Abstract
Introduction:
Subtle neurocognitive deficits have been recently observed in Acute Lym-phoblastic Leukemia (ALL) survivors.
Aim:
We aim to assess the neurocognitive functions of ALL survivors who had been treated with chemotherapy only using two different protocols, and to identify treatment-related risk factors.
Patients and Methods:
We carried a multicenter study involving 3 pediatric oncology centers on 100 children who were treated for ALL. Fifty patients were treated by the modified Children’s Cancer Group (CCG) 1991 protocol with low dose methotrexate and 50 children were treated by Total XV protocol with high dose methotrexate. Fifty healthy children were included as a control group. Psy-chometric assessment using Arabic version of Wechsler intelligence scale for children (WISC III) was performed for all patients and controls.
Results:
Patients had significantly lower mean full scale IQ, performance IQ and verbal IQ than con-trols. Patients ≤ 5 years at diagnosis had significantly lower mean full scale IQ and performance IQ than patients>5 years at diagnosis, while the verbal IQ showed no significant difference between both age groups. Female patients had significantly lower mean full scale IQ, performance IQ and verbal IQthan males. Patients who received Total XV protocol with high dose methotrexate had significantly lower mean full scale IQ, performance IQ and verbal IQ than patients who received modified CCG 1991 protocol with low dose methotrexate.
Conclusions:
CNS directed chemotherapy might appear to affect neurocognitive functions in chil-dren with ALL, which is more significant in young children at diagnosis, in girls and in those receiv-ing high dose methotrexate.
Keywords: Neurocognitive, ALL, chemotherapy, children, survivors, leukemia
1. INTRODUCTION
ALL is the most common cancer diagnosed in children and represents approximately 25% of cancer diagnoses among children younger than 15 years [1]. The improvement in the overall survival pediatric ALL is considered a real success story in modern clinical oncology with an 80% overall survival. This improvement has been attributed to the development of new chemotherapeutic drugs together with enhanced support services and evolution of risk-adapted therapy [2].
Unfortunately, a significant increase in morbidity has accompanied this development and many drawbacks were associated with the intensive anticancer treatment regimens [2]. Impaired neurocognitive functioning has been observed as a significant long-term consequence of ALL treatment [3] with clear effects on learning abilities, academic achievement, emotional development, emotional regulation and capacity for coping [4].
Cranial irradiation has been reported to have more severe long-term neurocognitive and psychosocial effects than CNS directed therapy [5]. However, there is growing evidence that chemotherapy alone may have long- term neuropsychological sequlae [6].
We herein aimed to evaluate the neurocognitive functions of ALL pediatric survivors who have been treated with chemotherapy only using two different protocols, and to identify treatment-related risk factors that can lead to neurocognitive impairment.
2. MATERIALS AND METHODS
We carried a multicenter cross-sectional study on those children in the pediatric oncology unit of Zagazig University, National Cancer Institute and Benha pediatric special hospital. Patients: The study included 100 children who were treated for ALL.
Patients were subgrouped according to the protocol of treatment into: 1. Group1: 50 children treated by modified Children’s Cancer Group (CCG) 1991 protocol for standard risk ALL with intravenous Vincristine (VCR), dexamethasone, L-asparginase (L Asp), Ara C and Doxorubicin (Dox) with low dose intrathecal Methotrexate (MTX) as induction therapy. Followed by consolidation with 6-mercaptopurine (6MP) and VCR. Then interim maintenanace phase I, delayed intensification, interim maintenance phase II, and maintenance cycles. 2. Group 2: 50 children treated by Total XV protocol with prednisilone, VCR, L Asp, Dox, ARA C, 6MP and cyclophosphamide as induction phase. Followed by high dose methotrexate as consolidation phase. Then continuation therapy follows with reind uction I and II phases.
Inclusion Criteria: 1- Patients who finished chemotherapy regimens for ALL (modified CCG 1991 or Total XV protocol). 2- Patients who survived for at least 12 months after finishing their chemotherapy regimens. 3- Patients with age from 5 to 15 years old.
Exclusion Criteria: 1- Patients with any known mental, psychological, or motor impairment 2- Patients with previous or current CNS disorders. 3- Patients less than 5 and more than 15 years old. 4- Patients who received cranial irradiation.
Controls: Fifty healthy children matched for gender, age, language and socioeconomic status and with normal development were included as a control group.
3. Methods
Psychometric assessment using Arabic version of Wechsler intelligence scale for children [WISC III] [7] was performed for all patients and controls.
It yields verbal, performance, and a combined full scale IQ tests and consists of six verbal subtests and six performance subtests as follows [7]:
3.1. Verbal IQ
It is calculated from six subsets scores: 1- Information subtest: General knowledge testing. It includes questionnaire about literature and geography. 2- Digit span subtest: Test child ability to repeat strings of digits recited by the examiner. 3- Vocabulary subset: Test the child's vocabulary. 4- Arithmetic subtest: Test the arithmetic skills of the child. 5- Comprehension subtest: Test the ability of the child to solve practical problems and explain the meaning of simple proverbs. 6-Similarities subtest: Test the ability of the child to describe the similarities between pairs of items, for example that oranges and apples are both fruits.
3.2. Performance IQ
It is derived from scores of six subtests. Scores of the performance subtests are based on the number of correct answers and the speed of response.
1- Picture completion subtest: Test child ability to complete pictures with missing elements. 2- Picture arrangement subtest: Test child ability to arrange pictures in order to tell a story 3- Block design subtest: Test the child’s ability to use blocks to make certain designs. 4- Object assembly subtest: test the child’s ability toput together pieces in such a way as to construct an entire object. 5- Coding subtest: Test child ability to make pairs from a series of shapes or numbers 6- Mazes subtest: Test child ability tosolve maze puzzles of increasing difficulty.
3.3. Administration Time
Around one hour to one and half hours. The child can complete the test in two separate sessions.
3.4. Ethics
The current study was in accordance with the Helsinki Declaration 2013 [8] and was approved by the research and ethical committees of Zagazig University, Benha University and National cancer institute.
3.5. Consent
Written informed consents were obtained from the parents or other legal guardians of the contributing children for contribution of their children in the current study.
3.6. Statistical Analysis
Data were analyzed using SPSS version 11(SPSS Inc., Chicago, IL, USA). For quantitative variables; mean ± standard deviation were used while for qualitative ones; number and percentage were used. Unpaired student t-test and Pearson coefficient of correlation (r) were used when appropriate. P-valuesis considered significant if less or equal to 0.05 and highly significant if less or equal to 0.001.
4. RESULTS
The current study included; 56 females and 44 males with mean age of 10. 75 ± 2.685 years. Patients had significantly lower mean full scale IQ, verbal IQ and performance IQ than controls (91. 20, 85.60 and 97.28 versus 111.7, 103.5 and 115.7 respectively, p value <0.05).
Additionally, there was significant difference between patients and controls in all verbal and performance IQ subtests (Table 1). Patients ≤ 5 years at diagnosis had significantly lower mean full scale IQ and performance IQ than patients>5 years at diagnosis (69.48, 45.96 versus 92.45, 98.41 respectively, p value <0.05), while there was no significant difference between both age groups as regards verbal IQ (p value >0.05). Also, significant difference exists, between both age groups in almost all performance IQ subsets except picture completion and mazes (Table 2).
Table 1.
IQ in patients and controls.
| - | Patients (n = 100) | Controls (n= 50) | P value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| F IQ | 91.20 | 13.15 | 111.7 | 18.45 | <0.001 |
| V IQ | 85.60 | 15.97 | 103.5 | 18.24 | <0.05 |
| P IQ | 97.28 | 16.21 | 115.7 | 12.72 | <0.05 |
| Verbal IQ subtests | |||||
| Information | 7.84 | 2.71 | 10.4 | 3.53 | <0.05 |
| Comprehension | 5.00 | 2.85 | 9.50 | 4.88 | <0.05 |
| Arithmetic | 4.40 | 2.51 | 8.60 | 1.65 | <0.05 |
| Similarities | 5.90 | 3.05 | 8.70 | 3.56 | <0.05 |
| Vocabulary | 4.04 | 3.18 | 12.20 | 5.07 | <0.001 |
| Digital span | 5.42 | 2.24 | 9.40 | 2.55 | <0.05 |
| Performance IQ subtests | |||||
| Picture completion | 8.34 | 3.07 | 11.60 | 1.90 | <0.05 |
| Picture arrangement | 6.18 | 2.95 | 12.30 | 0.95 | <0.05 |
| Block design | 5.36 | 2.93 | 9.50 | 2.07 | <0.05 |
| Object assembly | 3.36 | 2.81 | 7.90 | 2.42 | <0.05 |
| Coding | 11.16 | 4.42 | 15.90 | 4.25 | <0.05 |
| Mazes | 7.82 | 2.33 | 13.30 | 4.03 | <0.05 |
SD: Standard Deviation; IQ: Intelligence Quotient; FIQ: Full Scale IQ; PIQ: Performance IQ; VIQ: Verbal IQ.
Table 2.
IQ in patients in relation to age at diagnosis.
| - | Patients ≤ 5 years (n = 46) | Patients > 5 years (n = 54) | P value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| F IQ | 69.48 | 7.65 | 92.45 | 11.35 | <0.05 |
| V IQ | 86.48 | 11.86 | 84.85 | 18.99 | >0.05 |
| P IQ | 45.96 | 12.49 | 98.41 | 18.98 | <0.05 |
| Verbal IQ subtests | |||||
| Information | 8.39 | 3.20 | 7.37 | 2.17 | >0.05 |
| Comprehension | 5.00 | 1.68 | 5.00 | 3.60 | >0.05 |
| - | Patients ≤ 5 years (n = 46) | Patients > 5 years (n = 54) | P value | ||
| Mean | SD | Mean | SD | ||
| Arithmetic | 10.13 | 2.70 | 8.78 | 2.19 | >0.05 |
| Similarities | 7.00 | 2.94 | 6.81 | 3.19 | >0.05 |
| Vocabulary | 3.65 | 3.02 | 4.37 | 3.33 | >0.05 |
| Digital span | 7.78 | 2.26 | 7.11 | 2.23 | >0.05 |
| Performance IQ subtests | |||||
| Picture completion | 8.22 | 3.50 | 8.44 | 2.71 | >0.05 |
| Picture arrangement | 2.83 | 2.13 | 6.48 | 3.51 | <0.05 |
| Block design | 2.60 | 4.30 | 8.50 | 4.20 | <0.01 |
| Object assembly | 5.80 | 2.40 | 8.00 | 2.70 | <0.05 |
| Coding | 7.35 | 4.97 | 13.00 | 4.05 | <0.05 |
| Mazes | 9.78 | 2.47 | 8.95 | 2.25 | >0.05 |
SD: Standard Deviation; IQ: Intelligence Quotient; FIQ: Full Scale IQ; PIQ: Performance IQ; VIQ: Verbal IQ
Female patients had significantly lower mean full scale IQ, verbal IQ and performance IQ than male patients (86.43, 80.43 and 92.46 versus 97.27, 92.18 and 103.41 respectively, p value <0.05). Also, there was significant difference between the female patients and male patients in almost all verbal and performance IQ subtests except vocabulary and picture arrangementsubsets (Table 3).
Table 3.
IQ in patients in relation to gender.
| - | Boys (n = 44) | Girls (n = 56) | P value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| F IQ | 97.27 | 13.936 | 86.43 | 10.44 | <0.05 |
| V IQ | 92.18 | 15.117 | 80.43 | 14.908 | <0.05 |
| P IQ | 103.41 | 13.129 | 92.46 | 16.906 | <0.05 |
| Verbal IQ subtests | |||||
| Information | 7.95 | 2.380 | 5.75 | 2.989 | <0.05 |
| Comprehension | 8.59 | 3.459 | 4.54 | 2.219 | <0.05 |
| Arithmetic | 9.45 | 2.425 | 7.36 | 2.614 | <0.05 |
| Similarities | 8.82 | 3.231 | 5.18 | 2.73 | <0.05 |
| Vocabulary | 4.91 | 3.804 | 3.36 | 2.453 | >0.05 |
| Digital span | 8.32 | 2.124 | 3.71 | 2.106 | <0.05 |
| Performance IQ subtests | |||||
| Picture completion | 9.32 | 1.912 | 5.36 | 3.773 | <0.05 |
| Picture arrangement | 6.77 | 3.366 | 5.71 | 2.537 | >0.05 |
| Block design | 9.14 | 2.748 | 5.75 | 2.977 | <0.01 |
| Object assembly | 9.82 | 2.954 | 5.00 | 2.694 | <0.05 |
| Coding | 13.82 | 4.159 | 7.64 | 4.676 | <0.01 |
| Mazes | 8.59 | 1.992 | 5.21 | 2.425 | <0.05 |
SD: Standard Deviation; IQ: Intelligence Quotient; FIQ: Full Scale IQ; PIQ: Performance IQ; VIQ: Verbal IQ.
Patients who received Total XV protocol with high dose methotrexate had significantly lower mean full scale IQ, verbal IQ and performance IQ than patients who received modified CCG 1991 protocol with low dose methotrexate (82.88, 77.04 and 88.76 versus 99.52, 94.16 and 105.80 respectively, p value <0.05). Also, significant difference exists, between both treatment groups in almost all verbal and performance IQ subtests except picture completion and coding subsets (Table 4).
Table 4.
IQ in patients in relation to treatment protocol.
| - | CCG- 1991 Protocol (n = 44) | Total XV Protocol (n = 56) | P value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| F IQ | 99.52 | 12.118 | 82.88 | 7.865 | <0.001 |
| V IQ | 94.16 | 13.484 | 77.04 | 13.655 | <0.001 |
| P IQ | 105.80 | 10.985 | 88.76 | 16.256 | <0.05 |
| Verbal IQ subtests | |||||
| Information | 8.88 | 2.297 | 6.80 | 2.739 | <0.05 |
| Comprehension | 6.36 | 3.264 | 3.64 | 1.440 | <0.05 |
| Arithmetic | 10.32 | 2.673 | 8.48 | 1.982 | <0.05 |
| Similarities | 7.84 | 3.158 | 5.96 | 2.669 | <0.05 |
| Vocabulary | 5.12 | 3.420 | 2.96 | 2.557 | <0.05 |
| Digital span | 8.36 | 2.018 | 6.48 | 2.084 | <0.05 |
| Performance IQ subtests | |||||
| Picture completion | 8.56 | 2.663 | 8.12 | 3.468 | >0.05 |
| Picture arrangement | 7.52 | 3.356 | 4.84 | 1.650 | <0.01 |
| Block design | 9.52 | 2.104 | 7.20 | 3.215 | <0.01 |
| Object assembly | 6.28 | 3.103 | 4.44 | 2.181 | <0.05 |
| Coding | 13.88 | 3.822 | 12.44 | 4.976 | >0.05 |
| Mazes | 8.56 | 1.417 | 7.08 | 2.812 | <0.05 |
SD: Standard Deviation; IQ: Intelligence Quotient; FIQ: Full Scale IQ; PIQ: Performance IQ; VIQ: Verbal IQ.
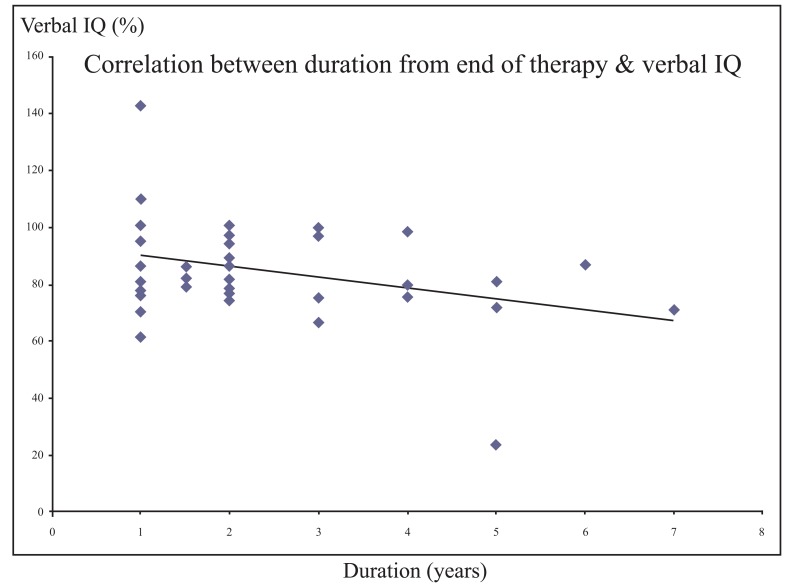
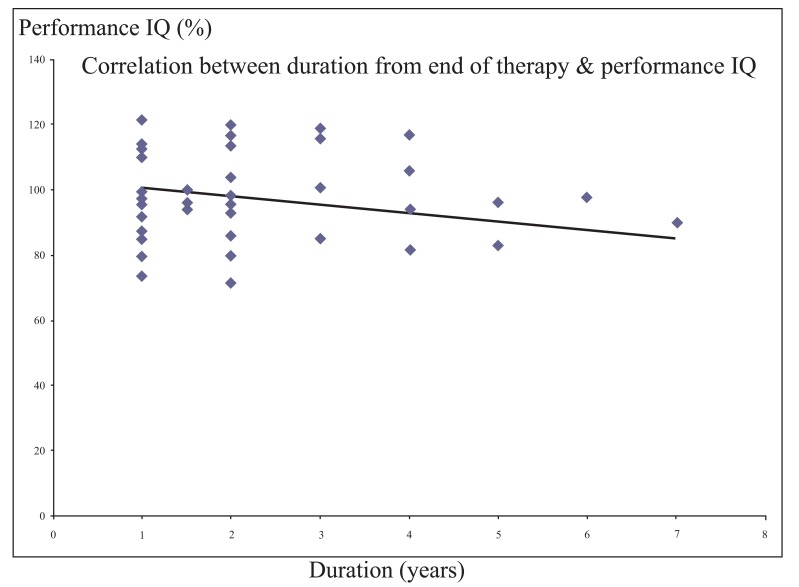
There was a significant negative correlation between each of verbal and performance IQ and the duration from the end of therapy (r = - 0.355 and - 0.329 respectively, p value <0.05) (Figs. 1 and 2).
Fig. (1).
Significant negative correlation between the duration from the end of therapy and verbal IQ.
Fig. (2).
Significant negative correlation between the duration from the end oftherapy andperformance IQ.
5. DISCUSSION
It was proved that cranial irradiation has long term detrimental impact of on intellectual functions and learning abilities and as a result for this, it has been largely replaced by CNS directed chemotherapy for treatment of childhood ALL.
Growing evidence is emerging that chemotherapy alone may impair activities of the brain, such as thinking, attention, memory, speech and flexible thought [9]. It is thought that CNS infiltration, mostly subclinical, is present in the majority of children with ALL and without preventive therapy directed at the CNS; up to 80% of these children develop CNS leukemia [10] with considerably increased rates of morbidity [11] and mortality [12].
In the current study, patients had significantly lower mean full scale IQ, verbal IQ and performance IQ than controls. Additionally, there was significant difference between patients and controls, in all verbal and performance IQ subtests.
In the majority of studies, children who have survived leukemia typically obtain lower IQ scores than children treated for solid tumors outside the CNS or matched healthy controls.
Children with solid tumors do not receive prophylactic CNS treatment [13-15]. Our results are in agreement with many other studies investigating the cognitive consequences of CNS chemotherapy given without cranial irradiation where they reported lower verbal and full Scale IQ scores, impaired performance on tasks involving simultaneous processing, deficits in the areas of motor performance, attention, and symbolic manipulation, memory problems and poorer academic performance [16-19].
Moleski [20] in his review of neuropsychological, neuroanatomical and neurophysiological consequences of CNS chemotherapy in ALL concluded that two-thirds of studies reported impaired intellectual functioning in ALL survivors receiving chemotherapy compared to controls. It has been documented that intrathecal methotrexate, even without radiation therapy, may be linked to white matter changes, leukoencephalopathy, cortical atrophy, calcifications, and seizures [20].
Peterson et al. [3] in his meta-analysis of the neurocognitive sequelae of chemotherapy in childhood ALL, reported worse functioning in ALL survivors in multiple domains of intelligence and academic achievement; Verbal memory; processing speed; executive functioning and fine motor skills.
On the contrary, other studies have failed to find deficits in neurocognitive functioning, academic difficulties or any evidence of lower IQ scores in childhood ALL survivors [21-27]. This discrepancy in the effects of CNS chemotherapy can be attributed to the different designs of these studies. The studies differ in many aspects including allocation of participants to treatment; comparison groups; and the outcome measures [21].
In our study, patients ≤ 5 years at diagnosis had significantly lower mean full scale IQ and performance IQ than patients>5 years at diagnosis, while there was no significant difference between both age groups as regards verbal IQ.
Young age at diagnosis was proved to be an important risk factor for cognitive dysfunction in children with ALL treated with chemotherapy only in many studies [6, 28, 29]. This may be explained based on the susceptibility of the developing brain to damage because of the higher metabolic activity and lower stability of the newly synthesized myelin, making it more vulnerable to the toxic effects of chemotherapy [30].
Castellino et al. [31] and Krull et al. [32] reported that young age at diagnosis was one of the most important risk factors for cancer-related cognitive dysfunction in children.
Other studies have not found CNS-directed chemotherapy without cranial irradiation to interact with age with respect to cognitive abilities, but follow up of the patients was not long enough time for these findings to be conclusive [16, 33].
Peterson et al. [3] in his meta-analysis reported intellectual dysfunction in ALL patients, even without irradiation, particularly in the areas of working memory, processing speed and perceptual reasoning skills. On the other hand, verbal subtests, were not significantly different between the study groups, suggesting that they may be spared in ALL survivors.
Our results showed that female patients had significantly lower mean full scale IQ, verbal IQ and performance IQ than male patients with significant difference between females and males in almost all subsets of verbal and performance IQ subtests.
This was consistent with Waber et al. [34] who assessed cognitive processing in 51 children previously treated for ALL with CNS prophylaxis and who were continuously disease-free for 5 to 12 years where they found females more severely affected than males.
Copeland and her colleagues [23] did not find any correlation between female sex and cognitive dysfunction in patients receiving intrathecal chemotherapy without cranial irradiation. However, intrathecal methotrexate may exert a deleterious synergic effect when administered concurrently with cranial irradiation, especially in girls [23].
Bleyer and coworkers referred this greater vulnerability in girls to the females’ more rapid brain growth and development during childhood. White matter increase during childhood has been documented to be less in girls than in boys which might make girls more susceptible to neurotoxic effects of chemotherapy [35]. In the present study, patients who received Total XV protocol with high dose methotrexate had significantly lower mean full scale IQ, verbal IQ and performance IQ than patients who received modified CCG 1991 protocol with low dose methotrexate.
Also, significant difference exists, between both treatment groups in almost all verbal and performance IQ subtests. Our results were matched with Waber et al. [36] and Eden [37], where they reported that increased dose intensity of intravenous methotrexate was found to be associated with lower IQ in children.
Similarly, Buizer et al. [6], in their multicenter study on 36 children with ALL, found that the deleterious neurocognitive effects were mostly limited to children who received higher doses of systemic methotrexate. In the current work, there was a significant negative correlation between each of the verbal and performance IQ and the duration from the end of therapy.
This observation means that cognitive impairment occurs as a late sequel in children with ALL. This was supported by the recent studies of Armstrong [38] and Krull [32, 39] where persistent neurocognitive deficits and progressive decline in intellectual function have been observed in adult cohorts who were treated for ALL during their childhood and were associated with reduced educational achievement and unemployment.
Also, Krull et al. [32] in their large study on adult survivors of childhood ALL found worsening in executive function skills to be increased with time since diagnosis.
CONCLUSION
CNS directed chemotherapy appears to affect neurocognitive functions in children with ALL, which is more significant in young children at diagnosis, in girls and in those receiving high dose methotrexate.
Strict follow up and monitoring of ALL survivors by health professionals is the cornerstone for early identification and treatment of the neurocognitive problems that may emerge over time.
Acknowledgements
Declared none.
Ethics Approval and Consent to Participate
The study was approved by the research and ethical committees of Zagazig University, Benha University and National cancer institute.
Human and Animal Rights
No animals were used in this research, All humans procedures were in accordance with the ethical standards of the committee responsible for human experimentation (institutional national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/).
Consent for Publication
Written informed consent was obtained from the patients or their legal guardians for participation in the study.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Howlader N., Noone A.M., Krapcho M., et al., editors. SEER Cancer Statistics Review, 1975-2010. Bethesda, Md: National Cancer Institute; 2013. Childhood cancer.https://seer.cancer.gov/archive/csr/1975_2010/ [Google Scholar]
- 2.Pui C.H., Evans W.E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Peterson C.C., Johnson C.E., Ramirez L.Y., et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2008;51(1):99–104. doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- 4.Compas B.E. Psychobiological processes of stress and coping: Implications for resilience in childhood and adolescence. Ann. N. Y. Acad. Sci. 2006;1094:226–234. doi: 10.1196/annals.1376.024. [DOI] [PubMed] [Google Scholar]
- 5.Lange B.J., Bostrom B.C., Cherlow J.M., et al. Double-delayed intensification improves event-free survival for children with intermediate-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2002;99(3):825–833. doi: 10.1182/blood.v99.3.825. [DOI] [PubMed] [Google Scholar]
- 6.Buizer A.I., de Sonneville L.M., van den Heuvel-Eibrink M.M., Veerman A.J. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr. Blood Cancer. 2005;45(3):281–290. doi: 10.1002/pbc.20397. [DOI] [PubMed] [Google Scholar]
- 7.Ismail M.E., Melika L.K. Wechsler Intelligence Scale for Children: Arabic Manual. 7th ed. Cairo, Egypt: El-Nahda Press; 1999. [Google Scholar]
- 8.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 9.Buizer A.I., de Sonneville L.M., Veerman A.J. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: a critical review of the literature. Pediatr. Blood Cancer. 2009;52(4):447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- 10.Evans A.E., Gilbert E.S., Zandstra R. The increasing incidence of central nervous system leukemia in children. (Children’s Cancer Study Group A). Cancer. 1970;26(2):404–409. doi: 10.1002/1097-0142(197008)26:2<404::aid-cncr2820260222>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Ochs J.J., Rivera G., Aur R.J., Hustu H.O., Berg R., Simone J.V. Central nervous system morbidity following an initial isolated central nervous system relapse and its subsequent therapy in childhood acute lymphoblastic leukemia. J. Clin. Oncol. 1985;3(5):622–626. doi: 10.1200/JCO.1985.3.5.622. [DOI] [PubMed] [Google Scholar]
- 12.George S.L., Ochs J.J., Mauer A.M., Simone J.V. The importance of an isolated central nervous system relapse in children with acute lymphoblastic leukemia. J. Clin. Oncol. 1985;3(6):776–781. doi: 10.1200/JCO.1985.3.6.776. [DOI] [PubMed] [Google Scholar]
- 13.Anderson V., Smibert E., Ekert H., Godber T. Intellectual, educational, and behavioural sequelae after cranial irradiation and chemotherapy. Arch. Dis. Child. 1994;70(6):476–483. doi: 10.1136/adc.70.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowell R.E., Jr, Copeland D.R., Francis D.J., Fletcher J.M., Stovall M. Absence of synergistic effects of CNS treatments on neuropsychologic test performance among children. J. Clin. Oncol. 1991;9(6):1029–1036. doi: 10.1200/JCO.1991.9.6.1029. [DOI] [PubMed] [Google Scholar]
- 15.Said J.A., Waters B.G., Cousens P., Stevens M.M. Neuropsychological sequelae of central nervous system prophylaxis in survivors of childhood acute lymphoblastic leukemia. J. Consult. Clin. Psychol. 1989;57(2):251–256. doi: 10.1037//0022-006x.57.2.251. [DOI] [PubMed] [Google Scholar]
- 16.Brown R.T., Madan-Swain A., Pais R., et al. Cognitive status of children treated with central nervous system prophylactic chemotherapy for acute lymphocytic leukemia. Arch. Clin. Neuropsychol. 1992;7(6):481–497. [PubMed] [Google Scholar]
- 17.Brown R.T., Madan-Swain A., Pais R., Lambert R.G., Sexson S., Ragab A. Chemotherapy for acute lymphocytic leukemia: cognitive and academic sequelae. J. Pediatr. 1992;121(6):885–889. doi: 10.1016/s0022-3476(05)80333-6. [DOI] [PubMed] [Google Scholar]
- 18.Mulhern R.K., Wasserman A.L., Fairclough D., Ochs J. Memory function in disease-free survivors of childhood acute lymphocytic leukemia given CNS prophylaxis with or without 1,800 cGy cranial irradiation. J. Clin. Oncol. 1988;6(2):315–320. doi: 10.1200/JCO.1988.6.2.315. [DOI] [PubMed] [Google Scholar]
- 19.Ochs J., Mulhern R., Fairclough D., et al. Comparison of neuropsychologic functioning and clinical indicators of neurotoxicity in long-term survivors of childhood leukemia given cranial radiation or parenteral methotrexate: a prospective study. J. Clin. Oncol. 1991;9(1):145–151. doi: 10.1200/JCO.1991.9.1.145. [DOI] [PubMed] [Google Scholar]
- 20.Moleski M. Neuropsychological, neuroanatomical, and neurophysiological consequences of CNS chemotherapy for acute lymphoblastic leukemia. Arch. Clin. Neuropsychol. 2000;15(7):603–630. [PubMed] [Google Scholar]
- 21.Raymond-Speden E., Tripp G., Lawrence B., Holdaway D. Intellectual, neuropsychological, and academic functioning in long-term survivors of leukemia. J. Pediatr. Psychol. 2000;25(2):59–68. doi: 10.1093/jpepsy/25.2.59. [DOI] [PubMed] [Google Scholar]
- 22.Copeland D.R., Dowell R.E., Jr, Fletcher J.M., et al. Neuropsychological effects of childhood cancer treatment. J. Child Neurol. 1988;3(1):53–62. doi: 10.1177/088307388800300113. [DOI] [PubMed] [Google Scholar]
- 23.Copeland D.R., Moore B.D., III, Francis D.J., Jaffe N., Culbert S.J. Neuropsychologic effects of chemotherapy on children with cancer: a longitudinal study. J. Clin. Oncol. 1996;14(10):2826–2835. doi: 10.1200/JCO.1996.14.10.2826. [DOI] [PubMed] [Google Scholar]
- 24.MacLean W.E., Jr, Noll R.B., Stehbens J.A., et al. Neuropsychological effects of cranial irradiation in young children with acute lymphoblastic leukemia 9 months after diagnosis. Arch. Neurol. 1995;52(2):156–160. doi: 10.1001/archneur.1995.00540260060017. [DOI] [PubMed] [Google Scholar]
- 25.Mulhern R.K., Ochs J., Fairclough D. Deterioration of intellect among children surviving leukemia: IQ test changes modify estimates of treatment toxicity. J. Consult. Clin. Psychol. 1992;60(3):477–480. doi: 10.1037//0022-006x.60.3.477. [DOI] [PubMed] [Google Scholar]
- 26.Kingma A., Van Dommelen R.I., Mooyaart E.L., Wilmink J.T., Deelman B.G., Kamps W.A. No major cognitive impairment in young children with acute lymphoblastic leukemia using chemotherapy only: a prospective longitudinal study. J. Pediatr. Hematol. Oncol. 2002;24(2):106–114. doi: 10.1097/00043426-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers J., Marckus R., Kearns P., Windebank K. Attentional ability among survivors of leukaemia treated without cranial irradiation. Arch. Dis. Child. 2003;88(2):147–150. doi: 10.1136/adc.88.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lähteenmäki P.M., Holopainen I., Krause C.M., Helenius H., Salmi T.T., Heikki L.A. Cognitive functions of adolescent childhood cancer survivors assessed by event-related potentials. Med. Pediatr. Oncol. 2001;36(4):442–450. doi: 10.1002/mpo.1108. [DOI] [PubMed] [Google Scholar]
- 29.Intallectual outcome in children and adolescents with acute lymphoblastic leukemia treated with chemotherapy alone: age- and sex-related differences. Eur. J. Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 30.Hill D.E., Ciesielski K.T., Hart B.L., Jung R.E. MRI morphometric and neuropsychological correlates of long-term memory in survivors of childhood leukemia. Pediatr. Blood Cancer. 2004;42(7):611–617. doi: 10.1002/pbc.20004. [DOI] [PubMed] [Google Scholar]
- 31.Castellino S.M., Ullrich N.J., Whelen M.J., et al. Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. J. Natl. Cancer Inst. 2014;106(8):dju186. doi: 10.1093/jnci/dju186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krull K.R., Brinkman T.M., Li C., et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J. Clin. Oncol. 2013;31(35):4407–4415. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowell R.E., Copeland D.R., Judd B.W. Neuropsychological effects of chemotherapeutic agents. Dev. Neuropsychol. 1989;5:17–24. [Google Scholar]
- 34.Waber D.P., Gioia G., Paccia J., et al. Sex differences in cognitive processing in children treated with CNS prophylaxis for acute lymphoblastic leukemia. J. Pediatr. Psychol. 1990;15(1):105–122. doi: 10.1093/jpepsy/15.1.105. [DOI] [PubMed] [Google Scholar]
- 35.De Bellis M.D., Keshavan M.S., Beers S.R., et al. Sex differences in brain maturation during childhood and adolescence. Cereb. Cortex. 2001;11(6):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 36.Waber D.P., Bernstein J.H., Kammerer B.L., et al. Neuropsychological diagnostic profiles of children who received CNS treatment for acute lymphoblastic leukemia. The systemic approach to assessment. Dev. Neuropsychol. 1992;8:1–28. [Google Scholar]
- 37.Eden O.B. Central nervous system-directed therapy in acute lymphoblastic leukemia. Pediatr. Hematol. Oncol. 1995;12(6):525–530. doi: 10.3109/08880019509030766. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong G.T., Reddick W.E., Petersen R.C., et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J. Natl. Cancer Inst. 2013;105(12):899–907. doi: 10.1093/jnci/djt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krull K.R., Zhang N., Santucci A., et al. Long-term decline in intelligence among adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiation. Blood. 2013;122(4):550–553. doi: 10.1182/blood-2013-03-487744. [DOI] [PMC free article] [PubMed] [Google Scholar]