Abstract
Objective:
While the survival of extremely premature infants has improved over the past decades, the rate of complications – especially for bronchopulmonary dysplasia (BPD) – remains un-acceptably high. Over the past 50 years, no safe therapy has had a substantial impact on the incidence and severity of BPD.
Methods:
This may stem from the multifactorial disease pathogenesis and the increasing lung imma-turity. Mesenchymal Stromal Cells (MSCs) display pleiotropic effects and show promising results in neonatal rodents in preventing or rescuing lung injury without adverse effects. Early phase clinical trials are now underway to determine the safety and efficacy of this therapy in extremely premature infants.
Results and Conclusion:
This review summarizes our current knowledge about MSCs, their mecha-nism of action and the results of preclinical studies that provide the rationale for early phase clinical trials and discuss remaining gaps in our knowledge
Keywords: Therapeutic potential, stem cells, bronchopulmonary, dysplasia, MSCs, angiogenesis
1. Introduction
1.1. Bronchopulmonary Dysplasia: Recognizing the Complexity and Challenge to Treat
Great advances in perinatal medicine have improved the survival of extreme low birth weight infants but this success has been tempered by the fact that these infants have an unacceptably high risk for developing complications such as Bronchopulmonary Dysplasia (BPD). Recent cohort studies report that the incidence of BPD in extremely low birth weight infants ranging between 30 to 70% [1-5]. It has been almost half a century since BPD was first described by Northway and colleagues in 1967 [6] but a cure for BPD still remains unavailable. The complexity of this debilitating chronic lung disease has hampered the discovery of efficient therapies. BPD remains the most common cause of morbidity and mortality in this group of infants [7-9].
Over the past five decades, as a result of the increasing survival of more premature infants, the disease pathogenesis has changed: Compared to the lung histology observed in premature infants before the surfactant era, the “new” BPD is characterized by a significant reduction in alveolarisation and vascularization. BPD now represents a combination of interruption of normal developmental signaling during early stages of lung development and a dysregulated repair process in response to perinatal insults (including inflammation, oxidative stress, and others) [10-12].
Postnatal corticosteroids, vitamin A, caffeine, lung-protective ventilation strategies, have been explored in large clinical trials but proven to be of limited success or unsafe in preventing BPD. Follow-up studies raised concerns about certain therapies such as dexamethasone due to its side effects especially on the premature brain [13-15]. Recent insights into stem cell biology have highlighted the potential use of these cells in regenerative medicine. Indeed, cell based-therapies have now advanced to be one of the most promising avenues for tissue repair including the preterm lung. More recent evidence suggesting dysfunction of resident lung stem cells in human lung diseases and animal models of BPD [16] provide further rationale for exogenous cell-based therapies to supplement the impaired endogenous repair mechanisms.
2. Stem Cells: The Concept of Self-renewal and Differentiation
Discovered by Till and McCulloch in 1961 [17], stem cells are generally defined as clonogenic cells capable of both self-renewal and multilineage differentiation (potency). Totipotent stem cells are the earliest cells in the ontogeny and extend from the zygote to the inner cell mass of the blastocyst. They are capable of differentiating into all adult, embryonic and extraembryonic tissues. Embryonic Stem Cells (ESC) are pluripotent stem cells capable of differentiating into derivatives of all three germ cell layers (ectoderm, mesoderm and endoderm). Multipotent stem cells are more restricted and give rise to multiple cell types of one lineage, such as Hematopoietic Stem Cells (HSCs). Residual pools of multipotent or unipotent stem cells are hypothesized to reside in almost all adult organs, contributing to their ability to repair and regenerate after injury [12, 17-20].
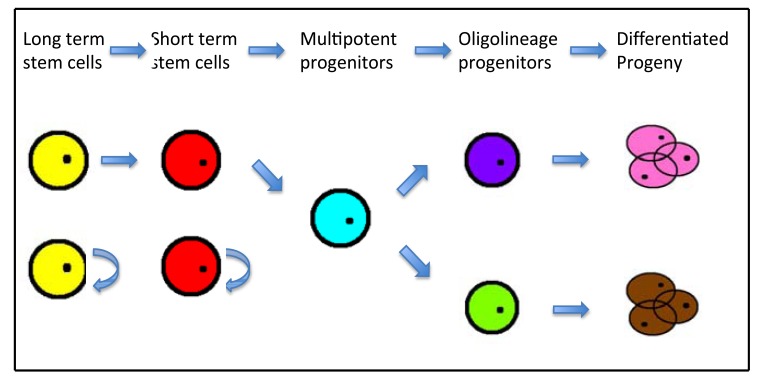
Long term (LT) stem cells: highly self-renewing cells that reconstitute an animal for its entire lifespan, for example: LT-HSCs (Thy-1.1loLin-Sca1+c-Kit+)
Short term (ST) stem cells: self-renewing cells that cannot give rise to LT stem cells but reconstitute an animal for a limited period, for example: ST-HSCs (Thy-1loLin-Mac1loSca1+c-Kit+)
Multipotent progenitors (MPP): arise from ST stem cells, for example: MPP-HSCs (Thy-1.1loLin-Sca1+c-Kit+Mac1loCD4lo)
Oligolineage progenitors: Oligolineage-restricted cells, for example: common lymphocyte progenitors (CLP) and common myeloerythroid progenitors, alveolar epithelial progenitor cells such as alveolar type(AT)2 pneumocytes, dual-lineage bronchioalveolar stem cells [12].
Differentiated progeny: for example: CLP give rise to T lymphocytes, B lymphocytes and natural killer cells progeny, AT2 pneumocytes give rise to AT1 and mature AT2 pneumocytes progeny.
Their ability to self-renew and regenerate after damage maybe limited naturally due to advancing age and extent of the disease [21]. This can occur secondary to depletion of the residual stem cell pools or as a consequence of genetic or microenvironmental changes that prevent proper stem cell renewal and function. These changes can potentially be reversed via stimulation of the endogenous stem cell pools or the replacement/supplementation of exogenous stem cells. Such exogenous stem cell replacement therapies have been used for decades for hematological disorders using Bone Marrow (BM) derived HSCs and increasingly for treating debilitating metabolic disorders [22-27].
HSCs are dependent on the proper function of their niche cells: mesenchymal stromal cells (MSCs). Over the past two decades, the recognition of the repair capabilities of these cells, has advanced MSCs as the most promising cell therapy for organ regeneration. This is in part due to their pleiotropic effects, including immune modulation [28, 29], angiogenesis [30, 31], and anti-oxidant activity [32, 33], appealing properties for the treatment of a multifactorial disease, such as BPD.
3. MSCS: From Niche Cells to Potent Repair Cells
MSCs were first described by Friedenstein and coworkers as adherent, fibroblast-like cells isolated from BM, stroma of the spleen and thymus [34, 35]. MSCs can differentiate into mesodermal lineage cells including osteoblasts, adipocytes, and chondrocytes in vitro and have the capability to self-renew. Since, MSCs have been isolated from almost every tissue, including adipose tissue [36], skeletal muscles [37], synovium [38], circulatory system [39], dental pulp [40], spleen, liver, kidney [41], umbilical cord [42], amniotic fluid [43], fetal blood, lung, liver and BM [44, 45].
Since its discovery in 1970s, MSCs have generated increasing interest but reported studies used different methods of isolation and expansion and different approaches to characterize the cells. In 2006, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) proposed minimal criteria to define human MSCs [46]:
MSC must be plastic adherent when maintained in standard culture conditions using tissue culture flasks.
-
Phenotype:
≥ 95% of the MSC population must express CD105, CD73, and CD90, as measured by flow cytometry.
Must lack expression (≤ 2% positive) of CD45, CD34, CD14, or CD11b, CD79α or CD19 and HLA class II.
Cells must be able to differentiate to osteoblasts, adipocytes and chondroblasts under standard in vitro differentiating conditions.
While the more detailed characterization of MSCs remains a topic of intense investigation, studies on the repair potential of MSCs have been equally intense. Extensive preclinical experiments have demonstrated the capacity of MSCs to ameliorate tissue damage and to improve organ function after injury.
A milestone indicating the potential of MSC replacement in organ repair occurred in 1999 when Makino S. and colleagues isolated a Cardiomyogenic Cell Line (CMG) from murine BM stromal cells. After treatment with 5-azacytidine, these cells changed morphology and acquired cardiomyocyte-like ultrastructure, including synchronous beating rhythm and expression of atrial natriuretic peptide and brain natriuretic peptide [47]. Shake J. G. and colleagues showed that MSCs engrafted in host myocardium, expressed muscle specific proteins and improved contractile dysfunction in the swine model of left ventricular wall infarction [48]. Although it was subsequently recognized that tissue engraftment is not the main mechanism of action of MSCs, their therapeutic benefit has been demonstrated extensively in acute lung injury [49, 50], acute and chronic kidney injuries [51-53], acute pancreatitis [54], liver fibrosis [55] and autoimmune encephalitis [56, 57].
Currently, there are more than six hundred clinical trials utilizing MSCs being conducted for adult diseases, such as in heart failure, inflammatory bowel disease, asthma, acute respiratory distress syndrome, cystic fibrosis, idiopathic lung fibrosis, osteoarthritis, diabetes mellitus, and various neurological diseases.
4. MSCS and preclinical studies in BPD
4.1. Animal Models
In order to determine the beneficial effect of MSCs in human premature lung, various animal models have been explored to mimic the pathological features of BPD [58]. Rodents (rat and mice) exposed to hyperoxia have been widely used. While these rodents are not delivered preterm and are otherwise healthy, the advantages of this model include birth at the saccular stage (equivalent to 26-28weeks of human gestation) of human lung development. [59, 60], low cost, fast turnaround, low maintenance, making this model ideal for rapid proof of concept experiments. Premature rabbits, lambs and piglets have also been employed to mimic BPD. The need for preterm delivery requires the need for mechanical ventilation and other life-sustaining interventions, making these models more clinically relevant, but labor intense and expensive [61-64]. The other interesting model using prematurely delivered baboon at 125 days (equivalent to 27 weeks of human gestation) and mechanically ventilated for 2 weeks offers great opportunity to test the effect of MSCs therapy in a model very close to the human setting [65]. These large animal models allow ultimate feasibility, safety and efficacy studies that may be required for regulatory approval of cell products.
4.2. Proof of Concept in Rodent Models of BPD for Testing the Therapeutic Benefits of MSCs
In 2007, Tian and colleagues has shown that Intravenous (IV) injection of 5x 104 (approximately 5 x 106/kg) BM derived MSCs (BM-MSCs) improves radial alveolar count and reduces inflammatory cytokines [Tumor Necrosis Factor Alpha (TNFα) and Transforming Growth Factor Beta-1 (TGFβ-1)] in the rat model exposed to 95% O2 [66]. This finding is supported by Aslam and colleagues in 2009, who showed normalized lung structure with attenuation in vascular remodeling in hyperoxic mice by IV BM-MSC injection [67]. Subsequent studies confirmed the same beneficial effects in the hyperoxic rat model [68-71].
Besides the IV route, intratracheal (IT) administration has been investigated by van Haaften and colleagues in 2009. In this prevention study, BM-MSCs improve survival, exercise tolerance and lung structure and reduce vascular changes secondary to pulmonary hypertension [72]. The benefits of this route are further supported by Chang and colleagues in 2011, 2013, Ahn and colleagues in 2015, Sung and colleagues in 2015 and Kim and colleagues in 2016 [73-77]. Most of these animal studies seem to indicate that early MSC replacement confers better lung protection than late therapy.
The efficacy and safety of Human Umbilical Cord/Cord Blood (HUC/B) derived MSCs in these animal models (xenogeneic therapy) suggest that allogenic transplantation is feasible in the clinical setting. The concept of harvesting HUC/B-MSCs after extreme premature delivery for autologous transplantation later to prevent or rescue BPD is worth considering, without jeopardizing the benefits of delayed cord clamping or umbilical cord milking. Alternatively, a ready to use, off-the-shelf MSC product may be logistically easier. Interestingly, Di Bernado and colleagues found that placenta derived MSCs (PL-MSCs) are as potent as BM-MSCs in terms of increasing alveolar surface area, branching morphogenesis and stimulating surfactant production in an in vitro study [78]. In an in vivo study using the combined perinatal inflammation and hyperoxic lung injury rat model, IT PL-MSCs reduces inflammatory cytokines and improves lung structure and vascular density [79]. The beneficial effects of MSCs are not only limited to the lung as IT injection of HUCB-MSCs has been shown to reduce brain inflammation and neuronal apoptosis [77].
4.3. Long Term Effects of MSCs
It is prudent to consider the potential long-term side effects of MSCs replacement therapy in the preclinical setting before pursuing clinical trials. In 2013, Pierro and colleagues have studied the short- and long-term effects of IT injection of both HUCB-MSCs and HUC derived perivascular cells (HUC-PCs, putative precursors of MSCs) as both preventive or rescue therapy in the hyperoxic rat model. Their study shows improved alveolar growth, improved lung compliance and reduced pulmonary hypertension in the short term, with long term preserved exercise tolerance and alveolar structure at 6 months of age. There were no observed side effects including tumor formation at 6 months [80]. The same safety profile is observed in the studies by Sutsko and colleagues at postnatal day 100 and by Ahn and colleagues at postnatal day 70 [81, 82].
4.4. Paracrine Effect of MSCs: Medicinal Signaling Cells [83] with Pleiotropic Effects
4.4.1. Low Cell Engraftment
The initial working hypothesis stipulated that MSCs would replace injured cells by engrafting and differentiation into the target cell type (heart, kidney, lung etc). However, contrary to this cell replacement hypothesis, very few engrafted cells could be found [84]. In the newborn rat BPD model, a low number of MSCs engraft as visualized by deconvolution microscopy and quantified by qRT-PCR, respectively [72, 80]. The observed engraftment of MSCs in the lung ranges from 0 to 20 percent in the majority of studies [52-55]. There is emerging evidence that most of the beneficial effects of stem cell therapy are due to cell-derived paracrine factors, rather than cell engraftment [85]. Tropea and colleagues in 2012 show that treatment of bronchoalveolar stem cells with MSCs derived conditioned media increases their growth efficiency, which might contribute to lung repair after injury [68]. However it is still unknown whether utilizing conditioned-media replacement alone will be superior to MSCs transplantation.
4.4.2. MSCs as Potent Immune Modulators
MSCs interact with cells of the innate and adaptive immune systems [86]. MSCs suppress inflammation, via their soluble factors, including interleukin(IL)-6, M-CSF, IL-10, TGFβ and prostaglandin E2 [87-89]. MSCs upregulate anti-inflammatory Th2 cytokines, including IL-3, IL-5, IL-10 and IL-13 and downregulate proinflammatory Th1 cytokines, including IL-1α and β, interferon-gamma(IFNγ) and TNFα [90, 91], culminating in reduced fibrosis. In the series of in vivo studies by Prockop et al., MSCs secrete TNFα stimulating gene/protein (TSG)-6 that reduces the inflammatory response [92-95]. Furthermore, MSCs display important anti-apoptotic properties in vital organ injury [96]. This immune modulation effect has been shown in most of the rodent studies of BPD [66, 67, 69, 71, 73, 76, 77, 79, 85] and is particularly important to attenuate inflammation and cell deaths in BPD and to restore lung and vascular growth.
4.4.3. MSCs are Pro-angiogenic
In addition, prostaglandin E2 enhances the secretion of vascular endothelial growth factor resulting in improved angiogenesis [86]. HUC-MSCs attenuate remodeling after myocardial infarction by pro-angiogenic, anti-apoptotic and endogenous cell-activation mechanisms. All these effects were paracrine mediated [97, 98]. A recent study by Reiter, J. and colleagues showed that this pro-angiogenic effect is enhanced by stromal derived factor-1 that mediates lung regeneration in the rodent model of BPD [99, 100].
4.4.4. MSCs Enhance Bacterial Clearance
There are also exciting findings regarding the bactericidal properties of MSCs via the secretion of bioactive molecules which are antimicrobial in vitro against Pseudomonas aeruginosa, Staphylococcal aureus and Streptococcal pneumonia [101]. In vivo, BM-MSCs infused into septic mice [102] and HUC-MSC into a neonatal Escherichia Coli sepsis model in rats, improve survival and enhance bacterial clearance in part through the production of the antimicrobial peptide LL-37 [103].
4.4.5. MSCs Display Anti-oxidant Activity
Anti-oxidant effects have been shown to ameliorate various organ injuries such as connective tissues, intestines and spinal cord [104-106]. Brief hyperoxic pre-conditioning of BM-MSC increases the production of the anti-oxidant stanniocalcin-1 and enhances lung protection in the rodent model of BPD [107].
These promising preclinical study results have prompted early phase clinical trials to examine the feasibility and safety of MSC therapy in preterm infants at risk of developing BPD.
5. Clinical trials using MSC for BPD
In 2014, Chang and colleagues conducted the first Phase 1 dose-escalation clinical trial in 9 premature infants (mean gestational age 25weeks, mean birth weight 793g) at high risk of developing moderate to severe BPD (NCT01632475). They administered 1x107 or 2x107 allogeneic HUCB-MSCs via IT route from postnatal day 7 to 14 and observed no immediate or short-term toxicity or adverse events compared to a historical control group [108]. Study participants were subsequently followed up for two years (NCT02023788) and no adverse effects in respiratory status, growth and neurodevelopment were reported [109]. There are currently 3 Phase I and 1 Phase II trials being conducted to assess the safety and efficacy of HUCB-MSCs in preterm infants.
Even though early phase of clinical trials have begun, it is critical to further our knowledge on MSC biology to better understand their exact mechanisms of action and thus optimize the manufacturing process of MSCs.
6. Remaining challenges for the clinical translation
6.1. Uniformity and Safety of MSC Products
There is abundant evidence in the animal literature that MSCs are lung protective, and it is timely to perform well-designed early phase clinical trials to advance our understanding about the safety and therapeutic potential of MSCs in preterm infants. A challenge is the careful characterization of clinical-grade MSCs to be used in these trials, because of the many steps in the manufacturing of a cell product [110, 111]. Causes of variations– impacting efficacy and safety – are plentiful and include amongst others: cell source, isolation process, number of passages/doubling times, freezing method, storage, thawing and administration procedure. For example, cryopreservation and thawing can impede MSC function [112] and bio-distribution [113], whereas a 24-hour culture period post-thawing or IF-γ licensing restores MSC function [114]. These findings highlight just one of the multiple gaps in our understanding of the biology of MSCs and how to harness their healing potential in the clinic. It also emphasizes the importance for the need of reliable potency assays that predict the therapeutic potential of a given cell product before release and infusion into patients.
6.2. Potency Assays and Therapeutic Efficacy
In order to further characterize clinical grade MSCs, efforts are focused on redefining MSCs products based on their potency in biological assays rather than their phenotypic characteristics and/or composition [115, 116]. So far, these biological assays have failed to link stem/progenitor properties and effector functions of MSCs and are limited to the anti-inflammatory effect of MSCs [117-119]. Recently, a Clinical Indications Prediction (CLIP) scale has been proposed to predict the therapeutic efficacy of different human MSC isolates for a given disease indication based on TWIST1 expression levels [120]. The CLIP scale overcomes the aforementioned limitations as it predicts differences in growth, survival, stem/progenitor, and effector function of MSCs. The CLIP scale is a promising attempt that can be further expanded to increase its predictability for the therapeutic efficacy of clinical grade MSC lots for different disease indications in the future. Currently the ISCT proposes a “focused analysis of selected MSC markers robustly deployed by in vitro licensing and metricized with a matrix of assays” to assess a MSC product’s biological potency [121]. This step is vital to ensure consistent validity of stem cells clinical trials so those results can be interpreted and compared with confidence.
6.3. Cell versus Cell-Free Products
Most of the MSCs’ therapeutic effects are mediated via a paracrine activity, as the cell-conditioned media exerts similar (and sometimes superior) therapeutic benefit [85]. Increasing evidence suggests that MSCs derived Extra-cellular Vesicles (EVs) are important players in mediating these paracrine effects [122, 123]. MSCs derived EVs have been shown to promote organ regeneration via its anti-apoptotic activities in kidney [124], liver [125], heart [126], suppressing graft-versus-host disease via its immune-suppressive effects [127], and ameliorating stroke-associated neurological deficits via promoting neurogenesis and angiogenesis [128]. MSCs derived EVs also attenuate pulmonary arterial hypertension in adult mice [129]. Restoration of lung function in the hyperoxia rat BPD model by MSCs derived EVs has also been demonstrated recently [130]. EVs’ therapeutic effects are mediated in part via miRNAs [131, 132]. These cell-free therapies may serve as a safer alternative cell-free therapy to ameliorate lung injury and restore lung and vascular growth in BPD. However, similar to the cell products, challenges in standardization of EV isolation, purification, industrial scale-up and good manufacturing practice compliance, will need to be overcome.
Conclusion
BPD still remains the most challenging complication of preterm birth even half a century after its first description. The multifactorial pathological process that leads to BPD makes small molecules and biologics difficult to tackle. MSC therapy emerges as a promising therapy as these cells are able to interact with their microenvironment and display pleiotropic effects by dispensing a variety of injury modulating substances to orchestrate the repair process [111, 133]. Promising preclinical data and the overall safety profile of MSCs have led to first clinical trial in preterm infants even so current cell products are still imperfect. Manufacturing of therapeutic MSCs and/or MSC free products will need to be standardized with its biological effects being tested and verified with approved potency assays before being released for clinical use.
Fig. (1).
Schematic diagram to illustrate the subsets of stem cells [18]:
Table 1.
Summary of MSCs clinical trials in BPD.
| Phase | Design | Number of Participants (Expected or Final) | Cell Origin | NCT ID |
|---|---|---|---|---|
| I | Open | 10 | MSCs (not specified) | NCT02443961 |
| I, II | Open | 12 | HUCB-MSCs | NCT02381366 |
| I | Randomized, placebo-controlled, double-blind | 10 | HUC-MSCs | NCT01207869 |
| I | Open | 9 | HUCB-MSCs | NCT01297205 |
| II | Randomized, placebo-controlled, double-blind | 70 | HUCB-MSCs | NCT01828957 |
| II | Randomized, placebo-controlled double-blind | 70 | HUCB-MSCs | NCT01897987 |
| I | Open, observational | 9 | HUCB-MSCs | NCT01632475 |
| I | Open, observational (2 year follow up from study NCT01632475) |
9 | HUCB-MSCs | NCT02023788 |
HUCB-MSCs: human umbilical cord blood derived MSCs.
HUC-MSCs: human umbilical cord derived MSCs.
Table 2.
Summary of the preclinical studies using MSCs/ MSCs derived conditioned media (MSCs-CM).
| Experimental Model | Therapeutic Cell or Product | Therapeutic Outcomes | Suggested Mechanisms | Ref. |
|---|---|---|---|---|
| Tian 2007 Hyperoxic-95% rat |
IV 5 x104 BM-MSCs | Increased Radial alveolar count | Engraftment Reduced TNFα and TGFβ1 |
[66] |
| Aslam 2009 Hyperoxic-75% mice |
IV 5x104 BM-MSCs or MSC-CM at PN4 | Both groups: Normalized alveolar number, improved vascular density, Reduced PHT vascular remodeling, Reduced macrophage and neutrophils count Reduced IL-5, IL-17, TNFα in MSC-CM |
Minimal engraftment Paracrine |
[67] |
| van Haaften 2009 Hyperoxic-95% rat |
IT1x105 BM-MSCs at PN4 and PN14 |
In vivo: Improved survival Improved exercise tolerance Reduced MLI and improved vascular density and PHT In vitro: MSC-CM prevented O2 induced AEC2 apoptosis, accelerated AEC2 wound healing, Enhanced endothelial cord formation |
Low engraftment Paracrine effect PN 4 better than PN14 therapy |
[72] |
| Chang 2011 Hyperoxic-95% rat |
IT UCB-MSCs comparing 5x 103, 5x104, 5x105 At PN5 |
For doses of 5x104 and x105 Reduced mean linear intercept, mean alveolar volume, collagen level Reduced MPO activity, mRNA levels of TNF-α, IL-1β, IL-6, TGF-β, reduced cytosolic and membrane p47 |
Engraftment Paracrine effect-aninflammatory and modulation oxidative stress |
[73] |
| Experimental Model | Therapeutic Cell or Product | Therapeutic Outcomes | Suggested Mechanisms | Ref. |
| Waszak 2012 Hyperoxic-95% rat |
IP BM-MSC-CM or preconditioned CM after 24hrs BM-MSC exposed to 95% O2 for 21d | Both Improved alveolar growth, MSC-O2CM PHT prevention Higher level of antioxidant stanniocalcin-1 in MSC-O2CM |
Paracrine and preconditioning More effect seen with MSC-O2CM in PHT prevention |
[107] |
| Tropea 2012 Hyperoxic-75% mice |
IV 5x104 MSCs at PN4 and IV MSC-CM and control at PN4 | Increased Bronchoalveolar Stem cells in vivo Improved alveolar density and surface area |
MSC and MSC-CM are equally effective in promoting BASC growth | [68] |
| Zhang 2012 Hyperoxic-95% rat |
IV 1x 105 BM-MSC PN10 | Improved weight Increased in radial alveolar count Reduced in TNFα and TGF β-1 but increased in IL-10 |
Engraftment Paracrine effect Immunomodulation effect |
[69] |
| Hansmann 2012 Hyperoxic-75% mouse |
IV BM-MSC-CM | Reversed parenchymal fibrosis, peripheral PA devascularization, partially reversed alveolar injury, normalized lung function, fully reversed the moderate PH and RVH | Paracrine effect via CM | [134] |
| Pierro 2013 Hyperoxic-95% rat Short term, long term, paracrine effect |
In vivo cell: IT 3x105 or 6x105 HUC-MSC or HUC-PCs at PN4 (EP P22) or PN14 (EP P35) Long term treated at PN4 and harvested at 6m In vivo CM: IP 7ul/kg CM from PN4-21 (EP P22) and PN14-28 (EP P35) Long term Treated PN4-21 and harvested at 6m |
Preserved alveolar growth Improved lung compliance Reduced pulm hypertension Long term: Improved exercise capacity Normalized alveolar structure |
Low engraftment Both HUC-MSC/HUC-PC are effective Both preventive/rescue therapy are effective Long term safety up to 6m |
[80] |
| Chang 2013 Hyperoxic -90% rat |
IT HUC-MSCs 5x105 at PN3, PN10, PN3+10 | In PN3 and PN3+10 group: Reduced MLI and mean alveolar volume Reduced TNFα, IL1a, IL-1b, IL-6, TIMP, CXCL7, RANTES, L-selectin, sICAM-1 Reduced cell apoptosis Reduced MPO activity and collagen deposition Increased VEGF and HGF |
Early MSC replacement confers better lung protection Engraftment Paracrine effect |
[74] |
| Sutsko 2013 Hyperoxic-90% rat Long term effect |
IT 2x106 BM-MSCs and MSC-CM at PN9 | Even at P100 (other than P16, P30): Increased vascular density and alveolar area Reduced MLI Reduced RVSP and RVH Reduced IL-6, IL-1β |
Minimal engraftment Paracrine |
[81] |
| Experimental Model | Therapeutic Cell or Product | Therapeutic Outcomes | Suggested Mechanisms | Ref. |
| Ahn 2013 Long term P70 safety and outcome Hyperoxic-90% rat |
IT HUC-MSC 5x105 at PN5 | At P70: Reduced MLI, increased vWF, reduced macrophages No abn in heart, liver, spleen |
Paracrine effect | [82] |
| Zhang 2013 Hyperoxic-95% rat |
IV 1x105 BM-MSC PN10 | Increased survival Increased SP-C expression and AE1 cells survival Inhibit lung apoptosis Increased VEGF expression |
Stimulation of potent mediators Reduced apoptosis |
[70] |
| Di Bernado 2014 | Incubation of fetal lung with 5x105 PL-MSC, BM-MSC and control | Increased lung surface area, terminal bud formation, branching morphogenesis Increased SP-B, SP-C and AQP-5 in PL-MSC only |
PL-MSC as potent as BM-MSC Paracrine effect |
[78] |
| Liu 2014 Hyperoxic-90% mice |
Intranasally vs. IP 0.1, 0.5, 1x106 HUC-MSCs |
Intranasal no effect IP 1x 106 has effect on Restoration of lung compliance, elastance, and tissue recoil but assoc with alveolar septal widening |
Paracrine modulation of interstitial matrix. Systemically administered MSCs are more beneficial |
[135] |
| Luan 2015 Hyperoxic-60% mice |
IV 1x106 BM-MSCs | Increased radial alveolar count Increased VEGF, reduced TGF-b1 Differentiation of MSC into vascular endothelial cells |
Engraftement Paracrine effect |
[71] |
| Ahn 2015 Hyperoxic-90% rat and A549 cells |
IT 5x105 HUC-MSC HA-MSC HUC-MNC at PN5 |
In vivo: Increased survival HUC/HA-MSC In vitro: MLI – reduced in HUC/HA-MSC Angiogenesis improved in HUC-MSC Inflmy cytokines: IL-1α, IL-1β, IL-6 attenuated in HUC-MSC VEGF and HGF recovered by HUC/HA-MSC |
HUC-MSC better than HA-MSC to protect hyperoxic lung injury Paracrine effect |
[75] |
| Sung 2015 Hyperoxic-90% rat |
IT 5x105 vs. IV 2x106 HUC-MSCs | In IT group: Greater decrease in MAV, MLI and ED-1/macrophage pos cells Reduced apoptosis Significant engraftment Reduced MIP-1α, TNFα, IL-6 Downregulate genes assoc with inflammation, cell death, fibrosis and upregulate genes assoc with angiongensis VEGF and HGF expression |
IT better than IV HUC-MSCs Engraftment Paracrine |
[76] |
| Gulasi 2016 Hyperoxic-85-95% rat |
IT PN11 with NS, CM, RCM, BM-MSC | Increased number of alveoli and decreased in alveolar diameter in BM-MSC group but not CM group Expression of aSMA reduced in BM-MSC |
Engraftment | [136] |
| Experimental Model | Therapeutic Cell or Product | Therapeutic Outcomes | Suggested Mechanisms | Ref. |
| Chou 2016 IP LPS to pregnant rat +Hyperoxic 85% rat |
IT PN5 3x105 and 1x106 PL-MSCs |
Reduced TNFα in high dose Reduced IL-6 in both doses Reduce MLI in both doses Reduced collagen density Increase vascular density Increased VEGF and reduced CTGF |
By VEGF and reducing CTGF and cytokines (paracrine effect) | [79] |
| Sammour 2016 Hyperoxic-85% rat |
IT male or female derived BM-MSCs 1x106 | Female MSCs has higher VEGF and IL-10 than Male MSC Both: Reduced MLI and increased RAC Improved vascular density Female MSCs is more superior in Reducing RVSP Reducing vasc remodeling Reduced IL-1β and increased IL-10 |
Female MSC has more impact in terms of protection from PHT | [137] |
| Kim 2016 Hyperoxic-90% rat |
IT 5x105 HUC-MSC at PN5 | Reduced neuronal apoptosis Reduced MLI Reduced TNFα, IL-1α, IL-1β, IL-6 Increased VEGF No statistical significance in short term neurofunctional outcome |
HUC-MSCs reduced lung and brain injury simultaneously | [77] |
| Zhang 2016 Hyperoxic-60% mice |
IV 1x106 BM-MSCs and IP 5000u/kg rHuEPO 1hr before and at 7d post hyperoxia | Increased RAC Improved BW Increased VEGF Reduced MMP9 |
More marked response in BM-MSC plus EPO Paracrine, anti-inflammatory effect Low engraftment |
[138] |
Acknowledgements
Bernard Thebaud holds a University of Ottawa Partnership Research Chair in Regenerative Medicine and is supported by the Canadian Health Research Institute (CIHR), Canadian Stem Cell Network, and the Ontario Institute for Regenerative Medicine and the Heart and Stroke Foundation of Canada.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Stoll B.J., Hansen N.I., Bell E.F., et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poindexter B.B., Feng R., Schmidt B., et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann. Am. Thorac. Soc. 2015;12(12):1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costeloe K.L., Hennessy E.M., Haider S., Stacey F., Marlow N., Draper E.S. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ancel P.Y., Goffinet F., Kuhn P., et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169(3):230–238. doi: 10.1001/jamapediatrics.2014.3351. [DOI] [PubMed] [Google Scholar]
- 5.Shah P.S., Sankaran K., Aziz K., et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J. Perinatol. 2012;32(2):132–138. doi: 10.1038/jp.2011.68. [DOI] [PubMed] [Google Scholar]
- 6.Northway W.H., Jr, Rosan R.C., Porter D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 7.Abman S.H. Pulmonary vascular disease and bronchopulmonary dysplasia: Evaluation and treatment of pulmonary hypertension. Neoreviews. 2011;12(11):e645–e651. [Google Scholar]
- 8.del Cerro M.J., Sabaté Rotés A., Cartón A., et al. Pulmonary hypertension in bronchopulmonary dysplasia: Clinical findings, cardiovascular anomalies and outcomes. Pediatr. Pulmonol. 2014;49(1):49–59. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer V., Junker D., Griesmaier E., Schocke M., Kiechl-Kohlendorfer U. Bronchopulmonary dysplasia is associated with delayed structural brain maturation in preterm infants. Neonatology. 2015;107(3):179–184. doi: 10.1159/000369199. [DOI] [PubMed] [Google Scholar]
- 10.Kinsella J.P., Greenough A., Abman S.H. Bronchopulmonary dysplasia. Lancet. 2006;367(9520):1421–1431. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 11.Bancalari E., Polin R.A. Bancalari E, Polin RA. The newborn lung : neonatology questions and controversies. 2nd Philadelphia: Elsevier/Saunders 2012. xiv, 446 pp. 2012. [Google Scholar]
- 12.Alphonse R.S., Rajabali S., Thébaud B. Lung injury in preterm neonates: the role and therapeutic potential of stem cells. Antioxid. Redox Signal. 2012;17(7):1013–1040. doi: 10.1089/ars.2011.4267. [DOI] [PubMed] [Google Scholar]
- 13.Jarreau P.H., Fayon M., Baud O., et al. The use of postnatal corticosteroid therapy in premature infants to prevent or treat bronchopulmonary dysplasia: current situation and recommendations. Arch. Pediatr. 2010;17(10):1480–1487. doi: 10.1016/j.arcped.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Doyle L.W., Ehrenkranz R.A., Halliday H.L. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 2014;(5):CD001146. doi: 10.1002/14651858.CD001146.pub4. [DOI] [PubMed] [Google Scholar]
- 15.Ordyan N.E., Pivina S.G., Rakitskaya V.V., Shalyapina V.G. The neonatal glucocorticoid treatment-produced long-term changes of the pituitary-adrenal function and brain corticosteroid receptors in rats. Steroids. 2001;66(12):883–888. doi: 10.1016/s0039-128x(01)00123-4. [DOI] [PubMed] [Google Scholar]
- 16.Möbius M.A., Thébaud B. Bronchopulmonary Dysplasia - Where have all the Stem Cells gone? Origin and (potential) function of resident lung stem cells. Chest. 2017;152(5):1043–1052. doi: 10.1016/j.chest.2017.04.173. [DOI] [PubMed] [Google Scholar]
- 17.Till J.E., McCulloch E.A., Siminovitch L. A Stochastic Model of Stem Cell Proliferation, Based on the Growth of Spleen Colony-Forming Cells. Proc. Natl. Acad. Sci. USA. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissman I.L. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 19.Modlinski J.A. The fate of inner cell mass and trophectoderm nuclei transplanted to fertilized mouse eggs. Nature. 1981;292(5821):342–343. doi: 10.1038/292342a0. [DOI] [PubMed] [Google Scholar]
- 20.Donovan P.J., Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414(6859):92–97. doi: 10.1038/35102154. [DOI] [PubMed] [Google Scholar]
- 21.Akram K.M., Patel N., Spiteri M.A., Forsyth N.R. Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. Int. J. Mol. Sci. 2016;17(1):E128. doi: 10.3390/ijms17010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teofili L., Sica S., Pierelli L., et al. Autologous peripheral blood stem cell transplantation in hematological malignancies. Haematologica. 1990;75(Suppl. 1):70–73. [PubMed] [Google Scholar]
- 23.Reiffers J., Marit G., Vezon G., et al. Autologous blood stem cell grafting in hematological malignancies. Present status and future directions. Transfus. Sci. 1992;13(4):399–405. doi: 10.1016/0955-3886(92)90024-B. [DOI] [PubMed] [Google Scholar]
- 24.Farah S.B., Simpson T.J., Golbus M.S. Hematopoietic stem cells for the treatment of genetic disease. Clin. Obstet. Gynecol. 1986;29(3):543–550. [PubMed] [Google Scholar]
- 25.Kirsch M., Heese O., Westphal M., Schackert G. Stem cells in neuro-oncology--development, regeneration and treatment. Acta Neurochir. Suppl. (Wien) 2003;88:143–151. doi: 10.1007/978-3-7091-6090-9_20. [DOI] [PubMed] [Google Scholar]
- 26.Angelini A., Castellani C., Ravara B., et al. Stem-cell therapy in an experimental model of pulmonary hypertension and right heart failure: role of paracrine and neurohormonal milieu in the remodeling process. J. Heart Lung Transplant. 2011;30(11):1281–1293. doi: 10.1016/j.healun.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y.X., Chen L., Wang R., et al. Mesenchymal stem cell therapy for diabetes through paracrine mechanisms. Med. Hypotheses. 2008;71(3):390–393. doi: 10.1016/j.mehy.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.W., Fang X., Gupta N., Serikov V., Matthay M.A. Allogeneic human mesenchymal stem cells for treatment of E coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis N.E., Hamilton D., Fontaine M.J. Harnessing the immunomodulatory and tissue repair properties of mesenchymal stem cells to restore β cell function. Curr. Diab. Rep. 2012;12(5):612–622. doi: 10.1007/s11892-012-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuchroo P., Dave V., Vijayan A., Viswanathan C., Ghosh D. Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev. 2015;24(4):437–450. doi: 10.1089/scd.2014.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen C., Lie P., Miao T., et al. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 2015;12(1):20–30. doi: 10.3892/mmr.2015.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dey R., Kemp K., Gray E., Rice C., Scolding N., Wilkins A. Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich’s ataxia. Cerebellum. 2012;11(4):861–871. doi: 10.1007/s12311-012-0406-2. [DOI] [PubMed] [Google Scholar]
- 33.Liu H., McTaggart S.J., Johnson D.W., Gobe G.C. Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy. 2012;14(2):162–172. doi: 10.3109/14653249.2011.613927. [DOI] [PubMed] [Google Scholar]
- 34.Fridenshteĭn A.Ia., Chaĭlakhian R.K., Lalykina K.S. Fibroblast-like cells in cultures of guinea pig hematopoietic tissue. Tsitologiia. 1970;12(9):1147–1155. [PubMed] [Google Scholar]
- 35.Friedenstein A.J., Gorskaja J.F., Kulagina N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 36.Zuk P.A., Zhu M., Mizuno H., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 37.Williams J.T., Southerland S.S., Souza J., Calcutt A.F., Cartledge R.G. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am. Surg. 1999;65(1):22–26. [PubMed] [Google Scholar]
- 38.De Bari C., Dell’Accio F., Tylzanowski P., Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.Di Meglio F., Castaldo C., Nurzynska D., et al. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J. Mol. Cell. Cardiol. 2010;49(5):719–727. doi: 10.1016/j.yjmcc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klingemann H., Matzilevich D., Marchand J. Mesenchymal Stem Cells - Sources and Clinical Applications. Transfus. Med. Hemother. 2008;35(4):272–277. doi: 10.1159/000142333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erices A., Conget P., Minguell J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 43.In ’t Anker P.S., Scherjon S.A., Kleijburg-van der Keur C., et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 44.Fan C.G., Tang F.W., Zhang Q.J., et al. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14(5):311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- 45.Campagnoli C., Roberts I.A., Kumar S., Bennett P.R., Bellantuono I., Fisk N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 46.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 47.Makino S., Fukuda K., Miyoshi S., et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. 2002. [DOI] [PubMed]
- 49.Iyer S.S., Co C., Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51(1):5–16. [PubMed] [Google Scholar]
- 50.Matthay M.A., Goolaerts A., Howard J.P., Lee J.W. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit. Care Med. 2010;38(Suppl. 10):S569–S573. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y., Yu J., Yin L., et al. MicroRNA-146b, a sensitive indicator of mesenchymal stem cell repair of acute renal injury. Stem Cells Transl. Med. 2016;5(10):1406–1415. doi: 10.5966/sctm.2015-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoja C., Garcia P.B., Rota C., et al. Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in adriamycin-induced nephropathy. Am. J. Physiol. Renal Physiol. 2012;303(9):F1370–F1381. doi: 10.1152/ajprenal.00057.2012. [DOI] [PubMed] [Google Scholar]
- 53.Morigi M., Imberti B., Zoja C., et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004;15(7):1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 54.Tang D.Q., Cao L.Z., Burkhardt B.R., et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53(7):1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju S., Teng G.J., Lu H., et al. In vivo differentiation of magnetically labeled mesenchymal stem cells into hepatocytes for cell therapy to repair damaged liver. Invest. Radiol. 2010;45(10):625–633. doi: 10.1097/RLI.0b013e3181ed55f4. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J., Li Y., Chen J., et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 2005;195(1):16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 57.Li M., Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013;2013:132642. doi: 10.1155/2013/132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nardiello C., Mižíková I., Morty R.E. Looking ahead: where to next for animal models of bronchopulmonary dysplasia? Cell Tissue Res. 2017;367(3):457–468. doi: 10.1007/s00441-016-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Reilly M., Thébaud B. Animal models of bronchopulmonary dysplasia. The term rat models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307(12):L948–L958. doi: 10.1152/ajplung.00160.2014. [DOI] [PubMed] [Google Scholar]
- 60.Berger J., Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307(12):L936–L947. doi: 10.1152/ajplung.00159.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Angio C.T., Ryan R.M. Animal models of bronchopulmonary dysplasia. The preterm and term rabbit models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307(12):L959–L969. doi: 10.1152/ajplung.00228.2014. [DOI] [PubMed] [Google Scholar]
- 62.Albertine K.H. Utility of large-animal models of BPD: chronically ventilated preterm lambs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308(10):L983–L1001. doi: 10.1152/ajplung.00178.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arrindell E.L., Jr, Krishnan R., van der Merwe M., et al. Lung volume recruitment in a preterm pig model of lung immaturity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;309(10):L1088–L1092. doi: 10.1152/ajplung.00292.2015. [DOI] [PubMed] [Google Scholar]
- 64.Caminita F., van der Merwe M., Hance B., et al. A preterm pig model of lung immaturity and spontaneous infant respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308(2):L118–L129. doi: 10.1152/ajplung.00173.2014. [DOI] [PubMed] [Google Scholar]
- 65.Yoder B.A., Coalson J.J. Animal models of bronchopulmonary dysplasia. The preterm baboon models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307(12):L970–L977. doi: 10.1152/ajplung.00171.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian Z.F., Du J., Wang B., Hong X.Y., Feng Z.C. Intravenous infusion of rat bone marrow-derived mesenchymal stem cells ameliorates hyperoxia-induced lung injury in neonatal rats. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(11):1692–1695. [PubMed] [Google Scholar]
- 67.Aslam M., Baveja R., Liang O.D., et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 2009;180(11):1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tropea K.A., Leder E., Aslam M., et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302(9):L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H., Fang J., Su H., et al. Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr. Transplant. 2012;16(6):589–598. doi: 10.1111/j.1399-3046.2012.01709.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhang H., Fang J., Wu Y., Mai Y., Lai W., Su H. Mesenchymal stem cells protect against neonatal rat hyperoxic lung injury. Expert Opin. Biol. Ther. 2013;13(6):817–829. doi: 10.1517/14712598.2013.778969. [DOI] [PubMed] [Google Scholar]
- 71.Luan Y., Ding W., Ju Z.Y., Zhang Z.H., Zhang X., Kong F. Bone marrow-derived mesenchymal stem cells protect against lung injury in a mouse model of bronchopulmonary dysplasia. Mol. Med. Rep. 2015;11(3):1945–1950. doi: 10.3892/mmr.2014.2959. [DOI] [PubMed] [Google Scholar]
- 72.van Haaften T., Byrne R., Bonnet S., et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am. J. Respir. Crit. Care Med. 2009;180(11):1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang Y.S., Choi S.J., Sung D.K., et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20(11-12):1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 74.Chang Y.S., Choi S.J., Ahn S.Y., et al. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS One. 2013;8(1):e52419. doi: 10.1371/journal.pone.0052419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn S.Y., Chang Y.S., Sung D.K., et al. Cell type-dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy. 2015;17(8):1025–1035. doi: 10.1016/j.jcyt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Sung D.K., Chang Y.S., Ahn S.Y., et al. Optimal route for human umbilical cord blood-derived mesenchymal stem cell transplantation to protect against neonatal hyperoxic lung injury: Gene expression profiles and histopathology. PLoS One. 2015;10(8):e0135574. doi: 10.1371/journal.pone.0135574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim Y.E., Park W.S., Sung D.K., et al. Intratracheal transplantation of mesenchymal stem cells simultaneously attenuates both lung and brain injuries in hyperoxic newborn rats. Pediatr. Res. 2016;80(3):415–424. doi: 10.1038/pr.2016.88. [DOI] [PubMed] [Google Scholar]
- 78.Di Bernardo J., Maiden M.M., Jiang G., Hershenson M.B., Kunisaki S.M. Paracrine regulation of fetal lung morphogenesis using human placenta-derived mesenchymal stromal cells. J. Surg. Res. 2014;190(1):255–263. doi: 10.1016/j.jss.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Chou H.C., Li Y.T., Chen C.M. Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am. J. Transl. Res. 2016;8(2):342–353. [PMC free article] [PubMed] [Google Scholar]
- 80.Pierro M., Ionescu L., Montemurro T., et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68(5):475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 81.Sutsko R.P., Young K.C., Ribeiro A., et al. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr. Res. 2013;73(1):46–53. doi: 10.1038/pr.2012.152. [DOI] [PubMed] [Google Scholar]
- 82.Ahn S.Y., Chang Y.S., Kim S.Y., et al. Long-term (postnatal day 70) outcome and safety of intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells in neonatal hyperoxic lung injury. Yonsei Med. J. 2013;54(2):416–424. doi: 10.3349/ymj.2013.54.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caplan A.I. Mesenchymal Stem Cells: Time to change the name! Stem Cells Transl. Med. 2017;6(6):1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotton D.N., Fabian A.J., Mulligan R.C. Failure of bone marrow to reconstitute lung epithelium. Am. J. Respir. Cell Mol. Biol. 2005;33(4):328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fung ME, Thebaud B. Stem cell-based therapy for neonatal lung disease: it is in the juice Pediatr Res. 2014. [DOI] [PMC free article] [PubMed]
- 86.Patel S.A., Sherman L., Munoz J., Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch. Immunol. Ther. Exp. (Warsz.) 2008;56(1):1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 87.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 88.Beyth S., Borovsky Z., Mevorach D., et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 89.Ramasamy R., Fazekasova H., Lam E.W., Soeiro I., Lombardi G., Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 90.Potian J.A., Aviv H., Ponzio N.M., Harrison J.S., Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J. Immunol. 2003;171(7):3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 91.Zhao M.M., Cui J.Z., Cui Y., et al. Therapeutic effect of exogenous bone marrow-derived mesenchymal stem cell transplantation on silicosis via paracrine mechanisms in rats. Mol. Med. Rep. 2013;8(3):741–746. doi: 10.3892/mmr.2013.1580. [DOI] [PubMed] [Google Scholar]
- 92.Oh J.Y., Roddy G.W., Choi H., et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. USA. 2010;107(39):16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ko J.H., Lee H.J., Jeong H.J., et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc. Natl. Acad. Sci. USA. 2016;113(1):158–163. doi: 10.1073/pnas.1522905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yun Y.I., Park S.Y., Lee H.J., et al. Comparison of the anti-inflammatory effects of induced pluripotent stem cell-derived and bone marrow-derived mesenchymal stromal cells in a murine model of corneal injury. Cytotherapy. 2017;19(1):28–35. doi: 10.1016/j.jcyt.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 95.Prockop D.J. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016;51:7–13. doi: 10.1016/j.matbio.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song Y.S., Joo H.W., Park I.H., et al. Bone marrow mesenchymal stem cell-derived vascular endothelial growth factor attenuates cardiac apoptosis via regulation of cardiac miRNA-23a and miRNA-92a in a rat model of myocardial infarction. PLoS One. 2017;12(6):e0179972. doi: 10.1371/journal.pone.0179972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Santos Nascimento D., Mosqueira D., Sousa L.M., et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 2014;5(1):5. doi: 10.1186/scrt394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang Y.L., Zhao Q., Qin X., et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann. Thorac. Surg. 2005;80(1):229–236. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 99.Reiter J., Drummond S., Sammour I., et al. Stromal derived factor-1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir. Res. 2017;18(1):137. doi: 10.1186/s12931-017-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang Y.S., Ahn S.Y., Jeon H.B., et al. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am. J. Respir. Cell Mol. Biol. 2014;51(3):391–399. doi: 10.1165/rcmb.2013-0385OC. [DOI] [PubMed] [Google Scholar]
- 101.Sutton MT, Fletcher D, Ghosh SK, et al. Antimicrobial properties of mesenchymal stem cells: Therapeutic potential for cystic fibrosis infection, and treatment. . stem Cells Int. 2016. [DOI] [PMC free article] [PubMed]
- 102.Németh K., Leelahavanichkul A., Yuen P.S., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu Y., Xu L., Collins J.J.P., et al. Human umbilical cord mesenchymal stromal cells improve survival and bacterial clearance in neonatal sepsis in rats. Stem Cells Dev. 2017;26(14):1054–1064. doi: 10.1089/scd.2016.0329. [DOI] [PubMed] [Google Scholar]
- 104.Maria A.T., Toupet K., Bony C., et al. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCl-induced systemic sclerosis. Arthritis Rheumatol. 2016;68(4):1013–1025. doi: 10.1002/art.39477. [DOI] [PubMed] [Google Scholar]
- 105.Inan M., Bakar E., Cerkezkayabekir A., et al. Mesenchymal stem cells increase antioxidant capacity in intestinal ischemia/reperfusion damage. J. Pediatr. Surg. 2017;52(7):1196–1206. doi: 10.1016/j.jpedsurg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 106.Kim Y., Jo S.H., Kim W.H., Kweon O.K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015;6:229. doi: 10.1186/s13287-015-0236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waszak P., Alphonse R., Vadivel A., Ionescu L., Eaton F., Thébaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012;21(15):2789–2797. doi: 10.1089/scd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 108.Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial J Pediatr. 2014. [DOI] [PubMed]
- 109.Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. Two-year followup outcomes of premature infants enrolled in the phase I Trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J Pediatr . 2017;185:49–54. doi: 10.1016/j.jpeds.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 110.Viswanathan S., Hematti P. Mesenchymal stromal cells : Translation pathways to clinical adoption. AP, Academic Press is an imprint of Elsevier 2017. Amsterdam: Elsevier/ xix, 346 pages p . 2017. [Google Scholar]
- 111.Möbius M.A., Thébaud B. Stem cells and their mediators - next generation therapy for bronchopulmonary dysplasia. Front. Med. (Lausanne) 2015;2:50. doi: 10.3389/fmed.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pollock K., Sumstad D., Kadidlo D., McKenna D.H., Hubel A. Clinical mesenchymal stromal cell products undergo functional changes in response to freezing. Cytotherapy. 2015;17(1):38–45. doi: 10.1016/j.jcyt.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chinnadurai R., Garcia M.A., Sakurai Y., et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports. 2014;3(1):60–72. doi: 10.1016/j.stemcr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chinnadurai R., Copland I.B., Garcia M.A., et al. Cryopreserved mesenchymal stromal cells are susceptible to t-cell mediated apoptosis which is partly rescued by IFNγ licensing. Stem Cells. 2016;34(9):2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boregowda S.V., Booker C.N., Phinney D.G. Mesenchymal stem cells: The moniker fits the science. Stem Cells. 2017;36(1):7–10. doi: 10.1002/stem.2713. [DOI] [PubMed] [Google Scholar]
- 116.Boregowda S.V., Phinney D.G. Quantifiable metrics for predicting MSC therapeutic efficacy. J. Stem Cell Res. Ther. 2016;6(11):365. doi: 10.4172/2157-7633.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bloom D.D., Centanni J.M., Bhatia N., et al. A reproducible immunopotency assay to measure mesenchymal stromal cell-mediated T-cell suppression. Cytotherapy. 2015;17(2):140–151. doi: 10.1016/j.jcyt.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lehman N., Cutrone R., Raber A., et al. Development of a surrogate angiogenic potency assay for clinical-grade stem cell production. Cytotherapy. 2012;14(8):994–1004. doi: 10.3109/14653249.2012.688945. [DOI] [PubMed] [Google Scholar]
- 119.Lee R.H., Yu J.M., Foskett A.M., et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. USA. 2014;111(47):16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boregowda S.V., Krishnappa V., Haga C.L., Ortiz L.A., Phinney D.G. A clinical indications prediction scale based on TWIST1 for human mesenchymal stem cells. EBioMedicine. 2015;4:62–73. doi: 10.1016/j.ebiom.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Galipeau J., Krampera M., Barrett J., et al. International society for cellular therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lener T., Gimona M., Aigner L., et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yáñez-Mó M., Siljander P.R., Andreu Z., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruno S., Grange C., Deregibus M.C., et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009;20(5):1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herrera M.B., Fonsato V., Gatti S., et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J. Cell. Mol. Med. 2010;14(6B):1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gnecchi M., He H., Liang O.D., et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 127.Kordelas L., Rebmann V., Ludwig A.K., et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 128.Chen J., Shehadah A., Pal A., et al. Neuroprotective effect of human placenta-derived cell treatment of stroke in rats. Cell Transplant. 2013;22(5):871–879. doi: 10.3727/096368911X637380. [DOI] [PubMed] [Google Scholar]
- 129.Chen J.Y., An R., Liu Z.J., et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol. Sin. 2014;35(9):1121–1128. doi: 10.1038/aps.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Willis G.R., Fernandez-Gonzalez A., Anastas J., Vitali S.H., Liu X., Ericsson M., et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2017;197(1):104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xin H., Li Y., Liu Z., et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Feng Y., Huang W., Wani M., Yu X., Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9(2):e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fischbach M.A., Bluestone J.A., Lim W.A. Cell-based therapeutics: the next pillar of medicine. Sci. Transl. Med. 2013;5(179):179ps7. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hansmann G., Fernandez-Gonzalez A., Aslam M., et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm. Circ. 2012;2(2):170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu L., Mao Q., Chu S., et al. Intranasal versus intraperitoneal delivery of human umbilical cord tissue-derived cultured mesenchymal stromal cells in a murine model of neonatal lung injury. Am. J. Pathol. 2014;184(12):3344–3358. doi: 10.1016/j.ajpath.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 136.Gülaşı S., Atıcı A., Yılmaz S.N., et al. Mesenchymal stem cell treatment in hyperoxia-induced lung injury in newborn rats. Pediatr. Int. (Roma) 2016;58(3):206–213. doi: 10.1111/ped.12764. [DOI] [PubMed] [Google Scholar]
- 137.Sammour I., Somashekar S., Huang J., et al. The effect of gender on Mesenchymal Stem Cell (MSC) efficacy in neonatal hyperoxia-induced lung injury. PLoS One. 2016;11(10):e0164269. doi: 10.1371/journal.pone.0164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Z.H., Pan Y.Y., Jing R.S., et al. Protective effects of BMSCs in combination with erythropoietin in bronchopulmonary dysplasia-induced lung injury. Mol. Med. Rep. 2016;14(2):1302–1308. doi: 10.3892/mmr.2016.5378. [DOI] [PubMed] [Google Scholar]