Abstract
Biosensors are powerful diagnostic tools defined as having a biorecognition element for analyte specificity and a transducer for a quantifiable signal. There are a variety of different biorecognition elements, each with unique characteristics. Understanding the advantages and disadvantages of each biorecognition element and their influence on overall biosensor performance is crucial in the planning stages to promote the success of novel biosensor development. Therefore, this review will focus on selecting the optimal biorecognition element in the preliminary design phase for novel biosensors. Included is a review of the typical characteristics and binding mechanisms of various biorecognition elements, and how they relate to biosensor performance characteristics, specifically sensitivity, selectivity, reproducibility and reusability. The goal is to point towards language needed to improve the design and development of biosensors towards clinical success.
Graphical Abstract
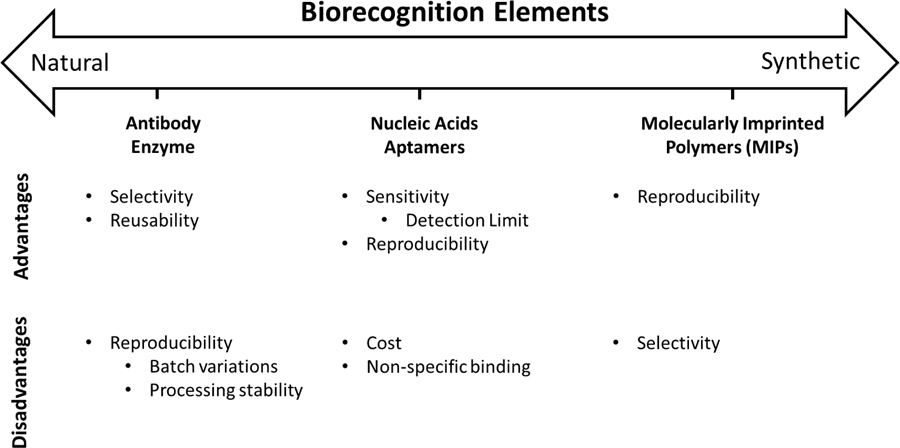
Introduction
The first biosensor, developed by Leland Clark over 55 years ago, combines glucose oxidase with an amperometric oxygen sensor.1 Since then, there has been an outbreak of activity and progress towards using biosensor technology in diagnostic applications, specifically towards point-of-care analysis of biomarkers.2–5 Biosensors are defined as having both a biorecognition element and a transducer. The biorecognition element is used for the specific sequestration of the target bioanalyte, and the transducer then creates a measurable signal for analysis. The first blood glucose biosensor has been a staple of the community, setting a standard of success for the development of novel biosensors, because of its simplicity, high sensitivity, selectivity, and reproducibility.1
Since the original glucose biosensor, many researchers across disciplines have advanced bioanalyte sensing using novel paradigms with improved biorecognition elements or transducer technology.6–10 Many biosensor review articles focus on signal transduction methods, or provide a detailed overview of biosensing capabilities and mechanisms of each biorecognition element paradigm.11–13 Whereas, this review will serve as a guide for the optimal selection of a biorecognition element in the initial design phase based on the biosensor characteristics: selectivity, sensitivity, reproducibility and reusability.
Traditionally, a researcher will first select a biorecognition element design based on their training, and then apply the biosensor towards appropriate applications for the selected paradigm. Rather than relying on previous training, we propose this review as a guide for researchers to select a biosensor paradigm based on the desired application. An early emphasis on the clinical application during the biosensor design phase has the potential to enhance patient-centric point-of-care devices and device accessibility in low-resource regions.
Within this review we focus on the advantages and limitations of each biorecognition element defined by the following biosensor characteristics: selectivity, sensitivity, reproducibility, and reusability. High sensitivity is a large measurable change in biosensor signal as a function of small changes in bioanalyte concentration. High selectivity indicates the sensor will only respond to the target bioanalyte, ignoring all potential competing analytes in the sample. High reproducibility indicates the ability to fabricate multiple identical sensors, with each sensor providing the same predictable response. High reusability indicates the ability to reuse a single sensor multiple times with a consistent sensor response. These biosensor characteristics where chosen to provide a framework to understand the capabilities of each biorecognition element, and ultimately, how the biosensor performance is influenced by the selection of biorecognition element. Ideally, it is best to have high sensitivity, selectivity, reproducibility, and reusability; however, typically biosensor development requires a tradeoff on these characteristics. Therefore, understanding the fundamental limitations of each biorecognition elements is needed to better inform decisions for biorecognition element selection in the preliminary design phase leading to the development of more robust biosensors.
Biorecognition Elements
The primary purpose of a biorecognition element is to provide analyte specificity for a biosensor. Specificity requires a strong and selective affinity between the biorecognition element and target bioanalyte. Several classes of biorecognition elements exist, giving rise to distinct structures that uniquely influence biosensor performance characteristics. Therefore, a fundamental understanding of the inherent characteristics of each biorecognition element is first needed before an in-depth analysis of biosensor performance may occur.
Numerous biorecognition elements exist ranging from naturally occurring to synthetic constructs. Naturally occurring biorecognition elements, such as antibodies and enzymes, are biologically derived constructs that take advantage of naturally-evolved physiological interactions to achieve analyte specificity. Synthetic biorecognition elements are artificially engineered structures developed to mimic physiologically defined interactions. However, at the cross roads of natural and synthetic biorecognition elements, there are pseudo-natural modalities possessing characteristics of both natural and synthetic recognition approaches. Pseudo-natural biorecognition elements are artificially engineered supramolecular structures using natural subunits. Each class of biorecognition element is comprised of several different types of recognition structures, all of which cannot be discussed within this review. Instead, prominent biorecognition elements from each category will be briefly summarized to serve as a representative of each category.
Antibody
Antibodies are naturally occurring 3D protein structures, typically ~150 kDa in size, that can be identified within biochemical pathways and purified for biosensor applications.14 The 3D protein structure of antibodies creates a unique recognition pattern with high specificity and accuracy for the bioanalyte. Antibodies share a general structural trend of a “Y” shaped 3D conformation, each comprised of a light chain and a heavy chain, with analyte binding domains located on the arms, seen in Figure 1. Antibody biorecognition elements can be classified as affinity-based, the biosensor signal is dependent on the binding event to form an antibody-antigen immunocomplex. The binding event is often monitored using colorimetric or piezometric transduction methods.15–18 Typically, antibodies are immobilized via covalent linkage to a sensor surface, forming a brush-like array.19,20
Figure 1:
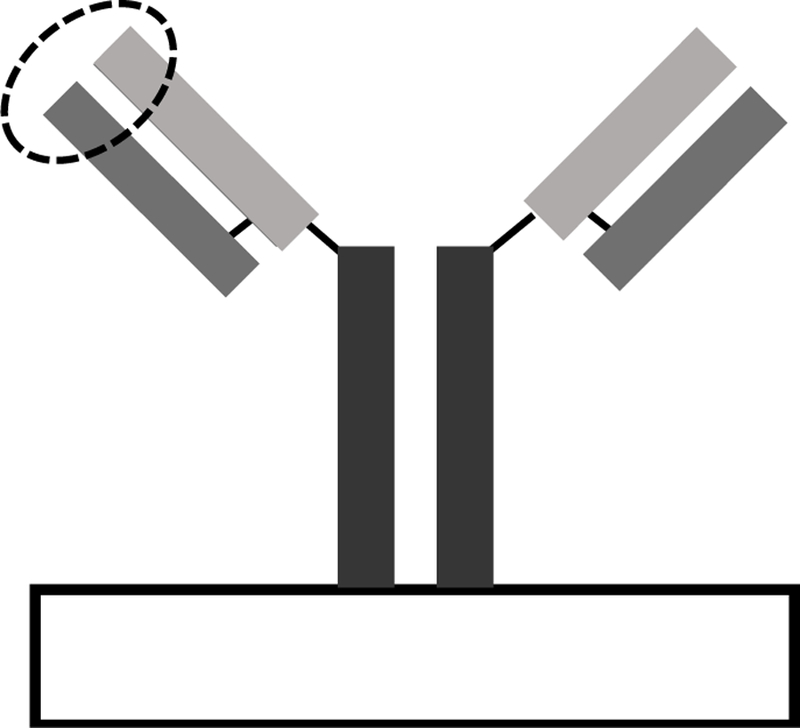
3D confirmation of antibodies is “Y” shaped with binding domains, circled above, typically located on the distal end.
Antibodies remain a staple in the biosensor community despite the widely known and accepted limitations of this biorecognition element. For example, antibody production requires experimentation with animals which is a costly and time-consuming process, which limits the discovery of new antibodies.4,21 Further, once an antibody is discovered, the isolation and purification procedures can be costly.
Enzymes
Enzymes achieve bioanalyte specificity with binding cavities buried within their 3D structure, using hydrogen-bonding, electrostatics, and other non-covalent interactions to form recognition patterns.22 Enzymatic biosensors are biocatalytic in nature, meaning the enzyme captures and catalytically converts the target bioanalyte to a measurable product, frequently monitored via amperometric or electrochemical transduction methods.23 Following bioanalyte sequestration an intermediate complex is formed before release of the measurable end product, shown in Figure 2.23,24 Enzymes are often embedded within surface structures, allowing for short diffusion pathways between biorecognition element and transducer.25–27
Figure 2:
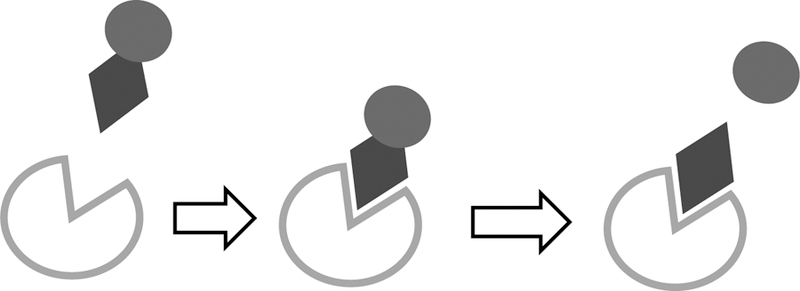
Target bioanalyte binds active site (left) to form bioanalyte-enzyme complex (middle) where a catalytic reaction converts target bioanalyte to measurable reaction product (dark circle, right).
Nucleic Acid
Nucleic acid biosensors, termed genosensors, take advantage of the complementary binding motif of DNA to achieve bioanalyte specificity.28 Once a DNA target sequence has been identified, a DNA fragment can be artificially designed and immobilized at the sensor surface as a biorecognition element.29–31 Specificity is achieved through the unique complementary recognition pattern between the immobilized DNA fragment and the target sequence.28,32 Recent advances in using nucleic acid recognition elements include locked nucleic acids (LNA) and peptide nucleic acids (PNA).33–35 LNA “locks” the ribose in the 3’-endo confirmation, which reduces the conformational flexibility and therefore improves binding with the complementary nucleic acid target. PNA is a synthetic oligonucleotide, comprised of a repeating aminoethyl-glycine unit linked by peptide; PNA is uncharged, and therefore, creates a higher stability in nucleic acid binding. Overall, nucleic acid biorecognition elements are extremely limited in their range of applications as their use is only optimal for biosensor applications targeting nucleic acids.36–39
Aptamer
Aptamer biorecognition elements, and other pseudo-natural modalities, provide a much wider range of biosensor applications with the ability to target various bioanalytes, including metal ions, small molecules, proteins, and more complex targets (e.g. whole cells).40 Aptamers are single-stranded oligonucleotides designed through a combinatorial selection process called Systemic Evolution of Ligands by Exponential Enrichment (SELEX).40 SELEX is an iterative process to search a library of randomly generated oligonucleotide sequences for strong binding affinities between the target analyte and oligonucleotide sequences, ensuring a selective and strong interaction pair shown in Figure 3. The SELEX cycle starts with incubation of the target bioanalyte with an oligonucleotide library containing all potential aptamer sequences. Unbound aptamer sequences are then removed only retaining bound aptamer sequences for polymerase chain reaction (PCR) amplification to regenerate the oligonucleotide library for the next SELEX round. Aptamers are typically 100 base pairs in length compromised of a 20–70 randomized base pair binding region in the center with constant primer binding regions at both ends.40
Figure 3:
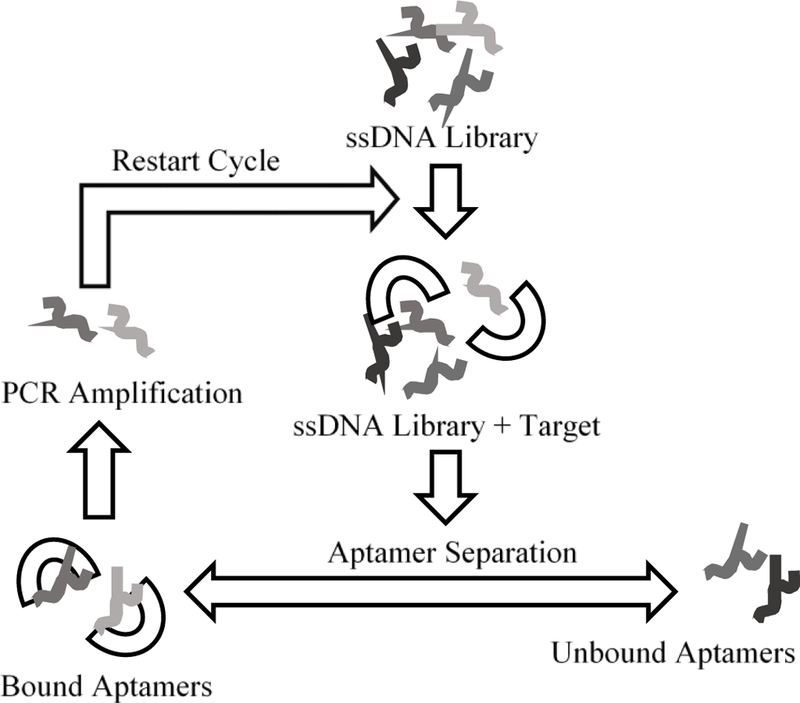
SELEX cycle starts with incubation of target bioanalyte with oligonucleotide. Unbound aptamers are removed retaining only bound aptamer sequences for PCR amplification to regenerate the oligonucleotide library and restart cycle.
SELEX is a beneficial biorecognition element discovery tool providing researchers with the ability to tailor a sequence for a target bioanalyte. A major drawback is that the SELEX method is costly, requiring multiple iterations using a large library of oligonucleotides each time. However, cost is an obstacle that could be mitigated with further research and development.14,41
Molecularly Imprinted Polymers
Molecularly imprinted polymers (MIPs) are synthetic biorecognition elements using a templated polymer matrix to achieve analyte specificity through patterns of non-covalent bonding, electrostatic interactions, or size inclusion/exclusion.42 Tunability of MIPs comes from the choice of functional monomer, crosslinker, target bioanalyte, and solvent.43 MIPs are designed to encapsulate the target bioanalyte, effectively forming synthetic recognition patterns between the bioanalyte and polymer matrix shown in Figure 4. Unlike natural biorecognition elements, MIP biorecognition elements are synthetically fabricated for each unique target bioanalyte. In other words, the synthetic polymer-based biorecognition element is designed, often in situ, around the bioanalyte.44 Therefore, a major benefit of MIPs is that a specific biorecognition element-bioanalyte pairing does not need to be biochemically identified.
Figure 4:
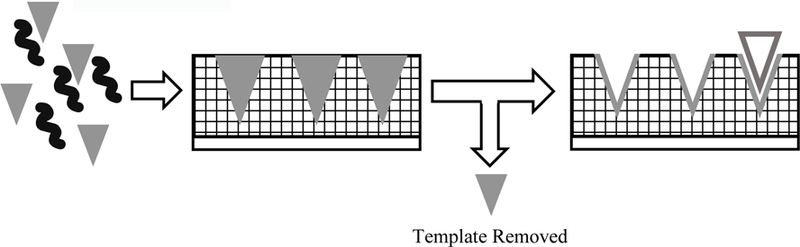
Synthetic polymerization encapsulating the target bioanalyte forms an analyte binding site.
Other Sensor Paradigms
The biggest obstacle for natural biorecognition elements (e.g. enzyme, antibody) paradigms is the initial identification of a biorecognition element paired to a target analyte. In addition to aptamers, many researchers use protein-based phage display as a pseudo-natural modality to identify protein-protein interactions.45,46 Phage display inserts a DNA fragment that encodes the desired protein target into a phage coat protein gene. A random-peptide library is used to search for high affinity interactions to the target protein sequence.47,48 Eventually the phage DNA can be extracted and encoded to be grown recombinantly in bacteria.48,49 With phage display, the potential for discovering new biorecognition elements is growing, but it can be limited similarly to other natural biorecognition element paradigms.
Another approach to detecting bioanalytes is to use synthetic surface nanostructures to catalytically activate a bioanalyte without the need of a natural biorecognition element.50–55 As an example, nanozymes mimic enzymatic catalytic function by interfacing with a recognition element mimic and an inorganic catalysts.25,50,56 The nanostructured surface can have high sensitivity but poor specificity, as multiple bioanalytes can be simultaneously catalytically activated.57–59 Even though progress has been made to improve the selectivity of biorecognition element mimics with nanozymes, this sensor paradigm remains less selective than the other discussed paradigms within this review.25 One promising application of nanostructured surfaces includes integrating multiple catalytic surfaces to create a cross-reactive (e.g. profile-based) sensors.60–63 However, clinicians struggle with profile-based sensing because it does not identify specific analytes in the traditional biosensor paradigm and definition.64
Other recognition elements, beyond those described in this review, do exist, and it is helpful to investigate alternatives prior to designing a biosensor. For example in protein detection, a protein biorecognition element can be immobilized on a surface, and protein-docking can be detected through the affinity interactions, similar to enzymes and antibodies.65 An advantage of protein specific recognition elements, for example maltose binding proteins which bind with glucose, is that they can be grown recombinantly in E. coli which reduces the costs of development and production.66 Also, some binding proteins have the ability to transport across cellular walls creating live-cell-based biosensors.67 Alternatively, proteins recognition elements can be engineered via phage display or SELEX aptamer processes. Yet, for the purposes of this review, we keep to the basic antibody, enzyme, nucleic acids (natural), aptamer (pseudo-natural), and MIP (synthetic) biorecognition elements as we compare and contrast biosensor characteristics.
Biosensor Characteristics
Selectivity
Selectivity is the ability of a biosensor to generate a positive result only from interactions with the target bioanalyte. A false positive is defined as a positive biosensor result generated from a negative sample; meaning the target bioanalyte is not present in the sample, but an inaccurate positive result is generated. Biosensors with poor selectivity tend to have high false positive rates preventing success in clinical applications. Selectivity is essential for the development of robust point-of-care biosensors especially because biological samples are complex and comprised of numerous competing analytes. Selectivity is often first published with more common competing analogs in a simplified buffered solution, which is used to artificially to mimic a biological matrix. However, this simplified approach still does not fully capture the impact of the matrix on biosensor selectivity. Therefore, we encourage skepticism until publications demonstrate selectivity of competing analogs in complex biofluid matrices from patient samples.
Naturally occurring biorecognition elements, such as antibodies or enzymes, are optimal for biosensor applications when selectivity is the most important biosensor characteristic. Antibodies achieve specificity through binding domains located on the arms of their “Y” conformational shape.68–70 Enzymes have binding pockets with unique hydrogen bonding and electrostatic biorecognition patterns to achieve analyte specificity.71,72 The shared high selectivity of antibody and enzyme biorecognition elements stems from their biologically evolved role requiring high specificity for the success of immunological and other physiological processes.73
The selectivity of nucleic acid or aptamer biorecognition elements is hindered by non-specific electrostatic interactions. The inherent negative charge of DNA causes non-specific electrostatic interactions with competing analytes, which can be overcome to some degree using peptide nucleic acids.32,35 Aptamer biorecognition elements are also comprised of oligonucleotide subunits which can also results in non-specific binding. However, post-synthesis chemical modifications of aptamers can help to reduce non-specific binding to improve biosensor selectivity.4,74
Synthetic modalities, such as MIPs, are beneficial biorecognition elements in terms of cost, stability, and ease of development but result in poor biosensor selectivty.43,44 MIP selectivity is hindered by non-specific binding of bioanalytes with similar structures and functional groups due to the heterogeneity of interactions within the binding cavity. Non-specific binding is more common when investigating large molecules, specifically for analytes greater than 1.5 kDa.75–77 Increasing the amount of polymer crosslinking can both preserve the binding cavity and reduce non-specific binding.78,79 However, extensive crosslinking can create a highly dense polymer construct, leading to the permanent entrapment of the bioanalyte template and reduction in diffusion of the bioanalyte through the construct.75 Therefore, highly crosslinked MIPs typically result in effectively low binding affinities and slow response times.79,80
Despite the tunability in the design for MIPs, their success in clinical applications is severely hindered by the poor selectivity of this class of biorecognition elements. Polymer chemists continue to employee techniques to improve the selectivity of MIPs, but often, the sensing paradigm is approached with a negative stigma that is often too difficult to overcome. Despite the history of MIPs in the biosensor community with commercial applications and products already available, many publications are limited to proof-of-concept applications feeding this stigma.75 Two major improvements are needed towards this biorecognition paradigm: (1) more MIP products tested in complex matrices and real bioanalyte samples, (2) more literature on the optimization point of all the variables involved in MIP development.77,81 Other synthetic modalities, such as nanozymes, are just starting to be tested in complex biofluid matrices,25 and we encourage careful development with discussion on the optimization selectivity in biofluid matrices for these evolving technologies.
Sensitivity
Biosensor sensitivity is defined by the relationship between changing bioanalyte concentration and transduced signal intensity. Highly sensitive biosensors can generate a biosensor response with small fluctuations in bioanalyte concentrations. Sensitivity is also often related to the biosensor range defined by the upper and lower limits of detection, indicating the maximum and minimum bioanalyte concentrations that can be accurately measured. Many publications solely push for the lowest possible detection limit, but improved biosensor sensitivity within physiological relevant concentration ranges is more desirable.82–85 Typically, the desired physiologically relevant bioanalyte concentration range is often in the picomolar to nanomolar scale.86–89 Improvements in biosensor sensitivity facilitates the ability to accurately measure small variations in biomarker concentrations to allow for earlier disease diagnosis and treatment intervention.
Biosensor sensitivity and range is primarily dictated by the number of available binding sites per surface area, equilibrium dissociation constant, and steric hindrances. A high surface loading density of a biorecognition element is desired to maximize the number of available analyte binding sites. A major advantage of aptamer biorecognition elements for biosensor sensitivity is their small size. For example, antibodies are ~10–15 nm in size compared to aptamers which are only ~1–2 nm in size.14 Therefore, aptamers can achieve higher density surface coverage with more available binding sites per surface area of the biosensor.14
Surface loading is not the sole influence on biosensor sensitivity; steric hindrances from adjacent biorecognition elements must be considered, especially for antibodies. Steric effects from adjacent antibodies result in a conformational change resulting in inaccessibility of the binding site, shown in Figure 5.90–93 To mitigate some of the obstacles associated with antibody biorecognition elements, single chain formats of antibodies, referred to as nanobodies, are growing increasingly popular.94 Discovered in 1993 in camels, camelid heavy-chain antibodies (HcAb) are devoid of the light chain.95,96 Therefore, nanobodies are smaller than conventional antibodies and have simplified “Y” conformational shape binding domains.
Figure 5:
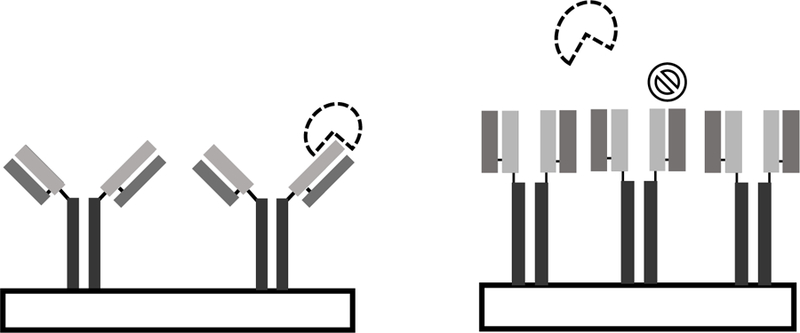
Steric hinderances occur when antibody recognition elements are too closely packed resulting in a conformational change making binding sites inaccessible.
Sensitivity range of traditional nucleic acid biorecognition elements is limited due to steric hindrances, which is overcome with the use of locked nucleic acids.30,31,33 The influence of surface loading and steric effects on aptamers has been recently studied, which leads to greater accessibility of binding domains, decreasing the possibility of sensor signal saturation, and increasing the functional concentration biosensing range.31,97–100
Enzyme and MIP biorecognition elements are integrated differently into a signal transduction platform. Instead of covalent linkage to a surface, enzyme and MIP biorecognition elements are often imbedded in a surface construct. The depth and density of the surface structure dictates the biosensor sensitivity, range, and response time.25,81,101 Thicker surface constructs allow for more biorecognition elements per unit area, and therefore, more binding sites for the target bioanalyte. However, the increase in thickness can also result in a loss of sensitivity, lower concentration limits and, poor response time.102 Therefore, sensitivity and range for enzyme and MIP biorecognition elements can be optimized with alterations to the surface construct.
Reproducibility
Reproducibility is defined as the ability to fabricate multiple identical biosensors that will produce the same response to a target bioanalyte. An understanding of the biorecognition element structure, behavior and production process can improve biosensor reproducibility. Reproducibility can be limited through various development processes, for example, in the construction of the recognition element (e.g. batch-to-batch variability), attachment of the biorecognition element to surface (e.g. surface loading), or variability in surface constructs. Reproducibility is often left unreported, and we propose that biosensor publications should include how many different sensors were fabricated and used in the publication or show data over multiple sensors to demonstrate reproducible variability.
Although antibody and enzyme based biosensors are the most represented on the clinical market, the production of these biosensors needs to be carefully controlled to prevent irreproducible results.103,104 Antibodies and enzymes are sensitive to degradation, from pH or temperature variations, making industrial processing and production difficult, especially in low-resource regions.21,105 Activity of the biorecognition element is dependent on storage and transport conditions, making a consistent biosensor response difficult to achieve.106 Despite their prevalence in the community, quality control and environmental instability are known and well defined problems for antibodies and enzymes.
Aptamers are optimal biorecognition elements to ensure highly reproducible biosensors.107–109 Chemical synthesis of aptamers is a well-defined and highly reproducible process leading to the manufacture of robust biorecognition elements. Post-synthesis modifications of aptamers is easily achieved to enhance stability and decrease non-specific binding to improve biosensor reproducibility and selectivity.4 Typically, aptamers interface with the sensor surface through thiol chemistry, which is a well understood and characterized process.31
MIP biorecognition elements experience similar reproducibility to aptamer biorecognition elements due to their well characterized chemical synthesis process.110–112 Synthetic polymer constructs (e.g. MIPs) are predictable and well understood reproducible paradigms. Additionally, translation from benchtop to point-of-care applications can easily be achieved due to the predictable nature of polymer processing, stability, and extended self-life of MIPs compared to natural biorecognition elements.110–113 Industrial production of MIPs is simple, cost-effective, and typically lacks batch-to-batch variations resulting in highly reproducible biosensors.113
Reusability
Reusability is not often quantitatively evaluated in biosensor literature. Tied to the stability and binding kinetics of the biorecognition element, reusability is defined as the ability to reuse a sensor multiple times.114,115 Instead of studying reusability, single-use biosensors, most commonly paper-based biosensors, are growing more popular in funding and literature.116–120 While single-use sensors have their applications and advantages, reusable biosensors have potential to improve accessibilities of biosensor technologies in low-resource regions.
Typically, the dissociation equilibrium constant is reported for bioanalyte-biorecognition complex kinetics, but biosensor use over multiple assays is unclear unless the literature directly specifies the result. Therefore, we encourage researchers to publish details of how often a sensor needs to be replaced to obtain the desired results. In addition, we encourage researchers to also discuss the off-kinetics of the bioanalyte-biorecognition complex or details to regenerate the biorecognition element. Regeneration of a biosensor occurs when the target bioanalyte dissociates from the biorecognition element reopening the binding site. Improved characterization of the off-kinetics, part of the dissociation constant, will provide a better understanding of how to effectively regenerate the biosensor surface. Further, regeneration of a biosensor surface should be considered for future publications, including chemical, thermal, or electrochemical regeneration methods of various biosensor paradigms.65,121–123
Understanding the forces mediating the bioanalyte-biorecognition element interaction is important when considering biosensor regeneration and reusability. Enzymatic biosensors are a prime candidate for reusable biosensors, as enzymes are not consumed or altered during catalytic reactions, therefore, the binding site is preserved maintaining activity after use.24 This process is often referred to as passive regeneration, as no extra action is needed to initiate regeneration and it is a spontaneous process. Though not extensively reviewed in the literature, regeneration of antibody, aptamer, or DNA biorecognition elements can achieve biosensor reusability.
Affinity based interactions, common for many biorecognition elements, are dominated by thermodynamic forces in the form of enthalpy and entropy. Charge mediated interactions are dominated by enthalpy forces while entropy drives hydrophobic interactions.121 The solvent environment influences these forces, and therefore, can be altered to achieve biosensor regeneration for reuse. For example, the charge state of amino acids will differ depending on the solvent pH, and placing the biosensor in a different pH buffer could disrupt protein based interactions.121 The goal of biosensor regeneration is to successfully unravel the bioanalyte-biorecognition element complex while maintaining the integrity of the biorecognition element. Biosensor reusability is challenged by the complex nature of numerous symbiotic forces mediating bioanalyte-biorecognition element interactions.
Choosing a Biorecognition Element
Blood glucose monitoring continues to set a standard of success within the biosensor community due to the simplicity of design, ease of use, accuracy, and quick response time. While blood glucose monitoring remains a gold standard for biosensors, this sensing paradigm is not appropriate for all bioanalytes and applications. Instead, there are countless biosensor configurations, each with a unique combination of biorecognition element, transducer, and target bioanalyte. One method to perpetuate the standard of success set by glucose monitoring is through greater care in the selection of the correct biorecognition element paradigm during the preliminary design phase.
Understanding the advantages and disadvantages of each biorecognition element in terms of biosensors performance is important during developmental stages. Very rarely will a biosensor paradigm lead to the most sensitive, selective, reproducible, and reusable sensor. Instead, a trade-off on biosensor characteristics is necessary for specific applications. For some applications, sensitivity and a low limit of detection is necessary to monitor a specific analyte. In alternative applications, selectivity is very important to distinguish the analyte from other similar competing analogs, and therefore, a small sacrifice in sensitivity can occur. However, emphasizing one biosensor characteristics does not indicate that other biosensor characteristics are unimportant. For these reasons, it is important to first understand the bioanalyte target and intended biosensor application prior to building your biosensor. The provided decision map, Figure 6, serves as a simplified guide for the selection of a biorecognition element in the initial design phase after the target bioanalyte and biosensor performance characteristics are clearly defined for the intended application.
Figure 6:
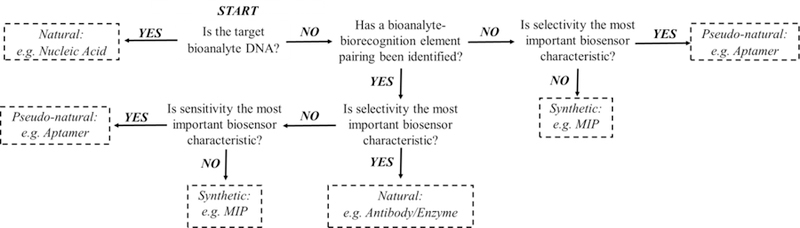
Decision map that can be used to choose a recognition element in the initial design of biosensors.
The decision map, Figure 6, is only used as a starting point for further investigation of the selected biorecognition element. This decision map also serves as a tool for biosensor redesign if obstacles arise during preliminary testing of the first design. Based on observations during preliminary biosensor tests, a different biorecognition element may be selected using the decision map to assist in biosensor redesign. Overall this systematic approach to the development and evaluation of new biosensors will promote the success of creative biosensor designs and facilitate the translation from bench-top to point-of-care applications.
Summary and Conclusions
Over the past 10 years, biosensor technology has significantly grown from the integration of different, interdisciplinary, scientific disciplines, leading to unique and novel biosensor designs and applications.124–127 Understanding the optimal role of each type of biorecognition element in the community is important to ensure successful advancements in novel biosensor technology. Novice biosensor researchers and collaborators can benefit from a brief overview of multiple paradigms in selecting which biorecognition element and transducer will best target the desired bioanalyte and clinical goals. The language used herein is proposed as a guide in collaborative discussions to understand the advantages and limitations of each biorecognition element when designing a new biosensor.
Further, published literature often uses quantitative evaluation of sensitivity and selectivity of a biosensor as the singular metric of success. As the community grows, a set standard of communication and evaluation, beyond sensitivity and selectivity of biosensor success, is important. The full potential of currently researched biorecognition elements is limited due to the lack of reproducibility and reusability biosensor performance characteristics in literature. Not openly discussing the limitations of reusability, reproducibility, and selectivity in complex matrices limits successful transition of biosensors technologies from bench-top to clinical applications. Often, the lack of reporting on reproducibility and reusability leads to false promises and over-exaggeration of existing technology. For these reasons we encourage researchers to publish results on reproducibility and reusability, even if the results are not positive, demonstrating the ability to generate the same results over multiple sensors and the ability to regenerate a singular sensor for reuse. The development of an evaluation standard for reproducibility and reusability is needed to fully grasp the potential of biosensor technologies. A guide for the optimization of biorecognition element selection, as well as, subsequent characterization will help push the community toward the development of more robust biosensor technologies.
Acknowledgments
MAM acknowledges the financial support from NSF (CBET 1638896). JMH acknowledges the financial support from NIH (P20 GM113131). Both authors acknowledge the support from Caroline Ladegard, the College of Engineering and Physical Sciences, and the Surface Enhanced Electrochemical Diagnostic Sensors Laboratory at the University of New Hampshire.
References
- (1).Giardi MT, and Piletska EV (2006) Biotechnological Applications of Photosynthetic Proteins: Biochips, Biosensors and Biodevices Springer US, Boston, MA. [Google Scholar]
- (2).Yu HLL, Maslova A, and Hsing I-M (2017) Rational design of electrochemical DNA biosensors for point-of-care applications. ChemElectroChem 4, 795–805. [Google Scholar]
- (3).Brazaca LC, Ribovski L, Janegitz BC, and Zucolotto V (2017) Nanostructured materials and nanoparticles for point of care (POC) medical biosensors, in Medical Biosensors for Point of Care (POC) Applications, pp 229–254. Elsevier. [Google Scholar]
- (4).Justino CIL, Freitas AC, Pereira R, Duarte AC, and Rocha Santos TAP (2015) Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem 68, 2–17. [Google Scholar]
- (5).Mittal S, Kaur H, Gautam N, and Mantha AK (2017) Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron 88, 217–231. [DOI] [PubMed] [Google Scholar]
- (6).Jeong Y, Kook Y-M, Lee K, and Koh W-G (2018) Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron 111, 102–116. [DOI] [PubMed] [Google Scholar]
- (7).Meshram BD, Agrawal AK, Adil S, Ranvir S, and Sande KK (2018) Biosensor and its application in food and dairy industry: A Review. Int. J. Curr. Microbiol. Appl. Sci 7, 3305–3324. [Google Scholar]
- (8).Swamy NK, Sandeep S, and Santhosh AS (2017) Conductive polymers and their nanohybrid transducers for electrochemical biosensors applications : A brief review. J. Adv. Chem. Sci S2, 6–9. [Google Scholar]
- (9).Kassal P, Steinberg MD, and Steinberg IM (2018) Wireless chemical sensors and biosensors: A review. Sensors Actuators B Chem 266, 228–245. [Google Scholar]
- (10).Ali J, Najeeb J, Asim Ali M, Farhan Aslam M, and Raza A (2017) Biosensors: Their fundamentals, designs, types and most recent impactful applications: A review. J. Biosens. Bioelectron 08. [Google Scholar]
- (11).Singh R, Mukherjee M Das, Sumana G, Gupta RK, Sood S, and Malhotra BD (2014) Biosensors for pathogen detection: A smart approach towards clinical diagnosis. Sensors Actuators B Chem 197, 385–404. [Google Scholar]
- (12).Ahmed MU, Saaem I, Wu PC, and Brown AS (2014) Personalized diagnostics and biosensors: A review of the biology and technology needed for personalized medicine. Crit. Rev. Biotechnol 34, 180–196. [DOI] [PubMed] [Google Scholar]
- (13).Chambers JP, Arulanandam BP, Matta LL, Weis A, and Valdes JJ (2008) Biosensor recognition elements. Curr. Issues Mol. Biol 10, 1–12. [PubMed] [Google Scholar]
- (14).Crivianu-Gaita V, and Thompson M (2016) Aptamers, antibody scFv, and antibody Fab’ fragments: An overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens. Bioelectron 85, 32–45. [DOI] [PubMed] [Google Scholar]
- (15).Corry B, Uilk J, and Crawley C (2003) Probing direct binding affinity in electrochemical antibody-based sensors. Anal. Chim. Acta 496, 103–116. [Google Scholar]
- (16).Scarano S, Mascini M, Turner APF, and Minunni M (2010) Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron 25, 957–966. [DOI] [PubMed] [Google Scholar]
- (17).Skottrup PD, Nicolaisen M, and Justesen AF (2008) Towards on-site pathogen detection using antibody-based sensors. Biosens. Bioelectron 24, 339–348. [DOI] [PubMed] [Google Scholar]
- (18).Su X-L, and Li Y (2004) A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of Escherichia coli O157:H7. Biosens. Bioelectron 19, 563–574. [DOI] [PubMed] [Google Scholar]
- (19).Caelen I, Gao H, and Sigrist H (2002) Protein density gradients on surfaces. Langmuir 18, 2463–2467. [Google Scholar]
- (20).Veiseh M, Zareie MH, and Zhang M (2002) Highly selective protein patterning on gold−silicon substrates for biosensor applications. Langmuir 18, 6671–6678. [Google Scholar]
- (21).Miller E, and Sikes HD (2015) Addressing barriers to the development and adoption of rapid diagnostic tests in global health. Nanobiomedicine 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Oue S, Okamoto A, Yano T, and Kagamiyama H (1999) Redesigning the substrate specificity of an enzyme by cumulative effects of the mutations of non-active site residues 274, 2344–2349. [DOI] [PubMed] [Google Scholar]
- (23).Gaudin V (2017) Advances in biosensor development for the screening of antibiotic residues in food products of animal origin – A comprehensive review. Biosens. Bioelectron 90, 363–377. [DOI] [PubMed] [Google Scholar]
- (24).Zhao WW, Xu JJ, and Chen HY (2017) Photoelectrochemical enzymatic biosensors. Biosens. Bioelectron 92, 294–304. [DOI] [PubMed] [Google Scholar]
- (25).Zhou Y, Liu B, Yang R, and Liu J (2017) Filling in the gaps between nanozymes and enzymes: Challenges and opportunities. Bioconjug. Chem 28, 2903–2909. [DOI] [PubMed] [Google Scholar]
- (26).Amine A, Mohammadi H, Bourais I, and Palleschi G (2006) Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens. Bioelectron 21, 1405–1423. [DOI] [PubMed] [Google Scholar]
- (27).Lange U, Roznyatovskaya NV, and Mirsky VM (2008) Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 614, 1–26. [DOI] [PubMed] [Google Scholar]
- (28).Li C, Karadeniz H, Canavar E, and Erdem A (2012) Electrochemical sensing of label free DNA hybridization related to breast cancer 1 gene at disposable sensor platforms modified with single walled carbon nanotubes. Electrochim. Acta 82, 137–142. [Google Scholar]
- (29).Fan C, Plaxco KW, and Heeger AJ (2003) Electrochemical interrogation of conformational changes as a reagentless method for the sequence-specific detection of DNA. Proc. Natl. Acad. Sci 100, 9134–9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kang D, Parolo C, Sun S, Ogden NE, Dahlquist FW, and Plaxco KW (2018) Expanding the scope of protein-detecting electrochemical DNA “scaffold” sensors. ACS Sensors 3, 1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Dauphin-Ducharme P, and Plaxco KW (2016) Maximizing the signal gain of electrochemical-DNA sensors. Anal. Chem 88, 11654–11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zhu B, Booth MA, Shepherd P, Sheppard A, and Travas-Sejdic J (2015) Distinguishing cytosine methylation using electrochemical, label-free detection of DNA hybridization and ds-targets. Biosens. Bioelectron 64, 74–80. [DOI] [PubMed] [Google Scholar]
- (33).Ferapontova EE (2018) DNA electrochemistry and electrochemical sensors for nucleic acids. Annu. Rev. Anal. Chem 11, 197–218. [DOI] [PubMed] [Google Scholar]
- (34).Teengam P, Siangproh W, Tuantranont A, Henry CS, Vilaivan T, and Chailapakul O (2017) Electrochemical paper-based peptide nucleic acid biosensor for detecting human papillomavirus. Anal. Chim. Acta 952, 32–40. [DOI] [PubMed] [Google Scholar]
- (35).D’Agata R, Giuffrida M, and Spoto G (2017) Peptide nucleic acid-based biosensors for cancer diagnosis. Molecules 22, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Odenthal KJ, and Gooding JJ (2007) An introduction to electrochemical DNA biosensors. Analyst 132, 603. [DOI] [PubMed] [Google Scholar]
- (37).Palchetti I, and Mascini M (2008) Nucleic acid biosensors for environmental pollution monitoring. Analyst 133, 846. [DOI] [PubMed] [Google Scholar]
- (38).Piunno PAE, and Krull UJ (2005) Trends in the development of nucleic acid biosensors for medical diagnostics. Anal. Bioanal. Chem 381, 1004–1011. [DOI] [PubMed] [Google Scholar]
- (39).Wang J (2000) Survey and summary: From DNA biosensors to gene chips. Nucleic Acids Res 28, 3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zhou W, Jimmy Huang P-J, Ding J, and Liu J (2014) Aptamer-based biosensors for biomedical diagnostics. Analyst 139, 2627. [DOI] [PubMed] [Google Scholar]
- (41).Darmostuk M, Rimpelova S, Gbelcova H, and Ruml T (2015) Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv 33, 1141–1161. [DOI] [PubMed] [Google Scholar]
- (42).Cieplak M, and Kutner W (2016) Artificial biosensors: How can molecular imprinting mimic biorecognition? Trends Biotechnol 34, 922–941. [DOI] [PubMed] [Google Scholar]
- (43).Wackerlig J, and Schirhagl R (2016) Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: A review. Anal. Chem 88, 250–261. [DOI] [PubMed] [Google Scholar]
- (44).Sharma PS, Iskierko Z, Pietrzyk-Le A, D’Souza F, and Kutner W (2015) Bioinspired intelligent molecularly imprinted polymers for chemosensing: A mini review. Electrochem. commun 50, 81–87. [Google Scholar]
- (45).Lamboy JA, Tam PY, Lee LS, Jackson PJ, Avrantinis SK, Lee HJ, Corn RM, and Weiss GA (2008) Chemical and genetic wrappers for improved phage and RNA display. ChemBioChem 9, 2846–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Sawada T, Okeya Y, Hashizume M, and Serizawa T (2013) Screening of peptides recognizing simple polycyclic aromatic hydrocarbons. Chem. Commun 49, 5088. [DOI] [PubMed] [Google Scholar]
- (47).Takakusagi Y, Takakusagi K, Sugawara F, and Sakaguchi K (2010) Use of phage display technology for the determination of the targets for small-molecule therapeutics. Expert Opin. Drug Discov 5, 361–389. [DOI] [PubMed] [Google Scholar]
- (48).Van Dorst B, Mehta J, Rouah-Martin E, Blust R, and Robbens J (2012) Phage display as a method for discovering cellular targets of small molecules. Methods 58, 56–61. [DOI] [PubMed] [Google Scholar]
- (49).Labib M, Sargent EH, and Kelley SO (2016) Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev 116, 9001–9090. [DOI] [PubMed] [Google Scholar]
- (50).Wei H, and Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev 42, 6060. [DOI] [PubMed] [Google Scholar]
- (51).Fimiani C, Goina E, and Mallamaci A (2015) Upregulating endogenous genes by an RNA-programmable artificial transactivator. Nucleic Acids Res 43, 7850–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Roham M, Halpern JM, Martin HB, Chiel HJ, and Mohseni P (2008) Wireless amperometric neurochemical monitoring using an integrated telemetry circuit. IEEE Trans. Biomed. Eng 55, 2628–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Halpern JM, Cullins MJ, Chiel HJ, and Martin HB (2010) Chronic in vivo nerve electrical recordings of Aplysia californica using a boron-doped polycrystalline diamond electrode. Diam. Relat. Mater 19, 178–181. [Google Scholar]
- (54).Halpern JM, Xie S, Schreiber JL, and Martin HB (2007) Kinetic and adsorption studies of biogenic amine neurotransmitters at polycrystalline diamond microelectrodes. ECS Trans 3, 47–57. [Google Scholar]
- (55).Halpern JM, Xie S, Sutton GP, Higashikubo BT, Chestek C. a., Lu H, Chiel HJ, and Martin HB (2006) Diamond electrodes for neurodynamic studies in Aplysia californica. Diam. Relat. Mater 15, 183–187. [Google Scholar]
- (56).Lin Y, Huang Y, Ren J, and Qu X (2014) Incorporating ATP into biomimetic catalysts for realizing exceptional enzymatic performance over a broad temperature range. NPG Asia Mater 6, e114–e114. [Google Scholar]
- (57).Yang N, Chen X, Ren T, Zhang P, and Yang D (2015) Carbon nanotube based biosensors. Sensors Actuators B Chem 207, 690–715. [Google Scholar]
- (58).Thompson G, Marnoto S, and Halpern JM (2017) Proper controls to electrochemically evaluate carotenoids using β-cyclodextrin modified surfaces. ECS Trans 80, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Jayanth Babu K, Sasya M, Nesakumar N, Shankar P, Gumpu MB, Ramachandra BL, Kulandaisamy AJ, and Rayappan JBB (2017) Non-enzymatic detection of glucose in fruits using TiO2–Mn3O4 hybrid nano interface. Appl. Nanosci 7, 309–316. [Google Scholar]
- (60).Le ND, Rana S, and Rotello VM (2013) Chemical nose sensors: An alternative strategy for cancer diagnosis. Expert Rev. Mol. Diagn 13, 111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Broza YY, Zuri L, and Haick H (2015) Combined volatolomics for monitoring of human body chemistry. Sci. Rep 4, 4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Halpern JM, Wang B, and Haick H (2015) Controlling the sensing properties of silicon nanowires via the bonds nearest to the silicon nanowire surface. ACS Appl. Mater. Interfaces 7, 11315–11321. [DOI] [PubMed] [Google Scholar]
- (63).Barash O, Zhang W, Halpern JM, Hua Q-L, Pan Y-Y, Kayal H, Khoury K, Liu H, Davies MPA, and Haick H (2015) Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget 6, 44864–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Mazzone P (2009) Sniffing out lung cancer. Nat. Nanotechnol 4, 621–622. [DOI] [PubMed] [Google Scholar]
- (65).Karlsson R, and Fält A (1997) Experimental design for kinetic analysis of protein-protein interactions with surface plasmon resonance biosensors. J. Immunol. Methods 200, 121–133. [DOI] [PubMed] [Google Scholar]
- (66).Boos W, and Gordon AS (1971) Transport properties of the galactose-binding protein of Escherichia coli. Occurrence of two conformational states. J. Biol. Chem 246, 621–8. [PubMed] [Google Scholar]
- (67).Jeon H, Lee E, Kim D, Lee M, Ryu J, Kang C, Kim S, and Kwon Y (2018) Cell-based biosensors based on intein-mediated protein engineering for detection of biologically active signaling molecules. Anal. Chem 8, acs.analchem.8b01481. [DOI] [PubMed] [Google Scholar]
- (68).Dormitzer PR (2002) The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J 21, 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Gilles-Gonzalez M-A, and Gonzalez G (2005) Heme-based sensors: defining characteristics, recent developments, and regulatory hypotheses. J. Inorg. Biochem 99, 1–22. [DOI] [PubMed] [Google Scholar]
- (70).Wu H, Nie Y, Huse WD, and Watkins JD (1999) Humanization of a murine monoclonal antibody by simultaneous optimization of framework and CDR residues1. J. Mol. Biol 294, 151–162. [DOI] [PubMed] [Google Scholar]
- (71).Kuah E, Toh S, Yee J, Ma Q, and Gao Z (2016) Enzyme mimics: Advances and applications. Chem. - A Eur. J 22, 8404–8430. [DOI] [PubMed] [Google Scholar]
- (72).Golub E, Albada HB, Liao W-C, Biniuri Y, and Willner I (2016) Nucleoapzymes: Hemin/G-quadruplex DNAzyme–aptamer binding site conjugates with superior enzyme-like catalytic functions. J. Am. Chem. Soc 138, 164–172. [DOI] [PubMed] [Google Scholar]
- (73).Price CP (2001) Application of chemistry to in vitro diagnostic tests. Chem. Soc. Rev 30, 1–7. [Google Scholar]
- (74).Yao C, Zhu T, Qi Y, Zhao Y, Xia H, and Fu W (2010) Development of a quartz crystal microbalance biosensor with aptamers as bio-recognition element. Sensors 10, 5859–5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Verheyen E, Schillemans JP, van Wijk M, Demeniex M-A, Hennink WE, and van Nostrum CF (2011) Challenges for the effective molecular imprinting of proteins. Biomaterials 32, 3008–3020. [DOI] [PubMed] [Google Scholar]
- (76).Ge Y, and Turner APF (2008) Too large to fit? Recent developments in macromolecular imprinting. Trends Biotechnol 26, 218–224. [DOI] [PubMed] [Google Scholar]
- (77).Kryscio DR, and Peppas NA (2012) Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater 8, 461–473. [DOI] [PubMed] [Google Scholar]
- (78).Spivak DA (2005) Optimization, evaluation, and characterization of molecularly imprinted polymers. Adv. Drug Deliv. Rev 57, 1779–94. [DOI] [PubMed] [Google Scholar]
- (79).Yilmaz E, Mosbach K, and Haupt K (1999) Influence of functional and cross-linking monomers and the amount of template on the performance of molecularly imprinted polymers in binding assays. Anal. Commun 36, 167–170. [Google Scholar]
- (80).Piletsky SA, Panasyuk TL, Piletskaya EV, Nicholls IA, and Ulbricht M (1999) Receptor and transport properties of imprinted polymer membranes–a review. J. Memb. Sci 157, 263–278. [Google Scholar]
- (81).Whitcombe MJ, Chianella I, Larcombe L, Piletsky SA, Noble J, Porter R, and Horgan A (2011) The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev 40, 1547–1571. [DOI] [PubMed] [Google Scholar]
- (82).Saha K, Agasti SS, Kim C, Li X, and Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem. Rev 112, 2739–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Sepúlveda B, Angelomé PC, Lechuga LM, and Liz-Marzán LM (2009) LSPR-based nanobiosensors. Nano Today 4, 244–251. [Google Scholar]
- (84).Storhoff JJ, Lucas AD, Garimella V, Bao YP, and Müller UR (2004) Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat. Biotechnol 22, 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Wang Y, Knoll W, and Dostalek J (2012) Bacterial pathogen surface plasmon resonance biosensor advanced by long range surface plasmons and magnetic nanoparticle assays. Anal. Chem 84, 8345–8350. [DOI] [PubMed] [Google Scholar]
- (86).Madsen R, Lundstedt T, and Trygg J (2010) Chemometrics in metabolomics—A review in human disease diagnosis. Anal. Chim. Acta 659, 23–33. [DOI] [PubMed] [Google Scholar]
- (87).Bro R, Kamstrup-Nielsen MH, Engelsen SB, Savorani F, Rasmussen MA, Hansen L, Olsen A, Tjønneland A, and Dragsted LO (2015) Forecasting individual breast cancer risk using plasma metabolomics and biocontours. Metabolomics 11, 1376–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Qiu Y, Zhou B, Su M, Baxter S, Zheng X, Zhao X, Yen Y, and Jia W (2013) Mass spectrometry-based quantitative metabolomics revealed a distinct lipid profile in breast cancer patients. Int. J. Mol. Sci 14, 8047–8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Patel S, and Ahmed S (2015) Emerging field of metabolomics: Big promise for cancer biomarker identification and drug discovery. J. Pharm. Biomed. Anal 107, 63–74. [DOI] [PubMed] [Google Scholar]
- (90).Wujcik EK, Wei H, Zhang X, Guo J, Yan X, Sutrave N, Wei S, and Guo Z (2014) Antibody nanosensors: A detailed review. RSC Adv 4, 43725–43745. [Google Scholar]
- (91).Liu Y, and Yu J (2016) Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 183, 1–19. [Google Scholar]
- (92).Trilling AK, Beekwilder J, and Zuilhof H (2013) Antibody orientation on biosensor surfaces: A minireview. Analyst 138, 1619. [DOI] [PubMed] [Google Scholar]
- (93).Ronkainen NJ, Halsall HB, and Heineman WR (2010) Electrochemical biosensors. Chem. Soc. Rev 39, 1747. [DOI] [PubMed] [Google Scholar]
- (94).Huang L, Muyldermans S, and Saerens D (2010) Nanobodies ®: Proficient tools in diagnostics. Expert Rev. Mol. Diagn 10, 777–785. [DOI] [PubMed] [Google Scholar]
- (95).Singh A, Pasha SK, Manickam P, and Bhansali S (2016) Single-domain antibody based thermally stable electrochemical immunosensor. Biosens. Bioelectron 83, 162–168. [DOI] [PubMed] [Google Scholar]
- (96).Gonzalez-Sapienza G, Rossotti MA, and Tabares-da Rosa S (2017) Single-domain antibodies as versatile affinity reagents for analytical and diagnostic applications. Front. Immunol 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Seok Kim Y, Ahmad Raston NH, and Bock Gu M (2016) Aptamer-based nanobiosensors. Biosens. Bioelectron 76, 2–19. [DOI] [PubMed] [Google Scholar]
- (98).Ohno Y, Maehashi K, and Matsumoto K (2010) Label-free biosensors based on aptamer-modified graphene field-effect transistors. J. Am. Chem. Soc 132, 18012–18013. [DOI] [PubMed] [Google Scholar]
- (99).Tothill IE (2009) Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol 20, 55–62. [DOI] [PubMed] [Google Scholar]
- (100).Zhang Z, Oni O, and Liu J (2017) New insights into a classic aptamer: binding sites, cooperativity and more sensitive adenosine detection. Nucleic Acids Res et al. 45, 7593–7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Iyer PV, and Ananthanarayan L (2008) Enzyme stability and stabilization-Aqueous and non-aqueous environment. Process Biochem 43, 1019–1032. [Google Scholar]
- (102).Zhu C, Yang G, Li H, Du D, and Lin Y (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem 87, 230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Newman JD, and Turner APF (2005) Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron 20, 2435–2453. [DOI] [PubMed] [Google Scholar]
- (104).Turner APF (2013) Biosensors: sense and sensibility. Chem. Soc. Rev 42, 3184. [DOI] [PubMed] [Google Scholar]
- (105).Iyer PV, and Ananthanarayan L (2008) Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem 43, 1019–1032. [Google Scholar]
- (106).Weller MG (2016) Quality issues of research antibodies. Anal. Chem. Insights 2016, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Iliuk AB, Hu L, and Tao WA (2011) Aptamer in bioanalytical applications. Anal. Chem 83, 4440–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Song S, Wang L, Li J, Fan C, and Zhao J (2008) Aptamer-based biosensors. TrAC Trends Anal. Chem 27, 108–117. [Google Scholar]
- (109).Tombelli S, Minunni M, and Mascini M (2005) Analytical applications of aptamers. Biosens. Bioelectron 20, 2424–2434. [DOI] [PubMed] [Google Scholar]
- (110).Boysen RI, Schwarz LJ, Nicolau DV, and Hearn MTW (2017) Molecularly imprinted polymer membranes and thin films for the separation and sensing of biomacromolecules. J. Sep. Sci 40, 314–335. [DOI] [PubMed] [Google Scholar]
- (111).Sharma PS, Pietrzyk-Le A, D’Souza F, and Kutner W (2012) Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem 402, 3177–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Soldatkin AP, Dzyadevych SV, Korpan YI, Sergeyeva TA, Arkhypova VN, Biloivan OA, Soldatkin OO, Shkotova LV, Zinchenko OA, Peshkova VM, Saiapina OY, Marchenko SV, and El’skaya AV (2013) Biosensors. A quarter of a century of R&D experience. Biopolym. Cell 29, 188–206. [Google Scholar]
- (113).Sellergren B, and Hall AJ (2012) Molecularly Imprinted Polymers, in Supramolecular Chemistry (Gale P, and Steed J, Eds.). John Wiley & Sons, Ltd, Chichester, UK. [Google Scholar]
- (114).Radi A-E, Acero Sánchez JL, Baldrich E, and O’Sullivan CK (2006) Reagentless, reusable, ultrasensitive electrochemical molecular beacon aptasensor. J. Am. Chem. Soc 128, 117–124. [DOI] [PubMed] [Google Scholar]
- (115).Lu H-C, Chen H-M, Lin Y-S, and Lin J-W (2000) A reusable and specific protein A-coated piezoelectric biosensor for flow injection immunoassay. Biotechnol. Prog 16, 116–124. [DOI] [PubMed] [Google Scholar]
- (116).de Araujo WR, Reddy SM, and Paixão TRLC (2017) Introduction of materials used in chemical sensors, in Materials for Chemical Sensing, pp 1–5. Springer International Publishing, Cham. [Google Scholar]
- (117).Cinti S, and Arduini F (2017) Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron 89, 107–122. [DOI] [PubMed] [Google Scholar]
- (118).Delaney JL, Hogan CF, Tian J, and Shen W (2011) Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem 83, 1300–1306. [DOI] [PubMed] [Google Scholar]
- (119).Li C, Thostenson ET, and Chou T-W (2008) Sensors and actuators based on carbon nanotubes and their composites: A review. Compos. Sci. Technol 68, 1227–1249. [Google Scholar]
- (120).Liana DD, Raguse B, Gooding JJ, and Chow E (2012) Recent advances in paper-based sensors. Sensors 12, 11505–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Goode JA, Rushworth JVH, and Millner PA (2015) Biosensor regeneration: A review of common techniques and outcomes. Langmuir 31, 6267–6276. [DOI] [PubMed] [Google Scholar]
- (122).Balamurugan S, Obubuafo A, Soper SA, and Spivak DA (2008) Surface immobilization methods for aptamer diagnostic applications. Anal. Bioanal. Chem 390, 1009–1021. [DOI] [PubMed] [Google Scholar]
- (123).Homola J (2008) Surface plasmon resonance sensors for detection of chemical and biological Species. Chem. Rev 108, 462–493. [DOI] [PubMed] [Google Scholar]
- (124).Zaripova VM, and Petrova IY (2017) System of Automated Design of Biosensors, in Creativity in Intelligent Technologies and Data Science (Kravets A, Shcherbakov M, Kultsova M, and Groumpos P, Eds.), pp 479–489. Springer International Publishing. [Google Scholar]
- (125).Bellah MM (2017) The emergence of interdisciplinary research in cancer diagnostics. J. Nanomedicine Res 6. [Google Scholar]
- (126).Kulkarni SJ (2016) A review on studies and research on biosensors: An interdisciplinary pursuit. Int. J. Sci. Res. Comput. Sci 1, 19–23. [Google Scholar]
- (127).Maduraiveeran G, Sasidharan M, and Ganesan V (2018) Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron 103, 113–129. [DOI] [PubMed] [Google Scholar]


