Abstract
Salivary gland cancers (SGCs), categorized as head and neck cancers (HNCs), constitute about 6% of head and neck cancer diagnoses based on estimate by American Head and Neck Society. Salivary gland tumors originate from different glandular cell types and are thus morphologically diverse. These tumors arise from any of the three major and various minor salivary glands. The incidence of SGCs has slowly increased during the last four decades. The etiology of SGCs is mostly unknown; however, specific gene mutations are associated with certain types of salivary tumors. Treatment options include surgical resection, radiation therapy (RT), chemotherapy, and multimodality therapy. HNC patients treated with RT often develop xerostomia and salivary hypofunction due to damaged salivary glands. In this review, we discuss etiology of SGCs, present findings on the role of autophagy in salivary tumorigenesis, review adverse effects of radiation treatment, and examine remedies for restoration of salivary function.
Keywords: salivary gland cancer, autophagy, radiation therapy, xerostomia
I. INTRODUCTION
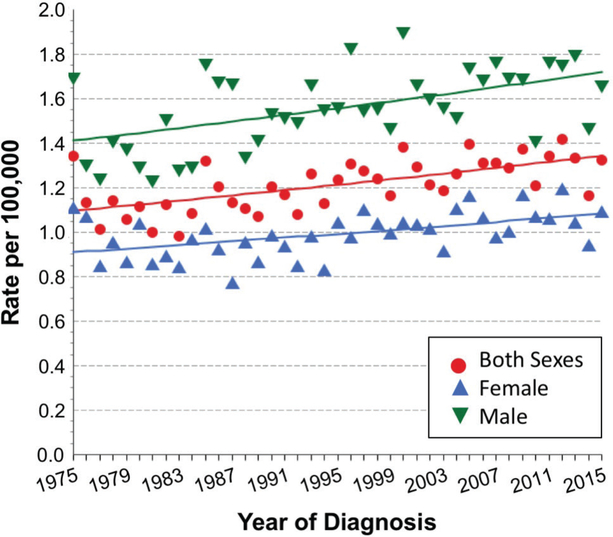
Salivary glands are exocrine organs that produce and release saliva into the oral cavity. In humans and rodents, three pairs of major salivary glands (parotid, submandibular, and sublingual) and hundreds of minor salivary glands are found throughout the lining of the oral cavity. In 2017, the World Health Organization (WHO) classified tumors of salivary glands into more than 30 malignant and benign histologic subtypes, based on cytology as an initial tool for assessment rather than originating anatomical site.1 Based on the Surveillance, Epidemiology, and End Results (SEER) program of the National Institutes of Health, salivary gland cancer (SGC) incidence in the United States (US) during 1975–2015 trended upward from 1.1 to 1.3 cases/100,000 individuals (Fig. 1), with a male-to-female ratio of ~ 1.6:1. Incidence rate accelerated after age 50 to more than seven cases per 100,000 aged 70 years and older.2,3 Year 2011 to 2015 SEER data registered 11% invasive cases of SGC among all histologically confirmed cancers of the oral cavity and pharynx.3 In the same data set, major histology types of SGC included adenocarcinoma (62%) and epidermoid carcinoma (22%). Salivary adenocarcinoma is further classified into adenoid cystic carcinoma (AdCC), acinic cell carcinoma (AciCC), polymorphous adenocarcinoma, and salivary duct carcinoma (SDC), among others.1 Except for AdCC and AciCC, which carries a female predilection, overall incidence of SGCs from 1975 to 2015 in the US was consistently greater in males.3 Moreover, stage distribution was > 50% localized in females, whereas regional and distant distributions were dominant in males.3 Since 1974, treatment approaches for managing salivary cancer have gradually shifted from surgery only to a combination of surgery and radiotherapy/chemotherapy.4 Patients with high-grade and/or locally advanced malignant major SGC who receive adjuvant radiation therapy (RT) have a significant survival advantage compared to patients who undergo surgery alone.5 However, xerostomia and hyposalivation are adverse effects of radiotherapy that affect quality of life in treated patients.6 In this article, we review promising remedies for salivary hypofunction due to RT.
FIG. 1:
Rising long-term SEER incident rates of SGC spanning 40 yr, from 1975 to 2015. Data were extracted from the SEER*Explorer interactive website2 (https://seer.cancer.gov/explorer/; updated April 16, 2018) of the National Cancer Institute. All ethnicities and age groups of both sexes were included in nine SEER areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta). Rates are per 100,000 individuals, and annual percent-change estimates were calculated from underlying rates using Joinpoint Trend Analysis software (https://surveillance.cancer.gov/joinpoint; version 4.6, February 2018, National Cancer Institute; created by SEER. cancer.gov/explorer/application.php on 07/23/2018).
II. ETIOLOGY AND PATHOLOGY OF SALIVARY TUMORS
The etiology of salivary gland tumors (SGTs) remains mostly unknown. Risk factors for SGC include age, radiation or radioactive substance exposure, and environmental and occupational exposure to chemicals and sawdust (see https://www.cancer.net/cancer-types/salivary-gland-cancer/risk-factors). The identification of molecular signatures in salivary tumor subtypes aids diagnosis, and these molecules serve as potential therapeutic targets.7 This review also focuses on pathology and genetic profiles of selected malignancy subtypes in salivary glands.
A. Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma (MEC), one of the most common primary salivary gland malignancies, is characterized by components of mucin-producing, intermediate-type, and squamoid cells with cystic and solid growth patterns.1 CRTC1 (or MECT1)-MAML2 gene fusion is identified in a large number of MEC tumors and may be associated with low-grade tumors,8 is a favorable prognosticator,9 but may not be correlated to either.10 In contrast, gene amplification of HER2 or an increased gene copy number of either HER2 or epidermal growth factor receptor (EGFR) are associated with high-grade MEC.11 The genes basic leucine zipper and W2 domains 1 (BZW1)/eukaryotic initiation factor (eIF)5-mimic protein 2 (5MP2) are highly expressed in MEC. Down-regulation of BZW1 arrests cell cycle in Mc3 in vitro and reduces MEC cell tumorigenicity in vivo.12 In addition, it has been shown that BZW1 interacts with eIF2 and eIF3 to induce expression of ATF4, which in turn enhances survival of MEC cells under stress.13 In addition to BZW1, components of the interleukin (IL)-6 signaling pathway are also highly expressed in the MEC cell line University of Michigan–human epidermoid carcinoma (UM-HMC) cells, and treatment with the humanized antihuman IL-6R antibody tocilizumab enhances antitumor effects of conventional chemotherapy in preclinical MEC models.14 Furthermore, it has been shown that MEC cells are efficiently eliminated using a combination treatment of nuclear factor (NF)-κB and histone deacetylase (HDAC) inhibitors.15 Thus, BZW1, the IL-6 pathway, NF-κB, and HDAC may represent viable therapeutic targets for MEC therapy.
B. AdCC
AdCC is a tumor of parotid, submandibular, and mostly minor salivary glands, accounting for ~ 10% of all salivary malignancies.16 AdCC tumors, comprised of both epithelial and myoepithelial neoplastic cells, are generally slow-growing salivary gland malignancies. Although females have greater AdCC incidence than males (1.5:1), they also have better prognosis than males.17 The translocation of the proto-oncogene MYB with transcription factor NFIB, as demonstrated by the MYB-NFIB fusion transcript, was detected in 25 of 29 (86%) AdCC tumor samples in one study18 and 21 of 82 (25.6%) tumors in another investigation.19 Driver mutations for AdCC include KDM6A, CREBBP, PIK3CA, SMARCA2, and TP53, among others,20 whereas therapeutic targets include c-KIT, NF-κB, EGFR, and HER2/Neu (for a review, see Ref. 21). Furthermore, it has been shown in an athymic nu/nu mouse model that the metastatic potential of human AdCC and metastatic AdCC (ACCM) cells is associated with expression of DNA-binding protein inhibitor ID1. Down-regulation of ID1 expression leads to a significant reduction in lung metastasis in mice injected intravenously with ACCM cells.22
C. AciCC
AciCC is one of the more common salivary malignancies, accounting for ~ 10%–12% of all SGCs.23 AciCC, comprised of cancer cells with acinar features, most commonly arises in the parotid gland.24 Of note, current WHO salivary tumor classification has branched out a subset of AciCC tumors, namely, mammary analog secretory carcinomas that are zymogen granule poor, mammaglobin positive, and contain a ETV6-NRTK3 translocation feature.25–27 A recent National Cancer Database retrospective analysis of 2362 AciCC cases diagnosed between 2004 and 2012 revealed female dominance (61.3%), with median age at diagnosis of 54 years.23 All patients received surgery as primary treatment, and 43% also received adjuvant RT or chemoradiotherapy (CRT); however, these adjuvant therapies did not improve 5-yr survival rate significantly when compared to cases receiving surgery only among patients with high-grade AciCCs.23
D. Polymorphous Adenocarcinoma
The name of polymorphous adenocarcinoma (PAC) was formerly known as polymorphous low-grade adenocarcinoma, but the current WHO classification of PAC is now used.1,28 PAC, a malignant epithelial tumor that commonly originates in minor salivary glands of the oral cavity, is mostly low grade and characterized by cytological uniformity and histological diversity.29,30 Genetic variations, including rearrangements of protein kinase D (PRKD) family members 1–331 and the activating mutation (E710D) of PRKD1,32 have been reported in cases of PAC. In a SEER study of 460 PAC cases, complete surgical resection was the primary treatment choice, and surgery with radiotherapy was second.33
E. SDC
Histologically, SDC resembles ductal breast carcinoma. SDC is estimated to account for 5%–10% of salivary gland malignancies1,34 but constitutes < 1.8% of all major SGTs in the SEER database.35 Although incidence is low, SDC is one of the highest-grade and aggressive salivary malignancies. In the SEER database between 1973 and 2008, 228 cases of SDC were identified.35 Among these, more than two-thirds of cases were advanced disease (stage III or IV), and about half of the cases had lymph node involvement at diagnosis. Similarly, based on 495 SDC cases diagnosed between 2004 and 2013 in the National Cancer Database, more than half were at advanced stage and had a male-to-female ratio of 2:1.36 Schmitt et al. summarized the literature on genetic aberrations in SDCs and found overexpression/gene amplification of ErbB2 (38%), EGFR (53%), and AR (83%), whereas mutations were detected in HRAS/NRAS (29%), PIK3CA (26%), and TP53 (53%) (see Ref. 37 for a review, and references therein). In addition, Kirsten rat sarcoma (KRAS) amplification was found in 100% of 37 SDC cases studied by Ku et al.38 These are thus potential target genes for therapies. However, a 25-yr study of 177 cases of SDCs in The Netherlands found that although AR and HER2 were immunopositive in 96% and 29% of SDC tumors, respectively, they presented no prognostic value.39 In a multivariable analyses, total amount of lymph node involvement was the only significant prognostic factor for poor overall survival and distant metastasis-free survival.39
III. MOUSE AS MODEL: ROLE OF AUTOPHAGY IN SALIVARY TUMOR DEVELOPMENT
Few animal models are appropriate for the study of SGTs. We established and characterized a conditional, inducible SDC mouse model using ductal activation of oncogenic KRAS.40 We found that SDC develops in submandibular glands following elastase-driven and tamoxifen-inducible Cre recombinase (Ela-CreERT)–mediated activation of LGL-KRasG12V. Mice had a median survival of 4 wk after tumor induction by tamoxifen.40 In addition, a mouse AciCC model (MMTV-Cre/Apcflox/flox/Ptenflox/flox), in which both Pten and Apc genes were inactivated through conditional mouse mammary tumor virus MMTV-Cre–mediated recombination, developed SGTs with 100% penetrance, and these tumors showed morphologic similarity to human AciCC.41 Both canonical Wnt and mammalian target of rapamycin (mTOR) signaling pathways were constitutively activated in this mouse model, and treatment with mTOR inhibitor rapamycin effectively inhibited tumor cell proliferation, resulting in tumor regression. This study was further substantiated by findings showing that activation of components in the PI3K/AKT/mTOR pathway was better associated with AciCC, compared to other salivary gland malignancies.42 Furthermore, aberrant activation of the receptor activator of the NF-κB ligand (RANKL) in an MMTV-RANKL transgenic mouse model induced SGTs that resembled poorly differentiated MEC.43 Recently, Cao et al.44 established an inducible keratin 5–driven conditional (K5CrePR1) Pten and Smad4 knockout mouse model. The 11 double knockout mice developed salivary AdCC (six cases), SDC (three cases), and salivary adenosquamous cell carcinoma (two cases) after salivary gland–specific delivery of progesterone receptor antagonist RU486, and tumors exhibited both mTOR activation and TGFβ1 overexpression. It is clear that SGT subtype development in these mouse models depends on specificity of driving promoter, but aberrant pathways that are involved in tumorigenesis determine latency, penetrance, and other tumor growth characteristics.
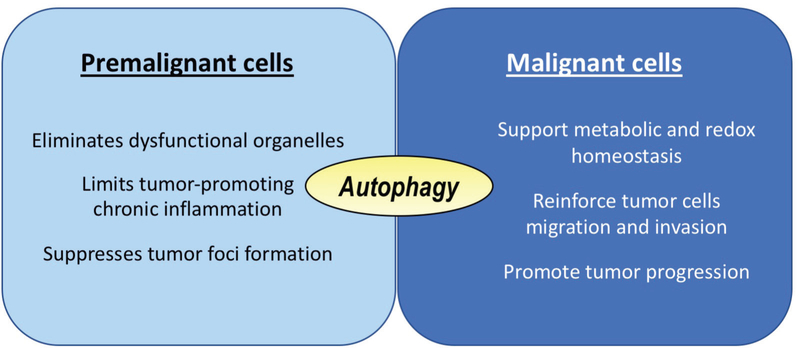
Dysregulation of autophagy has been implicated in a variety of human diseases, including those that are vascular, infectious, metabolic, intestinal, pulmonary, and neurodegenerative, as well as in aging and a variety of cancers.45–47 The association of autophagy and cancer development may be stage dependent; autophagy may initially serve a preventive role against cancer, but in later stages of tumor development may serve to protect cancer cells.48,49 An essential protein required for formation of autophagosome during macroautophagy is autophagy related 5 (ATG5).50 In an animal study of the Atg5f/f;Aqp5-Cre mouse model, in which autophagy was constitutively inactivated due to Atg5 inactivation in salivary acinar cells by aquoporin5-driven Cre recombinase, we found no differences in apoptosis, proliferation, or carbachol-induced salivary secretion, but Atg5 inactivation led to a moderate increase in acinar cell hypertrophy and accumulation of secretory granules in aging mice.51 Under stress of salivary excretory duct ligation, however, delayed apoptosis in autophagy-compromised salivary acinar cells occurred.52 To study the effect of autophagy on salivary tumor development, we crossed LGL-KRasG12V; Ela-Cre-ERT mice40 with Atg5f/f mice,53 and tamoxifen treatment simultaneously activated oncogenic KRas and disrupted Atg5 expression (atg5-KO).54 Compared to the autophagy-competent and oncogenic KRAS-induced SDC tumor-bearing mice, autophagy impairment extended survival of oncogenic KRAS-driven tumor-bearing mice by 38%. In addition, atg5-KO tumors spread more slowly during late tumorigenesis, despite having a quicker onset. Autophagy disruption impairs mitochondrial bioenergetics, as reflected by a reduced oxygen consumption rate, and dysregulates metabolite profiles, as revealed by a decrease in intracellular concentrations of 12 essential and nonessential amino acids in autophagy-deficient tumor cells.54 Furthermore, overexpression of asparagine synthase, a key enzyme for de novo asparagine synthesis, has been shown to be linked to poor clinical outcome in multiple cancers, including those of the head and neck.54 Therefore, consistent with the literature on oncogenic RAS-induced pancreatic,55 lung,56 and colorectal57 cancers, autophagy displays a differential role during tumor initiation and progression (Fig. 2).
FIG. 2:
Role of autophagy during tumor initiation and progression. Autophagy suppresses but does not prevent tumor initiation in oncogenic KRAS-induced SDC. Once premalignant cells progress to malignant cells, autophagy has a protective role against unfavorable tumor microenvironment, such as hypoxia and poor nutrient supply, to promote tumor progression. Similar observations have been reported in oncogenic KRAS-induced pancreatic,55 lung,56 and colorectal57 cancers.
Based on the context-dependent role of autophagy in tumorigenesis, autophagy inhibitors and inducers have been exploited as targets for anticancer therapy, depending on their role in promoting tumor suppression or progression, respectively.58,59 AdCC is one of the most common malignancies of salivary glands. Treatment of salivary AdCC cells with anticancer drugs, including cisplatin, mTOR inhibitor temsirolimus, obatoclax, YM155, isoliquiritigenin, and zoledronic acid, reportedly induces autophagy but produces different outcomes.60 Among these agents, autophagy plays a protective part in AdCC cells treated with cisplatin-based adjuvant chemotherapy, such that autophagy inhibition by 3-methyladenine, chloroquine, or Beclin-1 small-interfering RNA sensitized AdCC cells toward cisplatin-induced cytotoxicity.61,62 In contrast, autophagy inhibition attenuates zoledronic acid-63 and isoliquiritigenin-64induced autophagic and/or apoptotic cell death; thus, autophagy has a prodeath role in AdCC cells treated with these chemotherapeutic agents. Additionally, hypoxia-induced autophagy has been shown to contribute to AdCC incursion, so that inhibition of autophagy by chloroquine attenuates tumor invasion in AdCC cells.65 On the contrary, treatment of ACCM cells with antitumor compound silibinin induces autophagy but inhibits proliferation and lung metastasis.66 Furthermore, MEC cells treated with the antitumor HDAC inhibitor apicidin and HDAC7-silenced MEC cells both display growth inhibition through G2/M-phase cell-cycle arrest and exhibit induction of apoptosis and autophagy.67,68 It is hypothesized that HDAC7 silencing or apicidin-induced autophagy has a prosurvival role, because inhibition of autophagy enhances apicidin-induced apoptosis in MEC cells.67,68 In summary, the role of autophagy in tumor growth, invasion, and metastasis is evidently context dependent, and targeting autophagy in cancer cells as an anticancer therapy requires careful evaluation.
IV. RADIATION AND OTHER TREATMENT FOR SALIVARY TUMORS
Selection of the course of treatment must consider tumor grade, tumor-node–metastasis stage, localization, extension, and histological features. Greater than 30 histological subtypes of SGC recognized by the WHO add a level of complexity for identifying standard of care. Primary treatment is typically surgery, with selection of adjuvant therapies predominantly based on head and neck cancer (HNC) studies69 or retrospective studies of SGC.70 Very few prospective trials and the rarity and heterogeneity of SGC further complicate design of randomized control trials.70 Adjuvant radiotherapy using 60–66 Gy delivered at 2 Gy/d fractions during 6 wk was used in high-risk patients with higher than stage 3 tumors in a postoperative setting.70 Tumors were frequently high grade and undifferentiated, but radiotherapy improved 5-yr survival rates by up to 51%.70 When considering only stage 4 SGC treated with adjuvant RT, the Danish Head and Neck Cancer Group reported a 5-yr survival rate of 30%, and a meta-analysis of parotid adenocarcinoma revealed a 35% 5-yr survival rate in the high-grade group.71 For unresectable tumors, photon-based radiation of 70 Gy is recommended.72 Concurrent CRT (usually with platinum-based therapies) in a metastatic or recurrent setting showed limited evidence of efficacy and is not recommended by the National Comprehensive Cancer Network.73 Treatment of AdCC in particular uses combination chemotherapies comprised of cisplatin and one to three other compounds, with the addition of doxorubicin and cyclophosphamide producing highest response rates.70,74 Doublet therapy is preferred due to increasing toxicities with combined regimens, although the majority of response rates have been < 50%. Combination therapy for MEC uses evaluated doublet regimens of carboplatinum and paclitaxel or cisplatin and gemcitabine with 26% and 24% response rates, respectively.72,74
A number of prognostic features indicative of poor outcomes has been identified in SGC, including poorly differentiated, high-grade perineural invasion and extracapsular spread.71 One of the most predictive indicators of recurrence is neck metastasis.75 Patients with positive cervical lymph node metastasis at diagnosis are recommended to receive therapeutic neck dissection during surgical resection of SGC. Elective neck dissection is indicated when risk of occult lymph node metastasis exceeds 15%. Factors increasing risk include high-grade advanced tumor stage, histological type, and poorly differentiated tumors.76
Distinct therapies have been evaluated in specific types of SGC based on genotype or histological indications. SDCs can exhibit increased histological staining for HER/neu or androgen receptor; therefore, herceptin- and androgen-deprivation therapy, respectively, have been evaluated in SGC patients.74,77 With AdCC, 85% of patients experience increased EGFR; however, gefitinib therapy resulted in minimal benefit in a phase-II single-agent open-label study.78 In addition, 65%–95% of AdCC cases revealed elevated c-kit expression, but imatinib was ineffective.74
The lack of therapeutic options for SGC has led to evaluation of emerging treatments in an effort to improve 5-yr survival rates. Antiangiogenic therapy (sorafenib) in recurrent and/or metastatic SGCs appears to be less effective in AdCC cases compared to non-AdCC patients.79 Vorinostat inhibits HDACs and, as a single agent, demonstrated modest activity in MEC. Further investigation of its mechanism of action suggested that vironostat might influence expression of programmed cell death protein 1 PD-1 and its ligand PD-L1. A clinical trial (NCT02538510) combining vorinostat and pembrolizumab is currently underway in advanced SGCs, with anticipated completion in June 2019.74 The presence of gene fusion oncogenes in SGCs provides an avenue for future advancement in precision therapeutics that target novel proteins generated from these translocations.80
V. SIDE EFFECTS AND THERAPEUTIC INTERVENTION OF RADIATION-INDUCED DAMAGE TO SALIVARY GLANDS
A significant amount of knowledge has been gained regarding radiosensitivity of salivary glands during HNC treatment. Similarly to SGC, locally advanced HNC treatment involves surgery and adjuvant radiation or CRT.81 Concurrent CRT of HNC produces significant benefits in locoregional control and survival.71 In patients with head and neck tumors without nodal involvement, most salivary glands are frequently spared from the radiation field; however, increasing nodal involvement markedly negatively affects salivary glands. A majority of studies focus on increased radiosensitivity of the parotid gland, and treatment plans limiting cumulative radiation mean dose to < 26–30 Gy show greater likelihood for retaining some function.82 A mean dose of < 39 Gy is recommended for submandibular glands, but positive results may be difficult to achieve with tumors located at the base of the tongue.82 Collateral damage to noncancerous salivary glands leads to xerostomia and salivary gland hypofunction. Xerostomia is a subjective parameter that stems from patient-perceived dryness of the mouth. Salivary gland hypofunction is a quantitative measure of unstimulated or stimulated saliva reduction. Xerostomia may occur in the absence of salivary gland hypofunction and is likely influenced by saliva protein composition. Therefore, salivary gland dysfunction is utilized to incorporate both conditions.
Reduction in salivary flow is observed within 1 wk of commencing radiotherapy, with continued reduction observed throughout treatment. Patients frequently exhibit salivary gland dysfunction for months, years, or permanently, depending in part on radiation dose delivered to salivary glands.83 In addition to reduced total volume of secretion, salivary composition is also modulated following radiotherapy.84 Elevated sodium and chloride along with decreases in amylase, bicarbonate, and mucins collectively lead to reduced buffering capacity and a perception of “sticky” saliva. Histological evaluation of irradiated salivary glands reveals a selective loss of acinar parenchyma and continued presence of major ductal structures, large nerves, and myoepithelial cells.84 Lymphocytic infiltrate and fibrosis are commonly observed in patients at chronic time points following radiotherapy. A number of imaging techniques have been evaluated to assess salivary gland damage following radiation, including computed tomography, magnetic resonance imaging, and ultrasound. Yang et al. took an additional step to quantitatively model sonogram images to identify patients with acute versus late toxicities.85
A. Clinical Remedies for Xerostomia
A number of therapeutic interventions have been proposed due to the significant effects of xerostomia in HNC patients. Importantly, some evidence of residual salivary function is often a criterion for clinical studies. Here, we focus on clinical study outcomes; preclinical models are reviewed elsewhere.86–88
B. Pharmacological Therapies
Amifostine was the first pharmacologic therapy used to prevent radiation-induced salivary gland hypofunction. Amifostine is an organic thiophosphate that facilitates scavenging of free radicals that are generated by radiation treatment. A recent Cochrane review assessing 11 clinical trials with more than 1000 patients concluded that amifostine reduced the risk of moderate to severe (greater than grade 2) xerostomia when measured at the end of radiotherapy and again at 3 mo posttreatment. In contrast, there was insufficient evidence showing that amifostine reduced xerostomia or improved salivary flow rates at 12 mo postradiotherapy. These studies also reported a significant increase in side effects from amifostine, including nausea and vomiting, hypotension, and allergic responses.83
Saliva secretion requires innervation of salivary glands by the sympathetic and parasympathetic nervous systems. Therefore, parasympathetic agonists such as pilocarpine have been hypothesized to stimulate secretion from remaining salivary glands following radiotherapy. Despite considerable favorable evidence in rodent models of radiation-induced salivary gland dysfunction, a recent Cochrane review that assessed 12 clinical trials with more than 900 patients concluded that there was insufficient evidence showing that pilocarpine improved xerostomia, salivary gland hypofunction, or quality of life.83 Assessment occurred at the end of radiotherapy and again at 3 and 6 mo posttreatment. Although most patients reported side effects (nausea, sweating, vasodilation, and headaches) of pilocarpine to be mild, a small number of subjects (6%–15%) elected to discontinue its use due to the severe degree of side effects.89 In contrast, a meta-analysis of six clinical trials involving 369 patients reported that administration of pilocarpine during radiotherapy improved unstimulated salivary flow rates immediately after radiotherapy and at 3 mo, although patient-reported complaints of xerostomia did not differ among treatment groups. Six months after radiotherapy, unstimulated salivary flow rates did not decrease nor differ from levels at the end of radiotherapy; however, patients reported improvements in perceptions of xerostomia.90 Therefore, the jury is still out as to whether pilocarpine should be used to treat radiation-induced xerostomia.
Palifermin is a recombinant human keratinocyte growth factor that is approved for the treatment of radiation-induced mucositis. Based on its efficacy, palifermin was evaluated for secondary use in prevention of radiation-induced xerostomia. A recent Cochrane review evaluated three trials of 471 patients receiving palifermin or placebo and determined that there was insufficient evidence for a difference in the occurrence of greater than grade 2 xerostomia among treatment groups at 12 mo postradiotherapy.83
Saliva stimulants and substitutes are frequently used as palliative remedies for salivary gland dysfunction. Saliva stimulants include items such as chewing gum, organic acids, or mints, and saliva substitutes may be water or artificial saliva. Some of these agents can provide some relief, but efficacy is transient, frequent reapplication is required, and the products do not provide all of the beneficial functions of saliva.83,86,91
C. Nonpharmacological Therapies
A few studies have evaluated the potential of nonpharmacological therapies to improve xerostomia after radiotherapy. The greatest amount of work has evaluated hyperbaric oxygen and acupuncture, with a smaller number of studies investigating the use of Chinese herbs or trace minerals (zinc or selenium).83 Hyperbaric oxygen therapy (HBOT) involves patient treatment in specialized chambers with atmospheric conditions of 100% oxygen. This results in elevated oxygen levels delivered to tissues and is hypothesized to aid in wound-healing responses, especially with necrotic damage. A systematic review of seven studies involving 246 patients determined that HBOT reduced symptoms of xerostomia.92 Electrostimulation and acupuncture have also been investigated in treatment of xerostomia; however, a Cochrane review concluded that there was insufficient evidence that these interventions provided objective relief.93 The salivary gland transfer technique involves surgical movement of one submandibular gland to the submental region, contralateral to the head and neck tumor. This process results in a 95% reduction in total radiation dose delivered to submandibular glands and retains blood flow through facial vessels.94 A meta-analysis of six clinical trials involving 177 patients reported that salivary gland transfer preserved a greater level of salivary secretion at acute time points after radiotherapy, and follow-up at 1–2 yr later revealed sustained salivary function.94 Despite these promising results, concerns regarding complications with surgical procedure appear to prohibit widespread adoption.
D. Gene Therapy
A phase-I/II clinical trial involving 11 squamous cell carcinoma patients previously treated with radiation determined safety and initial efficacy of adenoviral gene therapy encoding the aquaporin 1 gene (AdhAQP1). Five patients from this trial exhibited objective response in the first 42 d after viral transduction, as defined by at least a 50% increase in salivary flow rate and improvement in subjective parameters (perception of oral dryness and amount of saliva present using visual analog scale assessments).95 Longer-term follow-up of these responders revealed continued improvement in salivary flow rates, although levels were lower than peak values that were observed acutely after the gene therapy procedure.96 Next-generation vectors use adeno-associated virus serotype 2 (AAV2) to promote longer expression of the gene of interest and reduced immunological reactions.97,98
E. Future Directions
We have previously demonstrated in a mouse model that induction of autophagy is important to prevent radiation-induced salivary gland hypofunction.99 We extended this work to evaluate the ability of a rapalog (CCI-779, temsirolimus) to restore salivary gland function to previously irradiated salivary glands. Administration of CCI-779 led to increased salivary secretion and amylase production within 30 d of radiation treatment, but irradiated glands exhibited reduced levels of both.100 A clinical pilot study is underway to evaluate the ability of the orally available rapalog everolimus to improve salivary gland function and xerostomia in HNC patients receiving definitive radiotherapy with curative intent.
VI. CONCLUSIONS
SGC is a rare form of HNC with many subtypes, depending on tumor-originating cells. Currently, gene translocations and mutations have been detected in major subtypes of SGC; however, cancer etiology is largely unknown. Treatment of SGC is mainly surgical, and depending on stage at diagnosis, adjuvant radiation and chemotherapy are also indicated for high-grade tumors. Autophagy has been investigated as a target for chemotherapeutics. Radiotherapy for treatment of HNC often causes xerostomia and affects quality of life in patients. Many unanswered questions and studies remain for future research.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants R01DE10742, R21DE023298, and R01DE026304 (H.H.L. and D.K.A.); R01DE023534 (K.H.L. and D.K.A.); and P30CA033572 (supporting research carried out in core facilities).
ABBREVIATIONS:
- AciCC
acinic cell carcinoma
- AdCC
adenoid cystic carcinoma
- MEC
mucoepidermoid carcinoma
- RAS
rat sarcoma
- RT
radiation therapy
- SDC
salivary duct carcinoma
- SGC
salivary gland cancer
- SGT
salivary gland tumor
REFERENCES
- 1.Tumours of salivary glands In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017. pp. 159–202. [Google Scholar]
- 2.SEER*Explorer: An Interactive Website for SEER Cancer Statistics [database on the Internet]. Surveillance Research Program, National Cancer Institute; c2018. [cited 2017 Apr 14]. Available from: https://seer.cancer.gov/explorer/. [Google Scholar]
- 3.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute; April 2018. Available from: https://seer.cancer.gov/csr/1975_2015/. [Google Scholar]
- 4.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–16. [DOI] [PubMed] [Google Scholar]
- 5.Mahmood U, Koshy M, Goloubeva O, Suntharalingam M. Adjuvant radiation therapy for high-grade and/or locally advanced major salivary gland tumors. Arch Otolaryngol Head Neck Surg. 2011;137(10):1025–30. [DOI] [PubMed] [Google Scholar]
- 6.Wong WY, Pier M, Limesand KH. Persistent disruption of lateral junctional complexes and actin cytoskeleton in parotid salivary glands following radiation treatment. Am J Physiol Regul Integr Comp Physiol. 2018. DOI: 10.1152/ajpregu.00388.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis AG, Tong T, Maghami E. Diagnosis and management of malignant salivary gland tumors of the parotid gland. Otolaryngol Clin North Am. 2016;49(2):343–80. [DOI] [PubMed] [Google Scholar]
- 8.Jee KJ, Persson M, Heikinheimo K, Passador-Santos F, Aro K, Knuutila S, Odell EW, Makitie A, Sundelin K, Stenman G, Leivo I. Genomic profiles and CRTC1-MAML2 fusion distinguish different subtypes of mucoepidermoid carcinoma. Mod Pathol. 2013;26(2):213–22. [DOI] [PubMed] [Google Scholar]
- 9.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–21. [DOI] [PubMed] [Google Scholar]
- 10.Birkeland AC, Foltin SK, Michmerhuizen NL, Hoesli RC, Rosko AJ, Byrd S, Yanik M, Nor JE, Bradford CR, Prince ME, Carey TE, McHugh JB, Spector ME, Brenner JC. Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncol. 2017;68:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano T, Yamamoto H, Hashimoto K, Tamiya S, Shiratsuchi H, Nakashima T, Nishiyama K, Higaki Y, Komune S, Oda Y. HER2 and EGFR gene copy number alterations are predominant in high-grade salivary mucoepidermoid carcinoma irrespective of MAML2 fusion status. Histopathology. 2013;63(3):378–92. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Chai Z, Li Y, Liu D, Bai Z, Li Y, Li Y, Situ Z. BZW1, a novel proliferation regulator that promotes growth of salivary muocepodermoid carcinoma. Cancer Lett. 2009;284(1):86–94. [DOI] [PubMed] [Google Scholar]
- 13.Kozel C, Thompson B, Hustak S, Moore C, Nakashima A, Singh CR, Reid M, Cox C, Papadopoulos E, Luna RE, Anderson A, Tagami H, Hiraishi H, Slone EA, Yoshino K-I, Asano M, Gillaspie S, Nietfeld J, Perchellet J-P, Rothenburg S, Masai H, Wagner G, Beeser A, Kikkawa U, Fleming SD, Asano K. Overexpression of eIF5 or its protein mimic 5MP perturbs eIF2 function and induces ATF4 translation through delayed re-initiation. Nucleic Acids Res. 2016;44(18):8704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mochizuki D, Adams A, Warner KA, Zhang Z, Pearson AT, Misawa K, McLean SA, Wolf GT, Nor JE. Anti-tumor effect of inhibition of IL-6 signaling in mucoepidermoid carcinoma. Oncotarget. 2015;6(26):22822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner VP, Martins MD, Martins MAT, Almeida LO, Warner KA, Nor JE, Squarize CH, Castilho RM. Targeting histone deacetylase and NFκB signaling as a novel therapy for mucoepidermoid carcinomas. Sci Rep. 2018;8(1):2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley PJ. Adenoid cystic carcinoma of the head and neck: A review. Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):127–32. [DOI] [PubMed] [Google Scholar]
- 17.Ellington CL, Goodman M, Kono SA, Grist W, Wadsworth T, Chen AY, Owonikoko T, Ramalingam S, Shin DM, Khuri FR, Beitler JJ, Saba NF. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973–2007 surveillance, epidemiology, and end results data. Cancer. 2012;118(18):4444–51. [DOI] [PubMed] [Google Scholar]
- 18.Brill LB, 2nd, Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T, Stenman G, Frierson HF, Jr. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–76. [DOI] [PubMed] [Google Scholar]
- 19.Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ, Zhang L, Mitani M, Weber RS, Lippman SM, Caulin C, El-Naggar AK. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: Association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17(22):7003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, Ramaswami D, Walsh LA, Eng S, Huse JT, Zhang J, Dolgalev I, Huberman K, Heguy A, Viale A, Drobnjak M, Leversha MA, Rice CE, Singh B, Iyer NG, Leemans CR, Bloemena E, Ferris RL, Seethala RR, Gross BE, Liang Y, Sinha R, Peng L, Raphael BJ, Turcan S, Gong Y, Schultz N, Kim S, Chiosea S, Shah JP, Sander C, Lee W, Chan TA. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45(7):791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A, Haigentz M Jr., Takes RP, Mondin V, Teymoortash A, Thompson LD, Ferlito A. Adenoid cystic carcinoma of the head and neck—An update. Oral Oncol. 2015;51(7):652–61. [DOI] [PubMed] [Google Scholar]
- 22.Murase R, Sumida T, Kawamura R, Onishi-Ishikawa A, Hamakawa H, McAllister SD, Desprez PY. Suppression of invasion and metastasis in aggressive salivary cancer cells through targeted inhibition of ID1 gene expression. Cancer Lett. 2016;377(1):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherl C, Kato MG, Erkul E, Graboyes EM, Nguyen SA, Chi AC, Morgan PF, Day TA. Outcomes and prognostic factors for parotid acinic cell carcinoma: A National Cancer Database study of 2362 cases. Oral Oncol. 2018;82:53–60. [DOI] [PubMed] [Google Scholar]
- 24.Patel NR, Sanghvi S, Khan MN, Husain Q, Baredes S, Eloy JA. Demographic trends and disease-specific survival in salivary acinic cell carcinoma: An analysis of 1129 cases. Laryngoscope. 2014;124(1):172–8. [DOI] [PubMed] [Google Scholar]
- 25.Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36(3):343–50. [DOI] [PubMed] [Google Scholar]
- 26.Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, Wang L, Hyman DM, Hechtman J, Wei G, Cam NR, Christiansen J, Luo D, Maneval EC, Bauer T, Patel M, Liu SV, Ou SH, Farago A, Shaw A, Shoemaker RF, Lim J, Hornby Z, Multani P, Ladanyi M, Berger M, Katabi N, Ghossein R, Ho AL. What hides behind the MASC: Clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol. 2016;27(5):920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vander Poorten V, Triantafyllou A, Thompson LD, Bishop J, Hauben E, Hunt J, Skalova A, Stenman G, Takes RP, Gnepp DR, Hellquist H, Wenig B, Bell D, Rinaldo A, Ferlito A. Salivary acinic cell carcinoma: Reappraisal and update. Eur Arch Otorhinolaryngol. 2016;273(11):3511–31. [DOI] [PubMed] [Google Scholar]
- 28.Seethala RR, Stenman G. Update from the 4th edition of the World Health Organization classification of head and neck tumours: Tumors of the salivary gland. Head Neck Pathol. 2017;11(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatura K Polymorphous low grade adenocarcinoma. J Oral Maxillofac Pathol. 2015;19(1):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent SD, Hammond HL, Finkelstein MW. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1994;77(1):41–7. [DOI] [PubMed] [Google Scholar]
- 31.Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B, Clarke BA, Skalova A, Chiosea SI, Seethala RR, Waggott D, Boutros PC, How C, Liu FF, Irish JC, Goldstein DP, Gilbert R, Ud Din N, Assaad A, Hornick JL, Thompson LD, Antonescu CR. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromo Cancer. 2014;53(10):845–56. [DOI] [PubMed] [Google Scholar]
- 32.Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B, Harding NJ, Alfaro J, Chu KC, Viale A, Fusco N, da Cruz Paula A, Marchio C, Sakr RA, Lim R, Thompson LD, Chiosea SI, Seethala RR, Skalova A, Stelow EB, Fonseca I, Assaad A, How C, Wang J, de Borja R, Chan-Seng-Yue M, Howlett CJ, Nichols AC, Wen YH, Katabi N, Buchner N, Mullen L, Kislinger T, Wouters BG, Liu FF, Norton L, McPherson JD, Rubin BP, Clarke BA, Weigelt B, Boutros PC, Reis-Filho JS. Hotspot activating PRKD1 somatic mutations in polymorphous lowgrade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel TD, Vazquez A, Marchiano E, Park RC, Baredes S, Eloy JA. Polymorphous low-grade adenocarcinoma of the head and neck: A population-based study of 460 cases. Laryngoscope. 2015;125(7):1644–9. [DOI] [PubMed] [Google Scholar]
- 34.Yin LX, Ha PK. Genetic alterations in salivary gland cancers. Cancer. 2016;122(12):1822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayaprakash V, Merzianu M, Warren GW, Arshad H, Hicks WL Jr., Rigual NR, Sullivan MA, Seshadri M, Marshall JR, Cohan DM, Zhao Y, Singh AK. Survival rates and prognostic factors for infiltrating salivary duct carcinoma: Analysis of 228 cases from the Surveillance, Epidemiology, and End Results database. Head Neck. 2014;36(5):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osborn V, Givi B, Lee A, Sheth N, Roden D, Schwartz D, Schreiber D. Characterization, treatment and outcomes of salivary ductal carcinoma using the National Cancer Database. Oral Oncol. 2017;71:41–6. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt NC, Kang H, Sharma A. Salivary duct carcinoma: An aggressive salivary gland malignancy with opportunities for targeted therapy. Oral Oncol. 2017;74:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ku BM, Jung HA, Sun JM, Ko YH, Jeong HS, Son YI, Baek CH, Park K, Ahn MJ. High-throughput profiling identifies clinically actionable mutations in salivary duct carcinoma. J Transl Med. 2014;12:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boon E, Bel M, van Boxtel W, van der Graaf WTA, van Es RJJ, Eerenstein SEJ, Baatenburg de Jong RJ, van den Brekel MWM, van der Velden LA, Witjes MJH, Hoeben A, Willems SM, Bloemena E, Smit LA, Oosting SF, Group P, Jonker MA, Flucke UE, van Herpen CML. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands. Int J Cancer. 2018. August 15;143(4):758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Cruz-Monserrate Z, Helen Lin H, Chung Y, Ji B, Lin SM, Vonderfecht S, Logsdon CD, Li CF, Ann DK. Ductal activation of oncogenic KRAS alone induces sarcomatoid phenotype. Sci Rep. 2015;5:13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diegel CR, Cho KR, El-Naggar AK, Williams BO, Lindvall C. Mammalian target of rapamycin-dependent acinar cell neoplasia after inactivation of Apc and Pten in the mouse salivary gland: Implications for human acinic cell carcinoma. Cancer Res. 2010;70(22):9143–52. [DOI] [PubMed] [Google Scholar]
- 42.Ettl T, Schwarz-Furlan S, Haubner F, Muller S, Zenk J, Gosau M, Reichert TE, Zeitler K. The PI3K/AKT/mTOR signalling pathway is active in salivary gland cancer and implies different functions and prognoses depending on cell localisation. Oral Oncol. 2012;48(9):822–30. [DOI] [PubMed] [Google Scholar]
- 43.Szwarc MM, Kommagani R, Jacob AP, Dougall WC, Ittmann MM, Lydon JP. Aberrant activation of the RANK signaling receptor induces murine salivary gland tumors. PLoS One. 2015;10(6):e0128467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Y, Liu H, Gao L, Lu L, Du L, Bai H, Li J, Said S, Wang XJ, Song J, Serkova N, Wei M, Xiao J, Lu SL. Cooperation between Pten and Smad4 in murine salivary gland tumor formation and progression. Neoplasia. 2018;20(8):764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368(7):651–62. [DOI] [PubMed] [Google Scholar]
- 46.Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider JL, Cuervo AM. Autophagy and human disease: Emerging themes. Curr Opin Genet Dev. 2014;26:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25(5):1037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. The autophagic network and cancer. Nat Cell Biol. 2018;20(3):243–51. [DOI] [PubMed] [Google Scholar]
- 50.Codogno P, Meijer AJ. Atg5: More than an autophagy factor. Nat Cell Biol. 2006;8:1045. [DOI] [PubMed] [Google Scholar]
- 51.Morgan-Bathke M, Lin HH, Chibly AM, Zhang W, Sun X, Chen CH, Flodby P, Borok Z, Wu R, Arnett D, Klein RR, Ann DK, Limesand KH. Deletion of ATG5 shows a role of autophagy in salivary homeostatic control. J Dent Res. 2013;92(10):911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin HH, Lin SM, Chung Y, Vonderfecht S, Camden JM, Flodby P, Borok Z, Limesand KH, Mizushima N, Ann DK. Dynamic involvement of ATG5 in cellular stress responses. Cell Death Dis. 2014;5:e1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885. [DOI] [PubMed] [Google Scholar]
- 54.Lin HH, Chung Y, Cheng C-T, Ouyang C, Fu Y, Kuo C-Y, Chi KK, Sadeghi M, Chu P, Kung H-J, Li C-F, Limesand KH, Ann DK. Autophagic reliance promotes metabolic reprogramming in oncogenic KRAS-driven tumorigenesis. Autophagy. 2018. DOI: 10.1080/15548627.2018.1450708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, Deng J, Hai J, Yang S, Wong KK, Kimmelman AC. Autophagy sustains pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Cancer Discov. 2018;8(3):276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo JY, Teng X, Laddha SV, Ma S, Van Nostrand SC, Yang Y, Khor S, Chan CS, Rabinowitz JD, White E. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 2016;30(15):1704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alves S, Castro L, Fernandes MS, Francisco R, Castro P, Priault M, Chaves SR, Moyer MP, Oliveira C, Seruca R, Corte-Real M, Sousa MJ, Preto A. Colorectal cancer-related mutant KRAS alleles function as positive regulators of autophagy. Oncotarget. 2015;6(31):30787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santana-Codina N, Mancias JD, Kimmelman AC. The role of autophagy in cancer. Ann Rev Cancer Biol. 2017;1(1):19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan-Bathke M, Lin HH, Ann DK, Limesand KH. The role of autophagy in salivary gland homeostasis and stress responses. J Dent Res. 2015;94(8):1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang L, Huang S, Zhang D, Zhang B, Li K, Li W, Zhang S, Zhang W, Zheng P. Inhibition of autophagy augments chemotherapy in human salivary adenoid cystic carcinoma. J Oral Pathol Med. 2014;43(4):265–72. [DOI] [PubMed] [Google Scholar]
- 62.Ma B, Liang LZ, Liao GQ, Liang YJ, Liu HC, Zheng GS, Su YX. Inhibition of autophagy enhances cisplatin cytotoxicity in human adenoid cystic carcinoma cells of salivary glands. J Oral Pathol Med. 2013;42(10):774–80. [DOI] [PubMed] [Google Scholar]
- 63.Ge XY, Yang LQ, Jiang Y, Yang WW, Fu J, Li SL. Reactive oxygen species and autophagy associated apoptosis and limitation of clonogenic survival induced by zoledronic acid in salivary adenoid cystic carcinoma cell line SACC-83. PLoS One. 2014;9(6):e101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, Hu X, Zhang W, Xu N, Wang FQ, Jia J, Zhang WF, Sun ZJ, Zhao YF. Mammalian target of rapamycin regulates isoliquiritigenin-induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis. 2012;17(1):90–101. [DOI] [PubMed] [Google Scholar]
- 65.Wu H, Huang S, Chen Z, Liu W, Zhou X, Zhang D. Hypoxia-induced autophagy contributes to the invasion of salivary adenoid cystic carcinoma through the HIF-1α/BNIP3 signaling pathway. Mol Med Rep. 2015;12(5):6467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang C, Jin S, Jiang Z, Wang J. Inhibitory effects of silibinin on proliferation and lung metastasis of human high metastasis cell line of salivary gland adenoid cystic carcinoma via autophagy induction. Onco Targets Ther. 2016;9:6609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahn M-Y, Yoon J-H. Histone deacetylase 7 silencing induces apoptosis and autophagy in salivary mucoepidermoid carcinoma cells. J Oral Pathol Med. 2017;46(4):276–83. [DOI] [PubMed] [Google Scholar]
- 68.Ahn MY, Ahn JW, Kim HS, Lee J, Yoon JH. Apicidin inhibits cell growth by downregulating IGF-1R in salivary mucoepidermoid carcinoma cells. Oncol Rep. 2015;33(4):1899–907. [DOI] [PubMed] [Google Scholar]
- 69.Blasco MA, Svider PF, Raza SN, Jacobs JR, Folbe AJ, Saraf P, Eloy JA, Baredes S, Fribley AM. Systemic therapy for head and neck squamous cell carcinoma: Historical perspectives and recent breakthroughs. Laryngoscope. 2017;127(11):2565–9. [DOI] [PubMed] [Google Scholar]
- 70.Cerda T, Sun XS, Vignot S, Marcy PY, Baujat B, Baglin AC, Ali AM, Testelin S, Reyt E, Janot F, Thariat J. A rationale for chemoradiation (vs radiotherapy) in salivary gland cancers? On behalf of the REFCOR (French rare head and neck cancer network). Crit Rev Oncol Hematol. 2014;91(2):142–58. [DOI] [PubMed] [Google Scholar]
- 71.Mifsud MJ, Tanvetyanon T, McCaffrey JC, Otto KJ, Padhya TA, Kish J, Trotti AM, Harrison LB, Caudell JJ. Adjuvant radiotherapy versus concurrent chemoradiotherapy for the management of high-risk salivary gland carcinomas. Head Neck. 2016;38(11):1628–33. [DOI] [PubMed] [Google Scholar]
- 72.Carlson J, Licitra L, Locati L, Raben D, Persson F, Stenman G. Salivary gland cancer: An update on present and emerging therapies. Am Soc Clin Oncol Educ Book. 2013:257–63. DOI: 10.1200/EdBook_AM.2013.33.257. [DOI] [PubMed] [Google Scholar]
- 73.Rosenberg L, Weissler M, Hayes DN, Shockley W, Zanation A, Rosenman J, Chera B. Concurrent chemoradiotherapy for locoregionally advanced salivary gland malignancies. Head Neck. 2012;34(6):872–6. [DOI] [PubMed] [Google Scholar]
- 74.Alfieri S, Granata R, Bergamini C, Resteghini C, Bossi P, Licitra LF, Locati LD. Systemic therapy in metastatic salivary gland carcinomas: A pathology-driven paradigm? Oral Oncol. 2017;66:58–63. [DOI] [PubMed] [Google Scholar]
- 75.Yoo SH, Roh JL, Kim SO, Cho KJ, Choi SH, Nam SY, Kim SY. Patterns and treatment of neck metastases in patients with salivary gland cancers. J Surg Oncol. 2015;111(8):1000–6. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Luo Y, Li M, Yan H, Sun M, Fan T. Management of salivary gland carcinomas: A review. Oncotarget. 2017;8(3): 3946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta R, Balasubramanian D, Clark JR. Salivary gland lesions: Recent advances and evolving concepts. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(6):661–74. [DOI] [PubMed] [Google Scholar]
- 78.Jakob JA, Kies MS, Glisson BS, Kupferman ME, Liu DD, Lee JJ, El-Naggar AK, Gonzalez-Angulo AM, Blumenschein GR Jr. Phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck. 2015;37(5):644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Locati LD, Perrone F, Cortelazzi B, Bergamini C, Bossi P, Civelli E, Morosi C, Lo Vullo S, Imbimbo M, Quattrone P, Dagrada GP, Granata R, Resteghini C, Mirabile A, Alfieri S, Orlandi E, Mariani L, Saibene G, Pilotti S, Licitra L. A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: Translational analyses and clinical impact. Eur J Cancer. 2016;69:158–65. [DOI] [PubMed] [Google Scholar]
- 80.Stenman G Fusion oncogenes in salivary gland tumors: Molecular and clinical consequences. Head Neck Pathol. 2013;7(Suppl 1):S12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–96. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Eisbruch A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J Radiat Res. 2016;57(Suppl 1):i69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. 2017;7:CD012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Redman RS. Histologic changes in the salivary glands following radiation therapy In: Cha S, editor. Salivary gland development and regeneration. 1st ed. Berlin: Springer International Publishing; 2017. pp. 75–91. [Google Scholar]
- 85.Yang X, Tridandapani S, Beitler JJ, Yu DS, Chen Z, Kim S, Bruner DW, Curran WJ, Liu T. Diagnostic accuracy of ultrasonic histogram features to evaluate radiation toxicity of the parotid glands: A clinical study of xerostomia following head-and-neck cancer radiotherapy. Acad Radiol. 2014;21(10):1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, Elting LS, Langendijk JA, Coppes RP, Reyland ME. Clinical management of salivary gland hypofunction and xerostomia in head and neck cancer patients: Successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78(4):983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baum BJ, Alevizos I, Chiorini JA, Cotrim AP, Zheng C. Advances in salivary gland gene therapy: Oral and systemic implications. Exp Opin Biol Ther. 2015;15(10):1443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: From animal models to therapies. J Dent Res. 2009;88(10):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davies AN, Thompson J. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Cochrane Database Syst Rev. 2015;(10):CD003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang WF, Liao GQ, Hakim SG, Ouyang DQ, Ringash J, Su YX. Is pilocarpine effective in preventing radiation-induced xerostomia? A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(3):503–11. [DOI] [PubMed] [Google Scholar]
- 91.Lovelace TL, Fox NF, Sood AJ, Nguyen SA, Day TA. Management of radiotherapy-induced salivary hypofunction and consequent xerostomia in patients with oral or head and neck cancer: Meta-analysis and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):595–607. [DOI] [PubMed] [Google Scholar]
- 92.Fox NF, Xiao C, Sood AJ, Lovelace TL, Nguyen SA, Sharma A, Day TA. Hyperbaric oxygen therapy for the treatment of radiation-induced xerostomia: A systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(1):22–8. [DOI] [PubMed] [Google Scholar]
- 93.Furness S, Bryan G, McMillan R, Birchenough S, Worthington HV. Interventions for the management of dry mouth: Non-pharmacological interventions. Cochrane Database Syst Rev. 2013;(9):CD009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sood AJ, Fox NF, O’Connell BP, Lovelace TL, Nguyen SA, Sharma AK, Hornig JD, Day TA. Salivary gland transfer to prevent radiation-induced xerostomia: A systematic review and meta-analysis. Oral Oncol. 2014;50(2):77–83. [DOI] [PubMed] [Google Scholar]
- 95.Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, Nottingham LK, Rudy SF, Van Waes C, Whatley MA, Brahim JS, Chiorini JA, Danielides S, Turner RJ, Patronas NJ, Chen CC, Nikolov NP, Illei GG. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA. 2012;109(47):19403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alevizos I, Zheng C, Cotrim AP, Goldsmith CM, McCullagh L, Berkowitz T, Strobl SL, Malyguine A, Kopp WC, Chiorini JA, Nikolov NP, Neely M, Illei GG, Baum BJ. Immune reactivity after adenoviral-mediated aquaporin-1 cDNA transfer to human parotid glands. Oral Diseases. 2017;23(3):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Momot D, Zheng C, Yin H, Elbekai RH, Vallant M, Chiorini JA. Toxicity and biodistribution of the serotype 2 recombinant adeno-associated viral vector, encoding aquaporin-1, after retroductal delivery to a single mouse parotid gland. PLoS One. 2014;9(3):e92832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao R, Yan X, Zheng C, Goldsmith CM, Afione S, Hai B, Xu J, Zhou J, Zhang C, Chiorini JA, Baum BJ, Wang S. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 2011;18(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morgan-Bathke M, Hill GA, Harris ZI, Lin HH, Chibly AM, Klein RR, Burd R, Ann DK, Limesand KH. Autophagy correlates with maintenance of salivary gland function following radiation. Sci Rep. 2014;4:5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morgan-Bathke M, Harris ZI, Arnett DG, Klein RR, Burd R, Ann DK, Limesand KH. The rapalogue, CCI-779, improves salivary gland function following radiation. PLoS One. 2014;9(12):e113183. [DOI] [PMC free article] [PubMed] [Google Scholar]