Abstract
Background:
There are studies demonstrating an increased expression of cyclooxygenase (COX) in keloids and hypertrophic scars, suggesting that anti-inflammatory drugs could be used in their treatment. However, a precise relationship between COX and pathological scarring has not been established in the literature yet. This study aims to evaluate the immunohistochemical expression of COXs in these scars.
Methods:
Prospective study, including 54 patients (aged 18–60 years) undergoing scar excision: 18 normal scars (group 1), 18 hypertrophic scar (group 2), and 18 keloids (group 3). The group classification was performed by clinical criteria. Scars samples were collected and anatomopathological examination (through hematoxylin-eosin method) was performed to confirm the scar type. Immunohistochemistry was performed to assess the expression of COX1 and COX2 in epidermis and dermis. Results were compared among all groups and between group I versus II and III together (abnormal scars).
Results:
For COX1, in the epidermis, there was no significant difference in the immunohistochemical expression when comparing the 3 groups. In the dermis, groups 2 and 3 had greater expression than group 1, with a significant difference being found when comparing all groups (P = 0.014), and in the comparison between normal versus abnormal scars (P = 0.004). For COX2, there was no significant difference between the groups in both the epidermis and dermis.
Conclusions:
The immunohistochemical expression of COX1 was greater in the dermis of abnormal scars when compared with normal scars. Future studies can be performed involving COX blockade as a perspective of these scars treatment.
INTRODUCTION
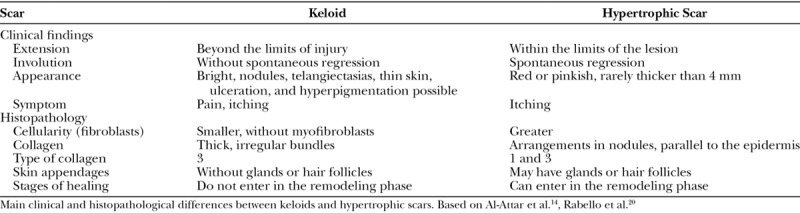
Keloids and hypertrophic scars (HS) are disorders characterized by excessive accumulation of extracellular matrix produced by fibroblasts.1,2 Keloids are often confused with HS. Clinically, HS do not extend the lesion’s border, often regress, and have a better prognosis than the keloids.3 Although there are no exact criteria for its histopathological differentiation, keloids have less cellularity and thick collagen bundles with irregular patterns, whereas the HS have more fibroblast proliferation and collagen fibers in nodules parallel to the epidermis (Table 1).4,5
Table 1.
Differences between Keloids and Hypertrophic Scars
The inflammatory phase of the healing may be related to the formation of these pathologic scars. The derivatives of arachidonic acid (AA), mainly prostaglandins (PGs) and leukotrienes, play a fundamental role in this process.6 The metabolism of the AA follows the pathway indicated by the enzyme that initiates its reaction: cyclooxygenase (COX) and lipoxygenase (Fig. 1).7,8 COX, also known as prostaglandin-endoperoxide synthase (PGHS) catalyzes the conversion of AA into PGs G2 and H2. The PGH2 is then converted into eicosanoids, such as PGE2, that promotes the recruitment of inflammatory cells, which release TGFβ, activating the fibroblasts and inducing the production of the extracellular matrix.6,9 The nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit COX and therefore the synthesis of PGs.6,7 There are at least 2 COX isoforms: COX1 and COX2.10 Both catalyze the same reaction. However, almost all normal tissues show an expression of COX1, which has a mainly homeostatic function, and low levels of COX2.11,12 COX2 is mainly induced by inflammatory stimuli. Therefore, specific inhibitors of COX2 have been developed to inhibit inflammation without blocking the protective effects of the constituent PGs. Studies on the distribution of COXs in skin are scarce. Rossiello et al.13 concluded that in normal skin COX1 is expressed both in the epidermis and the dermis, while COX2 is rarely found.
Fig. 1.
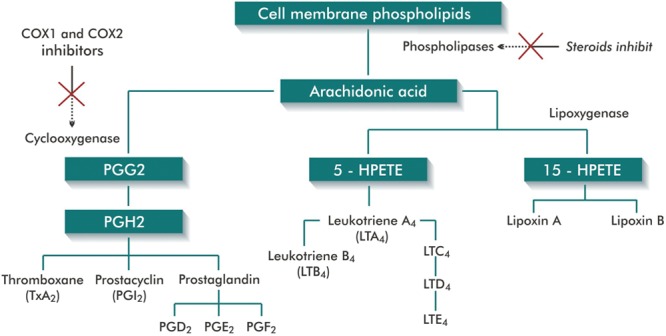
Metabolism of AA and lipoxygenase-cyclooxygenase pathways (Figure produced by the author, based on Stitham et al.8 and Kumar et al.7).
Several methods are described as treatment of pathologic scars, such as compression, massage, excision, topical or injectable corticosteroids, silicone gel, radiotherapy, cryotherapy, CO2 laser, intense pulsed light, 5-fluorouracil, mitomycin, bleomycin, and antihistamines. Most of these therapies have a high recurrence rate.14–16 Studies have suggested that pharmacological blockade of COX could be an adjuvant in the treatment of pathological scars.13,17,18 An experimental study showed a 50% reduction in PGE2 levels in wound healing with the application of celecoxib, with less scar tissue formation.18 It was also demonstrated that the immunohistochemical (IHC) expression of COXs in HS and keloids is greater than in normal scars. Kössi et al.19 found different COX1 and COX2 gene expressions in normal and abnormal scars. Therefore, COX activity may influence scar formation.13,17,18
The objective of this study was to compare the IHC expression of COXs in normal scars, HS, and keloids.
METHODOLOGY
A prospective study was conducted at the Universidade Federal de Ciências Médicas de Porto Alegre (UFCSPA), Rio Grande do Sul, Brazil. Fifty-four (54) consecutive patients (aged 18–60 years) were included and underwent excision of scars (18 normal, 18 hypertrophic, and 18 keloids) in the period from January 2014 to January 2015. The participants signed an informed consent form, which as approved along with the study, by the Research Ethics Committee UFCSPA, registered under number 24680913.3.0000.5345.
The excision of the scars was performed under local anesthesia with 2% lidocaine with epinephrine (1:200,000 - Xylestesin, from the brand Cristália, Itapira, Brazil). Fragments of the scars were collected and immediately stored in a 10% buffered formalin solution. Two examinations were performed:
Anatomopathological examination: hematoxylin-eosin (HE), to differentiate keloids, HS and normal scars.
IHC examination, to assess and quantify the expression of COX1 and COX2 in skin samples.
The examinations were performed in the Research Laboratory of the Postgraduate Program in Pathology at UFCSPA. The inclusion in the groups was performed according to the type of scar, by the clinical criteria (Table 1) confirmed by the anatomopathological examination.4,5,14,20 Group I: normal scars. Group II: HS. Group III: keloids. The clinical data collected were age, sex, skin type, cause, time, and location of the scar. Considering that the vast miscegenation hinders the inclusion of the various skin tones into static classifications, based on other studies, we opt to subdivide patients according to the Fitzpatrick scale, rather than classifying them by ethnicity or race.3,21
The IHC technique for COX1 and COX2 was performed according to the standard routines.22 The antibodies used were COX1: Clone RPR5866 from Abcam. As a positive control, human skin was used (dilution 1/200). COX2: Clone SP21 from Abcam. Positive Control - Breast carcinoma (diluiton 1/50). Secondary and tertiary antibody kit - universal Vectastain - Elite ABC kit.
The slides were analyzed using the Olympus BX51 microscope (Olympus DP72-optical), digitized with Olympus DP2-BSW 2.2 software. For each slide at high magnification, 5 fields were randomly chosen for the epidermis and 5 for the dermis, containing images of good quality. The percentage of positive cells was determined by counting 100 cells. It was considered a positive IHC expression when at least 30% of cells presented moderate to strong nuclear immunoreactivity.
Data Analysis
The data were analyzed with the software SPSS-22.0 (IBM, Armonk, N.Y.), by a professional statistician in the department of research support at UFCSPA. Values of P ≤ 0.05 were considered statistically significant. The analysis consisted of comparison between groups with respect to age, sex, skin type, cause, time, and location of the scar. The variables age and time with scar were analyzed using the analysis of variance test and the remaining variables by the χ2 test. For COX1 and COX2, a comparison of the IHC expression at epidermis and dermis was performed between the groups I, II, and III. Another analysis was performed comparing group I with pathological scars (groups II and III together). Statistical analysis was performed using Fisher’s exact test.
RESULTS
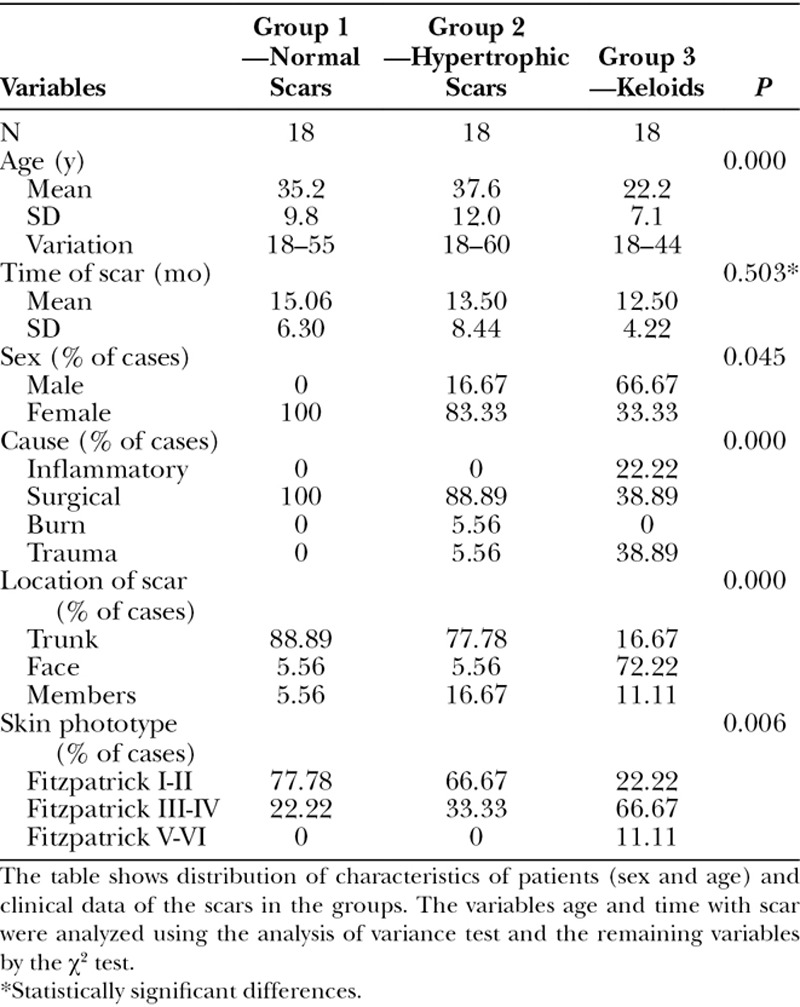
The distribution of the patients by age, sex, location of the scar, cause of the scar, time of the scar, and Fitzpatrick phototype can be seen in Table 2. The mean age of the patients obtained for group III (keloids) was significantly lower than in the other groups. No difference was obtained between the groups in relation to the time with scar. Group III had the highest concentration of the male sex. Regarding the cause of the scars, cases of inflammatory origin (acne) only occurring in the group of patients with keloids. Normal and HS occurred more in the torso, while keloids more in the face, because this group including a significant number of keloids in the earlobes. Regarding skin phototype, the groups of patients with Fitzpatrick I-II and III-IV presented more normal scars and HS than the V-VI types that had greater number of keloids.
Table 2.
Patient Characteristics and Clinical Data
The classification of the cases into the groups, initially based on the clinical criteria, was confirmed by HE staining in all cases.
The results of the IHC expression of the COXs are seen in Tables 3, 4 and charts of Figures 2, 3.
Table 3.
IHC Expression of COX1 in Skin Samples
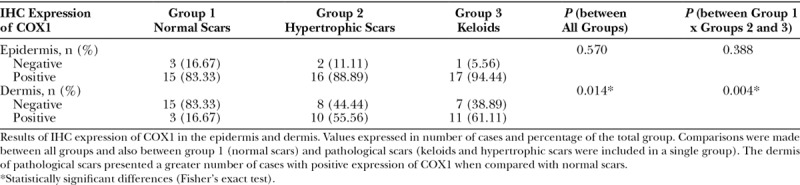
Fig. 2.
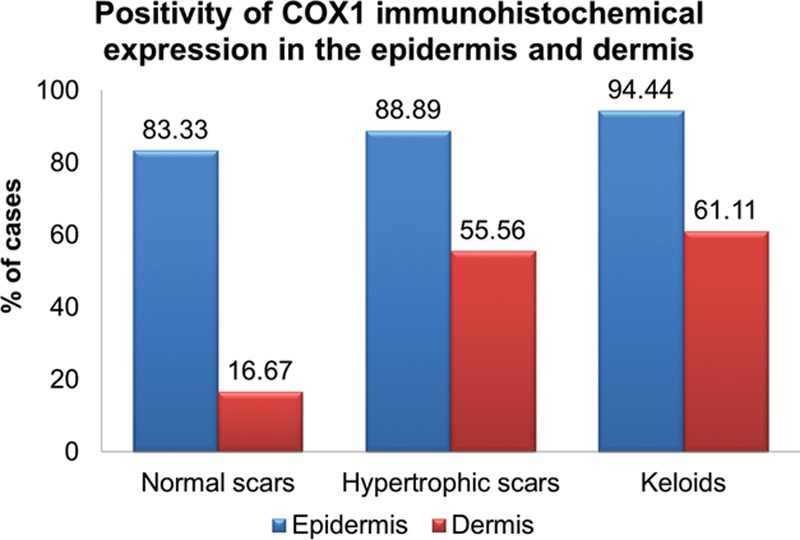
The graph shows the percentage of cases in each group in which the IHC expression of COX1 was positive. It is observed that, in the dermis there were more positive cases for COX1 in the groups of the hypertrophic and keloid scars compared with the group of normal scars.
Fig. 3.
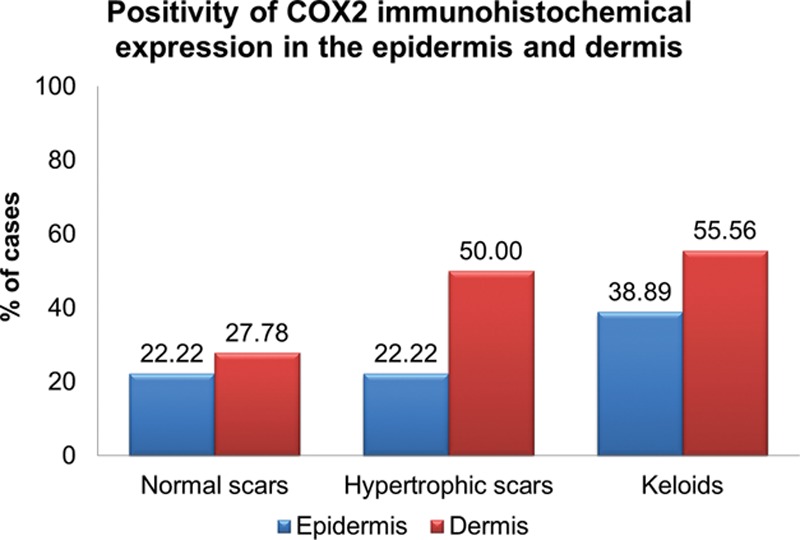
Graph showing the percentage of cases in each group with positive COX2 IHC expression. There was no statistically significant difference among the 3 groups in both the epidermis and the dermis.
Table 3 shows the results of the comparison of the IHC expression of the COX1 in the epidermis and dermis between all groups and between group 1 (normal scars) and groups 2 and 3 together (pathological scarring). There was no significant difference in the expression of COX1 in the epidermis in any comparison. In the dermis, however, a significant difference was obtained both in the comparison among all the groups (P = 0.014) and in the comparison between normal and pathological scars (P = 0.004): groups 2 and 3 had more cases with a positive expression of COX1 when compared with normal scars. Figures 4, 5 show IHC reaction with a positive expression of COX1 in the epidermis and dermis, respectively.
Fig. 4.
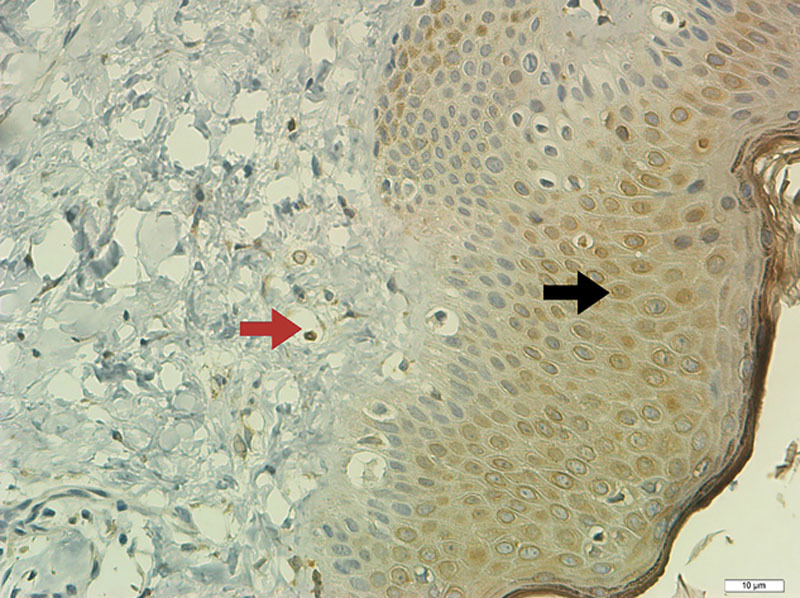
Field of view of a slide stained of hypertrophic scar (IHC technique for COX1) in the epidermis (×400). The black arrow shows cells with brown staining, demonstrating positive IHC expression for COX1. The red arrow indicates section of a vessel containing hemacy, not considered a positive count.
Fig. 5.
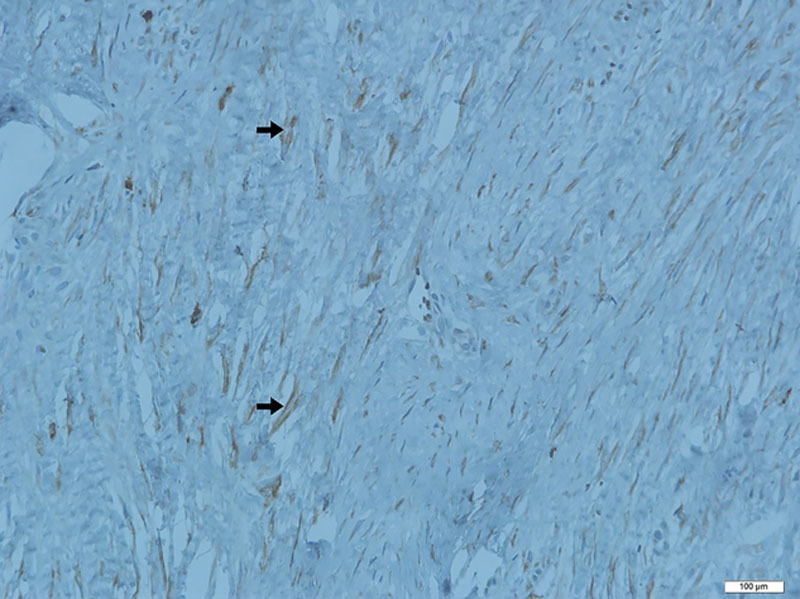
Sample of histological section of hypertrophic scar (IHC for COX1). Multiple fibroblasts (arrows) with brown staining showing a positive reaction in the dermis (×400).
Table 4 shows the results of the comparison of the IHC expression of the COX2 in the epidermis and dermis among all groups and between group 1 and groups 2 and 3 together. There was no statistical difference in the COX2 expression in any comparison, whether in the epidermis or the dermis. In the comparison between group 1 and groups 2 and 3 together, the data suggest a tendency toward greater positivity in the COX2 expression in the dermis for groups 2 and 3, although with P = 0.081. Figure 6 shows negative IHQ expression for COX2 in both epidermis and dermis of keloid scar.
Table 4.
IHC Expression of COX2 in Skin Samples
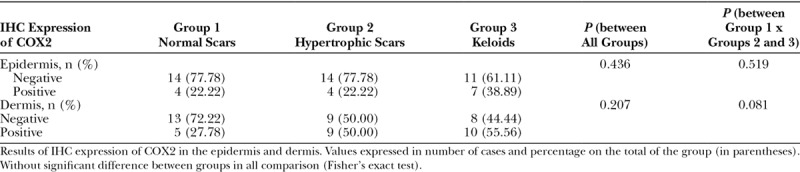
Fig. 6.
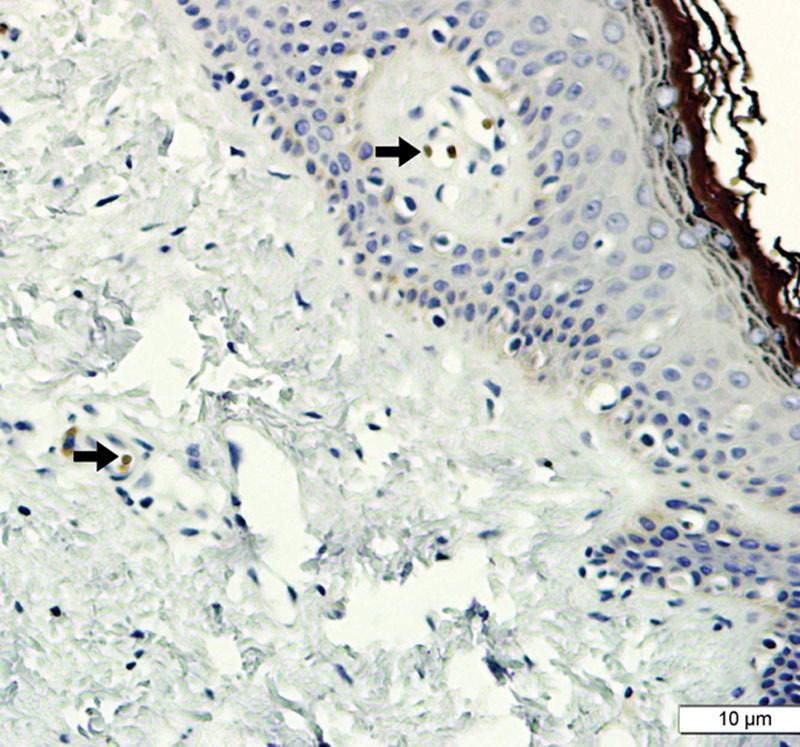
Image showing histological cut (IHC reaction for COX2), including dermis and epidermis (keloid type scar; ×400). No staining compatible with positivity is observed, except in blood vessels (arrows).
An intra-group comparison of IHQ expression of the COXs was also analyzed. In the epidermis, a positive COX1 expression was greater than that of COX2 in all types of scars. In the dermis, however, there was no significant difference between the COX1 and COX2 expression, in any comparison.
DISCUSSION
The pathologic scars represent frequent complications as a result of invasive procedures in certain medical specialties such as thoracic surgery, general surgery, gynecology, head and neck surgery, and dermatology.23–26 However, plastic surgery presents the greatest problems with keloids and HS, as the scar itself is an integral part of the outcome of the performed treatments. In addition to the aesthetic and functional disorders, these complications lead to lawsuits, resulting from dissatisfaction with the outcome obtained (Figs. 7, 8).
Fig. 7.
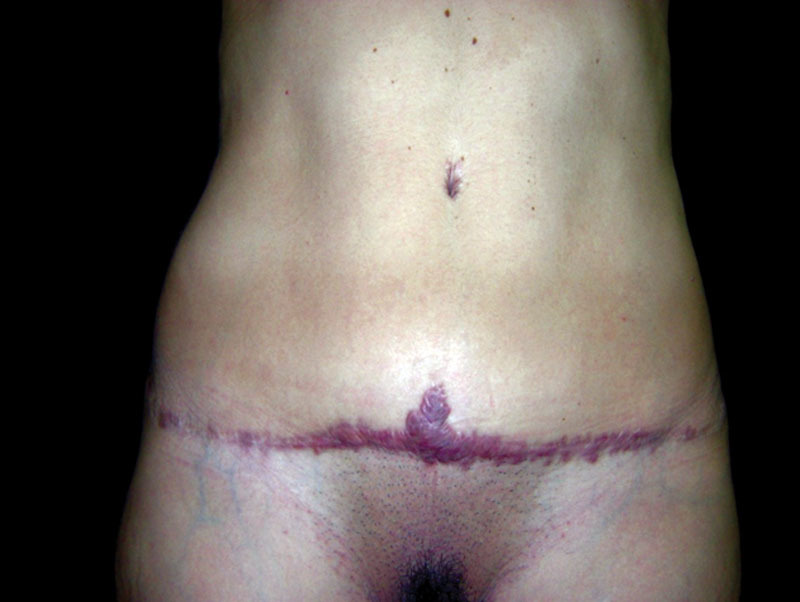
Keloid scar after-abdominoplasty, which extends beyond the limits of the surgical incision (photograph of the author’s collection). Aesthetic result highly compromised by the poor quality of the scar.
Fig. 8.
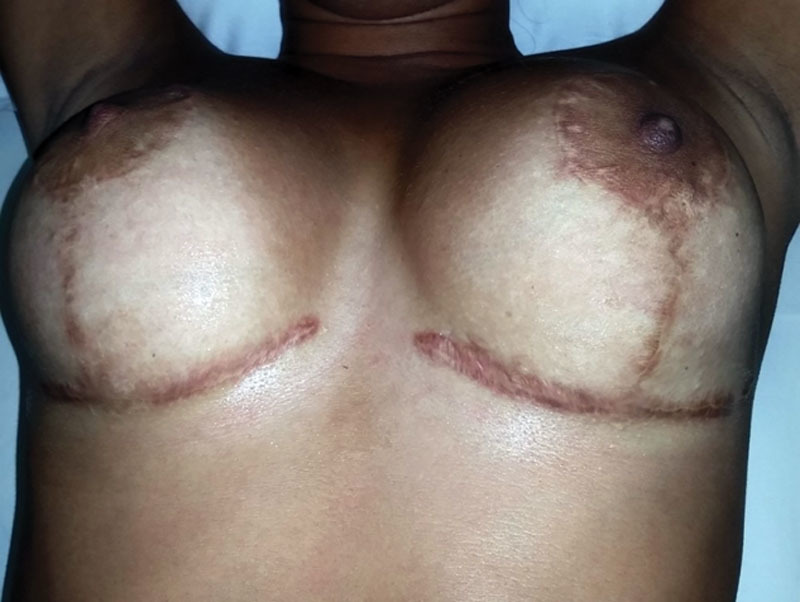
Hypertrophic scars - postmammoplasty (photograph of the author’s collection). The image exemplifies the impact of hypertrophic scars on results of plastic surgeries and the consequent risks of lawsuits.
The pathogenesis of keloids and HS is not fully understood. Alterations in growth factor regulation, failure in collagen remodeling, genetic disorders, and immunological dysfunctions have been implicated.14 Other causes may be endocrine and neural factors. Psychiatric diseases could also be involved.3,27,28 Although the role of PGs in healing is not definitely established, it is known that they can induce the in vitro proliferation of fibroblasts and collagen production in wounds, in vivo. Knowledge of wound healing without scars in fetuses suggests that the PGs and the respective inflammatory response induced control, at least in part, of the amount of fibrosis formed after skin injury and repair.9,29,30 Skin lesions produced in the first and second halves of fetal life can evolve without scars, with regeneration of normal skin, including the growth of skin appendages. It is noteworthy that this phenomenon of healing without scars occurs in the absence of inflammation. It is assumed, therefore, that the inflammatory phase of wound repair results in the production of scar tissue.30–32 Wilgus et al.32 studied fetal healing in mice. The COX2 expression and the ability of exogenous PGE2 to alter the wound healing was examined. The authors concluded that the COX2 pathway is involved in fetal wound healing, and treatments targeted at its blockade could limit the formation of skin scars in adults.32 The same authors demonstrated scar reduction in mice with the topical administration of a COX2 blocker. Celecoxib inhibited various parameters of inflammation at the wound site, leading to the reduction of scar tissue. The authors suggested that adult wounds could have a reduction in the inflammatory stage and that NSAIDs could improve the healing process.18 It is important to reiterate that keloids occur exclusively in humans3,14 and therefore the results of this study, conducted on mice, could not be safely extrapolated to our species. In our study, the IHC expression of COX2 was not significantly different between the groups, and we could infer that the topical application of COX2 blocker may not be beneficial in the treatment of these scars.
One possible disadvantage of the use of celecoxib would be its potential influence on delayed healing of surgical wounds.33 Su et al.34 consider that the NSAIDs, inhibitors of COX2, could, by reducing PGE2 production, exacerbate the formation of excessive scarring, especially when used in the final period of the proliferative phase.
Similar to our study, Abdou et al.17 evaluated the pattern and location of COX1 and COX2 in scars. Forty patients were included (15 HS, 15 keloids, and 10 normal scars). The immunoreactivity was considered positive when any expression was identified. The intensity of expression was evaluated subjectively as light, moderate, or strong. The difference in the COX1 expressions in normal scars, HS, and keloids (40%, 53.3% and 100%, respectively) was statistically significant. There was no significant difference in any comparison in the COX2 expression. These data are similar to those obtained in our study. It is relevant to point out that in the research of Abdou et al.,17 the group of normal scars was composed of 10 cases retrieved from the pathology department’s files. It is known that IHC reaction is sensitive to a vast number of factors, including the solution’s buffering method for the preservation of the tissue sample.35 Conversely, in our study, all samples of skin were handled and processed by the same methodology, in an effort to minimize errors. Another difference between our study and that of Abdou et al.17 is that they performed the cell count including fibroblasts, endothelial cells, and inflammatory cells. We chose to disregard the vascular endothelium cell count as our goal was to evaluate only the cell responsible for the fibroplasia, the fibroblast (Fig. 9).
Fig. 9.
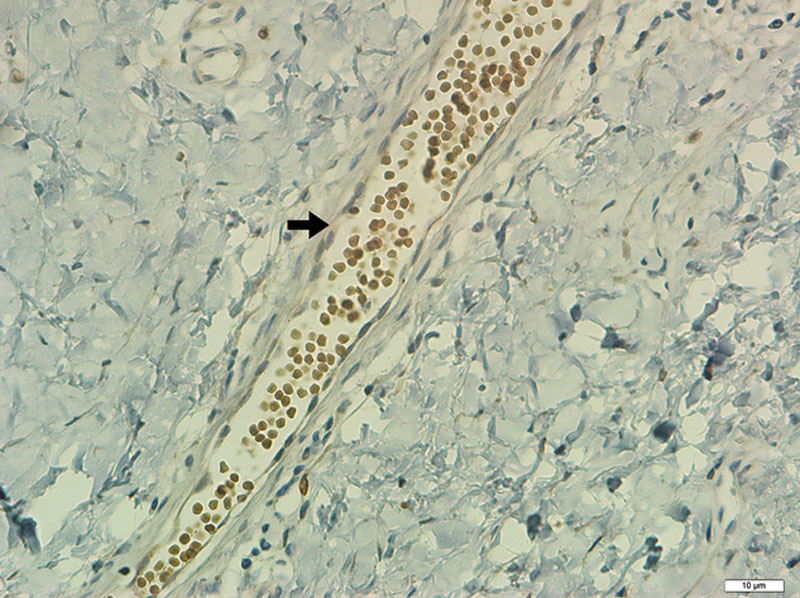
Histologic section of dermis with blood vessel (not considered in the evaluation of IHC expression), surrounded by connective tissue (COX2, ×400).
In our study, although we observed greater IHC expression of COX1 in pathological scars if compared with normal scars, we did not identify significant differences between keloids and HS. In turn, Rossiello et al.13 found differences in this comparison. They studied 36 cases of patients with keloids, 32 cases of HS, and 25 cases of normal skin, aiming to define the location and expression of COX1 and COX2. The results showed an increased expression of COX1 in HS when compared with normal skin and keloids and an increased expression of COX2 in keloids when compared with normal skin and HS. The authors concluded that COX1 is involved in the formation of HS and COX2 in the formation of keloids. In this study, the cases of pathological scars were compared with normal skin, rather than normal scars, as carried out in our study.
We believe that some variables could influence the results of studies. The IHC reaction comprises diverse and complex processes at all stages, and therefore with potential errors. Without standardization, the technique can result in nonreproducible and unreliable data. The methodology used for evaluating the intensity of the IHC expression is critical. In addition to the technical details involved with its execution, the variety of criteria adopted as the cutoff for positivity can make studies incomparable.36 Other factors that could influence the results would be the inherent difficulty to categorize the patients in the groups. In our study, patients were included consecutively, according to the type of scar, disregarding of other variables. The group of patients with keloids had lower mean age and higher concentration of men than other groups, since it included a higher number of posttraumatic scars, more prevalent in this population range. The highest concentration of patients with keloids and HS in patients with Fitzpatrick III-VI phototypes is compatible with the frequency described for these diseases. In agreement with our findings, in the study performed by Abdou et al.,17 the distribution of the patients in groups was also unequal in relation to the mean age and the cause of the scars. Patients with normal scars had the surgical incisions as their main cause, while HS keloids are predominantly caused by trauma or burns.17 The study of Rossiello et al.13 did not describe the characteristics of the groups and therefore did not analyze their possible differences.
Even though there is a tendency in the studies to show a positive relationship between the increased expression of the COXs and the HS and keloids, the data are not conclusive. In general, the inducible COX2, is known to be involved in pathological processes, whereas COX1 is in physiological processes. However, in our study, the enzyme that was shown to have the greatest expression in pathological scars was COX1. The number of studies is small with heterogeneous results. New researches could even use alternative methods for the IHC reaction. Although it is not possible to precisely qualify and quantify the relationship between COX and scarring, their expressions are not the same in the different types of scars, demonstrating that there is certain influence of them in this process.
CONCLUSIONS
The comparison of the IHC expression of the COX1 in the epidermis of scars did not show significant difference among groups. However, in the dermis, we obtained a significant difference: the groups of pathologic scars had more cases with a positive expression of the COX1 when compared with group of normal scars. The results of the IHC expression of the COX2 in the epidermis and dermis did not show any statistical difference when compared.
Footnotes
Published online 12 February 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Louw L. The keloid phenomenon: progress toward a solution. Clin Anat. 2007;20:3–14. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Bock O, Bayat A, et al. Decreased expression of inhibitory SMAD6 and SMAD7 in keloid scarring. J Plast Reconstr Aesthet Surg. 2006;59:221–229. [DOI] [PubMed] [Google Scholar]
- 3.Hochman B, Farkas CB, Isoldi FC, et al. Keloid and hypertrophic scar distribution according to Fitzpatrick skin phototypes. Rev Bras Cir Plast. 2012;27:4. [Google Scholar]
- 4.Lee JY, Yang CC, Chao SC, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004;26:379–384. [DOI] [PubMed] [Google Scholar]
- 5.Jumper N, Paus R, Bayat A. Functional histopathology of keloid disease. Histol Histopathol. 2015;30:1033–1057. [DOI] [PubMed] [Google Scholar]
- 6.Henry G, Garner WL. Inflammatory mediators in wound healing. Surg Clin North Am. 2003;83:483–507. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Abbas A, Aster J. Inflammation and repair. Robins Basic Pathology. 20139th ed Philadelphia, Pa.: Elsevier Saunders. [Google Scholar]
- 8.Stitham J, Midgett C, Martin KA, et al. Prostacyclin: an inflammatory paradox. Front Pharmacol. 2011;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talwar M, Moyana TN, Bharadwaj B, et al. The effect of a synthetic analogue of prostaglandin E2 on wound healing in rats. Ann Clin Lab Sci. 1996;26:451–457. [PubMed] [Google Scholar]
- 10.Holtzman MJ, Turk J, Shornick LP. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J Biol Chem. 1992;267:21438–21445. [PubMed] [Google Scholar]
- 11.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 12.Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des. 2004;10:577–588. [DOI] [PubMed] [Google Scholar]
- 13.Rossiello L, D’Andrea F, Grella R, et al. Differential expression of cyclooxygenases in hypertrophic scar and keloid tissues. Wound Repair Regen. 2009;17:750–757. [DOI] [PubMed] [Google Scholar]
- 14.Al-Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286–300. [DOI] [PubMed] [Google Scholar]
- 15.Janis JE, Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2016;138:9S–17S. [DOI] [PubMed] [Google Scholar]
- 16.Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg. 2014;67:1017–1025. [DOI] [PubMed] [Google Scholar]
- 17.Abdou AG, Maraee AH, Saif HF. Immunohistochemical evaluation of COX-1 and COX-2 expression in keloid and hypertrophic scar. Am J Dermatopathol. 2014;36:311–317. [DOI] [PubMed] [Google Scholar]
- 18.Wilgus TA, Vodovotz Y, Vittadini E, et al. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003;11:25–34. [DOI] [PubMed] [Google Scholar]
- 19.Kössi J, Peltonen J, Uotila P, et al. Differential effects of hexoses and sucrose, and platelet-derived growth factor isoforms on cyclooxygenase-1 and -2 mRNA expression in keloid, hypertrophic scar and granulation tissue fibroblasts. Arch Dermatol Res. 2001;293:126–132. [DOI] [PubMed] [Google Scholar]
- 20.Rabello FB, Souza CD, Farina Júnior JA. Update on hypertrophic scar treatment. Clinics (Sao Paulo). 2014;69:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Leeuwen MC, van der Wal MB, Bulstra AE, et al. Intralesional cryotherapy for treatment of keloid scars: a prospective study. Plast Reconstr Surg. 2015;135:580–589. [DOI] [PubMed] [Google Scholar]
- 22.Alves VAV, Bacchi CE, Vassallo J. Manual de Imuno-Histoquimica. 1999São Paulo, Brazil: Sociedade Brasileira de Patologia. [Google Scholar]
- 23.Freuschle A, Brom J, Hörmann K, et al. [Functional and aesthetic therapy concepts of keloids in the head- and neck area]. Laryngorhinootologie. 2014;93:309–315. [DOI] [PubMed] [Google Scholar]
- 24.Furtado F, Hochman B, Ferrara SF, et al. What factors affect the quality of life of patients with keloids? Rev Assoc Med Bras (1992). 2009;55:700–704. [DOI] [PubMed] [Google Scholar]
- 25.Sakuraba M, Takahashi N, Akahoshi T, et al. Experience of silicone gel sheets for patients with keloid scars after median sternotomy. Gen Thorac Cardiovasc Surg. 2010;58:467–470. [DOI] [PubMed] [Google Scholar]
- 26.Young WG, Worsham MJ, Joseph CL, et al. Incidence of keloid and risk factors following head and neck surgery. JAMA Facial Plast Surg. 2014;16:379–380. [DOI] [PubMed] [Google Scholar]
- 27.Hochman B, Isoldi FC, Furtado F, et al. New approach to the understanding of keloid: psychoneuroimmune-endocrine aspects. Clin Cosmet Investig Dermatol. 2015;8:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochman B, Nahas FX, Sobral CS, et al. Nerve fibres: a possible role in keloid pathogenesis. Br J Dermatol. 2008;158:651–652. [DOI] [PubMed] [Google Scholar]
- 29.Durant S, Duval D, Homo-Delarche F. Effect of exogenous prostaglandins and nonsteroidal anti-inflammatory agents on prostaglandin secretion and proliferation of mouse embryo fibroblasts in culture. Prostaglandins Leukot Essent Fatty Acids. 1989;38:1–8. [DOI] [PubMed] [Google Scholar]
- 30.Lupulescu A. Effect of prostaglandins on protein, RNA, DNA and collagen synthesis in experimental wounds. Prostaglandins. 1975;10:573–579. [DOI] [PubMed] [Google Scholar]
- 31.Smith PD, Kuhn MA, Franz MG, et al. Initiating the inflammatory phase of incisional healing prior to tissue injury. J Surg Res. 2000;92:11–17. [DOI] [PubMed] [Google Scholar]
- 32.Wilgus TA, Bergdall VK, Tober KL, et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol. 2004;165:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairweather M, Heit YI, Buie J, et al. Celecoxib inhibits early cutaneous wound healing. J Surg Res. 2015;194:717–724. [DOI] [PubMed] [Google Scholar]
- 34.Su WH, Cheng MH, Lee WL, et al. Nonsteroidal anti-inflammatory drugs for wounds: pain relief or excessive scar formation? Mediators Inflamm. 2010;2010:413238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin F, Chen Z. Standardization of diagnostic immunohistochemistry: literature review and geisinger experience. Arch Pathol Lab Med. 2014;138:1564–1577. [DOI] [PubMed] [Google Scholar]
- 36.Leong AS. Quantitation in immunohistology: fact or fiction? A discussion of variables that influence results. Appl Immunohistochem Mol Morphol. 2004;12:1–7. [DOI] [PubMed] [Google Scholar]


