Abstract
Background:
Vascularized submental lymph node flap transfer to the wrist is an effective treatment for breast cancer–related lymphedema. Dorsal placement was hypothesized to offer superior outcomes due to favorable venous drainage; however, the flap is more visible in this position compared with the volar side and was a cosmetic concern for patients. This study compared the treatment response of breast cancer–related lymphedema with the placement of vascularized submental lymph node flaps at the wrist, between dorsal and volar recipient sites.
Methods:
A retrospective longitudinal study examined 15 patients receiving vascularized submental lymph node flaps at the wrist performed by a single surgeon with a mean follow-up of 17 months. Clinical and biometric analyses, including quality of life questionnaires, circumference measurements, and number of infections were conducted.
Results:
All patients showed improvements in quality of life, reduced episodes of cellulitis, and reduced limb circumference measurements compared with preoperative data. Dorsal placement (n = 7) delivered significant reductions in limb circumference at all levels after 1 year (P = 0.04) and in overall function domains in the Lymphedema Specific Quality of Life Questionnaires (P = 0.04) compared with volar placement (n = 8). Venous outflow was greater in the dorsal recipient veins (P < 0.0001).
Conclusions:
Patients electing to undergo vascularized lymph node transfer to the wrist should be aware that when both options are effective, dorsal placement offers improvement in outcomes despite reduced cosmesis. These results have been incorporated into an evidence-based treatment algorithm that can inform the patient and physician on the decision-making in the breast and plastic surgical spheres.
INTRODUCTION
Breast cancer–related lymphedema is a debilitating result of breast cancer, or its treatment, causing long-term morbidity to patients who have otherwise undergone successful oncologic treatment. Thanks to advances in breast cancer care, many such patients have high functional demands in their professional and recreational lives. Therefore, strategies to prevent or otherwise ameliorate this condition are highly sought after.1–6 The pathophysiology of breast cancer–related lymphedema is clear. Treatment involves a multidisciplinary approach, with conservative management as the mainstay. Such treatments are focused on symptom control and not a cure. Surgical modalities include excisional and physiologic techniques, and the latter have become more popular in recent years, reflecting advances in microsurgery and supermicrosurgery.7–9 Vascularized lymph node transfer (VLNT) has been shown to be an effective treatment for extremity lymphedema. By transplanting healthy lymph nodes into the affected limb, lymph fluid is drained into the venous system via natural lymphovenous connections inside the flap.10–14 Good outcomes have been achieved; however, the optimal donor flap, recipient site, and patient selection criteria have yet to be defined.11,12,15 It is, therefore, of great importance that an energetic discourse among all caregivers in the multidisciplinary treatment of lymphedema occurs, and that evidence-based treatment algorithms, such as that proposed in this paper, are updated as new findings are identified. Preclinical studies have shown that the success of VLNT is dependent on the number of lymph nodes included in the transferred flap.16–18 It is hypothesized that the diameter, and therefore the flow rate, of the draining veins of the flap and recipient vessels are further determinants of outcome.
This team has achieved success using a submental vascularized lymph node flap placed distally in the upper limb, at the wrist. Placing the flap distally prevents an unfavorably distorted and scarred anatomy of the previously operated axilla and benefits from the dependency on aiding fluid return.19 These advantages are borne from clinical outcome studies comparing this method with placement at the elbow.11 The flap can be inset on the dorsal surface using the dorsal branch of the radial artery, or the volar surface using the ulnar artery.
The principal concern with this approach is the visibility of the flap at the dorsal wrist that patients may consider to be not cosmetically appealing. Placing the flap on the volar surface partially conceals the flap and is a potential solution. However, the less favorable anatomy of the recipient veins in this location may not be as conducive to off-loading fluid from the limb. The aim of this study, therefore, was to establish whether there was a difference in treatment outcome between dorsal and volar recipient sites of VLNT at the wrist for breast cancer–related lymphedema.
METHODS
The Institutional Review Board of Chang Gung Memorial Hospital, Taoyuan, Taiwan, reviewed and retrospectively approved this study. Patients were recruited between January 2014 and June 2015. Written consent for participation was obtained in all cases.
Preoperative Evaluation and Patient Selection
All patients underwent the same preoperative evaluation, including lymphoscintigraphy and indocyanine green lymphography, Doppler ultrasound of the recipient venous system, and donor lymph node basin and magnetic resonance imaging (MRI) to determine the pedicle course and quantity of lymph nodes at the donor site.
Patient Inclusion and Exclusion Criteria
Included were patients who had a diagnosis of upper extremity lymphedema after mastectomy and sentinel lymph node biopsy or axillary clearance, as confirmed by lymphoscintigraphy, who were to undergo VLNT using a submental flap to the wrist. Excluded were patients with primary lymphedema, less than 1 year of follow-up; those who were unable to comply with the follow-up protocol; and those who had additional surgery for other indications performed on the lymphedematous upper limb.
Outcome Measures
Patients were assessed clinically and by patient-reported outcome measures, both preoperatively and postoperatively. This enabled treatment planning, established a baseline, and enabled comparison of outcomes and elucidation of treatment response. Time points for assessment were preoperation and 3, 6, and 12 months postoperative.
Clinical assessment of demographic data included age, body mass index (BMI), duration of symptoms, and episodes of cellulitis. Clinical photographs were taken at each time point. Measurements were made of the upper limb circumference taken 10 cm above and below the elbow. Skin changes were noted. Circumferential difference and reduction rate were calculated from these circumference measurements. Circumferential difference was defined as the circumference of the affected limb subtracted from that of the normal limb and divided by that of the normal limb. Circumferential reduction rate was defined as the preoperative difference between the circumferences of the affected and normal limbs minus the postoperative difference, and divided by the preoperative difference.
Radiological assessments included preoperative upper limb lymphoscintigraphy to confirm the diagnosis. Preoperative duplex ultrasonography and MRI of the neck were performed to identify the number of submental lymph nodes, and to aid in flap selection and dissection. Duplex ultrasound of the wrist was performed to check the patency and diameters of the recipient vessels and to determine any venous compromise.
Patient-reported outcome measures were in the form of the Lymphedema Specific Quality of Life Questionnaires (LYMQOL), which were assessed preoperatively and 1 year postoperatively.20
Group Allocation
The research coordinator presented all patients who met the inclusion criteria with a standardized information pack to enable them to choose their site of preference, either volar or dorsal, at the wrist.
Surgical Technique
The submental lymph node flap was raised according to the procedure previously described and published.12,21,22 Figure 1 shows intraoperative photographs of the flap procurement and inset for the dorsal and volar recipient sites. A detailed reiteration of the dissection is beyond the scope of this paper but is summarized as follows. The side of flap harvest was chosen according to patient choice and by preoperative imaging studies suggesting which location was most favorable in terms of lymph node size and number. A skin paddle of 9 × 2.5 cm was marked with the long axis parallel to the inferior border of the mandible centered over the intraoperative Doppler location of the submental artery. The submental vessels, originating from the facial vessels, were used as the donor pedicle. Care was taken to preserve the marginal mandibular nerve.
Fig. 1.
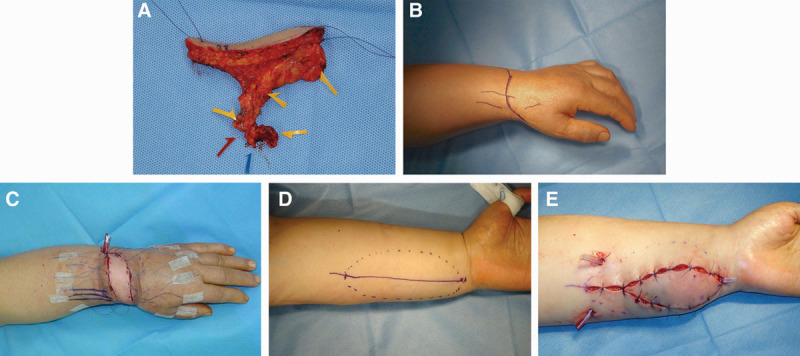
Flap inset surgical technique. A, The submental flap following harvest; blue arrow denotes the vein, red arrow denotes the facial artery, and yellow arrows denote sizable lymph nodes. B, Surgical marking for dorsal inset. C, Dorsal flap inset. D, Surgical marking for volar inset. E, Volar flap inset.
The recipient site was prepared in all cases according to the technique previously described.11,19,21 In both dorsal and volar placement, access was achieved through a “lazy-S” incision and enough space was dissected to create a pocket of adequate size to accommodate the flap. The dorsal side the radial artery was divided at the level of the snuffbox, taking care to preserve the superficial radial nerve. An end-to-end anastomosis was performed with the radial artery and cephalic vein. Volarly, the skin was incised longitudinally, and the ulnar artery and accompanying venae comitantes or basilic vein were also utilized in an end-to-end manner (the arterial anastomosis was occasionally performed in an end-to-side manner).
Flap inset was performed by means of multiple horizontal mattress sutures that were secured with adhesive tape. The sutures were tied at postoperative day 7, when there was no venous compression and swelling had subsided. This technical tip has been adopted as a means of minimizing compression on the flap to prevent venous compromise. No skin grafts were required to cover exposed areas of flap at the inset. The patients were monitored in the microsurgical intensive care unit for 5 days and were discharged on postoperative day 7. Unrestricted finger movement was encouraged from postoperative day 3. No compression garments were worn at any stage postoperatively. Massage of the flap and manual lymphatic drainage from proximal to distal are recommended 3 times a day for both groups. Patients are advised to return normal activity gradually as tolerated. Patients were routinely followed up at monthly outpatient review visits.
Statistical Analysis
SPSS 20.0 software (SPSS, Inc., Chicago, Ill.) was used to analyze the data. The Kolmogorov–Smirnov test was used to assess the normal distribution; the nonparametric Mann–Whitney–Wilcoxon test was used to assess the continuous variables, and the chi-squared test was used to assess the categorical data between dorsal and volar wrist groups. P values of <0.05 were considered significant.
RESULTS
A total of 15 patients met the inclusion criteria for this study. Vascularized lymph nodes were transferred to the dorsal wrist in 7 cases (46.7%) and to the volar wrist in 8 cases (53.3%). Table 1 summarizes the demographic details of the patients and the site of choice in each case. There were no significant differences between two groups with respect to the mean age, BMI, and duration of symptoms. The means of these variables were 53.6 ± 8.4 and 55.1 ± 5.1 years (P = 0.5), 26.6 ± 3.8 and 25.4 ± 1.7 kg/m2 (P = 0.3), and 25.6 ± 26.9 and 31.8 ± 26.2 months (P = 0.4), respectively (Table 1). All cases were classified as Cheng’s lymphedema, grade 2 or higher (P = 0.06) (Table 1).21
Table 1.
Demographic Data and Grade of Lymphedema

There were no flap failures in either group. One flap in each group required early reexploration due to evidence of venous compromise, and in each case, salvage was successful. Mean arterial size was 2.5 ± 0.5 mm for the dorsal and 2.2 ± 1.2 mm for the volar (P = 0.7) groups. Mean dorsal vein size was 2.8 ± 0.9 mm, and volar vein size was 2.0 ± 1.2 mm (P = 0.5). Based on Poiseuille’s law calculating the flow rate as proportional to the radius to the power 4, there was significantly more flow in the recipient veins on the dorsal side (P < 0.0001, unpaired t test).
The incidence of cellulitis was significantly reduced in both groups; mean preoperative episodes were 6 annually and were reduced to 0.3 annually (P = 0.04). There was no difference between the dorsal and volar groups (Table 1).Circumferential difference was significantly reduced at 12 months postoperatively in the above-elbow measurements in the dorsal [32.4% ± 12.8% to 13.5% ± 10.1% (P = 0.03)] and volar groups [34.5% ± 18.4% to 18.8% ± 8.2% (P = 0.03)], and in the below-elbow measurements in the dorsal [32.6% ± 10.2% to 14.1% ± 18.9% (P = 0.04)] and volar [30.4% ± 10.9% to 19.5% ± 14.5% (P = 0.04)] groups, respectively (Table 2). At a 12-month follow-up, the mean circumferential reduction rate was 40.2% ± 37.1% at above elbow and 31.9% ± 23.1% at below elbow in the dorsal group, and 28.1% ± 33.9% at above elbow and 28.2% ± 24.3% at below elbow in the volar group (Table 2). Both circumferential difference and circumferential reduction rates were improved in the both groups (P = 0.04, P = 0.04, P = 0.04, P = 0.04) (Table 2). Moreover, there was a significant difference between dorsal and volar groups, with a statistically greater circumferential difference and reduction rates in the dorsally placed flaps in the above-elbow and below-elbow measurements, respectively (P = 0.04, 0.04, 0.04, and 0.04) (Table 2).
Table 2.
Treatment Outcomes: Clinical Measurements

Figures 2 and 3 show preoperative and postoperative clinical photographs of representative cases for the dorsal and volar recipient sites, respectively. In both cases, the skin paddles of the flaps were excised 1 year after initial surgery. On each occasion, indocyanine green lymphoscintigraphy was repeated, demonstrating that lymphatic flow was drained from proximal to distal, as described in previous literature.23
Fig. 2.
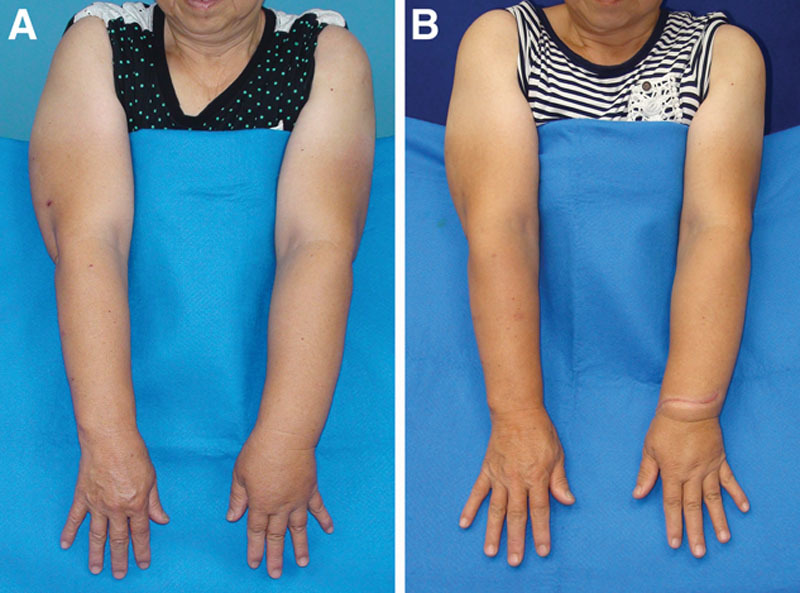
Dorsal inset preoperatively and postoperatively. A, Preoperative. B, One year postoperative, following revision of the flap skin paddle. Reduction in circumference was 40% above the elbow and 30% below the elbow.
Fig. 3.
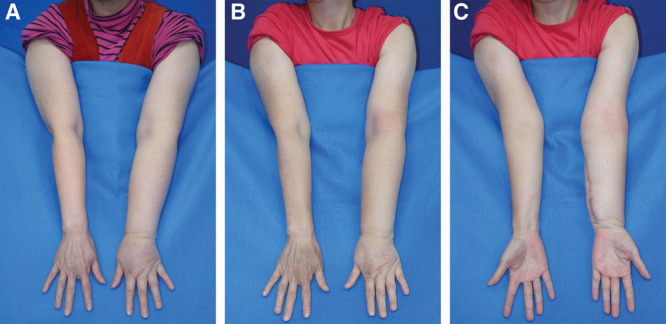
Volar inset preoperatively and postoperatively. A, Preoperative. B, One-year postoperative circumference reduction was 20% above the elbow and 25% below the elbow. C, One year postoperative, volar view, following the excision of the skin paddle.
The LYMQOL completed 1 year after surgery showed a significant improvement for both groups in overall satisfaction (dorsal P = 0.04, volar P = 0.04), function (dorsal P = 0.03, volar P = 0.04), appearance (dorsal P = 0.04, volar P = 0.04), symptoms (dorsal P = 0.03, volar P = 0.04), and mood (dorsal P = 0.03, volar P = 0.04) (Table 3).
Table 3.
Treatment Outcomes: LYMQOL Scores

Furthermore, the dorsally placed group showed significant improvement compared with the volar group in the overall satisfaction (P = 0.04) and function domains (P = 0.02). The dorsal group also showed improvements in the symptoms and mood domains; however, these improvements did not reach statistical significance (P = 0.06, P = 0.06). The appearance domain showed a significant improvement for the volar group over the dorsal group (P = 0.04) (Table 3). It is also noted that no patients required compression garments for postoperative symptomatic control in either group.
DISCUSSION
This study reiterated the effectiveness of VLNT, using the submental flap placed at the wrist, in treating breast cancer–related lymphedema. Additionally, this study has shown that dorsal placement of the flap provides outcomes superior to volar placement. This finding is important as it informs physician and patient choice and decision-making. An algorithm defining this teams’ approach to the investigation and treatment of breast cancer–related lymphedema has been modified according to the findings of this study to reflect the relative benefits of flap placement on functional outcome and cosmesis (Fig. 4). These findings highlight the importance of both technetium-99 lymphoscintigraphy and indocyanine green lymphography in the evaluation of the patency of remaining lymphatic channels, which is the key determinant of the potential efficacy of lymphovenous anastomosis versus vascularized lymph node transplant treatments.17,18,22–24 The role of conservative treatment for mild disease, as a patient preference, or due to contraindications to physiologic lymphatic surgery, is included. Referring practitioners who may consider surgical intervention appropriate for their patients may find this protocol of great utility.
Fig. 4.
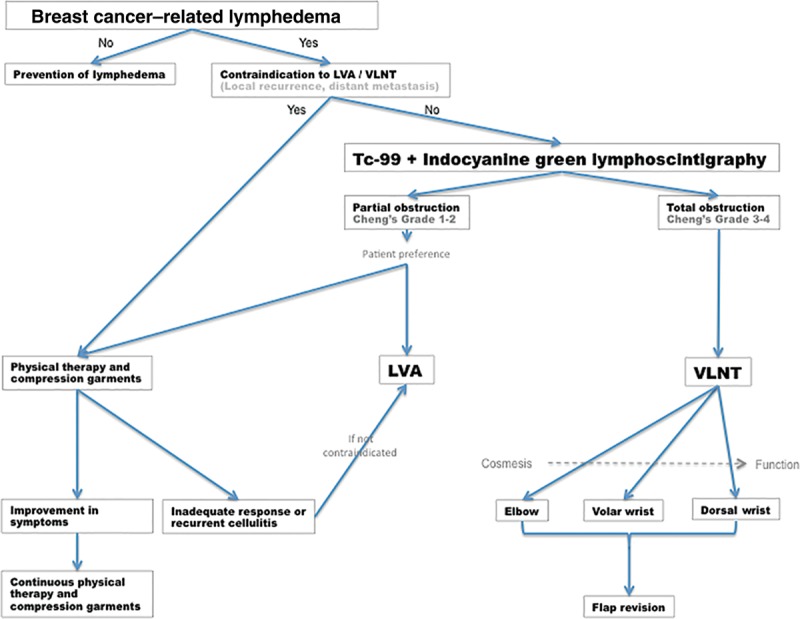
Treatment algorithm for breast cancer–related lymphedema. LVA indicates lymphovenous anastomosis; Tc-99, technetium-99; VLNT, vascularized lymph node transfer.
A concern of patients considering undergoing VLNT to the wrist is the likelihood of poor cosmetic outcome associated with the visibility of the flap; volar placement offers a potential improvement in this respect. Volar placement should be discussed with patients who are likely to see improvements in their symptoms, even though dorsal placement confers a superior outcome. Patients can be counseled that the skin paddle of the flap can be excised at a later date; in our practice, this is usually 1 year postoperatively. Finally, the finding that flow rates are significantly greater in the dorsal recipient veins supports the hypothesis that the venous outflow rate from the vascularized lymph node flap is an independent predictor of outcome. These findings suggest that effectiveness of nonanatomically placed lymph node flaps is dependent on gravity and the size of the recipient vein. Further investigation with a greater number of cases to study how the number of lymph nodes affects the functional outcome is mandated.
The submental flap has been found to have reliable vascular anatomy and consistently sized, numerous lymph nodes.12,16,21,22 The flap has supple handling characteristics, a concealed donor scar, and reduced concerns with donor site lymphedema. Donor site lymphedema is a dreaded complication in VLNT surgery. Strategies to avoid this have included the use of abdominal flaps; however, these have the disadvantage of requiring an abdominal procedure to procure the flap and possibly a combined team approach. The use of groin, thoracodorsal, or axillary nodes combined with reverse lymphatic mapping to identify critical limb draining nodes has been used with some success, but the risk remains.4,25–27 To date, no such complication has been reported following the use of a submental lymph node flap.
This study adds to the growing body of evidence supporting the effectiveness of VLNT for the treatment of breast cancer–related lymphedema.9,23,24,26–30 Furthermore, the “lymphatic pump” hypothesis of the physiologic mechanism of action of VLNT is emphasized.23,24,31 Distal, nonanatomic placement was described by Lin et al.19 and applies the principles of this latter theory to enable the uptake of lymphatic fluid, aided by gravity and dependent positioning, by healthy lymph nodes, and the return to systemic circulation by venous anastomosis. The finding of superior outcomes when comparing wrist placed flaps with those placed at the elbow provides additional credence to this theory.11 In addition, the process of lymph accumulation and lymph channel damage may be partially reversed once the chronic inflammatory state is ameliorated.24 Anatomic placement of vascularized lymph node flaps to re-create a lymphatic circulatory bridge across the site of lymphatic excision is conceptually exciting and good outcomes have been reported. The mechanism of action may also include lymphangiogenesis in response to growth factors secreted by the lymph nodes themselves. Proponents of this approach use the opportunity to operate on the scarred axilla to release the irradiated or surgical contractures and find the concealed location to be cosmetically acceptable. However, published results have reported the need for additional procedures to be performed distally and for the continued use of compression garments.29 Further investigation into the relative importance of these mechanisms in the combined approach for breast cancer–related lymphedema management is mandated. We think both the size and number of lymph nodes are important. We routinely performed preoperative MRI for the neck to determine the donor site of flap harvest, and the donor site is usually the one having greater number of lymph nodes. We had been published an experimental paper regarding the lymph drainage in the lymph node flap or nonlymph node flap, which had statistical difference16 and a clinical paper regarding transfer of ≥3 lymph nodes provided significantly better outcome regarding limb circumference reduction than the transfer of ≤2 lymph nodes.18 To prove that, we need a comparative study between lymph node factor and clinical improvement in lymphedema because there was no difference between both groups in this study. The direction of the transferred flap was different in 2 recipient sites, longitudinal in volar site and transverse in dorsal site. In our experience, the direction of the flap or scar does not affect the long-term outcomes either the circumferential difference or episodes of cellulitis because the skin paddle of the transferred flap is eventually excised at the revisional surgery, usually 1 year postoperatively.
This study is limited by an inability to randomize patients to treatment options; despite a high degree of equipoise between the 2 treatments, it was not considered adequate to permit formal randomization, and patients chose the position of the flap inset themselves following a standardized informed consent process. Majority of the lymphedema patients preferred the approach with greater functional recovery rather than cosmetic concern. Blinding of investigators was also impossible due to the externally visible position of the flap. Finally, for the reasons stated above, gold standard outcome measurements in lymphedema treatment have not been defined. Circumference measurements in particular may be affected by the weather, changes in body weight, lack of skin elasticity in severe cases and in elderly patients, the use of compression garments, the time of day of measurement, and the activity levels of the patient. They do not necessarily reflect symptomatic tightness that the patient may complain of. Nevertheless, our soon to be published data, comparing limb circumference measurements to limb composition measured by computed tomography analysis, have shown a useful correlation. The use of patient-reported outcome measures in this study is, therefore, considered to be a more robust approach, along with the quantitative measurement of infective complications, to evaluating symptomatic relief achieved by this intervention. Additionally, no patient in this study required compression garments following treatment, which is one of the key factors in determining quality of life (QoL) of patients.
CONCLUSIONS
VLNT, using the submental lymph node flap placed at the wrist, is effective in treating breast cancer–related lymphedema. Dorsal placement of the VLNT flap provides superior outcomes to volar placement. However, cosmesis may be improved in the latter position. Venous outflow from the VLNT is a critical determinant of clinical response.
ACKNOWLEDGMENTS
The authors thank Miffy Chia-yu Lin, MSc, and Chin-Yu Yang, MSc, for their invaluable assistance in preparing the data, illustrations, and manuscript for publication.
Footnotes
Published online 20 February 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
References
- 1.Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69–72. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed RL, Prizment A, Lazovich D, et al. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26:5689–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornblith AB, Herndon JE, 2nd, Weiss RB, et al. Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer. 2003;98:679–689. [DOI] [PubMed] [Google Scholar]
- 4.Pasko JL, Garreau J, Carl A, et al. Axillary reverse lymphatic mapping reduces patient perceived incidence of lymphedema after axillary dissection in breast cancer. Am J Surg. 2015;209:890–895. [DOI] [PubMed] [Google Scholar]
- 5.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–4321. [DOI] [PubMed] [Google Scholar]
- 6.Schünemann H, Willich N. Lymphoedema of the arm after primary treatment of breast cancer. Anticancer Res. 1998;18:2235–2236. [PubMed] [Google Scholar]
- 7.Campisi C, Davini D, Bellini C, et al. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery. 2006;26:65–69. [DOI] [PubMed] [Google Scholar]
- 8.Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126:752–758. [DOI] [PubMed] [Google Scholar]
- 9.Merchant SJ, Chen SL. Prevention and management of lymphedema after breast cancer treatment. Breast J. 2015;21:276–284. [DOI] [PubMed] [Google Scholar]
- 10.Chen HC, O’Brien BM, Rogers IW, et al. Lymph node transfer for the treatment of obstructive lymphoedema in the canine model. Br J Plast Surg. 1990;43:578–586. [DOI] [PubMed] [Google Scholar]
- 11.Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013;131:1286–1298. [DOI] [PubMed] [Google Scholar]
- 12.Cheng MH, Huang JJ, Nguyen DH, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol. 2012;126:93–98. [DOI] [PubMed] [Google Scholar]
- 13.Fu K, Izquierdo R, Vandevender D, et al. Transplantation of lymph node fragments in a rabbit ear lymphedema model: a new method for restoring the lymphatic pathway. Plast Reconstr Surg. 1998;101:134–141. [DOI] [PubMed] [Google Scholar]
- 14.Slavin SA, Upton J, Kaplan WD, et al. An investigation of lymphatic function following free-tissue transfer. Plast Reconstr Surg. 1997;99:730–741; discussion 742–743. [DOI] [PubMed] [Google Scholar]
- 15.Althubaiti GA, Crosby MA, Chang DW. Vascularized supraclavicular lymph node transfer for lower extremity lymphedema treatment. Plast Reconstr Surg. 2013;131:133e–135e. [DOI] [PubMed] [Google Scholar]
- 16.Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg. 2014;133:192e–198e. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DH, Chou PY, Hsieh YH, et al. Quantity of lymph nodes correlates with improvement in lymphatic drainage in treatment of hind limb lymphedema with lymph node flap transfer in rats. Microsurgery. 2016;36:239–245. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson J, Chu SY, Chan WH, et al. Correlation between Quantity of Transferred Lymph Nodes and Outcome in Vascularized Submental Lymph Node Flap Transfer for Lower Limb Lymphedema. Plast Reconstr Surg. 2018;142:1056–1063. [DOI] [PubMed] [Google Scholar]
- 19.Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg. 2009;123:1265–1275. [DOI] [PubMed] [Google Scholar]
- 20.Keeley V, Crooks S, Locke J, et al. A quality of life measure for limb lymphoedema (LYMQOL). J Lymphoedema. 2010; 5:26–37. [Google Scholar]
- 21.Allen RJ, Jr, Cheng MH. Lymphedema surgery: Patient selection and an overview of surgical techniques. J Surg Oncol. 2016;113:923–931. [DOI] [PubMed] [Google Scholar]
- 22.Patel KM, Chu SY, Huang JJ, et al. Preplanning vascularized lymph node transfer with duplex ultrasonography: an evaluation of 3 donor sites. Plast Reconstr Surg Glob Open. 2014;2:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel KM, Lin CY, Cheng MH. From theory to evidence: long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg. 2015;31:26–30. [DOI] [PubMed] [Google Scholar]
- 24.Silva AK, Chang DW. Vascularized lymph node transfer and lymphovenous bypass: Novel treatment strategies for symptomatic lymphedema. J Surg Oncol. 2016;113:932–939. [DOI] [PubMed] [Google Scholar]
- 25.Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg. 2015;135:277–285. [DOI] [PubMed] [Google Scholar]
- 26.Raju A, Chang DW. Vascularized lymph node transfer for treatment of lymphedema: a comprehensive literature review. Ann Surg. 2015;261:1013–1023. [DOI] [PubMed] [Google Scholar]
- 27.Basta MN, Gao LL, Wu LC. Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg. 2014;133:905–913. [DOI] [PubMed] [Google Scholar]
- 28.Aschen SZ, Farias-Eisner G, Cuzzone DA, et al. Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow. Plast Reconstr Surg. 2014;133:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobbia D, Semple J, Baker A, et al. Experimental assessment of autologous lymph node transplantation as treatment of postsurgical lymphedema. Plast Reconstr Surg. 2009;124:777–786. [DOI] [PubMed] [Google Scholar]
- 31.Shesol BF, Nakashima R, Alavi A, et al. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast Reconstr Surg. 1979;63:817–823. [PubMed] [Google Scholar]


