Supplemental Digital Content is available in the text.
Keywords: chronic hepatitis C, long-term follow-up, sustained virological response
Abstract
Objectives
Curing of hepatitis C virus (HCV) infection primarily aims to prevent severe liver complications. Our objectives were to investigate the long-term presence and impact of occult HCV infection (OCI) and to study the outcomes in terms of liver disease after virological cure.
Patients and methods
A total of 97 patients with achieved sustained virological response (SVR) during 1990–2005 were followed either by a clinical follow-up (FU) visit with blood sampling and liver elastography (n=54) or through national registries for outcomes (n=43). To diagnose OCI among patients with SVR, a highly sensitive method was used to detect HCV-RNA traces in whole blood. The FU duration was a median of 10.5 years, with samples up to 21.5 years after the end of treatment (EOT).
Results
The majority of patients [52 (96%)] were HCV-RNA negative at FU, and regression of fibrosis was statistically significant. OCI was found in two (4%) of them at 8 and 9 years after EOT. These patients had F1 and F2 fibrosis before treatment and F2 at FU, but no other abnormal findings. Three previously noncirrhotic men were diagnosed with hepatocellular carcinoma 8–11 years after EOT.
Conclusion
Occult infection could be detected many years after the achievement of SVR but was not associated with serious liver disease. The majority had persistent viral eradication and regression of fibrosis after SVR. However, an increased risk of hepatocellular carcinoma may persist in the long term after SVR even in noncirrhotic patients. Further studies with FU after direct-acting antiviral therapy and on the long-term impact after cure are needed.
Introduction
Curing of hepatitis C virus (HCV) infection primarily aims to prevent severe liver complications, but also to avoid transmission of the virus and to increase the quality of life. The previous interferon (IFN)-based therapy led to the cure of ~50% of treated patients and treatment response, or sustained virological response (SVR), differed depending on the viral genotype and stage of liver fibrosis 1. During the last few years, combinations of new direct-acting antivirals (DAA) have improved SVR to more than 95% 2.
Occult HCV infection (OCI) in patients considered to be cured of HCV infection has been described as HCV-RNA negativity in serum, but detectable traces of HCV-RNA in the liver or in peripheral blood mononuclear cells (PBMCs) using highly sensitive PCR methods 3. Two types of OCI have been proposed: (i) either anti-HCV positive and HCV-RNA negative after resolved infection, or (ii) anti-HCV negative and HCV-RNA negative (cryptogenic HCV infection) 4. It has been suggested that the virus is not fully eradicated after achievement of SVR, and a major concern is whether occult HCV may cause liver disease in the long term or reactivate immunosuppression 5,6. The clinical significance of OCI is uncertain 4.
Because of the slow progression of hepatitis C, it has been difficult to report the preventive effect of SVR on long-term complications. Studies have reported short-term benefits with rapid normalization of liver enzymes, undetectable HCV-RNA in plasma and reduction of liver fibrosis 7–12. After SVR, noncirrhotic patients are usually discharged from further follow-up (FU), whereas cirrhotic patients still have an increased risk of HCC and HCC surveillance by ultrasound is recommended 13–19. Alcohol can contribute toward the progression of liver fibrosis 20.
The aim of this study was to investigate the presence and impact of OCI in the long term and to analyze the outcome in terms of liver disease after SVR.
Patients and methods
Patients
All HCV-infected patients who received IFN-based therapy and achieved SVR between 1990 and 2005 at Örebro University Hospital, Sweden, were identified from the local patient register and through medical record search. In total, 237 patients were treated at least once and 97 of them achieved SVR, defined as negative HCV-RNA 24 weeks after the end of treatment (EOT). These 97 patients with SVR were included in this study (Fig. 1). Among these, five patients had died and the cause of death was determined from the Swedish Cause of Death Register. The other 92 were contacted by mail with an information letter and an invitation to participate in the study. This was followed by a second letter to those who did not answer. In total, 54 patients agreed and came for a FU visit. Informed written consents were signed, followed by a self-completion questionnaire, clinical examination, transient elastography (TE) using (Fibroscan; Echosens, Paris, France) and blood sampling for biochemical tests and detection of OCI. FU visits were performed from August 2013 to April 2014 by one investigator (C.L.) who also performed all TE examinations.
Fig. 1.
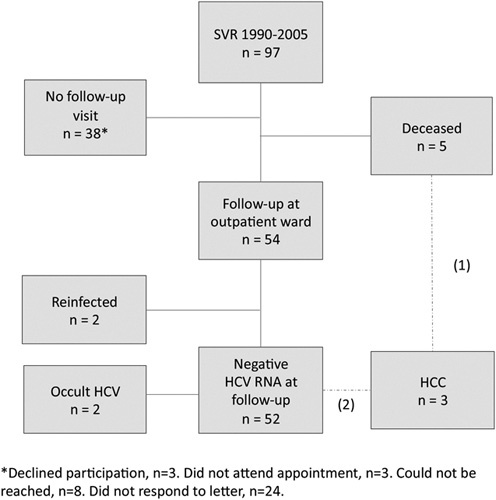
Patient flow chart. Enrollment and follow-up of the patients. HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SVR, sustained virological response.
For the 38 patients who did not participate, the Ethical Review Board approved access to FU data on incident HCC, HCV diagnosis and drug-related diagnoses from the National Cancer Register and Patient Register 21. Linkage was possible using personal identification numbers. Register data were anonymized. The patients were followed from EOT to the end of 2013.
The study was approved by the Regional Ethical Review Board at Uppsala University, Sweden, approval registration number 2013/127 and 2013/127/1, and carried out according to the ethical guidelines of the Helsinki Declaration.
Biochemical and histological data
Baseline data (the latest data collected before the start of treatment leading to SVR), such as HCV genotype, liver biopsy results (fibrosis stage F0–F4 according to the Batts–Ludwig scoring system), laboratory data [alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelets, international normalized ratio (INR), albumin, hemoglobin, and thyroid hormone levels] and antiviral treatment regimen, were obtained from medical records.
At the FU visit, blood samples were obtained for biochemical and virological analyses comparable to baseline data. Stage of fibrosis was established with TE or in one case with a liver biopsy. Liver stiffness cutoff values for different fibrosis stages were as follows: F0–F1: <7.0 kPa, F2: 7.0–9.5 kPa, F3: 9.6–12.5 kPa, and F4≥12.5 kPa 22. The estimated fibrosis stage at FU was compared with pretreatment liver biopsies.
HCV-RNA detection
At FU, all patients were screened for HCV-RNA by PCR using the routine method of the MagNA Pure LC system/COBAS TaqMan HCV test (Roche Diagnostics, Mannheim, Germany), with a sensitivity of 15 IU/ml for the HCV WHO Standard.
To detect OCI, a combined ultracentrifugation/RT-PCR method (described in Supplementary Materials, Supplemental digital content 1, http://links.lww.com/EJGH/A368) with a sensitivity of 0.74 IU/ml was used to detect HCV-RNA traces in whole blood in all patients who had negative HCV-RNA with the Roche COBAS TaqMan test (n=52).
Questionnaire
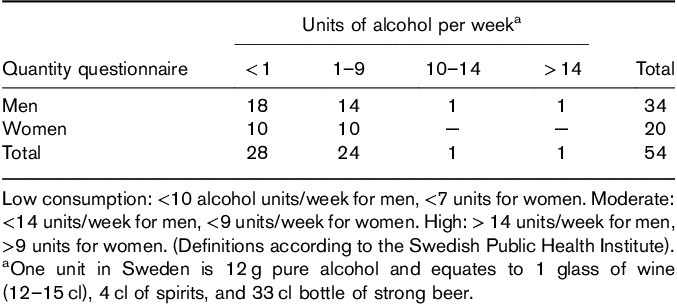
Before the medical examination, all patients completed a questionnaire about comorbidity, use of therapeutic drugs, injecting drug use (IDU), and quantity and frequency of alcohol consumption after SVR. Alcohol consumption was defined according to the Swedish Public Health Institute as low (<10 alcohol units/week for men and <7 units for women), moderate (<14 units/week for men, <9 units/week for women), or high (>14 units/week for men, >9 units for women; Table 4) 23.
Table 4.
Alcohol consumption among patients with cured hepatitis C (number of patients)
Statistical analysis
Continuous variables are presented as means with SD or median with range. Other variables are presented as frequencies and percentages. Continuous patient data measured at baseline and at FU were compared using the paired-samples t-test for dependent variables. An exact sign test was used to compare the differences in fibrosis stage before treatment and at FU. A P value less than 0.05 was considered statistically significant.
The incidence rate of HCC was calculated by dividing the number of incident cases by person-years (PY) of FU, including all 97 patients with SVR. Years of FU were calculated from EOT to the FU visit, death or end of 2013. The expected incidence of HCC in the study population (all 97 patients) was calculated using the PY of FU (calculated from EOT) in each 5-year age stratum and the age-specific and sex-specific incidence rates in the general population according to the National Cancer register. The expected incidence was compared with the observed incidence.
All statistical analyses were carried out using SPSS v.22.0 (IBM Corp., Armonk, New York, USA).
Results
Patient characteristics
In total, 97 patients with SVR were available for FU (Fig. 1). The median FU time for all 97 patients was 10.0 years (range: 5.5–21.5 years) after EOT.
The demographic and baseline characteristics of the 54 patients who came for FU visits are shown in Table 1. The median FU time after EOT was 10.5 years, with FU time up to 21.5 years (minimum: 7.5 years). Stage of fibrosis at baseline was low; only three (6%) patients were cirrhotic (F4) before treatment.
Table 1.
Demographics and baseline characteristics of patients enrolled for examination (number of patients)
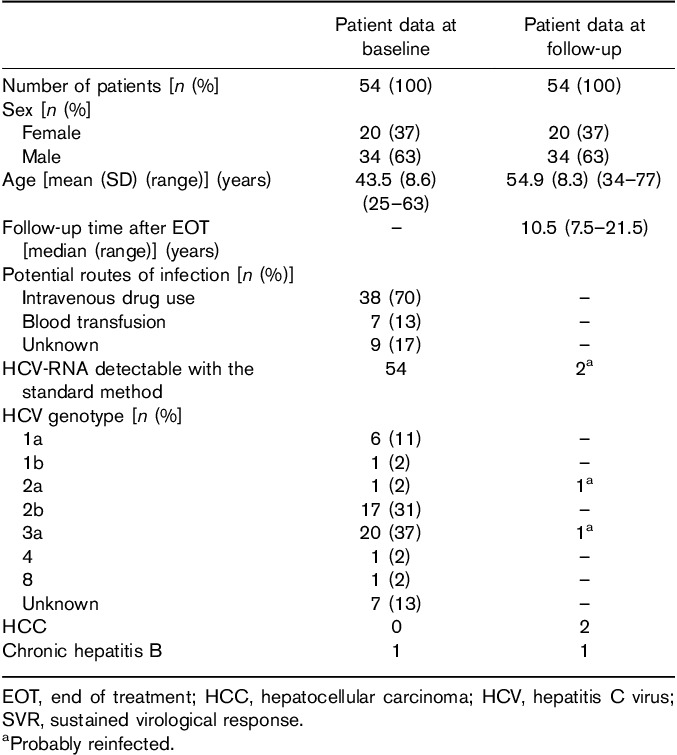
Among the five deceased patients, two women and three men, one died from HCC (described later). For the others, the cause of death was not liver related (three) or was unknown (one).
For the 38 patients without FU visit, data from Patient register and Swedish Cause of Death Register did not show any HCC. One of them had an HCV diagnosis and five had a drug-related diagnosis more than 2 years after SVR (after 2007).
Recurrence of HCV viremia
Positive HCV-RNA in serum was found in two of 54 (4%) patients at FU. Both reported IDU after SVR and had been diagnosed with reinfection before entering the study. One of them had a different HCV genotype at FU (2b at baseline, 1a at FU) and the other had the same genotype (3a). Another patient had been reinfected through IDU, but retreated with SVR in 2010; thus, HCV-RNA was negative at FU. The two patients with reinfection were excluded from further analyses of liver disease in patients with SVR.
Liver fibrosis
For the 52 patients with nondetectable HCV-RNA with standard methods, the biochemical tests (at a group level) indicated a statistically significant improvement (Table 2). The number of patients with fibrosis score F0–F1 increased from 19 before treatment to 41 at long-term FU. Comparisons were not possible in seven patients because of missing biopsy data at baseline (n=4) or missing TE/biopsy at FU (n=3; Table 2). Overall, the assessed fibrosis stages were significantly lower at FU (P=0.001; Fig. 2).
Table 2.
Clinical outcomes of patients enrolled for examination (n=52, two reinfected patients excluded)
Fig. 2.
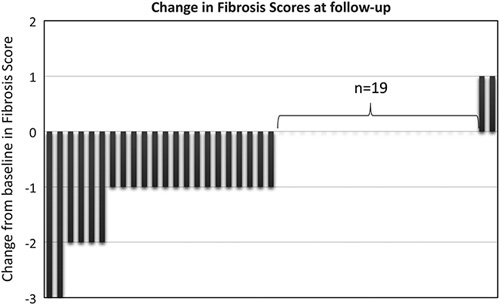
Change in fibrosis scores (F0–F1, F2, F3, and F4) in 42 patients with sustained virological response and assessed stage of fibrosis before treatment (baseline=0) and at follow-up. Nine patients were excluded because of missing paired data (n=7) or hepatocellular carcinoma (n=2).
Fibrosis stage was higher at FU in four patients. For two of them, fibrosis stage had increased from F1 to F2 (TE values 9.3 and 7.3 kPa, respectively, at FU); one of them had a detectable virus with highly sensitive PCR methods (OCI). The other two patients had very high TE values (>75 and 42.2 kPa) and both were diagnosed with large HCCs, with extrahepatic spread.
Occult HCV infection
Among the 52 patients with negative HCV-RNA by the routine method, OCI was detected in two patients (Fig. 3). Both were men and had normal laboratory tests (ALT, AST, platelets, INR, albumin, and hemoglobin) at FU, but TE values corresponded to F2. They showed no signs of HCC, although radiological imaging was not performed and they were not under HCC surveillance.
Fig. 3.
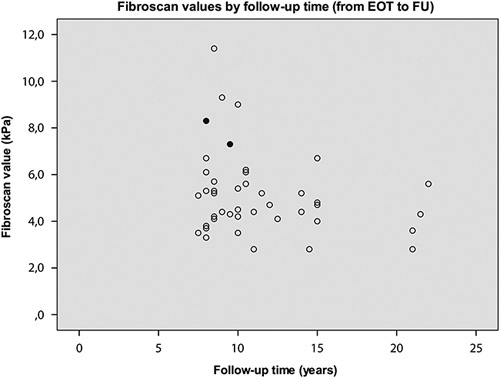
Long-term FU with liver elasticity measurement using Fibroscan and testings of HCV-RNA with a highly sensitive method in patients with a sustained virological response. The values of two patients with positive HCV-RNA at FU, indicative of occult infection, are shown with filled circles. EOT, end of treatment; FU, follow-up; HCV, hepatitis C virus.
For one of them, both baseline biopsy and TE at FU showed F2 (TE median: 8.3 kPa) 8 years after EOT. This patient had low alcohol consumption, no comorbidity, and was on no medication, whereas data on BMI are missing. A second and third highly sensitive HCV-RNA test were obtained 5 and 9 months after the first test, where the second sample was negative, but the third was again HCV-RNA positive. The genotype was the same before treatment and at FU (genotype 1) and the RNA sequences were identical in both samples at FU.
The other patient had F1 at baseline, whereas the TE value at FU was median 7.3 kPa (F2) 9 years after EOT. He had a BMI of 24, no comorbidity, was receiving no medication and did not consume any alcohol. Second and third highly sensitive HCV-RNA tests were obtained 7 and 11 months after the first test and were both negative. This patient had genotype 2 before treatment but genotype 1 at FU. The RNA sequence was different from the one obtained from the first patient.
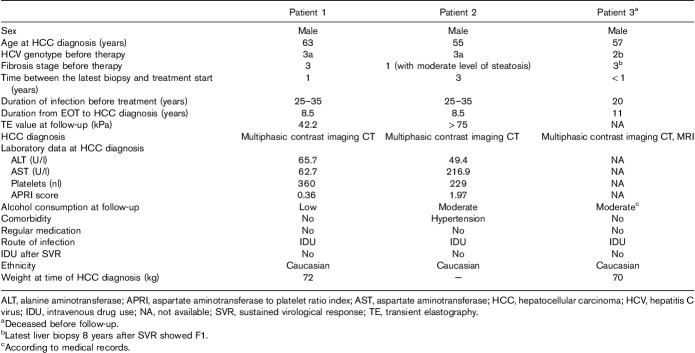
Hepatocellular carcinoma
In total, three patients (all men) were diagnosed with HCC. One had died before and two were found in relation to the FU visit because of unexpectedly high TE values (probably measuring the elasticity of HCC) and were examined by ultrasound and computed tomography scan, revealing large HCCs (Table 3). The diagnosis of HCC was established at 8.5, 8.5, and 11 years after EOT, respectively, at ages 55–63 years. None of them had cirrhosis before treatment and thus were not under HCC surveillance. The tumors were found at a very late stage, and all three patients died within a year from diagnosis. Two patients had HCV genotype 3a and one patient had genotype 2b before SVR. None of them had diabetes and none were obese. In the deceased patient, a liver biopsy (to follow fibrosis) had been performed 8 years after SVR, showing regression of fibrosis to F1, and this was only 3 years before HCC diagnosis. The two patients who were examined at FU had no biochemical signs of cirrhosis, with normal ALT, platelets, INR, albumin and hemoglobin values, except that one of them had an elevated AST, around four times the upper level of normal, resulting in an AST to platelet index score of 1.96. One patient reported low and the other patient reported moderate alcohol consumption. The patient who died before FU had moderate alcohol consumption according to medical records.
Table 3.
Characteristics of patients diagnosed with hepatocellular carcinoma after achievement of a sustained virological response
There were three patients with HCC in our cohort, which yields a standardized incidence ratio of 82.0 compared with the 0.037 expected HCCs in an age-adjusted and sex-adjusted general population cohort. The incidence rate of HCC in our cohort was 280 per 100 000 PY (95% confidence interval: 60–830). The annual incidence rate for men between 55 and 59 years in the general population is 10–14 per 100 000.
Alcohol consumption
All 54 patients completed the questionnaire. In total, 12 (22%) patients answered that they were careful with alcohol because of their former HCV infection to avoid further liver damage (Table 4). No or very low consumption was reported by 52% of the patients, and 44% reported low consumption. The difference between the mean TE value for patients with no or very low alcohol consumption (5.1 kPa, SD: 1.9) and patients with low to moderate consumption (5.3 kPa, SD: 1.8) was not statistically significant when excluding patients with HCC (probably erroneous TE values) or HCV reinfection (data not shown).
Discussion
The long-term FU (median: 10.5 years after EOT) in this study shows that SVR after HCV treatment generally leads to normal liver function tests, undetectable HCV-RNA with standard method tests and regression of fibrosis. We could, however, observe the presence of OCI in two patients: one with detectable HCV-RNA at two time points. This was not associated with serious liver damage or development of HCC during this long-term FU. Whether the OCI was the cause for the remaining F2 liver fibrosis in these patients needs to be further investigated. Importantly, three patients with SVR, all without pretreatment cirrhosis, developed HCC at age 55–63, 8–11 years after EOT, and about 20–35 years after the primary infection.
Among the patients with SVR in this cohort, two (4%) had detectable HCV-RNA in whole blood with a highly sensitive method 8 and 9 years, respectively, after SVR, indicative of OCI. Both patients were negative in a second sample, but one patient had detectable HCV-RNA in a third sample (identical sequence in the first and third samples) 9 months after the first sample was obtained. This is in line with previous studies of OCI where the appearance of RNA sequences has fluctuated over time, often to disappear some years after SVR 24. One of the patients had another HCV genotype at FU than before treatment, which may be explained by coinfection with two genotypes before treatment. We could not analyze pretreatment samples to look for the genotype 1 strain. We presume that OCI has been prevalent in both patients from EOT until FU. There might have been more patients with OCI at an earlier time point in this cohort.
Previous studies of OCI, with median FU time from 1 to 10 years after SVR, have found detection rates of HCV-RNA in PBMCs that vary from 0 to 50% and detection rates in liver biopsy specimens from 0 to 83% 4,5. The large variations are partly because of the lack of a standardized universal sensitive method for detecting HCV-RNA 5. Residual HCV-RNA seems to become negative if several consecutive tests are taken, indicating that HCV eventually is cleared 4,9,25–27. An association between OCI and progression of liver disease has not been proven and there is no reported human case proving transmission of HCV from a patient with OCI 25,28,29. It is speculated that the prevalence of OCI could increase in the future as a result of the shorter duration of therapy with DAAs and in the absence of IFN 5.
In the current study, we found no conclusive evidence of the progress of the liver disease in patients with OCI. However, and unlike the majority of patients in this study, both patients had fibrosis stage two at FU and no signs of regression of fibrosis. As the changes were very small after a long FU time, the clinical importance of these observations is probably low or at least unclear. None of the patients with HCC had OCI at FU.
A major observation in this study is that HCC could be diagnosed many years after SVR. The patients who developed HCC were diagnosed at age 55 years or older. Surprisingly, none of these patients had cirrhosis before treatment and no indication of cirrhosis at FU, except an elevated AST in one patient, which may have been associated with the large tumor. This patient had fibrosis stage 1 in pretreatment biopsy. The tumors were detected at a very late stage, without curative treatment options. It was not possible to calculate the exact incidence of HCC in this cohort as the study was not designed to look for HCC, and ultrasound of the liver was only performed in the patients with unexpectedly high TE values at FU. However, at least three of 97 patients with SVR developed HCC, representing an 82 times higher incidence than expected in a similar cohort in the general population. This indicates that noncirrhotic patients also have an increased risk of HCC a long time after cure from chronic HCV infection. One of the largest studies of the persistent risk of HCC long term after SVR was carried out in the USA using data from the Veterans Affairs HCV Clinical Case Registry. Among 10 800 patients with SVR and a mean FU time of 2.8 years, the overall annual HCC risk was 0.33%. Patients with cirrhosis before treatment had an annual risk of 1.39%, and for noncirrhotic patients, the risk was 0.16%. The annual risk for patients treated after the age of 65 was 0.95%, irrespective of cirrhosis 15.
Previously shown risk factors for HCC in HCV-infected individuals are liver cirrhosis, male sex, heavy alcohol consumption, diabetes mellitus and older age at the time of SVR 16,18–20,30. In the HCC patients in this study, the possible common risk factors were alcohol consumption (low or moderate consumption were reported), male sex and age older than 55 years; however, these patients achieved SVR at age 45–53 years. None of them were obese or had diabetes. The association between obesity and HCC is not clear; diverse results have been found in previous reports 31,32.
Our findings raise the question of whether HCC surveillance should also include patients without cirrhosis, especially those with risk factors or after a certain age. In patients with chronic hepatitis B, HCC surveillance is recommended when the incidence is higher than 0.2 per 100 PY 33,34. More studies are needed to investigate the long-term risk of HCC in both noncirrhotic and cirrhotic patients with SVR.
A history of alcohol abuse is common among patients with HCV infection in Sweden 35. In this study, the reported alcohol consumption after SVR was low in most patients. Many patients reported that they were sober alcoholics at FU and probably had higher alcohol consumption earlier in life. However, patients with heavy alcohol consumption were usually not eligible for treatment with IFN in 1990–2005. This, together with the self-reported alcohol consumption, indicates that a heavy drinking problem during treatment and after SVR was rare among these patients. Only one patient reported high alcohol consumption at FU. There was no significant difference in liver stiffness between those with no/very low consumption and those with low/moderate consumption.
In this study, the rate of reinfection was low (6%) after a long FU time, despite the fact that the majority of patients had contracted HCV through IDU. In a Norwegian study, 94 individuals with a history of IDU before treatment were followed for a median of seven years after SVR to assess HCV reinfection. Thirty-nine percent had relapsed into IDU, and 27% of these patients were reinfected with HCV 36. In our study, 5.6% of 54 patients had relapsed into IDU, all of them reinfected. It is possible that relapse into IDU was more common in the group that did not attend the FU study. For the 38 patients not available for FU visit, register data showed a diagnosis related to illicit drugs in five (13%) patients and an HCV diagnosis in one patient after 2007.
The advantages of this study are the long FU time of patients with SVR, and that all patients who had achieved SVR in the predetermined time period were invited to participate, without exclusions. A majority of these patients had FU visits and the remaining patients were followed through national registers, resulting in no persons lost to FU after a median of 10 years. The FU visits enabled blood analyses for OCI in patients with long FU duration, up to 21.5 years after EOT, and in a rather large number of patients, compared with previous studies of OCI.
In contrast with the rather large study of OCI, a potential limitation of the study of liver disease before and after SVR is the somewhat small size of the study cohort. However, the patients were thoroughly examined at FU visits and the FU time is longer than that in most studies. The surprisingly high number of HCC diagnoses would probably not have been detected with a shorter FU. The primary aim of the study was not to assess the risk for HCC and we did not have any control group for comparison. However, the finding of three HCCs in this small group of noncirrhotic patients in the long term after SVR was higher than expected, and a surprise, which raises questions about the long-term risk of HCC in an aging patient group long time after SVR. The patients in the study are those who were treated first of all in the 1990s and there are limited data on the long-term outcome after SVR in this population. We did not include patients treated after 2005 to solely include those with long-term FU.
Another limitation is that the fibrosis stage was estimated with TE at FU and compared with liver biopsy results at baseline. It has been suggested that TE overestimates the regression of fibrosis after SVR 37. However, TE is a noninvasive, rapid method without risk for the patient, and has become the preferred method for fibrosis staging in HCV-infected patients, and therefore the most suitable method for this study.
In this study of patients with SVR, genotypes 2 and 3 dominated and the patients were mostly noncirrhotic before treatment. This indicates that the IFN-based therapy almost only cured those with mild disease and genotypes 2 or 3 and that patients with genotype 1 (the most common in Sweden) and cirrhotic patients were more likely to fail treatment 38. A long-term FU after DAA therapy may show different results, both for the risk of HCC and for the occurrence of OCI. It is not yet known how effective DAAs are at clearing OCI.
Conclusion
SVR is associated with clinical cure and regression of liver fibrosis in most patients after 10 years of FU. However, occult HCV infection can be detected in some patients many years after achievement of SVR; this may have a negative effect on the regression of liver fibrosis, but more studies are needed. Future studies are planned to determine whether the viable virus can be recovered from PBMC. If so, this raises the question of whether those repetitively positive for HCV in PBMC should be retreated. Further, the risk of HCC is not eliminated in all noncirrhotic patients with SVR. Studies of the long-term outcome more than 10 years after IFN therapy and eventually after DAA therapy are needed.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com.
Acknowledgements
This study was supported by the Research Committee of Region Örebro County Council (Grant numbers: OLL-522501 and OLL-590001).
M.S. received research support from the Stockholm County Council, The Swedish Science Council and The Swedish Cancer Foundation.
Conflicts of interest
A.-S. D. has served as a speaker and/or consultant for AbbVie, BMS, Gilead, Janssen, Medivir, MSD and Roche. S.A. has served as a speaker and/or consultant for AbbVie, BMS, Gilead, Janssen, MSD/Merck, Medivir, Roche, and Tillotts Pharma, and has received research grants from AbbVie and Gilead. E.D.B. is currently an employee of Medivir AB (Stockholm, Sweden), although not at the time when the study was conducted. For the remaining authors, there are no conflicts of interest.
References
- 1.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med 2006; 355:2444–2451. [DOI] [PubMed] [Google Scholar]
- 2.Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int 2014; 34 (Suppl 1): 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham TN, Coffin CS, Michalak TI. Occult hepatitis C virus infection: what does it mean? Liver Int 2010; 30:502–511. [DOI] [PubMed] [Google Scholar]
- 4.Welker MW, Zeuzem S. Occult hepatitis C: how convincing are the current data? Hepatology 2009; 49:665–675. [DOI] [PubMed] [Google Scholar]
- 5.Attar BM, Van Thiel D. A new twist to a chronic HCV infection: occult hepatitis C. Gastroenterol Res Pract 2015; 2015:579147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeff LB. Sustained virologic response: is this equivalent to cure of chronic hepatitis C? Hepatology 2013; 57:438–440. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002; 122:1303–1313. [DOI] [PubMed] [Google Scholar]
- 8.Chavalitdhamrong D, Tanwandee T. Long-term outcomes of chronic hepatitis C patients with sustained virological response at 6 months after the end of treatment. World J Gastroenterol 2006; 12:5532–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology 2009; 49:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med 1997; 127:875–881. [DOI] [PubMed] [Google Scholar]
- 11.Maruoka D, Imazeki F, Arai M, Kanda T, Fujiwara K, Yokosuka O. Longitudinal changes of the laboratory data of chronic hepatitis C patients with sustained virological response on long-term follow-up. J Viral Hepat 2012; 19:e97–e104. [DOI] [PubMed] [Google Scholar]
- 12.Wang JH, Changchien CS, Hung CH, Tung WC, Kee KM, Chen CH, et al. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: Longitudinal study using FibroScan. J Gastroenterol Hepatol 2010; 25:964–969. [DOI] [PubMed] [Google Scholar]
- 13.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–943. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi S, Takeda T, Enomoto M, Tamori A, Kawada N, Habu D, et al. Development of hepatocellular carcinoma in patients with chronic hepatitis C who had a sustained virological response to interferon therapy: a multicenter, retrospective cohort study of 1124 patients. Liver Int 2007; 27:186–191. [DOI] [PubMed] [Google Scholar]
- 15.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology 2016; 64:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis 2013; 57:230–236. [DOI] [PubMed] [Google Scholar]
- 17.Nagaoki Y, Aikata H, Nakano N, Shinohara F, Nakamura Y, Hatooka M, et al. Development of hepatocellular carcinoma in patients with hepatitis C virus infection who achieved sustained virological response following interferon therapy: a large-scale, long-term cohort study. J Gastroenterol Hepatol 2016; 31:1009–1015. [DOI] [PubMed] [Google Scholar]
- 18.Hedenstierna M, Nangarhari A, Weiland O, Aleman S. Diabetes and cirrhosis are risk factors for hepatocellular carcinoma after successful treatment of chronic hepatitis C. Clin Infect Dis 2016; 63:723–729. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda H, Kumada T, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, et al. Risk factors of hepatocellular carcinoma development in non-cirrhotic patients with sustained virologic response for chronic hepatitis C virus infection. J Gastroenterol Hepatol 2015; 30:1183–1189. [DOI] [PubMed] [Google Scholar]
- 20.Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med 2001; 134:120–124. [DOI] [PubMed] [Google Scholar]
- 21.Socialstyrelsen. The National Board of Health and Welfare: homepage Sweden; 2016. Available at: http://www.socialstyrelsen.se/english. [Accessed 14 November 2018].
- 22.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008; 48:835–847. [DOI] [PubMed] [Google Scholar]
- 23.Andreasson S, Allebeck P, Leifman H, Rosengren A, Norström T, Adolfsson J, et al. Alcohol and health. An evaluation of the knowledge of the positive and negative health effects of alcohol; 2005. Available at: http://www.riskbruk.se/WebControls/Upload/Dialogs/Download.aspx?ID=3036. [Accessed 14 November 2018].
- 24.Castillo I, Bartolome J, Quiroga JA, Barril G, Carreno V. Long-term virological follow up of patients with occult hepatitis C virus infection. Liver Int 2011; 31:1519–1524. [DOI] [PubMed] [Google Scholar]
- 25.Hedenstierna M, Weiland O, Brass A, Bankwitz D, Behrendt P, Uhnoo I, et al. Long-term follow-up of successful hepatitis C virus therapy: waning immune responses and disappearance of liver disease are consistent with cure. Aliment Pharmacol Ther 2015; 41:532–543. [DOI] [PubMed] [Google Scholar]
- 26.Veerapu NS, Raghuraman S, Liang TJ, Heller T, Rehermann B. Sporadic reappearance of minute amounts of hepatitis C virus RNA after successful therapy stimulates cellular immune responses. Gastroenterology 2011; 140:676–685; e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morishima C, Morgan TR, Everhart JE, Wright EC, Apodaca MC, Gretch DR, et al. Interpretation of positive transcription-mediated amplification test results from polymerase chain reaction-negative samples obtained after treatment of chronic hepatitis C. Hepatology 2008; 48:1412–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carreno V, Pardo M, Lopez-Alcorocho JM, Rodriguez-Inigo E, Bartolome J, Castillo I. Detection of hepatitis C virus (HCV) RNA in the liver of healthy, anti-HCV antibody-positive, serum HCV RNA-negative patients with normal alanine aminotransferase levels. J Infect Dis 2006; 194:53–60. [DOI] [PubMed] [Google Scholar]
- 29.Radkowski M, Horban A, Gallegos-Orozco JF, Pawelczyk A, Jablonska J, Wilkinson J, et al. Evidence for viral persistence in patients who test positive for anti-hepatitis C virus antibodies and have normal alanine aminotransferase levels. J Infect Dis 2005; 191:1730–1733. [DOI] [PubMed] [Google Scholar]
- 30.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002; 36:1206–1213. [DOI] [PubMed] [Google Scholar]
- 31.Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer 2007; 109:2490–2496. [DOI] [PubMed] [Google Scholar]
- 32.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009; 136:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology 1998; 27:273–278. [DOI] [PubMed] [Google Scholar]
- 35.Stokkeland KDA, Montgomery SM, Franck J, Hultcrantz R. Alcohol dependence increases the mortality risk in patients with hepatitis C. Hepatology 2013; 58:1284A. [Google Scholar]
- 36.Midgard H, Bjoro B, Maeland A, Konopski Z, Kileng H, Damas JK, et al. Hepatitis C reinfection after sustained virological response. J Hepatol 2016; 64:1020–1026. [DOI] [PubMed] [Google Scholar]
- 37.D’Ambrosio R, Aghemo A, Fraquelli M, Rumi MG, Donato MF, Paradis V, et al. The diagnostic accuracy of Fibroscan for irrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol 2013; 59:251–256. [DOI] [PubMed] [Google Scholar]
- 38.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com.