Abstract
Background:
Ciprofloxacin is usually used in the treatment of lower respiratory tract infections (LRTIs). Recent studies abroad have shown ciprofloxacin is inadequately dosed and might lead to worse outcomes. The aim of this study was to perform pharmacokinetic and pharmacodynamic analyses of ciprofloxacin in elderly Chinese patients with severe LRTIs caused by Gram-negative bacteria.
Methods:
From September 2012 to June 2014, as many as 33 patients were empirically administered beta-lactam and ciprofloxacin combination therapy. Patients were infused with 200 or 400 mg of ciprofloxacin every 12 h, which was determined empirically by the attending physician based on the severity of the LRTI and the patient's renal condition. Ciprofloxacin serum concentrations were determined by high-performance liquid chromatography. Bacterial culture was performed from sputum samples and/or endotracheal aspirates, and the minimum inhibitory concentrations (MICs) of ciprofloxacin were determined. The ratios of the area under the serum concentration-time curve to the MIC (AUC/MIC) and of the maximum serum concentration of the drug to the MIC (Cmax/MIC) were calculated. The baseline data and pharmacokinetic parameters were compared between clinical success group and clinical failure group, bacteriologic success group and bacteriologic failure group.
Results:
Among the 33 patients enrolled in the study, 17 were infected with Pseudomonas aeruginosa, 14 were infected with Acinetobacter baumannii, and two were infected with Klebsiella pneumoniae. The mean age of the patients was 76.9 ± 6.7 years. Thirty-one patients (93.4%) did not reach the target AUC/MIC value of >125, and 29 patients (87.9%) did not reach the target Cmax/MIC value of >8. The AUC/MIC and Cmax/MIC ratios in the clinical success group were significantly higher than those in the clinical failure group (61.1 [31.7–214.9] vs. 10.4 [3.8–66.1], Z = −4.157; 9.6 [4.2–17.8] vs. 1.3 [0.4–4.7], Z = −4.018; both P < 0.001). The AUC/MIC and Cmax/MIC ratios in the patients for whom the pathogens were eradicated were significantly higher than those in the patients without the pathogens eradicated (75.3 [31.7–214.9] vs. 10.5 [3.8–66.1], Z = −3.938; 11.4 [4.2–17.8] vs. 1.4 [0.4–5.4], Z = −3.793; P < 0.001 for both). Receiver operating characteristic curve analysis showed that the AUC/MIC and Cmax/MIC values were closely associated with clinical and bacteriologic efficacies (P < 0.001 in both).
Conclusions:
Ciprofloxacin is inadequately dosed against Gram-negative bacteria, especially for those with relatively high MIC values. Consequently, the target values, AUC/MIC > 125 and Cmax/MIC > 8, cannot be reached.
Keywords: Ciprofloxacin, Lower respiratory tract infection, Pharmacokinetics
Introduction
Lower respiratory tract infections (LRTIs), including pneumonia, are a major cause of morbidity and mortality worldwide, including in China, especially among the elderly. Antibiotic therapy in elderly patients is particularly challenging for clinicians due to the progressive age-dependent changes in the absorption, distribution, metabolism, and excretion of antibacterial drugs.[1] LRTIs caused by Gram-negative bacteria such as Pseudomonas aeruginosa are especially common in elderly patients and are frequently found in patients with chronic lung or heart disease, associated with a substantially higher mortality rate (upward of 40%).[1,2,3]
Ciprofloxacin is a third-generation quinolone with a broad spectrum of antimicrobial activity against Gram-negative bacteria and shows strong activity against P. aeruginosa. Accordingly, ciprofloxacin is widely used for treating patients with infections in hospital wards and intensive care units (ICUs).[4,5] Since it is a concentration-dependent antibiotic, the pharmacokinetic (PK) and pharmacodynamic (PD) indices for predicting the antibacterial efficacy of ciprofloxacin are the 24-h area under the serum concentration-time curve (AUC0–24h), the maximum serum concentration of the drug (Cmax), and the minimum inhibitory concentration (MIC) against the pathogen.[6] Ciprofloxacin efficacy indices have been reported at AUC/MIC > 100–125 and Cmax/MIC > 8–10, which are used as thresholds for predicting the drug's clinical and bacteriologic efficacies.[6,7]
However, in recent years, several international studies have shown that ciprofloxacin may be inadequately dosed in patients with severe infections.[8,9] In China, ciprofloxacin is widely used in hospital wards and ICUs; however, there have been few studies to determine the ciprofloxacin AUC/MIC and Cmax/MIC ratios. Therefore, we performed a population PK and PD analysis in patients infected with Gram-negative bacteria in a Chinese hospital and evaluated whether ciprofloxacin could reach the reported target values of AUC/MIC > 125 and Cmax/MIC > 8 based on the conventional dosing and regimen applied.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Peking University Third Hospital (No: IRB00006761-2012068). All subjects signed informed consent.
Enrollment criteria
From September 2012 to June 2014, patients hospitalized in the Respiratory Intensive Care Unit (RICU) and ICU of Peking University Third Hospital, Beijing, aged ≥60 years, with community- or hospital-acquired LRTIs,[10] and a high risk of Gram-negative bacterial infection such as P. aeruginosa [5] that were empirically treated with intravenous ciprofloxacin were assessed for eligibility for inclusion in the study.[11] Patients were ultimately enrolled in this study when the infection was proven to be caused by Gram-negative bacteria isolated from the sputum and/or endotracheal aspirates. Patients were excluded if they had a positive HIV antibody titer, or had known or suspected tuberculosis or other infections caused by Gram-positive cocci, viruses, or fungi at baseline.
Key antimicrobial agent
The ciprofloxacin hydrochloride reference substance (84.9% purity; batch number: 30451-200302) was obtained from the National Institutes for Food and Drug Control, Beijing, China.
Data collection
The patients’ demographic data, including sex, age, race, weight, height, and underlying disease, were collected from medical records. Other data collected included the levels of alanine aminotransferase, aspartate aminotransferase, albumin, blood urea nitrogen, creatinine, serum creatinine clearance rate (CCR), and Acute Physiology and Chronic Health Evaluation (APACHE) II score. The CCR was calculated based on the Cockcroft-Gault equation.[12]
PK analysis of ciprofloxacin
Drugs were administered empirically by the attending physician based on the severity of the LRTI and renal function. Ciprofloxacin was administered as an intravenous infusion at a routine dose of 200 mg every 12 h, or 400 mg every 12 h if assessed as severe LRTI.[10] The ciprofloxacin dosage in patients with renal insufficiency was adjusted according to the package insert. In seven patients with a CCR of <30 mL/min, the ciprofloxacin dose was reduced to 200 mg once every 24 h. The infusion time for 200 mg of ciprofloxacin was 0.5 h, and that for 400 mg of ciprofloxacin was 1 h. Both ciprofloxacin doses were combined with beta-lactams. Blood samples were collected at 1, 2, 3, 4, 6, 8, and 12 h following ciprofloxacin administration on the fourth day of treatment, respectively. One milliliter of venous blood was collected at each time point and the samples were centrifuged at 3000×g for 5 min within 2 h of collection. The separated serum samples were stored in a –80°C freezer. The ciprofloxacin serum concentration was determined by high-performance liquid chromatography (Agilent 1100, Agilent Technologies, Santa Clara, CA, USA), and a nonlinear mixed-effects modeling approach was used to create a population pharmacokinetic (PPK) model, as described in our previous report.[11] The AUC0–24h values were calculated for each patient based on the corresponding individual concentration-time curve estimated by the PPK model. The drug concentration obtained at the end of infusion was regarded as the Cmax. The AUC0–24h/MIC and Cmax/MIC ratios were then calculated for each patient. The MIC was determined using the automated VITEK 2 identification system (bioMérieux, Craponne, France).
Microbial culture and ciprofloxacin sensitivity of Gram-negative bacteria
Cultures of a tracheal aspirate or sputum were performed for all patients. The respiratory specimens had to include <10 squamous epithelial cells and >25 neutrophils in each high-power field of vision for accurate analysis. Microbial culture and identification were performed according to the third edition of the National Guide for Clinical Laboratory Procedures. Microorganisms were identified using a VITEK 2 identification system, and the sensitivity to ciprofloxacin was tested according to MIC values of ≤0.25, 0.5, 1, 2, and ≥4 mg/L, respectively.
Clinical and bacteriologic efficacy evaluations
The clinical efficacy was evaluated based on the clinical response, categorized into a clinical success and clinical failure. Clinical success referred to the cure or improvement of signs and symptoms of infection such as fever, sputum, and dyspnea in patients, along with the improvement of other, non-microbiologic indicators determined by chest imaging and laboratory tests. Clinical failure referred to the persistence or deterioration of signs and symptoms of infection in patients, as well as the development of new signs and symptoms of infection and/or the need to switch to another antimicrobial treatment for treating the infection. The bacteriologic efficacy was evaluated based on the bacteriologic responses, also categorized into a bacteriologic success and bacteriologic failure. Bacteriologic success referred to complete pathogen eradication in patients or presumed pathogen eradication if tracheal secretions disappeared following symptom improvement. Bacterial failure referred to the absence of either proven or presumed pathogen eradication in the blood or tracheal secretions of patients.
Statistical analysis
Data analysis was performed using the SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). Quantitative data with a normal distribution are presented as the mean ± standard deviation, and non-normal distributed data are presented as the median (maximum–minimum). A P value <0.05 was considered statistically significant. When comparing the baseline data and PK parameters of the patients in the clinical success group vs. those in the clinical failure group and the patients in the bacteriologic success group vs. those in the bacteriologic failure group, continuous data with a normal distribution were tested using an independent sample t test, and non-normally distributed data were tested using the non-parametric Mann-Whitney U test. A Chi-squared test was used to compare categorical data. Receiver operating characteristic (ROC) curves were constructed to determine the thresholds for clinical and bacteriologic efficacy, as well as the sensitivity, specificity, positive predictive value, and negative predictive value.
Results
Demographic data
A total of 43 elderly patients with LRTI and a high risk of Gram-negative bacterial infection were assessed and received combination therapy of ciprofloxacin and beta-lactams. One patient died of severe respiratory failure on the third day of treatment, without relevant PK and PD data available. Two patients were diagnosed as having smear-positive pulmonary tuberculosis and were therefore excluded from the study. Among the other 40 patients, the infections of 33 patients were ultimately proven to be caused by Gram-negative bacteria isolated from sputum of tracheal aspirates [Figure 1].
Figure 1.
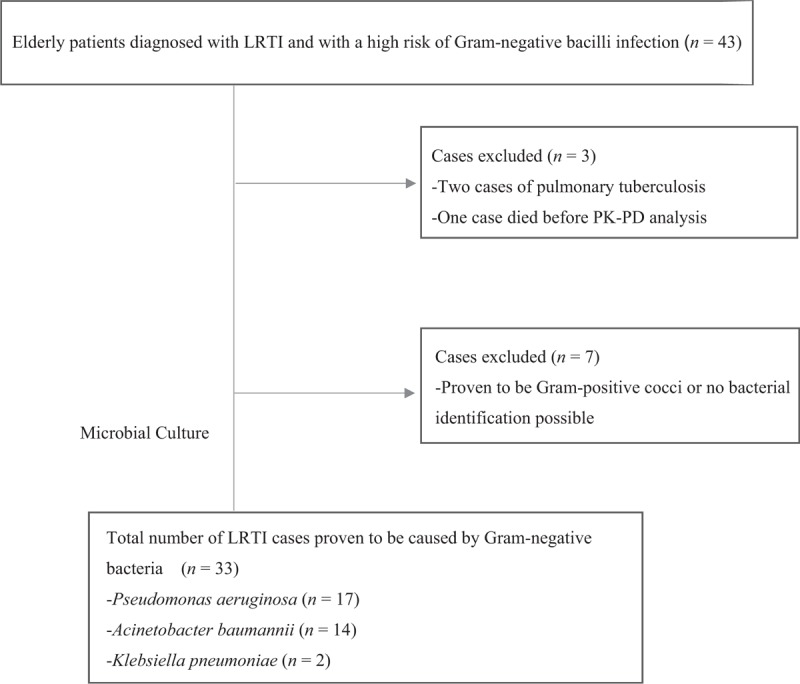
Case selection for Gram-negative lower respiratory tract infections (LRTIs). PK-PD: Pharmacokinetic-pharmacodynamic.
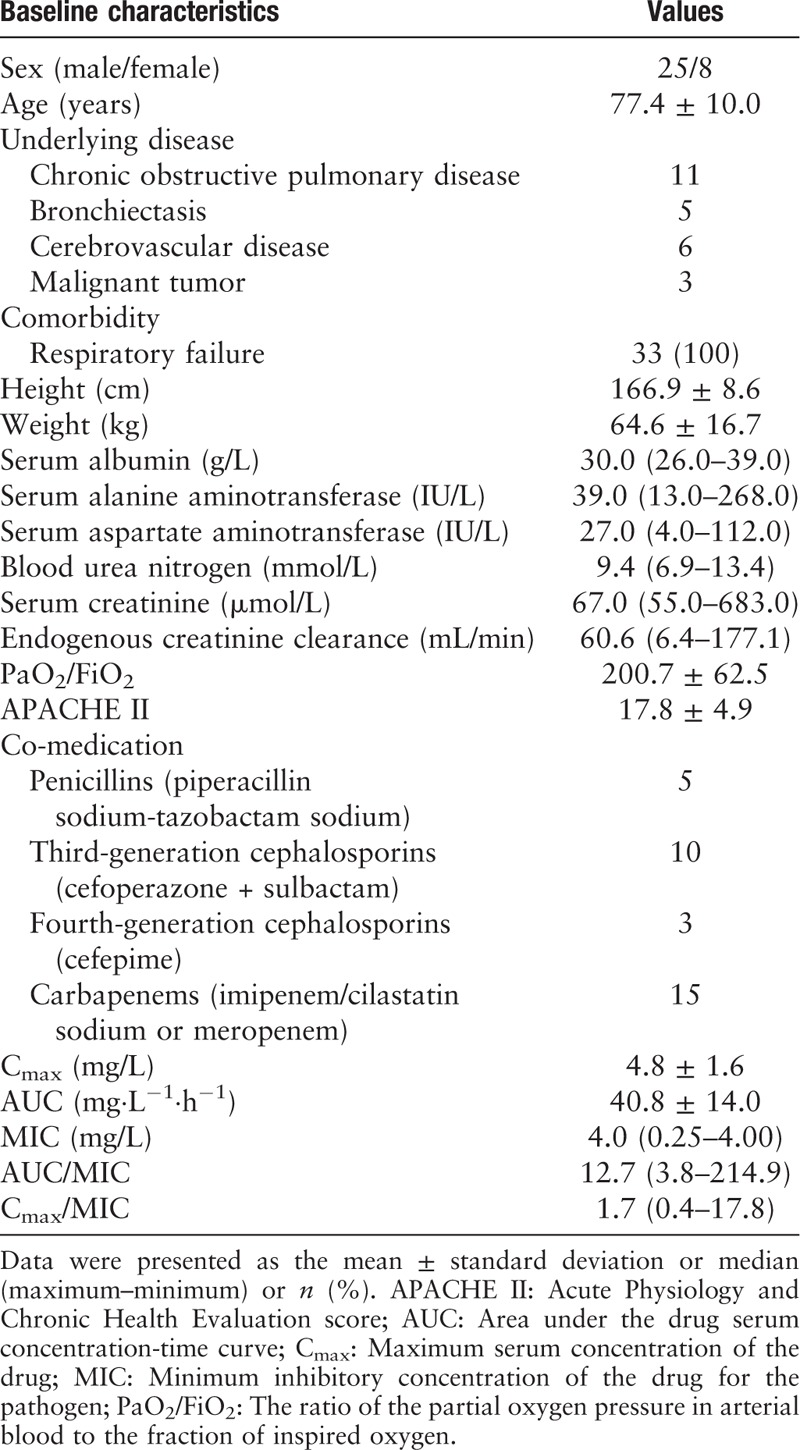
These 33 enrolled patients included 25 males and eight females, with a mean age of 76.9 ± 6.7 years. The baseline characteristics of the enrolled patients are shown in Table 1. The pathogens isolated included 17 strains of P. aeruginosa (MICs: four strains, 0.25 mg/L; four strains, 1 mg/L; two strains, 2 mg/L; and seven strains, 4 mg/L), 14 strains of Acinetobacter baumannii (MICs: one strain, 0.5 mg/L; one strain, 1 mg/L; one strain, 2 mg/L; and 11 strains, 4 mg/L), and two strains of Klebsiella pneumoniae (MICs: 0.5 and 1 mg/L, respectively). Twenty-nine pathogen strains were isolated from tracheal aspirate cultures, and four were isolated from sputum cultures.
Table 1.
Baseline characteristics of the 33 patients enrolled in the study.
PK and PD analysis
A total of 180 blood samples were collected from the 33 patients. For each patient, two to seven serum drug concentrations were obtained, which were analyzed with our previously developed two-compartment ciprofloxacin PPK model.[11] The mean AUC value, mean Cmax value, and median AUC/MIC ratio of this group of patients were 40.8 ± 14.0 mg·L−1·h−1, 4.8 ± 1.6 mg/L, and 12.7 (3.8–214.9), respectively. The target value of AUC/MIC (≥125) was reached in only two patients, with a clinical success rate of 100%. The median Cmax/MIC value was 1.7 (0.4–17.8), and only four patients reached the target value of >8 with a 100% bacteriologic success rate.
Efficacy analysis
Clinical efficacy
Comparison of the clinical data and PD indices between the clinical success and clinical failure groups showed that among the 33 patients infected with Gram-negative bacteria, eight were considered to have achieved clinical success, and 25 were considered as a clinical failure. Among the eight patients in the clinical success group, six had a P. aeruginosa infection (MICs of ciprofloxacin were 0.25 mg/L for four strains and 1 mg/L for two strains), one had an A. baumannii infection (MIC: 0.5 mg/L), and one patient had a K. pneumoniae infection (MIC: 0.5 mg/L). Among the 25 patients in the clinical failure group, 11 patients had P. aeruginosa infections (MICs: 1 mg/L for two strains, 2 mg/L for two strains, and 4 mg/L for seven strains), 13 patients had A. baumannii infections (MICs: 1 mg/L for one strain, 2 mg/L for one strain, and 4 mg/L for 11 strains), and one patient had a K. pneumoniae infection (MIC: 1 mg/L).
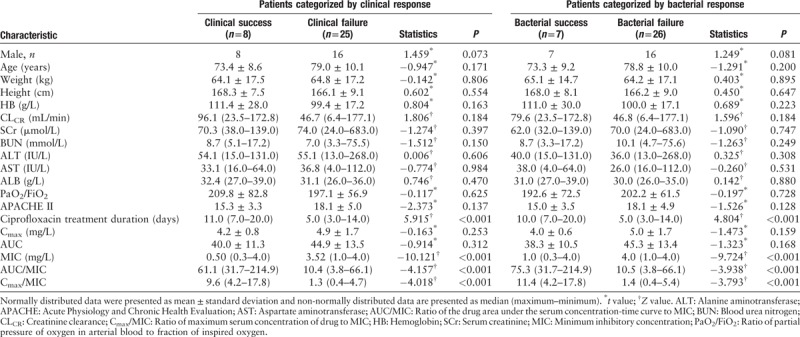
Among the 25 patients in the clinical failure group, six showed partial improvement, and 19 patients showed no improvement. All 25 patients were switched to other antibacterial drugs. At the end of the study, 18 patients were considered a clinical success, and seven patients died. Compared with that in the clinical failure group, the median MIC value in the clinical success group was significantly lower (P < 0.001). The AUC/MIC and Cmax/MIC ratios in the clinical success group were significantly higher than those in the clinical failure group (61.1 [31.7–214.9] vs. 10.4 [3.8–66.1], Z = −4.157; 9.6 [4.2–17.8] vs. 1.3 [0.4–4.7], Z = −4.018; both P < 0.001) [Table 2].
Table 2.
Demographic and clinical data of patients categorized by clinical response and bacterial response.
To determine the value of AUC/MIC in predicting the clinical efficacy, a ROC curve was established using the clinical response as a target scalar and AUC/MIC as the test variable. The area under the ROC curve was 0.966, indicating a good predictive value of AUC/MIC for clinical response. Based on the ROC curve, the AUC/MIC threshold was determined to be 40.9, with a sensitivity and specificity of 85.7% and 91.3%, respectively, in predicting the clinical response [Figure 2A].
Figure 2.
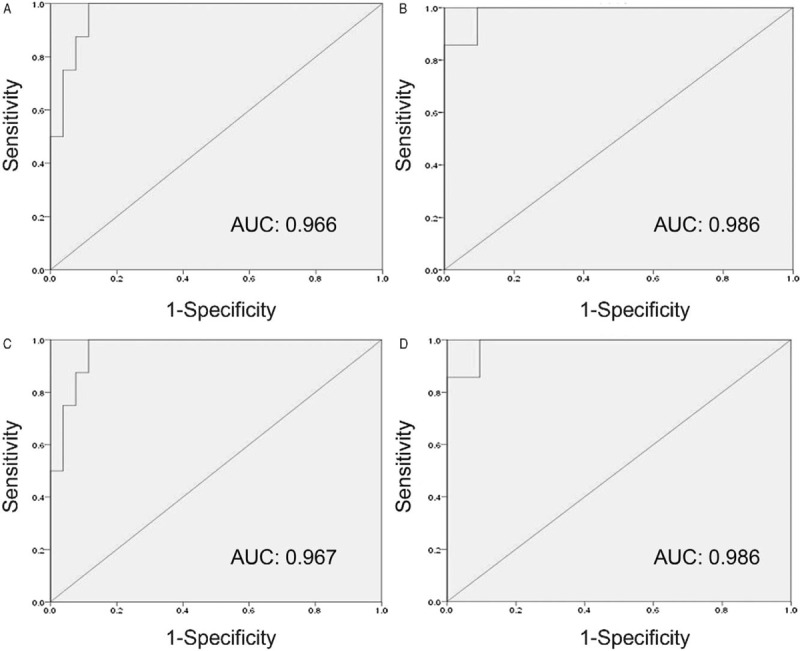
Receiver operating characteristic curve analysis of AUC/MIC and Cmax/MIC in predicting the efficacy. (A) ROC curve of AUC/MIC as a predictor of clinical efficacy. (B) ROC curve of Cmax/MIC as a predictor of clinical efficacy. (C) ROC curve of AUC/MIC as a predictor of bacteriologic efficacy. (D) ROC curve of Cmax/MIC as a predictor of bacteriologic efficacy. AUC: Area under the drug serum concentration-time curve; Cmax: Maximum serum concentration of the drug; MIC: Minimum inhibitory concentration of the drug for the pathogen; ROC: Receiver operating characteristic.
Similarly, the area under the ROC curve established using the clinical response as a target scalar and Cmax/MIC as the test variable was 0.986, indicating the good predictive value of Cmax/MIC for clinical response. Based on the ROC curve, the Cmax/MIC threshold was 3.7, with 100% sensitivity and 90.5% specificity in predicting the clinical response [Figure 2B].
Bacteriologic efficacy analysis
Comparison of the clinical data and PD indices between the bacteriologic success and bacteriologic failure groups showed that among the eight patients in the clinical success group, seven patients also achieved a bacteriologic success, while one was considered a bacteriologic failure. In addition, among the eight patients in the clinical success group, one patient achieved a clinical success without achieving a bacteriologic success. The pathogen isolated from the tracheal aspirate culture of this patient was identified as P. aeruginosa, with a MIC value of 1 mg/L, an AUC/MIC ratio of 54.9, and a Cmax value of 5.4 mg/L. Among the 25 patients in the clinical failure group, 18 patients did not achieve a bacteriologic success, and seven patients were presumed to have not achieved a bacteriologic success. Table 2 shows the clinical data and PK/PD parameters in the bacteriologic success and bacteriologic failure groups.
The AUC/MIC and Cmax/MIC ratios in the patients for whom the pathogens were eradicated were significantly higher than those in the patients without the pathogens eradicated (75.3 [31.7–214.9] vs. 10.5 [3.8–66.1], Z = −3.938; 11.4 [4.2–17.8] vs. 1.4 [0.4–5.4], Z = −3.793; P < 0.001 for both) [Table 2].
The area under the ROC curve established using the bacteriologic response as a target scalar and AUC/MIC as the test variable was 0.967 (95% confidence interval [CI]: 0.976–1.000; P < 0.001), indicating the predictive value of AUC/MIC for a bacteriologic response [Figure 2C]. Based on the ROC curve, when the AUC/MIC threshold was 40.9, the sensitivity and specificity for predicting pathogen eradication were 85.7% and 92.3%, respectively.
The area under the ROC curve established using the bacteriologic response as a target scalar and Cmax/MIC as the test variable was 0.986 (95% CI: 0.944–1.000; P < 0.001), indicating the predictive value of Cmax/MIC for a bacteriologic response [Figure 2D]. Based on the ROC curve, the Cmax/MIC threshold was 3.7, and the sensitivity and specificity for predicting pathogen eradication were 100% and 90.5%, respectively.
Discussion
Antibiotic therapy in elderly patients is particularly challenging for clinicians due to the progressive age-dependent changes, and high frequency of accompanying chronic diseases such as chronic obstructive pulmonary disorder, chronic heart failure, or other underlying diseases, and polytherapy. Thus, the mortality rate of elderly patients with LRTIs remains relatively high,[13] especially among those infected with Gram-negative bacteria such as P. aeruginosa. Moreover, in recent years, the bacterial drug-resistance rate has been increasing, and finding effective antibacterial treatment is a clinical challenge.[14] Ciprofloxacin is a third-generation quinolone with antimicrobial activity against Gram-negative bacteria, particularly P. aeruginosa, and is commonly used in RICUs. In this study, we performed PK and PD analysis of ciprofloxacin in elderly patients with LTRIs caused by Gram-negative bacteria and evaluated the ciprofloxacin clinical and bacteriologic efficacy. The results showed that the AUC/MIC and Cmax/MIC values were significantly higher in the patients considered a clinical and bacteriologic success than in those considered a clinical and bacteriologic failure. ROC curve analysis further showed that the AUC/MIC and Cmax/MIC values were closely associated with the clinical and bacteriologic efficacy, which is consistent with the characteristics of dose-dependent antibiotics.
Most of the patients in this study did not achieve the target drug values AUC/MIC > 100 to 125 and Cmax/MIC > 8 to 10, and patients in the clinical failure group showed significantly lower values than the thresholds. These results implied that ciprofloxacin was inadequately dosed, and this inadequacy was particularly obvious when treating drug-resistant bacteria, in which the drug concentrations were all significantly lower than the target values, resulting in clinically ineffective antibacterial treatment.
Studies from other countries have indicated AUC/MIC > 100 to 125 and Cmax/MIC > 8–10 as the thresholds for predicting the clinical and bacteriologic efficacy of ciprofloxacin.[6,7,15,16] Forrest et al [7] analyzed the ciprofloxacin efficacy in 74 seriously ill patients, the majority of which had LRTIs. Among these patients, 82% were infected with Gram-negative bacteria and 15% were infected with Staphylococcus aureus. All patients received intravenous infusions of ciprofloxacin at 200 mg every 12 h or 400 mg every 8 h. They found that patients with AUC/MIC < 125 had clinical and bacteriologic success rates of 42% and 26%, respectively, whereas patients with AUC/MIC ≥ 125 had clinical and bacteriologic success rates of 80% (P < 0.005) and 82% (P < 0.001), respectively, suggesting that the AUC/MIC ratio is a reliable predictor of the ciprofloxacin efficacy.
With the increasing number of drug-resistant bacteria recorded in recent years, continuous evaluation of the rational use of antimicrobial drugs has attracted worldwide research attention. Most researchers have used the standard thresholds suggested by Forrest et al [7] to evaluate whether the ciprofloxacin dosing reached the target values in patients.[17,18] Haeseker et al [17] monitored the serum drug concentrations in 80 hospitalized patients using the ciprofloxacin AUC/MIC value of ≥125 as the index for predicting the antibacterial efficacy. Their results indicated that 21% and 75% of the patients did not reach the target value of >125 with MIC values of 0.25 and 0.5 mg/L, respectively, suggesting inadequate ciprofloxacin dosing. Perreiter et al [18] investigated ciprofloxacin dosing in a tertiary acute care medical center, including 76 patients with acute infections, 34% of whom had an AUC/MIC value <100. Most of these patients (79%) had received a total daily dose of 800 mg of ciprofloxacin as an intravenous infusion, whereas only 8% of the patients received a total daily dose of 1200 mg of ciprofloxacin as an intravenous infusion, which was the recommended dose for most severe infections, according to some guidelines used in many countries.[4] The authors concluded that based on the efficacy indices, only 26% of the patients were accurately dosed, while the remaining patients were underdosed.
Inappropriate ciprofloxacin dosing not only affects the therapeutic efficacy but may also lead to the development of selective drug resistance. Khachman et al [8] evaluated the ciprofloxacin drug concentrations in ICU patients using the fAUC0–24h/MIC (where f is the free drug concentration) value of ≥90 (equivalent to AUC0–24h/MIC ≥ 125) as a target value for predicting clinical efficacy, and the time inside the mutant selection window (TMSW) between 0 and 24 h of ≤20% as a target value for selective drug resistance. The results showed that the standard ciprofloxacin dosing regimen of 400 mg administered two or three times daily was not sufficient to achieve the target TMSW values when treating P. aeruginosa and A. baumannii infections. The fAUC0–24h/MIC target value was also not ideal. When the MIC values of the pathogens were 0.5 and 1 mg/L, and only 18% of the patients achieved the target TMSW value, suggesting inadequate ciprofloxacin dosing, which might lead to a poor antibacterial effect and the development of drug-resistant bacteria. In the past 20 years, other countries have adjusted the maximum ciprofloxacin dose to 1200 mg, that is, 400 mg every 8 h, based on PK/PD results.[4] However, in China, the maximum ciprofloxacin dose remains at 800 mg. Therefore, there is an urgent need to conduct more in-depth research on ciprofloxacin dosing regimens and its effectiveness in China using larger sample sizes.
The present ROC curve analysis showed that AUC/MIC > 40.9 could predict a good anti-infective effect, which largely differs from the previously suggested AUC/MIC threshold of >125.[6,7] This discrepancy may be due to the fact that in vitro study[14] or the few clinical studies reported on the AUC/MIC threshold, such as that conducted by Forrest et al [7] were based on ciprofloxacin monotherapy, whereas in this study, ciprofloxacin was combined with beta-lactams in all patients. Among the eight patients in the clinical success group, six had a P. aeruginosa infection. All six patients had an AUC/MIC value of >40.9, but only two patients had an AUC/MIC value of >100 (178.7 and 214.9, respectively). Both patients had a MIC value of 0.25 mg/L and were considered both clinical and bacteriologic success. The other three patients had MIC values of 0.25, 0.25, and 1.0 mg/L, and AUC/MIC values of 96.0, 89.4, and 45.8, respectively, and all were considered both clinical and bacteriologic success. The remaining patients had a MIC value of 1 mg/L and an AUC/MIC value of 54.9. Although also considered a clinical success, the patient did not achieve bacteriologic success. Using a MIC value of <1 mg/L as a threshold for sensitive P. aeruginosa strains, these results suggest that after the MIC value of the infecting bacterial strain is determined, ciprofloxacin can be used as monotherapy with an appropriate dosing regimen.
One of the limitations of this study was the small number of patients included, which could lead to a sample size bias. The thresholds of AUC/MIC and Cmax/MIC for predicting clinical efficacy were the same as those used for predicting bacteriologic efficacy, which may be related to the small sample. In addition, this study focused on the combination therapy of ciprofloxacin and beta-lactams, which, unlike ciprofloxacin monotherapy, might have interfered with analysis of the clinical and bacteriologic efficacy. In other words, bacterial growth might have been affected by both antimicrobials and on time-kill curves. However, during the study design and implementation phase, we considered that combination therapy might have a better effect than monotherapy in reducing the mortality rates in patients with Gram-negative bacterial infections, especially in those with P. aeruginosa infection.[19] Nevertheless, most of the Gram-negative bacteria identified in this study were found to be resistant to the combined beta-lactams in the sensitivity test. Specifically, in the eight clinical responders, three patients received a combination of cefoperazone/sulbactam (all MICs > 4 mg/L), three were treated with piperacillin/sulbactam (two strains, MIC < 4 mg/L; one strain, MIC > 4 mg/L), one was treated with cefepime (MIC > 4 mg/L), and one patient received ceftazidime (MIC > 4 mg/L). In vitro studies have demonstrated the synergistic effects of ciprofloxacin and beta-lactams,[19,20] which might explain why some patients in this study achieved clinical success, even though the target AUC/MIC value of >125 was not reached.
In conclusion, this study shows that the AUC/MIC and Cmax/MIC ratios are important indicators for determining the clinical and bacteriologic efficacy of ciprofloxacin in elderly patients with LRTIs. These results are consistent with the characteristics of dose-dependent antibiotics. Thus, ciprofloxacin dosing might be inadequate against pathogens with relatively high MIC values. The current treatment approach for treating ciprofloxacin-sensitive P. aeruginosa infections allows the achievement of a good clinical outcome. However, treatments of bacterial pathogenic strains that have higher MIC values should be based on an assessment of the PPK model and patient-specific MIC values to optimize dosing regimens.
Funding
The study was supported by a grant from the Capital Development Fund Program (No. 2009–2025).
Conflicts of interest
None.
Footnotes
How to cite this article: Gai XY, Bo SN, Shen N, Zhou QT, Yin AY, Lu W. Pharmacokinetic-pharmacodynamic analysis of ciprofloxacin in elderly Chinese patients with lower respiratory tract infections caused by Gram-negative bacteria. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000136
Xiao-Yan Gai and Shi-Ning Bo contributed equally to this work.
References
- 1. Arnold FW. How antibiotics should be prescribed to hospitalized elderly patients with community-acquired pneumonia. Drugs Aging 2017; 34:13–20. doi: 10.1007/s40266-016-0423-9. [DOI] [PubMed] [Google Scholar]
- 2. Ekren PK, Ranzani OT, Ceccato A, Li Bassi G, Munoz Conejero E, Ferrer M, et al. Evaluation of the 2016 Infectious Diseases Society of America/American Thoracic Society Guideline Criteria for risk of multidrug-resistant pathogens in patients with hospital-acquired and ventilator-associated pneumonia in the ICU. Am J Respir Crit Care Med 2018; 197:826–830. doi: 10.1164/rccm.201708-1717LE. [DOI] [PubMed] [Google Scholar]
- 3. Claeys KC, Zasowski EJ, Trinh TD, Lagnf AM, Davis SL, Rybak MJ. Antimicrobial stewardship opportunities in critically ill patients with gram-negative lower respiratory tract infections: a multicenter cross-sectional analysis. Infect Dis Ther 2018; 7:135–146. doi: 10.1007/s40121-017-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiem U, Heppner HJ, Pientka L. Elderly patients with community-acquired pneumonia: optimal treatment strategies. Drugs Aging 2011; 28:519–537. doi: 10.2165/11591980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6. Wright DH, Brown GH, Peterson ML, Rotschafer JC. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother 2000; 46:669–683. [DOI] [PubMed] [Google Scholar]
- 7. Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 1993; 37:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khachman D, Conil JM, Georges B, Saivin S, Houin G, Toutain PL, et al. Optimizing ciprofloxacin dosing in intensive care unit patients through the use of population pharmacokinetic-pharmacodynamic analysis and Monte Carlo simulations. J Antimicrob Chemother 2011; 66:1798–1809. doi: 10.1093/jac/dkr220. [DOI] [PubMed] [Google Scholar]
- 9. Conil JM, Georges B, de Lussy A, Khachman D, Seguin T, Ruiz S, et al. Ciprofloxacin use in critically ill patients: pharmacokinetic and pharmacodynamic approaches. Int J Antimicrob Agents 2008; 32:505–510. doi: 10.1016/j.ijantimicag.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 10. Woodhead M. New guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2011; 38:1250–1251. doi: 10.1183/09031936.00105211. [DOI] [PubMed] [Google Scholar]
- 11. Gai X, Shen N, He B, Zhou Q, Bo S, Li X, et al. Population pharmacokinetics of ciprofloxacin in Chinese elderly patients with lower respiratory tract infection (in Chinese). Natl Med J China 2015; 95:1581–1585. doi: 10.3760/cma.j.issn.0376-2491.2015.20.008. [PubMed] [Google Scholar]
- 12. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13. Alan M, Grolimund E, Kutz A, Christ-Crain M, Thomann R, Falconnier C, et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6-year prospective follow-up study. J Intern Med 2015; 278:174–184. doi: 10.1111/joim.12341. [DOI] [PubMed] [Google Scholar]
- 14. DiMondi VP, Townsend ML, Drew RH. Risk factors associated with unfavorable short-term treatment outcome in patients with documented Pseudomonas aeruginosa infection. Int J Clin Pharm 2015; 37:348–354. doi: 10.1007/s11096-015-0067-6. [DOI] [PubMed] [Google Scholar]
- 15. Shams WE, Evans ME. Guide to selection of fluoroquinolones in patients with lower respiratory tract infections. Drugs 2005; 65:949–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aminimanizani A, Beringer P, Jelliffe R. Comparative pharmacokinetics and pharmacodynamics of the newer fluoroquinolone antibacterials. Clin Pharmacokinet 2001; 40:169–187. doi: 10.2165/00003088-200140030-00003. [DOI] [PubMed] [Google Scholar]
- 17. Haeseker M, Stolk L, Nieman F, Hoebe C, Neef C, Bruggeman C, et al. The ciprofloxacin target AUC: MIC ratio is not reached in hospitalized patients with the recommended dosing regimens. Br J Clin Pharmacol 2013; 75:180–185. doi: 10.1111/j.1365-2125.2012.04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perreiter A, Nix DE, Matthias KR. Appropriateness of ciprofloxacin dosing based on a population pharmacokinetic model. Hospital Pharmacy 2015; 45:237–243. [Google Scholar]
- 19. Kmeid JG, Youssef MM, Kanafani ZA, Kanj SS. Combination therapy for Gram-negative bacteria: what is the evidence? Expert Rev Anti Infect Ther 2013; 11:1355–1362. doi: 10.1586/14787210.2013.846215. [DOI] [PubMed] [Google Scholar]
- 20. Kanellakopoulou K, Sarafis P, Galani I, Giamarellou H, Giamarellos-Bourboulis EJ. In vitro synergism of beta-lactams with ciprofloxacin and moxifloxacin against genetically distinct multidrug-resistant isolates of Pseudomonas aeruginosa . Int J Antimicrob Agents 2008; 32:33–39. doi: 10.1016/j.ijantimicag.2008.02.019. [DOI] [PubMed] [Google Scholar]


