ABSTRACT
Although studies have suggested that milk and milk-product consumption may influence growth during childhood and puberty, results are inconsistent. This meta-analysis was performed to evaluate the available evidence of randomized controlled trials (RCTs) assessing whether milk and milk-product consumption could affect growth and body composition among children and adolescents aged 6–18 y. PubMed, EMBASE, Web of Science, and The Cochrane Library databases were systematically searched for all RCTs published up to December 2017 that investigated milk and milk-product consumption (≥12 wk) on growth and body composition among participants (aged 6–18 y) without undernourishment or diseases. Study screening and data extraction by 2 reviewers followed established PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The Cochrane Collaboration's tool was used to assess the quality of the trials. Data were pooled using a random-effects model. Seventeen trials with 2844 children and adolescents were included. Milk and milk-product interventions resulted in a greater increase in body weight (0.48 kg; 95% CI: 0.19, 0.76 kg; P = 0.001), lean mass (0.21 kg; 95% CI: 0.01, 0.41 kg; P = 0.04), and attenuated gain in percentage body fat (−0.27%; 95% CI: −0.45%, −0.09%; P = 0.003) compared with control groups. However, there were no significant changes in fat mass, height, or waist circumference in the intervention groups compared with the control groups (P ≥ 0.05). In subgroup analyses, the baseline weight and age, and the duration of intervention were associated with the efficacy of milk and milk-product intake on the change in lean mass, percentage body fat, and waist circumference, respectively (test for subgroup differences: P < 0.05). Children and adolescents aged 6–18 y consuming milk and milk products are more likely to achieve a lean body phenotype. This meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42018086850.
Keywords: milk, weight, lean mass, meta-analysis, body phenotype
Introduction
Childhood and puberty are critical phases for growth and development and milk and milk products are natural, rich sources of a wide range of essential nutrients (1–3). Milk and milk products may be important modifiable dietary factors in managing a healthy body weight and body composition due to their richness in protein, vitamins, and minerals (particularly calcium) (4, 5). Consuming adequate amounts of some nutrients—such as calcium, potassium, and vitamin D—may be more difficult without including dairy foods in the diet (6).
Studies investigating the association between milk and milk-product consumption and body growth, such as height, weight, and body composition, during childhood and puberty have reported inconsistent results (7–11). Evidence from a 1-y family-centered lifestyle intervention study investigating the impact of milk and milk-product consumption on prepubertal children with obesity reported that fat mass percentage was significantly decreased among children who consumed 4 servings milk and milk products/d compared with a control group (10). However, a longitudinal cohort study in children from ages 10 to 13 y reported less-favorable changes in body weight and body fat for normal or overweight children consuming flavored milk compared with nonconsumers over a 2-y period (11).
Recently, an updated meta-analysis of 37 randomized controlled trials (RCTs) suggested that a high-dairy intervention increased body weight and lean mass and decreased body fat and waist circumference among adults (12). In addition, a systematic review of prospective cohort studies found that dairy consumption was inversely and longitudinally associated with the risk of childhood overweight and obesity (13). However, to our knowledge, there is no meta-analysis summarizing results from RCTs for the effects of milk and milk-product consumption on quality of growth during childhood and puberty. Therefore, this meta-analysis was conducted to evaluate the available evidence of RCTs assessing whether milk and milk-product consumption could affect growth among children and adolescents aged 6–18 y, primarily weight, height, lean mass, and fat mass.
Methods
This meta-analysis was conducted according to the recommendations and standards set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (14).
Study search
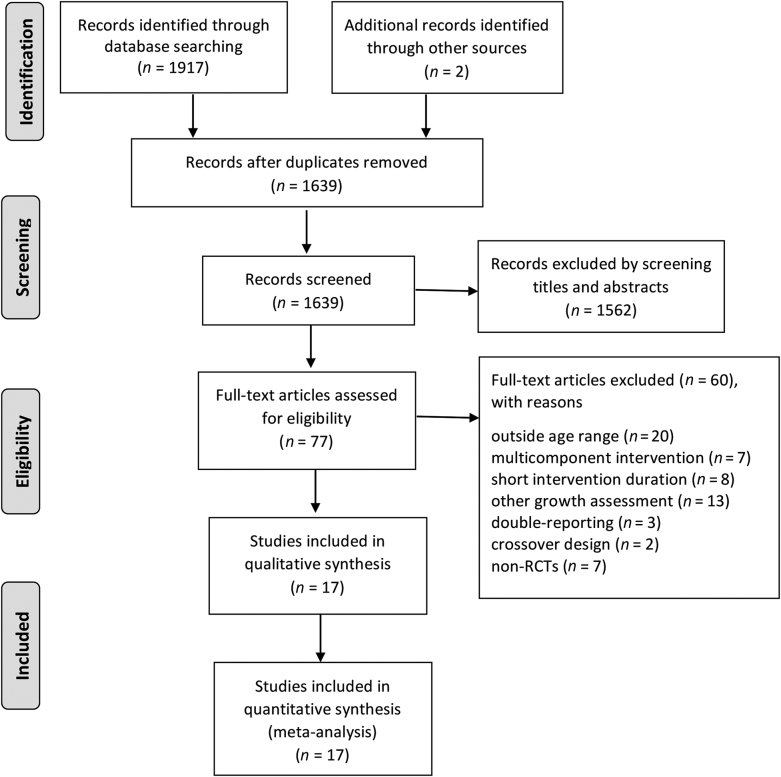
PubMed, EMBASE, Web of Science, and The Cochrane Library databases were systematically searched for all RCTs investigating the effects of milk and milk-product consumption on growth among children and adolescents aged 6–18 y published up to December 2017. Three comprehensive search themes were specified. The first theme identified relevant terms for milk by combining exploded versions of the Medical Subject Heading (MeSH) terms dairy products, dairy, milk, cheese, or yogurt and corresponding keywords in titles and abstracts. The second theme identified expanded the MeSH terms by combining body height, body weight, weight loss, weight change, weight reduction, body composition, body fat, body fat percentage, fat mass, fat body mass, percentage of fat body mass, lean mass, fat free mass, fat-free mass, lean body mass, or percentage of lean body mass and corresponding keywords in titles and abstracts. The third theme identified terms related to children and adolescents by combining child, childhood, adolescent, teens, teen, teenagers, teenager, youths, or youth and corresponding keywords in titles and abstracts. The 3 themes were then combined and further filtered by “human” and the publication type of RCT, with no language restrictions. The initial search identified 1917 potentially relevant articles through databases and 2 records from other sources (reference lists). After removing duplicates and screening the titles and abstracts, full texts of 77 articles were screened. Figure 1 provides detailed search selection, and the complete search algorithm is detailed in Supplemental Table 1.
FIGURE 1.
PRISMA flow diagram of the study selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial.
Study selection
Studies were included if they met the following criteria: 1) they were RCTs with a parallel controlled design, 2) participants age range was 6–18 y, 3) milk or milk products were used as the main intervention but not as part of a multicomponent dietary supplement in either the intervention or control groups, 4) participants consumed dairy products for ≥12 wk, and 5) measurements of the outcome related to growth and body composition were reported. Studies were excluded if 1) they were not randomized designs, 2) participants were undernourished or diseased, or 3) milk and milk products were supplied in the same quantity for both experimental and control groups. Studies that supplied milk and milk products to control groups were not excluded from this meta-analysis because milk and milk products are a recommended food group and their impact on growth is well elucidated, especially in childhood through adolescence. In addition, we included studies categorizing intervention groups with different doses of milk and milk products.
Relevant studies were identified in duplicate by 2 independent reviewers (KK and OFS) by first screening the titles and abstracts followed by the full text against inclusion and exclusion criteria. Any disagreement was resolved by consensus with the senior reviewer (HAW) experienced in the field of nutrition interventions and child health. When multiple articles for a single study were present, we used the latest publication and supplemented it, if necessary, with data from the most complete publication.
Data extraction and quality assessment
From each study, information was extracted including the following: first author, publication year, geographic location, study design (duration, randomization procedures, blinding), participant information (sample size, sex distribution, age, inclusion and exclusion criteria), details with regard to intervention and control regimens (category of milk and milk products, mean intakes per day, and proportion of nutrients), source of milk supplement, method of outcome ascertainment, outcome results (change in body height, weight, fat mass, lean mass, and waist circumference), and corresponding 95% CIs, SEs, or exact P values. When multiple time points were reported, only the end of the intervention was used.
Because differences in study populations and design might cause variations in results, the Cochrane Collaboration's tool was used to assess the quality of the trials, which contained 7 risk-of-bias items: random-sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias (15). If all features were adequate, the study was allocated a low risk of bias. If ≥1 features were unclear, the risk of bias was unclear. If ≥1 features were inadequate or negative, the study was allocated a high risk of bias (16).
Statistical analysis and exploration of heterogeneity
Data pooling was performed with the use of classical meta-analytic methodology using Review Manager 5.3 (Cochrane Informatics and Knowledge Management Department). Significance was set at P < 0.05, and 95% CIs were calculated for each investigation and for each outcome variable. Data were extracted from the text, tables, and figures of the original published articles. To include data from as many trials as possible, missing SD data for one trial (17) were imputed from SD data from all other trials using the same measure (18). When estimating the analysis indexes, the weighted mean difference was used as the effect size of the continuous variable.
Before calculating the standardized mean effect for all trials, statistical heterogeneity was evaluated by using the I2 statistic and P values, which assessed the appropriateness of pooling the individual study results (19). The I2 value provided an estimate of the amount of variance across studies because of heterogeneity rather than chance (20). I2 values of 25%, 50%, and 75% corresponded to low, moderate, and high levels of heterogeneity, respectively (21). If P ≥ 0.05, the heterogeneity was not substantial. Thus, a fixed-effects model was used to calculate forest plots with weighted mean difference and 95% CIs. If P < 0.05, however, the heterogeneity was considered substantial. Then, combined results were tested with a random-effects model of DerSimonian-Laird methods and inverse variance weighting.
Subgroups were categorized according to sex (males compared with females), age (6–12 y compared with 13–18 y), baseline body weight (normal weight compared with overweight or obesity) and intervention duration (<12 compared with ≥12 mo). The subgroup analysis was assessed using chi-square statistic, with P < 0.05 taken to indicate significance. Sensitivity analyses were conducted to evaluate the effect of removing any single study from the meta-analysis. The risk of publication bias was assessed by rendering funnel plots. This meta-analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration ID: CRD42018086850).
Results
Study characteristics
The search strategy and selection of studies for this meta-analysis are shown in Figure 1. In total, 2844 children and adolescents aged between 6 and 18 y from 17 RCTs (22–38) were included. Doses of milk and milk products in the included studies ranged from 190 to 1000 mL/d, and the intervention duration varied from 3 to 24 mo. Six trials investigated participants with overweight and obese (23, 28, 32, 34, 36, 37). The types of intervention included fluid milk (22–26, 28–38), cheese (26–28, 33, 34, 37), yogurt (26–28, 32–34, 37), whey (23), or casein (23). With regard to the source of intervention, most of them were provided free to the participants by the study. The characteristics of the selected trials are presented in Table 1.
TABLE 1.
Characteristics of the trials included in the meta-analysis1
| Study, year, country (ref) | Age,2 y | No. of participants (no. in intervention) 3 | Percentage of girls; population characteristics | Intervention | Control | Duration, mo | Source of milk supplement | Main outcomes | Design |
|---|---|---|---|---|---|---|---|---|---|
| Albala et al., 2008, Chile (22) | 8–10 | 93 (47) | 47.3; prepubertal | 3 servings milk/d (∼200 g/serving provided 80 kcal, 8 g protein, 3 g fat, 11 g carbohydrate, and 320 mg Ca) | Usual diet | 4 | Delivered to home weekly | Weight, height, body composition (DXA), FFQ | R, P |
| Arnberg et al., 2012, Denmark (23) | 12–15 | 193 (48/48/47) | 62; age- and sex-adjusted BMI (kg/m2) >25 | 1 L skim milk/d, 1 L whey/d, or 1 L casein/d | 1 L water/d | 3 | Provided at the start of and halfway through the intervention | Weight, waist circumference, fasting plasma insulin, HOMA, and plasma C-peptide | R, B, P |
| Baker et al., 1980, UK (24) | 7–8 | 520 (281) | 48.7; families with ≥4 children | One-third of a pint (190 mL) milk/d | Usual diet | 21 | Provided free at school | Weight, height | R, B, P |
| Cadogan et al., 1997, UK (25) | 12.2 ± 0.34 | 82 (44) | 100; white | 568 mL (1 pint) whole or reduced-fat milk/d | Usual diet | 18 | Delivered to home every morning | Height, weight, pubertal staging, bone mass, body composition (DXA), biochemical values | R, P |
| Chan et al., 1995, USA (26) | 9–13 | 48 (22) | 100; Tanner stage II | Allowance of 1200 mg Ca/d of dairy products (milk, cheese, and yogurt) | Usual diet | 12 | Delivered weekly | Bone mineral content and density, body composition (DXA), serum or urinary biochemical values | R, P |
| Cheng et al., 2005, Finland (27) | 10–12 | 77 (39) | 100; Tanner stage I–II, dietary calcium intakes <900 mg/d | Dairy products (low-fat cheese and lactose-reduced yogurt; 1000 mg Ca/d) | Habitual diet (calcium intakes >900 mg/d) | 24 | Donated by VALIOOy, Helsinki, Finland | Height, weight, pubertal staging, bone mass, body composition (DXA), biochemical values | R, DB, PC |
| Cohen et al., 2016, Canada (28) | 6–8.5 | 73 (23/24) | 57; obese, prepubertal | 2 or 4 servings milk and alternatives/d, preferably with lower %MF (i.e., 1%MF milk, 15%–18%MF cheese, 1%–2%MF yogurt) | Usual diet | 12 | Self-support | Anthropometry, BAZ, waist circumference, body composition (DXA), biochemical values | R, P |
| Du et al., 2004, China (29) | 10–12 | 346 (111/113) | 100 | 330 mL UHT milk/d (560 mg Ca); 330 mL UHT milk/d (560 mg Ca and 5 or 8 mg cholecalciferol) | Habitual diet | 24 | The UHT milk was specially formulated by Murray Goulburn Co-operative Co. Ltd. (Brunswick, Australia) | Height, weight, bone mass, body composition (DXA), biochemical values | R, P |
| Gibbons et al., 2004, New Zealand (30) | 8–10 | 154 (74) | 51.3; prepubertal | High-calcium milk provided 600 mg Ca per 40-g serving or 1200 mg/d | A control drink reconstituted with water | 18 | Delivered to school fortnightly or to home monthly | Height, weight, bone mineral density, bone mineral content, body composition (DXA) | R, B, P |
| Lambourne et al., 2013, USA (31) | 13.6 | 74 (36) | 66; RT (3 d/wk) | 24 oz fat-free chocolate milk or low-fat white milk/d on RT days: 16 fl oz fat-free chocolate milk (16 g protein, 280 kcal) immediately after completion of RT, and 8 fl oz low-fat white milk (8 g protein, 100 kcal) at lunch; non-RT days: 16 fl oz low-fat white milk before their first class and 8 fl oz fat-free chocolate milk at lunch | Bottled water | 6 | Provided at school | Height, weight, body composition (DXA), waist circumference, muscle strength, daily physical activity, energy/macronutrient intake | R, B, P |
| Lappe et al., 2017, USA (32) | 13–14 | 274 (136) | 100; overweight, intakes of ≤600 mg/d | Low-fat milk (skim, 1%, or 2%) or yogurt servings providing ≥1200 mg Ca/d | Usual diet | 12 | Participants refunded for yogurt and milk purchased after receipt submission | Anthropometry, bone mineral density, bone mineral content, body composition (DXA), biochemical values | R, P |
| Merrilees et al., 2000, New Zealand (33) | 15–16 | 91 (45) | 100; postpubertal | Dairy food products (milk, flavored milk, dairy dessert, cheese, or yogurt; low-fat options were available; >1000 mg Ca/d) | Usual diet | 24 | Delivered fortnightly | Height, weight, bone mineral density, bone mineral content, body composition (DXA), biochemical values | R, P |
| Mobarhan et al., 2009, Iran (34) | 12–18 | 96 (28/33) | Not mentioned; Overweight or obese | A calorie-restricted diet providing 500 kcal/d with 3 or 4 servings of dairy products/d | A calorie-restricted diet providing 500 kcal/d | 3 | Not mentioned | Height, weight, waist circumference, body composition (BIA), biochemical values | R, P |
| Rahmani et al., 2011, Iran (35) | 6–9 | 469 (235) | 51 | 250 mL 2.5% milk/d | Usual diet | 3 | Provided at school | Weight, height, midarm circumference | R, P |
| St-Onge et al., 2009, USA (36) | 8–10 | 45 (21) | 80; overweight | 3 × 236 mL of skim milk and 1 × 236 mL of 1% low-fat chocolate milk/d | Usual diet with 3 × 200 mL of sugar-sweetened beverage and 236 mL of milk/d | 4 | Provided weekly | Height, weight, % body fat, waist and hip circumferences, MRI | R, P |
| Vogel et al., 2017, USA (37) | 8–15.9 | 181 (overweight 47; healthy weight 55) | 64; early pubertal, low amounts of dairy (<800 mg Ca/d) | 3 servings (equivalent to ∼900 mg Ca)/d of milk, yogurt, or cheese | Habitual diet | 18 | Provided biweekly | Height, weight, waist circumference, pubertal staging, bone mass, body composition (DXA), biochemical values | R, P |
| Volek et al., 2003, USA (38) | 13–17 | 28 (14) | 0; RT (3 d/wk) | 3 servings (708 mL or 24 fl oz) 1% fluid milk/d | Habitual diet with unfortified juice | 3 | Provided weekly | Anthropometric measures, body composition (DXA), bone density, dietary intakes, performance measures | R, P |
B, blind; BAZ, BMI-for-age z-score; BIA, bioimpedance analysis; DB, double-blind; fl, fluid; HOMA, homeostatic model assessment; oz, ounces; P, parallel; PC, placebo-controlled; R, randomized; ref, reference; RT, resistance training; UHT, ultra-high temperature; %MF, percentage of milk fat.
The mean age range is provided for studies that presented mean age separately for each group (control and intervention).
The multiple intervention numbers represent the number of participants in different intervention groups.
Mean ± SD.
Change in growth
Body weight
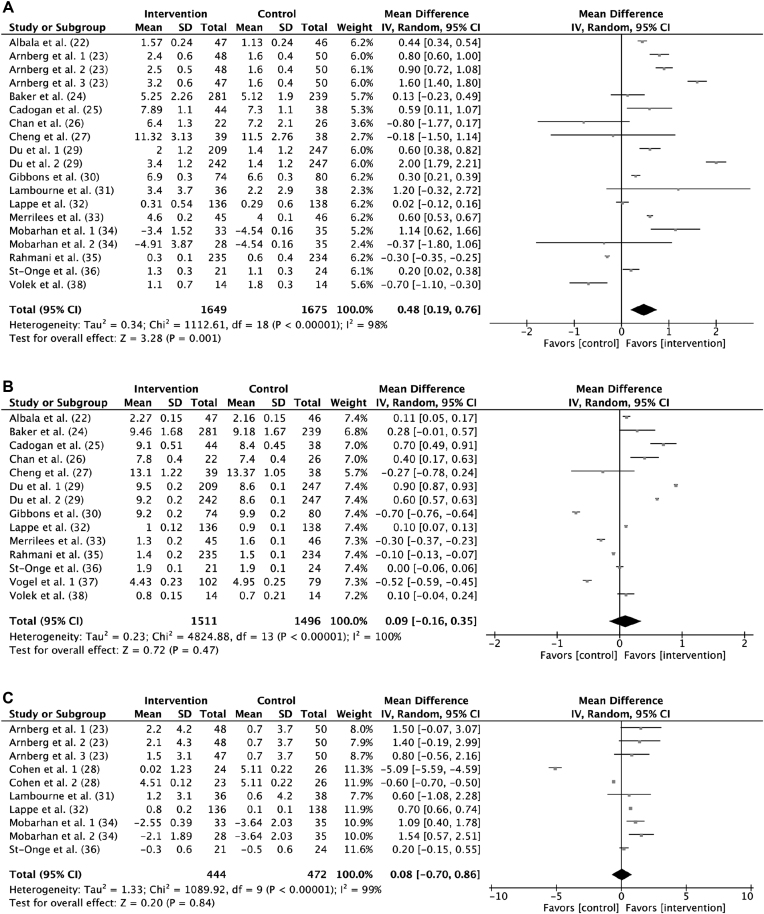
In the analysis for change in body weight, 15 studies (22–27, 29–36, 38) (n = 3324 participants) were included. There was a significant difference in body-weight change between the intervention and control groups (0.48 kg; 95% CI, 0.19, 0.76 kg; P = 0.001; Figure 2A) However, significant heterogeneity was observed for change in body weight (I2 = 98%; P < 0.001). Thus, subgroup analysis was performed by categorizing subgroups by sex (males compared with females), age (6–12 y compared with 13–18 y), baseline body weight (normal weight compared with overweight or obesity) and intervention duration (<12 compared with ≥12 mo). In all subgroup analyses, the impact of milk and milk products on change in body weight was not significantly different between the categories for any subgroup (test for subgroup differences: P ≥ 0.05; Supplemental Figure 1).
FIGURE 2.
Forest plot of change in body weight (kilograms) (A), height (centimeters) (B), and waist circumference (centimeters) (C) between intervention and control groups: random-effects model. IV, inverse variance.
Height
In the analysis for the change in height, 13 studies (22, 24–27, 29, 30, 32, 33, 35–38) in 3007 participants were included. There was no significant difference in height change between the intervention and control groups (0.09 cm; 95% CI: −0.16, 0.35 cm; P = 0.47; Figure 2B). However, significant heterogeneity was observed for height (I2 = 100%; P < 0.001). In all subgroup analysis, the effect of milk and milk products showed no significant difference on change in body height between the categories for any subgroup (test for subgroup differences: P ≥ 0.05; Supplemental Figure 2).
Waist circumference
Six studies (23, 28, 31, 32, 34, 36) (n = 916 participants) evaluated the effect of milk and milk-product consumption on change in waist circumference, and the analysis suggested there was no significant difference in waist circumference change between the intervention and control groups (0.08 cm; 95% CI: −0.70, 0.86 cm; P = 0.84; Figure 2C). Moreover, heterogeneity was significant (I2 = 99%; P < 0.001). In subgroup analysis, the effectiveness of milk and milk-product consumption in reducing gain in waist circumference was more pronounced among participants with a short-term intervention (<12 mo; 0.90 cm; 95% CI: 0.36, 1.44 cm; P = 0.001) than in those with a long-term intervention (≥12 mo; −1.62 cm; 95% CI: −3.00, −0.23 cm; P = 0.02) (test for subgroup differences: I2 = 90.9%; P < 0.001; Supplemental Figure 3). However, there were no significant differences between sex, age, baseline body weight, and waist circumference change, respectively (test for subgroup differences: P ≥ 0.05; Supplemental Figure 3).
Change in body composition
Lean mass
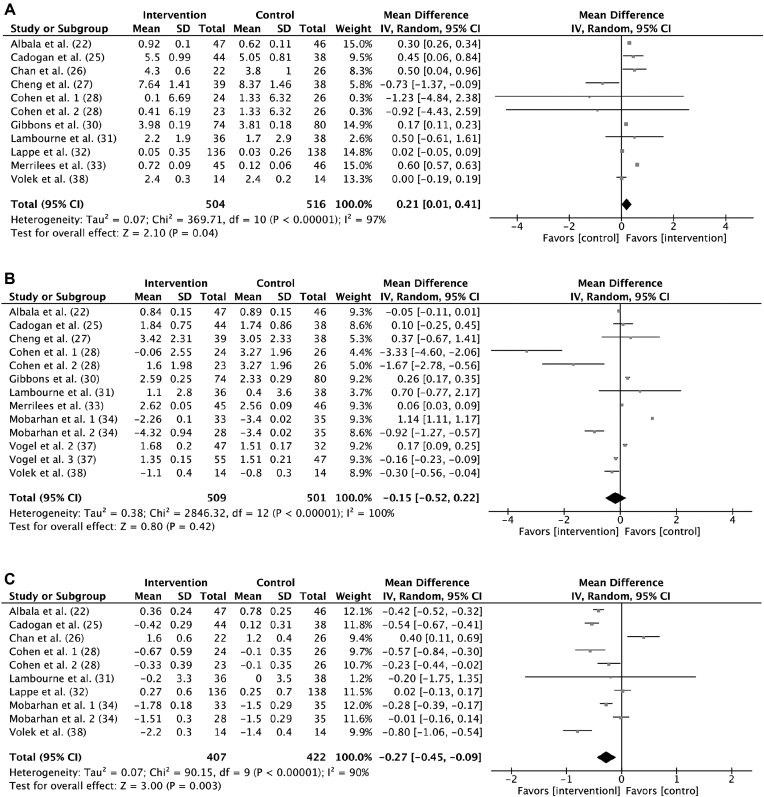
Ten studies (22, 25–28, 30–33, 38) (n = 1020 participants) evaluated the effect of milk and milk-product consumption on change in lean mass. A significant increase that favored milk and milk-product consumption on lean mass was shown (0.21 kg; 95% CI: 0.01, 0.41 kg; P = 0.04; Figure 3A). With regard to the significant heterogeneity (I2 = 97%; P < 0.001), subgroup analysis was performed. The effectiveness of milk and milk-product consumption in increasing lean mass was more pronounced (test for subgroup differences: I2 = 79.5%; P = 0.03) in participants with a baseline weight within the normal range (0.26 kg; 95% CI: 0.06, 0.46 kg; P = 0.01) than in those who were overweight or obese (0.02 kg; 95% CI: −0.05, 0.09 kg; P = 0.61). There were no significant differences on change in lean mass between the categories for sex, age, and intervention duration, respectively (test for subgroup differences: P ≥ 0.05; Supplemental Figure 4).
FIGURE 3.
Forest plot of change in lean mass (kilograms) (A), fat mass (kilograms) (B), and percentage body fat (C) between intervention and control groups: random-effects model. IV, inverse variance.
Fat mass
For fat mass (kilograms), 10 studies (22, 25, 27, 28, 30, 31, 33, 34, 37, 38) (n = 1010 participants) were included in the analysis and the heterogeneity was high (I2 = 100%; P < 0.001). There was no significant difference in fat mass between the intervention and control groups (−0.15 kg; 95% CI: −0.52, 0.22 kg; P = 0.42; Figure 3B). Furthermore, in all subgroup analyses, the impact of milk and milk products on change in fat mass was not significantly different between the categories for any subgroup (test for subgroup differences: P ≥ 0.05; Supplemental Figure 5).
Percentage body fat
There were 8 studies (22, 25, 26, 28, 31, 32, 34, 38) in 829 participants that mentioned the change in percentage body fat, and the heterogeneity between them was significant (I2 = 90%; P < 0.001). Participants in the intervention group gained less percentage body fat than did those in control groups (−0.27%; 95% CI: −0.45%, −0.09%; P = 0.003; Figure 3C). In subgroup analysis, the effectiveness of milk and milk-product consumption in reducing gain in percentage body fat was more pronounced among children (6–12 y; −0.44%; 95% CI: −0.56%, −0.32%; P < 0.001) compared with adolescents (13–18 y; −0.14%; 95% CI: −0.40%, 0.13%; P = 0.31) (test for subgroup differences: I2 = 75.3%; P = 0.04). There were no significant differences on change in percentage body fat between the categories for sex, baseline body weight, and intervention duration, respectively (test for subgroup differences: P ≥ 0.05; Supplemental Figure 6).
Sensitivity analysis and quality assessment
To test the robustness of the results, we removed one study each time in the pooled analysis and found that no single study substantially influenced the pooled association of interest. Risk of bias for each domain in each included study is presented in Supplemental Figure 7. On the basis of the funnel plots (Supplemental Figure 8), it could be concluded that studies that evaluated changes in growth rarely had publication bias with the symmetric figures, except for change in waist circumference.
Discussion
Findings from this meta-analysis of 17 RCTs suggest that milk and milk-product interventions resulted in higher lean mass and body weight and attenuated gain in percentage body fat compared with control groups among children and adolescents aged 6–18. This result is consistent with the conclusion of a meta-analysis by Geng et al. (12) that a high-dairy intervention increased body weight and lean mass among adults. Furthermore, another meta-analysis by Abargouei et al. (39), including 883 adults aged 18–85 y, showed that increased dairy consumption without energy restriction might not lead to a significant change in weight or body composition, whereas the inclusion of dairy products in energy-restricted weight-loss diets significantly resulted in a greater reduction in weight, fat mass, and waist circumference and gain in lean mass compared with that in the usual weight-loss diets. Chen et al. (40) also found that dairy products may have modest benefits in facilitating weight loss when energy is restricted, but this effect seems to be short and not sustainable. In addition, the results of this meta-analysis showed that milk and milk-product consumption did not lead to change in fat mass, height, and waist circumference in the intervention groups compared with the control groups.
In this meta-analysis, high statistical heterogeneity was detected in the primary outcome, and several solutions were applied to minimize this. Sensitivity analyses were adopted, and the subgroup analyses investigated the effect of sex (males compared with females), age (6–12 y compared with 13–18 y), baseline body weight (normal weight compared with overweight or obesity), and intervention duration (<12 compared with ≥12 mo). Most of the subgroups were effective at reducing the heterogeneity. This analysis showed that the baseline weight and age and the duration of intervention were associated with the effectiveness of milk and milk-product intake on the change in lean mass, percentage body fat, and waist circumference, respectively. In addition, the asymmetry funnel plot suggested that possible publication bias existed between studies that evaluated change in waist circumference, which may be attributed to the limited number of trials included in the comparison.
The types of milk and milk-product interventions contained fluid milk, cheese, yogurt, whey, and casein in this meta-analysis. The potential mechanisms underlying the impact of milk and milk-product consumption on the regulation of body weight and body composition have not been elucidated. Milk and milk products are rich in protein and calcium. These 2 nutrients have been linked to growth status in childhood and puberty (41). Our meta-analysis of RCTs showed that milk and milk-product intake increased body weight and lean mass among children and adolescents. A potential mechanism is that the consumption of dairy protein may reduce appetite (42). Trials (22, 28) observed a significant reduction in energy intake among the milk intervention group compared with the control group. Moreover, studies suggest that a high intake of dietary protein generally associates with body-composition regulation by diet-induced thermogenesis, increasing satiety and decreasing hunger and preserving or increasing lean mass (43–46). For example, leucine, which is concentrated in milk and milk products, has been found to have a beneficial effect on protein synthesis and maintenance of lean mass (47); and the stimulation of protein synthesis might cause a repartitioning of energy from fat mass into lean mass (48). In addition, it has also been suggested that calcium may have an important role in weight and body-composition regulation in several different ways, such as decreasing de novo lipogenesis, increasing lipolysis by suppressing the formation of 1,25-dihydroxyvitamin D and secretion of parathyroid hormone or calciotropic hormones (49), interfering with FA absorption (50) by forming insoluble soaps (51, 52), or controlling intracellular calcium (49). More trials are necessary to further illustrate potential mechanisms of milk products on body growth.
This meta-analysis focused on evaluating the correlations between milk and milk-product intake and body growth among children and adolescents aged 6–18 y, in which the corresponding subgroups, on the basis of participants’ age, sex, baseline body weight, and the duration of the intervention, were also assessed. However, there were some potential limitations. First, 11 of the total included RCTs (22–24, 26–28, 31, 32, 34, 36, 37) of milk and milk-product supplementation had height, weight, or body composition as a key outcome, with the remaining studies having bone health as the key outcome with change in height, weight, or body compositions as the secondary outcomes. Thus, it could be argued that these studies were not designed to evaluate body growth for height, weight, or body-composition changes. However, weight and height changes are easy to measure and are reproducible, which are necessary for the interpretation of studies evaluating bone outcomes. The technique of measuring bone outcomes in those studies was DXA, which is also accurate for evaluating body composition, such as lean mass, fat mass, and percentage body fat, and reference data exist from the NHANES (53). Therefore, the fact that it was not the primary measure should not influence these results. Second, the heterogeneity between all of the included studies meant that only the random-effects model could be used, which affected the efficiency of the study. Third, double blinding was almost not possible in this type of intervention trial (54), which could affect the quality assessment. Fourth, differences in effects of milk and milk-product type (e.g., amount of fat, fermented compared with nonfermented, liquid compared with solid/semisolid) were not evaluated. Another potential limitation may be other confounding factors influencing the relation between milk and growth, which were not accounted for, such as physical activity and other dietary factors.
In summary, our pooled analysis indicates that children and adolescents aged 6–18 y who consume milk and milk products are more likely to achieve a lean body phenotype. This is presumably caused by improving body composition, particularly increasing lean mass and consequently decreasing gain in percentage body fat. Because milk and milk products are considered constituting a package of healthy nutrients for child and adolescent development, in addition to the evidence generated from this meta-analysis, children and adolescents should be encouraged to add milk and milk products to their diet in sufficient amounts to reach healthy intakes. Future studies are needed to further investigate types of milk products (e.g., milk with different fat percentages or fermented milk) in relation to growth in children and adolescents.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—KK: conceptualized and designed the study; collected and statistically analyzed the data; and drafted, edited, and critically reviewed and revised the manuscript; OFS: conceptualized and designed the study, collected the data; and edited, critically reviewed, and revised the manuscript; HAW: conceptualized and designed the study and edited, critically reviewed, revised, and supervised the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Dairy Farmers of Canada and the Dairy Research Cluster Initiative (Agriculture and Agri-Food Canada, Dairy Farmers of Canada, and the Canadian Dairy Commission).
Author disclosures: KK, OFS, and HAW, no conflicts of interest.
Supplemental Table 1 and Supplemental Figures 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
References
- 1. Weinberg LG, Berner LA, Groves JE. Nutrient contributions of dairy foods in the United States, Continuing Survey of Food Intakes by Individuals, 1994–1996, 1998. J Am Diet Assoc. 2004;104:895–902. [DOI] [PubMed] [Google Scholar]
- 2. Moore LL, Bradlee ML, Gao D, Singer MR. Effects of average childhood dairy intake on adolescent bone health. J Pediatr. 2008;153:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prentice AM. Dairy products in global public health. Am J Clin Nutr. 2014;99(Suppl):1212S–6S. [DOI] [PubMed] [Google Scholar]
- 4. Rockell JE, Williams SM, Taylor RW, Grant AM, Jones IE, Goulding A. Two-year changes in bone and body composition in young children with a history of prolonged milk avoidance. Osteoporos Int. 2005;16:1016–23. [DOI] [PubMed] [Google Scholar]
- 5. Pallister T, Haller T, Thorand B, Altmaier E, Cassidy A, Martin T, Jennings A, Mohney RP, Gieger C, MacGregor A et al.. Metabolites of milk intake: a metabolomic approach in UK twins with findings replicated in two European cohorts. Eur J Nutr. 2017;56:2379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weaver CM. Role of dairy beverages in the diet. Physiol Behav. 2010;100:63–6. [DOI] [PubMed] [Google Scholar]
- 7. Fayet F, Ridges LA, Wright JK, Petocz P. Australian children who drink milk (plain or flavored) have higher milk and micronutrient intakes but similar body mass index to those who do not drink milk. Nutr Res. 2013;33:95–102. [DOI] [PubMed] [Google Scholar]
- 8. Murphy MM, Douglass JS, Johnson RK, Spence LA. Drinking flavored or plain milk is positively associated with nutrient intake and is not associated with adverse effects on weight status in US children and adolescents. J Am Diet Assoc. 2008;108:631–9. [DOI] [PubMed] [Google Scholar]
- 9. Berkey CS, Rockett HR, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Arch Pediatr Adolesc Med. 2005;159:543–50. [DOI] [PubMed] [Google Scholar]
- 10. Cohen TR, Hazell TJ, Vanstone CA, Rodd C, Weiler HA. Bone health is maintained, while fat mass is reduced in pre-pubertal children with obesity participating in a 1-year family-centered lifestyle intervention. Calcif Tissue Int. 2017;101:612–22. [DOI] [PubMed] [Google Scholar]
- 11. Noel SE, Ness AR, Northstone K, Emmett P, Newby PK. Associations between flavored milk consumption and changes in weight and body composition over time: differences among normal and overweight children. Eur J Clin Nutr. 2013;67:295–300. [DOI] [PubMed] [Google Scholar]
- 12. Geng T, Qi L, Huang T. Effects of dairy products consumption on body weight and body composition among adults: an updated meta-analysis of 37 randomized control trials. Mol Nutr Food Res. 2018;62:e1700410. [DOI] [PubMed] [Google Scholar]
- 13. Lu L, Xun P, Wan Y, He K, Cai W. Long-term association between dairy consumption and risk of childhood obesity: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70:414–23. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A et al.. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. In: Methods guide for effectiveness and comparative effectiveness reviews. Rockville (MD): Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 17. Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. [DOI] [PubMed] [Google Scholar]
- 18. Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the number needed to treat? An empirical study of summary effect measures in meta-analyses. Int J Epidemiol. 2002;31:72–6. [DOI] [PubMed] [Google Scholar]
- 19. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA et al.. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–302. [DOI] [PubMed] [Google Scholar]
- 22. Albala C, Ebbeling CB, Cifuentes M, Lera L, Bustos N, Ludwig DS. Effects of replacing the habitual consumption of sugar-sweetened beverages with milk in Chilean children. Am J Clin Nutr. 2008;88:605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnberg K, Molgaard C, Michaelsen KF, Jensen SM, Trolle E, Larnkjaer A. Skim milk, whey, and casein increase body weight and whey and casein increase the plasma C-peptide concentration in overweight adolescents. J Nutr. 2012;142:2083–90. [DOI] [PubMed] [Google Scholar]
- 24. Baker IA, Elwood PC, Hughes J, Jones M, Moore F, Sweetnam PM. A randomised controlled trial of the effect of the provision of free school milk on the growth of children. J Epidemiol Commun H. 1980;34:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cadogan J, Eastell R, Jones N, Barker M. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ. 1997;315:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan GM, Hoffman K, McMurry M. Effects of dairy products on bone and body composition in pubertal girls. J Pediatr. 1995;126:551–6. [DOI] [PubMed] [Google Scholar]
- 27. Cheng S, Lyytikäinen A, Kröger H, Lamberg-Allardt C, Alén M, Koistinen A, Wang QJ, Suuriniemi M, Suominen H, Mahonen A et al.. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10–12-y-old girls: a 2-y randomized trial. Am J Clin Nutr. 2005;82:1115–26.; quiz: 1147–8. [DOI] [PubMed] [Google Scholar]
- 28. Cohen T, Hazell T, Vanstone C, Rodd C, Weiler H. A family-centered lifestyle intervention for obese six- to eight-year-old children: results from a one-year randomized controlled trial conducted in Montreal, Canada. Can J Public Health. 2016;107:e453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X, Fraser DR, Greenfield H. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr. 2004;92:159–68. [DOI] [PubMed] [Google Scholar]
- 30. Gibbons MJ, Gilchrist NL, Frampton C, Maguire P, Reilly PH, March RL, Wall CR. The effects of a high calcium dairy food on bone health in pre-pubertal children in New Zealand. Asia Pac J Clin Nutr. 2004;13:341–7. [PubMed] [Google Scholar]
- 31. Lambourne K, Washburn RA, Lee J, Betts JL, Thomas DT, Smith BK, Gibson CA, Sullivan DK, Donnelly JE. A 6-month trial of resistance training with milk supplementation in adolescents: effects on body composition. Int J Sport Nutr Exerc Metab. 2013;23:344–56. [DOI] [PubMed] [Google Scholar]
- 32. Lappe JM, McMahon DJ, Laughlin A, Hanson C, Desmangles JC, Begley M, Schwartz M. The effect of increasing dairy calcium intake of adolescent girls on changes in body fat and weight. Am J Clin Nutr. 2017;105:1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merrilees MJ, Smart EJ, Gilchrist NL, Frampton C, Turner JG, Hooke E, March RL, Maguire P. Effects of dairy food supplements on bone mineral density in teenage girls. Eur J Nutr. 2000;39:256–62. [DOI] [PubMed] [Google Scholar]
- 34. Mobarhan GM, Sahebkar A, Vakili R, Safarian M, Nematy M, Lotfian, Khorashadizadeh M, Tavallaie S, Dahri M, Ferns G. Investigation of the effect of high dairy diet on body mass index and body fat in overweight and obese children. Indian J Pediatr. 2009;76:1145–50. [DOI] [PubMed] [Google Scholar]
- 35. Rahmani K, Djazayery A, Habibi MI, Heidari H, Dorosti-Motlagh AR, Pourshahriari M, Azadbakht L. Effects of daily milk supplementation on improving the physical and mental function as well as school performance among children: results from a school feeding program. J Res Med Sci. 2011;16:469–76. [PMC free article] [PubMed] [Google Scholar]
- 36. St-Onge MP, Goree LL, Gower B. High-milk supplementation with healthy diet counseling does not affect weight loss but ameliorates insulin action compared with low-milk supplementation in overweight children. J Nutr. 2009;139:933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vogel KA, Martin BR, McCabe LD, Peacock M, Warden SJ, McCabe GP, Weaver CM. The effect of dairy intake on bone mass and body composition in early pubertal girls and boys: a randomized controlled trial. Am J Clin Nutr. 2017;105:1214–29. [DOI] [PubMed] [Google Scholar]
- 38. Volek JS, Gómez AL, Scheett TP, Sharman MJ, French DN, Rubin MR, Ratamess NA, McGuigan MM, Kraemer WJ. Increasing fluid milk favorably affects bone mineral density responses to resistance training in adolescent boys. J Am Diet Assoc. 2003;103:1353–6. [DOI] [PubMed] [Google Scholar]
- 39. Abargouei AS, Janghorbani M, Salehi-Marzijarani M, Esmaillzadeh A. Effect of dairy consumption on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Obes (Lond). 2012;36:1485–93. [DOI] [PubMed] [Google Scholar]
- 40. Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. [DOI] [PubMed] [Google Scholar]
- 42. Vien S, Luhovyy BL, Patel BP, Panahi S, El Khoury D, Mollard RC, Hamilton JK, Anderson GH. Pre- and within-meal effects of fluid dairy products on appetite, food intake, glycemia, and regulatory hormones in children. Appl Physiol Nutr Metab. 2017;42:302–10. [DOI] [PubMed] [Google Scholar]
- 43. Dove ER, Hodgson JM, Puddey IB, Beilin LJ, Lee YP, Mori TA. Skim milk compared with a fruit drink acutely reduces appetite and energy intake in overweight men and women. Am J Clin Nutr. 2009;90:70–5. [DOI] [PubMed] [Google Scholar]
- 44. Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr. 2013;4:418–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soerensen KV, Thorning TK, Astrup A, Kristensen M, Lorenzen JK. Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am J Clin Nutr. 2014;99:984–91. [DOI] [PubMed] [Google Scholar]
- 46. Law M, Huot PSP, Lee YT, Vien S, Luhovyy BL, Anderson GH. The effect of dairy and nondairy beverages consumed with high glycemic cereal on subjective appetite, food intake, and postprandial glycemia in young adults. Appl Physiol Nutr Metab. 2017;42:1201–9. [DOI] [PubMed] [Google Scholar]
- 47. Zemel MB, Bruckbauer A. Effects of a leucine and pyridoxine-containing nutraceutical on body weight and composition in obese subjects. Diabetes Metab Syndr Obes. 2013;6:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teegarden D. The influence of dairy product consumption on body composition. J Nutr. 2005;135:2749–52. [DOI] [PubMed] [Google Scholar]
- 49. Palacios C, Bertran JJ, Rios RE, Soltero S. No effects of low and high consumption of dairy products and calcium supplements on body composition and serum lipids in Puerto Rican obese adults. Nutrition. 2011;27:520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenblum JL, Castro VM, Moore CE, Kaplan LM. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. Am J Clin Nutr. 2012;95:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buchowski MS, Aslam M, Dossett C, Dorminy C, Choi L, Acra S. Effect of dairy and non-dairy calcium on fecal fat excretion in lactose digester and maldigester obese adults. Int J Obes (Lond). 2010;34:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, Tremblay A, Astrup A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev. 2009;10:475–86. [DOI] [PubMed] [Google Scholar]
- 53. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group. Methods and processes of the CONSORT group: example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med. 2008;148:W60–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.