Abstract
Background
The inability to have children affects 10% to 15% of couples worldwide. A male factor is estimated to account for up to half of the infertility cases with between 25% to 87% of male subfertility considered to be due to the effect of oxidative stress. Oral supplementation with antioxidants is thought to improve sperm quality by reducing oxidative damage. Antioxidants are widely available and inexpensive when compared to other fertility treatments, however most antioxidants are uncontrolled by regulation and the evidence for their effectiveness is uncertain. We compared the benefits and risks of different antioxidants used for male subfertility. This review did not examine the use of antioxidants in normospermic men.
Objectives
To evaluate the effectiveness and safety of supplementary oral antioxidants in subfertile men.
Search methods
The Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, and two trials registers were searched on 1 February 2018, together with reference checking and contact with study authors and experts in the field to identify additional trials.
Selection criteria
We included randomised controlled trials (RCTs) that compared any type, dose or combination of oral antioxidant supplement with placebo, no treatment or treatment with another antioxidant, among subfertile men of a couple attending a reproductive clinic. We excluded studies comparing antioxidants with fertility drugs alone and studies that included fertile men attending a fertility clinic because of female partner infertility.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary review outcome was live birth. Clinical pregnancy, adverse events and sperm parameters were secondary outcomes.
Main results
We included 61 studies with a total population of 6264 subfertile men, aged between 18 and 65 years, part of a couple who had been referred to a fertility clinic and some of whom were undergoing assisted reproductive techniques (ART). Investigators compared and combined 18 different oral antioxidants. The evidence was of 'low' to 'very low' quality: the main limitation was that out of the 44 included studies in the meta‐analysis only 12 studies reported on live birth or clinical pregnancy. The evidence is current up to February 2018.
Live birth: antioxidants may lead to increased live birth rates (OR 1.79, 95% CI 1.20 to 2.67, P = 0.005, 7 RCTs, 750 men, I2 = 40%, low‐quality evidence). Results suggest that if in the studies contributing to the analysis of live birth rate, the baseline chance of live birth following placebo or no treatment is assumed to be 12%, the chance following the use of antioxidants is estimated to be between 14% and 26%. However, this result was based on only 124 live births from 750 couples in seven relatively small studies. When studies at high risk of bias were removed from the analysis, there was no evidence of increased live birth (Peto OR 1.38, 95% CI 0.89 to 2.16; participants = 540 men, 5 RCTs, P = 0.15, I2 = 0%).
Clinical pregnancy rate: antioxidants may lead to increased clinical pregnancy rates (OR 2.97, 95% CI 1.91 to 4.63, P < 0.0001, 11 RCTs, 786 men, I2 = 0%, low‐quality evidence) compared to placebo or no treatment. This suggests that if in the studies contributing to the analysis of clinical pregnancy, the baseline chance of clinical pregnancy following placebo or no treatment is assumed to be 7%, the chance following the use of antioxidants is estimated to be between 12% and 26%. This result was based on 105 clinical pregnancies from 786 couples in 11 small studies.
Adverse events Miscarriage: only three studies reported on this outcome and the event rate was very low. There was no difference in miscarriage rate between the antioxidant and placebo or no treatment group (OR 1.74, 95% CI 0.40 to 7.60, P = 0.46, 3 RCTs, 247 men, I2 = 0%, very low‐quality evidence). The findings suggest that in a population of subfertile men with an expected miscarriage rate of 2%, the chance following the use of an antioxidant would result in the risk of a miscarriage between 1% and 13%.
Gastrointestinal: antioxidants may lead to an increase in mild gastrointestinal upsets when compared to placebo or no treatment (OR 2.51, 95% CI 1.25 to 5.03, P = 0.010, 11 RCTs, 948 men, I2 = 50%, very low‐quality evidence). This suggests that if the chance of gastrointestinal upsets following placebo or no treatment is assumed to be 2%, the chance following the use of antioxidants is estimated to be between 2% and 9%. However, this result was based on a low event rate of 35 out of 948 men in 10 small or medium‐sized studies, and the quality of the evidence was rated very low and was high in heterogeneity.
We were unable to draw any conclusions from the antioxidant versus antioxidant comparison as insufficient studies compared the same interventions.
Authors' conclusions
In this review, there is low‐quality evidence from seven small randomised controlled trials suggesting that antioxidant supplementation in subfertile males may improve live birth rates for couples attending fertility clinics. Low‐quality evidence suggests that clinical pregnancy rates may also increase. Overall, there is no evidence of increased risk of miscarriage, however antioxidants may give more mild gastrointestinal upsets but the evidence is of very low quality. Subfertilte couples should be advised that overall, the current evidence is inconclusive based on serious risk of bias due to poor reporting of methods of randomisation, failure to report on the clinical outcomes live birth rate and clinical pregnancy, often unclear or even high attrition, and also imprecision due to often low event rates and small overall sample sizes. Further large well‐designed randomised placebo‐controlled trials reporting on pregnancy and live births are still required to clarify the exact role of antioxidants.
Plain language summary
Antioxidants for male subfertility
Review question Do supplementary oral antioxidants compared with placebo, no treatment or another antioxidant improve fertility outcomes for subfertile men?
Background A couple may be considered to have fertility problems if they have been trying to conceive for over a year with no success. Many subfertile men undergoing fertility treatment also take dietary supplements in the hope of improving their fertility. Fertility treatment can be a very stressful time for men and their partners. It is important that these couples have access to high‐quality evidence that will allow them to make informed decisions on whether to take a supplemental antioxidant. This is especially important, as most antioxidant supplements are uncontrolled by regulation. This review aimed to assess whether supplements with oral antioxidants, taken by the subfertile men, would increase the chances of a couple to achieve a (clinical) pregnancy confirmed by ultrasound and ultimately the birth of a baby (live birth). This review did not examine the use of antioxidants in men with normal sperm.
Study characteristics
Cochrane authors conducted a review including 61 randomised controlled trials comparing 18 different antioxidants with placebo, no treatment or another antioxidant in a total population of 6264 subfertile men. The age range of the participants was 18 to 65 years; they were part of a couple who had been referred to a fertility clinic and some were undergoing fertility treatment. The evidence is current to February 2018.
Main results Antioxidants may be associated with an increased live birth and clinical pregnancy rate. Based on the studied population for live birth, we would expect that out of 100 subfertile men not taking antioxidants, 12 couples would have a baby, compared with between 14 and 26 couples per 100 who would have a baby if taking antioxidants. If studies with high risk were removed from the analysis, there was no evidence of increased live birth. In the people who were studied for clinical pregnancy, we would expect that out of 100 subfertile men not taking antioxidants, seven couples would have a clinical pregnancy, compared with between 12 and 26 couples per 100 who would have a clinical pregnancy if taking antioxidants. Adverse events were poorly reported. However based on three studies, we could conclude that miscarriage did not occur more often if taking antioxidants. The use of antioxidants could give more gastrointestinal upsets, meaning that we expect that out of 100 subfertile men not taking antioxidants, two would have gastrointestinal upsets compared to between two and nine men if taking antioxidants.
Authors' conclusion and quality of the evidence
Antioxidant supplementation taken by subfertile males of a couple attending a fertility clinic may increase the chance of a live birth, however the overall quality of evidence was low from only seven small randomised controlled trials. Low‐quality evidence also suggests that clinical pregnancy rates may increase. Overall, there is no evidence of increased risk of miscarriage, however evidence of very low quality suggest that antioxidants may give more mild gastrointestinal upsets. Subfertile couples should be advised that overall the current evidence is inconclusive due to the poor reporting of methods, failure to report on the clinical outcomes live birth rate and clinical pregnancy, and furthermore imprecision due to often low event rates, high number of dropouts and small study group sizes. Further large well‐designed randomised placebo‐controlled trials reporting on pregnancy and live births are still required to clarify the exact role of antioxidants.
Summary of findings
Summary of findings for the main comparison. Antioxidants compared to placebo or no treatment for patients with male subfertility.
| Antioxidants compared to placebo or no treatment for patients with male subfertility | ||||||
| Patient or population: patients with male subfertility Setting: clinic Intervention: antioxidants Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with antioxidants | |||||
| Live birth rate per couple randomised | 117 per 1000 | 192 per 1.000 (138 to 262) | OR 1.79 (1.20 to 2.67) | 750 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 | |
| Clinical pregnancy rate per couple randomised | 69 per 1000 | 180 per 1.000 (124 to 255) | OR 2.97 (1.91 to 4.63) | 786 (11 RCTs) | ⊕⊕⊝⊝ LOW 1 | |
| Adverse events ‐ Miscarriage | 19 per 1000 | 33 per 1.000 (8 to 129) | OR 1.74 (0.40 to 7.60) | 247 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| Adverse events ‐ Gastrointestinal | 18 per 1000 | 45 per 1.000 (23 to 86) | OR 2.51 (1.25 to 5.03) | 948 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Peto Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels for serious risk of bias: lack of allocation concealment, lack of blinding and incomplete accounting of patients and outcome events
2 Downgraded one level for serious imprecision: crossing the line of no effect
3 Downgraded one level for serious imprecision: incomplete accounting of patients and outcome events
Background
Description of the condition
It is believed that 48.5 to 186 million people worldwide are affected by the inability to have children (Boivin 2007; Inhorn 2015; Mascarenhas 2012), with delayed conception affecting 10% to 15% of couples trying to conceive (Evers 2002). The International Glossary on Infertility and Fertility Care (Zegers‐Hochschild 2017) defines infertility as a disease characterised by the failure to establish a clinical pregnancy after 12 months of regular, unprotected intercourse and is used interchangeably with the term subfertility (Zegers‐Hochschild 2017). Subfertility generally describes any form of grade of reduced fertility in couples unsuccessfully trying to conceive (Gnoth 2005).
In 2010 it was stated in a World Health Organization (WHO) report (Mascarenhas 2012) that worldwide, measured in 190 countries, 1.9% of child‐seeking women were unable to have a first live birth (primary infertility) and 10.5% with a prior live birth were unable to have an additional live birth (secondary infertility). However, the distribution of male and female causes of infertility have not been well defined. Based on a WHO multicentre study from the 1980s, it is suggested that 20% of cases are solely attributed to the male, 38% to the female, 27% to both, and 15% not clearly to either (Comhaire 1987). Surprisingly, the most recent studies are from the 1990s and still more than two decades old. However, these data show similar percentages, though mainly based on national databases (ESHRE Guidelines 1996; Thonneau 1991).
In the literature, it is suggested that a male factor is involved in up to 50% of infertility cases (Irvine 1998; Winters 2014). However, the true extent of male infertility is likely to be underestimated due to the lack of male evaluation in infertile couples and the heterogeneity of studies (Barratt 2017; Winters 2014). In the past decades, oxidative stress (OS) has been commonly investigated and found to play a role in 25% to 87% of male factor subfertility (Aitken 1987; Aitken 1989; Aitken 1992; Iwasaki 1992; Mazzilli 1994; Shekarriz 1995; Zini 1993).
In all cells using oxygen to survive, toxins are produced as a consequence. These toxic end‐products are better known as free radicals, atoms with unpaired electrons. Some free radicals are characterised by having higher reactive activity than molecular oxygen, and are therefore called reactive oxygen species (ROS). ROS can act as mediators and regulators of cell metabolism and apoptosis (Mirończuk‐Chodakowska 2018). The three major ROS are superoxide anion (O2‐), hydroxyl radical (ºOH), and hydrogen peroxide (H2O2). Excessive production of ROS can lead to cell damage. Therefore, the human body has developed a defence system in which antioxidants play an important role. Antioxidants are capable of reducing the production of free radicals, slowing or preventing the oxidation and repairing the damage (Mirończuk‐Chodakowska 2018).
The increased levels of ROS are thought to be due to either exogenous or endogenous factors. Exogenous factors could be environmental such as high temperatures, pesticides and pollution or more due to lifestyle factors such as alcohol consumption, smoking, poor nutrition and obesity. Endogenous factors are infections, chronic disease, auto‐immune disease and in the male reproductive tract the occurrence of more immature spermatozoa and varicocele (Alvarez 2003; Tremellen 2008).
In conclusion, OS occurs when ROS production overwhelms the antioxidant defence mechanisms leading to cellular damage (Sikka 1995).
Description of the intervention
Antioxidants are substances that inhibit or delay the oxidation of biologically relevant molecules, either by directly scavenging free radicals or by chelation of redox metals (Valko 2006). However, the definition is very general and does not specify how a compound may act as an antioxidant (Huang 2018). In general, non‐enzymatic antioxidants play a substantial role in first‐line defence by preventing the formation of ROS by binding ions and enzymatic antioxidants that regulate the gene expression of oxidative enzymes.
The predominant supplementary antioxidants that are studied in male subfertility clinical trials are vitamin E, vitamin C, carotenoids, carnitines, coenzyme Q10 (ubiquinol), cysteine and the micronutrients folate, selenium and zinc (Eskenazi 2005; Majzoub 2017). Antioxidants can be administered orally as a single or combined supplement. They are widely available and inexpensive when compared to other fertility treatments. However cost‐benefit analysis is beyond the scope of this review.
In contrary to the previous versions of this review, pentoxifylline is no longer included as it is a conventional medicine or over‐the‐counter drug and not a dietary supplement.
Substances with direct antioxidant action
Arginine Arginine, or L‐arginine, is an amino acid that is required for normal spermatogenesis. It plays a role in the inflammatory response and directly protects against oxidative damage by being a free radical scavenger. Arginine can be derived from meat products, dairy, nuts and seeds. Significant adverse events have not been observed, however contraindication for people with a history of genital or oral herpes, asthma or cancer (Appleton 2002).
Carnitines Carnitine is an antioxidant, with the two most important isomers being called l‐carnitine (LC) and l‐acetylcarnitine (LAC). In the male genital tract carnitines are found in the epididymis, seminal plasma and in spermatozoa (Bøhmer 1978). Carnitines assist sperm metabolism by positively affecting sperm motility and maturation. There might be an association between the concentration of LAC and male fertility (Agarwal 2004a). Animal products like meat, fish, poultry and dairy are the best sources for carnitines. Doses above 3 g/day can give gastrointestinal side effects and malodorous effects (Annals of the New York Academy of Science 2004).
Carotenoids Carotenoids are pigments found in plants. One of the most important carotenoids is β‐carotene (Ross 2006), a provitamin A, which can directly scavenge ROS. Other carotenoids found in food are lycopene, lutein, and zeaxanthin, however these are not converted into vitamin A. Both in vivo and in vitro, β‐carotene has been shown to protect isolated lipid membranes from peroxidation (Bendich 1989). Healthy young men with a higher carotenoid intake have higher sperm motility, and higher lycopene intake is associated with better sperm morphology (Zareba 2013). However, a review by Grune and colleagues (Grune 2010) stated that there are conflicting results whether β‐carotene has antioxidant properties. Carotenoids come from leafy green vegetables, fruits, and some vegetable oils (Ross 2006). Excess intake of preformed vitamin A can lead to toxicity (hypervitaminosis A). However, excessive ingestion of provitamins such as carotenoids are not associated with vitamin A toxicity, the only side effect is carotenaemia (yellow‐tinged skin).
Coenzyme Q10 Coenzyme Q10 (CoQ10) is a fat‐soluble antioxidant synthesised endogenously and an essential component of the mitochondrial energy metabolism. In its reduced form, CoQH2, ubiquinol, it inhibits protein and DNA oxidation and lipid peroxidation (Littarru 2007). CoQ10 seminal fluid levels are significantly correlated to sperm count and motility, except in men with varicocele (Mancini 1994). Meat, fish, nuts and some oils are the most important dietary sources of CoQ10 due to their relatively high level of fats and mitochondria (Pravst 2010). Reported side effects are mild gastrointestinal symptoms (Bhagavan 2006).
Cysteine Cysteine plays an important role in glutathione synthesis. N‐acetylcysteine (NAC) is a precursor of the amino acid cysteine and a direct scavenger of ROS. Glutathione becomes depleted when there is OS, and this can be reversed by NAC supplementation (Atkuri 2007). NAC is less toxic and less susceptible to oxidation compared to cysteine itself. Oral administration of NAC up to 8000 mg/day is not known to cause significant adverse events (Atkuri 2007). Less is known about ethylcysteine, however in vivo and animal studies have shown anti‐oxidative effects (Hsia 2016).
Micronutrients (folate, selenium, zinc) Folate, also known as vitamin B9, is a micronutrient important for the synthesis of DNA, transfer RNA and the amino acids cysteine and methionine. Folic acid, the synthetic form, can scavenge oxidising free radicals and it inhibits lipid peroxidation (Joshi 2001). Folate is present in green‐leafy vegetables, liver, bread, yeast and fruits (Ebisch 2007). Folic acid doses of 5 mg/day and over can cause abdominal cramps, diarrhoea and rash. Higher doses can even cause altered sleep patterns, irritability, confusion, exacerbation of seizures and nausea (Rogovik 2009).
Zinc is involved in DNA transcription and protein synthesis and has extensive antioxidants properties (Ebisch 2007). Zinc has an important role in testes development, sperm physiological functions and decrease of zinc in seminal plasma is associated with sperm quality (Colagar 2009a). Zinc, like selenium, is absorbed from the soil into plants. Dietary sources rich of zinc are meat products, wheat and seeds.
Magnesium and selenium are different than other antioxidant nutrients because they are involved in the mechanisms of cellular antioxidant defence by increasing the activity of the antioxidant enzyme glutathione peroxidase, and not by directly reacting with oxidant molecules (Burk 2002; Yavuz 2013). It is suggested that both magnesium and selenium deficiency would make humans more susceptible to oxidative injury. Selenium is furthermore essential for normal spermatogenesis (Boitani 2008). Selenium is derived from fish, meat products, diary and soil absorption by plants (Navarro‐Alarcon 2008). Early indicators of excess intake are a garlic odour in the breath and a metallic taste in the mouth. The most common clinical signs of chronically high selenium intakes are gastrointestinal symptoms, fatigue, hair loss, joint pain and nail problems (MacFarquhar 2010). Magnesium is derived from green leafy vegetables, nuts, beans, and cereals (McNeill 1985).
Vitamin E Vitamin E, also known as the bioactive form α‐tocopherol, has a principal role by being the first defence against oxidant‐induced membrane injury (Traber 2007). Vitamin E is found in vegetable oils and there is a given upper daily limit based on the possible increased bleeding risk (Institute of Medicine 2000).
Vitamin C Vitamin C, also known as ascorbic acid, is able to diminish DNA damage directly by scavenging free radicals and decreasing formation of lipid hydroperoxides (Padayatty 2003). Ascorbic acid concentrations are 10‐fold higher in seminal plasma compared to blood plasma. Low levels of seminal plasma ascorbic acid are directly related to decreased amount of normal morphology of spermatozoa and increased sperm DNA damage (Colagar 2009). Vitamin C is mainly found in fruits and vegetables.
Substances with antioxidant properties
Myo‐inositol Inositol is a polyalcohol, naturally occurring as nine stereoisomers including myo‐inositol (MYO). Myo‐inositol, a "pseudovitamin" and previously known as vitamin B8, plays an important roll in cell membrane formation and lipid synthesis. The highest concentration in the genital tract is within the seminiferous tubules, and myo‐inositol is produced by Sertoli cells in response to follicle‐stimulating hormone (FSH) (Lewin 1976). Myo‐inositol is a precursor for the phosphatidyl‐inositol (PtdIns) signalling pathway and directly involved in regulation of motility, capacitation and acrosome reaction (Bevilacqua 2015). Myo‐inositol has a role as a possible antioxidant agent by increasing endogenous antioxidant enzymes and directly affecting the mitochondria leading to an increase of the membrane potential (Colone 2010; Condorelli 2017).
Polyunsaturated fatty acids (PUFAs) Polyunsaturated fatty acids (PUFAs) are classified into omega‐3 (docosahexaenoic acid, DHA), omega‐6 and omega‐9. Omega‐9 is synthesised by animals, but omegas‐3 and ‐6 needs to be supplemented in the diet. The main sources of these are vegetable and fish oils (Wathes 2007). PUFAs increase the plasma fluidity of the sperm membrane. However, this fluidity makes the sperm susceptible to ROS and lipid peroxidation that can damage the sperm. Wathes states that "It appears that PUFAs are a two edged sword ‐ some are essential, but too many are potentially harmful" (Wathes 2007, page 198). It seems to be that PUFAs have a pro‐oxidant rather than a direct antioxidant effect. Although it is suggested that omega 3 might have a free radical‐scavenging potential (Giordano 2014; Richard 2008).
Resveratrol Resveratrol is a natural phytoalexin with antioxidant properties. Several in vitro studies with human cryopreserved sperm and in vivo studies in animal models suggest that resveratrol improves sperm motility and enhances antioxidant defences (Branco 2010; Collodel 2011; Ourique 2013). It is naturally found in our diet in the form of grapes, berries, several nuts and wine (Ourique 2013). Worldwide, resveratrol is better known from research on the effect of daily intake of red wine, "the "Mediterranean diet", in cardiovascular disease (Bertelli 2009). Reversible gastrointestinal side effects are reported, however evidence on side effects is limited (Hausenblas 2014).
Vitamin B (complex) Vitamin B is a water‐soluble vitamin and consists of several precursor and coenzymes such as thiamine (B1), riboflavin (B2) and cobalamin (B12). Vitamin B plays an important role in the homocysteine metabolism. It is suggested that total plasma homocysteine may have a pro‐oxidant effect and a role in the release of ROS (Hankey 1999). Increased intake of vitamin B has an homocysteine‐lowering effect, which is the strongest for folate, but vitamins B6, B12, and B2 have all been shown to be independently predictive of plasma homocysteine. Vitamin B is mainly found in meat products, other examples of food sources are beans, potatoes, bananas and mushrooms.
Vitamin D Vitamin D is a fat‐soluble vitamin, with the natural main source being dermal synthesis (sun light). The active form of vitamin D is 1,25‐dihydroxyvitamin D, also called vitamin D3. Halicka and colleagues suggest that vitamin D3 has antioxidant activity, mainly by inducing the antioxidant protein superoxide dismutase (Halicka 2012). However, there are no other studies about the antioxidant properties of vitamin D in male fertility. Clearly, vitamin D plays an important role in male fertility and serum levels of vitamin D are positively associated with semen quality (de Angelis 2017). However, most of the studies do not mention the antioxidant properties of vitamin D, but rather relate the effect to the synthesis of sex steroids or the regulation of calcium.
How the intervention might work
In the second half of the 20th century it was found that semen leukocytes (white blood cells) and, mostly immature, spermatozoa are major sources of ROS production in the male reproductive tract (Aitken 1987; Aitken 1990; Iwasaki 1992). Additionally, the existence of a varicocele leads to increased scrotal temperature, reflux of blood flow and a damaged microcirculation, all of which act to increase both germ cell death and levels of ROS. This ultimately decreases semen quality and sperm function (Zini 2011). However, a low production of ROS is physiological and needed for adequate sperm functioning by supporting capacitation, maturation and hyperactivation (Aitken 1994).
In most body cells, ROS are directly inactivated and their damage repaired by cytoplasmic antioxidant enzymes such as catalase, superoxide dismutase or glutathione peroxidase (Aitken 1994; Ebisch 2007). However, spermatozoa differ from other cells as a substantial proportion of their cytoplasm is removed during the final stages of spermatogenesis. The lack of cytoplasma and therefore enzymatic antioxidants makes them very vulnerable. Furthermore, spermatozoal membranes are rich in PUFA which makes them susceptible for lipid peroxidation resulting in decreased flexibility of the sperm membrane and reduction of tail motion (Jones 1973). For these two reasons, spermatozoa are dependant on seminal plasma, which is rich in antioxidants (Smith 1996; Zini 1993).
In general, it can be stated that OS can cause fertility problems in two ways; firstly by damaging the sperm membrane thus affecting the sperm motility and ability to break down the oocyte membrane, and secondly by apoptosis and direct alteration of the sperm DNA (Kodama 1997; Lewis 2013) Deceivingly, men with sperm DNA damage can still have normal seminal parameters, but have a poor chance of natural conception (Aktan 2013; Intasqui 2015). Sperm DNA damage or integrity can be measured in a number of ways, either direct or indirect (Agarwal 2017). The most current used sperm DNA fragmentation (SDF) testS are terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling (TUNEL), the COMET assay and sperm chromatin structure assay (SCSA). Other options are measurement of the byproduct of DNA oxidation, 8‐hydroxydeoxyguanosine (8‐OHdG) or by chemoluminescence assays using luminol or lucigenin.There are experts within the field who state that SDF testing should be part of a standard assessment of the male partner when a couple presents with subfertility (Agarwal 2016; Boe‐Hansen 2006). Women undergoing intrauterine insemination with a sperm DNA fragmentation index < 30%, as measured by the SCSA, were seven times more likely to achieve a pregnancy than those couples where the male partner had a higher degree of sperm DNA damage (Bungum 2004). Furthermore, multiple meta‐analyses show an association between the sperm DNA fragmentation test and live birth or clinical pregnancy after in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment (Collins 2008; Evenson 2006; Li 2006; Osman 2015; Zhang 2015; Zhao 2018). However, a recent meta‐analysis showed that an association does not imply that SDF tests have an actual predictive value (Cissen 2016). An explanation for the little predictive value of SDF testing in assisted reproductive techniques (ART) is the heterogeneity of tests. Most of them are expensive, complex and lack standardisation and validation (Borini 2017; Cissen 2016).
Multiple studies in the past showed that men of a subfertile couple have higher levels of ROS and lower antioxidant levels in their semen compared to fertile men (Aktan 2013; Bykova 2007; Zini 1993). Furthermore, there is evidence that sperm with high percentages of fragmented DNA have less potential of natural conception, with levels above 30% being mentioned as the cut‐off value (Evenson 1999; Spanò 2000). However when fertilisation does occur, spermatozoa releasing ROS could expose oocytes and lead to impaired oocyte function, including its capacity to repair sperm DNA fragmentation post fertilisation (Shimura 2002). The negative impact of damaged paternal DNA could be manifested by impaired embryo development and an association is reported on sperm DNA integrity and early pregnancy loss (Robinson 2012; Simon 2014). On the contrary, there are also some studies suggesting that sperm DNA damage and oxidative stress do not exist in male idiopathic infertility (Hughes 1996; Verit 2006).
If oxidative stress is at the heart of the increased sperm DNA damage and the lowering of pregnancy and live birth rates, then supporting the antioxidant defence system with exogenous antioxidants would seem logical. An extra dietary intake of antioxidants or a healthy diet in general has shown to be strongly associated with semen quality in healthy men (Eskenazi 2005; Irvine 1998; Lewis 1997; Mendiola 2010; Pasqualotto 2001; Salas‐Huetos 2017; Zareba 2013). In conclusion, there is a fine balance between preventing oxidative stress by antioxidants, removing excessive amounts of ROS, and maintaining a small amount of ROS for their physiological effect on sperm functions. Since "reductive stress" as a rebound effect has been reported, large or high doses of antioxidants might better be avoided (Dattilo 2016; Ghyczy 2001).
Why it is important to do this review
In an effort to enhance fertility, couples are increasingly resorting to ART. However, these techniques are expensive and do not cure the causes of subfertility, but rather overcome some of its barriers. Since integrity of sperm DNA is one of the major determinants of normal fertilisation and embryo growth in natural and assisted conception (Agarwal 2003; Aitken 2010; Evenson 2006), there is a clear rationale for antioxidant therapy.
One of the other reasons for this review, apart from finding out if antioxidant therapy can overcome some of the barriers of subfertility, is that the global vitamin and supplement market has grown exponentially over the last years. The market value is expected to reach 278 billion USD by 2024 (Grand View Research 2016). The low costs of supplements and relative risk are appealing to both patients and healthcare providers. However, most antioxidants are uncontrolled by regulation and the evidence for their effectiveness is not based on randomised clinical studies. Vitamins and supplements are dispensed through various retail outlets, including health food shops, online retailers, health centres, fitness clubs, supermarkets and pharmacies (Showell 2017).
The purpose of this Cochrane Review is to assess the effectiveness and safety of different antioxidants and dosages used by men of subfertile couples, by means of improvement of live birth rates, clinical pregnancy rates and adverse events. This is an update of a review first published in 2008 (Showell 2008) and updated in 2014 (Showell 2014).
Objectives
To evaluate the effectiveness and safety of supplementary oral antioxidants compared with placebo, no treatment or another antioxidant in subfertile men.
Search methods
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Randomised controlled trials (RCTs)
Cross‐over trials are included: however, we only used first‐phase data in the analysis. Achieving outcomes such as pregnancy and live birth would preclude entry of couples into the next trial phase (Dias 2006)
Exclusion criteria
Any quasi‐randomised trials
Types of participants
Inclusion criteria
Studies that included subfertile men (male factor subfertility) part of a couple who had been referred to a fertility clinic and might or might not be undergoing assisted reproductive techniques (ART), such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI), or intrauterine insemination (IUI)
In situations where individuals were randomised again following failed cycles, the data would not be pooled in a meta‐analysis unless individual data could be excluded.
Exclusion criteria
Studies enrolling only men attending a fertility clinic exclusively as the result of female partner or idiopathic infertility
Studies enrolling men taking any other fertility enhancing drugs
Studies enrolling men who had chemotherapy treatment in the past
Types of interventions
Inclusion criteria
Any type or dose of oral antioxidant supplementation (individual or combined) that can be obtained without prescription and is not regulated as a pharmaceutical drug, versus placebo or no treatment
Any type or dose of oral antioxidant supplementation (individual or combined) versus another type or dose of oral antioxidant (head‐to‐head)
Interventions were considered 'combined antioxidants' if they included three or more antioxidants in the intervention arm.
Exclusion criteria
Interventions that included plant extracts (for example garlic) or herbal substances
Studies that included antioxidants plus a plant extract (for example garlic) were included if the antioxidant agent was the main focus of the investigation.
Definition of antioxidant in male fertility: a substance that has the ability to protect spermatozoa against endogenous oxidative damage by directly neutralising hydroxyl, superoxide, and hydrogen peroxide radicals, chelation of redox metals or by functioning as a component of an antioxidant enzyme.
Types of outcome measures
Primary outcomes
Live birth rate per couple randomised, defined as delivery of a live fetus after 20 completed weeks of gestation
Secondary outcomes
Clinical pregnancy rate per couple, defined as evidence of a gestational sac confirmed by ultrasound
Any adverse event (including miscarriage) reported by the study
Level of sperm DNA fragmentation, defined as percentage (%) of sperm with abnormal DNA integrity estimated by either toluidine blue (TB) staining, sperm chromatin structure assay (SCSA) or terminal transferase dUTP nick end labelling (TUNEL) assay)
Total sperm motility: any sperm movement in any direction (progressive plus forward plus non progressive motility), provided as percentage (%)
Progessive sperm motility: sperm with forward progression, defined as WHO category A + B, provided as percentage (%)
Sperm concentration:number of sperm (106)/mL
Search methods for identification of studies
We searched for all published and unpublished RCTs investigating oral antioxidant supplementation for subfertile men, without language restriction and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist(MGS).
Electronic searches
We searched the following electronic databases for relevant trials.
The Cochrane Gynaecology and Fertility Group's (CGF) Specialised Register of Controlled Trials, PROCITE platform (searched 1 February 2018) (Appendix 1)
The Cochrane Central Register of Controlled Trials; via the Cochrane Register of Studies Online (CRSO Web platform) ( searched 1 February 2018) (Appendix 2)
MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations) Ovid platform (searched from 1946 to 1 February 2018) (Appendix 3)
Embase Ovid platform (searched from 1980 to 1 February 2018) (Appendix 4)
CINAHL EBSCO platform (Cumulative Index to Nursing and Allied Health Literature) (searched from 1961 to 1 February 2018) (Appendix 5)
PsycINFO Ovid platform (searched from 1806 to 1 February 2018) (Appendix 6)
The MEDLINE search was limited by the Cochrane highly sensitive search strategy filter for identifying randomised trials which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.1.0, Chapter 6, 6.4.11) (Higgins 2011). The Embase, PsychINFO and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random).
Searching other resources
The following other resources were searched (last search February 2018).
International trial registers: the ClinicalTrials database, a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home) and the World Health Organization International Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx) (Appendix 7; Appendix 8)
Google scholar, using the keywords 'antioxidants male infertility' and 'antioxidants sperm random'
Database for Abstracts of Reviews of Effects (DARE) for other reviews on this topic
'Grey' literature (unpublished and unindexed), through the openGREY database (www.opengrey.eu/) (Appendix 9)
ProQuest Dissertations and Theses (http://search.proquest.com.ezproxy.auckland.ac.nz/pqdtft/advanced?accountid=8424) was also searched (Appendix 10)
Web of Knowledge for conference proceedings and published trials (Appendix 11)
Appropriate journals were handsearched for trial conference abstracts in the year 2017 (not included in CGF search). These journals included Human Reproduction, which contains abstract supplements for the European Society of Human Reproduction and Embryology (ESHRE), and Fertility and Sterility that contains abstract supplements for the 'American Society for Reproductive Medicine' (ASRM).
We handsearched reference lists of relevant trials and systematic reviews retrieved by the search and contacted experts in the field to obtain additional data.
Data collection and analysis
Selection of studies
Review authors RS and RM‐P did an initial screen of titles and abstracts retrieved by the search. The search was conducted by MGS and RS. We retrieved the full texts of all potentially eligible studies. Two review authors (RS and RM‐P) independently examined these full‐text articles for compliance with the inclusion criteria and selected eligible studies. We corresponded with study investigators as required, to clarify study eligibility. Disagreements were resolved by discussion. If any reports required translation, we described the process used for data collection. We documented the selection process with a “PRISMA” flow chart (see Figure 1).
1.
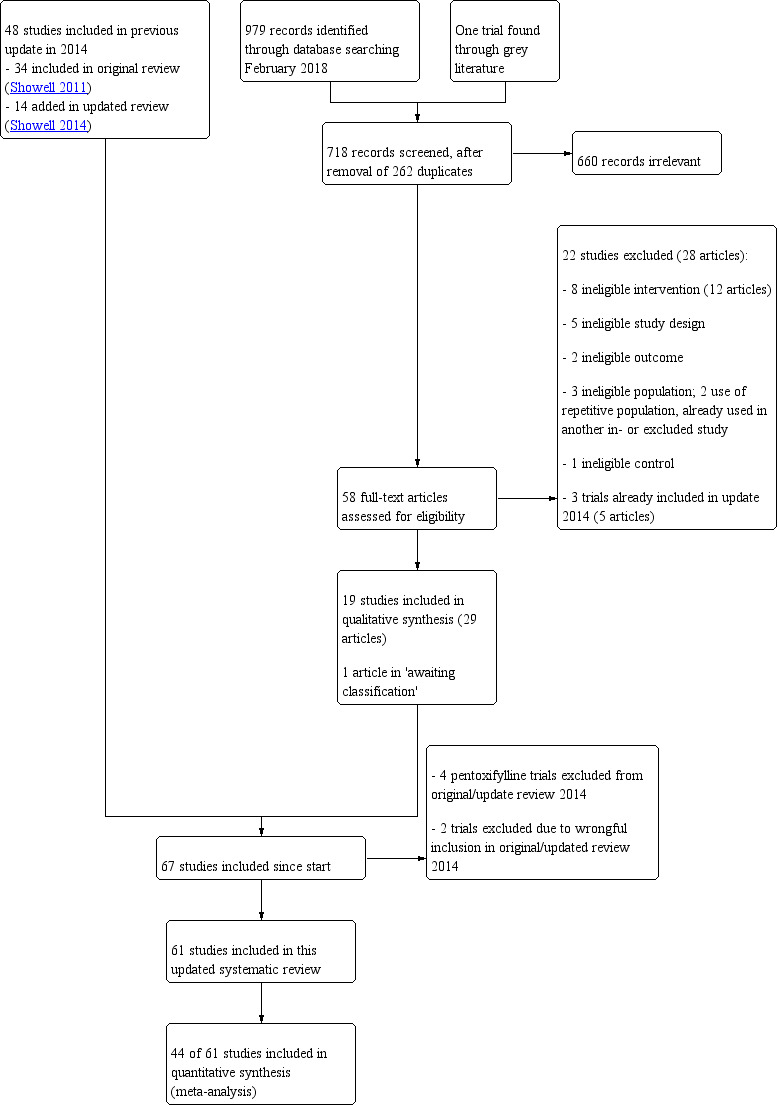
Study flow diagram.
Data extraction and management
Two review authors (RS and RM‐P) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the authors. Any disagreements were resolved by discussion. Data extracted included study characteristics and outcome data (see data extraction table for details, Characteristics of included studies and Characteristics of excluded studies). Where studies had multiple publications, the review authors collated the multiple reports under a single study ID with multiple references.
We corresponded with study investigators for further data on methods and/or results, as required.
Assessment of risk of bias in included studies
Two review authors (RS and RM‐P) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other potential sources of bias (Higgins 2011). Judgements were assigned as recommended in the Cochrane Handbook for Systematic Reviews of Interventions Section 8.5 (Higgins 2011). Disagreements were resolved by discussion; when needed we consulted a third party to achieve agreement (MGS or VJ). We described all judgements fully and present the conclusions in the 'Risk of bias' table (Characteristics of included studies), which is incorporated in the interpretation of review findings by means of sensitivity analyses (see below). We sought published protocols.
We took care to search for within‐study selective reporting, for example, trials failing to report outcomes such as live birth or reporting them in insufficient detail to allow inclusion. Where protocols were available, we assessed studies for differences between study protocols and published results.
In cases where included studies failed to identify the primary outcome of live birth, but did report pregnancy rates, we carried out an informal assessment to determine whether pregnancy rates were similar to those in studies that reported live birth.
We considered that the blinding status of participants could influence findings for the outcomes of live birth, pregnancy and adverse events, as antioxidants are easily available and it would be possible for participants to self‐medicate. Therefore, if the participants were not blinded or the study was not placebo‐controlled, or both, we considered the study to be at high risk of bias.
Measures of treatment effect
We collected dichotomous data for live birth, pregnancy rate, miscarriage and adverse events and for the continuous data for sperm quality measurements we collected mean differences (MDs) and the associated standard deviations (SDs).
Sperm parameter outcomes were analysed at the time points of three, six and nine months post‐randomisation. All studies were analysed in this way regardless of whether the participants were treated for three, six or nine months.
Unit of analysis issues
The primary analysis of the outcomes of live birth, pregnancy and adverse events was per couple randomised, counting multiple births as one live birth event. The sperm outcome analyses were per man randomised. Only the first‐phase data from cross‐over trials were included.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (i.e. including all randomised participants in analyses, in the groups to which they were randomised). Attempts were made to obtain missing data from the original trialists and the results of author contact are reported in Characteristics of included studies. When data were unobtainable, we undertook imputation of individual values for live birth only: live birth was assumed not to have occurred in participants without a reported outcome. For other outcomes, we analysed only the available data. Any imputation undertaken was subjected to sensitivity analysis (see below).
If studies reported sufficient detail to calculate MDs but gave no information on an associated SD, we assumed the outcome to have a SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2. If an I2 was 50% or higher, we assumed high heterogeneity, and conducted a sensitivity analysis. A high I2 statistic suggests that variations in effect estimates may be due to differences between trials rather than to chance alone (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We conducted statistical analysis of the data using Review Manager 5 (RevMan 2014). We expressed the dichotomous data for live birth, pregnancy rate, miscarriage and adverse events as Peto odds ratios (ORs) with 95% confidence intervals (CIs) and combined them in a meta‐analysis with Review Manager 5 software using the Peto method and a fixed‐effect model (Higgins 2011). A random‐effects model was used on sperm outcomes because we suspected high heterogeneity in these outcomes based on the previous review versions. The Peto OR has mathematically sound properties that are consistent with benefit or harm and work well in small samples with rare events. This effect measure is appropriate when considering subfertility. For continuous data (for example sperm quality measurements) MDs between treatment groups were calculated with associated SDs and 95% CIs. The results were displayed on forest plots, where possible.
We considered pregnancy outcomes to be positive, and higher pregnancy rates of benefit. We considered the outcomes of miscarriage and adverse events to be negative effects, and higher numbers harmful. We combined data for the following comparisons.
Antioxidants versus placebo or no treatment
Antioxidants versus antioxidants (head‐to‐head)
Adverse events as reported in the studies were included in the two comparisons above.
The total sperm motility, progressive sperm motility and concentration outcomes were divided into three groups: measurement after starting treatment, at three, six and nine months or more as reported by the studies. Studies were analysed together if they reported these outcomes at the same point in time, for example a study that stopped treatment at three months but measured at six or nine months was measured in the same analysis as those that were treated for six or nine months.
We displayed increases in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. adverse events), graphically in meta‐analyses to the right of the centre line, and decreases in the odds of a particular outcome to the left of the centre line.
The aim was to define analyses that were comprehensive and mutually exclusive, so that we could slot all eligible study results into one stratum only. We specified comparisons so that any studies falling within each stratum could be pooled for meta‐analysis. Stratification allowed for consideration of effects within each stratum, as well as or instead of an overall estimate for comparison.
If individuals had been randomly re‐assigned after failed cycles, we did not pool the data in a meta‐analysis.
Statistical analysis was performed using Review Manager 5.3 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analyses to determine the separate evidence within the following subgroups.
Studies that included different types of antioxidant ((for the outcomes of live birth and clinical pregnancy)
Studies that included couples who were also receiving IVF/ICSI treatment (for the outcomes of live birth and clinical pregnancy)
Studies using no treatment as control group compared to placebo (for outcomes of live birth and clinical pregnancy)
As‐treated analysis
Over time analysis for sperm outcomes of motility and concentration, at three, six and nine months
If we detected substantial heterogeneity, we explored possible explanations in subgroup analyses (e.g. differing populations) and/or sensitivity analyses (e.g. differing risk of bias). We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We conducted sensitivity analyses (using the random‐effects model in RevMan software) on the primary outcomes if we detected a high degree of heterogeneity (I2 = 50% or more), excluding studies to assess if there is a change in effect:
with a high risk of bias, or
enrolling men who are part of a couple undergoing IUI, or
enrolling men with varicocele, or
for studies that reported both live birth and clinical pregnancy rate in order to assess any overestimation of effect and reporting bias, or
for studies where results had been imputed, or
for studies that reported remarkably low SDs as the review authors considered that these data were potentially erroneous (a post hoc sensitivity analysis).
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings; table using GRADEpro (GRADEpro GDT 2015) and Cochrane methods (Higgins 2011). This table evaluates the overall quality of the body of evidence for the main review outcomes (live birth, clinical pregnancy, and the adverse events) for the main review comparison (antioxidant compared with placebo or no treatment). We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate, low or very low) were made by two review authors (RS and RM‐P) working independently, with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome.
We extracted study data, formatted our comparisons in data tables and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
2011 version of review
We assessed 590 abstracts for inclusion from the title and abstract found in a search dated from inception to August 2010. The MEDLINE search produced 406 abstracts; there were six abstracts from CENTRAL, three from CINAHL, 62 from Embase, 107 from the CGF database and three from PsycINFO. Two conference abstracts were found from handsearching the conference proceedings of the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM). One title was found from reference lists in reviews. After removal of inappropriate and duplicate studies, we retrieved the full texts of 53 studies. Five non‐English studies were assessed for inclusion: two Chinese, one Bulgarian, one Japanese and one Iranian. The two Chinese studies (Li 2005; Li 2005a), the Japanese study (Akiyama 1999) and the Iranian study (Peivandi 2010) were included in the analysis. The Bulgarian study (Nikolova 2007) was excluded as it did not use random allocation (see Characteristics of excluded studies). We excluded 15 articles and found four ongoing studies in searches of the clinical trial registers.
A total of 34 studies were included in the 2011 version of the review (Showell 2011).
2014 update
We assessed 483 abstracts for inclusion from the title and abstract found in a search dated from 1 August 2010 to 30 January 2014. After duplicates were removed 338 remained. We assessed 34 of these papers in full text.
Eleven of the full‐text reports assessed studies were in a language other than English and required translation, five of these were in Chinese, two in Persian and one each in Japanese, Russian, Italian, and Portuguese (see Acknowledgements for those who helped with translation). Five of the Chinese studies were excluded: three (Chen 2012; Tang 2011; Wang 2010a) due to an inappropriate intervention, one was not randomised (Wu 2012), and one had an inappropriate population (Lu 2010). The Portuguese study (Verzeletti 2012) was excluded as it used a herbal intervention. Five non‐English studies were included: one in Persian (Eslamian 2013), one Japanese (Kumamoto 1988), one Italian (Morgante 2010), one Russian (Sivkov 2011) and one Chinese (Wang 2010).
We excluded 20 articles, and included 14 articles. An updated search was run in August 2014 where six studies (Anarte 2013; Gopinath 2013; Iacono 2014;Nadjarzadeh 2014; Nashivochnikova 2014; Nematollahi‐Mahani 2014) were placed in 'Studies awaiting assessment'. There were six ongoing studies found in the new searches.
We included 14 new trials in the 2014 update: Attallah 2013; Azizollahi 2013; Dimitriadis 2010; Eslamian 2013; Kumamoto 1988; Martinez‐Soto 2010; Morgante 2010; Nadjarzadeh 2011; Poveda 2013; Pryor 1978; Safarinejad 2011; Safarinejad 2012; Sivkov 2011; Wang 2010.
A total of 48 studies were included in the 2014 update (Showell 2014).
2018 update
We assessed 979 abstracts for inclusion from the title and abstract found in a search dated from January 2014 until February 2018. One extra study was found through the grey literature search. After duplicates were removed, 718 articles remained. We assessed 58 of these papers in full text. One of the full‐text articles assessed studies was in Chinese (Deng 2014) and one in Russian (Gamidov 2017); both required translation. We excluded 22 studies (28 articles), and included 19 studies (29 articles). See the PRISMA flow chart (Figure 1)
Of the new included studies, one was from the six studies placed in 'Awaiting classification' in the 2014 update of this review (Gopinath 2013). The remaining studies awaiting classification were all found ineligible after screening of title and abstract or excluded after reading the full text.
In the current update, none of the eight previously 'ongoing studies' were included. Five of these ongoing studies were found ineligible after screening of title and abstract or excluded after reading the full text. Three studies remained as 'ongoing studies' (CTRI/2013/02/003431; NCT00975115; NCT01828710) with the status of still recruiting. We added nine new ongoing studies (DRKS00011616; IRCT2016111830947N1; IRCT2017012432153N1; NCT01407432; NCT01846325; NCT02310087; NCT02421887; NCT03104998; NCT03337360). In this 2018 update, a total of 12 studies are classified as 'ongoing studies' (Characteristics of ongoing studies).
We removed and excluded four pentoxifylline studies that were previously included in the 2014 update and the original review (Merino 1997; Micic 1988; Safarinejad 2011; Wang 1983). Furthermore, we removed two previously included studies due to the discovery that the population did not meet the inclusion criteria: they included men with idiopathic infertility with normal sperm parameters, and no male factor infertility. (Ciftci 2009; Keskes‐Ammar 2003).
We included 19 new trials in this update: Barekat 2016; Blomberg Jensen 2018; Boonyarangkul 2015; Busetto 2018; Cyrus 2015; Deng 2014; Ener 2016; Exposito 2016; Gamidov 2017; Gopinath 2013; Haghighian 2015; Haje 2015; Martinez 2015; Mehni 2014; Micic 2017; Pourmand 2014; Raigani 2014; Sharifzadeh 2016; Sofikitis 2016.
A total of 61 studies have been included in this update (Characteristics of included studies). A total of 59 studies were excluded (Characteristics of excluded studies).
Included studies
Study design and setting
The studies came from 28 different countries. Fourteen studies were from Iran (Azizollahi 2013; Barekat 2016; Cyrus 2015; Eslamian 2013; Haghighian 2015; Mehni 2014; Nadjarzadeh 2011; Peivandi 2010; Pourmand 2014; Raigani 2014; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012; Sharifzadeh 2016). Ten studies were based in Italy (Balercia 2005; Balercia 2009; Biagiotti 2003; Busetto 2018; Cavallini 2004; Galatioto 2008; Lenzi 2003; Lenzi 2004; Lombardo 2002; Morgante 2010). Four studies were from China (Deng 2014; Li 2005; Li 2005a; Wang 2010), three from Japan (Akiyama 1999; Dimitriadis 2010; Kumamoto 1988), and three from the UK (Kessopoulou 1995; Pryor 1978; Scott 1998). Two studies each were from Kuwait (Omu 1998; Omu 2008), Russia (Gamidov 2017; Sivkov 2011), Spain (Exposito 2016; Martinez‐Soto 2010), and the USA (Dawson 1990; Sigman 2006). A single study was set in each of the following countries: Australia (Tremellen 2007), Belgium (Zalata 1998), Canada (Conquer 2000), Denmark (Blomberg Jensen 2018), Egypt (Attallah 2013), France (Greco 2005), Germany (Rolf 1999), Greece (Sofikitis 2016), Hungary (Zavaczki 2003), India (Gopinath 2013), Iraq (Haje 2015), Mexico (Martinez 2015), the Netherlands (Wong 2002), Panama (Poveda 2013), Saudi Arabia (Suleiman 1996), Serbia (Micic 2017), Thailand (Boonyarangkul 2015), Tunisia (Nozha 2001) and Turkey (Ener 2016).
All included studies were randomised. Five studies had a randomised cross‐over design (Akiyama 1999; Kessopoulou 1995; Lenzi 2003; Peivandi 2010; Pryor 1978). In the meta‐analysis only the first phase data were used as all studies reported first and second phase data separately. The remaining 56 studies used a randomised parallel group design. One study (Li 2005) had a large imbalance between the intervention and control groups at the randomisation stage; 150 men were randomised, 90 into the treatment group and 60 into the control group. This appeared to be a blocked 3:2 allocation ratio. This method of randomisation was not explained in the report. Attempts were made to contact the author but there has been no reply. Thirteen studies (Biagiotti 2003; Cavallini 2004; Conquer 2000; Dawson 1990; Gamidov 2017; Gopinath 2013; Kumamoto 1988; Martinez 2015; Mehni 2014; Raigani 2014; Scott 1998; Sofikitis 2016; Zalata 1998) were three‐armed and eight (Azizollahi 2013; Balercia 2005; Boonyarangkul 2015; Haje 2015; Omu 2008; Poveda 2013; Safarinejad 2009; Wong 2002) were four‐armed.
The duration of the treatment period ranged from three weeks with a three‐week follow up (Dawson 1990) to 12 months treatment (Ener 2016). The longest follow‐up periods were in the studies by Blomberg Jensen and Safarinjad with respectively a five‐month (Blomberg Jensen 2018) and six and a half‐month (Safarinejad 2009a) treatment duration and both with 14 months of follow‐up. Seven studies reporting on either live birth rate or clinical pregnancy rate, only mentioned follow‐up consultations during their treatment, however they did not report the length of follow‐up after treatment (Azizollahi 2013; Attallah 2013; Barekat 2016; Busetto 2018; Kessopoulou 1995; Omu 1998; Suleiman 1996).
Funding sources were stated by 23 studies (Barekat 2016; Blomberg Jensen 2018; Busetto 2018; Conquer 2000; Deng 2014; Eslamian 2013; Haghighian 2015; Kessopoulou 1995; Lenzi 2003; Lombardo 2002; Martinez‐Soto 2010; Mehni 2014; Micic 2017; Nadjarzadeh 2011; Omu 1998; Peivandi 2010; Poveda 2013; Raigani 2014; Rolf 1999; Safarinejad 2012; Sharifzadeh 2016; Wang 2010; Zavaczki 2003). Five of these studies stated that funding was from a commercial source (Busetto 2018; Conquer 2000; Martinez‐Soto 2010; Micic 2017; Safarinejad 2012), and the remaining 18 obtained funding through non‐commercial avenues or university grants. Five studies reported specifically no funding (Cyrus 2015; Gopinath 2013; Haje 2015; Lombardo 2002; Pourmand 2014). Thirty‐three studies did not mention any funding sources.
Participants
The 61 studies included 6264 subfertile men, 3803 in the intervention groups and 2461 men in the control groups. The age range of the participants was 18 to 65 years. Studies included couples who had attended a fertility clinic, with a fertile partner and had been trying to conceive with regular intercourse for over one year. Most men in the included studies had a deficient level of spermatozoa in the seminal fluid (oligospermia) or a low motility of sperm in the seminal fluid (asthenospermia). Two studies also included fertile (Wong 2002) or normospermic men (Exposito 2016) with subgroup analysis. Studies excluded men with any inflammatory disease, antibody problems or chromosomal problems; and most studies stated that they did not enrol men who smoked, took any additional medication or drank alcohol.
Two studies enrolled men with varicocele (Busetto 2018; Cavallini 2004), six studies enrolled men post‐varicocelectomy (Azizollahi 2013; Barekat 2016; Cyrus 2015; Ener 2016; Gamidov 2017; Pourmand 2014), and one study enrolled men with chronic prostatitis (Sivkov 2011). Four studies (Exposito 2016; Kessopoulou 1995; Sigman 2006; Tremellen 2007) enrolled men who, as part of a couple, were undergoing in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI), and one study (Attallah 2013) enrolled men who were part of a couple undergoing intrauterine insemination (IUI).
Further details of inclusion and exclusion criteria are available in Characteristics of included studies.
Interventions
A wide variety of antioxidants were used in the included studies. Comparisons covered antioxidants versus placebo or no treatment and head‐to‐head comparisons (antioxidant versus antioxidant)
The comparison 'antioxidants versus placebo or no treatment' included the following antioxidants: arginine, carnitines (L‐carnitine, L‐acetyl carnitine, L‐carnitine plus L‐acetyl carnitine), carotenoids (β‐carotene), coenzyme Q10 (CoQ10), cysteines (ethylcysteine and N‐acetylcysteine (NAC)), folic acid, magnesium, polyunsaturated fatty acids (PUFAs) (alpha‐lipoic‐acid and docosahexaenoic acid (DHA)), resveratrol, selenium, vitamin B, vitamin C, vitamin D with calcium, vitamin E and zinc. Combined antioxidants were used in 10 studies. They were labelled as Proxeed Plus (Busetto 2018; Micic 2017), Menevit (Tremellen 2007), Selznic (Sivkov 2011), SpermActin‐forte (Gamidov 2017) and Spermotrend (Poveda 2013). Four of these 10 studies used combined antioxidants without any brand name or labelling; "N‐acetylcysteine (NAC) with vitamins and micronutrients" (Galatioto 2008), selenium plus vitamin A/C/E (Scott 1998), a fixed dose combination (FDC) of coenzyme Q10, L‐carnitine, lycopene and zinc (Gopinath 2013), and "essential fatty acid (EFA) mixture combined with α‐tocopherol (vitamin E) and β‐carotene, acetylcysteine and other antioxidants" (Zalata 1998).
The second comparison, head‐to‐head, included seven studies. The head‐to‐head comparisons were included in an attempt to assess whether one antioxidant was more effective than another.They looked at effects of ethylcysteine versus vitamin E, zinc versus folic acid versus zinc plus folic acid, L‐carnitine versus L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine, vitamin E plus selenium versus vitamin B, L‐carnitine plus acetyl‐L‐carnitine versus vitamin E plus vitamin C, L‐carnitine versus vitamin E plus vitamin C, vitamin D plus calcium versus vitamin C plus vitamin E, L‐carnitine plus vitamin E versus vitamin E, acetyl‐cysteine versus essential fatty acid (EFA) plus α‐tocopherol (vitamin E) plus β‐carotene versus acetylcysteine plus EFA plus antioxidants.
In summary:
26/61 studies compared antioxidants with placebo;
7/61 studies compared antioxidants with no treatment;
7/61 studies compared one antioxidant with another antioxidant (head‐to‐head);
21/61 multi‐arm studies: 16 of these compared antioxidants versus placebo and five compared antioxidants versus no treatment.
Outcomes
The primary outcome for this review was as follows.
Live birth per couple. Seven studies reported data for live birth in the antioxidant versus placebo or no treatment comparison (Balercia 2005; Balercia 2009; Blomberg Jensen 2018; Kessopoulou 1995; Omu 1998; Suleiman 1996; Tremellen 2007). One of these studies could also be included in the head‐to‐head comparison of live birth rate (Balercia 2005).
Secondary outcomes for this review were as follows.
Clinical pregnancy rate per couple, as reported by 11 studies in the antioxidant versus placebo or no treatment comparison (Attallah 2013; Azizollahi 2013; Balercia 2005; Balercia 2009; Barekat 2016; Busetto 2018; Kessopoulou 1995; Omu 1998; Suleiman 1996; Tremellen 2007; Zavaczki 2003). One of these studies could also be included in the head‐to‐head comparison of clinical pregnancy rate (Balercia 2005); one more study in the head‐to‐head comparison reported on clinical pregnancy rate (Deng 2014). Data for biochemical and undefined pregnancy can be seen in Table 2.
1. Data for undefined or biochemical pregnancy.
| Undefined or biochemical pregnancy | Antioxidant | Control | Peto OR [CI] | ||
| Antioxidant(s) versus placebo or no treatment | |||||
| Combined antioxidants | Events | Total | Events | Total | |
| Galatioto 2008 | 1 | 20 | 0 | 22 | 8.17 [0.16 to 413.39] |
| Gopinath 2013 | 13 | 92 | 2 | 46 | 2.72 [0.88 to 8.46] |
| Arginine | |||||
| Pryor 1978 | 2 | 35 | 2 | 29 | 0.82 [0.11 to 6.16] |
| Carnitines | 25 | 154 | 3 | 145 | |
| Sigman 2006 | 1 | 12 | 1 | 9 | 0.74 [0.04 to 13.02] |
| Peivandi 2010 | 3 | 15 | 0 | 15 | 8.57 [0.82 to 89.45] |
| Lenzi 2004 | 4 | 30 | 0 | 26 | 7.20 [0.95 to 54.34] |
| Lenzi 2003 | 6 | 43 | 0 | 43 | 8.37 [1.61 to 43.58] |
| Cavallini 2004 | 9 | 39 | 1 | 47 | 7.50 [2.01 to 27.98] |
| Coenzyme Q10 | 6 | 136 | 3 | 136 | |
| Safarinejad 2009a | 0 | 106 | 0 | 106 | Not estimable |
| Balercia 2009 | 6 | 30 | 3 | 30 | 2.16 [0.53 to 8.82] |
| Nadjarzadeh 2011 | 0 | 23 | 0 | 24 | Not estimable |
| Vitamin C + Vitamin E | |||||
| Rolf 1999 | 0 | 15 | 0 | 16 | Not estimable |
| Vitamin E | |||||
| Ener 2016 | 5 | 28 | 5 | 28 | 1.00 [0.26 to 3.88] |
| Head‐to‐head antioxidant(s) | Events | Total | Events | Total | |
| L‐acetyl carnitine + L‐carnitine vs Vitamin E + Vitamin C | |||||
| Li 2005 | 10 | 85 | 2 | 53 | 2.72 [0.81 to 9.14] |
| L‐carnitine + Vitamin E versus Vitamin E | |||||
| Wang 2010 | 21 | 68 | 3 | 67 | 6.01 [2.49 to 14.47] |
Adverse events (miscarriage, gastrointestinal upsets, euphoria and ectopic pregnancy) were reported by 13 studies (Busetto 2018; Cavallini 2004; Gamidov 2017; Gopinath 2013; Kessopoulou 1995; Omu 1998; Pourmand 2014; Safarinejad 2009a; Sharifzadeh 2016; Sigman 2006; Suleiman 1996; Tremellen 2007; Zavaczki 2003) in the antioxidant versus placebo or no treatment comparison. Adverse events were not reported as an outcome in any of the studies in the head‐to‐head comparisons, except that the study by Li (Li 2005) reported that no side effects were found in either the treatment or control groups.
DNA fragmentation was reported by six studies (Barekat 2016; Boonyarangkul 2015; Gamidov 2017; Greco 2005; Martinez‐Soto 2010; Raigani 2014), comparing antioxidants versus placebo or no treatment. Data from two studies were not usable because of the use of COMET assay and DNA tail length (Boonyarangkul 2015), or use of medians with interquartile ranges (Gamidov 2017)(Analysis 1.10). This outcome was not reported in the head‐to‐head comparison.
Total sperm motility at three months or less was reported by 16 studies in the antioxidants versus placebo or no treatment comparison (Azizollahi 2013; Balercia 2005; Barekat 2016; Conquer 2000; Dimitriadis 2010; Ener 2016; Gopinath 2013; Greco 2005; Martinez‐Soto 2010; Morgante 2010; Nadjarzadeh 2011; Omu 2008; Peivandi 2010; Scott 1998; Sigman 2006; Zavaczki 2003), by eight studies in the head‐to‐head comparison (Akiyama 1999; Azizollahi 2013; Balercia 2005; Conquer 2000; Dawson 1990; Li 2005; Omu 2008; Scott 1998).
Total sperm motility at six months was reported by 13 studies in the antioxidants versus placebo or no treatment comparison (Azizollahi 2013; Balercia 2005; Balercia 2009; Blomberg Jensen 2018; Busetto 2018; Ener 2016; Gopinath 2013; Lenzi 2004; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012; Sigman 2006; Suleiman 1996). Three studies reported this in the head‐to‐head comparison (Azizollahi 2013; Balercia 2005; Safarinejad 2009).
Total sperm motility at nine months or more was reported by five studies in the antioxidants versus placebo or no treatment comparison (Balercia 2005; Balercia 2009; Ener 2016; Safarinejad 2009a; Safarinejad 2012). One study reported this in the head‐to‐head comparison (Balercia 2005).
Progressive sperm motility at three months or less was reported by 14 studies in the antioxidants versus placebo or no treatment comparison (Attallah 2013; Azizollahi 2013; Balercia 2005; Boonyarangkul 2015; Cyrus 2015; Dawson 1990; Haghighian 2015; Martinez‐Soto 2010; Mehni 2014; Morgante 2010; Nadjarzadeh 2011; Peivandi 2010; Rolf 1999; Sharifzadeh 2016) Five studies reported this in the head‐to‐head comparison (Balercia 2005; Deng 2014; Li 2005; Li 2005a; Wang 2010).
Progressive sperm motility at six months was reported by five studies in the antioxidants versus placebo or no treatment comparison (Azizollahi 2013; Balercia 2005; Balercia 2009; Blomberg Jensen 2018; Boonyarangkul 2015). One study reported this in the head‐to‐head comparison (Balercia 2005).
Progressive sperm motility at nine months or more was reported by two studies in the antioxidants versus placebo or no treatment comparison (Balercia 2005; Balercia 2009). One study reported this in the head‐to‐head comparison (Balercia 2005).
Sperm concentration at three months or less was reported by 21 studies in the antioxidants versus placebo or no treatment comparison (Attallah 2013; Azizollahi 2013; Balercia 2005; Barekat 2016; Boonyarangkul 2015; Conquer 2000; Cyrus 2015; Dimitriadis 2010; Ener 2016; Gopinath 2013; Greco 2005; Haghighian 2015; Martinez‐Soto 2010; Mehni 2014; Morgante 2010; Nadjarzadeh 2011; Peivandi 2010; Rolf 1999; Scott 1998; Sharifzadeh 2016; Zavaczki 2003), and seven in the head‐to‐head comparison (Akiyama 1999; Azizollahi 2013; Balercia 2005; Conquer 2000; Li 2005a; Scott 1998; Wang 2010) .
Sperm concentration at six months was reported as an outcome by 11 studies in the antioxidants versus placebo or no treatment comparison (Azizollahi 2013; Balercia 2005; Balercia 2009; Boonyarangkul 2015; Busetto 2018; Ener 2016; Gopinath 2013; Lenzi 2004; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012), and three studies in the head‐to‐head comparison (Azizollahi 2013; Balercia 2005; Safarinejad 2009) .
Sperm concentration at nine months or more was reported by five studies in the antioxidants versus placebo or no treatment comparison (Balercia 2005; Balercia 2009; Ener 2016; Safarinejad 2009a; Safarinejad 2012), and one study in the head‐to‐head comparison (Balercia 2005).
1.10. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 10 Sperm DNA fragmentation (data not suitable for meta‐analysis).
| Sperm DNA fragmentation (data not suitable for meta‐analysis) | |||
|---|---|---|---|
| Study | Intervention | Control | P‐value |
| Folic acid | |||
| Boonyarangkul 2015 |
Folic acid DNA tail length, COMET assay 3 month: Mean = 4.04 (n = 15) SE = 0.94 6 month: Mean = 6.01 SE = 1.49 |
Placebo DNA tail length, COMET assay 3 month: Mean = 10.08 (n = 15) SE = 3.39 6 month: Mean = 8.69 SE = 4.28 |
Not provided |
| Combined antioxidants | |||
| Gamidov 2017 |
SpermActin‐forte (acetyl‐L‐carnitine, L‐carnitine fumarate and alpha‐lipoic acid) Median = 24 (18.2 ‐ 28.6) (n = 38) Median (interquartile range) |
No treatment Median 20.3 (12.7 ‐ 21.5) (n = 38) Median (interquartile range) |
Not provided |
| Gamidov 2017 |
SpermActin‐forte + Vitamin complex 'Man's formula' Median = 25 (20.5 ‐ 29.2) (n = 38) Median (interquartile range) |
No treatment Median 20.3 (12.7 ‐ 21.5) (n = 38) Median (interquartile range) |
Not provided |
Data were extracted from 44 of the included studies. The 17 remaining studies either did not report any data or the continuous data were reported in medians or ranges (Biagiotti 2003; Eslamian 2013; Exposito 2016; Galatioto 2008; Haje 2015; Kumamoto 1988; Lenzi 2003; Lombardo 2002; Martinez 2015; Micic 2017; Nozha 2001; Poveda 2013; Pryor 1978; Sivkov 2011; Sofikitis 2016; Wong 2002; Zalata 1998). Another study reported data for a treatment duration of three to six months, but did not specify this any further and therefore data could not be used in the meta‐analysis (Haje 2015).
See Characteristics of included studies and the analyses 'data not usable for meta‐analysis'(Analysis 1.10; Analysis 1.12; Analysis 1.14; Analysis 1.18; Analysis 1.20; Analysis 1.24; Analysis 1.26; Analysis 2.5; Analysis 2.12). Table 3 also described the outcomes and conclusions of all included studies. Attempts were made to contact all authors of the included studies for further details and clarification.
1.12. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 12 Total sperm motility at 3 months or less (data not suitable for meta analysis).
| Total sperm motility at 3 months or less (data not suitable for meta analysis) | |||
|---|---|---|---|
| Study | Intervention | Control | P value |
| Carnitines | |||
| Cavallini 2004 |
L‐carnitine + Acetyl‐carnitine Median = 22.3 (n = 39) Interquartile range = 28.4 ‐ 15.2 |
Placebo Median = 14.0 (n = 47) Interquartile range = 17.4 ‐ 5.1 |
Not provided |
| Folic acid | |||
| Raigani 2014 |
Folic acid Median = 35 (15 ‐ 50) (n = 20) Median (25th ‐ 75h percentile) 16 weeks |
Placebo Median = 35 (21 ‐ 42.5) (n = 18) Median (25th ‐ 75h percentile) 16 weeks |
Not provided |
| Folic acid + Zinc | |||
| Raigani 2014 |
Folic acid + Zinc Median = 35 (26.3 ‐ 50) (n = 21) Median (25th ‐ 75h percentile) 16 weeks |
Placebo Median = 35 (21 ‐ 42.5) (n = 18) Median (25th ‐ 75h percentile) 16 weeks |
Not provided |
| Vitamin E | |||
| Kessopoulou 1995 |
Vitamin E Median = 7 (n = 15) Min/max = ‐27 ‐ 34 |
Placebo Median = 7 (n = 15) Min/max = ‐33 ‐ 36 |
Not provided |
| Zinc | |||
| Raigani 2014 |
Zinc Median = 35 (17 ‐ 50) (n = 24) Median (25th ‐ 75h percentile) 16 weeks |
Placebo Median = 35 (21 ‐ 42.5) (n = 18) Median (25th ‐ 75h percentile) 16 weeks |
Not provided |
| Combined antioxidants | |||
| Galatioto 2008 |
N‐acetylcysteine (NAC) 600 mg + vitamins‐minerals % of motile sperm (Class A WHO) = 58% (n = 20) |
No treatment % of motile sperm (Class A WHO) = 51% (n = 22) |
P = 0.847 |
1.14. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 14 Total sperm motility at 6 months(data not suitable for meta analysis).
| Total sperm motility at 6 months(data not suitable for meta analysis) | |||
|---|---|---|---|
| Study | Intervention | Control | P value |
| Carnitines | |||
| Cavallini 2004 |
L‐carnitine + Acetyl‐carnitine Median = 23.6 (n = 39) Interquartile range = 28.9 ‐ 16.0 |
Placebo Median = 13.2 (n = 47) Interquartile range = 18.6 ‐ 9.0 |
Not provided |
| Folic acid | |||
| Wong 2002 |
Folic acid Median = 35 (n = 22) Range = 5 ‐ 65 |
Placebo Median = 30 (n = 25) Range = 5 ‐ 80 |
Not provided |
| Zinc | |||
| Wong 2002 |
Zinc Median = 35 (n = 23) Range = 10 ‐ 65 |
Placebo Median = 30 (n = 25) Range = 5 ‐ 80 |
Not provided |
| Zinc + Folic acid | |||
| Wong 2002 |
Zinc + Folic acid Median = 35 (n = 24) Range 5 ‐ 70 |
Placebo Median = 30 (n = 25) Range = 5 ‐ 80 |
Not provided |
| Combined antioxidants | |||
| Micic 2017 |
Proxeed Plus Median = 31.0 (20.0 ‐ 41.0) (n = 125 ) Median (interquartile range) Progressive sperm motility |
Placebo Median 29.0 (15.5 ‐ 35.5) (n = 50) Median (interquartile range) Progressive sperm motility |
Not provided |
1.18. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 18 Progressive sperm motility at 3 months (data not usable for meta‐analysis).
| Progressive sperm motility at 3 months (data not usable for meta‐analysis) | |||
|---|---|---|---|
| Study | Intervention | Control | P value |
| Combined antioxidants | |||
| Gamidov 2017 |
SpermActin‐forte + Vitamin complex 'Man's formula' Median = 36.5 (26 ‐ 47) (n = 38 ) Median (interquartile range) |
No treatment Median = 34.5 (27 ‐ 40) (n = 38) Median (interquartile range) |
Not provided |
| Gamidov 2017 |
SpermActin‐forte (acetyl‐L‐carnitine, L‐carnitine fumarate and alpha‐lipoic acid) Median = 30.5 (26 ‐ 37) (n = 38) Median (interquartile range) |
No treatment Median = 34.5 (27 ‐ 40) (n = 38) Median (interquartile range) |
Not provided |
| Micic 2017 |
Proxeed Plus Median = 30.0 (12.0 ‐ 39.0) (n = 125) Median (interquartile range) |
Placebo Median 28.5 (11.5 ‐ 32.0) (n = 50) Median (interquartile range) |
Not provided |
1.20. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 20 Progessive sperm motility at 6 months (data not usable for meta‐analysis).
| Progessive sperm motility at 6 months (data not usable for meta‐analysis) | |||
|---|---|---|---|
| Study | Intervention | Control | P value |
| Combined antioxidants | |||
| Micic 2017 |
Proxeed Plus Median = 31.0 (20.0 ‐ 41.0) (n = 125) Median (interquartile range) |
Placebo Median 29.0 (15.5 ‐ 35.5) (n = 50) Median (interquartile range) |
Not provided |
1.24. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 24 Sperm concentration at 3 months or less (data not suitable for meta analysis).
| Sperm concentration at 3 months or less (data not suitable for meta analysis) | |||
|---|---|---|---|
| Study | Intervention | Control | P value |
| Carnitines | |||
| Cavallini 2004 |
L‐carnitine + Acetyl‐carnitine Median = 20.9 (n = 39) Interquartile range = 25.6 ‐ 14.8 |
Placebo Median = 12.3 (n = 47) Interquartile range = 16.0 ‐ 9.1 |
Not provided |
| Lenzi 2003 |
L‐carnitine Mean = 9 (1st phase data) (n = 43) No SD given |
Placebo Mean = 5.3 (n = 43) No SD given |
P = 0.03 |
| Vitamin E | |||
| Kessopoulou 1995 |
Vitamin E Median = ‐15 (n = 15) Min/max = ‐58 ‐ 59 |
Placebo Median = 0 (n = 15) Min/max = ‐37 ‐ 160 |
Not provided |
| Folic acid | |||
| Raigani 2014 |
Folic acid Median 15 (9.7 ‐ 24) (n = 20) Median (25th ‐ 75th percentile) 16 weeks |
Placebo Median 12 (7.5 ‐ 27.3) Median (25th ‐ 75th percentile) 16 weeks |
Not provided |
| Zinc | |||
| Raigani 2014 |
Zinc Median 13.2 (7 ‐ 27) (n = 24) Median (25th ‐ 75th percentile) 16 weeks |
Placebo Median 12 (7.5 ‐ 27.3) Median (25th ‐ 75th percentile) 16 weeks |
Not provided |
| Folic acid + Zinc | |||
| Raigani 2014 |
Folic acid + Zinc Median 10.5 (8.06 ‐ 17.7) (n = 21) Median (25th ‐ 75th percentile) 16 weeks |
Placebo Median 12 (7.5 ‐ 27.3) Median (25th ‐ 75th percentile) 16 weeks |
Not provided |
| Combined antioxidants | |||
| Gamidov 2017 |
SpermActin‐forte (acetyl‐L‐carnitine, L‐carnitine fumarate and alpha‐lipoic acid) Median = 26.5 (2.3 ‐ 48) Median (interquartile range) |
No treatment Median = 22 (11.5 ‐ 26.6) Median (interquartile range) |
Not provided |
| Gamidov 2017 |
SpermActin‐forte + Vitamin complex 'Man's formula' Median = 23.5 (10 ‐34.5) (n = 38) Median (interquartile range) |
No treatment Median = 22 (11.5 ‐ 26.6) (n = 38) Median (interquartile range) |
Not provided |
1.26. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 26 Sperm concentration at 6 months(data not suitable for meta analysis).
| Sperm concentration at 6 months(data not suitable for meta analysis) | |||
|---|---|---|---|
| Study | Intervention | Control | P value |
| Carnitines | |||
| Cavallini 2004 |
L‐carnitine + Acetyl‐carniitne Median = 20.6 (n = 39) Interquartile range = 24.9 ‐ 15.1 |
Placebo Median = 10.9 (n = 47) Interquartile range = 15.1 ‐ 9.0 |
Not provided |
| Folic acid | |||
| Wong 2002 |
Folic acid Median = 14 (n = 22) Range = 0.9 ‐ 130 |
Placebo Median = 9 (n = 25) Range = 0.8 ‐ 80 |
Not provided |
| Zinc | |||
| Wong 2002 |
Zinc Median = 16 (n = 23) Range = 0.6 ‐ 80 |
Placebo Median = 9 (n = 25) Range = 0.8 ‐ 80 |
Not provided |
| Zinc + Folic acid | |||
| Wong 2002 |
Zinc + Folic acid Median = 12 (n = 24) Range = 0.5 ‐ 180 |
Placebo Median = 9 (n = 25) Range = 0.8 ‐ 80 |
Not provided |
| Vitamin D + Calcium | |||
| Blomberg Jensen 2018 |
Vitamin D + Calcium Median = 12.8 (n = 133) 25th, 75th percentiles = 3.4, 32.3 At 5 months. |
Placebo Median = 13.3 (n = 136) 25th, 75th percentiles = 4.2, 38.5 At 5 months |
Not provided |
2.5. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 5 Total sperm motility at 6 months (data not suitable for meta analysis).
| Total sperm motility at 6 months (data not suitable for meta analysis) | |||
|---|---|---|---|
| Study | Antioxidant A | Antioxidant B | P value |
| Folic acid vs Zinc + Folic acid | |||
| Wong 2002 |
Folic acid Median = 35 Range = 5 ‐ 65 "Forward motile sperm" |
Zinc + Folic acid Median = 35 Range = 5 ‐ 70 "Forward motile sperm" |
Not provided |
| Zinc vs Folic acid | |||
| Wong 2002 |
Zinc Median = 35 Range = 10 ‐ 65 "Forward motile sperm" |
Folic acid Median = 35 Range = 5 ‐ 65 "Forward motile sperm" |
Not provided |
| Zinc vs Zinc + Folic acid | |||
| Wong 2002 |
Zinc Median = 35 Range = 10 ‐ 65 "Forward motile sperm" |
Zinc + Folic acid Median = 35 Range = 5 ‐ 70 "Forward motile sperm" |
Not provided |
2.12. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 12 Sperm concentration at 6 months (data not suitable for meta analysis).
| Sperm concentration at 6 months (data not suitable for meta analysis) | |||
|---|---|---|---|
| Study | Antioxidant A | Antioxidant B | P value |
| Zinc vs Folic acid | |||
| Wong 2002 |
Zinc Median = 16 Range = 0.6 ‐ 80 |
Folic acid Median = 14 Range = 0.9 ‐ 130 |
Not provided |
| Zinc vs Zinc + Folic acid | |||
| Wong 2002 |
Zinc Median = 16 Range = 0.6 ‐ 80 |
Zinc + Folic acid Median = 12 Range = 0.5 ‐ 180 |
Not provided |
| Folic acid vs Zinc + Folic acid | |||
| Wong 2002 |
Folic acid Median = 14 Range = 0.9 ‐ 130 |
Zinc + Folic acid Median = 12 Range = 0.5 ‐ 180 |
Not provided |
2. Outcomes and conclusions from all included studies.
| Study ID | Design, population | Outcomes described in methods section | Outcomes reported on in results | In meta‐analysis Y or N | Results |
Conclusions + = positive effect ‐ = negative or no effect |
| Akiyama 1999 | Cross‐over, head‐to‐head Infertile men, high ROS levels N = 10 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | Ethylcystein did not improve sperm density and motility but "sperm function" increased and ROS levels decreased, compared to vitamin E | + Ethylcysteine shown to be effective for improvement of sperm parameters when compared to vitamin E |
| Attallah 2013 | Parallel, no treatment Idiopathic athenozospermia, IUI N = 30 Conference abstract |
Sperm parameters, chemical and clinical pregnancy | Sperm parameters, chemical and clinical pregnancy | Y ‐ sperm parameters Y ‐ pregnancy rate, clinical |
NAC increased sperm concentration and motility Clinical pregnancy was not significantly different between the groups |
+ NAC improves semen quality and improves pregnancy rates prior to IUI, no improvement of pregnancy rate |
| Azizollahi 2013 | Multiple arm trial Men post‐varicocelectomy N = 160 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters Y ‐ pregnancy rate, clinical |
Mild improvement in sperm parameters with the use of antioxidants zinc, folic acid or both | + Co‐administration of zinc and folic acid improved sperm parameters and increased varicocelectomy outcomes, only zinc an improvement in pregnancy rate |
| Balercia 2005 | Multiple arm, placebo Infertile men N = 60 |
Sperm parameters | Sperm parameters, pregnancy rate | Y ‐ sperm parameters Y ‐ pregnancy rate, clinical Y ‐ live birth |
Improvement in motility in LAC group. | + Long‐term carnitine is effective in increasing sperm motility. No evidence of increased live birth or clinical pregnancy. |
| Balercia 2009 | Parallel, placebo Infertile and unexplained N = 60 |
Sperm parameters | Sperm parameters, pregnancy rate | Y ‐ sperm parameters Y ‐ pregnancy rate, clinical |
Co enzyme Q10 increased sperm motility. | + Q10 effective in improving sperm kinetic features in asthenospermia. No evidence of increased live birth or clinical pregnancy. |
| Barekat 2016 | Parallel, no treatment Subfertile men with varicocele N = 40 |
Sperm parameters, DNA fragmentation | Sperm parameters, DNA fragmentation, clinical spontaneous pregnancies | Y ‐ sperm parameters Y ‐ DNA fragmentation Y ‐ pregnancy rate, clinical (SEs converted to SDs) |
Sperm parameters significantly improved after surgery compared to before surgery in both the NAC and control groups. NAC might have an additional value by improving sperm motility post‐varicocelectomy | + The results of this study revealed that NAC improved chromatin integrity and pregnancy rate when administered as adjunct therapy post‐varicocelectomy |
| Biagiotti 2003 | Multiple arm, no treatment Severe idiopathic oligoasthenospermia N = 42 Conference abstract |
Sperm parameters | Sperm parameters | N ‐ no data available | A significant improvement in morphology concentration, motility in the carnitine group No side effects |
+ Quality of semen is positively associated with fertilisation and implantation rates in assisted reproduction |
| Blomberg Jensen 2018 | Parallel, placebo Infertile men with impaired semen quality N = 307 |
Sperm parameters, reproductive hormones, live birth rate | Sperm parameters, reproductive hormones, live birth rate | Y ‐ sperm parameters Y ‐ live birth rate |
Vitamin D was not associated with changes in semen parameters, although spontaneous pregnancies tended to be higher in couples in which the man was in the treatment group | ± Vitamin D did not improve semen quality. The positive impact of vitamin D supplementation on live birth rate and serum inhibin B in oligozoospermic and vitamin D–deficient men may be of clinical importance and warrant verification by others. |
| Boonyarangkul 2015 | Multiple arm, placebo, tamoxifen excluded Men with abnormal semen analysis N = 68 |
Sperm parameters, DNA damage (Comet assay) | Sperm parameters, DNA tail length | Y ‐ sperm parameters | Folate alone significantly decreased DNA tail length at 3‐months. Sperm motility was significantly increased after 3‐months Folate alone. | + Our study indicated that folate in combination with Tamoxifen citrate could improve sperm quality including semen parameters and sperm DNA integrity |
| Busetto 2018 | Parallel, placebo Infertile men with OAT, 50% included with varicocele N = 104 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | Y ‐ sperm parameters Y ‐ pregnancy rate, clinical |
Sperm concentration, total sperm count, progressive and total motility were significantly increased in supplemented (Proxeed Plus) patients. Increased pregnancy rate | + Supplementation with metabolic and antioxidant compounds could be efficacious when included in strategies to improve fertility |
| Cavallini 2004 | Multiple arm, placebo Idiopathic OAT men with varicocele N = 325 |
Sperm parameters, pregnancy rate, adverse events | Sperm parameters, pregnancy rate, adverse events | N ‐ sperm parameters, only medians given in full text. Means in conference abstract but no data given for placebo group and data for group 3 (carnitine + cinoxacin) versus group 2 (carnitines) unable to be used as 3 includes cinoxacin an anti‐inflammatory drug. Analysis 1.12; Analysis 1.14; Analysis 1.24; Analysis 1.26 N ‐ pregnancy rate, unclear if clinical Table 2 Y ‐ adverse events |
Significant increase in sperm parameters for carnitines when compared to placebo. Carnitine groups had a significantly higher pregnancy rate than placebo group |
+ The antioxidant plus anti‐inflammatory group was more effective in improving sperm parameters and pregnancy than those of carnitines alone or placebo however carnitines alone were more effective than placebo |
| Conquer 2000 | Multiple arm, placebo Asthenozoospermic men N = 28 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters (SEs converted to SDs) |
DHA showed no effect on sperm motility or concentration | ± DHA supplementation increased DHA levels in the sperm but not motility or concentration |
| Cyrus 2015 | Parallel, placebo Infertile men with varicocele N = 115 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | Vitamin C was not effective on sperm count but improved sperm motility and morphology significantly | + Ascorbic acid can play a role as adjuvant treatment after varicocelectomy in infertile men |
| Dawson 1990 | Multiple arm, placebo Men with sperm agglutination N = 30 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters (SEs converted to SDs) |
The group receiving 1000 mg of AA showed more improvement in parameters than the 200mg group and the placebo | + Vitamin C can improve sperm parameters, especially dosage of 1000 mg. |
| Deng 2014 | Head‐to‐head Men with idiopathic oligoasthenozoospermia N = 86 |
Sperm parameters, adverse reactions, pregnancy rate | Sperm parameters, adverse reactions, pregnancy rate | Y ‐ sperm parameters Y ‐ clinical pregnancy rate |
Vitamin D is a safe option for the treatment of idiopathic oligoasthenozoospermia and can effectively improve the semen quality especially the progressive sperm motility |
+ Vitamin D can improve forward movement sperm number and percentage, improve the woman's clinical pregnancy rate, and is well tolerated |
| Dimitriadis 2010 | Multiple arm, no treatment, vardenafil/sildenafil arms excluded Men with oligoasthenospermia N = 75 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | An improvement in sperm concentration with carnitine versus no treatment | + Enhancement of Leydig cell secretory function may increase sperm concentration and motility |
| Ener 2016 | Parallel, no treatment Infertile men with varicocele N = 56 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | Y ‐ sperm parameters N ‐ pregnancy rate, unknown if clinical Table 2 |
The administration of vitamin E increased all of the parameters; however not statistically significant | ‐ Vitamin E supplementation does not improve the sperm parameters after varicocelectomy |
| Eslamian 2013 | Parallel, placebo Asthenoszoospermic men N = 50 |
Sperm parameters | sperm parameters, sperm membrane and serum fatty acids | N ‐ sperm parameters, data not usable, no continuous data but categories from 'significantly improvement' to 'worsened' | Sperm parameters improved with DHA + vitamin E supplementation | + Sperm parameters improve with DHA + vitamin E supplementation |
| Exposito 2016 | Parallel, placebo Normozoospermig, oligozoospermic and asthenozoospermic men N = 113 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | N ‐ sperm parameters N ‐ pregnancy rate Both not included because data included normospermic men |
50% of oligozoospermic men improved sperm concentration and sperm count to normozoospermic levels. This trend was also observed in asthenozoospermic men, but nog significantly | + Vitamin E treatment by oral administration improves semen parameters |
| Galatioto 2008 | Parallel, no treatment Men with persistent oligospermia after embolisation of varicocele N = 42 |
Sperm parameters, pregnancy rate, adverse events | Sperm parameters, pregnancy rate, adverse events | N ‐ sperm parameters, only medians given N ‐ pregnancy, unclear if clinical Table 2 N ‐ adverse events |
Significant difference in sperm count in combined antioxidant group but not in motility. One pregnancy in the NAC group No significant adverse effects |
± NAC does not improve pregnancy rate, no significant adverse events, but do significantly increase sperm count |
| Gamidov 2017 | Multiple arm, no treatment Men with varicocele N = 114 |
Sperm parameters, DNA fragmentation, adverse events | Sperm parameters, DNA fragmentation, adverse events | N ‐ sperm parameters, only medians with IQR Analysis 1.18; Analysis 1.24; Analysis 1.24 N ‐ DNA fragmentation, only medians with IQR Analysis 1.10 Y ‐ adverse events |
SpermActine (SA) resulted in a 22.3% decrease in the level of sperm DNA fragmentation at 3 months. SA + vitamin complex resulted in a 27% increase in the sperm concentration at 3 months. There were no side effects of pharmacotherapy. | + Antioxidant therapy leads to an improvement in the basic sperm parameters (sperm concentration and motility) and a decrease in the level of sperm DNA fragmentation in the short term. There were no side effects |
| Gopinath 2013 | Multiple arm, placebo Idiopathic OAT men N = 138 |
Sperm parameters, pregnancy rate, adverse events | Sperm parameters, pregnancy rate, adverse events | Y ‐ sperm parameters N ‐ pregnancy rate, not clinical Table 2 Y ‐ adverse events |
Combined antioxidant significantly improved sperm count and total motility in both treatment arms (1 vs 2 tablets). Mild adverse events were reported, no severe. | + Exogenous administration of fixed dose combination of antioxidants is safe and effective therapy in improving the male subfertility regarding sperm parameters. Only mild adverse events when using combined antioxidants |
| Greco 2005 | Parallel, placebo Infertile males with high DNA fragmentation N = 64 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | No significant difference in concentration or motility however DNA fragmentation was significantly reduced in the vitamin C + E when compared to placebo | + A short oral treatment of Vitamin C + E can reduce DNA fragmentation |
| Haghighian 2015 | Parallel, placebo Men with idiopathic asthenozoospermia N = 48 |
Sperm parameters, adverse events | Sperm parameters, adverse events | Y ‐ sperm parameters N ‐ adverse events, reported "none", however not clear which side effects they aimed for |
Sperm parameters were significantly higher in ALA group. No side effects due to the oral administration of ALA were observed in any participants. | + Medical therapy of asthenoteratospermia with ALA supplement could improve quality of semen parameters |
| Haje 2015 | Multiple arm, placebo, tamofixen arms excluded Infertile men with idiopathic OAT N = 128 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | N ‐ sperm parameters, range of treatment 3 ‐ 6 months and not divided N ‐ pregnancy rate, unclear if pregnancy and no numbers but percentage |
L‐carnitine no improvement of sperm count or motility. Only tamoxifen or tamofixen + L‐carnitine improved pregnancy rate, not significantly. | ± Administration of tamoxifen or L‐carnitine can improve sperm parameters and ICSI outcomes. Combining those result in maximum therapeutic effect |
| Kessopoulou 1995 | Cross‐over, placebo Male infertility N = 30 |
Sperm parameters, adverse events, live birth | Sperm parameters, adverse effects, live birth | N ‐ sperm parameters, only medians given Analysis 1.12; Analysis 1.24 Y ‐ pregnancy rate, clinical Y ‐ live births Y ‐ adverse events |
No differences in sperm outcomes were seen between the groups. 1 pregnancy in the vitamin E group and nil in the placebo (first phase data) | + No difference in semen parameters. There is evidence of increased live birth and clinical pregnancy rate. |
| Kumamoto 1988 | Multiple arm, placebo Men with abnormal sperm count or motility N = 396 |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, only scales given | No statistical difference in sperm outcomes in vitamin B 12 groups or placebo | ‐ No improvement in sperm parameters after use of vitamin B12 |
| Lenzi 2003 | Cross‐over, placebo Infertile men with OAT N = 100 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | Y ‐ sperm parameters N ‐ pregnancy rate, no definition of pregnancy given see Table 2 |
The patient groups showed no differences in sperm outcomes between therapy (carnitine) and placebo groups. Six pregnancies in the carnitine group and nil in the placebo (first phase) |
+ The pregnancies obtained during the carnitine therapy period could suggest that carnitines may also lead to improvement in sperm function and fertilisation |
| Lenzi 2004 | Parallel, placebo Infertile men with OAT N = 60 |
Sperm parameters, pregnancy rate, adverse events | Sperm parameters, pregnancy rate, adverse events | Y ‐ sperm parameters N ‐ pregnancy rate, no definition of pregnancy given Table 2 N ‐ adverse events |
Four participants taking carnitine induced a pregnancy in their partner and nil in the placebo | + No evidence of improved sperm parameters |
| Li 2005 | Head‐to‐head Infertile men with OAT N = 150 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | Y ‐ sperm parameters N ‐ pregnancy rate, no definition given Table 2 |
L‐carnitine and acetyl carnitine more effective than vitamin E + vitamin C for pregnancy, sperm parameters and no evidence of adverse events | + L‐carnitine and acetyl carnitine more effective than vitamin E + vitamin C for pregnancy, sperm parameters and no evidence of adverse events |
| Li 2005a | Head‐to‐head Infertile men with OAT N = 80 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | Statistical significance for carnitines over vitamin E + C | + Improvement of sperm parameters for carnitines compared to vitamin E + C |
| Lombardo 2002 | Cross‐over Infertile men with OAT N = 100 Conference abstract |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, no data available | Sperm parameters (concentration, motility) carnitines versus placebo | + Improvement of sperm parameters |
| Martinez 2015 | Multiple arm, placebo, SG1002 arm excluded Men with idiopathic OAT N = 54 |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, no SDs given | Resveratrol treatment did not significantly affect any of the parameters. | ‐ Resveratrol treatment did not significantly affect any of the parameters. SG1002 may reverse oligoasthenozoospermia. It seems to be more potent antioxidant than resveratrol |
| Martinez‐Soto 2010 | Parallel, placebo Infertile men N = 50 Conference abstract + manuscript from author |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | No differences were found in traditional sperm parameters or lipid composition of the sperm membrane after DHA treatment, only reduction in the percentage of spermatozoa with DNA damage | + Positive effect only on DNA fragmentation |
| Mehni 2014 | Multiple arm, placebo, pentoxifylline arms excluded Infertile men with OAT N = 235 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | L‐carnitine only improved sperm motility, combined with pentoxifylline it improves all sperm parameters. | + Positive effect only sperm motility |
| Micic 2017 | Parallel, placebo Men with OAT N = 175 Conference abstract |
Sperm motility | Sperm motility | N ‐ sperm motility, data given in medians with IQR Analysis 1.18 | Proxeed Plus significantly improved progressive sperm motility | + Proxeed Plus significant improvement in percentage of progressive sperm motility after six months of therapy and also underlines the importance of duration of therapy (3 and 6 months) |
| Morgante 2010 | Parallel, no treatment Infertile men with idiopathic asthenospermia N = 180 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | Significant improvement in sperm motility. | + Improvement of sexual satisfaction Significant improvement in sperm motility |
| Nadjarzadeh 2011 | Parallel, placebo Men with Idiopathic OAT N = 60 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | Non‐significant changes in semen parameters of CoQ10 group. | ‐ CoQ10 further evidence suggesting that supplementation is associated with alleviating oxidative stress, although it does not show any significant effects on sperm concentration, motility and morphology |
| Nozha 2001 | Head‐to‐head Men with OAT N = unclear, 20? |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, no data available | Vitamin E + selenium significantly improves sperm motility | + Vitamin E + selenium associated with improved sperm motility when compared with vitamin B |
| Omu 1998 | Parallel, no treatment Men with asthenozoopermia N = 100 |
Sperm parameters | Sperm parameters, pregnancy, live birth |
N ‐ sperm parameters, only % increase or decrease, not usable Y ‐ pregnancy rate, clinical Y ‐ live birth |
Significant improvement in sperm quality by zinc therapy | + Zinc has a role in improving sperm parameters. Significant increase in pregnancy, not live birth |
| Omu 2008 | Multiple arm, no treatment Men with asthenozoospermia N = 100 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | Zinc therapy alone, in combination with vitamin E or with vitamin E+C were associated with comparably improved sperm parameters and less sperm DNA fragmentation | + Zinc therapy reduces asthenozoospermia |
| Peivandi 2010 | Cross‐over, placebo Infertile men N = 30 |
Sperm parameters | Sperm parameters, pregnancy rate | Y ‐ sperm parameters N ‐ pregnancy rate, no defined as clinical Table 2 |
Significant improvements in mean sperm concentration and progressive sperm motility upon two months of L‐carnitine intake but no significant changes were found in sperm volume or morphology. | + Sperm outcomes and biochemical pregnancies. L‐carnitine intake effectively improved the mean sperm count and progressive sperm motility |
| Pourmand 2014 | Parallel, no treatment Men with male factor infertility and varicocele N = 100 |
Sperm parameters, DNA fragmentation, adverse events | Sperm parameters, DNA fragmentation, adverse events | N ‐ sperm parameters, no SD given
N ‐ DNA fragmentation, no SD given Y ‐ adverse events |
No statistical difference between the two groups (varicocelectomy with L‐carnitine or with no adjuvant therapy). | ‐ Addition of 750 mg of L‐carnitine orally daily to standard inguinal varicocelectomy does not add any extra benefit in terms of improvement in semen analysis parameters or DNA damage |
| Poveda 2013 | Multiple arm, placebo Infertile men N = 60 Conference abstract |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, data not available | L‐carnitine significantly improves sperm concentration, Spermotrend and Maca improve sperm motility. | + Sperm concentration with L‐carnitine and motility with combined antioxidant Spermotrend |
| Pryor 1978 | Cross‐over, placebo Men with severe oligozoospermia N = 64 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | N ‐ sperm parameters, bar graph of % patients showing an increase in motility and density N ‐ pregnancy rate, not clear if clinical. Included in biochemical analysis Table 2 |
Arginine was no more effective than placebo for sperm parameters and biochemical pregnancy rates | ‐ There was no difference in the conception rates of the wives or changes in the quality of the semen during each period of treatment |
| Raigani 2014 | Multiple arm, placebo Men with proven male factor infertility N = 83 |
Sperm parameters, DNA fragmentation | Sperm parameters, DNA fragmentation | N ‐ sperm parameters, data provided in medians with IQR Y ‐ DNA fragmentation (mean with SD) |
Sperm concentration, DNA fragmentation not significantly improved in either group | ‐ Zinc sulphate and folic acid supplementation did not ameliorate sperm quality in infertile men with severely compromised sperm parameters, OAT |
| Rolf 1999 | Asthenospermia (N = 33) |
Sperm parameters, pregnancy rates, adverse events | Sperm parameters, pregnancy rate, adverse events | Y ‐ sperm parameters N ‐ pregnancy rate, not stated as clinical pregnancy N ‐ adverse events, not clear which side effects aimed for |
No adverse events or pregnancies in either group | ‐ Overall no difference vitamin E + C versus placebo |
| Safarinejad 2009 | Multiple arm, placebo Men with idiopathic OAT N = 468 |
Sperm parameters, adverse events | Sperm parameters, adverse events | Y ‐ sperm parameters N ‐ adverse events, not specified which adverse events aimed for |
All semen parameters significantly improved with selenium and N‐acetyl‐cysteine treatment. Administering selenium plus N‐acetyl‐cysteine resulted in additive beneficial effects. Zero adverse events | + Supplemental selenium and N‐acetyl‐cysteine improve semen quality. Zero adverse events |
| Safarinejad 2009a | Parallel, placebo Men with idiopathic OAT N = 212 |
Sperm parameters, adverse events | Sperm parameters, adverse events | Y ‐ sperm parameters N ‐ adverse events, not specified which adverse events aimed for |
Significant improvement in sperm density and motility after coenzyme Q10 therapy. Zero adverse events | + Coenzyme Q10 supplementation resulted in a statistically significant improvement in certain sperm parameters. Zero adverse events |
| Safarinejad 2012 | Parallel, placebo Infertile men N = 228 |
Sperm parameters | Sperm parameters | Y ‐ sperm parameters | Sperm parameters improved significantly after coenzyme Q10 | + Coenzyme Q10 was significantly effective in men with unexplained oligoasthenoteratozoospermia for improving sperm density, sperm motility and sperm morphology |
| Scott 1998 | Multiple arm, placebo Men with subfertility and low sperm motility N = 69 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | Y ‐ sperm parameters N ‐ pregnancy rate, not usable due to pooling of data in the two intervention groups Table 2 |
Sperm motility increased in both selenium‐treated groups, only significant if both treatment groups were combined. Sperm density unaffected | ± Selenium supplementation in subfertile men with low selenium status can improve sperm motility and the chance of successful conception. However, not all patients responded; 56% showed a positive response to treatment |
| Sharifzadeh 2016 | Parallel, placebo Idiopathic subfertile men N = 114 |
Sperm parameters, adverse events | Sperm parameters, adverse events | Y‐ sperm parameters Y ‐ adverse events |
Significant increase in concentration in zinc group | + Normal sperm percentage and total sperm concentration increased after zinc sulphate treatment |
| Sigman 2006 | Parallel, placebo Infertile men with low sperm motility N = 26 |
Sperm parameters, pregnancy rate | Sperm parameters, pregnancy rate | Y ‐ sperm parameters N ‐ pregnancy rate, biochemical Table 2 |
No statistically significant or clinically significant increase in motility or total motile sperm counts between baseline, 12 week, or 24 weeks in the carnitine or placebo arms. | ‐ Carnitine supplementation demonstrated no clinically or statistically significant effect on sperm motility or total motile sperm counts. No difference in pregnancy rate |
| Sivkov 2011 | Parallel, placebo Men with chronic prostatitis and infertility N = 30 |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, no SD given Analysis 1.12 | One‐month course of therapy produced no side effects, had a positive effect on low fertility of ejaculate. | + Selenium + zinc improve |
| Sofikitis 2016 | Multiple arm, no treatment, Avanafil excluded Oligoasthenospermic infertile men N = 39 Abstract only |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, no data available | No significant difference in L‐carnitine group regarding sperm parameters | ‐ No direct conclusion made about L‐carnitine. From result section concluded: no impact on sperm parameters after use of L‐carnitine |
| Suleiman 1996 | Parallel, placebo Asthenospermic men N = 110 |
Sperm parameters | Sperm parameters, pregnancy rate, live birth, miscarriage | Y ‐ sperm parameters Y ‐ pregnancy rate, clinical Y ‐ live birth Y ‐ adverse events: miscarriage |
Vitamin E significantly decreased the MDA concentration in spermatozoa and improved sperm motility. Significant increase pregnancy/live birth rate | + Vitamin E increases sperm motility, pregnancy rate and live birth rate compared to placebo |
| Tremellen 2007 | Parallel, placebo Male factor infertility N = 60 |
Pregnancy rate, adverse events | Pregnancy rate, adverse events, live birth provided by author | Y ‐ pregnancy rate, clinical Y ‐ live birth Y ‐ adverse events |
Antioxidant group recorded a statistically significant improvement in viable pregnancy rate. Side‐effects on the Menevit antioxidant were rare (8%) and mild in nature. | + Menevit antioxidant appears to be a useful ancillary treatment that significantly improves pregnancy rates in couples undergoing IVF‐ICSI treatment. Side‐effects on the Menevit antioxidant were rare (8%) and mild in nature. |
| Wang 2010 | Head‐to‐head Infertile men with asthenozoospermia N = 135 |
Sperm parameters, pregnancy rate, adverse events | Sperm parameters, pregnancy rate, adverse events | Y ‐ sperm parameters N ‐ pregnancy rate, not clear if clinical Table 2 N ‐ adverse events, zero found, however not clear which they aimed for |
Significant increase in L‐carnitine + vitamin E group for sperm motility, no difference for sperm density and morphology. Pregnancy rate significantly higher in L‐carnitine + vitamin E group | + L‐carnitine (+vitamin E) significantly improves sperm motility and pregnancy rate |
| Wong 2002 | Multiple arm, placebo Fertile and subfertile men N = 103 |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, only medians provided Analysis 1.14; Analysis 1.26; Analysis 2.5; Analysis 2.12 | Subfertile men demonstrated a significant 74% increase in total normal sperm count and a minor increase of 4% abnormal spermatozoa | + Total normal sperm count increases after combined zinc sulphate and folic acid treatment in both subfertile and fertile men |
| Zalata 1998 | Head‐to‐head, pilot Men attending andrology clinic N = 22 Conference abstract |
Sperm parameters | Sperm parameters | N ‐ sperm parameters, only before and after median data given | No significant difference in sperm parameters after treatment (acetyl‐cysteine or DHA). DNA damage measured by oh8dG (fmol/ug) was significantly decreased after supplementation | ‐ No improvement of sperm parameters |
| Zavaczki 2003 | Parallel, placebo Men with idiopathic infertility N = 20 |
Sperm parameters, clinical pregnancy, adverse events | Sperm parameters, clinical pregnancy, adverse events | Y ‐ sperm parameters Y ‐ pregnancy rate, clinical Y ‐ adverse events |
No significant changes in sperm characteristics were detected | ‐ Magnesiumt neither leads to a significant improvement of sperm variables nor does it increase the pregnancy rates |
DHA: docosahexaenoic acid; IUI: intrauterine insemination; NAC: N‐acetylcysteine; OAT:oligoasthenoteratozoospermia; ROS: reactive oxygen species
Excluded studies
We retrieved the full text of studies that were identified as potentially eligible (see Figure 1). In this update we excluded 22 studies (28 full‐text articles) and two ongoing studies, in total we excluded 59 studies. The most common reasons for exclusions were ineligible due to use of a different intervention, study design or population. See details in Characteristics of excluded studies.
In summary:
21/59 ineligible based on different intervention such as an added sperm wash or herbal extract; also pentoxifylline studies were excluded;
13/59 ineligible based on different study design, they were not randomised;
15/59 ineligible based on different population, either normospermic men or used the exact same population as other already included studies; in the search of this update; three of the studies were already included in the previous 2014 update;
3/59 ineligible based on different outcome;
5/59 ineligible based on different control group, fertile men without treatment;
2 previously 'ongoing studies' were placed in excluded studies because they were terminated due to insufficient recruiting (NCT01075334; NCT01520584).
Ongoing studies
Eight studies were 'ongoing studies' in the 2014 update. In the current update, only one of the eight previously ongoing studies was included (Blomberg Jensen 2018).The former ongoing study Righospitalet 2011 was a duplicate registration of this study. The former ongoing study AGUNCO 2012 (NCT01560065) became the article Gulino 2016, which was excluded because of the use of a wrong comparator with fertile men of a subfertile couple undergoing IVF. The former ongoing studies Sadeghi 2008 and Sadeghi 2009 became respectively the already previously included study Nadjarzadeh 2011 and excluded study Nadjarzadeh 2014. Three studies remained as ongoing studies (CTRI/2013/02/003431; NCT00975115; NCT01828710) with the status of still recruiting.
We added nine new ongoing studies (DRKS00011616; IRCT2016111830947N1; IRCT2017012432153N1; NCT01407432; NCT01846325; NCT02310087; NCT02421887; NCT03104998; NCT03337360). In this 2019 update, a total of 12 studies are classified as 'ongoing studies'.
Awaiting classification
Six studies were 'awaiting classification' in the 2014 update of this review. One study was included in the 2018 update (Gopinath 2013). The remaining studies awaiting classification were all found to be ineligible after screening of title and abstract or excluded after reading the full text. The former Anarte 2013a conference abstract was the same study as the conference abstract Anarte 2013, which was already excluded in the 2014 update. Nadjarzadeh 2014 was excluded due to using the same study population as already included Nadjarzadeh 2011. The article of Nashivochnikova 2014 was dismissed after a quick translation from Russian of the methods section due to the use of a non‐randomised design. Nematollahi‐Mahani 2014 was excluded due to reporting outcomes not of interest to our review; they reported on seminal antioxidant levels and hormone levels but not on semen parameters or pregnancy outcomes. Furthermore, they used the same study population as the included Azizollahi 2013.
One article from the updated 2018 search was placed in Studies awaiting classification, waiting for an answer from the authors after requesting the full text (Goswami 2015).
Risk of bias in included studies
See Figure 2 for a summary of risk of bias in individual studies, and Figure 3 for a summary of each risk of bias item across all included studies.
Allocation
Sequence generation
All of the 61 included studies were randomised, six of these were cross‐over studies (Akiyama 1999; Kessopoulou 1995; Lenzi 2003; Lombardo 2002; Peivandi 2010; Pryor 1978) and the remaining studies were parallel design.
Only 27 studies described their methods of sequence generation and were rated as low risk in this domain (Azizollahi 2013; Balercia 2005; Barekat 2016; Biagiotti 2003; Blomberg Jensen 2018; Busetto 2018; Cavallini 2004; Cyrus 2015; Eslamian 2013; Exposito 2016; Galatioto 2008; Gamidov 2017; Gopinath 2013; Haghighian 2015; Kessopoulou 1995; Martinez‐Soto 2010; Micic 2017; Nadjarzadeh 2011; Rolf 1999; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012; Scott 1998; Sharifzadeh 2016; Sigman 2006; Tremellen 2007; Wong 2002) (see Figure 2 and Figure 3).
2.
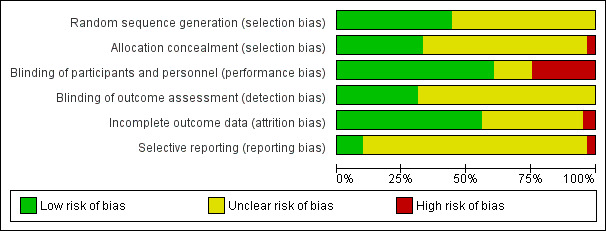
Methodological risk of bias graph: review authors' judgements about each methodological bias item presented as percentages across all included studies.
3.
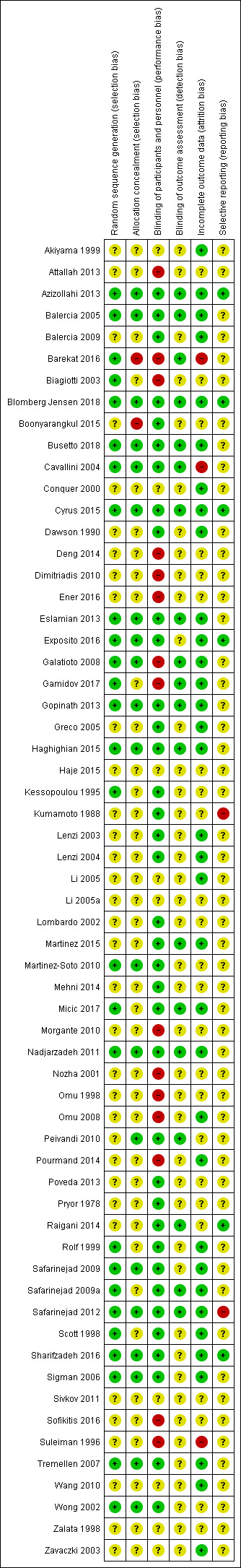
Methodological risk of bias summary: review authors' judgements about each methodological bias item for each included study.
The remaining 34 studies were rated as unclear risk (Akiyama 1999; Attallah 2013; Balercia 2009; Boonyarangkul 2015; Conquer 2000; Dawson 1990; Deng 2014; Dimitriadis 2010; Ener 2016; Greco 2005; Haje 2015; Kumamoto 1988; Lenzi 2003; Lenzi 2004; Li 2005; Li 2005a; Lombardo 2002; Martinez 2015; Mehni 2014; Morgante 2010; Nozha 2001; Omu 1998; Omu 2008; Peivandi 2010; Pourmand 2014; Poveda 2013; Pryor 1978; Raigani 2014; Sivkov 2011; Sofikitis 2016; Suleiman 1996; Wang 2010; Zalata 1998; Zavaczki 2003).
The predominant method of randomisation was by computer‐generated blocks. Tremellen 2007 reported a 2:1 ratio randomisation schedule, Cyrus 2015 reported a 3:2 randomisation schedule, Li 2005 appeared to have a blocked 3:2 allocation, and Micic 2017 appeared to have a 5:2 ratio.
Allocation concealment
The methods of allocation concealment were generally quite poorly described in the included studies. Twenty studies described both their methods of randomisation and allocation concealment and were rated as low risk in this domain (Azizollahi 2013; Balercia 2005; Blomberg Jensen 2018; Busetto 2018; Cavallini 2004; Cyrus 2015; Eslamian 2013; Exposito 2016; Galatioto 2008; Gopinath 2013; Haghighian 2015; Martinez‐Soto 2010; Nadjarzadeh 2011; Peivandi 2010; Safarinejad 2009; Safarinejad 2012; Sharifzadeh 2016; Sigman 2006; Tremellen 2007; Wong 2002).
There were two studies with a high risk of allocation concealment: one due to the use of a randomisation table by the doctor (Barekat 2016); and one due to great baseline imbalance for sperm parameters between the intervention and control group (Boonyarangkul 2015)
The remaining 39 studies were rated as unclear risk (Akiyama 1999; Attallah 2013; Balercia 2009; Biagiotti 2003; Conquer 2000; Dawson 1990; Deng 2014; Dimitriadis 2010; Ener 2016; Gamidov 2017; Greco 2005; Haje 2015; Kessopoulou 1995; Kumamoto 1988; Lenzi 2003; Lenzi 2004; Li 2005; Li 2005a; Lombardo 2002; Martinez 2015; Mehni 2014; Micic 2017; Morgante 2010; Nozha 2001; Omu 1998; Omu 2008; Pourmand 2014; Poveda 2013; Pryor 1978; Raigani 2014; Rolf 1999; Safarinejad 2009a; Scott 1998; Sivkov 2011; Sofikitis 2016; Suleiman 1996; Wang 2010; Zalata 1998; Zavaczki 2003). The methods of allocation concealment included anonymous coloured boxes, sealed opaque envelopes, and numbered bottles.
Blinding
Performance bias
Thirty‐four studies were described as randomised, double‐blind controlled trials in which clinicians and participants were blinded (Azizollahi 2013; Balercia 2005; Balercia 2009; Blomberg Jensen 2018; Boonyarangkul 2015; Busetto 2018; Cavallini 2004; Cyrus 2015; Dawson 1990; Exposito 2016; Gopinath 2013; Greco 2005; Kessopoulou 1995; Kumamoto 1988; Lenzi 2003; Lenzi 2004; Lombardo 2002; Martinez 2015; Martinez‐Soto 2010; Mehni 2014; Micic 2017; Nadjarzadeh 2011; Poveda 2013; Pryor 1978; Raigani 2014; Rolf 1999; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012; Scott 1998; Sharifzadeh 2016; Sigman 2006; Tremellen 2007; Wong 2002). In two studies investigators, clinicians and participants were blinded (Eslamian 2013; Haghighian 2015). A total of thirty‐six studies were rated as low risk (see Figure 2 and Figure 3). In one of the low risk studies (Dawson 1990), it was stated that a placebo was used as the control but only the participants were blinded.
Fifteen other studies were rated high risk (Attallah 2013; Barekat 2016; Biagiotti 2003; Deng 2014; Dimitriadis 2010; Ener 2016; Galatioto 2008; Gamidov 2017; Morgante 2010; Nozha 2001; Omu 1998; Omu 2008; Pourmand 2014; Sofikitis 2016; Suleiman 1996;) Of these high‐risk studies, 12 studies used 'no treatment' as their comparator. Two studies were head‐to‐head trials and open‐labelled (Deng 2014; Nozha 2001). The double‐blinded trial Suleiman 1996 used a placebo, however they reported that if a couple became pregnant then "the treatment was stopped; otherwise it was continued for 6 months. The placebo was given for 6 months" This does appear that they did not stop the placebo. This could suggest that the investigators had knowledge of whether the participants were in the placebo or antioxidant group, therefore this study was rated as high risk.
Nine studies did not give a statement regarding blinding and were rated as unclear risk of bias (Akiyama 1999; Conquer 2000; Haje 2015; Li 2005; Li 2005a; Sivkov 2011; Wang 2010; Zalata 1998; Zavaczki 2003). Three of these studies used a placebo as the control but did not discuss blinding (Conquer 2000; Zavaczki 2003; Sivkov 2011).
Detection bias
The methods of blinding outcome assessment were generally poorly described in the included studies. Only 19 studies reported this aspect of blinding and were therefore classified as low risk (Azizollahi 2013; Balercia 2005; Barekat 2016; Blomberg Jensen 2018; Busetto 2018; Cavallini 2004; Cyrus 2015; Eslamian 2013; Galatioto 2008; Gamidov 2017; Gopinath 2013; Haghighian 2015; Martinez 2015; Micic 2017; Nadjarzadeh 2011; Peivandi 2010; Raigani 2014; Safarinejad 2009a; Safarinejad 2012).
The other 42 studies were rated as unclear risk due to the lack of information (Akiyama 1999; Attallah 2013; Balercia 2009; Biagiotti 2003; Boonyarangkul 2015; Conquer 2000; Dawson 1990; Deng 2014; Dimitriadis 2010; Ener 2016; Exposito 2016; Greco 2005; Haje 2015; Kessopoulou 1995; Kumamoto 1988; Lenzi 2003; Lenzi 2004; Li 2005; Li 2005a; Lombardo 2002; Martinez‐Soto 2010; Mehni 2014; Morgante 2010; Nozha 2001; Omu 1998; Omu 2008; Pourmand 2014; Poveda 2013; Pryor 1978; Rolf 1999; Safarinejad 2009; Scott 1998; Sharifzadeh 2016; Sigman 2006; Sivkov 2011; Sofikitis 2016; Suleiman 1996; Tremellen 2007; Wang 2010; Wong 2002; Zalata 1998; Zavaczki 2003).
Incomplete outcome data
Thirty‐four studies were rated as low risk for incomplete outcome data (Akiyama 1999; Azizollahi 2013; Balercia 2005; Balercia 2009; Blomberg Jensen 2018; Busetto 2018; Conquer 2000; Cyrus 2015; Dawson 1990; Eslamian 2013; Exposito 2016; Gopinath 2013; Galatioto 2008; Gamidov 2017; Greco 2005; Haghighian 2015; Lenzi 2003; Lenzi 2004; Li 2005; Martinez 2015; Micic 2017; Nadjarzadeh 2011; Omu 2008; Pourmand 2014; Rolf 1999; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012; Scott 1998; Sharifzadeh 2016; Sigman 2006; Tremellen 2007; Wang 2010; Zavaczki 2003).
Twenty‐four studies were rated as unclear, most of them did report the number of drop outs, but did not provide the reasons (Attallah 2013; Biagiotti 2003; Boonyarangkul 2015; Deng 2014; Dimitriadis 2010; Ener 2016; Haje 2015; Kessopoulou 1995; Kumamoto 1988; Li 2005a; Lombardo 2002; Martinez‐Soto 2010; Mehni 2014; Morgante 2010; Nozha 2001; Omu 1998; Peivandi 2010; Poveda 2013; Pryor 1978; Raigani 2014; Sivkov 2011; Sofikitis 2016; Wong 2002; Zalata 1998).
Three studies were rated as high risk of attrition bias due to lack of compliance directly related to treatment and high drop‐out rates (20" to 42%) (Barekat 2016; Cavallini 2004; Suleiman 1996).
Only five studies (Balercia 2009; Blomberg Jensen 2018; Busetto 2018; Galatioto 2008; Pryor 1978) actually stated that they used intention‐to‐treat (ITT) in their analysis. However, Pryor 1978 stated they had used ITT but the data were not presented. Most of the other included studies accounted for the participants that withdrew from their studies and then analysed the groups in an ITT.
Three studies (Azizollahi 2013; Barekat 2016; Wang 2010) did not use ITT, however the numbers of dropouts were given for each intervention and control group and therefore we were able to use ITT in the data analysis by making the assumption of no event for the binary outcomes. No imputation was carried out on the continuous outcome data these were analysed as they were reported in the studies.
Six studies had over 20% withdrawal from their studies. Cavallini 2004 had a 30% dropout rate and reasons were provided for only 53 out of the 55 dropouts; these reasons included refusal due to the chance of taking a placebo and preference for assisted reproduction techniques (ARP). There also remained some confusion in this study on the total numbers randomised and analysed. Azizollahi 2013 had a 30% dropout rate; Li 2005a; Suleiman 1996, Nadjarzadeh 2011, and Barekat 2016 had slightly over 20% withdrawal from their studies.
One study (Suleiman 1996) had a large imbalance in numbers. There were found to be 52 in the treatment group and 35 in the placebo once the code had been broken at the end of the study. There was no indication of how the randomisation was performed. The reasons given for dropout were only accounted for broadly: many couples had left the region and some simply failed to continue, no numbers were given for individual dropout reasons (see Figure 2 and Figure 3). The numbers of participants that were initially randomised to each group were not available, so ITT for the dichotomous outcomes was not possible.
Selective reporting
Study protocols were only available for six out of the 61 included studies (Azizollahi 2013; Blomberg Jensen 2018; Cyrus 2015; Exposito 2016; Raigani 2014; Sharifzadeh 2016).
Two studies were rated at high risk of reporting bias; Kumamoto 1988 performed subgroup analysis post‐treatment and Safarinejad 2012 did not pre‐specify outcomes. Six studies were rated as unclear risk as they were conference abstracts (Attallah 2013; Biagiotti 2003; Lombardo 2002; Micic 2017; Sofikitis 2016; Zalata 1998), and two studies were rated as unclear as it was possible that these were two publications of the same study that were reporting on different intervention arms (Li 2005; Li 2005a). Obtaining help with Chinese translation did not clarify this and attempts to contact the authors were unsuccessful. The remaining 45 studies were rated as unclear risk in this domain because there were no published study protocols available.
Other potential sources of bias
One study reported great baseline imbalance for sperm parameters between the intervention and control group (Boonyarangkul 2015).
Effects of interventions
See: Table 1
1 Antioxidants versus placebo or no treatment (natural conception and undergoing fertility treatment)
1.1 Live birth; type of antioxidant
See Analysis 1.1 and Figure 4.
1.1. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 1 Live birth; type of antioxidant.
4.
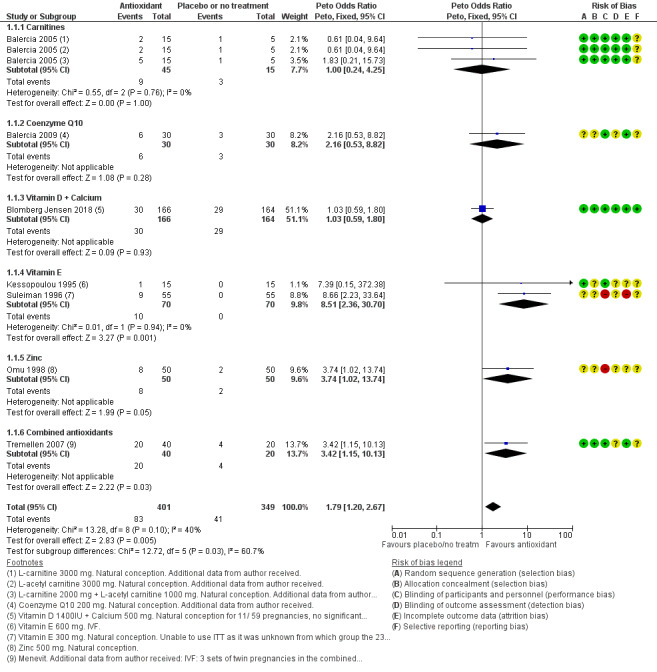
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.1 Live birth; type of antioxidant.
Only seven studies reported on live birth; four of these had methodological inadequacies as they did not describe their methods of randomisation or allocation concealment. Three studies reported that all clinical pregnancies led to a live birth (Balercia 2005; Balercia 2009; Kessopoulou 1995). The meta‐analysis of the seven studies showed that antioxidants were associated with an increased live birth rate compared with placebo or no treatment (Peto odds ratio (OR) 1.79, 95% confidence interval (CI) 1.20 to 2.67, 750 men, 7 RCTs, P = 0.005, I2 = 40%, low‐quality evidence). This meant that within this studied population of subfertile men with a baseline expected live birth rate of 12%, use of an antioxidant increased this rate to between 14% and 26% (Table 1).
1.1.1 One study reported on this outcome comparing carnitines versus placebo (Balercia 2005). There was no evidence of increased live birth rate (Peto OR 1.00, 95% CI 0.24 to 4.25; 60 men, P = 1.00, I2 = not applicable).
1.1.2 One study reported on this outcome comparing coenzyme Q10 versus placebo (Balercia 2009). There was no evidence of increased live birth rate (Peto OR 2.16, 95% CI 0.53 to 8.82; 60 men, P = 0.28, I2 = not applicable).
1.1.3 One study reported on this outcome comparing vitamin D plus calcium versus placebo (Blomberg Jensen 2018). There was no evidence of increased live birth rate (Peto OR 1.03, 95% CI 0.59 to 1.80, 330 men, P = 0.93, I2 = not applicable).
1.1.4 Two studies reported on this outcome comparing vitamin E versus placebo (Kessopoulou 1995; Suleiman 1996). There was evidence of increased live birth rate (Peto OR 8.51, 95% CI 2.36 to 30.70, 140 men, 2 RCTs, P = 0.001, I2 = 0%).
1.1.5 One study reported on this outcome comparing zinc versus no treatment (Omu 1998). There was no evidence of increased live birth rate (Peto OR 3.74, 95% CI 1.02 to 13.74, 100 men, P = 0.05, I2 = not applicable).
1.1.6 One study reported on this outcome comparing combined antioxidants versus placebo (Tremellen 2007). There was evidence of increased live birth rate (Peto OR 3.42, 95% CI 1.15 to 10.13, 60 men, P = 0.03 I2 = not applicable). The results from this study also included three sets of twins in the combined antioxidant group and nil in the placebo group. Each twin birth was counted as one event as stated in the methods section in the review protocol.
There was evidence that different antioxidants had differing effects (test for subgroup differences Chi² = 12.72, P = 0.03).
1.2 Live birth; placebo or no treatment
Only one study (Omu 1998) used 'no treatment' as the control. When this study was removed from the analysis, evidence of increased live birth remained when compared with placebo only (Peto OR 1.65, 95% CI 1.08 to 2.52, 650 men, 6 RCTs, P = 0.02, I2 = 41%).
There was no evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 1.05, P = 0.31).
1.3 Live birth; in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI)
See Analysis 1.3.
1.3. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 3 Live birth; IVF/ICSI.
There were only two studies in women undergoing IVF/ICSI which reported on live birth (Kessopoulou 1995; Tremellen 2007). There was evidence of increased live birth rate, in those couples undergoing IVF/ICSI, with antioxidant use when compared with placebo (Peto OR 3.61, 95% CI 1.27 to 10.29, 2 RCTs, 90 men, P = 0.02, I2 = 0%).
Sensitivity analysis for studies reporting live birth and clinical pregnancy
The seven studies that reported live birth had an OR for live birth of 1.79, and in these same studies the OR for clinical pregnancy was 2.96. When we pooled all 11 studies reporting the clinical pregnancy rate there was a comparable OR 2.97. This suggest that there is no overestimation of live birth. However, the true effect is unknown unless all studies reporting on clinical pregnancy rate also reported on live birth rate.
Sensitivity analysis for studies rated as high risk of bias
When the two studies (Omu 1998; Suleiman 1996) rated with a high risk of bias were removed from the analysis, there was no evidence of association between antioxidants and an increased live birth rate when compared with placebo (Peto OR 1.38, 95% CI 0.89 to 2.16; participants = 540 men, 5 RCTs, P = 0.15, I2 = 0%).
1.4 Live birth; as‐treated analysis
See Analysis 1.4.
1.4. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 4 Live birth; as‐treated analysis.
When an as‐treated analysis was done, there was evidence of increased live birth rate when antioxidants were compared with placebo (Peto OR 1.71, 95% CI 1.13 to 2.58, 649 men, 7 RCTs, P = 0.01, I2 = 26%).
1.5 Clinical pregnancy; type of antioxidant
See Analysis 1.5 and Figure 5 and Figure 6.
1.5. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 5 Clinical pregnancy; type of antioxidant.
5.
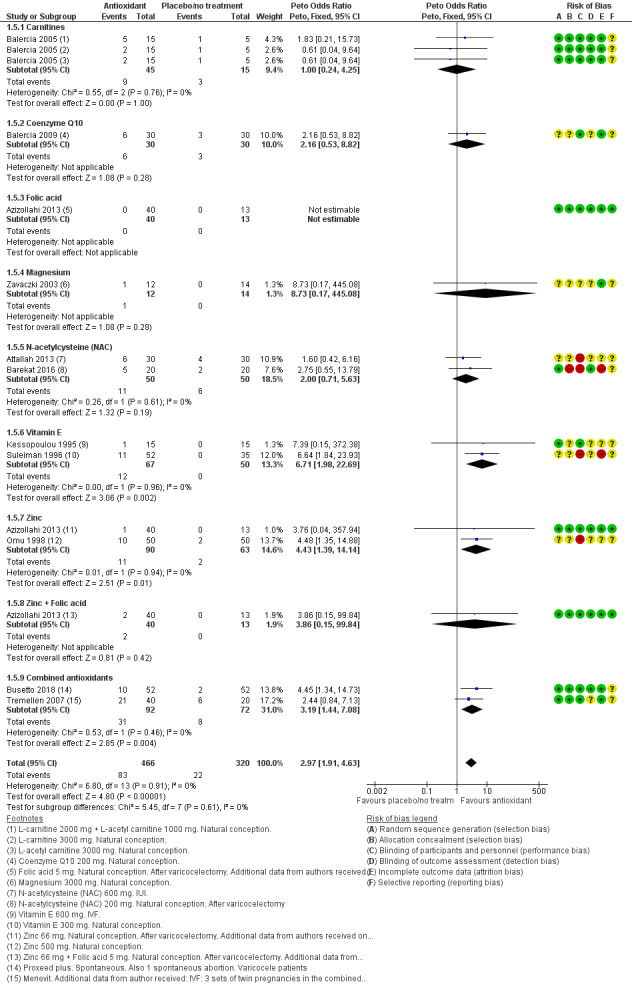
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.5 Clinical pregnancy; type of antioxidant.
6.
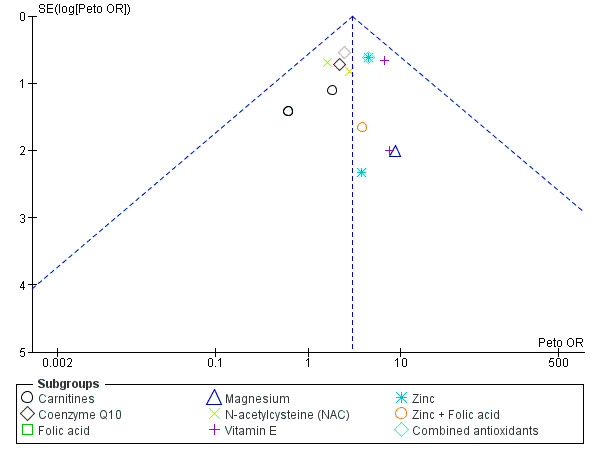
Funnel plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.5 Clinical pregnancy; type of antioxidant.
Only 11 studies (with 15 treatment arms) reported on clinical pregnancy rate; four of these had methodological inadequacies with high risk of bias for methods of randomisation, allocation concealment or blinding. The meta‐analysis of these studies showed that antioxidants were associated with an increased clinical pregnancy rate when compared to placebo or no treatment (Peto OR 2.97, 95% CI 1.91 to 4.63, 786 men, 15 RCTs, P < 0.001, I2 = 0%, low‐quality evidence). This meant that within this population of subfertile men with the expected clinical pregnancy rate of 7%, use of an antioxidant increased this rate to between 12% and 26% (Table 1).
1.5.1 One study reported on this outcome comparing carnitines versus placebo (Balercia 2005). There was no evidence of increased clinical pregnancy rate (Peto OR 1.00, 95% CI 0.24 to 4.25, 60 men, 3 RCTs, P = 0.76, I2 = not applicable).
1.5.2 One study reported on this outcome comparing coenzyme Q10 versus placebo (Balercia 2009). There was no evidence of increased clinical pregnancy rate (Peto OR 2.16, 95% CI 0.53 to 8.82, 60 men, 1 RCT, P = 0.28, I2 = not applicable).
1.5.3 One study reported on this outcome comparing folic acid versus placebo (Azizollahi 2013). There was no OR estimable due to the occurrence of zero pregnancies in both groups.
1.5.4 One study reported on this outcome comparing magnesium versus placebo (Zavaczki 2003). There was no evidence of increased clinical pregnancy rate (Peto OR 8.73, 95% CI 0.17 to 445.08, 1 RCT, 26 men, P = 0.28, I2 = not applicable).
1.5.5 Two studies reported on this outcome comparing N‐acetylcysteine versus placebo or no treatment (Attallah 2013; Barekat 2016). There was no evidence of increased clinical pregnancy rate (Peto OR 2.00, 95% CI 0.71 to 5.63, 100 men, 2 RCTs, P = 0.19, I2 = 0%).
1.5.6 Two studies reported on this outcome comparing vitamin E versus placebo (Kessopoulou 1995; Suleiman 1996). There was an increased clinical pregnancy rate (Peto OR 6.71, 95% CI 1.98 to 22.69, 2 RCTs, 117 men, P = 0.002, I2 = 0%).
1.5.7 Two studies reported on this outcome comparing zinc versus placebo or no treatment (Azizollahi 2013; Omu 1998). There was an increased clinical pregnancy rate (Peto OR 4.43, 95% CI 1.39 to 14.14, 2 RCTs, 153 men, P = 0.01, I2 = 0%).
1.5.8 One study reported on this outcome comparing zinc with folic acid versus placebo (Azizollahi 2013). There was no evidence of increased clinical pregnancy rate (Peto OR 3.86, 95% CI 0.15 to 99.84, 53 men, 1 RCT, P = 0.42, I2 = not applicable).
1.5.9 Two studies reported on this outcome comparing combined antioxidants versus placebo (Busetto 2018; Tremellen 2007). There was an increased clinical pregnancy rate (Peto OR 3.19, 95% CI 1.44 to 7.08, 164 men, 2 RCTs, P = 0.004, I2 = 0%).
There was no evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 5.45, P = 0.61).
Sensitivity analysis for studies rated as high risk of bias
When the four studies rated with a high risk of bias, were removed from the analysis there remained an association between antioxidants and an increased clinical pregnancy rate (Peto OR 2.57, 95% CI 1.42 to 4.67, 499 men, 7 RCTs, P = 0.002, I2 = 0%) (Attallah 2013; Barekat 2016; Omu 1998; Suleiman 1996).
Sensitivity analysis for studies enrolling men with varicocele
When the studies that enrolled men with varicocele or after varicocelectomy were removed from the analysis, antioxidants remained associated with increased clinical pregnancy rate when compared to placebo or no treatment (Peto OR 2.76, 95% CI 1.65 to 4.59, 483 men, 15 RCTs, P < 0.0001, I2 = 0%) (Azizollahi 2013; Barekat 2016; Busetto 2018).
Sensitivity analysis for studies enrolling men in couples undergoing intrauterine insemination (IUI)
Only one study Attallah 2013 reported on men in couples undergoing IUI. When this study was removed from the analysis there remained an association between the use of antioxidants and increased clinical pregnancy rate when compared to no treatment (OR 3.20, 95% CI 2.00 to 5.13, 726 men, 15 RCTs, P < 0.0001, I2 = 0%).
1.6 Clinical pregnancy: placebo or no treatment
See Analysis 1.6.
1.6. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 6 Clinical pregnancy; placebo or no treatment.
Antioxidants were associated with an increase in clinical pregnancy rate in the studies that compared antioxidants with placebo (Peto OR 3.01, 95% CI 1.81 to 5.03, 626 men, 9 RCTs, 13 intervention arms, P < 0.001, I2 = 0%) (Azizollahi 2013; Balercia 2005; Balercia 2009; Barekat 2016; Busetto 2018; Kessopoulou 1995; Suleiman 1996; Tremellen 2007; Zavaczki 2003). Antioxidants were also associated with an increase in clinical pregnancy rate in those studies that compared antioxidants versus no treatment (Peto OR 2.84, 95% CI 1.16 to 6.96, 160 men, 2 RCTs, P = 0.02, I2 = 20%) (Attallah 2013; Omu 1998).
There was no evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 0.01, P = 0.91).
1.7 Clinical pregnancy; IVF/ICSI
See Analysis 1.7.
1.7. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 7 Clinical pregnancy; IVF/ICSI.
There were only two studies in women undergoing IVF/ICSI which reported on clinical pregnancy rate (Kessopoulou 1995;Tremellen 2007). There was no evidence of an increase in clinical pregnancy in those couples undergoing IVF/ICSI, when antioxidant use was compared with placebo (Peto OR 2.64, 95% CI 0.94 to 7.41, 90 men, 2 RCTs,P = 0.07, I2 = 0%).
1.8 Adverse events
See Analysis 1.8 and Figure 7.
1.8. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 8 Adverse events.
7.
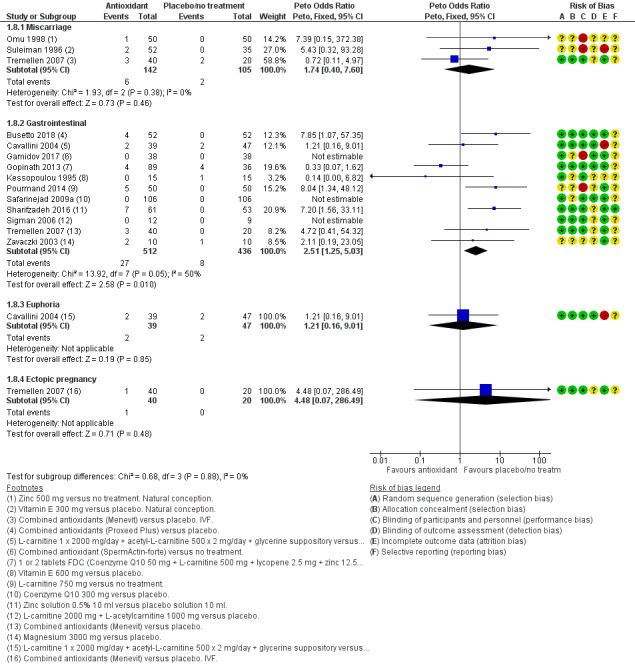
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.8 Adverse events.
The only adverse events reported in the studies were miscarriage, gastrointestinal disorders, euphoria and ectopic pregnancy.
1.8.1 Miscarriage. Only three studies reported on miscarriage and the event rate was very low (eight miscarriages from 247 couples) (Omu 1998; Suleiman 1996; Tremellen 2007). The analysis of these three studies showed no evidence of increased miscarriage between the use of antioxidants when compared to placebo or no treatment (Peto OR 1.74, 95% CI 0.40 to 7.60, 3 RCTs, 247 men, P = 0.46, I2 = 0%, very low‐quality evidence). This meant that within this population of subfertile men, with an expected miscarriage rate of 2%, the chances of having a miscarriage lay between 1% and 13% with the use of an antioxidant (Table 1).
1.8.2 Gastrointestinal. The analysis of 11 studies showed an increase between the use of antioxidants and gastrointestinal upsets when compared to placebo or no treatment (Peto OR 2.51, 95% CI 1.25 to 5.03, 948 men, 11 RCTs, P =0.010, I2 = 50%, very low‐quality evidence) (Busetto 2018; Cavallini 2004; Gamidov 2017; Gopinath 2013; Kessopoulou 1995; Pourmand 2014; Safarinejad 2009a; Sharifzadeh 2016; Sigman 2006; Tremellen 2007; Zavaczki 2003). However, the event rate was very low so we could not be sure of these results. Three of these 11 studies reported no events, therefore a Peto OR could not be estimated and a funnel plot was not created.
1.8.3 Euphoria. Only one study (Cavallini 2004) reported on this adverse event and there was no evidence of increased occurrence of euphoria when antioxidants were compared to placebo (Peto OR 1.21, 95% CI 0.16 to 9.01,1 RCT, 86 men, P = 0.85, I2 = not applicable).
1.8.4 Ectopic pregnancy. Only one study (Tremellen 2007) reported on this adverse event and there was no evidence of increase of ectopic pregnancy when antioxidants were compared to placebo (Peto OR 4.48, 95% CI 0.07 to 286.49,1 RCT, 60 men, P = 0.48, I2 = not applicable).
It was unlikely that these last two adverse events, euphoria and ectopic pregnancy, were related to intake of antioxidants especially with the reported extreme low event rate. Therefore these outcomes were not included in the 'Summary of findings' table.
1.9 Sperm DNA fragmentation; type of antioxidant
See Analysis 1.9, Analysis 1.10 and Figure 8
1.9. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 9 Sperm DNA fragmentation; type of antioxidant.
8.
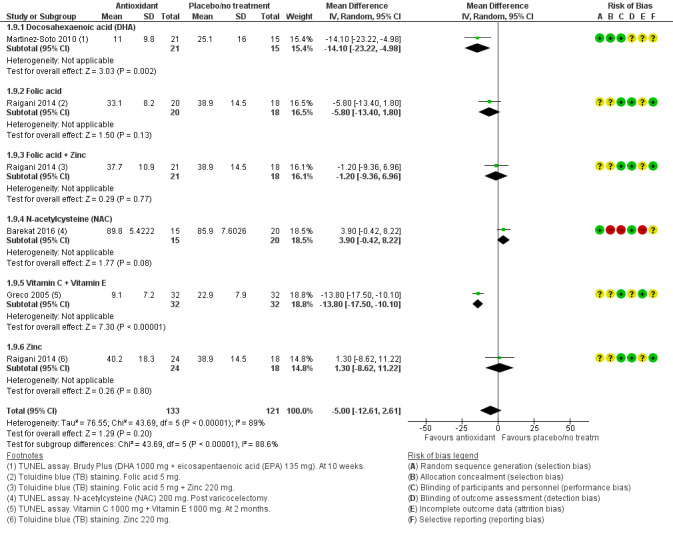
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.9 Sperm DNA fragmentation; type of antioxidant.
Four studies reported on DNA fragmentation and found that there was a lower DNA fragmentation rate when antioxidants were compared with placebo or no treatment (mean difference (MD) ‐5.00, 95% CI ‐12.61 to 2.61, 254 men, 4 RCTs, six intervention arms, P < 0.0001, I2 = 89%) (Barekat 2016; Greco 2005; Martinez‐Soto 2010; Raigani 2014).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 43.69, P < 0.00001).
Sensitivity analysis for studies enrolling men with varicocele
In the literature it is reported that men with varicocele have higher levels of DNA fragmentation. Only one study reported on men with varicocele. When this study was removed from the analysis there remained an association between the use of antioxidants and lower DNA fragmentation rate when compared to no treatment (MD ‐10.05, 95% CI ‐12.86 to ‐7.25, 219 men, 6 RCTs, P < 0.001, I2 = 74%) (Barekat 2016).
1.10 Data not usable for meta‐analysis
Two studies reported on DNA fragmentation, but could not be included in the forest plots of the meta‐analysis. Boonyarangkul 2015 because of the use of Comet assay, and Gamidov 2017 only reported medians and interquartile ranges (Analysis 1.10). Both studies showed an improvement (decrease) in DNA fragmentation after the use of antioxidants. However, in Boonyarangkul 2015 this was only in the (excluded) arm tamoxifen plus folate.
1.11 Total sperm motility at three months or less; type of antioxidant
See Analysis 1.11 and Figure 9
1.11. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 11 Total sperm motility at 3 months or less; type of antioxidant.
9.
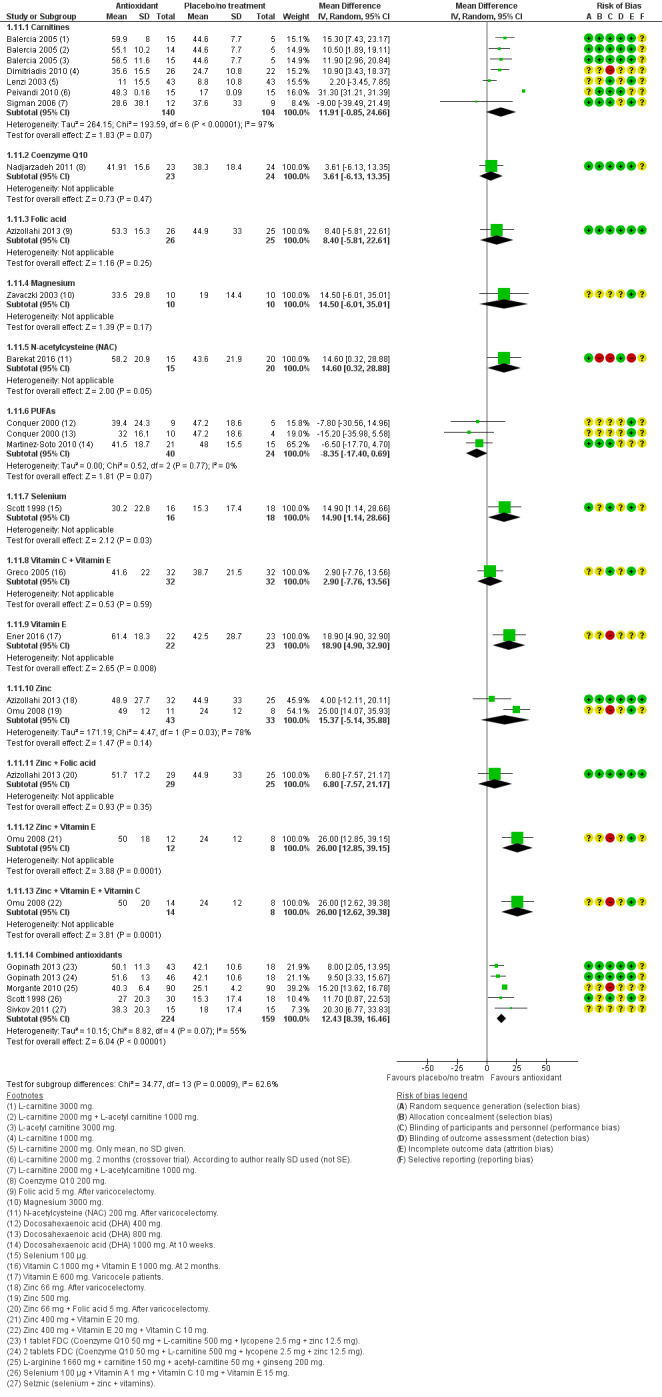
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.11 Total sperm motility at 3 months or less; type of antioxidant.
We analysed this outcome using a random‐effects model and used subtotals as pooling was not possible.
1.11.1 Five studies (seven intervention arms) comparing carnitines with placebo or no treatment did not show an increase in total sperm motility (Balercia 2005; Dimitriadis 2010; Lenzi 2003; Peivandi 2010; Sigman 2006) (MD 11.91, 95% CI ‐0.85 to 24.66, 244 men, 5 RCTs, 7 intervention arms, P = 0.07, I2 = 97%). One study (Lenzi 2003) did not report standard deviations (SDs); we assumed the outcome to have an SD equal to the highest SD from other studies within this analysis. The heterogeneity was extremely high due to the fact that one study (Peivandi 2010) had very small SDs when compared to data in the other studies but the authors confirmed, when contacted, that they are indeed SDs and not standard errors (SEs). When these two studies were removed from the analysis carnitines did show an increase in total sperm motility when compared with placebo or no treatment, with low heterogeneity (MD 11.83, 95% CI 7.78 to 15.87, 128 men, 3 RCTs, 5 intervention arms, P < 0.00001, I2 = 0%).
1.11.2 Coenzyme Q10 did not show evidence of an increase in total sperm motility compared with placebo (Nadjarzadeh 2011) (MD 3.61, 95% CI ‐6.13 to 13.35, 47 men, 1 RCT, P = 0.47, I2 = not applicable).
1.11.3 Folic acid did not show evidence of an increase in total sperm motility compared with placebo (Azizollahi 2013) (MD 8.40, 95% CI ‐5.81 to 22.61, 51 men, 1 RCT, P = 0.25, I2 = not applicable).
1.11.4 Magnesium did not show evidence of an increase in total sperm motility compared with placebo (Zavaczki 2003) (MD 14.50, 95% CI ‐6.01 to 35.01, 20 men, 1 RCT, P = 0.17, I2 = not applicable).
1.11.5 N‐acetylcysteine (NAC) did not show evidence of an increase in total sperm motility compared with placebo (Barekat 2016) (MD 14.60, 95% CI 0.32 to 28.88, 35 men, P = 0.05, I2 = not applicable).
1.11.6 Two studies (three intervention arms) compared polyunsaturated fatty acids (PUFAs) with placebo and did not show evidence of an increase in total sperm motility (Conquer 2000; Martinez‐Soto 2010) (MD ‐8.35, 95% CI ‐17.40 to 0.69, 64 men, 3 RCT, P = 0.07, I2 = 0%).
1.11.7 Selenium did show an increase in total sperm motility compared with placebo (Scott 1998) (MD 14.90, 95% CI 1.14 to 28.66, 34 men, 1 RCT, P = 0.03, I2 = not applicable).
1.11.8 Vitamin C plus Vitamin E did not show evidence of an increase in total sperm motility compared with placebo (Greco 2005) (MD 2.90, 95% CI ‐7.76 to 13.56, 64 men, 1 RCT, P = 0.59, I2 = not applicable).
1.11.9 Vitamin E did show an increase in total sperm motility compared with no treatment (Ener 2016) (MD 18.90, 95% CI 4.90 to 32.90, 45 men, 1 RCT, P = 0.08, I2 = not applicable).
1.11.10 Two studies compared zinc with placebo or no treatment and did not show evidence of an increase in total sperm motility (Azizollahi 2013; Omu 2008). As the heterogeneity was high (78%) we have not reported the pooled analysis; individually their results were:
Azizollahi 2013 showed did not show evidence of an increase in total sperm motility at three months when compared to placebo (MD 4.00, 95% CI ‐12.11 to 20.11, 57 men);
Omu 2008 showed an increase in total sperm motility at three months when compared to no treatment (MD 25.00, 95% CI 14.07 to 35.93, 19 men).
1.11.11 Zinc plus folic acid did not show evidence of an increase in total sperm motility compared with placebo (Azizollahi 2013) (MD 6.80, 95% CI ‐7.57 to 21.17, 54 men,1 RCT, P = 0.93, I2 = not applicable).
1.11.12 Zinc plus vitamin E did show an increase in total sperm motility compared with no treatment (Omu 2008) (MD 26.00, 95% CI 12.85 to 39.15, 20 men, 1 RCT, P = 0.0001 I2 = not applicable)
1.11.13 Zinc plus vitamin E plus vitamin C did show an increase in total sperm motility compared with no treatment (Omu 2008) (MD 26.00, 95% CI 12.62 to 39.38, 22 men, 1 RCT, P ‐ 0.0001, I2 = not applicable).
1.11.14 Four studies (five intervention arms) compared combined antioxidants with placebo or no treatment (Gopinath 2013; Morgante 2010; Scott 1998; Sivkov 2011). There was an increase in total sperm motility (MD 12.43, 95% CI 8.39 to 16.46, 383 men, 4 RCTs, P < 0.00001, I2 = 55%). However, there was high heterogeneity of 55%. One study (Morgante 2010) had not described the method of randomisation and carried 40.8% of the weight in this analysis; a sensitivity analysis for this risk of bias still showed an increase in total sperm motility, however now with low heterogeneity (MD 10.02, 95% CI 6.20 to 13.84, 203 men, 3 RCTs, P < 0.00001, I2 = 0%).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 853.44, P < 0.00001).
1.12 Data not usable for meta‐analysis
Four studies (Cavallini 2004; Galatioto 2008; Kessopoulou 1995; Raigani 2014) provided data as medians or percentages, and therefore they could not be used in the forest plot. Three of these studies (Galatioto 2008; Kessopoulou 1995; Raigani 2014) found no difference between the intervention and control or no treatment for this outcome. Two studies (Cavallini 2004; Lenzi 2003) indicated that there might be some improvement in sperm motility in the intervention group when measured at three months, however these data were not rigorous and no conclusions could be made.
1.13 Total sperm motility at six months or less; type of antioxidant
See Analysis 1.13.
1.13. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 13 Total sperm motility at 6 months; type of antioxidant.
We analysed this outcome using a random‐effects model and used subtotals as pooling was not possible.
1.13.1 Three studies compared carnitines with placebo (Balercia 2005; Lenzi 2004; Sigman 2006) . As the heterogeneity was high (78%) we have not reported the pooled analysis for these studies; individually their results were:
Balercia 2005 (three arms) showed an increased total sperm motility at six months when compared to placebo (MD 21.13, 95% CI 14.58 to 27.68, 30 men, P < 0.00001);
Lenzi 2004 showed no evidence of increased total sperm motility at six months when compared to placebo (MD 1.50, 95% CI‐4.56 to 7.56, 56 men, P = 0.63);
Sigman 2006 showed no evidence of increased total sperm motility at six months when compared to placebo (MD ‐7.70, 95% CI ‐33.24 to 17.84, 21 men, P = 0.55).
1.13.2 Three studies compared coenzyme Q10 with placebo (Balercia 2009; Safarinejad 2009a; Safarinejad 2012). As the heterogeneity was extremely high (99%) we we have not reported the pooled analysis; individually their results were:
Balercia 2009 did show an increased total sperm motility when compared to placebo ( MD 4.50, 95% 0.74 to 8.26, 60 men, P = 0.02);
Safarinejad 2009a did show an increased total sperm motility when compared to placebo (MD 4.50, 95% CI 3.89 to 5.11, 194 men, P < 0.000001);
Safarinejad 2012 did show an increased total sperm motility when compared to placebo (MD 10.40, 95% CI 9.77 to 11.03, 225 men, P < 0.000001).
1.13.3 Folic acid did not show evidence of increased total sperm motility when compared to placebo (MD 1.70, 95% CI ‐8.49 to 11.89, 51 men, P = 0.74, I2 = not applicable) (Azizollahi 2013).
1.13.4 N‐acetylcysteine (NAC) did show increased total sperm motility when compared to placebo (MD 1.90, 95% CI 1.20 to 2.60, 211 men, P ≤ 0.0001, I2 = not applicable) (Safarinejad 2009).
1.13.5 Selenium did show increased total sperm motility when compared to placebo (MD 3.20, 95% CI 2.50 to 3.90, 211 men, P ≤ 0.00001, I2 = not applicable) (Safarinejad 2009).
1.13.6 Selenium plus N‐acetylcysteine did show increased total sperm motility when compared to placebo (MD 6.30, 95% CI 5.60 to 7.00, 210 men, P ≤ 0.00001, I2 = not applicable) (Safarinejad 2009).
1.13.7 Vitamin D plus calcium did not show evidence of increased total sperm motility when compared to placebo (MD ‐4.00, 95% CI ‐9.57 to 1.57, 260 men, P = 0.16, I2 = not applicable) (Blomberg Jensen 2018).
1.13.8 Vitamin E did show increased total sperm motility when compared to placebo or no treatment (MD 11.20, 95% CI 4.70 to 17.70, 132 men, 2 RCTs, P = 0.0007, I2 = 16%) (Ener 2016; Suleiman 1996).
1.13.9 Zinc did not show evidence of increased total sperm motility when compared to placebo (MD 0.00, 95% CI ‐10.19 to 10.19, 57 men, P = 1.00, I2 = not applicable) (Azizollahi 2013).
1.13.10 Zinc plus folic acid did not show evidence of increased total sperm motility when compared to placebo (MD 2.60, 95% CI ‐8.82 to 14.02, 54 men, P = 0.66, I2 = not applicable) (Azizollahi 2013).
1.13.11 Combined antioxidants did not show evidence of increased total sperm motility when compared to placebo or no treatment (Busetto 2018; Gopinath 2013). As the heterogeneity was high (80%) we we have not reported the pooled analysis; individually their results were:
Busetto 2018 did show increased total sperm motility when compared to placebo (MD 4.40, 95% CI 1.49 to 7.31, 104 men, P = 0.003);
Gopinath 2013 with three arms, did show increased total sperm motility when compared to placebo(MD 12.44, 95% CI 8.29 to 16.59, 125 men, P < 0.00001).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 239.07, P < 0.00001).
1.14 Data not usable for meta‐analysis
See Analysis 1.14.
Three studies (Cavallini 2004; Micic 2017; Wong 2002) provided data as medians, no SDs or percentages, and therefore could not be used in the forest plot. All studies indicated that there might be some increase in sperm motility in the intervention group when measured at six months, however these data are not rigorous and no conclusions could be made.
1.15 Total sperm motility at nine months or more; type of antioxidant
See Analysis 1.15.
1.15. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 15 Total sperm motility at 9 months or more; type of antioxidant.
We analysed this outcome using a random‐effects model and used subtotals as pooling was not possible.
1.15.1 One study reported on carnitines, and did show increased total sperm motility when compared to placebo (Balercia 2005):
L‐carnitine did show increased total sperm motility when compared to placebo (MD 11.54, 95% CI 1.66 to 21.42, 19 men, P = 0.02);
L‐acetyl carnitine did not show evidence of increased total sperm motility when compared to placebo (MD 7.84, 95% CI ‐1.41 to 17.09, 20 men, P = 0.10);
L‐carnitine + L‐acetyl carnitine did not show evidence of increased total sperm motility when compared to placebo (MD 6.27, 95% CI ‐3.36 to 15.90, 20 men, P = 0.20).
1.15.2 Three studies reported on coenzyme Q10 (Balercia 2009; Safarinejad 2009a; Safarinejad 2012). As the heterogeneity was extremely high (98%) we we have not reported the pooled analysis; individually their results were:
Balercia 2009 did not show evidence of increased total sperm motility when compared to placebo (MD ‐2.30, 95% CI ‐5.94 to 1.34, 60 men, P = 0.22);
Safarinejad 2009a did show increased total sperm motility when compared to placebo (MD 1.40, 95% CI 0.79 to 2.01, 194 men, P < 0.00001);
Safarinejad 2012 did show increased total sperm motility when compared to placebo (MD 5.40, 95% CI 4.80 to 6.00, 225 men, P < 0.00001).
1.15.4 Vitamin E did not show evidence of increased total sperm motility when compared to no treatment (Ener 2016) (MD 2.20, 95% CI ‐8.48 to 12.88, 45 men, 1 RCT, P = 0.69, I2 = not applicable).
There was no evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 3.42, P = 0.18).
1.16 Total sperm motility over time
See Analysis 1.16.
1.16. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 16 Total sperm motility over time.
This analysis was only useful in directly comparing the same studies reporting at the three time points and not in comparing results of meta‐analyses that included different subsets of studies.
1.16.1 Total sperm motility at three months or less. We analysed this outcome using a random‐effects model (MD 10.19, 95% CI 4.35 to 16.04, 1105 men, 18 RCTs, 27 intervention arms, P = 0.006, I2 = 97%) and used subtotals (Attallah 2013; Azizollahi 2013; Balercia 2005; Barekat 2016; Conquer 2000; Dimitriadis 2010; Ener 2016; Gopinath 2013; Greco 2005; Lenzi 2003; Martinez‐Soto 2010; Morgante 2010; Nadjarzadeh 2011; Omu 2008; Peivandi 2010; Scott 1998; Sigman 2006; Zavaczki 2003).
1.16.2 Total sperm motility at six months. We analysed this outcome using a random‐effects model (MD 6.00, 95% CI 3.92 to 8.09, 1768 men,13 RCTs, 20 intervention arms, P < 0.000001, I2 = 95%) and used subtotals (Azizollahi 2013; Balercia 2005; Balercia 2009; Blomberg Jensen 2018; Busetto 2018; Ener 2016; Gopinath 2013; Lenzi 2004; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012; Sigman 2006; Suleiman 1996).
1.16.3 Total sperm motility at nine months or more. We analysed this outcome using a random‐effects model (MD 3.29, 95% CI 0.36 to 6.23, 583 men, 5 RCTs, seven intervention arms, P = 0.03 I2 = 94%) and used subtotals (Balercia 2005; Balercia 2009; Ener 2016; Safarinejad 2009a; Safarinejad 2012).
Two of the studies included in the analysis of the semen parameter outcomes (Safarinejad 2009; Safarinejad 2009a) had consistently reported SDs very much smaller than those reported by most of the other included studies. The review authors considered that these were potentially erroneous, but an attempt to check with the study authors was unsuccessful. One other study (Peivandi 2010) also had very small SDs when compared to data in the other studies, but the authors confirmed, when contacted, that they are indeed SDs and not SEs. We tried to manage these analyses in two different ways: firstly we assumed the outcome to have a SD equal to the highest SD from other studies within the same analysis and secondly by treating the data as SEs and converting back to SDs, however heterogeneity remained high in both situations so for the final analyses we reverted to the SDs as reported in the studies. The low SDs may have been due to the strict inclusion and exclusion criteria indicating that the study was homogenous in nature, however we were unable to carry out a sensitivity analysis on these studies as pooling was not possible due to high heterogeneity.
1.17 Progressive sperm motility at three months or less; type of antioxidant
See Analysis 1.17 and Figure 10.
1.17. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 17 Progressive sperm motility at 3 months or less; type of antioxidant.
10.
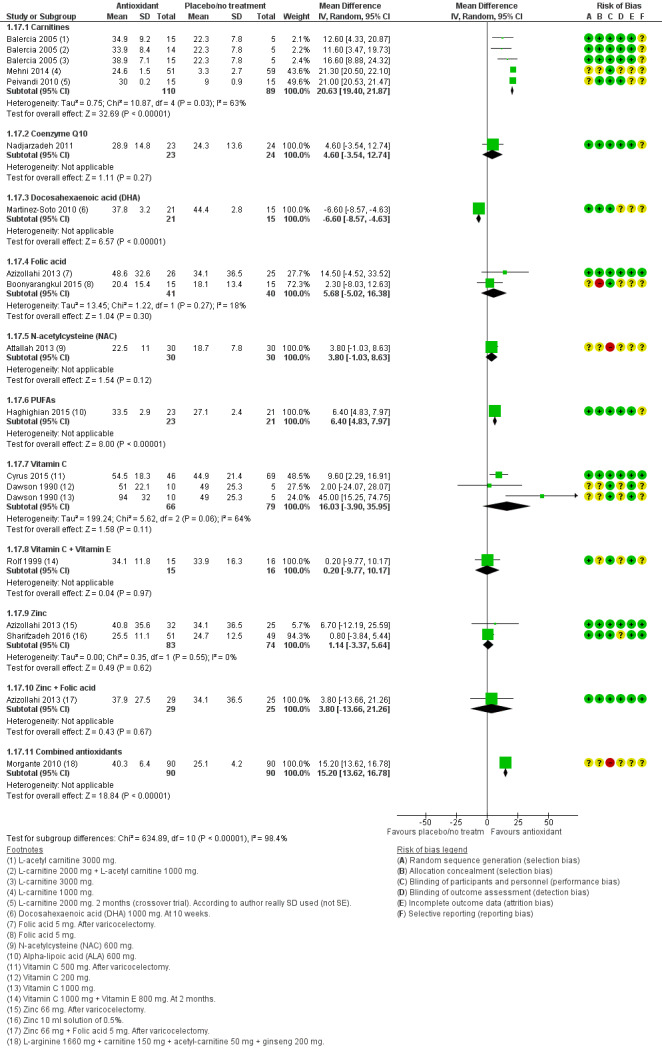
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.17 Progressive sperm motility at 3 months or less; type of antioxidant.
We analysed this outcome using a random‐effects model and used subtotals as pooling was not possible.
1.17.1 Three studies with carnitines reported an increase in progressive sperm motility when compared to placebo (Balercia 2005; Mehni 2014; Peivandi 2010). As the heterogeneity was moderately high (63%), we have not reported the pooled analysis; individually their results were:
Balercia 2005 showed an increase in progressive sperm motility when compared to placebo (MD 13.72, 95% CI 9.08 to 18.35, 59 men, P < 0.00001);
Mehni 2014 showed an increase in progressive sperm motility when compared to placebo (MD 21.30, 95% CI 20.50 to 22.10, 110 men, P < 0.00001);
Peivandi 2010 showed an increase in progressive sperm motility when compared to placebo (MD 21.00, 95% CI 20.53 to 21.47, 30 men, P < 0.00001).
1.17.2 Coenzyme Q10 did not show evidence of increased progressive sperm motility when compared to placebo (Nadjarzadeh 2011) (MD 4.60, 95% CI ‐3.54 to 12.74, 47 men, 1 RCT, P = 0.27, I2 = not applicable).
1.17.3 Docosahexaenoic (DHA) did show an increase in progressive sperm motility when compared to placebo (Martinez‐Soto 2010) (MD ‐6.60, 95% CI ‐8.57 to ‐4.63, 36 men, 1 RCT, P< 0.00001, I2 = not applicable).
1.17.4 Two studied with folic acid did not show evidence of increased progressive sperm motility when compared to placebo (Azizollahi 2013; Boonyarangkul 2015) (MD 5.68, 95% CI ‐5.02 to 16.38, 81 men, 2 RCTs, P = 0.3, I2 = 18%).
1.17.5 N‐acetylcysteine (NAC) did not show evidence of increased progressive sperm motility when compared to no treatment (Attallah 2013) (MD 3.80, 95% CI ‐1.03 to 8.63, 60 men, 1 RCT, P = 0.12, I2 = not applicable).
1.17.6 PUFAs did not show evidence of increased progressive sperm motility when compared to placebo (Haghighian 2015) (MD 6.40, 95% CI 4.83 to 7.97, 44 men,1 RCT, P < 0.00001, I2 = not applicable).
1.17.7 Two studies with vitamin C did show an increase in progressive sperm motility when compared to placebo (Cyrus 2015; Dawson 1990). As the heterogeneity was high (64%) we have not reported the pooled analysis; individually their results were:
Cyrus 2015 showed an increase in progressive sperm motility when compared to placebo (MD 9.60, 95% CI 2.29 to 16.91, 115 men, P = 0.01);
Dawson 1990 showed an increase in progressive sperm motility when vitamin C 1000 mg was compared to placebo (MD 45.00, 95% CI 15.25 to 74.75,15 men, P = 0.03);
Dawson 1990 did not show evidence of increased progressive sperm motility when vitamin C 200 mg was compared to placebo (MD 2.00, 95% CI ‐24.07 to 28.07, 15 men, P = 0.88).
1.17.8 Vitamin C plus vitamin E did not show evidence of increased progressive sperm motility when compared to placebo (Rolf 1999) (MD 0.20, 95% CI ‐9.77 to 10.17, 31 men, 1 RCT, P = 0.97, I2 = not applicable).
1.17.9 Two studies with zinc did not show evidence of increased progressive sperm motility when compared to placebo (Azizollahi 2013; Sharifzadeh 2016) (MD 1.14, 95% CI ‐3.37 to 5.64, 157 men, 2 RCTs, P = 0.62, I2 = 0%).
1.17.10 Zinc plus folic acid did not show evidence of increased progressive sperm motility when compared to placebo (Azizollahi 2013) (MD 3.80, 95% CI ‐13.66 to 21.26, 54 men, 1 RCT, P = 0.67, I2 = not applicable).
1.17.11 Combined antioxidants did not show evidence of increased progressive sperm motility when compared to placebo (Morgante 2010) (MD 15.20, 95% CI 13.62 to 16.78, 180 men, 1 RCT, P < 0.00001, I2 = not applicable).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 1152.65, P < 0.00001).
1.18 Data not usable for meta‐analysis
See Analysis 1.18.
Two studies provided data as medians with interquartile ranges and therefore could not be used in the forest plot (Gamidov 2017; Micic 2017). These data are not rigorous and no conclusions could be made.
1.19 Progressive sperm motility at six months; type of antioxidant
See Analysis 1.19.
1.19. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 19 Progressive sperm motility at 6 months; type of antioxidant.
We analysed this outcome using a random‐effects model and used subtotals as pooling was not possible.
1.19.1 Carnitines did show an increase in progressive sperm motility when compared with placebo (Balercia 2005) (MD 15.94, 95% CI 11.01 to 20.87, 59 men, 1 RCT, 3 intervention arms, P < 0.00001, I2 = not applicable).
1.19.2 Coenzyme Q10 did show an increase in progressive sperm motility when compared to placebo (Balercia 2009) (MD 5.00, 95% CI 2.13 to 7.87, 60 men,1 RCT, P = 0.0006, I2 = not applicable).
1.19.3 Two studies with folic acid did not show evidence of increased progressive sperm motility when compared to placebo (Azizollahi 2013; Boonyarangkul 2015) (MD ‐1.77, 95% CI ‐10.21 to 6.67, 81 men, 2 RCTs, P = 0.68, I2 = 0%).
1.19.4 Vitamin D with calcium did not show evidence of increased progressive sperm motility when compared to placebo (Blomberg Jensen 2018) (MD ‐4.00, 95% CI ‐9.59 to 1.59, 260 men, P = 0.16, I2 = not applicable).
1.19.5 Zinc did not show evidence of increased progressive sperm motility when compared to placebo (Azizollahi 2013) (MD 2.00, 95% CI ‐13.56 to 17.56, 57 men, 1 RCT, P = 0.80, I2 = not applicable).
1.19.6 Zinc plus folic acid did not show evidence of increased progressive sperm motility when compared to placebo (Azizollahi 2013) (MD 2.70, 95% CI ‐14.58 to 19.98, 54 men, 1 RCT, P = 0.76, I2 = not applicable).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 31.49, P < 0.000001).
1.20 Data not usable for meta‐analysis
See Analysis 1.20.
One study provided data as medians with interquartile range and therefore could not be used in the forest plot (Micic 2017). Results indicated that there might be increased progressive sperm motility in the intervention group when measured at six months, however these data are not rigorous and no conclusions could be made.
1.21 Progressive sperm motility at nine months or more; type of antioxidant
See Analysis 1.21.
1.21. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 21 Progressive sperm motility at 9 months or more; type of antioxidant.
We analysed this outcome using a random‐effects model and used subtotals as pooling was not possible.
1.21.1 Carnitines did show an increase in progressive sperm motility when compared to placebo (Balercia 2005) (MD 7.77, 95% CI 2.68 to 12.87, 59 men, 1 RCT, 3 intervention arms, P = 0.003, I2 = not applicable).
1.21.2 Coenzyme Q10 did not show evidence of increased progressive sperm motility when compared to placebo (Balercia 2009) (MD ‐0.90, 95% CI ‐2.68 to 0.88, 60 men, 1 RCT, P = 0.32, I2 = not applicable).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 9.93, P = 0.002).
1.22 Progressive sperm motility over time
See Analysis 1.22.
1.22. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 22 Progressive sperm motility over time.
This analysis was only useful in directly comparing the same studies reporting at the three time points and not in comparing results of meta‐analyses that included different subsets of studies.
1.22.1 Progressive sperm motility at three months or less. We analysed this outcome using a random‐effects model and used subtotals (Attallah 2013; Azizollahi 2013; Balercia 2005; Balercia 2009; Boonyarangkul 2015; Cyrus 2015; Dawson 1990; Haghighian 2015; Martinez‐Soto 2010; Mehni 2014; Morgante 2010; Nadjarzadeh 2011; Peivandi 2010; Rolf 1999).
1.22.2 Progressive sperm motility at six months. We analysed this outcome using a random‐effects model (MD 6.11, 95% CI 0.57 to 11.66, 521 men, 5 RCTs, 9 intervention arms, P = 0.03, I2 = 76%) and used subtotals (Azizollahi 2013; Balercia 2005; Balercia 2009; Blomberg Jensen 2018; Boonyarangkul 2015).
1.22.3 Progressive sperm motility at nine months or more. We analysed this outcome using a random‐effects model (MD 4.64, 95% CI ‐1.67 to 10.95, 119 men, 2 RCTs, 4 intervention arms, P = 0.15) and used subtotals (Balercia 2005; Balercia 2009).
1.23 Sperm concentration at three months or less; type of antioxidant
See Analysis 1.23 and Figure 11.
1.23. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 23 Sperm concentration at 3 months or less; type of antioxidant.
11.
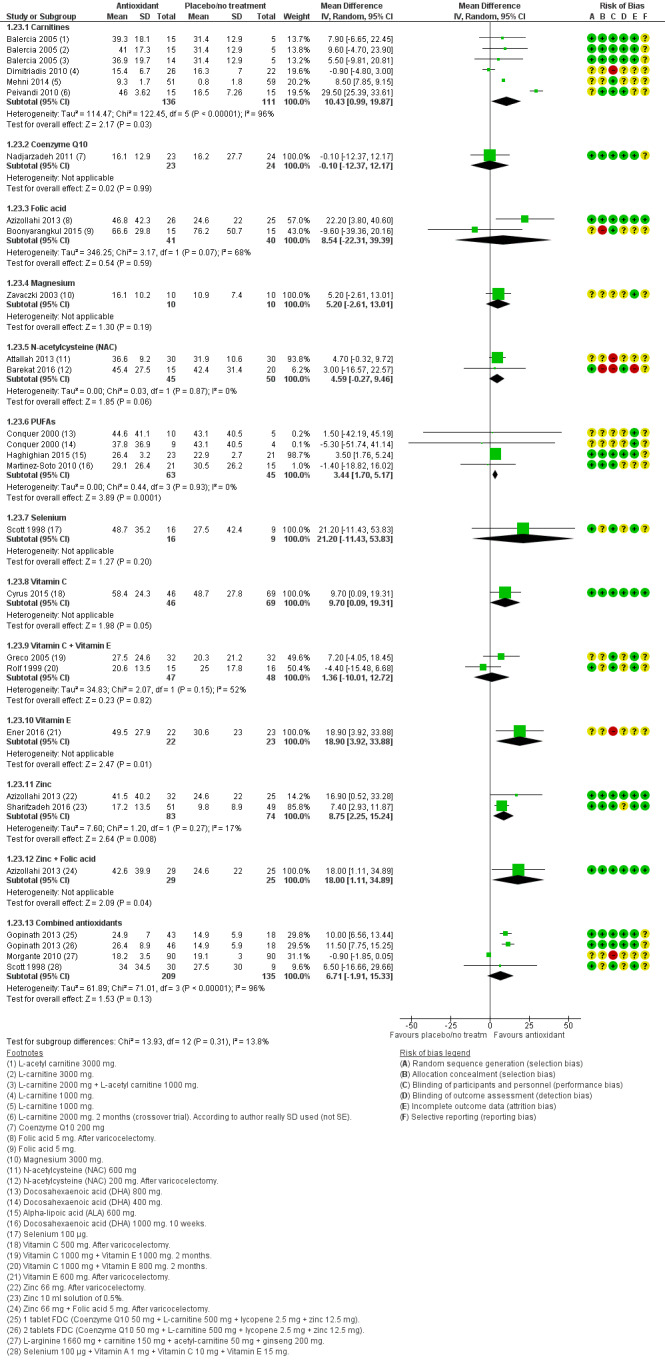
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.23 Sperm concentration at 3 months or less; type of antioxidant.
We analysed this outcome using a random‐effects model. We used only subtotals in this analysis.
1.23.1 Four studies (six intervention arms) compared carnitines with placebo or no treatment and showed an increase in sperm concentration (Balercia 2005; Dimitriadis 2010; Mehni 2014; Peivandi 2010). As the heterogeneity was extremely high (96%) we have not reported the pooled analysis; individually their results were:
Balercia 2005 did not show evidence of increased sperm concentration when compared to placebo (MD 7.76, 95% CI ‐0.73 to 16.25, 59 men, P = 0.07);
Dimitriadis 2010 did not show evidence of increased sperm concentration when compared to no treatment (MD ‐0.90, 95% CI ‐4.80 to 3.00, 48 men, P = 0.65);
Mehni 2014 showed an increase in sperm concentration when compared to placebo (MD 8.50, 95% CI 7.85 to 9.15, 110 men, P < 0.00001);
Peivandi 2010 showed an increase in sperm concentration when compared to placebo (MD 29.50, 95% CI 25.39 to 33.61, 30 men, P < 0.00001).
1.23.2 Coenzyme Q10 did not show evidence of increased sperm concentration when compared to placebo (Nadjarzadeh 2011) (MD ‐0.10, 95% CI ‐12.37 to 12.17, 47 men, 1 RCT, P = 0.99, I2 = not applicable).
1.23.3 Two studies compared folic acid with placebo and did not show evidence of increased sperm concentration (Azizollahi 2013; Boonyarangkul 2015). As the heterogeneity was high (68%) we have not reported the pooled analysis; individually their results were:
Azizollahi 2013 showed an increase in sperm concentration when compared to placebo (MD 22.20, 95% CI 3.80 to 40.60, 51 men, P = 0.02);
Boonyarangkul 2015 did not show evidence of increased sperm concentration when compared to placebo (MD ‐9.60, 95% CI ‐39.36 to 20.16, 30 men, P = 0.53). However, in this study there was great baseline imbalance for sperm parameters between the intervention and control group.
1.23.4 Magnesium did not show evidence of increased sperm concentration when compared to placebo (Zavaczki 2003) (MD 5.20, 95% CI ‐2.61 to 13.01, 20 men, 1 RCT, P = 0.19, I2 = not applicable).
1.23.5 Two studies did not show evidence of increased sperm concentration when N‐acetylcysteine (NAC) was compared with placebo or no treatment (Attallah 2013; Barekat 2016) (MD 4.59, 95% CI ‐0.27 to 9.46, 95 men, 2 RCTs, P = 0.06, I2 = 0%).
1.23.6 Three studies showed an increase in sperm concentration when PUFAs were compared to placebo or no treatment (Conquer 2000; Haghighian 2015; Martinez‐Soto 2010) (MD 3.44, 95% CI 1.70 to 5.17, 108 men, P = 0.0001, I2 = 0%).
1.23.7 Selenium did not show evidence of increased sperm concentration when compared to placebo (Scott 1998) (MD 21.20, 95% CI ‐11.43 to 53.83, 25 men, 1 RCT,P = 0.20, I2 = not applicable).
1.23.8 Vitamin C did not show evidence of increased sperm concentration when compared to placebo (Cyrus 2015) (MD 9.70, 95% CI 0.09 to 19.31, 115 men, 1 RCT,P = 0.05, I2 = not applicable).
1.23.9 Two studies did not show evidence of increased sperm concentration when Vitamin C plus vitamin E was compared to placebo (Greco 2005; Rolf 1999). As the heterogeneity was high (52%) we have not reported the pooled analysis; individually their results were:
Greco 2005 did not show evidence of increased sperm concentration when compared to placebo (MD 7.20, 95% CI ‐4.05 to 18.45, 64 men, P = 0.21);
Rolf 1999 did not show evidence of increased sperm concentration when compared to placebo (MD ‐4.40, 95% CI ‐15.48 to 6.68, 31 men, P = 0.44).
1.23.10 Vitamin E showed an increase in sperm concentration when compared to no treatment (Ener 2016) (MD 18.90, 95% CI 3.92 to 33.88, 45 men, 1 RCT, P = 0.01, I2 = not applicable).
1.23.11 Two studies showed an increase in sperm concentration when zinc was compared to placebo (Azizollahi 2013; Sharifzadeh 2016) (MD 8.75, 95% CI 2.25 to 15.24, 157 men, 2 RCTs, P = 0.008, I2 = 17%).
1.23.12 Zinc plus folic acid showed an increase in sperm concentration when compared to placebo (Azizollahi 2013) (MD 18.00, 95% CI 1.11 to 34.89, 54 men, 1 RCT, P = 0.04, I2 = not applicable).
1.23.13 Three studies (four intervention arms) showed an increase in sperm concentration when combined antioxidants were compared to placebo or no treatment (Gopinath 2013; Morgante 2010; Scott 1998). As the heterogeneity was extremely high (96%) we have not reported the pooled analysis; individually their results were:
Gopinath 2013 showed an increase in sperm concentration when compared to placebo (MD 10.69, 95% CI 8.15 to 13.22, 125 men, P < 0.00001);
Morgante 2010 showed an increase in sperm concentration when compared to no treatment (MD ‐0.90, 95% CI ‐1.85 to 0.05, 180 men, P = 0.06);
Scott 1998 did not show evidence of increased sperm concentration when compared to placebo (MD 6.50, 95% CI ‐16.66 to 29.66, 39 men, P = 0.58).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 236.53, P < 0.00001).
Data not usable for meta‐analysis
See Analysis 1.24.
Four studies (Cavallini 2004; Gamidov 2017; Kessopoulou 1995; Raigani 2014) provided data as medians and interquartile ranges or percentiles and therefore could not be used in the forest plot. These studies might indicate some improvement in sperm concentration in the intervention group when measured at three months, however these data were not rigorous and no conclusions could be made. One study (Lenzi 2003) provided data as the mean with no SD, the P value was 0.03 indicating that there may have been an association between L‐carnitine and improved sperm concentration at three months.
1.25 Sperm concentration at six months; type of antioxidant
See Analysis 1.25.
1.25. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 25 Sperm concentration at 6 months; type of antioxidant.
We analysed this outcome using a random‐effects model. We used only subtotals in this analysis.
1.25.1 Two studies (four intervention arms) did not show evidence of increased sperm concentration when carnitines were compared with placebo (Balercia 2005; Lenzi 2004) (MD 2.60, 95% CI ‐3.13 to 8.33, 115 men, 2 RCTs, 4 intervention arms, P = 0.37, I2 = 0%).
1.25.2 Three studies showed an increase in sperm concentration when coenzyme Q10 was compared with placebo (Balercia 2009; Safarinejad 2009a; Safarinejad 2012). As the heterogeneity was extremely high (96%) we have not reported the pooled analysis; individually their results were:
Balercia 2009 did not show evidence of increased sperm concentration when compared to placebo (MD ‐1.50, 95% CI ‐11.39 to 8.39, 60 men, P = 0.77);
Safarinejad 2009a showed an increase in sperm concentration when compared to placebo (MD 5.60, 95% CI 4.38 to 6.82, 194 men, P < 0.00001);
Safarinejad 2012 showed an increase in sperm concentration when compared to placebo (MD 11.90, 95% CI 10.72 to 13.08, 225 men, P < 0.00001).
1.25.3 Two studies compared folic acid with placebo did not show evidence of increased sperm concentration (Azizollahi 2013; Boonyarangkul 2015). As the heterogeneity was high (78%) we have not reported the pooled analysis; individually their results were:
Azizollahi 2013 showed an increase in sperm concentration when compared to placebo(MD 19.20, 95% CI 12.24 to 26.16, 51 men, P < 0.00001);
Boonyarangkul 2015 did not show evidence of increased sperm concentration when compared to placebo (MD ‐22.80, 95% CI ‐60.44 to 14.84, 30 men, P = 0.24). However, in this study there was great baseline imbalance for sperm parameters between the intervention and control group.
1.25.4 N‐acetylcysteine (NAC) showed an increase in sperm concentration when compared to placebo (Safarinejad 2009) (MD 3.30, 95% CI 1.80 to 4.80, 211 men, 1 RCT, P < 0.0001, I2 = not applicable).
1.25.5 Selenium showed an increase in sperm concentration when compared to placebo (Safarinejad 2009) (MD 4.10, 95% CI 2.45 to 5.75, 211 men, 1 RCT, P < 0.0001, I2 = not applicable).
1.25.6 Selenium plus N‐acetylcysteine (NAC) showed an increase in sperm concentration when compared to placebo (Safarinejad 2009) (MD 8.60, 95% CI 6.89 to 10.31, 210 men, 1 RCT, P < 0.0001 I2 = not applicable).
1.25.7 Vitamin E did not show evidence of increased sperm concentration when compared to no treatment (Ener 2016) (MD 5.90, 95% CI ‐10.83 to 22.63, 45 men, 1 RCT, P = 0.49, I2 = not applicable).
1.25.8 Zinc did not show evidence of increased sperm concentration when compared to placebo (Azizollahi 2013) (MD 9.70, 95% CI ‐7.00 to 26.40, 57 men, 1 RCT, P = 0.26, I2 = not applicable).
1.25.9 Zinc plus folic acid did not show evidence of increased sperm concentration when compared to placebo (Azizollahi 2013) (MD 17.70, 95% CI ‐1.88 to 37.28, 54 men, 1 RCT< P = 0.08, I2 = not applicable).
1.25.10 Two studies (three intervention arms) did not show evidence of increased in sperm concentration when combined antioxidants were compared to placebo (Busetto 2018; Gopinath 2013). As the heterogeneity was high (74%) we have not reported the pooled analysis; individually their results were:
Busetto 2018 showed an increase in sperm concentration when compared to placebo (MD 7.70, 95% CI 2.41 to 12.99, 104 men, P = 0.004);
Gopinath 2013 showed an increase in sperm concentration when compared to placebo (MD 16.48, 95% CI 13.08 to 19.87, 125 men, P < 0.00001).
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 82.87, P < 0.00001)
Data not usable for meta‐analysis
Three studies (Blomberg Jensen 2018; Cavallini 2004; Wong 2002) provided data as medians with interquartile ranges or percentages with no SDs, and therefore could not be used in the forest plot. The last two mentioned of these studies indicated that there might be some improvement in sperm concentration in the intervention group when measured at six months.
1.27 Sperm concentration at nine months; type of antioxidant
See Analysis 1.27.
1.27. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 27 Sperm concentration at 9 months; type of antioxidant.
We analysed this outcome using a random‐effects model. We used only subtotals in this analysis.
1.27.1 Carnitines (three intervention arms) did not show evidence of increased sperm concentration when compared to placebo (Balercia 2005) (MD 4.17, 95% CI ‐1.71 to 10.06, 59 men, 1 RCT, 3 intervention arms, P = 0.16, I2 = not applicable).
1.27.2 Three studies showed an increase in sperm concentration when coenzyme Q10 was compared to placebo (Balercia 2009; Safarinejad 2009a; Safarinejad 2012). As the heterogeneity was extremely high (95%) we have not reported the pooled analysis; individually their results were:
Balercia 2009 did not show evidence of increased sperm concentration when compared to placebo (MD ‐5.40, 95% CI ‐15.75 to 4.95, 60 men, P = 0.31);
Safarinejad 2009a showed an increase in sperm concentration when compared to placebo (MD 1.60, 95% CI 0.53 to 2.67, 194 men, P = 0.003);
Safarinejad 2012 showed an increase in sperm concentration when compared to placebo (MD 6.20, 95% CI 5.17 to 7.23, 225 men, P < 0.00001).
1.27.3 Vitamin E did not show evidence of increased sperm concentration when compared to no treatment (Ener 2016) (MD 11.40, 95% CI ‐2.56 to 25.36, 45 men, 1 RCT, P = 0.11, I2 = not applicable).
There was no evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 1.10, P = 0.58).
1.28 Sperm concentration over time
See Analysis 1.28.
1.28. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 28 Sperm concentration over time.
This analysis was only useful in directly comparing the same studies reporting at the three time points and not in comparing results of meta analyses that included different subsets of studies.
1.28.1 Total sperm concentration at three months or less. We analysed this outcome using a random‐effects model (MD 7.51, 95% CI 4.23 to 10.79, 1244 men, 20 RCTs, P < 0.000001, I2 = 95%) and used subtotals (Attallah 2013; Azizollahi 2013; Balercia 2005; Balercia 2009; Barekat 2016; Boonyarangkul 2015; Conquer 2000; Cyrus 2015; Dimitriadis 2010; Ener 2016; Gopinath 2013; Greco 2005; Haghighian 2015; Martinez‐Soto 2010; Mehni 2014; Morgante 2010; Nadjarzadeh 2011; Peivandi 2010; Rolf 1999; Scott 1998; Zavaczki 2003).
1.28.2 Total sperm concentration at six months. We analysed this outcome using a random‐effects model (MD 7.49, 95% CI 4.76 to 10.23, 1430 men, 11 RCTs, P < 0.0001, I2 = 87%) and used subtotals (Azizollahi 2013; Balercia 2005; Balercia 2009; Busetto 2018; Boonyarangkul 2015; Ener 2016; Gopinath 2013; Lenzi 2004; Safarinejad 2009; Safarinejad 2009a; Safarinejad 2012).
1.28.3 Total sperm concentration at nine months or more. We analysed this outcome using a random‐effects model (MD 3.61, 95% CI 0.17 to 7.06, 583 men, 5 RCTs, seven intervention arms, P = 0.04, I2 = 86%) and used subtotals (Balercia 2005; Balercia 2009; Ener 2016; Safarinejad 2009a; Safarinejad 2012).
2 Head‐to‐head antioxidants (natural conception and undergoing fertility treatment)
The studies included in this comparison did not report on adverse events or sperm DNA fragmentation.
2.1 Live birth; type of antioxidant
See Analysis 2.1.
2.1. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 1 Live birth; type of antioxidant.
Totals were not used in this analysis as there were data for one study only per subgroup, and therefore pooling was not possible.
2.1.1 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased live birth rate when compared to L‐acetyl carnitine (Balercia 2005) (Peto OR 1.00, 95% CI 0.13 to 7.92, 30 men, 1 RCT, P = 1.00).
2.1.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine.There was evidence of the use of L‐carnitine and increased live birth rate when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (Peto OR 0.34, 95% CI 0.06 to 1.79, 30 men, 1 RCT, P = 0.20).
2.1.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine.There was no evidence of the use of L‐acetyl carnitine and increased live birth rate when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (Peto OR 0.34, 95% CI 0.06 to 1.79, 30 men, 1 RCT, P = 0.20).
There was no evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 0.79, P = 0.67)
2.2 Clinical pregnancy; type of antioxidant
See Analysis 2.2.
2.2. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 2 Clinical pregnancy; type of antioxidant.
Totals were not used in this analysis as there were data for one study only per subgroup, and therefore pooling was not possible.
2.2.1. L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased clinical pregnancy rate when compared to L‐acetyl carnitine (Balercia 2005) (Peto OR 1.00, 95% CI 0.13 to 7.92, 30 men, 1 RCT, P = 1.00).
2.2.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased clinical pregnancy rate when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (Peto OR 0.34, 95% CI 0.06 to 1.79, 30 men,1 RCT, P = 0.20).
2.2.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased clinical pregnancy rate when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (Peto OR 0.34, 95% CI 0.06 to 1.79, 30 men,1 RCT, P = 0.20).
2.2.4 Vitamin D plus calcium versus vitamin E plus vitamin C. There was an association between the use of vitamin D plus calcium and increased clinical pregnancy rate when compared to vitamin E plus vitamin C (Deng 2014) (Peto OR 5.13, 95% CI 1.21 to 21.79, 86 men, P = 0.03)
There was evidence that different antioxidants had differing effects (test for subgroup differences: Chi² = 8.15, P = 0.04)
2.3 Total sperm motility at three months or less; type of antioxidant
See Analysis 2.3.
2.3. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 3 Total sperm motility at 3 months or less; type of antioxidant.
Totals were not used in this analysis as, of the eight studies included, there were data for one study only per subgroup, and therefore pooling was not possible.
2.3.1 Docosahexaenoic acid (DHA) 400 mg versus DHA 800 mg. There was no evidence of the use of DHA 400 g/day and increased sperm motility when compared to DHA800 mg/day (Conquer 2000) (MD 7.40, 95% CI ‐11.35 to 26.15, 19 men, P = 0.44).
2.3.2 Ethylcysteine versus vitamin E. There was no evidence of the use of ethyl cysteine and increased sperm motility when compared to vitamin E (Akiyama 1999) (MD ‐1.90, 95% CI ‐41.97 to 38.17, 10 men, P = 0.93).
2.3.3 L acetyl carnitine plus L carnitine versus vitamin E plus vitamin C. There was an association between the use of L acetyl carnitine + L carnitine and increased sperm motility when compared to vitamin E + vitamin C (Li 2005) (MD 23.10, 95% CI 20.14 to 26.06, 138 men, P < 0.00001).
2.3.4 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm motility when compared to L‐acetyl carnitine (Balercia 2005) (MD 3.40, 95% CI ‐3.73 to 10.53, 30 men, P = 0.35).
2.3.5 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 4.80, 95% CI ‐1.76 to 11.36, 30 men, P = 0.15).
2.3.6 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 1.40, 95% CI ‐6.42 to 9.22, 30 men, P = 0.73).
2.3.7 Selenium versus combined antioxidants. There was no evidence of the use of selenium and increased sperm motility when compared to combined antioxidants (Scott 1998) (MD 3.20, 95% CI ‐10.13 to 16.53, 46 men, P = 0.64).
2.3.8 Vitamin C 200 mg/day versus vitamin C 1000 mg/day. There was an association between the use of ascorbic acid 200 mg/day and decreased sperm motility when compared to ascorbic acid 1000 mg/day (Dawson 1990) (MD ‐43.00, 95% CI ‐67.10 to ‐18.90, 20 men, P = 0.0005).
2.3.9 Zinc versus folic acid. There was no evidence of the use of zinc and increased sperm motility when compared to folic acid (Azizollahi 2013) (MD ‐4.40, 95% CI ‐14.21 to 5.41, 80 men, P = 0.38).
2.3.10 Zinc versus zinc plus folic acid. There was no evidence of the use of zinc and increased sperm motility when compared to zinc plus folic acid (Azizollahi 2013) (MD ‐2.80, 95% CI ‐12.91 to 7.31, 80 men, P = 0.59).
2.3.11 Zinc plus folic acid versus folic acid. There was no evidence of the use of zinc plus folic acid and increased sperm motility when compared to folic acid alone (Azizollahi 2013) (MD ‐0.60, 95% CI ‐7.74 to 6.54, 80 men, P = 0.87).
2.3.12 Zinc versus zinc plus vitamin E. There was no evidence of the use of zinc and increased sperm motility when compared to zinc plus vitamin E (Omu 2008) (MD ‐1.00, 95% CI ‐15.00 to 13.00, 18 men, P = 0.89).
2.3.13 Zinc versus zinc plus vitamin E plus vitamin C. There was no evidence of the use of zinc and increased sperm motility when compared to zinc plus vitamin E plus vitamin C (Omu 2008) (MD ‐1.00, 95% CI ‐19.66 to 17.66, 12 men, P = 0.89).
2.3.14 Zinc plus vitamin E versus zinc plus vitamin E plus vitamin C. There was no evidence of the use of zinc plus vitamin E and increased sperm motility when compared to zinc plus vitamin E plus vitamin C (Omu 2008) (MD ‐0.00, 95% CI ‐18.97 to 18.97, 18 men, P = 1.00).
2.4 Total sperm motility at six months or less; type of antioxidant
See Analysis 2.4.
2.4. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 4 Total sperm motility at 6 months; type of antioxidant.
Pooling was not possible in this analysis as of the four studies included in this analysis there were data for one study per subgroup.
2.4.1 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm motility when compared to L‐acetyl carnitine (Balercia 2005) (MD 4.10, 95% CI ‐2.70 to 10.90, 30 men, P = 0.2).
2.4.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 3.40, 95% CI ‐2.87 to 9.67, 30 men, P = 0.29).
2.4.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD ‐0.70, 95% CI ‐7.73 to 6.33, 30 men, P = 0.85).
2.4.4 N‐acetylcysteine versus selenium plus NAC. There was an association between the use of NAC and decreased sperm motility when compared to selenium plus NAC (Safarinejad 2009) (MD ‐4.40, 95% CI ‐5.14 to ‐3.66, 234 men, P < 0.00001).
2.4.5 Selenium versus N‐acetylcysteine (NAC). There was an association between the use of selenium and increased sperm motility when compared to NAC (Safarinejad 2009) (MD 1.30, 95% CI 0.56 to 2.04, 234 men, P = 0.0006).
2.4.6 Selenium versus selenium plus N‐acetylcysteine (NAC). There was an association between the use of selenium and decreased sperm motility when compared to selenium plus NAC (Safarinejad 2009) (MD ‐3.10, 95% CI ‐3.85 to ‐2.35, 232 men, P < 0.00001).
2.4.7 Zinc versus folic acid. There was no evidence of the use of zinc and increased sperm motility when compared to folic acid (Azizollahi 2013) (MD ‐1.70, 95% CI ‐6.42 to 3.02, 80 men, P = 0.48).
2.4.8 Zinc plus folic acid versus folic acid. There was no evidence of the use of zinc plus folic acid and increased sperm motility when compared to folic acid (Azizollahi 2013) (MD 0.90, 95% CI ‐5.45 to 7.25, 80 men, P = 0.78).
2.4.9 Zinc versus zinc plus folic acid. There was no evidence of the use of zinc and increased sperm motility when compared to zinc plus folic acid (Azizollahi 2013) (MD ‐2.60, 95% CI ‐9.13 to 3.93, 80 men, P = 0.44).
Data not usable for meta‐analysis
See Analysis 2.5.
Zinc versus folic acid, zinc versus zinc plus folic acid, folic acid versus zinc plus folic acid. One study Wong 2002 reported data as medians and ranges for these three subgroups. There was no indication of any difference in effect for total sperm motility at six months between the intervention and control groups, however these data were not rigorous and no conclusions could be made.
2.6 Total sperm motility at nine months or more; type of antioxidant
See Analysis 2.6.
2.6. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 6 Total sperm motility at 9 months or more; type of antioxidant.
Pooling was not possible in this analysis as it included only one study.
2.6.1 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm motility when compared to L‐acetyl carnitine (Balercia 2005) (MD 3.70, 95% CI ‐1.69 to 9.09, 30 men, P = 0.18).
2.6.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 5.30, 95% CI ‐0.73 to 11.33,30 men, P = 0.08).
2.6.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 1.60, 95% CI ‐3.29 to 6.49, 30 men, P = 0.52).
2.7 Progressive sperm motility at three months or less; type of antioxidant
See Analysis 2.7.
2.7. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 7 Progessive sperm motility at 3 months or less; type of antioxidant.
Pooling was not possible in this analysis as of the four studies included in this analysis there were data for one study per subgroup.
2.7.1 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased progressive sperm motility when compared to L‐acetyl carnitine (Balercia 2005) (MD 4.00, 95% CI ‐1.88 to 9.88, 30 men, P = 0.18).
2.7.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased progressive sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 5.00, 95% CI ‐0.68 to 10.68, 29 men, P = 0.08)
2.7.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased progressive sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 1.00, 95% CI ‐5.41 to 7.41, 29 men, P = 0.76).
2.7.4 L‐acetyl carnitine versus L‐carnitine plus vitamin E plus vitamin C. There was an association between the use of L‐acetyl carnitine and increased progressive sperm motility when compared to L‐carnitine plus vitamin E plus vitamin C (Li 2005) (MD 13.30, 95% CI 11.21 to 15.39, 138 men, P < 0.00001).
2.7.5 L‐carnitine versus vitamin E plus vitamin C. There was an association between the use of L‐carnitine and increased progressive sperm motility when compared to vitamin E plus vitamin C (Li 2005a) (MD 30.50, 95% CI 27.70 to 33.30, 63 men, P < 0.00001).
2.7.6 L‐carnitine plus vitamin E versus vitamin E. There was an association between the use of L‐carnitine plus vitamin E and increased progressive sperm motility when compared to vitamin E (Wang 2010) (MD 14.10, 95% CI 10.11 to 18.09, 113 men, P < 0.00001).
2.7.7 Vitamin D plus calcium versus vitamin E plus vitamin C. There was an association between the use of vitamin D plus calcium and increased progressive sperm motility when compared to vitamin E plus vitamin C (Deng 2014) (MD 6.90, 95% CI 5.38 to 8.42, 86 men, P < 0.000001).
2.8 Progressive sperm motility at six months; type of antioxidant
See Analysis 2.8.
2.8. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 8 Progressive sperm motility at 6 months; type of antioxidant.
Pooling was not possible in this analysis as it included only one study.
2.8.1 L‐carnitine versus L‐acetyl carnitine. There was an association between the use of L‐carnitine and increased progressive sperm motility when compared to L‐acetyl carnitine (Balercia 2005) (MD 6.30, 95% CI 0.42 to 12.18, 30 men, P = 0.04).
2.8.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was an association between the use of L‐carnitine and increased progressive sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 5.70, 95% CI 0.10 to 11.30, 29 men, P = 0.05).
2.8.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased progressive sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD ‐0.60, 95% CI ‐6.93 to 5.73, 29 men, P = 0.85).
2.9 Progressive sperm motility at nine months or more; type of antioxidant
See Analysis 2.9.
2.9. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 9 Progressive sperm motility at 9 months; type of antioxidant.
Pooling was not possible in this analysis as it included only one study.
2.9.1 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased progressive sperm motility when compared to L‐acetyl carnitine (Balercia 2005) (MD 3.80, 95% CI ‐1.50 to 9.10, 30 men, P = 0.16).
2.9.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased progressive sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 5.50, 95% CI ‐0.11 to 11.11,29 men, P = 0.05).
2.9.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased progressive sperm motility when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 1.70, 95% CI ‐4.17 to 7.57, 29 men, P = 0.57).
2.10 Sperm concentration at three months or less; type of antioxidant
See Analysis 2.10.
2.10. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 10 Sperm concentration at 3 months or less; type of antioxidant.
Pooling was not possible in this analysis as the six studies included in this analysis reported on single subgroups.
2.10.1 Docosahexaenoic acid (DHA) 400 mg versus DHA 800 mg. There was no evidence of the use of DHA 400 mg and increased sperm concentration when compared to DHA 800 mg (Conquer 2000) (MD ‐6.80, 95% CI ‐41.87 to 28.27, 19 men, P = 0.70).
2.10.2 Ethyl cysteine versus vitamin E. There was no evidence of the use of ethyl cysteine and increased sperm concentration when compared to vitamin E (Akiyama 1999) (MD 2.20, 95% CI ‐16.65 to 21.05, 10 men, P = 0.82).
2.10.3 L‐carnitine versus vitamin E plus vitamin C. There was an association between the use of L‐carnitine and increased sperm concentration when compared to vitamin E plus vitamin C (Li 2005a) (MD 15.50, 95% CI 12.49 to 18.51, 63 men, P < 0.00001).
2.10.4 L‐carnitine plus vitamin E versus vitamin E. There was no evidence of the use of L‐carnitine plus vitamin E and increased sperm concentration when compared to vitamin E (Wang 2010) (MD 1.90, 95% CI ‐10.52 to 14.32, 113 men, P = 0.76).
2.10.5 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm concentration when compared to L‐acetyl carnitine (Balercia 2005) (MD 1.70, 95% CI ‐10.97 to 14.37, 30 men, P = 0.79).
2.11.6 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm concentration when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 4.10, 95% CI ‐9.17 to 17.37, 30 men, P = 0.54).
2.10.7 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased sperm concentration when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 2.40, 95% CI ‐11.14 to 15.94, 30 men, P = 0.73).
2.10.8 Selenium versus combined antioxidants. There was no evidence of the use of selenium and increased sperm concentration when compared to combined antioxidants (Scott 1998) (MD 14.70, 95% CI ‐6.51 to 35.91, 46 men, P = 0.17).
2.10.9 Zinc versus folic acid. There was no evidence of the use of zinc and increased sperm concentration when compared to folic acid (Azizollahi 2013) (MD ‐5.30, 95% CI ‐23.38 to 12.78, 80 men, P = 0.57).
2.10.10 Zinc plus folic acid versus folic acid. There was no evidence of the use of zinc plus folic acid and increased sperm concentration when compared to folic acid alone (Azizollahi 2013) (MD ‐4.20, 95% CI ‐22.21 to 13.81, 80 men, P = 0.65).
2.10.11 Zinc versus zinc plus folic acid. There was no evidence of the use of zinc and increased sperm concentration when compared to zinc plus folic acid (Azizollahi 2013) (MD ‐1.10, 95% CI ‐18.63 to 16.43, 80 men, P = 0.90)
2.11 Sperm concentration at six months or less; type of antioxidant
See Analysis 2.11.
2.11. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 11 Sperm concentration at 6 months; type of antioxidant.
Pooling was not possible in this analysis as of the three studies included in this analysis there were data for only one study per subgroup.
2.11.1 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm concentration when compared to L‐acetyl carnitine (Balercia 2005) (MD 5.90, 95% CI ‐8.92 to 20.72, 30 men, P = 0.44).
2.11.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm concentration when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 8.10, 95% CI ‐5.54 to 21.74, 30 men, P = 0.24).
2.11.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased sperm concentration when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 2.20, 95% CI ‐10.89 to 15.29, 30 men, P = 0.74).
2.11.4 N‐acetylcysteine (NAC) versus selenium plus NAC. There was an association between the use of NAC and decreased sperm concentration when compared to selenium plus NAC (Safarinejad 2009) (MD ‐5.30, 95% CI ‐6.86 to ‐3.74, 234 men, P < 0.00001).
2.11.5 Selenium versus N‐acetylcysteine (NAC). There was no evidence of the use of selenium and increased sperm concentration when compared to NAC (Safarinejad 2009) (MD 0.80, 95% CI ‐0.71 to 2.31, 234 men, P = 0.30).
2.11.6 Selenium versus selenium plus N‐acetylcysteine (NAC). There was an association between the use of selenium and decreased sperm concentration when compared to selenium plus NAC (Safarinejad 2009) (MD ‐4.50, 95% CI ‐6.20 to ‐2.80, 232 men, P < 0.00001).
2.11.7 Zinc versus folic acid. There was no evidence of the use of zinc and increased sperm concentration when compared to folic acid (Azizollahi 2013) (MD ‐9.50, 95% CI ‐20.31 to 1.31, 80 men, P = 0.08).
2.11.8 Zinc plus folic acid versus folic acid. There was no evidence of the use of zinc plus folic acid and increased sperm concentration when compared to folic acid (Azizollahi 2013) (MD ‐1.50, 95% CI ‐15.06 to 12.06, 80 men, P = 0.83).
2.11.9 Zinc versus zinc plus folic acid. There was no evidence of the use of zinc and increased sperm concentration when compared to zinc plus folic acid (Azizollahi 2013) (MD ‐8.00, 95% CI ‐23.69 to 7.69, 80 men, P = 0.32).
Data not usable for meta‐analysis
See Analysis 2.12.
One study Wong 2002 reported data as medians and ranges for these three subgroups. There may have been an association with improved sperm concentration at six months for the intervention groups when compared to the control groups, however these data were not rigorous and no conclusions could be made.
2.13 Sperm concentration at nine months or more; type of antioxidant
See Analysis 2.13.
2.13. Analysis.
Comparison 2 Head‐to‐head antioxidant(s), Outcome 13 Sperm concentration at 9 months or more; type of antioxidant.
Pooling was not possible in this analysis as only one study reported on two subgroups.
2.13.1 L‐carnitine versus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm concentration when compared to L‐acetyl carnitine (Balercia 2005) (MD 8.20, 95% CI ‐0.07 to 16.47, 30 men, P = 0.05).
2.13.2 L‐carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐carnitine and increased sperm concentration when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD 6.10, 95% CI ‐3.74 to 15.94, 30 men, P = 0.22).
2.13.3 L‐acetyl carnitine versus L‐carnitine plus L‐acetyl carnitine. There was no evidence of the use of L‐acetyl carnitine and increased sperm concentration when compared to L‐carnitine plus L‐acetyl carnitine (Balercia 2005) (MD ‐2.10, 95% CI ‐10.24 to 6.04, 30 men, P = 0.61).
Funnel plot
We assessed publication bias by using a funnel plot. Only the outcome of clinical pregnancies included 10 studies. There was no clear evidence of publication bias. We did not have enough studies to look at each of the subgroups for publication bias (Figure 6). However, the majority of the other studies (33) included in this review reported only on sperm parameters. Only 30 of the 61 studies reported on pregnancy. Only six studies reported live birth (Balercia 2005; Blomberg Jensen 2018; Kessopoulou 1995; Omu 1998; Suleiman 1996; Tremellen 2007). The author of Balercia 2005 provided live birth data for this update. No new studies in the update reported on live birth. Twelve studies reported on clinical pregnancy (Attallah 2013; Azizollahi 2013; Balercia 2005; Barekat 2016; Busetto 2018; Deng 2014; Haje 2015; Kessopoulou 1995; Omu 1998; Suleiman 1996; Tremellen 2007; Zavaczki 2003). Seventeen studies reported on biochemical pregnancy or undefined pregnancy (Balercia 2009; Cavallini 2004; Ener 2016; Exposito 2016; Galatioto 2008; Gopinath 2013; Lenzi 2003; Lenzi 2004; Li 2005; Nadjarzadeh 2011; Peivandi 2010; Pryor 1978; Rolf 1999; Safarinejad 2009a; Scott 1998; Sigman 2006; Wang 2010) (Table 2). Six of these studies reported on pregnancy rates even though this was not stated a priori in the methods sections of the papers (Balercia 2005; Balercia 2009; Barekat 2016; Kessopoulou 1995; Lenzi 2004; Omu 1998) (Table 3). Six studies were included in both the clinical pregnancy and the live birth analyses (Balercia 2005; Balercia 2009; Kessopoulou 1995; Omu 1998; Suleiman 1996; Tremellen 2007). Failure to report live birth or pregnancy is common and of great loss as ultimately for couples these are the most meaningful outcomes.
Discussion
Summary of main results
Effectiveness of antioxidants versus placebo or no treatment
Live birth
The findings of this review suggest that for subfertile men, the use of antioxidants may be effective in increasing a couple's chances of having a live birth when compared to placebo or no treatment. It was found that within the studies that contributed to the analysis of live birth rate, the population of subfertile men had a baseline or expected live birth rate of 12% and with the use of antioxidant this would increase to between 14% and 26%. However, there were only seven studies with a total of 750 couples reporting on live birth and the quality of this evidence was considered to be low (Table 1). The methods were not well explained in two out of seven of these studies, Suleiman 1996 had a significant number of participants who dropped out of the study and Omu 1998 used 'no treatment' as control which introduced a degree of performance bias. We were unaware of how many of the dropouts were from the treatment or control groups. When these high‐risk studies were removed from the analysis, there was no evidence of association between the use of antioxidants and increased live birth.
The apparent benefit from antioxidants persisted when analyses were restricted to placebo‐controlled studies and studies enrolling men undergoing assisted reproductive techniques (ART) (in vitro fertilisation (IVF)/intracytoplasmic sperm injection(ICSI)).
Clinical pregnancy
The findings of this review also suggest that for subfertile men the use of antioxidants may be effective in increasing a couple's chances of clinical pregnancy rate when compared to placebo or no treatment. It was found that within the studies that contributed to the analysis of clinical pregnancy, the population of subfertile men had a baseline or expected clinical pregnancy rate of 7%,and with the use of antioxidants this would increase to between 12% and 26%. However there were only 11 studies with a total of 786 men reporting on clinical pregnancy and the quality of this evidence was considered to be low (Table 1). The methods were not well explained in four of the 11 studies, with two of these studies having a significant number of participants who dropped out of the study (Barekat 2016; Suleiman 1996). Furthermore, four of the 15 analyses (one trial had three arms) crossed the line of no effect with wide confidence intervals.
The apparent benefit from antioxidants persisted when analyses were restricted to studies at lower risk of bias, studies of men not undergoing ART, and studies of men post‐varicocelectomy. This benefit was not seen in the men undergoing IVF/ICSI.
Adverse events
There is no evidence that antioxidants used by the subfertile male lead to an increased miscarriage risk when compared to placebo or no treatment. It was found that within this population of subfertile men with an expected miscarriage rate of 2%, the use of an antioxidant would increase the chances of having a miscarriage to between 1% and 13%. However, there were only three studies with a total of 247 men reporting on miscarriage and the quality of this evidence was very low quality (Table 1). The event rate in this analysis was very low with only eight miscarriages reported in three studies, furthermore there was a high risk of bias within these studies.
The use of antioxidants by subfertile men may increase the occurrence of mild gastrointestinal complaints when compared to placebo or no treatment. It was found that within this population of subfertile men with an expected gastrointestinal event rate of 2%, the use of an antioxidant would increase the chances of having gastrointestinal complaints to between 2% and 9%. However, there were only 11 studies with a total of 948 men reporting on gastrointestinal complaints and the quality of this evidence was very low (Table 1). The event rate in this analysis was low with only 35 events reported; furthermore there was a high risk of bias within these studies.
There was no evidence that the risk of other adverse events, such as euphoria and ectopic pregnancy differed between antioxidant or control group.
Sperm DNA fragmentation
Only four studies (254 men) reported on sperm DNA fragmentation. Antioxidant use showed a lowered sperm DNA fragmentation when compared to placebo. One study reported substantial higher DNA fragmentation rates (> 80%), which could be explained by enrolment of post‐varicocelectomy participants (Barekat 2016).
Sperm parameters
The findings for total sperm motility, progressive sperm motility and concentration at three, six and nine months were unreliable as heterogeneity was extremely high in each analysis. The only subgroups within the analyses with low heterogeneity reported the following.
Carnitines (three studies, five intervention arms, 128 men) showed evidence of increased total sperm motility at three months when compared to placebo or no treatment
PUFAs (two studies, three intervention arms, 64 men) did not show evidence of increased total sperm motility at three months when compared to placebo
Combined antioxidants (three studies, four intervention arms, 203 men) showed evidence of increased total sperm motility at three months when compared to placebo or no treatment
Zinc (two studies, 157 men) did not show evidence of increased progressive sperm motility at three months when compared to placebo
Folic acid (two studies, 81 men) did not show evidence of increased progressive sperm motility when compared to placebo
N‐acetylcysteine (two studies, 95 men) did not show evidence of increased sperm concentration at three months when compared to placebo or no treatment
PUFAs (three studies, 108 men) showed evidence of increased sperm concentration at three months when compared to placebo or no treatment
Zinc (two studies, 157 men) showed evidence of increased sperm concentration at three months when compared to placebo
Carnitines (two studies, four intervention arms, 115 men) did not show evidence of increased sperm concentration at six months when compared to placebo
Comparisons for each parameter over time showed an improvement after the use of antioxidants, especially after three and six months of use. The slight decrease of this positive effect after nine months of use could be explained by a possible decrease in therapy compliance or less living up to influencing lifestyle factors such as smoking and alcohol use.
Effectiveness of antioxidants versus antioxidants ( head‐to‐head)
In the head‐to‐head studies only two studies reported on live birth and/or clinical pregnancy, one study with different types of carnitines in multiple arms (versus placebo) and one study comparing vitamin D plus calcium with vitamin E plus vitamin C. Only vitamin D plus calcium showed an association. However, due to the small study size no direct conclusions can be drawn. The head‐to‐head studies did not report adverse events.
Overall completeness and applicability of evidence
Of the 61 studies included in this review only seven reported on the primary outcome of live birth, and only 12 reported on clinical pregnancy rate. Live birth and clinical pregnancy rate are the outcomes of most interest to subfertile couples and until these are robustly reported by all subfertility studies we will not be able to draw clear conclusions for the use of antioxidants for subfertile men. We believe that the lower baseline rate for clinical pregnancy than the baseline rate for live birth could be due to the difference in included populations. In the clinical pregnancy analysis (11 studies) there were three studies including men with varicocele; those studies did not report live birth and were therefore not included in the live birth rate analysis (seven studies). Adverse events such as miscarriage, ectopic pregnancy, euphoria and gastrointestinal side effects appear to be poorly reported. The high heterogeneity may be an artefact caused by some of the studies reporting very small and potentially erroneous standard deviations (SDs). This undermines the credibility of the data.
Two of the trials included in the analysis of the semen parameter outcomes (Safarinejad 2009; Safarinejad 2009a) had consistently reported SDs very much smaller than those reported by most of the other included trials. The review authors considered that these were potentially erroneous, but an attempt to check with the study authors was unsuccessful. One other trial (Peivandi 2010), also had very small SDs when compared to data in the other trials but the authors confirmed, when contacted, that they are indeed SDs and not standard errors (SEs). We tried to manage these analyses in two different ways: firstly by imputing SDs from studies of a similar size and secondly by treating the data as SEs and converting back to SDs, however heterogeneity remained high in both situations so for the final analyses we reverted to the SDs as reported in the studies. The low SDs may have been due to the strict inclusion and exclusion criteria indicating that the trial was homogenous in nature, however we were unable to carry out a sensitivity analysis on these trials as pooling was not possible due to high heterogeneity.
Sixteen of the 61 included trials were very small in size (randomising < 50 men), 25 of 61 included trials were small in size (randomising between 50 to 100 men) and only 20 of 61 included trials included more then 100 men. The estimates of the intervention effect tend to be more beneficial in smaller studies. Smaller studies also may not be as rigorous as the larger studies in their methodology (Higgins 2011).
We tried to assess which type of antioxidant might have a beneficial effect on the outcomes of interest in this review, however only three studies at the most could be pooled in any antioxidant subgrouping. Five studies (Busetto 2018; Gopinath 2013; Morgante 2010; Scott 1998; Tremellen 2007) used combined antioxidants versus placebo or no treatment but only Tremellen 2007 reported on live birth and clinical pregnancy rate. The other studies reported on total or progressive sperm motility and concentration.
The head‐to‐head comparison does not provide constructive information as we could not pool direct comparisons. Subgrouping of antioxidants, or different doses of antioxidants, was unable to be performed in the treatment versus treatment groups as there were only single studies analysing these differences. Therefore, this review was unable to show any difference in effect between different antioxidants or different doses of the same antioxidant.
There were 24 studies that contained data that were unusable in the analysis, with either some or all of their data (Biagiotti 2003; Boonyarangkul 2015; Cavallini 2004; Eslamian 2013; Exposito 2016; Gamidov 2017; Galatioto 2008; Haje 2015; Kessopoulou 1995; Kumamoto 1988; Lenzi 2003; Lombardo 2002; Martinez 2015; Micic 2017; Nozha 2001; Omu 1998; Pourmand 2014; Poveda 2013; Pryor 1978; Raigani 2014; Sivkov 2011; Sofikitis 2016; Wong 2002; Zalata 1998). The reasons for this were baseline imbalance, presentation of medians, percentages or ranges, and in some cases no SDs or SEs were given (Analysis 1.10; Analysis 1.12; Analysis 1.14; Analysis 1.18; Analysis 1.20; Analysis 1.24; Analysis 1.26; Analysis 2.5; Analysis 2.12). Attempts were made to contact these authors regarding the data. There was no clear evidence of publication bias
Quality of the evidence
The evidence was graded as low to very low quality. The main limitation of this review was that out of the 44 included studies in the meta‐analysis, only 13 studies reported on live birth or clinical pregnancy. Other limitations included poor reporting of study methods, imprecision, the number of small studies, reporting bias and lack of data about adverse events.There was no clear evidence of publication bias.
Figure 2 shows the review authors' judgements about the risk of bias of the studies included in this review. All included studies were described as randomised, however only less than 50% gave information on how the randomisation was achieved. Allocation concealment was described in only 31% of the studies. Blinding was better described with over 56% of the studies being double‐blinded or occasionally single‐blinded; 8% of studies stated that there was no blinding and 21% of included studies used no treatment as a control. Dropout rates were high in some studies and dropout rates tended to be higher in the control groups, which created a potential for differential follow‐up with better reporting of clinical pregnancies in the intervention groups. Reporting bias was unclear in 87% of studies.
Potential biases in the review process
There may have been some potential for bias in the review process, as there were some changes compared to the protocol. These included additions and deletions to exclusion criteria such as the removal of pentoxifylline, and adding the new outcome progressive sperm motility. Some bias in the review process may have arisen due to the inclusion of studies that have had a dropout of participants of > 20%, with subsequent imbalances in the number of participants between the treatment and control groups.
Agreements and disagreements with other studies or reviews
The results of our review are in agreement with those of other published systematic reviews. Two other reviews described the effects of L‐carnitine and L‐acetylcarnitine on subfertile men. The systematic review and meta‐analysis by Zhou and colleagues (Zhou 2007) compared L‐carnitine and L‐acetylcarnitine therapy versus placebo treatment and found improvements in pregnancy rate and total sperm motility. Our review was unable to pool the results of the carnitine studies due to inconsistencies between the studies. The descriptive review by Patel and Sigman (Patel 2008) discusses the improvement in pregnancy rates with oral intake of antioxidants, however Patel states that randomised controlled trials (RCTs) have not shown an effect on sperm motility and that there is a need for more RCTs in men with oxidative stress. Furthermore, Garg 2016 discusses in a review the effect of antioxidants in men with varicocele. They conclude that antioxidant therapy is a potential option as primary treatment or adjunct after surgical repair of varicocele.
Agarwal and colleagues discussed in both an overview of the literature (Agarwal 2004) and systematic review (Majzoub 2018), the effectiveness of antioxidants. In the 2004 overview Agarwal notes that vitamin E and a combination of vitamin E with other antioxidants such as N‐acetylcysteine, vitamin A and fatty acids appears to improve pregnancy rates in asthenozoospermic men. This is in agreement with our review. However, their conclusion that carnitines also appear to have an effect on pregnancy rates could not be confirmed. In the systematic review Majzoub 2018 included 29 studies, of which there were 19 RCTs and 10 prospective studies. In 26 studies they found a significant positive effect on basic semen parameters, advanced sperm function tests, ART outcomes or live birth rate. Specifically, a positive effect was seen on live birth rate and fertilisation rate when using vitamin E, vitamin C, carnitines, coenzyme Q10 and zinc. A difference between differing antioxidants was not seen in our study.
Another review (Ross 2010) showed improvement in pregnancy rate and sperm quality after antioxidant therapy. This is in agreement with our review, although we are uncertain of the sperm parameter outcomes due to the extreme heterogeneity. A systematic review (Lafuente 2013) looking at the effect of coenzyme Q10 and male subfertility found an association between this antioxidant and improved pregnancy rate, sperm concentration and motility. We did agree on the effect of coenzyme Q10 on sperm motility and concentration at six months, however we could not draw clear conclusions due to the heterogeneity in these analyses. A more recent systematic review with meta‐analysis studied the effectiveness of folate and folate plus zinc on sperm parameters in subfertile men (Irani 2017). They concluded that folate alone was only effective on sperm concentration, and folate plus zinc only on sperm concentration and morphology. Both interventions did not have any effect on sperm motility. This effect of zinc plus folate or folate alone could be confirmed with our review.
The above‐mentioned systematic reviews mainly reported on overall pregnancy rates, whereas this updated Cochrane Review reported specifically on clinical pregnancy rates (as confirmed by the identification of a gestational sac on ultrasound) so fewer studies were available for analysis.
A Cochrane Review of antioxidants for female subfertility has been published (Showell 2017) showing that there is limited evidence for a beneficial effect of antioxidants for subfertile women. Furthermore, a recent systematic review and meta‐analysis looking at the effect of micronutrient supplementation, in both male and females, on IVF outcomes showed a positive influence on clinical outcomes in terms of pregnancy rate and/or live birth rate (Kofi Arhin 2017). However, only five RCTs could be included, with significant heterogeneity among the interventions and study designs.
Authors' conclusions
Implications for practice.
In this review, there is low‐quality evidence from seven small randomised controlled trials suggesting that antioxidant supplementation in subfertile males may improve live birth rates for couples attending fertility clinics. Low‐quality evidence suggests that clinical pregnancy rates may also increase. Overall, there is no evidence of increased risk of miscarriage, however antioxidants may give more mild gastrointestinal upsets but the evidence is of very low quality. Subfertilte couples should be advised that overall the current evidence is inconclusive based on serious risk of bias due to poor reporting of methods of randomisation, failure to report on the clinical outcomes live birth rate and clinical pregnancy, often unclear or even high attrition, and also imprecision due to often low event rates and small overall sample sizes. Further large well‐designed randomised placebo‐controlled trials reporting on pregnancy and live births are still required to clarify the exact role of antioxidants.
Implications for research.
In this review there were only seven small studies reporting on live birth, the most important outcome from the perspective of the couple experiencing difficulty with conception, and the number of events was very small. Strangely, most of the trials in our meta‐analysis reporting on live birth are from before 2008. Despite our recommendations in the original review and 2014 update on this topic, principal investigators of clinical trials seem not to have taken clinical outcomes into consideration, which leads to a great gap in evidence. Only four studies reported on DNA fragmentation. A low degree of DNA fragmentation is thought to increase the likelihood of achieving a pregnancy. Further large well‐designed placebo‐controlled randomised trials with live birth as primary outcome are needed.
Five studies (Busetto 2018; Gopinath 2013; Morgante 2010; Scott 1998; Tremellen 2007) used combined antioxidants (three or more antioxidants) versus control but reported on different outcomes. The results were generally in favour of the antioxidant over the control. However, there is a need for more randomised controlled trials in order to make any conclusions on whether a combination of antioxidants would have a statistically significant benefit over a single antioxidant versus placebo.
If evidence emerges from placebo‐controlled randomised trials which shows that antioxidant supplements improve clinical outcomes (pregnancy and live birth) then randomised head‐to‐head trials will be needed to assess whether one antioxidant is more effective than another in terms of size of benefit. A network meta‐analysis could be of interest.
There is also a gap in the evidence as to whether different doses of an antioxidant have different effects. This review was only able to include single studies measuring different doses and therefore meta‐analysis of this comparison was not possible.
Evidence to date shows that few studies reported side effects. According to the studies that did report, the side‐effect profile of antioxidants was low and mild. However, more data are required to evaluate fully any adverse events and the side effect profile of these supplements.
What's new
| Date | Event | Description |
|---|---|---|
| 4 December 2018 | New search has been performed | Nineteen new studies were added in this update (Barekat 2016; Blomberg Jensen 2018; Boonyarangkul 2015; Busetto 2018; Cyrus 2015; Deng 2014; Ener 2016; Exposito 2016; Gamidov 2017; Gopinath 2013; Haghighian 2015; Haje 2015; Martinez 2015; Mehni 2014; Micic 2017; Pourmand 2014; Raigani 2014; Sharifzadeh 2016; Sofikitis 2016). There is one study placed in awaiting classification (Goswami 2015). All pentoxifylline studies were excluded. Two previously included studies were excluded for containing an ineligible study population. |
| 4 December 2018 | New citation required and conclusions have changed | Pentoxifylline was removed from the review due to the fact that it is a prescription drug and not an 'over‐the‐counter' supplement. Progressive sperm motility was added as a secondary outcome; this is an outcome with more clinical importance than total sperm motility. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 10 February 2015 | Amended | Correction of some analysis graph labels. |
| 28 November 2014 | New citation required and conclusions have changed | Comparisions were restructured into a more logical framework. Clinical pregnancy rate data were used in this update rather than the undefined pregnancy rate data of the original review as this is more clinically meaningful when considering the evidence for use of antioxidants. |
| 28 November 2014 | New search has been performed | 14 new studies were added in this update (Attallah 2013, Azizollahi 2013, Dimitriadis 2010, Eslamian 2013, Kumamoto 1988, Martinez‐Soto 2010, Morgante 2010, Nadjarzadeh 2011, Poveda 2013, Pryor 1978, Safarinejad 2011b, Safarinejad 2012, Sivkov 2011, Wang 2010). The search was updated in August 2014 and six studies were placed in awaiting classification (Anarte 2013a; Gopinath 2013; Iacono 2014; Nadjarzadeh 2014; Nashivochnikova 2014a; Nematollahi‐Mahani 2014). |
| 7 December 2011 | Feedback has been incorporated | Change of emphasis to conclusions, additional sensitivity analysis performed, Risk of Bias, Summary of Findings Table and Discussion sections edited to increase this review's focus on clinical outcomes of pregnancy and live birth. |
| 3 May 2011 | Amended | 2.1 Analysis edited to fixed effect Peto. The conclusions remain the same. |
| 8 March 2011 | Amended | Changed summary of findings table to reflect quality of studies |
| 21 December 2010 | Amended | Minor edits made ‐ no changes to conclusions |
| 4 May 2007 | New citation required and major changes | Substantive amendment |
Acknowledgements
Cochrane Gynaecology and Fertility group. I would like to make special mention of the editors who were very thorough and helpful in editing this review.
Many thanks to the translators of the non‐English studies: Ichiro Omori, Shaofu Li, Ivan Sola, Pawel Kanturski, Dr Peviandi, Shaofu Li, Farhad Shokraneh, Taixiang Wu, Juliane Reid, Roberto D'Amico, Vasily Vlassov, Liu Qin, Jianping Liu, Guoyan Yang, Gustavo Porfi, Valter Silva, Maíra Parra, Dr Tomoko Kumaga, Tan Wantao and Andrew Dubovyi. A special thank you to Juliane Reid and Helen Nagels for putting us in touch with many of our translators.
Thanks also to Stephan Bontekoe who kindly helped with some of the text in the original review.
We acknowledge comments sent by Tina Kold Jensen, Niels Erik Skakkebaek, Niels Jørgensen, Martin Blomberg Jensen, Anders Juul, Peter Gøtzsche, Department of Growth and Reproduction, and The Nordic Cochrane Centre, Rigshospitalet, Denmark. Our formal response was published in December 2011 and the points made have been addressed.
The authors of the 2018 review thank Professor Roger Hart for his contributions to all previous version of this review.
Further information for the studies was received from:
Dr N Adel (Adel 2015)
Dr Ovchinnikov (Gamidov 2017)
Dr Zavari (Gopinath 2013)
Dr Kabir (Cyrus 2015)
Professor Matorras (Exposito 2016)
Dr Balercia (Balercia 2005; Balercia 2009)
Dr Busetto (Busetto 2018)
Dr Nasr‐Esfahani (Barekat 2016)
Dr Irge (NCT01520584)
Dr Dimitriadis (Sofikitis 2016)
Dr Agarwal and ms. Micic (Micic 2017)
Dr Norouzi (Sharifzadeh 2016)
Dr Hekmatdoost (NCT01846325)
Dr Mathieu‐d'Argent (NCT01407432)
Dr Kamath (CTRI/2013/02/003431)
Dr Pinter (NCT02310087)
Dr Nematollahi‐mahani (Azizollahi 2013),
Associate Professor Kelton Tremellen (Tremellen 2007).
Dr Kamath (CTRI/2013/02/003431)
Dr Peivandi (Peivandi 2010)
Dr El Gindy (Elgindy 2008)
Dr M Sigman (Sigman 2006)
Professor Niewchlag (Rolf 1999)
Dr Cavallini (Cavallini 2004)
Dr Wang (Wang 1983)
Dr Martinez‐Soto (Martinez‐Soto 2010)
Dr Morgante (Morgante 2010)
Dr Nadjarzadeh (Nadjarzadeh 2011)
Dr Safarinejad (Safarinejad 2009; Safarinejad 2009a).
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
Searched 1 Febuary 2018
PROCITE platform
Keywords CONTAINS "antioxidants" or "antioxidant levels" or "vitamin" or "vitamin A" or "vitamin B" or "Vitamin‐B‐12" or "Vitamin‐B‐12‐Therapeutic‐Use" or "vitamin B6" or "vitamin C" or "Vitamin D" or "vitamin E" or "vitamins" or "selenium" or "folic acid" or "glutathione" or "Menevit anti‐oxidant" or "carnitene" or "ascorbic acid" or "zinc" or "fatty acids" or "oil" or "fish oils" or "plant extracts" or "flavonoids" or "L‐arginine" or "pycnogenol" or "folate" or "ubiquinol "or "coenzyme Q10"or "L‐carnitin" or "L‐carnitine" or "multivitamins" or "beta‐caritine" or "N‐acetyl cysteine" or "L‐acetyl‐carnitine" or "acetyl L‐carnitine" or "acetylcysteine" or "ethylcysteine" or "alpha tocopherol" or "pentoxifylline" or "omega‐3"or "omega‐6 fatty acid" or "inositol" or "Myo‐inositol" or "d‐chiro‐inositol" or "melatonin" or "docosahexaenoic acid" or "Magnesium" or "nutritional supplement" or "nutritional supplements" or Title CONTAINS "antioxidants" or "antioxidant levels" or "vitamin" or "vitamin A" or "vitamin B" or "Vitamin‐B‐12" or "Vitamin‐B‐12‐Therapeutic‐Use" or "vitamin B6" or "vitamin C" or "nutritional supplement" or "nutritional supplements"
AND
Keywords CONTAINS "idiopathic asthenospermia" or "idiopathic oligozoospermia" or "IVF" or "ICSI" or "Intrauterine Insemination" or "ART" or "Sperm" or "sperm DNA integrity" or "sperm damage" or "sperm quality" or "sperm parameters" or "oligo‐asthenozoospermia" or "Oligoasthenospermia" or "oligoasthenoteratozoospermia" or "oligospermia" or "oligozoospermia" or "asthenospermia" or "asthenozoospermia" or "assisted reproduction techniques" or "azoospermia" or "Male" or "male subfertility" or Title CONTAINS "idiopathic asthenospermia" or "idiopathic oligozoospermia" or "Sperm" or "sperm DNA integrity" or "sperm damage" or "sperm quality" or "sperm parameters" or "oligo‐asthenozoospermia" or "Oligoasthenospermia" or "oligoasthenoteratozoospermia" or "oligospermia" or "oligozoospermia" or "asthenospermia" or "asthenozoospermia" or "assisted reproduction techniques" or "azoospermia" or "Male" (318 hits)
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 1 Febuary 2018
Web platform
#1 MeSH descriptor: [Infertility, Male] explode all trees 664
#2 asthenozoospermia or oligospermia or azoospermia:ti,ab,kw 462
#3 Asthenospermia or Teratospermia:ti,ab,kw 75
#4 MeSH descriptor: [Spermatozoa] explode all trees 440
#5 Sperm*:ti,ab,kw 3994
#6 male subfertility:ti,ab,kw 197
#7 male infertility:ti,ab,kw 1604
#8 subfertile men:ti,ab,kw 48
#9 infertile men:ti,ab,kw 265
#10 semen:ti,ab,kw 1255
#11 oligoasthenoteratozoospermia:ti,ab,kw 25
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 5016
#13 MeSH descriptor: [Antioxidants] explode all trees 4250
#14 antioxidant*:ti,ab,kw 8353
#15 radical scavenger*:ti,ab,kw 687
#16 MeSH descriptor: [Vitamins] explode all trees 2263
#17 vitamin*:ti,ab,kw 19677
#18 MeSH descriptor: [Zinc] explode all trees 1393
#19 zinc:ti,ab,kw 4285
#20 MeSH descriptor: [Selenium] explode all trees 584
#21 Selenium:ti,ab,kw 1456
#22 Glutathione or folate:ti,ab,kw 4279
#23 ubiquin$ or folic acid:ti,ab,kw 3446
#24 coenzyme q10:ti,ab,kw 524
#25 MeSH descriptor: [Carnitine] explode all trees 559
#26 carnitine$ or carotenoid 1792
#27 astaxanthin$ or lycopene 564
#28 menevit 3
#29 multivitamin$ 904
#30 betacarotene$ or beta carotene$ 1694
#31 ascorbic acid 3534
#32 acetylcysteine 1587
#33 MeSH descriptor: [Acetylcysteine] explode all trees 738
#34 Acetylcysteine 1587
#35 cysteine or ethylcysteine 1083
#36 alpha‐tocopherol$ 2596
#37 fish oil$ 2286
#38 omega$ 4656
#39 MeSH descriptor: [Fatty Acids] explode all trees 19683
#40 fatty acid$ 10373
#41 arginine or flavonoid or carotenoid or riboflavin 5416
#42 pycnogenol$ or lutein$ or lipoic acid$ or Inositol 1626
#43 MeSH descriptor: [Inositol] explode all trees 340
#44 myoinositol or mesoinositol or melatonin 1767
#45 cysteine or docosahexaenoic or magnesium 9176
#46 nutritional supplement$ 2441
#47 nutraceutical$ 383
#48 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 73432
#49 #12 and #48 419
Appendix 3. MEDLINE search strategy
Searched 1946 to 1 Febuary 2018
OVID platform
1 exp male infertility/ (25443) 2 (asthenozoospermia or oligospermia or azoospermia).tw. (6718) 3 Asthenospermia.tw. (319) 4 Teratospermia.tw. (157) 5 exp Spermatozoa/ (61935) 6 Sperm$.tw. (123530) 7 (male$ adj2 subfertil$).tw. (716) 8 (male$ adj2 infertil$).tw. (10031) 9 (subfertil$ adj2 men).tw. (493) 10 (infertil$ adj2 men).tw. (4006) 11 (male$ adj2 fertility).tw. (5274) 12 semen.tw. (26643) 13 oligoasthenoteratozoospermi$.tw. (360) 14 or/1‐13 (153999) 15 exp antioxidants/ or free radical scavengers/ (411638) 16 (antioxidant$ or radical scavengers).tw. (161958) 17 exp vitamins/ or exp ascorbic acid/ or exp dehydroascorbic acid/ or exp vitamin a/ or exp vitamin e/ or exp vitamin u/ or exp alpha‐tocopherol/ or exp beta carotene/ or exp beta‐tocopherol/ or exp gamma‐tocopherol/ (318144) 18 vitamin$.tw. (184483) 19 exp Zinc/ (55556) 20 exp Selenium/ (18842) 21 (Glutathione$ or folate).tw. (134066) 22 exp Glutathione Peroxidase/ or exp folic acid/ (52442) 23 exp Ubiquinone/ (8226) 24 (ubiquin$ or folic acid).tw. (25733) 25 coenzyme q10.tw. (2906) 26 exp Carnitine/ (8935) 27 (carnitine$ or carotenoid$).tw. (30261) 28 (astaxanthin$ or lycopene$).tw. (5723) 29 menevit.tw. (3) 30 multivitamin$.tw. (3391) 31 (betacarotene$ or beta carotene$).tw. (12411) 32 ascorbic acid.tw. (28266) 33 n‐acetylcysteine.tw. (9954) 34 exp Acetylcysteine/ (11959) 35 Acetylcysteine.tw. (10732) 36 Acetyl cysteine.tw. (3094) 37 Acetyl‐carnitine.tw. (168) 38 ethylcysteine.tw. (62) 39 alpha‐tocopherol$.tw. (14639) 40 (fish adj2 oil$).tw. (9589) 41 omega$.tw. (44863) 42 exp fatty acids/ or exp fish oils/ or exp cod liver oil/ or exp fatty acids, omega‐3/ or exp plant oils/ (448639) 43 fatty acid$.tw. (185377) 44 (plant adj4 oil$).tw. (2449) 45 arginine.tw. (88608) 46 flavonoid$.tw. (32547) 47 carotenoid$.tw. (17021) 48 riboflavin$.tw. (9284) 49 pycnogenol$.tw. (345) 50 lutein$.tw. (36115) 51 lipoic acid$.tw. (3967) 52 exp Inositol/ (22263) 53 (Inositol or myoinositol).tw. (35152) 54 mesoinositol.tw. (36) 55 melatonin.tw. (21226) 56 n acetyl cysteine.tw. (3045) 57 docosahexaenoic acid.tw. (10078) 58 magnesium.tw. (51372) 59 nutritional supplement$.tw. (5235) 60 (diet$ adj3 supplement$).tw. (36417) 61 nutraceutical$.tw. (4403) 62 or/15‐61 (1623704) 63 randomized controlled trial.pt. (452080) 64 controlled clinical trial.pt. (92108) 65 randomized.ab. (400977) 66 placebo.tw. (190947) 67 clinical trials as topic.sh. (182333) 68 randomly.ab. (283901) 69 trial.ti. (176948) 70 (crossover or cross‐over or cross over).tw. (75086) 71 or/63‐70 (1155842) 72 (animals not (humans and animals)).sh. (4385913) 73 71 not 72 (1063162) 74 14 and 62 and 73 (559)
Appendix 4. Embase search strategy
Searched 1980 to 1 Febuary 2018
OVID platform
1 exp male infertility/ (36830) 2 (asthenozoospermia or oligospermia or azoospermia).tw. (8576) 3 Asthenospermia.tw. (404) 4 Teratospermia.tw. (196) 5 exp Spermatozoa/ (40046) 6 Sperm$.tw. (134402) 7 (male$ adj2 subfertil$).tw. (924) 8 (male$ adj2 infertil$).tw. (13888) 9 (subfertil$ adj2 men).tw. (607) 10 (infertil$ adj2 men).tw. (5561) 11 (male$ adj2 fertility).tw. (6397) 12 semen.tw. (31205) 13 oligoasthenoteratozoospermi$.tw. (501) 14 or/1‐13 (168218) 15 vitamin$.tw. (224591) 16 exp Zinc/ (96319) 17 exp Selenium/ (33745) 18 (zinc or selenium).tw. (136357) 19 (Glutathione$ or folate).tw. (154128) 20 exp Ubiquinone/ (7428) 21 ubiquin$.tw. (8388) 22 coenzyme q10.tw. (4117) 23 exp Carnitine/ (13571) 24 (carnitine$ or carotenoid$).tw. (34331) 25 (astaxanthin$ or lycopene$).tw. (6806) 26 menevit.tw. (12) 27 multivitamin$.tw. (4547) 28 (betacarotene$ or beta carotene$).tw. (14287) 29 ascorbic acid.tw. (31008) 30 n‐acetylcysteine.tw. (12585) 31 exp acetylcysteine/ (31271) 32 acetylcysteine.tw. (13657) 33 Acetyl cysteine.tw. (4153) 34 ethylcysteine.tw. (61) 35 alpha‐tocopherol$.tw. (15603) 36 (fish adj2 oil$).tw. (12046) 37 omega$.tw. (44717) 38 fatty acid$.tw. (208396) 39 (plant adj4 oil$).tw. (3474) 40 arginine.tw. (95239) 41 flavonoid$.tw. (47169) 42 carotenoid$.tw. (18326) 43 riboflavin$.tw. (9563) 44 pycnogenol$.tw. (439) 45 lutein$.tw. (36273) 46 lipoic acid$.tw. (4844) 47 exp antioxidant/ (165170) 48 free radical scavengers/ (20469) 49 (antioxidant$ or radical scavengers).tw. (209843) 50 exp vitamin/ or exp ascorbic acid/ or exp carotenoid/ or exp multivitamin/ or vitamin b group/ (560638) 51 exp edible oil/ or exp castor oil/ or exp lyprinol/ or exp olive oil/ or exp safflower oil/ or exp essential fatty acid/ or exp arachidonic acid/ or exp linoleic acid/ or exp linolenic acid/ or exp gamma linolenic acid/ or exp unsaturated fatty acid/ or exp omega 6 fatty acid/ or exp polyunsaturated fatty acid/ (170403) 52 exp fatty acid/ (499866) 53 exp vegetable oil/ (71261) 54 exp fish oil/ (15511) 55 exp cod liver oil/ (1108) 56 exp omega 3 fatty acid/ (26841) 57 exp inositol/ (10949) 58 docosahexaenoic acid.tw. (12438) 59 magnesium.tw. (57498) 60 (Inositol or myoinositol).tw. (38014) 61 mesoinositol.tw. (10) 62 melatonin.tw. (25584) 63 nutritional supplement$.tw. (7123) 64 nutraceutical$.tw. (5817) 65 or/15‐64 (1906582) 66 Clinical Trial/ (963424) 67 Randomized Controlled Trial/ (481441) 68 exp randomization/ (76750) 69 Single Blind Procedure/ (30162) 70 Double Blind Procedure/ (142753) 71 Crossover Procedure/ (53922) 72 Placebo/ (303506) 73 Randomi?ed controlled trial$.tw. (170913) 74 Rct.tw. (26655) 75 random allocation.tw. (1715) 76 randomly allocated.tw. (28704) 77 allocated randomly.tw. (2277) 78 (allocated adj2 random).tw. (789) 79 Single blind$.tw. (20147) 80 Double blind$.tw. (177989) 81 ((treble or triple) adj blind$).tw. (733) 82 placebo$.tw. (259893) 83 prospective study/ (418782) 84 or/66‐83 (1845002) 85 case study/ (51632) 86 case report.tw. (343900) 87 abstract report/ or letter/ (1015495) 88 or/85‐87 (1402788) 89 84 not 88 (1798045) 90 14 and 65 and 89 (1401)
Appendix 5. CINAHL search strategy
Searched from 1961 to 1 February 2018
EBSCO platform
| # | Query | Results |
| S43 | S25 AND S42 | 100 |
| S42 | S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 1,203,002 |
| S41 | TX allocat* random* | 8,211 |
| S40 | (MH "Quantitative Studies") | 18,214 |
| S39 | (MH "Placebos") | 10,641 |
| S38 | TX placebo* | 49,628 |
| S37 | TX random* allocat* | 8,211 |
| S36 | (MH "Random Assignment") | 45,438 |
| S35 | TX randomi* control* trial* | 142,613 |
| S34 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) | 13,112 |
| S33 | TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) | 923,796 |
| S32 | TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) | 291 |
| S31 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 224 |
| S30 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 224 |
| S29 | "or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )" | 224 |
| S28 | TX clinic* n1 trial* | 219,940 |
| S27 | PT Clinical trial | 85,642 |
| S26 | (MH "Clinical Trials+") | 233,936 |
| S25 | S20 AND S24 | 373 |
| S24 | S21 OR S22 OR S23 | 4,567 |
| S23 | TX sperm* | 4,329 |
| S22 | (MH "Sperm Motility") OR (MH "Spermatozoa") OR (MH "Sperm Count") OR "sperm" | 3,066 |
| S21 | "male infertility" | 500 |
| S20 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 | 108,659 |
| S19 | TX docosahexaenoic acid | 2,993 |
| S18 | TX magnesium | 6,027 |
| S17 | TX acetyl cysteine | 351 |
| S16 | TX melatonin | 2,528 |
| S15 | TX chiro inositol | 42 |
| S14 | TX myoinositol | 85 |
| S13 | TX Inositol | 839 |
| S12 | TX fatty acid | 22,457 |
| S11 | TX omega 3 | 7,555 |
| S10 | TX Pentoxifylline | 504 |
| S9 | TX Acetylcysteine | 1,656 |
| S8 | TX menevit | 3 |
| S7 | TX coenzyme q10 | 579 |
| S6 | TX Selenium | 2,579 |
| S5 | TX Zinc | 6,947 |
| S4 | TX carnitine | 1,529 |
| S3 | TX vitamin* | 45,611 |
| S2 | TX antioxidant* | 22,539 |
| S1 | (MH "Antioxidants+") or (MH "Berries+") or (MH "Chlorophyll") or (MH "Flavonoids+") or (MH "Lycopene") or (MH "Polyphenols+") | 25,644 |
Appendix 6. PsycINFO search strategy
Searched from 1806 to 1 Febuary 2018
OVID platform
1 exp Infertility/ (2007) 2 (asthenozoospermia or oligospermia or azoospermia).tw. (41) 3 exp Sperm/ (831) 4 Sperm$.tw. (2981) 5 (male$ adj2 subfertil$).tw. (8) 6 (male$ adj2 infertil$).tw. (202) 7 (subfertil$ adj2 men).tw. (1) 8 (infertil$ adj2 men).tw. (94) 9 (male$ adj2 fertility).tw. (143) 10 semen.tw. (439) 11 oligoasthenoteratozoospermi$.tw. (2) 12 Asthenospermia.tw. (2) 13 Teratospermia.tw. (0) 14 or/1‐13 (5309) 15 vitamin$.tw. (6685) 16 exp Zinc/ (780) 17 exp Antioxidants/ (2458) 18 (zinc or selenium).tw. (2256) 19 (Glutathione$ or folate).tw. (3469) 20 ubiquin$.tw. (98) 21 coenzyme q10.tw. (195) 22 (carnitine$ or carotenoid$).tw. (745) 23 (astaxanthin$ or lycopene$).tw. (76) 24 menevit.tw. (0) 25 multivitamin$.tw. (229) 26 (betacarotene$ or beta carotene$).tw. (139) 27 ascorbic acid.tw. (416) 28 n‐acetylcysteine.tw. (347) 29 exp Cysteine/ (628) 30 acetylcysteine.tw. (357) 31 alpha‐tocopherol$.tw. (219) 32 (fish adj2 oil$).tw. (278) 33 omega$.tw. (2433) 34 fatty acid$.tw. (4091) 35 (plant adj4 oil$).tw. (40) 36 l‐arginine$.tw. (1068) 37 arginine$.tw. (2842) 38 flavonoid$.tw. (382) 39 carotenoid$.tw. (352) 40 riboflavin$.tw. (196) 41 pycnogenol$.tw. (13) 42 lutein$.tw. (1544) 43 lipoic acid$.tw. (175) 44 (antioxidant$ or radical scavengers).tw. (4920) 45 Inositol.tw. (1411) 46 myoinositol.tw. (130) 47 mesoinositol.tw. (0) 48 acetyl cysteine.tw. (146) 49 melatonin.tw. (4242) 50 or/15‐49 (30905) 51 random.tw. (52089) 52 control.tw. (401965) 53 double‐blind.tw. (21242) 54 clinical trials/ (10777) 55 placebo/ (5057) 56 exp Treatment/ (705267) 57 or/51‐56 (1095872) 58 14 and 50 and 57 (34)
Appendix 7. 'The World Health Organization International Clinical Trials Registry Platform' search portal
Searched 1 Febuary 2018
Web platform
1) Antioxidant* AND men
2) Vitamins* AND men
3) Antioxidant* AND male
4) Vitamin* AND male
5) Infertility AND men
6) Infertility AND male
Appendix 8. 'ClinicalTrials.gov' trials register
Searched 1 Febuary 2018
Web platform
1) Antioxidants (clinical condition: infertility)
2) Vitamins (clinical condition: infertility)
Appendix 9. OpenGrey
Searched 1 Febuary 2018
Web platform
1) Antioxidant*
2) Vitamin*
3) Infertility AND Men
4) Antoxidant AND fertility
Appendix 10. ProQuest Dissertations & Theses database
Searched 1 Febuary 2018
Web platform
1) Antioxidants AND sperm AND (men OR male) AND (fertility or infertility) AND random*
2) Antoxidants AND sperm AND (men OR male) AND (fertility or infertility)
Appendix 11. Web of Science
Searched 1 Febuary 2018
Web platform
1) Antioxidants AND sperm AND male AND (fertility OR infertil*) limited by 'clinical trial'
Data and analyses
Comparison 1. Antioxidant(s) versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth; type of antioxidant | 7 | 750 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [1.20, 2.67] |
| 1.1 Carnitines | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.24, 4.25] |
| 1.2 Coenzyme Q10 | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.16 [0.53, 8.82] |
| 1.3 Vitamin D + Calcium | 1 | 330 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.59, 1.80] |
| 1.4 Vitamin E | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.51 [2.36, 30.70] |
| 1.5 Zinc | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.74 [1.02, 13.74] |
| 1.6 Combined antioxidants | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.42 [1.15, 10.13] |
| 2 Live birth; placebo or no treatment | 7 | 750 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [1.20, 2.67] |
| 2.1 Placebo | 6 | 650 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.08, 2.52] |
| 2.2 No treatment | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.74 [1.02, 13.74] |
| 3 Live birth; IVF/ICSI | 2 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.61 [1.27, 10.29] |
| 4 Live birth; as‐treated analysis | 7 | 649 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [1.13, 2.58] |
| 4.1 Carnitines | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.25, 4.41] |
| 4.2 Coenzyme Q10 | 1 | 55 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.10 [0.51, 8.64] |
| 4.3 Vitamin D + Calcium | 1 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.57, 1.81] |
| 4.4 Vitamin E | 2 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.44 [1.72, 24.04] |
| 4.5 Zinc | 1 | 97 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.67 [1.00, 13.51] |
| 4.6 Combined antioxidants | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.34 [1.04, 10.76] |
| 5 Clinical pregnancy; type of antioxidant | 11 | 786 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.97 [1.91, 4.63] |
| 5.1 Carnitines | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.24, 4.25] |
| 5.2 Coenzyme Q10 | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.16 [0.53, 8.82] |
| 5.3 Folic acid | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Magnesium | 1 | 26 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.73 [0.17, 445.08] |
| 5.5 N‐acetylcysteine (NAC) | 2 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.00 [0.71, 5.63] |
| 5.6 Vitamin E | 2 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.71 [1.98, 22.69] |
| 5.7 Zinc | 2 | 153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.43 [1.39, 14.14] |
| 5.8 Zinc + Folic acid | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.86 [0.15, 99.84] |
| 5.9 Combined antioxidants | 2 | 164 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.19 [1.44, 7.08] |
| 6 Clinical pregnancy; placebo or no treatment | 11 | 786 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.97 [1.91, 4.63] |
| 6.1 Placebo | 9 | 626 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [1.81, 5.03] |
| 6.2 No treatment | 2 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.84 [1.16, 6.96] |
| 7 Clinical pregnancy; IVF/ICSI | 2 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.64 [0.94, 7.41] |
| 8 Adverse events | 13 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 Miscarriage | 3 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.40, 7.60] |
| 8.2 Gastrointestinal | 11 | 948 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [1.25, 5.03] |
| 8.3 Euphoria | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.16, 9.01] |
| 8.4 Ectopic pregnancy | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.48 [0.07, 286.49] |
| 9 Sperm DNA fragmentation; type of antioxidant | 4 | 254 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐12.61, 2.61] |
| 9.1 Docosahexaenoic acid (DHA) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐14.10 [‐23.22, ‐4.98] |
| 9.2 Folic acid | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐5.80 [‐13.40, 1.80] |
| 9.3 Folic acid + Zinc | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐9.36, 6.96] |
| 9.4 N‐acetylcysteine (NAC) | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐0.42, 8.22] |
| 9.5 Vitamin C + Vitamin E | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐13.80 [‐17.50, ‐10.10] |
| 9.6 Zinc | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐8.62, 11.22] |
| 10 Sperm DNA fragmentation (data not suitable for meta‐analysis) | Other data | No numeric data | ||
| 10.1 Folic acid | Other data | No numeric data | ||
| 10.2 Combined antioxidants | Other data | No numeric data | ||
| 11 Total sperm motility at 3 months or less; type of antioxidant | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Carnitines | 5 | 244 | Mean Difference (IV, Random, 95% CI) | 11.91 [‐0.85, 24.66] |
| 11.2 Coenzyme Q10 | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 3.61 [‐6.13, 13.35] |
| 11.3 Folic acid | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 8.40 [‐5.81, 22.61] |
| 11.4 Magnesium | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 14.5 [‐6.01, 35.01] |
| 11.5 N‐acetylcysteine (NAC) | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 14.60 [0.32, 28.88] |
| 11.6 PUFAs | 2 | 64 | Mean Difference (IV, Random, 95% CI) | ‐8.35 [‐17.40, 0.69] |
| 11.7 Selenium | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 14.9 [1.14, 28.66] |
| 11.8 Vitamin C + Vitamin E | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐7.76, 13.56] |
| 11.9 Vitamin E | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 18.9 [4.90, 32.90] |
| 11.10 Zinc | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 15.37 [‐5.14, 35.88] |
| 11.11 Zinc + Folic acid | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐7.57, 21.17] |
| 11.12 Zinc + Vitamin E | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 26.0 [12.85, 39.15] |
| 11.13 Zinc + Vitamin E + Vitamin C | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 26.0 [12.62, 39.38] |
| 11.14 Combined antioxidants | 4 | 383 | Mean Difference (IV, Random, 95% CI) | 12.43 [8.39, 16.46] |
| 12 Total sperm motility at 3 months or less (data not suitable for meta analysis) | Other data | No numeric data | ||
| 12.1 Carnitines | Other data | No numeric data | ||
| 12.3 Folic acid | Other data | No numeric data | ||
| 12.4 Folic acid + Zinc | Other data | No numeric data | ||
| 12.5 Vitamin E | Other data | No numeric data | ||
| 12.6 Zinc | Other data | No numeric data | ||
| 12.7 Combined antioxidants | Other data | No numeric data | ||
| 13 Total sperm motility at 6 months; type of antioxidant | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Carnitines | 3 | 136 | Mean Difference (IV, Random, 95% CI) | 11.73 [1.87, 21.60] |
| 13.2 Coenzyme Q10 | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 6.59 [1.80, 11.37] |
| 13.3 Folic acid | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐8.49, 11.89] |
| 13.4 N‐acetylcysteine (NAC) | 1 | 211 | Mean Difference (IV, Random, 95% CI) | 1.90 [1.20, 2.60] |
| 13.5 Selenium | 1 | 211 | Mean Difference (IV, Random, 95% CI) | 3.20 [2.50, 3.90] |
| 13.6 Selenium + N‐acetylcysteine (NAC) | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 6.30 [5.60, 7.00] |
| 13.7 Vitamin D + Calcium | 1 | 260 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐9.57, 1.57] |
| 13.8 Vitamin E | 2 | 132 | Mean Difference (IV, Random, 95% CI) | 11.20 [4.70, 17.70] |
| 13.9 Zinc | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐10.19, 10.19] |
| 13.10 Zinc + Folic acid | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐8.82, 14.02] |
| 13.11 Combined antioxidants | 2 | 229 | Mean Difference (IV, Random, 95% CI) | 9.35 [3.19, 15.51] |
| 14 Total sperm motility at 6 months(data not suitable for meta analysis) | Other data | No numeric data | ||
| 14.1 Carnitines | Other data | No numeric data | ||
| 14.2 Folic acid | Other data | No numeric data | ||
| 14.3 Zinc | Other data | No numeric data | ||
| 14.4 Zinc + Folic acid | Other data | No numeric data | ||
| 14.5 Combined antioxidants | Other data | No numeric data | ||
| 15 Total sperm motility at 9 months or more; type of antioxidant | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Carnitines | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 8.54 [3.01, 14.07] |
| 15.2 Coenzyme Q10 | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐1.56, 5.36] |
| 15.3 Vitamin E | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐8.48, 12.88] |
| 16 Total sperm motility over time | 26 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Total sperm motility at 3 months or less | 18 | 1105 | Mean Difference (IV, Random, 95% CI) | 10.19 [4.35, 16.04] |
| 16.2 Total sperm motility at 6 months | 13 | 1768 | Mean Difference (IV, Random, 95% CI) | 6.00 [3.92, 8.09] |
| 16.3 Total sperm motility at 9 months or more | 5 | 583 | Mean Difference (IV, Random, 95% CI) | 3.29 [0.36, 6.23] |
| 17 Progressive sperm motility at 3 months or less; type of antioxidant | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Carnitines | 3 | 199 | Mean Difference (IV, Random, 95% CI) | 20.63 [19.40, 21.87] |
| 17.2 Coenzyme Q10 | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐3.54, 12.74] |
| 17.3 Docosahexaenoic acid (DHA) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.60 [‐8.57, ‐4.63] |
| 17.4 Folic acid | 2 | 81 | Mean Difference (IV, Random, 95% CI) | 5.68 [‐5.02, 16.38] |
| 17.5 N‐acetylcysteine (NAC) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐1.03, 8.63] |
| 17.6 PUFAs | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 6.40 [4.83, 7.97] |
| 17.7 Vitamin C | 2 | 145 | Mean Difference (IV, Random, 95% CI) | 16.03 [‐3.90, 35.95] |
| 17.8 Vitamin C + Vitamin E | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐9.77, 10.17] |
| 17.9 Zinc | 2 | 157 | Mean Difference (IV, Random, 95% CI) | 1.14 [‐3.37, 5.64] |
| 17.10 Zinc + Folic acid | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐13.66, 21.26] |
| 17.11 Combined antioxidants | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 15.20 [13.62, 16.78] |
| 18 Progressive sperm motility at 3 months (data not usable for meta‐analysis) | Other data | No numeric data | ||
| 18.1 Combined antioxidants | Other data | No numeric data | ||
| 19 Progressive sperm motility at 6 months; type of antioxidant | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Carnitines | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 15.94 [11.01, 20.87] |
| 19.2 Coenzyme Q10 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 5.0 [2.13, 7.87] |
| 19.3 Folic acid | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐10.21, 6.67] |
| 19.4 Vitamin D + Calcium | 1 | 260 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐9.59, 1.59] |
| 19.5 Zinc | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐13.56, 17.56] |
| 19.6 Zinc + Folic acid | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐14.58, 19.98] |
| 20 Progessive sperm motility at 6 months (data not usable for meta‐analysis) | Other data | No numeric data | ||
| 20.1 Combined antioxidants | Other data | No numeric data | ||
| 21 Progressive sperm motility at 9 months or more; type of antioxidant | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Carnitines | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 7.77 [2.68, 12.87] |
| 21.2 Coenzyme Q10 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.68, 0.88] |
| 22 Progressive sperm motility over time | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Progressive sperm motility at 3 months or less | 13 | 884 | Mean Difference (IV, Random, 95% CI) | 9.75 [5.26, 14.24] |
| 22.2 Progressive sperm motility at 6 months | 5 | 521 | Mean Difference (IV, Random, 95% CI) | 6.11 [0.57, 11.66] |
| 22.3 Progressive sperm motility at 9 months or more | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 4.64 [‐1.67, 10.95] |
| 23 Sperm concentration at 3 months or less; type of antioxidant | 21 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 Carnitines | 4 | 247 | Mean Difference (IV, Random, 95% CI) | 10.43 [0.99, 19.87] |
| 23.2 Coenzyme Q10 | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐12.37, 12.17] |
| 23.3 Folic acid | 2 | 81 | Mean Difference (IV, Random, 95% CI) | 8.54 [‐22.31, 39.39] |
| 23.4 Magnesium | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 5.20 [‐2.61, 13.01] |
| 23.5 N‐acetylcysteine (NAC) | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 4.59 [‐0.27, 9.46] |
| 23.6 PUFAs | 3 | 108 | Mean Difference (IV, Random, 95% CI) | 3.44 [1.70, 5.17] |
| 23.7 Selenium | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 21.20 [‐11.43, 53.83] |
| 23.8 Vitamin C | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 9.70 [0.09, 19.31] |
| 23.9 Vitamin C + Vitamin E | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐10.01, 12.72] |
| 23.10 Vitamin E | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 18.9 [3.92, 33.88] |
| 23.11 Zinc | 2 | 157 | Mean Difference (IV, Random, 95% CI) | 8.75 [2.25, 15.24] |
| 23.12 Zinc + Folic acid | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 18.0 [1.11, 34.89] |
| 23.13 Combined antioxidants | 3 | 344 | Mean Difference (IV, Random, 95% CI) | 6.71 [‐1.91, 15.33] |
| 24 Sperm concentration at 3 months or less (data not suitable for meta analysis) | Other data | No numeric data | ||
| 24.1 Carnitines | Other data | No numeric data | ||
| 24.2 Vitamin E | Other data | No numeric data | ||
| 24.3 Folic acid | Other data | No numeric data | ||
| 24.4 Zinc | Other data | No numeric data | ||
| 24.5 Folic acid + Zinc | Other data | No numeric data | ||
| 24.6 Combined antioxidants | Other data | No numeric data | ||
| 25 Sperm concentration at 6 months; type of antioxidant | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 25.1 Carnitines | 2 | 115 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐3.13, 8.33] |
| 25.2 Coenzyme Q10 | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 6.87 [1.18, 12.55] |
| 25.3 Folic acid | 2 | 81 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐37.87, 42.75] |
| 25.4 N‐acetylcysteine (NAC) | 1 | 211 | Mean Difference (IV, Random, 95% CI) | 3.30 [1.80, 4.80] |
| 25.5 Selenium | 1 | 211 | Mean Difference (IV, Random, 95% CI) | 4.10 [2.45, 5.75] |
| 25.6 Selenium + N‐acetylcysteine (NAC) | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 8.60 [6.89, 10.31] |
| 25.7 Vitamin E | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 5.90 [‐10.83, 22.63] |
| 25.8 Zinc | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 9.70 [‐7.00, 26.40] |
| 25.9 Zinc + Folic acid | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 17.70 [‐1.88, 37.28] |
| 25.10 Combined antioxidants | 2 | 229 | Mean Difference (IV, Random, 95% CI) | 13.68 [8.06, 19.31] |
| 26 Sperm concentration at 6 months(data not suitable for meta analysis) | Other data | No numeric data | ||
| 26.1 Carnitines | Other data | No numeric data | ||
| 26.2 Folic acid | Other data | No numeric data | ||
| 26.3 Zinc | Other data | No numeric data | ||
| 26.4 Zinc + Folic acid | Other data | No numeric data | ||
| 26.5 Vitamin D + Calcium | Other data | No numeric data | ||
| 27 Sperm concentration at 9 months; type of antioxidant | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 27.1 Carnitines | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 4.17 [‐1.71, 10.06] |
| 27.2 Coenzyme Q10 | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 2.74 [‐1.57, 7.05] |
| 27.3 Vitamin E | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 11.40 [‐2.56, 25.36] |
| 28 Sperm concentration over time | 26 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.1 Sperm concentration at 3 months or less | 20 | 1244 | Mean Difference (IV, Random, 95% CI) | 7.51 [4.23, 10.79] |
| 28.2 Sperm concentration 6 months | 11 | 1430 | Mean Difference (IV, Random, 95% CI) | 7.49 [4.76, 10.23] |
| 28.3 Sperm concentration at 9 months or more | 5 | 583 | Mean Difference (IV, Random, 95% CI) | 3.61 [0.17, 7.06] |
1.2. Analysis.
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 2 Live birth; placebo or no treatment.
Comparison 2. Head‐to‐head antioxidant(s).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth; type of antioxidant | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.13, 7.92] |
| 1.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.06, 1.79] |
| 1.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.06, 1.79] |
| 2 Clinical pregnancy; type of antioxidant | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.13, 7.92] |
| 2.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.06, 1.79] |
| 2.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.06, 1.79] |
| 2.4 Vitamin D + Calcium vs Vitamin E + Vitamin C | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.13 [1.21, 21.79] |
| 3 Total sperm motility at 3 months or less; type of antioxidant | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Docosahexaenoic acid (DHA) 400 mg vs Docosahexaenoic acid 800 mg | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 7.40 [‐11.35, 26.15] |
| 3.2 Ethylcysteine vs Vitamin E | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐41.97, 38.17] |
| 3.3 L‐acetyl carnitine + L‐carnitine vs Vitamin E + Vitamin C | 1 | 138 | Mean Difference (IV, Random, 95% CI) | 23.10 [20.14, 26.06] |
| 3.4 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐3.73, 10.53] |
| 3.5 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐1.76, 11.36] |
| 3.6 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐6.42, 9.22] |
| 3.7 Selenium vs combined antioxidants | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐10.13, 16.53] |
| 3.8 Vitamin C 200mg vs Vitamin C 1000mg | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐43.0 [‐67.10, ‐18.90] |
| 3.9 Zinc vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.40 [‐14.21, 5.41] |
| 3.10 Zinc vs Zinc + Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐12.90, 7.30] |
| 3.11 Zinc + Folic acid vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐7.73, 6.53] |
| 3.12 Zinc vs Zinc + Vitamin E | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐13.00, 13.00] |
| 3.13 Zinc vs Zinc + Vitamin E + Vitamin C | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐19.66, 17.66] |
| 3.14 Zinc + Vitamin E vs Zinc + Vitamin E + Vitamin C | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐18.97, 18.97] |
| 4 Total sperm motility at 6 months; type of antioxidant | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.10 [‐2.70, 10.90] |
| 4.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐2.87, 9.67] |
| 4.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐7.73, 6.33] |
| 4.4 N‐acetylcysteine (NAC) vs Selenium + N‐acetylcysteine (NAC) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐4.40 [‐5.14, ‐3.66] |
| 4.5 Selenium vs N‐acetylcysteine (NAC) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.56, 2.04] |
| 4.6 Selenium vs Selenium + N‐acetylcysteine (NAC) | 1 | 232 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐3.85, ‐2.35] |
| 4.7 Zinc vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐6.42, 3.02] |
| 4.8 Zinc + Folic acid vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐5.46, 7.26] |
| 4.9 Zinc vs Zinc + Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐9.13, 3.93] |
| 5 Total sperm motility at 6 months (data not suitable for meta analysis) | Other data | No numeric data | ||
| 5.1 Folic acid vs Zinc + Folic acid | Other data | No numeric data | ||
| 5.2 Zinc vs Folic acid | Other data | No numeric data | ||
| 5.3 Zinc vs Zinc + Folic acid | Other data | No numeric data | ||
| 6 Total sperm motility at 9 months or more; type of antioxidant | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 3.70 [‐1.69, 9.09] |
| 6.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐0.73, 11.33] |
| 6.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐3.29, 6.49] |
| 7 Progessive sperm motility at 3 months or less; type of antioxidant | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐1.88, 9.88] |
| 7.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐0.68, 10.68] |
| 7.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐5.41, 7.41] |
| 7.4 L‐acetyl carnitine + L‐carnitine vs Vitamin E + Vitamin C | 1 | 138 | Mean Difference (IV, Random, 95% CI) | 13.30 [11.21, 15.39] |
| 7.5 L‐carnitine vs Vitamin E + Vitamin C | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 30.50 [27.70, 33.30] |
| 7.6 L‐carnitine + Vitamin E vs Vitamin E | 1 | 113 | Mean Difference (IV, Random, 95% CI) | 14.10 [10.11, 18.09] |
| 7.7 Vitamin D + Calcium vs Vitamin E + Vitamin C | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 6.90 [5.38, 8.42] |
| 8 Progressive sperm motility at 6 months; type of antioxidant | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 6.30 [0.42, 12.18] |
| 8.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 5.70 [0.10, 11.30] |
| 8.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐6.93, 5.73] |
| 9 Progressive sperm motility at 9 months; type of antioxidant | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐1.50, 9.10] |
| 9.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 5.50 [‐0.11, 11.11] |
| 9.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐4.17, 7.57] |
| 10 Sperm concentration at 3 months or less; type of antioxidant | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Docosahexaenoic acid (DHA) 400 mg vs Docosahexaenoic acid (DHA) 800 mg | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐6.80 [‐41.87, 28.27] |
| 10.2 Ethylcysteine vs Vitamin E | 1 | 10 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐16.65, 21.05] |
| 10.3 L‐carnitine vs Vitamin E + Vitamin C | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 15.5 [12.49, 18.51] |
| 10.4 L‐carnitine + Vitamin E vs Vitamin E | 1 | 113 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐10.52, 14.32] |
| 10.5 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐10.97, 14.37] |
| 10.6 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.10 [‐9.17, 17.37] |
| 10.7 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐11.14, 15.94] |
| 10.8 Selenium vs combined antioxidants | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 14.70 [‐6.51, 35.91] |
| 10.9 Zinc vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐23.38, 12.78] |
| 10.10 Zinc + Folic acid vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐22.22, 13.82] |
| 10.11 Zinc vs Zinc + Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐18.65, 16.45] |
| 11 Sperm concentration at 6 months; type of antioxidant | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 5.90 [‐8.92, 20.72] |
| 11.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 8.10 [‐5.54, 21.74] |
| 11.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐10.89, 15.29] |
| 11.4 N‐acetylcysteine (NAC) vs Selenium + N‐acetylcysteine (NAC) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐6.86, ‐3.74] |
| 11.5 Selenium vs N‐acetylcysteine (NAC) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.71, 2.31] |
| 11.6 Selenium vs Selenium + N‐acetylcysteine (NAC) | 1 | 232 | Mean Difference (IV, Random, 95% CI) | ‐4.5 [‐6.20, ‐2.80] |
| 11.7 Zinc vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐9.5 [‐20.29, 1.29] |
| 11.8 Zinc + Folic acid vs Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐15.06, 12.06] |
| 11.9 Zinc vs Zinc + Folic acid | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐23.69, 7.69] |
| 12 Sperm concentration at 6 months (data not suitable for meta analysis) | Other data | No numeric data | ||
| 12.1 Zinc vs Folic acid | Other data | No numeric data | ||
| 12.2 Zinc vs Zinc + Folic acid | Other data | No numeric data | ||
| 12.3 Folic acid vs Zinc + Folic acid | Other data | No numeric data | ||
| 13 Sperm concentration at 9 months or more; type of antioxidant | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 L‐carnitine vs L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 8.2 [‐0.07, 16.47] |
| 13.2 L‐carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 6.10 [‐3.74, 15.94] |
| 13.3 L‐acetyl carnitine vs L‐carnitine + L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐10.24, 6.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akiyama 1999.
| Methods | Randomised single‐centre cross‐over trial Duration of study: 8 months |
|
| Participants | Country: Japan Population: infertile men, N = 10 Mean age: 36 years (treatment group age range 24 to 49 years, control age range 30 to 37 years) Inclusion criteria: male infertility (ROS > 5 x 10,000 counts/10,000,000 viable spermatozoa) Exclusion criteria: azoospermia, pyospermia |
|
| Interventions | Ethylcysteine 600 mg (n = 5) versus Vitamin E 600 mg (n = 5) Duration of treatment: 3 months, with a one month wash out, then cross‐over for another 3 months. Only data from the first phase were used in data analysis |
|
| Outcomes | Sperm parameters, blood serum and seminal plasma levels of ethylcysteine and vitamin E | |
| Notes | In Japanese. Data extraction translated by Ichiro, a colleague of Samantha Roberts, 29.01.2009 Author contacted 'no further information is available' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were divided randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Sperm parameters reported. No protocol available. |
Attallah 2013.
| Methods | Randomised controlled open‐label trial Duration of the study: unclear |
|
| Participants | Country: Egypt Population: men with isolated idiopathic athenozospermia, prior to intrauterine insemination (IUI), N = 60 Mean age: unknown, quote "both treatment groups were homogenous at the time of randomisation regarding the type and duration of infertility" Inclusion criteria: couples with idiopathic athenozospermia (progressive motility < 32%) with normal other seminal criteria and normal infertility workup for female partner Exclusion criteria: unclear |
|
| Interventions | N‐acetylcysteine (NAC) 600 mg (n = 30) versus No treatment (n = 30) Duration of treatment: 12 weeks |
|
| Outcomes | Sperm concentration, progressive sperm motility, clinical pregnancy rate | |
| Notes | Conference abstract, no full text. Attempted to contact authors 04.02.2014, unable to find e‐mail address. Letter posted 12.02.2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Couples were randomised" Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open‐labelled" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Unknown ‐ conference abstract |
Azizollahi 2013.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: from May 2008 to November 2010 |
|
| Participants | Country: Iran Population: infertile men with varicocele grade III, N = 160 (only 112 completed the study) Mean age: age range from 20 to 43 (mean ± SD: 29.07 ± 6.8) years Inclusion criteria: the presence of a grade III varicocele assessed by clinical parameters and was confirmed by Doppler ultrasound scanning Exclusion criteria: evidence of leukocytospermia, low testicular volume < 15 mL, congenital urogenital abnormalities and urogenital infections |
|
| Interventions | Zinc 66 mg (n = 32) versus Folic acid 5 mg (n = 26) versus Zinc 66 mg + Folic acid 5 mg (n = 29) versus Placebo (n = 25) Duration of treatment: 6 months, after varicocelectomy |
|
| Outcomes | Sperm parameters; number, morphology, halo formation rate, motility, forward progressive motility, chromomycin A3 positivity | |
| Notes | Trial registration: IRCT138802261910N1 E‐mailed the author 03.03.2014 (nematollahimahani@yahoo.com / nnematollahi@kmu.ac.ir). Author replied 06.03.2014 with information included in the ROB table. Author e‐mailed again to ask about pregnancy data and dropouts from which group. The author informed us that Azizollahi 2011 was part of this trial and gave pregnancy and dropout data (there were originally 40 in each group). Quote: "At that time we observed 2 pregnancies in zinc/folic acid group, 1 pregnancy in zinc group, and no pregnancy in placebo and folic acid group. These data were just 6 months after the start of the trial." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For randomisation we used a table with 200 numbers (1 to 200). Before the trial we gave each group a number between 1 and 4 and allocated each group into the table. By this method the first, fifth, ninth, 13th and ... patients were allocated into the group 1 and the same manner was applied to the other groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "We used sealed containers with the randomisation number on them. Drugs or placebo were in opaque capsules" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Our study was double blind. Neither the urologist nor the patient or examiner in the lab were aware of the arrangement of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Our study was double blind. Neither the urologist nor the patient or examiner in the lab were aware of the arrangement of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information gained from communication with the author explained the dropout numbers |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate data gained from email correspondence with the author. Protocol available. |
Balercia 2005.
| Methods | Randomised double‐blind trial Duration of study: 9 months, follow‐up 3 months |
|
| Participants | Country: Italy Population: infertile men with idiopathic asthenozoospermia, N = 60 Mean age: 30 (range 24 to 38) years Inclusion criteria: primary infertility > 2 years after regular intercourse with a fertile woman, 20 to 40 years of age, normal rheologic characteristics, sperm count > 20 x 106 /mL, sperm motility < 50%, normal sperm morphological features > 30%, seminal WBC < 1 x 106 /mL, negative sperm culture and chlamydia and mycoplasma urealyticum, normal serum gonadotropins, T, E2 and PRL, absence of infectious or genital disease, no anatomic abnormalities of the genital tract, absence of systemic diseases or treatment with other drugs within the 3 months before enrolment in the study, absence of smoking, alcohol or recreational drug use or of occupational chemical exposure |
|
| Interventions | L‐carnitine 3 g (n = 15) versus L‐acetyl carnitine 3 g (n = 15) versus L‐carnitine 2 g + L‐acetyl carnitine 1 g (n = 14) versus Placebo (n = 15) Duration of treatment: 6 months |
|
| Outcomes | Sperm parameters | |
| Notes | 2018: email sent on 07.03.2018 to author Balercia (g.balercia@aoumbertoprimo.marche.it: error, found new email: g.balercia@univpm.it) to ask if pregnancy rate were clinical pregnancies, how they were conceived, methods of randomisation and blinding Reply from author on 12.03.2018: Quote: "Pregnancies were clinical pregnancies, spontaneously conceived. I had at this time no data about the weekly progression, but the outcome of all pregnancies was newborn babies." New information added to RoB table. Added data in meta‐analysis on clinical pregnancy, live birth and progressive motility ('Antioxidants vs placebo/no treatment' and 'head to head') |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from email): "The randomisation was made by blinded key" |
| Allocation concealment (selection bias) | Low risk | Quote (from email): "sealed opaque envelopes provided by the monitor" (reply email) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from email): "The randomisation was made by a blinded key, sealed opaque envelopes provided by the monitor, without any access for the researchers (except the hypothesis of adverse events). The key of randomization was available just at the end of the study." (reply email) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal from the L carnitine 2 g/day + L acetyl carnitine 1 g/day group Quote (from email): "as far your last question, I can confirm the results concerning the drop‐out has not be considered in data analysis" (reply email) Conclusion: no ITT. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Balercia 2009.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: 10 months, follow‐up 3 months |
|
| Participants | Country: Italy Population: infertile men with idiopathic asthenozoospermia, N = 60 Mean age: 32 (range 27 to 32) years Inclusion criteria: age 20 to 40 years, infertility > 2 years, regular sexual intercourse with a potentially fertile female, normal rheologic characteristics (appearance, consistency and liquefaction) of semen and volume and pH in normal range, sperm count > 20 x 106 /mL, sperm motility < 50% (WHO 1999), normal morphology > 30%, seminal WBC < 1 x 106 /mL and a negative sperm culture and chlamydia and Mycoplasma urealyticum (M.urealyticum) detection, normal levels of gonadotropins, absence of genital disease and anatomical abnormalities of the genital tract including variocoele and antibodies, absence of systemic disease or treatment with other drugs within 3 months of being enrolled in the study, absence of smoking, alcohol and drug addiction and exposure to occupational chemicals Exclusion criteria: transient decrease in semen quality during run in and those who had sudden improvement in semen parameters during run in |
|
| Interventions | Coenzyme Q10 200 mg (n = 30) versus Placebo (n = 30) Duration of treatment: 6 months |
|
| Outcomes | Primary: sperm parameters, variations of coenzyme Q10 and ubiqiunol concentrations in seminal plasma and spermatozoa Secondary: pregnancy rate |
|
| Notes | 2018: added data on progressive sperm motility Email sent to author (g.balercia@staff.univpm.it) to ask if pregnancies were clinical and if he has live birth rates Reply of author Balercia on 29.03.2018: Quote: "Like the other study, I can confirm that pregnancies were clinical pregnancies, spontaneously conceived, but I had no data about the weekly progression (our outcome was another and we just reported the pregnancies as “collateral” data). All pregnancies gave newborn babies (patient/parent contacted us to share the joyful moment”)". Data added. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | At end of trial the paper mentions ‐ quote: "after opening randomisation list" page 1789 |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Semen quality was assessed by the same biologist" Blinding not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "5 patients dropped out of the study", 2 from the treatment group and 3 from the placebo group; this was discovered after opening the randomisation list at the end of the study. ITT was carried out |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Barekat 2016.
| Methods | Randomised clinical trial Duration of study: unclear, from 2011 to 2013 |
|
| Participants | Country: Iran Population: subfertile men with varicocele grade 2‐3, N = 40 Mean age: 30.1 ± 4.4 (range: 22‐45) years Inclusion criteria: age < 45 years, primary infertility, left‐sided varicocele (grade 2‐3) diagnosed by palpation and Doppler duplex ultrasound. Female partner with age < 35 years, normal ovulatory cycles and patent tubes (confirmed by hysterosalpingography or laparoscopy). Exclusion criteria: varicocele grade I, azoospermia, recurrent varicocele, leukocytospermia, urogenital infections, testicular size discrepancy, abnormal hormonal profile, anatomical disorders, Klinefelter’s syndrome, cancer, fever in the 90 days prior to sugery, seminal sperm antibodies, excessive alcohol and drug consumption, previous history of scrotal trauma or surgery, occupational exposure. Female partner with endometriosis, cycle irregularity, or gross anatomical abnormalities |
|
| Interventions | N‐acetylcysteine (NAC) 200 mg (n = 20) versus No treatment (n = 20) Duration of treatment: 3 months, directly after varicocelectomy |
|
| Outcomes | Sperm parameters, DNA‐fragmentation (TUNEL), protamine deficiency, ROS levels | |
| Notes | Email sent to last author Nasr‐Esfahani (mh.nasr‐esfahani@royaninstitute.org) on 06.03.2018 to ask about the allocation concealment, sequence generation and definition of pregnancies and method of conceiving. Reply the same day from author (06.03.2018): Quote: "Clinical, spontaneous, pregnancies confirmed by heartbeat." Rest of information in RoB. Authors replied on 04.04.18 answering that data was presented with SEM |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from email):"Randomisation done by table. We used computer‐generated or random allocation software and with one block" |
| Allocation concealment (selection bias) | High risk | Quote (from email): "Dr would prescribe the NAC based on randomization table" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or health care providers (control is no treatment) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from email): “All parameters assessed in this study were carried out by a single trained individual unaware of treatment assignment.” "Lab collected the sample based on a table of allocation and handed the sample over to the researcher that carried out the semen analysis and sperm functional tests and was unaware to randomization. A third person called the patients and enquired about pregnancy and whether it was confirmed by heartbeat. Finally, the data gathered and analyzed independently of Dr or researchers" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “In this study, five individuals were excluded from the treatment group due to lack of compliance with NAC use, according to the study protocol" Lack of compliance directly related to treatment, furthermore 25% dropout is high. No ITT. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Biagiotti 2003.
| Methods | Randomised trial Duration of study: unclear |
|
| Participants | Country: Italy Population: men with severe idiopathic oligoasthenospermia (sperm concentration < 5000 /μl), N = 42 Mean age: group A and B 35 (range 30 to 40) years, Group C 31 (range 24 to 34) years Inclusion criteria: severe idiopathic oligoasthenospermia (sperm concentration < 5000 /μl) Exclusion criteria: genomic, hormonal or inflammatory diseases |
|
| Interventions | Acetyl‐carnitine 1 g + L‐carnitine 2 g + Cinnoxicam (n = 14) versus Acetyl‐carnitine 1 g + L‐carnitine 2 g (n = 14) versus No treatment (n = 14) Duration of treatment: unclear |
|
| Outcomes | Sperm parameters | |
| Notes | Conference abstract. No full text or data given. Contacted authors but no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised (1patient = 1 block) analysis of variance" Was this at the time of sequence generation or at data analysis? |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is no treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Unclear conference abstract |
Blomberg Jensen 2018.
| Methods | Randomised single‐centre,triple‐blinded, clinical trial Duration of study: from January 2011 to August 2014, follow‐up 14 months |
|
| Participants | Country: Denmark Population: men part of an infertile couple with impaired semen quality, N = 307 Mean age: 34.8 ± 6.6 years Inclusion criteria: impaired semen quality (determined by WHO criteria) and vitamin D insufficient (25 OHD level #50 nmol/L) Exclusion criteria: serious comorbidities |
|
| Interventions | Vitamin D 1400 IU + calcium 500 mg (n = 151) plus vitamin D 300,000 IU oil once orally versus Placebo (n = 156) plus placebo oil once orally Duration of treatment: 150 days (5 months) |
|
| Outcomes | Sperm parameters, reproductive hormones, live birth rate | |
| Notes | Power calculation performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infertile men were randomly assigned 1:1 (in blocks of 10) to either placebo or.." "Included men were given a specific trial identity number determined by minimization using the computer program Minim (21). Minimization was done using four groups based on serum 25OHD, sperm concentration, body mass index (BMI) and serum inhibin B" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization and manufacture of the high initial dose of vitamin D and placebo were performed by Glostrup Apotek." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "triple‐blinded", "To avoid unblinding, the principal investigator gave the necessary clinical information to the sponsor, who had a list of numbers headed by X or Y. This ensured that both the principal investigator and the sponsor were unaware whether the patient was allocated to the vitamin D plus calcium (active) group or the placebo group (i.e., double blinding)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The trial remained blinded until all biochemical analyses, data handling, and statistical analyses by an independent statistician had been completed (i.e., triple blinding)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twenty men in the placebo group and 18 in the vitamin D plus calcium group were lost to follow‐up. In total, 269 of 307 men (87.6%) completed the study (Fig. 1). By counting returned tablets, it was evident that one man in the vitamin D group and three in the placebo group were noncompliant; however, all data from these four men were included in all the analyses." Quote: "Twenty‐nine of the 269 men completing the trial reported their partner was pregnant before start of the intervention, whereas five men lost their partner during the study period, leaving 235 with the possibility of effecting a pregnancy." ITT. No explanation given for lost to follow‐up? Therefore unclear risk |
| Selective reporting (reporting bias) | Low risk | All the outcomes from the protocol were reported |
Boonyarangkul 2015.
| Methods | Randomised double‐blind controlled trial Duration of study: from May 2013 to October 2014 |
|
| Participants | Country: Thailand Population: men with abnormal semen analysis, N = 68 Mean age: treatment group (folate only) 26.08 ± 0.76 years, control group 24.7 ± 10.84 years Inclusion criteria: abnormal semen analysis of at least one parameter according to WHO Criteria 2010(13) (concentration < 15 million/ml, motility < 40%, or morphology < 4%), failure of the female partner to conceive after one year of regular unprotected sexual intercourse, no history of tamoxifen and folate allergy Exclusion criteria: use of tamoxifen and folate within three months before recruitment, use of other medicines or vitamin during study period |
|
| Interventions | Placebo (n = 15) versus Tamoxifen citrate 20 mg (n = 15) versus Folate 5 mg (n = 15) versus Tamoxifen citrate 20 mg + Folate 5 mg (n = 15) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters, hyaluronan binding assay, hypo‐osmotic swelling test and DNA damage (Comet assay, tail length) | |
| Notes | Only folate and placebo arm included. Email sent to author on 06.03.2018 to Boonyarangkul (doctor_artit@yahoo.co.th) to ask about the randomisation process, blinding of outcome assessment, drop‐out rate and funding of trial. Reminder email sent on 22.03.2018 to authors Boonyarangkul and Chiamchanya (doctor_artit@yahoo.co.th; charoenchai12@hotmail.com). No reply to date (19.04.2018) Data used in meta‐analysis, however a sensitivity analysis was performed due to great baseline imbalance between these two groups, especially sperm concentration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | High risk | Baseline imbalance in concentration control versus folate group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Eight patients were excluded from the study (three patients declined to participate and five patients stop medication before completing the trial)" Unclear in which groups they participated. Data analysis by the authors was done without the 8 dropouts |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Busetto 2018.
| Methods | Randomised double‐blind placebo‐controlled study Duration of study: from December 2014 to June 2015, follow‐up unclear |
|
| Participants | Country: Italy Population: infertile men with oligo‐ and/or astheno‐ and/or teratozoospermia, N = 104, divided in two clusters, 52 patients with varicocele grade I‐III and 52 patients without varicocele Mean age: 32.5 ± 6.7 years Inclusion criteria: age 18 – 50 years, oligo‐, astheno‐ and/or teratozoospermia, with or without varicocele, having a history of infertility for more than 12 months, varicocele patients were not surgically treated before and during the treatment, patients without varicocele were suffering from idiopathic male infertility, no other previous history of diseases affecting fertility. Fertile female partners were required with regular menstrual cycles, age <40 and couples not looking for fertility‐related procedures (IVF/ICSI/IUI) for the next 90 days Exclusion criteria: known hypersensitivity to any of the treatment compounds, history of undescended testes or cancer, endocrine disorders, history of post‐pubertal mumps, genitourinary surgery, obstructive azoospermia or obstructive pathology of the urogenital system, autoimmune disease, cystic fibrosis, history of taking any therapy affecting fertility within last 3 months, excessive consumption of alcohol or regular use of illicit or “recreational” drugs, positive serology for HIV, participants following any special diet, any condition which in the opinion of the investigator might put the participanr at risk by participating in this study, participants involved in any other clinical trials |
|
| Interventions | Proxeed Plus 2 sachets (n = 52) (l‐carnitine 1000 mg, fumarate 725 mg, acetyl‐l‐carnitine 500 mg, fructose 1000 mg, CoQ10 20 mg, vitamin C 90 mg, zinc 10 mg, folic acid 200 μg and vitamin B12 1.5 μg) versus Placebo 2 sachets (n = 52) Duration of treatment: 6 months |
|
| Outcomes | Sperm parameters, pregnancy rate | |
| Notes | Power calculation performed. Email sent to author Busetto (gianmaria.busetto@uniroma1.it) on 07.03.2018 to ask about allocatian concealment, blinding of outcome assessment and if the pregnancies were clinical and spontaneous conceived. Reply from author on 07.03.2018: Quote: "All natural pregnancies, spontaneously conceived, confirmed by ultrasound and we had just one abortion." See RoB. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The block randomisation method was used to randomise subjects into groups resulting in equal sample sizes to ensure a balance across the groups over time." Quote (from email): "Randomisation schedule (nQuery Advisor nTerim 2.0 (2012) program)" |
| Allocation concealment (selection bias) | Low risk | Quote (from email): "The randomization was done by an external company (non‐pharmaceutical)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from email): "We used a double blind system and so researched didn't know anything about the randomization". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from email): "An external statistician evaluated everything external" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Ten patients dropped out from the study leaving 45 patients with varicocele and 49 without varicocele." "As for the ANCOVA, the p‐values refer to the intention‐to‐treat population (ITT). The last observation carried forward (LOCF) method was used for replacing the missing data" Reasons for dropout not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Cavallini 2004.
| Methods | Randomised controlled trial Duration of study: follow‐up 9 months |
|
| Participants | Country: Italy Population: idiopathic men with variocoele or idiopathic oligo‐asthenospermia (OAT), N = 325 Mean age: 34 (range 27 to 40) years Inclusion criteria: men with OAT and with deficiencies in all sperm patterns whose chief complaint was primary couple infertility > 12 months with regular intercourse. Normal sperm appearance, consistency, liquefaction, volume, pH. Female partner without fertility problems. Varicoceles. Exclusion criteria: azoospermia, seminal WBC concentration more than 1000,000/mL, positive urethral chlamydia swab test, oligospermia < 5,000,000 /mL, hormonal alterations, age > 40 years, presence of anti‐sperm antibodies, drug, tobacco or alcohol abuse, ongoing medical treatments, presence of hydrocoele, diabetes,hypertension, x‐ray exposure in previous 8 months, peptic ulcer, unexplained gastric pain, previous hypersensitivity to NSAIDS or carnitines, carnitine metabolism deficiency, bilateral variocoele, prostate abnormalities, previous or current testicular pathology, testicle echographic abnormalities |
|
| Interventions | Placebo starch tablets 2 times/day + glycerine suppository (1 every 4 days) (n = 118) versus L‐carnitine 1 x 2 g/day + acetyl‐L‐carnitine 500 x 2 mg/day + glycerine suppository (n = 101) versus L‐carnitine 1x 2 g/day + acetyl‐L‐carnitine 500 x 2 mg/day + glycerine suppository + cinnoxicam suppository 1 x 30 mg (every 4 days) (n = 106) Duration of treatment: 6 months |
|
| Outcomes | Primary: sperm parameters Secondary: pregnancy, side effects |
|
| Notes | Cinnoxicam is a NSAID, therefore the third arm was not included in meta‐analysis as per protocol Continuous data taken from Cavallini 2004a 'excluded conference abstract' no data for placebo group Unit of analysis variocoele therefore cannot extract data that were presented as median (interquartile range) Author contacted regarding uneven numbers and missing placebo and continuous data Author replied that raw data were not available due to computer crash |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "casual random tables" |
| Allocation concealment (selection bias) | Low risk | Quote: "drug placebos identical in appearance", "anonymized carnitine and cinnoxicam and glycerine suppository containers; and filled and sealed anonymous color coded boxes", "the color code was disclosed to physicians by pharmacists and by IRB at the end of the research" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study personnel and participants were blinded to treatment assignment for the duration of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All study personnel and participants were blinded to treatment assignment for the duration of the study" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 325 randomised but only 185 accounted for; 55 dropouts from 185 (42%), 53 reasons given for the dropouts |
| Selective reporting (reporting bias) | Unclear risk | Sperm parameters as primary outcome. Intention to collect biochemical pregnancy data as secondary outcome recorded in the methods. No protocol available. |
Conquer 2000.
| Methods | Randomised placebo‐controlled trial Duration of study: unclear |
|
| Participants | Country: Canada Population: healthy asthenozoospermic men who were patients of an infertility clinic, N = 28 Mean age: placebo group 35.2 years, treatment group 400 mg 38.3 years and treatment group 800 mg 34.4 years Inclusion criteria: asthenozoospermic, sperm motility < 50% of total sperm Exclusion criteria: unclear |
|
| Interventions | Docosahexaenoic acid (DHA) 400 mg (n = 9) versus Docosahexaenoic acid (DHA) 800 mg(n = 10) versus Placebo (n = 9) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters | |
| Notes | Data with SEs converted to SDs. Placebo arms split | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 28 subjects were randomly assigned to ..." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All men randomised were in the analysis, no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Cyrus 2015.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: from February 2010 to May 2011 |
|
| Participants | Country: Iran Population:infertile men with palpable varicocele grade 2‐3, N = 115 Mean age: 27.6 ± 5.3 years. Inclusion criteria: a palpable varicocele in physical examination and accompanying abnormalities in count, motility, or morphology of sperm in two separate semen analyses (according WHO criteria 1999), age range between 18 and 50, weight between 50 kg and 100 kg, being married Negative inclusion criteria:
Exclusion criteria: missed follow‐up, incorrect usage of the capsules, demonstrating side effects due to vitamin C, commencement of smoking or opium addiction during the follow‐up period, delayed complications of varicocelectomy such as: hydrocele, recurrence of varicocele, and testicular atrophy. |
|
| Interventions | Vitamin C 500 mg (n = 46) versus Placebo (n = 69) Duration of treatment: 3 months, after varicocelectomy |
|
| Outcomes | Primary: mean sperm count, motility (mean perc ent of type A plus type B divided by all motility types) , morphology index (before and after surgery) Secondary: complications of surgery, varicocele grade, age and weight |
|
| Notes | Trial registration: IRCT201103042134N2 Email sent to author on 06.03.2018 to dr Kabir (aikabir@yahoo.com) to ask about funding and if the new matched cases were randomised. Reply on 23.03.2018 with all questions answered (see RoB) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomization method using Excel 2010 software (Microsoft Corporation, Washington, USA) by RANDBETWEEN(0;1000000)”function." Quote: "Five patients from the intervention group and eight patients from controls did not show‐up for the follow‐up visits and were substituted with matched new cases" Reply from authors by email: new cases were randomised |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was produced by our statistician and was delivered to our pharmacist. Participants were enrolled by the two executive urologists who were unaware of the results of the allocation table. Then based on the number in the sequence being odd or even each new patient after varicocele surgery was assigned to intervention or placebo group by our pharmacist who supplied the drugs. The ratio of placebo to intervention group was 1.5" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Analyzed in a reference laboratory (Sina Laboratory of Arak) by an experienced specialist in pathology and clinical laboratory medicine. Complications of surgery, varicocele grade, age and weight were determined" Reply from authors by email: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Five patients from the intervention group and eight patients from controls did not show‐up for the follow‐up visits and were substituted with matched new cases" Quote (from email): "We were able to have access to some of these drop‐out cases. None of them mentioned disease‐, medication‐, or study‐related causes for loss to follow up. Moving out from the city, changing their mind for participating in the study immediately after accepting to participate, personal secret causes and so on were among some of these reasons." |
| Selective reporting (reporting bias) | Low risk | Quote: "Our secondary complications were rare and they were excluded from the study and only those with clinically cured varicocele were selected for the final analysis. If there was any other unaccounted factor from Ivanissevich method that could affect the results, since both groups had the same type of operation, it would be balanced in the two groups" All the outcomes from the aim of the study and methods were reported. |
Dawson 1990.
| Methods | Randomised controlled trial Duration of study: 4 weeks |
|
| Participants | Country: USA Population: men with sperm agglutination, N = 30 Mean age: range 25 to 45 years Inclusion criteria: sperm agglutination over 25%, negative sperm antibodies, physically normal, no inflammatory disease Exclusion criteria: unclear |
|
| Interventions | Ascorbic acid (vitamin C) 1000 mg (n = 10) versus Ascorbic acid (vitamin C) 200 mg (n = 10) versus Placebo (n = 10) Duration of treatment: 3 weeks |
|
| Outcomes | Seminal parameters | |
| Notes | Placebo numbers split by 2. Data were given in SE converted to SD New comment 2018: progressive forward motility instead of total motility, data total sperm motility moved to outcome progressive sperm motility |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "By random selection, three groups of 10 subjects each.." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each subject was told he was receiving AA and expected improvement in sperm quality" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | All specified outcomes were reported. No protocol available. |
Deng 2014.
| Methods | Randomised controlled trial Duration of study: from January 2013 to February 2014 |
|
| Participants | Country: China Population: men with idiopathic oligoasthenozoospermia (N = 86) Mean age: treatment group 31.5 ± 3.7 years, control group, 32.0 ± 4.1 years Inclusion criteria: 18 to 45‐year‐old male infertility patients, no contraception after marriage and infertility more than 12 months, normal sex life, no abnormal fertility of the women. According to WHO requirements 5 × 106/mL <s perm concentration < 20 × 106/mL, 10% < forward motility sperm percentage < 50%. Exclusion criteria: severe oligozoospermia; dead sperm disease due to erectile dysfunction (ED) or retrograde ejaculation or non‐ejaculation; drug, uncontrolled bacterial prostatitis, fever and other factors affecting fertility; taking drugs that may affect sperm function; congenital malformations, fine tract obstruction, testicular atrophy; tuberculosis, liver, kidney and haematopoietic system of severe primary disease, mental illness. |
|
| Interventions | Vitamin D 200 IU + calcium 600 mg chewable tablet once daily (n = 43) versus Vitamin E 100 mg + vitamin C 100 mg three times a day (n = 43) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters, adverse reactions, pregnancy rate | |
| Notes | Email sent on 23.07.2018 to dr Deng (dengxiaolin@hsc.pku.edu.cn) with questions regarding the randomisation, blinding, outcome data assessment. No reply to date | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "86 patients were randomly divided into treatment group and control group" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded: treatment A once daily chewable tablets, treatment B tablets three times a day |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Dimitriadis 2010.
| Methods | Randomised controlled trial Duration of study: unclear |
|
| Participants | Country: Japan Population: infertile men with oligoasthenospermia, N = 96 Mean age: unclear Inclusion criteria: unclear Exclusion criteria: unclear |
|
| Interventions | Vardenafil 10 mg (n = 23) versus Sildenafil 50 mg (n = 25) versus L‐carnitine 1000 mg (n = 26) versus No treatment (n = 22) Duration of treatment: 12 weeks |
|
| Outcomes | Seminal parameters | |
| Notes | Excluded were vardenafil (n = 23) and sildenafil (n = 25) Tried multiple times to contact authors for randomisation details and methods. No response. Last contacted in Feburary 2014. E‐mail addresses tried: saitomo@kochi‐u.ac.jp, akrosnin@hotmail.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control no treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or lost to follow‐up mentioned. |
| Selective reporting (reporting bias) | Unclear risk | All data points accounted for. No protocol available. |
Ener 2016.
| Methods | Randomised controlled trial Duration of study: unclear |
|
| Participants | Country: Turkey Population: infertile men with a left‐sided clinical varicocele, N = 56 Mean age: 25.8 ± 4.6 years Inclusion criteria: males diagnosed with a left‐sided clinical varicocele in the urology polyclinic, and for whom subinguinal varicocelectomy was planned Exclusion criteria: the use of alcohol, tobacco or any drugs including vitamins |
|
| Interventions | Vitamin E 600 mg (n = 22) versus No treatment (n = 23) Duration of treatment: 12 months, start after varicocelectomy |
|
| Outcomes | Sperm parameters, pregnancy rate | |
| Notes | Power calculation performed Email sent to author on 06.03.2018 to dr Ener (kemalener75@yahoo.com) to ask about funding, the randomisation process, blinding of outcome assessment and if the reported pregnancies were clinical pregnancies and how they were conceived. Reminder email sent to Ener and Ozayar (eozayar@yahoo.com.tr) on 22.03.2018. No reply to date (19.04.2018), data on pregnancy not used, unknown if clinical |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group is no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "A total of 45 patients were included in the study." Quote: "Of note, our cohort was not without limitation. During the study set‐up, the sample size was calculated as 56. However, 11 patients who could not use vitamin E regularly, or did not come to visit in control periods, were excluded from the study." Not clear in which groups drop‐outs belonged |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Eslamian 2013.
| Methods | Randomised controlled triple‐blinded trial Duration of study: 12 weeks |
|
| Participants | Country: Iran Population: asthenozoospermic infertile men, N = 50 Mean age: unclear Inclusion criteria: patients interest in contribution aged 20‐45 who have passed at least one year from the date they have decided to have a baby, not to using pregnancy protection methods, affected by idiopathic asthenozoospermia based on WHO criteria, normal serum gonadotropin, testosterone and prolactin values Exclusion criteria: affected by genital system infection or taking drug for the infection during past three months, affected by anatomical anomalies in genital system such as varicocoele, surgical history on testicles and vasdeferane |
|
| Interventions | Docosahexaenoic acid (DHA) 465 mg + vitamin E 600 IU (n = 25) versus Placebo (n = 25) Duration of treatment: 12 weeks |
|
| Outcomes | Sperm parameters, serum fatty acid concentration and sperm membrane fatty acid concentration | |
| Notes | In Arabic, translated. Tried multiple times to contact authors for further study details with no response. Last tried to contact Feburary 2014: janati@avicenna.ac.ir | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Cans containing capsules marked as A1, A2, B1, B2 and patients, researchers and physician were unaware of the types of drugs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Cans containing capsules marked as A1, A2, B1, B2 and patients, researchers and physician were unaware of the types of drugs" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Triple‐blinded" "Cans containing capsules marked as A1, A2, B1, B2 and patients, researchers and physician were unaware of the types of drugs" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and exclusions: Intervention group (3 withdrawals): one man could not refer to the clinic in sixth week, the wife of the other one got pregnant, and another one was excluded because he have not taken more than 10% of the capsules Control group (6 withdrawals): two men could not refer to the clinic in sixth week, one man could not refer to the clinic in 12th week. One man used complementary Coenzyme Q10, and another one was excluded because he have not taken more than 10% of the capsules |
| Selective reporting (reporting bias) | Unclear risk | Sperm parameters reported. No protocol available. |
Exposito 2016.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: quote: "from January 2010 to July 2014" (information from email) |
|
| Participants | Country: Spain Population: men from infertile couples participating in an IVF/ICSI program, N = 113 according to final manuscript and authors, grouped into three categories: normozoospermic, oligozoospermic and asthenozoospermic. Mean age: 37.6 ± 3.8 years Inclusion criteria: duration of infertility of at leat 12 months and female age less than 40, as this a mandatory criterion in all Spanish public hospitals Exclusion criteria: quote: "the patient does not sign the informed consent" (information from email) |
|
| Interventions | Vitamin E (α‐tocopherol) 400 mg (n = 55, n = 50 completed treatment) versus Placebo (n = 59, n = 51 completed treatment) Duration of treatment: 3 months |
|
| Outcomes | Sperm concentration, sperm count, progressive motility (A+B%), pregnancy rate | |
| Notes | Conference abstract. Trial registration: EudraCT 2007‐000960‐25 Email sent to author Exposito (antonia.expositonavarro@osakidetza.eus;) and Matorras (JOSEROBERTO.MATORRASWEINIG@osakidetza.eus) on 20.02.2018 and 07.03.2018 to request full text or data regarding the outcomes in the OAT/azoospermic group Reply from author Matorras on 13.03.2018, received draft of manuscript.("we hope we are able to submit it for publication in two months") and asked some more questions about design/methods and data (means with SD) on the subgroup of men with male factor (so without the normospermic men). Reply on 24.03.2018: see RoB. Data not usable in meta‐analysis due to the fact that is data for all the 3 categories (normozoospermic, oligozoospermic and asthenozoospermic) together. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from email): "To maintain the blindness to the investigator and the subject, the investigator receives the information of the treatment allocation number from the computer system." Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Quote (from email): "To maintain the blindness to the investigator and the subject, the investigator receives the information of the treatment allocation number from the computer system. The subject receives his study medication package from the study site of the institution." Investigator receives a number belonging to a study medication package |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind". Placebo used. Quote (from email): "All the active and placebo capsules are identical in appearance, shape, smell and taste" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from email): "At the end 101 couples completed the treatment (placebo group N=51 and vitamin E group N=50). Nine couples withdrew from this study before completing their 3 months of treatment due to IVF cycle cancelled or a lack of continuing interest(8%) (five of the placebo group and four of the vitamin E group)(N=104) .Three couples achieved spontaneous pregnancy at 50, 60 and 90 days of treatment;two of them belonged to placebo group and the other belonged to the vitamin E group (2.7%)" Quote (from email): "The data analysis was done with the people who completed the study (n=101)" No ITT. Reasons for drop‐out well explained and balanced. |
| Selective reporting (reporting bias) | Low risk | All the outcomes from the aim of the study and methods were reported |
Galatioto 2008.
| Methods | Randomised controlled, intention‐to‐treat, single centre study. Duration of study: 12 months, from January 2003 to June 2005 |
|
| Participants | Country: Italy Population: men with persistent oligospermia (5 to 20 m/ml), N = 42 Mean age: treatment group 32 (27.5 to 35.5) years, control 33 (23 to 36) years Inclusion criteria: having performed a retrograde embolization with concomitant oligospermia, persistent oligospermia and infertility > 12 months Exclusion criteria: smoking, alcohol consumption, taking any fertility drugs within 3 months prior to the study, serious medical or psychiatric condition, abnormal hormonal profile, sperm infection |
|
| Interventions | N‐acetylcysteine (NAC) 600 mg + vitamins‐minerals (vitamin C, vitamin E, vitamin A, thiamine, riboflavin, piridoxin, nicotinamide, pantothenate, biotin, cyanocobalamin, ergocalciferol, calcium, magnesium, phosphate, iron, manganese, copper, zinc) (n = 20) versus No treatment (n = 22) Duration of treatment: 90 days |
|
| Outcomes | Primary: seminal parameters Secondary: pregnancy (undefined) and adverse effects |
|
| Notes | Power calculation performed. Attempted to contact author regarding median data. No response yet (2014) 2018: motility reported as WHO Class A motile sperm instead of total motility, added to table 'data not usable for meta‐analysis' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned to either antioxidant therapy or no medical therapy. Randomisation number was assigned by random allocation software using a block randomisation design" |
| Allocation concealment (selection bias) | Low risk | Quote: "All steps of randomisation process were performed blindly in the pharmacy of our hospital" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All ejaculate analysis was analyzed blindly with respect to the treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "intention to treat" |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
Gamidov 2017.
| Methods | 'Open perspective randomised' study Duration of study: unclear |
|
| Participants | Country: Russia Population: men with varicocele, N = 114 Mean age: 34.1 ± 12.1 years Inclusion criteria: aged 25‐45 years, participants’ wives had not become pregnant in the last 12 months or more, despite regular unprotected sexual intercourse between the partners; oligo‐,asteno‐ and/or teratozoospermia, varicocele evident upon palpation confirmed by Doppler ultrasonography of scrotum blood vessels, normal constitutional development as determined by the physical exam Exclusion criteria: previously established genetic causes of infertility (Klinefelter syndrome, microdeletions AZF, CFTR), azoospermia, clinical and laboratory evidence for inflammatory changes to sex glands, pyospermia, follicle‐stimulating hormone (FSH) overproduction, immunologic infertility (MAR‐test IgG > 10%), pronounces somatic pathology, psychosexual or ejaculatory disfunction |
|
| Interventions | SpermActin‐forte (acetyl‐L‐carnitine, L‐carnitine fumarate and alpha‐lipoic acid) (n = 38) versus SpermActin‐forte + Vitamin complex 'Man's formula' (n = 38) versus No treatment (n = 38) Duration of treatment: 3 months, after microsurgical varicocelectomy (MVE) |
|
| Outcomes | Sperm parameters, DNA fragmentation, side‐effects | |
| Notes | Article in Russian, translated by Andrew Dubovyi. Ethical approval and obtaining informed consent not mentioned in text. Email sent to author Ovchinnikov (r_ovchinnikov@oparina4.ru) on 29.03.2018 to ask about the randomisation process, blinding of outcome assesors, drop‐outs and which side‐effects they aimed for ("No side effects related to the pharmacological treatment were observed."). Reply on 11.04.18, see RoB. Data on adverse events used. Other data not usable due to the use of medians and interquartile ranges |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using adaptive dynamic randomization with stratification patients were assigned to one of three groups of 38 subjects" Quote (from email): "It was computer randomized block design" |
| Allocation concealment (selection bias) | Unclear risk | Quote (from email): "Randomization was done by the researchers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is no treatment, furthermore group A uses 1 tablet, group B uses 2 tablets Quote (from email):"The study was not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from email): question was the person who assed the outcomes blinded? "Yes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from email):"There were no lost to follow‐up participants (the samples were small)" |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. Quote (from email) when asking about which adverse events were aimed for: "We have not registered any side effects, including gastro‐intestinal, urological, neurological complications, etc" |
Gopinath 2013.
| Methods | Randomised placebo‐controlled double‐blind parallel three‐arm multicentre trial Duration of study: follow‐up 6 months |
|
| Participants | Country: India Population: Idiopathic oligoasthenozoospermia men, N = 138 (N = 125 completed the study) Mean age: 30.74 (range 24‐45) years Inclusion criteria: age 21‐50 years, infertility >1 year, sperm count less than 15 million/mL, sperm total motility < 40%, no history of taking therapy for infertility, no history of OAT, regular sexual intercourse with a potentially normal fertile female, willing to sign informed consent and likely to be available for all visits during follow‐up period Exclusion criteria: primary testicular disease, any organic cause for infertility including varicocele, prostate‐vesiculo‐epididymitis,genital infectious disease,planning for any other ART during study period, serum follicle‐stimulating hormone FSH >15 mIU/mL, abnormal serum levels of LH, testosterone, estradiol and prolactin, presence of antispermatozoa antibodies, severe oligospermia (< 2 million sperm/mL), azoospermia, seminal WBCs more than 1 x 106 mL, major hepatic and renal disease, myopathy, history of allergy to any ingredient of the formulation, not likely to be available for follow‐up, have participated in another clinical trial in the past 3 months, female partners with anatomic or physiological alterations causing subfertility |
|
| Interventions | Fixed doses combination (FDC) 2 tablets (Coenzyme Q10 50 mg + L‐carnitine 500 mg + lycopene 2.5 mg + zinc 12.5 mg) (n = 46) versus Fixed doses combination (FDC) 1 tablet + 1 Placebo tablet (n = 43) versus Placebo 2 tablets (n = 36) Duration of treatment: 180 days |
|
| Outcomes | Primary: improvement in sperm count, total sperm motility (90 and 180 days) Secondary: pregnancy rate, side effects |
|
| Notes | Email sent on 06.03.2018 to dr Zaveri (drhemantzaveri@gmail.com) to ask about the pregnancies (clinical? How conceived?), the randomisation process, blinding of outcome assessment and allocation of 13 dropouts. Reminder email sent on 27.03.2018. Reply on 30.03.2018 from author; see text in RoB. Pregnancy data not used, distribution in groups unknown, only reply from author quote: "No pregnancies were not followed up to stage 12 weeks. So no pregnancy was clinical. 9 pregnancies were conceived through ART 3 Conceived spontaneous" Numbers from text: 6 in FDC 2, 7 in FDC 1, 2 in Placebo. Pregnancy data used in table 1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from email): "Procedures were computer" |
| Allocation concealment (selection bias) | Low risk | Quote: "Centrally randomised to one of three treatment arms (arm 1‐3) in a 1:1:1 ratio" Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blinded". Placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from email): "Yes outcome assessment was blinded " |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 lost to follow‐up (dropout), quote: "at different stage during the study" Asked by email in which groups or what reasons. Quote (reply email): "5 in paternia BID, 6 in placebo, 2 in paternia BID" Data‐analysis only on the 125 who completed the study. Low risk because dropouts accounted for. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Greco 2005.
| Methods | Randomised controlled double‐blind trial Duration of study: unclear |
|
| Participants | Country: France Population: infertile males, N = 64 Mean age: unclear Inclusion criteria: TUNEL assay showed a presence of fragmented DNA ≥ 15% of ejaculated spermatozoa Exclusion criteria: variocele, genitourinary inflammation, infection, smoking |
|
| Interventions | Vitamin C 1000 mg + Vitamin E 1000 mg (n = 32) versus Placebo (n = 32) Duration of treatment: 2 months |
|
| Outcomes | Sperm parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was double‐blinded with both the authors and the patients unaware of which of the patients was in the treatment or control arm of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
Haghighian 2015.
| Methods | Randomised triple‐blind placebo‐controlled trial Duration of study: unclear, in 2014 | |
| Participants | Country: Iran Population: men with idiopathic asthenozoospermia, N = 48 Mean age: 33.56 ± 5.07 years Inclusion criteria: unwilling childlessness at least 24 months in duration with a female partner, no medical condition that could account for infertility, normal fertile female partner according to investigations, all patients were needed to have stopped all medical therapy R12 weeks before study initiation Exclusion criteria: the history of epididymo‐orchitis, prostatitis, genital trauma, testicular torsion, inguinal or genital surgery, urinary tract infection, or previous hormonal therapy, another genital disease (cryptorchidism, current genital inflammation or varicocele), severe general or central nervous system disease and endocrinopathy, use of cytotoxic drugs, immunosuppressants, anticonvulsants, androgens, or antiandrogens, recent history of sexually transmitted infection, psychologic or physiologic abnormalities that would impair sexual performance or the ability to provide semen samples, drug or alcohol abuse, hepatobiliary disease, significant renal insufficiency, occupational and environmental subjections to possible reproductive toxins, BMI of >30 kg/m2, participation in another investigational study, unlikely availability for follow‐up |
|
| Interventions | Alpha‐lipoic acid (ALA) 600 mg (n = 23) versus Placebo (n = 21) Duration of treatment: 12 weeks |
|
| Outcomes | Sperm parameters, markers of oxidative stress (total antioxidant capacity (TAC) and malondialdehyde (MDA)), side‐effects | |
| Notes | Email sent to last author Haidari (haidari58@gmail.com) on 06.03.2018 to ask what side effects they aimed for and reasons for lost to follow‐up. Reminder email sent on 22.03.2018 to Haidari and Dadfar (mdadfar@yahoo.com). No reply to date (19.04.2018). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each eligible patient received a randomization number which was determined by a computer‐generated schedule. Then a randomization table was generated by the method of random permuted blocks" |
| Allocation concealment (selection bias) | Low risk | Quote: "Persons who were operationally independent from the study investigator performed the study randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigator, clinician prescriber, and patients were blinded to the treatment condition" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients'data collected during this trial were kept confidential and locked in a secure area. Randomization codes of the study were opened only after all participants had completed the study protocol" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N = 48, quote: "44 completed the study, rest lost to follow‐up: data analysis with 23 of 24 in ALA group, 21 of 24 in placebo group" Reasons lost to follow‐up not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Haje 2015.
| Methods | Randomised controlled trial Duration of study: from January 2013 to June 2014 |
|
| Participants | Country: Iraq Population: infertile men with idiopathic oligozoospermia (OA), N = 128 (in flow chart "182") Mean age: 37.54 ± 2.46 years Inclusion criteria: repeated exhibition of OA without detectable cause (idiopathic OA) Exclusion criteria: leukocytospermia, altered testicular volume of a minimum of 20 ml as depicted by ultrasonography, varicocele as detected by clinical examination and ultrasonography, abnormal FSH levels, couples with combined male and female factors |
|
| Interventions | Tamoxifen 20 mg (n = 45) versus L‐carnitine 1000 mg (n = 20) versus Tamoxifen 20 mg + L‐carnitine 1000 mg (n = 34) versus Placebo (n = 29) Duration of treatment: 3 to 6 months followed by ICSI |
|
| Outcomes | Sperm parameters, fertility and pregnancy outcome following ICSI | |
| Notes | Email sent to author Haje on 06.03.2018 (milathaji@yahoo.com) to ask about randomisation, dropouts, amount of pregnancies (intead of %) and if they were clinical, and to provide raw data specified for amount of months treatment used? Reminder email sent on 22.03.2018. No reply to date (19.04.2018). Data not usable: range of treatment 3 ‐ 6 months, not specified as separates, pregnancy in % instead of numbers, unknown if clinical or not. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not mentioned. Furthermore baseline characteristics not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes are mentioned and provided. No protocol available. |
Kessopoulou 1995.
| Methods | Randomised double‐blinded placebo cross‐over trial Duration of study: unclear |
|
| Participants | Country: UK Population: men with high levels of reactive oxygen species (ROS) of a couple undergoing IVF, N = 30 Mean age: unclear, median age 32 years Inclusion criteria: attending fertility clinic, high levels of ROS in semen. Female partner has patent tubes and is ovulating Exclusion criteria: men with antisperm antibodies, > 20% spermatozoa with Ig (immunoglobulin A) or IgG antibodies and sperm concentration < 5 x 106 mL |
|
| Interventions | Vitamin E 600 mg (n = 15) versus Placebo (n = 15) Duration of treatment: 3 months, 1 month wash‐out, 3 more months after cross‐over |
|
| Outcomes | Primary outcomes: sperm parameters Secondary outcomes: adverse effects, live birth |
|
| Notes | Power calculation performed. Attempted to contact author regarding median data, no response as yet (2014). Only first phase data used in analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was a randomised double blind placebo controlled trial". "The randomisation was performed by the manufacturer" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation was performed by the manufacturer" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the code was blind for the researcher and patients. The code was broken at the end of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "30 patients completed the study over 2 years" Changed to unclear risk in 2018 (was low risk); not reported how many were randomised to start with, or how many drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported as stated in the methods section. No protocol available. |
Kumamoto 1988.
| Methods | Randomised double‐blind parallel trial Duration of study: from January 1985 to June 1986 |
|
| Participants | Country: Japan, 25 centres Population: men with abnormal sperm count or motility, N = 375 Mean age: unclear, average 32.8 (SD 4.8) years Inclusion criteria: average sperm count ≤ 40 × 106 /mL measured on ≥ 2 occasions OR average sperm count ≥ 40 count ≤ 40 × 106 /mL measured on ≥ 2 occasions AND sperm motility < 50% Exclusion criteria: sperm count only measured at 1 occasion, average sperm count ≤ 2 × 106/mL, sperm motility = 0%, testicular size < 8 mL using orchidometer bilaterally, use of hormone or anti‐hormone drug within preceding 3 months before the study period, WBC > 5/HPF in the semen or the presence of possible genito‐urinary infection, presence of hypoganadism or endocrine disease, presence of undescended testes, genito‐uninary tract obstruction, varicocele or any other serious associated condition also included concomitant use of anti‐hormonal and hormonal treatment and the 2 patients with polypharmacy were excluded from the data analysis |
|
| Interventions | Mecobalamin (vitamin B12) 6.000 mcg (n = 125) versus Mecobalamin (vitamin B12) 1.500 mcg (n = 124) versus Placebo (n = 126) Duration of treatment: 12 weeks |
|
| Outcomes | Sperm concentration, sperm motility | |
| Notes | Article in Japanese, translated by Dr Tomoko Kumaga and Tan Wantao. No contact details available for authors. No useable data available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 396 patients were divided into 3 groups (6000ug/day, 1500ug/day, placebo) by randomisation. The implementation of randomisation and allocation concealment was carried out by two people (Doctor Yamamoto, Doctor Shimizu) |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT. 21 lost to follow‐up; 19 dropouts, 2 polypharmacy 2018 Change in RoB to unclear. Not sure in which groups dropouts belonged. |
| Selective reporting (reporting bias) | High risk | Subgroup analysis performed as an addition post‐treatment |
Lenzi 2003.
| Methods | Randomised placebo‐controlled, double‐blind cross‐over trial Duration of study: 10 months |
|
| Participants | Country: Italy Population: infertile men with oligoasthenoteratozoospermia (OAT), N = 100 Mean age: unclear, range: 20 to 40 years Inclusion criteria: age between 20 to 40 years with infertility lasting longer than 2 years, regular sexual intercourse with a gynaecologically normal female partner with no female infertility, absence of endocrine disease, genital infections, obstructive cryptorchism, antisperm antibodies, normal sperm parameters with no significant differences after 3 tests, mild oligospermia with perm concentration 10 to 20 x 106/mL and motility 10% to 30% Exclusion criteria: unclear |
|
| Interventions | L‐carnitine 2 g (n = 43) versus Placebo (n = 43) Duration of treatment: 2 months of washout, 2 months of therapy/placebo, 2 more months of washout, 2 more months of placebo/therapy |
|
| Outcomes | Sperm parameters, pregnancy rate | |
| Notes | Power calculation performed First phase data: attempted to contact author regarding standard deviations, how many were in each group for the first phase and how many of the 4 who went to assisted reproduction did so in the first phase and what do they mean by 172 cycles. No response yet (2014). Added to outcome data 'not usable for meta‐analysis' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blinded", "seemingly identical placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 withdrew ‐ 4 went onto assisted reproduction, 6 did not return for second period and 4 due to pregnancy in first phase. Therefore should only be ?4 at the most lost from first phase. No ITT All withdrawals accounted for for whole trial however how many were lost in the first phase in first phase |
| Selective reporting (reporting bias) | Unclear risk | All outcomes are reported. No protocol available. |
Lenzi 2004.
| Methods | Randomised placebo‐controlled, double‐blind trial Duration of study: 8 months |
|
| Participants | Country: Italy Population: infertile men with OAT, N = 60 Mean age: unclear, range 20 to 40 years Inclusion criteria: oligoasthenoteratospermia, age between 20 to 40 years, infertility > 2 years with regular intercourse, no endocrine disease, cryptorchidism, genital infections or obstructions, variocoele or testicular hypertrophy, antisperm antibodies Exclusion criteria: none |
|
| Interventions | L‐carnitine 2 g + L‐acetyl‐carnitine 1000 mg (n = 30) versus Placebo (n = 26) Duration of treatment: 6 months |
|
| Outcomes | Sperm parameters, pregnancy rate | |
| Notes | Power calculation performed Attempted to contact author regarding 8‐month follow‐up data. No reply as yet (2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Mentions coding: quote: "When codes were broken at the end of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 men withdrew from the placebo group. 60 randomised 56 analysed. No ITT |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Li 2005.
| Methods | Randomised double‐blinded parallel trial Duration of study: 3 months |
|
| Participants | Country: Eastern China Population: infertile men with oligoasthenospermia, N = 150 Mean age: treatment group 30 ± 5.5 (23 to 45) years, control group 32 ± 3.5 (24 to 46) years Inclusion criteria: no smoking or alcohol use, any fertility medication needed to be stopped 2 weeks before Exclusion criteria: none |
|
| Interventions | L‐carnitine 2 g + acetyl‐L‐carnitine 1 g (n = 85) (90 with ITT) versus Vitamin E 200 mg + vitamin C 200 mg (n = 53) (60 with ITT) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters, pregnancy rate | |
| Notes | Article in Chinese, translated by Shaofu Li 10.11.2008. Contact author regarding methods of randomisation, concealment and whether SD or SEs used and query that this is the same trial as Li 2005a 2018: added data on progressive motility |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind" but unclear who is blinded as the control is another antioxidant i.e. not placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition explained. Withdrawal: 5 from treatment group and 7 from control |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
Li 2005a.
| Methods | Randomised trial Duration: unclear |
|
| Participants | Country: Eastern China Population: infertile men with oligoasthenospermia, N = 80 Mean age: 29 ± 3.5 (23 to 40) years Inclusion criteria: no smoking or alcohol, any fertility medication needed to be stopped 2 weeks before Exclusion criteria: none |
|
| Interventions | L‐carnitine 2 g (n = 40) versus Vitamin E 100 mg + Vitamin C 200 mg (n = 40) Duration of treatment: 3 months |
|
| Outcomes | Seminal parameters, pregnancy rate | |
| Notes | Article in Chinese, translated by Shaofu Li 10.11.2008. Attempted to contact author re methods of randomisation, concealment and whether SD or SEs used and whether this is the same trial as Li 2005. Also asked whether there were any data on pregnancy rate. Translator replied 22.09.2009 no pregnancy data were available in the text of the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal: 8 from treatment (n = 32) and 9 from control (n = 31). 21% loss to follow‐up. No ITT |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
Lombardo 2002.
| Methods | Randomised controlled cross‐over trial Duration of study: 10 months |
|
| Participants | Country: Italy Population: infertile men with oligoasthenospermia, N = 100 Mean age: unclear, range 20 to 40 years Inclusion criteria: age 20 to 40 years,infertility > 2 years, 3 baseline semen analysis demonstrating concentration 10 to 20 106/mL, 10% to 30% total motility, forward progression < 15%, abnormal morphological forms < 70%, curvilinear velocity 10 to 30 /second + linearity < 4 Exclusion criteria: unclear |
|
| Interventions | L‐carnitine 2 g (n = ?) versus Placebo (n = ?) Duration of treatment: 2 months |
|
| Outcomes | Sperm parameters | |
| Notes | Abstract only Attempted to contact author re first phase data, outcomes, randomisation, concealment and whether there was a full publication of the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 86 patients completed the trial out of 100. Need to see full trial for the reasons for withdrawals and ITT |
| Selective reporting (reporting bias) | Unclear risk | Abstract only |
Martinez 2015.
| Methods | Randomised double‐blind controlled trial Duration of study: from July 2009 to September 2010 |
|
| Participants | Country: Mexico Population: men with idiopathic oligoasthenozoospermia, N = 54 Mean age: unclear Inclusion criteria: patient between the ages of 20 to 45 years with a diagnosis of idiopathic oligoasthenozoospermia. The diagnosis of oligoasthenozoospermia was reached by performing two semen analyses on different dates with an interval of three weeks between them. Exclusion criteria: infertile patients with normal findings on semen analysis, chronic smokers, antioxidants use in the last 6 months prior to the study, chronic degenerative diseases such as diabetes or high blood pressure Hormonal abnormalities |
|
| Interventions | Resveratrol (3,5,4´‐trihydroxystilbene) 25 mg + 725 mg microcrystalline cellulose (n = 18) versus SG1002 (hydrogen sulfide) 750 mg (n = 18) versus Placebo 750 mg microcrystalline cellulose (n = 18) Duration of treatment: 75 days |
|
| Outcomes | Sperm parameters (with A+B type sperm motility) | |
| Notes | SG1002 (hydrogen sulfide) excluded because it is a gaseous transmitter Email sent to second author Sordia‐Hernandez (luissordia@telmexmail.com) on 22.03.2018 to ask details about the randomisation process and for him to provide more data (SDs). Inconsistence in sentence about adverse events: 3 side effects in SG1002 group, however in the sentence before only 2 in this group? Data not usable, no SD's. No reply to date (19.04.2018). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind". Quote: "Bottles and capsules for each treatment were identical and identified by a code unknown to the researchers or subject." Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Sperm analysis performed by lab technicians, blinded to the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the seven subjects who did not complete the study (3 from the placebo group, 2 from the resveratrol treatment group and 2 from the SG1002 treatment group), none returned for follow‐up visits and therefore no data on sperm count, motility or abnormality was available and an intent to treat analysis could not be carried out. Four of these subjects were lost in follow‐up while the other three withdrew due to unpleasant smelling sweat (SG1002 treatment group), nausea and flatulence (SG1002 treatment group), and inconvenience (SG1002 treatment group)." Quote: "All study subjects who did not comply with medication given as prescribed, who discontinued the drug or were hypersensitive to it were eliminated" Reasons enough explained, all 3 in SG1002 due to side effects, however we did not include this arm |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Martinez‐Soto 2010.
| Methods | Randomised double‐blind controlled trial Duration of study: 10 weeks |
|
| Participants | Country: Spain Population: infertile men, N = 42 (abstract), N = 64 (from author) Mean age: treatment group 35.23 years, placebo 36.10 years, overall average age 35 years Inclusion criteria: men suffering from male factor infertility, according to the WHO guidelines (WHO 1999), and who were undergoing infertility evaluation during the period 2009 to 2011 Exclusion criteria: oncological patients, those suffering from metabolic disease, chromosomal or genetic alterations, and patients on anticoagulant treatment |
|
| Interventions | Brudy Plus 1500 mg of DHA‐enriched oil (DHA 1000 mg + eicosapentaenoic acid (EPA) 135 mg) (n = 35) versus Placebo (n = 29) Duration of treatment: 10 weeks |
|
| Outcomes | Sperm DNA fragmentation, seminal parameters, lipid composition, antioxidant capacity | |
| Notes | Conference abstract only. Contacted author multiple times by e‐mail (JuanCarlos.Martinez@ivi.es) for further study details. Clarified that the abstract details were different from that in the final study, a copy of the unofficial manuscript was submitted to the review authors. Last contact was on 26.02.2014 2018: added data on progressive sperm motility |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list with a computer program |
| Allocation concealment (selection bias) | Low risk | Closed and numerated envelopes with allocation group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants knew that they was included in group A or B but only Brudy technology knew the assignation to the control group or experimental group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Mehni 2014.
| Methods | Randomised double‐blind, placebo‐controlled trial Duration of study: from May 2008 to August 2012 |
|
| Participants | Country: Iran Population: infertile men with idiopathic OAT, N = 235 Mean age: treatment (L‐carnitine) group 30 ± 1.7 years, control group 30 ± 4.6 years Inclusion criteria: age 25 – 40 years, infertile men with OAT, healthy fertile wives Exclusion criteria: existence of genital abnormalities (undescended testes, varicocele, atrophy of testes), occupational chemical exposure history, systemic diseases, abnormal semen volume, pH, agglutination or viscosity, derum hormonal abnormalities (FSH, LH, testosterone, estradiol, prolactin), wives with known fertility risk factors confirmed by gynecologist |
|
| Interventions | Pentoxifylline 800 mg + L‐carnitine 1000 mg (n = 58) versus Pentoxifylline 800 mg + Placebo (n = 59) versus L‐carnitine 1000 mg + Placebo (n = 59) versus Placebo (n = 59) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters (progressive sperm motility), selection of type of assisted reproductive techniques (ART) | |
| Notes | Only data from L‐carnitine and placebo arm used. Email sent to author (dr.ketabchi@gmail.com) on 06.03.2018 to ask about the randomisation process and blinding of the outcome assessment Reminder email sent to Ketabchi on 22.03.2018. No reply to date (19.04.2018). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized by Bloch method to four groups" Bloch (block?) method, does this mean computerised? Insufficient explanation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "After intervention 23 patients excluded from study (3 patients for drug intolerance in group I, and 20 patients for uncooperative in group II and III)" Data‐analysis only with for those who completed the study (N = 212) According to figure 1: 5 patients (instead of 3 mentioned in text) dropped out due to drug intolerance in group I? Type error? Reasons and exact numbers for dropout not given for L‐carnitine arm specifically. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Micic 2017.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: unclear |
|
| Participants | Country: Serbia Population: men with oligo‐asthenozoospermia, N = 175 Mean age: unclear Inclusion criteria: men visiting the Andrology center, (18‐50 years) and with difficulty in conceiving > 12 months Exclusion criteria: unclear |
|
| Interventions | Proxeed Plus (L‐carnitine 2 g, acetyl‐L‐carinitine 1 g, vitamins and minerals) (n = 125) versus Placebo (n = 50) Duration of treatment: 6 months (and 2 months wash‐out) |
|
| Outcomes | Progressive motility, seminal plasma carnitine | |
| Notes | Conference abstract only. Email sent to last author Agarwal (AGARWAA@ccf.org) on 20.02.2018. Answer on 21.02.18 "this study is not published in a journal at this time" New email on 06.03.2018 to ask raw data (means with SD) and more information about randomisation/blinding outcome/dropout rates. Reply on 22.03.18 from Agarwal & Micic (savamicic2016@gmail.com) with more information in a word document. Only medians with IQR. See RoB. Data not usable, medians with IQR. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from email): "Random list was made using the nQuery Advisor nTerim 2.0 (2012) program" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from email): "This is a double blind study. Neither the patient, providers, nor investigators responsible for collecting data or analyzing laboratory specimens have been knowledgeable regarding the assignment of active or placebo product. A file has been maintained at each of the sites under the responsibility of the primary investigator which will provide product identification for each subject. Upon entry into the study, subjects have been assigned a unique study identification number." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from email): "Neither the patient, providers, nor investigators responsible for collecting data or analyzing laboratory specimens have been knowledgeable regarding the assignment of active or placebo product. " |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from email): "From the treated group (total 125 ) drop out was 6 subjects; 2 of them got flu with high temperature, 2 went form Serbia (new job), 2 stopped without reason. And from the placebo group ( total 5o ) drop out was 4; 2 drop out without explanation , 1 underwent abdominal surgery, and 1 divorced" |
| Selective reporting (reporting bias) | Unclear risk | Abstract only |
Morgante 2010.
| Methods | Randomised controlled trial Duration of study: 3 months |
|
| Participants | Country: Italy Population: infertile men with with asthenospermia, N = 180 Mean age: range 25 and 49 years Inclusion criteria: age between 28 and 45, sperm concentration < 20 x 106 spermatozoa /mL, sperm progressive motility < 30%, normal morphology < 30%, leucocyte < 1 x 106 /mL, no infections Exclusion criteria: men younger than 28 and over 45, sperm concentration > 20 x 106 spermatozoa /mL, sperm progressive motility > 30%, normal morphology > 30%, leucocyte > 1 x 106 /mL, current infections, history of testicular pathology: cryptorchidism, varicocele, surgical operations, radiotherapy or chemotherapy, use of anabolic steroids, deficiency of hypothalamic‐pituitary‐gonadal axis, genital tract infections |
|
| Interventions | L‐arginine 1660 mg + carnitine 150 mg + acetyl‐carnitine 50 mg + ginseng 200 mg in one vial (n = 90) versus No treatment (n = 90) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters, sexual satisfaction | |
| Notes | Article in Italian, translated by Roberto D'Amico. Contacted author by email (giuseppe.morgante@unisi.it) to clarify study details, recruitment, randomisation, blinding, ethics approval, study population, withdrawals and to clarify progressive mortality. Last response was on 12.03.2014 Quote: "Total motility and progressive motility are similar terms for the same definition: all the spermatozoa that have progressive or not linear motility" 2018: motility data included as progressive motility |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Nadjarzadeh 2011.
| Methods | Randomised controlled trial Duration of study: 3 months |
|
| Participants | Country: Iran Population: infertile men with OAT who have been trying for pregnancy for > 1 year unprotected intercourse, N = 60 (analysed N = 47) Mean age: 34 years Inclusion criteria: seminal WBC < 1,000,000 /mL, absence of anatomical abnormalities of the genital tract, absence of infectious genital diseases or systemic diseases, absence of treatment with other drugs and dietary supplement during the 3 months before enrolling in the study, at last absence of smoking, drug, and alcohol use or occupational chemical exposure Exclusion criteria: seminal WBC > 1,000,000 /mL, presence of anatomical abnormalities of the genital tract, presence of infectious genital diseases or systemic diseases, presence of treatment with other drugs and dietary supplement during the 3 months before enrolling in the study, currently smoking, using drug, or alcohol use or occupational chemical exposure |
|
| Interventions | Coenzyme Q10 (CoQ10) 200 mg (n = 23) versus Placebo (n = 24) Duration of treatment: 3 months |
|
| Outcomes | Sperm motility and concentration, progression, total antioxidant capacity (TAC) | |
| Notes | Power calculation performed Contacted regarding methods, randomisation, allocation concealment, recruitment, blinding and dropouts. Response from Azadeh Nadjarzadeh (azmm1383@yahoo.com)in October 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from email):"Participants were randomised using block randomisation. It was done by Dr Motevallian who is epidemiologist and it has done before study" |
| Allocation concealment (selection bias) | Low risk | Quote (from email): "Before the trial a colleague, that had not role in the study, coded the bottles of Coenzyme Q10 and placebo (that were similar) in A and B and give them to one of the staff of Avicenna Research centre. Only that person has a list of randomisation and give A or B bottles to the participants according to their code" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from email): "Both participants and investigators blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from email): "The appearance and the bottles of capsules were similar and none of outcome assessors knew group, because everyone had a code after being allocated group A and B" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "13 dropped out for personal reasons" ‐ 22%: 7 from treatment group and 6 from the control group |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Nozha 2001.
| Methods | Randomised comparative study Duration of study: unclear |
|
| Participants | Country: Tunisia Population: infertile males with OAT, N = unclear Mean age: unclear Inclusion criteria: males with OAT. Exclusion criteria: unclear |
|
| Interventions | Vitamin E 400 mg + Selenium 200 μg (n = 12) versus Vitamin B2, B6 and B12 (n = 8) Duration of treatment: 3 months |
|
| Outcomes | Seminal parameters | |
| Notes | Abstract only Attempted to contact authors regarding methods of randomisation and data. No reply as yet (2014). No extractable data from the abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a prospective randomised comparative study" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Omu 1998.
| Methods | Randomised controlled open trial Duration of study: follow‐up 12 months |
|
| Participants | Country: Kuwait Population: men with asthenozoospermia attending infertility and andrology clinic, N = 100 Mean age: treatment group 37.8 ± 7.9 years, control group 38.1 ± 8.2 years Inclusion criteria: men with asthenozoospermia, spermatozoal motility impaired with >4 0% non‐motile sperm, have been trying to conceive for at least one year plus no obvious female factor Exclusion criteria: none mentioned |
|
| Interventions | Zinc 500 mg (n = 49) versus No treatment (n = 48) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters | |
| Notes | Attempted to contact authors regarding methods randomisation and concealment questioned. No reply as yet (2014). Data on sperm count/motility not used; only percentage of increase/decrease given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 100 men randomised, 97 analysed, dropouts are not accounted for |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Omu 2008.
| Methods | Randomised controlled open trial Duration of study: unclear |
|
| Participants | Country: Kuwait Population: men with asthenozoospermia attending infertility clinic in Kuwait, N = 45 Mean age: 35 ± 1 years Inclusion criteria: asthenozoospermia with normal sperm concentration (20 to 250 million/mL) but with 40% or more immotile sperm Exclusion criteria: asthenozoospermia but sperm concentration of < 20 million/mL |
|
| Interventions | Zinc 400 mg (n = 11) versus Zinc 400 mg + Vitamin E 20 mg (n = 12) versus Zinc 400 mg + Vitamin E 20 mg + Vitamin C 10 mg (n = 14) versus No treatment (n = 8) Duration of intervention: 3 months |
|
| Outcomes | Sperm parameters | |
| Notes | Attempted to contact author regarding methods of randomisation, it states that quote: "8 men served as non‐ therapy control". No reply as yet (2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is another antioxidant or no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes are reported. No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Peivandi 2010.
| Methods | Randomised double‐blind cross‐over trial Duration of study: unclear, from 2005 to 2006 |
|
| Participants | Country: Iran Population: infertile men, N = 30 Mean age: 29.5 (SD 5.48) years Inclusion criteria: at least two abnormal spermograms based on WHO criteria with a two‐week interval during four weeks, normal range of gonadotropins, testosterone an prolactin concentrations Exclusion criteria: variocoele, testicular atrophy, ejaculatory disorders, use of medications, azoospermia, endocrinological disorders, ICSI candidacy or other causes of infertility |
|
| Interventions | L‐carnitine 2 g (n = 15) versus Placebo (n = 15) Duration of treatment: 8 weeks, washout period of 8 weeks, changed intervention and use for 8 more weeks |
|
| Outcomes | Sperm parameters | |
| Notes | Abstract in English, full text in Arabic. Contacted the author and he is filling out the data extraction sheets. Author responded but data queries remain contacted again re SDs and pregnancies in first phase of cross‐over. Author responded saying that the data were given in SDs and there were 3 pregnancies in the first phase 2018: added data on progessive motility for first phase (2 months). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome assessor was blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "loss to follow up was not accounted for" |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Pourmand 2014.
| Methods | Randomised trial with add‐on intervention Duration of study: unclear |
|
| Participants | Country: Iran Population: men with male factor infertility and varicocele, N = 100 Mean age: treatment group 26.73 ± 6.25 years, control group 27.52 ± 5.23 years Inclusion criteria: left‐sided clinical or subclinical varicocele plus one of these factors: primary infertility, secondary infertility, or impaired semen analysis. Exclusion criteria: right‐ sided isolated varicocele, bilateral varicocele, and each side varicocele that did not decompress in lying position, or any medical or surgical history of male factor infertility ‐ Medical: opium or drug abuse, any prior medical treatment for infertility, recurrent urinary tract infection, sexually transmitted disease, prostatitis, mumps in childhood, epididymo‐orchitis, and so forth ‐ Surgical: cryptorchidism, orchiopexy, prior varicocelectomy repair, inguinal hernia repair, other inguinal surgeries, and so forth |
|
| Interventions | L‐carnitine 750 mg (n = 50) versus No treatment (n = 50) Duration of treatment: 6 months, after varicocelectomy |
|
| Outcomes | Sperm parameters, DNA damage (TUNEL, PDA test), adverse effects | |
| Notes | Email sent to last author Noori (m_noori560@yahoo.com) on 06.03.2018: Asked about the SD's for sperm motility (A+B%), concentration and DNA fragmentation. Asked about allocation concealment and blinding of outcome assessment. Reminder email sent to Noori and Pourmand (n.pourmand@yahoo.com) on 22.03.2018. Only data on adverse events used. No reply to date (19.04.2018). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomization was performed for controlling less probable variation in varicocelectomy technique or surgeon within the time of study" Not specified how block randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group is no treatment after varicocelectomy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See appendix, none lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes from the aim of the study and methods were reported. No protocol available. |
Poveda 2013.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: from January 2012 to March 2013 |
|
| Participants | Country: Panama Population: infertile healthy men, N = 60 (quote: "60 patients completed the study", how may were randomised?) Mean age: unclear Inclusion criteria: infertile healthy men without previous treatments, non smokers, no alcoholics or drug users Exclusion criteria: varicocele and leukocyte‐spermia were excluded |
|
| Interventions | L‐carnitine 1 g/12 hours (n = ?) versus Spermotrend (Catalysis) 1 x /8 hours (n = ?) versus Maca extract 1 g/12 hours (n = ?) versus Placebo 1x/12 hours (n = ?) Duration of treatment: 13 weeks |
|
| Outcomes | Sperm motility, sperm concentration, normal sperm morphology | |
| Notes | Conference abstract only. Letter written and posted regarding methods and data 12.02.2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Pryor 1978.
| Methods | Randomised double‐blind cross‐over trial Duration of study: unclear |
|
| Participants | Country: UK (two centres) Population: men with severe oligozoospermia, N = 64 Mean age: unclear Inclusion criteria: sperm count of less than 10 million per ejaculate on each of 2 occasions immediately preceding the trial, no uncorrected varicoceles or testicular maldescent, testicular biopsy already performed (Johnsen 1970), no drugs taken in past 3 months which were known to affect spermatogenesis, no history of biliary disease owing to a suggestion that arginine might interfere with the metabolism of bile salts, the wives of all these men had been fully investigated with regard to fertility Exclusion criteria: men with varicocoele |
|
| Interventions | Arginine 4 g (n = 35) versus Placebo (n = 29) Duration of treatment: 12 weeks, than cross‐over without intervening wash‐out period |
|
| Outcomes | Total sperm motility, hormone levels | |
| Notes | No data available for sperm parameters. Unable to contact author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 withdrew reasons were given but unsure from which group, the paper stated that they used ITT but data not presented. The study did not report the outcomes for the different phases of the trial (i.e. not separated into phase 1 phase 2). Pregnancy data are separated into phase one data but probably biochemical and will be used in biochemical pregnancy table. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. Pregnancy not stated in the methods section as an outcome of interest but reported in the results. No protocol available. |
Raigani 2014.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: unclear |
|
| Participants | Country: Iran Population: men from infertile couples with proved male factor infertility, N = 83 Mean age: unclear Inclusion criteria: infertile men (OAT) with sperm concentrations of < 20 x 106 mL‐1, sperm motility < 50% (grades a, b, c) and sperm normal morphology < 30% Exclusion criteria: unclear |
|
| Interventions | Folic acid 5 mg + Placebo (n = 20) versus Folic acid 5 mg + Zinc sulphate 220 mg (n = 21) versus Zinc sulphate 220 mg + Placebo (n = 24) versus Placebo + Placebo (n = 18) Duration of treatment: 16 weeks |
|
| Outcomes | Sperm concentration, motility (grade A+B+C), morphology, sperm viability, sperm mitochondrial function, sperm chromatin status (DNA damage measured by staining methods), semen and blood folate/zinc/B12, total antioxidant capacity (TAC) and malondialdehyde (MDA) concentration | |
| Notes | Trial registration: IRCT138706091079N2 Email sent to last author Sadeghi (Sadeghi@avicenna.ac.ir) on 06.03.2018 to ask about the mean age, exclusion criteria, if there are means+SD instead of medians of the sperm concentration and sperm motility, randomisation process, dropouts/lost to follow‐ups Reminder email sent to Sadeghi on 22.03.2018. No reply to date (19.04.2018). Data on DNA fragmentation used (means+SD), however motility/concentration in medians (IQ range) so added in data outcome 'not suitable for meta‐analysis' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated into four treatment groups with different supplementations." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blinded". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Semen analysis and sperm function assays were assessed individually and blindly by two laboratory experts" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Low risk | Reported all the outcomes from the methods and protocol; trial registration (IRCT138706091079N2) |
Rolf 1999.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: 8 weeks |
|
| Participants | Country: Germany Population: men with infertility for over one year, N = 33 Mean age: treatment group 36.1 ± 5.0 years, control group 35.2 ± 4.8 years Inclusion criteria: asthenozoospermia (< 50% motile) diagnosed after 2 examinations, normal or reduced sperm concentration (> 20 x 106 per ejaculate) and without infection of access glands Exclusion criteria: unclear |
|
| Interventions | Vitamin C 1000 mg + Vitamin E 800 mg (n = 15) versus Placebo (n = 16) Duration of treatment: 8 weeks |
|
| Outcomes | Primary: sperm parameters Secondary: pregnancy rate and adverse effects |
|
| Notes | Power calculation performed. Contacted author about the allocation concealment and pregnancy and adverse effects were outcomes in their protocol. Author Rolf replied saying that pregnancy and adverse effects were stated in the protocol 2018: progressive forward motility instead of total motility, data total sperm motility moved to outcome progressive sperm motility |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed with random numbers without further stratification by the pharmacist and the code was withheld from researchers and patients" |
| Allocation concealment (selection bias) | Unclear risk | Pharmacist performing randomisation and code withheld from patients and researchers. However no mention of type of containers or envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double ‐ patients and researchers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported, 2 patients withdrew from the trial: quote: "results from two patients were rejected from analysis." 1 from the treatment group due to poor compliance and 1 from the placebo group due to genital tract infection. No ITT |
| Selective reporting (reporting bias) | Unclear risk | All semen outcomes reported and author states (e‐mail 22.09.09) that pregnancy and adverse effects were set a priori in the protocol. No protocol available. |
Safarinejad 2009.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: 56 weeks |
|
| Participants | Country: Iran Population: men with idiopathic oligoasthenoteratospermia, asthenospermia or teratospermia of 2 years duration, N = 468 (548 recruited) Mean age: 31 (25 to 48) years Inclusion criteria: sperm count > 5 x 106 /mL, over 2 years of failed conception, no female fertility problems, no history of possible cause for male infertility Exclusion criteria: abnormal testes, history of cancer or chemotherapy, testosterone or antiandrogen use, use of selenium or N‐acetylcystine supplements, abnormal hormone levels, genital disease, genital inflammation or variocoele, history of genital surgery, major surgery, central nervous system injury, a known sperm defect or retrograde ejaculation. Y chromosome abnormalities, sexually transmitted disease, genitourinary infection, leukocytospermia, smoking, any environmental exposures to reproductive toxins. Medical, neurological or psychological problems. A history of drug or alcohol abuse, hepatobiliary disease or significant renal insufficiency. Any endocrine abnormality, a b BMI of 30 kg/m2 or over, participation in another investigational study and a likelihood of being unavailable for follow‐up |
|
| Interventions | Selenium 200 µg (n = 116) versus N‐acetylcysteine (NAC) 600 mg (n = 118) versus Selenium 200 µg + N‐acetylcysteine (NAC) 600 mg (n = 116) versus Placebo (n = 118) Duration of treatment: 26 weeks or 6.5 weeks |
|
| Outcomes | Primary outcome: sperm parameters Secondary outcomes: spontaneously reported adverse events |
|
| Notes | Power calculation performed. Attempted to contact authors regarding side effect data that had not yet been added to the review due to the query of multiple comparisons. Also to ask whether data is in SD (as reported in the text) or SE, as requested by statistician 24.09.2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation table generated by the method of random permuted blocks. Patient randomisation numbers were allocated to each site in ascending sequence in blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignment to treatment groups was performed using a sealed envelope technique." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Eligible patients were randomly assigned to double blind.." Quote: "Placebo pills were coated with titanium oxide to ensure an identical appearance and smell." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed: n = 105 in selenium group (loss 11), n = 106 in placebo group (loss 12), n = 105 in N‐acetylcysteine group (loss 13) and n = 104 in selenium + N‐acetylcysteine group (loss 12) All withdrawals were accounted for in each treatment group. Withdrawal was mainly due to withdrawal of consent followed by lost to follow‐up and lastly for reasons of missing data. No ITT |
| Selective reporting (reporting bias) | Unclear risk | The published report includes all expected outcomes. No protocol available. |
Safarinejad 2009a.
| Methods | Randomised double‐blind controlled trial Duration of study: from February 2005 until October 2006, follow‐up 14 months |
|
| Participants | Country: Iran Population: infertile men with idiopathic oligoasthenoteratospermia, N = 212 (recruited 268) Mean age: treatment group 28 ± 9 years, placebo group 28 ± 10 years Inclusion criteria: minimum 2 years unprotected intercourse with 2 years unwilling childlessness. male infertility diagnosed if 1 or more standard semen parameters were below cutoff levels accepted by WHO. A fertile female partner. No known medical condition that could account for infertility, testicular volume 12 mL or greater. No medical therapy for at least 12 weeks before the study begins. Only patients seeking medical attention for infertility were included Exclusion criteria: azoospermia or severe oligospermia (sperm count less than 5 million/mL. An history of epypidymo‐orchitis, prostatitis, genital trauma, testicular torsion, inguinal or genital surgery. Any genital or central nervous system disease, endocrinopathy, cytotoxic drugs, immunosuppressants, anticonvulsives, androgens, antiandrogens, a recent history of Sexually transmitted disease. Psychological or physiological abnormalities that would impair sexual functioning or ability to produce sperm samples. Drug, alcohol or substance abuse. Liver disease, renal insufficiency or chromosome abnormalities. occupational and environmental exposures to reproductive toxins. A BMI of 30 kg/m2 or over, participation in another investigational study and a likelihood of being unavailable for follow‐up |
|
| Interventions | Coenzyme Q10 (CoQ10) 300 mg (n = 106) versus Placebo (n = 106) Duration of treatment: 26 weeks or 6.5 months |
|
| Outcomes | Primary outcomes: sperm parameters and testicular volume Secondary outcomes: adverse effects and hormone levels |
|
| Notes | Power calculation performed. Attempted to contact authors to ask whether data is in SD (as reported in the text) or SE, as requested by statistician 24.09.2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each eligible patient received a randomisation number, which was determined by a computer generated schedule. Therafter a randomisation table was generated by the method of random permuted blocks. Individuals who were geographically and operationally independent of the study investigator performed the study randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The clinician prescriber and the patients were blinded to the treatment condition. To maintain and guarantee blinding CoQ10 and placebo were identical in appearance." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participant data collected during this trial were kept confidential and locked in a secure office area. Randomisation codes were opened only after all patients had completed the whole study protocol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who dropped out of the trial were accounted for ‐ 8 from treatment group and 10 from placebo group for reasons such as withdrawal of consent, missing data and loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Safarinejad 2012.
| Methods | Randomised controlled trial Duration of study: from June 2010 to January 2011 |
|
| Participants | Country: Iran Population: infertile men with primary infertility for at least 2 years, N = 228 Mean age: treatment group 31 years, control group 32 years Inclusion criteria: history of primary infertility of more than 2 years, abnormal sperm count and motility according to WHO criteria, wife age between 20 and 40 years, documentation of fertile female partner, no known medical or surgical condition which can result in infertility Exclusion criteria: history of cancer chemotherapy or radiotherapy, history of genital disease such as cryptorchidism and varicocoele, history of genital surgery, BMI 30 kg/m2 or greater, any endocrinopathy, Y chromosome microdeletion or karyotype abnormalities, leukocytospermia (more than 106 WBC per mL), drug, alcohol or substance abuse, tobacco use, use of anticonvulsants, androgens or antiandrogens, significant liver (serum bilirubin greater than 2.0 mg/dL) or renal function (serum creatinine greater than 2.0 mg/dL) impairment, occupational and environmental exposure to reproductive toxins, severe oligozoospermia (less than 5 x 106 /mL), azoospermia and testicular volume less than 12 mL |
|
| Interventions | Coenzyme Q10 (Ubiquinol) 200 mg (n = 114) versus Placebo (n = 114) Duration of treatment: 26 weeks |
|
| Outcomes | Sperm volume, sperm density, sperm motility, sperm morphology, seminal plasma antioxidant status | |
| Notes | Power calculation performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | The randomisation codes were centrally assigned by the co‐ordination centre after checking the main eligibility criteria |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and study staff were blinded to treatment allocation during the whole study period, All of the participants were naive for treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators and study staff were blinded to treatment allocation during the whole study period, All of the participants were naive for treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 228 were randomised of 264 eligible Ubiquinol group – 13 excluded at end of treatment (3 protocol violations, 4 withdrawal of consent and 6 lost to follow‐up). At 12 weeks follow‐up a further 5 were lost to follow‐up Placebo group – 12 excluded at end of treatment (4 protocol violations, 4 withdrawal of consent, 6 lost to follow‐up. At 12 weeks follow‐up a further 7 were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | The authors do not pre‐specify which outcome measures will be reported. The primary outcome is a % change from baseline at the end of the treatment period |
Scott 1998.
| Methods | Randomised double‐blind trial Duration of study: 3 months and two weeks |
|
| Participants | Country: UK Population: men attending subfertility clinic with low sperm motility, N = 64 (recruited N = 69) Mean age: 33.3 ± 0.64 years Inclusion criteria: low sperm motility Exclusion criteria: not mentioned |
|
| Interventions | Selenium 100 µg (n = 16) versus Selenium 100 µg + Vitamin A 1 mg + Vitamin C 10 mg + Vitamin E 15 mg (n = 30) versus Placebo (n = 18) Duration of treatment: 3 months |
|
| Outcomes | Primary outcome: sperm parameters Secondary outcome: pregnancy rates |
|
| Notes | Uneven numbers, multivitamin numbers are double the other groups Asked author if they have separate numbers for pregnancy data. Currently have 5 pregnancies in the 2 treatment groups and none in placebo Furthermore; who was blinded, was the placebo identical when group 2 contained so many different vitamins. Was there any allocation concealment? Author has retired and is not able to be contacted. Data not added to table 'data for undefined or biochemical pregnancy' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "As the patients entered the trial they were randomly allocated to one of three treatments, which had in turn been randomised within each block of four numbers and 'blinded' using a numeric code." Unclear as to why the uneven nature of the numbers in the groups i.e. 30 in multivitamin group and 16 in selenium, 18 in placebo |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of withdrawals and reasons (non compliance) were reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Sharifzadeh 2016.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: from March 2015 to November 2015 |
|
| Participants | Country: Iran Population: Idiopathic subfertile men, N = 114 Mean age: Inclusion criteria: Idiopathic subfertile male with sperm rates 5 ‐ 20 million cells/mL, and according to failure of female to conceive after one year regular and unprotected intercourse Exclusion criteria: chromatically fertility disorder (Y chromosome deletions), use of zinc three months before recruitment |
|
| Interventions | Zinc sulphate 10 mL solution of 0.5% (n = 61) versus Placebo 10 ml (n = 53) Duration of treatment: 3 months |
|
| Outcomes | Sperm parameters, side‐effects, serum and semen plasma levels of zinc | |
| Notes | Trial registration: IR.IUMS.REC.1394.26155 Email sent to second author Norouzi (sr.norouzi@yahoo.com) on 06.03.2018 to ask if they can provide mean+SD instead of median, and if the motility is total motility or progressive motility. Reply on 11.03.2018: "yes we use SD for motility and total concentration, for both of them instead of a median. Motility means group A+ B (progressive motility)" New email on 12.03.2018 to ask if they can then provide mean + SD. Reply on 04.04.18 answering "In this study we used the SPSS software (SPSS, Inc., Chicago, IL, USA, version 20) for statistical analyses. After normality testing confirmed by Shapiro‐wilk test, quantitative data were reported as mean ± SD. Unfortunately there are some spelling and statistical errors in the final version of article. In the review process, some changes have been made in the manuscript and subtitle of the tables have been deleted. So all outcome are Mean ± SD." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In the current study males were divided into groups A and B by block randomized sampling." Quote: "sub fertile males were assigned according to a simple computer schedule into two groups to receive zinc sulfate or placebo." |
| Allocation concealment (selection bias) | Low risk | Quote: "Solutions were coded from 1 to 120 according to the randomization list by hospital pharmacy. Each code was given to one participant to receive one container of solution that according to their group called participates took zinc sulfate (0.5) or placebo." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind" Quote: "Containers of zinc solution and placebo were similar, and all of them had zinc syrup label. The secretary of infertility unit did not know about the box content and patients by showing their groups label could receive the medicine." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "seven subjects in the zinc group withdrew because of adverse gastrointestinal side effects, and three subjects in the zinc group and four subjects in the placebo group withdrew because of lack of motivation" Dropouts accounted for and reasons mentioned. No ITT |
| Selective reporting (reporting bias) | Low risk | Reported all the outcomes from the methods section and according to the protocol: trial registration (IR.IUMS.REC.1394.26155) |
Sigman 2006.
| Methods | Randomised double‐blind trial Duration of study: 24 weeks, follow‐up unclear |
|
| Participants | Country: USA Population: infertile men aged 18 to 65 years, N = 26 Mean age: 36.2 ± 5.8 years, 35.3 ± 7.5 years Inclusion criteria: males 18 to 65 years with infertility of at least six months duration, sperm concentration of at least 5 million sperm/mL, motility of 10% to 50%, absent pyospermia and normal FSH and testosterone levels Exclusion criteria: history of post‐pubertal mumps, cryptorchism, vasal or epididymal surgery, history of medication or chemotherapy. recent alcohol, chronic marijuana. Use of testosterone or steroids. Exposure to environmental toxins. Recent history of fever or diabetes, liver failure, renal failure, endocrine disorder, untreated variocoele, urogenital infection, or prior vasectomy reversal |
|
| Interventions | L‐carnitine 2000 mg + L‐acetylcarnitine 1000 mg (n = 12) versus Placebo (n = 9) Duration of treatment: 4 months |
|
| Outcomes | Primary outcome: sperm parameters Secondary outcomes: pregnancy rate |
|
| Notes | Author replied 21.09.2009 saying: Quote "The published 2006 trial is the published version of the 2003 abstract (Pryor 2003)" and giving details of randomisation and concealment. Author says he will try and find out about the 5 patients that dropped out. Why did ‐ "5 additional patients entered the study but dropped out before completion" ‐ when did these patients enter and were they randomised? Quote: "One of these 5 dropped out because of pregnancy three months after starting carnitine" Pryor paper excluded as it is the same study as Sigman, author also gave details of randomisation and allocation concealment, author will try to find info on 5 patients who dropped out. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised to receive carnitine or placebo" Quote: "The randomisation was done by a third party a company that oversaw the trial. We sent the patient number of new recruited patients in to them, they assigned them a study number that was associated with a collection of medication/placebo." The author replied to randomisation query 23.09.09 saying that the protocol stated that ‐ "treatments will be assigned randomly to a subject number. The numbers will range from 1‐84 for study centre 1 and 85‐168 for study centre 2. Randomisation of treatments for each centre will be done independently. One half of subject numbers will be placebo, the other half, active ingredient." |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigators and study sites had the study medication/placebo packets identified by number only. They were blinded to what was in the medication/placebo packets. We were sent the code at the conclusion of the trial." The author replied to a query on allocation concealment on 23.09.09 saying that the protocol stated that ‐ " Integrated Data Solutions, Inc. will keep the randomisation code in a separate sealed envelope for each site until the end of the study. The randomisation lists will be provided to the packaging company for packaging of the packets into patient medication boxes.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the investigators and the patient were blinded to the treatment arm assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "5 additional patients entered the study but dropped out before completion. One of these dropped out because of pregnancy three months after starting carnitine." Author replied to query re drop outs, quote: "I have data on one drop out at my site ‐ the drop out occurred after randomisation to carnitine. The drop out occurred before the first follow‐up study visit. The other four drop outs were from the other study site ‐ I am trying to get that data for you" (23.09.09) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes of interest were reported. No protocol available. |
Sivkov 2011.
| Methods | Randomised controlled open‐label trial Duration of study: unclear, from 2008 to 2009 |
|
| Participants | Country: Russia Population: men with chronic prostatitis and abnormal fertility for more than 6 months, N = 30 Mean age: unclear, range 18 to 40 years |
|
| Interventions | Selznic (selenium + zinc + vitamins) (n = 15) versus Placebo (n = 15) Duration of treatment: 3 months |
|
| Outcomes | Sperm motility, sperm concentration | |
| Notes | Article in Russian, translated by Vasya Vlassov. No SD available. Need to contact authors regarding methods, standard deviations, type of control and any pregnancy data. Author Vasya 17.02.14 saying that the control was placebo and SD's not given. Emailed the institution 18.02.2014 regarding methods and data, no reply as of 07.03.2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Open labelled". However placebo used, might be a translation problem |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Sofikitis 2016.
| Methods | Randomised controlled trial Duration of study: unclear |
|
| Participants | Country: Greece Population: oligoasthenospermic infertile (OAI) men, N = 39 Mean age: unclear Inclusion criteria: unclear Exclusion criteria: unclear |
|
| Interventions | Avanafil 150 mg (n = 13) versus L‐carnitine 1.5 g (n = 14) versus No treatment (n = 12) Duration of treatment: 12 weeks |
|
| Outcomes | Sperm parameters, length of sperm midpiece (LMP), outcome of hypoosmotic swelling test (%HPST), seminal plasma citrate concentration | |
| Notes | Abstract only. Email sent to Dimitriadis (helabio@yahoo.gr) on 21.02.2018 to ask for data/full text, reply the same day from the author: Quote: "This work has not been published as a full paper". New email sent on 26.02.2018 to ask if we could receive data (mean+SD) for the L‐carnitine and placebo group. Reminder email sent on 22.03.2018. No reply received to date (19.04.2018). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control is no treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Unclear, only abstract available |
Suleiman 1996.
| Methods | Randomised double‐blind controlled trial Duration of study: 6 months, follow‐up unclear |
|
| Participants | Country: Saudi Arabia Population: asthenozospermic men attending a fertility centre, N = 110 Mean age: treatment group 34.8 (27 to 52) years, control group 33.2 (22 to 45) years Inclusion criteria: asthenospermic (≥ 20 x 106 /mL). sperm motility ≤ 40%, normal sperm count, leucocyte concentration < 5%, normal fructose concentration, normal female Exclusion criteria: unclear |
|
| Interventions | Vitamin E 300 mg (n = 52) versus Placebo (n = 35) Duration of treatment: 6 months |
|
| Outcomes | Primary outcome: motility and MDA concentration Secondary outcome: live birth, pregnancy, miscarriage |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Either 100mg vitamin E or a placebo was prescribed in a random double blind fashion". Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"Double blinded". Placebo used. Quote: "If the semen sample improved and the patient's spouse became pregnant, the treatment was stopped; otherwise it was continued for 6 months. The placebo was given for 6 months" This could suggest that the investigators or clinicians had knowledge of whether the patients were in the placebo or antioxidant group, therefore this trial was rated as high risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The exact dropout figures for each group is unclear. Quote: "A total of 110 patients were enrolled in the study, but some of the patients dropped out and some left the region and failed to continue. When the experiment was terminated, 52 patients were found to have taken vitamin E and 35 patients to have taken the placebo." Assuming the groups were equal initially then the placebo group lost 20 men and the intervention lost 3. A drop out rate of >20% |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods were reported in results. No protocol available. |
Tremellen 2007.
| Methods | Randomised double‐blind controlled trial Duration of study: 1.5 years, follow‐up 13 weeks |
|
| Participants | Country: Australia Population: infertile men, couple undergoing IVF, N = 60 (recruited N = 82) Mean age: treatment group 37.1 ± 5.1 years, placebo group 35.5 ± 4.3 years Inclusion criteria: men with sperm samples showing oxidative stress and a significant level of DNA fragmentation (> 25% TUNEL positive) Exclusion criteria: female partner with diminished ovarian reserve or if the female partner is aged over 39 years |
|
| Interventions | Menevit (folate 0.5 mg + garlic 1000 mg + lycopene 6 mg + vitamin E 400 IU + vitamin C 100 mg + zinc 25 mg + selenium 26 μg + palm oil) (n = 40) versus Placebo (containing palm oil) (n = 20) Duraton of treatment: 3 months, prior to IVF cycle |
|
| Outcomes | Primary outcome: embryo quality Secondary outcomes: pregnancy, multiple pregnancy, fertilisation rate, side effects |
|
| Notes | Power calculation performed Associate Professor Tremellen provided live birth data in December 2014 "Only one pregnancy failed in the Menevit arm after 13 weeks (late miscarriage 19 weeks of male infant). All other pregnancies, including the twin pregnancies went on to live birth and all babies appear to be doing well from the records". There were three sets of twins in the combined antioxidants group and nil in the placebo group. Each twin pregnancy and live birth was counted as one event in the data analyses due to the protocol specifications of the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"The randomisation schedule was computer generated in blocks of six by Bayer Consumer Care Australia". Using a 2:1 ratio Quote: "There were no significant differences between the active and the placebo group in terms of important baseline prognostic characteristics..." |
| Allocation concealment (selection bias) | Low risk | Quote: "the appropriately numbered bottles of capsules delivered to the clinical site without any participant knowing the treatment sequence. Patients were allocated the next numerical treatment package (one to sixty as they became eligible for enrolment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind". Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals were accounted for, 2 from the intervention group, 4 from placebo all due to the couples not going through to embryo transfer |
| Selective reporting (reporting bias) | Unclear risk | All specified outcomes are reported. No protocol available. |
Wang 2010.
| Methods | Randomised controlled trial Duration of study: from August 2007 to August 2009 |
|
| Participants | Country: China Population: infertile men with asthenozoospermia, N = 135 Mean age: unclear, range 23 to 26 years Inclusion criteria: male asthenozoospermia patients, aged 23 to 26 years old, with a history of infertility for about 1 to 10 years, and with no contraception measures after marriage at least 12 months, has normal sex life, the wife’s fertility is normal., semen analysis for at least twice based on WHO criteria (Forward mobile sperm (a + b level) < 50%, and fast forward movement sperm (a level) < 25%, sperm density > 20 x 106 /mL), tests for peripheral blood chromosome and reproductive hormones (FSH, LH, PRL, T) were normal, the tests for semen ureaplasma mycoplasma and chlamydia trachomatis were negative, semen WBC < 1 x 106 /mL Exclusion criteria:cryptorchidism, testicular dysplasia, varicoceles, reproductive tract infection |
|
| Interventions | L‐carnitine 2 g + Vitamin E (n = 68) versus Vitamin E (n = 67) Duration of treatment: 3 months |
|
| Outcomes | Pregnancy rates, adverse effects, % forward motile sperm, sperm density, % sperm normal morphology | |
| Notes | Article in Chinese, translated by Liu Qi. E‐mailed Qin (translator) regarding pregnancy and adverse event data, then may need to write to the authors. No reply to date. 2018: added data on progressive sperm motility |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 135 patients with asthenozoospermia were randomly divided into Groups". Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 dropouts. Numbers from each group are given but no reasons are provided for the withdrawals. ITT not used in the trial analysis |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Wong 2002.
| Methods | Randomised double‐blind placebo‐controlled trial Duration of study: from July 1997 to August 1998 |
|
| Participants | Country: the Netherlands Population: fertile and subfertile men, N = 103 (recruited subfertile N = 258) Mean age: 34.3 ± 3.9 years Inclusion criteria for subfertile group: failure of the woman to conceive after 1 year regular unprotected intercourse and sperm concentration of 5 to 20 million/mL Exclusion criteria for subfertile group: chromosomal disorders, cryptorchidism, vasectomy, use of folic acid or zinc supplements in the previous 3 months, vitamin B deficiency |
|
| Interventions | Folic acid 5 mg (n = 22) versus Zinc sulphate 66 mg (n = 23) versus Zinc sulphate 66 mg + Folic acid 5 mg (n = 24) versus Placebo (n = 25) Duration of treatment: 26 weeks |
|
| Outcomes | Sperm parameters | |
| Notes | Data in median and range. Use of fertile and subfertile men. Attempted to contact authors regarding means and standard deviations. Letter returned to sender |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible fertile and subfertile men were randomly assigned according to a simple computer‐generated randomisation schedule in four blocks to receive folic acid and placebo, zinc sulphate and placebo, zinc sulphate and folic acid, or placebo and placebo, which resulted in eight subgroups." "At the end of the trial, the research fellow received the randomisation list that matched the codes from the hospital pharmacy." |
| Allocation concealment (selection bias) | Low risk | Quote: "capsules were coded by the hospital pharmacy according to the randomisation list." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind" Quote: "Neither the research fellow and the participants knew whether the participants received folic acid, zinc sulphate or placebo capsules" Quote: "Folic acid and placebo capsules were yellow and identical in appearance. Zinc sulphate and placebo capsules were white and identical in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 men withdrew from the subfertile arm of the trial, 1 due to side effects (gastrointestinal) and 8 due to lack of motivation. It is unclear which treatment groups these men were randomised to |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported. No protocol available. |
Zalata 1998.
| Methods | Randomised pilot study Duration of study: unclear |
|
| Participants | Country: Belgium Population: men attending andrology clinic, N = 22 Mean age: unclear Inclusion criteria: unclear Exclusion criteria: unclear |
|
| Interventions | Acetyl‐cysteine 600 mg (n = 5) versus Mixture of essential fatty acid (EFA) (DHA 1 g + y‐linolenic acid + arachidonic acid 100 mg) + α‐tocopherol (vitamin E) + β‐carotene (n = 12) versus Acetylcysteine + essential fatty acid (EFA) + antioxidants (n = 5) Duration of treatment: 4 to 6 months |
|
| Outcomes | Sperm parameters, DNA damage (oh8dG) | |
| Notes | Abstract only. No extractable data. Attempted to contact authors re availability of data as means, if published?, methods of randomisation and allocation concealment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Abstract only |
Zavaczki 2003.
| Methods | Randomised, placebo‐controlled trial Duration of study: 3 months |
|
| Participants | Country: Hungary Population: subfertile men, N = 20 (recruited N = 26) Mean age: treatment group 29.6 years, placebo group 28.3 years Inclusion criteria: unsuccessful attempt at pregnancy for over one year. A healthy female partner examined by a gynaecologist. Sperm volume < 2 mL and/or sperm concentration < 20 million/mL and/or morphology ratio < 30% and/or motility < 50%. No genital tract infection, no bacteria or fungi in urine or semen. Hormones are within physiological range. Intact renal function. No excessive magnesium intake Exclusion criteria: unclear |
|
| Interventions | Magnesium 3000 mg (n = 10) versus Placebo (n = 10) Duration of treatment: 90 days |
|
| Outcomes | Primary: sperm parameters Secondary: clinical pregnancy and side effects |
|
| Notes | Attempted to contact authors regarding methods of randomisation and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The members of Group P received the same number of placebo tablets which closely resembled the Magnerot tablets." Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 were randomised and 14 were analysed. Quote: "To date 26 patients have participated in the study and 20 men (10 in both groups) have completed the program of treatment. Six patients (2 in group M and 4 in group P were excluded from the program, including five cases for poor compliance, since they did not attend the control meeting at the end of treatment. One patient from Group M experienced severe diarrhoea and so his treatment was halted." |
| Selective reporting (reporting bias) | Unclear risk | All sperm data for outcomes in the trial were given, however clinical pregnancy only reported in the results section and not mentioned in methods. No protocol available. |
ART: assisted reproductive technique;BMI: body mass index;FSH: follicle‐stimulating hormone; ICSI: intracytoplasmic sperm injection; IgG: immunogobulin G;ITT: intenttion‐to‐treat; mg: milligram; IQR: interquartile range; IU: international unit; IUI: intrauterine insemination; IVF: in vitro fertilisation; MDA: malondialdehyde; NSAID: non‐steroidal anti‐inflammatory; OAT:oligoasthenoteratozoospermia; PRL: prolactin;RoB: risk of bias; ROS: reactive oxygen species; SD: standard deviation; SE: standard error; SEM: standard error of the mean; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling; WBC: white blood cell; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adel 2015 | Ineligible based on intervention: main intervention is oral Vitamin E. However there was also an in vitro Berberine wash added to the collected sperm in 10 random participants from both groups (treatment group with oral Vitamin E or untreated group) |
| Alahmar 2017 | Ineligible based on study design: "prospective randomised trial", however there was no control group, only comparison before and after treatment with antioxidants |
| Alizadeh 2018 | Ineligible based on intervention: Curcumin Nanomicelle is a herbal product |
| Alsalman 2018 | Ineligible based on control: subfertile men with zinc treatment versus fertile men without treatment |
| Anarte 2012 | Ineligible based on study population: normozoospermic men and donors |
| Anarte 2013 | Ineligible based on study population: normozoospermic men and donors |
| Azizollahi 2013a | Ineligible based on outcome: seminal antioxidant levels and endocrine parameters. Furthermore, same study population/group as Azizollahi 2013 which was already included in the 2014 update |
| Cai 2012 | Ineligible based on study population: not subfertile men |
| Calogero 2015 | Ineligible based on population: idiopathic infertile men, not male factor |
| Capece 2017 | Ineligible based on intervention: treatment with myo‐inositol plus herbal extracts (Tribulus Terrestris, Alga Ecklonia Bicyclis) |
| Chattopadhyay 2016 | Ineligible based on study design: not a randomised controlled trial |
| Chen 2012 | Ineligible based on intervention: includes fertility drugs like tamoxifen. Group A tamoxifen + vitamin E, Group B tamoxifen |
| Ciftci 2009 | Ineligible based on population: includes men with idiopathic infertility and normal sperm parameters. |
| Comhaire 2005 | Ineligible based on study design: used non‐randomised controls recruited from another unrelated trial |
| Ebisch 2003 | Ineligible based on study population: inappropriate population, polymorphisms |
| Elgindy 2008 | Ineligible based on study population: antioxidant given to the women |
| Ghafarizadeh 2018 | Ineligible based on intervention: in vitro selenium, no oral intake |
| Ghanem 2010 | Ineligible based on intervention: clomiphene + vitamin E versus placebo, fertility enhancing drug |
| Gulati 2015 | Ineligible based on study design: prospective cohort study, not a randomised controlled trial |
| Gulino 2016 | Ineligible based on control: healthy fertile patients with intervention or control group of healthy patients undergoing IVF for a female factor |
| Hafeez 2011 | Ineligible based on intervention: plant extracts, herbal formulation |
| Iacono 2014 | Ineligible based on intervention: fertility enhancing drug, protocol exclusion criteria. Group A Tamofixfen citrate with antioxidant, group B tamoifen alone and group C placebo. |
| Jawad 2013 | Ineligible based on study design: not randomised quote: "men were classified into groups". Numbers of men in the groups were uneven |
| Kanta Goswami 2017 | Ineligible based on study design: prospective study, not randomised |
| Keskes‐Ammar 2003 | Ineligible based on population: includes infertile men who are normospermic, oligospermic or azoospermic. No subpopulation with extraction data |
| Kim 2010 | Ineligible based on study population: female participants not men |
| Korosi 2017 | Ineligible based on intervention: oral myo‐inositol supplement with treatment of the semen with myo‐inositol incubation. The control group did not receive any form of treatment (no oral, no incubation). Not able to differentiate between effect due to oral supplement or incubation |
| Kumar 2011 | Ineligible based on intervention: used a herbo‐mineral supplement |
| Lenzi 1993 | Ineligible based on intervention: route of supplementation was intramuscular not oral |
| Lu 2010 | Ineligible based on study population: women |
| Martinez‐Soto 2016 | Ineligible based on study population: also included infertile men with normospermic parameters. No subgroup analysis |
| Merino 1997 | Ineligible based on intervention: pentoxifylline no longer included, fertility enhancing drug |
| Micic 1988 | Ineligible based on intervention: pentoxifylline no longer included, fertility enhancing drug |
| Micic 2001 | Ineligible based on study design: not randomised, 105 men in the treatment group and 35 in control. Abstract only |
| Movahedin 2014 | Ineligible based on (repetitive) study population: same study as Pourmand 2014, second author Movahedin |
| Nadjarzadeh 2014 | Ineligible based on (repetitive) study population: exact same population, including the baseline characteristics and period of inclusion, as Nadjarzadeh 2011. Different outcome parameters (seminal plasma levels of antioxidant enzymes and oxidative stress) |
| Nashivochnikova 2014 | Ineligible based on study design: no RCT, full‐text received from first author by email, after translation of full‐text (in Russian) to English found out there was no control group. |
| NCT01075334 | Ineligible based on no data to publish: study was terminated, not being able to recruit enough participants (contact with author) |
| NCT01520584 | Ineligible based on no data to publish: recruiting participants not successful (contact with author) |
| Nematollahi‐Mahani 2014 | Ineligible based on outcome: endocrine parameters and seminal antioxidant level. Furthermore, same study population as Azizollahi 2013 (included in update 2014) |
| Niederberger 2011 | Ineligible based on study design: a commentary on Ghanem 2010 |
| Nikolova 2007 | Ineligible based on study design: not randomised, allocation method is by alternation. Translated from Bulgarian by Ivan Sola. "50 of them were randomly invited to participate depending on their order of attendance to the clinic" |
| Pawlowicz 2001 | Ineligible based on study design: not randomised |
| Polak 2013 | Ineligible based on study population: women |
| Raigani 2010 | Ineligible based on outcome: measurement of MTHFR genotype. Furthermore, same study population as Raigani 2014 which is an included study |
| Safarinejad 2011 | Ineligible based on intervention: pentoxifylline no longer included, fertility enhancing drug |
| Safarinejad 2011a | Ineligible based on intervention: saffron, herbal not a supplement |
| Singh 2016 | Ineligible based on study design: not randomised, based on conference abstract |
| Soylemez 2012 | Ineligible based on study population: not subfertile men |
| Stanislavov 2009 | Ineligible based on study design: not randomised, the study uses alternate allocation, odd and even numbers. Appears to be a report of the study Nikolova 2007 |
| Stanislavov 2014 | Ineligible based on intervention: L‐arginine combined with herbal extract |
| Tang 2011 | Ineligible based on intervention: tamoxifen, protocol exclusion criteria (tamoxifen + Q10 versus tamoxifen). Quote: “trials that included men taking other fertility enhancing drugs” |
| Verzeletti 2012 | Ineligible based on intervention: Spirulina platensis (4 g) and Resveratrol (500 mg) are plant extracts not antioxidant supplements |
| Vicari 2001 | Ineligible based on control: inappropriate control (anti‐inflammatory) group. Treatment is not compared to placebo or another antioxidant |
| Vicari 2001a | Ineligible based on control: Inappropriate comparison. The same antioxidant is compared at different times ‐ L‐carnitine + acetyl‐carnitine versus L‐carnitine + acetyl‐carnitine |
| Vicari 2002 | Ineligible based on control: inappropriate control (anti‐inflammatory). Treatment is not compared to placebo or another antioxidant |
| Wang 1983 | Ineligible based on intervention: pentoxifylline no longer included, fertility enhancing drug |
| Wang 2010a | Ineligible based on intervention: fertility enhancing drug, protocol exclusion criteria. Group A L‐carnitine + tamoxifen, Group B L‐carnitine, Group C tamoxifen. No placebo or no treatment control |
| Wu 2012 | Ineligible based on study design: probably not randomised, no mention of randomisation in the abstract and uneven numbers between the groups, attempted to contact authors with no reply |
IVF: in vitro fertilisation; MTHFR: Methylene tetrahydrofolate reductas; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Goswami 2015.
| Methods | Prospective observational study Duration of study: from March 2013 to April 2015 |
| Participants | Country: India Population: men with idiopathic male infertility with high reactive oxygen species (ROS), N = 175 Inclusion criteria: unclear Exclusion criteria: unclear |
| Interventions | Diet rich in antioxidants and lifestyle changes (n = 80) versus Combined oral antioxidant (n = 95) versus Placebo (n = 75) Duration of treatment: unclear |
| Outcomes | Semen parameters, antioxidant concentrations (CoQ‐10, L‐carnitine, zinc), plasma total antioxidant capacity (TAC), total glutathione (GSH), sperm DNA fragmentation (TUNEL assay) |
| Notes | Conference abstract only. Not clear if it is a randomised clinical trial. Email sent to authors Goswami and Chakravarty (bncirm@gmail.com; syednkabir@yahoo.com) on 20.02.2018 and 06.03.2018. No reply to date (march 2018) |
Characteristics of ongoing studies [ordered by study ID]
CTRI/2013/02/003431.
| Trial name or title | Pre treatment with antioxidants versus no treatment for male partner in couples undergoing assisted reproductive technology (ART) for male infertility: a randomized controlled trial. |
| Methods | Interventional (clinical trial) Design Randomised: permuted block randomisation, variable method of allocation concealment: sequentially numbered, sealed, opaque envelopes Blinding and masking: open‐label |
| Participants | Inclusion criteria Couples undergoing ART due to male factor infertility with the following parameters
AND/OR
AND/OR
Exclusion criteria
|
| Interventions | Drug: tablet Vitamin C 500 mg, capsule Vitamin E 400 mg and tablet Zinc 140 mg Control: no treatment Duration: 3 months |
| Outcomes | Primary
Secondary
|
| Starting date | February 2013 |
| Contact information | Dr Mohan S Kamath, MS,DNB, Fellow ( Reproductive Medicine) Associate Professor Reproductive Medicine Unit Christian Medical College and Hospital Vellore 632004 India Telephone: 04162283301 Email: dockamz@gmail.com Affiliation: Christian Medical College and Hospital |
| Notes | Email sent 26.03.14. Dr Kamath replied 3.04.14 saying that they were still in the recruitment phase and were hoping to finish the trial in 2015. Email sent 07.02.18. Dr Kamath replied 08.02.18 saying that they are still recruiting and hope to complete the recruitment by Mid 2018 and results should be available by the end of 2018. They have recruited approximately 150‐160 participants. |
DRKS00011616.
| Trial name or title | Randomized, placebo‐controlled, double‐blind, multi‐centre pilot study to investigate the effect of AM019016 on male spermatogenesis in subjects with diagnosed unspecific (idiopathic) subfertility. |
| Methods | Interventional (clinical trial) Design Allocation: randomised controlled trial Masking: blinded (patient/participant, investigator/therapist) Control: placebo Assignment: parallel Study design purpose: treatment |
| Participants | Males with minimum age of 18 years Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: Taking AM019016 (verum), dietary food, 3 capsules once a day Ingredients: Vitamin D, E, C, B12, B6, B2, Folic Acid, L‐Carnithine, L‐Arginine, Coenzyme Q10, Zinc, Selenium, β‐carotene, Copper, Pigrafert (combination of pine bark, grape seed, green tea extract). Control: Taking AM019016 (placebo), 3 capsules once a day Ingredients Placebo: maltodextrin, release agent magnesium salts of feed fatty acids and dye E171 and hydropropylmethylcellulose in the capsule shell. Free of gluten and lactose. Duration: 12 weeks |
| Outcomes | Primary Parameters for the assessment of the benefit by preparation and evaluation of spermograms according to the WHO criteria (2010, 5th edition)
Secondary Parameters for the assessment of tolerability:
|
| Starting date | July 2017 |
| Contact information | Holger Baumgraß Urologische Praxis Förster‐Funke‐Allee 104 14532 Kleinmachnow Germany +49(0)33203 58 50 holger.baumgrass@t‐online.de |
| Notes | Secondary ID: S15(a)/2017 |
IRCT2016111830947N1.
| Trial name or title | The effect of oral vitamin D3 supplementation on spermogram quantitative and qualitative indicators in infertile male |
| Methods | Interventional (clinical trial) Design Randomisation: non‐randomised. Randomly by tossing coin. Blinding: triple‐blind Placebo: used Assignment: parallel Purpose: treatment |
| Participants | Males. Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: the supplement of vitamin D3 (each week 1 pill of supplement vitamin D3 for 8 weeks and in remaining 4 weeks 1 supplement vitamin D3 pill as a maintenance dose) Control: placebo of vitamin D3 (each week 1 pill of Placebo vitamin D3 for 8 weeks and in remaining 4 weeks? 1 pill of Placebo vitamin D3 as a maintenance dose) Duration: 12 weeks |
| Outcomes | Primary: spermogram qualitative indicators Secondary: hormonal markers related to spermatogenesis(LH? FSH? TT? FT? SHBG) |
| Starting date | February 2017 |
| Contact information | Afsaneh Talebi Iran University of Medical Sciences, School of Nursing and Midwifery Yasemi Rashid street, Valiasr street, Tehran. Iran, Islamic Republic Of 00982143651820 Talebi.a@tak.iums.ac.ir / AfsanehTalebi68@gmail.com |
| Notes |
IRCT2017012432153N1.
| Trial name or title | The effects of folic acid, vitamin E, selenium on semen parameters in infertile men |
| Methods | Interventional (clinical trial) Design Randomisation: randomised. Sampling based on table of random numbers. Blinding: single‐blind Placebo: used Assignment: parallel Purpose: treatment |
| Participants | Males. Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: Selenium tablets (200 micrograms), vitamin zahravi Manufacturing Co. (400IU), folic acid tablets (5 mg) Galinuse Manufacturing Co. ‐ all once‐daily, Control: placebo daily Duration: 12 weeks |
| Outcomes | Primary
|
| Starting date | April 2017 |
| Contact information | Azima Sara School of Nursing and Midwifery Nemazee squair, Shiraz, Iran 009871 36474254 Azimas@sums.ac.ir |
| Notes |
NCT00975115.
| Trial name or title | Assessment of the efficacy of dietary supplement Spermotrend in the treatment of male infertility |
| Methods | Interventional (clinical trial) Design Allocation: randomised Masking: triple‐blind (participant, caregiver, investigator) Placebo control Parallel assignment |
| Participants | Males, 19 years to 60 years Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: Spermotrend (vitamins plus other antioxidants) twice a day Control: placebo twice a day Duration: 12 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | September 2009 |
| Contact information | Miguel Aguilar Charara, MD "Ramón González Coro" Gynecologic and Obstetric Hospita 53 7 838 2626 ext 277 Gynecologic and Obstetric Hospital Havana, Cuba, 10400 miguel.aguilar@infomed.sld.cu |
| Notes | Email sent 08.02.2018 to miguel.aguilar@infomed.sld.cu |
NCT01407432.
| Trial name or title | Impact of folates in the care of the male infertility (FOLFIV) |
| Methods | Interventional (Clinical Trial). Phase 3 Design Allocation: randomised Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants | Males, 18 years to 60 years Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: Folic acid 15 mg per day (tablets of 5 mg) Control: Placebo of folic acid Duration: 3‐4 months |
| Outcomes | Primary
Secondary
|
| Starting date | November 2011 |
| Contact information | Mathieu‐d'Argent E Service of gynaecology‐obstetrics and medicine of the reproduction, Tenon Hospital ‐ APHP Paris, France, 75020 |
| Notes | Email sent 08.02.18 to emmanuelle.mathieu@aphp.fr. Received an answer 09.02.18 that the trial recruiting phase is completed. Submitting the results within a few weeks. |
NCT01828710.
| Trial name or title | Myo‐inositol on human semen parameters Official title: Effect of treatment with myo‐inositol on human semen parameters in patients undergoing In vitro fertilization cycles |
| Methods | Interventional (clinical trial), phase 2/3 Design Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: screening |
| Participants | Male 25 years to 65 years Inclusion criteria
Exclusion criteria
|
| Interventions | Sham arm (normospermic): 4000 mg/die of myo‐inositol + 400 µg of folic acid (phase 2) Active arm (OAT): myo‐inositol 4000 mg/die associated to 400 µg of folic acid (phase 3) Placebo arm (normospermic): 400 µg of folic acid Duration: three months |
| Outcomes | Primary
|
| Starting date | August 2012 |
| Contact information | Palumbo MA Division of Obstetrics and Gynaecology/Department of Surgery Center of Physiopathology of Human Reproduction S. Bambino Hospital / University of Catania Catania, Italy,95010 Other Study ID Numbers: INO‐2103‐GC |
| Notes | Email sent 07.02.18 to Gulino (docferdi@hotmail.it) to ask if this study correlates with the same study population of study NCT01560065 (Gulino 2016) |
NCT01846325.
| Trial name or title | The effects of administration of combined docosahexaenoic acid and vitamin E supplements on spermatogram and seminal plasma oxidative stress in infertile men with asthenozoospermia |
| Methods | Interventional (clinical trial) Design Allocation: randomised Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants | Males, 20 years to 45 years Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: docosahexaenoic acid (DHA) Groups Experimental: capsule DHA 460 mg + vitamin E 600 mg per day Active comparator: vitamin E 600 mg + placebo Active comparator: 460 mg DHA + placebo Placebo comparator: DHA‐shaped placebo + vitamin E‐shaped placebo |
| Outcomes | Primary
Secondary
|
| Starting date | December 2013 |
| Contact information | Dr Azita Hekmatdoost National Nutrition and Food Technology Institute a_hekmat2000@yahoo.com |
| Notes | Email sent 07.02.18 to a_hekmat2000@yahoo.com, reply on the same day: study completed. Not yet submitted the manuscript |
NCT02310087.
| Trial name or title | Oral astaxanthin and semen quality, fertilization and embryo development in assisted reproduction technique procedures (Astax‐ART) |
| Methods | Interventional (clinical trial) Design Allocation: randomised Intervention model: parallel assignment Masking: triple (participant, care provider, investigator) |
| Participants | Males, 18 years and older Inclusion criteria
Exclusion criteria
|
| Interventions | Astaxanthin with vitamin E Drug: four tablets of 4 mg astaxanthin with 10 mg vitamin E (Astasan), single daily dose Placebo: four tablets of placebo daily taken in single daily dose Duration: 3 months |
| Outcomes | Primary
Secondary
|
| Starting date | November 2014 |
| Contact information | Bojana Pinter, MD, PhD / Senka Imamovic Kumalic, MD Division of Ob/Gyn, University Medical Centre Ljubljana Ljubljana, Slovenia, 1000 bojana.pinter@kclj.si / senka81@gmail.com |
| Notes | Sent email 07.02.18 to bojana.pinter@kclj.si and senka81@gmail.com Received a reply on the same day from dr Pinter: still recruiting, expecting to finish the study in 2018 |
NCT02421887.
| Trial name or title | Males, antioxidants, and infertility trial (MOXI) |
| Methods | Interventional (Clinical Trial) Design Allocation: randomised Intervention model: parallel assignment Masking: triple (participant, care provider, investigator) |
| Participants | Males, 18 years and older Inclusion criteria Couple
Male
Female
Exclusion criteria Couple
Male
Female
|
| Interventions | Drug: antioxidant supplement Ingredients: Vitamin C, 500 mg; Vitamin D3, 1000 IU; Vitamin E, 400 IU; Folic Acid 1000 mcg; Zinc, 20 mg; Selenium 200 mcg; Lycopene, 10 mg; Capsule: Vitamin D3, 1000 IU, L‐Carnitine, 1000 mg Control: placebo |
| Outcomes | Primary
Secondary
|
| Starting date | December 2015 |
| Contact information | Anne Z Steiner, MD University of North Carolina Heping Zhang, Principal Investigator, Yale University |
| Notes | Still recruiting according to the Yale/Stanford site/Penn Medicine sites, February 2018 |
NCT03104998.
| Trial name or title | Neotililty trial: Effect of coenzyme Q10 on semen parameters in men with idiopathic infertility |
| Methods | Interventional (Clinical Trial) Design Intervention model: single‐group assignment Masking: none (open‐label) |
| Participants | Males, 20 years to 50 years Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: coenzyme Q10 200 mg daily Control: placebo daily Duration: 26 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | August 2017 |
| Contact information | Anum Siddiqui, PharmD / Masood Jawaid, MRCS,FCPS HillPark Hospital Karachi, Pakistan 9221‐34315195 anum.siddiqui@pharmevo.biz Sonia_naqvi@hotmail.com |
| Notes |
NCT03337360.
| Trial name or title | The impact of a nutritional supplement (Impryl®) on male fertility (SUMMER) |
| Methods | Interventional (Clinical Trial) Design Allocation: randomised Intervention model: multicentre, randomised double‐blind placebo‐controlled clinical trial/superiority study Masking: triple (participant, care provider, investigator) |
| Participants | Males, 18 years to 50 years Inclusion criteria
OR
OR
Furthermore
Exclusion criteria
|
| Interventions | Drug: Impryl, one tablet daily Ingredients: food supplement with betaine, cystine, zinc, niacin, folic acid (di5MTHF‐glucosamine), Vitamin B12 (cobalamin), Vitamin B6, Vitamin B2 (Riboflavin) Control: placebo, one tablet daily Duration: 6 months |
| Outcomes | Primary
Secondary
|
| Starting date | April 2018 |
| Contact information | Roos Smits, MD Radboud University Nijmegen, the Netherlands, 6500HB +31 (0) 651751244 roos.smits@radboudumc.nl |
| Notes |
ART: assisted reproductive technique;FSH: follicle‐stimulating hormone; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; OAT:oligoasthenoteratozoospermia; WHO: World Health Organization
Differences between protocol and review
In the 2011 full review, sperm outcomes of concentration and motility were added as these two sperm outcomes are thought to reflect the oxidative process. A study by El‐Taieb (El‐Taieb 2009) states that "increased ROS generation and reduced antioxidant capacity is negatively correlated with sperm concentration and motility in infertile men".
The comparisons 'antioxidant versus placebo' and 'antioxidants versus no treatment' were combined as the one comparison 'antioxidants versus control', and then it was stated in the sensitivity analysis whether exclusion of those that failed to use placebo would have altered the conclusions ‐ as per statistical advice in the editorial comments.
Subgrouping and sensitivity analysis were performed on the outcomes of live birth and pregnancy in order to assess the potential of overestimation of benefit and reporting bias.
Subgroup analysis was performed on studies that enrolled couples undergoing IVF/ICSI and a sensitivity analysis was performed on those studies enrolling men undergoing IUI.
Sensitivity analysis was performed to consider whether conclusions were any different if eligibility was restricted to those studies without risk of bias.
A post hoc sensitivity analysis was conducted to examine the effect of excluding from the analysis those studies which reported remarkably low standard deviations as the review authors considered that these data were potentially erroneous.
In the 2014 update of the review 'pregnancy rate per couple' was redefined to be 'clinical pregnancy rate'. Stillbirth as an outcome was removed; this will be reported as an adverse event, as reported by the studies. The outcome 'level of sperm DNA damage after treatment' was reworded as 'level of sperm DNA fragmentation'.
In the 2018 update, we decided to remove pentoxifylline due to the fact that it is a prescription drug and not an 'over‐the‐counter' or overall free available supplement. In the future, there will be a new Cochrane Review solely on this item. We added a new secondary outcome: progressive sperm motility. In past versions of this review we already noticed that four studies only reported on progressive sperm motility and not on total sperm motility. In this 2018 update, we noticed that eight more studies (out of the 17 new included) report only on progressive sperm motility. We came to the conclusion that progressive sperm motility is the motility outcome with more clinical importance. Furthermore, in the 2018 update we clarified that this review is (as the title implies) solely for subfertile men; men with abnormal semen parameters. In the previous updates it was said to include "men of a couple with male factor infertility or unexplained infertility". However, male factor infertility has always been the main focus of the search and the review. Broadening the focus of the review to also unexplained infertility would change the scope of the review. Therefore we changed the inclusion and exclusion criteria, which are now also more like those in the review 'Antioxidants for female subfertility' (Showell 2017). Other changes were made in regard with the 'Risk of bias' assessments of blinding: we decided to assess 'performance bias' and 'detection bias' separately.
Contributions of authors
RS: starting from the 2018 update: searched other sources, selected studies for inclusion, assessed quality, performed data extraction, entered data, updated and renewed the whole background text and wrote the final 2018 update review. Also provided clinical expertise. RM‐P: selected studies for inclusion in the 2014 and 2018 update, assessed quality, performed data extraction and commented on the final version of the update. In the 2014 update also assisted with background text updating and entered text into tables of characteristic. AY: co‐drafted the protocol and wrote the section concerning sperm DNA fragmentation for the background up to the 2014 update. Provided technical advice on all versions. MS: co‐drafted the protocol and provided technical advice on semen parameters. Commented on all versions. VJ: starting from the 2018 update: provided technical advice and commented on the final version of the update. MGS: initiated, conceptualised and wrote the protocol, performed the searches in all versions. Up to and including the 2014 update: selected studies for inclusion, assessed quality, performed data extraction, entered data and wrote the first review and the 2014 update. Commented on the final versions of the 2018 update.
Sources of support
Internal sources
Cochrane Gynaecology and Fertility Group, Other.
External sources
None, Other.
Declarations of interest
The institution of first author Roos M Smits received an unrestricted grant for conducting the trial NCT03337360, to cover the salary of the trial co‐ordinator Roos M Smits. This trial (NCT03337360) started in April 2018. No data have been extracted from this study. The trial NCT03337360 is submitted to 'Ongoing studies'. This matter was referred to Cochrane's Funding Arbiters who have confirmed that Dr Smits’ declared interest does not constitute a COI under the current policy.
The following authors have reported financial activities outside the submitted work:
AY is a member of the advisory board of MSD. He is a stockholder of Queensland Fertility Group and is director of research and development of that institution. The research foundation of Queensland Fertility Group has received research grants from Merck Serono, MSD and the AGES Society (unrestricted grant December 2018; research proposal for pragmatic trial on surgery vs IVF). AY has received travel and conference expenses from Merck Serono (February 2018) and Ferring (January 2016 and January 2018). AY advises that none of these companies manufacture or market any antioxidants.
MS has received travel and conference expenses from Merck Serono (July 2018), Finox (April 2017) and Ferring (January 2016).
VJ, MGS and RM‐P have no conflicts to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akiyama 1999 {published data only}
- Akiyama M. In vivo scavenging effect of ethylcysteine on reactive oxygen species in human semen. Japanese Journal of Urology 1999;90(3):421‐8. [DOI] [PubMed] [Google Scholar]
Attallah 2013 {published data only}
- Attallah D, Nashar IH, Mahmoud R, Shaaban OM, Salman SA. N‐acytelcysteine prior to intrauterine insemination in couples with isolated athenozospermia: a randomized controlled trial. Fertility and Sterility 2013;100(3 Suppl):S462. [Google Scholar]
Azizollahi 2013 {published data only}
- Azizollahi G, Azizollahi S, Babaei H, Kianinejad M, Baneshi MR, Nematollahi‐mahani SN. Effects of supplement therapy on sperm parameters, protamine content and acrosomal integrity of varicocelectomized subjects. Journal of Assisted Reproduction and Genetics 2013;30(4):593‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizollahi G, Azizollahi S, Babaei H, Kianinejad MA, Baneshi MR, Nematollahi‐Mahani SN. Effects of zinc sulfate and folic acid coadministration on sperm parameters, protamine content and acrosomal integrity of varicocelectomized patients. Iranian Journal of Reproductive Medicine 2013;1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizollahi S, Azizollahi G, Nematollahi SN, Babaei H, Rastegari A, Maghsudi S. The effect of folic acid administration on protamine deficiency, acrosomal activity and semen parameters in varicocelectomized patients. Human Reproduction 2011;26 Suppl 1 Abstract no: P‐57:i340. [Google Scholar]
- Azizollahi S, Nematollahi‐Mahani SN, Azizollahi G, Baneshi MR, Safari Z. The effect of folic acid and zinc sulfate on endocrine parameters and seminal antioxidant level, after variocelectomy. Iranian Journal of Reproductive Medicine 2013;1):36. [DOI] [PubMed] [Google Scholar]
Balercia 2005 {published data only}
- Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo‐controlled double‐blind randomized trial on the use of L‐carnitine, L‐acetylcarnitine, or combined L‐carnitine and L‐acetylcarnitine in men with idiopathic asthenozoospermia. Fertility and Sterility 2005;84(3):662‐71. [DOI] [PubMed] [Google Scholar]
Balercia 2009 {published data only}
- Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo‐controlled, double‐blind randomized trial. Fertility and Sterility 2009;91(5):1785‐92. [DOI] [PubMed] [Google Scholar]
Barekat 2016 {published data only}
- Barekat F, Tavalaee M, Deemeh MR, Bahreinian M, Azadi L, Abbasi H, et al. A Preliminary Study: N‐acetyl‐L‐cysteine Improves Semen Quality following Varicocelectomy. International Journal of Fertility & Sterility 2016;10(1):120‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biagiotti 2003 {published data only}
- Biagiotti G, Cavallini G, Modenini F, Vitali G, Magli C, Ferraretti A. Prostaglanadins pulsed down‐regulation enhances carnitine therapy performance in severe idiopathic oligoasthenospermia. Human Reproduction 2003;18 Suppl 1:202. [Google Scholar]
Blomberg Jensen 2018 {published data only}
- Blomberg Jensen M, Gerner Lawaetz J, Andersson A, Petersen JH, Nordkap L, Bang AK, et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Human Reproduction 2016;31(8):1875‐85. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jorgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones, and live birth rate: a randomized clinical trial. Journal of Clinical Endocrinology & Metabolism 2018;103(3):870‐81. [DOI] [PubMed] [Google Scholar]
Boonyarangkul 2015 {published data only}
- Boonyarangkul A, Vinayanuvattikhun N, Chiamchanya C, Visutakul P. Comparative study of the effects of tamoxifen citrate and folate on semen quality of the infertile male with semen abnormality. Journal of the Medical Association of Thailand 2015;98(11):1057‐63. [PubMed] [Google Scholar]
Busetto 2018 {published data only}
- Busetto G, Virmani A, Giudice F, Micic S, Agarwal A, Berardinis E. Varicocele and oligoasthenoteratozoo‐spermia: Evaluation of antioxidant supplementation effect on pregnancy rate and sperm quality. Fertility and Sterility 2017;108 (3 Supplement 1):e133. [Google Scholar]
- Busetto G, Virmani MA, Antonini G, Ragonesi G, Berardinis E, Agarwal A, et al. Effect of antioxidant supplementation on sperm parameters in oligoasthenoteratozoospermia, with and without varicocele: A DBPC study. Fertility and Sterility 2016;106 (Supplement 3):e46. [Google Scholar]
- Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Giudice F, et al. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo‐astheno‐teratozoospermia, with and without varicocele: a double‐blind placebo‐controlled study. Andrologia 2018;07:07. [DOI] [PubMed] [Google Scholar]
- Busetto GM, Virmani A, Antonini G, Ragonesi G, Giudice F, Agarwal A, et al. Effect of antioxidant supplementation on sperm parameters in oligo‐astheno‐teratozoospermia, with and without varicocele: A double blind place controlled (DBPC) study. Human Reproduction 2017;32 (Supplement 1):i142‐3. [DOI] [PubMed] [Google Scholar]
Cavallini 2004 {published data only}
- Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G. Cinnoxicam and L‐carnitine/acetyl‐L‐carnitine treatment for idiopathic and varicocoele associated oligoasthenospermia [see comment]. Journal of Andrology 2004; Vol. 25, issue 5:761‐70. [DOI] [PubMed]
- Cavallini G, Ferraretti AP, Gianaroli L, Magli MC, Biagiotti G, Vitali G. Cinnoxicam + carnitines for idiopathic dyspermia. The 20th Annual Meeting of the European Society of Human Reproduction and Embryology. Human Reproduction 2004:i23‐4.
Conquer 2000 {published data only}
- Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Effect of DHA supplementation on DHA status and sperm motility in asthenozoospermic males. Lipids 2000;35(2):149‐54. [DOI] [PubMed] [Google Scholar]
Cyrus 2015 {published data only}
- Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. International Brazilian Journal of Urology 2015; Vol. 41, issue 2:230‐8. [DOI] [PMC free article] [PubMed]
Dawson 1990 {published data only}
Deng 2014 {published data only}
- Deng XL, Li YM, Yang XY, Huang JR, Guo SL, Song LM. Efficacy and safety of vitamin D in the treatment of idiopathic oligoasthenozoospermia. [Chinese]. Zhonghua nan ke xue = National Journal of Andrology 2014; Vol. 20, issue 12:1082‐5. [PubMed]
- Deng XL, Li YM, Yang XY, Huang JR, Guo SL, Song LM. [Efficacy and safety of vitamin D in the treatment of idiopathic oligoasthenozoospermia]. Zhong Hua Nan Ke Xue 2014; Vol. 20, issue 12:1082‐5. [PubMed]
Dimitriadis 2010 {published data only}
- Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, et al. Effects of phosphodiesterase‐5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: A randomized trial. BJU International 2010;106(8):1181‐5. [DOI] [PubMed] [Google Scholar]
Ener 2016 {published data only}
- Ener K, Aldemir M, Isik E, Okulu E, Ozcan MF, Ugurlu M, et al. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: A randomised controlled study. Andrologia 2016;48(7):829‐34. [DOI] [PubMed] [Google Scholar]
Eslamian 2013 {published data only}
- Eslamian G, Amirjannati N, Sadeghi MR, Rashidkhani B, Pahlavan S, Hooshangi A, et al. The effects of combined supplementation of docosahexaenoic acid and vitamin E on fatty acid changes in sperm membrane in asthenozoospermic men. Iranian Journal of Nutrition Sciences & Food Technology 2013;8(1):23‐37. [Google Scholar]
- Hekmatdoost A. The effects of administration of combined docosahexaenoic acid and vitamin E supplements on spermatogram and seminal plasma oxidative stress in infertile men with asthenozoospermia. www.clinicaltrials.gov. [ClinicalTrials.gov: NCT01846325]
Exposito 2016 {published data only}
- Exposito A, Perez‐Sanz J, Crisol L, Aspichueta F, Quevedo S, Diaz‐Nunez M, et al. A prospective double‐blind randomized placebo‐controlled study of the effect of vitamin E on semen parameters in infertile men. Human Reproduction 2016; Vol. 31 (Supplement 1):i137‐8.
- Exposito A, Perez‐Sanz J, Crisol L, Aspichueta F, Quevedo S, Diaz‐Nunez M, et al. A prospective double‐blind randomized placebo‐controlled study of the effect of vitamin E on semen parameters in infetile men. Human Reproduction 2016; Vol. 31 (Supp1):i137‐i138 Abstract no: P‐018.
Galatioto 2008 {published data only}
Gamidov 2017 {published data only}
- Gamidov SI, Ovchinnikov RI, Popova AY, Avakyan AY, Sukhikh GT. Adjuvant antioxidant therapy in varicocele infertility. [Russian]. Urologiia 2017; Vol. 2:64‐72. [DOI] [PubMed]
- Gamidov SI, Ovchinnikov RI, Popova AY, Avakyan AY, Sukhikh GT. [Adjuvant antioxidant therapy in varicocele infertility]. Urologiia (Moscow, Russia) 2017, issue 2 (supplement):64‐72. [DOI] [PubMed]
Gopinath 2013 {published data only}
- Gopinath PM, Kalra B, Saxena A, Malik S, Kochhar K, Kalra S, et al. Fixed dose combination therapy of antioxidants in treatment of idiopathic oligoasthenozoospermia: Results of a randomized, double‐blind, placebo‐controlled clinical trial. International Journal of Infertility and Fetal Medicine 2013; Vol. 4, issue 1:6‐13.
Greco 2005 {published data only}
Haghighian 2015 {published data only}
- Haghighian HK, Haidari F, Mohammadi‐Asl J, Dadfar M. Randomized, triple‐blind, placebo‐controlled clinical trial examining the effects of alpha‐lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertility & Sterility 2015; Vol. 104, issue 2:318‐24. [DOI] [PubMed]
Haje 2015 {published data only}
- Haje M, Naoom K. Combined tamoxifen and L‐carnitine therapies for the treatment of idiopathic male infertility attending intracytoplasmic sperm injection: A randomized controlled trial. International Journal of Infertility and Fetal Medicine 2015; Vol. 6, issue 1:20‐4.
Kessopoulou 1995 {published data only}
- Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double‐blind randomized placebo cross‐over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertility and Sterility 1995; Vol. 64, issue 4:825‐31. [DOI] [PubMed]
Kumamoto 1988 {published data only}
- Kumamoto Y, Maruta H, Ishigami J, Kamidono S, Orikasa S, Kimura M, et al. Clinical efficacy of mecobalamin in the treatment of oligozoospermia‐‐results of double‐blind comparative clinical study. Hinyokika Kiyo. Acta Urologica Japonica 1988;34:1109‐32. [PubMed] [Google Scholar]
Lenzi 2003 {published data only}
Lenzi 2004 {published data only}
- Lenzi A, Sgro P, Salacone P, Paoli D, Gilio B, Lombardo F, et al. A placebo‐controlled double‐blind randomized trial of the use of combined l‐carnitine and l‐acetyl‐carnitine treatment in men with asthenozoospermia [see comment]. Fertility and Sterility 2004; Vol. 81, issue 6:1578‐84. [DOI] [PubMed]
Li 2005 {published data only}
- Li Z, Chen GW, Shang XJ, Bai WJ, Han YF, Chen B, et al. A controlled randomized trial of the use of combined L‐carnitine and acetyl‐L‐carnitine treatment in men with oligoasthenozoospermia. Zhong Hua Nan Ke Xue 2005; Vol. 11, issue 10:761‐4. [PubMed]
Li 2005a {published data only}
- Li Z, Gu R, Liu Y, Xiang Z, Cao X, Han Y, et al. Curative effect of L‐carnitine supplementation in the treatment of male infertility. Academic Journal of Shanghai Second Medical University 2005; Vol. 25, issue 3:292‐4.
Lombardo 2002 {published data only}
- Lombardo F, Gandini L, Agarwal A, Sgro P, Dondero F, Lenzi A. A prospective double blind placebo controlled cross over trial of carnitine therapy in selected cases of male infertility. Fertility and Sterility 2002; Vol. 78 Suppl 1:68‐9.
Martinez 2015 {published data only}
- Martinez AM, Sordia‐Hernandez LH, Morales JA, Merino M, Vidal O, Garcia Garza MR, et al. A randomized clinical study assessing the effects of the antioxidants, resveratrol or SC1002, a hydrogen sulfide prodrug, on idiopathic oligoasthenozoospermia. Asian Pacific Journal of Reproduction 2015; Vol. 4, issue 2:106‐11.
Martinez‐Soto 2010 {published data only}
- Martinez‐Soto JC, Domingo JC, Cardobilla LP, Pellicer A, Landeras J. Effect of dietary DHA supplementation on sperm DNA integrity. Fertility and Sterility September 2010;94(4):S235‐6. [Google Scholar]
Mehni 2014 {published data only}
- Mehni NM, Ketabchi AA, Hosseini E. Combination effect of pentoxifylline and L‐carnitine on idiopathic oligoasthenoteratozoospermia. Iranian Journal of Reproductive Medicine 2014; Vol. 12, issue 12:817‐24. [PMC free article] [PubMed]
Micic 2017 {published data only}
- Micic S, Lalic N, Bojanic N, Djordjevic D, Virmani A, Agarwal A. Assessment of sperm motility in oligoasthenospermic men, treated with metabolic and essential nutrients, in a randomized, double blind, placebo study. Human Reproduction 2016; Vol. 31 (Supplement 1):i151‐2.
- Micic S, Lalic N, Bojanic N, Djordjevic D, Virmani A, Agarwal A. DBPC study in oligoasthenospermic men treated with metabolic and essential nutrients showed that progressive sperm motility was correlated to seminal carnitine levels. Andrology 2016; Vol. 4 (Supplement 2):56.
- Micic S, Lalic N, Bojanic N, Djordjevic D, Virmani A, Agarwal A. DBPC study showed significant correlation of DNA fragmetation index (DFI) and seminal carnitine with progressive sperm motility in oligospermic men treated with metabolic and essential nutrients. Fertility and Sterility 2017; Vol. 108 (3 Supplement 1):e307‐8.
- Micic S, Lalic N, Bojanic N, Djordjevic D, Virmani A, Agarwal A. Oligoastenospermic men treated with proxeed plus showed correlation between sperm motility and seminal carnitine. Fertility and Sterility 2016; Vol. 106 (Supplement 3):e298‐9.
- Micic S, Lalic N, Djordjevic D, Bogavac‐Stanojevic N, Virmani A, Agarwal A. Seminal carnitine and DNA fragmentation index (DFI) impact progressive sperm motility in oligoasthenospermic men treated with metabolic and essential nutrients, with moderate accuracy. Human Reproduction 2017; Vol. 32 (Supplement 1):i132.
Morgante 2010 {published data only}
- Morgante G, Scolaro V, Tosti C, Sabatino A, Piomboni P, Leo V. Treatment with carnitine, acetyl carnitine, L‐arginine and ginseng improves sperm motility and sexual health in men with asthenopermia. Minerva Urologica e Nefrologica 2010;62(3):213‐8. [PubMed] [Google Scholar]
Nadjarzadeh 2011 {published data only}
- Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on seman in idiopathic oligoasthenoteratozoospermia: A randomized double‐ blind placebo controlled trial. Journal of Endocrinological Investigation 2011;34:e224‐8. [DOI] [PubMed] [Google Scholar]
Nozha 2001 {published data only}
- Nozha CF, Leila AK, Zouhir S, Hanen G, Khled Z, Tarek R. Oxidative stress and male infertility: comparative study of combined vitamin E/selenium treatment versus vitamin B. Human Reproduction 2001; Vol. 17, issue Abstract book 1:111‐2.
Omu 1998 {published data only}
Omu 2008 {published data only}
Peivandi 2010 {published data only}
- Peivandi S, Abasali K, Narges M. Effects of L‐carnitine on infertile men's spermogram; a randomised clinical trial. Journal of Reproduction and Infertility 2010;10(4):331. [Google Scholar]
Pourmand 2014 {published data only}
- Pourmand G, Movahedin M, Dehghani S, Mehrsai A, Ahmadi A, Pourhosein M, et al. Does L‐carnitine therapy add any extra benefit to standard inguinal varicocelectomy in terms of deoxyribonucleic acid damage or sperm quality factor indices: a randomized study. Urology 2014; Vol. 84, issue 4:821‐5. [DOI] [PubMed]
- Pourmand GH, Movahedin M, Dehghan S, Mehrsai A, Ahmadi A, Pourhosein M. Does L‐carnitine therapy add any extra benefit to standard inguinal varicocelectomy in terms of deoxyribonucleic acid damage or sperm quality factor indices: a randomized study. Iranian Journal of Reproductive Medicine. 2015, issue 4 suppl. 1:67. [DOI] [PubMed]
Poveda 2013 {published data only}
- Poveda C, Rodriguez R, Chu EE, Aparicio LE, Gonzales IG, Moreno CJ. A placebo‐controlled double‐blind randomized trial of the effect of oral supplementation with spermotrend, maca extract (lepidium meyenii) or L‐carnitine in semen parameters of infertile men. Fertility and Sterility 2013; Vol. 100, issue 3 Suppl:S440.
Pryor 1978 {published data only}
- Pryor JP, Blandy JP, Evans P, Chaput de Saintonge DM, Usherwood M. Controlled clinical trial of arginine for infertile men with oligozoospermia. British Journal of Urology 1978;50:47‐50. [DOI] [PubMed] [Google Scholar]
Raigani 2014 {published data only}
Rolf 1999 {published data only}
- Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high‐dose vitamin C and vitamin E: a randomized, placebo‐controlled, double‐blind study. Human Reproduction 1999;14(4):1028‐33. [DOI] [PubMed] [Google Scholar]
- Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high‐dose vitamin C and vitamin E: a randomized, placebo‐controlled, double‐blind study [see comment]. Human Reproduction 1999;14(4):1028‐33. [DOI] [PubMed] [Google Scholar]
Safarinejad 2009 {published data only}
- Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N‐acetyl‐cysteine for improving semen parameters in infertile men: a double‐blind, placebo controlled, randomized study. Journal of Urology 2009;181(2):741‐51. [DOI] [PubMed] [Google Scholar]
Safarinejad 2009a {published data only}
- Safarinejad ME. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. Journal of Urology 2009;182(1):237‐48. [DOI] [PubMed] [Google Scholar]
Safarinejad 2012 {published data only}
- Safarinejad MR, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: A double‐blind, placebo controlled, randomized study. Journal of Urology 2012;188(2):526‐31. [DOI] [PubMed] [Google Scholar]
Scott 1998 {published data only}
Sharifzadeh 2016 {published data only}
- Sharifzadeh F, Norouzi S, Ashrafi M, Aminimoghaddam S, Koohpayezadeh J. Effects of zinc sulfate on subfertility related to male factors: a prospective double‐blind, randomized, placebo‐controlled clinical trial. Journal of Obstetrics, Gynecology and Cancer Research 2016; Vol. 1, issue 2:e7242. [2476‐5848]
Sigman 2006 {published data only}
- Pryor JL, Stacy L, Glass S, Campagnone J, Sigman M. Randomized double blind placebo controlled trial of carnitine for the treatment of idiopathic asthenospermia. Fertility and Sterility 2003;80 Suppl 3:48. [DOI] [PubMed] [Google Scholar]
- Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the treatment of idiopathic asthenospermia: a randomized, double‐blind, placebo‐controlled trial. Fertility and Sterility 2006;85(5):1409‐14. [DOI] [PubMed] [Google Scholar]
Sivkov 2011 {published data only}
- Sivkov AV, Oshchepkov VN, Evdokimov VV, Keshishev NG, Skabko OV. Selzink plus study in patients with chronic non‐infectious prostatitis and abnormal fertility. [Russian]. Urologii 2011; Vol. 5:27‐33. [PubMed]
Sofikitis 2016 {published data only}
- Sofikitis N, Dimitriadis F, Skouros S, Stavrou S, Seminis G, Giannakis I, et al. Effects of avanafil on semen quality and sperm cytoskeleton in oligoasthenospermic infertile men: A randomized controlled trial. Journal of Sexual Medicine 2016; Vol. 2:S164‐5.
Suleiman 1996 {published data only}
- Suleiman SA, Ali ME, Zaki ZM, el‐Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. Journal of Andrology 1996; Vol. 17, issue 5:530‐7. [PubMed]
Tremellen 2007 {published data only}
- Tremellen K, Froiland D, Miari G, Thompson J. A randomised control trial examining the effect of an antioxidant on sperm function pregnancy outcome during IVF treatment. The Fertility Society of Australia 25th Annual scientific meeting. Australian & New Zealand Journal of Obstetrics & Gynaecology 2006;46 Suppl 2:A.2. [DOI] [PubMed] [Google Scholar]
- Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF‐ICSI treatment. Australian & New Zealand Journal of Obstetrics & Gynaecology 2007;47:216‐21. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang YX, Yang SW, Qu CB, Huo HX, Li W, Li JD, et al. L‐carnitine: safe and effective for asthenozoospermia. Zhonghua nan ke xue ‐ National Journal of Andrology 2010;16(5):420‐2. [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Ebisch IM, Pierik FH, Jong FH, Thomas CM, Steegers‐Theunissen RP. Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men?. International Journal of Andrology 2006;29(2):339‐45. [DOI] [PubMed] [Google Scholar]
- Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers‐Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double‐blind, randomized, placebo‐controlled trial. Fertility and Sterility 2002; Vol. 77, issue 3:491‐8. [DOI] [PubMed]
Zalata 1998 {published data only}
- Zalata A, Christophe A, Horrbin D, Dhooge W, Comhaire F. Protection of sperm function and DNA by essential fatty acids and antioxidants dietary supplementation. Fertility and Sterility 1998;70:287. [Google Scholar]
- Zalata A, Christophe A, Horrobin D, Dhooge W, Comhaire F. Effect of essential fatty acids and antioxidants dietary supplementation on the oxidative DNA damage of the human spermatozoa. ESHRE 14th annual meeting. Goteborg, 1998:270‐1.
Zavaczki 2003 {published data only}
- Zavaczki Z, Szollosi J, Kiss S, Koloszar S, Fejes I, Kovacs L, Pal A. Magnesium‐orotate supplementation for idiopathic infertile male patients: a randomized, placebo‐controlled clinical pilot study. Magnesium Research 2003;16(2):131‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Adel 2015 {published data only}
- Adel N, Maghraby H, Ghareeb D, Elmahdy M. Vitamin e and berberine counteract the adverse effects of ros on sperm and seminal parameters. Human Reproduction 2015;30 Suppl 1:i171 Abstract no: P‐113. [Google Scholar]
Alahmar 2017 {published data only}
- Alahmar AT. Effect of vitamin C, vitamin E, zinc, selenium, and coenzyme Q10 in infertile men with idiopathic oligoasthenozoospermia. International Journal of Infertility and Fetal Medicine 2017;8(2):45‐9. [Google Scholar]
Alizadeh 2018 {published data only}
- Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytotherapy Research 2018;32(3):514‐21. [DOI] [PubMed] [Google Scholar]
Alsalman 2018 {published data only}
- Alsalman AR, Almashhedy LA, Hadwan MH. Effect of oral zinc supplementation on the thiol oxido‐reductive index and thiol‐related enzymes in seminal plasma and spermatozoa of Iraqi asthenospermic patients. Biological Trace Element Research 2018;184(2):340‐9. [DOI] [PubMed] [Google Scholar]
Anarte 2012 {published data only}
- Anarte C, Calvo I, Domingo A, Presilla N, Aleman M, Bou R, et al. Effect of DHA supplementation on fatty acid composition of sperm and its relation to semen quality. Human Reproduction ‐ Abstract book of the 28th ESHRE Annual Meeting, Turkey 1‐4 July 2012. 2012; Vol. 27, issue 2:ii121‐ii50.
Anarte 2013 {published data only}
- Anarte C, Domingo A, Agirregoikoa JA, Pablo J, Barrenetxea G. Regular consumption of docosahexaenoic acid (DHA) improves semen quality. Fertility and Sterility 2013;100 Suppl 3:S443. [Google Scholar]
Azizollahi 2013a {published data only}
- Azizollahi S, Nematollahi‐Mahani SN, Azizollahi G, Baneshi MR, Safari Z. The effect of folic acid and zinc sulfate on endocrine parameters and seminal antioxidant level, after variocelectomy. Iranian Journal of Reproductive Medicine 2013;1):36. [DOI] [PubMed] [Google Scholar]
Cai 2012 {published data only}
- Cai T, Wagenlehner F, Mazzoli S, Meacci F, Mondaini N, Nesi G, et al. Semen quality in patients with Chlamydia trachomatis genital infection treated concurrently with prulifloxacin and a phytotherapeutic agent. Journal of Andrology 2012;33(4):615‐23. [DOI] [PubMed] [Google Scholar]
Calogero 2015 {published data only}
- Calogero AE, Gullo G, Vignera S, Condorelli RA, Vaiarelli A. Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: a prospective double‐blind randomized placebo‐controlled study. Andrology 2015;3(3):491‐5. [DOI] [PubMed] [Google Scholar]
Capece 2017 {published data only}
- Capece M, Romeo G, Ruffo A, Romis L, Mordente S, Lauro G. A phytotherapic approach to reduce sperm DNA fragmentation in patients with male infertility. Urologia (Treviso) 2017;84(2):79‐82. [DOI] [PubMed] [Google Scholar]
- Capece M, Romeo G, Ruffo A, Romis L, Mordente S, Lauro G. Alga Ecklonia Bicyclis, tribulus terrestris, myoinositol and biovistm reduce sperm DNA fragmentation in patients with oligo‐asthenoterato‐zoospermia (OAT) syndrome. Journal of Sexual Medicine 2015;12 (Supplement 5):365. [Google Scholar]
Chattopadhyay 2016 {published data only}
- Chattopadhyay R, Yasmin S, Chakravarty BN. Effect of continuous 6 months oral antioxidant combination with universally recommended dosage in idiopathic male infertility. International Journal of Infertility and Fetal Medicine 2016;7(1):1‐6. [Google Scholar]
Chen 2012 {published data only}
- Chen XF, Li Z, Ping P, Dai JC, Zhang FB, Shang XJ, et al. Efficacy of natural vitamin E on oligospermia and asthenospermia: a prospective multi‐centered randomized controlled study of 106 cases. Zhong Hua Nan Ke Xue 2012;18(5):428‐31. [PubMed] [Google Scholar]
Ciftci 2009 {published data only}
- Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N‐acetylcysteine on semen parameters and oxidative/antioxidant status. Urology 2009;74(1):73‐6. [DOI] [PubMed] [Google Scholar]
Comhaire 2005 {published data only}
- Comhaire FH, Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined conventional/antioxidant "Astaxanthin" treatment for male infertility: a double blind, randomized trial. Asian Journal of Andrology 2005;7(3):257‐62. [DOI] [PubMed] [Google Scholar]
Ebisch 2003 {published data only}
- Ebisch IM, Heerde WL, Thomas CM, Put N, Wong WY, Steegers‐Theunissen RP. C677T methylenetetrahydrofolate reductase polymorphism interferes with the effects of folic acid and zinc sulfate on sperm concentration. Fertility and Sterility 2003;80(5):1190‐4. [DOI] [PubMed] [Google Scholar]
Elgindy 2008 {published data only}
- Elgindy EA, El‐Huseiny AM, Mostafa MI, Gaballah AM, Amed TA. N‐Acetylcysteine: Could it be an effective adjuvant therapy in ICSI cycles. Fertility and Sterility 2008;90 Suppl 1(American Society for Reproductive Medicine 64th Annual Meeting. 8‐12 November 2008, San Francisco, CA):356 Abstract no: P738. [Google Scholar]
Ghafarizadeh 2018 {published data only}
- Ghafarizadeh AA, Vaezi G, Shariatzadeh MA, Malekirad AA. Effect of in vitro selenium supplementation on sperm quality in asthenoteratozoospermic men. Andrologia 2018;50(2):e12869. [DOI] [PubMed] [Google Scholar]
Ghanem 2010 {published data only}
- Ghanem H, Shaeer O, El‐Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertility and Sterility 2010;93(7):2232‐5. [DOI] [PubMed] [Google Scholar]
Gulati 2015 {published data only}
- Gulati S, Chattopadhyay R, Ghosh B, Yasmin S, Ghosh S, Bose G, et al. Treatment with combined antioxidant formulation before ICSI improves pregnancy rate in couples with obstructive azoospermia. Fertility and Sterility 2015;1):e241. [Google Scholar]
Gulino 2016 {unpublished data only}
- AGUNCO Obstetrics and Gynecology Centre (PI unknown). Effect of treatment with myo‐inositol on human semen parameters in patients undergoing in vitro fertilization cycles. https://clinicaltrials.gov.
- Gulino FA, Leonardi E, Marilli I, Musmeci G, Vitale SG, Leanza V, et al. Effect of treatment with myo‐inositol on semen parameters of patients undergoing an IVF cycle: in vivo study. Gynecological Endocrinology 2016;32(1):65‐8. [DOI] [PubMed] [Google Scholar]
Hafeez 2011 {published data only}
- Hafeez M, Ahmed A, Usmanghani K, Mohiuddin E, Asif HM, Akram M, et al. Clinical evaluation of herbal medicine for oligospermia. Pakistan Journal of Nutrition 2011;10(3):238‐40. [Google Scholar]
Iacono 2014 {published data only}
- Iacono F, Ruffo A, Prezioso D, Illiano E, Romeo G, Romis L, et al. Combination therapy with antiestrogen and a natural composite containing tribulus terrestris, alga ecklonia bicyclis, biovis and myo‐inositol in the treatment of male idiopathic infertility. Journal of Sexual Medicine 2014;1:92. [Google Scholar]
Jawad 2013 {published data only}
- Jawad HM. Zinc sulfate treatment of secondary male infertility associated with positive serum and seminal plasma anti‐sperm antibody test. Middle East Fertility Society Journal 2013;18(1):24‐30. [Google Scholar]
Kanta Goswami 2017 {published data only}
- Kanta Goswami S, Chattopadhyay R, Ghosh S, Ghosh B, Yasmin S, Goswami M, et al. Exogenous oral combination antioxidants in idiopathic infertile males, having higher DNA fragmentation index, before ICSI cycle may improve live birth rate. Human Reproduction 2017;32 (Supplement 1):i160. [Google Scholar]
Keskes‐Ammar 2003 {published data only}
- Keskes‐Ammar L, Feki‐Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Archives of Andrology 2003;49(2):83‐94. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim C‐H, Yoon J‐W, Ahn J‐W, Kang H‐J, Lee J‐W, Kang B‐M. The effect of supplementation with omega‐3‐polyunsaturated fatty acids in intracytoplasmic sperm injection cycles for infertile patients with a history of unexplained total fertilization failure. Fertility and Sterility 2010;94(4 Suppl 1):S242. [Google Scholar]
Korosi 2017 {published data only}
- Korosi T, Barta C, Rokob K, Torok T. Physiological Intra‐Cytoplasmic Sperm Injection (PICSI) outcomes after oral pretreatment and semen incubation with myo‐inositol in oligoasthenoteratozoospermic men: results from a prospective, randomized controlled trial. European Review for Medical & Pharmacological Sciences 2017;21(2 Suppl):66‐72. [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar R, Saxena V, Shamsi M, Venkatesh S, Dada R. Herbo‐mineral supplementation in men with idiopathic oligoasthenoteratospermia: A double blind randomized placebo‐controlled trial. Indian Journal of Urolology 2011;27(3):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenzi 1993 {published data only}
- Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F. Placebo‐controlled, double‐blind, cross‐over trial of glutathione therapy in male infertility. Human Reproduction 1993;8(10):1657‐62. [DOI] [PubMed] [Google Scholar]
Lu 2010 {published data only}
- Lu SM, Li X, Zhang HB, Hu JM, Yan JH, Liu JL, et al. [Use of L‐carnitine before percutaneous epididymal sperm aspiration‐intracytoplasmic sperm injection for obstructive azoospermia]. Zhonghua nan ke xue ‐ National Journal of Andrology 2010;16(10):919‐21. [PubMed] [Google Scholar]
Martinez‐Soto 2016 {published data only}
- Martinez‐Soto JC, Domingo JC, Cordobilla B, Nicolas M, Fernandez L, Albero P, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Systems Biology in Reproductive Medicine 2016;62(6):387‐95. [DOI] [PubMed] [Google Scholar]
Merino 1997 {published data only}
- Merino G, Martinez Chequer JC, Barahona E, Bermudez JA, Moran C, Carranza‐Lira S. Effects of pentoxifylline on sperm motility in normogonadotropic asthenozoospermic men. Archives of Andrology 1997;39(1):65‐9. [DOI] [PubMed] [Google Scholar]
Micic 1988 {published data only}
- Micic S, Hadzi‐Djokic J, Dotlic R, Tulic C. Pentoxifyllin treatment of oligoasthenospermic men. Acta Europaea Fertilitatis 1988;19(3):135‐7. [PubMed] [Google Scholar]
Micic 2001 {published data only}
- Micic S, Lalic N, Bojanic N, Nale DJ. Oligospermic men treated by carnitine. 17th World Congress on Fertility and Sterility. Melbourne, 2001:38.
Movahedin 2014 {published data only}
- Movahedin M, Mehrsai A, Noori M, Dehghani S, Pourmand G, Ahmadi A, et al. Does anti‐oxidant therapy add any extra benefit to standard inguinal varicocelectomy in terms of DNA damage or sperm quality factor indices: a randomized study. Urology 2014;4(suppl. 1):S252. [DOI] [PubMed] [Google Scholar]
Nadjarzadeh 2014 {published data only}
- Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double‐blind randomised clinical trial. Andrologia 2014;46(2):177‐83. [DOI] [PubMed] [Google Scholar]
Nashivochnikova 2014 {published data only}
- Nashivochnikova NA, Krupin VN, Selivanova SA. [Efficiency of spematon in male infertility]. Urologiia (Moscow, Russia: 1999) 2014;2:52‐4. [PUBMED: 24956674] [PubMed] [Google Scholar]
NCT01075334 {unpublished data only}
- Tsafrir A. Is a carnitine based food supplement (PorimoreTM) for infertile men superior to folate and zinc with regard to pregnancy rates in intrauterine insemination cycles?. https://clinicaltrials.gov.
NCT01520584 {unpublished data only}
- Irge D. Supplement intake in infertile men; the effect on sperm parameters, fertilization rate and embryo quality. https://clinicaltrials.gov.
Nematollahi‐Mahani 2014 {published data only}
- Nematollahi‐Mahani SN, Azizollahi GH, Baneshi MR, Safari Z, Azizollahi S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia 2014;46(3):240‐5. [DOI] [PubMed] [Google Scholar]
Niederberger 2011 {published data only}
- Niederberger C. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Journal of Urology 2011;185(1):252. [DOI] [PubMed] [Google Scholar]
Nikolova 2007 {published data only}
- Nikolova V, Stanislavov R, Vatev I, Nalbanski B, Punevska M. Sperm parameters in male idiopathic infertility after treatment with prelox. Akusherstvo i Ginekologiia 2007;46(5):7‐12. [PubMed] [Google Scholar]
Pawlowicz 2001 {published data only}
- Pawlowicz P, Stachowiak G, Bielak A, Wilczynski J. Administration of natural anthocyanins derived from chokeberry (aronia melanocarpa) extract in the treatment of oligospermia in males with enhanced autoantibodies to oxidized low density lipoproteins (oLAB). The impact on fructose levels. Ginekologia Polska 2001;72(12):983‐8. [PubMed] [Google Scholar]
Polak 2013 {published data only}
- Polak de Fried E, Bossi NM, Notrica JA, Vazquez Levin MH. Vitamin‐d treatment does not improve pregnancy rates in patients undergoing art: a prospective, randomized, double‐blind placebo‐controlled trial. Fertility and Sterility 2013;100 Suppl 3:S493‐4. [Google Scholar]
Raigani 2010 {published data only}
- Raigani M, Sadeghi MR, Akhondi MA, Amir Jannati N, Soleimani Badia M. Impacts of MTHFR polymorphism on the effects of folic acid and zinc sulfate supplementations in OAT men. Iranian Journal of Reproductive Medicine 2010;1):46. [Google Scholar]
Safarinejad 2011 {published data only}
- Safarinejad MR. Effect of pentoxifylline on semen parameters, reproductive hormones and seminal plasma antioxidant capacity in men with idiopathic infertility: a randomized double‐blind placebo‐controlled study. International Urology and Nephrology 2011;43:315‐28. [DOI] [PubMed] [Google Scholar]
Safarinejad 2011a {published data only}
- Safarinejad MR, Shafiei N, Safarinejad S. A prospective double‐blind randomized placebo‐controlled study of the effect of saffron (Crocus sativus Linn.) on semen parameters and seminal plasma antioxidant capacity in infertile men with idiopathic oligoasthenoteratozoospermia. Phytotherapy research: PTR 2011;25(4):508‐16. [DOI] [PubMed] [Google Scholar]
Singh 2016 {published data only}
- Singh AK, Deshpande SB. Role of nutraceuticals in treatment of azoospermia due to maturation arrest. Indian Journal of Physiology and Pharmacology 2016;60 (5 Supplement 1):6‐7. [Google Scholar]
Soylemez 2012 {published data only}
- Soylemez H, Kilic S, Atar M, Penbegul N, Sancaktutar AA, Bozkurt Y. Effects of micronised purified flavonoid fraction on pain, semen analysis and scrotal color Doppler parameters in patients with painful varicocele; results of a randomized placebo‐controlled study. International Urology and Nephrology 2012;44(2):401‐8. [DOI] [PubMed] [Google Scholar]
Stanislavov 2009 {published data only}
- Stanislavov R, Nikolova V, Rohdewald P. Improvement of seminal parameters with Prelox®: a randomized, double‐blind, placebo controlled, cross over trial. Phytotherapy Research 2009;23:297‐302. [DOI: ] [DOI] [PubMed] [Google Scholar]
Stanislavov 2014 {published data only}
- Stanislavov R, Rohdewald P. Sperm quality in men is improved by supplementation with a combination of L‐arginine, L‐citrullin, roburins and Pycnogenol. Minerva Urologica e Nefrologica 2014;66(4):217‐23. [PubMed] [Google Scholar]
- Stanislavov R, Rohdewald P. Sperm quality in men is improved by supplementation with a combination of L‐arginine, L‐citrulline, roburins and Pycnogenol. Minerva Urologica e Nefrologica 2014;66(4):217‐23. [PubMed] [Google Scholar]
- Stanislavov R, Rohdewald P. Sperm quality in men is improved by supplementation with a combination of L‐arginine, L‐citrulline, roburins and Pycnogenol. Minerva Urologica e Nefrologica 2014;66(4):217‐23. [PubMed] [Google Scholar]
Tang 2011 {published data only}
- Tang KF, Xing Y, Wu CY, Liu RZ, Wang XY, Xing JP. [Tamoxifen combined with coenzyme Q10 for idiopathic oligoasthenospermia]. Zhong Hua Nan Ke Xue 2011;17(7):615‐8. [PubMed] [Google Scholar]
Verzeletti 2012 {published data only}
- Verzeletti FB, Poletto RS, Bertolin TE, Fornari F. Evaluation of sperm quality in adults after use spirulina platensis and resveratrol. Jornal Brasileiro de Reproducao Assistida 2012;16(5):271‐7. [Google Scholar]
Vicari 2001 {published data only}
Vicari 2001a {published data only}
- Vicari E, Rubino C, Palma A, Longo G, Lauretta M, Consoli S, et al. Antioxidant therapeutic efficiency after the use of carnitine in infertile patients with bacterial or non bacterial prostato‐vesiculo‐epididymitis. Archivio Italiano di Urologia, Andrologia 2001;73(1):15‐25. [PubMed] [Google Scholar]
Vicari 2002 {published data only}
- Vicari E, Vignera S, Calogero AE. Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti‐inflammatory compounds. Fertility and Sterility 2002;78(6):1203‐8. [DOI] [PubMed] [Google Scholar]
Wang 1983 {published data only}
- Wang C, Chan CW, Wong KK, Yeung KK. Comparison of the effectiveness of placebo, clomiphene citrate, mesterolone, pentoxifylline, and testosterone rebound therapy for the treatment of idiopathic oligospermia. Fertility and Sterility 1983;40(3):358‐65. [DOI] [PubMed] [Google Scholar]
Wang 2010a {published data only}
- Wang Y, Yang S, Cai W, Qu C, Li J, Chang X, et al. [Clinical efficacy of L‐carnitine combined with tamoxifen in treatment of oligoasthenozoospermia] LA: Chi. Zhonghua nan ke xue ‐ National Journal of Andrology 2010;16(5):420‐2. [PubMed] [Google Scholar]
Wu 2012 {published data only}
- Wu ZM, Lu X, Wang YW, Sun J, Tao JW, Yin FH, et al. Short‐term medication of L‐carnitine before intracytoplasmic sperm injection for infertile men with oligoasthenozoospermia. Zhong Hua Nan Ke Xue 2012;18(3):253‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Goswami 2015 {published data only}
- Goswami SK, Yasmin S, Chakraborty P, Chattopadhyay R, Ghosh S, Goswami M, et al. Role of dietary antioxidant supplementation in treatment of idiopathic male infertility: Promising evidence from a sub‐continental study. Fertility and Sterility 2015;1):e288. [Google Scholar]
References to ongoing studies
CTRI/2013/02/003431 {unpublished data only}
- Kamath MS. To compare the effectiveness of antioxidants versus no treatment for male partner for improving pregnancy rates in couples undergoing In vitro fertilization (IVF) for abnormal semen analysis. www.who.int/trialsearch.
DRKS00011616 {unpublished data only}
- Baumgraß H. Randomized, placebo‐controlled, double‐blind, multi‐centre pilot study to investigate the effect of AM019016 on male spermatogenesis in subjects with diagnosed unspecific (idiopathic) subfertility. www.who.int/trialsearch.
IRCT2016111830947N1 {unpublished data only}
- Talebi A, Amini L. The effect of oral vitamin D3 supplementation on spermogram quantitative and qualitative indicators in infertile male. www.who.int/trialsearch.
IRCT2017012432153N1 {unpublished data only}
- Azima S. The effects of folic acid, vitamin E, selenium on semen parameters in infertile men [Effect of folic acid, vitamin E, selenium on semen parameters]. www.who.int/trialsearch.
NCT00975115 {unpublished data only}
- Aguilar MM. Assessment of the efficacy of dietary supplement spermotrend in the treatment of male infertility. ClinicalTrials.gov.
NCT01407432 {unpublished data only}
- Mathieu‐d’Argent E. Impact of folates in the care of the male infertility (FOLFIV). https://clinicaltrials.gov.
NCT01828710 {unpublished data only}
NCT01846325 {unpublished data only}
- Hekmatdoost A. The effects of administration of combined docosahexaenoic acid and vitamin e supplements on spermatogram and seminal plasma oxidative stress in infertile men with asthenozoospermia. https://clinicaltrials.gov.
NCT02310087 {unpublished data only}
- Pinter B, Imamovic Kumalic S. Oral Astaxanthin and semen quality, fertilization and embryo development in Assisted Reproduction Techniques procedures. https://clinicaltrials.gov.
NCT02421887 {unpublished data only}
- Eisenberg E, Steiner AZ. Males, antioxidants, and infertility trial (MOXI). https://clinicaltrials.gov.
NCT03104998 {unpublished data only}
- Jawaid M. Neotililty trial: Effect of coenzyme Q10 on semen parameters in men with idiopathic infertility (neotility). https://clinicaltrials.gov.
NCT03337360 {unpublished data only}
- Smits RM, Fleischer K, Braat DDM. The impact of a nutritional supplement (Impryl®) on male fertility. https://clinicaltrials.gov. [DOI] [PMC free article] [PubMed]
Additional references
Agarwal 2003
- Agarwal A, Said T. Role of sperm chromatin abnormalities and DNA damage in male infertility. Human Reproduction Update 2003;9(4):331‐45. [DOI] [PubMed] [Google Scholar]
Agarwal 2004
- Agarwal A, Nallella KP, Allamaneni S, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reproductive Biomedicine Online 2004;8(6):616‐27. [DOI] [PubMed] [Google Scholar]
Agarwal 2004a
Agarwal 2016
- Agarwal A, Majzoub A, Esteves SC, Ko EY, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Translational Andrology and Urology 2016;5(6):935‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agarwal 2017
- Agarwal A, Majzoub A. Laboratory tests for oxidative stress. Indian Journal of Urology 2017;33(3):199‐206. [0970‐1591: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aitken 1987
- Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Journal of Reproduction and Fertility 1987;81(2):459‐69. [0022‐4251: (Print)] [DOI] [PubMed] [Google Scholar]
Aitken 1989
- Aitken RJ, Clarkson JS, Hargreave TB, Irvine DS, Wu FC. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. Journal of Andrology 1989;10(3):214‐20. [0196‐3635: (Print)] [DOI] [PubMed] [Google Scholar]
Aitken 1990
Aitken 1992
- Aitken RJ, Buckingham D, West K, Wu FC, Zikopoulos K, Richardson DW. Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. Journal of Reproduction and Fertility 1992;94(2):451‐62. [0022‐4251: (Print)] [DOI] [PubMed] [Google Scholar]
Aitken 1994
- Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 1994;16(4):259‐67. [0265‐9247: (Print)] [DOI] [PubMed] [Google Scholar]
Aitken 2010
- Aitken RJ, Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Human Reproduction 2010;25(10):2415‐26. [DOI] [PubMed] [Google Scholar]
Aktan 2013
- Aktan G, Dogru‐Abbasoglu S, Kucukgergin C, Kadioglu A, Ozdemirler‐Erata G, Kocak‐Toker N. Mystery of idiopathic male infertility: is oxidative stress an actual risk?. Fertility and Sterility 2013;99(5):1211‐5. [DOI] [PubMed] [Google Scholar]
Alvarez 2003
- Alvarez 2003. Nuture vs nature: How can we optimise sperm quality?. Journal of Andrology 2003;24(5):640‐8. [DOI] [PubMed] [Google Scholar]
Annals of the New York Academy of Science 2004
- Annals of the New York Academy of Science. Preface: Carnitine: Lessons from One Hundred Years of Research. Annals of the New York Academy of Science. Blackwell Publishing Ltd, 2004; Vol. 1033, issue 1:ix‐xi.
Appleton 2002
- Appleton J. Arginine: clinical potential of a semi‐essential amino acid. Alternative Medicine Review 2002;7(6):512‐22. [10895159] [PubMed] [Google Scholar]
Atkuri 2007
- Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N‐Acetylcysteine—a safe antidote for cysteine/glutathione deficiency. Cancer/Immunomodulation 2007;7(4):355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barratt 2017
- Barratt CLR, Bjorndahl L, Jonge CJ, Lamb DJ, Osorio Martini F, McLachlan R, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance‐challenges and future research opportunities. Human Reproduction Update 2017;23(6):660‐80. [1460‐2369: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bendich 1989
- Bendich A, Olson JA. Biological actions of carotenoids. The FASEB Journal 1989;3(8):1927‐32. [PubMed] [Google Scholar]
Bertelli 2009
- Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. Journal of Cardiovascular Pharmacology 2009;54(6):468‐76. [DOI] [PubMed] [Google Scholar]
Bevilacqua 2015
- Bevilacqua A, Carlomagno G, Gerli S, Montanino Oliva M, Devroey P, Lanzone A, et al. Results from the International Consensus Conference on myo‐inositol and D‐chiro‐inositol in Obstetrics and Gynecology – assisted reproduction technology. Gynecological Endocrinology 2015;31(6):441‐6. [DOI] [PubMed] [Google Scholar]
Bhagavan 2006
- Bhagavan HN, Chopra RK. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radical Research 2006;40(5):445‐53. [DOI] [PubMed] [Google Scholar]
Boe‐Hansen 2006
- Boe‐Hansen GB, Fedder J, Ersboll AK, Christensen P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Human Reproduction 2006;21(6):1576‐82. [DOI] [PubMed] [Google Scholar]
Boitani 2008
- Boitani C, Puglisi R. Selenium, a key element in spermatogenesis and male fertility. Molecular Mechanisms in Spermatogenesis. New York, NY: Springer New York, 2008:65‐73. [DOI] [PubMed] [Google Scholar]
Boivin 2007
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Human Reproduction 2007;22(6):1506‐12. [0268‐1161] [DOI] [PubMed] [Google Scholar]
Borini 2017
- Borini A, Tarozzi N, Nadalini M. Sperm DNA fragmentation testing in male infertility work‐up: are we ready?. Translational Andrology and Urology 2017;6(Suppl 4):S580‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branco 2010
- Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 2010;60(2):235‐7. [DOI] [PubMed] [Google Scholar]
Bungum 2004
- Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Human Reproduction 2004;19(6):1401‐8. [DOI] [PubMed] [Google Scholar]
Burk 2002
- Burk RF. Selenium, an antioxidant nutrient. Nutrition in Clinical Care 2002;5(2):75‐9. [DOI] [PubMed] [Google Scholar]
Bykova 2007
- Bykova M, Athayde K, Sharma R, Jha R, Sabanegh E, Agarwal A. Defining the reference value of seminal reactive oxygen species in a population of infertile men and normal healthy volunteers. Fertility and Sterility 2007;88 Suppl 1(P‐597):305. [Google Scholar]
Bøhmer 1978
- Bøhmer T, Hoel P, Purvis K, Hansson V. Carnitine levels in human accessory sex organs. Archives of Andrology 1978;1(1):53‐9. [DOI] [PubMed] [Google Scholar]
Cissen 2016
- Cissen M, Wely M, Scholten I, Mansell S, Bruin JP, Mol BW, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta‐analysis. PLOS One 2016;11(11):e0165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colagar 2009
- Colagar AH, Marzony ET. Ascorbic acid in human seminal plasma: determination and its relationship to sperm quality. Journal of Clinical Biochemistry and Nutrition 2009;45(2):144‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colagar 2009a
Collins 2008
- Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization?. Fertility and Sterility 2008;89(4):823‐31. [DOI] [PubMed] [Google Scholar]
Collodel 2011
- Collodel G, Federico MG, Geminiani M, Martini S, Bonechi C, Rossi C, et al. Effect of trans‐resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reproductive Toxicology 2011;31(2):239‐46. [DOI] [PubMed] [Google Scholar]
Colone 2010
- Colone M, Marelli G, Unfer V, Bozzuto G, Molinari A, Stringaro A. Inositol activity in oligoasthenoteratospermia‐‐an in vitro study. European Review for Medical and Pharmacological Sciences 2010;14(10):891‐6. [1128‐3602: (Print)] [PubMed] [Google Scholar]
Comhaire 1987
- Comhaire FH. Towards more objectivity in diagnosis and management of male infertility. International Journal of Andrology. Supplement. Oxford; Melbourne: Blackwell Scientific, 1987; Vol. 7.
Condorelli 2017
- Condorelli RA, Vignera S, Mongioi LM, Vitale SG, Lagana AS, Cimino L, et al. Myo‐inositol as a male fertility molecule: speed them up!. European Review for Medical and Pharmacological Sciences 2017;21(2 Suppl):30‐5. [2284‐0729: (Electronic)] [PubMed] [Google Scholar]
Dattilo 2016
- Dattilo M, Giuseppe D, Ettore C, Ménézo Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: mechanisms, clinical results, insights on gene‐environment interactions and the role of diet. Journal of Assisted Reproduction and Genetics 2016;33(12):1633‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Angelis 2017
- Angelis C, Galdiero M, Pivonello C, Garifalos F, Menafra D, Cariati F, et al. The role of vitamin D in male fertility: A focus on the testis. Reviews in Endocrine and Metabolic Disorders 2017;18(3):285‐305. [DOI] [PubMed] [Google Scholar]
Dias 2006
- Dias S, McNamee R, Vail A. Evidence of improving quality of reporting of randomised controlled trials in subfertility. Human Reproduction 2006;21(10):2617‐27. [DOI] [PubMed] [Google Scholar]
Ebisch 2007
- Ebisch IM, Thomas CM Peters WH, Braat DD, Steegers‐Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Human Reproduction Update 2007;13(2):163‐74. [DOI] [PubMed] [Google Scholar]
El‐Taieb 2009
- El‐Taieb MA, Herwig R, Nada EA, Greilberger J, Marberger M. Oxidative stress and epididymal sperm transport, motility and morphological defects. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;144 Suppl 1:199‐203. [DOI] [PubMed] [Google Scholar]
ESHRE Guidelines 1996
- The European Society of Human Reproduction and Embryology (ESHRE). Guidelines to the prevalence, diagnosis, treatment and management of infertility, 1996. Human Reproduction 1996;11(8):1775. [Google Scholar]
Eskenazi 2005
- Eskanazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobeck AJ. Antioxidant intake is associated with semen quality in healthy men. Human Reproduction 2005;20(4):1006‐12. [DOI] [PubMed] [Google Scholar]
Evenson 1999
- Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Human Reproduction 1999;14(4):1039‐49. [DOI] [PubMed] [Google Scholar]
Evenson 2006
- Evenson D, Wixon R. Meta‐analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reproductive Biomedicine Online 2006;12(4):466‐72. [DOI] [PubMed] [Google Scholar]
Evers 2002
- Evers JL. Female subfertility. Lancet 2002;360(9327):151‐9. [0140‐6736: (Print)] [DOI] [PubMed] [Google Scholar]
Garg 2016
- Garg H, Kumar R. An update on the role of medical treatment including antioxidant therapy in varicocele. Asian Journal of Andrology 2016;18(2):222‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghyczy 2001
- Ghyczy M, Boros M. Electrophilic methyl groups present in the diet ameliorate pathological states induced by reductive and oxidative stress: a hypothesis. British Journal of Nutrition 2001;85(4):409‐14. [DOI] [PubMed] [Google Scholar]
Giordano 2014
- Giordano E, Visioli F. Long‐chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostaglandins Leukotrienes and Essential Fatty Acids 2014;90(1):1‐4. [DOI] [PubMed] [Google Scholar]
Gnoth 2005
- Gnoth C, Godehardt E, Frank‐Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human Reproduction 2005;20(5):1144‐7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro. GRADEpro Guideline Development Tool. McMaster University: developed by Evidence Prime, Inc., 2015.
Grand View Research 2016
- Grand View Research. Dietary Supplements Market Analysis By Ingredient (Botanicals, Vitamins, Minerals, Amino Acids, Enzymes), By Product (Tablets, Capsules, Powder, Liquids, Soft Gels, Gel Caps), By Application (Additional Supplement, Medicinal Supplement, Sports Nutrition), By End‐Use (Infant, Children, Adults, Pregnant Women, Old‐Aged) And Segment Forecasts To 2024. https://www.grandviewresearch.com 2016:0‐200.
Grune 2010
- Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, et al. β‐Carotene Is an Important Vitamin A Source for Humans1–3. Journal of Nutrition 2010;140(12):2268S‐85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halicka 2012
- Halicka HD, Zhao H, Li J, Traganos F, Studzinski GP, Darzynkiewicz Z. Attenuation of constitutive DNA damage signaling by 1,25‐dihydroxyvitamin D3. Aging. Impact Journals, LLC, 2012; Vol. 4, issue 4:270‐8. [DOI] [PMC free article] [PubMed]
Hankey 1999
Hausenblas 2014
- Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—systematic review and meta‐analysis. Molecular Nutrition & Food Research 2014;59(1):147‐59. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org. Chichester UK: John Wiley and Sons Ltd.
Hsia 2016
- Hsia T, Yin M. Post‐intake of S‐Ethyl Cysteine and S‐Methyl Cysteine improved LPS‐induced acute lung injury in mice. Nutrients 2016;8(8):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2018
- Huang D, Tocmo R. Assays based on competitive measurement of the scavenging ability of reactive oxygen/nitrogen species. In: Apak R, Capanoglu E, Shahidi F editor(s). Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2018. [Google Scholar]
Hughes 1996
- Hughes CM, Lewis SE, McKelvey‐Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Molecular Human Reproduction 1996;2(8):613‐9. [1360‐9947: (Print)] [DOI] [PubMed] [Google Scholar]
Inhorn 2015
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update 2015;21(4):411‐26. [1460‐2369: (Electronic)] [DOI] [PubMed] [Google Scholar]
Institute of Medicine 2000
- Institute of Medicine. Vitamin E. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press, 2000. [PubMed] [Google Scholar]
Intasqui 2015
- Intasqui P, Antoniassi M, Camargo M, Nichi M, Carvalho VM, Cardozo KHM, et al. Differences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parameters. Fertility and Sterility 2015;104(2):292‐301. [DOI] [PubMed] [Google Scholar]
Irani 2017
- Irani M, Amirian M, Sadeghi R, Lez JL, Latifnejad Roudsari R. The effect of folate and folate plus zinc supplementation on endocrine parameters and sperm characteristics in sub‐fertile men: a systematic review and meta‐analysis. Urology Journal August 29 2017;14(5):4069‐78. [PubMed] [Google Scholar]
Irvine 1998
- Irvine DS. Epidemiology and aetiology of male infertility. Human Reproduction 1998;13 Suppl 1:33‐44. [0268‐1161: (Print)] [DOI] [PubMed] [Google Scholar]
Iwasaki 1992
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertility and Sterility 1992;57(2):409‐16. [0015‐0282: (Print)] [DOI] [PubMed] [Google Scholar]
Jones 1973
- Jones R, Mann T. Lipid peroxidation in spermatozoa. Proceedings of the Royal Society Series B‐Biological Sciences 1973;184(1074):103‐7. [0080‐4649] [DOI] [PubMed] [Google Scholar]
Joshi 2001
- Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radical Biology & Medicine 2001;30(12):1390‐9. [DOI] [PubMed] [Google Scholar]
Kodama 1997
- Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertilty and Sterility 1997;68(3):519‐24. [0015‐0282: (Print)] [DOI] [PubMed] [Google Scholar]
Kofi Arhin 2017
- Kofi Arhin S, Zhao Y, Lu XS, Chetry M, Lu JQ. Effect of micronutrient supplementation on IVF outcomes: a systematic review of the literature. Reproductive BioMedicine Online 2017;35(6):715‐22. [DOI] [PubMed] [Google Scholar]
Lafuente 2013
- Lafuente R, Gonzalez‐Comadran M, Sola I, Lopez G, Brassesco M, Carreras R, et al. Coenzyme Q10 and male infertility: a meta‐analysis. Journal of Assisted Reproduction and Genetics. 2013;30(9):1147‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewin 1976
- Lewin LM, Yannai Y, Sulimovici S, Kraicer PF. Studies on the metabolic role of myo‐inositol. Distribution of radioactive myo‐inositol in the male rat. Biochemical Journal 1976;156(2):375‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1997
- Lewis SE, Sterling ES, Young IS, Thompson W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertility and Sterility 1997;67(1):142‐7. [DOI] [PubMed] [Google Scholar]
Lewis 2013
Li 2006
- Li Z, Wang L, Cai J, Huang H. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta‐analysis. Journal of Assisted Reproduction and Genetics 2006;23(9‐10):367‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Littarru 2007
- Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Molecular Biotechnology 2007;37(1):31‐7. [DOI] [PubMed] [Google Scholar]
MacFarquhar 2010
- MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, et al. Acute selenium toxicity associated with a dietary supplement. Archives of Internal Medicine 2010;170(3):256‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Majzoub 2017
- Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian Journal of Urology 2017;33(3):207‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Majzoub 2018
- Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live‐birth rate. Arab Journal of Urology 2 January 2018;16(1):113‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascarenhas 2012
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLOS Medicine 2012;9(12):e1001356. [1549‐1676: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzilli 1994
- Mazzilli F, Rossi T, Marchesini M, Ronconi C, Dondero F. Superoxide anion in human semen related to seminal parameters and clinical aspects. Fertility and Sterility 1994;62(4):862‐8. [0015‐0282: (Print)] [DOI] [PubMed] [Google Scholar]
McNeill 1985
- McNeill DA, Ali PS, Song YS. Mineral analyses of vegetarian, health, and conventional foods: magnesium, zinc, copper, and manganese content. Journal of the American Dietetic Association 1985;85(5):569‐72. [0002‐8223: (Print)] [PubMed] [Google Scholar]
Mendiola 2010
- Mendiola J, Torres‐Cantero AM, Vioque J, Moreno‐Grau JM, Ten J, Roca M, et al. A low intake of antioxidant nutrients is associated with poor semen quality in patients attending fertility clinics. Fertility and Sterility 2010;93(4):1128‐33. [DOI] [PubMed] [Google Scholar]
Mirończuk‐Chodakowska 2018
- Mirończuk‐Chodakowska I, Witkowska AM, Zujko ME. Endogenous non‐enzymatic antioxidants in the human body. Advances in Medical Sciences 2018;63(1):68‐78. [DOI] [PubMed] [Google Scholar]
Navarro‐Alarcon 2008
- Navarro‐Alarcon M, Cabrera‐Vique C. Selenium in food and the human body: A review. Science of The Total Environment 2008;400(1):115‐41. [DOI] [PubMed] [Google Scholar]
Osman 2015
- Osman A, Alsomait H, Seshadri S, El‐Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta‐analysis. Reproductive Biomedicine Online 2015;30(2):120‐7. [DOI] [PubMed] [Google Scholar]
Ourique 2013
- Ourique GM, Finamor IA, Saccol EM, Riffel AP, Pês TS, Gutierrez K, et al. Resveratrol improves sperm motility, prevents lipid peroxidation and enhances antioxidant defences in the testes of hyperthyroid rats. Reproductive Toxicology 2013;37:31‐9. [DOI] [PubMed] [Google Scholar]
Padayatty 2003
- Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee J, et al. Vitamin C as an antioxidant: evaluation of Its role in disease prevention. Journal of the American College of Nutrition 2003;22(1):18‐35. [DOI] [PubMed] [Google Scholar]
Pasqualotto 2001
- Pasqualotto FF, Sharma RK, Kobayashi H, Nelson DR, Jr AJT, Agarwal A. Oxidative stress in normospermic men undergoing infertility evaluation. Journal of Andrology 2001;22(2):316‐22. [PubMed] [Google Scholar]
Patel 2008
- Patel SR, Sigman M. Antioxidant therapy in male infertility. Urologic Clinics of North America. 2008;35(2):319‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pravst 2010
- Pravst I, Žmitek K, Žmitek J. Coenzyme Q10 contents in foods and fortification strategies. Critical Reviews in Food Science and Nutrition 2010;50(4):269‐80. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richard 2008
- Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacological Research 2008;57(6):451‐5. [DOI] [PubMed] [Google Scholar]
Robinson 2012
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta‐analysis. [Review]. Human Reproduction 2012;27(10):2908‐17. [DOI] [PubMed] [Google Scholar]
Rogovik 2009
- Rogovik AL, Vohra S, Goldman RD. Safety considerations and potential interactions of vitamins: should vitamins be considered drugs?. Annals of Pharmacotherapy 2009;44(2):311‐24. [DOI] [PubMed] [Google Scholar]
Ross 2006
- Ross AC. Vitamin A and Carotenoids. In: Shils ME SM, Ross AC, Caballero B, Cousins R editor(s). Modern Nutrition in Health and Disease. Vol. 10th edition, Lippincott Williams & Wilkins, 2006:351‐75. [Google Scholar]
Ross 2010
- Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, El‐Toukhy T. A systematic review of the effect of oral antioxidants on male infertility. Reproductive Biomedicine Online 2010;20(6):711‐23. [PUBMED: 20378409] [DOI] [PubMed] [Google Scholar]
Salas‐Huetos 2017
- Salas‐Huetos A, Bulló M, Salas‐Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Human Reproduction Update 1 July 2017;23(4):371–89. [DOI] [PubMed] [Google Scholar]
Shekarriz 1995
- Shekarriz M, Thomas AJ Jr, Agarwal A. Incidence and level of seminal reactive oxygen species in normal men. Urology 1995;45(1):103‐7. [0090‐4295: (Print)] [DOI] [PubMed] [Google Scholar]
Shimura 2002
- Shimura T, Toyoshima M, Taga M, Shiraishi K, Uematsu N, Inoue M, et al. The novel surveillance mechanism of the Trp53‐dependent s‐phase checkpoint ensures chromosome damage repair and preimplantation‐stage development of mouse embryos fertilized with x‐irradiated sperm. Radiation Research 2002;158(6):735‐42. [DOI] [PubMed] [Google Scholar]
Showell 2017
- Showell MG, Mackenzie‐Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD007807.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sikka 1995
- Sikka S, Rajasekaran M, Hellstrom W. Role of oxidative stress and antioxidants in male infertility. Journal of Andrology 1995;16(6):464‐8. [PubMed] [Google Scholar]
Simon 2014
- Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Human Reproduction 2014;29(11):2402‐12. [DOI] [PubMed] [Google Scholar]
Smith 1996
- Smith R, Vantman D, Ponce J, Escobar J, Lissi E. Total antioxidant capacity of human seminal plasma. Human Reproduction 1996;11(8):1655‐60. [DOI] [PubMed] [Google Scholar]
Spanò 2000
- Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertility and Sterility 2000;73(1):43‐50. [DOI] [PubMed] [Google Scholar]
Thonneau 1991
- Thonneau P, Marchand S, Tallec A, Ferial M, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988–1989)*. Human Reproduction 1991;6(6):811‐6. [DOI] [PubMed] [Google Scholar]
Traber 2007
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biology and Medicine 2007;43(1):4‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tremellen 2008
- Tremellen K. Oxidative stress and male infertility ‐ a clinical perspective. Human Reproduction Update 2008;14(3):243‐58. [DOI] [PubMed] [Google Scholar]
Valko 2006
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress‐induced cancer. Chemico‐Biological Interactions 2006;160(1):1‐40. [DOI] [PubMed] [Google Scholar]
Verit 2006
- Verit FF, Verit A, Kocyigit A, Ciftci H, Celik H, Koksal M. No increase in sperm DNA damage and seminal oxidative stress in patients with idiopathic infertility. Archives of Gynecology and Obstetrics 2006;274(6):339‐44. [DOI] [PubMed] [Google Scholar]
Wathes 2007
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biology of Reproduction 2007;77(2):190‐201. [DOI] [PubMed] [Google Scholar]
Winters 2014
- Winters BR, Walsh TJ. The epidemiology of male infertility. Urologic Clinics of North America 2014;41(1):195‐204. [1558‐318X: (Electronic)] [DOI] [PubMed] [Google Scholar]
Yavuz 2013
- Yavuz Y, Mollaoglu H, Yurumez Y, Ucok K, Duran L, Tunay K, et al. Therapeutic effect of magnesium sulphate on carbon monoxide toxicity‐mediated brain lipid peroxidation. European Review for Medical and Pharmacological Sciences 2013;17 Suppl 1:28‐33. [1128‐3602: (Print)] [PubMed] [Google Scholar]
Zareba 2013
- Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jorgensen N, Mendiola J, et al. Semen quality in relation to antioxidant intake in a healthy male population. Fertility and Sterility 2013;100(6):1572‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers‐Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Human Reproduction 2017;32(9):1786‐1801. [1556‐5653: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2015
- Zhang Z, Zhu L, Jiang H, Chen H, Chen Y, Dai Y. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta‐analysis. Journal of Assisted Reproduction and Genetics 2015;32(1):17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2018
- Zhao J, Huang X, Xu B, Yan Y, Zhang Q, Li Y. Whether vitamin D was associated with clinical outcome after IVF/ICSI: a systematic review and meta‐analysis. Reproductive Biology and Endocrinology 2018;16(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2007
- Zhou X, Liu F, Zhai S. Effect of L‐carnitine and/or L‐acetyl‐carnitine in nutrition treatment for male infertility: a systematic review. Asia Pacific Journal of Clinical Nutrition 2007;16 Suppl 1:383‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Zini 1993
Zini 2011
- Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? [Review]. Fertility and Sterility 2011;96(6):1283‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Showell 2008
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007411] [DOI] [PubMed] [Google Scholar]
Showell 2011
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007411.pub2] [DOI] [PubMed] [Google Scholar]
Showell 2014
- Showell MG, Mackenzie‐Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD007411.pub3] [DOI] [PubMed] [Google Scholar]


