Abstract
Background:
Significant blood loss is still one of the most frequent complications in spinal surgery, which often necessitates blood transfusion. Massive perioperative blood loss and blood transfusion can create additional risks. Aprotinin, tranexamic acid (TXA), and epsilon-aminocaproic acid (EACA) are antifibrinolytics currently offered as prophylactic agents to reduce surgery-associated blood loss. The aim of this study was to evaluate the efficacy and safety of aprotinin, EACA, and low/high doses of TXA in spinal surgery, and assess the use of which agent is the most optimal intervention using the network meta-analysis (NMA) method.
Methods:
Five electronic databases were searched, including PubMed, Cochrane Library, ScienceDirect, Embase, and Web of Science, from the inception to March 1, 2018. Trials that were randomized and compared results between TXA, EACA, and placebo were identified. The NMA was conducted with software R 3.3.2 and STATA 14.0.
Results:
Thirty randomized controlled trial (RCT) studies were analyzed. Aprotinin (standardized mean difference [SMD]=−0.65, 95% credibility intervals [CrI;−1.25, −0.06]), low-dose TXA (SMD = −0.58, 95% CrI [−0.92, −0.25]), and high-dose TXA (SMD = −0.70, 95% CrI [−1.04, −0.36]) were more effective than the respective placebos in reducing intraoperative blood loss. Low-dose TXA (SMD = −1.90, 95% CrI [−3.32, −0.48]) and high-dose TXA (SMD = −2.31, 95% CrI [−3.75, −0.87]) had less postoperative blood loss. Low-dose TXA (SMD = −1.07, 95% CrI [−1.82, −0.31]) and high-dose TXA (SMD = −1.07, 95% CrI [−1.82, −0.31]) significantly reduced total blood loss. However, only high-dose TXA (SMD = −2.07, 95% CrI [−3.26, −0.87]) was more effective in reducing the amount of transfusion, and was significantly superior to low-dose TXA in this regard (SMD = −1.67, 95% CrI [−3.20, −0.13]). Furthermore, aprotinin (odds ratio [OR] = 0.16, 95% CrI [0.05, 0.54]), EACA (OR = 0.46, 95% CrI [0.22, 0.97]) and high dose of TXA (OR = 0.34, 95% CrI [0.19, 0.58]) had a significant reduction in transfusion rates. Antifibrinolytics did not show a significantly increased risk of postoperative thrombosis. Results of ranking probabilities indicated that high-dose TXA had the greatest efficacy and a relatively high safety level.
Conclusions:
The antifibrinolytic agents are able to reduce perioperative blood loss and transfusion requirement during spine surgery. And the high-dose TXA administration might be used as the optimal treatment to reduce blood loss and transfusion.
Keywords: Antifibrinolytics, Spine surgery, Blood loss, Transfusion, Network meta-analysis
Introduction
Significant blood loss is still one of the most frequent complications in spinal surgery and is especially common for complex spinal surgeries with prolonged operative times.[1] Massive intraoperative and postoperative blood loss may cause: anemia, organ damage, particularly cardiac, pulmonary, renal, and coagulopathy. Excessive blood loss inevitably requires aggressive blood transfusions, which increases the additional risk of transfusion-transmissible infections,[2] immunological transfusion reactions, and mis-transfusion,[3] in addition to increasing long-term mortality rates. Furthermore, the direct costs of the blood products and intraoperative blood salvage technology and indirect costs of prolonged patient hospitalization and complication management associated with major blood loss escalate the burden of health-care costs.[4] Therefore, the control of perioperative bleeding of spine surgery is an important issue. The efficacy of multidisciplinary approaches to blood conservation in spinal surgery has been shown.[1,5]
In spine surgery, increased fibrinolysis has been implicated as a contributing factor in excessive blood loss.[6] Antifibrinolytics, such as tranexamic acid (TXA), epsilon-aminocaproic acid (EACA), and aprotinin, through the inhibition of clot degradation to decrease blood loss and transfusion requirements, have generally gained popularity to use in spine surgery since the 1990s.[7] There is a considerable difference between the mechanism of action of TXA, EACA, and the serine protease inhibitor aprotinin. EACA and TXA are both small molecules with similar mechanisms that saturate the lysine binding sites of plasminogen inhibiting plasminogen and plasmin from binding to the surface of fibrin to suppress fibrinolysis.[6] While aprotinin inhibits serine protease to inactivate free plasmin, aprotinin has little effect on bound plasmin.[8] There have been numerous studies and meta-analyses on the use of antifibrinolytics to reduce the blood loss and transfusion in spinal surgery, however, the results are in conflict with each other.[7,9,10]
Although plenty of research studies have compared these antifibrinolytic agents in spinal surgeries, there is no study comparing all of the above approaches with direct and indirect evidence. By using a network meta-analysis (NMA), we gathered current information on 3 drugs together, and then made comparisons with the direct and indirect evidence. NMA utility and value have been acknowledged widely. The aim of this study was to evaluate the efficacy and safety of aprotinin, EACA, low and high dose of TXA in spinal surgery, and assess which agents is the most optimal intervention by applying the NMA method.
Methods
Search strategy
Electronic literature searches, both manual and computer assisted, were conducted using PubMed, Embase, Cochrane library database, ScienceDirect, and Web of Science from the date of inception to March 2018. Search terms used were: “antifibrinolytics,” “antifibrinolytic agents,” “anti-fibrinolytic,” “aprotinin,” “tranexamic acid,” “epsilon-aminocaproic acid,” “spine surgery,” “spinal surgery,” “spine,” “lumbar surgery,” “thoracic surgery,” “cervical surgery,” “randomized controlled trials,” “controlled clinical trial,” “randomized,” “controlled trial,” “random,” “placebo groups.” The literature search was refined to randomized controlled trials (RCTs) in spine surgery. Reference lists in studies, reviews, and previous meta-analyses were checked to identify any initially omitted studies. Two investigators independently reviewed the title, abstract, and the full text of all articles. The recommended PRISMA statement and guidelines were followed for the present systematic review and meta-analysis[11] and the network meta-analysis was registered in PROSPERO (CRD42018094389).
Study eligibility criteria and exclusion criteria
Studies were included if they met the following criteria: (1) RCTs; (2) patients underwent cervical, thoracic, or lumbar spinal surgeries irrespective of anterior or posterior approach; (3) aprotinin, tranexamic acid, or epsilon-aminocaproic acid was compared with placebo and/or each other; (4) studies evaluated the efficacy or safety of antifibrinolytic agents using at least one of the following endpoints: (a) amounts of blood losses (intraoperative, postoperative, or total), (b) transfusion requirements, (c) blood transfusion rate, (d) operative time, (e) postoperative hemoglobin (Hb) or hematocrit (Hct) value, or (f) number of postoperative thrombosis events. The following criteria were used for data exclusion: (1) retrospectively designed trials or trials of low quality; (2) letters, case reports, comments, meta-analyses, reviews, and meeting abstracts; (3) data were unavailable on odds ratios (OR) or standardized mean difference (SMD).
Assessment of risk of bias
According to the Cochrane Handbook for Systematic Reviews of Interventions,[12] 2 independent reviewers assessed methodological quality. The following criterions were evaluated: random sequence generation, allocation concealment, blind experimental design of participants and personnel, blind outcome assessment, incomplete outcome data, selective reporting, and other biases. Based on the report and appropriateness of methods, the included studies were graded accordingly: (1) low risk (methods were indicated and proper), (2) moderate risk (methods were not indicated), and (3) high risk (methods were indicated, but improper). Disagreements on risk of bias ratings were regularly resolved through discussion by the 2 reviewers or consultation with a third team member.
Data extraction
We compared the effects of aprotinin, epsilon-aminocaproic acid (EACA), low or high dose of TXA on blood losses, blood transfusion volume and rate, operative durations, postoperative Hb and Hct value, and the incidence of thromboembolism. According to Hui et al,[13] low dose of TXA (TXA1 group) was defined as bolus dose of no more than 10 mg/kg, followed by maintenance dose of no more than 10 mg·kg−1·h−1. High dose (TXA2 group) was defined as bolus dose of 10 to 100 mg/kg, followed by a maintenance dose of greater than 10 mg·kg−1·h−1. Two investigators independently performed the data extraction. The data extracted included both study characteristics and measuring outcomes from the included studies. Study characteristics included the first author's name, year of publication, sample size, intervention, dose, transfusion indication, and surgical procedure. Intraoperative blood loss, postoperative blood loss, total blood loss, blood transfusion volume, transfusion rate, postoperative hemoglobin (Hb) and hematocrit (Hct) values were recorded as the measured outcomes of effectiveness, while the incidence of thromboembolism was extracted as the measured outcomes of safety. When data were incomplete or unclear, attempts were made to contact the investigators for clarification.
Statistical analysis
Traditional pairwise meta-analysis was performed first, with SMD or odds ratios (ORs) and 95% confidence intervals (CI) of the endpoints as effect sizes. The fixed-effects model (Mantel-Haenszel method) was used for cases with no significant heterogeneity (P > 0.1 and I2 < 50%); otherwise, the random-effects model was used. We used network meta-analysis methods to compare different incorporating evidence on both direct and indirect comparisons. Network meta-analysis was performed using STATA version 14.0 (Stata Corp LP, College Station, TX, USA) to calculate SMDs or ORs with 95% credibility intervals (CrI) and generate forest plots using Bayesian models compared the effect estimates of different therapies relative to comparator. STATA command metan was used to obtain the forest plot, and command netfunnel and networkplot were used to obtain the network funnel plots and the network plots. Rank probabilities were generated to determine the rank of therapies in which the given treatment ranked first as the most effective therapy, second, and so on.
Heterogeneity within pairwise comparisons was assessed quantitatively using Mantel-Haenszel χ2 based test and I2 statistic, with values over 50% indicating substantial heterogeneity.[14] Loop inconsistency, that is, the difference between direct and indirect estimates for 3 treatments within a loop, was evaluated by the inconsistency factor (IF) for the loop. Within each loop, the IF value was defined as IF = Edirect − Eindirect (E: estimate). We also derived a 95% confidence interval (CI) and a Z-test for IF. We rejected the null hypothesis that the evidence is consistent (H0: IF = 0), when P > 0.05 or IF was significantly greater or smaller than 0.[15] Funnel plot analysis was performed to assess small study effects or publication bias of pairwise estimates. All tests were 2-tailed. P < 0.05 was considered to indicate a statistically significant difference.
Results
Description of study and the risks of bias assessment
The initial database search yielded 602 citations (PubMed = 61, Embase = 273, Cochrane library = 56, ScienceDirect = 38, Web of Science = 174) and 41 citations from additional sources. Following elimination of duplicates, a total of 466 records were screened with 379 of the studies then being excluded by screening their titles and abstracts. The full manuscripts were obtained and analyzed, and we ultimately selected 30 RCT studies[16–45] with 2087 patients for the analysis using the pre-defined inclusion and exclusion criteria. Of the 30 RCTs, 26 were 2-arm controlled trials comparing active intervention (4 RCTs of aprotinin,[40–42,45] 3 of EACA,[34,39,44] 9 of TXA1,[16,18–20,22,23,32,35,37] 10 of TXA2[17,21,24,25,29–31,33,36,38]) to placebo; 1 was a 2-arm trial comparing EACA to TXA2,[28] 3 were 3-arm trials (2 RCTs of TXA1 vs. EACA vs. placebo,[26,27,43] 1 was EACA vs. aprotinin vs. placebo[43]). Figure 1 shows the flowchart for study selection process. The characteristics of included studies are presented in Table 1 .
Figure 1.
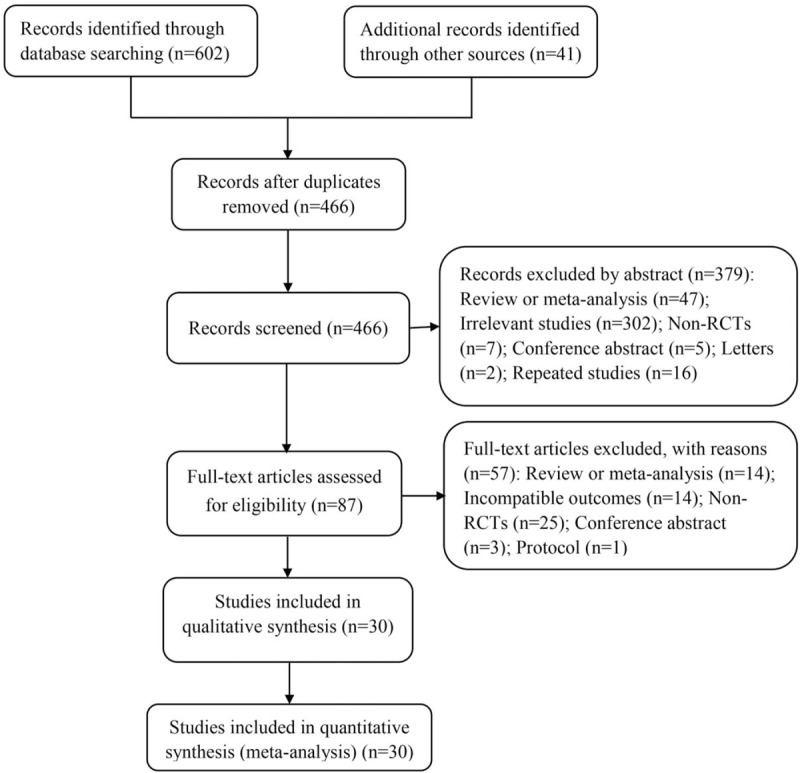
Flow diagram for study selection process in this network meta-analysis. RCTs: randomized controlled trials.
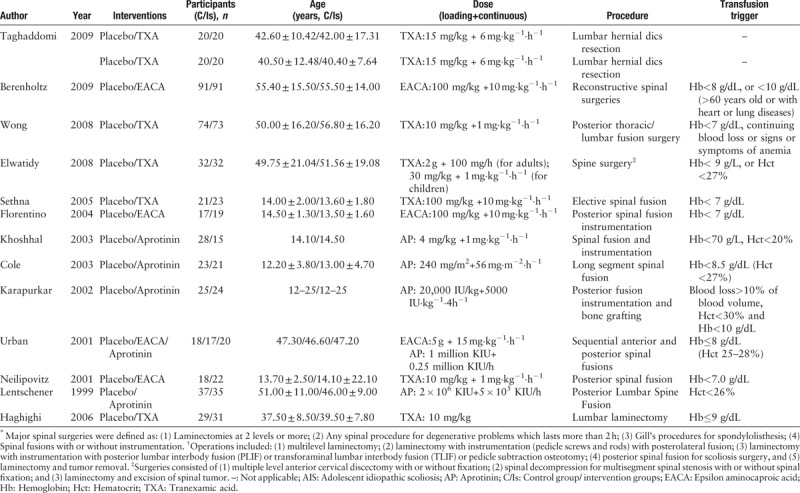
Table 1.
Characteristics of the included studies.
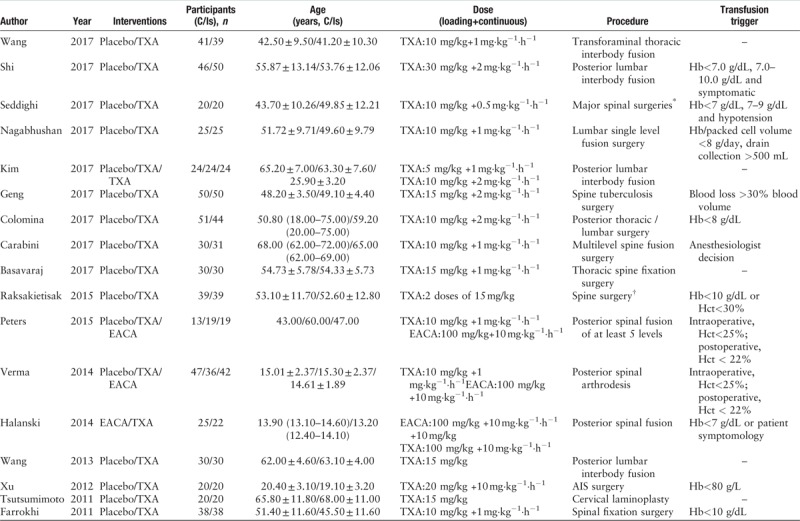
Table 1 (Continued).
Characteristics of the included studies.
Quality assessment was performed according to the Cochrane Collaboration tool for assessing risk of bias; overall, the studies were placed at low-moderate risk of bias. Nineteen of 30 included studies had adequate randomization, 2 of the trials were randomized by medical record number, one used odd and even numbers, and the remaining trials did not specifically describe the method of sequence generation. Nonetheless, there are only 13 articles reported the allocation concealment. However, more than half of qualified research studies used a blind experimental set-up. Attrition bias and reporting bias were performed in the included trials. Furthermore, the overall and study-level quality assessments were summarized [Figure 2A and 2B], as well as were a network plot of all outcomes [Figure 3]. Node sizes and edge widths in the network plot indicate the number of interventions being compared and the available direct comparisons between pairs of interventions, respectively.
Figure 2.
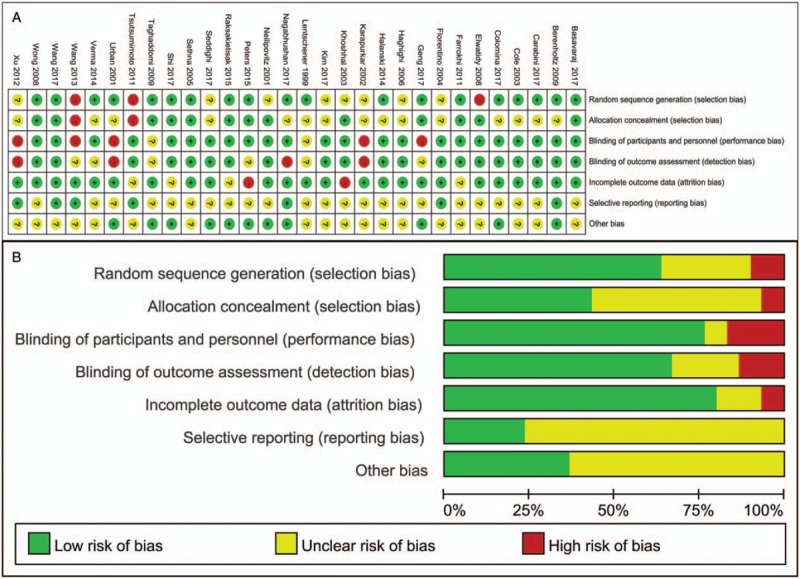
Risk of bias graph and summary of the included studies. (A) Reviewers’ judgments about each risk of bias item for eligible studies. (B) The judgments about each risk of bias item presented as percentages across all eligible studies. Green, low risk of bias; Red, high risk of bias; Yellow, unclear of risk of bias.
Figure 3.
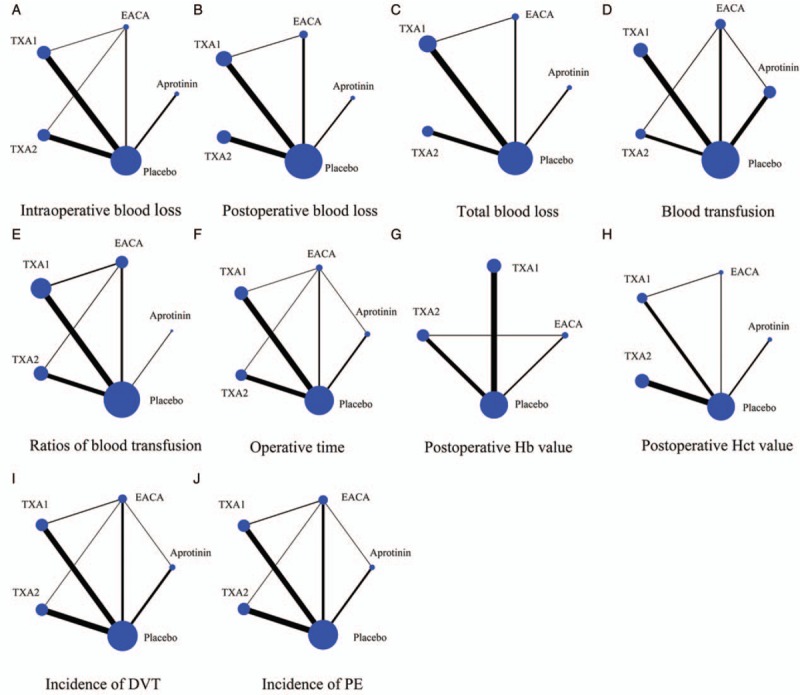
Network map of the clinical efficacy and safety of aprotinin, EACA, TXA1, and TXA2. (A) Intraoperative blood loss, (B) postoperative blood loss, (C) total blood loss, (D) blood transfusion, (E) ratios of blood transfusion, (F) operative time, (G) postoperative Hb value, (H) postoperative Hct value, (I) incidence of DVT, (J) incidence of PE. Node size and line width are based on the number of intervention studies included in the meta-analysis. Larger nodes and thicker lines indicate a higher frequency of intervention with the indicated drug. DVT: Deep vein thrombosis; EACA: Epsilon-aminocaproic acid; Hb: Hemoglobin; Hct: Hematocrit; PE: Pulmonary embolism; TXA1: Low dose of tranexamic acid; TXA2: High dose of tranexamic acid.
Network meta-analysis of the efficacy and safety of antifibrinolytic agents
Intraoperative blood loss
Twenty-eight studies (n = 2014 patients) were included in the NMA for the intraoperative blood loss [Figure 3A]. Pooled results of the NMA indicated that aprotinin (SMD = −0.65, 95% CrI [−1.25, −0.06]), TXA1 (SMD = −0.58, 95% CrI [−0.92, −0.25]), and TXA2 (SMD = −0.70, 95% CrI [−1.04, −0.36]) were more effective than the placebo in reducing intraoperative blood loss, while, EACA did not have a significant effect (SMD = −0.44, 95% CrI [−1.00, 0.12]). There were no significant differences when comparing aprotinin, EACA, TXA1, and TXA2 with each other for decreasing intraoperative blood loss [Figure 4A]. Furthermore, probabilities of rank plot were as follows: TXA2, TXA1, EACA, aprotinin, and placebo ranked 1 to 5, respectively, with rank 1 as the best and rank 5 as the worst [Supplementary Figure 1A and Table 1].
Figure 4.
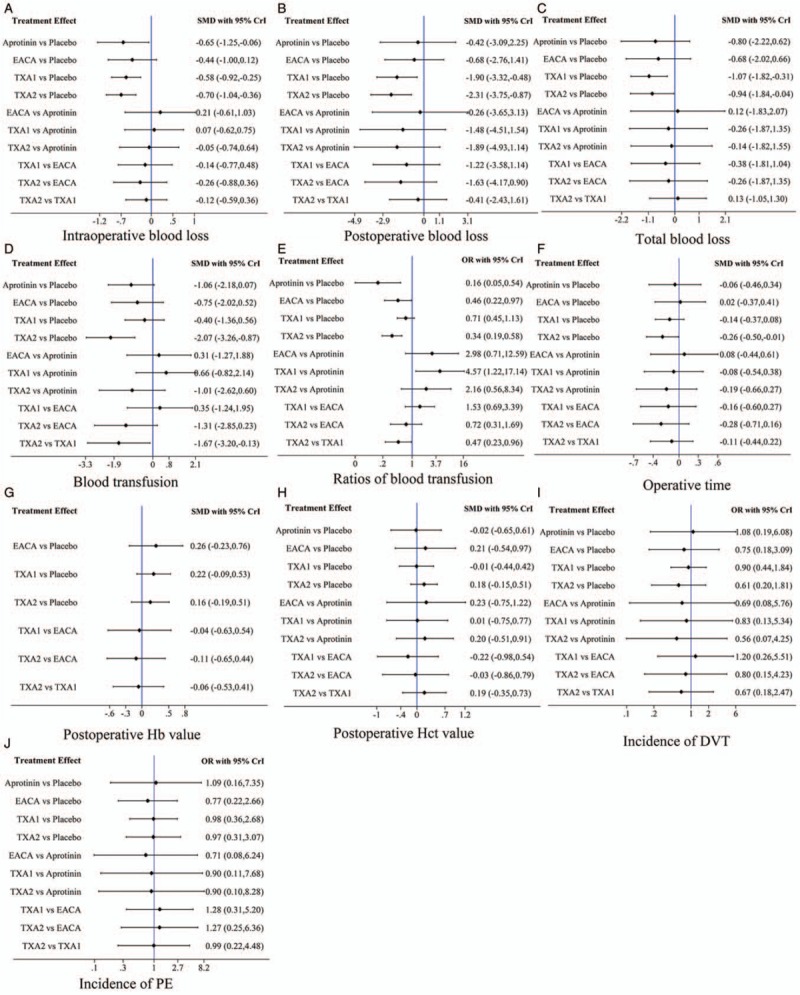
Network of the comparisons for the Bayesian network meta-analysis. (A) Intraoperative blood loss, (B) postoperative blood loss, (C) total blood loss, (D) blood transfusion, (E) ratios of blood transfusion, (F) operative time, (G) postoperative Hb value, (H) postoperative Hct value, (I) incidence of DVT, (J) incidence of PE. CrI: Credibility intervals; DVT: Deep vein thrombosis; EACA: Epsilon-aminocaproic acid; Hb: Hemoglobin; Hct: Hematocrit; OR: Odds ratio; PE: Pulmonary embolism; SMD: Standardized mean difference; TXA1: Low dose of tranexamic acid; TXA2: High dose of tranexamic acid.
Postoperative blood loss
A total of 17 studies (n = 1375 patients) provided data for postoperative blood loss outcomes [Figure 3B]. TXA1 had less postoperative blood loss than that of the placebo (SMD = −1.90, 95% CrI [−3.32, −0.48]), and TXA 2 had the same effect as the TXA1 (SMD = −2.31, 95% CrI [−3.75, −0.87]). Aprotinin (SMD = −0.42, 95% CrI [−3.09, 2.25]) and EACA (SMD = −0.68, 95% CrI [−2.76, 1.41]) did not significantly reduce the postoperative bleeding compared with the placebo. Furthermore, there was no significant difference when comparing the 4 groups with each other [Figure 4B]. In the rank-probability test, TXA1 had the highest rank for reducing postoperative blood loss, followed by TXA2, EACA, placebo, and aprotinin ranked 2 to 5, respectively [Supplementary Figure 1B and Table 1].
Total blood loss
In terms of total blood loss, 1078 patients in 14 studies were included in the NMA [Figure 3C], with pooled results, indicating that TXA1 group (SMD = −1.07, 95% CrI [−1.82, −0.31]) and TXA2 group (SMD = −1.07, 95% CrI [−1.82, −0.31]) were significantly better than placebo groups. However, the aprotinin group (SMD = −0.80, 95% CrI [−2.22, 0.62]) and EACA group (SMD = −0.68, 95% CrI [−2.02, 0.66]) were not significantly different from the placebo group in reducing the total blood loss. Furthermore, no difference in total blood loss was observed between antifibrinolytics using TXA1, TXA2, EACA, and aprotinin [Figure 4C]. TXA1 was observed to reduce most total blood loss, followed by TXA2, EACA, placebo, and aprotinin, ranks 2 to 5, respectively [Supplementary Figure 1C and Table 1].
Blood transfusion
Blood transfusion requirement was available in 20 studies including 1267 patients [Figure 3D]. Only the high dose TXA (TXA2) was more effective than placebo in reducing the amount of blood transfusion (SMD = −2.07, 95% CrI [−3.26, −0.87]) and it was significantly superior to TXA1 (SMD = −1.67, 95% CrI [−3.20, −0.13]). Pooled results did not reveal a significant reduction in blood transfusion by aprotinin and EACA administration compared with placebo. Furthermore, compared with aprotinin, the other 3 antifibrinolytics had no difference in reducing the amount of transfusion. In addition, there was no difference between TXA and EACA [Figure 4D]. Probabilities of rank plot were: TXA2, aprotinin, EACA, TXA1, placebo ranked 1 to 5, respectively [Supplementary Figure 1D and Table 1].
Ratios of blood transfusion
The ratios of blood transfusion were reported in 17 studies (n = 1264) [Figure 3E]. Pooled results indicated that aprotinin (odds ratio [OR] = 0.16, 95% CrI [0.05, 0.54]), EACA (OR = 0.46, 95% CrI [0.22, 0.97]), and high dose of TXA (TXA2) (OR = 0.34, 95% CrI [0.19, 0.58]) had an insignificant reduction in the proportion of patients who need transfusion. Furthermore, TXA2 decreased the ratio of blood transfusion more than TXA1 (OR = 0.47, 95% CrI [0.23, 0.96]), while TXA1 significantly increased transfusion ratio than aprotinin and was no significant difference with placebo. Comparison of EACA and TXA2 with aprotinin, and TXA1 and TXA2 with EACA showed no significant differences [Figure 4E]. Probabilities of rank plot were as follows: aprotinin, TXA2, EACA, TXA1, and placebo ranked 1 to 5, respectively [Supplementary Figure 1E and Table 1].
Operative time
Data on duration of surgery were available in 27 studies with a total of 1735 patients [Figure 3F]. The operation duration from NMA and TXA2 administration appeared to have a statistically significant reduction in surgery duration compared with the placebo (SMD = −0.26, 95% CrI [−0.50, −0.01]). Whereas, the operation duration of other 3 administrations were not significantly different from placebo; there was no significant difference between all of 4 groups when compared with each other [Figure 4F]. TXA2 was ranked shortest in the rank probability test for operation duration, followed by TXA1, placebo, aprotinin, and EACA in order [Supplementary Figure 1F and Table 1].
Postoperative Hb and Hct value
Based on the data of fifteen studies (n = 1144 patients), with TXA1 included in 6 studies [Figure 3G], TXA2 in 6 studies, and EACA in 3 studies, the antifibrinolytics were not significantly different when compared with the placebo and each other for the value of Hb postoperatively [Figure 4G]. Probabilities of rank plot were as follows: EACA, TXA1, TXA2, and placebo ranked 1 to 4, respectively [Supplementary Figure 1E and Table 1). A total of 13 component studies (929 patients] was analyzed for the postoperative Hct value [Figure 3H], however, none of those antifibrinolytics had a statistically significant difference when compared with placebo and one another [Figure 4H]. Probabilities of rank plot were: EACA, TXA2, placebo, TXA1, and aprotinin ranked 1 to 5, respectively [Supplementary Figure 1E and Table 1].
Incidence of thromboembolic event
There were 29 studies (n = 2026 patients) and 27 studies (n = 1932 patients) that reported incidence of postoperative DVT and PE, respectively [Figure 3I and J]. This meta-analysis did not show a significantly increased risk of postoperative thrombosis event with antifibrinolytic agents administration [Figure 4I and 4J]. Probabilities of rank plot are detailed in Supplementary Figure 1I and 1J, and Table 1.
Consistency, convergence analysis, and publication bias
We found no significant inconsistency existed in this research, with the P value were lower than 0.05. The result of the consistency model was reliable. Moreover, all the potential scale reduction factor (PSRF) values of the different parameters were limited to 1 and it demonstrated that this research achieved good convergence efficiency. Funnel plots revealed that all included studies were symmetrical in terms of standard error of the effect size and the effect size centered at the comparison-specific pooled effect for all endpoints. This indicates that there was minimal publication bias.
Discussion
The past 2 decades had the steepest volume rise in total number of spinal surgeries[46,47] and for complex spinal surgeries.[48] Unfortunately, reconstructive, multilevel procedures, as well as single-level spinal surgery can be complicated by significant blood loss, which often necessitates blood transfusion.[19,23,34] Massive transfusions can result in metabolic abnormalities, electrolyte disturbances, clotting abnormalities, and hypothermia. Moreover, patients undergoing elective spinal surgery, even after they received a single blood transfusion, have been shown to have an increased length of hospital stay and higher postoperative morbidity.[49] Because blood transfusions carry their own costs and risks, it is important to control perioperative bleeding. In order to reduce blood loss and blood transfusion requirements, many methods have been applied, such as controlled hypotension, preoperative use of erythropoietin, varying surgical procedure, use of cell salvage, bedside coagulation management, substitution of coagulation factors, and the use of antifibrinolytic drugs.[1,27,40,50]
Aprotinin, TXA, and EACA are 3 commonly used antifibrinolytics. Aprotinin is a polypeptide serine protease inhibitor that inhibits kallikrein, plasmin, and platelet-activation factors. The drug was used primarily for the treatment of hyperfibrinolysis conditions such as acute pancreatitis. After Royston et al[51] reported aprotinin could reduce blood loss and the need for transfusion in cardiac surgery, the medical indications of aprotinin was expanded to use in cardiac, hepatic, and orthopedic surgeries to decrease bleeding and transfusion.[40–42,52] In an observational study involving 4374 patients undergoing revascularization, Mangano et al[53] reported that aprotinin was associated with an increased risk of renal failure, myocardial infarction, heart failure, stroke, encephalopathy, and mortality. Because of safety concerns, the United States Food and Drug Administration announced that the manufacturer of aprotinin had halted its production in November 2007. And now aprotinin is only allowed for myocardial revascularization,[8] so there are only 5 RCTs[40–43,45] included 115 patients about aprotinin in our analysis. EACA, a synthetic derivative of the amino acid lysine known by the brand name of Amicar, binds reversibly to the kringle domain of the enzyme plasminogen to block fibrin binding, which inhibits plasminogen activation and its transformation into plasmin. EACA also inhibits the proteolytic activity of plasmin and preserves the structural and functional integrity of the platelet receptor. It has been widely used in cardiac bypass procedures, hematologic disorders, abruptio placentae, hepatic cirrhosis, and neoplastic disease.[6] The action mechanism of TXA and EACA is similar, however, TXA is 7 to 10 times more potent than EACA and binds more strongly to the plasminogen molecule,[26] and higher doses of TXA have been used safely in numerous studies.[17,38] Based on previous studies and reviews, those antifibrinolytics have been demonstrated to be effective for reducing blood loss and transfusions in spinal surgery. However, among the antifibrinolytics, the best strategy for application in spine surgery is still unclear. Thus, it is necessary to make a comprehensive comparison among different antifibrinolytics to improve our understanding of their various therapeutic effects and safeties.
To the best of our knowledge, this research was the first comprehensive comparison of those 3 antifibrinolytics. In our NMA, a total of 30 high-quality RCTs including 2087 patients met the inclusion criteria. Both direct and indirect comparisons were carried out to evaluate antifibrinolytics in spine surgery. We aimed to find out the best intervention that could balance both efficacy and safety. As for efficacy, we evaluate these drugs by 8 points, including intraoperative blood loss, post-operative blood loss, total blood loss, blood transfusion, ratios of blood transfusion, operative time, postoperative Hb value, and postoperative Hct value. Furthermore, the incidence of DVT and PE was used to assess the safety. From the result of NMA, we concluded that: (1) TXA, both in low and high dose, had a significant efficacy and ranked top 2 in reducing blood loss in spine surgery, for intraoperative and postoperative as well as for total blood loss. Whereas, aprotinin could only significantly reduce intraoperative blood loss. As for EACA, compared with the placebo group, there was no significant difference in reducing the perioperative blood loss. Meanwhile, no obvious difference was observed between aprotinin, EACA, TXA1, and TXA2 [Figure 4]. (2) With respect to transfusion, the high dose of TXA, ranking first in reducing blood transfusion requirement, was significantly more effective than the placebo and low dose of TXA. However, aprotinin, EACA, and low dose of TXA had no obvious differences when compared with the placebo and each other. Moreover, aprotinin, EACA, and high dose of TXA were associated with a significantly lower ratio of transfusion comparing with placebo. Aprotinin ranked best at reducing the transfusion need, followed by high dose of TXA, with both were superior to low dose of TXA. (3) A statistically significant reduction in surgery duration was only observed in high TXA dose administration compared with placebo. The results revealed that there was no obvious difference in postoperative Hb and Hct value between antifibrinolytics and placebo. (4) This NMA did not show a significantly increased risk of a postoperative thrombosis event with antifibrinolytics administration.
Although we demonstrated that high-dose TXA was the best administration for controlling blood loss and transfusion in spine surgery, the results still needed to be discussed. TXA plays an antifibrinolytic role by acting as a reversible blockade of lysine-binding sites of plasminogen molecules. However, data on a TXA concentration-effect relationship are rather rare. A pharmacodynamic study showed the need for target TXA plasma concentrations was greater than 10 μg/mL. While in a cardiac study, high dose (30 mg/kg bolus followed by 16 mg·kg–1·h–1 infusion) was more effective than a low dose (10 mg/kg bolus followed by 1 mg·kg–1·h–1 infusion) to decrease transfusion needs, blood loss.[54] There were no dose-response studies available in spinal surgery, and a relationship between TXA doses in controlling blood loss was not well established. However, previous studies have suggested that high doses of TXA were more effective than smaller doses,[13] and the mechanism still needs further research for molecular evidence. Based on our network results and ranking results, we recommended high-dose TXA as the optimal administration that had the best efficacy and safety. For efficacy, high-dose TXA could effectively reduce perioperative blood loss, transfusion, and operative time, and ranked the best in terms of intraoperative, postoperative blood loss, transfusion requirement, and operative time. Low-dose TXA could also reduce perioperative blood loss, but it only ranked first in total blood loss. Furthermore, low dose of TXA had no significant role in transfusion. Although our NMA and previous traditional meta-analyses indicated high dose TXA was more effective,[13,55] there is still no consensus on the optimal exact dosage of perioperative TXA administration. When it came to safety, high-dose TXA did not increase the incidence of DVT and PE of the patients. According to Wang et al[9], aprotinin could significantly reduce intraoperative, total blood loss and transfusion requirement and rate but had no effect on postoperative blood loss in scoliosis surgery, and EACA had less total blood loss and transfusion. However, Li et al[7] conducted a traditional meta-analysis concluding aprotinin and EACA could reduce postoperative blood loss, but these 2 drugs had on effect in reducing intraoperative, total blood loss, and transfusion requirement. In our NMA, we concluded that aprotinin could reduce intraoperative bleeding and both aprotinin and EACA had lower transfusion rates.
Overall, there were several strengths in our research: (1) comprehensive retrieval strategy was applied to reduce the risk of publication bias; (2) our study was the first comparison of direct and indirect approaches, which incorporated all available data to evaluate the interventions more precisely; (3) the docimastic probabilities of rank plot were utilized to distinguish the differences among all surgeries; (4) all of eligible RCTs had described random sequence generation; (5) operative time and postoperative Hb and Hct values were pooled as measuring outcomes. Nevertheless, our meta-analysis does have certain limitations which needed to be addressed. Firstly, the enrolled ages, diagnoses, surgical procedures, fusion levels, transfusion triggers, intraoperative mean arterial pressure (MAP), antifibrinolytics dosages, and subsequent infusion rate varied from one study to another, which may introduce considerable bias in the analyses. Secondly, the detailed blind methods and allocation concealment were not described in some RCTs and that may affect the validity of overall findings. Despite these limitations, the present NMA supports the efficacy of TXA in reducing blood loss and transfusion in spine surgery.
Conclusion
In conclusion, this NMA suggests that the high-dose TXA might be the optimal administration with high efficacy and safety when compared with low-dose TXA, EACA, aprotinin, and placebo in spinal surgeries, which significantly reduces the intraoperative, postoperative, perioperative blood losses, the blood transfusion requirement and rates, and the operative time. There is no evidence that use of antifibrinolytic agents is a risk factor for thromboembolism event in spine surgery. However, considering limitations of this NMA, efforts still should be paid to eliminate the heterogeneity and additional high-quality studies are needed to further evaluate the outcomes.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Yuan L, Zeng Y, Chen ZQ, Zhang XL, Mai S, Song P, Tao LY. Efficacy and safety of antifibrinolytic agents in spinal surgery: a network meta-analysis. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000108
References
- 1.Theusinger OM, Spahn DR. Perioperative blood conservation strategies for major spine surgery. Best Pract Res Clin Anaesthesiol 2016; 30:41–52. doi: 10.1016/j.bpa.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Janssen SJ, Braun Y, Wood KB, Cha TD, Schwab JH. Allogeneic blood transfusions and postoperative infections after lumbar spine surgery. Spine J 2015; 15:901–909. doi: 10.1016/j.spinee.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth 2005; 95:33–42. doi: 10.1093/bja/aeh290. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann A, Ozawa S, Farrugia A, Farmer SL, Shander A. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol 2013; 27:59–68. doi: 10.1016/j.bpa.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi R, Puvanesarajah V, Jain A, Hassanzadeh H. Perioperative management of blood loss in spine surgery. Clin Spine Surg 2017; 30:383–388. doi: 10.1097/BSD.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 6.Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg 2010; 18:132–138. doi: 10.5435/00124635-201003000-00002. [PubMed] [Google Scholar]
- 7.Li G, Sun TW, Luo G, Zhang C. Efficacy of antifibrinolytic agents on surgical bleeding and transfusion requirements in spine surgery: a meta-analysis. Eur Spine J 2017; 26:140–154. doi: 10.1007/s00586-016-4792-x. [DOI] [PubMed] [Google Scholar]
- 8.Royston D. The current place of aprotinin in the management of bleeding. Anaesthesia 2015; 70 suppl 1:46–49. e17. doi: 10.1111/anae.12907. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Zheng XF, Jiang LS. Efficacy and safety of antifibrinolytic agents in reducing perioperative blood loss and transfusion requirements in scoliosis surgery: a systematic review and meta-analysis. PLoS One 2015; 10:e0137886.doi: 10.1371/journal.pone.0137886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan C, Zhang H, He S. Efficacy and safety of using antifibrinolytic agents in spine surgery: a meta-analysis. PLoS One 2013; 8:e82063.doi: 10.1371/journal.pone.0082063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162:777–784. doi: 10.7326/m14-2385. [DOI] [PubMed] [Google Scholar]
- 12.Higgins PT, Green S, Altman GD. Chapter 8: Assessing risk of bias in included studies. In: Green S, Higgins JPT, eds. Handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011. Available from http://handbook.cochrane.org/ [Accessed on July 12, 2018]. [Google Scholar]
- 13.Hui S, Xu D, Ren Z, Chen X, Sheng L, Zhuang Q, et al. Can tranexamic acid conserve blood and save operative time in spinal surgeries? A meta-analysis. Spine J 2017; 18:1325–1337. doi: 10.1016/j.spinee.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwendicke F, Tu Y-K, Hsu L-Y, Göstemeyer G. Antibacterial effects of cavity lining: A systematic review and network meta-analysis. J Dent 2015; 43:1298–1307. doi: 10.1016/j.jdent.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Duan K, Ma M, Jiang Y, Liu T, Liu J, et al. Tranexamic acid decreases visible and hidden blood loss without affecting prethrombotic state molecular markers in transforaminal thoracic interbody fusion for treatment of thoracolumbar fracture-dislocation. Spine 2017; 43:E734–E739. doi: 10.1097/brs.0000000000002491. [DOI] [PubMed] [Google Scholar]
- 17.Shi H, Ou Y, Jiang D, Quan Z, Zhao Z, Zhu Y. Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: a randomized trial. Medicine 2017; 96:e5718.doi: 10.1097/MD.0000000000005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seddighi A, Nikouei A, Seddighi AS, Zali A, Tabatabaei SM, Yourdkhani F, et al. The role of tranexamic acid in prevention of hemorrhage in major spinal surgeries. Asian J Neurosurg 2017; 12:501–505. doi: 10.4103/1793-5482.165791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mn R, Shetty AP, Dumpa SR, Subarmaniam B, Kanna RM, Shanmuganathan R. Effectiveness and safety of batroxobin, tranexamic acid and a combination in reduction of blood loss in lumbar spinal fusion surgery. Spine 2017; 43:E267–E273. doi: 10.1097/BRS.0000000000002315. [DOI] [PubMed] [Google Scholar]
- 20.Kim KT, Kim CK, Kim YC, Juh HS, Kim HJ, Kim HS, et al. The effectiveness of low-dose and high-dose tranexamic acid in posterior lumbar interbody fusion: a double-blinded, placebo-controlled randomized study. Eur Spine J 2017; 26:2851–2857. doi: 10.1007/s00586-017-5230-4. [DOI] [PubMed] [Google Scholar]
- 21.Geng T, Chen Y, Zhang L. Safety and efficacy of tranexamic acid in the application of spinal tuberculosis surgery. Int J Clin Exp Med 2017; 10:3561–3567. [Google Scholar]
- 22.Colomina MJ, Koo M, Basora M, Pizones J, Mora L, Bago J. Intraoperative tranexamic acid use in major spine surgery in adults: a multicentre, randomized, placebo-controlled trialdagger. Br J Anaesth 2017; 118:380–390. doi: 10.1093/bja/aew434. [DOI] [PubMed] [Google Scholar]
- 23.Carabini LM, Moreland NC, Vealey RJ, Bebawy JF, Koski TR, Koht A, et al. A randomized controlled trial of low-dose tranexamic acid versus placebo to reduce red blood cell transfusion during complex multilevel spine fusion surgery. World Neurosurg 2017; 110:E572–E579. doi: 10.1016/j.wneu.2017.11.070. [DOI] [PubMed] [Google Scholar]
- 24.Basavaraj K, Hegde R. A randomized prospective study of efficacy of tranexamicacid on perioperative blood loss in thoracicspine fixation. Sri Lankan J Anaesthesiol 2017; 25:13–18. doi: 10.4038/slja.v25i1.8182. [Google Scholar]
- 25.Raksakietisak M, Sathitkarnmanee B, Srisaen P, Duangrat T, Chinachoti T, Rushatamukayanunt P, et al. Two doses of tranexamic acid reduce blood transfusion in complex spine surgery: a prospective randomized study. Spine 2015; 40:E1257–E1263. doi: 10.1097/BRS.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 26.Peters A, Verma K, Slobodyanyuk K, Cheriyan T, Hoelscher C, Schwab F, et al. Antifibrinolytics reduce blood loss in adult spinal deformity surgery: a prospective, randomized controlled trial. Spine 2015; 40:E443–E449. doi: 10.1097/BRS.0000000000000799. [DOI] [PubMed] [Google Scholar]
- 27.Verma K, Errico T, Diefenbach C, Hoelscher C, Peters A, Dryer J, et al. The relative efficacy of antifibrinolytics in adolescent idiopathic scoliosis: a prospective randomized trial. J Bone Joint Surg Am 2014; 96:e80.doi: 10.2106/jbjs.l.00008. [DOI] [PubMed] [Google Scholar]
- 28.Halanski MA, Cassidy JA, Hetzel S, Reischmann D, Hassan N. The efficacy of amicar versus tranexamic acid in pediatric spinal deformity surgery: A prospective, randomized, double-blinded pilot study. Spine Deform 2014; 2:191–197. doi: 10.1016/j.jspd.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Liu J, Fan R, Chen Y, Yu H, Bi Y, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J 2013; 22:2035–2038. doi: 10.1007/s00586-013-2836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C, Wu A, Yue Y. Which is more effective in adolescent idiopathic scoliosis surgery: batroxobin, tranexamic acid or a combination? Arch Orthop Trauma Surg 2012; 132:25–31. doi: 10.1007/s00402-011-1390-6. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine 2011; 36:1913–1918. doi: 10.1097/BRS.0b013e3181fb3a42. [DOI] [PubMed] [Google Scholar]
- 32.Farrokhi MR, Kazemi AP, Eftekharian HR, Akbari K. Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol 2011; 23:290–296. doi: 10.1097/ANA.0b013e31822914a1. [DOI] [PubMed] [Google Scholar]
- 33.Taghaddomi RJ, Mashhadiezhad H, Attar ARS, Peivandi A. The effect of intravenous tranexamic acid on blood loss in lumbar hernial disc resection under inhalation and total intravenous anesthesia. Iran Red Crescent Med J 2009; 11:265–270. [Google Scholar]
- 34.Berenholtz SM, Pham JC, Garrett-Mayer E, Atchison CW, Kostuik JP, Cohen DB, et al. Effect of epsilon aminocaproic acid on red-cell transfusion requirements in major spinal surgery. Spine 2009; 34:2096–2103. doi: 10.1097/BRS.0b013e3181b1fab2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, et al. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg 2008; 107:1479–1486. doi: 10.1213/ane.0b013e3181831e44. [DOI] [PubMed] [Google Scholar]
- 36.Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine 2008; 33:2577–2580. doi: 10.1097/BRS.0b013e318188b9c5. [DOI] [PubMed] [Google Scholar]
- 37.Haghighi M. The effect of tranexamic acid on bleeding during lumbar laminectomy. Iran Red Crescent Med J 2006; 8:46–50. [Google Scholar]
- 38.Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology 2005; 102:727–732. doi: 10.1097/00000542-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Florentino-Pineda I, Thompson GH, Poe-Kochert C, Huang RP, Haber LL, Blakemore LC. The effect of amicar on perioperative blood loss in idiopathic scoliosis: the results of a prospective, randomized double-blind study. Spine 2004; 29:233–238. doi:10.1097/01.BRS.0000109883.18015.B9. [DOI] [PubMed] [Google Scholar]
- 40.Khoshhal K, Mukhtar I, Clark P, Jarvis J, Letts M, Splinter W. Efficacy of aprotinin in reducing blood loss in spinal fusion for idiopathic scoliosis. J Pediatr Orthop 2003; 23:661–664. doi: 10.1097/01241398-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Cole JW, Murray DJ, Snider RJ, Bassett GS, Bridwell KH, Lenke LG. Aprotinin reduces blood loss during spinal surgery in children. Spine 2003; 28:2482–2485. doi: 10.1097/01.BRS.0000090835.45437.7F. [DOI] [PubMed] [Google Scholar]
- 42.Karapurkar A, Kudalkar A, Naik L. Aprotinin, To reduce perioperative blood loss in scoliosis surgery. Indian J Anaesth 2002; 46:378–1378. [Google Scholar]
- 43.Urban MK, Beckman J, Gordon M, Urquhart B, Boachie-Adjei O. The efficacy of antifibrinolytics in the reduction of blood loss during complex adult reconstructive spine surgery. Spine 2001; 26:1152–1156. doi: 10.1097/00007632-200105150-00012. [DOI] [PubMed] [Google Scholar]
- 44.Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg 2001; 93:82–87. doi: 10.1097/00000539-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Lentschener C, Cottin P, Bouaziz H, Mercier FJ, Wolf M, Aljabi Y, et al. Reduction of blood loss and transfusion requirement by aprotinin in posterior lumbar spine fusion. Anesth Analg 1999; 89:590–597. doi: 10.1213/00000539-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi K, Ando K, Nishida Y, Ishiguro N, Imagama S. Epidemiological trends in spine surgery over 10 years in a multicenter database. Eur Spine J 2018; 26:1698–1703. doi: 10.1007/s00586-018-5513-4. [DOI] [PubMed] [Google Scholar]
- 47.Hughey AB, Lesniak MS, Ansari SA, Roth S. What will anesthesiologists be anesthetizing? Trends in neurosurgical procedure usage. Anesth Analg 2010; 110:1686–1697. doi: 10.1213/ANE.0b013e3181cbd9cc. [DOI] [PubMed] [Google Scholar]
- 48.Cheng JS, Vohra KP, Wong CC, McGirt MJ. The future of the use of spine surgery. Neurosurgery 2013; 60 suppl 1:34–40. doi: 10.1227/01.neu.0000430316.94197.c6. [DOI] [PubMed] [Google Scholar]
- 49.Seicean A, Alan N, Seicean S, Neuhauser D, Weil RJ. The effect of blood transfusion on short-term, perioperative outcomes in elective spine surgery. J Clin Neurosci 2014; 21:1579–1585. doi: 10.1016/j.jocn.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine 2010; 35 (26 Suppl):S322–S330. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 51.Royston D, Bidstrup BP, Taylor KM, Sapsford RN. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet 1987; 2:1289–1291. doi: 10.1016/S0140-6736(87)91190-1. [DOI] [PubMed] [Google Scholar]
- 52.Port RJ, Molenaar IQ, Begliomini B, Groenland THN, Januszkiewicz A, Lindgren L, et al. Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double-blind study. The Lancet 2000; 355:1303–1309. doi: 10.1016/S0140-6736(00)02111-5. [DOI] [PubMed] [Google Scholar]
- 53.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med 2006; 354:353–365. doi: 10.1056/NEJMoa051379.doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 54.Sigaut S, Tremey B, Ouattara A, Couturier R, Taberlet C, Grassin-Delyle S, et al. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology 2014; 120:590–600. doi: 10.1097/ALN.0b013e3182a443e8. [DOI] [PubMed] [Google Scholar]
- 55.Yuan QM, Zhao ZH, Xu BS. Efficacy and safety of tranexamic acid in reducing blood loss in scoliosis surgery: a systematic review and meta-analysis. Eur Spine J 2017; 26:131–139. doi: 10.1007/s00586-016-4899-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


