Abstract
Background
Various pharmacological treatments have been suggested to treat osteonecrosis of the femoral head. However, their practicability remains a controversial issue.
Methods
We systemically reviewed articles published during last 20 years to assess the efficacy and safety of the pharmacological treatments.
Results
To date, enoxaparin, statins, bisphosphonates, iloprost and acetylsalicylic acid have been practiced for the treatment of osteonecrosis. However, none of them were proven to be effective by high level studies, and most of them have adverse reactions.
Conclusions
No pharmacological prevention or treatment of osteonecrosis is recommendable at this moment.
Keywords: Bone remodeling, Diphosphonates, Drug therapy, Osteonecrosis
INTRODUCTION
Osteonecrosis of the femoral head frequently leads to a collapse of the necrotic portion and subsequent degenerative osteoarthritis of the hip. The disease is the most common diagnosis leading to total hip arthroplasty (THA) in young adults.[1,2] It is becoming more prevalent due to increasing use of steroids in the adjuvant therapy for malignant neoplasms and management of organ transplantation.[3,4,5] In the US, 10,000 to 20,000 new patients are affected with the disease.[6] In nationwide surveys from South Korea, the annual prevalence was more than 10,000.[7] The disease is a socioeconomic burden world widely.
To determine the efficacy of pharmacological treatments for osteonecrosis, we searched literatures published from January 1999 and to December 2018. To date, there had been 2 meta-analysis regarding medical treatment of osteonecrosis, but they were restricted to the efficacy of bisphosphonate.[8,9]
Other studies were level III/IV studies, which included small number of patients. Due to a lack of study number and paucity of level I/II study, meta-analysis of other pharmacological treatments were not conductible. Thus, we systemically reviewed articles published during last 20 years and described the current knowledge on pathomechanism and various pharmacological treatments of femoral head osteonecrosis.
ETIOLOGY AND PATHOGENESIS
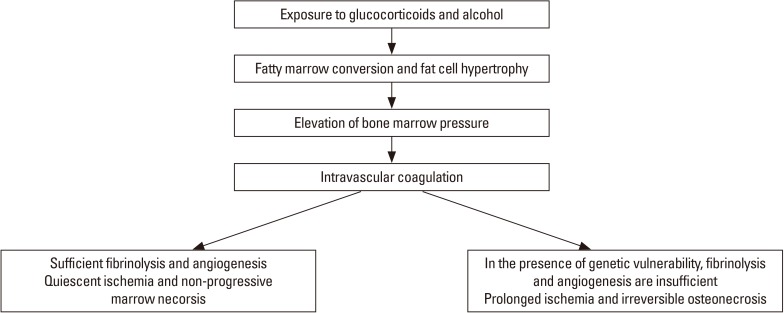
Femoral head osteonecrosis has a multifactorial etiology including genetic predispositions and exposure to risk factors, which play roles together in the development of the disease. Ischemia due to a disruption of blood flow seems to be the most probable cause of the disease. The pathomechanism is an evolutionary process involving (1) marrow necrosis and osteocyte death; (2) reparative process around the necrotic zone; and (3) collapse of necrotic bone and subsequent degenerative arthritis of the hip (Fig. 1).[10]
Fig. 1. Pathogenesis of femoral head osteonecrosis.
Steroids and alcohol induce hypertrophy of marrow fat cell, intraosseous hypertension, compression of venous sinusoids and intravascular coagulation (IC) in the proximal femur. The coagulation leads to an ischemia and marrow necrosis. Various hereditary and genetic factors are related with thrombophilia/hypofibrinolysis and impaired angiogenesis. In the absence of these factors, a complete fibrinolysis and sufficient angiogenesis occur and the ischemic insult is reversible and not progressive. However, when the marrow necrosis is associated with these factors, the ischemia is prolonged and the necrosis is progressive to a sequestrum. When the sequestrum is larger than 1 cm, creeping substitution does not occur. Instead, a foreign body reaction occurs around the sequestrum. Histiocytes and giant cells aggregate and forms a fibrous capsule, which appears as a band lesion on magnetic resonance imaging (MRI). In the sequestrum dead marrow releases fatty acid, which saponifies with calcium to form a soap. The dead bone and saponified marrow do not attain the previous mechanical strength and a fatigue fracture occurs at the subchondral portion (crescent sign), which leads to collapse and degenerative arthritis of the hip. The progression of ischemia to osteonecrosis depends on the restoration of vascular perfusion by fibrinolysis and reparative angiogenesis, which affects the viability of osteocytes.
PHARMACOLOGICAL TREATMENTS
According to this pathomechanism, various medical treatments have been suggested and practiced during decades.
1. Enoxaparin
To date, 2 human studies reported the efficacy of enoxaparin in early stage osteonecrosis. Glueck et al.[11] postulated that enoxaparin facilitates fibrinolysis of intravascular thrombi, improves blood flow, reverses hypoxia, and allows healing of dead bone. They reported a pilot study including 16 patients (25 hips), who had primary osteonecrosis associated with thrombophilic-hypofibrinolytic disorders, and 12 patients (15 hips), who had secondary osteonecrosis associated with alcohol or steroid. Patients were treated with enoxaparin for (60 mg/day) for 3 months. Femoral head collapse occurred in only 12% of patients with primary osteonecrosis. However, in patients with secondary osteonecrosis, enoxaparin did not stop the progression of the disease. Additionally, Glueck et al.[12] reported that, after enoxaparin trial, 6 patients with familial thrombophilia received long-term oral anticoagulant therapy and had no radiographic progression for 4 to 16 years.
Chotanaphuti et al.[13] retrospectively reviewed 36 patients who had early stage osteonecrosis of the femoral head. Eighteen patients (26 hips) received 6,000 units of enoxaparin daily for 12 weeks, while the remaining 18 patients (23 hips) did not receive the medication. At a minimum follow-up of 2-years, 15 hips (57.7%) in the study group and 5 hips (21.7%) in the control group remained in the pre-collapse stage (P=0.042).
Although there were no serious adverse effects including bleeding in these studies, we should consider the balance of bleeding risk versus benefit in most osteonecrosis patients without any clear evidence of thrombophilia.
2. Lipid lowering agents
Glucocorticoids increase the fat content in the bone marrow and the risk for osteonecrosis.
Statins are lipid-lowering agents, which are used to decrease the risk of cardiovascular diseases. Several studies showed that statins may have a protective effect against osteonecrosis in steroid-users.[14,15]
Pritchett [14] reviewed 284 statin-user patients who were treated with high-dose steroids. Those patients were followed for a minimum of 5 years, and osteonecrosis developed in only 1% of them. This incidence was much lower than reportedly known 3% to 20% incidence of osteonecrosis develop in patients receiving high-dose steroids.
Ajmal et al.[16] analyzed their database of 2,881 renal transplantation patients to determine whether statin reduces the incidence of glucocorticoid-associated osteonecrosis. Among 338 statin-users, 15 (4.4%) developed osteonecrosis, while 180 of 2,543 (7%) non-users developed the disease. The osteonecrosis-free survival was similar in patients with and without statin exposure.
These few human studies are level 3 or 4 evidence studies, and there are no randomized controlled trials on the preventive effects of statins on osteonecrosis.
Despite their safety and popularity, statins are known to have some degree of risk. Long-term use of statin might increase the incidence of diabetes, damage the liver and induce cognitive disorders.[17]
Randomized controlled trials and risk-benefit assessment are required before the use of statins for the prevention of osteonecrosis in steroid users.
3. Bisphosphonates
Bisphosphonates inhibit the activity of the osteoclasts. Early studies showed that alendronate significantly reduced the incidence of femoral head collapse in osteonecrotic hips. In 2005, Lai et al.[18] reasoned that alendronate might delay or even prevent collapse of osteonecrotic femoral head. They randomized 40 patients (54 hips) with early stage osteonecrosis of the femoral head osteonecrosis into alendronate group and control group. They prescribed 70 mg of oral alendronate every week during 25 weeks for the test group. As of 2 year follow-up, collapse occurred in only 2 of 29 femoral heads in the alendronate group, whereas 19 of 25 femoral heads in the control group collapsed. One hip in the alendronate group and 16 hips in the control group underwent THA.[18]
Agarwala et al.[19] medicated 60 patients (100 osteonecrotic hips) with alendronate (10 mg/day or 70 mg/week) along with 500 to 1,000 mg of daily calcium and vitamin D supplements. Patients had a significant reduction in pain and significant increase in walking time. All hip movements improved at 1 year with an insignificant decline after that. On radiological evaluation, the disease were stabilized or progressed by 1 grade. MRI showed a decrease in marrow edema in most cases. Only 6 patients (10 hips) required surgery.
Nishii et al.[20] reported similar results. In their study, collapse of the necrotic femoral head occurred in 6 of 13 hips in the control group and in 1 of 20 hips in the alendronate group.
However, later studies showed that alendronate has no effect on reducing disease progression or preventing the necessity for THA. Chen et al.[21] performed a 2-year multicenter, prospective, randomized, double-blind, placebo-controlled study on 64 patients with osteonecrosis. Sixty-five hips of 52 patients were assessed in final analysis. Four of 32 hips in the alendronate group and 5 of 33 hips in the placebo group underwent THA. There were no differences in disease progression, Harris Hip Scores, or Short Form 36 scores between the 2 groups.
Lee et al.[22] conducted a 2-year prospective, randomized, open-label, multicenter study on 110 patients with early stage osteonecrosis and a medium to large necrotic area (>30%) to determine whether zoledronate prevents collapse and reduces the need for THA. Fifty-five patients in the zoledronate group received 5 mg of zoledronate intravenously per year for 2 years, while 55 patients in the control group did not receive this medication. During the 2-year follow-up, 29 femoral heads in the zoledronate group and 22 in the control group collapsed. Nineteen hips in the zoledronate group and 20 in the control group underwent THA. In their study, zoledronate neither prevented the femoral head collapse nor reduced the need for THA.
There were 2 meta-analysis on the efficacy of bisphosphonate.[8,9] In a meta-analysis, Yuan et al.[8] identified 5 eligible trials involving 329 subjects with 920.9 patient-years of follow-up. Bisphosphonate did not improve Harris hip score, did not prevent femoral head collapse, and did not reduce the rate of THA.
Li et al.[9] identified 16 animal studies and 7 human trials on the efficacy of bisphosphonates. They found that bisphosphonates improved bone remodeling in the animal studies. However, bisphosphonates were not effective in terms of hip pain, Harris score, the occurrence rate of femoral head collapse, and the necessity of THA in the human trials.
The esophageal or gastric irritation is an established adverse effect of the oral bisphosphonates. Other known adverse effects are osteonecrosis of the jaw and insufficiency fractures of the femur.[23] Because of their unclear preventive effect and known adverse drug reactions, bisphosphonates are not recommended in patients with femoral head osteonecrosis.
4. Iloprost
Iloprost is a synthetic analogue of prostaglandin I2, which dilates systemic and pulmonary vascular beds. Recently, it has been used for the treatment of osteonecrosis.
Jager et al.[24] treated 95 patients (186 bones) with iloprost for bone marrow edema syndrome/osteonecrosis. At an average follow-up of 33 months, pain levels reduced and functional scores improved in the course of treatment. However, they included bone marrow edema patients as well as osteonecrosis patients and their diagnostic and inclusion criteria were uncertain.
Claβen et al.[25] treated 108 patients with 136 osteonecrosis of various joints using iloprost. Their patients were followed for a mean of 50 months. Most of them (74.8%) had a decrease of Visual Analog Scale pain scores after the treatment. However, 20% of the joints with Association for Research on Osseous Circulation (ARCO) stage 2, 71% with ARCO 3 and 100% with ARCO 4 underwent subsequent total joint arthroplasty. One limitation of their study included heterogeneous patient population. Moreover, they did not consider the size of necrotic portion, which is the major determinant for further collapse.
Common adverse reactions after the use of iloprost are flushing, cough, headache, flu-like syndrome, nausea, hypotension, insomnia, and syncope. Serious adverse events include congestive heart failure, supraventricular tachycardia, and renal failure.[26]
Although the study of Jager et al.[24] and that of Claβen et al.[25] suggested that iloprost is effective in bone marrow edema syndrome and early stage osteonecrosis, previous study showed that bone marrow edema syndrome and ischemic lesion with marrow necrosis do not progress to definite osteonecrosis.[10] Thus, further studies on the safety and efficiency of iloprost are mandatory.
5. Acetylsalicylic acid (ASA)
ASA binds to acetylates serine residues in cyclooxygenases and inhibits the biosynthesis of prostaglandins. It also inhibits platelet aggregation and prevents arterial and venous thrombosis.
Albers et al.[27] treated 10 patients (12 hips), who had precollapse osteonecrosis of the femoral head, with daily 81 mg of ASA. Collapse of the femoral head occurred only in 1 patient (8%) at a mean follow-up of 3.7 years.
However, their study involved only 10 patients, and 9 of their patients had Japanese Investigation Committee type B or C1 lesions.[28] These medium size lesions are at a moderate risk of further collapse without any intervention.
CONCLUSIONS
The efficacy of any phamacological treatment should be determined cautiously. The natural course of pain in early stage osteonecrosis and the size of necrotic portion should be counted in the evaluation. In the very early stage of osteonecrosis after acute ischemic attack, marrow edema develops around the necrotic portion of the femoral head and patient suffers severe pain even prior to collapse. The pain improves spontaneously according to the resolution of edema without any treatment.[29] This spontaneous improvement of pain might be considered as an effect of medication. In small medially located lesions, collapse of the femoral head does not develop even without any medical or surgical intervention.[28,30,31]
Various medical treatments have been introduced during decades. However, whether these treatments really retard or reverse the disease progression is still unclear. Considering risk and lack of evidence, no pharmacological prevention or treatment of osteonecrosis is recommendable at this moment.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Schneider W, Knahr K. Total hip replacement in younger patients: survival rate after avascular necrosis of the femoral head. Acta Orthop Scand. 2004;75:142–146. doi: 10.1080/00016470412331294385. [DOI] [PubMed] [Google Scholar]
- 2.Hungerford DS. Treatment of osteonecrosis of the femoral head: everything's new. J Arthroplasty. 2007;22:91–94. doi: 10.1016/j.arth.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Shibatani M, Fujioka M, Arai Y, et al. Degree of corticosteroid treatment within the first 2 months of renal transplantation has a strong influence on the incidence of osteonecrosis of the femoral head. Acta Orthop. 2008;79:631–636. doi: 10.1080/17453670810016641. [DOI] [PubMed] [Google Scholar]
- 4.Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992;(277):12–21. [PubMed] [Google Scholar]
- 5.Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford) 2011;50:2023–2028. doi: 10.1093/rheumatology/ker277. [DOI] [PubMed] [Google Scholar]
- 6.Hungerford DS. Osteonecrosis: avoiding total hip arthroplasty. J Arthroplasty. 2002;17(4 Suppl 1):121–124. doi: 10.1054/arth.2002.33300. [DOI] [PubMed] [Google Scholar]
- 7.Kang JS, Park S, Song JH, et al. Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J Arthroplasty. 2009;24:1178–1183. doi: 10.1016/j.arth.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Yuan HF, Guo CA, Yan ZQ. The use of bisphosphonate in the treatment of osteonecrosis of the femoral head: a meta-analysis of randomized control trials. Osteoporos Int. 2016;27:295–299. doi: 10.1007/s00198-015-3317-5. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Yang Z, Wei Z, et al. Efficacy of bisphosphonates in the treatment of femoral head osteonecrosis: A PRISMA-compliant meta-analysis of animal studies and clinical trials. Sci Rep. 2018;8:1450. doi: 10.1038/s41598-018-19884-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo KH, Jeong ST, Jones JP., Jr Borderline necrosis of the femoral head. Clin Orthop Relat Res. 1999;(358):158–165. [PubMed] [Google Scholar]
- 11.Glueck CJ, Freiberg RA, Sieve L, et al. Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res. 2005;(435):164–170. doi: 10.1097/01.blo.0000157539.67567.03. [DOI] [PubMed] [Google Scholar]
- 12.Glueck CJ, Freiberg RA, Wissman R, et al. Long term anticoagulation (4–16 years) stops progression of idiopathic hip osteonecrosis associated with familial thrombophilia. Adv Orthop. 2015;2015:138382. doi: 10.1155/2015/138382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chotanaphuti T, Thongprasert S, Laoruengthana A. Low molecular weight heparin prevents the progression of precollapse osteonecrosis of the hip. J Med Assoc Thai. 2013;96:1326–1330. [PubMed] [Google Scholar]
- 14.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;(386):173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Pengde K, Fuxing P, Bin S, et al. Lovastatin inhibits adipogenesis and prevents osteonecrosis in steroid-treated rabbits. Joint Bone Spine. 2008;75:696–701. doi: 10.1016/j.jbspin.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Ajmal M, Matas AJ, Kuskowski M, et al. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009;40:235–239. doi: 10.1016/j.ocl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. 2012. [cited by 2019 Jan 7]. Available from: http://www.fda.gov/drugs/drugsafety/ucm293-101.htm#dose.
- 18.Lai KA, Shen WJ, Yang CY, et al. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 19.Agarwala S, Shetty V, Karumuri LK, et al. Patellar resurfacing versus nonresurfacing with patellaplasty in total knee arthroplasty. Indian J Orthop. 2018;52:393–398. doi: 10.4103/ortho.IJOrtho_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishii T, Sugano N, Miki H, et al. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin Orthop Relat Res. 2006;443:273–279. doi: 10.1097/01.blo.0000194078.32776.31. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Chang JK, Lai KA, et al. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:1572–1578. doi: 10.1002/art.33498. [DOI] [PubMed] [Google Scholar]
- 22.Lee YK, Ha YC, Cho YJ, et al. Does zoledronate prevent femoral head collapse from osteonecrosis? A prospective, randomized, open-label, multicenter study. J Bone Joint Surg Am. 2015;97:1142–1148. doi: 10.2106/JBJS.N.01157. [DOI] [PubMed] [Google Scholar]
- 23.Pazianas M, Abrahamsen B. Safety of bisphosphonates. Bone. 2011;49:103–110. doi: 10.1016/j.bone.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Jager M, Zilkens C, Bittersohl B, et al. Efficiency of iloprost treatment for osseous malperfusion. Int Orthop. 2011;35:761–765. doi: 10.1007/s00264-010-0998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claßen T, Becker A, Landgraeber S, et al. Long-term clinical results after iloprost treatment for bone marrow edema and avascular necrosis. Orthop Rev (Pavia) 2016;8:6150. doi: 10.4081/or.2016.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pountos I, Giannoudis PV. The role of Iloprost on bone edema and osteonecrosis: safety and clinical results. Expert Opin Drug Saf. 2018;17:225–233. doi: 10.1080/14740338.2018.1424828. [DOI] [PubMed] [Google Scholar]
- 27.Albers A, Carli A, Routy B, et al. Treatment with acetylsalicylic acid prevents short to mid-term radiographic progression of nontraumatic osteonecrosis of the femoral head: a pilot study. Can J Surg. 2015;58:198–205. doi: 10.1503/cjs.016814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugano N, Atsumi T, Ohzono K, et al. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–605. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 29.Koo KH, Ahn IO, Kim R, et al. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999;213:715–722. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 30.Ha YC, Jung WH, Kim JR, et al. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88(Suppl 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]