Supplemental Digital Content is available in the text.
Abstract
Background:
Bacterial contamination of breast implants causes infection, can lead to capsular contracture, and is implicated in breast implant-associated anaplastic large cell lymphoma. Bacteria, however, also colonize clinically benign breast implants and little is known about the biologic signals that trigger the switch from a benign to pathologic state.
Methods:
Explanted smooth as well as Biocell and Siltex textured breast implants associated with clinically normal and pathologic conditions were analyzed in this observational study. Immunofluorescence and bacterial culture techniques were performed. To avoid sampling bias, implant surfaces >25 sq cm were analyzed.
Results:
Bacteria were detected on 9 of 22 clinically normal explanted devices or periprosthetic capsules, including 40% of Biocell tissue expanders and 75% of Biocell textured implants. Staphylococcus epidermidis was identified in 67% of the bacteria-positive capsular contractures. Fibrinogen was present on 17 of 18, and collagen on 13 of 18 analyzed breast implants. S. epidermidis co-localized with collagen, while group B streptococci and Klebsiella pneumoniae co-localized with fibrinogen.
Conclusions:
Bacteria are often detectable on clinically benign breast implants when a multimodal approach is applied to a substantial proportion of the device surface to avoid sampling bias. The impact of bacteria on breast implant pathology should be studied in the presence of an adequate negative control group to account for clinically benign bacteria. Disruption of the interaction of bacteria with matrix proteins coating the surface of breast implants may represent a nonantibiotic strategy for the prevention of breast implant bacterial contamination.
INTRODUCTION
Bacterial contamination of breast implants can cause infection,1 capsular contracture (CC),2–4 and has been linked to breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).5 Bacteria can also be identified on clinically benign breast implants, however, as they indefinitely abut parenchymal tissue laden with a diverse array of microbes.6–10 Staphylococcus epidermidis is the most common bacterium found on both pathologic and nonpathologic implants, yet why complications manifest in some women and not others remains unknown.11 To establish the impact of bacterial contamination, including differing bacterial species, strains, abundance, or virulence factors, on breast implant pathology, detailed characterization of bacteria on clinically benign breast implants is needed to establish a negative control against which pathology can be compared.
The majority of bacterial infections of medical devices are associated with biofilms. Hallmarks of these infections include increased resistance to antibiotics and the host immune system, resulting in chronic infection, treatment failure, and often surgical intervention.12 For breast implants bacterial biofilm formation is a major concern. There is a large unmet need to understand the mechanisms by which bacteria colonize breast implants to form biofilms to develop effective drugs that can eradicate biofilm-associated infections. The extent to which bacteria become associated with breast implants is influenced by the surface characteristics of the device.13–15 Textured devices, whose contoured surfaces have increased surface area available for bacterial colonization, harbor significantly more bacteria than do smooth breast implant surfaces.16 However, recent studies show that medical devices become coated with host proteins that can be exploited by bacterial pathogens for colonization and biofilm formation.11,17 For example, Staphylococcus aureus, which causes the majority of implant-associated infections (IAI), utilizes a fibrinogen binding adhesin to colonize devices that become coated with fibrinogen, a common occurrence after placement of most kinds of medical devices.18,19 For breast prostheses, additional host ligands are deposited on the devices in the form of a collagen-rich capsule and this likely facilitates bacterial colonization.20,21 A granular understanding of the host-pathogen interactions that lead to breast implant colonization and the biological signals that trigger the switch from a benign to a pathologic state will inform future strategies to optimize breast implant design and establish antibiotic-sparing therapies that prevent problematic bacterial contamination.19,22–25
The purpose of this observational study is 2-fold: (1) we examined the extent of which bacteria were associated with a series of explanted breast prostheses, with different surface characteristics, obtained from normal and pathologic clinical scenarios and used immunofluorescence techniques to characterize the bacteria associated with the devices; and (2) we evaluate, for the first time, matrix protein deposition on the breast device surface to determine the potential repertoire of available bacterial binding ligands. Characterization of bacterial binding mechanisms to breast implants may lead to the development of nonantibiotic antibacterial therapeutics for downstream clinical translation.
METHODS
Study Population
Cosmetic or reconstructive breast prostheses, either implants or tissue expanders (TE), explanted between March 2017 and March 2018 were analyzed under protocol #201703063 at the Washington University School of Medicine. We identified patients with CC, double capsules, seroma, and infection. Benign breast prostheses consisted of TE removed at the time of planned device exchange as well as the contralateral breast implant in patients with unilateral pathology where both devices were explanted. Breast prostheses and capsules were sharply removed using sterile technique, the surface between capsule and implant was marked with a suture, and samples were sectioned and immediately placed in a sterile container. In all cases, the entire breast prosthesis was removed, while the entire capsule was removed in cases of CC and double capsules, while the submuscular capsule was entirely removed but the acellular dermal matrix sling maintained in cases of submuscular TEs. In this manner, >25 sq cm of capsule and implant surface was made available for analysis. Device type was confirmed to be consistent with the medical record. Duration of implantation, device type, and clinical presentation were recorded.
Bacterial Culture and Identification
Explanted devices were divided into 3 sections, the largest section (>25 sq cm) was fixed for immunofluorescence staining and the 2 smaller pieces (~4–25 sq mm) were cultured for bacterial growth. One piece was sonicated for 10 minutes in phosphate buffered saline, plated on the rich media Brain Heart Infusion agar, and grown aerobically and anerobically at 37°C for 48 hours. Individual bacterial species were evaluated for colony size, morphology, color, and bacterial load. The second piece was submerged in BHI and grown for 48 hours at 37°C. Cultures with visible microbial growth were restreaked onto BHI agar for single colonies. A representative isolate of each bacterial species was selected for identification via 16S sequencing, as described previously.18
Immunofluorescence Staining
Immunofluorescence staining was performed as previously described.18 Briefly, the largest piece of the patient device was fixed, blocked, washed, and incubated with primary antibodies. Devices were then washed and incubated with secondary antibodies, which were then washed, dried, and imaged. All antibodies were tested against each isolated bacterial species to determine the optimal concentration for immunofluorescence detection. Infrared signal was examined using the Odyssey Imaging System (LI-COR Biosciences, Lincoln, Nebraska). Controls for auto-fluorescence included small pieces of each respective device in the absence of primary antibody were performed.
RESULTS
Study Population
Twenty-two clinically benign and 18 clinically pathologic breast prostheses were explanted from 33 women. Half of the breast prostheses placed were for reconstructive cases and the other half were for cosmetic purposes. Duration of implantation ranged from 3 months for some TEs to 540 months for severely contracted smooth, shaped, saline filled Dow Corning breast implants. Pocket irrigation with 50% Betadine26 was utilized in the TE cases, but this information was not reliably available for the other implants collected.
Bacteria Cultured from Benign Breast Implants
Patient samples were cultured to determine the bacterial abundance (Table 1), and 16S sequencing was utilized to identify the species (Fig. 1 and Supplemental Digital Content 1) colonizing uncomplicated devices (see figure, Supplemental Digital Content 1, which displays presence, absence, and species of bacteria identified on breast implants explanted from women in the absence of clinical pathology. Results from analyses for bacteria from TEs, permanent breast implants (I), capsules (CAP), http://links.lww.com/PRSGO/A930). Bacteria were cultured from 9 of 22 clinically normal explanted devices or periprosthetic capsules, including 6 of 15 (40%) Biocell TEs and 3 of 4 (75%) Biocell textured implants. In instances where bacteria were recovered, more than one species was identified in 6 of 9 breasts (Supplemental Digital Content 1). Only Gram-positive bacteria, and specifically coagulase-negative staphylococci (CNS), were detected on clinically normal breast implants. In contrast, clinically normal TE were colonized by a broader array of both Gram-positive and Gram-negative bacteria. CNS, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterobacter, were recovered from 4 Biocell textured TE implanted for 124, 157, 180, and 372 days (Supplemental Digital Content 1). Bacteria were not detected on the 3 clinically normal saline and Siltex textured breast implants evaluated (Fig. 1 and Supplemental Digital Content 1). Additionally, while capsular tissue was not routinely harvested for all specimens, there were 2 instances, one with a TE and one with an implant, where bacteria were retrieved from the prosthesis but not the capsule (Supplemental Digital Content 1).
Table 1.
Bacterial Load Recovered from Clinically Normal and Complicated Patient Devices
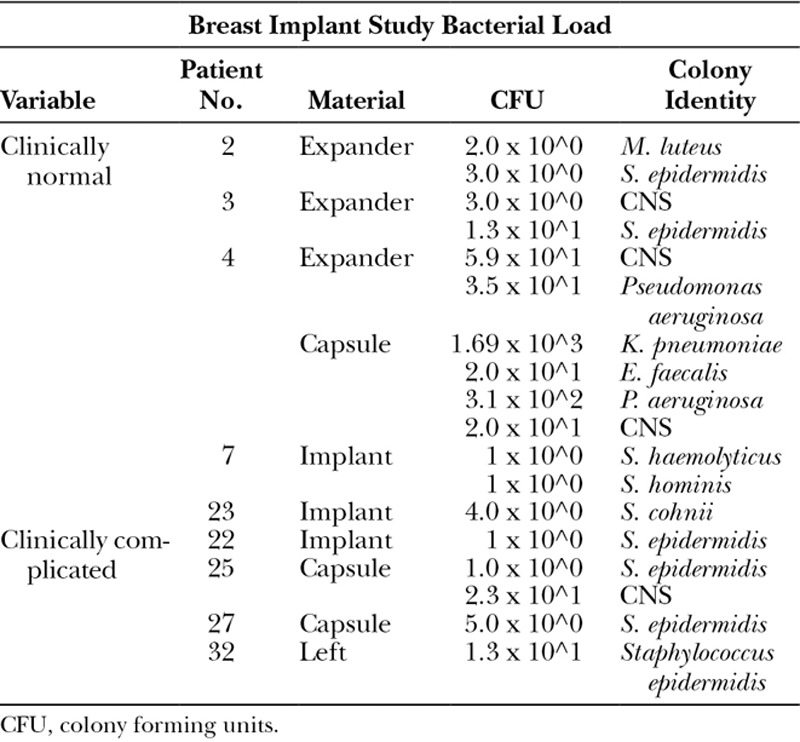
Fig. 1.
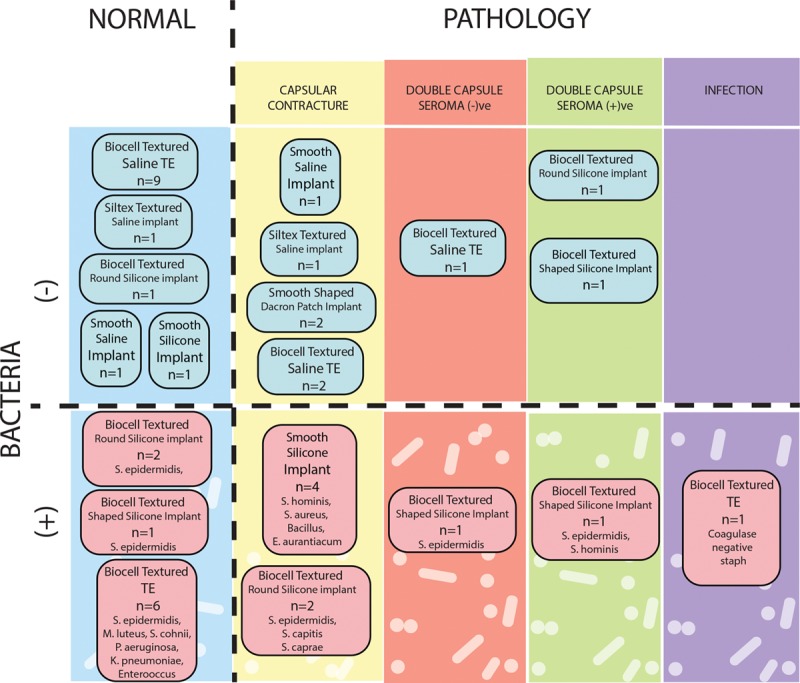
Schematic categorization of study implants by presence or absence of pathology, presence or absence of implant or capsular bacteria, and implant type. Pathology includes CC, double capsule with or without intervening seroma, and infection requiring explantation.
Bacteria Cultured from Pathologic Breast Implants
Clinically complicated samples were cultured and bacterial abundance was assessed (Table 1) and species colonizing pathologic prostheses were determined via 16S sequencing (Fig. 1 and Supplemental Digital Content 1). CCs were noted in 12 breast prostheses collected from 8 women (Fig. 1 and Supplemental Digital Content 2) and S. epidermidis was identified in the majority (67%) of these cases (see figure, Supplemental Digital Content 2, which displays presence, absence, and species of bacteria identified on breast implants explanted from women in the absence of clinical pathology. Pathology categorized as CC, double capsule without seroma (seroma (-)ve), double capsule with seroma (seroma (+)ve), or infection requiring explantation, http://links.lww.com/PRSGO/A990). Other Gram-positive bacteria, including other CNS, were found colonizing the rest (Supplemental Digital Content 2). Smooth-surface and Siltex and Biocell textured devices (both saline and silicone) were represented in the CCs analyzed (Fig. 1). Bacteria were inconsistently identified in smooth and textured devices complicated by CC (Fig. 1). Double capsules—defined as 2 distinct capsules between the device and the soft-tissue space with 1 capsule tenaciously adherent to the device surface—were exclusively identified in patients with Biocell textured prostheses (Fig. 1). CNS were identified in 2 of the 5 double capsules with or without seroma (Fig. 1 and Supplemental Digital Content 2). One TE was explanted for infection, and CNS was isolated (Fig. 1 and Supplemental Digital Content 2). Interestingly, the microbes isolated from the complicated prostheses were exclusively Gram-positive bacteria.
Matrix Protein Deposition on Breast Implants
Complicated (n = 5) and normal (n = 13) devices without any detectable bacteria were immunofluorescently stained for the presence of host proteins, including fibrinogen, a protein known to be deposited on other medical devices,18,22,27 and collagen type I and type III, proteins that make up the implant capsule.21,28,29 Fibrinogen was present on 5/5 and 12/13 clinically complicated and normal devices (Table 2 and Fig. 2). Collagen was detected on 4/5 and 9/13 clinically complicated and normal devices. All analyzed textured devices, including 14 Biocell and 2 Siltex, were coated with fibrinogen. Smooth surfaced breast implants4 included 1 that lacked matrix protein deposition, 1 coated with fibrinogen, and 2 coated with fibrinogen and collagen.
Table 2.
Clinically Normal Breast Implants without Detectable Bacteria Stained for Deposited Fibrinogen and Collagen
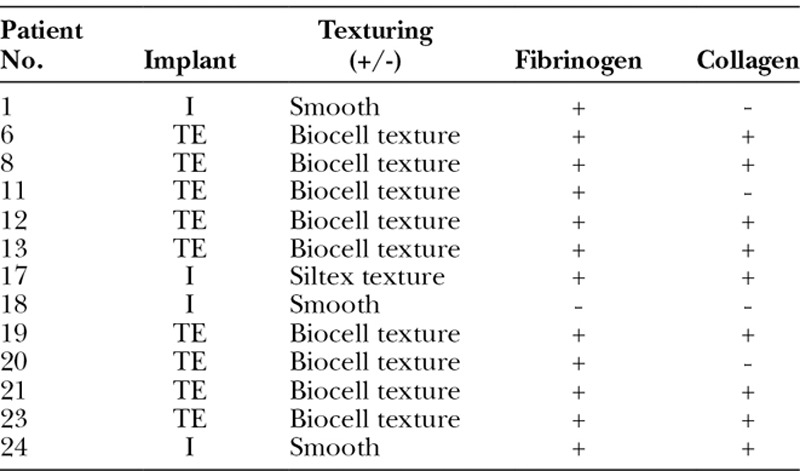
Fig. 2.
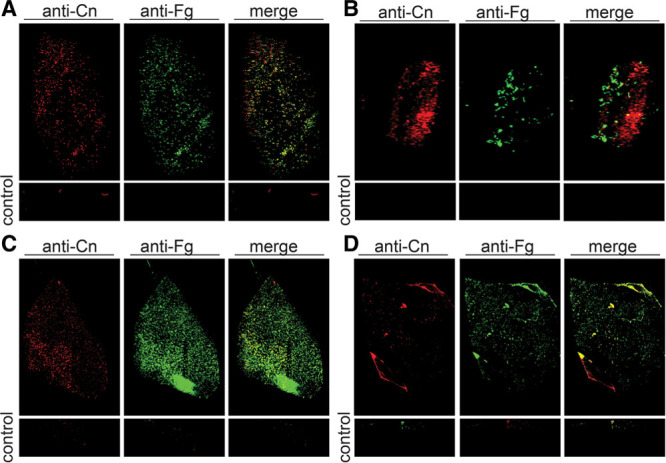
Representative images of clinically normal and clinically complicated breast implants immunofluorescently stained for fibrinogen and collagen. All devices imaged had no culturable bacteria. Staining revealed collagen (Cn) and fibrinogen (Fg) were present on the majority of clinically uncomplicated (A and B) and clinically complicated (C and D) patient devices. Controls are implant pieces treated the same but without primary antibody. Commercially available primary antibodies: goat antifibrinogen (Cat # F8512); rabbit anticollagen (Cat. #234169, Calbiochem).
Bacteria Co-localize with Deposited Matrix Proteins
Breast prostheses with detectable bacteria16 were immunofluorescently stained with commercially available antibodies for the respective microbe (antibodies were not available for Micrococcus, Bacillus, or Exiguobacterium). S. epidermidis was detected on all devices from which the bacteria were isolated (Table 3). Additionally, since staphylococcal-collagen interactions have been implicated in breast IAI,11 we simultaneously stained the samples for collagen. S. epidermidis predominantly co-localized with collagen (Tables 4, 5, Fig. 3). Furthermore, group B strep was detected on the device from patient 9 (Supplemental Digital Content 2 and Fig. 3) and K. pneumoniae was detected on the prostheses from patient 23 (Supplemental Digital Content 2 and Fig. 3). Interestingly, we found that group B Strep and K. pneumoniae co-localized with fibrinogen (Table 4). Bacteria were not detected on the other 10 devices via immunofluorescence staining. Overall, of the bacteria that could be detected on both clinically normal and pathologic Biocell textured and smooth surfaced implants, all were found to co-localize with matrix proteins (Tables 4, 5, Fig. 3).
Table 3.
Breast Implants with Pathology without Detectable Bacteria Stained for Deposited Fibrinogen and Collagen
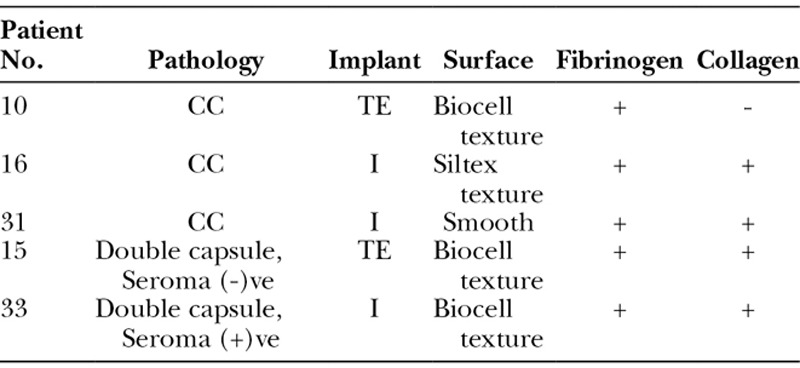
Table 4.
Bacteria Can Be Detected on Clinically Normal Breast Implants and Bacteria Co-localizes with Host Matrix Protein Deposition
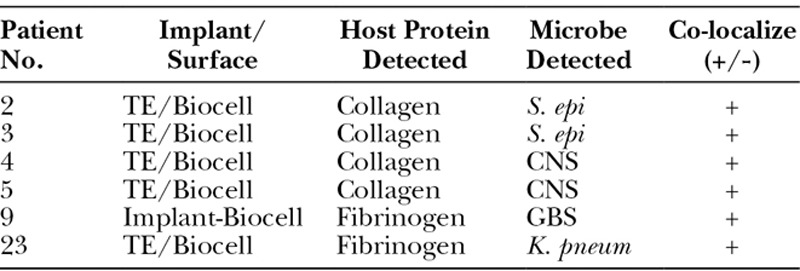
Table 5.
Bacteria Can Be Detected on Breast Implants with Pathology and Bacteria Co-localizes with Host Collagen Deposited
Fig. 3.
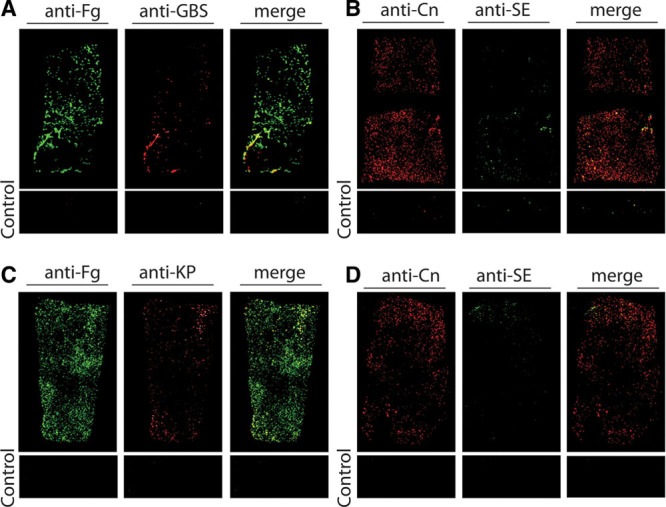
Representative images of devices immunofluorescently stained for cultured bacteria and host proteins. A, Group B streptococcus (GBS) was cultured from the Biocell-textured silicone breast implant of patient 9, which was clinically normal. GBS was detected via immunofluorescence staining and it co-localized with the deposited host protein fibrinogen (Fg). Staphylococcus epidermidis (SE) was cultured from patient 22’s Biocell-textured silicone implant, which was clinically complicated by a double capsule without seroma. SE was visible on the device and co-localized with collagen (Cn). K. pneumoniae (KP) and SE were cultured from patients 23’s Biocell-textured TE, which was clinically normal. KP (C) and SE (D) were both visible on the device and co-localized with deposited Fg and Cn, respectively. Controls were pieces of each device treated the same, but without primary antibody. Commercially available primary antibodies: goat antifibrinogen (Cat # F8512); rabbit anticollagen (Cat. #234169, Calbiochem); mouse anti-S. epidermidis (Cat. # MA1-35788, ThermoFisher Scientific); rabbit anti-Pseudomonas (Cat. # ab68538, Abcam); rabbit anti-Protein A (for S. aureus) (P3775, Sigma-Aldrich); rabbit antienterococcus (Cat. # PA1-73120); rabbit anti-Klebsiella,58 and rabbit antigroup B streptococcus.22,59 Secondary antibodies: IRDye 800CW donkey antigoat, IRDye 800LT donkey antimouse, and IRDye 680LT donkey antirabbit (LI-COR Biosciences).
DISCUSSION
Bacteria cause IAI,1 have a role in CC,2,3,30–32 and may play a role in the etiology of BIA-ALCL5; however, the microbial species responsible and the host-pathogen interactions that result in these diverse complications are still being investigated. We have previously shown that the predominant Gram-positive and Gram-negative bacterial causes of breast IAI and explantation are S. aureus and P. aeruginosa, respectively.1 Ralstonia pickettii has also been identified in a disproportionately high percentage of breast implants from patients with BIA-ALCL.5 Additionally, while there is a growing body of evidence implicating bacterial colonization of devices in the development of CC, including a strong correlation between Baker grade and positive breast implant and TE cultures3 and a reduction in CC rates after breast implant placement with the use of antibiotic pocket irrigation or impregnated mesh strategies,32–35 these findings are not universally consistent among reports,36,37 and the latter are limited to a mean of 2 years of follow-up or less.33–35 An elegant swine model, however, has demonstrated causation between S. epidermidis infection and CC.2 Together, these studies highlight the need to better understand the host-pathogen interactions that facilitate the development of pathologic implants in patients, including CC and BIA-ALCL, to implement truly effective interventions.
To understand how bacteria influence the development of breast implant-associated complications, it is critical to know which bacteria are present in a clinically benign scenario. However, data from this “negative control” group are scant, as evaluating normal breast implant colonization is challenging since it requires assessing either temporary TE38 or permanent implants explanted at the time of less common revision surgery due to malposition or when managing pathology on the contralateral side. Of the few small studies that address this question, Pajkos et al.4 identified bacteria in 1 of 8 (12.5%) clinically benign breast implants, Rieger et al.3 identified bacteria in 4 of 21 (19%) patients with Baker grade I and II capsules, and Hu et al.5 identified 7.6 × 105 bacteria/mg tissue in 3 clinically normal breast implants. Significantly, more bacteria were detected on pathologic implants than uncomplicated ones in these studies, suggesting bacterial abundance impacts the development of complications. The presence of bacteria on benign implants, though, requires further study to determine whether bacterial species or strains, virulence factor production, or interactions with other bacteria or the host contribute to the development of complications.19,24,39–41
While this study is not adequately designed or powered to compare the bacterial abundance between clinically normal and complicated breast implants, it does provide important insights into the potential bacterial reservoir on uncomplicated devices. In this study, we detected bacteria in 41% of the clinically normal breast implants and/or surrounding capsules analyzed—a higher proportion of bacteria-positive, normal breast prostheses than previous reports.3–5 This is likely due to our combining standard sonication and plating, similar to previous studies, with liquid culturing techniques, to detect low colonization levels and/or bacteria firmly adherent to the implant. Of the clinically normal implants that were bacteria-positive, all had a Biocell-textured surface and were colonized exclusively by Gram-positive bacteria, with CNS the chief microorganism identified. For clinically normal TE, all colonized devices also had Biocell-textured surfaces; however, Gram-negative bacteria, including K. pneumoniae and P. aeruginosa, were found in addition to Gram-positive microorganisms. The wider array of bacteria present on TE may be due to the fact that the implant reconstruction paradigm differs significantly from aesthetic breast augmentation, with the traumatic dispersion of parenchymal and ductal bacteria from the breast and skin microbiome during mastectomy and reconstruction, longer operative times, and the potential for disease- or chemotherapy-induced immunosuppression.1,42 Recognizing the greater likelihood for bacterial contamination following TE breast reconstruction,42 a higher incidence of bacterial contamination with a more diverse group of microbes is not unexpected. Notably, the routine use of betadine irrigation of the skin and postmastectomy pocket before insertion of a TE with acellular dermal matrix may have been sufficient to reduce the contaminating bacteria below a clinically problematic threshold in these patients.13,26 Interestingly, similarly to what we found for normal implants, only Gram-positive bacteria, and primarily CNS, were found colonizing CC (50%) and double capsule (40%) specimens. While we cannot discount that some of the patients with colonized, clinically normal devices would eventually go on to develop complications like infection or CC, future studies elucidating the host-pathogen interactions that lead to pathologic implants will be critical for understanding why some women with colonized implants develop complications while others do not.
The presence of CNS, which are known skin colonizers, on both pathologic and benign implants supports the dogma that breast prosthesis contamination primarily occurs through contact with the skin microbiota during placement. However, even if skin contact can be minimized,43,44 breast implants are still susceptible to bacteria that reside in the breast parenchyma, which also contain CNS, among other Gram-positive bacteria.6–10 Thus, the breast represents a clean-contaminated surgical site.9 Importantly, the breast microbiome has greater microbial diversity than the skin,6 and is formed and evolved over time through the translocation of bacteria through mucosal membranes of the gut, oropharynx, and urogenital tract.7,45,46 Recent evidence suggests that the composition of the breast microbiome varies based on depth of biopsy, suggesting the important contributions of the nipple and skin microbiome to bacteria within the breast.10 Given the proximity of a breast implant to the colonized breast parenchyma, there is ample opportunity, even years past the time of initial device insertion, for bacteria to contact the implant surface and/or capsule. Thus, it is reasonable to expect that the bacteria contaminating implants is similar to the breast parenchyma microbiome because parenchymal microbes have the most direct pathway to the implant surface after placement.
Our previous work detailing the molecular mechanisms that result in catheter-associated urinary tract infections (CAUTI) has provided important insights into the host-pathogen interactions that facilitate disease and may lead to the development of therapeutics to treat these recalcitrant infections.18 This work showed that fibrinogen, which is recruited to the bladder following catheter-induced damage to the urothelium, is deposited on the urinary catheter surface and provides a critical binding substrate for Enterococcus faecalis and S. aureus adherence and biofilm formation.18,22 By developing a vaccine that specifically blocked the ability of the adhesive tip of the E. faecalis Ebp pilus to interact with fibrinogen, we could effectively prevent and treat enterococcal CAUTI in a mouse model.22 Thus, by identifying bacterial virulence mechanisms and dissecting the host-pathogen interactions that lead to CAUTI, it may be possible to design and develop effective therapeutics. Herein, we begin to translate this work to the breast implant paradigm. We found that fibrinogen was deposited on 17/18 and collagen on 13/18 of the breast implants analyzed in both clinically normal and abnormal cohorts (Tables 2, 3). Fibrinogen deposition likely stems from surgical manipulation of the breast parenchyma, which stimulates inflammation.47,48 Importantly, the deposition of host proteins, including fibrinogen and collagen, on breast prostheses has important implications for bacterial adherence and infection, as it is becoming increasingly clear that many bacteria encode proteins that bind to these factors to facilitate disease. Notably, we found that CNS co-localized with collagen on explanted breast implants. S. epidermidis elaborates SdrF49 and GehD, which interact with collagen,50 thus providing a potential mechanism for S. epidermidis breast implant adherence or biofilm formation. Interestingly, K. pneumoniae51 and Group B Strep52 that express pili that are reported to play an important roles in biofilm formation19,53 co-localized with fibrinogen in our explanted implants. Together, these data provide important insights into potential targets for the development of nonantibiotic therapeutics, including small molecule inhibitors or vaccines that specifically block host-pathogen interactions that facilitate disease.24,40 Immediate next steps will include further characterization of matrix protein binding to various breast implant surfaces and the predilection of particular bacteria to them.
CONCLUSIONS
We readily detected bacteria and matrix protein deposition on the smooth and textured surfaces of clinically normal and pathologic implants, explanted months to decades after insertion. Bacteria co-localized with matrix proteins, thus suggesting bacteria may preferentially adhere to host proteins instead of abiotic surfaces. Future studies examining bacteria-related breast implant pathology should analyze a sufficient percentage of the implant surface to avoid sampling bias, and include an adequately powered control group. Finally, several knowledge gaps in the field of breast implant bacteria require further study including identifying the signals, bacterial, or host, that trigger the transition from a normal, uncomplicated implant to a pathologic state and the role of matrix proteins, like collagen and fibrinogen, in implant contamination. The answers to these questions may lead to the development of novel nonantibiotic therapeutic strategies.
ACKNOWLEDGMENTS
The authors are grateful to Allergan for funding this work. The authors would also like to thank the clinical team, without whom this work would not have been possible.
Supplementary Material
Footnotes
Published online 8 February 2019.
Supported by an investigator-initiated grant from Allergan Inc. to Dr. Myckatyn (IIT-2017–10074). Dr. Myckatyn has received grant funding, consultant, and advisory board fees from Allergan, investigator-initiated grant funding and consultant fees from LifeCell, investigator-initiated grant funding and consultant fees from RTI, and advisory board fees from Viveve.
Disclosure: This study was funded by an investigator-initiated grant from Allergan Inc. to Dr. Myckatyn (IIT-2017-10074). Dr. Myckatyn has received grant funding, consultant, and advisory board fees from Allergan, investigator-initiated grant funding and consultant fees from LifeCell, investigator-initiated grant funding and consultant fees from RTI, and advisory board fees from Viveve. None of the other authors have relevant disclosures. The Article Processing Charge was paid for by Allergan Medical.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Cohen JB, Carroll C, Tenenbaum MM, et al. Breast implant-associated infections: the role of the National Surgical Quality Improvement Program and the local microbiome. Plast Reconstr Surg. 2015;136:921–929. [DOI] [PubMed] [Google Scholar]
- 2.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126:835–842. [DOI] [PubMed] [Google Scholar]
- 3.Rieger UM, Mesina J, Kalbermatten DF, et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100:768–774. [DOI] [PubMed] [Google Scholar]
- 4.Pajkos A, Deva AK, Vickery K, et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659–1669. [DOI] [PubMed] [Google Scholar]
- 6.Hieken TJ, Chen J, Hoskin TL, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. 2016;6:30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbaniak C, Burton JP, Reid G. Breast, milk and microbes: a complex relationship that does not end with lactation. Womens Health (Lond). 2012;8:385–398. [DOI] [PubMed] [Google Scholar]
- 8.Urbaniak C, Cummins J, Brackstone M, et al. Microbiota of human breast tissue. Appl Environ Microbiol. 2014;80:3007–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartsich S, Ascherman JA, Whittier S, et al. The breast: a clean-contaminated surgical site. Aesthet Surg J. 2011;31:802–806. [DOI] [PubMed] [Google Scholar]
- 10.Galdiero M, Larocca F, Iovene MR, et al. Microbial evaluation in capsular contracture of breast implants. Plast Reconstr Surg. 2018;141:23–30. [DOI] [PubMed] [Google Scholar]
- 11.Barbieri R, Pesce M, Franchelli S, et al. Phenotypic and genotypic characterization of staphylococci causing breast peri-implant infections in oncologic patients. BMC Microbiol. 2015;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantine RS, Constantine FC, Rohrich RJ. The ever-changing role of biofilms in plastic surgery. Plast Reconstr Surg. 2014;133:865e–872e. [DOI] [PubMed] [Google Scholar]
- 13.Jacombs A, Tahir S, Hu H, et al. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstr Surg. 2014;133:471e–480e. [DOI] [PubMed] [Google Scholar]
- 14.Paek LS, Giot JP, Tétreault-Paquin JO, et al. The impact of postoperative expansion initiation timing on breast expander capsular characteristics: a prospective combined clinical and scanning electron microscopy study. Plast Reconstr Surg. 2015;135:967–974. [DOI] [PubMed] [Google Scholar]
- 15.Giot JP, Paek LS, Nizard N, et al. The double capsules in macro-textured breast implants. Biomaterials. 2015;67:65–72. [DOI] [PubMed] [Google Scholar]
- 16.Brown T. Surface areas of textured breast implants: implications for the biofilm theory of capsule formation. Plast Reconstr Surg Glob Open. 2018;6:e1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabaté Brescó M, Harris LG, Thompson K, et al. Pathogenic mechanisms and host interactions in Staphylococcus epidermidis device-related infection. Front Microbiol. 2017;8:1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker JN, Flores-Mireles AL, Pinkner CL, et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc Natl Acad Sci U S A. 2017;114:E8721–E8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores-Mireles AL, Walker JN, Caparon M, et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiEgidio P, Friedman HI, Gourdie RG, et al. Biomedical implant capsule formation: lessons learned and the road ahead. Ann Plast Surg. 2014;73:451–460. [DOI] [PubMed] [Google Scholar]
- 21.Moyer KE, Ehrlich HP. Capsular contracture after breast reconstruction: collagen fiber orientation and organization. Plast Reconstr Surg. 2013;131:680–685. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Mireles AL, Pinkner JS, Caparon MG, et al. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci Transl Med. 2014;6:254ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Flores-Mireles AL, Cusumano ZT, et al. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. NPJ Biofilms Microbiomes. 2017;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaulding CN, Klein RD, Schreiber HL, 4th, et al. Precision antimicrobial therapeutics: the path of least resistance? NPJ Biofilms Microbiomes. 2018;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalas V, Hibbing ME, Maddirala AR, et al. Structure-based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc Natl Acad Sci U S A. 2018;115:E2819–E2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams WP., Jr. Commentary on: surgical site irrigation in plastic surgery: what is essential? Aesthet Surg J. 2018;38:276–278. [DOI] [PubMed] [Google Scholar]
- 27.Flores-Mireles AL, Walker JN, Bauman TM, et al. Fibrinogen release and deposition on urinary catheters placed during urological procedures. J Urol 2016;196(2):416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfram D, Dolores W, Rainer C, et al. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J Autoimmun. 2004;23:81–91. [DOI] [PubMed] [Google Scholar]
- 29.Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg. 2007;120:275–284. [DOI] [PubMed] [Google Scholar]
- 30.Rieger UM, Pierer G, Lüscher NJ, et al. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast Surg. 2009;33:404–408. [DOI] [PubMed] [Google Scholar]
- 31.Deva AK, Adams WP, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319–1328. [DOI] [PubMed] [Google Scholar]
- 32.Jacombs A, Allan J, Hu H, et al. Prevention of biofilm-induced capsular contracture with antibiotic-impregnated mesh in a porcine model. Aesthet Surg J. 2012;32:886–891. [DOI] [PubMed] [Google Scholar]
- 33.Adams WP, Jr., Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;118:46S–52S. [DOI] [PubMed] [Google Scholar]
- 34.Giordano S, Peltoniemi H, Lilius P, et al. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative study. Aesthet Surg J. 2013;33:675–680. [DOI] [PubMed] [Google Scholar]
- 35.Burkhardt BR, Dempsey PD, Schnur PL, et al. Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg. 1986;77:919–932. [PubMed] [Google Scholar]
- 36.Drinane JJ, Bergman RS, Folkers BL, et al. Revisiting triple antibiotic irrigation of breast implant pockets: a placebo-controlled single practice cohort study. Plast Reconstr Surg Glob Open. 2013;1:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drinane JJ, Kortes MJ, Bergman RS, et al. Evaluation of antibiotic irrigation versus saline irrigation in reducing the long-term incidence and severity of capsular contraction after primary augmentation mammoplasty. Ann Plast Surg 2016. Jan;77(1):32–6. [DOI] [PubMed] [Google Scholar]
- 38.Poppler L, Cohen J, Dolen UC, et al. Histologic, molecular, and clinical evaluation of explanted breast prostheses, capsules, and acellular dermal matrices for bacteria. Aesthet Surg J. 2015;35:653–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kane TL, Carothers KE, Lee SW. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr Drug Targets. 2018;19:111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paharik AE, Schreiber HLt, Spaulding CN, et al. Narrowing the spectrum: the new frontier of precision antimicrobials. Genome Med. 2017;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moser C, Pedersen HT, Lerche CJ, et al. Biofilms and host response—helpful or harmful. APMIS. 2017;125:320–338. [DOI] [PubMed] [Google Scholar]
- 42.Dolen UC, Schmidt AC, Um GT, et al. Impact of neoadjuvant and adjuvant chemotherapy on immediate tissue expander breast reconstruction. Ann Surg Oncol. 2016;23:2357–2366. [DOI] [PubMed] [Google Scholar]
- 43.Deva AK. Commentary on: does implant insertion with a funnel decrease capsular contracture? A preliminary report. Aesthet Surg J. 2016;36:557–558. [DOI] [PubMed] [Google Scholar]
- 44.Flugstad NA, Pozner JN, Baxter RA, et al. Does implant insertion with a funnel decrease capsular contracture? A preliminary report. Aesthet Surg J. 2016;36:550–556. [DOI] [PubMed] [Google Scholar]
- 45.Perez PF, Doré J, Leclerc M, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–e732. [DOI] [PubMed] [Google Scholar]
- 46.Albesharat R, Ehrmann MA, Korakli M, et al. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst Appl Microbiol. 2011;34:148–155. [DOI] [PubMed] [Google Scholar]
- 47.Zdolsek J, Eaton JW, Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med. 2007;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellis TM, Hardt NS, Campbell L, et al. Cellular immune reactivities in women with silicone breast implants: a preliminary investigation. Ann Allergy Asthma Immunol. 1997;79:151–154. [DOI] [PubMed] [Google Scholar]
- 49.Arrecubieta C, Lee MH, Macey A, et al. SdrF, a Staphylococcus epidermidis surface protein, binds type I collagen. J Biol Chem. 2007;282:18767–18776. [DOI] [PubMed] [Google Scholar]
- 50.Bowden MG, Visai L, Longshaw CM, et al. Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin? J Biol Chem. 2002;277:43017–43023. [DOI] [PubMed] [Google Scholar]
- 51.Gerlach GF, Clegg S, Allen BL. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989;171:1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samen U, Heinz B, Boisvert H, et al. Rga is a regulator of adherence and pilus formation in Streptococcus agalactiae. Microbiology. 2011;157:2319–2327. [DOI] [PubMed] [Google Scholar]
- 53.Spaulding CN, Schreiber HLt, Zheng W, et al. Functional role of the type 1 pilus rod structure in mediating host-pathogen interactions. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen DA, Pinkner JS, Jones JM, et al. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect Immun. 2008;76:3337–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flores-Mireles AL, Walker JN, Potretzke A, et al. Antibody-based therapy for enterococcal catheter-associated urinary tract infections. MBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]


