Abstract
Dominant mutations in MFN2 cause a range of phenotypes, including severe, early-onset axonal neuropathy, “classical CMT2”, and late-onset axonal neuropathy. We found a novel MFN2 mutation - c.283A>G (p.Arg95Gly) - that results in an axonal neuropathy with variable clinical severity in a multigenerational family. In affected family members, electromyography showed moderate to severe, chronic denervation in distal muscles. Such variable clinical severity illuminates the need to do careful assessments of at risk individuals when assessing MFN2 variants.
Keywords: Charcot-Marie-Tooth disease Type 2, CMT2A, multigenerational affection, variable penetrance, late-onset axonal neuropathy
Introduction
Dominant mutations in MFN2 cause Charcot-Marie-Tooth type 2A (CMT2A), which is the most common, dominantly inherited axonal neuropathy.(3),(14) More than 100 different, disease-associated mutations have been reported to date, as well as many variants of unknown significance. The clinical manifestations of dominant MFN2 mutations are strikingly varied – ranging from severe, early-onset axonal neuropathy (SEOAN),(1) to “classical CMT2”, and even late-onset axonal neuropathy.(13) Motor impairment often predominates the clinical picture, and some patients have additional features, such as optic neuropathy, myelopathy, and white matter changes in imaging. Here we describe a family with a previously unreported p.Arg95Gly mutation that results in strikingly variable severity.
Materials and Methods
We evaluated 12 family members – 6 affected and 6 unaffected – from a large family (Fig. A). The index patient and 10 others were evaluated at the University of Pennsylvania (by S.S.S.) with the CMT Neuropathy Score version 2,(10) which uses the MRC scale for strength testing, a Rydel-Seiffer tuning fork, and clinical electrophysiology (Table). The proband’s son was seen at the Nemours Alfred I. duPont Hospital for Children (by M.S.). After obtaining informed consent, blood samples were collected and sent to the University of Miami, where DNA was extracted, and whole-exome sequencing (WES) was performed on the proband and analyzed by GATK software packages and the variant analysis tool GENESIS 2.0 (tgp-foundation.org).(4),(8) Sanger sequencing was done in Miami on the family members shown in Figure A, and confirmed in CLIA-approved commercial laboratories.
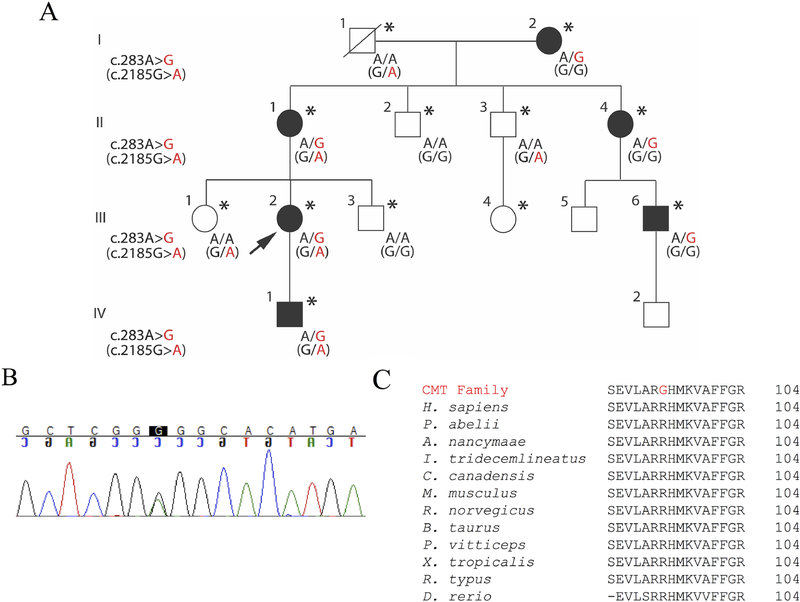
Figure.
c.283A>G variant in MFN2 segregates with CMT, and is highly conserved, while the c.2185G>A variant in AARS does not segregate.
(A) All evaluated affected individuals have the c.283A>G variant. The index patient is indicated by an arrow; genetic results are shown when available; patients who were examined are indicated by an asterisk. The AARS variant, indicated by parentheses, is present in unaffected family members and thus does not segregate.
(B) Sanger sequencing in exon 4 of the MFN2 in the proband.
(C) BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) sequence alignments show complete conservation of Arg95 (PhastCons score> 0.9 and phyloP score> 1.5) and nearby amino acids in the genes encoding MFN2 and orthologous proteins across divergent vertebrate species.
Table 1.
Summary of nerve conductions for affected adult family members.
| Age at EMG | Ulnar MNCV ≥49 m/s | Ulnar CMAP ≥6 mV | Median MNCV ≥49 m/s | Median CMAP ≥4 mV | Peroneal MNCV ≥41 m/s | Peroneal CMAP EDB ≥2 mV | Tibial MNCV ≥41 m/s | Tibial CMAP ≥4 mV | Ulnar SNAP ≥7 μV (O) | Median SNAP ≥10 μV (O) | Radial SNAP ≥15 μV (A) | Sural SNAP ≥6 μV (A) | CMTNS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I.2 | 85 | 51 | 11.2 | 51 | 4.5 | - | - | - | - | .8 | 10.8 | 15.3 | - | 5 |
| II.1 | 60 | 54 | 9.6 | - | - | 44 | 4.4 | 42 | 5.1 | - | - | 24.7 | 13.5 | 3 |
| II.4 | 52 | 65 | 9.6 | 50 | 7.4 | 43 | 2.0 | 42 | 3.8 | 6.0 | 5.7 | 6.5 | 2.9 | 6 |
| III.2 | 26 | 56 | 8.7 | 60 | 6.7 | 43 | 6.2 | 44 | 13.3 | 9.4 | 10.4 | 20.1 | 12.6 | 11 |
| III.6 | 23 | 55 | 9.7 | 52 | 8.0 | NR | NR | 34 | 1.5 | 10.3 | 12.4 | 7.8 | 4.7 | 18 |
MNCV = motor nerve conduction velocity; CMAP = compound muscle action potential; SNAP = sensory nerve action potential; O = orthodromic; A = antidromic; CMTNS = CMT Neuropathy Score version 2 [10]
Results
Clinical description
At age 26, our index patient (III.2) reported that she had always been clumsy, unable to run, with decreased feeling in her feet, and sensations of bugs crawling on her legs; these symptoms had slowly progressed. In her early adolescence, she began to experience restless legs, and was diagnosed with periodic limb movement disorder at age 23. Her weakness worsened during her pregnancy at age 19, to the point that she had difficulty climbing stairs, with some improvement after physical therapy. At age 26, strength, bulk and tone were normal in proximal and distal muscles in her arms, but ankle plantar flexion (4−), ankle dorsiflexion (4+), and extensor hallucis longus (EHL; 4) were weak. She could not stand on her toes. Reflexes were absent at the ankles, and 3+ at the knees, and 2+ at the biceps. Vibration was reduced (2 toes, 3 ankles, 4 knees), and pinprick was reduced to below the knees. Nerve conductions were normal (Table), but electromyography (EMG) showed severe, chronic denervation distal leg muscles. Her CMTNS was 11.
The index patient’s son (IV.1) was initially evaluated at age 7. He had always experienced difficulty in running, but had no problems in hand function. His exam at that time showed minimal distal weakness, but wearing ankle foot orthotics improved his ability to run and jump, as well as his level of fatigue. At age 8, distal weakness had increased and he had begun to experience foot drops. His intrinsic hand muscles (4), EHL (4), ankle dorsiflexion (4), and ankle plantar flexion (4) were weak. His sensory exam was not documented and he has not had clinical neurophysiological testing. His exam presently remains the same, at age 11. Like his mother, he has restless legs.
The index patient’s cousin (III.6) was in his usual state of health until age 10, when he noticed difficulty walking. At ages 12, 15, and 21, he had surgeries on his feet and ankles. At age 23, he had normal strength, bulk, and tone in the proximal muscles of his arms and legs, and was weak in the first dorsal interosseous (FDI; 4+R, 4L), abductor pollicis brevis (APB; 4), ankle plantar flexion (4−), and dorsiflexion (3R, 0L). Reflexes were absent at the ankles and present at the knees. Vibration was 2, 2, and 5 at the toes, ankles, and knees, respectively. Pinprick was reduced to above the knees. Nerve conductions and EMG showed severe, chronic, motor > sensory polyneuropathy (Table). His CMTNS was 18.
The index patient’s mother (II.1) was seen at age 60. She believed that she had a mild, non-progressive neuropathy her entire life. In her youth, she had weak grip, could not do pull-ups, and had trouble going up stairs. She had normal strength in her arms and legs, except for mild weakness and atrophy in her APB (4+), EHL (4R, 4+L), and ankle dorsiflexion (4+). Ankle plantar flexion was normal. Vibration was 4 at the toes, and 5 at the ankles. Pinprick was reduced to above the knees. Nerve conductions in an arm and leg were normal, and EMG showed moderate-to-severe chronic denervation in distal leg muscles (Table). Her CMTNS was 3.
At age 52, the index patient’s maternal aunt (II.4) reported that she had a propensity to roll her ankles since adolescence, and that this had worsened after she received chemotherapy (cyclophosphamide, paclitaxel, and adriamycin) for breast cancer 10 years prior. After chemotherapy, she developed such severe discomfort on the bottoms of her feet that she stopped riding horses. Strength, bulk, and tone were normal in the arms and legs, except for EHL (4+). The right ankle reflex was absent; the other deep tendon reflexes were present. Vibration was 3 at the toes and 5 at the ankles. Pinprick was reduced to below the knees. The sensory responses had reduced or absent amplitudes, the motor responses were normal, and EMG showed moderate-to-severe chronic denervation in distal leg muscles (Table). Her CMTNS was 6.
The index patient’s maternal grandmother (I.2) was seen at age 85, and was unaware that she had neuropathy, although she had had falls since her 50s, and walking and balance issues in her 80s. She had normal strength in her arms and legs, except for APB (4+), EHL (4), and ankle dorsiflexion (4+). Reflexes were absent in the legs, but present at the biceps. Vibration was 3, 4, and 5 at the toes, ankles, and knees, respectively. Pinprick was normal. The sensory and motor responses were normal, and there was severe, chronic denervation in distal muscles of the left arm (Table). Her CMTNS was 5.
Five unaffected family members (II.2, II.3, III.1, III.3, and III.4) had normal strength and sensation in their legs. The index patient’s grandfather (I.1), at 87, had a history and exam consistent with known lumbar stenosis and diabetic neuropathy, which he had had for 10 and 20 years, respectively.
Genetic testing
Two missense mutations were identified in the WES of the index patient - c.2185C>T (p.Arg729Trp) in AARS and c.283A>G (p.Arg95Gly) in MFN2; both were confirmed by Sanger sequencing (Fig. B). The p.Arg729Trp AARS mutation is predicted to be disruptive/damaging by Mut-taster, Provean, and SIFT, but its prevalence in gnomAD (0.062%) is too high to be a Mendelian cause of dominantly inherited CMT. Further, the mutation was absent in three affected family members (I.2, II.4, and III.6), and was present in II.3, who did not have neuropathy at age 53. Thus, the p.Arg729Trp AARS variant is a polymorphism. (7) The p.Arg95Gly MFN2 mutation was absent in gnomAD, was predicted to be disruptive/damaging by Mut-taster, Metalr, FATHMM, Provean, LRT, and Metasvm, and segregated in this family. Further, Arg95 is highly conserved across divergent vertebrates (Fig. C), and it is located in the GTPase protein domain (amino acids 94–265) (InterPro: https://www.ebi.ac.uk/interpro/entry/IPR030381/proteins-matched) and essential for GTP-dependent mitochondrial fusion.(2),(12)
Discussion
To date, at least 74 non-synonymous variants have been noted in the GTPase domain of MFN2 (http://hihg.med.miami.edu/neuropathybrowser). Although the clinical consequences of some variants are inadequately described, and at least 7 have been described to cause more than one phenotype, these variants have been reported to cause SEOAN (16), CMT2 (42), late-onset, mild neuropathy (3), and benign polymorphisms (9). The p.Arg95Gly mutation is a clear example of the clinical variability that can be found within a single family, ranging from a CMT2 phenotype to a minimal, late-onset neuropathy but not including a SEOAN phenotype. Other mutations in the GTPase domain (p.Leu146Phe(5) and p.Asp210Val(11)) and two outside of it (p.Val273Gly(6) and p.Ala383Val, (9)) have also been reported to cause a comparable variable phenotype. This variability raises two important practical issues: (1) that one cannot assume that a MFN2 variant found in an individual with a late-onset axonal neuropathy is an incidental finding, and that it will produce a similarly mild phenotype in another individual, and (2) that a MFN2 variant found in a clinically unaffected person must be benign. These uncertainties underscore the importance of the careful clinical, electrophysiological, and genetic investigation of at risk patients in a segregation analysis, and the sharing of such data in the Inherited Neuropathy Variant Browser.
Highlights.
We describe a previously unreported p.Arg95Gly mutation in MFN2
Variable clinical severity is seen across four generations
Segregation analysis requires careful evaluation of at risk patients
Acknowledgements
This work was supported by the Inherited Neuropathy Consortium (INC), Rare Disease Clinical Research Consortium funded by the National Institutes of Health (NINDS/ORD), the Neurogenetics Translational Center of Excellence, Department of Neurology, University of Pennsylvania, and the Judy Seltzer Levenson Memorial Fund for CMT Research. The INC (U54 NS065712) is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between NCATS and the NINDS. We thank the family for participating in our work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feely SME, Laura M, Siskind CE, Sottile S, Davis M, Gibbons VS, et al. MFN2 mutations cause severe phenotypes in most patients with CMT2A. Neurology. 2011;76(20):1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco A, Kitsis RN, Fleischer JA, Gavathiotis E, Kornfeld OS, Gong G, et al. Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature. 2016;540(7631):74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridman V, Bundy B, Reilly MM, Pareyson D, Bacon C, Burns J, et al. CMT subtypes and disease burden in patients enrolled in the Inherited Neuropathies Consortium natural history study: a cross-sectional analysis. J Neurol Neurosurg Psychiatry. 2015;86(8):873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez M, Falk MJ, Gai X, Postrel R, Schule R, Zuchner S. Innovative genomic collaboration using the GENESIS (GEM.app) platform. Hum Mutat. 2015;36(10):950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein C, Kimmel G, Pittock S, Engelstad J, Cunningham J, Wu Y, & Dyck P Large Kindred Evaluation of Mitofusin 2 Novel Mutation, Extremes of Neurologic Presentations, and Preserved Nerve Mitochondria. Archives of Neurology. 2011;68(10):1295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson V, Graham B, Flanigan K. Clinical and electrophysiologic features of CMT2A with mutations in the mitofusin 2 gene. Neurology. 2005;65(2):197–204. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin HM, Sakaguchi R, Giblin W, Program NCS, Wilson TE, Biesecker L, et al. A recurrent loss-of-function alanyl-tRNA synthetase (AARS) mutation in patients with Charcot-Marie-Tooth disease type 2N (CMT2N). Hum Mutat. 2012;33(1):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motley WW, Palaima P, Yum SW, Gonzalez MA, Tao F, Wanschitz JV, et al. De novo PMP2 mutations in families with type 1 Charcot-Marie-Tooth disease. Brain. 2016;139(Pt 6):1649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muglia M, Vazza G, Patitucci A, Milani M, Pareyson D, Taroni F, et al. A novel founder mutation in the MFN2 gene associated with variable Charcot-Marie-Tooth type 2 phenotype in two families from Southern Italy. J Neurol Neurosurg Psychiatry. 2007;78(11):1286–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy SM, Herrmann DN, McDermott MP, Scherer SS, Shy ME, Reilly MM, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011;16(3):191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouzier C, Bannwarth S, Chaussenot A, Chevrollier A, Verschueren A, Bonello-Palot N, et al. The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy ‘plus’ phenotype. Brain. 2012;135(Pt 1):23–34. [DOI] [PubMed] [Google Scholar]
- 12.Santel A, Fuller M. Control of mitochondrial morphology by a human mitofusin. Journal of cell science. 2001;114:867–74. [DOI] [PubMed] [Google Scholar]
- 13.Verhoeven K, Claeys KG, Zuchner S, Schroder JM, Weis J, Ceuterick C, et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129(Pt 8):2093–102. [DOI] [PubMed] [Google Scholar]
- 14.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36(5):449–51. [DOI] [PubMed] [Google Scholar]
Web Resources
- BLAST: (https://blast.ncbi.nlm.nih.gov/Blast.cgi)
- GENESIS 2.0: (http://tgp-foundation.org), Last accessed: August 3, 2018
- Inherited Neuropathy Variant Browser: (http://hihg.med.miami.edu/neuropathybrowser), Last accessed: August 3, 2018 [DOI] [PMC free article] [PubMed]
- InterPro: (https://www.ebi.ac.uk/interpro/entry/IPR030381/proteins-matched), Last accessed: August 3, 2018