To the Editor: Although the causes and mechanisms of lung cancer (LC) are still not clear, with the advances in science and technology and the progress in research methods, it has been shown that tumor cells aggregate with platelets (PLTs) and escape being recognized by the immune system. They adhere to distant vascular endothelial cells and continue to metastasize, invade and grow through the blood circulation.[1] Therefore, the hypercoagulable state and enhancement of PLT functions promote tumor growth, invasion, and metastasis.[2,3] Therefore, the effective reduction in PLT counts and their activities can help reduce the progression and prognosis of LC. We conducted this research to explore the effect of the tumor on circulating platelets in patients with LC.
Forty patients with LC (LC group) hospitalized in the 96th Hospital of the People's Liberation Army of China were selected for the study. This study follows relevant ethical regulations and is approved by the Ethics Committee of the 96th Hospital of the People's Liberation Army of China (2016 Number 40, the Jinan Military Region General Hospital). All the patients provided written informed consent. The group included 28 men and 12 women (mean age: 58.08 ± 10.22 years). Among these patients, 12, 26 and 2 had squamous cell carcinoma, adenocarcinoma, and small cell carcinoma, respectively. During the same period, 50 healthy individuals formed the control group and included 32 men and 18 women (mean age: 58.52 ± 11.93 years). Fasting venous blood samples were collected in the morning from both the LC and control group subjects who were not given hemostasis and anticoagulant medication for up to two weeks prior to the blood sampling. Thromboelastograms were drawn using a thromboelastograph, and PLT counts were determined using an automatic blood analyzer.
From the initial study sample, 30 NSCLC patients (17 men and 13 women, mean age: 61.27 ± 7.90 years) and 20 control individuals (11 men and nine women, mean age: 57.00 ± 10.08 years) were selected for determination of CD62P positivity.
Peripheral blood samples (2 mL) were centrifuged at 2000 r/min for 10 min. Using a straw-type pipette 100 μL of the intermediate gray cloudy interfacial layer, containing PLT-rich plasma was removed (making sure not to recover other layers) and transferred into a flow tube. PLT-rich plasma (5 μL each) was taken into test tube A (negative control tube) and test tube B (study tube). While CD61-FITC + IgG1-PE antibody (5 μL) was added to tube A, CD61-FITC + CD62P-PE antibody (5 μL) was added to tube B and incubated in the dark at room temperature for 20 min. Cells were washed once with 5 volumes of phosphate-buffered saline (PBS) solution, centrifuged at 2000 r/min for 5 min, and discarded the supernatant. CD62P positive rate = (CD61 + CD62P + count/CD61 + count) × 100%.
Compared with the control group, the LC group showed lower values of reaction time (R), K time (K), and PLT distribution width (PDW). However, values of angle, maximum amplitude (MA), coagulation composite index (CI), PLT, mean PLT volume (MPV) and FIB were higher in the LC group compared to the control group.
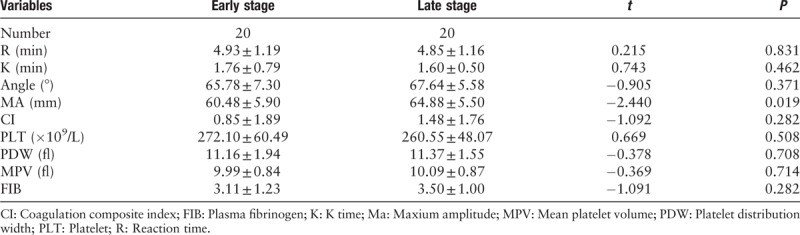
Based on the 2017 version of clinical TNM staging, patients were divided into two groups: early stage (I/II) and late stage (III/IV). The differences in MA values were statistically significant between the two groups [Table 1].
Table 1.
Comparison of the parameters between patients with different TNM stage cancers.
The PLT counts in the LC group was (250.60 ± 57.09) × 109/L, which was significantly higher than that in the control group (192.35 ± 43.37) ×109/L. At the same time, patients with LC also had a significantly higher rate (%) of CD62P positive expression (50.42 ± 15.42), compared to the control group (27.31 ± 18.16).
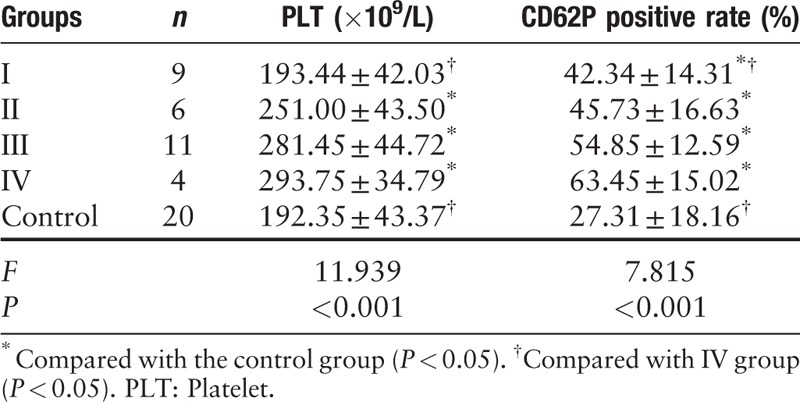
LC patients were classified as stage I (nine cases), stage II (six cases), stage III (11 cases), and stage IV (four cases). Compared to the control group, while the PLT counts in stage I patients (193.44 ± 42.03)×109/L were not significantly different, those in stages II, III, and IV patients were significantly higher and also increased with the stage of the disease. Similarly, the rate of CD62P positivity in stage I, II, III, and IV patients was significantly higher compared to the control group, and increased with advancing stage. The CD62P expression in stage IV patients was significantly higher than that in stage I patients [Table 2].
Table 2.
Comparison of expression of CD62P on PLT surface with different TNM staging.
High PLT numbers and expression of fibrinogen are often observed in NSCLC patients with local regional or distant metastasis.[4] In this study, evaluation of thromboelastography (TEG) plots showed an increase in the MA. A larger MA value reflects increased PLT aggregation. The series of changes are indicative of the abnormal changes in coagulation, fibrinolysis, and PLTs caused by the tumor.
PLTs play an important and multifaceted role in cancer progression.[5] We analyzed findings of blood tests and found that PLT numbers and their morphology in patients with LC were significantly different from the control group.
It has been reported that MA values in the group with later stage cancer were significantly increased, indicating a direct relationship between tumor stage and PLT function. TEG and PLT counts were, therefore, evaluated to assess if the patients are in a hypercoagulable state to determine the TNM stage and prognosis.
Structural changes in PLTs have been shown to play an important role in their functional changes. The main functions of PLTs include adhesion and aggregation (white thrombosis), secretion and release (release of particulate matter), enhancement of coagulation factors and endothelial cell function, and deformation (morphological changes). Receptor activation results in a series of physiological and chemical changes, which constitute the pathophysiological basis of PLT-dependent thrombosis.[6]
We first analyzed that, compared to the control group, PLT counts in stage I patients were not significantly different, while those in stages II, III, and IV patients were all comparable and significantly higher. Our findings suggest that the PLTs count does not change significantly in the early stages of LC, but increase as the stage of the disease advances. The PLT counts in phase IV were significantly higher than those in phase I. It can be seen that in the early stage, especially stage I, the PLT count does not clearly reflect the existence of LC. The longer the disease course and later the disease stage, the more pronounced is the increase in PLT counts,[7] which is consistent with our findings.
We detected the viscoelasticity of coagulation clots during coagulation by TEG and found that the tumor causes an increase in the aggregation function of PLTs in peripheral blood. Flow cytometric analysis revealed that the rate of CD62P positivity on PLT membranes in the LC group (50.42 ± 15.42%) was significantly higher than in the control group (27.31 ± 18.16%). The rate of CD62P positivity in stages I, II, III, and IV was significantly higher than that in the control group. It is suggested that as the tumor size increases with increasing stage, the adhesion capacity of PLTs also increase. Treatment of early stage (I) patients for inhibition of PLT function should, therefore, be considered. In general, stage IV disease often has distant metastatic lesions which are difficult to be cured surgically. It is suggested that the rate of CD62P positivity in LC patients is significantly associated with the occurrence, development, and distant metastasis of LC. We also found that the rate of CD62P positivity in stage IV was significantly higher than that in stage I.
More and more evidence show that compared with healthy control subjects, the PLT counts in LC patients are significantly increased, they are in an activated state, and their membrane glycoproteins are significantly altered and overexpressed, which are important factors in promoting thrombosis. This study confirmed that LC is an important factor in increasing the PLT counts and enhancing their adhesion and aggregation function, especially in patients with late-stage cancer and lymph node metastasis. With the increase of the stage, the PLT counts and their functional activation is increased, which promotes and metastasis of LC cells.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. They understand that the patient's name and initials will not be published, and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang SS, Zhang M, Yuan L, Zou ZQ. Analysis of platelet parameters and activation markers in hematologic metastases of lung cancer. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000138
References
- 1. Chen J, Li GD. Pathology. 2nd edition. 2010; Beijing: People's Medical Publishing House, 146–147. [Google Scholar]
- 2. Kim KH, Park TY, Lee JY, Lee SM, Yim JJ, Yoo CG, et al. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci 2014; 29:507–511. doi: 10.3346/jkms.2014.29.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tormoen GW, Haley KM, Levine RL, McCarty OJ. Do circulating tumor cells play a role in coagulation and thrombosis? Front Oncol 2012; 2:115 doi: 10.3389/fonc.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu JF, Cai L, Zhang XW, Wen YS, Su XD, Rong TH, et al. High plasma fibrinogen concentration and platelet count unfavorably impact survival in non-small cell lung cancer patients with brain metastases. Chin J Cancer 2014; 33:96–104. doi: 10.5732/cjc.012.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim Y, Seok JY, Hyun KY, Lee GH, Choi SC. Correlation of Glasgow prognostic score or procalcitonin to clinical variables in patients with pretreatment lung cancer. Biomed Sci Lett 2016; 22:9–17. doi.org/10.15616/BSL.2016.22.1.9. [Google Scholar]
- 6. Yang C, Wang JX, Han Y. Research advance in platelet function assays. J Exp Hematol 2007; 15:1130–1134. doi: 10.3969/j.issn.1009-2137.2007.05.048. [PubMed] [Google Scholar]
- 7. Gu LY, Wang X, Wang Y, Zhong D. Relationship between thrombocytosis and effect of chemotherapy, prognosis in patients with advanced non-small cell lung cancer. Cancer Res Clinic 2015; 27:35–38. doi: 10.3760/cma.j.issn.1006-9801.2015.01.009. [Google Scholar]


