Abstract
Bone turnover markers (BTMs) have important role in the management of osteoporosis. Recently the clinical application of BTMs has achieved significant progress and measurement of BTMs give us better understanding of pathogenesis of osteoporosis. However, the use of BTMs is still insufficient in Korea. We summarized the available methods and standard interval of the BTMs in Korea. Also we reviewed published literatures on pre-analytical variability in the measurement of BTMs and provided recommendations for standardized sample handling and patient preparation for reducing those pre-analytical variabilities. The clinical application of BTMs in patients with chronic kidney disease who have a higher fracture risk than the general population is summarized.
Keywords: Biomarkers, Bone remodeling, Bone turnover markers, Chronic kidney disease, Osteoporosis
INTRODUCTION
Osteoporosis is a major health problem worldwide. Osteoporosis is defined as a disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and consequent increase in the risk of fracture.[1] The bone strength is determined by bone mass and bone quality. Bone mass is expressed mainly in bone mineral density (BMD), and bone quality is composed of microarchitecture, bone turnover rate, mineralization, and microdamage accumulation.[2] The BMD measurement using dual energy X-ray absorptiometry (DXA) is the most commonly used diagnostic criteria for osteoporosis.[3] Although BMD is used for treatment strategy determination and evaluation of bone loss rate or treatment response, it might not completely capture the osteoporotic fracture risk. Also, changes in BMD are reflected at a slow rate.
In bone, bone turnover, which removes old bones by bone resorption, and forms new bones by bone formation, is continuously occurring.[4,5] The change of bone turnover rate could affect the bone quality. Bone turnover markers (BTMs) are an index reflecting the rate of bone turnover and samples to measure BTMs can be taken from urine and blood easily.[6] Considering the limitations of BMD and the characteristic of BTMs reflecting bone quality, interest in the clinical potential of BTM to predict fracture risk and monitor treatment is steadily increasing.
BTMs are classified as either bone formation markers or bone resorption makers. The BTMs have not yet to be proven to make diagnosis and treatment decision for osteoporosis. But, the clinical usefulness of BTMs to predict bone loss and fracture risk, and to monitor the response of osteoporosis treatment have been shown in several studies.[7,8,9] Also, the measurement of BTMs give us better understanding of pathogenesis of osteoporosis. However, the value of the BTMs might be influenced by physiological and pathological factors, and, in some cases, multiple methodologies used for the same analyte. Theses strength and weakness of BTMs in clinical practice have been considered by several national societies and guidelines development groups and have resulted in differing recommendation.[10,11]
The use of BTMs is still insufficient in Korea. The Korean Society of Bone Mineral Society organized the bone turnover working group to investigate on the application of BTMs and provide recommendations on their clinical use to physicians in Korea.
The aim of this paper is to investigate the available methods and standard reference intervals of BTMs in Korea, and review the standardization of sampling to decrease the pre-analytical issue. The patients with chronic kidney disease (CKD) have a higher fracture risk than the general population. Bone disease related to advanced CKD features a large spectrum of clinical phenotype. The clinical application of BTMs in those population summarized separately.
THE AVAILABLE ASSAY METHODS FOR MEASUREMENT OF BTMS IN KOREA
1. Bone formation markers
Bone formation markers include osteocalcin, bone-specific alkaline phosphatase (BSALP), carboxy-terminal propeptide of type I collagen (P1CP), and aminoterminal propeptide of type I collagen (P1NP). BSALP and osteocalcin released by osteoblasts and plays a major role in bone mineralization. P1CP and P1NP cleaved from procollagen type I during collagen synthesis. In Korea, osteocalcin and BSALP are most commonly used as bone formation markers. Several kits have been used to measure osteocalcin and BSALP depending on the assay methods. For osteocalcin, 1 radioimmunoassay (RIA; BGP [BRAHMS Diagnostica, Berlin, Germany]), 2 immunoradiometric assay (IRMA; DIAsource hOST-IRMA or CISBIO osteo-RIACT [DIAsource Immunoassays S.A., Nivelles, Belgium]) and 1 electrochemiluminescence assay (ECLIA; Elecsys N-MID Osteocalcin [Roche Diagnostics, Mannheim, Germany]) were used. For BSALP, 1 chemiluminescence assay (CLIA; Beckman Coulter Inc., Sacramento, CA, USA) and 1 enzyme immunoassay (EIA; MicroVue BAP EIA [Quidel Corporation, San Diego, CA, USA]) were used. Also, it can be measure by electrophoresis assay (Spife ALP-20; Helena Laboratories, Beaumont, TX, USA). Although P1NP has not been widely used yet, P1NP can be measured by the ECLIA using Elecsys total P1NP (Roche). In Korea, total P1NP (Roche Elecsys) is covered by insurance in osteoporotic patients since 2018. Reference interval and median value of P1NP in Korean population was published.[12]
2. Bone resorption markers
Bone resorption markers are serum C-terminal telopeptides of type I collagen (CTX), urinary N-terminal telopeptide of collagen type I (NTx), free and total pyridinoline (PYD) and free and total deoxypyridinoline (DPD). These are collagen cross-links.
In Korea, CTX, NTx, and DPD can be measured as bone resorption markers. In Korea, CTX is most commonly used as bone resorption markers. Several kits have been used to measure bone resorption markers depending on the assay methods. Currently for CTX, 1 automated immunoassay is available: Beta-CrossLaps Roche Elecsys (ECLIA [Roche Diagnostics]). For NTx, enzyme-linked immunosorbent assay (ELISA) is used for urine and serum: OSTEOMARK (Alere Inc., Scarborough, ME, USA). CLIA (Ortho-Clinical Diagnostics, Inc., High Wycombe, UK) is also available. For DPD, EIA (Qulidelmetra, San Diego, CA, USA) and CLIA are available.
INTERPRETATION ON THE REFERENCE INTERVALS OF BTMS
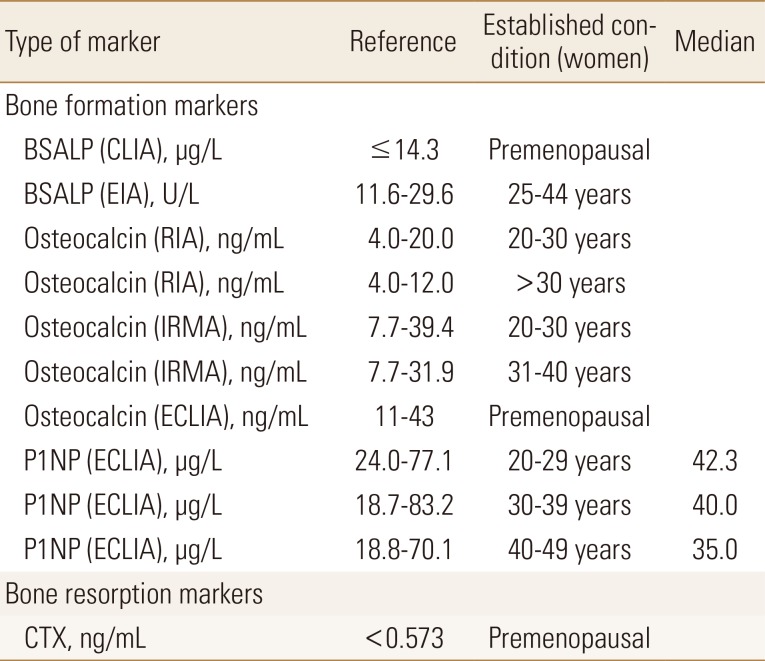
The reference intervals of BTMs are useful for interpreting the results in osteoporosis patients. Several prospective studies have reported the presence of increased BTMs have an additive effect on fracture risk in women.[7] The very high BTM values (>3 standard deviation above the mean of the reference values) during the initial assessment suggests other metabolic disease. However, sufficient consensus has not been achieved for cut-point of BTMs that increase fracture risk or assess response of treatment. It is considered necessary to establish reference intervals for different geographic area and ethnicities. Although reference intervals of P1NP in Korean population were reported,[12] only reference of manufacture's data is available for other BTMs (Table 1).
Table 1. The reference intervals and median values of bone turnover markers.
PremenopausalBSALP, bone-specific alkaline phosphatase; CLIA, chemiluminescence assay; EIA, enzyme immunoassay; RIA, radioimmunoassay; IRMA, immunoradiometric assay; P1NP, aminoterminal propeptide of type I collagen; ECLIA, electrochemiluminescence assay; CTX, C-terminal telopeptides of type I collagen.
STANDARDIZATION OF PATIENT SAMPLE COLLECTION PROCEDURE
1. Importance of standardized patient sample collection procedure
The primary challenge to the adoption of many BTMs in routine practice has been poor within-subject and between-lab reproducibility. For BTMs to be useful, the pre-analytical sources of variability, along with the underlying disease process, must be identified, minimized, and controlled through carefully standardized patient preparation and sample handling procedures.[13,14]
Although BTMs except CTX are relatively stable and very modestly affected by pre-analytical variables, several BTMs are frequently measured together on automated platforms within one sample.[15] Therefore, it is important that the conditions that ensure CTX integrity may be maintained when measuring BTMs. Moreover, serum P1NP has recently been recommended as a reference BTM along with serum CTX.[16] Therefore, proper sample collection procedures for both CTX and P1NP are focused in this section.
2. Sources of pre-analytical variability sample handling
1) Sample type
Although either serum or plasma sample may be used for CTX and PINP measurements, EDTA plasma has the advantage of superior sample stability for CTX compared with serum.[17,18,19] The same sample type such as serum or plasma should be used consistently when monitoring a patient.
2) Sample collection
CTX exhibits a circadian rhythm in blood. CTX level peaks during the early morning hours (2—5 a.m.) and reaches a nadir between 11 a.m. and 2 p.m. The only known modulator having a major effect on this circadian pattern is food intake. Overnight fasting markedly reduced the circadian variation of CTX. Therefore, blood samples for CTX measurement must be collected following an overnight fast during the morning between 7:30 a.m. and 10:00 a.m.[20,21,22] Other BTMs have minimal circadian rhythm and are minimally affected by food intake.
3) Sample processing
Samples destined for CTX measurement should be frozen at ≤−20℃ preferably within 2 hr of collection.[23] If measurement is to be made within 8 hr of phlebotomy, the sample may be stored at 4℃. Moderate hemolysis (0.5 g/dL) should be avoided for CTX measurement.[19,24]
4) Sample stability
CTX is more stable in EDTA plasma than in serum, regardless of measurement methods.[23] For Roche package inserts, CTX has stability of 24 hr at room temperature (RT; 20–25℃), 8 days at 2 to 8℃ in EDTA plasma, and 6 hr at RT and 8 hr at 4℃ in serum.[25] P1NP is more stable compared to CTX with stability of at least 24 hr at RT and 5 days at 4℃ in both EDTA plasma and serum.[26] For long term storage, stability for 3 months for CTX and 6 months for P1NP at ≤−20℃ is ensured for all methods. For longer term researches, the recommendation is aliquots of samples at −70℃ or below to allow to analysis of all samples in a single batch at a later time.[19,25,26]
5) Freeze/thaw cycle
Multiple freeze-thaw cycles are reported to be acceptable for CTX and P1NP.[19,24]
3. Sources of pre-analytical variability: patient related factors
The patient related factors are divided into controllable and uncontrollable factors. Controllable factors include the menstrual cycle, seasonal variation, and physical activities. The optimal time to collect samples in pre-menopausal women is the early-mid-follicular phase.[27] There is a minor but detectable seasonal variation for CTX in older adults and those with severe vitamin D deficit.[28,29] Intensive physical training (e.g., elite soccer players) moderately increases serum CTX and slightly decreases PINP. Vigorous exercise should be avoided the day prior to sampling.[30] Uncontrollable factors include age, sex, pregnancy, geography, renal function, and specific diseases and medications.[14]
USE IN PATIENTS WITH CKD
The patients with CKD have a higher fracture risk than the general population. In patients with CKD, secondary hyperparathyroidism, adynamic bone, hemodialysis associated amyloidosis, vitamin D deficiency, hypocalcemia, changes in the bone architecture, nutritional disturbance, and increase in oxidative stress could increase fracture risk.[31] Because some BTMs are influenced by the renal function, there are limitations to use BTMs to predict and evaluate the fracture risk in patients with CKD. The frequency of monitoring serum calcium, phosphate, and parathyroid hormone (PTH) as well as BTMs are recommended to evaluate the presence and magnitude of abnormalities, and the rate of progression of CKD.[32]
As bone formation markers, BSALP, P1NP, and P1CP are independent of the renal function status. But, osteocalcin is influenced by the renal function.[33,34] Combining a low PTH (<150 pg/mL) and a low BSALP (<27 IU/L) improved the specificity of diagnosing adynamic bone disease in 103 dialysis patients with bone biopsy results.[35] In the newer automated Ostase bone specific alkaline phosphatase (ALP) assay, cut-off <20 IU/L is used. The Kidney Disease, Improving Global Outcomes (KDIGO) guidelines suggest that measurements of serum PTH or BSALP can be used to evaluate bone disease because markedly high or low values predict underlying bone turnover in patients with CKD G3a (estimated glomerular filtration rate, 45–59 mL/min/1.73m2)-G5D (end-stage renal disease patients who undergo chronic dialysis).[32] Since osteocalcin is cleared by kidney, its use in CKD patients is limited. The combination of osteocalcin (<41 ng/L) and BSALP (<23 U/L) improved the positive predictive value for diagnosing adynamic bone disease in a CKD-5 cohort to 77%.[33] The proportion of the monomeric form of P1NP is elevated in patients with CKD, whereas the apparent concentration of P1NP is unaffected by glomerular filtration rate in kidney disease patients when an intact assay for P1NP is used.[34] P1NP monomers are not cleared by conventional dialysis sessions and the least significant change is of 32% for the intact assay.[36]
As bone resorption markers, CTX is excreted by kidney and accumulates in CKD patients. CTX is cleared by dialysis and therefore predialysis sampling is required for longitudinal monitoring.[33] PYD, DPD and NTx are influenced by the renal function status. Therefore, the KDIGO guidelines recommended that the bone derived markers of collagen synthesis and breakdown should not be routinely measured in patients with CKD stages 3 to 5D.[32] Levels of tartrate-resistant acid phosphatase 5b correlates with PTH and ALP and are unaffected by renal function. Its use is limited by availability of automated assays.[33]
The limitations of our study is that few Korean data is available. However, all available methods in Korea were surveyed. This data will support upcoming study for standardization and reference intervals of BTMs can be used for decision and monitoring of treatment.
CONCLUSIONS
BTMs could be used to predict the fracture risk prediction and monitor treatment response. In 2017, P1NP and CTX were flagged for standardization by the International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine. But, these BTMs are not widely used in clinical practice. The data on standardized reference interval of PINP in Koreans are available. Further researches for CTX assay in Koreans are needed. The standardized patient preparation and sample handling procedures is important to decrease analytical sources of variability. In the patients with CKD, use of BTMs is limited. When used in combination with PTH, BSALP can predict adynamic bone disease. This review support standardization and clinical use in the management of patients of osteoporosis.
Footnotes
No potential conflict of interest relevant to this article was reported.
- Conceptualization: Hong S, Park SY, Ahn SH, Yoo JI, Chung YJ, Jeon YK, Yoon BH, Kim HY, Lee SH, Lee J.
- Wrote first draft of manuscript: Hong S, Park SY, Ahn SH, Yoo JI, Chung YJ, Jeon YK, Yoon BH, Kim HY, Lee SH, Lee J.
- Comment and revise manuscript: Hong S, Park SY, Ahn SH, Yoo JI, Chung YJ, Jeon YK, Yoon BH, Kim HY, Lee SH, Lee J.
- Approved final version: Hong S, Park SY, Ahn SH, Yoo JI, Chung YJ, Jeon YK, Yoon BH, Kim HY, Lee SH, Lee J.
References
- 1.Peck WA. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein RS. True strength. J Bone Miner Res. 2000;15:621–625. doi: 10.1359/jbmr.2000.15.4.621. [DOI] [PubMed] [Google Scholar]
- 3.Blake GM, Fogelman I. Role of dual-energy X-ray absorptiometry in the diagnosis and treatment of osteoporosis. J Clin Densitom. 2007;10:102–110. doi: 10.1016/j.jocd.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmas PD, Eastell R, Garnero P, et al. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int. 2000;11(Suppl 6):S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 7.Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 8.Garnero P, Sornay-Rendu E, Claustrat B, et al. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 9.Ross PD, Kress BC, Parson RE, et al. Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int. 2000;11:76–82. doi: 10.1007/s001980050009. [DOI] [PubMed] [Google Scholar]
- 10.Nishizawa Y, Nakamura T, Ohta H, et al. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004) J Bone Miner Metab. 2005;23:97–104. doi: 10.1007/s00774-004-0547-6. [DOI] [PubMed] [Google Scholar]
- 11.Morris HA, Eastell R, Jorgensen NR, et al. Clinical usefulness of bone turnover marker concentrations in osteoporosis. Clin Chim Acta. 2017;467:34–41. doi: 10.1016/j.cca.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Yoo JI, Park AJ, Lim YK, et al. Age-related reference intervals for total collagen-I-N-terminal propeptide in healthy Korean population. J Bone Metab. 2018;25:235–241. doi: 10.11005/jbm.2018.25.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishizawa Y, Ohta H, Miura M, et al. Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition) J Bone Miner Metab. 2013;31:1–15. doi: 10.1007/s00774-012-0392-y. [DOI] [PubMed] [Google Scholar]
- 14.Szulc P, Naylor K, Hoyle NR, et al. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28:2541–2556. doi: 10.1007/s00198-017-4082-4. [DOI] [PubMed] [Google Scholar]
- 15.Bauer D, Krege J, Lane N, et al. National bone health alliance bone turnover marker project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int. 2012;23:2425–2433. doi: 10.1007/s00198-012-2049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 17.Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the serum CrossLaps one step ELISA, a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998;44:2290–2300. [PubMed] [Google Scholar]
- 18.Garnero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. 2001;47:694–702. [PubMed] [Google Scholar]
- 19.Morovat A, Catchpole A, Meurisse A, et al. IDS iSYS automated intact procollagen-1-N-terminus pro-peptide assay: method evaluation and reference intervals in adults and children. Clin Chem Lab Med. 2013;51:2009–2018. doi: 10.1515/cclm-2012-0531. [DOI] [PubMed] [Google Scholar]
- 20.Qvist P, Christgau S, Pedersen BJ, et al. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 21.Redmond J, Fulford AJ, Jarjou L, et al. Diurnal rhythms of bone turnover markers in three ethnic groups. J Clin Endocrinol Metab. 2016;101:3222–3230. doi: 10.1210/jc.2016-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clowes JA, Hannon RA, Yap TS, et al. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30:886–890. doi: 10.1016/s8756-3282(02)00728-7. [DOI] [PubMed] [Google Scholar]
- 23.Stokes FJ, Ivanov P, Bailey LM, et al. The effects of sampling procedures and storage conditions on short-term stability of blood-based biochemical markers of bone metabolism. Clin Chem. 2011;57:138–140. doi: 10.1373/clinchem.2010.157289. [DOI] [PubMed] [Google Scholar]
- 24.Rosenquist C, Fledelius C, Christgau S, et al. Serum CrossLaps one step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem. 1998;44:2281–2289. [PubMed] [Google Scholar]
- 25.Roche Diagnostics. Elecsys β-Crosslaps/serum (B-CTX in serum) immunoassay cobas package insert (V 14.1) Manheim, DE: Roche Diagnostics; 2014. [Google Scholar]
- 26.Roche Diagnostics GmbH. Total PINP (2014) (total procollagen type I amino-terminal propeptide) immunoassay Cobas package insert (V11.0) Mannheim, DE: Roche Diagnostics GmbH; 2014. [Google Scholar]
- 27.Gass ML, Kagan R, Kohles JD, et al. Bone turnover marker profile in relation to the menstrual cycle of premenopausal healthy women. Menopause. 2008;15:667–675. doi: 10.1097/gme.0b013e31815f8917. [DOI] [PubMed] [Google Scholar]
- 28.Bhattoa HP, Nagy E, More C, et al. Prevalence and seasonal variation of hypovitaminosis D and its relationship to bone metabolism in healthy Hungarian men over 50 years of age: the HunMen Study. Osteoporos Int. 2013;24:179–186. doi: 10.1007/s00198-012-1920-2. [DOI] [PubMed] [Google Scholar]
- 29.Pasco JA, Henry MJ, Kotowicz MA, et al. Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:752–758. doi: 10.1359/JBMR.040125. [DOI] [PubMed] [Google Scholar]
- 30.Weiler R, Keen R, Wolman R. Changes in bone turnover markers during the close season in elite football (soccer) players. J Sci Med Sport. 2012;15:255–258. doi: 10.1016/j.jsams.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Nitta K, Yajima A, Tsuchiya K. Management of osteoporosis in chronic kidney disease. Intern Med. 2017;56:3271–3276. doi: 10.2169/internalmedicine.8618-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isakova T, Nickolas TL, Denburg M, et al. KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Am J Kidney Dis. 2017;70:737–751. doi: 10.1053/j.ajkd.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Chiang C. The use of bone turnover markers in chronic kidney disease-mineral and bone disorders. Nephrology (Carlton) 2017;22(Suppl 2):11–13. doi: 10.1111/nep.13014. [DOI] [PubMed] [Google Scholar]
- 34.Ueda M, Inaba M, Okuno S, et al. Clinical usefulness of the serum N-terminal propeptide of type I collagen as a marker of bone formation in hemodialysis patients. Am J Kidney Dis. 2002;40:802–809. doi: 10.1053/ajkd.2002.35692. [DOI] [PubMed] [Google Scholar]
- 35.Couttenye MM, D'Haese PC, Van Hoof VO, et al. Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant. 1996;11:1065–1072. [PubMed] [Google Scholar]
- 36.Cavalier E, Delanaye P, Moranne O. Variability of new bone mineral metabolism markers in patients treated with maintenance hemodialysis: implications for clinical decision making. Am J Kidney Dis. 2013;61:847–848. doi: 10.1053/j.ajkd.2012.12.013. [DOI] [PubMed] [Google Scholar]