ABSTRACT
Human brown adipose tissue (BAT) has attracted clinical interest not only because it dissipates energy but also for its potential capacity to counteract obesity and related metabolic disorders (e.g., insulin resistance and dyslipidemia). Cold exposure is the most powerful stimulus for activating and recruiting BAT, and this stimulatory effect is mediated by the transient receptor potential (TRP) channels. BAT can also be activated by other receptors such as the G-protein–coupled bile acid receptor 1 (GPBAR1) or β-adrenergic receptors. Interestingly, these receptors also interact with several dietary components; in particular, capsinoids and tea catechins appear to mimic the effects of cold through a TRP-BAT axis, and they consequently seem to decrease body fat and improve metabolic blood parameters. This systematic review critically addresses the evidence behind the available human studies analyzing the effect of several dietary components (e.g., capsinoids, tea catechins, and ephedrine) on BAT activity. Even though the results of these studies are consistent with the outcomes of preclinical models, the lack of robust study designs makes it impossible to confirm the BAT-activation capacity of the specified dietary components. Further investigation into the effects of dietary components on BAT is warranted to clarify to what extent these components could serve as a powerful strategy to treat obesity and related metabolic disorders.
Keywords: brown fat, obesity, dietary components, TRP channels, 18F-FDG PET/CT
Introduction
Brown adipose tissue (BAT) generates heat via nonshivering thermogenesis (NST) to maintain a constant core body temperature at low ambient temperatures (1–4). Among other mechanisms (5), NST occurs via the action of uncoupling protein 1 (UCP1), a molecular hallmark of BAT (6). This protein is expressed in both brown adipocytes (classical BAT) and brite adipocytes (brown-like adipocytes emerging in white adipose depots, also known as beige adipocytes) (7). The sympathetic nervous system (SNS) is the primary regulator of BAT activity, releasing norepinephrine through terminal neurons (8). The surfaces of BAT adipocytes are rich in β-adrenergic receptors, which bind to norepinephrine (9). These β-adrenergic receptors are coupled to a Gs protein system that activates the enzyme adenylyl cyclase and leads to the formation of cAMP as a secondary messenger (10). cAMP activates protein kinase A, which leads to the activation of the thermogenic response (9). Both intracellular fatty acids and fatty acids from the bloodstream are the primary substrates of BAT mitochondria (11). Circulating glucose is also a fuel for brown adipocytes, which allows for the use of imaging techniques to trace human BAT activity via labelled glucose (12). However, a set of techniques based on lipid metabolism are being postulated as an alternative method for measuring BAT activity (13).
Several studies have demonstrated a negative association of human BAT activity and/or volume with BMI (14), fat mass (15–17), glucose concentrations (16, 18), total cholesterol, and TGs (19, 20) and the incidence of type 2 diabetes (21). Thus, since its “rediscovery” in humans in 2009 (4, 22–24), BAT has been postulated as a potential target tissue in the treatment of obesity and related diseases. Cold exposure, which is the primary BAT-activating stimulus (25), stimulates the transient potential receptor (TRP) channels that activate the SNS response (26). Acute or chronic cold exposure effectively increases BAT volume and activity in humans, and improves overall metabolic health in healthy, obese, and diabetic patients (27–30). However, cold acclimation is difficult to implement in clinical practice and is unpleasant for patients (26, 31).
Interestingly, TRP channels not only mediate temperature stimuli but also function as chemesthetic receptors of substances naturally present in food and herbal plants (32). TRP vanilloid 1 (TRPV1), TRP ankyrin 1 (TRPA1), and TRP melastin 8 appear to be the most relevant TRP channels for BAT activation, as their stimulation is associated with increased BAT activity (33). Currently, it is known that several thermogenic food ingredients (hereafter referred to as dietary components) mimic cold exposure through the activation of TRP channels, consequently stimulating BAT (26). The activation of TRPV1, TRPA1, and TRP melastin 8 (34) may be protective against obesity and cardiovascular disease risk by preventing dietary-induced body fat gain and inhibiting proinflammatory pathways (35–37).
In addition, some of these dietary components can increase cold-induced thermogenesis (CIT) as well as diet-induced thermogenesis (38). These effects may be partially explained by BAT, as diet-induced thermogenesis is higher in BAT-positive subjects than in BAT-negative subjects (39), and by the association between cold-induced BAT activation and CIT, independently of age and fat-free mass (40).
To date, there is increasing scientific evidence suggesting that dietary components may play a role in human BAT volume and activity (41). We systematically reviewed the available human intervention studies analyzing the effect of dietary components on BAT to further understand the potential clinical relevance of this promising strategy.
Methods
We conducted a systematic search of articles of interest in PubMed and the Web of Science. Our search strategy included articles from 1 January 2007, the year of publication of the first article suggesting that BAT was metabolically active in adult humans, until 1 February 2018 (42).
Search strategy
Search terms related to studies of brown fat in humans were combined in the following strategy in PubMed: (((((((“Adipose Tissue, Brown” [Mesh] OR “Brown Fat” OR “Brown adipose tissue”))) OR ((“Adipose tissue, beige” [Mesh] OR “beige adipose tissue” OR “Brite fat” OR “beige fat”))))) NOT (((((((((((“Mice” [Mesh]) OR “Rats” [Mesh]) OR “Animal Experimentation” [Mesh]) OR “Models, Animal” [Mesh])) OR (“rats” OR “mouse”))) OR “mice”)) OR “rat”))) NOT “Review” [Publication Type]; and in Web of Science: ((“Brown adipose tissue” OR “Brown fat” OR “Brite adipose” OR “Beige adipose” OR “Beige fat” OR “brite fat”) NOT (“Mice” OR Rat* OR (Experiment* AND Animal*) OR (Research* AND Animal*) OR “mouse” OR (model* AND animal*))). File type: (ARTICLE OR CLINICAL TRIAL OR CASE REPORT).
Study selection
The inclusion criteria were as follows: 1) dietary components (those components that met the dietary component definition criteria, i.e., any nonartificially synthetized or pharmaceutical chemical component of biological origin able to elucidate a significant thermogenic response when administered orally or injected); 2) human studies; 3) original studies (no reviews); and 4) articles written in the English language. Studies that included cancer reports such as pheochromocytoma or hibernoma were excluded. After discarding the duplicates found in both databases, eligibility for inclusion was evaluated based on the following: 1) reading the title and abstract and 2) reading the full text.
Data collection process
The following data were collected from each included study: 1) dietary compound; 2) source; 3) dose; 4) year; 5) country; 6) season or month; 7) study design; 8) BMI; 9) participants’ sex; 10) main findings; 11) BAT activity measurement technique; 12) 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) combined with computed tomography (CT)(18F-FDG PET/CT) measurement point; and 13) reference.
Results
General results
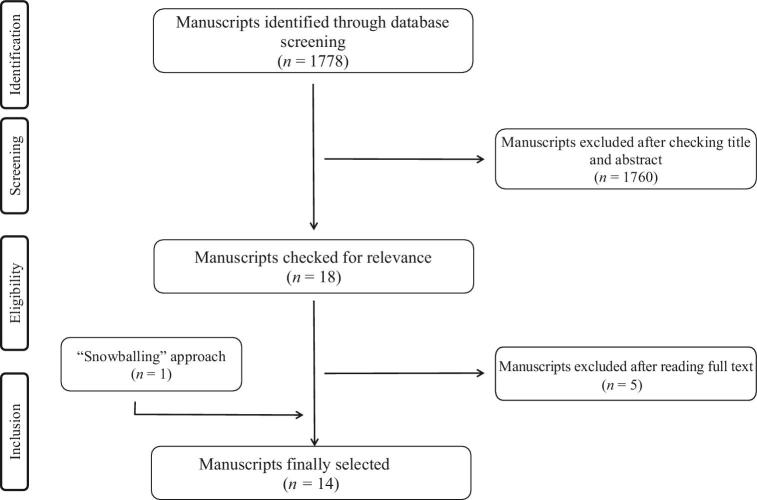
A total of 1778 studies were identified after duplicates were discarded (Figure 1). No additional information was retrieved after repeating the search in Scopus (information not included in the flowchart). A total of 14 manuscripts were finally included after applying the aforementioned inclusion and exclusion criteria. Two of the manuscripts (43, 44) included 2 studies in the same manuscript, resulting in a total of 16 studies. We found no studies conducted in participants with metabolic syndrome or type 2 diabetes. Notably, some studies did not report information regarding the season in which the study was conducted (45–47), whereas others were conducted completely (40, 43, 44, 48–50) or partially (51–53) in winter. Due to the high heterogeneity of the identified studies, no quality-assessment scale systems were used to evaluate the quality of our eligible studies.
FIGURE 1.
Flowchart showing the literature search and article selection process.
The most-studied dietary components were capsinoids (n = 6 studies) (40, 43, 48, 49, 54), followed by tea catechins (n = 3 studies) (44, 50) and ephedrine (n = 3 studies) (45, 51, 52). Other studies focused on bile acids and various extracts of plants and seaweed (46, 47, 53, 55). All studies were published between 2012 and 2018. The Japanese group headed by Dr. Saito conducted nearly half of the studies in this field (n = 6; 35%) (40, 44, 46–48). A total of n = 12 (75%) of the studies were performed in Asians (40, 43, 44, 46–50, 54, 55) and 25% (n = 4) (45, 51–53) were conducted in Caucasians. Notably, only 3 (43, 52, 55) of the 16 studies conducted 18F-FDG PET/CT scans before and after the intervention (Table 1). Another 3 studies (19%) performed an 18F-FDG PET/CT scan only after the intervention (45, 51, 53). A total of 13 (81%) studies used 18F-FDG PET/CT scans to quantify BAT activity and/or volume before and/or after the intervention (Table 1). Two studies (43, 50) (n = 2; 12.5%) used near-infrared time-resolved spectroscopy (NIRTRS), whereas only 1 study used infrared thermography (n = 1; 6%) (49) to quantify BAT activity. No study conducted biopsies. All studies were conducted in adults under the age of 35 y (40, 43–53, 55); 14 studies were conducted in healthy and lean humans (40, 43, 44, 46–53); and 2 studies were conducted in obese humans (45, 55).
TABLE 1.
Summary of studies found in the systematic review that reported an increase or decrease in BAT activity after the ingestion or administration of a dietary component1
| Dietary component | Source | Dose | Year | Country | Season or month | Study design | BMI,2 kg/m2 | n | Age,2 y | Sex | Main findings | BAT activity measurement technique | 18F-FDG PET/CT measurement point | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsinoids | CH-19 Sweet (Capsicum annuum L.) | 9 mg (acute effect) | 2012 | Japan | January–March (winter) | Single-blind, randomized, placebo-controlled, crossover | 21.3 ± 0.4 | 18 | 22.8 ± 0.7 | Male | RMR significantly increased by 15.2 ± 2.6 kJ/h in the BAT-positive group and by 1.7 ± 3.8 kJ/h in the BAT-negative group after 1 h of the oral ingestion of capsinoids | 18F-FDG PET/CT | Before administration | (48) |
| Capsinoids | CH-19 Sweet (C. annuum L.) | 9 mg/d for 6 wk | 2013 | Japan | January–March (winter) | Single-blind, randomized, placebo-controlled, crossover | 22.0 ± 0.4 | 10 | 24.4 ± 0.5 | Male | CIT after capsinoid treatment (200.0 ± 33.9 kcal/d) was significantly higher than before capsinoid treatment (20.6 ± 43.0 kcal/d) and after placebo treatment (81.0 ± 32.5 kcal/d) | 18F-FDG PET/CT | Before administration | (40) |
| Capsinoids | CH-19 Sweet (C. annuum L.) | 9 mg/d for 6 wk | 2016 | Japan | December–March (winter) | Single-blind, crossover | Not reported | 3 | Not reported | Male | SUVmax increased by 48.8% (from 2.2 ± 0.3 to 3.3 ± 1.3) after capsinoid ingestion | 18F-FDG PET/CT | Before and after administration | (43) |
| Capsinoids | CH-19 Sweet (C. annuum L.) | 9 mg/d for 8 wk | 2016 | Japan | December–March (winter) | Double-blind, randomized, placebo-controlled, crossover | 21.7 ± 1.4 | 20 | 20.8 ± 1.1 | Male and female | Supraclavicular total-Hb increased by 46.4% after capsinoid treatment (from 70.4 ± 14.8 μM to 102.2 ± 27.2 μM) | NIRTRS | No measurement | (43) |
| Capsinoids | CH-19 Sweet (C. annuum L.) | 9 mg (acute effect) | 2016 | Japan | October–June (autumn to summer) | Double-blind, randomized, placebo-controlled, crossover | 20.4 ± 0.3 | 24 | 23 ± 0.4 | Male | RMR was significantly higher (66.90%) in subjects who had ingested capsinoids vs. placebo in the high-BAT group | IRT | No measurement | (49) |
| Capsinoids | CH-19 Sweet (C. annuum L.) | 12 mg (acute effect) | 2018 | Japan | August–March (summer to winter) | Nonblind, crossover | 21.7 ± 0.6 | 20 | 25.9 ± 0.9 | Male and female | Capsinoids did not stimulate BAT above the PET SUV-threshold of 2.0 SUVmean, whereas mild cold did | 18F-FDG PET/CT | After administration | (54) |
| Tea catechins | Green tea extract (Camellia sinensis) | 540 mg/d for 12 wk | 2016 | Japan | December–March (winter) | Double-blind, randomized, placebo-controlled, crossover | 21.1 ± 1.5 | 21 | 20.8 ± 2.1 | Female | There was a significant increase in total-Hb after 12 wk of catechin beverage ingestion (from 67.9 ± 20.4 μM to 80.6 ± 24.3 μM) | NIRTRS | No measurement | (50) |
| Tea catechins and caffeine | Green tea extract (C. sinensis) | 615 mg tea catechins and 77 mg caffeine (acute effect) | 2017 | Japan | January–March (winter) | Single-blind, randomized, placebo-controlled, season-matched, crossover | 21.4 ± 0.7 | 15 | 23.1 ± 0.6 | Male | RMR was significantly higher after the ingestion of the catechin beverage vs. the placebo at 0–90 and 0–180 min (quantification of AUC expressed as figures not reported) | 18F-FDG PET/CT | Before administration | (44) |
| Tea catechins and caffeine | Green tea extract (C. sinensis) | 615 mg tea catechins + 77 mg caffeine for 5 wk | 2017 | Japan | January–March (winter) | Single-blind, randomized, placebo-controlled, season-matched, crossover | 20.7 ± 0.5 | 20 | 22.7 ± 0.7 | Male | CI fat oxidation was significantly higher after the treatment with the catechin beverage (33.7 ± 4.8 mg/min) than before the treatment (22.8 ± 3.8 mg/min) and significantly higher than after the placebo (15.2 ± 5.8 mg/min) | 18F-FDG PET/CT | Before administration | (44) |
| Ephedrine | Ephedra extract | 2.5 mg/kg (acute effect) | 2012 | Australia | Not reported | Double-blind, randomized, placebo-controlled, crossover | 22 ± 1 (lean), 36 ± 1 (obese) | 18 | 25 ± 1 (lean), 27 ± 1 (obese) | Male | There was a significant increase in supraclavicular SUVmax by 145% ± 4.8% in lean, but not in obese participants (4.0% ± 8.3%) | 18F-FDG PET/CT | After administration | (45) |
| Ephedrine | Ephedra extract | 1 mg/kg (acute effect) | 2012 | United States | February–March (throughout the year) | Double-blind, randomized, placebo-controlled | 23.7 ± 1.4 | 10 | 27.1 ± 1.7 | Male and female | Ephedrine treatment did not reach a measurable effect on detection of BAT activity, whereas cold exposure did | 18F-FDG PET/CT | After administration | (51) |
| Ephedrine | Ephedra extract | 1.5 mg · kg–1 · d–1 for 4 wk | 2015 | Australia | March–October (autumn to spring) | Double-blind, randomized, placebo-controlled | 24.5 ± 1.5 | 23 | 22.5 ± 1.5 | Male | Both SUVmax (placebo: −3% ± 6%; ephedrine: −13% ± 7%) and SUVmean values (placebo: −3% ± 7%; ephedrine: −22% ± 6%) decreased after the ephedrine treatment | 18F-FDG PET/CT | Before and after administration | (52) |
| 6-Paradol, 6-gingerol, and 6-shogaol | Grains of Paradise (Aframomum melegueta) | 40 mg (acute effect) | 2013 | Japan | Not reported | Single-blind, randomized, placebo- controlled, crossover | 21.3 ± 0.6 | 19 | 22.6 ± 0.7 | Male | EE response to extract ingestion during the 2-h period expressed as AUC was 24.7 ± 6.1 kJ/h in the BAT-positive group, which was significantly higher than that in the BAT-negative group (23.2 ± 10.1 kJ/h) | 18F-FDG PET/CT | Before administration | (46) |
| 3,5,7,4′-Tetramethoxy-flavone, dimethoxy-flavone, and pentame-thoxyflavone | Kaempferia parviflora extract | 100 mg (acute effect) | 2014 | Japan | Not reported | Single-blind, randomized, placebo-controlled, crossover | 21.2 ± 0.3 | 20 | 24.1 ± 0.4 | Male | An increase in EE after ingestion was observed in the high-BAT group (351 ± 50 kJ/d at 60 min), but not in the low-BAT activity group | 18F-FDG PET/CT | Before administration | (47) |
| Xanthigen (fucoxanthin + punicic acid) | Undaria pinnatifida | 600 mg fucoxanthin/d for 3 mo | 2015 | Korea | August–November (summer to autumn) | Pilot study | 36.6 | 2 | 32.5 | Female | Xanthigen visually increased BAT activity in 1 of the 2 participants | 18F-FDG PET/CT | Before and after administration | (55) |
| Chenodeoxycholic acid | Synthetic product | 15 mg/kg for 2 d (acute effect) | 2015 | Netherlands | February–June (winter to spring) | Double-blinded, randomized, placebo-controlled, crossover | 21.9 ± 1.6 | 12 | 22 ± 3 | Female | Chenodeoxycholic acid increased both BAT SUVmean (from 0.8 ± 0.3 to 1.2 ± 0.3) and SUVmax values (from 1.0 ± 0.4 to 1.6 ± 0.4) | 18F-FDG PET/CT | After administration | (53) |
BAT, brown adipose tissue; CI, cold induced; CIT, cold-induced thermogenesis; EE, energy expenditure; 18F-FDG PET/CT, fluorodeoxyglucose positron emission tomography combined with computed tomography, Hb, hemoglobin; IRT, infrared thermography; NIRTRS, near-infrared time-resolved spectroscopy; RMR, resting metabolic rate; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value.
BMI and age values are means ± SDs.
Capsinoids
All studies on capsinoids used the oral administration of 9 mg capsinoids/d extracted from Capsicum annuum L. (CH-19 sweet chili pepper), except for a single study (54) that administered 12 mg/d (acute effect). There were 3 chronic-effect studies that used 9 mg capsinoids/d (between 6 and 8 wk) (40, 43) and 3 acute-effect studies (48, 49).
Yoneshiro et al. (40) conducted a chronic-effect study (Table 1) in which the participants were selected according to their BAT activity. After 6 wk of capsinoid treatment, the participants who had received capsinoids exhibited a significant increase in CIT capacity compared with the control group (40). Nirengi et al. (43) reported that 6 wk of capsinoid treatment induced an increase in BAT activity as measured by 18F-FDG PET/CT scan (43). The same group (43) studied the effect of daily capsinoid ingestion over 8 wk and showed that the total hemoglobin (total-Hb) change (assessed by NIRTRS every 2 wk at 27°C in the supraclavicular region) was significantly greater in the capsinoid group (43).
Yoneshiro et al. (48) reported that acute-effect ingestion of capsinoids significantly increased resting metabolic rate (RMR) and supraclavicular skin temperature in the high-BAT activity group (48). The observed increase in RMR was associated with an increase in BAT activity (48). Moreover, Ang et al. (49) concluded that a single ingestion of capsinoids elicited a significant increase in RMR and skin temperature in the cervical-supraclavicular region measured by infrared thermography in the capsinoid group (49). Finally, Sun et al. (54) used the highest dose of capsinoids (12 mg/d) in healthy adults and compared this treatment with mild cold exposure: no effect of capsinoid ingestion on BAT stimulation was observed, whereas a mild cold exposure did stimulate BAT (54).
Tea catechins
All studies on tea catechins used green tea extract beverages from Camellia sinensis, and 2 of these studies also used caffeine supplementation (44). Nirengi et al. (50) administered a beverage containing 540 mg catechins plus 77 mg caffeine/d for 12 wk and demonstrated an increase in BAT density evaluated by NIRTRS (50). Yoneshiro et al. (44) administered a beverage containing 615 mg catechins plus 77 mg caffeine twice daily for 5 wk. BAT activity was measured by 18F-FDG PET/CT scan before the intervention with a previously fixed cooling protocol (2 h; 19°C) to select participants with low BAT activity (44). The authors demonstrated an increase in CIT in the catechin group relative to the control group (44). The results of the experimental group (44) also revealed that a single ingestion of the beverage containing catechins plus caffeine induced a significant increase in RMR in the catechin group compared with the placebo group.
Ephedrine
The only available chronic-effect study determined that BAT activity was significantly reduced after a 28-d ephedrine treatment (1.5 mg ephedrine hydrochloride · kg–1 · d–1) in the experimental group compared with the control group (52). The findings of the acute-effect studies are contradictory. Carey et al. (45) measured BAT activity 60 min after ingestion of 2.5 mg ephedrine/kg, and revealed an increase in BAT activity in lean humans but not in obese humans. In contrast, Cypess et al. (51) detected no increase in BAT activity after an intramuscular injection of 1 mg ephedrine/kg.
Other dietary components
Three studies used plant extracts with pungent activity (46, 47, 55), and 1 study used bile acids (53). Only 1 study evaluated the chronic effect of the dietary component (55) and 3 studies evaluated the acute effects (46, 47, 53).
One study used a seaweed extract containing fucoxanthin on 2 obese adult women (55). The women were instructed to take 2 pills of Xanthigen (600 mg contained in 2 capsules; PLT Health Solutions) with fucoxanthin (3 mg) and punicic acid (174 mg) every day for 12 wk. The 18F-FDG PET/CT scan analyses, which were conducted before and after the 12-wk intervention, reported a visual (not quantitative) increase in BAT activity in 1 of the participants. Sugita et al. (46) used a single dose of 40 mg from Aframomum melegueta. The participants were divided into BAT-positive and BAT-negative groups. After the oral ingestion of the dietary components, a significant increase in RMR was found in the BAT-positive group (46). Matsushita et al. (47) used a single 100-mg dose of Kaempferia parviflora extract. All participants were men and were previously divided into high-BAT-activity and low-BAT-activity groups. A significant increase in RMR in the high-BAT group was demonstrated. The acute effect of chenodeoxycholic acid (15 mg · kg–1 · d–1 for 2 d) was tested in young and lean women (53). The authors reported a significant increase in BAT activity and an increase in RMR in the chenodeoxycholic acid group (53).
Discussion
We analyzed all available human studies that investigated the effect of both chronic and acute ingestion of dietary components on BAT activity, as measured by 18F-FDG PET/CT, and on CIT, RMR, supraclavicular total-Hb, and supraclavicular skin temperature. In general, the study designs were not robust, because only 7 studies (47%) (43, 45, 49–53) used a double-blind, randomized, placebo-controlled design, and only 3 studies (43, 52, 55) conducted an 18F-FDG PET/CT scan before and after the intervention. There is a lack of information on the effect of the dietary component at the molecular level in BAT, white adipose tissue, or muscle measured in vivo through biopsy analysis. The results of many studies (40, 43–50, 53–55) suggest that it seems plausible to activate and recruit human BAT through the ingestion of certain dietary components in healthy adults, yet the current level of evidence precludes a definitive conclusion. Further studies are warranted to confirm this hypothesis.
Capsinoids
Capsinoids are substances naturally present in chili peppers, and they are particularly abundant in C. annuum L. or “CH-19 Sweet” (56). Capsinoids include capsiate, dihydrocapsiate, and nordihydrocapsiate (57). Although capsinoids are structurally similar to capsaicin, they are 1000 times less pungent but are as potent as capsaicin in increasing thermogenesis and RMR (48).
The thermogenic activation pathways of capsinoids include TRPV1 and TRPA1, which have possible mechanisms of action on BAT activity, because capsinoids activate both receptors (58, 59). In mice, intragastric administration of capsinoids has been shown to elicit an increase in temperature in the intrascapular BAT region, whereas this effect was attenuated in TRPV1-deficient animals (60). The thermogenic response is also impaired in humans with a mutation affecting TRPV1 function (61). Furthermore, capsiate is an enhancer of UCP1 expression (62). Consequently, it is likely that capsinoids activate BAT through the TRPV1-SNS-BAT axis in humans. Only 1 study on capsinoids analyzed BAT activity before and after the intake of dietary components (43) and only 1 study analyzed BAT activity after the capsinoid ingestion. Notably, the first study (43) had a low sample size (n = 3; single-blind and crossover study design), whereas the second study (54) had a nonblind design. Neither study (43, 54) performed a personalized cooling protocol before the 18F-FDG PET/CT scan, and the glucose standardized uptake values were not individualized (43) and did not meet the recommendations for BAT analysis and quantification (63).
Tea catechins
Tea catechins are polyphenolic components present in green tea. The most abundant and bioactive component is epigallocatechin gallate, which is one of the most thoroughly studied dietary components present in green tea. Therefore, epigallocatechin gallate may be the best choice if only 1 catechin is encapsulated to test its properties on BAT activity. The thermogenic effect of tea catechins has repeatedly been shown in humans (64, 65). Regarding the mechanism of action, epigallocatechin gallate and its auto-oxidation products have been shown to be TRPA1 and TRPV1 agonists (66, 67). Catechins can activate and recruit BAT via TRP channels located in the sensory neurons of the gastrointestinal tract (68). There is no solid evidence that tea catechins can activate and recruit BAT in humans, because there are no studies in which 18F-FDG PET/CT scans were conducted before and after the dietary component administration. However, it seems biologically plausible that tea catechins plus caffeine activate human BAT in both chronic and acute treatments, because this combination has been shown to increase CIT and RMR in humans (44). Certain studies suggest that CIT may be proportional to BAT-dependent thermogenic capacity (69). Therefore, the significant increase in CIT observed after the tea catechin treatment may be due to an increase in BAT activity. Interestingly, there is strong evidence that skeletal muscle is a major contributor of CIT, so we cannot discard the possibility that the effects of catechins are muscle-mediated and not BAT-mediated (70, 71). Nirengi et al. (50) quantified the change in total-Hb in the supraclavicular region after a chronic intake of catechins under thermoneutral conditions, which was found to be significantly higher in the catechin group (50). This finding is in agreement with the results of a previous experiment performed by the same research group in which they demonstrated that total-Hb values under thermoneutral conditions were positively correlated with BAT activity (72). Thus, it seems feasible that the increase in total-Hb in the supraclavicular area may be directly correlated with an increase in BAT activity, and that tea catechins could activate BAT even under thermoneutral conditions in healthy humans.
An acute-effect study (44) showed an increase in RMR after the ingestion of a tea catechin beverage in subjects with detectable BAT activity but not in subjects with undetectable BAT activity. Even though this increase in RMR was likely due to an increase in BAT activity, no 18F-FDG PET/CT scan was conducted after the intervention to confirm the finding.
In vitro studies using tea catechins have revealed an inhibition of the catecholamine-degrading enzyme catechol-O-methyltransferase (73), which could explain the SNS-BAT connection as due to an increase in norepinephrine life span. Nonetheless, catechol-O-methyltransferase activity was not impaired by high doses of epigallocatechin gallate in humans (74). Regarding the thermogenic effects of caffeine, these effects may occur through the inhibition of phosphodiesterase (an enzyme that degrades cAMP) (75). In addition, a synergistic interaction has been proposed between tea catechins and caffeine, with the latter increasing adrenergic and lipolysis activity (76, 77). Additional studies are warranted to clarify whether the inhibition of catechol-O-methyltransferase and phosphodiesterase is responsible, in part, for the thermogenic effect of tea catechins and to verify to what extent TRPA1 and TRPV1 activation can enhance human BAT activity.
Ephedrine
The dietary component ephedrine is a sympathomimetic amine found in plants of the Ephedra genus, which can bind to adrenergic receptors. The mechanism of action of ephedrine does not involve activation of the TRP receptor, but rather, stimulation of SNS activity and thermogenic pathways boosts BAT activity. Historically, ephedrine has been used to increase energy expenditure in humans (78), and it has been linked to an increase in 18F-FDG BAT uptake in mice (79); thus it seems plausible that ephedrine itself could activate BAT in humans. Carey et al. (45) showed that BAT can be activated in healthy, lean adults with a single dose of ephedrine. Interestingly, the same treatment administered to obese patients did not significantly increase BAT activity, suggesting that, at least in response to sympathomimetic dietary components, BAT activity may be impaired in obese humans. This finding is consistent with previous cold exposure studies that did not detect a significant increase in BAT in obese humans (4, 15, 24). Conversely, another acute-effect study performed by Cypess et al. (51) failed to stimulate BAT activity after ephedrine administration. However, this study used a single intramuscular dose of 1 mg ephedrine/kg, which was a lower dose (and a different route of administration) than that used by Carey et al. (45) (a single oral dose of 2.5 mg ephedrine/kg); hence, we cannot discern to what extent the difference between intramuscular injection and oral ingestion affected the outcome. Moreover, the analyses were performed over a wide period of time (>1 y considering all the interventions and measurements of all participants) so the seasonal variations in BAT activity could have introduced a bias.
Chronic effects of ephedrine treatments appear to reduce BAT activity. Carey et al. (52) showed that BAT activity was reduced after 28 d of ephedrine treatment. Interestingly, this intervention was performed from spring to autumn, far from the winter season, which means that the warmer outdoor temperatures might have also inhibited BAT activity (29). Further studies are needed to determine whether BAT activation could be due to ephedrine itself, what the ideal dose and route of administration (intramuscular or orally ingested) are, and to what extent the duration of the treatment impairs BAT activity.
Other dietary components
A. melegueta seeds, also known as “Grains of Paradise,” are used as a spice for flavoring food and are known to have anti-inflammatory properties (80). These seeds are rich in 6-gingerol, 6-paradol, and 6-shogaol (all of which are nonvolatile with pungent activity) (81). It seems feasible that these dietary components may exert their effects by binding TRPV1 (68). Sugita et al. (46) demonstrated an increase in RMR in BAT-positive individuals compared with BAT-negative individuals after an intervention with an extract of A. melegueta (46). However, the effect of A. melegueta on BAT activity was not tested and is therefore unknown because the 18F-FDG PET/CT scan was performed only before the intervention. The volatile components existing in the A. melegueta extract have a vanilloid moiety, which can activate TRPV1 (involved in the thermic effects of capsinoids and catechins, as previously described). Therefore, if the increase in RMR after the ingestion of A. melegueta is confirmed by a parallel augmentation in BAT activity assessed after ingestion of the dietary component, then the activation of the TRPV1-SNS-BAT axis may be the underlying mechanism of action.
Regarding human BAT activity after oral ingestion of the K. parviflora extract, RMR increased in the BAT-positive group (46). K. parviflora has been demonstrated to have antiobesity effects in type 2 diabetic obese mice (82). Dietary supplementation with K. parviflora prevented not only body weight increase and body fat accumulation but also glucose intolerance (82, 83). Yoshino et al. (84) showed that K. parviflora ingestion increased urinary excretion of noradrenaline, UCP1 expression, and RMR in mice. Thus, it could be expected that K. parviflora, similar to capsinoids, activates and recruits BAT. Nevertheless, studies with better methodological designs are warranted to determine whether K. parviflora truly enhances BAT activity in humans.
Xanthigen is a weight-management ingredient combining punicic acid (from pomegranate) and fucoxanthin from the brown edible seaweed Undaria pinnatifida. The combination of Xanthigen with punicic acid appears to have positive effects on weight loss, body fat, and liver fat content in obese nondiabetic women (85). In addition, fucoxanthin from U. pinnatifida has exhibited an antiobesity effect through the enhancement of UCP1 expression in murine white adipose tissue (86). Although Kim et al. (55) performed a before-and-after assessment of BAT, the sample size of their study (n = 2) precludes any firm conclusion. Moreover, these authors reported a visual increase (not quantified) in BAT activity according to PET/CT (55). Thus, even though the results appear to support the evidence of previous studies demonstrating that Xanthigen increases RMR in obese patients (85) and could therefore be useful as a therapy against diabetes (87), a larger sample size and a better study design are needed.
Chenodeoxycholic acid is one of the primary bile acids produced by the liver in humans, and its supplemental use has been demonstrated to be safe in humans and easily administered orally (88). Chenodeoxycholic acid activates the G-protein–coupled bile acid receptor 1 (GPBAR1), which results in an increase in the concentration of intracellular cAMP. This secondary messenger, cAMP, activates type 2 deiodinase, an enzyme that drives the conversion of the inactive thyroid hormone to the active form (T3). Thus, T3 is the final activator of BAT, which also increases RMR (89). It also appears to increase BAT activity and enhance RMR in murine models (90). Moreover, there is strong evidence supporting a correlation between circulating bile acids, BAT activity, and NST (89). Broeders et al. (53) showed that an administration of 15 mg chenodeoxycholic acid/d for 2 d increases BAT activity measured by 18F-FDG PET/CT scan under thermoneutral conditions. Therefore, it would be of clinical interest to study the effects of chronic chenodeoxycholic acid supplementation on BAT activity.
General limitations of the studies included in the review
Homogeneous composition of dietary component extracts
To enable interstudy comparisons, there is a need to standardize the use of dietary component extracts. For example, Yoneshiro et al. (44) and Nirengi et al. (50) used different concentrations of catechins in their beverages; therefore, it is not possible to determine to what extent the results between these studies are comparable.
18F-FDG limitations
Even though 18F-FDG uptake is a marker of BAT activity, this parameter is not directly proportional to energy consumption, because glucose is not the major fuel in BAT metabolism (91). Indeed, fatty acids, which are the main substrate for BAT mitochondria, are mostly provided from the inner depots, but are also provided from the bloodstream via the action of the lipoprotein lipase (12). Hence, we should use additional approaches to quantify total BAT activity, such as measuring the oxygen consumption (70), tracking other fatty acid derivatives such as 11C-acetate (92), and using MRI (93–96).
Ethnicity
There is evidence that BAT volume is dependent on ethnicity (97). The majority of the studies included in the review were conducted in South Asians (75%; n = 12) (40, 43, 44, 46–50, 55), compared with 25% in Caucasians (n = 4) (45, 51–53). Therefore, caution must be used when translating the results from one ethnic group to another.
Seasonality
Seasonal changes in BAT and CIT may be due to environmental factors such as outdoor temperatures (98) and photoperiod (99). In addition, not only CIT but also cold-induced fat oxidation have been shown to be increased in winter compared with summer, and this change is more notable in high-BAT subjects than in low-BAT subjects (100). Considering all this evidence, the involvement of BAT in the seasonal variations of CIT in healthy humans is another variable that warrants consideration to optimize study designs (i.e., crossover with washout for acute-effect studies; control group with placebo for chronic-effect studies with placebo).
Dose adjustment
Only the chenodeoxycholic acid (53) and ephedrine (45, 51, 52) studies adjusted the dietary component dose according to the weight of each participant.
Authorship
Notably, all studies using capsinoids and tea catechins were conducted by Japanese researcher groups (40, 43, 44, 46–50), with Dr. Saito leading a significant fraction of the studies (n = 6; 35%) (40, 44, 46–48). To date, there is no confirmation of the results of these studies by an independent research group, except for Sun et al. (54), who studied capsinoids.
Conclusions
Although it is biologically plausible that the ingestion of dietary components increases human BAT activity, the current level of evidence supporting this hypothesis is low. More and better-designed studies (e.g., double-blind, randomized, placebo-controlled, and season-matched, with a personalized cooling protocol prior to PET/CT or MRI scan) are needed to understand whether dietary components are an effective treatment to activate and recruit human BAT.
Acknowledgments
We are grateful to Carmen Sainz-Quinn for assistance with the English language. All authors read and approved the final manuscript.
Notes
Supported by Spanish Ministry of Economy and Competitiveness, Fondo de Investigación Sanitaria del Instituto de Salud Carlos III grant PI13/01393 and Retos de la Sociedad grant DEP2016-79512-R, by the Fondos Estructurales de la Unión Europea (FEDER), by Spanish Ministry of Education grants FPU 13/04365 (to GS-D) and 16/02828 (to FJO-P), by the Fundación Iberoamericana de Nutrición (FINUT), by Redes Temáticas de Investigación Cooperativa RETIC grant Red SAMID RD16/0022, by the AstraZeneca HealthCare Foundation by Cátedra Real Madrid-Universidad Europea, Escuela Universitaria Real Madrid–Universidad Europea, and by the University of Granada, Plan Propio de Investigación 2016, Excellence actions: Units of Excellence; Scientific Unit of Excellence on Exercise and Health (UCEES).
Author disclosures: FJO-P, BM-T, GS-D, CMA, JL-S, DA-R, AS-C, and JRR, no conflicts of interest.
This study is part of a PhD thesis conducted within the Biomedicine Doctoral Studies of the University of Granada, Spain.
Abbreviations used: BAT, brown adipose tissue; CIT, cold-induced thermogenesis; CT, computed tomography; FDG, fluorodeoxyglucose; NIRTRS, near-infrared time-resolved spectroscopy; NST, nonshivering thermogenesis; PET, positron emission tomography; RMR, resting metabolic rate; SNS, sympathetic nervous system; total-Hb, total hemoglobin; TRP, transient receptor potential channel; TRPA1, TRP ankyrin 1; TRPV1, TRP vanilloid 1; UCP1, uncoupling protein 1.
References
- 1. Devlin MJ. The “skinny” on brown fat, obesity, and bone. Am J Phys Anthropol 2015;156:98–115. [DOI] [PubMed] [Google Scholar]
- 2. Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol 2003;88:141–8. [DOI] [PubMed] [Google Scholar]
- 3. Nedergaard J, Cannon B, Jaenicke R. Mammalian hibernation [and discussion]. Philos Trans R Soc B Biol Sci 1990;326:669–86. [DOI] [PubMed] [Google Scholar]
- 4. van Marken Lichtenbelt WD. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–8. [DOI] [PubMed] [Google Scholar]
- 5. Betz MJ, Enerbäck S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol 2018;14(2):77–87. [DOI] [PubMed] [Google Scholar]
- 6. Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bargut TCL, Aguila MB, Mandarim-de-Lacerda CA. Brown adipose tissue: updates in cellular and molecular biology. Tissue Cell 2016;48:452–60. [DOI] [PubMed] [Google Scholar]
- 8. Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol 2014;537:199–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AWC, Stasch J-P, Bloch W, Friebe A, Heeren J et al.. Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nat Commun 2015;6:7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blondin DP, Tingelstad HC, Noll C, Frisch F, Phoenix S, Guérin B, Turcotte ÉE, Richard D, Haman F, Carpentier AC. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun 2017;8:14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson CM, Kazantzis M, Wang J, Venkatraman S, Goncalves RLS, Quinlan CL, Ng R, Jastroch M, Benjamin DI, Nie B et al.. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep 2015;10:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schilperoort M, Hoeke G, Kooijman S, Rensen PCN. Relevance of lipid metabolism for brown fat visualization and quantification. Curr Opin Lipidol 2016;27:242–8. [DOI] [PubMed] [Google Scholar]
- 14. Brendle C, Werner MK, Schmadl M, la Fougère C, Nikolaou K, Stefan N, Pfannenberg C. Correlation of brown adipose tissue with other body fat compartments and patient characteristics. a retrospective analysis in a large patient cohort using PET/CT. Acad Radiol 2017;25:102–10. [DOI] [PubMed] [Google Scholar]
- 15. Vijgen GHEJ, Bouvy ND, Teule GJJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One 2011;6:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee P, Greenfield JR, Ho KKY, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010;299:E601–6. [DOI] [PubMed] [Google Scholar]
- 17. Vijgen GHEJ, Bouvy ND, Teule GJJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 2012;97:E1229–33. [DOI] [PubMed] [Google Scholar]
- 18. Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes 2014;38:812–17. [DOI] [PubMed] [Google Scholar]
- 19. Hoeke G, Kooijman S, Boon MR, Rensen PCN, Berbeé JFP. Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ Res 2016;118:173–82. [DOI] [PubMed] [Google Scholar]
- 20. Chondronikola M, Volpi E, Børsheim E, Porter C, Saraf MK, Annamalai P, Yfanti C, Chao T, Wong D, Shinoda K et al.. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 2016;23:1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011;96:192–9. [DOI] [PubMed] [Google Scholar]
- 22. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y-H, Doria A et al.. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto N, Enerbäck S et al.. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–25. [DOI] [PubMed] [Google Scholar]
- 24. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K et al.. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brychta RJ, Chen KY. Cold-induced thermogenesis in humans. Eur J Clin Nutr 2017;71:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saito M, Yoneshiro T, Matsushita M. Activation and recruitment of brown adipose tissue by cold exposure and food ingredients in humans. Best Pract Res Clin Endocrinol Metab 2016;30:537–47. [DOI] [PubMed] [Google Scholar]
- 27. Hanssen MJW, Hoeks J, Brans B, van der Lans AAJJ, Schaart G, van den Driessche JJ, Jörgensen JA, Boekschoten MV, Hesselink MKC, Havekes B et al.. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 2015;21:863–5. [DOI] [PubMed] [Google Scholar]
- 28. Hanssen MJW, van der Lans AAJJ, Brans B, Hoeks J, Jardon KMC, Schaart G, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 2016;65:1179–89. [DOI] [PubMed] [Google Scholar]
- 29. Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 2014;63:3686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Lans AAJJ, Vosselman MJ, Hanssen MJW, Brans B, van Marken Lichtenbelt WD. Supraclavicular skin temperature and BAT activity in lean healthy adults. J Physiol Sci 2016;66:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karjalainen S. Thermal comfort and gender: a literature review. Indoor Air 2012;22:96–109. [DOI] [PubMed] [Google Scholar]
- 32. Saito M, Yoneshiro T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol 2013;24:71–7. [DOI] [PubMed] [Google Scholar]
- 33. Saito M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv Food Nutr Res 2015;76:1–28. [DOI] [PubMed] [Google Scholar]
- 34. Sakellariou P, Valente A, Carrillo AE, Metsios GS, Nadolnik L, Jamurtas AZ, Koutedakis Y, Boguszewski C, Andrade CMB, Svensson PA et al.. Chronic L-menthol-induced browning of white adipose tissue hypothesis: a putative therapeutic regime for combating obesity and improving metabolic health. Med Hypotheses 2016;93:21–6. [DOI] [PubMed] [Google Scholar]
- 35. Hochkogler CM, Lieder B, Rust P, Berry D, Meier SM, Pignitter M, Riva A, Leitinger A, Bruk A, Wagner S et al.. A 12-week intervention with nonivamide, a TRPV1 agonist, prevents a dietary-induced body fat gain and increases peripheral serotonin in moderately overweight subjects. Mol Nutr Food Res 2017;61:1600731. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Cui L, Xu H, Liu S, Zhu F, Yan F, Shen S, Zhu M. TRPV1 agonism inhibits endothelial cell inflammation via activation of eNOS/NO pathway. Atherosclerosis 2017;260:13–19. [DOI] [PubMed] [Google Scholar]
- 37. Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci 2008;29:29–36. [DOI] [PubMed] [Google Scholar]
- 38. Westerterp KR, de Jonge L, Bray G, Granata G, Brandon L, Weststrate J, Reed G, Hill J, Tataranni P, Westerterp K et al.. Diet induced thermogenesis. Nutr Metab (Lond) 2004;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hibi M, Oishi S, Matsushita M, Yoneshiro T, Yamaguchi T, Usui C, Yasunaga K, Katsuragi Y, Kubota K, Tanaka S et al.. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes 2016;40:1655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013;123:3404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonet ML, Mercader J, Palou A. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie 2017;134:99–117. [DOI] [PubMed] [Google Scholar]
- 42. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. AJP Endocrinol Metab 2007;293:E444–52. [DOI] [PubMed] [Google Scholar]
- 43. Nirengi S, Homma T, Inoue N, Sato H, Yoneshiro T, Matsushita M, Kameya T, Sugie H, Tsuzaki K, Saito M et al.. Assessment of human brown adipose tissue density during daily ingestion of thermogenic capsinoids using near-infrared time-resolved spectroscopy. J Biomed Opt 2016;21:091305. [DOI] [PubMed] [Google Scholar]
- 44. Yoneshiro T, Matsushita M, Hibi M, Tone H, Takeshita M, Yasunaga K, Katsuragi Y, Kameya T, Sugie H, Saito M. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am J Clin Nutr 2017;105:873–81. [DOI] [PubMed] [Google Scholar]
- 45. Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MH, Kingwell BA. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 2013;56:147–55. [DOI] [PubMed] [Google Scholar]
- 46. Sugita J, Yoneshiro T, Hatano T, Aita S, Ikemoto T, Uchiwa H, Iwanaga T, Kameya T, Kawai Y, Saito M. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br J Nutr 2013;110(4):733–8. [DOI] [PubMed] [Google Scholar]
- 47. Matsushita M, Yoneshiro T, Aita S, Kamiya T, Kusaba N, Yamaguchi K, Takagaki K, Kameya T, Sugie H, Saito M. Kaempferia parviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. J Nutr Sci Vitaminol (Tokyo) 2015;61:79–83. [DOI] [PubMed] [Google Scholar]
- 48. Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr 2012;95:845–50. [DOI] [PubMed] [Google Scholar]
- 49. Ang QY, Goh HJ, Cao Y, Li Y, Chan S-P, Swain JL, Henry CJ, Leow MK-S. A new method of infrared thermography for quantification of brown adipose tissue activation in healthy adults (TACTICAL): a randomized trial. J Physiol Sci 2017;67:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nirengi S, Amagasa S, Homma T, Yoneshiro T, Matsumiya S, Kurosawa Y, Sakane N, Ebi K, Saito M, Hamaoka T. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. Springerplus 2016;5:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cypess AM, Chen Y-C, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM et al.. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA 2012;109:10001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carey AL, Pajtak R, Formosa MF, Van Every B, Bertovic DA, Anderson MJ, Eikelis N, Lambert GW, Kalff V, Duffy SJ et al.. Chronic ephedrine administration decreases brown adipose tissue activity in a randomised controlled human trial: implications for obesity. Diabetologia 2015;58:1045–54. [DOI] [PubMed] [Google Scholar]
- 53. Broeders EPM, Nascimento EBM, Havekes B, Brans B, Roumans KHM, Tailleux A, Schaart G, Kouach M, Charton J, Deprez B et al.. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 2015;22:418–26. [DOI] [PubMed] [Google Scholar]
- 54. Sun L, Camps SG, Goh HJ, Govindharajulu P, Schaefferkoetter JD, Townsend DW, Verma SK, Velan SS, Sun L, Sze SK et al.. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan. Am J Clin Nutr 2018;107:62–70. [DOI] [PubMed] [Google Scholar]
- 55. Kim KM, Kim SM, Cho DY, Park SJ, Joo NS. The effect of xanthigen on the expression of brown adipose tissue assessed by 18F-FDG PET. Yonsei Med J 2016;57:1038–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kawabata F, Inoue N, Yazawa S, Kawada T, Inoue K, Fushiki T. Effects of CH-19 sweet, a non-pungent cultivar of red pepper, in decreasing the body weight and suppressing body fat accumulation by sympathetic nerve activation in humans. Biosci Biotechnol Biochem 2006;70:2824–35. [DOI] [PubMed] [Google Scholar]
- 57. Luo X, Peng J, Li Y. Recent advances in the study on capsaicinoids and capsinoids. Eur J Pharmacol 2011;650:1–7. [DOI] [PubMed] [Google Scholar]
- 58. Shintaku K, Uchida K, Suzuki Y, Zhou Y, Fushiki T, Watanabe T, Yazawa S, Tominaga M. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br J Pharmacol 2012;165:1476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Iida T, Moriyama T, Kobata K, Morita A, Murayama N, Hashizume S, Fushiki T, Yazawa S, Watanabe T, Tominaga M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 2003;44:958–67. [DOI] [PubMed] [Google Scholar]
- 60. Kawabata F, Inoue N, Masamoto Y, Matsumura S, Kimura W, Kadowaki M, Higashi T, Tominaga M, Inoue K, Fushiki T. Non-pungent capsaicin analogs (capsinoids) increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci Biotechnol Biochem 2009;73:2690–7. [DOI] [PubMed] [Google Scholar]
- 61. Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, Takahashi M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr 2009;89:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Masuda Y, Haramizu S, Oki K, Ohnuki K, Watanabe T, Yazawa S, Kawada T, Hashizume S, Fushiki T. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J Appl Physiol 2003;95:2408–15. [DOI] [PubMed] [Google Scholar]
- 63. Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA, Enerbäck S, Kinahan PE, van Marken Lichtenbelt W, Lin FI et al.. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab 2016;24:210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gosselin C, Haman F. Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br J Nutr 2013;110:282–8. [DOI] [PubMed] [Google Scholar]
- 65. Hursel R, Westerterp-Plantenga MS. Catechin- and caffeine-rich teas for control of body weight in humans. Am J Clin Nutr 2013;98:1682–93. [DOI] [PubMed] [Google Scholar]
- 66. Kurogi M, Miyashita M, Emoto Y, Kubo Y, Saitoh O. Green tea polyphenol epigallocatechin gallate activates TRPA1 in an intestinal enteroendocrine cell line, STC-1. Chem Senses 2012;37:167–77. [DOI] [PubMed] [Google Scholar]
- 67. Kurogi M, Kawai Y, Nagatomo K, Tateyama M, Kubo Y, Saitoh O. Auto-oxidation products of epigallocatechin gallate activate TRPA1 and TRPV1 in sensory neurons. Chem Senses 2015;40:27–46. [DOI] [PubMed] [Google Scholar]
- 68. Yoneshiro T, Saito M. Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management. Curr Opin Clin Nutr Metab Care 2013;16:625–31. [DOI] [PubMed] [Google Scholar]
- 69. Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, Herscovitch P, Millo CM, Remaley A, Lee P et al.. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab 2013;98:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 2013;54:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blondin DP, Labbé SM, Phoenix S, Guérin B, Turcotte ÉE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol 2015;593:701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nirengi S, Yoneshiro T, Sugie H, Saito M, Hamaoka T. Human brown adipose tissue assessed by simple, noninvasive near-infrared time-resolved spectroscopy. Obesity (Silver Spring) 2015;23:973–80. [DOI] [PubMed] [Google Scholar]
- 73. Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol 2005;69:1523–31. [DOI] [PubMed] [Google Scholar]
- 74. Lorenz M, Paul F, Moobed M, Baumann G, Zimmermann BF, Stangl K, Stangl V. The activity of catechol-O-methyltransferase (COMT) is not impaired by high doses of epigallocatechin-3-gallate (EGCG) in vivo. Eur J Pharmacol 2014;740:645–51. [DOI] [PubMed] [Google Scholar]
- 75. Stohs SJ, Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phyther Res 2016;30:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes (Lond) 2009;33:956–61. [DOI] [PubMed] [Google Scholar]
- 77. Ferreira MA, Silva DM, de Morais ACJ, Mota JF, Botelho PB. Therapeutic potential of green tea on risk factors for type 2 diabetes in obese adults – a review. Obes Rev 2016;17(12):1316–28. [DOI] [PubMed] [Google Scholar]
- 78. Shekelle PG. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 2003;289:1537–45. [DOI] [PubMed] [Google Scholar]
- 79. Baba S, Tatsumi M, Ishimori T, Lilien DL, Engles JM, Wahl RL. Effect of nicotine and ephedrine on the accumulation of 18F-FDG in brown adipose tissue. J Nucl Med 2007;48:981–6. [DOI] [PubMed] [Google Scholar]
- 80. Ilic NM, Dey M, Poulev AA, Logendra S, Kuhn PE, Raskin I. Anti-inflammatory activity of grains of paradise (Aframomum Melegueta Schum) extract. J Agric Food Chem 2014;62:10452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Connell DW, McLachman R. Examination of the gingerols, shogaols, paradols and related compounds by thin-layer and gas chromatography. J Chromatogr 1972;67:29–35. [Google Scholar]
- 82. Akase T, Shimada T, Terabayashi S, Ikeya Y, Sanada H, Aburada M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J Nat Med 2011;65:73–80. [DOI] [PubMed] [Google Scholar]
- 83. Shimada T, Horikawa T, Ikeya Y, Matsuo H, Kinoshita K. Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia 2011;82:1272–8. [DOI] [PubMed] [Google Scholar]
- 84. Yoshino S, Kim M, Awa R, Kuwahara H, Kano Y, Kawada T. Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci Nutr 2014;2:634–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metab 2010;12:72–81. [DOI] [PubMed] [Google Scholar]
- 86. Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun 2005;332:392–7. [DOI] [PubMed] [Google Scholar]
- 87. Maeda H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: a review. J Oleo Sci 2015;64:125–32. [DOI] [PubMed] [Google Scholar]
- 88. Chiang J. Bile acid metabolism and signalling. Compr Physiol 2013;3:1191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T et al.. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006;439:484–9. [DOI] [PubMed] [Google Scholar]
- 90. Teodoro JS, Zouhar P, Flachs P, Bardova K, Janovska P, Gomes AP, Duarte FV, Varela AT, Rolo AP, Palmeira CM et al.. Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int J Obes (Lond) 2014;38:1027–34. [DOI] [PubMed] [Google Scholar]
- 91. Halpern B, Mancini MC, Halpern A. Brown adipose tissue: what have we learned since its recent identification in human adults. Arq Bras Endocrinol Metabol 2014;58:889–99. [DOI] [PubMed] [Google Scholar]
- 92. Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during cold exposure in humans. J Clin Invest 2012;122:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Deng J, Schoeneman SE, Zhang H, Kwon S, Rigsby CK, Shore RM, Josefson JL. MRI characterization of brown adipose tissue in obese and normal-weight children. Pediatr Radiol 2015;45:1682–9. [DOI] [PubMed] [Google Scholar]
- 94. Hu HH, Perkins TG, Chia JM, Gilsanz V. Characterization of human brown adipose tissue by chemical-shift water-fat MRI. Am J Roentgenol 2013;200:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reddy NL, Jones TA, Wayte SC, Adesanya O, Sankar S, Yeo YC, Tripathi G, McTernan PG, Randeva HS, Kumar S et al.. Identification of brown adipose tissue using MR imaging in a human adult with histological and immunohistochemical confirmation. J Clin Endocrinol Metab 2014;99:E117–21. [DOI] [PubMed] [Google Scholar]
- 96. Paulus A, van Marken Lichtenbelt W, Mottaghy FM, Bauwens M. Brown adipose tissue and lipid metabolism imaging. Methods 2017;130:105–13. [DOI] [PubMed] [Google Scholar]
- 97. Bakker LEH, Boon MR, van der Linden RAD, Arias-Bouda LP, van Klinken JB, Smit F, Verberne HJ, Jukema JW, Tamsma JT, Havekes LM et al.. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol 2014;2:210–17. [DOI] [PubMed] [Google Scholar]
- 98. Kern PA, Finlin BS, Zhu B, Rasouli N, McGehee RE, Westgate PM, Dupont-Versteegden EE. The effects of temperature and seasons on subcutaneous white adipose tissue in humans: evidence for thermogenic gene induction. Obstet Gynecol Surv 2015;70:180–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kooijman S, van den Berg R, Ramkisoensing A, Boon MR, Kuipers EN, Loef M, Zonneveld TCM, Lucassen EA, Sips HCM, Chatzispyrou IA et al.. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci USA 2015;112:6748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yoneshiro T, Matsushita M, Nakae S, Kameya T, Sugie H, Tanaka S, Saito M, Au-Yong I, Thorn N, Ganatra R et al.. Brown adipose tissue is involved in the seasonal variation of cold-induced thermogenesis in humans. Am J Physiol Regul Integr Comp Physiol 2016;310:R999–1009. [DOI] [PubMed] [Google Scholar]