ABSTRACT
Chronic kidney disease (CKD) has been associated with changes in gut microbial ecology, or “dysbiosis,” which may contribute to disease progression. Recent studies have focused on dietary approaches to favorably alter the composition of the gut microbial communities as a treatment method in CKD. Resistant starch (RS), a prebiotic that promotes proliferation of gut bacteria such as Bifidobacteria and Lactobacilli, increases the production of metabolites including short-chain fatty acids, which confer a number of health-promoting benefits. However, there is a lack of mechanistic insight into how these metabolites can positively influence renal health. Emerging evidence shows that microbiota-derived metabolites can regulate the incretin axis and mitigate inflammation via expansion of regulatory T cells. Studies from animal models and patients with CKD show that RS supplementation attenuates the concentrations of uremic retention solutes, including indoxyl sulfate and p-cresyl sulfate. Here, we present the current state of knowledge linking the microbiome to CKD, we explore the efficacy of RS in animal models of CKD and in humans with the condition, and we discuss how RS supplementation could be a promising dietary approach for slowing CKD progression.
Keywords: resistant starch, high-amylose maize starch, chronic kidney disease, diabetic nephropathy, microbiota, microbiome, short-chain fatty acids, uremic retention solutes
Introduction
Chronic kidney disease (CKD) affects ≤13% of the population worldwide (1); however, <1 in 10 affected individuals are aware they have the condition (2). Despite its under-diagnosis, the prevalence of CKD is increasing, particularly in low- and middle-income countries facing epidemic growths in obesity and diabetes and increased life expectancy. In the 2015 Global Burden of Disease Study, kidney disease was the 12th most common cause of mortality, accounting for 1.1 million deaths worldwide, and overall CKD mortality has increased by 31.7% over the last 10 y (3). In the United States, the treatment costs of CKD exceed $48 billion/y (4), placing a considerable burden on the health care system.
The 2 major clinical features seen in CKD are the progressive loss of kidney function over time and the development of cardiovascular disease (CVD). CKD is associated with a 2- to 3-fold increased risk of CVD mortality, and individuals with CKD are at higher risk of death from ischemic heart disease or stroke than they are of progressing to end-stage renal disease (ESRD) (5). Modifiable risk factors for CKD include overweight and obesity, diabetes, hypertension, and cigarette smoking (6); and preventative lifestyle intervention strategies to encourage healthy eating, physical activity, and smoking cessation are of vital importance. As kidney function declines, excess fluid, electrolytes, and nitrogen-based waste products accumulate in the circulation and contribute to the complications and end-organ damage associated with CKD. Current treatment methods for ESRD, such as dialysis, attempt to remove these wastes from the circulation. Many nitrogenous uremic toxins are of gut bacterial origin, and increasing evidence indicates that the intestinal microbiota is a potential therapeutic target for interventions to limit the effects of CKD.
Dysbiosis in CKD
Many diseases have been associated with alterations in the gut microbiota including liver disease (7), hypertension (8), obesity (9), and insulin resistance (10). It has recently been recognized that in patients with CKD there is a change in gut microbial ecology that may contribute to the pathogenesis of the disease (11). Early studies in hemodialysis patients showed lower viable counts of beneficial bacterial genera such as Bifidobacteria, and greater viable counts of Clostridium perfringens and aerobic bacteria including Enterobacteriaceae and Enterococci species compared with control subjects (12). When compared with healthy controls, patients with ESRD were found to have large increases in Actinobacteria, Firmicutes (especially the class Clostridia), and Proteobacteria (primarily Gammaproteobacteria) phyla (13). A follow-up study utilizing this same cohort identified that there were increases in bacterial families that possess uricase, urease, p-cresol–forming, and indole-forming enzymes (14) and hemodialysis patients have been shown to have higher fecal concentrations of indole and p-cresol than healthy controls (15). Several studies have identified that ESRD patients have a decrease in bacterial families possessing enzymes for the formation of SCFAs compared with healthy controls (14, 16). A recent pilot study comparing hemodialysis and peritoneal dialysis patients confirmed that ESRD patients have an altered microbiome compared with controls and also identified that the microbiome composition was different between modes of dialysis (17). These findings highlight that dysbiosis occurs during CKD and provide insights into the potential mechanisms that may be targeted through dietary interventions, which will be explored in greater detail below.
Prebiotics
Prebiotics are substrates that promote the growth of beneficial bacteria. The term “prebiotic” was first introduced in 1995 (18) and early attempts at defining prebiotics focused on those compounds that had beneficial effects on health-promoting groups of bacteria, namely Lactobacilli and Bifidobacteria, within the colon (19, 20). This definition of prebiotics led to 2 broadly characterized types of prebiotics: inulin-type fructans (inulin, oligofructose, and fructo-oligosaccharides) and galactans (galacto-oligosaccharides) (21). With regards to food labeling, these prebiotics are often classified as dietary fibers (22); however, there is no agreed-upon universal definition for the term “dietary fiber,” which is sometimes used as an umbrella term that is inclusive of nonstarch polysaccharides and certain oligosaccharides (20). As the knowledge of the human gut microbiota increased it became apparent that there was more to healthy gut microbiota than just Lactobacilli and Bifidobacteria, and it was suggested that the definition of prebiotics be expanded to reflect this knowledge (23). A consensus statement has recently been published on the subject, defining a prebiotic as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (24) and expanding the list of substrates that may be considered prebiotics to include noncarbohydrate food components such as polyphenols, PUFAs, and conjugated linoleic acids.
Resistant Starch
Starch is a polymeric carbohydrate produced by most plants for the purpose of energy storage. Starch granules are composed of 2 polysaccharides: amylose, which is a linear molecule, and amylopectin, which is highly branched, making amylopectin easily accessible to digestive enzymes in the small intestine (25). Resistant starches (RSs) contain α-linked glucose molecules that are resistant to hydrolysis in the human small intestine, due to being either physically or chemically resistant to digestive α-amylases (26), and are categorized depending on the mechanism of enzymatic resistance. RS type 1 (RS1) is found in whole grains and legumes and is inaccessible to digestive enzymes because it is surrounded by a protective barrier. RS type 2 (RS2) products contain dense ungelatinized starch granules, such as high-amylose maize starch and raw potatoes. Retrograded starches, such as cooked and cooled potatoes, are categorized as RS type 3 (RS3). Retrogradation occurs when starches are first heated to undergo gelatinization and then cooled to form a crystalline structure. RS type 4 (RS4) is formed via chemical cross-linking of starch by the addition of esters and ether groups. These starches are often found in breads and cakes (27). Last, RS type 5 (RS5) forms when amylose and long branch chains of amylopectin form single-helical complexes with fatty acids and fatty alcohols, preventing enzymatic access to the starch (28). The different types of RS are outlined in Table 1. As RS passes into the large intestine it becomes available for fermentation by health-promoting colonic bacteria (28), which has led some commentators to refer to RS as a potential prebiotic (29). RS is a soluble, nonviscous fiber that is preferentially metabolized in the large intestine by saccharolytic bacteria (30) and consumption of RS represents a potential strategy for beneficially altering the human gut microbiome.
TABLE 1.
Categories of RS1
| RS type | Description | Examples |
|---|---|---|
| RS1 | Physically inaccessible | Coarsely milled grains or seeds, legumes |
| RS2 | Ungelatinized starch | Raw potato, unripe banana, high-amylose maize starch |
| RS3 | Retrograded starch | Cooked, cooled foods (potatoes, pasta, rice), corn flakes |
| RS4 | Chemically modified starch | Cross-linked starch and octenyl succinate starch |
| RS5 | Amylose–lipid complex | Stearic acid–complexed high-amylose starch |
1RS, resistant starch.
Metabolic effects of RS
Adequate dietary fiber consumption is associated with several health benefits, including reduced risk of obesity, metabolic syndrome, type 2 diabetes (T2D), CVD, colon cancer, and constipation (31–37). Soluble, highly viscous forms of dietary fiber such as β-glucan and psyllium are well known for their glucose- and cholesterol-lowering effects, as the gel-forming ability of these fibers slows gastric emptying and increases bile acid excretion (38). However, the association between RS consumption (a nonviscous, soluble fiber) and improvements in glucose and lipid metabolism is unclear. Human RS intervention studies performed to date have been of short duration, contained small sample sizes, and were of moderate to low methodological quality. Although some studies have found that RS supplementation is associated with reductions in total (39, 40) and LDL (39) cholesterol, the majority of human clinical trials investigating the impact of RS on lipid concentrations have found no effects in healthy individuals, people who are overweight or obese, those with the metabolic syndrome, or subjects with T2D (41–49).
Digestible starch contributes 17 kJ/g to energy intake, whereas RS contributes ∼8–10 kJ/g of metabolizable energy (50). Therefore, if RS replaces the same weight of digestible starch in a food product, it would be plausible to hypothesize that RS might assist with weight reduction. However, this does not appear to be the case in human trials, where participants have been observed to unconsciously compensate by increasing their kilojoule intake of other foods in order to remain weight-stable (51–53). Some researchers have found that RS consumption increases subjective satiety ratings (54, 55), although others have found no effect of RS on appetite and satiety ratings (51, 56, 57). A small number of animal studies have found that RS supplementation results in significant reductions in body weight, but the quantity of RS provided to rodents (≤500 g/kg diet) has far exceeded what is tolerable or realistic in humans (58–60). In summary, RS is unlikely to induce significant reductions in appetite or body weight.
RS and diabetes
Diabetes is a significant contributor to the development of CKD and it has been suggested that nondigestible carbohydrates, such as RS, may limit the progression of CKD via improvements in glycemic control (28). RS has been shown to attenuate postprandial glucose excursions in single-meal studies involving healthy-weight and obese subjects (61–63). Viscous soluble fibers lower postprandial glucose and insulin responses by dose-dependently increasing the viscosity of chyme in the stomach, slowing gastric emptying, and reducing the rate of carbohydrate digestion and absorption in the small intestine (64). However, RS is a nonviscous soluble fiber, so its contribution to postprandial glucose reductions is primarily due to the lower energy density of the RS in comparison to the digestible starch control. The majority of acute RS feeding studies performed to date have not provided the same quantities of available carbohydrate between treatment and control groups, so it has not been possible to determine whether postprandial glucose and insulin responses were reduced due to a unique property of the RS or simply due to the lower energy density and available carbohydrate in the test meals. In the small number of trials that have controlled for metabolizable energy by providing isoenergetic meals, RS supplementation significantly reduced postprandial blood glucose concentrations in healthy individuals and people with T2D (65–67). Further research is now needed in order to determine the mechanism whereby RS exerts its postmeal effects and the optimal RS type, dosage, and patient populations required to maximize glycemic improvements.
Whereas no reductions in fasting blood glucose concentrations have been observed in individuals consuming diets supplemented with RS (40, 42, 43, 45, 47, 48, 52, 68–71), improvements in insulin sensitivity have frequently been reported in trial participants who are overweight or obese, people with the metabolic syndrome, and those with T2D (53, 68–70, 72). However, studies using gold-standard techniques to assess insulin sensitivity (euglycemic-hyperinsulinemic clamp) have been unable to confirm these findings (52, 65). The effects of RS consumption on glycated hemoglobin (HbA1c) in individuals with T2D are contradictory. In a parallel randomized controlled trial, obese individuals with T2D consumed 24 g RS2/d for 4 wk (43). Although treatment resulted in significant reductions in body weight and improved insulin sensitivity compared with controls, there was no change in HbA1c, which could be partially due to the brief study duration. A longer (12-wk) trial providing 40 g RS2/d also failed to show any significant change in HbA1c in people with T2D (65). In contrast, 1 study undertaken in women with T2D who consumed only 10 g RS2/d for 8 wk reported a significant reduction in HbA1c (70, 73). Given the ability of RS to beneficially modify the composition of the gut bacteria and potentially improve some metabolic health abnormalities, it is interesting to consider whether RS may have beneficial applications in the management of CKD and the potential mechanisms for its action.
RS and CKD
An RS-supplemented diet has been shown to reduce plasma urea concentrations in both healthy animals (74, 75) and animal models of CKD (76); however, it should be noted that in the study by Younes et al. (76) the control group received a fiber-free diet. Compared with normal corn starch, supplementing the diet with 5%, 10%, or 20% RS did not ameliorate albuminuria in a streptozotocin-induced diabetes model (77). Supplementation with 20% RS did lead to reductions in urinary albumin concentrations, compared with normal corn starch, in the Zucker diabetic fatty rat, an obese model of T2D (78). Furthermore, RS led to an improvement in renal histopathological score, suggesting a renoprotective effect in the setting of diabetes (78). In a rat model of adenine-induced CKD, a diet containing 59% high-amylose maize starch led to improvements in creatinine clearance, serum creatinine, renal inflammation, and interstitial fibrosis, compared with animals fed a low-fiber diet containing 12.8% cellulose (79). Limited research has been completed in humans, with a 2014 study finding no difference in albuminuria or blood urea concentrations after 6 wk of RS supplementation; however, there were marked reductions in uremic retention solutes, as discussed below (80). This study provided RS as a powder in sachets and instructed patients to mix the supplements in with food and drinks, with the placebo group receiving sachets containing highly digestible corn starch (80). A recent study showed that after 8 wk of RS supplementation, provided as biscuits, hemodialysis patients had no change in albuminuria; however, there were reductions in serum urea, creatinine, IL-6, and TNF-α concentrations (81). The placebo in this study consisted of biscuits prepared using regular wheat flour. The evidence from animal and human studies using RS in the context of CKD is summarized in Table 2. Whereas there is a paucity of evidence in humans for the use of RS to ameliorate CKD, results from animal trials are promising and suggest that it would be beneficial to trial longer-duration studies in humans.
TABLE 2.
Summary of trials using RS interventions and measuring renal parameters1
| Nitrogen | Plasma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (reference) | Intervention | Population | Group size, n | Study duration | Albu-minuria | CrCl | BUN | Urinary | Fecal | Cecal NH4 | IS | PCS | Other findings |
| Healthy animals | |||||||||||||
| Kalmokoff et al. (74) | 5% HAMSRS2 vs. cellulose | Male BioBreeding control rats | 8 | 4 wk | — | — | ↓ | — | — | — | — | — | — |
| Younes et al. (75) | 25% RS (crude potato starch) vs. digestible starch | Male Wistar rats | 10 | 3 wk | — | — | ↓ | ↓ | ↑ | ↓ | — | — | ↑ urea transferred to cecum, ↑ nitrogen balance |
| Diabetic animals | |||||||||||||
| Koh et al. (77) | 5%, 10%, or 20% HAMSRS2-supplemented diet vs. normal corn starch | Male STZ-induced T1D SD rats | 8 | 4 wk | ↔ | ↔ | — | — | — | — | — | — | |
| Koh et al. (78) | 20% HAMSRS2-supplemented diet vs. normal corn starch | Male Zucker diabetic fatty rats | 8 | 6 wk | ↓ | — | — | — | — | — | — | — | ↓ renal histopathology scores ↓ kidney weight |
| CKD animals | |||||||||||||
| Younes et al. (76) | 20% RS (crude potato starch) vs. fiber-free diet | Unilateral nephrectomized male Wistar rats | 10 | 17 d | — | — | ↓ | ↓ | ↑ | ↓ | — | — | |
| Vaziri et al. (79)2 | 59% HAMSRS2 vs. low fiber | Adenine-induced CKD male SD rats | 9 | 3 wk | ↓ | ↑ | ↔ | — | — | — | — | — | ↓ interstitial fibrosis ↓ renal NF-κB p65, MCP-1 |
| Kieffer et al. (82)2 | 59% HAMSRS2 vs. low fiber | Adenine-induced CKD male SD rats | 9 | 3 wk | — | — | — | — | — | — | ↓ | — | ↓ urinary IS, p-cresol, hippurate ↑ serum IAA, ↓ serum uric acid |
| Human studies | |||||||||||||
| Sirich et al. (80) | 15–30 g/d HAMSRS2 vs. control starch | HD patients (male and female) | 20 | 6 wk | ↔ | — | ↔ | — | — | — | ↓ | ↓ | |
| Tayebi Khosroshahi et al. (81) | 20–25 g/d HAMSRS2 vs. regular wheat flour biscuits | HD patients (male and female) | 22 | 8 wk | ↔ | — | ↓ | — | — | — | — | — | ↓ serum creatinine ↓ serum uric acid |
1BUN, blood urea nitrogen; CKD, chronic kidney disease; CrCl, creatinine clearance; HAMS, high-amylose maize starch; HD, hemodialysis; IAA, indole acetic acid; IS, indoxyl sulfate; MCP-1, monocyte chemoattractant protein 1; PCS, p-cresyl sulfate; RS, resistant starch; SD, Sprague-Dawley; STZ, streptozotocin; T1D, type 1 diabetes; ↑, increased; ↓, decreased; ↔, no change; —, not reported.
2Using the same cohort.
RS and the Microbiome
It is generally accepted that dietary fibers, including RS, are able to modify the gut microbiota by acting as a digestible substrate to be utilized by the saccharolytic bacteria of the colon. Results from animal studies have shown that RS supplementation increases abundance of Bacteroides (83, 84), Bifidobacteria (74, 83–87), and Lactobacilli (84, 86–88). However, not all studies have shown this effect, with 2 wk, 8 wk, or 6 mo of feeding RS (9% potato starch) having no effect on Lactobacilli (89). Human studies have shown that RS supplementation increases abundance of Bifidobacteria (90, 91) and a double-blind crossover study in 10 healthy humans showed that 3 wk of supplementation with RS4, although not RS2, was associated with an increase in Bacteroidetes and a decrease in Firmicutes, suggesting that different types of RS may have functional differences in how they affect the human gut microbiota (92). The general consensus among the scientific literature is that RS supplementation is associated with increases of Bifidobacteria and Lactobacilli.
Further research using 16S ribosomal RNA sequencing has identified particular bacterial species that are affected by RS feeding. Kalmokoff et al. (74) showed that, in rats, RS2 increases Bacteroides uniformis concentrations. Interestingly, RS4 feeding in humans has also been associated with an increase in B. uniformis (93). Another species that has been shown to be upregulated with RS in humans is Eubacterium rectale (92, 94, 95), a well-recognized butyrate producer (96). Several studies have shown that Ruminococcus bromii is increased with RS supplementation in animals (74, 97, 98) and humans (92, 94, 99, 100). Recent genetic analyses demonstrate that R. bromii has a unique, specialized organization of extracellular amylases that give it an exceptional ability to ferment RS (101), suggesting it may be a “keystone” species for the degradation of RS (102). R. bromii is not a butyrate producer; however, it was recently shown that R. bromii may promote the growth of Anaerostipes hadrus, which is not a starch degrader but is a butyrate producer (103). Although the specific mechanism is still under investigation, it is known that RS supplementation affects the microbiota by promoting the selective growth of certain bacterial species.
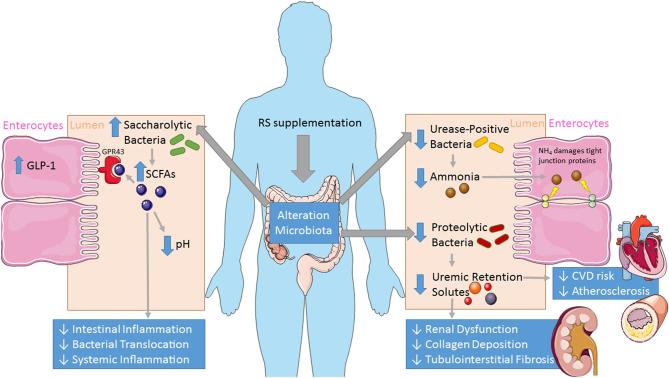
Although it is known that patients with CKD have an altered microbiota, and that RS promotes the growth of beneficial bacteria, there is a paucity of studies that have specifically looked at the effects of RS on the microbiota in the context of CKD. Kieffer et al. (82) utilized the adenine-induced Sprague-Dawley rat as a model of CKD and found that RS supplementation altered bacterial composition, notably by a decrease in the Firmicutes phylum, an increase in the Bacteroidetes-to-Firmicutes ratio, and increases in Bifidobacteria. A study in healthy rats supplemented with 5% RS reported changes in the gut microbiota, notably increases in Bacteroidetes, Bifidobacteria, R. bromii, Porphyromonadaceae, and B. uniformis that were associated with a decrease in blood urea nitrogen concentrations, which may give us some indication of effects on renal function, outside of the context of CKD (74). Although there is growing consensus that RS represents a potential therapeutic avenue to target the microbiota of patients with CKD (Figure 1), there is a lack of evidence, particularly in human trials, that RS alters the microbiome in the context of CKD. The authors note that researchers at the University of Arkansas have recently registered a clinical trial (NCT03356990) that will hopefully shed further light on this question.
FIGURE 1.
Effects of RS on the gut microbiota and microbial metabolites. Blue arrows indicate the effects of RS. CVD, cardiovascular disease; GLP, glucagon-like peptide; GPR43, G-protein-coupled receptor 43; RS, resistant starch.
RS and SCFAs
SCFAs are carboxylic acids containing two- to six-carbon molecules that are naturally produced in small quantities in the liver, but their primary site of production is in the colon by specific gut microbiota. Acetate (C2), propionate (C3), and butyrate (C4) are the major SCFAs generated through bacterial fermentation of dietary fibers and are released in the proximal colon in high concentrations (70–140 mM), whereas their concentrations are lower in the distal colon (20–70 mM) and the distal ileum (20–40 mM) (104). Butyrate is primarily utilized locally by colonocytes, whereas small concentrations of propionate and acetate travel through the portal vein to the liver, where propionate is metabolized by hepatocytes and acetate is utilized by the liver and released to peripheral tissues (25). The ability of SCFAs to alter gene expression through histone deacetylase inhibition and activation of G-protein coupled receptors (GPCRs), including GPR41, GPR43, GPR109a, and olfactory receptor 78 (Olfr78), throughout the body makes them important mediators in multiple cellular and molecular processes involving immunity, inflammation, glucose and lipid metabolism, and gastrointestinal health (105).
Animal studies have shown that RS feeding increases cecal concentrations of SCFAs in rats (87, 88, 106–108) and pigs (86, 98). RS supplementation increases fecal SCFA concentrations in rats (74, 87), pigs (109), and humans (93, 99, 110, 111). Results from animal (83, 89) and human (100, 110) studies have indicated that RS feeding is associated particularly with production of butyrate, with less of an effect seen on propionate concentrations. However, the effect of RS on particular SCFAs has been inconsistent, with 1 study finding increases in propionate, but neither acetate nor butyrate, concentrations in the distal colon of rats (97); another study finding increases in propionate and acetate, but not butyrate, in the cecum and colon of pigs (98); whereas another study in rats showed increases in acetate but not propionate or butyrate (85). Historically there has been a lack of standardized methodology for the measurement of SCFAs, which may account for some of the inconsistencies between studies. Recent advances in stable-isotope techniques may provide some clarity (112). Production of SCFAs by gut microbial fermentation of RS lowers the pH of the cecum (107) and colon (86, 99, 106), favoring the proliferation of saccharolytic bacteria. The general consensus is that RS feeding increases the production of butyrate, which can occur from direct fermentation by butyrate-producing species, such as E. rectale (113), or indirectly by cross-feeding between members of the gut microbiome (114). For example, Bifidobacteria lack the enzymes for butyrate production but ferment RS to produce substrates that are metabolized by butyrate-producing colon bacteria (115). SCFAs have been shown to mediate anti-inflammatory effects (116) and it is suggested that improvements in renal function can be attributed, in part, to RS promoting the proliferation of SCFA-producing bacteria and the subsequent rise in SCFA concentrations mediating the attenuation of the inflammatory sequelae (79).
Glucagon-like peptide 1
Engagement of GPR43 by SCFAs stimulates the secretion of the incretin hormone glucagon-like peptide 1 (GLP-1) from intestinal L cells. GLP-1 has been shown to be protective against diabetic nephropathy, by inhibiting NAD(P)H oxidase and subsequently reducing renal oxidative stress (117). GLP-1 also dose-dependently stimulates natriuresis and the renal excretion of other electrolytes such as calcium, phosphate, and chloride (118, 119). In animal studies, GLP-1 attenuates hypertension induced by angiotensin II, the main effector hormone of the renin–angiotensin system, by blockade of angiotensin II–induced superoxide formation (120, 121), and enhances glucose-stimulated insulin secretion from pancreatic β cells and inhibits glucagon release from α cells, which reduces hepatic glycogenolysis and enhances peripheral glucose uptake (122). Animal models have shown an increase in serum GLP-1 with RS supplementation (123–126); however, no such changes have been detected in human trials (56, 65).
Metabolic endotoxemia
LPSs are endotoxins found in the cell membrane of gram-negative bacteria. Upon entry into the bloodstream from the gut within chylomicrons, LPS activates a proinflammatory cascade by binding to the Toll-like receptor-4 on macrophages and monocytes. This promotes the transcription of cytokines, chemokines, adhesion molecules, and reactive oxygen species (ROS), resulting in low-grade chronic inflammation, enhanced oxidative stress, and insulin resistance (127). Increased concentrations of LPS in the circulation have been referred to as “metabolic endotoxemia,” which is observed in people with obesity, insulin resistance, T2D, and CKD (128, 129). Feeding of dietary prebiotics, and the subsequent production of SCFAs, has been shown to reduce metabolic endotoxemia by maintaining the integrity of the gastrointestinal barrier. SCFAs upregulate the production of intestinal glucagon-like peptide 2 (GLP-2), tight-junction proteins (occludin, zonula occludens-1), and intectin, which together reinforce the health and structure of the intestinal endothelium and maintain optimal gut permeability (130, 131). GLP-2 also upregulates the transcription of tight-junction proteins (claudins, occludins), which connect the actin cytoskeletons of adjacent colonocytes (132, 133). Butyrate upregulates the mucin-associated genes responsible for generating production of the thick mucin layer that plays an important role in maintaining the integrity of the intestinal mucosal barrier (134). Modification of gut microbial composition, gastrointestinal permeability, or chylomicron absorption could have future applications in preventing the progression of CKD.
Although the majority of studies investigating the effects of RS on the production of proinflammatory cytokines have been performed in rodents, a few RS supplementation studies conducted in humans with T2D demonstrated significant reductions in TNF-α (65, 68) and IL-6 (68) and increased total antioxidant capacity (70). However, an equal number of trials found no effect of RS on inflammatory markers in healthy or overweight individuals (53, 71, 135).
Regulatory T cells
Regulatory T cells (Tregs) are suppressive lymphocytes, which prevent excessive immune responses to foreign antigens in order to prevent autoimmune activation (136). Butyrate enhances the number and function of Tregs and has ameliorated a number of autoimmune inflammatory conditions in mice (137). People with ESRD have lower quantities of Tregs due to elevated urea concentrations in the circulation, and excessive oxidative stress may induce early cell-cycle arrest and premature apoptosis of Tregs (138, 139). In the absence of Treg-induced immune cell suppression, inappropriate T cell responses in people with CKD can result in renal tissue injury, hypersensitivity reactions, and glomerulonephritis (140). RS supplementation has been shown to increase expansion of Tregs and reduce inflammatory injury in mouse models of colitis (141) and inflammatory bowel disease (142). GPR109a is highly expressed in adipocytes, intestinal epithelial cells, dendritic cells, and macrophages (143) and engagement of butyrate with GPR109a receptors on immune cells stimulates the production of the anti-inflammatory cytokine IL-10, which supports the production of Tregs from naïve T cells while repressing the generation of proinflammatory T-helper (Th) 17 cells (144). The use of RS to increase colonic SCFA production and encourage the expansion of Tregs represents a potential therapeutic mechanism for the restoration of immune homeostasis and reduction of renal injury in the context of CKD.
RS and Uremic Retention Solutes
We have already discussed that RS may have beneficial effects in CKD by promoting bacteria that produce health-promoting metabolites (i.e., SCFAs); it is also recognized that RS may assist in decreasing nitrogenous loads and the concentrations of nitrogenous compounds that accumulate in patients with CKD.
Urea and ammonia
Urea accumulates in the blood of patients with kidney disease due to insufficient renal excretion, translocates into the intestinal lumen, and is associated with an increase in the population of urease-positive bacteria (145), which convert urea into ammonia, altering intestinal pH (146). Ammonia degrades the tight epithelial junctions of the gut, leading to bacterial translocation from the gut lumen into the systemic circulation, which stimulates an inflammatory response that is thought to contribute to the progression of CKD (147, 148). Hemodialysis patients have elevated circulating IL-6, high-sensitivity C-reactive protein, and endotoxin concentrations compared with controls (149); and gut-derived microbial DNA has been detected in the circulation of patients with CKD (150). Andersen et al. (151) utilized a Col4α3-deficient mouse model of CKD (Alport nephropathy) to demonstrate that uremic mice had elevated endotoxin concentrations, which were accompanied by alterations in the gut microbiota and activation of splenic T lymphocytes. When antibiotics were used to eradicate facultative anaerobic microbes in mice with CKD, markers of systemic inflammation were reduced to the same levels as those seen in nonuremic mice, highlighting a crucial role for the microbiome linking uremia and inflammation (151).
RS supplementation decreases plasma urea concentrations associated with increases in fecal nitrogen excretion in rats (75, 76) and humans (152, 153). Dietary intake of fermentable fibers increases fecal bacterial mass and nitrogen output while decreasing blood urea nitrogen concentrations (154), suggesting that the decrease in plasma urea seen with RS supplementation is due to nitrogen being utilized for microbial growth. In hemodialysis patients, 8-wk supplementation with RS was associated with a decrease in uremia, an effect that was not seen after 4 or 6 wk (80, 81). During CKD there is an increase in the number of urease-positive bacteria and a decrease in saccharolytic bacteria (14) and RS has been shown to decrease cecal ammonia concentrations (75, 84) while increasing the relative abundance of saccharolytic bacteria (74, 85). It has been suggested that this increase in saccharolytic bacteria causes competitive colonization of the colon that attenuates the number of urease-possessing bacteria (155). Targeting the gut microbiota with RS may prevent or slow CKD progression by altering the abundance of microbial communities that produce nitrogenous waste compounds.
P-cresyl sulfate and indoxyl sulfate
Two of the most well-studied uremic retention solutes, p-cresyl sulfate (PCS) and indoxyl sulfate (IS), are formed from dietary amino acids by colonic bacteria that possess p-cresol– and indole-forming enzymes, respectively. Wikoff et al. (156) used a targeted MS metabolomics screening to reveal that many protein-bound uremic toxins were dependent on the presence of the gut microbiota. The colonic source of these microbial metabolites has also been confirmed in hemodialysis patients who had undergone colectomy; these metabolites were significantly lowered compared with patients with an intact colon (157). There is an increase in serum IS and PCS in patients with CKD due to an increase in the bacterial populations that produce these toxic metabolites (14, 158) in tandem with a reduction in renal clearance (159). Furthermore, these compounds bind to albumin in the blood, limiting removal during hemodialysis (160). Both IS and PCS are associated with the progression of CKD (161) and with inflammatory markers in CKD patients (162), and serum concentrations of these uremic toxins have been shown to predict the progression of nephropathy (163).
Serum PCS concentrations are predictive of coronary artery disease in patients with diabetic nephropathy (164) and multiple studies have shown that plasma PCS is an independent predictor of CVD and mortality in hemodialysis (165–167) and predialysis CKD (168) patients. The association between plasma PCS and mortality in CKD patients has been confirmed in a recent meta-analysis (169). A nested case-control study performed a metabolomics screen of patients with T2D and found that those who progressed to ESRD had higher baseline plasma PCS, but not IS, than those who did not (170). In CKD patients not yet requiring dialysis, serum concentrations of p-cresol, the precursor to PCS, were shown to predict cardiovascular events, independently of kidney function or Framingham risk score (171). Urinary PCS has also been reported to independently predict cardiovascular events in patients with mild to moderate CKD (172). Mechanistically, PCS increases the expression of inflammatory genes (173), impairs mitochondrial function (174), and promotes epithelial-to-mesenchymal transition (175) in renal proximal tubular cells. PCS has a proapoptotic and proinflammatory effect on human proximal tubular epithelial cells (176); has been shown to increase oxidative stress in leukocytes (177), cardiomyocytes (178), endothelial cells, and smooth muscle cells (179, 180); and in vivo superfusion at uremic concentrations increases the number of rolling leukocytes on the vascular endothelium (181). In vitro PCS has been shown to suppress the immune response of Th1 cells (182) and alter macrophage cytokine production in response to LPS, with an increase in IL-10 production (183). Work in animal models has identified enhanced NAD(P)H oxidase activity leading to increased oxidative stress and subsequent induction of inflammatory cytokines that contribute to renal fibrosis (184); and in vitro knockdown of Nox4, an NAD(P)H oxidase isoform, ameliorates PCS-induced ROS production and monocyte chemoattractant protein 1 (MCP-1) expression (180).
Serum IS concentrations are associated with coronary atherosclerosis (185), coronary artery disease (164), heart failure (186), and all-cause mortality (187) and are a predictor of CVD (188) and cardiovascular mortality (189) in CKD patients. IS promotes CKD progression (190) and chronic IS exposure in mice is associated with worsening glomerulosclerosis (191) and an increase in the urinary albumin-to-creatinine ratio (192). In vitro studies have demonstrated that IS induces ROS production (193, 194), which contributes to endoplasmic reticulum stress (195), oxidative stress (196, 197), and activation of the NF-κB pathway leading to increased expression of profibrotic genes in proximal tubular cells (198) and intercellular adhesion molecule 1 (ICAM-1) and MCP-1 in vascular endothelial cells (199). Furthermore, this NF-κB activation leads to the production of proinflammatory cytokines, leading to increased macrophage infiltration into the kidneys of rats (200), and IS has been shown in vitro to promote TNF-α and IL-1β production by THP-1–derived macrophages (201). IS promotes epithelial-to-mesenchymal transition in vitro in renal proximal tubular cells (175, 202, 203) and in vivo in a hypertensive rat model (203). In vitro and in vivo studies have demonstrated that mitochondrial function is impaired by IS, which may be ameliorated by antioxidants that reduce oxidative stress (174, 204). IS acts as a ligand for the aryl hydrocarbon receptor (AhR) (205), a transcriptional factor that regulates inflammation, detoxification, and carcinogenesis (206). AhR is expressed by mouse podocytes (192, 207) and in vitro exposure of mouse podocytes to IS increases proinflammatory gene expression and decreases cell viability (192).
Targeting the microbiota to reduce uremic retention solutes
Given the pathogenic effect of uremic retention solutes, there has been significant interest in the use of dietary therapeutic interventions to alter the microbiome and alter the concentrations of these metabolites of microbial origin (208, 209). A cross-sectional study in patients with stage 4–5 CKD found that dietary fiber intake was inversely associated with serum PCS (210) and interventional human studies demonstrate that prebiotic fibers targeting the gut microbiota reduce urinary (211, 212) and plasma PCS (213, 214) concentrations. In mice that had undergone a five-sixths nephrectomy, arabino-xylo-oligosaccharide reduced PCS concentrations and insulin resistance (215), whereas galacto-oligosaccharide reduced indole-positive bacteria, serum IS, tubulointerstitial injury, and tubular ER stress and apoptosis (216). Synbiotics (combinations of pre- and probiotics) trials in human populations have shown beneficial effects and reduced plasma concentrations of p-cresol (217, 218) and PCS (219). Those trials that did measure IS showed no change with synbiotic treatment (218, 219), suggesting that these uremic toxins may be regulated by different pathways. There is limited evidence to indicate that probiotics by themselves play a role in reducing uremic toxins—this is covered in a recent review by Briskey et al. (220). Feeding rats with adenine-induced CKD an RS2-supplemented diet decreases proteinuria, albeit not to the level of controls (79), and a follow-up study using an untargeted metabolomics screen demonstrated that RS was associated with a decrease in serum IS and p-cresol (103). In healthy volunteers, RS supplementation significantly reduced fecal concentrations of p-cresol, although no change was observed in urinary p-cresol concentrations (153); and more recently, Sirich et al. (80) demonstrated RS supplementation in hemodialysis patients decreased IS and PCS. These data indicate that nondigestible carbohydrates, including RSs, may play an important role in retarding CKD progression by limiting the production of uremic retention solutes.
Emerging uremic retention solutes
Although most of the research to date has been focused on the uremic retention solutes PCS and IS, a total of 88 chemicals have been identified that are deemed uremic retention solutes (221), some of which may play a role in CKD development. This increased rate of discovery of uremic retention solutes has been ascribed to the implementation of newer technologies which have improved sensitivity for detecting metabolites (222). Furthermore, the production of several of these retention solutes has been shown to be dependent on the gut microbiota (156), indicating that dietary interventions that alter the microbiome may represent a potential therapeutic strategy in CKD.
Phenylalanine metabolites
Dietary phenylalanine is able to be converted by the gut microbiota into a variety of products that may have implications for kidney disease. Decarboxylation of phenylalanine produces phenylethylamine, a reaction that is catalyzed by the enzyme tyrosine decarboxylase (223), which is present in gram-positive bacteria and has been found to be particularly prevalent among lactic acid bacteria (224). The majority of phenylethylamine undergoes oxidation to form phenylacetic acid (225), which is increased in the plasma of patients with ESRD (221, 226). In vitro work has shown that phenylacetic acid increases ROS formation (227), impairs mitochondrial metabolism (228), and can directly inhibit renal tubular efflux pumps (229). Another microbial pathway for phenylalanine leads to the production of m-tyramine, which undergoes further enzymatic conversion to 3-hydroxyphenylacetic acid (230), which is elevated in the plasma of hemodialysis patients (231). Presence of phenylacetic acid in plasma is dependent on the gut microbiota (156) and rats with adenine-induced CKD receiving an RS-supplemented diet had reductions in the concentration of 3-hydroxyphenylacetic acid by 71% in cecal digesta and 64% in the serum (103), suggesting that targeting of the gut microbiota may prove a useful therapy.
Phenylacetic acid undergoes hepatorenal glutamine conjugation to form phenylacetylglutamine (PAG) (232). Serum PAG concentrations are >100 times higher in hemodialysis patients than in healthy volunteers (233), inversely associated with estimated glomerular filtration rate (GFR) in a healthy population (234), positively associated with progression to ESRD in patients with diabetes (170), and associated with risk of CVD in predialytic (235) and dialysis-requiring (236) CKD patients. Serum concentrations of PAG were 14 times greater in dialysis patients with an intact colon than in those who had undergone a colectomy, demonstrating the role of the microbiota in the production of PAG (157), and PAG concentrations have been shown to correlate with the relative abundance of bacterial families from the Clostridiales order (235). It remains unclear whether PAG is a uremic toxin per se (235) as it undergoes tubular secretion (237), and elevated PAG concentrations may be reflective of increased tubular dysfunction. It is known that the products of the microbial metabolism of phenylalanine are increased in patients with CKD, and animal studies show these are reduced with RS supplementation; however, further studies are required to assess the clinical implications of these findings.
Hippurate
Dietary aromatic compounds are metabolized by the gut microbiota, forming benzoates that undergo hepatorenal conjugation with glycine, leading to the formation of hippurate (11). Patients with ESRD have plasma concentrations of hippurate that are >100 times higher than those of healthy controls (233). Hippurate can directly inhibit renal tubular efflux transporters (229), and in rats that had undergone a subtotal nephrectomy, 18 wk of treatment with 500 mg hippurate · kg–1 · d–1 in drinking water induced a reduction in GFR and an increase in glomerulosclerotic index (238). ROS production was observed when endothelial cells were treated with hippurate in the absence of albumin; however, this was not observed when albumin was present, suggesting that this effect may not be physiologically relevant (193). Furthermore, plasma hippurate concentrations were not associated with progression to ESRD in a cohort with T2D (170), nor did they correlate with CVD risk in dialysis patients (236). Results from animal studies indicate the gut microbiota is required for the production of hippurate (156) and adenine-induced CKD rats fed with RS had a 74% reduction in the urinary concentration of hippurate (103). However, there was no difference in plasma concentrations of hippurate between dialysis patients with and without a colon (157), and it is suggested that the majority of hippurate in humans may come from precursors that are absorbed in the small intestine (239). Reduced clearance of hippurate is associated with an increased risk of mortality independent of estimated GFR in a population with CKD not requiring dialysis (240); however, given that hippurate is cleared by tubular secretion, this may simply be a marker of tubular dysfunction. The role of hippurate in the progression of kidney disease is unclear; however, it may be an interesting metabolite to consider including in future studies looking at the gut–kidney axis.
Tryptophan metabolites
The most widely studied product of microbial tryptophan metabolism is IS, a well-established nephrotoxic compound that is formed by production of indole from tryptophan by tryptophanase-expressing bacteria and that is conjugated to sulfate in the liver (discussed in detail above). Bacterial metabolism of tryptophan may undertake other routes to produce compounds such as indole acetic acid (IAA), another protein-bound uremic retention solute (241). Serum IAA concentrations are increased in CKD populations, correlate with worsening stage of disease (242), and predict mortality and CVD in patients with CKD (156, 242). In an animal model of CKD, treatment of IAA in drinking water contributed to a reduction in GFR (measured by inulin clearance), increased glomerulosclerosis, and interstitial fibrosis (238). Mechanistically, IAA has been shown to dose-dependently activate NF-κB and promote free radical production in human proximal tubule cells (197). IAA is able to activate AhR (241), which has been shown in vitro to increase expression of the proinflammatory cyclo-oxygenase 2 (COX-2) enzyme, activate NF-κB via p38 phosphorylation, and increase ROS production (242).
Interestingly, in a rat model of adenine-induced CKD, rats supplemented with RS had serum concentrations of IAA that were 615% higher than those of rats fed a low-fiber diet (103). Importantly, these rats had an improvement in creatinine clearance with RS feeding (79) despite these large increases in IAA, although it is important to note that RS reduced IS (103). In conventionally raised mice, the cecal concentration of indole is ∼20 times greater than the concentration of IAA (243), and the composition of the gut microbiota plays an important role in determining the metabolites produced from microbial metabolism of tryptophan. One possible hypothesis, albeit one that requires experimental testing, is that RS supplementation alters the microbial composition, resulting in a “shunting” of tryptophan bacterial metabolism, resulting in greater IAA and less indole production, subsequently leading to lower IS production.
Trimethylamine-N-oxide
Trimethylamine-N-oxide (TMAO) is a uremic retention solute of microbial origin that has been implicated in CVD. Dietary phosphatidylcholine, primarily found in animal products including liver, eggs, milk, red meat, poultry, fish, and shellfish, is a major dietary source of choline (244), which undergoes deamination by the microbial enzyme choline trimethylamine lyase (CutC) to produce trimethylamine (245), which is oxidized by hepatic flavin monooxygenase (FMO) to produce circulating TMAO (246). Another dietary precursor to TMAO is l-carnitine, a nutrient found abundantly in red meat, which has a similar chemical structure to choline and also undergoes microbial metabolism to form trimethylamine (247). Wang et al. (248) undertook a metabolomics screen of >2000 compounds and found that TMAO, choline, and betaine (another TMAO precursor) were associated with CVD risk and the progression of atherosclerosis. TMAO is cleared by the kidneys and serum TMAO concentrations are elevated in patients with ESRD (249–251) and stage 3–4 CKD (252) and have recently been shown to correlate with renal function and inflammation in CKD patients (253). Plasma TMAO concentrations are a predictor of coronary atherosclerosis (254), CVD (255), and mortality (253, 254, 256) in CKD patients. Animal studies have shown that dietary supplementation of TMAO, or its precursor choline, is associated with increased serum TMAO concentrations, tubulointerstitial fibrosis, collagen deposition, SMAD3 phosphorylation, and progressive renal dysfunction (256). The gut microbiota plays an important role in TMAO production, with antibiotic treatment of mice preventing choline-induced increases in TMAO and atherosclerosis (248), and transplanting fecal samples from patients with CKD into antibiotic-treated mice increased TMAO concentrations (249). Given the important effect of TMAO on CKD and CVD, and the clear role that the gut microbiota plays in the production of TMAO, it may be prudent to investigate whether dietary interventions such as RS alleviate disease risk by alterations in TMAO concentrations.
To date, the authors are aware of no studies that have measured the effects of RS supplementation on TMAO concentrations in patients with CKD. Two recent studies have shown that 2-wk feeding of an RS-rich diet was associated with increases in plasma concentrations of TMAO in healthy (42) and insulin-resistant (95) individuals. Intriguingly, another study using a targeted metabolomics approach found that TMAO was one of the most increased metabolites after consumption of a low-glycemic-load diet (257). This is in contrast to epidemiologic studies that have found in men, but not women, that high-glycemic-load diets are associated with increased risk of CVD (258). Further research is required to determine whether the use of dietary carbohydrate sources that are associated with an increase in plasma TMAO concentrations translates to negative long-term cardiovascular consequences, particularly in the context of CKD.
Conclusions
In light of the increasing recognition of the role of microbial dysbiosis in the progression of CKD, there has been much interest in using dietary modification to favorably alter the microbiome of patients with CKD. RS is a prebiotic compound that is resistant to digestion by human α-amylases and becomes available for fermentation in the colon, favoring the proliferation of health-promoting bacteria including Bifidobacteria and Lactobacilli. This change in the microbiota is associated with an increase in SCFAs and a decrease in uremic retention solutes that are produced by the microbiota. SCFAs confer health benefits by a number of mechanisms including ligating with metabolite-sensing GPCRs to promote GLP-1 secretion, promotion of gastrointestinal barrier integrity, and enhancing the number and function of Tregs. Uremic retention solutes are produced by the colonic flora, contribute to the progression of CKD, and are reduced by supplementation with RS. Recent research has identified a number of emerging gut-derived uremic retention solutes. Phenylacetic acid and phenylacetylglutamine are 2 products that arise from the microbial metabolism of the amino acid phenylalanine that are increased in CKD and reduced by RS supplementation. Hippurate concentrations are increased in patients with ESRD and reduced by RS; however, there is insufficient evidence for a causative role in CKD progression. IAA has been shown experimentally to contribute to the development of CKD; however, RS supplementation was associated with large increases in serum concentrations despite improvements in renal function. IAA and IS are both products of tryptophan metabolism, so a possible explanation is that RS shunts the tryptophan microbial metabolism pathway; however, this requires experimental testing. TMAO, a nephrotoxic compound of microbial origin, was increased in healthy volunteers after RS supplementation. Further research is required to elucidate the implications of RS supplementation relative to TMAO in the context of CKD. Diet is used as an adjunct therapy in CKD, and RS can be added to conventional foods to create functional foods that confer a health benefit. Overall, RS represents a promising avenue for providing therapeutic benefit to patients with CKD by targeting the microbiome.
Acknowledgments
All authors read and approved the final manuscript.
Notes
Supported by an Australian Postgraduate Award (to MS) and a Career Development Fellowship from the "JDRF" Australian Type 1 Diabetes Clinical Research Network, a special initiative of the Australian Research Council (to MTC).
Author disclosures: MS, NJK, and MTC, no conflicts of interest.
Abbreviations used: AhR, aryl hydrocarbon receptor; CKD, chronic kidney disease; CVD, cardiovascular disease; ESRD, end-stage renal disease; GFR, glomerular filtration rate; GLP-1/2, glucagon-like peptide 1/2; HbA1c, glycated hemoglobin; IAA, indole acetic acid; IS, indoxyl sulfate; MCP-1, monocyte chemoattractant protein 1; PAG, phenylacetylglutamine; PCS, p-cresyl sulfate; ROS, reactive oxygen species; RS, resistant starch; T2D, type 2 diabetes; TMAO, trimethylamine-N-oxide; Treg, regulatory T cell.
References
- 1. Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One 2016;11(7):e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tuot DS, Plantinga LC, Judd SE, Muntner P, Hsu C-y, Warnock DG, Gutiérrez OM, Safford M, Powe NR, McClellan WM. Healthy behaviors, risk factor control and awareness of chronic kidney disease. Am J Nephrol 2013;37(2):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM et al.. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet North Am Ed 2016;388(10053):1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol 2013;24(9):1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004;15(5):1307–15. [DOI] [PubMed] [Google Scholar]
- 6. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80(1):17–28. [DOI] [PubMed] [Google Scholar]
- 7. Llorente C, Schnabl B. The gut microbiota and liver disease. Cell Mol Gastroenterol Hepatol 2015;1(3):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B et al.. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harakeh SM, Khan I, Kumosani T, Barbour E, Almasaudi SB, Bahijri SM, Alfadul SM, Ajabnoor GMA, Azhar EI. Gut microbiota: a contributing factor to obesity. Front Cell Infect Microbiol 2016;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010;5(2):e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis 2016;67(3):483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin®, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996;74(2):349–55. [DOI] [PubMed] [Google Scholar]
- 13. Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen T-H, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013;83(2):308–15. [DOI] [PubMed] [Google Scholar]
- 14. Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014;39(3):230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P, D'Haese P, Evenepoel P, Verbeke K, Meijers B. The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol 2016;27(5):1389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang S, Xie S, Lv D, Wang P, He H, Zhang T, Zhou Y, Lin Q, Zhou H, Jiang J et al.. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep 2017;7:2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stadlbauer V, Horvath A, Ribitsch W, Schmerböck B, Schilcher G, Lemesch S, Stiegler P, Rosenkranz AR, Fickert P, Leber B. Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Sci Rep 2017;7(1):15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibson G, Roberfroid M. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995;125(6):1401–12. [DOI] [PubMed] [Google Scholar]
- 19. Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 2004;17(2):259–75. [DOI] [PubMed] [Google Scholar]
- 20. Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013;5(4):1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B et al.. Prebiotic effects: metabolic and health benefits. Br J Nutr 2010;104(Suppl2):S1–S63. [DOI] [PubMed] [Google Scholar]
- 22. Coussement PAA. Inulin and oligofructose: safe intakes and legal status. J Nutr 1999;129(7):1412S–17S. [DOI] [PubMed] [Google Scholar]
- 23. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 2015;12:303. [DOI] [PubMed] [Google Scholar]
- 24. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD et al.. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017;14:491–502. [DOI] [PubMed] [Google Scholar]
- 25. Annison G, Topping DL. Nutritional role of resistant starch: chemical structure vs physiological function. Annu Rev Nutr 1994;14:297–320. [DOI] [PubMed] [Google Scholar]
- 26. Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch—a review. Compr Rev Food Sci Food Saf 2006;5(1):1–17. [DOI] [PubMed] [Google Scholar]
- 27. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients 2010;2(12):1266–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Birt DF, Boylston T, Hendrich S, Jane J-L, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M et al.. Resistant starch: promise for improving human health. Adv Nutr 2013;4(6):587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fuentes-Zaragoza E, Sánchez-Zapata E, Sendra E, Sayas E, Navarro C, Fernández-López J, Pérez-Alvarez JA. Resistant starch as prebiotic: a review. Starch 2011;63(7):406–15. [Google Scholar]
- 30. Bindels LB, Walter J, Ramer-Tait AE. Resistant starches for the management of metabolic diseases. Curr Opin Clin Nutr Metab Care 2015;18(6):559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aldoori WH, Giovannucci EL, Rockett HRH, Sampson L, Rimm EB, Willett WC. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr 1998;128(4):714–19. [DOI] [PubMed] [Google Scholar]
- 32. Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Stampfer MJ, Rosner B, Speizer FE, Willett WC. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med 1999;340(3):169–76. [DOI] [PubMed] [Google Scholar]
- 33. Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, Buring JE. Fruit and vegetable intake and risk of cardiovascular disease: the Women's Health Study. Am J Clin Nutr 2000;72(4):922–8. [DOI] [PubMed] [Google Scholar]
- 34. Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, Willett WC. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr 2002;76(3):535–40. [DOI] [PubMed] [Google Scholar]
- 35. McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004;27(2):538–46. [DOI] [PubMed] [Google Scholar]
- 36. Pereira MA, O'Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P et al.. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med 2004;164(4):370–6. [DOI] [PubMed] [Google Scholar]
- 37. Yao B, Fang H, Xu W, Yan Y, Xu H, Liu Y, Mo M, Zhang H, Zhao Y. Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol 2014;29(2):79–88. [DOI] [PubMed] [Google Scholar]
- 38. Bernstein A, Titgemeier B, Kirkpatrick K, Golubic M, Roizen M. Major cereal grain fibers and psyllium in relation to cardiovascular health. Nutrients 2013;5(5):1471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park OJ, Kang NE, Chang MJ, Kim WK. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J Nutr Sci Vitaminol 2004;50(2):93–9. [PubMed] [Google Scholar]
- 40. Nichenametla SN, Weidauer LA, Wey HE, Beare TM, Specker BL, Dey M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double-blind controlled crossover intervention. Mol Nutr Food Res 2014;58(6):1365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Behall KM, Howe JC. Effect of long-term consumption of amylose vs amylopectin starch on metabolic variables in human subjects. Am J Clin Nutr 1995;61(2):334–40. [DOI] [PubMed] [Google Scholar]
- 42. Bergeron N, Williams PT, Lamendella R, Faghihnia N, Grube A, Li X, Wang Z, Knight R, Jansson JK, Hazen SL et al.. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br J Nutr 2016;116(12):2020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ble-Castillo JL, Aparicio-Trápala MA, Francisco-Luria MU, Córdova-Uscanga R, Rodríguez-Hernández A, Méndez JD, Díaz-Zagoya JC. Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int J Environ Res Public Health 2010;7(5):1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bodinham CL, Smith L, Wright J, Frost GS, Robertson MD. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS One 2012;7(7):e40834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gower BA, Bergman R, Stefanovski D, Darnell B, Ovalle F, Fisher G, Sweatt SK, Resuehr HS, Pelkman C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: a randomized, controlled trial. Nutr Metab (Lond) 2016;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heijnen ML, van Amelsvoort JM, Deurenberg P, Beynen AC. Neither raw nor retrograded resistant starch lowers fasting serum cholesterol concentrations in healthy normolipidemic subjects. Am J Clin Nutr 1996;64(3):312–18. [DOI] [PubMed] [Google Scholar]
- 47. Noakes M, Clifton PM, Nestel PJ, Le Leu R, McIntosh G. Effect of high-amylose starch and oat bran on metabolic variables and bowel function in subjects with hypertriglyceridemia. Am J Clin Nutr 1996;64(6):944–51. [DOI] [PubMed] [Google Scholar]
- 48. Robertson MD, Wright JW, Loizon E, Debard C, Vidal H, Shojaee-Moradie F, Russell-Jones D, Umpleby AM. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab 2012;97(9):3326–32. [DOI] [PubMed] [Google Scholar]
- 49. Stewart ML, Nikhanj SD, Timm DA, Thomas W, Slavin JL. Evaluation of the effect of four fibers on laxation, gastrointestinal tolerance and serum markers in healthy humans. Ann Nutr Metab 2010;56(2):91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baer DJ, Stote KS, Henderson T, Paul DR, Okuma K, Tagami H, Kanahori S, Gordon DT, Rumpler WV, Ukhanova M et al.. The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J Nutr 2014;144(7):1023–9. [DOI] [PubMed] [Google Scholar]
- 51. de Roos N, Heijnen ML, de Graaf C, Woestenenk G, Hobbel E. Resistant starch has little effect on appetite, food intake and insulin secretion of healthy young men. Eur J Clin Nutr 1995;49(7):532–41. [PubMed] [Google Scholar]
- 52. Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 2005;82(3):559–67. [DOI] [PubMed] [Google Scholar]
- 53. Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med 2010;27(4):391–7. [DOI] [PubMed] [Google Scholar]
- 54. Willis HJ, Eldridge AL, Beiseigel J, Thomas W, Slavin JL. Greater satiety response with resistant starch and corn bran in human subjects. Nutr Res 2009;29(2):100–5. [DOI] [PubMed] [Google Scholar]
- 55. Guerin-Deremaux L, Pochat M, Reifer C, Wils D, Cho S, Miller LE. The soluble fiber NUTRIOSE induces a dose-dependent beneficial impact on satiety over time in humans. Nutr Res 2011;31(9):665–72. [DOI] [PubMed] [Google Scholar]
- 56. Emilien CH, Hsu WH, Hollis JH. Effect of resistant wheat starch on subjective appetite and food intake in healthy adults. Nutrition 2017;43–44:69–74. [DOI] [PubMed] [Google Scholar]
- 57. Maziarz MP, Preisendanz S, Juma S, Imrhan V, Prasad C, Vijayagopal P. Resistant starch lowers postprandial glucose and leptin in overweight adults consuming a moderate-to-high-fat diet: a randomized-controlled trial. Nutr J 2017;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aziz AA, Kenney LS, Goulet B, Abdel-Aal E-S. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J Nutr 2009;139(10):1881–9. [DOI] [PubMed] [Google Scholar]
- 59. Belobrajdic DP, King RA, Christophersen CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr Metab (Lond) 2012;9(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lerer-Metzger M, Rizkalla SW, Luo J, Champ M, Kabir M, Bruzzo F, Bornet F, Slama G. Effects of long-term low-glycaemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats. Br J Nutr 1996;75(5):723–32. [DOI] [PubMed] [Google Scholar]
- 61. Reader DM, O'Connell BS, Johnson ML, Franz M. Glycemic and insulinemic response of subjects with type 2 diabetes after consumption of three energy bars. J Am Diet Assoc 2002;102(8):1139–42. [DOI] [PubMed] [Google Scholar]
- 62. Behall KM, Scholfield DJ, Hallfrisch JG, Liljeberg-Elmståhl HGM. Consumption of both resistant starch and β-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care 2006;29(5):976–81. [DOI] [PubMed] [Google Scholar]
- 63. Luhovyy BL, Mollard RC, Yurchenko S, Nunez MF, Berengut S, Liu TT, Smith CE, Pelkman CL, Anderson GH. The effects of whole grain high-amylose maize flour as a source of resistant starch on blood glucose, satiety, and food intake in young men. J Food Sci 2014;79(12):H2550–6. [DOI] [PubMed] [Google Scholar]
- 64. McRorie JW Jr, McKeown NM. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet 2017;117(2):251–64. [DOI] [PubMed] [Google Scholar]
- 65. Bodinham CL, Smith L, Thomas EL, Bell JD, Swann JR, Costabile A, Russell-Jones D, Umpleby AM, Robertson MD. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr Connect 2014;3(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Al-Tamimi EK, Seib PA, Snyder BS, Haub MD. Consumption of cross-linked resistant starch (RS4XL) on glucose and insulin responses in humans. J Nutr Metab 2010:651063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stewart ML, Zimmer JP. A high fiber cookie made with resistant starch type 4 reduces post-prandial glucose and insulin responses in healthy adults. Nutrients 2017;9(3):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aliasgharzadeh A, Dehghan P, Gargari BP, Asghari-Jafarabadi M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: a randomised controlled clinical trial. Br J Nutr 2015;113(2):321–30. [DOI] [PubMed] [Google Scholar]
- 69. Dainty SA, Klingel SL, Pilkey SE, McDonald E, McKeown B, Emes MJ, Duncan AM. Resistant starch bagels reduce fasting and postprandial insulin in adults at risk of type 2 diabetes. J Nutr 2016;146(11):2252–9. [DOI] [PubMed] [Google Scholar]
- 70. Karimi P, Farhangi MA, Sarmadi B, Gargari BP, Zare Javid A, Pouraghaei M, Dehghan P. The therapeutic potential of resistant starch in modulation of insulin resistance, endotoxemia, oxidative stress and antioxidant biomarkers in women with type 2 diabetes: a randomized controlled clinical trial. Ann Nutr Metab 2016;68(2):85–93. [DOI] [PubMed] [Google Scholar]
- 71. Maki KC, Pelkman CL, Finocchiaro ET, Kelley KM, Lawless AL, Schild AL, Rains TM. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 2012;142(4):717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ble-Castillo JL, Aparicio-Trapala MA, Cervantes-Toache MC, Rodriguez-Hernandez A, Martinez-Bricaire RL, Cordova-Uscanga R, Juarez-Rojop IE, Jimenez-Dominguez G, Mondragon-Camara F, Ramon-Frias T et al.. Effects of native banana resistant starch on body weight and insulin resistance in non-diabetic obese women. Can J Diabetes 2009;33(3):303–4.25998601 [Google Scholar]
- 73. Gargari BP, Namazi N, Khalili M, Sarmadi B, Jafarabadi MA, Dehghan P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement Ther Med 2015;23(6):810–15. [DOI] [PubMed] [Google Scholar]
- 74. Kalmokoff M, Zwicker B, O'Hara M, Matias F, Green J, Shastri P, Green-Johnson J, Brooks SPJ. Temporal change in the gut community of rats fed high amylose cornstarch is driven by endogenous urea rather than strictly on carbohydrate availability. J Appl Microbiol 2013;114(5):1516–28. [DOI] [PubMed] [Google Scholar]
- 75. Younes H, Demigné C, Behr S, Rémésy C. Resistant starch exerts a lowering effect on plasma urea by enhancing urea N transfer into the large intestine. Nutr Res 1995;15(8):1199–210. [Google Scholar]
- 76. Younes H, Remesy C, Behr S, Demigne C. Fermentable carbohydrate exerts a urea-lowering effect in normal and nephrectomized rats. Am J Physiol Gastrointest Liver Physiol 1997;272(3):G515–G21. [DOI] [PubMed] [Google Scholar]
- 77. Koh GY, Rowling MJ, Schalinske KL, Grapentine K, Loo YT. Consumption of dietary resistant starch partially corrected the growth pattern despite hyperglycemia and compromised kidney function in streptozotocin-induced diabetic rats. J Agric Food Chem 2016;64(40):7540–5. [DOI] [PubMed] [Google Scholar]
- 78. Koh GY, Whitley EM, Mancosky K, Loo YT, Grapentine K, Bowers E, Schalinske KL, Rowling MJ. Dietary resistant starch prevents urinary excretion of vitamin D metabolites and maintains circulating 25-hydroxycholecalciferol concentrations in Zucker diabetic fatty rats. J Nutr 2014;144(11):1667–73. [DOI] [PubMed] [Google Scholar]
- 79. Vaziri ND, Liu S-M, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 2014;9(12):e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 2014;9(9):1603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tayebi Khosroshahi H, Vaziri ND, Abedi B, Asl BH, Ghojazadeh M, Jing W, Vatankhah AM. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: a randomized clinical trial. Hemodial Int 2018. [DOI] [PubMed] [Google Scholar]
- 82. Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, Nazertehrani S, Moore ME, Marco ML, Martin RJ et al.. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol 2016;310(9):F857–F71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang X, Brown IL, Khaled D, Mahoney MC, Evans AJ, Conway PL. Manipulation of colonic bacteria and volatile fatty acid production by dietary high amylose maize (amylomaize) starch granules. J Appl Microbiol 2002;93(3):390–7. [DOI] [PubMed] [Google Scholar]
- 84. Silvi S, Rumney CJ, Cresci A, Rowland IR. Resistant starch modifies gut microflora and microbial metabolism in human flora-associated rats inoculated with faeces from Italian and UK donors. J Appl Microbiol 1999;86(3):521–30. [DOI] [PubMed] [Google Scholar]
- 85. Paturi G, Nyanhanda T, Butts CA, Herath TD, Monro JA, Ansell J. Effects of potato fiber and potato-resistant starch on biomarkers of colonic health in rats fed diets containing red meat. J Food Sci 2012;77(10):H216–H23. [DOI] [PubMed] [Google Scholar]
- 86. Bird AR, Vuaran M, Brown I, Topping DL. Two high-amylose maize starches with different amounts of resistant starch vary in their effects on fermentation, tissue and digesta mass accretion, and bacterial populations in the large bowel of pigs. Br J Nutr 2007;97(1):134–44. [DOI] [PubMed] [Google Scholar]
- 87. Kleessen B, Stoof G, Proll J, Schmiedl D, Noack J, Blaut M. Feeding resistant starch affects fecal and cecal microflora and short-chain fatty acids in rats. J Anim Sci 1997;75(9):2453–62. [DOI] [PubMed] [Google Scholar]
- 88. Le Blay GM, Michel CD, Blottière HM, Cherbut CJ. Raw potato starch and short-chain fructo-oligosaccharides affect the composition and metabolic activity of rat intestinal microbiota differently depending on the caecocolonic segment involved. J Appl Microbiol 2003;94(2):312–20. [DOI] [PubMed] [Google Scholar]
- 89. Le Blay G, Michel C, Blottière HM, Cherbut C. Enhancement of butyrate production in the rat caecocolonic tract by long-term ingestion of resistant potato starch. Br J Nutr 1999;82(5):419–26. [DOI] [PubMed] [Google Scholar]
- 90. Bouhnik Y, Raskine L, Simoneau G, Vicaut E, Neut C, Flourié B, Brouns F, Bornet FR. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr 2004;80(6):1658–64. [DOI] [PubMed] [Google Scholar]
- 91. Alfa MJ, Strang D, Tappia PS, Graham M, Van Domselaar G, Forbes JD, Laminman V, Olson N, DeGagne P, Bray D et al.. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin Nutr 2018;37(3):797–807. [DOI] [PubMed] [Google Scholar]
- 92. Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One 2010;5(11):e15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Upadhyaya B, McCormack L, Fardin-Kia AR, Juenemann R, Nichenametla S, Clapper J, Specker B, Dey M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci Rep 2016;6:28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A et al.. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2010;5:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, Lamendella R, McDermott JE, Bergeron N, Heinzmann SS et al.. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio 2017;8(5):e01343–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol 2014;87(1):30–40. [DOI] [PubMed] [Google Scholar]
- 97. Abell GCJ, Christophersen CT, McOrist AL, Clarke JM. Dietary resistant and butyrylated starches have different effects on the faecal bacterial flora of azoxymethane-treated rats. Br J Nutr 2011;105(10):1480–5. [DOI] [PubMed] [Google Scholar]
- 98. Haenen D, Zhang J, Souza da Silva C, Bosch G, van der Meer IM, van Arkel J, van den Borne JJGC, Pérez Gutiérrez O, Smidt H, Kemp B et al.. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr 2013;143(3):274–83. [DOI] [PubMed] [Google Scholar]
- 99. Abell GCJ, Cooke CM, Bennett CN, Conlon MA, McOrist AL. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol 2008;66(3):505–15. [DOI] [PubMed] [Google Scholar]
- 100. Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016;4(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ze X, Ben David Y, Laverde-Gomez JA, Dassa B, Sheridan PO, Duncan SH, Louis P, Henrissat B, Juge N, Koropatkin NM et al.. Unique organization of extracellular amylases into amylosomes in the resistant starch-utilizing human colonic Firmicutes bacterium Ruminococcus bromii. mBio 2015;6(5):e01058–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 2012;6(8):1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ze X, Le Mougen F, Duncan SH, Louis P, Flint HJ. Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 2013;4(3):236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. In: Frederick WA, editor. Advances in immunology. San Diego, CA: Academic Press; 2014. p. 91–119. [DOI] [PubMed] [Google Scholar]
- 105. Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015;7(4):2839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Morita T, Kasaoka S, Oh-hashi A, Ikai M, Numasaki Y, Kiriyama S. Resistant proteins alter cecal short-chain fatty acid profiles in rats fed high amylose cornstarch. J Nutr 1998;128(7):1156–64. [DOI] [PubMed] [Google Scholar]
- 107. Conlon MA, Kerr CA, McSweeney CS, Dunne RA, Shaw JM, Kang S, Bird AR, Morell MK, Lockett TJ, Molloy PL et al.. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a western diet. J Nutr 2012;142(5):832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ferguson LR, Tasman-Jones C, Englyst H, Harris PJ. Comparative effects of three resistant starch preparations on transit time and short-chain fatty acid production in rats. Nutr Cancer 2000;36(2):230–7. [DOI] [PubMed] [Google Scholar]
- 109. Brown I, Warhurst M, Arcot J, Playne M, Illman RJ, Topping DL. Fecal numbers of bifidobacteria are higher in pigs fed Bifidobacterium longum with a high amylose cornstarch than with a low amylose cornstarch. J Nutr 1997;127(9):1822–7. [DOI] [PubMed] [Google Scholar]
- 110. McOrist AL, Miller RB, Bird AR, Keogh JB, Noakes M, Topping DL, Conlon MA. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr 2011;141(5):883–9. [DOI] [PubMed] [Google Scholar]
- 111. Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin 2009;25(8):1919–30. [DOI] [PubMed] [Google Scholar]
- 112. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sheridan PO, Martin JC, Lawley TD, Browne HP, Harris HMB, Bernalier-Donadille A, Duncan SH, O'Toole PW, Scott KP, Flint HJ. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microbial Genomics 2016;2(2):e000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 2006;72(5):3593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. De Vuyst L, Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol 2011;149(1):73–80. [DOI] [PubMed] [Google Scholar]
- 116. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity 2014;40(6):833–42. [DOI] [PubMed] [Google Scholar]
- 117. Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ et al.. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 2014;85(3):579–89. [DOI] [PubMed] [Google Scholar]
- 118. Gutzwiller J-P, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B et al.. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 2004;89(6):3055–61. [DOI] [PubMed] [Google Scholar]
- 119. Skov J, Dejgaard A, Frøkiær J, Holst JJ, Jonassen T, Rittig S, Christiansen JS. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 2013;98(4):E664–E71. [DOI] [PubMed] [Google Scholar]
- 120. Hirata K, Kume S, Araki S-i, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M et al.. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun 2009;380(1):44–9. [DOI] [PubMed] [Google Scholar]
- 121. Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 2013;19:567. [DOI] [PubMed] [Google Scholar]
- 122. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest 2017;127(12):4217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tachon S, Zhou J, Keenan M, Martin R, Marco ML. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol Ecol 2013;83(2):299–309. [DOI] [PubMed] [Google Scholar]
- 124. Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto–Kakizaki rat. Mol Nutr Food Res 2011;55(10):1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, Brown IL, Birkett A, Newman SS, Skaf J, Hegsted M, Tulley RT et al.. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: a microarray study. J Nutrigenet Nutrigenomics 2012;5(1):26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab 2008;295(5):E1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kim JJ, Sears DD. TLR4 and insulin resistance. Gastroenterol Res Pract 2010:212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Feroze U, Kalantar-Zadeh K, Sterling KA, Molnar MZ, Noori N, Benner D, Shah V, Dwivedi R, Becker K, Kovesdy CP et al.. Examining associations of circulating endotoxin with nutritional status, inflammation and mortality in hemodialysis patients. J Ren Nutr 2012;22(3):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med 2011;62:361–80. [DOI] [PubMed] [Google Scholar]
- 130. Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009;15(13):1546–58. [DOI] [PubMed] [Google Scholar]
- 131. Cani PD. Gut microbiota—at the intersection of everything? Nat Rev Gastroenterol Hepatol 2017;14(6):321–2. [DOI] [PubMed] [Google Scholar]
- 132. Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 2015;3(3):207–15. [DOI] [PubMed] [Google Scholar]
- 133. Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y et al.. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017;357(6351):570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jung TH, Park JH, Jeon WM, Han KS. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract 2015;9(4):343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nilsson AC, Johansson-Boll EV, Bjorck IM. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: a randomised cross-over study in healthy middle-aged subjects. Br J Nutr 2015;114(6):899–907. [DOI] [PubMed] [Google Scholar]
- 136. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133(5):775–87. [DOI] [PubMed] [Google Scholar]
- 137. Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One 2017;12(2):e0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hendrikx TK, van Gurp EAFJ, Mol WM, Schoordijk W, Sewgobind VDKD, IJzermans JNM, Weimar W, Baan CC. End-stage renal failure and regulatory activities of CD4 + CD25 bright+ FoxP3 + T-cells. Nephrol Dial Transplant 2009;24(6):1969–78. [DOI] [PubMed] [Google Scholar]
- 139. Meier P. FOXP3+ regulatory T-cells in chronic kidney disease: molecular pathways and clinical implications. Adv Exp Med Biol 2009;665:163–70. [DOI] [PubMed] [Google Scholar]
- 140. Wang YM, Hu M, Wang Y, Polhill T, Zhang GY, Wang Y, Lee VWS, Harris DCH, Alexander SI. Regulatory T cells in renal disease. Int J Clin Exp Med 2008;1(4):294–304. [PMC free article] [PubMed] [Google Scholar]
- 141. Praengam K, Sahasakul Y, Kupradinun P, Sakarin S, Sanitchua W, Rungsipipat A, Rattanapinyopituk K, Angkasekwinai P, Changsri K, Mhuantong W et al.. Brown rice and retrograded brown rice alleviate inflammatory response in dextran sulfate sodium (DSS)-induced colitis mice. Food Funct 2017;8(12):4630–43. [DOI] [PubMed] [Google Scholar]
- 142. Bassaganya-Riera J, DiGuardo M, Viladomiu M, de Horna A, Sanchez S, Einerhand AWC, Sanders L, Hontecillas R. Soluble fibers and resistant starch ameliorate disease activity in interleukin-10–deficient mice with inflammatory bowel disease. J Nutr 2011;141(7):1318–25. [DOI] [PubMed] [Google Scholar]
- 143. Wanders D, Graff EC, Judd RL. Effects of high fat diet on GPR109A and GPR81 gene expression. Biochem Biophys Res Commun 2012;425(2):278–83. [DOI] [PubMed] [Google Scholar]
- 144. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH et al.. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014;40(1):128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Vaziri ND. Gut microbial translocation in the pathogenesis of systemic inflammation in patients with end-stage renal disease. Dig Dis Sci 2014;59(9):2020–2. [DOI] [PubMed] [Google Scholar]
- 146. Lau WL, Vaziri ND. Urea, a true uremic toxin: the empire strikes back. Clin Sci (Lond) 2017;131(1):3–12. [DOI] [PubMed] [Google Scholar]
- 147. Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol 2013;37(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Anders H-J, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 2013;83(6):1010–16. [DOI] [PubMed] [Google Scholar]
- 149. Shi K, Wang F, Jiang H, Liu H, Wei M, Wang Z, Xie L. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci 2014;59(9):2109–17. [DOI] [PubMed] [Google Scholar]
- 150. Bossola M, Sanguinetti M, Scribano D, Zuppi C, Giungi S, Luciani G, Torelli R, Posteraro B, Fadda G, Tazza L. Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients. Clin J Am Soc Nephrol 2009;4(2):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Andersen K, Kesper MS, Marschner JA, Konrad L, Ryu M, Kumar VR S, Kulkarni OP, Mulay SR, Romoli S, Demleitner J et al.. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD–related systemic inflammation. J Am Soc Nephrol 2016;28(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Younes H, Egret N, Hadj-Abdelkader M, Rémésy C, Demigné C, Gueret C, Deteix P, Alphonse J-C. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Ren Nutr 2006;16(1):67–74. [DOI] [PubMed] [Google Scholar]
- 153. Birkett A, Muir J, Phillips J, Jones G, O'Dea K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am J Clin Nutr 1996;63(5):766–72. [DOI] [PubMed] [Google Scholar]
- 154. Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr 1996;63(3):392–8. [DOI] [PubMed] [Google Scholar]
- 155. Moraes C, Borges NA, Mafra D. Resistant starch for modulation of gut microbiota: promising adjuvant therapy for chronic kidney disease patients? Eur J Nutr 2016;55(5):1813–21. [DOI] [PubMed] [Google Scholar]
- 156. Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009;106(10):3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Aronov PA, Luo FJ-G, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW. Colonic contribution to uremic solutes. J Am Soc Nephrol 2011;22(9):1769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant 2015;30(6):924–33. [DOI] [PubMed] [Google Scholar]
- 159. Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 2009(114):S12–19. [DOI] [PubMed] [Google Scholar]
- 160. Viaene L, Annaert P, de Loor H, Poesen R, Evenepoel P, Meijers B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm Drug Dispos 2013;34(3):165–75. [DOI] [PubMed] [Google Scholar]
- 161. Huang ST, Shu KH, Cheng CH, Wu MJ, Yu TM, Chuang YW, Chen CH. Serum total p-cresol and indoxyl sulfate correlated with stage of chronic kidney disease in renal transplant recipients. Transplant Proc 2012;44(3):621–4. [DOI] [PubMed] [Google Scholar]
- 162. Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, Weston KS, Hawley CM, McWhinney BC, Ungerer JPJ et al.. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3–4 chronic kidney disease. Arch Med Res 2014;45(4):309–17. [DOI] [PubMed] [Google Scholar]
- 163. Wu IW, Hsu K-H, Lee C-C, Sun C-Y, Hsu H-J, Tsai C-J, Tzen C-Y, Wang Y-C, Lin C-Y, Wu M-S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 2011;26(3):938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chiu C-A, Lu L-F, Yu T-H, Hung W-C, Chung F-M, Tsai IT, Yang C-Y, Hsu C-C, Lu Y-C, Wang C-P et al.. Increased levels of total p-cresylsulphate and indoxyl sulphate are associated with coronary artery disease in patients with diabetic nephropathy. Rev Diabet Stud 2010;7(4):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 2006;69(6):1081–7. [DOI] [PubMed] [Google Scholar]
- 166. Meijers BKI, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 2008;73(10):1174–80. [DOI] [PubMed] [Google Scholar]
- 167. Wu IW, Hsu K-H, Hsu H-J, Lee C-C, Sun C-Y, Tsai C-J, Wu M-S. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrol Dial Transplant 2012;27(3):1169–75. [DOI] [PubMed] [Google Scholar]
- 168. Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010;25(4):1183–91. [DOI] [PubMed] [Google Scholar]
- 169. Lin C-J, Wu V, Wu P-C, Wu C-J. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 2015;10(7):e0132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J et al.. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int 2014;85(5):1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Meijers BKI, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 2010;5(7):1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Poesen R, Viaene L, Verbeke K, Augustijns P, Bammens B, Claes K, Kuypers D, Evenepoel P, Meijers B. Cardiovascular disease relates to intestinal uptake of p-cresol in patients with chronic kidney disease. BMC Nephrol 2014;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sun C-Y, Hsu H-H, Wu M-S. p-Cresol sulfate and indoxyl sulfate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol Dial Transplant 2013;28(1):70–8. [DOI] [PubMed] [Google Scholar]
- 174. Sun C-Y, Cheng M-L, Pan H-C, Lee J-H, Lee C-C. Protein-bound uremic toxins impaired mitochondrial dynamics and functions. Oncotarget 2017;8(44):77722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sun C-Y, Chang S-C, Wu M-S. Uremic toxins induce kidney fibrosis by activating intrarenal renin–angiotensin–aldosterone system associated epithelial-to-mesenchymal transition. PLoS One 2012;7(3):e34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Poveda J, Sanchez-Niño MD, Glorieux G, Sanz AB, Egido J, Vanholder R, Ortiz A. p-Cresyl sulphate has pro-inflammatory and cytotoxic actions on human proximal tubular epithelial cells. Nephrol Dial Transplant 2014;29(1):56–64. [DOI] [PubMed] [Google Scholar]
- 177. Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 2007;22(2):592–6. [DOI] [PubMed] [Google Scholar]
- 178. Han H, Zhu J, Zhu Z, Ni J, Du R, Dai Y, Chen Y, Wu Z, Lu L, Zhang R. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J Am Heart Assoc 2015;4(6):e001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gross P, Massy ZA, Henaut L, Boudot C, Cagnard J, March C, Kamel S, Drueke TB, Six I. Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling. J Cell Physiol 2015;230(12):2927–35. [DOI] [PubMed] [Google Scholar]
- 180. Watanabe H, Miyamoto Y, Enoki Y, Ishima Y, Kadowaki D, Kotani S, Nakajima M, Tanaka M, Matsushita K, Mori Y et al.. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol Res Perspect 2015;3(1):e00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Pletinck A, Glorieux G, Schepers E, Cohen G, Gondouin B, Van Landschoot M, Eloot S, Rops A, Van de Voorde J, De Vriese A et al.. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 2013;24(12):1981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Shiba T, Kawakami K, Sasaki T, Makino I, Kato I, Kobayashi T, Uchida K, Kaneko K. Effects of intestinal bacteria-derived p-cresyl sulfate on Th1-type immune response in vivo and in vitro. Toxicol Appl Pharmacol 2014;274(2):191–9. [DOI] [PubMed] [Google Scholar]
- 183. Shiba T, Makino I, Kawakami K, Kato I, Kobayashi T, Kaneko K. p-Cresyl sulfate suppresses lipopolysaccharide-induced anti-bacterial immune responses in murine macrophages in vitro. Toxicol Lett 2016;245:24–30. [DOI] [PubMed] [Google Scholar]
- 184. Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani S et al.. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int 2013;83(4):582–92. [DOI] [PubMed] [Google Scholar]
- 185. Hsu CC, Lu YC, Chiu CA, Yu TH, Hung WC, Wang CP, Lu LF, Chung FM, Lee YJ, Tsai IT. Levels of indoxyl sulfate are associated with severity of coronary atherosclerosis. Clin Invest Med 2013;36(1):E42–9. [DOI] [PubMed] [Google Scholar]
- 186. Cao X-S, Chen J, Zou J-Z, Zhong Y-H, Teng J, Ji J, Chen Z-W, Liu Z-H, Shen B, Nie Y-X et al.. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol 2015;10(1):111–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Melamed ML, Plantinga L, Shafi T, Parekh R, Meyer TW, Hostetter TH, Coresh J, Powe NR. Retained organic solutes, patient characteristics and all-cause and cardiovascular mortality in hemodialysis: results from the retained organic solutes and clinical outcomes (ROSCO) investigators. BMC Nephrol 2013;14(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lin C-J, Liu H-L, Pan C-F, Chuang C-K, Jayakumar T, Wang T-J, Chen H-H, Wu C-J. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res 2012;43(6):451–6. [DOI] [PubMed] [Google Scholar]
- 189. Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009;4(10):1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Niwa T. Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci 2010;72(1–2):1–11. [PMC free article] [PubMed] [Google Scholar]
- 191. Miyazaki T, Ise M, Seo H, Niwa T. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int Suppl 1997;62:S15–22. [PubMed] [Google Scholar]
- 192. Ichii O, Otsuka-Kanazawa S, Nakamura T, Ueno M, Kon Y, Chen W, Rosenberg AZ, Kopp JB. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl-hydrocarbon receptor ligand. PLoS One 2014;9(9):e108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y, Niwa T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem 2012;403(7):1841–50. [DOI] [PubMed] [Google Scholar]
- 194. Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Ren Nutr 2012;22(1):86–9. [DOI] [PubMed] [Google Scholar]
- 195. Kawakami T, Inagi R, Wada T, Tanaka T, Fujita T, Nangaku M. Indoxyl sulfate inhibits proliferation of human proximal tubular cells via endoplasmic reticulum stress. Am J Physiol Renal Physiol 2010;299(3):F568–F76. [DOI] [PubMed] [Google Scholar]
- 196. Palm F, Nangaku M, Fasching A, Tanaka T, Nordquist L, Hansell P, Kawakami T, Nishijima F, Fujita T. Uremia induces abnormal oxygen consumption in tubules and aggravates chronic hypoxia of the kidney via oxidative stress. Am J Physiol Renal Physiol 2010;299(2):F380–F6. [DOI] [PubMed] [Google Scholar]
- 197. Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-κB and free radical in proximal tubular cells. Kidney Int 2003;63(5):1671–80. [DOI] [PubMed] [Google Scholar]
- 198. Shimizu H, Bolati D, Adijiang A, Muteliefu G, Enomoto A, Nishijima F, Dateki M, Niwa T. NF-κB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am J Physiol Cell Physiol 2011;301(5):C1201–C12. [DOI] [PubMed] [Google Scholar]
- 199. Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-ĸB activation. Am J Nephrol 2010;31(5):435–41. [DOI] [PubMed] [Google Scholar]
- 200. Shimizu H, Bolati D, Higashiyama Y, Nishijima F, Shimizu K, Niwa T. Indoxyl sulfate upregulates renal expression of MCP-1 via production of ROS and activation of NF-κB, p53, ERK, and JNK in proximal tubular cells. Life Sci 2012;90(13):525–30. [DOI] [PubMed] [Google Scholar]
- 201. Matsuo K, Yamamoto S, Wakamatsu T, Takahashi Y, Kawamura K, Kaneko Y, Goto S, Kazama JJ, Narita I. Increased proinflammatory cytokine production and decreased cholesterol efflux due to downregulation of ABCG1 in macrophages exposed to indoxyl sulfate. Toxins 2015;7(8):3155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kim SH, Yu M-A, Ryu ES, Jang Y-H, Kang D-H. Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab Invest 2012;92:488. [DOI] [PubMed] [Google Scholar]
- 203. Bolati D, Shimizu H, Higashiyama Y, Nishijima F, Niwa T. Indoxyl sulfate induces epithelial-to-mesenchymal transition in rat kidneys and human proximal tubular cells. Am J Nephrol 2011;34(4):318–23. [DOI] [PubMed] [Google Scholar]
- 204. Lee W-C, Li L-C, Chen J-B, Chang H-W. Indoxyl sulfate-induced oxidative stress, mitochondrial dysfunction, and impaired biogenesis are partly protected by vitamin C and n-acetylcysteine. ScientificWorldJournal 2015:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S et al.. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 2010;49(2):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Barouki R, Aggerbeck M, Aggerbeck L, Coumoul X. The aryl hydrocarbon receptor system. Drug Metab Drug Interact 2012;27(1):3–8. [DOI] [PubMed] [Google Scholar]
- 207. Falahatpisheh MH, Nanez A, Ramos KS. AHR regulates WT1 genetic programming during murine nephrogenesis. Mol Med 2011;17(11–12):1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Rossi M, Klein K, Johnson DW, Campbell KL. Pre-, pro-, and synbiotics: do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol 2012:673631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. p-cresyl sulfate. Toxins 2017;9(2):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C, Campbell KL. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis 2015;25(9):860–5. [DOI] [PubMed] [Google Scholar]
- 211. Cloetens L, Broekaert WF, Delaedt Y, Ollevier F, Courtin CM, Delcour JA, Rutgeerts P, Verbeke K. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: a randomised, placebo-controlled cross-over study. Br J Nutr 2010;103(5):703–13. [DOI] [PubMed] [Google Scholar]
- 212. De Preter V, Vanhoutte T, Huys G, Swings J, Rutgeerts P, Verbeke K. Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment Pharmacol Ther 2008;27(6):504–13. [DOI] [PubMed] [Google Scholar]
- 213. Meijers BKI, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 2010;25(1):219–24. [DOI] [PubMed] [Google Scholar]
- 214. Salmean YA, Segal MS, Palii SP, Dahl WJ. Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J Ren Nutr 2015;25(3):316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y et al.. P-cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 2013;24(1):88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Furuse SU, Ohse T, Jo-Watanabe A, Shigehisa A, Kawakami K, Matsuki T, Chonan O, Nangaku M. Galacto-oligosaccharides attenuate renal injury with microbiota modification. Physiol Rep 2014;2(7):e12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, Barbato F, Cataldi M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis 2014;24(9):1043–9. [DOI] [PubMed] [Google Scholar]
- 218. Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant 2011;26(3):1094–8. [DOI] [PubMed] [Google Scholar]
- 219. Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto C-C, McWhinney BC, Ungerer JPJ, Campbell KL. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 2016;11(2):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Briskey D, Tucker P, Johnson DW, Coombes JS. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin Exp Nephrol 2017;21(1):7–15. [DOI] [PubMed] [Google Scholar]
- 221. Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work Group Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 2012;23(7):1258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Vanholder R, Boelaert J, Glorieux G, Eloot S. New methods and technologies for measuring uremic toxins and quantifying dialysis adequacy. Semin Dial 2015;28(2):114–24. [DOI] [PubMed] [Google Scholar]
- 223. Irsfeld M, Spadafore M, Prüß BM. β-phenylethylamine, a small molecule with a large impact. WebmedCentral 2013;4(9):4409. [PMC free article] [PubMed] [Google Scholar]
- 224. Marcobal A, De Las Rivas B, Landete JM, Tabera L, Muñoz R. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit Rev Food Sci Nutr 2012;52(5):448–67. [DOI] [PubMed] [Google Scholar]
- 225. Oberdoerster J, Guizzetti M, Costa LG. Effect of phenylalanine and its metabolites on the proliferation and viability of neuronal and astroglial cells: possible relevance in maternal phenylketonuria. J Pharmacol Exp Ther 2000;295(1):295–301. [PubMed] [Google Scholar]
- 226. Jankowski J, van der Giet M, Jankowski V, Schmidt S, Hemeier M, Mahn B, Giebing G, Tölle M, Luftmann H, Schlüter H et al.. Increased plasma phenylacetic acid in patients with end-stage renal failure inhibits iNOS expression. J Clin Invest 2003;112(2):256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Schmidt S, Westhoff TH, Krauser P, Zidek W, van der Giet M. The uraemic toxin phenylacetic acid increases the formation of reactive oxygen species in vascular smooth muscle cells. Nephrol Dial Transplant 2008;23(1):65–71. [DOI] [PubMed] [Google Scholar]
- 228. Mutsaers HAM, Wilmer MJG, Reijnders D, Jansen J, van den Broek PHH, Forkink M, Schepers E, Glorieux G, Vanholder R, van den Heuvel LP et al.. Uremic toxins inhibit renal metabolic capacity through interference with glucuronidation and mitochondrial respiration. Biochim Biophys Acta 2013;1832(1):142–50. [DOI] [PubMed] [Google Scholar]
- 229. Mutsaers HA, van den Heuvel LP, Ringens LH, Dankers AC, Russel FG, Wetzels JF, Hoenderop JG, Masereeuw R. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One 2011;6(4):e18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Xiong X, Liu D, Wang Y, Zeng T, Peng Y. Urinary 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, 3-hydroxyphenylacetic acid, and 3-hydroxyhippuric acid are elevated in children with autism spectrum disorders. BioMed Res Int 2016:9485412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Nowak PJ, Wilk R, Prymont-Przyminska A, Zwolinska A, Sarniak A, Wlodarczyk A, de Graft-Johnson J, Mamelka B, Zasowska-Nowak A, Bartnicki P et al.. Hemodialysis decreases the concentration of accumulated plant phenols in the plasma of patients on maintenance dialysis: influence of residual renal function. Ther Apher Dial 2017;21(6):572–85. [DOI] [PubMed] [Google Scholar]
- 232. Mokhtarani M, Diaz GA, Rhead W, Lichter-Konecki U, Bartley J, Feigenbaum A, Longo N, Berquist W, Berry SA, Gallagher R et al.. Urinary phenylacetylglutamine as dosing biomarker for patients with urea cycle disorders. Mol Genet Metab 2012;107(3):308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 2014;25(3):615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, Pascual J, Ley RE, Spector TD, Bell JT et al.. Gut-microbiota-metabolite axis in early renal function decline. PLoS One 2015;10(8):e0134311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Poesen R, Claes K, Evenepoel P, de Loor H, Augustijns P, Kuypers D, Meijers B. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol 2016;27(11):3479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Shafi T, Meyer TW, Hostetter TH, Melamed ML, Parekh RS, Hwang S, Banerjee T, Coresh J, Powe NR. Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: results from the Retained Organic Solutes and Clinical Outcomes (ROSCO) investigators. PLoS One 2015;10(5):e0126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 2013;84(3):585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Satoh M, Hayashi H, Watanabe M, Ueda K, Yamato H, Yoshioka T, Motojima M. Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp Nephrol 2003;95(3):e111–e18. [DOI] [PubMed] [Google Scholar]
- 239. Rechner AR, Kuhnle G, Hu H, Roedig-Penman A, van den Braak MH, Moore KP, Rice-Evans CA. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic Res 2002;36(11):1229–41. [DOI] [PubMed] [Google Scholar]
- 240. Suchy-Dicey AM, Laha T, Hoofnagle A, Newitt R, Sirich TL, Meyer TW, Thummel KE, Yanez ND, Himmelfarb J, Weiss NS et al.. Tubular secretion in CKD. J Am Soc Nephrol 2016;27(7):2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos 2015;43(10):1522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Dou L, Sallée M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N, Fallague K, Brunet P, Calaf R, Dussol B et al.. The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 2015;26(4):876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Jin U-H, Lee S-O, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 2014;85(5):777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Zeisel SH, Mar M-H, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133(5):1302–7. [DOI] [PubMed] [Google Scholar]
- 245. Bodea S, Funk MA, Balskus EP, Drennan CL. Molecular basis of C-N bond cleavage by the glycyl radical enzyme choline trimethylamine-lyase. Cell Chem Biol 2016;23(10):1206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Stock J. Gut microbiota: an environmental risk factor for cardiovascular disease. Atherosclerosis 2013;229(2):440–2. [DOI] [PubMed] [Google Scholar]
- 247. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L et al.. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M et al.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Xu K-Y, Xia G-H, Lu J-Q, Chen M-X, Zhen X, Wang S, You C, Nie J, Zhou H-W, Yin J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep 2017;7(1):1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant 2006;21(5):1300–4. [DOI] [PubMed] [Google Scholar]
- 251. Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O. Associations of trimethylamine-N-oxide (TMAO) with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr 2015;25(4):351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Mutsaers HAM, Engelke UFH, Wilmer MJG, Wetzels JFM, Wevers RA, van den Heuvel LP, Hoenderop JG, Masereeuw R. Optimized metabolomic approach to identify uremic solutes in plasma of stage 3–4 chronic kidney disease patients. PLoS One 2013;8(8):e71199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, Stenvinkel P, Bergman P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One 2016;11(1):e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD et al.. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 2016;27(1):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto C et al.. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int 2016;89(5):1144–52. [DOI] [PubMed] [Google Scholar]
- 256. Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015;116(3):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Barton S, Navarro SL, Buas MF, Schwarz Y, Gu H, Djukovic D, Raftery D, Kratz M, Neuhouser ML, Lampe JW. Targeted plasma metabolome response to variations in dietary glycemic load in a randomized, controlled, crossover feeding trial in healthy adults. Food Funct 2015;6(9):2949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Burger KNJ, Beulens JWJ, Boer JMA, Spijkerman AMW, van der A DL. Dietary glycemic load and glycemic index and risk of coronary heart disease and stroke in Dutch men and women: the EPIC-MORGEN Study. PLoS One 2011;6(10):e25955. [DOI] [PMC free article] [PubMed] [Google Scholar]