Abstract
Background:
The most common type of mucinous pancreatic cyst that may progress to pancreatic cancer is intraductal papillary mucinous neoplasm (IPMN). Low risk IPMN with low/moderate grade dysplasia may be safely watched whereas high risk IPMN with high grade dysplasia or invasive components should undergo resection. However, there is currently no reliable means of making this distinction. We hypothesize that blood concentrations of insulin resistance biomarkers may aid in the differentiation of low and high risk IPMN.
Methods:
Plasma/serum was collected from consented patients undergoing pancreatic resection. IPMN diagnosis and dysplastic grade were confirmed by surgical pathology. The study included 235 IPMN (166 low/moderate grade, 39 high grade, 30 invasive). Circulating levels of leptin, branched chain amino acids (BCAA), and retinol-binding protein-4 (RBP-4) were measured by enzyme-linked immunoassay and correlated with surgical pathology.
Results:
Circulating leptin levels (mean ± SE) were significantly higher in patients with low/moderate IPMN than in high grade/invasive IPMN (15,803±1686 vs. 10,275±1228 pg/ml; p=0.0086). Leptin levels were positively correlated with BMI (r=0.65, p<0.0001) and were higher in females (p<0.0001). Stratified analysis showed that mean leptin levels were significantly different between low/moderate and high/invasive IPMNs only in females (24,383±2748 vs. 16,295±2040 pg/ml; p=0.020). Conversely, circulating BCAA levels were lower in low/moderate IPMN than in high grade/invasive IPMN (0.38±0.007 vs. 0.42±0.01mM; p=0.011). No significant differences in RBP-4 levels were observed.
Conclusions:
Circulating leptin in females and BCAA correlate with IPMN dysplastic grade and if combined with clinical characteristics, have the potential to improve clinical decision-making.
Keywords: Leptin, BCAA, IPMN, Biomarker, Pancreatic cyst
Introduction:
Pancreatic cancer remains one of the deadliest cancers, surpassing breast cancer as the third leading cause of cancer related death in the United States. In 2018, the American Cancer Society estimates that pancreatic cancer will be diagnosed in 55,440 people and of these, 44,330 will die [1]. Although many cases of pancreatic cancer occur sporadically, two groups of patients are at increased risk of developing pancreatic cancer. One group is patients with familial or hereditary pancreatic cancer, and the other is patients with cystic lesions of the pancreas [2, 3]. Cystic lesions of the pancreas are diagnosed in increasing numbers due to the use of high resolution imaging and increased awareness of pancreas cyst symptoms [4]. In fact, greater than 2% of American adults undergoing imaging for an unrelated indication are incidentally found to harbor a pancreatic cyst.
Pancreatic cysts exhibit variable potential for malignant transformation. The most common type of mucinous pancreatic cyst that has considerable potential to progress to invasive pancreatic cancer is intraductal papillary mucinous neoplasm (IPMN). While low/moderate grade IPMN are low-risk and can be safely monitored, high grade and invasive IPMN are high-risk and should be resected in fit patients. However, there is currently no reliable means of predicting malignant potential preoperatively. Although branch versus main duct involvement can aid in assessing IPMN malignant risk, it is unreliable by itself. Additional clinical factors and imaging characteristics may also be useful in prediction of IPMN malignant potential but are not sufficient. Consensus guidelines incorporating clinical and radiologic features predict malignant IPMN with satisfactory sensitivity (>90%) but poor specificity (25–30%) [5–7]. Such low specificity results in an unacceptably high false positive rate. This may result in unnecessary resections of low risk IPMN with their concomitant risk of surgery-related morbidity and mortality. Thus, discovery of biomarkers that correlate with dysplastic grade and thereby differentiate low risk IPMN from high risk IPMN would greatly facilitate patient risk stratification and optimize clinical management.
Recent epidemiological and clinical evidence suggests a link between cancer and insulin resistance, a metabolic disorder characterized by increased circulating insulin levels and commonly associated with obesity and type 2 diabetes [8]. In particular, a high risk of pancreatic cancer is associated with sedentary lifestyle, obesity, diabetes, and elevated circulating levels of insulin and glucose [9–12]. We hypothesize that insulin resistance may also correlate with malignant progression of IPMN, a well-established precursor lesion to invasive pancreatic cancer. In the present study, three reported biomarkers of insulin resistance were evaluated in blood collected from patients diagnosed with IPMN. Leptin, an adipokine produced by adipose tissue, is involved in energy intake regulation and has been associated with insulin resistance [13, 14]. Branched chain amino acids (BCAA), comprised of leucine, isoleucine, and valine, positively correlate with risk of insulin resistance and type 2 diabetes [15, 16]. Retinol-binding protein-4 (RBP-4), another adipokine, also contributes to the development of health conditions related to insulin resistance [17]. In the present study, we present novel evidence that leptin and BCAA may hold promise as non-invasive blood biomarkers of high risk IPMN.
Materials and Methods:
Patient samples:
Blood samples were collected prospectively from IPMN or pancreatic cancer patients at the time of endoscopic ultrasound and/or pancreatic resection at Indiana University Health University Hospital between June 2003 and February 2017. The diagnosis was confirmed on surgical pathology by a University Hospital staff pathologist and subsequently reconfirmed by a pancreatic pathologist. Dysplasia grade was assessed according to the World Health Organization criteria. Demographic and clinical data for these patients were gathered by retrospective review of a prospectively generated database and supplemented through review of electronic medical records. Healthy control blood samples were obtained from friends or family accompanying patients being treated at Indiana University Health University Hospital. Demographic and anthropometric data from the healthy controls were collected by using a risk factor questionnaire. After procurement, blood samples were placed immediately on ice, processed to prepare plasma and/or serum, and then aliquoted for storage at −80o C. All patients and healthy controls provided informed consent in accordance with the Indiana University Institutional Review Board.
Assays:
Leptin, BCAA, and RBP-4 levels were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits from R&D (leptin, RBP-4; Minneapolis, MN) and Abcam (BCAA, same lot number used for all tests; Cambridge, MA). Samples were thawed on ice prior to assay. Tests to determine whether freeze/thawing or assaying plasma vs. serum samples affected the results were also performed. The majority of banked samples were plasma; however, in some cases, only serum was available. For a subset of patients from whom both serum and plasma were collected, we confirmed that serum and plasma showed similar concentrations for leptin as well as for BCAA so that serum/plasma results were analyzed together.
Statistical analysis:
Demographic, anthropometric, and clinic-pathologic characteristics of patients with low/moderate and high/invasive IPMN were compared with t-test for continuous variables and chi-square test for categorical variables. Differences in blood levels of BCAA, leptin (sex-specific and all), and RBP4 were examined with t-test or ANOVA for two-group or multiple-group comparisons, respectively. A p-value of <0.05 was considered statistically significant. Predictive accuracy of the biomarkers was determined by receiver operator characteristic (ROC) analyses.
Results:
A total of 235 patients with pathologically confirmed diagnosis of IPMN were analyzed in the present study. Of these, 166 were classified as low/moderate grade IPMN and 69 as high grade/invasive IPMN. There were no significant differences in the demographic and clinical characteristics of the patients, including age, sex, race, serum tumor marker CA19–9, and clinical pancreatitis, between the two groups (Table 1). A marginally significant difference in BMI was observed between the two groups (p=0.05). Radiographic main duct involvement was more common in high grade/invasive IPMN than in low/moderate grade IPMN (p<0.0001); main pancreatic duct diameter was significantly larger in high grade/invasive IPMN (p<0.0001).
Table 1.
Demographic and clinical characteristics of patients with intraductal papillary mucinous neoplasm (IPMN), Indiana University Health University Hospital, 2003–2017
| Low/Moderate Grade IPMN | High Grade/Invasive IPMN | ||||
|---|---|---|---|---|---|
| Characteristics | N | Mean (SE)/% | N | Mean (SE)/% | p-value |
| Age (year) | 166 | 66.5 (0.86) | 69 | 66.4 (1.40) | 0.96 |
| Sex (%) | 0.25 | ||||
| Male | 77 | 46.4 | 38 | 55.1 | |
| Female | 89 | 53.6 | 31 | 44.9 | |
| Race (%) | 1.0 | ||||
| White | 160 | 97.0 | 67 | 97.1 | |
| Others | 5 | 3.0 | 2 | 2.9 | |
| BMI (kg/m2) | 162 | 27.5 (0.42) | 68 | 26.0 (0.53) | 0.05 |
| Radiographic Duct involvement (%) | <0.0001 | ||||
| Branch duct | 96 | 58.5 | 16 | 23.1 | |
| Main duct | 68 | 41.5 | 53 | 76.8 | |
| Main pancreatic duct diameter (mm) | 152 | 5.0 (0.26) | 65 | 8.5 (0.60) | <0.0001 |
| CA19–9 (serum, U/ml) | 143 | 27.64 (4.7) | 60 | 461 (238) | 0.074 |
| Pancreatitis (%) | 1.0 | ||||
| Yes | 85 | 51.5 | 35 | 51.0 | |
| No | 80 | 48.5 | 34 | 49.0 | |
Circulating leptin levels (mean ± SE) were significantly higher in patients with low/moderate grade IPMN than in those with high grade/invasive IPMN (15,803 ± 1686 vs. 10,275 ± 1228 pg/ml, respectively; p=0.0086) (Figure 1a and Table 2). Additionally, leptin levels were higher in females than in males (p<0.0001) and positively correlated with BMI (r=0.65, p<0.0001). When stratified by sex, differences in mean leptin levels between low/moderate and high grade/invasive IPMNs were not statistically significant among 115 men (5886 ± 892 vs. 5365 ± 912 pg/ml, respectively; p=0.68) but were significant among 120 women (24,383 ± 2748 vs. 16,295 ± 2040 pg/ml, respectively; p=0.02) (Figures 1b-c and Table 2). When stratified by BMI, leptin levels differed significantly between low/moderate and high grade/invasive IPMN in individuals with BMI 30 or greater (34,733 ± 4928 vs. 14,796 ± 2413 pg/ml, respectively; p=0.0006) (Table 2).
Figure 1.
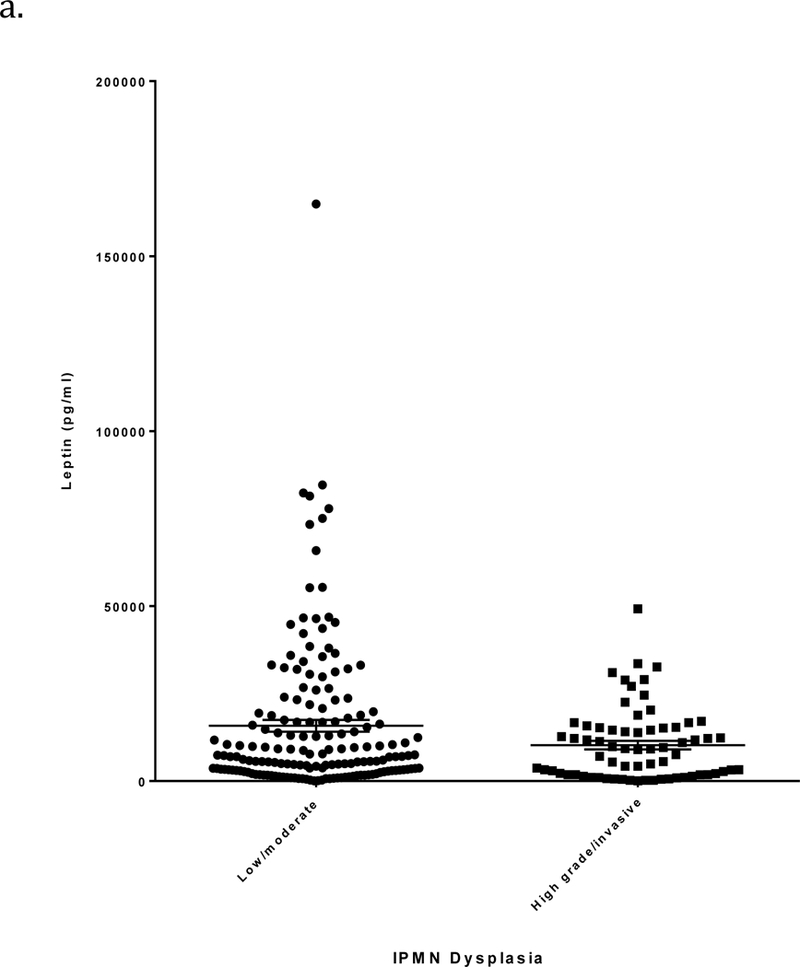
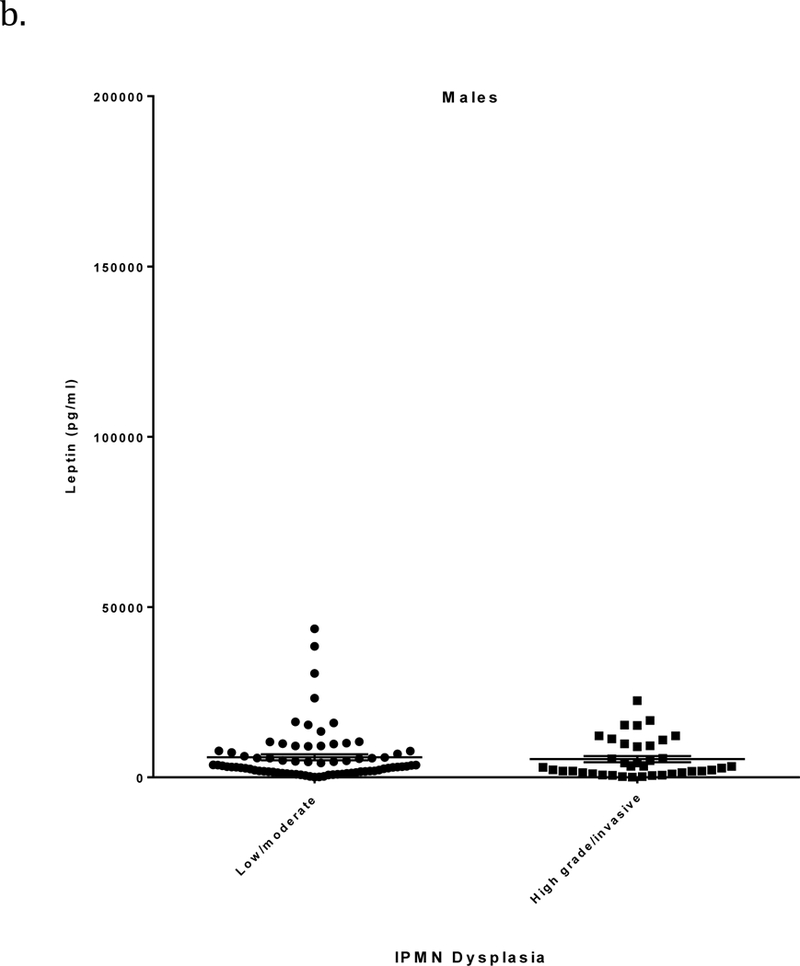
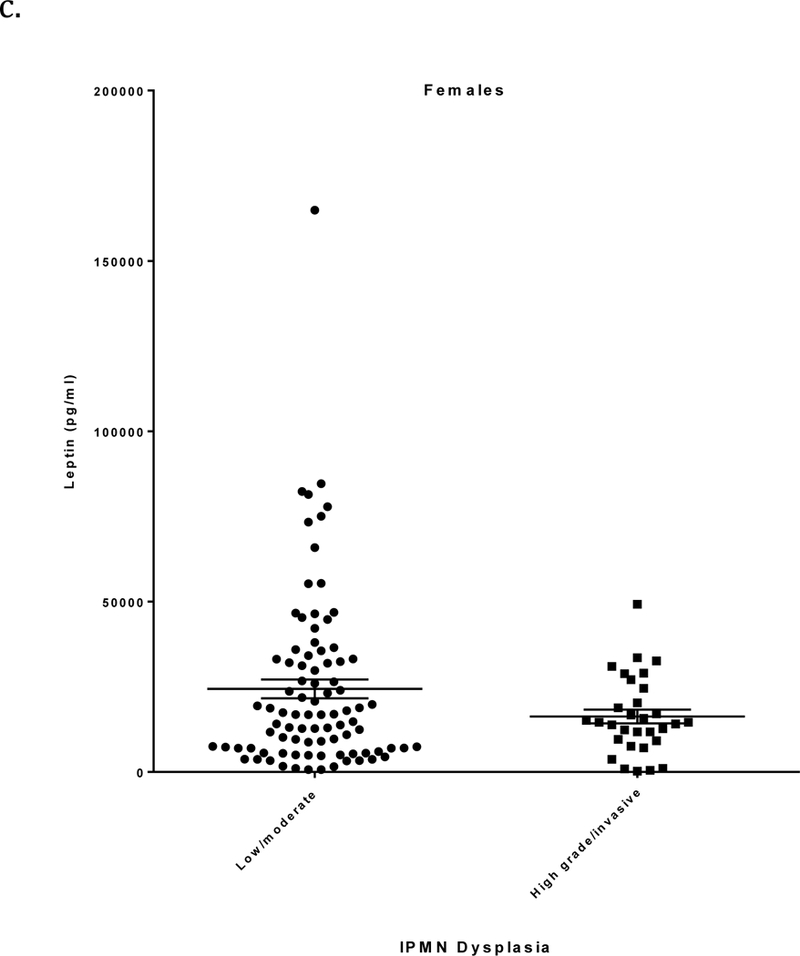
a) Leptin levels (pg/ml; mean ± SE, horizontal black lines) determined by ELISA in relation to IPMN dysplastic grade. Plasma/serum was obtained from patients with a pathologically confirmed diagnosis of low/moderate grade or high grade/invasive IPMN. b) Leptin levels in males in relation to IPMN dysplastic grade. c) Leptin levels in females in relation to IPMN dysplastic grade.
Table 2.
Circulating levels of insulin resistance biomarkers in patients with different dysplasia grade of IPMN, Indiana University Health University Hospital, 2003–2017
| Low/Moderate Grade IPMN | High Grade/Invasive IPMN | ||||
|---|---|---|---|---|---|
| Biomarkers | N | Mean (SE) | N | Mean (SE) | p-value |
| Leptin (pg/ml) | |||||
| All | 166 | 15,803 (1686) | 69 | 10,275 (1228) | 0.0086 |
| Male | 77 | 5886 (892) | 38 | 5365 (912) | 0.68 |
| Female | 89 | 24,383 (2748) | 31 | 16,295 (2040) | 0.02 |
| Normal (BMI<=25) | 53 | 5603 (1177) | 32 | 6728 (1334) | 0.54 |
| Overweight (25<BMI<30) | 70 | 11897 (1308) | 26 | 12729 (2447) | 0.75 |
| Obesity (BMI>=30) | 43 | 34733 (4928) | 11 | 14796 (2413) | 0.0006 |
| BCAA (mM) | |||||
| All | 166 | 0.38 (0.007) | 69 | 0.42 (0.01) | 0.01 |
| Normal (BMI<=25) | 53 | 0.37 (0.015) | 32 | 0.40 (0.017) | 0.35 |
| Overweight (25<BMI<30) | 70 | 0.37 (0.011) | 26 | 0.41 (0.022) | 0.026 |
| Obesity (BMI>=30) | 43 | 0.39 (0.015) | 11 | 0.46 (0.052) | 0.25 |
| RBP4 (ng/ml) | 50 | 41660 (2662) | 48 | 38,397 (2577) | 0.38 |
| HbA1C (%) | 95 | 6.19 (0.10) | 43 | 6.34 (0.22) | 0.56 |
BCAA, branch-chain amino acids; RBP4, retinol-binding protein 4
To further investigate whether leptin levels correlate with malignant progression, additional blood samples were collected from patients with conventional pancreatic ductal adenocarcinoma (PDAC) (n=103) and healthy controls (n=42) for comparison. Measured leptin levels were significantly different when all four groups were compared (P<0.0001) (Figure 2). Specifically, there was an overall decreasing trend in leptin levels (mean ± SE) across healthy controls (26,255 ± 4042 pg/ml), low/moderate grade IPMN (15,803 ± 1686 pg/ml), high grade/invasive IPMN (10,275 ± 1228 pg/ml), and PDAC (10,394 ± 1410 pg/ml), with similar levels observed in the last two groups.
Figure 2.
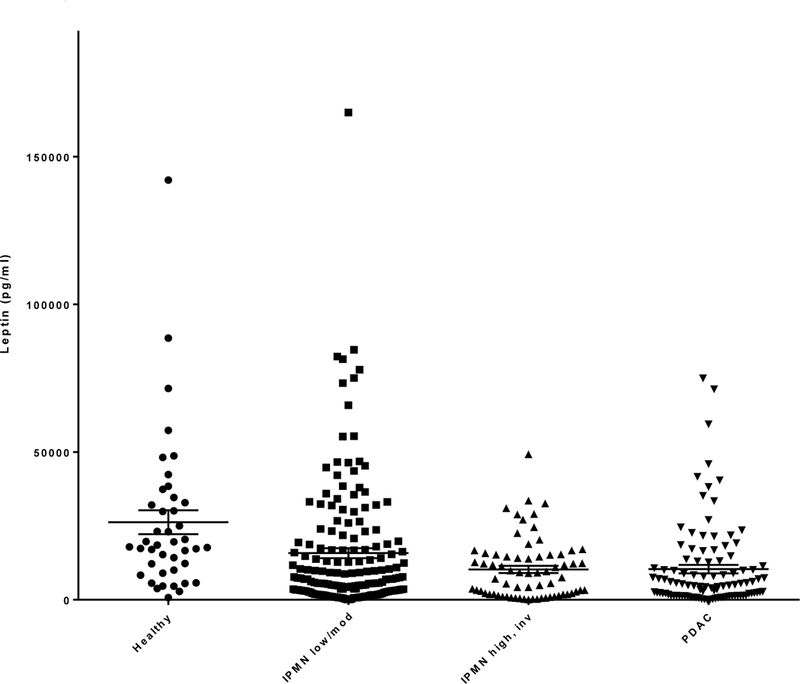
Circulating leptin levels (pg/ml; mean ± SE, horizontal black lines) compared among healthy controls, low/moderate dysplastic grade IPMN, high grade/invasive IPMN and pancreatic ductal adenocarcinoma (PDAC).
Circulating BCAA levels (mean ± SE) were significantly lower in low/moderate grade IPMN (0.38 ± 0.007 mM) than in high grade/invasive IPMN (0.42 ± 0.01mM) (p=0.01) (Figure 3 and Table 2). When further stratified by BMI, BCAA levels differed significantly between low/moderate and high grade/invasive IPMN in individuals with BMI greater than 25 but less than 30 (0.37 ± 0.011 vs. 0.41 ± 0.022 mM, respectively; p=0.026) (Table 2). In contrast, there was no significant difference in RBP-4 levels between the two groups (p=0.38) (Table 2). HbA1c, a measure of average blood glucose levels over 6–8 weeks and indicator of diabetes, did not significantly differ between the two groups (p=0.56) (Table 2). Similarly, no significant difference was observed for two additional biomarkers of insulin resistance, adiponectin and C-peptide, between the two groups (data not shown).
Figure 3.
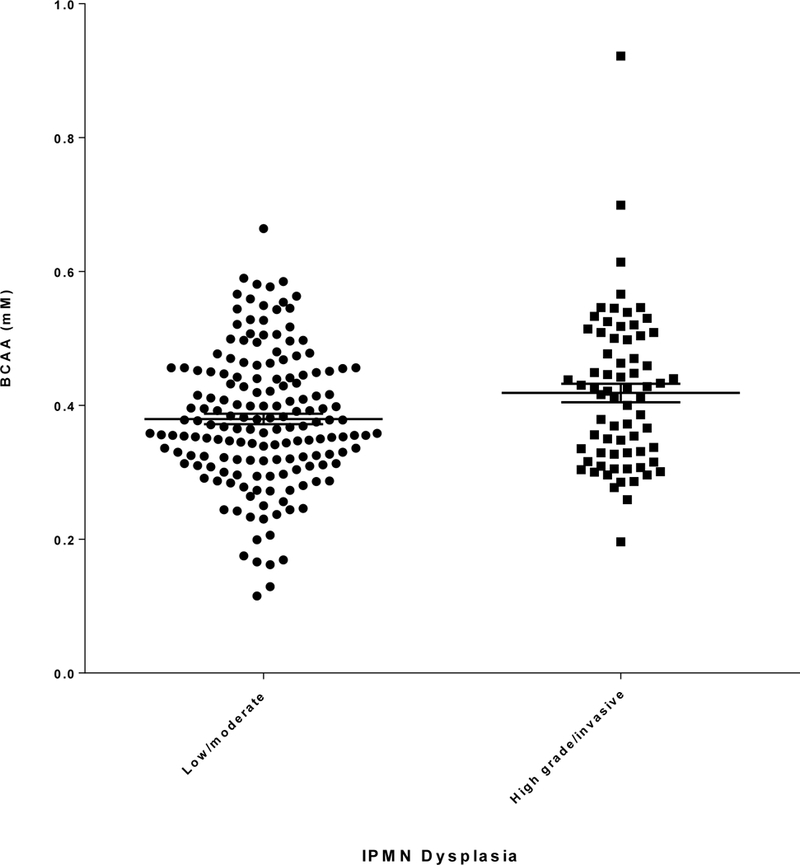
BCAA levels (mM; mean ± SE, horizontal black lines) determined by ELISA in plasma/serum collected from patients with low/moderate or high grade/invasive IPMN.
To assess the performance of combined leptin/BCAA as an indicator of IPMN dysplasia, ROC analysis was performed and revealed an AUC of 0.61 (Table 3). We also determined the performance of two relevant clinical markers, serum CA19–9 level and main pancreatic duct (MPD) diameter, which demonstrated individual AUCs of 0.62 and 0.75 respectively. Combining leptin/BCAA with either CA19–9 or MPD diameter resulted in improved AUCs of 0.65 or 0.77 respectively. An IPMN risk prediction model consisting of leptin, BCAA and both clinical markers exhibited the highest AUC of 0.81.
Table 3.
Predictive accuracy of Ieptin, BCAA, CA 19–9 and MPD diameter for IPMN dysplasia (high grade/invasive IPMN vs low/moderate grade IPMN)
| Prediction Models | N | AUCa | 95% CI |
|---|---|---|---|
| Lepti n, BCAA | 235 | 0.61 | 0.53 — 0.69 |
| CA19–9 (serum) | 204 | 0.62 | 0.53 — 0.71 |
| Main pancreatic duct (M PD) diameter | 217 | 0.75 | 0.69 — 0.82 |
| Leptin, BCAA, and CA19–9 | 204 | 0.65 | 0.57 — 0.73 |
| Leptin, BCAA, and M PD diameter | 217 | 0.77 | 0.70 — 0.83 |
| Leptin, BCAA, CA19–9, and M PD diameter | 187 | 0.81 | 0.74 — 0.87 |
Area Under the Receiver Operating Characteristic curve
Discussion:
Obesity, pre-diabetes, and type-2 diabetes are conditions that are commonly associated with insulin resistance, defined by an inadequate response in target tissues to normal circulating insulin levels [18]. Since insulin resistance has been associated with an elevated risk of pancreatic cancer, insulin resistance may also play a role in the malignant transformation of its precursor lesions [19, 12, 9]. In the present study, we investigated this clinically relevant question in 235 patients with a specific type of these precursor lesions, IPMN.
The insulin resistance biomarkers that were evaluated in this group of pathologically confirmed IPMN included leptin, BCAA, RBP-4, HbA1c, adiponectin, and c-peptide. We demonstrated that circulating leptin levels were significantly higher in low/moderate grade IPMN than in high grade/invasive IPMN. We further investigated the correlation between leptin and malignant progression by analyzing blood samples collected from patients diagnosed with pancreatic ductal adenocarcinoma and healthy controls. Although leptin level correlated with IPMN dysplastic grade specifically in females, this was not observed when the cancer and healthy groups were included in the analysis (data not shown). A significant correlation was only demonstrated when males and females were considered together, possibly due to the relatively small number of healthy controls that were available for this study. Nevertheless overall, our results revealed that mean circulating leptin levels were highest in healthy controls, intermediate in the low/mod IPMN group, and lowest in patients with high grade/invasive IPMN or pancreatic ductal adenocarcinoma. This stepwise decline suggests that leptin levels may reflect malignant progression from normal pancreatic tissue through precancerous lesions to invasive pancreatic cancer. In support, others have reported reduced plasma or serum leptin levels in pancreatic cancer patients compared to healthy controls or patients with type-2 diabetes [20, 21]. Adipose wasting accompanies muscle catabolism in cancer cachexia, and an association between lower leptin levels and cancer cachexia has also been described [22, 23]. To confirm whether circulating leptin is an indicator of IPMN malignant transformation, prospective cohort studies should be conducted in which leptin levels are determined in serial blood samples collected from patients under surveillance for presumed low risk IPMN, a percentage of whom will go on to develop high risk IPMN.
Our study also found that circulating BCAA levels were lower in low/moderate grade IPMN than in high grade/invasive IPMN. Significant differences in BCAA as well as leptin were observed between low and high risk IPMN in overweight (25<BMI<30) or obese (BMI>=30) subjects, respectively. In contrast, no differences in HbA1C, adiponectin or C-peptide were detected between the two risk groups. Interestingly, Mayers et al detected elevated plasma BCAA levels in pre-diagnostic pancreatic cancer samples, 2 to 5 years prior to diagnosis, compared to healthy controls [23]; in theory, these patients harbor a precursor lesion such as IPMN at some stage along the continuum of malignant transformation. The authors propose that the elevated plasma BCAA may be due to increased tissue protein breakdown during the early stages of PDAC development.
To our knowledge, this is the first report that circulating levels of leptin and BCAA are associated with dysplastic grade of IPMN. This represents an extension of our research work in this area as our laboratory has previously identified and validated a biomarker of IPMN dysplasia in pancreatic cyst fluid - prostaglandin E2 [24, 25]. A major advantage of blood-based biomarkers is that blood collection is relatively less invasive and more cost-effective compared with endoscopic ultrasound-guided fine needle aspiration (FNA) of IPMN for procurement of cyst fluid. An active search for biomarker candidates associated with IPMN malignant risk is ongoing at the DNA level as well in pancreatic cyst fluid and blood [26]. Other recently reported blood-based biomarkers of IPMN dysplasia include microRNAs (mi-RNA) and MUC5AC [27, 28]; however, these results need to be validated as they were obtained from a small number of patients (n<43 for both studies).
Since circulating levels of leptin and BCAA are likely influenced by individual body composition and metabolism, we hypothesize that a longitudinal comparison of these biomarkers in an individual patient may more accurately reflect their temporal change in response to dysplastic progression. These dynamic changes in an individual, rather than absolute cutoff values, may hold the most promise as reliable predictors of IPMN malignant transformation. Limitations of the present study include its cross-sectional nature and reliance on blood samples from a single institution. Further validation of our findings in a larger, prospective multi-institution study is warranted. Moreover, all study patients underwent surgical resection and therefore may not be representative of the overall population of both surveillance and surgical patients who might benefit from such testing. With respect to patients in the present study, the indications for surgery were largely based upon criteria outlined in the 2006 and subsequent 2012 Consensus Guidelines. Some patients resected earlier in our series would not be resected now because large branch-duct IPMN without other worrisome features are more likely to be followed based upon the current guidelines.
The work presented here does not support the use of the identified biomarkers as a stand-alone test at this stage. Therefore, we evaluated the diagnostic performance of leptin/BCAA together with relevant clinical characteristics, specifically serum CA19–9 and main pancreatic duct (MPD) diameter. CA19–9 is a known serum tumor marker associated with pancreatic cancer that has been shown to differentiate between invasive and benign IPMN [29]. Our laboratory has also recently reported that serum CA19–9 is a predictor of invasive progression in main-duct involved IPMN [30]. MPD diameter is another established risk factor in the 2012 International Consensus Guidelines [5]. We report an AUC of 0.81 for the IPMN risk prediction model consisting of leptin/BCAA, serum CA19–9, and MPD diameter.
Conclusion:
In conclusion, we have identified circulating leptin in females and BCAA as promising non-invasive biomarkers of malignant IPMN. We anticipate that longitudinal monitoring of these biomarkers in combination with clinical features can increase the accuracy of the preoperative risk stratification of patients with IPMN. Such a strategy will improve clinical management by limiting pancreatic resection in low risk IPMN and promoting timely resection of high risk IPMN in fit patients.
Acknowledgments
Support: This study was supported by the National Institutes of Health Grant #1R21CA209366–01, Indiana Clinical and Translational Sciences Institute funded in part by Grant #UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and National Center for Research Resources Construction Grant #RR020128.
Abbreviations:
- (IPMN)
intraductal papillary mucinous neoplasm
- (BCAA)
branched chain amino acids
- (RBP-4)
retinol-binding protein-4
- (ELISA)
enzyme-linked immunosorbent assay
- (PDAC)
pancreatic ductal adenocarcinoma
Footnotes
Conflict of Interest: None declared.
References
- 1.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. Accessed March 20, 2018. [Google Scholar]
- 2.Harinck F, Poley JW, Kluijt I, Fockens P, Bruno MJ. Is early diagnosis of pancreatic cancer fiction? Surveillance of individuals at high risk for pancreatic cancer. Dig Dis 2010;28:670–678. [DOI] [PubMed] [Google Scholar]
- 3.Pitman MB, Lewandrowski K, Shen J, Sahani D, Brugge W, Fernandez-del Castillo C. Pancreatic cysts: preoperative diagnosis and clinical management. Cancer Cytopathol 2010;118:1–13. [DOI] [PubMed] [Google Scholar]
- 4.Sheehan MK, Beck K, Pickleman J, Aranha GV. Spectrum of cystic neoplasms of the pancreas and their surgical management. Arch Surg 2003;138:657–660; discussion 660–652. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–197. [DOI] [PubMed] [Google Scholar]
- 6.Tang RS, Weinberg B, Dawson DW, Reber H, Hines OJ, Tomlinson JS, Chaudhari V, Raman S, Farrell JJ. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol 2008;6:815–819; quiz 719. [DOI] [PubMed] [Google Scholar]
- 7.Jang JY, Park T, Lee S, Kang MJ, Lee SY, Lee KB, Chang YR, Kim SW. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg 2014;101:686–692. [DOI] [PubMed] [Google Scholar]
- 8.Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res 2012;2012:789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, Albanes D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872–2878. [DOI] [PubMed] [Google Scholar]
- 10.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–929. [DOI] [PubMed] [Google Scholar]
- 11.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194–202. [DOI] [PubMed] [Google Scholar]
- 13.Nowak C, Sundstrom J, Gustafsson S, Giedraitis V, Lind L, Ingelsson E, Fall T. Protein Biomarkers for Insulin Resistance and Type 2 Diabetes Risk in Two Large Community Cohorts. Diabetes 2016;65:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paz-Filho G, Mastronardi C, Wong ML, Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J Endocrinol Metab 2012;16:S549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon MS. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016;8(7): 405. doi: 10.3390/nu8070405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Han Q, Liu Y, Sun C, Gang X, Wang G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. Journal Diabetes Res 2016;2016:2794591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–362. [DOI] [PubMed] [Google Scholar]
- 18.Korc M Update on diabetes mellitus. Dis Markers 2004;20:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res 1996;63:310–313. [DOI] [PubMed] [Google Scholar]
- 20.Krechler T, Zeman M, Vecka M, Macasek J, Jachymova M, Zima T, Zak A. Leptin and adiponectin in pancreatic cancer: connection with diabetes mellitus. Neoplasma 2011;58:58–64. [DOI] [PubMed] [Google Scholar]
- 21.Gasiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Malecka-Panas E. Role of adipocytokines and its correlation with endocrine pancreatic function in patients with pancreatic cancer. Pancreatology 2013;13:409–414. [DOI] [PubMed] [Google Scholar]
- 22.Mondello P, Lacquaniti A, Mondello S, Bolignano D, Pitini V, Aloisi C, Buemi M. Emerging markers of cachexia predict survival in cancer patients. BMC Cancer 2014;14:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, Davidson SM, Papagiannakopoulos T, Yang A, Dayton TL, Ogino S, Stampfer MJ, Giovannucci EL, Qian ZR, Rubinson DA, Ma J, Sesso HD, Gaziano JM, Cochrane BB, Liu S, Wactawski-Wende J, Manson JE, Pollak MN, Kimmelman AC, Souza A, Pierce K, Wang TJ, Gerszten RE, Fuchs CS, Heiden MGV, Wolpin BM. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 2014;20:1193-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt CM, Yip-Schneider MT, Ralstin MC, Wentz S, DeWitt J, Sherman S, Howard TJ, McHenry L, Dutkevitch S, Goggins M, Nakeeb A, Lillemoe KD. PGE(2) in pancreatic cyst fluid helps differentiate IPMN from MCN and predict IPMN dysplasia. J Gastrointest Surg 2008;12:243–249. [DOI] [PubMed] [Google Scholar]
- 25.Yip-Schneider MT, Carr RA, Wu H, Schmidt CM. Prostaglandin E2: A Pancreatic Fluid Biomarker of Intraductal Papillary Mucinous Neoplasm Dysplasia. J Am Coll Surg 2017;225:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moris D, Damaskos C, Spartalis E, Papalampros A, Vernadakis S, Dimitroulis D, Griniatsos J, Felekouras E, Nikiteas N. Updates and Critical Evaluation on Novel Biomarkers for the Malignant Progression of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Anticancer Res 2017;37:2185–2194. [DOI] [PubMed] [Google Scholar]
- 27.Permuth-Wey J, Chen DT, Fulp WJ, Yoder SJ, Zhang Y, Georgeades C, Husain K, Centeno BA, Magliocco AM, Coppola D, Malafa M. Plasma MicroRNAs as Novel Biomarkers for Patients with Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancer Prev Res (Phila) 2015;8:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maker AV, Katabi N, Gonen M, DeMatteo RP, D’Angelica MI, Fong Y, Jarnagin WR, Brennan MF, Allen PJ. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol 2011;18:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Zhang L, Chen L, Wei J, Sun Q, Xie Q, Zhou X, Zhou D, Huang P, Yang Q, Xie H, Zhou L, Zheng S. Serum carcinoembryonic antigen and carbohydrate antigen 19–9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Biomed Rep 2015;3:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roch AM, Ceppa EP, Al-Haddad MA, DeWitt JM, House MG, Zyromski NJ, Nakeeb A, Schmidt CM. The natural history of main duct-involved, mixed-type intraductal papillary mucinous neoplasm: parameters predictive of progression. Ann Surg 2014;260:680–688; discussion 688–690. [DOI] [PubMed] [Google Scholar]


