Carrillo LM, Zambrano EA, Ramirez R. Endothelial-mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium. 2005;12(4):193–200. doi: 10.1080/10623320500227283. [DOI] [PubMed] [Google Scholar]
- Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103(Pt 2):521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Developmental dynamics: an official publication of the American Association of Anatomists. 2009;238(2):431–442. doi: 10.1002/dvdy.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Developmental biology. 1999;208(2):530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11(11):1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak I, Lenna S, Stawski L, Trojanowska M. Interferon-gamma promotes vascular remodeling in human microvascular endothelial cells by upregulating endothelin (ET)-1 and transforming growth factor (TGF) beta2. J Cell Physiol. 2013;228(8):1774–1783. doi: 10.1002/jcp.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Developmental biology. 2003;264(1):275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Doerr M, Morrison J, Bergeron L, Coomber BL, Viloria-Petit A. Differential effect of hypoxia on early endothelial-mesenchymal transition response to transforming growth beta isoforms 1 and 2. Microvasc Res. 2016;108:48–63. doi: 10.1016/j.mvr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gao F, Al-Azayzih A, Somanath PR. Discrete functions of GSK3alpha and GSK3beta isoforms in prostate tumor growth and micrometastasis. Oncotarget. 2015;6(8):5947–5962. doi: 10.18632/oncotarget.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35(Pt 4):661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- Gerarduzzi C, Di Battista JA. Myofibroblast repair mechanisms post-inflammatory response: a fibrotic perspective. Inflamm Res. 2016 doi: 10.1007/s00011-016-1019-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43(2):161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Molecular biology of the cell. 2003;14(6):2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. The American journal of pathology. 2007;170(6):1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. The American journal of pathology. 2012;180(4):1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. The Journal of cell biology. 1998;141(3):805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuan JJ. Transforming growth factors beta. Br Med Bull. 1989;45(2):425–437. doi: 10.1093/oxfordjournals.bmb.a072332. [DOI] [PubMed] [Google Scholar]
- Jalali A, Zhu X, Liu C, Nawshad A. Induction of palate epithelial mesenchymal transition by transforming growth factor beta3 signaling. Dev Growth Differ. 2012;54(6):633–648. doi: 10.1111/j.1440-169X.2012.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6(6):1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. The American journal of pathology. 2009;175(4):1371–1373. doi: 10.2353/ajpath.2009.090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121(Pt 20):3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Experimental eye research. 2006;83(6):1309–1316. doi: 10.1016/j.exer.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Li C, Dong F, Jia Y, Du H, Dong N, Xu Y, Wang S, Wu H, Liu Z, Li W. Notch signal regulates corneal endothelial-to-mesenchymal transition. The American journal of pathology. 2013;183(3):786–795. doi: 10.1016/j.ajpath.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Li J, Bertram JF. Review: Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology (Carlton) 2010;15(5):507–512. doi: 10.1111/j.1440-1797.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. The Journal of cell biology. 2004;166(3):359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119(4):566–576. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- Manetti M, Guiducci S, Matucci-Cerinic M. The origin of the myofibroblast in fibroproliferative vasculopathy: does the endothelial cell steer the pathophysiology of systemic sclerosis? Arthritis Rheum. 2011;63(8):2164–2167. doi: 10.1002/art.30316. [DOI] [PubMed] [Google Scholar]
- Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Molecular biology of the cell. 2008;19(11):4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Potenta S, Kalluri R. Transforming growth factor-beta2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. The Biochemical journal. 2011;437(3):515–520. doi: 10.1042/BJ20101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16(12):1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montorfano I, Becerra A, Cerro R, Echeverria C, Saez E, Morales MG, Fernandez R, Cabello-Verrugio C, Simon F. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-beta1 and TGF-beta2-dependent pathway. Lab Invest. 2014;94(10):1068–1082. doi: 10.1038/labinvest.2014.100. [DOI] [PubMed] [Google Scholar]
- Morin P, Wickman G, Munro J, Inman GJ, Olson MF. Differing contributions of LIMK and ROCK to TGFbeta-induced transcription, motility and invasion. Eur J Cell Biol. 2011;90(1):13–25. doi: 10.1016/j.ejcb.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Developmental dynamics: an official publication of the American Association of Anatomists. 1997a;209(3):296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Miyazono K, Kato M, Takase M, Yamagishi T, Nakamura H. Extracellular fibrillar structure of latent TGF beta binding protein-1: role in TGF beta-dependent endothelial-mesenchymal transformation during endocardial cushion tissue formation in mouse embryonic heart. The Journal of cell biology. 1997b;136(1):193–204. doi: 10.1083/jcb.136.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258(2):119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Neilson EG. Mechanisms of disease: Fibroblasts--a new look at an old problem. Nat Clin Pract Nephrol. 2006;2(2):101–108. doi: 10.1038/ncpneph0093. [DOI] [PubMed] [Google Scholar]
- Nie L, Lyros O, Medda R, Jovanovic N, Schmidt JL, Otterson MF, Johnson CP, Behmaram B, Shaker R, Rafiee P. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: role of IL-1beta and TGF-beta2. Am J Physiol Cell Physiol. 2014;307(9):C859–877. doi: 10.1152/ajpcell.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. The American journal of pathology. 2011;179(3):1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto MT, Covas DT, Kashima S, Rodrigues CO. Endothelial Mesenchymal Transition: Comparative Analysis of Different Induction Methods. Biol Proced Online. 2016;18:10. doi: 10.1186/s12575-016-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. British journal of cancer. 2008;99(9):1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Vizan P, Das D, Chakravarty P, Vogt J, Rogers KW, Muller P, Hinck AP, Sapkota GP, Hill CS. TGF-beta uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. Elife. 2018:7. doi: 10.7554/eLife.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano LA, Runyan RB. Slug is an essential target of TGFbeta2 signaling in the developing chicken heart. Developmental biology. 2000;223(1):91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- Rosa F, Roberts AB, Danielpour D, Dart LL, Sporn MB, Dawid IB. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152(3):159–166. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- Santibanez JF, Quintanilla M, Bernabeu C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2011;121(6):233–251. doi: 10.1042/CS20110086. [DOI] [PubMed] [Google Scholar]
- Shi S, Kanasaki K, Koya D. Linagliptin but not Sitagliptin inhibited transforming growth factor-beta2-induced endothelial DPP-4 activity and the endothelial-mesenchymal transition. Biochem Biophys Res Commun. 2016;471(1):184–190. doi: 10.1016/j.bbrc.2016.01.154. [DOI] [PubMed] [Google Scholar]
- Sniegon I, Priess M, Heger J, Schulz R, Euler G. Endothelial Mesenchymal Transition in Hypoxic Microvascular Endothelial Cells and Paracrine Induction of Cardiomyocyte Apoptosis Are Mediated via TGFbeta(1)/SMAD Signaling. Int J Mol Sci. 2017;18(11) doi: 10.3390/ijms18112290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR, Kandel ES, Hay N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. The Journal of biological chemistry. 2007;282(31):22964–22976. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365(3):495–506. doi: 10.1007/s00441-016-2464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C, Murakami G, Fukuchi M, Shimanuki T, Shikauchi Y, Imamura T, Miyazono K. Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane. The Journal of biological chemistry. 2002;277(42):39919–39925. doi: 10.1074/jbc.M201901200. [DOI] [PubMed] [Google Scholar]
- Wermuth PJ, Li Z, Mendoza FA, Jimenez SA. Stimulation of Transforming Growth Factor-beta1-Induced Endothelial-To-Mesenchymal Transition and Tissue Fibrosis by Endothelin-1 (ET-1): A Novel Profibrotic Effect of ET-1. PLoS One. 2016;11(9):e0161988. doi: 10.1371/journal.pone.0161988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Nakajima Y, Nakamura H. Expression of TGFbeta3 RNA during chick embryogenesis: a possible important role in cardiovascular development. Cell Tissue Res. 1999;298(1):85–93. doi: 10.1007/s004419900073. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19(12):2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Strutz F, Muller GA. Role of fibroblast activation in inducing interstitial fibrosis. J Nephrol. 2000;13(Suppl 3):S111–120. [PubMed] [Google Scholar]
- Zeng L, Wang G, Ummarino D, Margariti A, Xu Q, Xiao Q, Wang W, Zhang Z, Yin X, Mayr M, Cockerill G, Li JY, Chien S, Hu Y, Xu Q. Histone deacetylase 3 unconventional splicing mediates endothelial-to-mesenchymal transition through transforming growth factor beta2. The Journal of biological chemistry. 2013;288(44):31853–31866. doi: 10.1074/jbc.M113.463745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1: TGFβ2 exhibits predominance in inducing EndMT. (A) Representative Western blot images and the corresponding bar graph of band densitometry showing a trend in the increased expression of the mesenchymal marker N-Cadherin in HMECs at 72 hours treated with TGFβ2 compared to that of control and TGFβ1 and TGFβ3. (B) Representative Western blot images and the corresponding bar graph of band densitometry showing decreased expression of the endothelial marker eNOS in HMECs at 72 hours treated with TGFβ2 compared to that of control and TGFβ1 and TGFβ3. Data are represented as mean ± SD. (n=3–5), *p<0.05.
FIGURE S2: TGFβ2 exhibits predominance in promoting expression of mesenchymal markers. Bar graphs of band densitometry showing a trend in the increased expression of the mesenchymal marker αSMA, Snail, P-P38 MAPK, P-Smad2/3 and TGFβ2 in HMECs at 72 hours treated with 5 ng/ml dose of TGFβ2 compared to that of control and 5 ng/ml dose of TGFβ1 and TGFβ3.
FIGURE S3: Replicate Western blot images indicating the predominant, dose-dependent effect of TGFβ2 on the expression of N-Cadherin, αSMA, and p-p38MAPK and decreased expression of eNOS compared to control, TGFβ1 and TGFβ3 treated cells after 72 hours of treatment.
FIGURE S4: Replicate Western blot images indicating the predominant, dose-dependent effect of TGFβ2 on the expression of p-Smad1/2, and Snail1 with no changes in the expression of VE-Cadherin compared to control, TGFβ1 and TGFβ3 treated cells after 72 hours of treatment. Replicate images of dose-dependent effect of TGFβ1 and TGFβ3 on increasing the level of TGFβ2 is also shown.