Abstract
The present study investigated the effects of children without disabilities on maternal physical and mental health in families with adolescents or adults with fragile X syndrome (FXS). Mothers with the FMR1 premutation (N = 87) reported on behavior problems and functional limitations of their adolescent or adult child with FXS and their own physical and mental health. Mothers also provided a blood sample to determine FMR1 CGG repeat length. The proportion of unaffected children in the family significantly buffered the effect of both child behavior problems and functional limitations on maternal self-rated health, such that having a higher proportion of unaffected children in the family had a protective effect on maternal health when the target child had more severe behavior problems and functional limitations. There was a similar buffering process for maternal depressive symptoms, but at a trend level. Additionally, maternal CGG repeat length had a significant curvilinear association with self-rated health, indicating that mothers with mid-range repeat lengths reported the poorest health, while mothers with lower and higher repeat lengths in the premutation range reported better health. The data suggest that unaffected children in the family may be an important resource for premutation carrier mothers. Findings are consistent with previous research indicating that mothers with varying levels of genetic liability have variable risk for health problems.
Keywords: fragile X syndrome, FMR1 premutation, siblings, maternal depressive symptoms, maternal health, behavior problems, functional limitations
Unaffected Siblings of Adolescents and Adults with Fragile X Syndrome: Effects on Maternal Well-Being
Fragile X syndrome (FXS), the most common inherited cause of intellectual disability, is a neurodevelopmental disorder caused by an expansion of CGG repeats on the FMR1 gene (Brown, 2002), which inhibits production of FMRP, a protein crucial for cognitive development. In addition to intellectual disability, individuals with FXS display behavior problems and functional limitations (Smith, Barker, Seltzer, Abbeduto, & Greenberg, 2012; Smith, Hong, Greenberg, & Mailick, 2016). The challenges associated with caring for a person with FXS across the lifespan can negatively impact parental physical and mental health (Abbeduto et al., 2004; Hartley, Seltzer, Head, & Abbeduto, 2012). Mothers may be particularly vulnerable given their own genetic status as premutation carriers. However, having children in the family who do not have a disability (whom we refer to as “unaffected siblings”) may mitigate the impact of these stressors on maternal health. This is particularly important in families carrying inherited genetic conditions, such as FXS, where each child is potentially but not necessarily affected. In this study, we investigated the effect of the proportion of unaffected children on maternal health (health rating and depressive symptoms) and its role as a buffer of the association between stressors (behavior problems and functional limitations of a child with FXS) and maternal health.
The behavior problems and functional limitations associated with FXS are often sources of stress for mothers. We consider these issues within the context of life course theory (Elder, 1998; Seltzer, Greenberg, Floyd, Pettee, & Hong, 2001) to address the cumulative effect of these challenges on mothers over time. Although the severity of behavior problems and functional limitations declines over the lifespan for individuals with FXS (Smith et al., 2016), both remain a significant source of maternal stress (Bailey et al., 2012; Hastings, 2002). (Smith et al., 2016).
In addition to experiencing stress related to parenting a child with FXS, premutation carriers may have a genetic liability for physical and mental health problems (Chan, Smith, Greenberg, Hong, & Mailick, 2017; Wheeler, Raspa, Hagerman, Mailick, & Riley, 2017). Most mothers of children with FXS are premutation carriers, with between 55 and 200 CGG repeats. Moreover, longer CGG repeat length is associated with higher levels of self-reported depressive symptoms (Johnston et al., 2001), but there is also data indicating a curvilinear association between CGG repeat length and maternal health. Premutation carrier mothers with mid-range CGG repeat lengths have higher risk for health problems than premutation mothers with lower or higher repeat lengths (Allen et al., 2007; Roberts et al., 2009; Seltzer et al., 2012). For premutation carrier mothers, genetic liability may have an impact on health, particularly in the context of other stressors (Hartley, Seltzer, Hong, et al., 2012). Therefore, it is important to account for CGG repeat length in research on premutation carriers.
According to a family systems perspective, it is crucial to study the ways that family members influence one another (Broderick, 1993). Parenting multiple children is associated with increased stress when children are typically developing (Adam & Gunnar, 2001) and with both stress and depressive symptoms when children have disabilities such as FXS (Hartley, Seltzer, Hong, et al., 2012), or autism spectrum disorder, which like FXS is accompanied by behavior problems (Orsmond, Lin, & Seltzer, 2007). However, in families of individuals with disabilities, having other children without disabilities does not have the same negative effect on marital relationships as it does for families where no children have disabilities (Namkung, Song, Greenberg, Mailick, & Floyd, 2015), and may even mitigate parental stress (Orsmond & Seltzer, 2000). Families with children with FXS present a unique family system for considering the impact of unaffected children on maternal well-being, given the variability in patterns of inheritance of FMR1 CGG repeats. For example, such families may have 1) other children with the FMR1 premutation, 2) other children unaffected by FMR1 conditions but who have different disabilities, and/or 3) unaffected children. The latter are the focus of this paper.
For this brief report, we investigated the extent to which the proportion of unaffected children in a family may affect maternal physical and mental health. Based on previous literature suggesting that characteristics of unaffected children can impact maternal well-being (Hall, Burns, & Reiss, 2007), and research suggesting that the presence of unaffected children in the family may ameliorate maternal stress (Namkung et al., 2015), we examined both direct and stress buffering effects on the association between stressors (i.e., behavior problems and functional limitations of a child with FXS) and maternal physical and mental health.
Methods
Participants
The sample for this study (N = 87) included mothers participating in an ongoing longitudinal study of families of adolescents and adults with FXS (Masked for review). Participants were premutation carrier biological mothers of a son or daughter with FXS who was 12 years or older (the target child) at the start of the study. Mothers and target children resided together or maintained at least weekly contact. If a mother had more than one child with FXS, she reported on the co-residing child. If there was more than one co-residing child, she reported on the child she viewed as most severely affected. The ongoing longitudinal study currently includes four waves of data spanning a decade. Data from the third wave were used in this study, as that was the wave for which the most comprehensive sibling data were available.
Mothers were in their fifties on average (Mage = 54.82 years, SD = 7.19, range 40 – 72 years), and nearly all were white/non-Hispanic (97.7%). The majority had some college education or more (88.5%) and were married (85.1%). The median household income was $90,000 to $99,999 (range $10,000 - $160,000+). Mothers had between one and six children (M = 2.32, SD = 1.12), and between one and five children diagnosed with a disability (M = 1.87, SD = .87). Mothers had between one and three children diagnosed with FXS (M = 1.46, SD = .63). Target children with FXS were mostly males (85.8%), in their twenties on average (Mage = 24.60, SD = 6.96, range 15 – 45 years), and most lived with their mothers (86.2%).
Procedure
Mothers provided data through self-administered questionnaires and telephone interviews, and provided a blood sample to determine CGG repeat length. For the present sample, CGG repeat length varied from 67 to 180 CGG repeats (M = 96.97, SD = 21.64). The Institutional Review Board at (Masked for review) approved the data collection protocol and written consent was obtained from all participants.
Measures
Maternal overall health.
Mothers were asked to rate their own current physical health on a scale from 0 (poor) to 3 (excellent). This scale has been shown to be a robust, reliable, and valid indicator of health and a predictor of mortality across 27 empirical studies (for a review, see Idler & Benyamini, 1997).
Maternal depressive symptoms.
The Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1977) is a 20-item measure used to index depressive symptoms. Mothers were asked to report how often in the previous week they experienced each symptom, on a scale from 0 (never) to 3 (5 to 7 days). A sum score was computed, with higher scores indicating more depressive symptoms. Scores of 16 or above are considered clinically significant. In this sample, the Cronbach’s alpha for the CES-D was .93.
Target Child’s Behavior Problems.
Mothers completed the Behavior Problems subscale of the Scales of Independent Behavior-Revised (SIB-R; Bruininks, Bradley, Weatherman, & Woodcock, 1996) on their target son or daughter with FXS. Mothers were asked about eight behavior problem types: (a) self-injurious behavior, (b) unusual or repetitive behaviors, (c) withdrawn or inattentive behavior, (d) behavior that is hurtful to others, (e) property destruction, (f) disruptive behavior, (g) socially offensive behavior, and (h) uncooperative behavior. If a behavior problem was endorsed, mothers rated frequency from 1 (less than once a month) to 5 (one or more times per hour) and severity from 1 (not serious) to 5 (extremely serious). Standardized algorithms (Bruininks et al., 1996) were used to create a general maladaptive behavior summary score, with higher scores indicating more severe maladaptive behaviors. Reliability and validity of this measure have been established by Bruininks et al. (1996).
Target Child’s Daily Living Skills.
Mothers completed the Waisman Activities of Daily Living Scale (W-ADL; Maenner et al., 2013; Smith, Maenner, & Seltzer, 2012) to rate their child’s level of independence on 17 items involving personal care, housekeeping, and meal-related activities. Each item was rated on a scale of 0 (does not perform the task at all), 1 (performs the task with help), or 2 (performs the task independently), and items were summed to create a total score where higher scores indicate more independence (i.e., fewer functional limitations). The correlation with the Vineland Daily Living Skills Scale is .82 (Maenner et al., 2013). Cronbach’s alpha for the W-ADL in this sample was .89.
Proportion of Unaffected Children.
We identified siblings who did not have any of the following based on mother-report: FXS full mutation or premutation, autism spectrum disorder, attention deficit disorder or attention deficit/hyperactivity disorder, learning disability, psychiatric disorder, genetic disorder, or a medical diagnosis. Mothers reported that 90.4% of siblings had undergone testing for FMR1 CGG expansions. Mothers had between zero and four unaffected children. Unaffected siblings were mostly males (59.0%) and in their thirties on average (Mage = 30.81, SD = 13.12, ranged in age from 9 to 50 years of age). Because the total number of children varied among families, we computed the proportion of unaffected children in the family and used this variable in analyses, in line with previous research (Cicirelli, 2009).
Analytic Plan
Hierarchical regression analyses were conducted to examine the direct effect of the proportion of unaffected children on maternal physical and mental health (step 1) and its role as a moderator of the association between stressors (target child’s behavior problems and daily living skills) and maternal health (step 2). We controlled for target child age, marital status (1 = married, 0 = other), CGG repeat number (linear and curvilinear), and household income. Analyses were conducted in SPSS Version 22.
Results
Descriptive Data on Family Constellations.
As shown in Table 1, 18 of 87 families had only one child (i.e., the target child with FXS), 40 had two children, 19 had three children, and 10 had four to six children. In 15 families all of the other children were unaffected, whereas in 41 all other children were affected by FMR1 conditions or other disabilities. There was at least one unaffected child in 28 families. In all families with four or more children, at least one other child had a disability. It was notable that only nine of the 87 families had a child with a premutation range CGG expansion.
Table 1.
Descriptive Statistics on Family Constellations.
| 2−Child Families | 3−Child Families | 4+Child Families | Total | |
|---|---|---|---|---|
| 1-Child Families | - | - | - | 18 |
| Any unaffected non-target child | 14 | 8 | 6 | 28 |
| All non-target children with any diagnosis | 26 | 11 | 4 | 41 |
| Total Number of Families | 40 | 19 | 10 | 87 |
| All non-target children are unaffected | 14 | 1 | 0 | 15 |
| Any non-target child with premutation | 2 | 3 | 4 | 9 |
| Any non-target child with FXS | 14 | 13 | 7 | 34 |
| Any non-target child with other diagnosis* | 16 | 15 | 9 | 40 |
Note. Ns represent number of families in each category. Ns within the shaded area of the table are non-mutually exclusive.
The “other diagnoses” (with number of children in parentheses) include learning disability (N = 29), attention deficit disorder or attention deficit/hyperactivity disorder (N = 29), psychiatric disorder (N = 29), intellectual disability (N = 22), medical diagnosis (N = 18), autism spectrum disorder (N = 7), other genetic disorder (N = 6).
Models Including Behavior Problems as Primary Stressor.
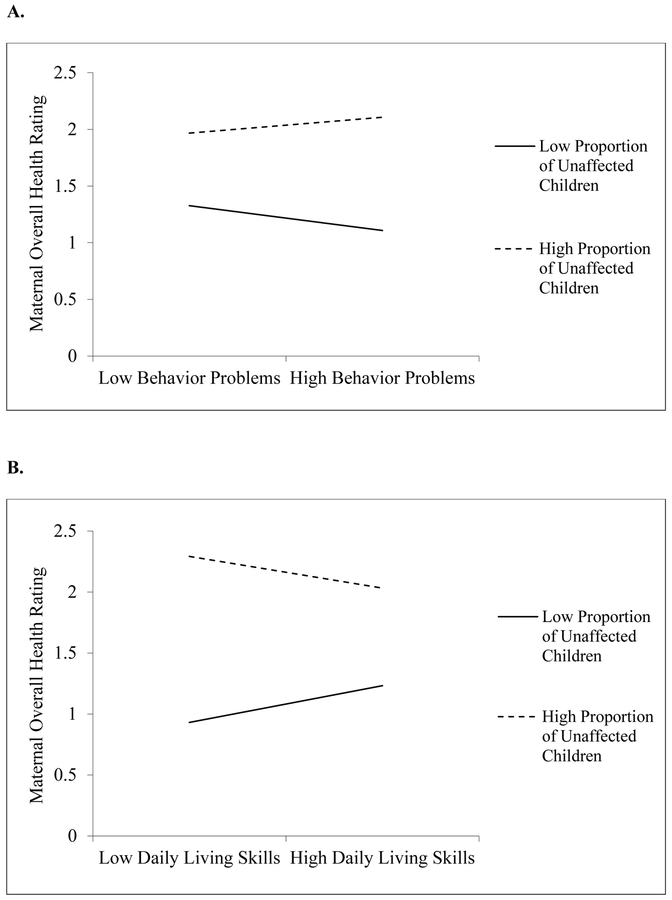
See Table 2. In Model 1, higher levels of target child behavior problems were significantly associated with lower levels of maternal overall health, p = .012. Model 1 accounted for 21% of the variance in overall health, F(7, 79) = 2.960, p = .008. In Model 2, there was a significant interaction of behavior problems with the proportion of unaffected children, p = .034. As displayed in Figure 1, when the target child with FXS displayed more behavior problems, mothers with a higher proportion of unaffected children reported better health than those with a low proportion. The proportion of unaffected children had less of an effect on maternal health when the target child had few behavior problems. Income, marital status, and linear CGG were not significant predictors of overall health. However, CGG repeat length had a significant curvilinear effect, p = .049, indicating that premutation mothers with mid-range repeat lengths reported the poorest overall health, while those with lower and higher repeat lengths reported better health. Model 2 accounted for 25% of the variance, F(8, 78) = 3.293, p = .003, ΔR2=.045, ΔF = 4.663, p = .034.
Table 2.
Two-step hierarchical regression analyses examining proportion of unaffected children in a family as a moderator of the association between target child’s behavior problems and maternal overall health rating and depressive symptoms.
| Maternal Overall Health Rating | Maternal Depressive Symptoms | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Predictor Variable | B(SE) | β | B(SE) | β | B(SE) | β | B(SE) | β |
| Target Age | .00(.0l) | −.01 | .00(.01) | −.03 | −.01(.16) | −.01 | .00(.16) | .00 |
| Household Income | .00(.02) | .00 | −.01(.02) | −.03 | −1.01(34) | −.32** | −.95(34) | −30** |
| Marital Status (1=Married) | .42(.23) | .20† | .41(.22) | .20† | −2.05(3.19) | −.07 | −2.02(3.16) | −.07 |
| CGG | −.0l(.00) | −.24† | −.01(.00) | −.20 | .05(.07) | .11 | .04(.07) | .08 |
| CGG2 | .00(.0) | .32* | .00(.00) | .26* | .00(.00) | −.22 | .00(.00) | −.17 |
| Target Behavior Problems | −.02(.01) | −.27* | −.02(.01) | −.21† | .19(.13) | .15 | .13(.13) | .10 |
| Unaffected Children | .35(33) | .11 | .42(.32) | .13 | −9.73(4.62) | −.21* | −10.59(4.59) | −.23* |
| Behavior Problems*Unaffected Children | .09(.04) | .23* | −1.05(.62) | −.18† | ||||
Note. SE = standard error. CGG number, target child behavior problems, and proportion of unaffected children were mean-centered.
p < .10,
p < .05,
p < .01.
Figure 1.
Significant interactions of child stressors (Panel A: behavior problems; Panel B: functional limitations) and proportion of unaffected children on maternal overall health.
In Model 1, higher proportions of unaffected children were significantly associated with lower levels of maternal depressive symptoms, p = .038. However, behavior problems were not directly associated with depressive symptoms. Model 1 accounted for 24% of the variance in depressive symptoms, F(7, 79) = 3.523, p = .002. In Model 2, there was a trend-level interaction of behavior problems with the proportion of unaffected children on maternal depressive symptoms, p = .091. Higher income was significantly associated with lower depressive symptoms, p = .006, while marital status and CGG were not significant covariates. Model 2 accounted for 27% of the variance, F(8, 78) = 3.524, p = .002, , ΔR2=.028, ΔF = 2.928, p = .091.
Models Including Daily Living Skills as Primary Stressor.
See Table 3. For overall health rating, in Model 1 there were no significant main effects. Model 1 accounted for 15% of the variance in overall health, F(7, 79) = 1.907, p = .079. As shown in Model 2, there was a significant interaction between the proportion of unaffected children and daily living skills on maternal overall health rating, p = .037 (See Figure 1). When the target child with FXS displayed a greater impairment in daily living skills, mothers with a higher proportion of unaffected children reported better health than those with a lower proportion. The proportion of unaffected children had less of an effect when children had better daily living skills. Model 2 accounted for 19% of the variance, F(8, 78) = 2.309, p = .028, ΔR2=.047, ΔF = 4.526, p = .037.
Table 3.
Two-step hierarchical regression analyses examining proportion of unaffected children in a family as a moderator of the association between target child’s daily living skills and maternal overall health rating and depressive symptoms.
| Maternal Overall Health Rating | Maternal Depressive Symptoms | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Predictor Variable | B(SE) | β | B(SE) | β | B(SE) | β | B(SE) | β |
| Target Age | .00(.0l) | .03 | .0l(.0l) | .06 | −.05(.16) | −.03 | −.09(.16) | −.06 |
| Household Income | .00(.03) | −.02 | −.01(.03) | −.02 | −.98(35) | −.31* | −.96(.35) | −.30** |
| Marital Status (1=Married) | .47(.24) | .23† | .43(.23) | .21† | −2.40(3.24) | −.08 | −1.96(3.20) | −.07 |
| CGG | −.0l(.00) | −.18 | −.01(.00) | −.17 | .04(.07) | .08 | .04(.06) | .07 |
| CGG2 | .00(.00) | .29* | .00(.00) | .28* | .00(.00) | −.20 | .00(.00) | −.19 |
| Target Daily Living Skills | .01(.01) | .06 | .00(.01) | .03 | −.10(.20) | −.05 | −.04(.20) | −.02 |
| Unaffected Children | .38(.34) | .12 | .53(.34) | .16 | −9.95(4.70) | −.22* | −11.73(4.72) | −.25* |
| Daily Living Skills*Unaffected Children | −.14(.07) | −.23* | 1.73(.91) | .20† | ||||
Note. SE = standard error. CGG number, target child daily living skills, and proportion of unaffected children were mean-centered.
p < .10,
p < .05,
p < .01.
Model 1 shows that a higher proportion of unaffected children was significantly associated with lower levels of maternal depressive symptoms, p = .015. Model 1 accounted for 22% of the variance in depressive symptoms, F(7, 79) = 3.166, p = .005. As shown in Model 2, there was a trend-level interaction between daily living skills and the proportion of unaffected children on maternal depressive symptoms, p = .060. Model 2 accounted for 25% of the variance, F(8, 78) = 3.319, p = .003, ΔR2=.035, ΔF = 3.646, p = .060.
Discussion
This study investigated the effects of unaffected children on the association between child-related stressors and maternal physical and mental health in families of adolescents and adults with FXS. Findings supported a stress buffering effect on maternal overall health, with the proportion of unaffected children significantly attenuating the negative impact of behavior problems and functional limitations. In families with higher proportions of unaffected children, child stressors had less of a negative effect on maternal overall health than in families with lower proportions, suggesting a protective effect of unaffected children on maternal self-rated health in the presence of stress. There was suggestion of a similar stress buffering process for depressive symptoms. Findings indicate that the presence of unaffected children impact maternal health in these families. Although parenting multiple young children may be stressful (Adam & Gunnar, 2001), there may be benefits later in life to having multiple children (Ward, Spitze, & Deane, 2009), particularly in families who have children with disabilities.
Examination of covariates revealed that higher income related to better health, as in the general population (Subramanian & Kawachi, 2004). With most mothers reporting being currently married, marital status was not associated with maternal health. Samples with greater variability may provide information on differential associations for mothers of different income or marital status. Consistent with research from our group and others (Masked for review; Loesch et al., 2015; Roberts et al., 2009), premutation mothers with mid-range CGG repeat lengths had poorer health than those with lower and higher lengths.
This study’s strengths included using data on siblings to consider family member impact on maternal health. This was possibly the first study of families of children with FXS to focus on the unaffected children in the family. We analyzed maternal CGG repeat length to consider genetic influences on maternal health, and we examined effects on both physical and mental health. However, analyses using a larger sample and longitudinal data would provide a more robust analysis. The study’s sample was limited in racial/ethnic minority group representation, which limit generalizability. There is a potential for shared method variance, as mothers reported on all variables (other than CGG repeat length, determined by a blood sample). Studies would benefit from multiple reporters, including other significant individuals in the lives of individuals with disabilities, such as fathers. There was no measure of the specific role of unaffected children or mother-child relationship quality. Future work to gain a better understanding of how unaffected children function in the family system would be informative.
Future research on the relationship between children with FXS and siblings could address additional questions regarding the family system in FXS. For example, does siblings’ emotional closeness relate to maternal health? Findings from this study highlight that family members operate both individually and together to impact mothers’ health, and suggest that an approach that incorporates studying the entire family is informative for studying premutation carrier mothers in families affected by FXS. Implications include evidence for interventions that offer support for complex family systems, as individuals’ adaptation is intertwined within families.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development to the IDDRC at the University of North Carolina (P30 HD003100-S1) to support a Fragile X Research Center at three additional sites (Research Triangle Institute International, the University of Wisconsin-Madison and University of Kansas). The present analysis was based on data collected by the UW-Madison Waisman Center site (M. Mailick, PI). Additional support was provided by the National Institute of Child Health and Human Development (T32 HD07489 and R01 HD082110, M. Mailick, PI) and the Waisman Center IDDRC (P30 HD03352 and U54 HD090256, A. Messing, PI). We are extremely grateful to the families who participated in this study; without their generous support and commitment, our research would not be possible.
Funding:
This work was supported by the National Institute of Child Health and Human Development to the IDDRC at the University of North Carolina (P30 HD003100-S1) to support a Fragile X Research Center at three additional sites (Research Triangle Institute International, the University of Wisconsin-Madison and University of Kansas). The present analysis was based on data collected by the UW-Madison Waisman Center site (M. Mailick, PI). Additional support was provided by the National Institute of Child Health and Human Development (T32 HD07489 and R01 HD082110, M. Mailick, PI) and the Waisman Center IDDRC (P30 HD03352 and U54 HD090256, A. Messing, PI).
Footnotes
Compliance with Ethical Standards:
Conflict of Interest:
The authors declare that they have no conflict of interest.
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Abbeduto L, Seltzer MM, Shattuck P, Krauss MW, Orsmond G, & Murphy MM (2004). Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. American Journal of Mental Retardation, 109(3), 237–254. [DOI] [PubMed] [Google Scholar]
- Adam EK, & Gunnar MR (2001). Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology, 26(2), 189–208. doi: 10.1016/S0306-4530(00)00045-7 [DOI] [PubMed] [Google Scholar]
- Allen E, Sullivan A, Marcus M, Small C, Dominguez C, Epstein M, … Sherman S (2007). Examination of reproductive aging milestones among women who carry the FMR1 premutation. Human reproduction, 22(8), 2142–2152. [DOI] [PubMed] [Google Scholar]
- Bailey DB Jr., Raspa M, Bishop E, Mitra D, Martin S, Wheeler A, & Sacco P (2012). Health and economic consequences of fragile X syndrome for caregivers. Journal of Developmental and Behavioral Pediatrics, 33(9), 705–712. doi: 10.1097/DBP.0b013e318272dcbc [DOI] [PubMed] [Google Scholar]
- Broderick CB (1993). Understanding family process: Basics of family systems theory: Sage. [Google Scholar]
- Brown WT (2002). The molecular biology of the fragile X mutation. Fragile Xsyndrome: Diagnosis, treatment and research, 3, 110–135. [Google Scholar]
- Bruininks RH, Bradley HK, Weatherman RF, & Woodcock RW (1996). SIB-R: Riverside publishing Company. [Google Scholar]
- Chan W, Smith LE, Greenberg JS, Hong J, & Mailick MR (2017). Executive functioning mediates the effect of behavioral problems on depression in mothers of children with developmental disabilities. American Journal on Intellectual and Developmental Disabilities, 122(1), 11–24. doi: 10.1352/1944-7558-122.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli VG (2009). Sibling death and death fear in relation to depressive symptomatology in older adults. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences, 64(1), 24–32. doi: 10.1093/geronb/gbn024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GH (1998). The life course as developmental theory. Child development, 69(1), 1–12. [PubMed] [Google Scholar]
- Hall SS, Burns DD, & Reiss AL (2007). Modeling family dynamics in children with fragile X syndrome. Journal of abnormal child psychology, 35(1), 29–42. doi: 10.1007/s10802-006-9081-4 [DOI] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Head L, & Abbeduto L (2012). Psychological well-being in fathers of adolescents and young adults with Down Syndrome, Fragile X syndrome, and autism. Family Relations: An Interdisciplinary Journal of Applied Family Studies, 61(2), 327–342. doi: 10.1111/j.1741-3729.2011.00693.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Hong J, Greenberg J, Smith LE, Almeida DM, … Abbeduto L (2012). Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. International Journal of Behavioral Development, 36(1), 53–61. doi: 10.1177/0165025411406857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings RP (2002). Parental stress and behaviour problems of children with developmental disability. Journal of Intellectual and Developmental Disability, 27(3), 149–160. [Google Scholar]
- Idler EL, & Benyamini Y (1997). Self-rated health and mortality: a review of twenty-seven community studies. Journal of health and social behavior, 21–37. [PubMed] [Google Scholar]
- Johnston C, Eliez S, Dyer-Friedman J, Hessl D, Glaser B, Blasey C, … Reiss A (2001). Neurobehavioral phenotype in carriers of the fragile X premutation. American Journal of Medical Genetics Part A, 103(4), 314–319. [PubMed] [Google Scholar]
- Loesch DZ, Bui MQ, Hammersley E, Schneider A, Storey E, Stimpson P, … Hessl D (2015). Psychological status in female carriers of premutation FMR1 allele showing a complex relationship with the size of CGG expansion. Clinical genetics, 87(2), 173–178. doi: 10.1111/cge.12347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Smith LE, Hong J, Makuch R, Greenberg JS, & Mailick MR (2013). Evaluation of an activities of daily living scale for adolescents and adults with developmental disabilities. Disability and Health Journal, 6(1), 8–17. doi: 10.1016/j.dhjo.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailick MR, Greenberg JS, Smith LE, Sterling A, Brady N, Warren SF, & Hong J (2014). Fragile X-associated disorders: How the family environment and genotype interact In Burack JA & Schmidt LA (Eds.), Cultural and Contextual Perspectives on Developmental Risk and Well-Being (pp. 221–253, Chapter xix, 295 Pages): Cambridge University Press, New York, NY. [Google Scholar]
- Mailick MR, Hong J, Greenberg JS, Smith LE, & Sherman SL (2014). Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 165b(8), 705–711. doi: 10.1002/ajmg.b.32277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung EH, Song J, Greenberg JS, Mailick MR, & Floyd FJ (2015). The relative risk of divorce in parents of children with developmental disabilities: Impacts of lifelong parenting. American Journal on Intellectual and Developmental Disabilities, 120(6), 514–526. doi: 10.1352/1944-7558-120.6.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsmond GI, Lin L-Y, & Seltzer MM (2007). Mothers of adolescents and adults with autism: Parenting multiple children with disabilities. Intellectual and developmental disabilities, 45(4), 257–270. doi: 10.1352/1934-9556(2Q07)45[257:MOAAAW]2.Q.CO;2 [DOI] [PubMed] [Google Scholar]
- Orsmond GI, & Seltzer MM (2000). Brothers and sisters of adults with mental retardation: Gendered nature of the sibling relationship. American Journal on Mental Retardation, 105(6), 486–507. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Roberts JE, Bailey DB Jr., Mankowski J, Ford A, Sideris J, Weisenfeld LA, … Golden RN (2009). Mood and anxiety disorders in females with the FMR1 premutation. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150B(1), 130–139. doi: 10.1002/aimg.b.30786 [DOI] [PubMed] [Google Scholar]
- Seltzer M, Barker ET, Greenberg JS, Hong J, Coe C, & Almeida D (2012). Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychology, 31(5), 612–622. doi: 10.1037/a0026528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer M, Greenberg JS, Floyd FJ, Pettee Y, & Hong J (2001). Life course impacts of parenting a child with a disability. American Journal on Mental Retardation, 106(3), 265–286. doi: [DOI] [PubMed] [Google Scholar]
- Smith LE, Barker ET, Seltzer M, Abbeduto L, & Greenberg J (2012). Behavioral phenotype of fragile X syndrome in adolescence and adulthood. American Journal on Intellectual and Developmental Disabilities, 117(1), 1–17. doi: 10.1352/1944-7558-117.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Hong J, Greenberg JS, & Mailick MR (2016). Change in the behavioral phenotype of adolescents and adults with FXS: Role of the family environment. Journal of Autism and Developmental Disorders, 46(5), 1824–1833. doi: 10.1007/s10803-016-2714-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Maenner MJ, & Seltzer M (2012). Developmental trajectories in adolescents and adults with autism: the case of daily living skills. Journal of the American Academy of Child & Adolescent Psychiatry, 51(6), 622–631. doi: 10.1016/j.jaac.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian SV, & Kawachi I (2004). Income Inequality and Health: What Have We Learned So Far? Epidemiologic Reviews, 26(1), 78–91. doi: 10.1093/epirev/mxh003 [DOI] [PubMed] [Google Scholar]
- Ward RA, Spitze G, & Deane G (2009). The more the merrier? Multiple parent-adult child relations. Journal of Marriage and Family, 71(1), 161–173. doi: 10.1111/j.1741-3737.2008.00587.x [DOI] [Google Scholar]
- Wheeler A, Raspa M, Hagerman R, Mailick M, & Riley C (2017). Implications of the FMR1 premutation for children, adolescents, adults, and their families. Pediatrics, 139(Supplement 3), S172–S182. [DOI] [PMC free article] [PubMed] [Google Scholar]