Abstract
Aims:
Mitochondria exist as a morphologically plastic network driven by cellular bioenergetic demand. Induction of fusion and fission machinery allows the organelle to regulate quality control and substrate flux. Physiologic stressors promote fragmentation of the mitochondrial network, a process implicated in the onset of metabolic disease, including type 2 diabetes and obesity. It is well known that exercise training improves skeletal muscle mitochondrial volume, number, and density. However, the effect of exercise training on muscle mitochondrial dynamics remains unclear.
Methods:
Ten sedentary adults (65.8 ± 4.6 years; 34.3 ± 2.4 kg/m2) underwent 12 weeks of supervised aerobic exercise training (5 day/wk, 85% of HRMAX). Body composition, cardio-metabolic testing, hyperinsulinemic-euglycemic clamps, and skeletal muscle biopsies were performed before and after training. MFN1, MFN2, OPA1, OMA1, FIS1, Parkin, PGC-1α, and HSC70 protein expression was assessed via Western blot.
Results:
Exercise training led to improvements in insulin sensitivity, aerobic capacity, and fat oxidation (all P<0.01), as well as reductions in bodyweight, BMI, fat mass and fasting glucose (all P<0.001). When normalized for changes in mitochondrial content, exercise reduced skeletal muscle FIS1 and Parkin (P<0.05), while having no significant effect on MFN1, MFN2, OPA1, and OMA1 expression. Exercise also improved the ratio of fusion to fission proteins (P<0.05), which positively correlated with improvements in glucose disposal (r2=0.59, P<0.05).
Conclusions:
Exercise training alters the expression of mitochondrial fusion and fission proteins, promoting a more fused, tubular network. These changes may contribute to the improvements in insulin sensitivity and substrate utilization that are observed after exercise training.
Keywords: mitochondrial dynamics, exercise training, insulin resistance, obesity, skeletal muscle
Introduction
Peripheral insulin resistance is implicated in numerous chronic diseases, including non-alcoholic fatty liver disease (NAFLD), polycystic ovarian syndrome (PCOS), central obesity, hypertension, dyslipidemia, and type 2 diabetes1–4. Yet, the mechanisms underlying the onset and development of insulin resistance remain unclear. A growing body of research has implicated mitochondrial dysfunction in the pathogenesis of insulin resistance5–11. However, the integrity, morphology, structure, and functionality of mitochondria in chronic disease states remains heavily disputed12–14.
Recent advances in molecular biology have redefined mitochondria as a morphologically plastic network sensitive to the bioenergetic demands of the cell. Mitochondria continuously undergo cycles of fission and fusion in order to balance input from mitochondrial biogenesis with output from damaged or poorly functioning mitochondria15. This process, referred to as mitochondrial dynamics, plays an essential role in cellular homeostasis, the extent of which has been reviewed elsewhere16. Induction of the cellular fusion and fission machinery allows the organelle to regulate mitochondrial quality control17,18, ion transport19, ATP synthesis20, membrane potential (Δψm)21, metabolic byproducts22, and substrate flux23.
Mitochondrial fusion occurs in two discrete yet coordinated events. Fusion of the outer mitochondrial membrane (OMM) is mediated by mitofusins 1 and 2 (MFN1 and MFN2, respectively)24. The inner mitochondrial membrane (IMM) of partially fused mitochondria is then coordinated by optic atrophy 1 (OPA1). OPA1-mediated IMM fusion is regulated by the peptidase OMA1 and i-AAA protease YME1L25. Mitochondrial fission is primarily regulated by dynamin-related protein 1 (DRP1), a cytosolic GTPase. Upon activation, DRP1 is phosphorylated by multiple known serine kinases, and is translocated to the mitochondria26. DRP1 is recruited to the OMM by a number of adapter proteins, the most well studied being mitochondrial fission 1 (FIS1)27. Damaged organelles may then be cleared by Parkin, an E3 ubiquitin-protein ligase, which mediates mitochondrial autophagy (mitophagy)28.
Bioenergetic stress arising from nutrient oversupply induces fragmentation of the mitochondrial network, resulting in uncontrolled fission and inhibition of fusion29. In addition, acute and chronic exposure to saturated fatty acids (SFA’s) and hyperglycemia fragment the mitochondrial network30, diminish Δψm and total ATP content30, enhance ROS generation22, and impair glucose uptake in β-cells31, adipocytes32, and myocytes30,33. Furthermore, impairments in mitochondrial fusion have been observed in obese rats and humans34. In contrast, cells exposed to nutrient deprivation develop elongated tubules, and are protected against mitophagy35. Collectively, these findings implicate mitochondrial dynamics as a potential regulatory mechanism linking excess nutrient intake to skeletal muscle insulin resistance.
It is well established that exercise training improves the volume, number, density, and function of skeletal muscle mitochondria36. However, little is known about the effect of exercise training on mitochondrial dynamics. We have previously reported that 12 weeks of exercise training reduces DRP1 activity at Serine 616 in human skeletal muscle, a known site of mitochondrial fission37. To our knowledge, exercise-induced adaptations to skeletal muscle mitochondrial dynamics in humans has been scarcely reported38,39.
Thus, the purpose of this investigation was to examine changes in human skeletal muscle mitochondrial dynamics after 12 weeks of aerobic training. We hypothesized that exercise training would enhance expression of fusion proteins, while inhibiting fission and mitophagy activation. It was also hypothesized that changes in mitochondrial dynamics would be associated with improvements in insulin sensitivity.
Results
12 weeks of exercise training significantly reduced body weight (p<0.001), BMI (P<0.001), body fat % (P=0.001), VAT (P<0.001), FPG (P=0.020), FPI (P=0.033), triglycerides (P=0.025), cholesterol (P=0.003), VLDL (P=0.019), LDL (P=0.006), and HOMA-IR (P=0.039) (Table 1). Furthermore, the intervention significantly improved VO2MAX (P<0.001), GDR (P<0.001), NOGD (P<0.0001), and M/I (P<0.001) (Table 1).
Table 1.
Changes in subject characteristics after 12 weeks of exercise training
| Characteristic | PRE | POST | p-value (2-tailed) |
||
|---|---|---|---|---|---|
| M | SEM | M | SEM | ||
| n (m/f) | 10 | (8/2) | |||
| Age (yrs) | 66.3 | 1.6 | - | - | - |
| Weight (kg) | 106.0 | 4.2 | 92.8 | 3.3 | <0.001 |
| BMI (kg/m2) | 34.5 | 0.7 | 30.2 | 0.8 | <0.001 |
| Body Fat % | 40.6 | 2.1 | 34.7 | 3.5 | 0.001 |
| VAT (cm2) | 107.7 | 17.2 | 60.1 | 17.8 | <0.001 |
| VO2MAX (ml/kg/min) | 23.8 | 1.2 | 32.0 | 2.5 | <0.001 |
| Blood Chemistry | |||||
| FPG (mg/dL) | 98.2 | 2.5 | 94.1 | 1.8 | 0.020 |
| FPI (μU/mL) | 14.1 | 1.5 | 10.9 | 1.2 | 0.033 |
| HOMA-IR | 3.1 | 0.5 | 2.3 | 0.4 | 0.019 |
| Triglycerides (mg/dL) | 154.0 | 24.6 | 96.2 | 15.6 | 0.025 |
| Cholesterol (mg/dL) | 209.5 | 12.2 | 174.2 | 9.5 | 0.003 |
| HDL (mg/dL) | 46.6 | 4.5 | 48.6 | 4.1 | 0.329 |
| VLDL (mg/dL) | 29.0 | 5.2 | 17.3 | 2.4 | 0.019 |
| LDL (mg/dL) | 133.9 | 9.2 | 108.3 | 8.8 | 0.006 |
| Insulin Sensitivity | |||||
| Glucose90–120 (mg/L) | 89.9 | 0.8 | 88.2 | 0.3 | 0.087 |
| Insulin90–120 (μU/mL) | 97.4 | 5.5 | 95.4 | 7.0 | 0.660 |
| M90–120 (mg/kg/min) | 2.6 | 0.4 | 4.7 | 0.4 | <0.0001 |
| GDR90–120 (mg/kg/min) | 2.5 | 0.3 | 4.9 | 0.4 | <0.001 |
| EGP (mg/kg/min) | 2.2 | 0.2 | 1.4 | 0.1 | 0.030 |
| Suppression of EGP (%) | 49.7 | 10.4 | 84.1 | 9.8 | 0.012 |
| NOGD (mg/kg/min) | 1.0 | 0.3 | 3.0 | 0.5 | <0.0001 |
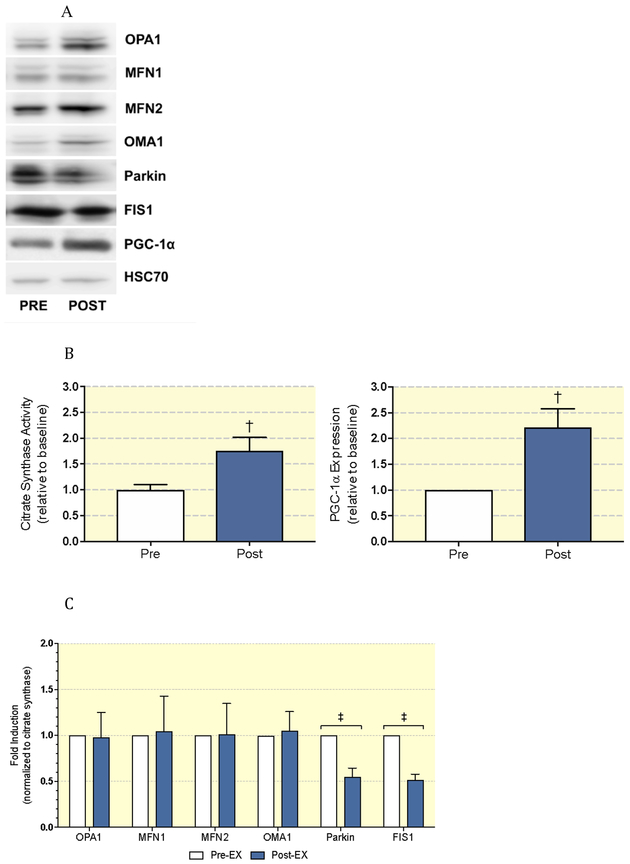
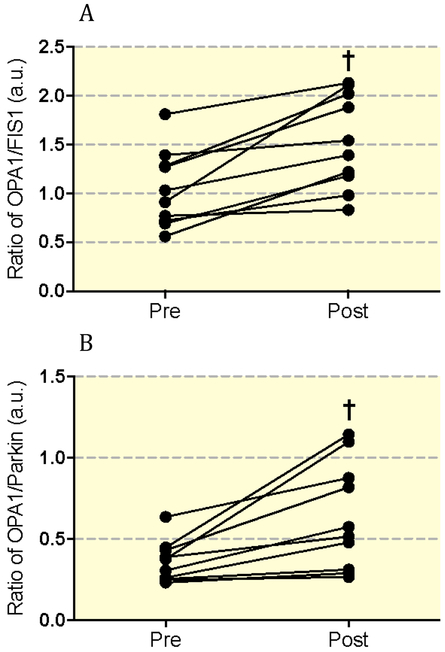
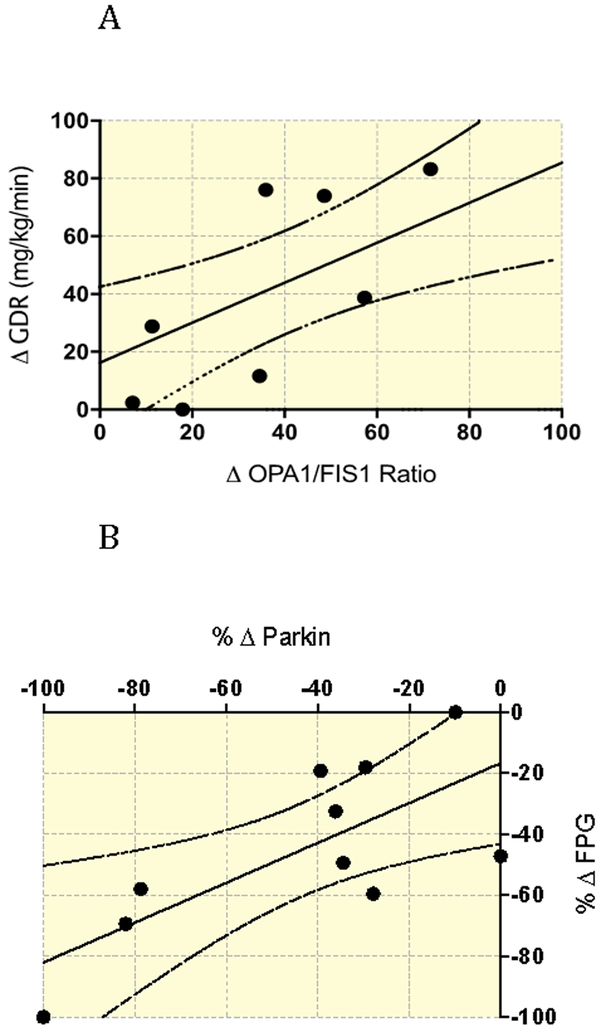
Next, we assessed differences in mitochondrial dynamics protein expression pre- and post-exercise training (Figure 1a). To assess the influence of biogenesis on mitochondrial dynamics, we additionally measured PGC-1α expression and citrate synthase activity. PGC-1α expression and citrate synthase activity significantly increased after exercise training (ALL P<0.01; Figure 1b). To account for these changes, we normalized protein expression data to citrate synthase activity, and observed significant reductions in Parkin (P<0.0001), and FIS1 (P<0.001), with no significant changes to MFN1, MFN2, OPA1, or OMA1 (Figure 1c). To further understand our findings in the context of the balance between fission and fusion, we generated ratios of OPA1/FIS1 and OPA1/Parkin pre- and post-training (Figure 2). Exercise training significantly increased the ratio of both OPA1/FIS1 (P=0.001; Figure 2a) and OPA1/Parkin (P=0.007; Figure 2b). Changes in the ratio of OPA1/FIS1 expression significantly correlated with improvements in glucose disposal rate (P=0.01, r2=0.59) (Figure 3a). Also, changes in Parkin expression significantly correlated with FPG (P=0.02, r2=0.54) (Figure 3b).
Figure 1:
(A) Representative immunoblots pre (−) to post (+) exercise training in human skeletal muscle. (B) Changes in citrate synthase activity and PGC-1α expression pre/post exercise training. (C) Densitometric analysis of mitochondrial dynamics proteins pre (white) to post (blue) exercise training.
Figure 2:
(A) Effect of exercise training on OPA1/FIS and (B) OPA1/Parkin expression ratios.
Figure 3:
(A) Significant correlation (P=0.01, R2=0.59) between Δ OPA1/FIS1 ratio (pre to post intervention) and Δ GDR (pre to post intervention). (B) Significant correlation (P<0.02, R2=0.54) between %Δ parkin (pre to post intervention) and %Δ FPG (pre to post intervention).
Discussion
We have previously reported that exercise reduces mitochondrial fission in insulin resistant skeletal muscle, which correlated with improvements in fatty acid oxidation, and insulin sensitivity37. Herein we extend those findings to demonstrate that 12 weeks of exercise training also reduces FIS1, a regulatory protein of the mitochondrial fission and mitophagy machinery. We also provide the first in vivo evidence that exercise reduces skeletal muscle Parkin activation, a protein directly implicated in the mitophagy pathway. These changes were observed in concert with improvements in oxidative capacity, glycolytic activity, and whole-body insulin sensitivity, as well as reductions in body fat, peripheral lipid and glucose accumulation. Taken together, these data suggest that exercise can enhance skeletal muscle mitochondrial dynamics and quality control, leading to increased whole-body insulin sensitivity via reductions in mitochondrial insults due to excess nutrient intake.
Exercise Training and Mitochondrial Dynamics
Few studies have examined changes in mitochondrial dynamics after prolonged exercise training. Konopka et al. reported a similar increase in skeletal muscle expression of MFN1 and MFN2 in both young and older adults after 12 weeks of aerobic exercise training38. Zampieri et al. reported increased OPA1 expression after 12 weeks of skeletal muscle electrical stimulation, but not resistance training39. Interestingly, further mitochondrial analysis revealed greater network integration and elongation after electrical stimulation, suggestive of enhanced fusion activity. In a pre-clinical model, after 7 days of exercise Sprague-Dawley rats displayed a pro-fusion shift similar to the results described herein40. Furthermore, prolonged sedentarism or aging induced pro-fission phenotypes, suggesting a relationship between skeletal muscle oxidative capacity and mitochondrial dynamics. Activation of fission and inhibition of fusion proteins have additionally been reported in muscle denervation models, further strengthening this notion41,42. However, generalizability of these findings is limited to due lack of normalization to changes in mitochondrial content.
It was previously shown that aerobic exercise training leads to a 60–120% increase in mitochondrial protein content43,44. To account for the contributions of biogenesis to changes in mitochondrial dynamics, we measured PGC-1α expression, a known transcriptional regulator of mitochondrial biogenesis45, and normalized protein expression to citrate synthase activity, a validated biomarker of mitochondrial content46. We found that citrate synthase activity accounted for exercise-induced changes in fusion activity (MFN1, MFN2, OPA1, OMA1). However, the reductions in fission and mitophagy (FIS1 and Parkin) appeared to be independent of changes in mitochondrial content. Pre-clinical models, in concert with data described herein, support the notion that exercise drives post-translational modifications in the mitochondria which contribute towards a pro-fusion phenotype.
The Mitophagic Response in Trained Skeletal Muscle
Data from Egan et al. suggest that an adequate mitophagic response is essential for balancing oxidative stress from nutrient metabolism, with mitochondrial network integrity, structure, and function47. This is further evidenced by studies demonstrating the onset of metabolic diseases with loss of autophagic function in the liver and pancreas48,49. For this reason, it has been postulated that enhancing mitochondrial quality control may be a critical adaptation to exercise50. In this regard, exercised muscle benefits from increased mitophagic rates, clearance and quality control as a result of increased skeletal muscle oxidation rates. This idea is largely supported by acute exercise studies in skeletal muscle, which have demonstrated upregulation in mitochondrial fission, with modality specific effects on fusion51–53.
However, our intervention data support the notion that exercise training may result in fewer and/or less frequent mitophagic events - a product of enhanced metabolic function and improved mitochondrial architecture and supporting machinery. In this view, exercise increases the pool of skeletal muscle mitochondria that can be integrated into larger tabulated networks. Kim et al. found that mice with a skeletal muscle mitophagy specific knockout were protected against high fat diet-induced insulin resistance, had greater energy expenditure, and enhanced insulin sensitivity compared to wild type mice54. This observation is further supported by the increased abundance of antioxidants and free radical scavengers in trained skeletal muscle55.
It appears therefore, that mitophagy in skeletal muscle is discrete and possibly more disposable than in other tissues such as the liver or pancreas. Thus, exercise may alter mitochondrial turnover via a biphasic mechanism. Acute exercise promotes enhanced mitochondrial turnover, priming the mitochondrial network for future bouts. Chronic exercise may then reduce mitochondrial turnover by improving the integrity of the mitochondrial network, or by increasing the size and abundance of intact mitochondrial networks. Clearly, further research is necessary to elucidate the effects of chronic exercise on skeletal muscle mitophagy.
Overall, these results highlight the role of exercise in the regulation of skeletal muscle mitochondrial dynamics with regards to the balance of fusion, fission, and quality control. Our data in concert with pre-clinical models demonstrate that chronic exercise alters expression of key mitochondrial proteins involved in the regulation of mitochondrial dynamics. Furthermore, we provide additional insight into the role of mitochondrial dynamics proteins in the regulation of skeletal muscle insulin sensitivity. Previous data from our group in conjunction with the findings described herein implicates altered mitochondrial fission and mitophagy in the pathogenesis of type 2 diabetes. Furthermore, exercise training carries the therapeutic potential to normalize or enhance mitochondrial dynamics, reducing fission and mitophagy and increasing fusion by input from mitochondrial biogenesis. The current investigation was limited by assessing proteins governing mitochondrial fission and fusion processes. More detailed studies to elucidate the mechanisms driving mitochondrial fission-induced insulin resistance and type 2 diabetes using imaging-based approaches are underway.
Materials and Methods
Subjects:
Ten obese, prediabetic adults completed a 12 week exercise training intervention. Medical screenings excluded individuals with heart, kidney, liver, thyroid, intestinal, and pulmonary diseases or individuals taking medications known to affect the outcome variables of the study. Resting 12-lead electrocardiograms and submaximal exercise stress tests excluded individuals with any contraindication to high intensity physical activity. All women were postmenopausal and not using hormone replacement therapy. Participants had also been weight stable for at least the previous 6 months. The study was approved by the Cleveland Clinic Institutional Review Board, and all subjects provided written informed consent in accordance with our guidelines for the protection of human subjects.
Exercise Intervention:
Subjects underwent 60 minutes of aerobic exercise 5 days/week for 12 weeks (treadmill walking and cycle ergometry) at ~85% (± 5 beats per minute) of their maximal heart rate (HRMAX). HRMAX was obtained at baseline, and at 4 week increments during the intervention and was recorded during an incremental maximal aerobic-exercise (VO2MAX) test using a modified Bruce protocol. All sessions were supervised by an exercise physiologist. The test was deemed to be maximal if at least three of the following criteria were satisfied: a respiratory exchange ratio of ≥1.10, a leveling off in oxygen consumption with increasing workloads, volitional fatigue, or a heart rate greater than or equal to age-predicted maximum. Subjects were instructed to maintain eucaloric intake and dietary records were collected at baseline, and 4 week increments to ensure compliance. Dietary analysis was performed with Nutritionist Pro software (Axxya Systems, Stafford, TX).
Inpatient control period:
All measures collected pre- and post-intervention were obtained during a 3-day in-patient stay in the Clinical Research Unit at the Cleveland Clinic. During the inpatient control periods, participants were provided with a weight maintenance isocaloric diet (total kcal/day = resting metabolic rate × 1.25; 55% carbohydrate, 35% fat, and 10% protein) derived from indirect calorimetry measures conducted at the beginning of the inpatient control period. All metabolic measurements were conducted during the inpatient control period and within 24 h of the last exercise bout.
Body Composition:
After a 12-hour overnight fast, height and weight were measured using standard techniques in a hospital gown. Dual-energy X-ray absorptiometry (Lunar iDXA; Madison, WI) was then used to determine whole body fat and lean mass. Estimation of fat and lean tissue content was obtained from iDXA software according to the manufacturer’s instructions. Computerized tomography scanning was additionally used to quantify visceral adipose tissue (VAT) with a SOMOTOM Sensation 16 Scanner (Siemens Medical Solutions, Malvern, PA), as previously described56. Non-contrast scans were performed locally in the lumbar region.
Insulin Sensitivity Testing:
Measurements of insulin sensitivity were obtained after an overnight fast using a four hour, euglycemic-hyperinsulinemic clamp (90 mg/dl, 40 mU·m−2·min−1), as described previously57,58. Briefly, a primed (3.28 mg/kg) continuous (0.036 mg·kg−1 ·min−1) infusion of D-[6,6-2H2]glucose began at −120 min and continued throughout the procedure to calculate endogenous glucose production (EGP). At 0 minutes, simultaneous infusion of insulin (constant) and 20% dextrose (variable) began. Arterialized heated-hand venous blood was sampled at 5 minute intervals (YSI 2300; STAT Plus, Yellow Springs, OH), and the glucose infusion rate (GIR) was adjusted according to the correction algorithm of DeFronzo et al.57. Insulin sensitivity was then calculated as insulin-stimulated glucose metabolism (M; mg·kg−1·min−1) during the last 30 minutes of the clamp. Plasma for assessing glucose kinetics was deproteinized, extracted and derivatized before analysis by gas chromatography-mass spectrometry as previously described37. The isotopic enrichment (mole percent excess) of the samples were obtained by comparing their peak area percentage (m/z 202)/(m/z 202 + m/z 200) with that of a standard curve. Endogenous glucose production (EGP) and the rate of glucose disposal (GDR) was then derived using the Steele equation59. Hepatic insulin resistance was estimated by the suppression of EGP after insulin stimulation. Homeostatic model assessment of insulin resistance (HOMA-IR) {[fasting plasma glucose (FPG) × fasting plasma insulin (FPI)] ÷ 405} was also calculated as an additional measure of insulin resistance. Whole body respiratory exchange ratio (RER) and substrate metabolism was assessed basally and under insulin-stimulated conditions via indirect calorimetry as previously described (Vmax Encore; Viasys, Yorba Linda, CA)60. Non-oxidative glucose disposal was estimated by subtracting the glucose oxidation rate from the GDR.
Percutaneous Muscle Biopsy:
Skeletal muscle tissue was obtained from the medial vastus lateralis using a modified Bergström technique as previously described61. Samples were obtained approximately 36 hours after the final exercise bout. Upon collection, samples were dissected of fat and connective tissue, and immediately frozen in liquid nitrogen. All muscle samples were then stored at −140°C until the time of analysis.
Tissue Homogenization, Western Blot Analysis, and Quantification:
Muscle homogenates were prepared by grinding muscle tissue in ice-cold lysis buffer (Invitrogen) in the presence of protease inhibitor cocktail, 5 mM phenylmethylsulfonyl fluoride (Sigma), and Phos-STOP (Roche Applied Sciences, Indianapolis, IN). The homogenates were then centrifuged for 10 min at 14,000 x g. The resulting supernatant was decanted and the tissue lysates were stored at −80°c until the time of analysis. Protein concentrations were measured using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). 32 μg’s (0.8 μg/mL) of muscle lysate were solubilized in Laemmli sample buffer containing 5% β-mercaptoethanol and boiled for 5 min. 40 μL’s of sample was then loaded into 4–12% Tris Glycine gels (Novex) and separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis at 125 volts for 1.5 hours (SDS-PAGE: Invitrogen).
The gels were transferred to polyvinylidene fluoride membranes (Bio-Rad), and blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline with 0.1% Tween-20 (PBST) for 1 hour. Membranes were then incubated overnight with anti-MFN1 (R & D Systems; Minneapolis, MN, catalog no. AF7880), anti-MFN2 (Cell Signaling Technology; Danvers, MA, catalog no. 9482), and anti-OPA1 (Abnova; Walnut, CA, catalog no. 12083), anti-OMA1 (Abcam; Cambridge, MA, ab154949), anti-FIS1 (Thermo Fisher Scientific; Waltham, MA, PA1–41082), anti-Parkin (Cell Signaling Technology; 4211), anti-PGC1α (Novus Biologicals; NBP1–04676) and anti-HSC70 (Santa Cruz Biotechnology; Dallas, TX, SC-7298) antibodies diluted 1:1000 in 5% BSA solution. Membranes were washed with PBST and incubated with species-specific horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ; catalog no. NA931) diluted 1:10,000 in 5% BSA solution. Immunoreactive proteins were visualized by enhanced chemiluminescence reagent (ECL Prime; GE Healthcare) and quantified by densitometric analysis using ImageJ software62. All antibodies were internally validated prior to use. All bands displayed were subject to quantification with the exception of MFN1 and OMA1. For MFN1, only the bottom band, which appears at the predicted molecular weight (90 kDa), was used. For OMA1, only the middle band, which appears at the predicted molecular weight (60 kDa), was used. Protein expression was normalized to changes in mitochondrial biogenesis by dividing the raw values by PGC-1α expression. Values were then expressed as fold induction relative to pre-intervention normalized to loading control. The fold induction ratios of OPA1/FIS and OPA1/Parkin were subsequently derived from the raw densitometric values.
Citrate Synthase Activity:
Citrate synthase activity was measured via commercially available kinetic assay (Sigma-Aldrich; CS0720). Briefly, ~5 mg of frozen muscle tissue were homogenized (FastPrep-24™; MP Biomedicals, LLC) in 500 μL’s of lysis buffer (Sigma-Aldrich; C3228) containing protease inhibitor cocktail (Sigma-Aldrich; P8340). Protein concentrations were measured using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). 8 μg’s of protein were then diluted in assay buffer, and assessed for endogenous net citrate synthase activity on a spectrophotometer.
Statistical Analysis:
Student’s paired samples t-test was used to assess differences pre- to post-exercise intervention. Normality of distribution was verified using the Shapiro-Wilks test. Equality of variance was assessed visually using a Q-Q plot. Correlations between study variables were assessed using Pearson’s r. If data sets were abnormally distributed, log transformation was used to approach normality, and reassessed using the Shapiro-Wilks test. Graph Pad Prism 5 was used for statistical analysis. R was used to generate the Q-Q plot. The level of significance was set at P<0.05. *,†,‡ Indicates a significant treatment effect at P<0.05, P<0.01, and P<0.001, respectively.
New & Noteworthy:
This study provides novel insight into changes in muscle mitochondrial fusion, fission, and mitophagy in humans before and after exercise training. Importantly, our data utilize adequate normalization to decipher contributions from mitochondrial dynamics, not content, in exercise-related cardiometabolic improvements.
ACKNOWLEDGEMENTS
This research was supported by NIH Grants RO1 DK-108089 (JPK) and NIH National Center for Research Resources, 1UL1RR024989, Cleveland, Ohio.
We thank the study volunteers, the Cleveland Clinical Foundation clinical research unit, and study staff for their considerable time, and effort on this project.
Footnotes
CONFLICT OF INTEREST
None of the authors have a conflict of interest related to this work.
References
- 1.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–90. [DOI] [PubMed] [Google Scholar]
- 2.Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48(4):634–642. [DOI] [PubMed] [Google Scholar]
- 3.Corbould A, Kim YB, Youngren JF, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288(5):E1047–1054. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. [DOI] [PubMed] [Google Scholar]
- 5.Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56(5):1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. [DOI] [PubMed] [Google Scholar]
- 7.Ortenblad N, Mogensen M, Petersen I, et al. Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta. 2005;1741(1–2):206–214. [DOI] [PubMed] [Google Scholar]
- 8.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuermann-Freestone M, Madsen PL, Manners D, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107(24):3040–3046. [DOI] [PubMed] [Google Scholar]
- 10.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50(1):113–120. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster BH. Mitochondrial deficiency is associated with insulin resistance. Diabetes. 2013;62(4):1032–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50(4):790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloszy JO. “Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes. 2013;62(4):1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund MT, Larsen S, Hansen M, et al. Mitochondrial respiratory capacity remains stable despite a comprehensive and sustained increase in insulin sensitivity in obese patients undergoing gastric bypass surgery. Acta Physiol (Oxf). 2018;223(1):e13032. [DOI] [PubMed] [Google Scholar]
- 15.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8(11):870–879. [DOI] [PubMed] [Google Scholar]
- 16.Archer SL. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369(23):2236–2251. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105(38):14503–14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13(5):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saotome M, Safiulina D, Szabadkai G, et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008;105(52):20728–20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodi R, Tonon C, Valentino ML, et al. Deficit of in vivo mitochondrial ATP production in OPA1-related dominant optic atrophy. Ann Neurol. 2004;56(5):719–723. [DOI] [PubMed] [Google Scholar]
- 21.Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13(12):4343–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103(8):2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79(2):341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 2014;19(4):630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1(4):515–525. [DOI] [PubMed] [Google Scholar]
- 27.Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24(5):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisler S, Holmstrom KM, Skujat D, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–131. [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Jin P, Yu L, et al. Impaired mitochondrial dynamics and bioenergetics in diabetic skeletal muscle. PLoS One. 2014;9(3):e92810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jheng HF, Tsai PJ, Guo SM, et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molina AJ, Wikstrom JD, Stiles L, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58(10):2303–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao CL, Zhu C, Zhao YP, et al. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2010;320(1–2):25–33. [DOI] [PubMed] [Google Scholar]
- 33.Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab. 2010;299(6):E1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bach D, Pich S, Soriano FX, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278(19):17190–17197. [DOI] [PubMed] [Google Scholar]
- 35.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108(25):10190–10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(4):831–838. [DOI] [PubMed] [Google Scholar]
- 37.Fealy CE, Mulya A, Lai N, Kirwan JP. Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. J Appl Physiol (1985). 2014;117(3):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2014;69(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zampieri S, Mammucari C, Romanello V, et al. Physical exercise in aging human skeletal muscle increases mitochondrial calcium uniporter expression levels and affects mitochondria dynamics. Physiol Rep. 2016;4(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve. 2013;48(6):963–970. [DOI] [PubMed] [Google Scholar]
- 41.Kitaoka Y, Takeda K, Tamura Y, Fujimaki S, Takemasa T, Hatta H. Nrf2 deficiency does not affect denervation-induced alterations in mitochondrial fission and fusion proteins in skeletal muscle. Physiol Rep. 2016;4(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham ZA, Harlow L, Bauman WA, Cardozo CP. Alterations in mitochondrial fission, fusion, and mitophagic protein expression in the gastrocnemius of mice after a sciatic nerve transection. Muscle Nerve. 2018. [DOI] [PubMed] [Google Scholar]
- 43.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemura A, Roy RR, Edgerton VR, Ishihara A. Biochemical Adaptations in a Slow and a Fast Plantarflexor Muscle of Rats Housed in Small Cages. Aerosp Med Hum Perform. 2016;87(5):443–448. [DOI] [PubMed] [Google Scholar]
- 45.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286(12):10605–10617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Larsen S, Nielsen J, Hansen CN, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590(14):3349–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu JJ, Quijano C, Chen E, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY). 2009;1(4):425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11(6):467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lira VA, Okutsu M, Zhang M, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27(10):4184–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding H, Jiang N, Liu H, et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800(3):250–256. [DOI] [PubMed] [Google Scholar]
- 52.Kruse R, Pedersen AJ, Kristensen JM, Petersson SJ, Wojtaszewski JF, Hojlund K. Intact initiation of autophagy and mitochondrial fission by acute exercise in skeletal muscle of patients with Type 2 diabetes. Clin Sci (Lond). 2017;131(1):37–47. [DOI] [PubMed] [Google Scholar]
- 53.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim KH, Jeong YT, Oh H, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19(1):83–92. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44(2):126–131. [DOI] [PubMed] [Google Scholar]
- 56.Solomon TP, Haus JM, Kelly KR, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92(6):1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–223. [DOI] [PubMed] [Google Scholar]
- 58.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297(1):E151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187(1):15–24. [DOI] [PubMed] [Google Scholar]
- 60.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):628–634. [DOI] [PubMed] [Google Scholar]
- 61.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14(1):101–102. [PubMed] [Google Scholar]
- 62.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]