Agricultural liming severely affects the use of strontium isotopes in prehistoric provenance and migration studies.
Abstract
The application of 87Sr/86Sr in prehistoric mobility studies requires accurate strontium reference maps. These are often based from present-day surface waters. However, the use of agricultural lime in low to noncalcareous soils can substantially change the 87Sr/86Sr compositions of surface waters. Water unaffected by agriculture in western Denmark has an average 87Sr/86Sr ratio of 0.7124 as compared to an average of 0.7097 in water from nearby farmland. The 87Sr/86Sr ratio obtained from samples over 1.5 km along a stream, which originates in a forest and flows through lime-treated farmland, decreased from 0.7131 to 0.7099. Thus, 87Sr/86Sr-based mobility and provenance studies in regions with low to noncalcareous soils should be reassessed. For example, reinterpreting the iconic Bronze Age women at Egtved and Skrydstrup using values unaffected by agricultural lime indicates that it is most plausible that these individuals originated close to their burial sites and not far abroad as previously suggested.
INTRODUCTION
In recent years, variations in strontium 87Sr/86Sr ratios have been applied to uncover migration patterns of prehistoric humans and animals with considerable success (1–6). The method is based on the observation that the 87Sr/86Sr ratios in soil substrates vary geographically, reflecting the lithology of the soil. Strontium is released from the substrate to groundwater and surface water and becomes bioavailable, and is taken up in plants and animals, with no change to the average 87Sr/86Sr ratio (4, 7–9). Thus, the mobility of a prehistoric human can be estimated by comparing the 87Sr/86Sr value of the remains of an individual to a reference map showing the distribution of bioavailable strontium in the study area. The success of the method strongly depends on the accuracy of the reference map (10–14). However, investigations of prehistoric human migration are generally based on comparisons with modern-day isotopic maps, and a large variety of geological and biological sample materials have been analyzed to provide the necessary data (10), notably surface water and vegetation (10, 15), which are two of the most widely used and probably most reliable materials.
On the basis of 192 samples of surface waters (15) and of data from animal remains (16), Frei et al. (5) calculated an 87Sr/86Sr baseline for Denmark (excluding the island of Bornholm) spanning from 0.708 to 0.711. The mean and 2 SDs of the dataset is 0.7096 ± 0.0015, and the full range is 0.7079 to 0.7128 (15). The baseline has since been used as a reference in several remarkable studies of prehistoric migration in Denmark, in particular, regarding female mobility during the Bronze Age. For example, Frei et al. (5) concluded that the iconic Egtved Girl excavated in Central Jutland spent her early years outside of Denmark, possibly in southern Germany. During the last 2 years of her life, she traveled back and forth between Denmark and a place outside of Denmark (likely here birthplace) before she died at Egtved at the age of 16 to 18. Another Bronze Age female from Jutland, the Skrydstrup Woman, appears to have first come to Denmark at the age of 12 to 13. Here, she died 4 years later and was buried in a mound at Skrydstrup (6).
The 87Sr/86Sr map generated by Frei and Frei (15) show an isotopic homogeneity at odds with the geologically varied Pleistocene glacial deposits that constitute the sediment cover of most of Denmark (15). The only distinct geological feature shown in this map is a zone across northern Jutland with noticeable lower 87Sr/86Sr values (~0.708). This zone represents an area where glacial deposits directly overlie Upper Cretaceous and lower Paleocene chalk and limestone formations, both of which are rich in strontium carrying an 87Sr/86Sr ratio of ~0.708 (15).
Considering the effect of chalk and limestone in northern Jutland, it was expected that the east-west shift across Jutland from the strongly calcareous soils in East Jutland to noncalcareous soils in West Jutland (17, 18) would be visible in the 87Sr/86Sr map, with lower ratios in East Jutland than in West Jutland. However, this is apparently not the case according to the baseline map of Frei and Frei (15), which indicates that the 87Sr/86Sr ratios change very little across Jutland. A possible explanation for this lack of correlation between the 87Sr/86Sr ratio and the calcium carbonate content in the sediments could be agricultural lime, which for more than a century has been used for soil improvement throughout most of Denmark.
To test whether agricultural lime can influence the 87Sr/86Sr ratios of surface waters, we compare 87Sr/86Sr ratios and strontium concentrations measured in surface water samples from “pristine” areas, which never have been subject to agricultural lime, with data from neighboring farmland, where lime has been added to the soils. The use of surface water as a sample medium has the advantage that it allows for the determination of strontium concentration to aid in the interpretation of the strontium isotopic ratios. Lime is the main subject of this study, but it is evident that anthropogenic activities such as the use of fertilizers (10) and several physical factors including rainwater, dust, and marine sea spray may also influence the strontium content in surface water (11).
The question of whether agricultural lime can affect the 87Sr/86Sr ratios of bioavailable strontium is of global importance, as sediments similar to the deposits studied here occur widespread and are commonly subjected to intensive farming and liming. If agricultural lime can significantly change the isotopic composition of bioavailable strontium, then it has serious implications for the development of 87Sr/86Sr baseline maps and for the use of 87Sr/86Sr in provenance and migration studies worldwide.
RESULTS
The study area comprises the middle part of Jutland (Fig. 1A). The area is divided into three zones by two glaciogenic boundaries: (i) the Main Stationary Line (MSL), separating West and Central Jutland, marks the maximum extent of the Scandinavian ice sheet during the last Glaciation (22 ka ago); and (ii) the East Jutland Line (EJL), separating Central and East Jutland, marks the maximum extent of the later East Jutland advance (17 ka ago) (Fig. 1A) (17). The periglacial deposits of West Jutland consist of Weichselian outwash plains surrounding older Saale landscapes (Fig. 1B). Both of these units are essentially noncalcareous (see fig. S1). The deposits of Central and East Jutland consist predominantly of clayey tills. Whereas the near-surface deposits of the tills of Central Jutland are predominantly noncalcareous, the tills of East Jutland are strongly calcareous (fig. S1) (17–19).
Fig. 1. Simplified Quaternary geological maps of Denmark with sample localities.
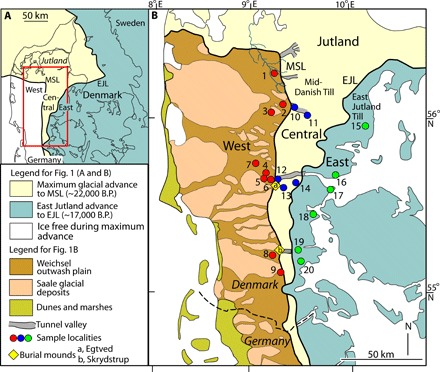
(A) Map showing the position of the MSL and the EJL, indicating the maximum extent of the Weichselian ice sheet in Denmark and the maximum advance of the East Jutland Till, respectively. The red rectangle marks the geographic location of the map in (B). (B) The MSL and EJL subdivide Jutland into three glaciogenic provinces: West Jutland (West), Central Jutland (Central), and East Jutland (East). The distribution is indicated in West Jutland of “Hill Islands” (Saale glacial deposits) and Weichsel outwash plains. Sample localities 1 to 20 are marked. Red dots denote samples obtained in West Jutland, blue dots denote those in Central Jutland, and green dots denote those in East Jutland. Maps are based on (17, 18). B.P., before the present.
A total of 84 samples of surface waters were analyzed for strontium concentration and isotopic composition (table S1). The sampling strategy was to follow streams from their sources in pristine areas to areas where agricultural lime has been applied. Where pristine streams are not available, lakes and ponds from pristine areas are compared to lakes and ponds from neighboring farmland areas. The identification of pristine and farmland areas was made a priori by overlaying maps and aerial photos of varying ages. A pond in a flat-lying area is considered to be pristine when there is a distance of at least 150 m to the nearest farmland. A stream is only regarded as “pristine” if it originates within the pristine area where it is sampled. Because streams generally occupy the lowest topographic points and tend to draw a wider catchment area than ponds, the buffer zone for pristine streams is set to 300 m. A full overview of localities and samples including geographic coordinates, sampling dates, distances between sampled pristine water bodies, and farmland and environmental affiliation is given in table S1. Two samples of stream water from locality 1 (K-13 and K-14) were collected closer to farmland than 300 m to examine the transition between pristine land and farmland. In table S1, they are classified as pristine.
The distance requirements for pristine ponds and streams are based primarily on Belgian studies of the effect of pesticides on farmland ponds (20, 21). These studies found that the levels of pesticides in ponds changed from a high degree of contamination at intensive land use with a 10-m buffer zone between pond and farmland to a very low degree of contaminations in more “pristine, natural” environments with a buffer zone of around 100 m (21). Overall, it was found that the load of a pesticide decreased to about a 20th when the buffer zone increased from 10 m to at least 100 m.
Denmark is a heavily cultivated country. About 57% of the total area is arable and is on a regular basis supplied with agricultural lime. Pristine areas in Denmark are therefore small and almost exclusively located in nature preserves or in old forests. When applying a buffer zone of 150 m, pristine areas (excluding coastal dune areas) make up about 5.6% of the total area of the country.
The samples obtained during the course of this study are distributed on 20 localities representing the three geological regions: (i) the noncalcareous West Jutland, (ii) the low to noncalcareous Central Jutland, and (iii) the calcareous East Jutland (Fig. 1B). This allows further examination of the effect of agricultural lime in relation to variations in the natural content of lime in the soil. In addition to surface water samples, a few samples of groundwater (well water) were analyzed. Maps and descriptions of most localities can be found in fig. S2.
Pristine samples versus farmland samples
The 87Sr/86Sr ratios of all 84 samples of surface waters vary from 0.7080 to 0.7150, while the strontium concentrations vary from 0.001 to 0.400 mg/liter (table S1). Sixty samples were obtained from pristine areas unaffected by farming, while 24 samples were obtained from farmland. Basic statistical information of the dataset is given in table S2.
The data from the noncalcareous West Jutland show a substantial difference between pristine and farmland samples (Fig. 2A). In the pristine samples, the 87Sr/86Sr values are high and variable (0.7104 to 0.7150), whereas in the farmland samples, the values are low and the range is narrower (0.7091 to 0.7115). By contrast, the strontium concentrations are low and relative variable in the pristine areas (0.002 to 0.038 mg/liter) and higher and less variable in the farmland areas (0.038 to 0.133 mg/liter). There is no overlap between the Sr concentration data from pristine and farmland areas (Fig. 2A).
Fig. 2. 87Sr/86Sr ratios and strontium concentrations for all surface water samples.
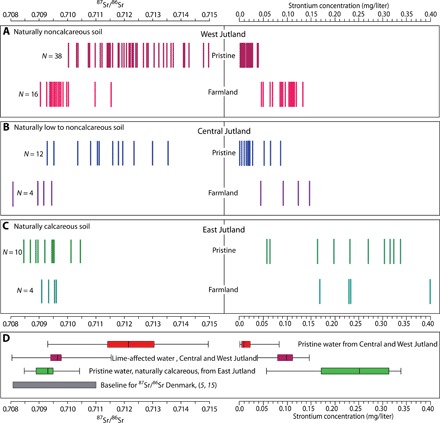
Each sample is represented by two vertical bars, one for the 87Sr/86Sr ratio and one for the strontium concentration. The samples are subdivided into three main groups following the geological division of the study area: (A) Samples from the noncalcareous West Jutland, (B) samples from the low to noncalcareous Central Jutland, and (C) samples from the calcareous East Jutland. Within each of these geological zones, samples from pristine areas and samples from farmland areas are distinguished. (D) Box and whisker plots (25, 50, 75, and 100%) of 87Sr/86Sr ratios and strontium concentrations, with pristine samples from the low to noncalcareous West and Central Jutland added together, farmland samples from the low to noncalcareous West and Central Jutland added together, pristine samples from calcareous East Jutland, and the 87Sr/86Sr baseline for Denmark of Frei and Frei (5, 15). N = number of samples in each category.
The results from Central Jutland resemble the results from West Jutland (Fig. 2, A and B). The 87Sr/86Sr ratios of the pristine samples exhibit a wide range of high values (0.7093 to 0.7136), whereas the farmland samples exhibit a narrower range of low values (0.7080 to 0.7095) (Fig. 2B). The Sr concentration data also show many similarities to the data from West Jutland. The strontium concentrations in the pristine samples are relatively low (0.001 to 0.084 mg/liter) but more variable than in West Jutland. The farmland samples have higher Sr concentrations, with a range from 0.045 to 0.146 mg/liter.
The strontium distribution pattern of the calcareous East Jutland is markedly different from those of West and Central Jutland, in that there is no significant difference between the pristine and the farmland samples (Fig. 2C). The 87Sr/86Sr ratios are lower and with a narrower range (0.7085 to 0.7104 as compared to 0.7104 to 0.7150 for West Jutland), whereas the Sr concentrations are higher and more variable (0.057 to 0.338 mg/liter as compared to 0.002 to 0.038 mg/liter for West Jutland). Furthermore, it is apparent that strontium concentration is lower (max, 0.15 mg/liter) in the farmland water samples from West and Central Jutland supplied with agricultural lime than in the naturally calcareous water samples from East Jutland (max, 0.35 mg/liter).
A plot of strontium concentrations versus 87Sr/86Sr ratios (Fig. 3) shows that there is no simple relationship between strontium concentration and isotopic composition of the pristine samples from the low to noncalcareous West and Central Jutland. The variability could reflect variable levels of rainwater dilution, different amounts of windblown dust, differing degrees of equilibrium between surface waters and soils, or variations in soil type. Several studies from northern Europe have shown that the strontium concentration in rainwater is very low, relative to the concentration in surface water (table S1) (15, 22, 23). Rainwater can thus have leverage on the strontium concentration in surface water but not on the strontium isotopic composition. Variations in grain size, lithology, and particularly in the cover of aeolian sand may explain why the pristine samples from the outwash plain at Kompedal Plantage (Fig. 1B, locality 1) show much smaller variation in strontium isotopic composition than the samples from the plain at Randbøl Hede (heathland) and Frederikshåb Plantage 60 km to the south (Fig. 1B, localities 4 and 5, and data in fig. S2, A and B). The southerly plain is overlain by a patchy cover of aeolian material, in contrast to the plain at Kompedal, where aeolian material is nearly absent.
Fig. 3. Strontium concentration versus 87Sr/86Sr ratio for Danish surface water samples.
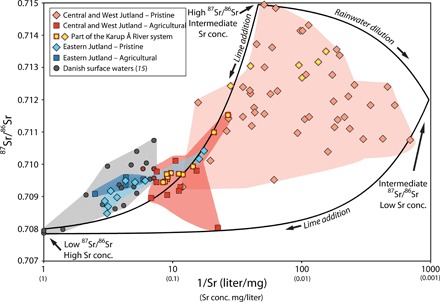
Samples are subdivided into two groups following the geological subdivisions of the study area: (i) the noncalcareous West Jutland and low to noncalcareous Central Jutland and (ii) the calcareous East Jutland. The samples are classified as pristine or agricultural. Previously published samples (15), marked with gray dots are classified as agricultural. Shaded areas show the range in strontium concentrations (Sr conc.) and 87Sr/86Sr ratios for each group. Samples from Karup River are marked with internal yellow circles. Hypothetical mixing lines are shown for three end-members: (i) low 87Sr/86Sr, high Sr concentration waters derived from limestone; (ii) intermediate 87Sr/86Sr, low Sr concentration waters derived from quartz sand or dominated by rainwater; and (iii) high 87Sr/86Sr intermediate Sr concentration waters derived from quartz sand with significant input from felsic rocks. Data for samples from (15) obtained from the same geographical locations as those obtained in the course of this study (n = 4) are each marked with a colored dot corresponding to geographical location and sample classification. Analytical uncertainties in the y-axis values are smaller than the data symbols. Analytical uncertainties in the x-axis values are about the size of the data symbols for low-concentration samples and are smaller for high-concentration samples.
Pristine samples versus farmland samples at individual localities
The differences between surface water from pristine areas and farmland areas are best detailed in the Vallerbæk stream–Karup River catchment area (Fig. 1B, locality 1, and Fig. 4). The Karup River is situated on the geologically homogeneous Karup outwash plain. The river is primarily sourced by groundwater, because of the coarse, highly permeable, sandy sediments. The Vallerbæk tributary originates in Kompedal Plantage (a 200-year-old extensively worked coniferous tree plantation with no soil improvement), where conditions are considered to be pristine, and runs in a shallow Holocene erosional valley (Fig. 4B). The uppermost sampling location (K-5) is a small pond. Between K-5 and K-3, the run is tiny and mostly dry. This part was not sampled. From K-3 to the farmland, the stream carries water, but the run is irregular and it frequently expands into swampy areas with no visible flow. At the beginning of the farmland at sampling location K-13, the flow is about 10 liters/s. After 1.5 km at sampling location K-4, the flow has increased to about 40 liters/s. The groundwater table in the plantation is 5 to 10 m below the forest floor, indicating that the water in this part of the stream is surface water with little interaction with groundwater. As the brook downstream erodes deeper into the outwash plain, the distance to the groundwater table decreases, such that in the farmland, the groundwater table and the water table of the stream are level, indicating that the stream water is primarily groundwater.
Fig. 4. Variability in 87Sr/86Sr ratio and strontium concentration in the Vallerbæk stream-Karup River catchment area.
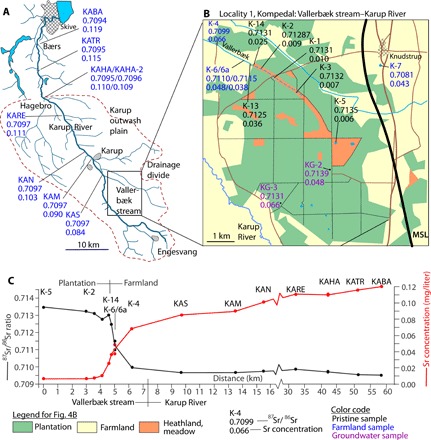
(A) Strontium data along the main run of Karup River with location of the Vallerbæk tributary indicated. (B) Detailed map of Kompedal Plantage and Vallerbæk tributary with sample positions and strontium data indicated. (C) Strontium concentration and 87Sr/86Sr ratios for Vallerbæk stream and Karup River as a function of distance from the head of Vallerbæk stream. Maps are based on data from “Styrelsen for Dataforsyning og Effektivisering, skærmkortet, WMS-tjeneste.”
The 87Sr/86Sr ratios of the surface water samples from within the tree plantation are high (>0.7130), whereas the Sr concentrations are low (<0.01 mg/liter) (Fig. 4C). Within 1.5 km downstream of the plantation, the 87Sr/86Sr ratio decreases to 0.7099, while the concentration increases to 0.066 mg/liter. Most of these changes occur over a distance of less than 500 m (Fig. 4C). For the next 57 km, the 87Sr/86Sr ratio remains nearly constant, while the Sr concentration increases gradually. The pristine groundwater in the plantation exhibits the same 87Sr/86Sr ratio as the surface water, but it has a much higher concentration (0.066 and 0.048 mg/liter as compared to <0.01 mg/liter for the surface water) (Fig. 4B).
Localities 3, 4, and 5 (Gludsted, Sepstrup Sande, and Nørlund) are also centered around streams flowing from pristine forests to farmland areas (Fig. 1B and fig. S2, C, D, and F), but they are located in regions with more variable geology. The results from these localities follow the pattern observed in the Vallerbæk stream–Karup River system with 87Sr/86Sr ratios decreasing and concentrations increasing downstream, as the stream flows from pristine heathland and woodland into farmland.
The difference in Sr isotopic composition between pristine heathland and woodland and farmland observed in streams is also apparent when comparing lakes and ponds, as illustrated by the samples from Bevtoft Plantage, which is situated on a noncalcareous outwash plain west of a major tunnel valley (Fig. 1B, locality 8, and fig. S2J). Two pristine samples give 87Sr/86Sr ratios of 0.7115 and 0.7141, as compared to a ratio of 0.7095 for a water sample taken in a pond on farmland just 800 m away (fig. S2J).
The effect of agricultural lime on the distribution of strontium isotopes in surface waters
The most notable characteristic of the 87Sr/86Sr data from the low to noncalcareous West and Central Jutland is the distinct difference between the high 87Sr/86Sr ratios in samples from pristine areas and the low 87Sr/86Sr ratios in samples from farmland, and the reverse pattern in the strontium concentrations (Fig. 2, A and B). Geologically, there are no indications of changes in lithology in connection with the transition from pristine land to farmland, and it is highly unlikely that the shift is related to changes in geology.
The only consistent environmental differences that may explain these changes appear to be differences in the degree of anthropogenic activity, from almost no activity in the pristine woodland and heathland to high activity in the farms. Without farming, the high 87Sr/86Sr ratios and the low strontium concentrations that characterize the upper pristine part of Vallerbæk stream would have been expected to have continued all the way to the mouth of Karup River at Skive (Fig. 4).
Agricultural activities that could affect the compositions of strontium in surface waters include the spreading of agricultural lime, fertilizers, manure, animal feed, and pesticides. To analyze the relative effect of each of these anthropogenic components, we performed a quantitative analysis of the input and output of strontium in the Vallerbæk stream–Karup River catchment area. This catchment area was selected because it is located on a geologically homogeneous, noncalcareous, outwash plain, and because the hydrology of the Karup River system is well established (24). The quantities of lime, fertilizer, and other sources used in the calculations have been provided by farmers from the Vallerbæk area and by local agricultural consultants.
Estimating the average strontium contribution from agricultural lime and the lime’s isotopic composition is relatively straightforward in the Vallerbæk catchment area. The lime applied in Jutland today consists of equal parts pure Upper Cretaceous chalk from northern Jutland and 2.5% magnesium chalk. The latter product is a mixture of Jutland chalk and Permian Magnesian Limestone imported from England. The average 87Sr/86Sr ratio of both products is 0.7079, while the strontium concentration varies from ~600 to 1055 mg/kg (table S3). These values are typical for northwest European chalk (25). Arable fields in the Vallerbæk catchment area are typically limed at a rate of 500 to 800 kg/ha per year of agricultural lime. The chalk is dissolved at a rate corresponding to the yearly supply. This means that about 720 g/ha per year of strontium with an 87Sr/86Sr ratio of 0.7079 is added to the farmland along Vallerbæk on the basis of agricultural lime alone.
Estimating the contributions from fertilizers and animal feed is much more complex, as several types of fertilizers and animal feed are used, and these have highly different strontium contents. In table S3, the 87Sr/86Sr ratios and strontium concentrations are listed for the various fertilizers and animal feeds used in the Vallerbæk area. On the basis of these values, and of the amount used of each type of fertilizer and animal feed, a total strontium contribution from fertilizers and animal feed of about 128 g/ha per year has been calculated. The application of pesticides in Denmark is very low (~1 kg/ha per year) compared to the supply of agricultural lime (~500 to 800 kg/ha per year). As the strontium concentration in the most common pesticides is very low [~10 parts per million (ppm)] compared to that of agricultural lime, it is apparent that pesticides are of no importance for the strontium budget in Danish soils.
Together, these numbers suggest that about 85 to 90% of the agricultural strontium added to the Vallerbæk catchment area every year come from agricultural lime, up to ~10% come from fertilizers, and up to ~10% come from manure (see calculations in table S4). About 20% of the strontium in the manure comes from nonlocal animal feed. These figures may change from year to year depending on the origin of the nonlocal food and on the components in the fertilizers. Because of the overwhelming importance of agricultural lime and for ease of terminology, all agricultural strontium sources are henceforth referred to as “agricultural lime.”
Whereas lime has a significant influence on the 87Sr/86Sr ratios and strontium concentrations in surface waters from the low to noncalcareous deposits of West and Central Jutland, it has apparently little or no effect in East Jutland (Fig. 2, A to C). The reason for this difference is the high natural content of lime in the glacial deposits of East Jutland (18, 19), where percentages of calcium carbonate between 5 and 22 are normal. A hypothetical soil, 20 cm thick containing 10% CaCO3, would naturally hold about 320 metric tons/ha of calcium carbonate. The addition of 500 kg/ha of agricultural lime would increase this amount with about 0.16%, which would have minimal influence on both the strontium concentration and isotopic composition. Simple modeling detailing the relationship between the effect of agricultural liming and soil thickness, Sr content, and original Sr isotopic composition is presented in fig. S3.
The data and data analysis presented above show that agricultural activity can have a strong impact on the 87Sr/86Sr ratio and strontium concentration in surface water in areas with low or noncalcareous soils. In Jutland, agricultural lime is by far the most important source of anthropogenic strontium. Fertilizers, animal feed, and other sources make up less than 15%. While the effect of agricultural lime seems not to have been investigated previously, the influence of fertilizers has been studied several times. Frei and Frei (15), in their investigation of strontium isotopes in Danish surface waters, found that the effect of fertilizers was negligible, but they used a study site with high calcium carbonate content in the soil. Other studies have found that fertilizers do have an effect (e.g., 10, 22, 26–31). However, environmental complexity combined with varied human activities has in most of these studies prevented a quantitative assessment of the contribution from fertilizers.
Modeling of the Vallerbæk stream–Karup River system
The variations in 87Sr/86Sr ratio and strontium concentration in the Vallerbæk stream–Karup River system can be modeled to quantify the amount of agricultural lime in the system using a simple three-component mixing model (Fig. 5). The average composition of the pristine surface water and pristine groundwater from Kompedal Plantage serve as two of the end-members, while a calculated agricultural runoff serves as the third (Fig. 5). The calculations for the agricultural runoff are based on information from local farmers on land use and amounts of lime, fertilizer, and manure applied to the fields. This yields an estimate of the total agricultural strontium and its isotopic composition (examples of these calculations are given in table S4), which can then be used to determine the strontium concentration of the agricultural runoff, by estimating the average runoff from rainfall.
Fig. 5. Mixing modeling of water in the Karup River system.
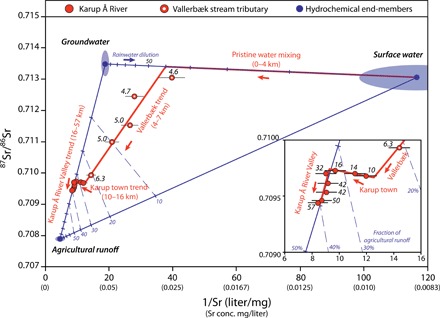
Three-component mixing of the inverse Sr concentration and 87Sr/86Sr ratios of water from the Karup River system (samples marked with yellow dots in Fig. 3). Hydrochemical end-member values for Kompedal surface water and groundwater are weighted averages of analyses, with the shaded area representing the 2σ uncertainty. The end-member value for agricultural runoff is calculated on the basis of the amounts of lime, manure, and fertilizer applied to the fields by local farmers (see the Supplementary Materials) and average runoff and rainfall values, and the shaded area around the points represents the estimated uncertainty in the values. The numbers at each data point indicate the distance in kilometer from the headwaters of Vallerbæk stream.
The evolution of the strontium isotopic composition and concentration in the Vallerbæk stream–Karup River system can be divided into four separate trends shown by the best-fit linear regression lines in Fig. 5. At the source of Vallerbæk stream in Kompedal Plantage, the surface water has the same 87Sr/86Sr ratios as the groundwater, but much lower strontium concentrations (Fig. 4B). The surface water is a mixture of groundwater and rainwater (Fig. 5, pristine water mixing trend). Where Vallerbæk becomes a permanent continuous stream, about 4 km from the head of the drainage basin, the water is about an even mixture of surface water and groundwater (Fig. 5). Upon entering the farmland, the composition of the stream water quickly changes toward the composition of agricultural runoff (Fig. 5, the Vallerbæk trend), and just before the outflow into Karup River, agricultural runoff makes up about 20% of the water in the stream. Vallerbæk stream joins Karup River a few kilometers south of the town of Karup. Through the town, the strontium trend changes sharply toward elevated strontium concentrations (Fig. 5, Karup town trend). This can be interpreted as an addition of a mixture of agricultural runoff and groundwater (wastewater from the municipality; population, 5600). After leaving the town and entering the sparsely populated and densely farmed Karup River Valley, the strontium changes behavior again, trending directly toward agricultural runoff (Fig. 5, Karup River Valley trend). When the river passes the last sampling site, shortly before it discharges into the Limfjord at Skive, the water in the river is composed of about 40% agricultural runoff and 60% groundwater.
At the Hagebro location (Fig. 4A), the discharge of the Karup River is 6600 liters/s, with almost no seasonal variation (24). Using the average measured strontium concentration of 0.11 mg/liter, this equates to 23 metric tons of dissolved strontium per year. The strontium concentration in the groundwater is 0.05 mg/liter, and if there were no agricultural input, then the total amount of dissolved strontium in Karup River would be expected to be 11 tons per year. The catchment area for Hagebro is 480 km2 (Fig. 4A), and assuming an average arable land fraction at the national average of 57%, the excess 12 tons of strontium in Karup River corresponds to an average rate of agricultural liming of 486 kg/ha per year. This is very close to the expected rate of 500 kg/ha per year and suggests that the anthropogenic input from the town of Karup and surrounding villages is trivial compared to that of farming. The calculations presented here indicate that farming and the use of agricultural lime are by far the largest sources of anthropogenic strontium in the Karup Å catchment area and that the addition of agricultural lime alone can explain the large observed decrease in 87Sr/86Sr ratio in the river system.
It is worth noting that there is a big difference in strontium isotopic values between the plants grown in the Karup River Valley and the water in the river, with the plants showing a pure lime signature (table S2) and the river a mixture between agricultural lime and groundwater. The multiple sources of strontium in an area such as this complicates the use of strontium isotopes as tracers in areas of research investigating modern strontium isotope variations, such as food and forensic sciences.
DISCUSSION
On the basis of these results, it can be inferred that all major streams and lakes in Jutland today are affected by agricultural lime, making them unsuitable for baseline maps of prehistoric bioavailable 87Sr/86Sr ratios. In Jutland, agricultural lime affects the strontium content of surface water by decreasing the 87Sr/86Sr ratio and increasing the strontium concentration. The data also show that the impact of agricultural lime depends on the natural content of calcium carbonate in a soil. The impact is high in water from low to noncalcareous soils and negligible in water from strongly calcareous soils. In Jutland, pristine areas are limited to larger forests and nature preserves, and they probably constitute only 5 to 6% of the total area of the peninsula.
The range of pristine 87Sr/86Sr values (0.7085 to 0.7150) obtained in this study is about double that of the baseline (0.708 to 0.711) for Denmark established by Frei and Frei (15) (Fig. 2D). That baseline is in fact much more similar to the range of the lime-affected samples (0.7080 to 0.7115) (Fig. 2D), suggesting that most of the samples used to create the baseline are affected by lime, either naturally or from farming. This assumption is supported by the fact that most of the baseline samples are from larger lakes and rivers, whose catchment areas contain large areas of farmland.
These findings have global implications for 87Sr/86Sr-based mobility and provenance studies. Deeply leached soils, resulting from natural processes or human activities, occur widely on all continents (32). Noncalcareous outwash plains composed mainly of meltwater sand and low to noncalcareous tills, comparable to the Danish deposits, are common in most northern European countries along the periphery of the Weichsel and Saale ice sheets from Russia in the east to Ireland in the west (33–35). Similar deposits occur widely in both the United States and Canada (36–38). Applying agricultural lime is the most economical way of improving these acidic soils and increasing crop yield. In most European and North American countries, the application of lime generally varies from 200 to 1000 kg/ha per year (32, 38–41). These levels are comparable to the rates of agricultural liming in Jutland, suggesting that the use of lime in these countries would have a similar effect on the strontium content in the environment.
An example of conditions similar to those in Denmark with respect to the impact of agricultural lime may be present on the North German Plain. The northeastern part of this plain, which stretches from Poland in the east to the Netherlands in the west, consists of moraine landscapes resembling the landscapes of eastern Denmark. The periglacial landscape south and west of the moraines is dominated by fluvial and aeolian sands, similar to and physically connected to periglacial outwash deposits of western Jutland (see Fig. 1). Most 87Sr/86Sr values from the North German Plain are low and fall within the baseline for Denmark, but several values especially from the sandy regions are high and well above the baseline (42). The source of these “anomalous” values is unknown (42).
By analogy with the situation in Jutland, the low values may be from areas with calcareous soils, while the high values may be from areas with noncalcareous soils. In the moraine landscape to the north, the soil is calcareous and 87Sr/86Sr values are therefore dominated by marine carbonates and are low. The periglacial sands to the south are geologically noncalcareous and should yield high values. However, most of the land here is farmed and supplied with agricultural lime. The natural variation in the 87Sr/86Sr ratio is therefore suppressed and skewed toward carbonate values, with higher values restricted to the remaining pristine areas unaffected by liming.
Implications for the origin and migration of the Egtved Girl and Skrydstrup Woman
The baseline of Frei and Frei (15) has been used as a reference in several studies of provenance and migration from Denmark. On the basis of the results presented here, two of the most important of these studies have been reevaluated, namely, the interpretations of the origins and migration histories of the two iconic Bronze Age women: the Egtved Girl and the Skrydstrup Woman (5, 6).
Frei et al. (5) analyzed eight nail, tooth, and hair samples from the Egtved Girl. Six samples produced 87Sr/86Sr values above the baseline for Denmark of 0.708 to 0.711, while two samples from the middle section of a 23-cm-long hair yielded values within the baseline interval. The data suggests that the Egtved Girl lived her early life outside of present-day Denmark, possibly in southern Germany. She came to Denmark about 13 months before her death. About 9 months after her arrival, she left Denmark and traveled to an area with higher strontium isotopic values, likely her birthplace, where she stayed for 4 to 6 months before returning to Denmark, shortly before her death at age 16 to 17. She was buried in an oak coffin at Egtved 1370 BCE (Fig. 1A). Eight pieces of woolly textiles and two portions of ox-hide hair from the oak coffin were also analyzed. Eight of these pieces, including each article of clothing, yielded 87Sr/86Sr values above the Danish baseline and thus were regarded to be of non-Danish origin (5).
The burial mound of the Skrydstrup Woman is located about 45 km south of the mound at Egtved (Fig. 1B). High 87Sr/86Sr values (0.7133 to 0.7138) obtained from samples of a tooth and the distal part of the Skrydstrup Woman’s hair suggested that she also originated outside of Denmark (6). Lower values (0.7086 to 0.7102) in the proximal part of the woman’s hair suggested that she moved to the Skrydstrup area at the age of 13 to 14 and remained there until her death about 4 years later.
In the present study, water samples were analyzed from 20 pristine lakes, ponds, and springs within a 10-km radius of the burial mound of the Egtved Girl (Fig. 6A and fig. S2B). The samples yielded a wide range of 87Sr/86Sr values spanning from 0.7093 to 0.7150. Seventeen of the 20 sites yielded 87Sr/86Sr ratios exceeding the Danish baseline (15), while six samples yielded values above the highest value measured in the remains of the Egtved Girl (Fig. 6A).
Fig. 6. Main landscape elements and 87Sr/86Sr ratios for lakes, ponds, and springs near the burial mounds of the Egtved Girl and the Skrydstrup Woman.
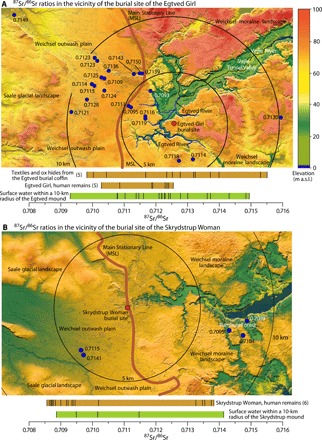
(A and B) Topographical maps with the locations of the two burial mounds marked with red dots. Only water samples regarded as pristine are shown. Circles with 5- and 10-km radii centered on the burial mounds are indicated. For ponds that have been analyzed twice during the fall of 2018, only the first analysis is shown. The range in the 87Sr/86Sr ratio is shown as bars below the maps together with data obtained from the Egtved Girl (5) and the Skrydstrup Woman (6). All results are listed in table S1 and shown in fig. S2. Maps are based on data from “Styrelsen for Dataforsyning og Effektivisering, skærmkortet, WMS-tjeneste.” m a.s.l., meters above sea level.
Because of a scarcity of pristine areas, only five water samples from the area around Skrydstrup (Fig. 6B and fig. S2J) were analyzed. Nevertheless, these five samples yield a range of 87Sr/86Sr values from 0.7089 to 0.7141, which is very close to the total range measured in the remains of the Skrydstrup Woman (0.7086 to 0.7138) (6).
On the basis of these results, it is evident that the 87Sr/86Sr ratios measured in the remains of the Egtved Girl and the Skrydstrup Woman could have been obtained locally within a radius of 10 km of their respective burial sites. The strontium data therefore provide no basis for claiming that the Egtved Girl and the Skrydstrup Woman originated far from Denmark or that the Egtved Girl traveled between Egtved and a place far outside of Denmark and back again during the last 2 years of her life. On the contrary, it seems far more plausible that the 87Sr/86Sr values measured in the two women were obtained from movements or migration within the immediate vicinity of their final resting place. It is also very likely that the textiles and ox hides from the burial coffin of the Egtved Girl are of local origin, although two pieces of wool have values slightly above the maximum values observed here (Fig. 6A).
The differing 87Sr/86Sr values measured in the remains of the two women may be explained by examining the geography surrounding the two burial mounds. The landscape around the mound at Egtved has two main features: the Vejle Tunnel Valley and the upland surrounding the tunnel valley (Fig. 6A). The pristine lakes, ponds, and springs from the upland are, with a few exceptions, characterized by high 87Sr/86Sr values of up to 0.7150. The valley, on the other hand, is distinguished by meadows and wetland with frequent springs that produce calcareous water (43) with low 87Sr/86Sr values, as exemplified by the large spring at Rosdam, which yields a low 87Sr/86Sr value of 0.7093 (Fig. 6A).
Assuming that the 87Sr/86Sr isotope ratios measured on pristine waters today reflect the prehistoric bioavailable strontium isotopic composition, the simplest explanation for the high 87Sr/86Sr values measured in the nail, tooth and, hair of the Egtved Girl is that she was born, lived most of her life, and died on the upland south and west of the tunnel valley, probably close to her final resting place (Fig. 6A). The only indications of a life elsewhere are the two low values obtained from the middle part of her hair and tentatively dated to 12 and 6 months before her death (5). These values could also have been obtained on the upland areas, where a small pond and a raised bog yield similar low values (0.7095 and 7109). A more likely alternative is that the low values were acquired in the tunnel valley, where numerous springs feed the Egtved River with calcareous water. The nearest of these springs is located about 1 km from the Egtved Girl’s burial mound. The shifts in 87Sr/86Sr values in the Egtved Girl’s remains could perhaps reflect a seasonal migration pattern, as those seen in transhumance farming, where shepherds spend months in temporary residences, often in close geographical distance to their primary residence.
Similarly, the landscape around Skrydstrup consists of two main features separated by the MSL (Fig. 6B). West of the line, the outwash plain deposits are naturally noncalcareous, and the surface waters exhibit high 87Sr/86Sr values (Fig. 6B and fig. S2, I and J). East of the line, the soils of the hilly moraines are mainly calcareous, and the 87Sr/86Sr values are low. The simplest explanation for the change in the 87Sr/86Sr values observed in the remains of the Skrydstrup Woman is that she moved from a place west of the MSL to a place east of the MSL at an age of 13 to 14 years. The move could have been only a few hundred meters, and any social implications remain unknown.
Implications for the creation of 87Sr/86Sr baseline maps
This study demonstrates the need for obtaining pristine samples to create an understanding of preindustrial and prehistorical strontium isotopic variations. The data generated here come from a varied glacial landscape in Jutland, Denmark, where more than three-quarters of the area is naturally low to noncalcareous. Today, about 57% of the land is arable and affected by the addition of agricultural lime. Only about 5 to 6% of the area is not influenced by farming activity or urbanized. All larger lakes, most streams, and all rivers are affected by agricultural lime. While land-use proportions may vary, this situation is common throughout the world in recently glaciated areas.
Because glacial soils often are intensively farmed, identification of pristine areas becomes essential. Sampling of surface waters is advantageous as it allows for the determination of both strontium concentrations and isotopic ratios. The combination is helpful to identify anthropogenically contaminated or rainwater-diluted samples. The ability of even modest amounts of agricultural liming to significantly change the strontium isotopic composition of surface waters suggests that many strontium baseline maps in areas with farming should be revised, in particular, where observed variations in strontium isotopic composition cannot be correlated with the underlying geology.
Examples of human migration studies where revised strontium maps have changed the interpretation are those of the Egtved Girl and the Skrydstrup Woman. Both women were buried close to boundaries between noncalcareous and calcareous soils, and the variable strontium isotopic values measured in their remains likely reflect movements back and forth across these boundaries. Especially in the case of the Egtved Girl, the repeated shift in Sr isotopic values suggests a seasonal pattern of migration—a potentially far more intriguing interpretation than the one based on the previous map.
MATERIALS AND METHODS
Sampling methods
Water samples were collected in acid-cleaned polyethylene bottles of 100- or 250-ml capacity. During field sampling, bottles were rinsed twice with sample water before sample collection. Most samples were taken at a water depth of 15 to 20 cm. Sampling was carried out from August 2017 to February 2018. The samples from October and later were collected following a period of heavy rain. Samples were stored in a cool room at a temperature of 2°C.
Laboratory methods
Sample preparation, handling, and analysis were done in the AGiR laboratories at the Institute of Geoscience, Aarhus University. An approximately 20-ml aliquot of a water sample was retrieved from the sample bottle for analysis using an acid-cleaned single-use polyethylene syringe and filtered (to remove particles) through an acid-cleaned 0.45-μm single-use nylon filter into a perfluoroalkoxy Teflon sample vial particulates. The filtered water samples were dried down and digested in 2 ml of aqua regia overnight, dried down again, and dissolved in nitric acid for strontium separation chemistry. Strontium was separated from the sample matrix using Eichrom (TrisKem International) Sr spec resin and analyzed for isotopic composition using a Nu Plasma II multicollector ICP-MS (inductively coupled plasma mass spectrometry). The four stable isotopes of strontium (84Sr, 86Sr, 87Sr, and 88Sr) were measured simultaneously, as were isotopes of Kr, Rb, Y, and doubly charged REE, to monitor and correct for interferences. Masses 82, 83, 84, 85, 86, 87, 88, and 89 were measured simultaneously, as well as half-masses 83.5, 84.5, 85.5, 86.5, and 87.5. Baselines were determined by on-peak zero, and each sample run consisted of 400 s of peak time. Data were fractionation-corrected using the exponential law and normalized to NBS SRM 987 (87Sr/86Sr = 0.71025), and samples of Holocene foraminifera (Baculogypsina sphaerulata), expected to give a modern-day seawater Sr isotope value (87Sr/86Sr = 0.70918), were processed along with the samples as a secondary standard. Six samples of foraminifera analyzed throughout the analytical campaign gave 87Sr/86Sr = 0.709177 ± 6 (8 ppm, 2σ). The reproducibility of surface water samples with lower Sr content is 20 ppm (2σ). Samples of limestone, fertilizer, and animal feed were digested in aqua regia before Sr purification and analysis following the same steps as outlined above for the water samples.
The standard-sample bracketing method of multicollector ICP-MS and the quantitative yield of the Sr separation procedure allow for fairly accurate Sr concentration determinations on the same unspiked sample analyzed for Sr isotopic composition. As a test for accuracy, 12 samples were analyzed for their Sr concentrations by both conventional quadrupole ICP-MS (Agilent 7900 at the AGiR laboratories) and standard-sample bracketed multicollector ICP-MS. This test is presented in fig. S4.
Supplementary Material
Acknowledgments
We thank the Danish Nature Agency for permission to collect water samples in forest and nature preserves managed by the agency. We are also indebted to several owners of private forests and properties for sampling access. We are grateful to farmers along Vallerbæk stream, F. Ø. Brauner, S.-E. Beier, L. Olesen, and S. Olesen for sharing information about use of lime, fertilizers, manure, and animal feed and for donating samples of the same products. We are indebted to Dankalk Aggersund and Dansk Landbrugs Grovvareselskab for donating lime and soy products. We thank S. B. Andersen, A. Clemmensen, C. Heilmann-Clausen, T. L. Rasmussen, H. C. Millgaard, and B. Lavesen for advice or assistance during field work. We also thank H. L. Andersen for creating the Lidar maps. The manuscript was improved by E. J. Rosenberg and two anonymous reviewers. Funding: We are grateful for financial support by the Torben and Alice Frimodt’s Foundation. Author contributions: E.T. conceived the study and collected all samples except for two that were collected by R.A. Laboratory analyses and modeling were conducted by R.A. E.T wrote the first draft of the manuscript. Both authors contributed equally to interpretation of the data and to the final version of the text. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav8083/DC1
Table S1. Strontium isotope and concentration data for surface water, groundwater, rainwater, and reference samples.
Table S2. Basic statistical data on strontium isotopic composition (87Sr/86Sr) and strontium concentration of pristine and farmland samples.
Table S3. Strontium isotope and concentration data for agricultural lime products, fertilizers, and animal feed.
Table S4. Calculated strontium isotope composition and concentration for two farms in the Vallerbæk area.
Fig. S1. Calcium carbonate distribution in glaciogenic deposits, middle Jutland.
Fig. S2. Simplified maps and description of localities.
Fig. S3. Influence of soil thickness and soil composition on the level of disturbance of the 87Sr/86Sr ratio from agricultural lime.
Fig. S4. Comparison of strontium concentrations measured by quadrupole ICP-MS and multicollector ICP-MS.
Reference (44)
REFERENCES AND NOTES
- 1.Ericson J. E., Strontium isotope characterization in the study of prehistoric human ecology. J. Hum. Evol. 14, 503–514 (1985). [Google Scholar]
- 2.Müller W., Fricke H., Halliday A. N., McCulloch M. T., Wartho J.-A., Origin and migration of the Alpine Iceman. Science 302, 862–866 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Haak W., Brandt G., de Jong H. N., Meyer C., Ganslmeier R., Heyd V., Hawkesworth C., Pike A. W. G., Meller H., Alt K. W., Ancient DNA, strontium isotopes, and osteological analyses shed light on social and kinship organization of the Later Stone Age. Proc. Natl. Acad. Sci. U.S.A. 105, 18226–18231 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery J., Passports from the past: Investigating human dispersals using strontium isotope analysis of tooth enamel. Ann. Hum. Biol. 37, 325–346 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Frei K. M., Mannering U., Kristiansen K., Allentoft M. E., Wilson A. S., Skals I., Tridico S., Nosch M. L., Willerslev E., Clarke L., Frei R., Tracing the dynamic life story of a Bronze Age female. Sci. Rep. 5, 10431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frei K. M., Villa C., Jørkov M. L., Allentoft M. E., Kaul F., Ethelberg P., Reiter S. S., Wilson A. S., Taube M., Olsen J., Lynnerup N., Willerslev E., Kristiansen K., Frei R., A matter of months: High precision migration chronology of a Bronze Age female. PLOS ONE 12, e0178834 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capo R. C., Stewart B. W., Chadwick O. A., Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma 82, 197–225 (1998). [Google Scholar]
- 8.Bentley R. A., Strontium isotopes from the earth to the archaeological skeleton: A review. J. Archaeol. Meth. Theor. 13, 135–187 (2006). [Google Scholar]
- 9.Montgomery J., Evans J. A., Cooper R. E., Resolving archaeological populations with Sr-isotope mixing models. Appl. Geochem. 22, 1502–1514 (2007). [Google Scholar]
- 10.Maurer A.-F., Galer S. J. G., Knipper C., Beierlein L., Nunn E. V., Peters D., Tütken T., Alt K. W., Schöne B. R., Bioavailable 87Sr/86Sr in different environmental samples—Effects of anthropogenic contamination and implications for isoscapes in past migration studies. Sci. Total Environ. 233, 216–229 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Evans J. A., Montgomery J., Wildman G., Bouton N., Spatial variations in biosphere 87Sr/86Sr in Britain. J. Geol. Soc. London 167, 1–4 (2010). [Google Scholar]
- 12.Grimstead D. N., Nugent S., Whipple J., Why a standardization of strontium isotope baseline environmental data is needed and recommendations for methodology. Advan. Archaeol. Practice 5, 184–195 (2017). [Google Scholar]
- 13.Willmes M., Bataille C. P., James H. F., Moffat I., McMorrow L., Kinsley L., Armstrong R. A., Eggins S., Grün R., Mapping of bioavailable strontium isotope ratios in France for archaeological provenance studies. Appl. Geochem. 90, 75–86 (2018). [Google Scholar]
- 14.Bataille C. P., von Holstein I. C. C., Laffoon J. E., Willmes M., Liu X.-M., Davies G. R., A bioavailable strontium isoscape for Western Europe: A machine learning approach. PLOS ONE (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frei K. M., Frei R., The geographic distribution of strontium isotopes in Danish surface waters – A base for provenance studies in archaeology, hydrology and agriculture. Appl. Geochem. 26, 326–340 (2011). [Google Scholar]
- 16.Frei K. M., Price T. D., Strontium isotopes and human mobility in prehistoric Denmark. Archaeol. Anthropol. Sci. 4, 103–114 (2012). [Google Scholar]
- 17.Kjær K. H., Houmark-Nielsen M., Richardt N., Ice-flow patterns and dispersal of erratics at the southwestern margin of the last Scandinavian ice sheet: Signature of palaeo-ice streams. Boreas 32, 130–148 (2003). [Google Scholar]
- 18.H. B. Madsen, A. H. Nørr, K. A. Holst, Atlas over Danmark (C. A. Reitzel Publisher, 1992). [Google Scholar]
- 19.Balstrøm T., Breuning-Madsen H., Krüger J., Jensen N. H., Greve M. H., A statistically based mapping of the influence of geology and land use on soil pH: A case study from Denmark. Geoderma 192, 453–462 (2013). [Google Scholar]
- 20.Mandiki S. N. M., Gillardin V., Martens K., Ercken D., De Roeck E., De Bie T., Declerck S. A. S., De Meester L., Brasseur C., Van der Heiden E., Schippo M.-L., Kestemont P., Effect of land use on pollution status and risk of fish endocrine disruption in small farmland ponds. Hydrobiologia 723, 103–120 (2014). [Google Scholar]
- 21.Declerck S., De Bie T., Ercken D., Hampel H., Schrijvers S., Wichelen J., Gillard V., Mandiki R., Losson B., Bauwens D., Keijers S., Vyverman W., Goddeeris B., De Meester L., Brendonck L., Martens K., Ecological characteristics of small farmland ponds: Associations with land use practices at multiple spatial scales. Biol. Conserv. 131, 523–532 (2006). [Google Scholar]
- 22.Zieliński M., Dopieralska J., Belka Z., Walczak A., Siepak M., Jakubowicz M., Sr isotope tracing of multiple water sources in a complex river system, Noteć River, central Poland. Sci. Total Environ. 548–549, 307–316 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Åberg G., The use of natural strontium isotopes as tracers in environmental studies. Water Air Soil Pollut. 79, 309–322 (1995). [Google Scholar]
- 24.Karup Å undersøgelsen. Miljø-projekter 51 (Miljøstyrelsen, 1993). [Google Scholar]
- 25.McArthur J. M., Thirlwall M. F., Engkilde M., Zinsmeister W. J., Howarth R. J., Strontium isotope profiles across K/T boundary sequences in Denmark and Antarctica. Earth Planet. Sci. Lett. 160, 179–192 (1998). [Google Scholar]
- 26.Négrel P., Deschamps P., Natural and anthropogenic budgets of a small watershed in the Massif Central (France): Chemical and strontium isotopic characterization of water and sediments. Aquat. Geochem. 2, l–27 (1996). [Google Scholar]
- 27.Böhlke J. K., Horan M., Strontium isotope geochemistry of groundwaters and streams affected by agriculture, Locust Grove, MD. Appl. Geochem. 15, 599–609 (2000). [Google Scholar]
- 28.Drouet T., Herbauts J., Demaiffe D., Long-term records of strontium isotopic composition in tree rings suggest changes in forest calcium sources in the early 20th century. Glob. Chang. Biol. 11, 1926–1940 (2005). [Google Scholar]
- 29.Hosono T., Nakano T., Igeta A., Tayasu I., Tanaka T., Yachi S., Impact of fertilizer on a small watershed of Lake Biwa: Use of sulfur and strontium isotopes in environmental diagnosis. Sci. Total Environ. 384, 342–354 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Kume T., Akca E., Nakano T., Nagano T., Kapur S., Watanabe T., Seasonal changes of fertilizer impacts on agricultural drainage in a salinized area in Adana, Turkey. Sci. Total Environ. 408, 3319–3326 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Shin W.-J., Ryu J.-S., Lee K.-S., Park Y., Identification of anthropogenic contaminant sources in urbanized streams using multiple isotopes. Environ. Earth Sci. 73, 8311–8324 (2015). [Google Scholar]
- 32.F. Adams, Soil Acidity and Liming, 2nd edition, Agronomy 12 (American Society of Agronomy/Crop Science Society of America/Soil Science Society of America, 1984). [Google Scholar]
- 33.J. Ehlers, Glacial Deposits in North-West Europe (A. A. Balkema, 1983). [Google Scholar]
- 34.Böse M., Lüthgens C., Lee J. R., Rose J., Quaternary glaciations of northern Europe. Quat. Sci. Rev. 44, 1–25 (2012). [Google Scholar]
- 35.Ballabio C., Panagos P., Monatanarella L., Mapping topsoil physical properties at European scale using the LUCAS database. Geoderma 26, 110–123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A. R. Gillespie, S. C. Porter, B. F. Atwater, in The Quaternary Period in the United States, Vol. 1 (Elsevier Science, 2003). [Google Scholar]
- 37.D. S. Fullerton, C. A. Bush, J. N. Pennell, Map of surficial deposits and materials in the eastern and central United States (East of 102° West Longitude). U.S. Geological Survey Geologic Investigation Series I-2789 (2004).
- 38.A. E. Kehew, B. B. Curry, Eds., Quaternary glaciation of the Great Lakes region: Process, landforms, sediments, and chronology. Geol. Soc. Am. Spec. Paper 530 (2018).
- 39.Goulding K. W. T., McGrath S. P., Johnston A. E., Predicting the lime requirement of soils under permanent grassland and arable crops. Soil Use Manag. 5, 54–58 (1989). [Google Scholar]
- 40.N. P. Anderson, J. M. Hart, D. M. Sullivan, N. W. Christensen, D. A. Horneck, G. J. Pirelli, in Applying Lime to Raise Soil pH for Crop Production (Western Oregon). EM9057. Oregon State, University Extension Service, 1–21 (2013).
- 41.Goulding K. W. T., Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 32, 390–399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price T. D., Meiggs D., Weber M.-J., Pike-Tay A., The migration of Late Pleistocene reindeer: Isotopic evidence from northern Europe. Archaeol. Anthropol. Sci. 9, 371–394 (2017). [Google Scholar]
- 43.Revideret Natura 2000-basisanalyse 2015–2021. Egtved Ådal Natura 2000-område nr. 238 Habitatområde H238 (Miljøministeriet, Naturstyrelsen, 2014). [Google Scholar]
- 44.Greve M. H., Greve M. B., Bøcher P. K., Balstrøm T., Breuning-Madsen H., Krogh L., Generating a Danish raster-based topsoil property map combining choropleth maps and point information. Geografisk Tidsskrift 107, 1–12 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav8083/DC1
Table S1. Strontium isotope and concentration data for surface water, groundwater, rainwater, and reference samples.
Table S2. Basic statistical data on strontium isotopic composition (87Sr/86Sr) and strontium concentration of pristine and farmland samples.
Table S3. Strontium isotope and concentration data for agricultural lime products, fertilizers, and animal feed.
Table S4. Calculated strontium isotope composition and concentration for two farms in the Vallerbæk area.
Fig. S1. Calcium carbonate distribution in glaciogenic deposits, middle Jutland.
Fig. S2. Simplified maps and description of localities.
Fig. S3. Influence of soil thickness and soil composition on the level of disturbance of the 87Sr/86Sr ratio from agricultural lime.
Fig. S4. Comparison of strontium concentrations measured by quadrupole ICP-MS and multicollector ICP-MS.
Reference (44)


