To the Editor: The incidence of esophageal atresia (EA) along with tracheoesophageal fistula (TEF) is approximately 1/3000 in newborns.[1] A large population study revealed that 46% of patients with TEF/EA had at least one of the other vertebral, anal, cardiac, tracheal, esophageal, renal, and limb (VACTERL) malformations.[2] Duodenal obstruction has an incidence of 1/7000 live births and can be caused by duodenal atresia (DA) or annular pancreas (AP).[3] AP is an uncommon congenital anomaly, and duodenal obstruction most commonly presents in the infancy or early childhood of patients.[4] The incidence of DA combined with EA varies between 3% and 6%,[5] and DA combined with EA is often associated with significant morbidity and mortality.[6] Combined presence of EA/TEF and duodenal obstruction always lead to several management challenges, such as determination of the treatment order and the treatment pattern (together or staged).[7] There is no relevant surgical strategy or literature for the complicated cases of combined digestive tract atresia in different parts of the chest and abdomen. The management protocol for this combination of anomalies is not well defined and controversial. A gastrostomy tube placed first for gastric decompression and staged repair (ideally within days, weeks) have been suggested by most surgeons.[8]
On the basis of long-term clinical work, this study summarized the experiences of diagnosis and treatment of complicated multiple digestive tract obstruction in four infants with combined TEF/EA and severe duodenal obstruction caused by DA or AP, who were treated at the Department of Pediatric Surgery, the Northwest Women's and Children's Hospital from January 2015 to January 2018. The clinical details of the four infants were shown in Table 1. Polyhydramnios was noted in common, and one of the babies had a delayed diagnosis of associated DA.
Table 1.
Clinical characteristics of four infants with esophageal and duoenal anomalies.
Patient 1: The patient was delivered due to cesarean section scar uterus, premature rupture of membranes and umbilical cord around neck for 360°. On prenatal ultrasound, too much amniotic fluid was noted. However, the double bubble sign and polyhydramnios were not observed. His Apgar score was 10 at 1 min, 10 at 5 min, and 10 at 10 min. His anus was patent, and renal ultrasound was within normal limits. Attempts to pass a nasogastric tube (NGT) were unsuccessful. Chest films demonstrated that the NGT was in the upper esophagus, suggesting that there was a presence of EA but no any vertebral anomalies [Figure 1A]. A cardiac echo revealed a patent ductus arteriosus (PDA) and acleistocardia. On the next day after birth, the patient received the first urgent surgery of right posterior lateral thoracotomy and extrapleural separation. The azygos vein was mobilized and divided. The intra-operative pathologic anatomy was shown in Figure 2A. Ligation of TEF and “diamond-shape” anastomosis of EA was performed for primary repair [Figure 2E–2H]. An extrapleural chest tube was not inserted into the right chest. Feedings were subsequently introduced but not tolerated, resulting in large bilious nasogastric aspirates. On the ninth day after the first operation for EA/TEF, a repeat upper gastrography confirmed the presence of esophageal anastomosis patency and a delayed diagnosis of the associated DA [Figure 1B]. Then the patient received exploratory laparotomy and duodenoduodenostomy for DA repair. A nasojejunal feeding tube was placed during the DA repair. Exploratory laparotomy on the 17th day ruled out malrotation but could not exclude duodenal obstruction. The patient started to accept enteral feedings via a NGT on the 22nd day. As oral feeding increased, feedings via the NGT was reduced. The patient reached full volume oral feeds on the 27th day when the NGT was removed. At the 3-month follow-up, the patient weighed 4250 g. A repeat upper gastrointestinal imaging confirmed the presence of esophageal and duodenal anastomosis patency well [Figure 1F]. At 20-month follow-up, the patient was in good health condition.
Figure 1.
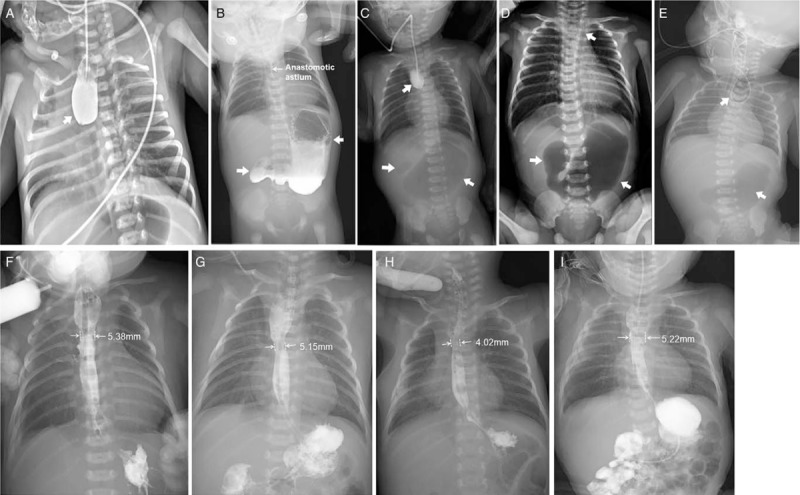
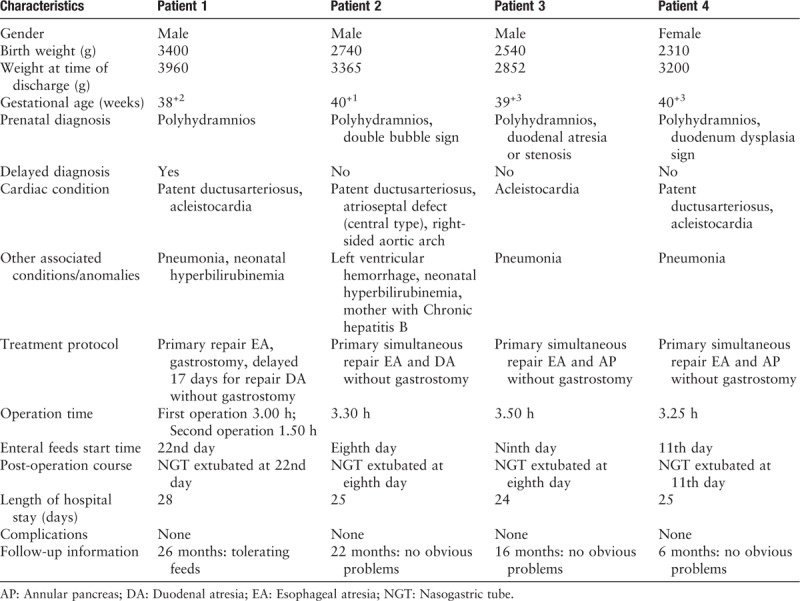
The X-ray series films of the 4 patients with esophageal and duoenal anomalies. Patient 1: esophageal radiography confirmed esophageal atresia (arrow; A); upper gastrography confirmed the presence of esophageal anastomosis patency on the ninth day after the first stage operation and demonstrated a large dilated stomach and the absence of distal bowel gas (arrows; B); and a repeat upper gastrointestinal imaging confirmed esophageal stoma after “diamond-shape” anastomosis during follow-up (F). Patient 2: esophageal radiography revealed esophageal atresia and a distended stomach with no distal bowel gas (arrows; C), suggesting duodenal obstruction and no vertebral anomalies; and a repeat upper gastrointestinal imaging confirmed esophageal stoma after “diamond-shape” anastomosis during follow-up (G). Patient 3: A preoperative X-ray film demonstrating showing combined coiled-up of NGT in the upper esophageal pouch and large gastric bubble with no distal bowel gas (arrows; D); and a repeat upper gastrointestinal imaging confirmed esophageal stoma after “diamond-shape” anastomosis during follow-up (H). Patient 4: a combined chest and abdominal X-ray demonstrated that the nasogastric tube was in the upper esophageal pouch. Vertebral anomalies and a large gastric bubble with no distal bowel gas were observed, suggesting tracheoesophageal fistula/esophageal atresia complicated with duodenal obstruction (E); and a repeat upper gastrointestinal imaging confirmed the presence of esophageal and duodenal anastomosis patency well during follow-up (I).
Figure 2.
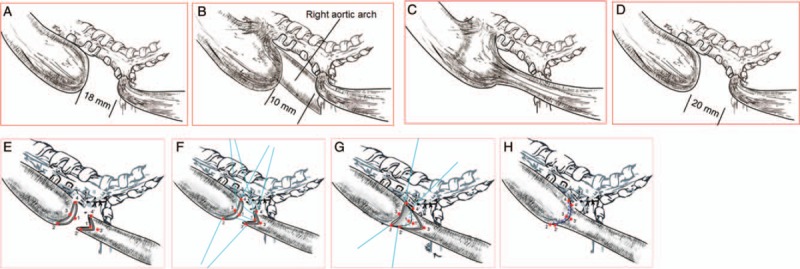
Schematic diagrams of gross relative positions taking their orientation of the EA/TEF in the 4 cases. (A) Esophageal atresia with a distal tracheoesophageal fistula, the most frequently encountered form of esophageal anomaly. (B) Atresia appearance seemed with a double (proximal and distal) fistula on gross, but the esophageal atresia was confirmed only with a distal tracheoesophageal fistula and a right-sided aortic arch be found in operation. (C) Appearance seemed with H-type fistula but the double ends were not connected and there was only distal tracheoesophageal fistula with atresia. (D) The intra-operative pathologic anatomy showed esophageal atresia with a distal tracheoesophageal fistula. (E–H) Schematic pictures of the intra-operative “diamond-shape” anastomosis of EA performed for primary repair in the 4 cases. The esophageal atresia proximal blind end with a transverse cut and distal end with longitudinal cut, to make 2 esophageal end surface in “diamond-shape”. The absorb suture for 1-1’, 2-2’, 3-3’ and 4-4’ point to point corresponding anastomosis. Complete the “diamond-shape” anastomosis of EA. EA: Esophageal atresia; TEF: Tracheoesophageal fistula.
Patient 2: The patient was delivered through vaginal delivery. His Apgar scores were 9, 10, and 10 at 1, 5, and 10 min, respectively. Mother had chronic hepatitis B (HBeAg), and the prenatal ultrasound demonstrated polyhydramnios and the double bubble sign. The initial esophageal radiography suggested EA, and abdominal X-rays showed no vertebral anomalies. However, a distended stomach with no distal bowel gas was observed, suggesting duodenal obstruction [Figure 1C]. The failure of passing a NGT confirmed the presence of EA and TEF. His anus was patent, and the renal ultrasound was within the normal limits. Postnatal echo showed a PDA with atrial septal defect (central type) and a right-sided aortic arch. At 24 h after birth, the patient received an emergency operation, in which an operation repairing the EA/TEF and duodenoduodenostomy repairing DA were synchronously performed. The intra-operative pathologic anatomy was shown in Figure 2B. The surgical procedure was the same as the first case, followed by a right thoracotomy with ligation of the TEF, and “diamond-shape” anastomosis for primary repair of EA [Figure 2E–2H]. The chest operation lasted for 1.6 h and chest tube was not placed in the right chest. The abdominal exploration operation was then immediately started during the same anesthesia. On the eighth day, a swallow test showed no esophageal or duodenal leakage. On the 10th day, enteral feedings were initiated, and the infant received full volume oral feedings by the16th day. At the 3-month follow-up, the patient showed adequate growth, without signs of reflux or respiratory problems. A repeat upper gastrointestinal imaging confirmed the presence of esophageal and duodenal anastomosis patency well [Figure 1G]. The patient was in good health at 16-month follow-up.
Patient 3: The patient was born via vaginal delivery. His Apgar scores were 8, 9 and 9 at 1, 5 and 10 min, respectively. The prenatal ultrasound demonstrated polyhydramnios. He was pink and vigorous on room air. An NGT could not be passed successfully. The chest and abdominal X-ray demonstrated that the NGT was in the upper esophageal pouch [Figure 1D], and mediastinal shift, vertebral anomalies and a large gastric bubble with no distal bowel gas were observed, suggesting it had TEF/EA complicated with duodenal obstruction and right lung hypoplasia. The duodenal obstruction was caused by AP. An emergency surgery was performed on the next day after birth. An operation repairing the EA/TEF and duodenoduodenostomy repairing AP were synchronously performed. The intra-operative pathologic anatomy was shown in Figure 2C. The operation procedure was the same as the first case [Figure 2E–2H]. On the ninth day, enteral feedings initiated, and the infant received full volume oral feedings by the 14th day. The NGT was extubated on the eighth day and oral feedings were started on the ninth day after the swallow test, which revealed no esophageal or duodenal leakage. A repeat upper gastrointestinal imaging confirmed the presence of esophageal and duodenal anastomosis patency well [Figure 1H]. The patient was in good health at 10-month follow-up.
Patient 4: The patient was delivered due to fetal distress. Prenatal ultrasound demonstrated polyhydramnios and duodenum dysplasia sign. Her Apgar score was 7 at 1 min, 8 at 5 min and 8 at 10 min. Her anus was patent, and renal ultrasound was within normal limits. Attempts to pass a NGT were unsuccessful. Chest films demonstrated that the NGT was in the upper esophagus, suggesting the presence of EA but without any vertebral anomalies [Figure 1E]. A cardiac echo revealed a patent ductusarteriosus (PDA) and acleistocardia. An NGT could not be passed successfully. A combined chest and abdominal X-ray demonstrated the NGT was in the upper esophageal pouch [Figure 1E]. Vertebral anomalies and a large gastric bubble with no distal bowel gas were observed, suggesting TEF/EA complicated with duodenal obstruction. An emergency surgery was performed and AP caused duodenal obstruction was confirmed on the next day after birth. An operation repairing the EA/TEF and duodenoduodenostomy repairing AP were performed at same time. The intra-operative pathologic anatomy was shown in Figure 2D. The operation procedure was the same as the second and third cases [Figure 2E–2H]. The patient started to accept enteral feedings via a NGT on the 11th day. As oral feeding increased, feedings via the NGT was reduced. The patient reached full volume oral feeds on the 20th day. At the 3-month follow-up, the patient weighed 6100 g. A repeat upper gastrointestinal imaging confirmed the presence of esophageal and duodenal anastomosis patency well [Figure 1I]. At the 6-month follow-up, the patient was in good health condition.
DA was well-described association of TEF/EA and the prevalence was approximately 6%, and this combined anomaly was associated with significant morbidity and mortality.[5,6] A study involving 17 patients with combined defects reported an overall survival rate of 88%.[7] Therefore, associated DA in babies with EA is a cause of increased morbidity. The combined occurrence of TEF, EA, and DA leads to several management challenges. On the one hand, the presence of TEF predisposes the patient to respiratory compromise from aspiration. TEF also fills the stomach with air that cannot traverse through the rest of the gastrointestinal tract due to the DA. This gastric distension also cannot be decompressed with a NGT due to the EA. Therefore, it is difficult to determine which condition should be addressed first and whether the defects should be treated together or in a staged manner.
Although the management of EA/TEF and DA as isolated lesions is well defined with an established trend of not performing gastrostomy in most patients, the treatment protocol for combined EA/TEF and DA varies in the published literature.[6] The authors also recommended a staged repair of EA/TEF with mandatory gastrostomy first, followed by duodenoduodenostomy a few days later. In the absence of an associated TEF, a primary duodenoduodenostomy and gastrostomy with a transanastomotic feeding tube is the approach of choice.
In this report, one of the patients underwent a delayed repair of DA. During the first operation, the EA-TEF repair was carried out via a posterolateral thoracotomy using an extrapleural approach. The other 3 newborns with combined EA/TEF and duodenal obstruction caused by DA or AP safely received one surgery to synchronously repair the anomalies. Our experience suggested that an early combined chest and abdomen X-ray examination was helpful for DA diagnosis. Two patients had esophageal secretions due to aspiration pneumonia. Besides this, none of the patients suffered significant pre- or post-operative complications, and none of the associated anomalies compromised the patient's recovery. There was no death. The average hospital stay length was 20 days. The follow-up data (between 7 and 28 months) of this study suggested that all patients eventually outgrew their reflux and respiratory symptoms. There were no patients with anastomotic strictures or severe gastroesophageal reflux disease in this study.
The gastrostomy might provide better decompression of the stomach and hence protect the oesophageal and duodenal anastomoses. However, this argument is disputed. Dave et al [9] concluded that a primary simultaneous repair of these anomalies without a gastrostomy was adequate without notable increase in the morbidity or mortality compared to a staged approach. In addition, they argued that the use of the Kimura diamond anastomosis for DA allowed for early functioning of the duodenal anastomosis and might preclude the use of a gastrostomy in patients with EA and DA. Although with the limited experience, we believed that the omission of a gastrostomy in neonates with combined EA/TEF and DA was justifiable and safe.
Simultaneous correction of combined major anomalies in neonates can be tried due to advances in neonatal surgery, anesthesia and intensive care. In this report, simultaneous repair (EA repair followed by AP repair) in the last three patients was performed. No mortality or significant additional morbidity was observed. None of our patients suffered significant pre-operative complications. At the end of follow-up, all patients were able to tolerate enteral feedings without significant sequelae from their congenital conditions or surgeries. Our results further confirmed the effectiveness of a simultaneous repair. We expected that the experience and lessons of the diagnosis and treatment of the four cases of complicated digestive tract multiple atresia in the newborn can provide some reference values for the peers. Due to the rareness, there was no consensus regarding the optimal treatment strategy for the combined TEF/EA and duodenal obstruction. We believed that in a stable baby, a primary simultaneous repair of the EA and AP should be attempted. An initial thoracotomy with fistula ligation and oesophageal repair through extrapleural separation should be performed. This might abolish the risk of aspiration and allow the laparotomy to proceed under stable ventilatory status. Surgical therapy of EA/TEF is evolving; thoracoscopic ligation is increasingly being performed.[10] In the future, we will try our best to complete the operation treatment of multiple malformations by combining thoracoscope and laparoscope.
In conclusion, we recommended a synchronous operation repairing the EA/TEF and performing duodenoduodenostomy in one surgery without gastrostomy for the treatment of combined EA/TEF and duodenal obstruction. It is of great importance to identify the anomalies in TEF/EA beyond the VACTERL spectrum and to develop new therapies in infants with multiple anomalies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients’ parents/guardians have given their consents for the images and other clinical information to be reported in the journal. The patients’ parents/guardians understand that the names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgements
We thank Dr. Rui Yan at Department of Radiology, the Northwest Women's and Children's Hospital for imaging examination. We are very grateful for the support and assistance of the staff of the medical record information management department for this study.
Funding
This work was supported by a grant from the National Natural Science Foundation of Shaanxi Province, China (No. 2014D85).
Conflicts of interest
None.
Footnotes
How to cite this article: Cao ZP, Li QF, Liu SQ, Niu JH, Zhao JR, Chen YJ, Wang DY, Li XS. Surgical management of newborns with combined tracheoesophageal fistula, esophageal atresia, and duodenal obstruction. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000102
Zhu-Ping Cao and Qi-Feng Li contributed equally to this study.
References
- 1. Fraser C, Baird PA, Sadovnick AD. A comparison of incidence trends for esophageal atresia and tracheoesophageal fistula, and infectious disease. Teratology 1987; 36:363–369. doi: 10.1002/tera.1420360313. [DOI] [PubMed] [Google Scholar]
- 2. McMullen KP, Karnes PS, Moir CR, Michels VV. Familial recurrence of tracheoesophageal fistula and associated malformations. Am J Med Genet 1996; 63:525–528. doi: 10.1002/tera.1420360313. [DOI] [PubMed] [Google Scholar]
- 3. Escobar MA, Ladd AP, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, 3rd, et al. Duodenal atresia and stenosis: long-term follow-up over 30 years. J Pediatr Surg 2004; 39:867–871. doi: 10.1016/j.jpedsurg.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 4. Yigiter M, Yildiz A, Firinci B, Yalcin O, Oral A, Salman AB. Annular pancreas in children: a decade of experience. Eurasian J Med 2010; 42:116–119. doi: 10.5152/eajm.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ein SH, Shandling B, Wesson D, Filler RM. Esophageal atresia with distal tracheoesophageal fistula: associated anomalies and prognosis in the 1980s. J Pediatr Surg 1989; 24:1055–1059. doi: 10.5152/eajm.2010.33. [DOI] [PubMed] [Google Scholar]
- 6. Spitz L, Ali M, Brereton RJ. Combined esophageal and duodenal atresia: experience of 18 patients. J Pediatr Surg 1981; 16:4–7. doi: 10.1016/S0022-3468(81)80105-4. [DOI] [PubMed] [Google Scholar]
- 7. Ein SH, Palder SB, Filler RM. Babies with esophageal and duodenal atresia: a 30-year review of a multifaceted problem. J Pediatr Surg 2006; 41:530–532. doi: 10.1016/j.jpedsurg.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 8. Nabzdyk CS, Chiu B, Jackson CC, Chwals WJ. Management of patients with combined tracheoesophageal fistula, esophageal atresia, and duodenal atresia. Int J Surg Case Rep 2014; 5:1288–1291. doi: 10.1016/j.ijscr.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dave S, Shi EC. The management of combined oesophageal and duodenal atresia. Pediatr Surg Int 2004; 20:689–691. doi: 10.1007/s00383-004-1274-8. [DOI] [PubMed] [Google Scholar]
- 10. Ma L, Liu YZ, Ma YQ, Zhang SS, Pan NL. Comparison of neonatal tolerance to thoracoscopic and open repair of esophageal atresia with tracheoesophageal fistula. Chin Med J 2012; 125:3492–3495. doi: 10.3760/cma.j.issn.0366-6999.2012.19.023. [PubMed] [Google Scholar]


