Abstract
Background:
Classification of the pulmonary neuroendocrine tumor (pNET) categories is a step-wise process identified by the presence of necrosis and number of mitoses per 2 mm2. In neuroendocrine tumor pathology, Ki-67 was first described as a prognostic factor in the pancreas and incorporated into the grading system of digestive tract neuroendocrine neoplasms in the 2010 WHO classification. However, the significance of Ki-67 in pNETs was still a controversial issue. This study was to investigate the potentially diagnostic value of Ki-67 in pNETs.
Methods:
We retrieved 159 surgical specimens of pNETs, including 35 typical carcinoids (TCs), 2 atypical carcinoid (ACs), 28 large-cell neuroendocrine carcinomas (LCNECs), 94 small-cell lung cancers (SCLCs). Manual conventional method (MCM) and computer-assisted image analysis method (CIAM) were used to calculate the Ki-67 proliferative index. In CIAM, 6 equivalent fields (500 × 500 μm) at 10× magnification were manually annotated for digital image analysis.
Results:
The Ki-67 index among the 4 groups with ranges of 0.38% to 12.66% for TC, 4.34% to 29.48% for AC, 30.67% to 93.74% for LCNEC, and 40.71% to 96.87% for SCLC. The cutoff value of Ki-67 index to distinguish low grade with high grade was 30.07%. For the univariate survival analyses in pNETs, both the overall survival and progression-free survival correlated with Ki-67 index. In addition, the Ki-67 index performed by CIAM was proved to be of great positive correlation with MCM.
Conclusions:
Ki-67 index counted by CIAM is a reliable method and can be a useful adjunct to classify the low- and high-grade NETs.
Keywords: Antigen Ki-67, Computer-assisted numerical analysis, Neuroendocrine tumor, Prognostic factor
Introduction
Neuroendocrine tumors of lung account for 20% to 25% of all invasive lung malignancies.[1] Small-cell lung cancer (SCLC) is the most aggressive tumor of the malignant neuroendocrine tumors, which represents the highest incidence of 15% to 20%, followed by large-cell neuroendocrine carcinoma (LCNEC) (3%), typical carcinoid (TC) (1–2%), and atypical carcinoid (AC) tumors (0.1–0.2%).[1] The distinction of these 4 different subtypes is mainly based on morphologic features, mitotic index, and presence or absence necrosis from 2015 WHO classification of lung.[2] Accordingly, high-grade SCLC and LCNEC are differentiated from intermediate-grade AC and low-grade TC. However, there exist a gray zone between AC and LCNEC which showed that high-grade neuroendocrine carcinomas with carcinoid morphology, but with mitotic figure >10/mm2.[3] Moreover, in biopsied samples, SCLC may be confused with TC sometimes.[4] Thus, an urgent strategy was needed to resolve the present situation.
Recently, there is much interest in evaluating proliferation markers particularly Ki-67 in neuroendocrine tumors arising from various sites to identify high-risk subsets that may behave more aggressively. Several studies have shown a correlation between high Ki-67 and poorer prognosis.[5–8] In neuroendocrine tumor pathology, Ki-67 was first clinically investigated as a diagnostic factor in the pancreas.[9] However, the value of Ki-67 in pulmonary neuroendocrine tumors (pNETs) remained a debated problem.[10]
In SCLC, the proliferative index (PI) as evaluated by Ki-67 immunohistochemistry is >50%, and the averaging ≥80%. In LCNEC, the PI ranges from 40% to 80%. In carcinoid tumors, low (<20%) Ki-67 labeling index was confirmed.[4] Accordingly, the guideline of Ki-67 proliferation rates are given for the first time in the fourth edition of the WHO classification of tumors of the lungs for SCLC as 50% to 100%, for LCNEC 40% to 80%, for AC up to 20% and for TC up to 5%.[11] However, the utility of Ki-67 to discriminate TC from AC of predict prognosis (with cutoff values ranging from 2.5% to 5.8%) within individual carcinoid tumors categories is not established.[12] In contrast, high Ki-67 score may help distinguish SCLC and LCNEC from pulmonary carcinoids, especially in small crushed biopsies,[4] while staining for Ki-67 still not part of the diagnostic criteria.
Because it is impractical to count positive nuclear cells manually for routine diagnostic purposes in calculating Ki-67 index, and visual estimates are subjective and dependent on observer experience. Computer-assisted image analysis method (CIAM) is becoming increasingly commonplace for the quantitation of biomarkers in formalin-fixed, paraffin-embedded tissue. The use of image analysis software to measure the nuclear staining index of Ki-67 in lymphomas has been examined.[13] Meanwhile, there exist studies which were focused on the measurement of PI and found that automated Ki-67 counts were similar to manual counts.[8]
The aim of this study was to evaluate the usefulness of Ki-67 index as well as traditional parameters, such as mitoses and necrosis for predicting prognosis in pNETs. In addition, the objective of the study was to attempt to separate the subtypes by using Ki-67 index and to develop a systematic counting method for Ki-67 estimation in pNETs and evaluate the accuracy of CIAM for calculating Ki-67 index. We hypothesized that Ki-67 antigen might be an important parameter for identifying high-risk subsets of patients and might be a useful adjuvant index to classify the pathologic grade in pNETs.
Methods
Study population
A search of electronic pathology database with the key words “carcinoid,” “small-cell lung cancer,” and “large-cell neuroendocrine carcinoma” of lung tumors. The study included resection specimens of tumor with or without lymph node dissection. A total of 159 cases (35 TCs, 2 ACs, 28 LCNECs, 94 SCLCs) originally diagnosed as neuroendocrine tumors of lung at Peking Cancer Hospital (Peking, China) from January 2010 to December 2015 were enrolled in our research. Patients’ consents have been obtained from all the participants before data collection.
Histologic and pathologic classification
All cases were reviewed by 2 experienced pathologists (ZW Li and DM Lin) to confirm the diagnosis based on the current WHO criteria for lung neuroendocrine tumors. TC was defined as well-differentiated neuroendocrine tumor with 0 to 1 mitoses per 2 mm2 and without necrosis. AC was classified as intermediate-grade neuroendocrine tumor and distinguished by 2 to 10 mitoses per 2 mm2 and/or focal necrosis. LCNEC and SCLC had >10 mitotic figures in 2 mm2, usually with large areas of necrosis; showed neuroendocrine morphology (nuclear palisading with nests, rosette-like, or ribbons of cells).
Immunohistochemistry
All the tumors showed immunohistochemical evidence of neuroendocrine differentiation (diffuse staining for either synaptophysin, chromogranin A, CD56, with or without MAP2). Ki-67 was applied to calculate the proliferation index. Immunohistochemical staining was performed using an automated immunostainer (Link 48; Dako, Carpinteria, CA, USA) for synaptophysin, CD56, and Ki-67. Chromogranin A was performed on an autostainer (BOND-MAX, LEICA; Leica Biosystems Newcastle Ltd., Newcastle, UK), while MAP2 was performed on a Ventana BenchMark ULTRA autostainer (Ventana Medical Systems, Oro Valley, AZ, USA). The following neuroendocrine and proliferative immunohistochemical markers were used: monoclonal mouse anti-human synaptophysin (clone DAK-SYNAP; Dako, Glostrup, Denmark), monoclonal rabbit anti-human chromogranin A (clone EP38; Zhongshan Golden Bridge Biotechnology, Beijing, China), monoclonal mouse anti-human CD56 (Clone 123C3; Dako), monoclonal mouse anti-human MAP2 (Clone AP18; Zhongshan Golden Bridge Biotechnology), and monoclonal mouse anti-human Ki-67 (clone MIB-1; Dako). The tumor staging was obtained from the 8th AJCC guideline.
Ki-67 PI by 2 methodologies: manual conventional method and CIAM
Quantification of Ki-67 expression has been accomplished on surgical resection specimens by manual conventional method (MCM) and a nuclear quantitation algorithm performed by CIAM, respectively. In the literature, the term hot spot has been applied to indicate tumor areas with the highest concentration of nuclear decoration for Ki-67. CIAM was performed on a validated nuclear algorithm (Aperio; Leica Biosystems, Buffalo Grove, IL, USA), where slides were scanned at 20× magnification using the Aperio Scanscope Console and the images were analyzed using ImageScope Nuclear v9 algorithm software that reports the total number of nuclei counted and the percentage of positive cell nuclei. In our study, digital image analysis was executed by manually annotating at least 6 representative equivalent (500 × 500 μm) non-necrotic tumor fields at 10× magnification which together contained at least total 2000 cells (or all tumor cells if <2000) in which automated analysis was to be performed. Positive staining was defined as faint nuclear positivity or greater (1+). Care was taken to exclude fields containing substantial numbers of non-tumor cells, such as endothelial cells and intratumoral lymphocytes by visual review of the artificially selected digital images and the corresponding hemotoxylin-and-eosin-stained scanned slide.
The mitotic index
The mitotic activity was manually quantitated in the most cellular areas on whole-slide images; eight 40× high power fields were calibrated as 2 mm2. The mitotic index was performed according to the method recommended in the 8th edition of the AJCC Cancer Staging Manual: enumerate mitoses in the most mitotically active area (“hot spot”) and then extend mitotic index to adjacent contiguous fields. If no mitotic activity is evident, random representative tumor fields were chosen.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 version statistical software (IBM Corporation, Armonk, NY, USA). The relationship between Ki-67 index expression and clinicopathologic parameters was performed using the Pearson Chi-squared and Fisher exact test. The receiver operating characteristic (ROC) curve analysis and Youden index were used to discriminate the appropriate cutoff value for pNETs. Overall survival was calculated from the date of pathologic diagnosis to time of death or last follow-up, and progression-free survival was calculated from date of pathologic diagnosis to time of last clinical evidence of recurrence, progression, or death. Overall survival curves and significance of survival and progression-free survival distributions were generated using Kaplan-Meier and Cox regression method. The correlations between the 2 Ki-67 methodologies (MCM and CIAM) were completed using the Pearson correlation coefficient. The 2-sided P value < 0.05 was considered statistically significant, and r value > 0.8 is considered as strong correlation.
Results
Patient clinicopathologic characteristics
The clinical and pathologic features of 159 patients diagnosed as pNETs are described in Tables 1 and 2. The pathologic patterns including TC, AC, LCNEC, and SCLC were represented. The median age was 59.5 years (range, 30–83). The cases included 109 males (68.6%) and 50 females (31.4%). A small minority (5.7%) had received neoadjuvant chemotherapy before surgery while 150 cases did not (see Table 3, which showed the characteristics of 9 patients who have received chemotherapy). The tumors from the 159 patients were classified as early stage (n = 127, 88.2%) and advanced stage (n = 17, 11.8%), which contained IA1 8, IA2 22, IA3 22, IB 14, IIA 4, IIB 27, IIIA 30, and IIIB 17. Among the 159 patients, lymph node metastasis was detected in 63 patients (43.2%). The median mitotic and Ki-67 index for TC, AC, LCNEC, and SCLC were 1 and 3.61%, 3.5 and 16.91%, 58 and 57.62%, 75 and 79.66%, respectively. The Ki-67 index by MCM and CIAM among the 4 groups with ranges of 1% to 15%, 0.38% to 12.66% for TC, 2% to 25%, 4.34% to 29.48% for AC, 30% to 85%, 30.67% to 93.74% for LCNEC, and 35% to 90%, 40.71% to 96.87% for SCLC. The mitotic index of the 4 groups with ranges of 0 to 1 for TC, 1 to 6 for AC, 11 to 138 for LCNEC, and 26 to 146 for SCLC. Approximately half of the 159 cases with tumor necrosis (n = 74, 46.5%). The majority of the tumors have no vascular invasion (n = 129, 81.1%). The morphologic and immunohistochemical characteristics of 4 tumor subtypes of pNETs are shown in Figures 1–4.
Table 1.
Relationship between Ki-67 index expression and clinicopathologic parameters, n (%).
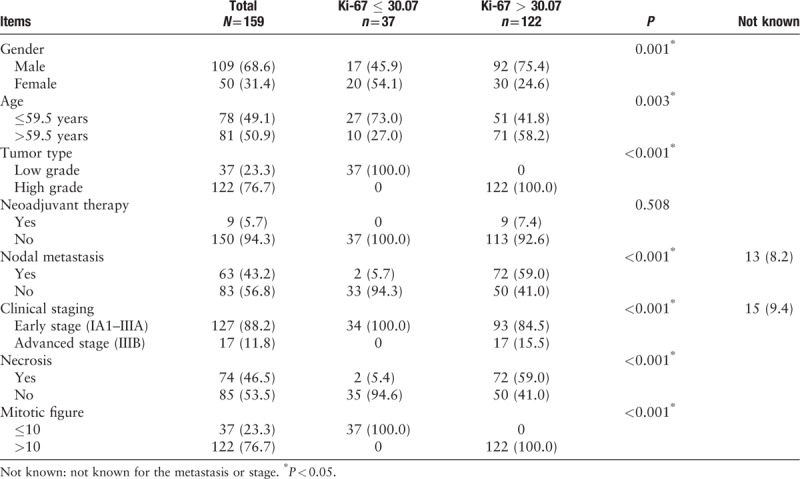
Table 2.
Pathologic characteristics in the 4 subtypes.
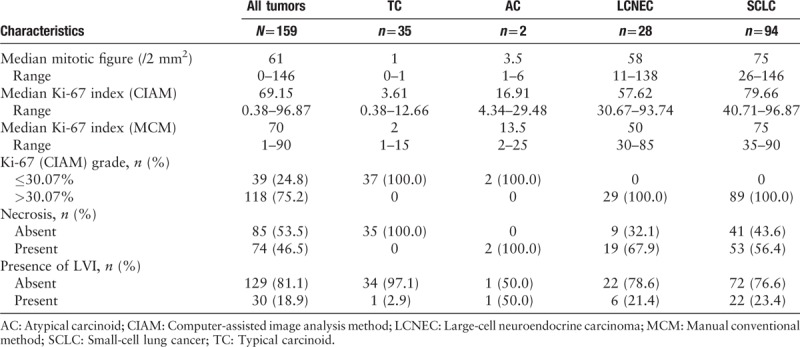
Table 3.
Characteristics of the 9 patients who have received chemotherapy.
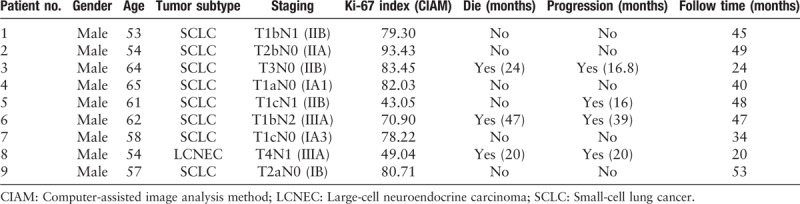
Figure 1.
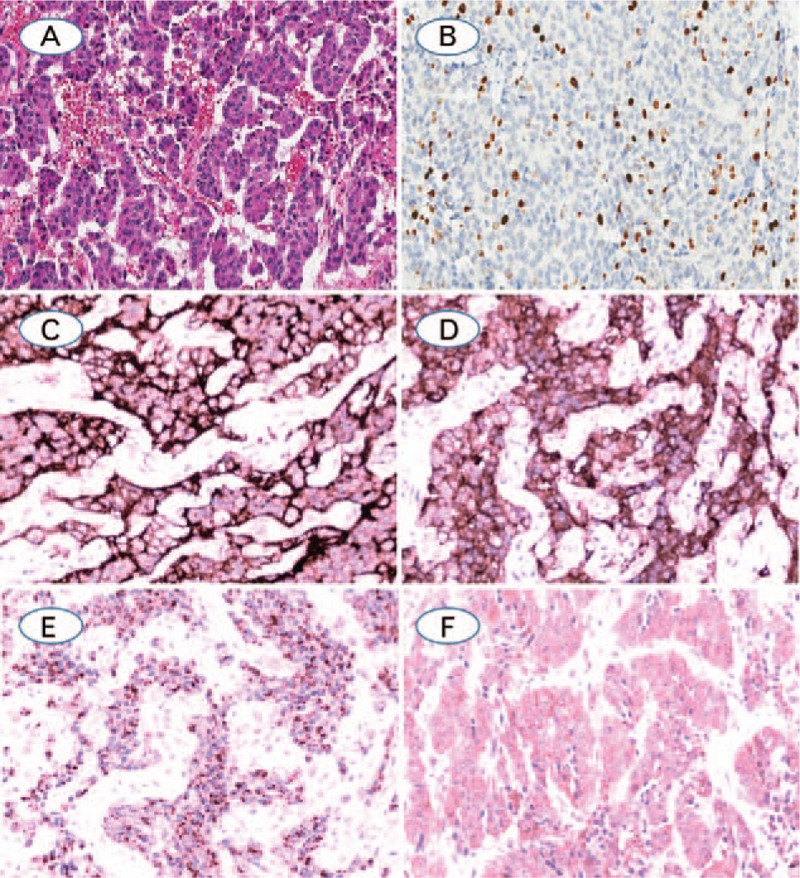
(A) Typical carcinoid, with trabecular pattern with delicate intervening vascular stroma, no mitoses or necrosis are identified; (B) Ki-67, showing 12.66% positivity by computer image analysis and 15% positivity by manual counting in the hot spot; (C–E). TC showed diffuse positive expression for CD56, synaptophysin, CgA and MAP2, respectively (A: Hematoxylin-eosin staining; B–E: Immunohistochemistry staining. The original magnification of A, B was 100×, magnification for remaining cases were 200×).
Figure 4.
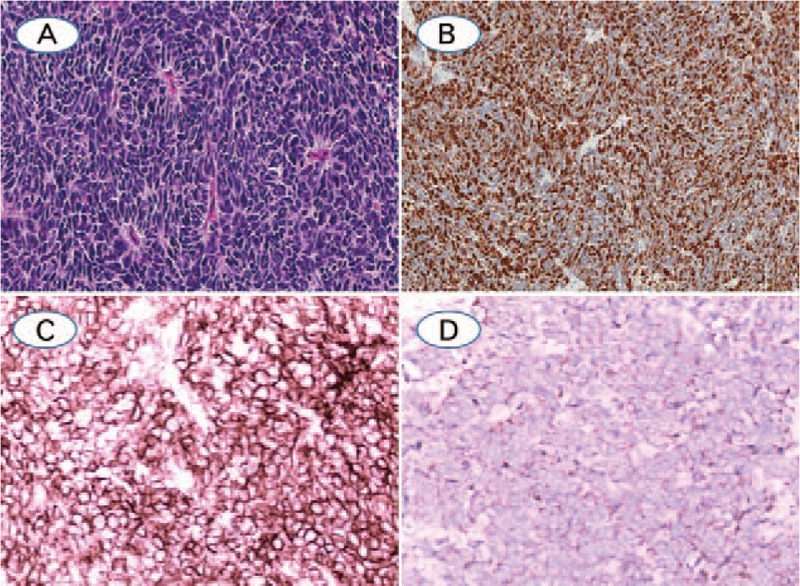
(A) SCLC with rosettes growth pattern and high mitotic count, with malignant small cells with scant cytoplasm and defined cell borders, fine granular nuclear chromatin, absence or inconspicuous nucleoli, necrosis was not showed; (B) Ki-67, showing 64.92% positivity by digital image analysis and 75% positivity by manual counting; (C) CD56 was diffuse positive expression in membrane; (D) The neuoroendocrine marker of Synaptophysin was weak to moderately positive in cytoplasm (A: Hematoxylin-eosin staining; B–D: Immunohistochemistry staining. The original magnification of A, B was 100×, magnification for remaining cases were 200×).
Figure 2.
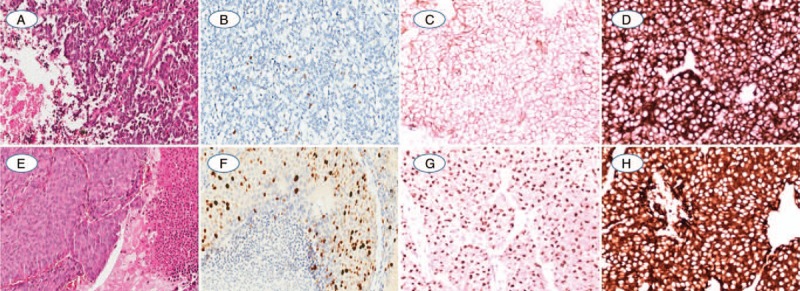
(A) One AC showed vascularized stroma and spotty necrosis in the peripheral tumor clusters; (B) The Ki-67 index was evaluated as 4.34% positivity by CIAM and 2% positivity by MCM; (C, D). AC was diffuse strong positive expression of CD56 and synaptophysin; (E) Another AC with kindly cell morphology, focal necrosis, and 6 mitosis per mm2; (F) Ki-67 index was counted as 29.48% by CIAM and 25% by MCM with weak to strong intensity; (G, H). The neuroendocrine marker of CgA and Synaptophysin were diffuse positive expression in cytoplasm (A and E: Hematoxylin-eosin staining; B–D and F–H: Immunohistochemistry staining. The original magnification of A, B and E, F was 100×, magnification for remaining cases were 200×).
Figure 3.
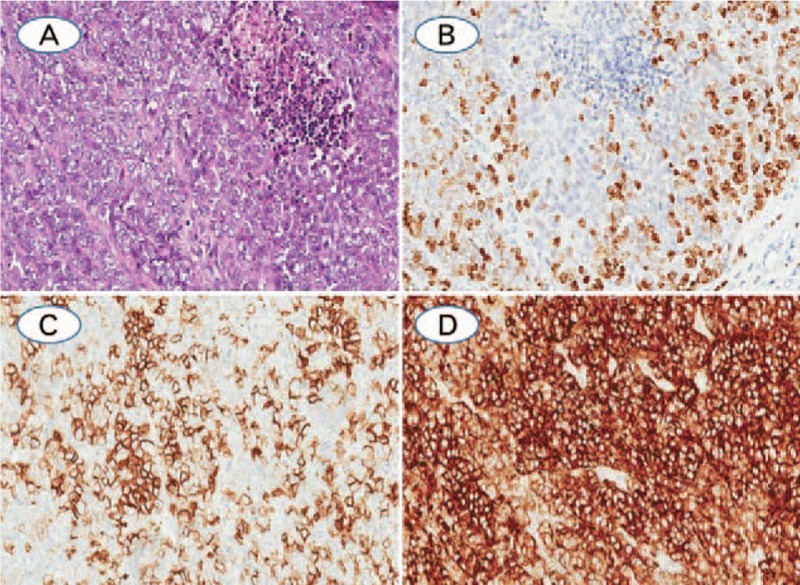
(A) Large cell neuroendocrine carcinoma with solid patterns, and the tumor cells are large, abundant cytoplasma, prominent nucleoli, and necrosis was observed; (B) Ki-67 index, which was evaluated as 54.48% by CIAM and 40% by MCM; (C) LCNEC was positive staining with the neuroendocrine markers of CD56; (D) Synaptophysin was diffuse positive expression in the cytoplasm (A: Hematoxylin-eosin staining; B–D: Immunohistochemistry staining. The original magnification of all cases were 200×).
Threshold for pNETs, and the relationship between Ki-67 index expression and clinicopathologic parameters
The ROC curve analysis and Youden index found that 30.07% of automated Ki-67 index be the adequate cutoff for pNETs [Figure 5]. Correlations between Ki-67 index expression and clinicopathologic parameters in pNETs are described in detail in Table 1. The expression of Ki-67 index was significantly associated with male patients (P = 0.001), elderly patients (P = 0.003), high histologic grade (P < 0.001), presence of nodal metastasis (P < 0.001), advanced clinical staging (P < 0.001), higher mitotic figure (P < 0.001), and presence of necrosis (P < 0.001), while no significant association between Ki-67 index and prior neoadjuvant therapy was observed (P = 0.508).
Figure 5.
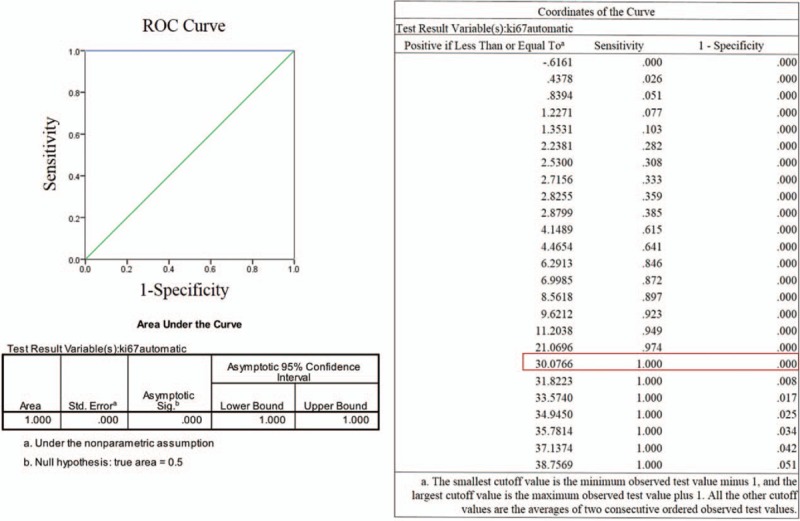
The ROC curve to identify the threshold for pNETs.
Correlation and discrepancy between the 2 methodologies: MCM and CIAM
We used both MCM and CIAM for counting Ki-67 index. The median and ranges of Ki-67 index by MCM and CIAM for TC, AC, LCNEC, and SCLC are described in detail in Table 2. The discrepancy of the results by 2 methodologies (MCM and CIAM) can be classified as 4 groups: ≤5%, from 6% to 10%, from 11% to 15%, and greater than 15% (>15%). More than half (57.2%) of the discrepancy between the two methods was within 5% [Figure 6]. Moreover, along with the difference value increased, the percentage of cases was decreased. In addition, the calculating methods of Ki-67 by CIAM were slightly higher than MCM in most cases (77.4%; 123/159). The detailed data are shown in Table 4.
Figure 6.
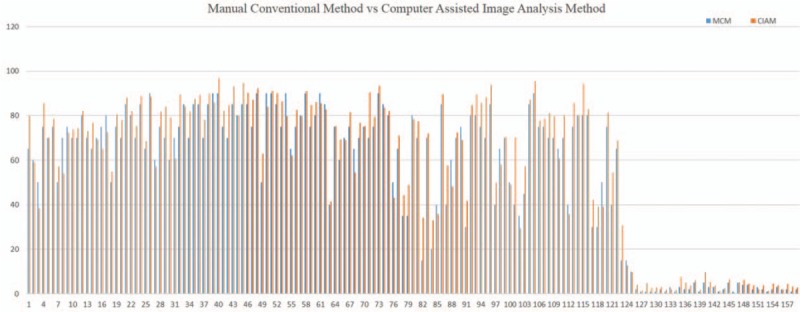
The detailed Ki-67 index of manual conventional method and computer-assisted image analysis method of all 159 pNETs.
Table 4.
The 2 methodologies (MCM and CIAM) by calculating Ki-67 index, n (%).
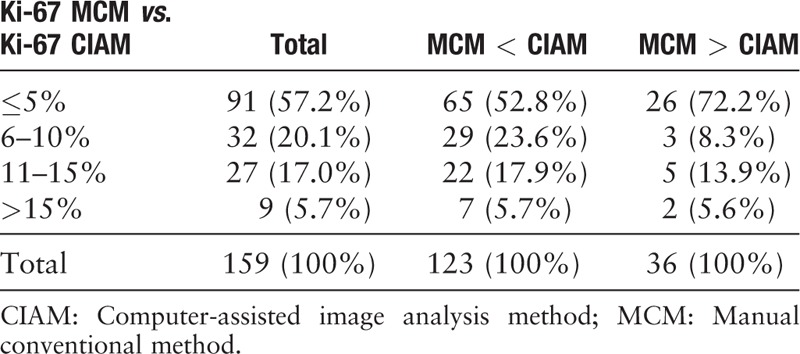
We found that there was a strong positive relationship between these 2 counting methods (r = 0.975, P = 0.000) [Table 5]. Moreover, a lineage relationship was described by the Scatter Diagram method [Figure 7], which indicated that the 2 methodologies showed no difference in calculating Ki-67 index.
Table 5.
The correlation between 3 Ki-67 index calculation methods.
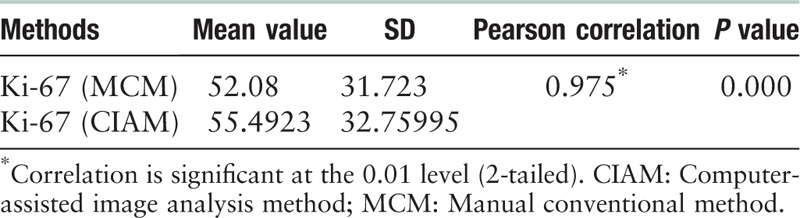
Figure 7.
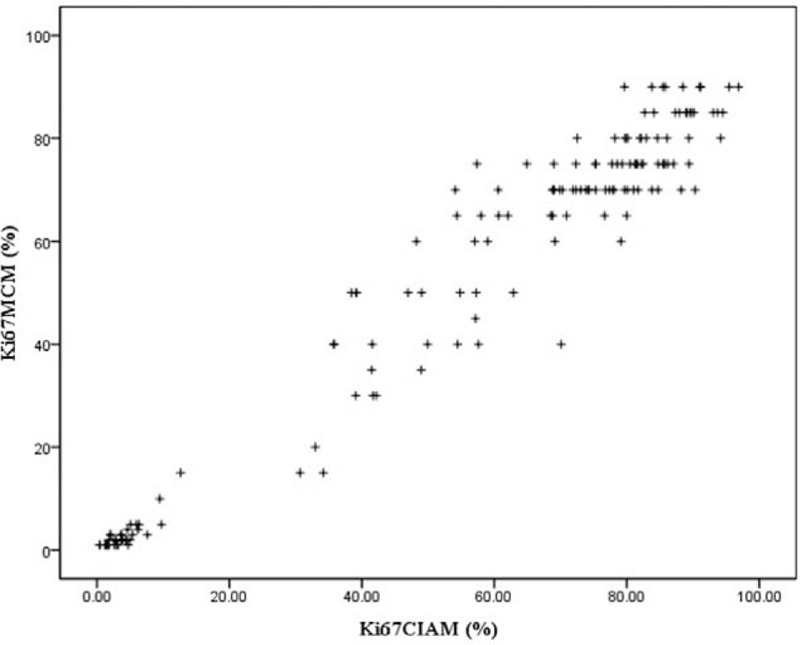
This plot diagram illustrates a linear relationship between computer image analysis and manual method for calculating Ki-67 index.
Association of Ki-67 expression with OS and PFS
First of all, we classified the tumor pathologic staging as 2 groups: early stage (IA1–IIIA) and advanced stage (IIIB) pNETs. Meanwhile, we confirmed that the early stage pNETs have an improved OS (P = 0.000) and PFS (P = 0.000) than advanced stage pNETs. Additionally, we identified the SCLC has the worst prognosis than LCNEC and low-grade NETs [Figure 8].
Figure 8.
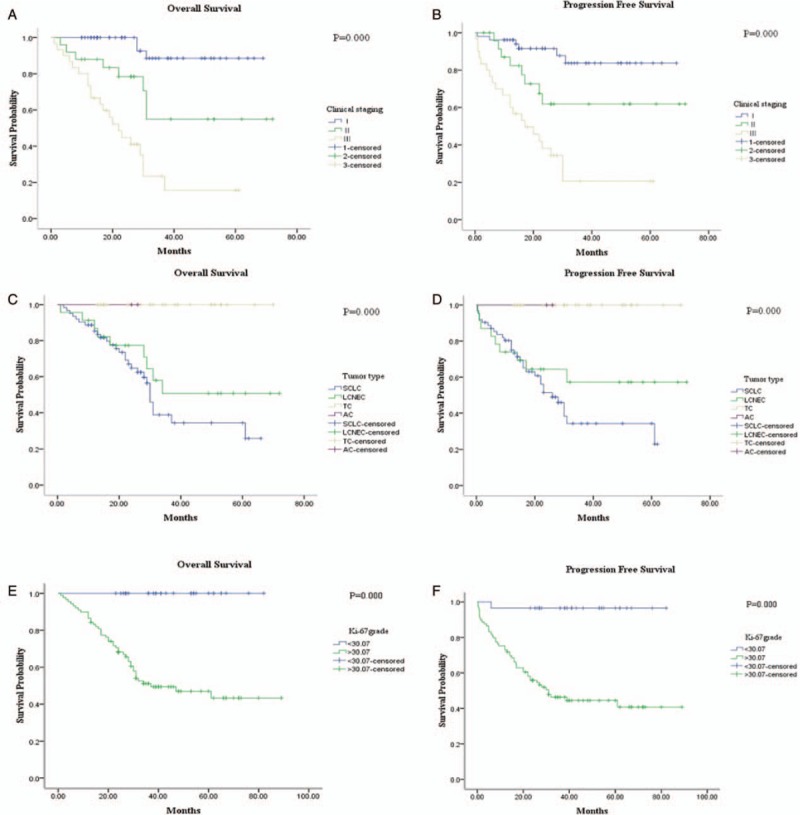
The relationship between OS and PFS with clinical staging were performed by the univiarate analysis. The early stage pNETs showed (A) better OS (P = 0.000) and (B) PFS (P = 0.000) with advanced cases. The Ki-67 index was classified as two groups by CAIM: ≤ 30.07% and > 30.07%. SCLC and LCNEC presented with worse OS (C) and PFS (D) compared with low grade NETs. The Ki-67 value ≤ 30.07% showed a (E) better OS (P = 0.000) and (F) better PFS (P = 0.000) than Ki-67 index > 30.07%.
In the univariate survival analyses, Ki-67 index is increased by 10% to carry out survival analysis. Then, we found that the presence of low Ki-67 proliferation index proved to be a better prognostic factor for OS and PFS. Moreover, the threshold of 30.07% also showed prognostic significance [Figure 8]. As the traditional grading criterion of pNETs, the higher mitotic index and presence of necrosis was associated with poorer OS and PFS (figure not shown). In addition, we analyzed the impact of chemotherapy on both OS (P = 0.676) and PFS (P = 0.922), while no correlation was observed between chemotherapy and survival status [Figure 9]. Meanwhile, the survival analysis of the 9 patients who have received chemotherapy was also performed. In this group, the cutoff value of Ki-67 index was set as 74.56% by using the receiver operating characteristic (ROC) curve analysis and Youden index method. We found that a higher Ki-67 index was correlated with better PFS (P = 0.024), and had a trend with improved OS (P = 0.149) [Figure 9].
Figure 9.
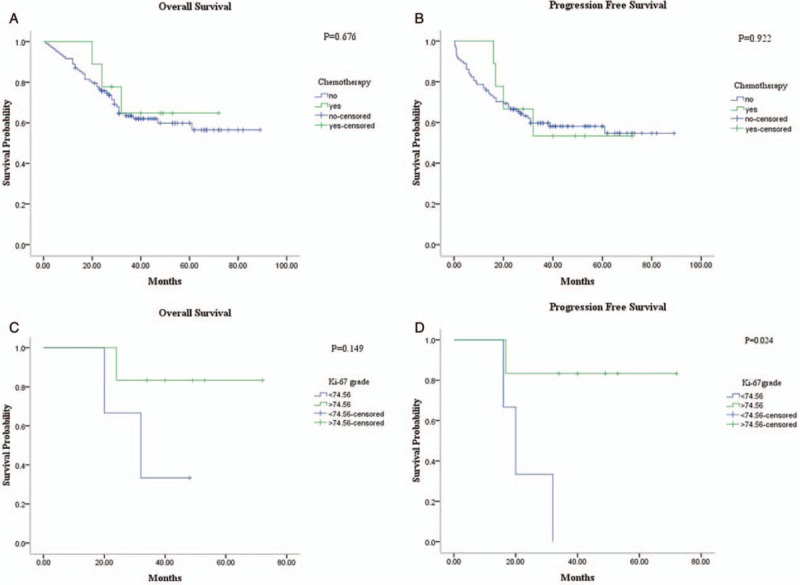
Univiarate analysis for the patients who have received chemotherapy before surgery. The survival curve showed that chemotherapy have no correlation with (A) OS (P = 0.676) and (B) PFS (P = 0.922). In addition, we also performed the relationship between Ki-67 index and OS and PFS in the 9 patients treated with chemotherapy. A higher Ki-67 index had a trend with (C) better OS (P = 0.149) and correlated with (D) improved PFS (P = 0.024).
The Cox proportional hazards model was also performed; since Ki-67 index and mitotic figure led to coefficients not converged, age, gender, necrosis, the status of lymph node, and clinical staging were included. Multivariate analysis revealed that the early pathologic staging was an independent prognostic factor for better OS (hazard ratio [HR] = 0.099; 95% confidence interval [CI], 0.040–0.247; P = 0.000) and PFS (HR = 0.091; 95% CI, 0.016–0.508; P = 0.006).
Discussion
Among a variety of potential prognostic parameters analyzed in numerous of studies, PI has consistently stood out as having strong prognostic value, along with mitotic index. Ki-67 antigen, which plays an essential role in the control and timing of cell proliferation, has been demonstrated as a reliable marker of PI and has been applied in practice for more than 2 decades. Moreover, Ki-67 index and mitotic count can divided pancreatic neuroendocrine tumors (PanNETs) into 3 tiers (G1, G2, and G3).[14] Therefore, Ki-67 antigen has become an essential part of classification of PanNETs.[15] In contrast, the guideline Ki-67 proliferation rates are given for the first time in the 4th edition of the WHO classification of tumors of the lungs for SCLC as 50–100%, for LCNEC 40–80%, for AC up to 20% and for TC up to 5%.[11] However, the Ki-67 index has not yet included for grading pNETs as in PanNETs. There is no consensus in separating pulmonary TC from AC, although reported ranges for TC are 2.30% to 4.15% and for AC are 9.0% to 17.8%.[12] In addition, there is no well-defined threshold to distinguish carcinoids from SCLC or LCNEC, though a wide range of cutoff values from 20% to 50% has been proposed. The average values of Ki-67 by different authors are presented in Table 6.[10,16–22] However, none of them have been widely accepted as clinically application.
Table 6.
Average values of Ki-67 (%) by different authors.
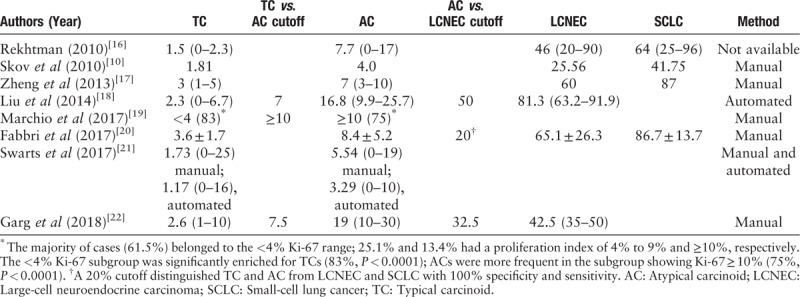
In our study, the Ki-67 index overlapped among the carcinoids, with ranges of 0.38% to 12.66% (median value of 3.61%) for TC, 4.34% to 29.48% (median value 16.91%) for AC. Though only 2 cases confirmed as AC were included in our study, and the results were with extensive ranges (4.34–29.48%), the number was reasonable in our overall cases with the percentage of 1.3% (2/159).[1] Actually, before we confirm the histology type, there exist 7 cases which was misdiagnosed as AC. After carefully examined by 2 experienced subspeciality professors, only 2 cases maintained former diagnosis (the remaining 5 cases were corrected to LCNEC). The 2 cases showed varying degrees of focal necrosis and the mitotic index ranged from 1 to 6. Though the wide range of Ki-67 index in AC indicated that no adequate cutoff value was existed, and the behavior between TC and AC is distinctive in some extent, related research have demonstrated that their pathogenesis mechanism was crossed and the treatment strategies were similar.[23]
Additionally, AC and LCNEC may overlap to some extent in morphology and prognosis. For LCNEC, the mitotic figure >10 and <20 per 2 mm2, which was called LCNEC in gray zone.[3,24] In our study, LCNEC in a total of 2 cases with mitotic figure of >10 and <20, which is similar to that of AC in cellular morphology. At this time, Ki-67 can be a useful adjunct to distinguish the 2 types of tumor with the threshold of Ki-67 to be 30.07%, which might be the most important value in Ki-67 index. All of the 2 cases were survived. Then we supposed that the LCNEC in the gray zone, which may be reclassified as AC in the future. Currently, although a few studies suggest the category of G3 AC in the lung may exist,[3] these cases are not a validated entity and not recognized in the 2015 WHO classification.[25] Therefore, at present, more cases were collected and multicenter research setting has been established, including patients in which full data of follow-up are available.
The Ki-67 index was also overlapped in the poorly differentiated carcinomas, with ranges of 30.67% to 93.74% (median value of 57.62%) for LCNEC, and 35.71% to 96.87% (median value of 79.66%) for SCLC. As the SCLC and LCNEC shared similar therapeutic strategies, separation of the 2 subtypes was of limited clinical impact. Although no appropriate threshold of Ki-67 to separate AC from TC or LCNEC from SCLC was observed,[23] the Ki-67 value of 30.07% can be an indicator to separate low grade from high-grade pNETs. In the diagnosis of SCLC, the differentiation of this tumor from carcinoid tumors remains a challenge due to crush artifacts and poor tissue preservation in small bronchial biopsies.[26] At this time, careful evaluation of hematoxylin and eosin sections was the most important tool for the differential diagnosis. In addition, evaluation of tumor cell proliferation by Ki-67 labeling index was also as the most useful ancillary technique for the distinction.[4]
Inclusion of the Ki-67 index in WHO classification criteria is prognostically effective[27] and is widely used as a prognostic factor in a variety of tumors including breast,[5] oropharyngeal squamous cell carcinoma,[28] hematolymphoid,[6] and other malignancies.[7,29] In our study, we take Ki-67 index of 30.07% as the cutoff value in pNETs, which indicated that the cases with lower Ki-67 PI had a better OS and PFS. However, in multivariate analysis, Ki-67 index was not an independent prognostic value for both OS and PFS, while the lower pathologic TNM staging was demonstrated to be an independent factor with improved OS and PFS. In other words, adding Ki-67 index to conventional morphologic features does not increase the prognostic prediction to morphologic criteria significantly, which was consistent with previous researches.[21,30] In addition, chemotherapy showed no correlation with both OS and PFS. While in the group of 9 cases with chemotherapy, a higher Ki-67 index was associated with better PFS and had a trend with improved OS, which indicate that higher Ki-67 index might associated with tumor sensitivity. In this point, our study was consistent with previous research.[31]
The adaptation of Ki-67 into daily practice has been fraught with challenges related to the counting methodology (manual and automated Ki-67 evaluation). In PanNETs, the most accurate, reproducible, and practical method for counting Ki-67 was the camera-captured printed images of tumor hot spots, and had the highest inter-observer agreement.[32] Warth et al[8] had reported that the agreement of manual and automated Ki-67 evaluation was good and was further increased when more than 1 participant evaluated a given case. In our study, the 2 methods (MCM and CIAM) of counting Ki-67 index showed a positively lineage relationship and the discrepancy of 2 methods in individual cases was limited within 5% in more than half of the cases. However, it is important to note that Ki-67 index calculated by CIAM was slightly higher than that by MCM for most cases (77.4%), which was inconsistent with the research written by Joseph et al.[33] This can be illustrated by the MCM was a method using “eye balling,” which was fast but had poor reliability and reproducibility. Moreover, the interpretation criterion (especially for the positivity of 1+) was difficult to understand, which made the cases with weak positive usually to be neglected. In addition, pNETs may mix together with lymphocytes, stromal, or endothelial cells, which lead difficult to differentiate by using CIAM.[34,35] Despite of these, Ki-67 staining still clearly outperforms mitotic count with respect to inter-observer agreement in pulmonary carcinoids, with the latter having an unacceptable low performance status.[8]
Our study has some limitations. First, this study was a retrospective design with a relatively small sample size drawn from a single institution; therefore, there might have an inherent selection bias. Moreover, mature survival information was limited as the follow-up duration in our study was not long enough to fully evaluate 5-year survival rates. Finally, the number of cases with AC and LCNEC in gray zone were small, and insufficient data were not enough to further explore the internal mechanism.
In this study, Ki-67 index was positively associated with high mitotic figure, necrosis, high histologic grade, presence of nodal metastasis, and advanced clinical staging. Although there was no adequate cutoff value to separate pulmonary AC from TC and no statistic difference of Ki-67 index between LCNEC and SCLC, the threshold of Ki-67 with 30.07% was demonstrated to be a useful tool to discriminate the pathologic grade, and had statistic significant values for OS and PFS. Moreover, the 2 calculating methods (MCM and CIAM) of Ki-67 labeling index was of great consistent and CIAM was proved to be a reliable method in our study. Therefore, Ki-67 is a feasible and potentially meaningful marker in lung neuroendocrine tumors. This study might contribute a reference data in the diagnostic criteria guidelines of low- and high-grade NETs.
Conflicts of interest
None.
Footnotes
How to cite this article: Wang HY, Li ZW, Sun W, Yang X, Zhou LX, Huang XZ, Jia L, Lin DM. Automated quantification of Ki-67 index associates with pathologic grade of pulmonary neuroendocrine tumors. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000109
References
- 1.Travis WD. Advances in neuroendocrine lung tumors. Annals of oncology: official journal of the European Society for Medical Oncology 2010; 21 suppl 7:vii65–vii71. doi:10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10:1243–1260. doi:10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Quinn AM, Chaturvedi A, Nonaka D. High-grade neuroendocrine carcinoma of the lung with carcinoid morphology: a study of 12 cases. Am J Surg Pathol 2017; 41:263–270. doi: 10.1097/PAS.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 4.Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 2005; 29:179–187. doi:10.1055/s-2008-1074452. [DOI] [PubMed] [Google Scholar]
- 5.Locker AP, Birrell K, Bell JA, Nicholson RI, Elston CW, Blamey RW, et al. Ki67 immunoreactivity in breast carcinoma: relationships to prognostic variables and short term survival. Eur J Surg Oncol 1992; 18:224–229. doi:10.1093/carcin/13.6.1047. [PubMed] [Google Scholar]
- 6.Gaudio F, Giordano A, Perrone T, Pastore D, Curci P, Delia M, et al. High Ki67 index and bulky disease remain significant adverse prognostic factors in patients with diffuse large B cell lymphoma before and after the introduction of rituximab. Acta Haematol 2011; 126:44–51. doi: 10.1159/000324206. [DOI] [PubMed] [Google Scholar]
- 7.Tretiakova MS, Wei W, Boyer HD, Newcomb LF, Hawley S, Auman H, et al. Prognostic value of Ki67 in localized prostate carcinoma: a multi-institutional study of >1000 prostatectomies. Prostate Cancer Prostatic Dis 2016; 19:264–270. doi:10.1038/pcan.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warth A, Fink L, Fisseler-Eckhoff A, Jonigk D, Keller M, Ott G, et al. Interobserver agreement of proliferation index (Ki-67) outperforms mitotic count in pulmonary carcinoids. Virchows Arch 2013; 462:507–513. doi:10.1007/s00428-013-1408-2. [DOI] [PubMed] [Google Scholar]
- 9.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst 2012; 104:764–777. doi:10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 10.Skov BG, Holm B, Erreboe A, Skov T, Mellemgaard A. ERCC1 and Ki67 in small cell lung carcinoma and other neuroendocrine tumors of the lung: distribution and impact on survival. J Thorac Oncol 2010; 5:453–459. doi:10.1097/JTO.0b013e3181ca063b. [DOI] [PubMed] [Google Scholar]
- 11.Schnabel PA, Junker K. Pulmonary neuroendocrine tumors in the new WHO 2015 classification: start of breaking new grounds? [in German]. Pathologe 2015; 36:283–292. doi:10.1007/s00292-015-0030-2. [DOI] [PubMed] [Google Scholar]
- 12.Pelosi G, Rindi G, Travis WD, Papotti M. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 2014; 9:273–284. doi:10.1097/JTO.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 13.Samols MA, Smith NE, Gerber JM, Vuica-Ross M, Gocke CD, Burns KH, et al. Software-automated counting of Ki-67 proliferation index correlates with pathologic grade and disease progression of follicular lymphomas. Am J Clin Pathol 2013; 140:579–587. doi:10.1309/AJCPTMA1F6LWYTQV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology 2018; 72:168–177. doi:10.1111/his.13408. [DOI] [PubMed] [Google Scholar]
- 15.Pape UF, Jann H, Muller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008; 113:256–265. doi:10.1002/cncr.23549. [DOI] [PubMed] [Google Scholar]
- 16.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010; 134:1628–1638. doi:10.1043/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 17.Zheng G, Ettinger DS, Maleki Z. Utility of the quantitative Ki-67 proliferation index and CD56 together in the cytologic diagnosis of small cell lung carcinoma and other lung neuroendocrine tumors. Acta Cytol 2013; 57:281–290. doi:10.1159/000346394. [DOI] [PubMed] [Google Scholar]
- 18.Liu SZ, Staats PN, Goicochea L, Alexiev BA, Shah N, Dixon R, et al. Automated quantification of Ki-67 proliferative index of excised neuroendocrine tumors of the lung. Diagn Pathol 2014; 9:174.doi:10.1186/s13000-014-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchio C, Gatti G, Massa F, Bertero L, Filosso P, Pelosi G, et al. Distinctive pathological and clinical features of lung carcinoids with high proliferation index. Virchows Arch 2017; 471:713–720. doi:10.1007/s00428-017-2177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabbri A, Cossa M, Sonzogni A, Papotti M, Righi L, Gatti G, et al. Ki-67 labeling index of neuroendocrine tumors of the lung has a high level of correspondence between biopsy samples and surgical specimens when strict counting guidelines are applied. Virchows Arch 2017; 470:153–164. doi:10.1007/s00428-016 -2062-2. [DOI] [PubMed] [Google Scholar]
- 21.Swarts DR, Rudelius M, Claessen SM, Cleutjens JP, Seidl S, Volante M, Ramaekers FC, et al. Limited additive value of the Ki-67 proliferative index on patient survival in World Health Organization-classified pulmonary carcinoids. Histopathology 2017; 70:412–422. doi:10.1111/his.13096. [DOI] [PubMed] [Google Scholar]
- 22.Garg R, Bal A, Das A, Singh H. Proliferation marker (Ki67) in sub-categorization of neuroendocrine tumours of the lung. Turk Patoloji Derg 2019; 35:15–21. doi:10.5146/tjpath.2018.01436. [DOI] [PubMed] [Google Scholar]
- 23.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018; 31:1770–1786. doi:10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.den Bakker MA, Thunnissen FB. Neuroendocrine tumours--challenges in the diagnosis and classification of pulmonary neuroendocrine tumours. J Clin Pathol 2013; 66:862–869. doi:10.1136/jclinpath-2012-201310. [DOI] [PubMed] [Google Scholar]
- 25.Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res 2016; 22:3618–3629. doi:10.1158/1078-0432.CCR-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslan DL, Gulbahce HE, Pambuccian SE, Manivel JC, Jessurun J. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol 2005; 123:874–878. doi:10.1309/QYV0-5VGE-GKUL-2RTT. [DOI] [PubMed] [Google Scholar]
- 27.Rindi G, Kloppel G, Couvelard A, Komminoth P, Korner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2007; 451:757–762. doi:10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Zhang M, Rose B, Veillard AS, Jones D, Zhang X, et al. Ki67 expression has prognostic significance in relation to human papillomavirus status in oropharyngeal squamous cell carcinoma. Ann Surg Oncol 2015; 22:1893–1900. doi:10.1245/s10434-014-4237-x. [DOI] [PubMed] [Google Scholar]
- 29.Tisell LE, Oden A, Muth A, Altiparmak G, Molne J, Ahlman H, et al. The Ki67 index a prognostic marker in medullary thyroid carcinoma. Br J Cancer 2003; 89:2093–2097. doi:10.1038/sj.bjc.6601453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walts AE, Ines D, Marchevsky AM. Limited role of Ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod Pathol 2012; 25:1258–1264. doi:10.1038/modpathol.2012.81. [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi N, Maebayashi T, Aizawa T, Sakaguchi M, Nishimaki H, Masuda S. Correlation between the Ki-67 proliferation index and response to radiation therapy in small cell lung cancer. Radiat Oncol 2017; 12:16.doi:10.1186/s13014-016-0744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 2015; 28:686–694. doi:10.1038/modpathol.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph MG, Shibani A, Panjwani N, Arab A, Shepherd J, Stitt LW, et al. Usefulness of Ki-67, mitoses, and tumor size for predicting metastasis in carcinoid tumors of the lung: a study of 48 cases at a tertiary care centre in Canada. Lung Cancer Int 2015; 4:1–7. doi:10.1155/2015/545601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsukuma K, Olson KA, Gui D, Gandour-Edwards R, Li Y, Beckett L. Synaptophysin Ki67 double stain: a novel technique that improves interobserver agreement in the grading of well-differentiated gastrointestinal neuroendocrine tumors. Mod Pathol 2017; 30:620–629. doi:10.1038/modpathol.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adsay V. Ki67 labeling index in neuroendocrine tumors of the gastrointestinal and pancreatobiliary tract: to count or not to count is not the question, but rather how to count. Am J Surg Pathol 2012; 36:1743–1746. doi: 10.1097/PAS.0b013e318272ff77. [DOI] [PubMed] [Google Scholar]


