ABSTRACT
Increased blood platelet activation, especially platelet aggregation, plays an important function in cardiovascular disease; however, various dietary components may inhibit platelet activation. Recent clinical and epidemiologic studies indicate that both fruits and vegetables, and their products, contain various phytoprotective substances possessing biological properties such as antiplatelet and antioxidant effects that may work synergistically to ameliorate the effect of cardiovascular disease. In addition, the consumption of vegetables and their products may also play an important role in prevention. However, the mechanisms involved have not been clearly defined. Various studies clearly indicate that certain vegetables (e.g., onions, garlic, and tomatoes) have beneficial effects on blood platelet hyperactivity, an important cardiovascular risk factor, and hence may offer new prophylactic and therapeutic possibilities for the treatment of blood platelet hyperactivation and cardiovascular disease. This mini-review evaluates the current literature on the relationship between the consumption of onion (Allium cepa L.), garlic (Allium sativum L.), tomato (Solanum lycopersicum L.), and beetroot (Beta vulgaris L.), and blood platelet activation, which may have important implications for the prophylaxis and treatment of cardiovascular disease.
Keywords: blood platelet, vegetable, platelet activation, aggregation
Introduction
Blood platelets are irregular, small (2–3 µm), anucleated elements produced from megakaryocytes. About 1011 blood platelets per day are generated, and their half-life ranges from 7 to 10 d in circulation (1). Blood platelets include 3 types of granules (dense, α, and lysosomal granules) composed of a wide range of components with various biological properties, such as adhesive proteins, coagulation factors, growth factors, and chemokines (2). The platelets can be activated by various physiologic agonists, stimulators, or activators, including ADP, thrombin, arachidonic acid, and collagen, which act as ligands that bind to receptors in platelet membrane. Platelet activation induced by these agonists involves a range of biochemical processes such as generation of reactive oxygen species (ROS) and reactive nitrogen species, phosphoinositide hydrolysis, and eicosanoid synthesis, which often produce secondary messengers (3–5). Blood platelets play an important function in many physiologic processes, including hemostasis, which regulates the flowing properties of blood, and dysregulation of platelet activation is associated with various diseases, particularly cardiovascular disease. In 2015, the WHO identified cardiovascular disease as the leading single cause of global mortality, being responsible for about one-third of deaths globally (6).
Lifestyle factors play an important part in the etiology and treatment of cardiovascular disease. One such factor is nutrition. Zheng et al. (7) reported that a diet rich in fruits has a significant influence, and studies by other authors found fruit consumption to lower the risk of cardiovascular disease by various mechanisms, one of which is the inhibition of blood platelet activation (8–10). In addition, recent evidence reveals that certain vegetables and their products (including oils) may also act as key mediators in the prevention and treatment of cardiovascular disease, including atherosclerosis, arteriosclerosis, and thrombosis, by reducing blood platelet activation, especially platelet aggregation (11, 12). A review of epidemiologic studies, experimental research, and clinical trials examining the effects of celery, lettuce, rape, pumpkin, carrot, broccoli, and pea on cardiovascular disease by Tang et al. (13) found the vegetables to demonstrate a range of cardioprotective effects, including improving endothelial function, lowering blood pressure, modifying lipid metabolism, and regulating blood glucose concentration, as well as possessing general antioxidant and anti-inflammation properties. However, this review did not provide any information about their effects on blood platelet function. Hence, the present minireview examines the current literature concerning the influence of selected vegetables, namely onion (Allium cepa L.), garlic (Allium sativum L.), tomato (Solanum lycopersicum L.), and beetroot (Beta vulgaris L.), on blood platelet activation, especially platelet aggregation. These findings may have important implications for the prophylaxis and treatment of cardiovascular disease.
Effect of Selected Vegetables on Blood Platelet Functions
Onion (Allium cepa L.)
Onion (A. cepa L.) is among the oldest of all cultivated plants and has been used medicinally for thousands of years. Its biological properties may be associated with its alkyl cysteine sulfoxide content as well as its collection of phenolic compounds, especially quercetin and its derivatives. Onions themselves and their preparations have demonstrated therapeutic activity against cardiovascular disease associated with hyperactivation of blood platelets in a number of studies, and are known to offer beneficial effects in preventing coronary thrombosis, atherosclerosis, and stroke (14–17). Onions have also been found to inhibit blood platelet aggregation stimulated by different agonists in vivo and in vitro (16, 18). For example, Briggs et al. (19) reported that onion juice reduces platelet aggregation induced by collagen in an in vitro study of dog whole blood. Ali et al. (20) reported that rabbit platelet aggregation induced by 2 µg/mL collagen was inhibited in a dose-dependent manner by a boiled aqueous extract of onion, with 50% inhibition observed at a concentration of 90 mg onion extract/mL blood plasma. Blood platelet aggregation was monitored by turbidimetry for platelet-rich plasma (PRP).
A recent study based on washed rat platelets and PRP by Ro et al. (21) found onion peel extract (50, 100, and 500 µg/mL) to have antiaggregation properties in vitro. The antiaggregation activity of the extract was correlated with that of its main component, a phenolic compound called quercetin. The extract was dissolved in 50% methanol, and then analyzed by HPLC. The content of quercetin was 16.7% ± 0.1% of tested extract. Ewald et al. (22), found quercetin to be one of the most abundant phenolic compounds in vegetables, including onions. Moreover, epidemiologic data suggest that a diet rich in quercetin may be associated with a reduced risk of cardiovascular disease (23, 24). Ro et al. (21) examined various parameters of blood platelet activation, including platelet aggregation, induced by 5 µg/mL collagen, Ca2+, thromboxane B2 (TXA2), cyclic adenosine 5′-monophosphate (cAMP), and cyclic guanosine 5′-monophosphate (cGMP), in washed platelets and in PRP. However, Lee et al. (25) reported that quercetin-rich onion peel extract does not affect platelet aggregation or the hemostatic activity of plasma in vivo, including prothrombin time and activated partial thromboplastin time (Table 1). In this experiment, after washing onion peels 3 times in water, onion peel extract was extracted with 60% aqueous ethanol solution. However, again the authors did not analyze the chemical content of the extract (25). A study of the antithrombotic effects of 80% methanol extracts of 10 different onion varieties (Kitamiko27, Toyohira, Kitawase3, Tsukisappu, Superkitamomiji, CS3–12, Tsukiko22, Rantaro, 2935A, and K83211) by Yamada et al. (26) found Toyohira to have antiplatelet activity in vitro and in vivo; however, no correlation was observed between the quercetin content of a variety and its biological activity. A study by Hubbard et al. (27) investigated the effects of quercetin ingestion from a dietary source, i.e. 2 soups containing either a low (5 mg) or high (69 mg) amount of quercetin, on collagen-stimulated human blood platelet aggregation. Following ingestion of the high-quercetin soup, aggregation was found to be inhibited in a time-dependent manner; Syk tyrosine phosphorylation was also significantly inhibited, and this finding was correlated with the amount of quercetin in plasma. In this study, the tested soups contained naturally occurring quercetin.
TABLE 1.
The effect of various vegetables on selected properties of blood platelets (in vitro and in vivo experiments)1
| Vegetable | Inhibition of platelet aggregation | Inhibition of platelet adhesion | Inhibition of TXA2 synthesis | Inhibition of cAMP production | Stimulation of platelet aggregation | Stimulation of platelet adhesion | Stimulation of TXA2 synthesis | Stimulation of cAMP production |
|---|---|---|---|---|---|---|---|---|
| In vitro experiments | ||||||||
| Onion (Allium cepa L.) | Rat platelets, concentration of onion peel extract (16.7% ± 0.1% quercetin in extract): 50, 100, and 500 µg/mL; agonist: 5 µg/mL collagen (22) | — | Rat platelets, concentration of onion peel extract (16.7% ± 0.1% quercetin in extract): 50, 100, and 500 µg/ml; agonist: 5 µg/mL collagen (21) | — | — | — | — | — |
| Rabbit platelets, concentration of boiled aqueous extract (chemical contents: undefined) of whole onion: 6, 12, 24, and 48 mg/mL; agonist: 2 µg/mL collagen (20) | — | — | — | — | — | — | — | |
| Rat and human platelets, concentration of aqueous extract (chemical contents: undefined) of whole onion: 0.05, 0.1, 0.5, and 1 g/mL; agonists: 6 µg/mL collagen, 0.4 U/mL thrombin, 100 µM arachidonic acid (18) | — | Rat and human platelet, concentration of aqueous extract (chemical contents: undefined) of whole onion: 0.05, 0.1, 0.5, and 1 g/mL; agonist: 6 µg/mL collagen (18) | — | — | — | — | — | |
| Human platelets, concentration of onion thiosulfinates: 0.1, 0.2, 0.4, 0.6, 0.8, and 1 mM; agonist: 5 µg/mL collagen (28) | — | — | — | — | — | — | — | |
| Human platelets, concentration of alk(en)yl thiosulfates from whole onion: 0.001, 0.01, 0.1, and 1 mM; agonist: 2 µM ADP (29) | — | — | — | — | — | — | — | |
| Human platelets, raw juice, after cooking (chemical contents: undefined); agonist: 1 µg/mL collagen (31) | — | — | — | — | — | — | — | |
| Welsh onion (Allium fistulosum L.) | Human platelets, concentration of raw extract (chemical contents: undefined) of Welsh whole onion: 0.1, 0.2, 0.5, 1, 2, and 4 mg/mL; agonist: 1 µM ADP (32, 33) | Adhesion to fibrinogen. Human platelets, concentration of raw extract (chemical contents: undefined) of Welsh whole onion: 0.1, 0.2, 0.5, 1, 2, and 4 mg/mL; agonist: 1 µM ADP (32, 33) | Human platelets, concentration of raw extract (chemical contents: undefined) of Welsh whole onion: 0.1, 0.2, 0.5, 1, 2, and 4 mg/mL; agonist: 1 µM ADP (32, 33) | — | Human platelets, concentration of boiled extract (chemical contents: undefined) of Welsh whole onion: 0.1, 0.2, 0.5, 1, 2, and 4 mg/mL; agonist: 1 µM ADP (32, 33) | — | — | — |
| In vivo experiments | ||||||||
| Onion (Allium cepa L.) | Raw onion homogenate (chemical contents: undefined) intragastrically, 2 g/kg, n = 6 dogs. Aggregation stimulated by different agonists (19) | — | — | — | 5% onion powder (chemical contents: undefined) for 4 wk; n = 40 hypercholesterolemic rats treated with simvastatin. Aggregation stimulated by 2 µM ADP (34) | — | — | — |
| Aqueous extract of whole onion (chemical contents: undefined), 0.5 g · mL–1 · kg–1 · d–1, for 4 wk, n = 5 normal and diabetic rats. Aggregation stimulated by different agonists (35) | — | Aqueous extract of whole onion (chemical contents: undefined), 0.5 g · mL–1 · kg–1 · d–1, for 4 wk, n = 5 normal and diabetic rats (35) | — | — | — | — | — | |
| Two onion soups contained a low (5 mg) and high (69 mg) amount of natural quercetin, normal men; agonist: 0.5 µg/mL collagen (27) | — | — | — | — | — | — | — | |
| In vitro experiments | ||||||||
| Garlic (Allium sativum L.) | Human and rat platelets, boiled aqueous extract (chemical contents: undefined): 1.5–12 mg/mL; agonists: 2 µg/mL collagen, 0.5 and 1 mM arachidonic acid (20) | — | — | — | — | — | — | — |
| Healthy beagle dog and human platelets, alk(en)yl thiosulfates derived from whole garlic: 0.001–1 mM; agonist: 2 and 20 µM ADP (29) | — | — | — | — | — | — | — | |
| Human platelets, organosulfur compounds from whole garlic (1 and 10 µg/mL); agonist: 1–5 µg/mL ADP (36) | — | — | — | |||||
| Human platelets, aged garlic extract 0.19–6.25%; the most abundant water-soluble organosulfur compound in the aged garlic extract was found to be S-allyl cysteine (1.47 g/L); agonist: 8 µM ADP (37) | — | — | Human platelets, aged garlic extract 0.19–6.25%; the most abundant water-soluble organosulfur compound in the aged garlic extract was found to be S-allyl cysteine (1.47 g/L), agonist: 8 µM ADP (37) | — | — | — | — | |
| — | — | — | Human platelets, 3.12%–12.5% aged garlic extract containing 305 g/l extracted solids and S-allyl cysteine, agonist: ADP (38) | — | — | — | — | |
| In vivo experiments | ||||||||
| Aged garlic extract (1, 2, and 5 g · kg body wt–1 · d–1) for 14 d, n = 10 rats 11 hydrophilic sulfur compounds in extract. Aggregation stimulated by collagen (10–40 µg/mL) and ADP (10–40 µM) (39) | — | Aged garlic extract (1, 2, and 5 g · kg body wt–1 · d–1) for 14 d, n = 10 rats 11 hydrophilic sulfur compounds in extract. Platelets stimulated by collagen (39) | — | |||||
| In vitro experiments | ||||||||
| Tomato (Solanum lycopersicum L.) | Human platelets, tomato extract (chemical contents: undefined): 5–50 µL at 450 µL PRP; agonists: 2 mg/L collagen, 10 µM ADP, and 0.5 mM arachidonic acid (40) | — | — | — | — | — | — | Human platelets, tomato extract: 5–50 µL at 450 µL PRP, agonists: 2 mg/L collagen, 10 µM ADP, and 0.5 mM arachidonic acid (40) |
| Human platelets, tomato phenolic extract: 1 mg/mL; agonists: 8 µM ADP, 1.5 µg/mL collagen, 30 µM TRAP, and 1 mM arachidonic acid (41) | — | — | — | — | — | — | — | |
| Human platelets, guanosine from tomatoes: 1–4 mM; agonists: 8 µM ADP and 1.5 µg/mL collagen (42) | Human platelets, guanosine from tomatoes: 0.2–2 mM; agonist: collagen (42) | — | — | — | — | — | — | |
| Human platelets, aqueous extract (chemical contents: undefined) of fresh tomato hybrids: 1 mg/mL; agonist: 8 µM ADP (43) | — | — | — | — | — | — | — | |
| In vivo experiments | ||||||||
| Fruitflow (DSM Nutritional Products), 3 capsules, n = 47 healthy adults. Aggregation stimulated by different agonists (44, 45) | — | Fruitflow (DSM Nutritional Products), 3 capsules, n = 47 healthy adults (44, 45) | — | — | — | — | — | |
| Fruitflow (DSM Nutritional Products), 150 mg Fruitflow/d, n = 18 prehypertensive males. Aggregation stimulated by ADP (46) | — | — | — | — | — | — | — | |
| Standardized tomato extract (ZAAX, Sequia), 213 mg orally in the morning for 4 wk, n = 82 high-risk hypertensive patients. Aggregation measured by VerifyNow analyzer (47, 48) | — | — | — | — | — | — | — | |
| Tomato pomace (chemical contents: undefined), 1 g · kg–1 · d–1 for 15 d, n = 5 rats. Aggregation stimulated by ADP (43) | — | — | — | — | — | — | — | |
1cAMP, cyclic AMP; PRP, platelet-rich plasma; TRAP, thrombin receptor–activated peptide, a peptide beginning with the SFLLRN sequence Ser-Phe-Leu-Leu-Arg-Asn; TXA2, thromboxane A2.
Elsewhere, Briggs et al. (28) reported that various onion thiosulfinates, including propyl propane-thiosulfinates and 2-propenyl 2-propene-thiolsulfinate (allicin), inhibit human platelet aggregation by ∼90% when administered at a concentration of 0.4 mM in vitro. Platelet aggregation was measured in whole blood. The tested onion thiosulfinates were more potent platelet inhibitors than aspirin, a commonly used antiplatelet drug, at equivalent concentrations. Chang et al. (29) observed that the administration of alk(en)yl thiosulfonates (sodium n-propyl thiosulfate and sodium 2-prepenyl thiosulfate) from onion at concentrations between 0.001 and 0.1 mM inhibited human platelet aggregation induced by 2 µM ADP in vitro. Makheja and Bailey (30) also reported that the components of onion, especially polysulfides, inhibit TXA2 biosynthesis in platelets by inhibiting cyclooxygenase activity in an in vitro model.
Cavagnaro and Galmarini (31) evaluated the effect of cooking on the antiaggregation properties of onions. Their study examines the influence of 2 different cooking systems, i.e., microwaves and convection ovens, at a range of times and temperatures, and the effect of using whole bulbs, quarters of bulbs, and completely crushed bulbs on the degradation thiosulfonates and other sulfur compounds. Platelet function was measured in whole blood isolated from healthy human donors by electrical impedance aggregometry, with 1 µg/mL collagen used as the agonist. The authors observed that heating may affect the antiaggregatory properties of onion. For example, they noted significant proaggregatory effects in the samples cooked in the oven for 20 and 30 min. These results demonstrate that the antiaggregatory activity of onion depends on the production process, including cooking.
Moon et al. (18) suggested that the antiplatelet properties of onions may act by inhibiting arachidonic acid release and TXA2 synthase activity, and by blocking the TXA2 and prostaglandin H2 receptors on the platelet surface. They examined the effects of aqueous of onion extract prepared from fresh onions at concentrations of 0.05, 0.1, 0.5, and 1 µg/mL against rat and human platelets in vitro. Collagen, thrombin, and arachidonic acid were used as platelet agonists.
Chen et al. (32, 33) found that Welsh onion (Allium fistulosum L.), one of the most important flavoring vegetables in Asian dishes, also influences platelet adhesion to fibrinogen, platelet aggregation, and TXA2 biosynthesis in human blood. Their findings indicate that boiled and raw onion extract had different effects on platelet function in vitro: the boiled extract induced blood platelet aggregation in a dose-dependent manner, whereas the raw extract inhibited blood platelet adhesion and aggregation stimulated by ADP.
Both in vitro studies and in vivo experiments have demonstrated that the consumption of onions and onion juice has beneficial effects on platelet aggregation (16, 19, 35). For example, rats treated with this aqueous extract of onion (500 mg/kg body weight) for 4 wk demonstrated lowered thromboxane B2 synthesis (16). In addition, Briggs et al. (19) reported that the consumption of raw onion reduces collagen-stimulated blood platelet aggregation in dogs, and Jung et al. (35) found consumption of aqueous extract of onion (0.5 g · mL–1 · kg–1 · d–1 for 4 wk) to have antiplatelet activity in both normal and diabetic rats, as measured by platelet aggregate formation and thromboxane B2 concentration (Table 1). On the other hand, Kim et al. (34) noted that onion acts not only as blood platelet inhibitor, but also as a stimulant of platelet aggregation in hypercholesterolemic rats treated with simvastatin. These results were obtained from a study of 40 rats who had consumed a diet of simvastatin plus 5% onion powder for 4 wk. In these studies, platelet aggregation function was measured in whole blood, and 2 µM ADP was used as agonist. In addition, a recent study by Ko et al. (49) found that various methanol fractions and flavonols extracted from onion have not only antiplatelet properties, including antiaggregatory action, but also antioxidant properties.
Osmont et al. (50) suggested that, as the platelet inhibitory activity of onion organosulfur compounds is time dependent, the temporary formation of organosulfur compounds should be taken into account during both in vivo and in vitro assessment of onion-induced antiplatelet properties (50).
Garlic (Allium sativum L.)
Various studies indicate that garlic and its active compounds effectively reduce the risk of cardiovascular disease by normalizing the amounts of plasma lipids, oxidized LDLs, and blood pressure (51–54). Preclinical, clinical, and in vitro studies have also demonstrated that garlic and its different preparations have a range of activities concerning platelets, especially antiaggregatory properties (20, 29, 55, 37, 36, 39). However, like fresh onion, there is no standard intake of raw garlic. Clinical studies demonstrate that the effective daily dosage of garlic powder ranges from 150 to 2400 mg; and for aged garlic intakes range from 0.25 to 7.2 g/d. Aged garlic extract is prepared by storing raw sliced garlic in 15–20% ethanol, which is then filtered and concentrated at low temperatures. The aging process modifies the harsh and irritating components found in raw garlic (56). Allison et al. (38) reported that 3.12–12.5% aged garlic extract containing 305 g/L extracted solids and S-allyl cysteine reduced the degree of platelet adhesion to fibrinogen. They attribute this reduction to an increased amount of intracellular cyclic adenosine monophosphate (cAMP) and inhibition of the interaction of αIIbβ3 integrin with fibrinogen in vitro. In this experiment, platelet activation was initiated by 8 µM ADP. In a study of blood platelets taken from 14 healthy participants, Rahman et al. (37) found 0.19–6.25% aged garlic extract to reduce platelet aggregation stimulated by ADP in vitro. In addition, this extract significantly reduced the binding of activated platelets to fibrinogen, preventing changes in blood platelet shape. The authors have also suggested that this inhibition of platelet aggregation is associated with increased amounts of cyclic nucleotides, i.e. cAMP and cGMP, probably via stimulation of soluble guanylyl cyclase and adenylyl cyclase, and inhibition of phosphodiesterase activity, in the presence of garlic extract. The most abundant water-soluble organosulfur compound in the aged garlic extract was found to be S-allyl cysteine (1.47 g/L). Fakhar and Hashemi Tayer (57) reported that the consumption of garlic pills (1200 and 2400 mg) reduced ADP-stimulated platelet aggregation in 36 healthy volunteers. However, the authors did not describe the chemical composition of the garlic pills. Karagodin et al. (58) also found that 14-d treatment with garlic powder pills inhibited ADP-induced platelet aggregation in men with cerebral atherosclerosis by ∼25%.
Morihara and Hino (39) reported that aged garlic extract, rich in water-soluble cysteinyl moieties, demonstrates antiaggregatory properties in rats. HPLC analysis identified 11 hydrophilic sulfur compounds in this extract, some of which were produced by the aging process. Characteristic sulfur compounds included S-methyl cysteine, S-allyl cysteine, S-1-propenyl cysteine, and S-allyl mercaptocysteine. This garlic extract (1, 2, or 5 g · kg body wt–1 · d–1) was administered orally to rats for 7 or 14 d at a dose of 10 ml/kg body wt. The treatment significantly reduced platelet aggregation after 14 d, but not after 7 d. Platelet aggregation was also found to be reduced in vitro. In addition, the antiaggregatory action was correlated with suppression of phosphorylation of collagen-induced p38, extracellular signal regulated kinase, and c-Jun N-terminal kinase. No change was observed in bleeding time (39). However, Ried et al. (59) reported that aged garlic extract does not influence blood platelet functions in 88 patients with uncontrolled hypertension who received aged garlic extract (1.2 g containing 1.2 mg S-allyl cysteine) daily for 12 wk. Treatment with aged garlic extract, a garlic preparation rich in water-soluble cysteinyl moieties, has been found to suppress blood platelet aggregation by changing the functional property of platelets to respond to collagen (10–40 µg/mL).
The antiaggregatory properties of garlic tablets and aspirin were compared in 62 healthy volunteers (20–50 y old) as part of a randomized clinical trial. The participants took 80 mg aspirin/d. After 1 mo, the volunteers were randomly assigned into 3 groups, and each received 1, 2, or 3 garlic tablets/d (garlic 1250 mg, odor control; Nature Made) for 1 mo. Following the aspirin and garlic tablet treatment, platelet aggregation was induced by 20 µM ADP, 20 µM epinephrine, 0.19 mg/mL collagen, or 0.5 mg/mL arachidonic acid. However, the authors did not identify the effective antiaggregatory dose of garlic equivalent to aspirin (60).
Fluorinated analogs of organosulfur compounds extracted from garlic, such as difluoroallicin, have been found to suppress platelet aggregation stimulated by collagen (1–5 µg/mL). Blood was obtained from 6 healthy human volunteers who had been supplementing their diet with these compounds for 2 wk. Platelet aggregation was then measured in whole blood by the impedance method (36).
Cooked, blanched garlic leaf juice inhibits blood platelet aggregation stimulated by ADP and collagen in vitro and in vivo. In one experiment, the juice of blanched garlic leaves was mixed with PRP, and then platelet aggregation was measured. In another experiment, 10 rabbits received cooked, blanched garlic leaf juice, and platelet aggregation was determined after 1, 3, 5, and 8 wk. Wang and Di (61) attributed inhibition of platelet aggregation to the blockage of the interaction between fibrinogen and its receptor. In addition, a review by Bradley et al. (56) reported that hydrogen sulfide (H2S) derived from organic polysulfides in garlic has also been found to have cardioprotective properties. Several papers suggest that dietary garlic intake or the use of garlic as supplement influences blood coagulation (62–64). Bedi et al. (65) found that dietary supplements containing garlic and fish oil have the potential to cause bleeding in patients undergoing surgery. However, although their results indicated the postoperative blood platelet count to be normal, they did not use platelet function tests.
Tomato (Solanum lycopersicum L.)
The tomato is the most widely consumed vegetable in the world (66). In addition, in vitro, ex vivo, and in vivo studies have found fresh tomatoes and tomato products, including tomato pasta and pomace, to have various biological properties, including the ability to inhibit blood platelet aggregation (Table 1) (40–45, 47, 48, 46). For example, a study conducted among 90 healthy human subjects found supplementation with tomato extract to result in a significant reduction in ADP- and collagen-stimulated platelet aggregation (67).
Fuentes et al. (68) reported that aqueous and methanolic tomato extracts have antiaggregatory activity, both in an in vitro model and in vivo in Wistar rats. They also found the highest antiaggregation activity to be demonstrated by one of the tomato extract fractions that did not contain lycopene; the extract was found to inhibit aggregation by ∼70% in vitro. Interestingly, chromatography analysis of the tested fractions presented 2 absorption peaks, one at 210 nm and another at 261 nm, which is compatible with the presence of nucleotides (68). Earlier, Dutta-Roy et al. (69) also observed that tomatoes contain antiplatelet compounds as well as adenosine. In addition, Fuentes et al. (42) reported that guanosine from tomatoes possesses antiplatelet properties in vitro and inhibits the secretion of platelet inflammatory mediator of atherosclerosis (sCD40L); various parameters (i.e. platelet ATP secretion, platelet aggregation induced by ADP and collagen, and platelet adhesion to collagen) were used to measure the effect of guanosine (0.2–2 mM) on platelet functions.
Rodríguez-Azúa et al. (43), in an in vitro study, examined the anti-platelet properties of 9 varieties of fresh hybrid tomatoes (Apt 410, H 9888, Bos 8066, Sun 6366, AB3, HMX 7883, H 9665, H 7709, and H 9997) as tomato pasta and pomace, containing mainly skin and seeds. The substances were fed to 15 rats, which ingested 0.1 and 1 g pomace/kg body wt each day. Neither the variety of the tomato hybrids nor the type of manufacture were found to have any significant effect on platelet activity. In addition, pomace intake of 1 g · kg–1 · d–1 also reduced blood platelet aggregation. In these experiments, platelet aggregation was monitored by light transmission turbidimetry, and 8 µM ADP was used as an agonist. Fuentes et al. (41) examined the effect of industrial processing (i.e. drying, heating, and pasteurizing) on the antiaggregation properties and phenolic compound profile of tomato products in vitro and in vivo. Platelet aggregation was induced by a range of agonists, including arachidonic acid, collagen, ADP, and TRAP (thrombin receptor-activated peptide, a peptide beginning with the SFLLRN sequence Ser-Phe-Leu-Leu-Arg-Asn). HPLC analysis of aqueous extracts from tomatoes and tomato products (juice, ketchup, sauce, and pomace) identified chlorogenic, ferulic, p-coumaric, and caffeic acids. Although all tested tomatoes and tomato products inhibited platelet aggregation in vitro, the pomace extract presented the best antiaggregatory activity; for example, 1 mg/mL pomace extract demonstrated 35% inhibition of ADP-stimulated aggregation, and 200 mg/kg pomace extract displayed antithrombotic activity.
O'Kennedy et al. (44, 45) found Fruitflow (DSM Nutritional Products), a commercially available water-soluble tomato extract, to demonstrate antiaggregatory activity and to possess various cardioprotective properties. Forty-seven healthy subjects received the extract daily, at a dose providing at least 65 mg of Fruitflow. The extract was found to demonstrate approximately one-third the anti-platelet efficacy of taking 75 mg aspirin/d. Similarly, Uddin et al. (46) report that 150 mg Fruitflow inhibited ADP-induced platelet aggregation and decreased blood pressure in 12 healthy adult males (aged 25–65 y) after 24 h. Article 13(5) of the European Health Claims Regulation 1924/2006 recommends that Fruitflow should be consumed daily in the following doses: 3 g for Fruitflow 1 (a syrup with >50% w/w tomato-derived carbohydrates, and ∼3% w/w of compounds with known antiplatelet activity), and 150 mg for Fruitflow 2 (a low-carbohydrate powder, with >55% w/w bioactive compounds dried to produce a tablet-grade powder). However, Fruitflow differs from typical antiplatelet drugs in the reversibility of its action. Typical antiplatelet drugs have an irreversible mechanism of action (44, 45). Similarly, ZAAX standardized tomato extract (Sequia) has also been found to possess antiaggregatory properties in high-risk hypertension patients: a study on a group of 82 patients aged 28–74 y who may be at high risk of cardiovascular disease found a dose of 213 mg orally in the morning to be effective at preventing aggregation (47), whereas Krasinska et al. (48) note a positive correlation between blood platelet activation and P2Y12 receptor activity, particularly in obese patients.
Some studies indicated that the bioactive component of tomatoes is lycopene, known to have antioxidant, antihypertensive, and hypolipidemic effects in vitro and in vivo (70, 71). In addition, although Thies et al. (72) reported that lycopene may also have an effect on blood platelet activation, none of the studies of Fruitflow or ZAAX tomato extract described the concentration of lycopene in the preparations (44, 45, 47, 48, 46).
More details about antiplatelet action of other selected vegetables are given in Table 1. Yamamoto et al. (73) indicated that various varieties of carrot have antiplatelet activity, and Li et al. (74) reported that steroidal saponins derived from dioscorea rhizomes, a common vegetable widely used in traditional Chinese medicine, inhibit blood platelet aggregation in rats. Gong et al. (75) also indicated that diosgenin extract from dioscorea has antiaggregatory effects in vitro and in vivo.
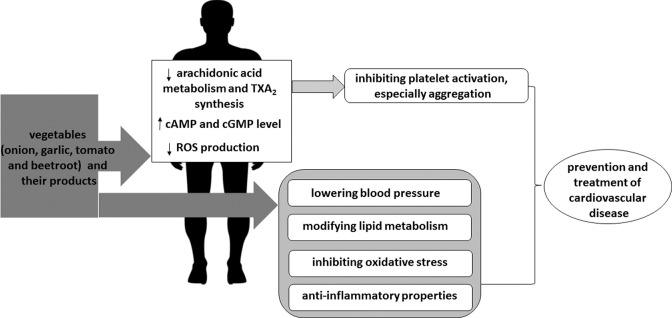
Figure 1 demonstrates the effects of vegetables and their products on a range of parameters which are important in the prophylaxis and treatment of cardiovascular disease, not only blood platelet activation.
FIGURE 1.
Experiments involving vegetables and their products in cardiovascular disease, showing their influence on selected cardiovascular parameters and the proposed mechanism of action of vegetables and their products on blood platelets. Vegetable components may inhibit the synthesis of TXA2 (a platelet agonist), inhibit ROS production, and increase the level of cAMP and cGMP in platelets. cAMP, cyclic adenosine 5′-monophosphate; cGMP, cyclic guanosine 5′-monophosphate; ROS, reactive oxygen species; TXA2, thromboxane A2.
Interestingly, some vegetables, such as beetroot, are also sources of inorganic nitrate or nitrite, which may be metabolized to produce nitric oxide, which controls blood platelet functions (5). Bondonno et al. (76), Raubenheimer et al. (77), and other authors (78) have suggested that the bioactive nitrate derived from vegetables such as beetroot and spinach may serve as an important bioactive cardioprotective component in a vegetable-rich diet by inhibiting blood platelet activation. Jackson et al. (79) also reported that inorganic nitrate and nitrite may play a positive role in cardiovascular disease by reducing platelet aggregation.
Conclusions
As therapy with antiplatelet drugs such as aspirin is often correlated with various side effects, the use of natural compounds may be a safer alternative approach. Recent studies have investigated the potential of vegetables, especially onion, garlic, and tomato, for modulating blood platelet activation, including their aggregation, and evaluated their role in the prophylaxis and treatment of cardiovascular disease. The in vitro and in vivo studies described in this manuscript are based on the examination of blood platelets isolated from healthy subjects and patients with cardiovascular risk factors.
Recent experiments suggest that actions of garlic and other vegetables against platelets, especially their antiaggregation and cardioprotective properties, are largely determined by the method of preparation. In addition, the beneficial health of vegetables on blood platelets are also dependent on multiple mechanisms, which may be mediated by the active compounds present in vegetables and their products, including phenolic compounds such as quercetin for onion, organosulfur compounds, and H2S for garlic, and adenosine for tomato. Figure 1 demonstrates that these active components may modulate the signal pathways associated with platelets in varied, and sometimes opposing ways; for example, some may inhibit ROS production whereas others may inhibit the biosynthesis of TXA2, a platelet agonist. However, as the chemical content of tested vegetable extracts and other products is not always well described, it is difficult to determine whether the antiaggregation effect associated with consuming the vegetables or their constituents, along with other effects on platelets, can be achieved at typical dietary amounts. As few clinical trials of the potential of vegetables and their products to treat cardiovascular disorders have been performed, the development of controlled and high-quality human clinical experiments is encouraged, especially to determine the prophylactic and therapeutic doses of vegetables and their constituents.
ACKNOWLEDGEMENTS
The sole author was responsible for all aspects of the manuscript.
Notes
The author reported no funding received for this study.
The author reports no conflict of interest.
Abbreviations used: cAMP, cyclic adenosine 5′-monophosphate; cGMP, cyclic guanosine 5′-monophosphate; PRP, platelet-rich plasma; ROS, reactive oxygen species; TRAP, thrombin receptor–activated peptide; TXA2, thromboxane A2.
References
- 1. George JN. Platelets. Lancet. 2000;355:1541–9. [DOI] [PubMed] [Google Scholar]
- 2. Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, Devone MT, Fuchs TA, Weyrich AS, Semple JW. T granules in human platelets function in TLR9 organization and signaling. J Cell Biol. 2012;198:561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blockmans D, Deckmyn H, Vermylen J. Platelet activation. Blood Rev. 1995;9:143–56. [DOI] [PubMed] [Google Scholar]
- 4. Ryningen A, Holmsen H. Biochemistry of platelet activation. In: Gundu H, Rao R, editors. Handbook of Platelet Physiology and Pharmacology. Norwell, MA: Kluwer Academic Publishers; 1999. p. 1–22. [Google Scholar]
- 5. Olas B, Wachowicz B. Role of reactive nitrogen species in blood platelet functions. Platelets. 2007;18:555–65. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. World Health Statistics, 2015, Cardiovascular Diseases. 2015. [Google Scholar]
- 7. Zheng J, Zhou Y, Li S, Zhang P, Zhou T, Xu DP, Li HB. Effects and mechanisms of fruit and vegetable juices on cardiovascular diseases. Int J Mol Sci. 2018;18:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olas B. The multifunctionality of berries toward blood platelets and the role of berry phenolics in cardiovascular disorder. Platelets. 2017; 28:540–9. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto J, Ijiri Y, Tamura Y, Iwasaki M, Murakami M, Okada Y. Reevaluation of antithrombotic fruits and vegetables: great variation between varieties. Drug Discover Therapeut. 2016;10:129–40. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch GE, Viecili PR, de Almeida AS, Nascimento S, Porto FG, Otero J, Schmidt A, da Silva B, Parisi MM, Klafke JZ. Natural products with antiplatelet action. Curr Pharm Des. 2017:23:1228–46. [DOI] [PubMed] [Google Scholar]
- 11. Ali M, Thomson M, Afzal M. Garlic and onions: their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot Essent Fatty Acids. 2000;62:55–73. [DOI] [PubMed] [Google Scholar]
- 12. Alali FQ, El-Elimat T, Khalid L, Hudaib R, Al-Shehabi TS, Eid AH. Garlic for cardiovascular disease: prevention or treatment?. Curr Pharm Des. 2017;23:1028–41. [DOI] [PubMed] [Google Scholar]
- 13. Tang GY, Meng X, Li Y, Zhao CN, Liu Q, Li HB. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients. 2017;9:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomson M, Mustafa T, Ali M. Thromboxane-B(2) levels in serum of rabbits receiving a single intravenous dose of aqueous extract of garlic and onion. Prostaglandins Leukot Essent Fatty Acids. 2000;63:217–21. [DOI] [PubMed] [Google Scholar]
- 15. Bordia A, Bansal HC, Arora SK, Singh SV. Effect of the essential oils of garlic and onion on alimentary hyperlipidemia. Atheroscl. 1975;21:15–9. [DOI] [PubMed] [Google Scholar]
- 16. Bordia A, Mohammed N, Thomson M, Ali M. An evaluation of garlic and onion as antithrombotic agents. Prostaglandins Leukot Essent Fatty Acids. 1996;54:183–6. [DOI] [PubMed] [Google Scholar]
- 17. Kawamoto E, Sakai Y, Okamura Y, Yamamoto Y. Effects of boiling on the antihypertensive and antioxidant activities of onion. J Nutr Sci Vitaminol (Tokyo). 2004;50:171–6. [DOI] [PubMed] [Google Scholar]
- 18. Moon CH, Jung YS, Kim MH, Lee SH, Baik EJ, Park SW. Mechanism for antiplatelet effect of onion: AA release inhibition, thromboxane A2 synthase inhibition and TXA2/PGH2 receptor blockade. Prostaglandins Leukot Essent Fatty Acids. 2000;62:277–83. [DOI] [PubMed] [Google Scholar]
- 19. Briggs WH, Folts JD, Osman HE, Goldman IL. Administration of raw onion inhibits platelet-mediated thrombosis in dogs. J Nutr. 2001;131:2619–22. [DOI] [PubMed] [Google Scholar]
- 20. Ali M, Bordia T, Mustafa T. Effect of raw versus boiled aqueous extract of garlic and onion on platelet aggregation. Prostaglandins Leukot Essent Fatty Acids. 1999;60:43–7. [DOI] [PubMed] [Google Scholar]
- 21. Ro JY, Ryu JH, Park HJ, Cho HJ. Onion (Allium cepa L.) peel extract has anti-platelet effects in rat platelets. SpringerPlus. 2015;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ewald C, Fjelkner-Modig S, Johansson K, Sjöholm I, Åkesson B. Effect of processing on major flavonoids in processed onions, green beans, and peas. Food Chem. 1999;64:231–5. [Google Scholar]
- 23. Glässer G, Graefe E, Struck F, Veit M, Gebhaerdt R. Comparison of antioxidative capacities and inhibitory effects on cholesterol biosynthesis of quercetin and potential metabolites. Phytomedicine. 2002;9:33–40. [DOI] [PubMed] [Google Scholar]
- 24. Kris-Etherton P, Lefevre M, Beecher G, Gross M, Keen C, Etherton T. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–38. [DOI] [PubMed] [Google Scholar]
- 25. Lee SM, Moon J, Chung JH, Cha YJ, Shin MJ. Effect of quercetin-rich onion peel extracts on arterial thrombosis in rats. Food Chem Toxicol. 2013;57:99–105. [DOI] [PubMed] [Google Scholar]
- 26. Yamada K, Naemura A, Sawashita N, Noguchi Y, Yamamoto J. An onion variety has natural antithrombotic effect as assessed by thrombosis/thrombolysis models in rodents. Thromb Res. 2004;114:213–20. [DOI] [PubMed] [Google Scholar]
- 27. Hubbard GP, Wolffram S, de Vos R, Bovy A, Gibbins JM, Lovegrove JA. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: a pilot study. Br J Nutr. 2006;96:482–8. [PubMed] [Google Scholar]
- 28. Briggs WH, Xiao H, Parkin KL, Shen C, Goldman IL. Differential inhibition of human platelet aggregation by selected Allium thiosulfinates. J Agric Food Chem. 2000;48:5731–5. [DOI] [PubMed] [Google Scholar]
- 29. Chang HS, Yamato O, Sakai Y, Yamasaki M, Maede Y. Acceleration of superoxide generation in polymorphonuclear leukocytes and inhibition of platelet aggregation by alk(en)yl thiosulfates derived from onion and garlic in dogs and humans. Prostaglandins Leukot Essent Fatty Acids. 2004;70:77–83. [DOI] [PubMed] [Google Scholar]
- 30. Makheja AN, Bailey JM. Antiplatelet constituents of garlic and onion. Agents Actions. 1990;290:360–3. [DOI] [PubMed] [Google Scholar]
- 31. Cavagnaro PE, Galmarini CR. Effect of processing and cooking conditions on onion (Allium cepa L.) induced antiplatelet activity and thiosulfinate content. J Agric Food Chem. 2012;60:8731–7. [DOI] [PubMed] [Google Scholar]
- 32. Chen JH, Chen HI, Tsai SJ, Jen CJ. Chronic consumption of raw but not boiled Welsh onion juice inhibits rat platelet function. J Nutr. 2000;130:34–7. [DOI] [PubMed] [Google Scholar]
- 33. Chen JH, Chen HI, Wang JS, Tsai SJ, Jen CJ. Effects of Welsh onion extracts on human platelet function in vitro. Life Sci. 2000;66:1571–9. [DOI] [PubMed] [Google Scholar]
- 34. Kim JL, Chae IS, Kang YH, Kang JS. Effect of onion and beet on plasma and liver lipids, platelet aggregation, and erythrocyte Na efflux in simvastatin treated hypercholesterolemic rats. Nutr Res Pract. 2008;2:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jung YS, Kim MH, Lee SH, Baik EJ, Park SW, Moon C-H. Antithrombotic effect of onion in streptozotocin-induced diabetic rat. Prostaglandins Leukot Essent Fatty Acids. 2002;66:453–8. [DOI] [PubMed] [Google Scholar]
- 36. Block E, Bechand B, Gundala S, Vattekkatte A, Wang K, Mousa SS, Godugu K, Yalcin M, Mousa SA. Fluorinated analogs of organosulfur compounds from garlic (Allium sativum): synthesis, chemistry and anti-angiogenesis and antithrombotic studies. Molecules. 2017;22:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Rahman K, Lowe GM, Smith S. Aged garlic extract inhibits human platelet aggregation by altering intracellular signaling and platelet shape change. J Nutr. 2016;146:410S–5S. [DOI] [PubMed] [Google Scholar]
- 38. Allison GL, Lowe GM, Rahman K. Aged garlic extract inhibits platelet activation by increasing intracellular cAMP and reducing the interaction of GPIIb/IIIa receptor with fibrinogen. Life Sci. 2012;91:1275–80. [DOI] [PubMed] [Google Scholar]
- 39. Morihara N, Hino A. Aged garlic extract suppresses platelet aggregation by changing the functional property of platelets. J Nat Med. 2017;71:249–56. [DOI] [PubMed] [Google Scholar]
- 40. Lazarus SA, Garg ML. Tomato extract inhibits human platelet aggregation in vitro without increasing basal cAMP levels. Int J Food Sci Nutr. 2004;55:249–56. [DOI] [PubMed] [Google Scholar]
- 41. Fuentes E, Forero-Doria O, Carrasco G, Maricán A, Santos LS, Alarcón M, Paloma I. Effect of tomato industrial processing on phenolic profile and antiplatelet activity. Molecules. 2013;18:11526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fuentes E, Alarcón M, Astudillo L, Valenzuela C, Gutiérrez M, Paloma I. Protective mechanism of guanosine from Solanum lycopersicum on agonist-induced platelet activation: role of sCD40L. Molecules. 2013;18:8120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodríguez-Azúa R, Treuer A, Moore-Carrasco R, Cortacáns D, Gutiérrez M, Astudillo L, Fuentes E, Paloma I. Effect of tomato industrial processing (different hybrids, paste, and pomace) in inhibition of platelet function in vitro, ex vivo, and in vivo. J Med Food. 2014;17:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Kennedy N, Raederstorff D, Duttaroy AK. Fruitflow®: the first European Food Safety Authority-approved natural cardio-protective functional ingredient. Eur J Nutr. 2017;56:461–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Kennedy N, Crosbie L, Song H-J, Zhang X, Horgan G, Duttaroy AK. A randomised controlled trial comparing a dietary antiplatelet, the water-soluble tomato extract Fruitflow, with 75 mg aspirin in healthy subjects. Eur J Clin Nutr. 2017;71:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uddin M, Biswas D, Ghosh A, O'Kennedy N, Duttaroy AK. Consumption of Fruitflow® lowers blood pressure in pre-hypertensive males: a randomised, placebo controlled, double blind, cross-over study. Int J Food Sci Nutr. 2018;69:494–502. [DOI] [PubMed] [Google Scholar]
- 47. Osinska AN, Begier-Krasinska B, Rzymski P, Krasinska A, Tykarski A, Krasinski Z. The influence of adding tomato extract and acetylsalicylic acid to hypotensive therapy on the daily blood pressure profiles of patients with arterial hypertension and high cardiovascular risk. Kardiochirurgia and Torakochirurgia Polska. 2017;14:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krasinska B, Osinska A, Krasinska A, Osinski M, Rzymski P, Tykarski A, Krasinski Z. Favorable hypotensive effect after standardised tomato extract treatment in hypertensive subjects at high cardiovascular risk: a randomized controlled trial. Kardiol Pol. 2018;76:388–95. [DOI] [PubMed] [Google Scholar]
- 49. Ko EY, Nile SH, Jung YS, Keum YS. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.). Biotech. 2018;8(3):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Osmont KS, Arnt CR, Goldman IL. Temporal aspects of onion-induced antiplatelet activity. Plant Foods Hum Nutr. 2003;58:27–40. [DOI] [PubMed] [Google Scholar]
- 51. Slevin M, Ahmed N, Wang Q, McDowell G, Badimon L. Unique vascular protective properties of natural products: supplements or future main-line drugs with significant anti-atherosclerotic potential. Vasc Cell. 2012;40:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chan JY, Yuen AC, Chan RY, Chan SW. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother Res. 2013;27:637–46. [DOI] [PubMed] [Google Scholar]
- 53. Khatua TN, Adela R, Banerjee SK. Garlic and cardioprotection: insights into the molecular mechanisms. Can J Physiol Pharmacol. 2013;91:448–58. [DOI] [PubMed] [Google Scholar]
- 54. Qudwai W, Ashfag T. Role of garlic usage in cardiovascular disease prevention: an evidence-based approach. Evid Based Complement Alternat Med. 2013;2013:125649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang XH, Shao DH, Liang GW, Zhang R, Xin Q, Zhang T, Cao QY. Cyclooxygenase inhibitors in some dietary vegetables inhibit aggregation function induced by arachidonic acid. Zhongoouo Shi Yan Xue Ye Xue Za Zhi. 2011;19:1260–3. [PubMed] [Google Scholar]
- 56. Bradley JM, Organ CL, Lefer DJ. Garlic-derived organic polysulfides and myocardial protection. J Nutr. 2016;146(2):403S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fahkar H, Hashemi Tayer A. Effect of the garlic pill in comparison with Plavix on platelet aggregation and bleeding time. Iran J Ped Hematol Oncol. 2012;2:146–52. [PMC free article] [PubMed] [Google Scholar]
- 58. Karagodin VP, Sobenin IA, Orekhov AN. Antiatherosclerotic and cardioprotective effects of time-released garlic powder pills. Curr Pharm Des. 2016;22:196–213. [DOI] [PubMed] [Google Scholar]
- 59. Ried K, Travica N, Sali A. The effect of aged garlic extract on blood pressure and other cardiovascular risk factors in uncontrolled hypertensives: the AGE at heart trial. Integr Blood Press Control. 2016;27:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shafiekhani M, Faridi P, Kojuri J, Namazi S. Comparison of antiplatelet activity of garlic tablets with cardioprotective dose of aspirin in healthy volunteers: a randomized clinical trial. Avicenna J Phytomed. 2016;6:550–7. [PMC free article] [PubMed] [Google Scholar]
- 61. Wang XH, Di YH. Mechanism of cooked blanched garlic leaves against platelet aggregation. Zhongoouo Shi Yan Xue Ye Xue Za Zhi. 2014;22:753–7. [DOI] [PubMed] [Google Scholar]
- 62. Borrelli F, Capassso R, Izzo AA. Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol Nutr Food Res. 2007;51:1386–97. [DOI] [PubMed] [Google Scholar]
- 63. Stanger MJ, Thompson LA, Young AJ, Lieberman HR. Anticoagulant activity of select dietary supplements. Nutr Rev. 2012;70:107–17. [DOI] [PubMed] [Google Scholar]
- 64. Wang CZ, Moss J, Yuan CS. Commonly used dietary supplements on coagulation function during surgery. Medicines. 2015;2:157–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bedi HS, Tewerson V, Negi K. Bleeding risk of dietary supplements: a hidden nightmare for cardiac surgeons. Indian Heart J. 2016;68:S249–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW Jr. The tomato as a functional food. J Nutr. 2005;135:1226–30. [DOI] [PubMed] [Google Scholar]
- 67. O'Kennedy N, Crosbie L, Whelan S, Luther V, Horgan G, Broom JI, Webb DJ, Duttaroy AK. Effects of tomato extract on platelet function: a double-blinded crossover study in healthy humans. Am J Clin Nutr. 2006;84:561–9. [DOI] [PubMed] [Google Scholar]
- 68. Fuentes EJ, Astudillo LA, Gutiérrez MI, Contreras SO, Bustamante LO, Rubio PI, Moore-Carrasco R, Alarcón MA, Fuentes JA, González DE et al.. Fractions of aqueous and methanolic extracts from tomato (Solanum lycopersicum L.) present platelet antiaggregant activity. Blood Coagul Fibrinolysis. 2012;23:109–17. [DOI] [PubMed] [Google Scholar]
- 69. Dutta-Roy AK, Crosbie L, Gordon MJ. Effects of tomato extract on human platelet aggregation in vitro. Platelets. 2001;12:218–27. [DOI] [PubMed] [Google Scholar]
- 70. Karimi G, Ramezani M, Abdi A. Protective effects of lycopene and tomato extract against doxorubicin-induced cardiotoxicity. Phytother Res. 2005;19:912–4. [DOI] [PubMed] [Google Scholar]
- 71. Armoza A, Haim Y, Bashiri A, Wolak T, Paran E. Tomato extract and the carotenoids lycopene and lutein improve endothelial function and attenuate inflammatory NF-κB signaling in endothelial cells. J Hypertens. 2013;31:521–9. [DOI] [PubMed] [Google Scholar]
- 72. Thies F, Mills LM, Moir S, Masson LF. Cardiovascular benefits of lycopene: fantasy or reality?. Proc Nutr Soc. 2017;76:122–9. [DOI] [PubMed] [Google Scholar]
- 73. Yamamoto J, Naemura A, Ijiri Y, Ogawa K, Suzuki T, Shimada Y, Giddings JC. The antithrombotic effects of carrot filtrates in rats and mice. Blood Coagul Fibrinolysis. 2008;19:785–92. [DOI] [PubMed] [Google Scholar]
- 74. Li H, Huang W, Wen Y, Gong G, Zhao Q, Yu G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis C.H. Wright. Fitoterapia. 2010;81:1147–56. [DOI] [PubMed] [Google Scholar]
- 75. Gong G, Qin Y, Huang W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis C.H. Wright in vitro and in vivo. Phytomed. 2011;18:458–63. [DOI] [PubMed] [Google Scholar]
- 76. Bondonno C, Blekkenhorst LC, Liu AH, Bondonno NP, Ward NC, Croft KD, Hodgson JM. Vegetable-derived bioactive nitrate and cardiovascular health. Mol Asp Med. 2018;61:83–91. [DOI] [PubMed] [Google Scholar]
- 77. Raubenheimer K, Hickey D, Leveritt M, Fassett R, de Zevallos Munoz JO, Allen JD, Briskey D, Parker TJ, Kerr G, Peake JM et al.. Acute effects of nitrate-rich beetroot juice on blood pressure, hemostasis and vascular inflammation markers in healthy older adults: a randomized, placebo-controlled crossover study. Nutrients. 2017;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rathod KS, Jones DA, Van-Eijl TJA, Tsang H, Warren H, Hamsshere SM, Kapil V, Jain AK, Deaner A, Poulter N et al.. Randomised, double-blind, placebo-controlled study investigating the effects of inorganic nitrate on vascular function, platelet reactivity and restenosis in stable angina: protocol of the NITRATE-OCT study. BMJ Open. 2016;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jackson JK, Patterson AJ, MacDonald-Wicks LK, Oldmedow C, MeEvoy MA. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: a systematic review and meta-analysis of human evidence. Nutr Rev. 2018;76:348–71. [DOI] [PubMed] [Google Scholar]