Abstract
Background:
Fatty acid oxidation (FAO) disorder is involved in the pathogenesis of some cases of preeclampsia (PE). Several studies show that mammalian target of rapamycin (mTOR) signaling pathway is related to FAO. Pravastatin (Pra) can promote FAO in Nω-nitro-L-arginine methyl ester (L-NAME) PE-like mouse model in our previous study. This study aimed to investigate the effect of mTOR signaling pathway in PE-like model treated with Pra.
Methods:
Pregnant mice were randomly injected with L-NAME as PE-like model group or saline as control group respectively, from gestational 7th to 18th day. Giving Pra (L-NAME + Pra, Control + Pra, n = 8) or normal saline (NS; L-NAME + NS, Control + NS, n = 8) from gestational 8th to 18th day, the mice were sacrificed on day 18 and their liver and placental tissues were collected. Then the activation of mTOR and its substrates in the liver and placenta were detected. And the association between mTOR activation and serum free fatty acid (FFA) levels and the expression of long-chain 3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD) were evaluated using Pearson correlation test. Differences between groups were analyzed using independent t-test or one-way analysis of variance (ANOVA).
Results:
Both in the maternal liver and placenta, the activation of mTOR protein and its effect on substrates increased significantly in the L-NAME + NS group and decreased significantly in the L-NAME + Pra group. The p-mTOR/mTOR protein ratio decreased in the L-NAME + Pra group significantly than that in the L-NAME + NS group both in liver and placenta (liver: 0.74 ± 0.08 vs. 0.85 ± 0.06, t = 2.95, P < 0.05; placenta: 0.63 ± 0.06 vs. 0.77 ± 0.06, t = 4.64, P < 0.05). The activation of mTOR protein in the liver and placenta negatively correlated with the expression of LCHAD in the L-NAME + NS group (liver: r = −0.745, P < 0.05; placenta: r = −0.833, P < 0.05) and that in the maternal liver negatively correlated with the expression of LCHAD (r = −0.733, P < 0.05) and positively with the serum FFA levels (r = 0.841, P < 0.05) in the L-NAME + Pra group.
Conclusion:
The inhibition of mTOR signaling pathway might be involved in the regulation of FAO in mouse model treated with Pra.
Keywords: Preeclampsia, mTOR signaling pathway, Pravastatin, Fatty acid oxidation
Introduction
Preeclampsia (PE) is a pregnancy complication with high blood pressure and is a major cause of maternal and fetal death. The measures of prevention and treatment for PE are limited because its exact pathogenesis remains unclear. The fatty acid oxidation (FAO) disorders exist in the pathogenesis of some PE cases.[1] Robinson et al [2] found that the plasma in PE can induce fatty deposition and cause a significant decrease in mitochondrial dehydrogenase activity in cultured human umbilical vein endothelial cells. A previous study showed that the long-chain L-3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD), which is important in fatty acid beta-oxidation, decreased in the placenta at the early onset of severe PE, which was related to the elevated level of serum free fatty acid (FFA).[3]
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase important in cell metabolism, growth, proliferation, and apoptosis under the stimulation of hormones, growth factors, nutritional status, energy levels, and various stress factors. It is closely related to many diseases, such as abnormal pregnancy, cancer, diabetes, obesity, and cardiovascular diseases. Sati et al [4] found increased activation of downstream substrates of the placental mTOR signaling pathway in patients with gestational diabetes mellitus (GDM), suggesting that the mTOR signaling pathway might be involved in the placental pathology of GDM. In patients with PE, the mTOR signaling pathway is important to the nutritional supply to the fetus. Aiko et al [5] showed that the protein expression of mTOR increased in pregnant women with fetal growth restriction and PE. In our previous study, the use of mTOR inhibitor rapamycin in PE-like mouse models reduced the serum FFA levels and the lipid deposition in maternal liver and placenta, indicating that the mTOR signaling pathway was involved in the onset of partial PE and had a close relationship with lipid metabolism.[6] An in vitro study showed that lovastatin induced vascular smooth muscle cell differentiation by inhibiting mTOR, which might be mediated through the inhibition of the activation of Ras homolog enriched in brain (Rheb).[7]
The preventive measures for PE, such as calcium, cod liver oil, antioxidants, low-dose aspirin, heparin, and diet or lifestyle interventions, showed potential but fewer benefits.[8] As statins, pravastatin (Pra) reduced the blood lipids by inhibiting the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and reduced the incidence and mortality of cardiovascular diseases. Further, Pra exerted many cholesterol-lowering independent effects, such as upregulation of nitric oxide synthase, anti-inflammatory, inhibition of anti-angiogenic factors, and dilatation of blood vessels, making Pra have the biological plausibility in PE prevention.[9]
The mechanism of Pra in PE mainly including regulation of angiogenesis factors, improvement in endothelial cell function, reduction of the level of oxidative stress, and regulation of immunity etc. The PE-like mouse model induced by Nω-nitro-L-arginine methyl ester (L-NAME), which is the nitric oxide synthase inhibitor, exhibited high blood pressure, proteinuria, and FAO disorders. Our previous studies already showed that the Pra in L-NAME PE-like models could alleviate the PE-like symptoms, increase the expression of LCHAD protein in the maternal liver and placenta, and decrease the serum level of FFA, indicating that Pra could improve the dysfunction of FAO in some PE.[10,11] However, the exact mechanism of Pra in regulating FAO in the L-NAME PE-like model is not yet clear. This study aimed to explore the changes in mTOR signaling pathway in PE-like model and the involvement of this pathway in the regulatory effect of Pra on FAO.
Methods
Ethical approval
Animal experiments were approved by the Animal Care Committee and Medical Ethics Committee of Peking University, and all procedures were conducted in strict accordance with the guidelines of the Principles of Laboratory Animal Care, promulgated by the National Institutes of Health.
Experimental animals
Adult wide-type C57BL/6J female mice aged 8 to 10 weeks and male mice aged 10 to 14 weeks were purchased from the Laboratory Animal Science Department of Peking University Health Science Center. The female mice were mated to males at a ratio of 2:1 and were inspected daily for vaginal plugs. The day when vaginal plugs were observed was designated as day 1 of pregnancy. Mice were randomly divided into two groups: (1) L-NAME group: PE-like mouse model established by injecting L-NAME according to our previous study;[12] (2) Control group: the normal pregnancy mice were simultaneously injected with saline as control group. Each group were divided into two subgroups treated with normal saline (NS) or Pra (Sigma-Aldrich, 5 mg·kg−1·d−1) by intragastric administration from gestational days 8 to 18. Eventually, there existed four groups: L-NAME + NS group, L-NAME + Pra group, Control + NS group and Control + Pra group. Each group consisted of eight pregnant mice.
The pregnant mice were sacrificed after anesthesia on day 18 of pregnancy as we previously described.[10] Part of the maternal liver and placental tissues were collected and frozen at −80°C for mRNA and protein detection, others were fixed with 4% neutral formaldehyde for immunohistochemistry (IHC).
Western blot analysis
Protein was extracted from maternal liver and placenta tissues with RIPA lysis buffer (Applygen, Beijing, China). A 50 μg of protein was used for electrophoresis in 8% or 10% polyacrylamide gels and transferred to a nitrocellulose filter membrane (Millipore, Bedford, MA, USA). The membrane was blocked in 5% non-fat milk at room temperature for 1 h, and then incubated at 4°C overnight, respectively, with rabbit anti-mouse primary antibodies mTOR (Cell Signaling Technology, Beverly, MA, USA; 1:1000), p-mTOR (Ser2448; Cell Signaling Technology, Beverly, MA, USA; 1:1000), S6K1 (Abcam, Cambridge, UK; 1:1000), p-S6K1 (Thr389; Cell Signaling, USA; 1:1000), p-S6K1 (Ser371; Cell Signaling, USA; 1:1000), 4EBP-1 (Thr389; Cell Signaling, USA; 1:1000), p-4EBP-1 (Thr37/46; Cell Signaling, USA; 1:1000), β-actin (Cell Signaling, USA; 1:1000). Membranes were washed at room temperature for 10 min × 3 times and then incubated with goat anti-rabbit IRDye 800 CW secondary antibody (Li-Cor Biosciences, Lincoln, NE, USA, 1:10,000) for 1 h and washed again for 10 min × 3 times. The bands were scanned on an Odyssey Infrared Imaging System (Li-Cor Bioscience, USA). The resulting images were analyzed with Image J software (National Institutes of Health, Bethesda, USA) and the data were standardized to β-actin.
RNA isolation and real-time quantitative polymerase chain reaction (qPCR): TRIzol reagent (Invitrogen, Grand Island, NY, USA) was used to extract total RNA from the liver and placenta. The concentration and purity of total RNA were detected by NanoDrop 2000 (ThermoFisher, Scientific, Wilmington, MA, USA). Five hundred nanogram total RNA was reverse-transcribed to cDNA by use of the FastKing RT kit (Tiangen Biotech, Beijing, China). The qPCR reaction system involved a Talent qPCR PreMix (SYBR Green) kit (Tiangen Biotech, Beijing, China) and PCR amplification involved the QuantStudio 5 Real-Time PCR System (Applied Biosystems, Forster City, CA, USA). The primer synthesis was completed by Sangon Biotech (China) with the primer sequences for β-actin, forward, 5′-GTGACGTTGACATCCGTAAAGA-3′, and reverse, 5′-GCCGGACTCATCGTACTCC-3′; mTOR, forward, 5′-CAGTTCGCCAGTGGACTGAAG-3′, and reverse, 5′-GCTGGTCATAGAAGCGAGTAGAC-3′. The β-actin was detected as the endogenous control. All reactions were carried out in triplicate. Statistical analysis of the results was performed with the ΔCt value (Ct gene of interest–Ct β-actin). The fold changes of target genes were calculated by the 2−ΔΔCT method.[13]
Immunohistochemistry
Maternal liver and placenta tissues fixed in neutral formaldehyde underwent dehydration, paraffin embedding and were cut into 5-μm slices. Sections were deparaffinized and antigen retrieval was performed with EDTA (pH 8.0) at 100°C for 2 min, and then were blocked in goat serum for 30 min. Sections were incubated with antibodies to mTOR (1:500) and p-mTOR (Ser2448) (1:500) at 4°C overnight and with horseradish peroxidase-conjugated secondary antibodies (Zsbio, Beijing, China) at room temperature for 30 min. After rinsing with PBS, sections were revisualized using a 3, 3′-diaminobenzidine (DAB) kit (Zsbio, China) and counterstained with hematoxylin. The images were analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Bethesda, MD, USA). Five randomly selected fields (original magnification × 400) were detected in each sample, the results of mean optical density (integral optical density/ total stained area) were collected, and the average of five results was analyzed for each sample.
Statistical analysis
All data were analyzed with SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation (SD). Differences between groups were analyzed using independent t-test or one-way analysis of variance (ANOVA). Pearson correlation test was used for comparing the association between the activation degree of mTOR, LCHAD expression, and serum FFA levels. Differences were statistically significant when P < 0.05.
Results
mTOR protein activation in PE-like model detected using western blot analysis
The expression of p-mTOR protein in the liver and placenta increased obviously in the L-NAME + NS group than in the Control + NS group, whereas the expression of mTOR protein had no significant difference [Figure 1A]. The ratio of expression of p-mTOR to mTOR represents the activation degree of mTOR protein. In liver and placenta, p-mTOR/mTOR ratio was elevated significantly in the L-NAME + NS group than in the Control + NS group (liver: 0.85 ± 0.06 vs. 0.53 ± 0.09, t = 0.81, P < 0.05; placenta: 0.77 ± 0.06 vs. 0.57 ± 0.07, t = 0.85, P < 0.05) [Figure 1B]. And in the L-NAME + Pra group, which was treated with Pra, the p-mTOR/mTOR protein ratio decreased significantly than that in the L-NAME + NS group both in liver and placenta (liver: 0.74 ± 0.08 vs. 0.85 ± 0.06, t = 2.95, P < 0.05; placenta: 0.63 ± 0.06 vs. 0.77 ± 0.06, t = 4.64, P < 0.05) [Figure 1B], and was still higher in the liver but had no significant difference in the placenta compared with the Control + NS group (liver: t = 4.78, P < 0.05; placenta: t = 1.89, P > 0.05) [Figure 1B].
Figure 1.
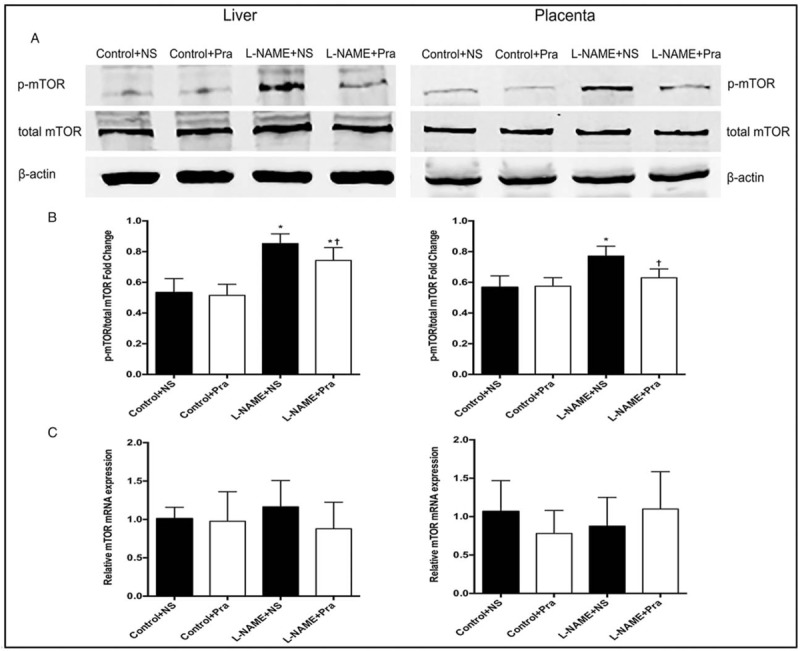
Effects of pravastatin on mTOR protein activation and mRNA expression in the maternal liver and placental tissues detected using Western blot analysis (n = 8). (A and B) The degree of phosphorylation of mTOR protein and statistical values. (C) mRNA expression levels of mTOR in each group. The left panel is liver tissue, and the right panel is placental tissue. ∗ P < 0.05, compared with the Control + NS group; + P < 0.05, compared with the L-NAME + NS group. NS: Normal saline; Pra: Pravastatin.
Distribution and activation of mTOR in the liver and placenta detected using immunohistochemistry
The results of IHC showed that the mTOR and p-mTOR distributed widely and evenly in maternal liver and placenta. The expression of p-mTOR increased in the cell membrane of sponge trophoblasts in placenta compared with the expression of mTOR. Pra had no effect on the distribution of maternal total and phosphorylated mTOR protein in maternal liver and placenta.
The changes in maternal liver and placenta were similar, the mTOR displayed no obvious difference, and the expression of p-mTOR was higher in the L-NAME + NS group than that in the Control + NS group. The mean optical density of p-mTOR/mTOR increased significantly in the L-NAME + NS group than that in the Control + NS group (liver: 1.10 ± 0.13 vs. 0.89 ± 0.12, t = 3.29, P < 0.05; placenta: 1.79 ± 0.09 vs. 1.55 ± 0.07, t = 6.24, P < 0.05) [Figure 2]. The p-mTOR/total mTOR ratio decreased significantly in the L-NAME + Pra group than that in the L-NAME + NS group (liver: 0.93 ± 0.09 vs. 1.10 ± 0.13, t = 3.01, P < 0.05; placenta: 1.64 ± 0.13 vs. 1.79 ± 0.09, t = 2.77, P < 0.05), with no significant difference compared with the Control + NS group (liver: t = 0.66, P > 0.05; placenta: t = 1.86, P > 0.05) [Figure 2].
Figure 2.
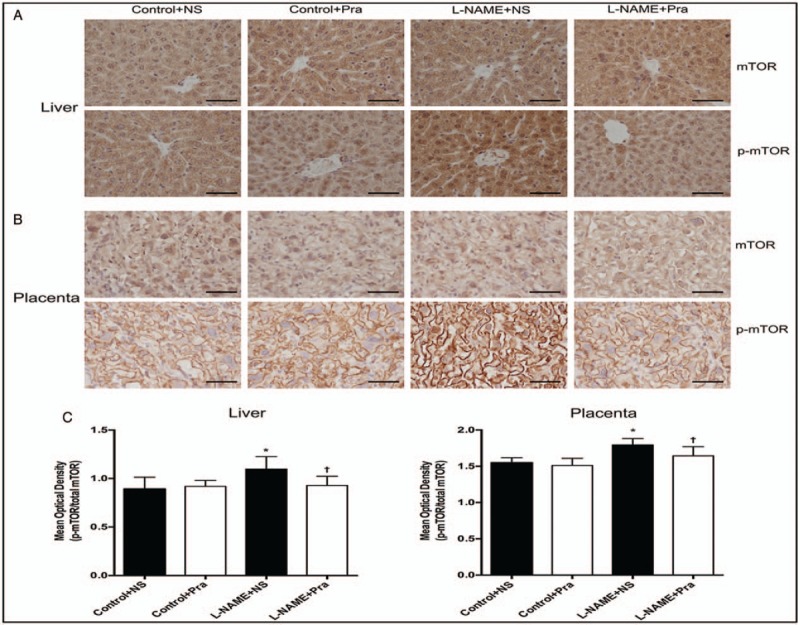
Effects of pravastatin on mTOR protein distribution and activation in the maternal liver and placental tissues detected using immunohistochemistry (n = 8). (A and B) Expression of mTOR protein and p-mTOR protein in liver and placenta; (C) The mean optical density of p-mTOR/mTOR ratio in the liver and placenta. ∗ P < 0.05, compared with the Control + NS group; † P < 0.05, compared with the L-NAME + NS group. Scale bars: 50 μm. L-NAME: Nω-nitro-L-arginine methyl ester; mTOR: Mammalian target of rapamycin; NS: Normal saline; Pra: pravastatin.
mTOR mRNA levels in the liver and placenta detected using qPCR
Levels of mTOR mRNA in the maternal liver and placenta had no obvious difference between the L-NAME + NS and Control + NS groups. Moreover, in the L-NAME + Pra group, the mRNA levels of mTOR in maternal liver and placenta had no significant difference compared with the L-NAME + NS or Control + NS group (liver: F = 1.122; placenta: F = 1.192, P > 0.05) [Figure 1C].
Activation of mTOR substrates in the maternal liver and placenta
Activation of mTOR substrates, including 4EBP1 and S6K1, which has two different phosphorylated sites p-S6K1 (Thr389) and p-S6K1 (Ser371), were detected to investigate the activation of mTOR signaling pathway.
The expression of S6K1 total protein had no significant difference in maternal liver and placenta in all groups. The p-S6K1 (Thr389)/S6K1 (liver: 0.69 ± 0.11 vs. 0.42 ± 0.07, t = 6.09, P < 0.05; placenta: 0.48 ± 0.07 vs. 0.30 ± 0.05, t = 5.99, P < 0.05) and p-S6K1 (Ser371)/S6K1 (liver: 1.16 ± 0.10 vs. 0.94 ± 0.15, t = 3.40, P < 0.05; placenta: 1.09 ± 0.16 vs. 0.90 ± 0.15, t = 2.52, P < 0.05) ratios increased significantly in the L-NAME + NS group than those in the Control + NS group in liver and placenta [Figure 3B]. The p-S6K1 (Thr389)/total S6K1 ratio decreased significantly in the L-NAME + Pra group than that in the L-NAME + NS group both in liver and placenta (liver: 0.59 ± 0.08 vs. 0.69 ± 0.11, t = 2.23, P < 0.05; placenta: 0.34 ± 0.09 vs. 0.48 ± 0.07, t = 2.52, P < 0.05). And when compared with Control + NS group, the p-S6K1 (Thr389)/total S6K1 ratio in the L-NAME + NS group still higher than that in the liver t = 4.64, P < 0.05) and had no significant difference in the placenta (t = 1.15, P > 0.05). The p-S6K1 (Ser371)/S6K1 ratio also decreased in the liver and had no significant difference in the placenta in the L-NAME + Pra group compared with that in the L-NAME + NS group (liver: 0.90 ± 0.13 vs. 1.16 ± 0.10, t = 4.49, P < 0.05; placenta: 0.96 ± 0.10 vs. 1.09 ± 0.16, t = 1.93, P > 0.05), and had no significant difference compared with the Control + NS group (liver: t = 0.58, P > 0.05; placenta: t = 1.05, P > 0.05) [Figure 3B]. The total protein expression of 4EBP1 remained no significantly different, and the p-4EBP1/total 4EBP1 ratio decreased obviously in the L-NAME + NS group compared with the Control + NS group (liver: 0.62 ± 0.11 vs. 0.89 ± 0.09, t = 5.35, P < 0.05; placenta: 0.98 ± 0.22 vs. 1.36 ± 0.07, t = 4.78, P < 0.05), and increased significantly in the L-NAME + Pra group compared with the L-NAME + NS group (liver: 0.79 ± 0.11 vs. 0.62 ± 0.11, t = 2.92, P < 0.05; placenta: 1.20 ± 0.13 vs. 0.98 ± 0.22, t = 2.48, P < 0.05). The ratio in the liver had no significant difference in the liver and still decreased in the placenta in the L-NAME + Pra group compared with that in the Control + NS group (liver: t = 2.02, P > 0.05; placenta: t = 3.20, P < 0.05) [Figure 3].
Figure 3.
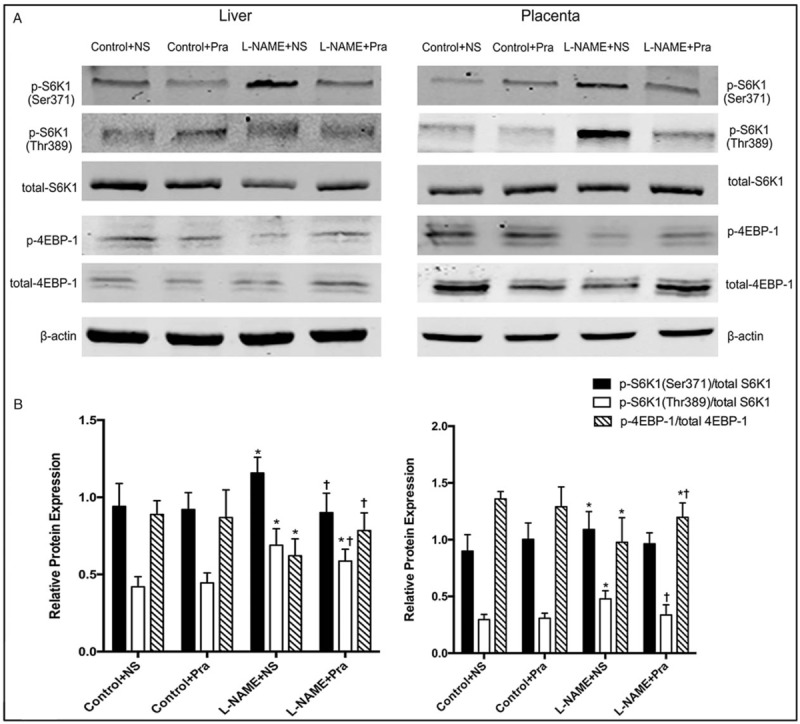
Activation of mTOR substrates S6K1 and 4EBP1 in the maternal liver and placenta in all groups. (A) Expression of phosphorylation and total S6K1 and 4EBP1 protein. (B) Statistical results of the activation of S6K1 and 4EBP1 (n = 8). The left panel is liver tissue, and the right panel is placental tissue. ∗ P < 0.05, compared with the Control + NS group; † P < 0.05, compared with the L-NAME + NS group. L-NAME: Nω-nitro-L-arginine methyl ester; mTOR: Mammalian target of rapamycin; NS: Normal saline; Pra: Pravastatin.
Correlation analysis of the mTOR activation and FAO-related indexes
The reduction of LCHAD protein expression and elevated serum FFA levels in the L-NAME group were reversed after treatment with Pra in our previous research.[10,11] We analyzed the correlation between the mTOR activation levels and the corresponding protein expression of LCHAD and that between the mTOR activation levels and serum FFA levels.
In the L-NAME + NS group, the activation levels of mTOR (p-mTOR/mTOR ratio) were negatively correlated with the LCHAD expression level in the liver and placental tissues (liver: r = −0.745, P < 0.05; placenta: r = −0.833, P < 0.05); no significant correlation existed with the serum FFA levels (liver: r = 0.602; placenta: 0.538, both P > 0.05).
In the L-NAME + Pra group, the activation of mTOR was negatively correlated with the protein expression of LCHAD in the liver, but no significant correlation existed between the two in the placental tissues (liver: r = −0.733, P < 0.05; placenta: r = −0.344, P > 0.05). The mTOR activation in the maternal liver had a positive correlation with the serum FFA level and had no significant correlation with the serum FFA level in placental tissues (liver: r = 0.841, P < 0.05; placenta: r = −0.358, P > 0.05) [Figure 4].
Figure 4.
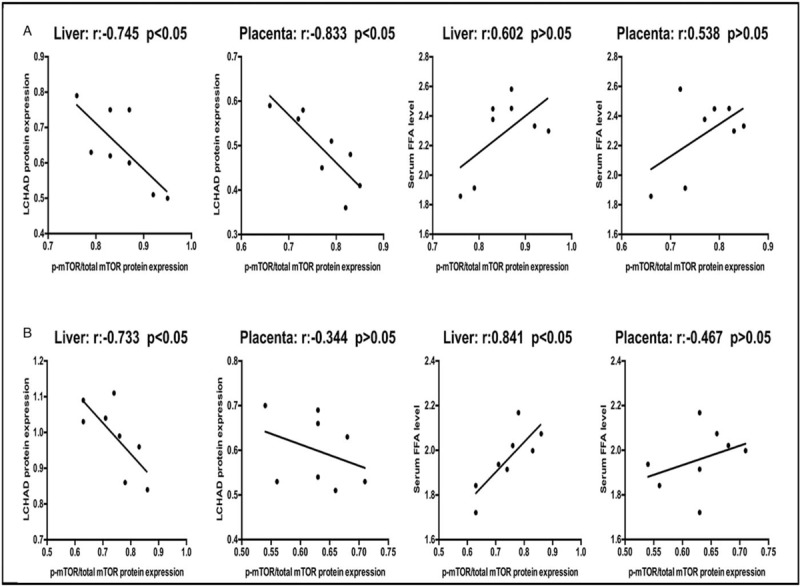
Correlation between the phosphorylation degree of mTOR in maternal liver and placenta and the corresponding protein expression of LCHAD and serum FFA levels (n = 8). (A) L-NAME + NS group. (B) L-NAME + Pra group. FFA: Free fatty acid; LCHD: Long-chain L-3-hydroxyacyl-coenzyme A dehydrogenase; L-NAME: Nω-nitro-L-arginine methyl ester; mTOR: Mammalian target of rapamycin; NS: Normal saline.
Discussion
In this study, an abnormal activation of mTOR signaling pathway was found in the L-NAME PE-like mouse model. Also, the inhibition of mTOR signaling pathway by Pra might be involved in the regulation of FAO in the PE-like mouse model.
Part of PE was found to have a close correlation with FAO dysfunction. The FAO disorders were important in the pathogenesis of PE and were closely related to pathological pregnancy such as acute fatty liver of pregnancy and hemolysis, elevated liver enzymes and low platelet (HELLP) syndrome.[14] In some patients with PE, fatty infiltration of liver and placenta and increased levels of serum triglycerides and FFA existed.[15] Minakami et al [16] performed Oil Red O staining in the liver tissue of 41 patients with PE and liver dysfunction, showing various degrees of fatty disposition in the livers of the patients. Hubel et al [17] found that compared with normal pregnancy, the serum levels of triglyceride and FFA in patients with PE increased nearly twice and were positively correlated with the concentration of lipid peroxide malondialdehyde. The exploration of the pathogenic mechanism of FAO disorders indicated the involvement of LCHAD, an important enzyme for the oxidation of long-chain fatty acids. Previous studies have shown that fetal LCHAD deficiency was often associated with maternal acute fatty liver or HELLP syndrome.[18] Both domestic and international studies have shown an abnormal expression of LCHAD gene in some patients with PE. A previous study found that the placental expression of LCHAD was significantly reduced in PE with liver damage, whose onset time was less than 28 weeks or 28 to 32 weeks, suggesting that the occurrence of early-onset severe PE was associated with FAO disorders.[3] This study also observed lipid deposition and decreased protein expression of LCHAD in the liver and placenta and elevated serum FFA levels in the L-NAME PE-like mouse model.
mTOR is an atypical serine/threonine protein kinase and a member of the phosphatidylinositol-related kinase protein family, which is relatively evolutionarily conserved and ubiquitous in all eukaryotes. Activation of mTOR protein, activates two substrates, ribosomal S6K1 and eukaryotic initiation factor 4EBP1, which are key regulators of protein translation. It has an important regulatory effect on cell metabolism, growth, proliferation, and apoptosis.[19] Recent studies showed that the mTOR signaling pathway was involved in the development of a variety of diseases and was activated in various cellular processes, such as tumor formation and angiogenesis, insulin resistance, adipogenesis, and T-lymphocyte activation.[20] The research on the involvement of mTOR signaling pathway in pregnancy-related diseases was limited. The present study showed the involvement of the mTOR signaling pathway in the pregnancy complications, such as GDM, PE, and fetal growth restriction, which was related to insulin resistance.[21] In the study, the levels of mTOR and its substrates activation increased in the liver and placenta of L-NAME PE-like mouse model compared with normal pregnancy. This indicated that the mTOR signaling pathway was abnormally activated, suggesting its involvement in the pathogenesis of this PE-like model; also its specific mechanism was further analyzed.
The mTOR signaling pathway is important in lipid homeostasis. Clinical studies have shown that the long-term use of rapamycin, an immunosuppressant, might have a risk of hyperlipidemia in patients undergoing renal transplantation.[22] mTOR can directly participate in the lipid metabolism mediated by the transcription factors sterol-regulatory element binding proteins (SREBPs) and peroxisome proliferator-activated receptor alpha (PPARα). SREBPs are a class of transcription factors that mediate the synthesis of lipids in the body and are mainly involved in fatty acid and cholesterol synthesis induced by insulin stimulation. mTOR can increase the protein expression of SREBPs, induce endoplasmic reticulum stress and unfolded protein response, and promote the modification of SREBPs in the Golgi.[23] PPARα is a nuclear receptor involved in the synthesis of ketones. The activation of mTOR could reduce the activity of PPARα and the production of ketones; knocking out the main structure of mTOR, Raptor, could reverse this effect.[24] Brown et al [25] used a primary rat liver cell model to determine the effect of rapamycin on liver FAO and found that rapamycin promoted the metabolism of saturated long-chain fatty acids in a dose-dependent manner. Moreover, the exogenous fatty acid esterification and lipid ab initio synthesis decreased. In the studies on skeletal muscle cells, the application of rapamycin significantly increased the rate of fatty acid palmitate oxidation in skeletal muscle cells.[26] However, the regulatory effects of mTOR signaling pathway in FAO are still unclear. In the adult mouse heart induced by doxycycline or heart-specific mTOR depletion, the mTOR signaling pathways were inhibited after insulin stimulation. The results showed that myocardial palmitate oxidation reduced and the expression of fatty acid-binding protein, LCHAD, and many other FAO-related enzymes decreased. mTOR deletion caused FAO disorders, suggesting that the activation of mTOR signaling pathway could increase the expression of several enzymes in the FAO and promote FAO.[27] This study suggested that in the PE-like mouse model, the activation of mTOR protein in the liver and placenta negatively correlated with LCHAD, suggesting that the decreased LCHAD levels might be associated with the abnormal activation of mTOR signaling pathway in the PE-like mouse model.
In this study, the level of phosphorylation of mTOR decreased in the maternal liver and placenta after the use of Pra in the L-NAME PE-like model. The promotion of S6K1 activation and the inhibition of 4EBP1 were also weakened, suggesting that the overall level of the mTOR signaling pathway was suppressed. A previous study showed that Pra could promote FAO in the L-NAME model, reduce the lipid deposition and up-regulate the protein expression of LCHAD, reduce the serum FFA levels, and finally alleviate the PE-like symptoms in the mouse model.[10,11] The abnormal activation of the mTOR signaling pathway was associated with FAO disorders in the PE-like model, but it was still necessary to further explore whether the regulation of FAO by Pra in this model was related to the mTOR signaling pathway. Pra, belonging to statins, is a competitive inhibitor of the rate-limiting enzyme HMG-CoA reductase in cholesterol synthesis, which reduced cholesterol, low-density lipoprotein, very low-density lipoprotein, and triglyceride levels in the body directly or indirectly, thereby regulating blood lipids levels.[28] Various studies found that Pra exerted various effects independent of the lipid-lowering function, such as upregulation of nitric oxide synthase, dilation of blood vessels, and anti-inflammatory and anti-angiogenic effects.[29] In clinical and animal model studies, the mechanism of Pra in PE mainly included regulation of angiogenesis balance, reduction of oxidative stress, anti-inflammatory effect, and immune regulation, but the specific molecular mechanism remained unclear.[9] Rheb is a small G protein in the Ras family, which is an important activator of mTOR. GTP-binding Rheb could bind to the catalytic domain, and then activate the mTOR. The upstream signaling could regulate the activation of mTOR by changing the state of GTP binding. After inhibiting the HMG-CoA reductase, statins could reduce the intermediate products in the cholesterol synthesis, such as mevalonic acid, farnesyl pyrophosphate, geranyldiphosphate, and so on, which are important for the posttranslational modification and activation of small G proteins. The small G proteins transferred from the cell to the cell membrane and then regulated cell function in many aspects after activation. Therefore, Pra might inhibit the mTOR signaling pathway by suppressing the activation of Rheb and play a further role in PE.[30,31] In this study, the activation of mTOR protein negatively correlated with the expression of LCHAD in the maternal liver and placenta in the L-NAME + Pra group. Also, a positive correlation existed with the level of serum FFA, indicating that the effects of Pra on serum FFA levels and liver and placental expression of LCHAD might be related to its regulation of mTOR signaling pathway.
The present study showed that the mTOR signaling pathway was related to the pathogenesis of FAO disorders in PE and might be involved in the regulatory effect of Pra on FAO. The findings help further understanding the pathogenesis and mechanism of action of Pra on FAO in PE. However, its specific mechanism remains to be further explored.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81370723), and Beijing Municipal Natural Science Foundation (No. 7132215).
Conflicts of interest
None.
Footnotes
How to cite this article: Huai J, Yang Z, Yi YH, Wang GJ. Role of mammalian target of rapamycin signaling pathway in regulation of fatty acid oxidation in a preeclampsia-like mouse model treated with pravastatin. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000129
References
- 1. Bartha JL, Visiedo F, Fernandez-Deudero A, Bugatto F, Perdomo G. Decreased mitochondrial fatty acid oxidation in placentas from women with preeclampsia. Placenta 2012; 33:132–134. doi: 10.1016/j.placenta.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 2. Robinson NJ, Minchell LJ, Myers JE, Hubel CA, Crocker IP. A potential role for free fatty acids in the pathogenesis of preeclampsia. J Hypertens 2009; 27:1293–1302. doi: 10.1097/HJH.0b013e328329fbfe. [DOI] [PubMed] [Google Scholar]
- 3. Li F, Yang Z, Zhang A, Sun X, Wang J, Meng R. The changes of LCHAD in preeclampsia with different clinical features and the correlation with NADPH P47-phox, p38MAPK-alpha, COX-2 and serum FFA and TG (In Chinese). Chin J Obstet Gynecol 2015; 50:92–100. doi: 10.3760/cma.j.issn.0529-567x.2015.02.002. [PubMed] [Google Scholar]
- 4. Sati L, Soygur B, Celik-Ozenci C. Expression of mammalian target of rapamycin and downstream targets in normal and gestational diabetic human term placenta. Reprod Sci 2016; 23:324–332. doi: 10.1177/1933719115602765. [DOI] [PubMed] [Google Scholar]
- 5. Aiko Y, Askew DJ, Aramaki S, Myoga M, Tomonaga C, Hachisuga T, et al. Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR. BMC Pregnancy Childbirth 2014; 14:181 doi: 10.1186/1471-2393-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yi YH, Yang Z, Han YW, Huai J. Effects of rapamycin on clinical manifestations and blood lipid parameters in different preeclampsia-like mouse models. Chin Med J 2017; 130:1033–1041. doi: 10.4103/0366-6999.204924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagner RJ, Martin KA, Powell RJ, Rzucidlo EM. Lovastatin induces VSMC differentiation through inhibition of Rheb and mTOR. Am J Physiol Cell Physiol 2010; 299:C119–C127. doi: 10.1152/ajpcell.00429.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol 2014; 10:531–540. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costantine MM, Cleary K. Pravastatin for the prevention of preeclampsia in high-risk pregnant women. Obstet Gynecol 2013; 121:349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huai J, Yang Z, Yi YH, Wang GJ. Different effects of pravastatin on preeclampsia-like symptoms in different mouse models. Chin Med J 2018; 131:461–470. doi: 10.4103/0366-6999.225058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huai J, Yang Z, Yi YH, Wang GJ, Xiang QQ. Regulation of pravastatin on long-chain fatty acid oxidative enzyme in pre-eclampsia-like mouse model (In Chinese). Chin J Obstet Gynecol 2018; 53:183–189. doi: 10.3760/cma.j.issn.0529-567X.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 12. Ding X, Yang Z, Han Y, Yu H. Correlation of long-chain fatty acid oxidation with oxidative stress and inflammation in pre-eclampsia-like mouse models. Placenta 2015; 36:1442–1449. doi: 10.1016/j.placenta.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14. Liu J, Ghaziani TT, Wolf JL. Acute fatty liver disease of pregnancy: updates in pathogenesis, diagnosis, and management. Am J Gastroenterol 2017; 112:838–846. doi: 10.1038/ajg.2017.54. [DOI] [PubMed] [Google Scholar]
- 15. Dani R, Mendes GS, Medeiros Jde L, Peret FJ, Nunes A. Study of the liver changes occurring in preeclampsia and their possible pathogenetic connection with acute fatty liver of pregnancy. Am J Gastroenterol 1996; 91:292–294. [PubMed] [Google Scholar]
- 16. Minakami H, Oka N, Sato T, Tamada T, Yasuda Y, Hirota N. Preeclampsia: a microvesicular fat disease of the liver? Am J Obstet Gynecol 1988; 159:1043–1047. doi: 10.1016/0002-9378(88)90407-3. [DOI] [PubMed] [Google Scholar]
- 17. Hubel CA, McLaughlin MK, Evans RW, Hauth BA, Sims CJ, Roberts JM. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am J Obstet Gynecol 1996; 174:975–982. doi: 10.1016/S0002-9378(96)70336-8. [DOI] [PubMed] [Google Scholar]
- 18. Ibdah JA, Bennett MJ, Rinaldo P, Zhao Y, Gibson B, Sims HF, et al. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med 1999; 340:1723–1731. doi: 10.1056/nejm199906033402204. [DOI] [PubMed] [Google Scholar]
- 19. Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol 2005; 17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009; 122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villalobos-Labra R, Silva L, Subiabre M, Araos J, Salsoso R, Fuenzalida B, et al. Akt/mTOR role in human foetoplacental vascular insulin resistance in diseases of pregnancy. J Diabetes Res 2017; 2017:5947859 doi: 10.1155/2017/5947859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brattstrom C, Wilczek H, Tyden G, Bottiger Y, Sawe J, Groth CG. Hyperlipidemia in renal transplant recipients treated with sirolimus (rapamycin). Transplantation 1998; 65:1272–1274. doi: 10.1097/00007890-199805150-00023. [DOI] [PubMed] [Google Scholar]
- 23. Bakan I, Laplante M. Connecting mTORC1 signaling to SREBP-1 activation. Curr Opin Lipidol 2012; 23:226–234. doi: 10.1097/MOL.0b013e328352dd03. [DOI] [PubMed] [Google Scholar]
- 24. Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010; 468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 25. Brown NF, Stefanovic-Racic M, Sipula IJ, Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism 2007; 56:1500–1507. doi: 10.1016/j.metabol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 26. Sipula IJ, Brown NF, Perdomo G. Rapamycin-mediated inhibition of mammalian target of rapamycin in skeletal muscle cells reduces glucose utilization and increases fatty acid oxidation. Metabolism 2006; 55:1637–1644. doi: 10.1016/j.metabol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 27. Zhu Y, Soto J, Anderson B, Riehle C, Zhang YC, Wende AR, et al. Regulation of fatty acid metabolism by mTOR in adult murine hearts occurs independently of changes in PGC-1alpha. Am J Physiol Heart Circ Physiol 2013; 305:H41–H51. doi: 10.1152/ajpheart.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McTavish D, Sorkin EM. Pravastatin. A review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs 1991; 42:65–89. doi: 10.2165/00003495-199142010-00005. [DOI] [PubMed] [Google Scholar]
- 29. Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med 2008; 14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu N, Guan S, Chen Z, Yu Y, Xie J, Pan FY, et al. The alteration of protein prenylation induces cardiomyocyte hypertrophy through Rheb-mTORC1 signalling and leads to chronic heart failure. J Pathol 2015; 235:672–685. doi: 10.1002/path.4480. [DOI] [PubMed] [Google Scholar]
- 31. Finlay GA, Malhowski AJ, Liu Y, Fanburg BL, Kwiatkowski DJ, Toksoz D. Selective inhibition of growth of tuberous sclerosis complex 2 null cells by atorvastatin is associated with impaired Rheb and Rho GTPase function and reduced mTOR/S6 kinase activity. Cancer Res 2007; 67:9878–9886. doi: 10.1158/0008-5472.can-07-1394. [DOI] [PubMed] [Google Scholar]


