Abstract
Background:
Tumor-infiltrating lymphocytes (TILs) are one of the major participants in the tumor microenvironment of pancreatic ductal adenocarcinoma (PDAC). However, the mechanism of interaction between TILs and tumors is complex and remains unclear.
Objective:
To evaluate the state of immunoreactions in PDAC tissues, and explore the prognostic value of these markers in a large sample, to provide a new theoretical basis for PDAC immunotherapy.
Method:
Immunohistochemical staining of CD4+ and CD8+T cells was performed in a tissue microarray (TMA) of 143 cases of PDAC. Two major variables for the spatial distributions of CD4+T and CD8+T cells in PDAC tissues, intraepithelial attack and intratumoral infiltration, were used to evaluate the state of immunoreactions, and the interrelationships with the clinicopathological variables were analyzed.
Results:
Our data showed that both the intraepithelial CD4+T and CD8+T attack were less frequent than the intratumoral infiltration. CD8+T intraepithelial attack and intratumoral infiltration were more intense than CD4+T. CD8+T intraepithelial attack was an independent favorable prognostic factor for overall survival, correlating negatively with vascular invasion and positively with CD4+T and CD8+T high intratumoral infiltration. CD8+T high intratumoral infiltration without CD8+T intraepithelial attack was a poor prognostic factor. CD8+T high intratumoral infiltration was accompanied by T stage progression. Conclusively, in PDAC progression, imbalances of T cells occurred in CD4+ and CD8+ immunoreactions. The CD8+T intraepithelial attack was an independent favorable prognostic indicator, however the intraepithelial attack of CD4+T and the both intratumoral infiltration of CD8+T and CD4+T played an ambiguous role.
Conclusion:
Our data suggested that it is a potential approach to increasing the number of intraepithelial attacking CD8+T cells for tumor immunotherapy, and exploring a new mechanism for immunosuppression in a tumor microenvironment with high T cell infiltration without attack.
Keywords: Pancreatic cancer, CD8+T lymphocytes, CD4+T lymphocytes, tumor microenvironment, prognostic indicator, PDAC
1. INTRODUCTION
Pancreatic cancer (pancreatic ductal adenocar-cinoma/PDAC) is one of the most malignant tumors. The rate of 5-year survival is only 7% [1]. This disease is difficult to detect and tumor metastasis has often occurred by the time of diagnosis; thus, the rate of successful surgical resection is only 20% [2]. PDAC often metastasizes and recurs after surgery. A growing body of research suggests that tumor biological behavior may be regulated by the tumor microenvironment [3]. A recent paper showed that abundant stromal components in pancreatic tumor tissue play an important role in tumorigenesis, development, and metastasis [4]. Tumor-infiltrating lymphocytes (TILs) are one of the major participants in the tumor microenvironment [5]. TILs and the secreted cytokines constitute the main components of the tumor immune microenvironment and play a role in the immune regulation of tumors.
TILs have been observed in various cancers, including pancreatic cancer. In the previous studies, many variables for the spatial distribution of TILs were used to evaluate the status of the anti-tumor immmunoreactions in the tumor microenvironment, such as intratumoral and peritumoral, intraepithelial and stroma. It was common that the intratumoral refers to lymphocytes in the tumor tissues; the peritumoral lymphocytes in the tumor adjacent normal tissue; the intraepithelial lymphocytes in the tumor nest contacting with tumor cells [6] (the distance less than 20μm [7]); and tumor stroma lymphocytes in the tumor stroma not contacting with tumor cells (the distance more than 20μm [7]).
However, the mechanism of interaction between TILs and tumors is complex and remains unclear. In previous studies, it was observed that the intratumoral CD8+T cell was an independent favorable prognostic factor in esophageal carcinomas [8], while another study showed that there was no significant difference in the overall survival (OS) or DFS rate between the low and high CD8+T infiltrate including the intratumoral and the peritumoral in the hepatocellular carcinomatissues [9]. In serous ovarian cancer, it was reported the higher number of CD3+ or CD8+T in the intraepithelial infiltration was an independent prognostic factor for the longer OS [10], while another study in the ovarian carcinosarcoma showed that the intraepithelial CD8+T did not had a statistical significance between and OS, but the stromal CD8+T infiltration had [11]. Currently, there are little studies about the spatial distribution of TILs in pancreatic tumor [6, 12]. In the present study, the major two variables, the intratumoral and the intraepithelial of TIL infiltration, were used to evaluate the state of immunoreactions in PDAC tissues, and explore the prognostic value of these markers in a large sample.
2. Materials and Methods
2.1. Patients and TMA
Two commercial tissue microarray (TMA) chips consisting of 60 and 90 paired pancreatic intratumoral tumor tissues and adjacent peritumoral normal tissues were used (catalog no. HPan-Ade120 Sur-01 & HPan-Ade180 Sur-02, Shanghai Outdo Biotech, China). Pathologists collected the tissues immediately after pancreatic duodenectomy (Whipple) treatments. Each tissue sample had a core of 1-mm diameter. HPan-Ade120 Sur-01 samples were collected from 2004 to 2008, while HPan-Ade180 Sur-02 samples were collected from 2009 to 2013. Patients were treated with conventional chemotherapy regimens after surgery and were followed regularly, including outpatient follow-up or telephone follow-up. The duration of patient follow-up ranged from 1.2 to 7 years after surgery. Overall survival (OS) refers to the interval between surgery and death or the time of last observation for surviving patients. The study was approved by the Ethics Committee of Changhai hospital, Second Military Medical University, China. Informed consent was signed by all patients or their families.
2.2. Immunohistochemistry Staining and Interpretation
Each section was stained according to a commercial protocol (Shanghai Outdo Biotech, China), and was then scanned with a ScanScope XT (Aperio). Briefly, clinicopathological morphology was stained with hematoxylin-eosin, and CD4 and CD8 were stained with rabbit anti-human CD4 (Product ID RMA-0620, Cloning SP35, Maixin Biotech, Fuzhou, China) and CD8 (Product IDRMA-0514, Cloning SP16, Maixin Biotech, Fuzhou, China) monoclonal antibodies.
The microscopic images were imported as digital photo files with an ImageScope Installer system. The TMAs were analyzed by two independent pathologists who had no knowledge of clinical and prognostic conditions. We proposed two major variables of the intratumoral and the intraepithelial for assessing the spatial distribution of TIL infiltration in PDAC tissues, as showed in Supplementary Fig. 1 (1.5MB, pdf) . The intratumoral TIL were defined as the lymphocytes located in intratumoral tissues, and the intraepithelial were defined as the lymphocytes located in intraepithelial tissues and contacted to epithelial cell directly (the distance less than 20μm [7]). To estimate the level of the TIL in the intratumoral, three representative fixed individual region (200-micron quadrant) containing the most tumor cells were selected according to the intensity of lymphocyte infiltration, refer to rank 1, rank 2 and rank 3, was occupied with software, as shown in Supplementary Fig. 2 (1.5MB, pdf) . Then, the mean number of three region TIL infiltration was obtained as each tissue sample’s intensity of TIL infiltration. Samples were divided into infiltration high and infiltration low (intratumoral infiltration high/low, Ihigh /low) by the intensity of TIL intratumoral infiltration. The mean value of the intensity of TIL intratumoral infiltration for all samples is taken as the cutoff value. In parallel, to estimate the level of the TIL in the intraepithelial, the whole tissue specimen was examined. If the number of intraepithelial lymphocytes was greater than or equal to three, we proposed that the lymphocyte attacking tumor cells were coded as Yes, otherwise lymphocyte attacking was coded as No (Attack yes/no, Ayes/no). Finally, the percentages of the lymphocyte attack yes and infiltration high are defined as the incidence rates of lymphocyte intraepithelial attack yes and intratumoral infiltration high.
2.3. Statistical Analysis
Comparisons of the incidence of high lymphocyte intratumoral infiltration and intraepithelial lymphocyte attack between the two groups were performed using the χ2 test. Correlations were assessed by Pearson correlation analysis or linear regression analysis. The OS analysis for univariate analyses was performed using the Kaplan-Meier method, and for multivariate analyses was performed using Cox regression analysis. P<0.05 was considered statistically significant. All data were analyzed with SPSS 19.0 statistical softwares.
3. Results
3.1. Spatial Distributions of CD4+T and CD8+T Cells in PDAC Tissues
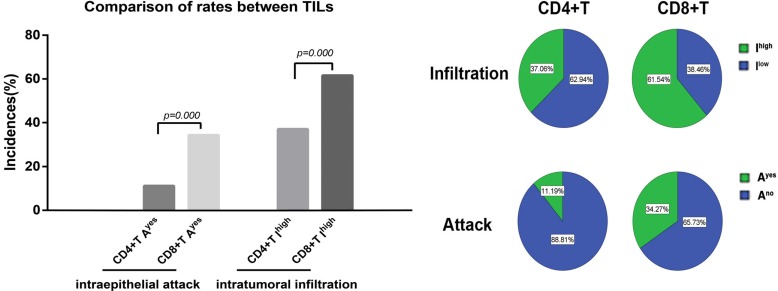
Two TMA chips including specimens from 150 patients were collected. 147 cases were identified as pancreatic ductal adenocarcinoma (PDAC), as confirmed by HE staining. For four of these cases, tumor tissue points fell off the tissue chip; 143 cases of PDAC were included in the final sample. Table 1 shows that clinical and pathological data of the patients with pancreatic cancer. (Supplementary Fig. 1 (1.5MB, pdf) ) displays the images of HE, CD4+, and CD8+ staining for two TMAs. As shown in Fig. 1, the incidence rates of CD4+T and CD8+T intratumoral infiltration high (Ihigh) were 37.06% (53/143) and 61.54% (88/143), respectively, while the incidence rates of CD4+T and CD8+T intraepithelial attack yes (Ayes) were 11.19% (16/143) and 34.27% (49/143), respectively. These differences were significant. The Ayes incidence rates of all CD4+T and CD8+T significantly lower than the Ihigh incidence rates. However, the incidence rates of CD4+T and CD8+ T high infiltration in peritumoral normal tissues were only 21.10% (23/109) and 21.49% (26/121), respectively. This difference was not significant. It was observed that there were significant more incidence rates of CD4+ and CD8+T intratumoral infiltration compared with peritumoral infiltration(CD4+: 37.06% vs. 21.10%, P=0.004%; CD8+: 61.54% vs. 21.49% P=0.000%).
Table 1.
Patient demographics and clinicopathologic factors.
| Variables | Category | No. of patients |
|---|---|---|
| Age, years | <70/≥70 | 102/41 |
| Sex | Male/ Female | 86/57 |
| Grade | 1/2/3 | 20/108/15 |
| Tumor size | ≤4/>4cm | 89/54 |
| Tumor site | Head/ others | 83/60 |
| V I | No/Yes | 81/62 |
| L I | No/Yes | 80/63 |
| T | 1/2/3/4 | 78/8/57 |
| N | N0/N1/N2 | 83/46/14 |
| M | M0/M1 | 137/6 |
| TNM stage* | I/II/III/IV | 47/76/14/6 |
| Smoking | No/Yes | 49/8 |
| Drink | No/Yes | 47/10 |
| Diabetes | No/Yes | 43/14 |
VI, Vascular invasion; LI, Lymphatic invasion
*, The TNM stage of patients with pancreatic adenocarcinoma, per the American Joint Commission on Cancer guidelines (8th edition).
Fig. (1).
Comparison of the incidence rates of CD4+ and CD8+T intraepithelial attack and intratumoral infiltration in PDAC tissues. Ihigh, intratumoral infiltration high. Ayes, intraepithelial attack yes.
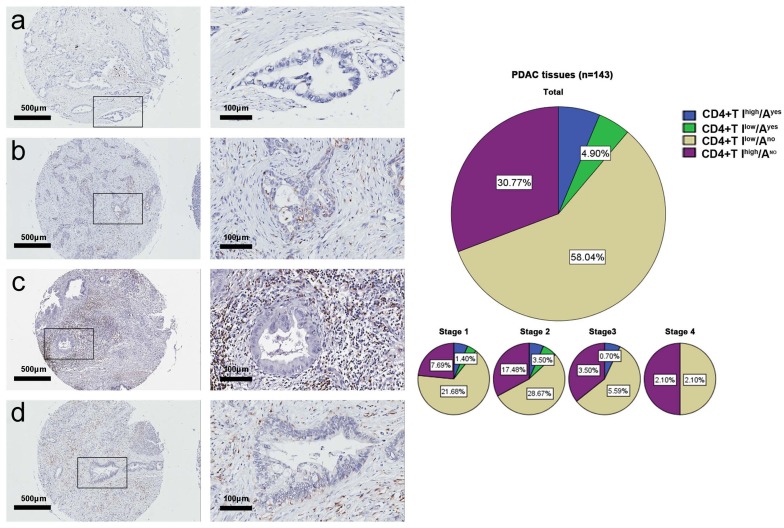
Combining the two variables of intratumoral infiltration and intraepithelial attack, it was observed that the spatial distributions of CD4+T, CD8+T cells in pancreatic cancer tissues had four patterns. Fig. 2 shows the four spatial distribution patterns of CD4+T cells and their incidence rates per TNM stages, as follows: 1) high CD4+T intratumoral infiltration and CD4+T intraepithelial attack (CD4+T Ihigh/Ayes), 6.3%, 2) low CD4+T intratumoral infiltration and CD4+T intraepithelial attack (CD4+T Ilow/Ayes), 4.9%, 3) low CD4+T intratumoral infiltration and no CD4+T intra-epithelial attack (CD4+T Ilow/Ano), 58.0%, and 4) high CD4+T intratumoral infiltration and no CD4+T intra-epithelial attack (CD4+T Ihigh/Ano), 30.8%. The incidence rate of the CD4+T Ilow/Ano was significantly greater than the sum rate of CD4+T Ihigh/Ayes, CD4+T Ilow/Ayes, and CD4+T Ihigh/Ano (58.0% vs. 42.0%, p<0.01).
Fig. (2).
CD4+ T cell spatial distribution patterns in the tumor tissue microarray. The representative photographs for CD4+T Ilow/Ano (a), CD4+T Ilow/Ayes (b), CD4+T Ihigh/Ano (c), and CD4+T Ihigh/Ayes (d). The incidence rates of the four spatial distribution patterns by TNM stage.
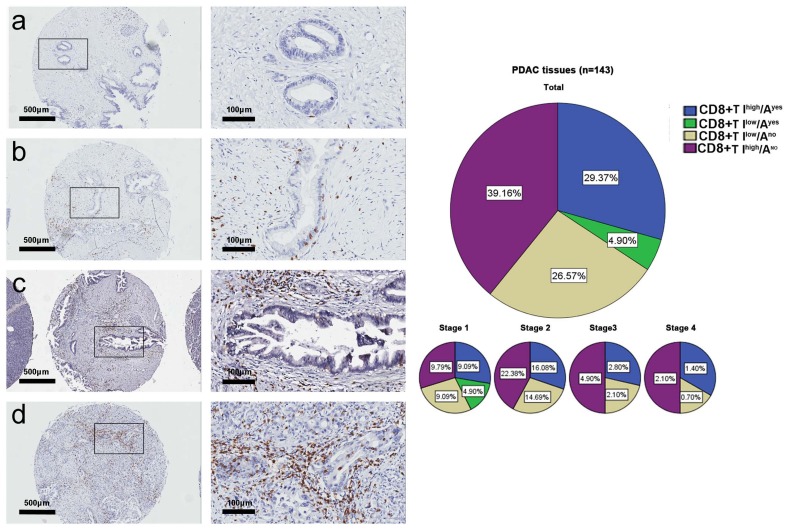
Fig. 3 showed the four spatial distribution patterns of CD8+ T cells and their incidence rates in the tumor tissue microarray: 1) high CD8+T intratumoral infiltration and CD8+T intraepithelial attack (CD8+T Ihigh/Ayes), 29.4%, 2) low CD8+T intratumoral infiltration and CD8+T intraepithelial attack (CD8+T Ilow/Ayes), 4.9%, 3) low CD8+T intratumoral infiltration and no intraepithelial CD8+T attack (CD8+T Ilow/Ano), 26.6%, and 4) high CD8+T intratumoral infiltration and no CD8+T intraepithelial attack (CD8+T Ihigh/Ano), 39.2%. The incidence rate of CD8+T Ilow/Ano was significantly less than the sum rate of CD8+T Ihigh/Ayes, CD8+T Ilow/Ayes, and CD8+T Ihigh/Ano (26.57% vs. 73.43%, p<0.01). Taken together, these data showed that: 1) the T cell intraepithelial immunoreactions of both CD4+T and CD8+T were significantly more frequent in intratumoral tissues than in peritumoral normal tissues, 2) the T cell intraepithelial attack immunoreactions of both CD4+T and CD8+T occurred less often than did CD4+T and CD8+T intratumoral infiltration, and 3) the CD8+T response to immunoreactions was more intense than that of CD4+T in the intratumoral tissues.
Fig. (3).
CD8+ T cell spatial distribution patterns in the tumor tissue microarray. The representative photographs for CD8+T Ilow/Ano (a), CD8+T Ilow/Ayes (b), CD8+T Ihigh/Ano (c), and CD8+T Ihigh/Ayes (d). The incidence rates of the four spatial distribution patterns by TNM stage.
3.2. Interrelationships Between the Spatial Distributions of CD4+ and CD8+T in PDAC Tissues and Clinicopathological Variables
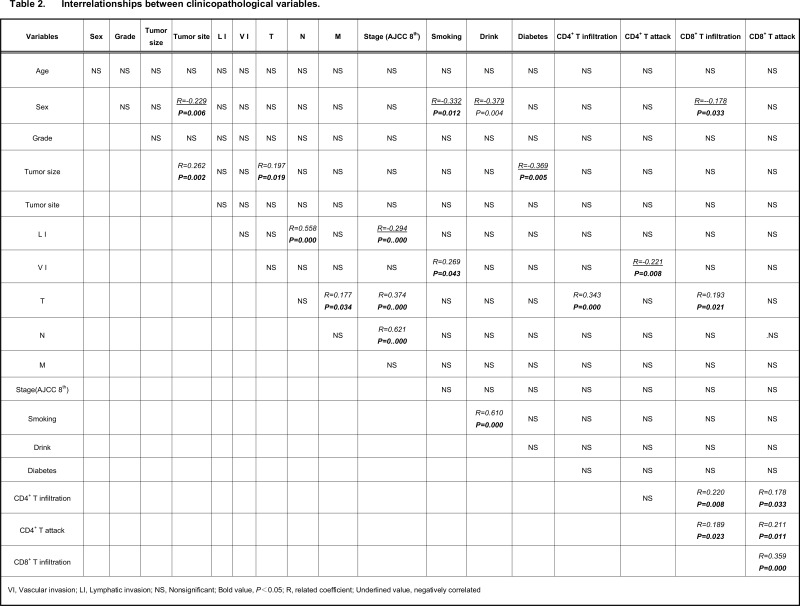
As shown in Table 2, CD4+T intratumoral infiltration had a significant positive correlation with CD8+T intratumoral infiltration, CD4+T intraepithelial attack, and T stage. In contrast, CD4+T intraepithelial attack had a significant positive correlation with CD8+T intratumoral infiltration and CD8+T intraepithelial attack, and a negative correlation with vascular invasion. Furthermore, CD8+T intratumoral infiltration had a significant positive correlation with CD8+T intraepithelial attack, male gender, and TNM stage. Taken together, the data suggest that both CD4+T and CD8+T high intratumoral infiltration were accompanied by T stage progression. CD8+T intraepithelial attack immunoreactions required CD4+T and CD8+T intratumoral infiltration assistance and had an anti-vascular invasion ability.
Table 2.
3.3. Prognostic Significance for the Spatial Distributions of CD4+ and CD8+T Cells in PDAC Tissues
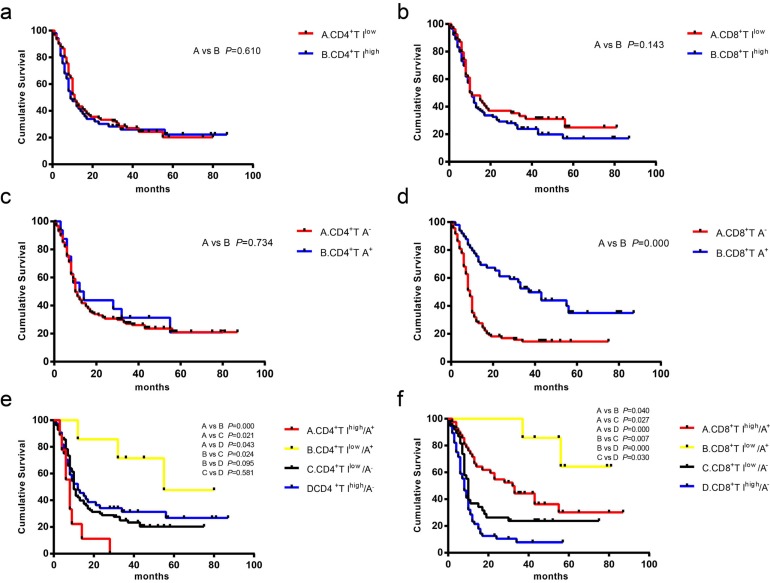
While OS was no significantly associated with CD4+T intratumoral infiltration, CD4+T intraepithelial attack, and CD8+T intratumoral infiltration (Fig. 4A, 4B and 4C), CD8+T attack had a better prognosis; the median OS of CD8+T Ayes was significantly longer than that of CD8+T Ano (median survival, 37.0 vs. 9.0 months; Fig. 4D). Analysis of the CD4+T four spatial distributions patterns showed that median overall survival was linked to intratumoral infiltration and intraepithelial attack spatial distribution patterns, as follows: CD4+T Ilow/Ayes (55.8 months), CD4+T Ihigh/Ano (12.5 months), CD4+T Ilow/Ano (10.7 months), and CD4+T Ihigh/Ayes (8.3 months), all p=0.013. There was no significant difference when CD4+T Ilow/Ayes and CD4+T Ihigh/Ano were compared, or when CD4+T Ilow/Ano and CD4+T Ihigh/Ano were compared (Fig. 4E).
Fig. (4).
Kaplan-Meier survival curves showed the comparison of overall survival among the different spatial distribution patterns of CD4+ and CD8+T cells in PDAC tissues. (a) between CD4+T Ilow and CD4+T Ihigh. (b) between CD8+T Ilow and CD8+T Ihigh. (c) between CD4+TAyes and CD4+T Ano. (d) between CD8+T Ayes and CD8+T Ano. (e) spatial distribution patterns among CD4+ T cells, including CD4+T Ihigh/Ayes, CD4+T Ilow/Ayes, CD4+T Ilow/Ano, and CD4+T Ilow/Ayes. (f), spatial distribution patterns among CD4+ T cells including CD8+T Ihigh/Ayes, CD8+T Ilow/Ayes, CD8+T Ilow/Ano, and CD8+T Ilow/Ayes.
Similarly, analysis of the CD8+T spatial distribution patterns showed that median overall survival differed by intratumoral infiltration and intraepithelial attack spatial distribution patterns, as follows: CD8+T Ilow/Ayes (81.0 months), CD8+ T Ihigh/Ayes (32.9 months), CD8+T Ilow/Ano (10.6 months) and CD8+T Ihigh/Ano (8.6 months), with significant differences between the patterns, all p<0.0001.Taken together, the findings indicated that CD8+T attack was a favorable factor for median overall survival. The combination of CD8+T intraepithelial attack with CD8+T low and high intratumoral infiltration, respectively, had the longest overall survival durations, while CD8+T high intratumoral infiltration without CD8+T intraepithelial attack was correlated with significantly shorter survival time.CD4+T intraepithelial attack combined with CD4+T low intratumoral infiltration had the best prognosis; however, CD4+T intraepithelial attack combined with CD4+T high intratumoral infiltration had the worst prognosis as PDAC progressed.
3.4. Prognostic Significance of the Combinations of the Spatial Distributions of CD4+ and CD8+T Cells in PDAC Tissues with Clinicopathological Variables
As shown in Table 3, lymphatic invasion, N stage, M stage and TNM stage were significantly poorly correlated with OS, while the CD8+T attack was associated with a better prognosis. Due to associations of lymphatic invasion, N stage, and M stage with TNM stage, only the TNM stage variable combined with CD8+T attack variable was put into the Cox regression analysis. The results showed that both TNM stage and CD8+T attack had significant associations with OS. After the stratification of TNM stages, the median OS of CD8+T Ayes was significantly longer than that of CD8+T Ano in Stage I (56.0 vs. 10.0) and II (52.0 vs. 10.0), while there was no significant difference in Stage III and IV (Supplementary Fig. 3 (1.5MB, pdf) ). In Stage I and II, CD8+T Ilow/Ayes and CD8+T Ihigh/Ayes had significantly better survival times at all TNM stages, with no significant difference between Stages III and IV. The results indicated that CD8+T attack was an independent favorable prognostic factor in PDAC progression, and its role was more prominent in the early stages of PDAC.
Table 3.
Univariate and multivariate analyses of spatial distributions of CD4+ and CD8+T cells in PDAC tissues as prognostic factors associated with the patients’ overall survival.
| Variables | Category | Univariate analyses | Multivariate analyses | |||
|---|---|---|---|---|---|---|
| Median survival time | P Value | HR | 95% CI | P Value | ||
| Sex | Male/Female | 10/11 | 0.703 | - | - | - |
| Age | <70/≥70 | 12/8 | 0.052 | 1.777 | 1.172-2.693 | 0.007 |
| Grade | 1/2&3 | 14/10 | 0.393 | - | - | - |
| Tumor size | ≤40/>40mm | 11/10 | 0.234 | - | - | - |
| Tumor site | Head/others | 10/12 | 0.267 | - | - | - |
| Vascular invasion | No/yes | 11/10 | 0.996 | - | - | - |
| Lymphatic invasion | No/yes | 15/10 | 0.000 | - | - | - |
| T | T1/T2/T3 | 11/9/10 | 0.551 | - | - | - |
| N | N0/N1/N2 | 15/10/8 | 0.001 | - | - | - |
| M | M0/M1 | 11/4 | 0.007 | - | - | - |
| Stage | I/II/III&IV | 28/10/7 | 0.000 | 1.874 | 1.374-2.555 | 0.000 |
| Drink | No/yes | 15/10 | 0.572 | - | - | - |
| Smoking | No/yes | 12/11 | 0.578 | - | - | - |
| Diabetes | No/yes | 15/10 | 0.334 | - | - | - |
| CD4+ T infiltration | Low/High | 11/9 | 0.610 | - | - | - |
| CD8+ T infiltration | Low/High | 11/10 | 0.143 | - | - | - |
| CD4+ T attack | No/yes | 10/12 | 0.734 | - | - | - |
| CD8+ T attack | No/yes | 9/37 | 0.000 | 0.371 | 0.238-0.577 | 0.000 |
4. Discussion
The tumor microenvironment of pancreatic cancer is extremely complex. In this study, the spatial distribution of TILs including CD4 and CD8 were investigated and their prognostic values were evaluated. According to our two major variables of the intratumoral and the intraepithelial, the PDAC tissues were divided into four spatial distribution pattern groups. Although there were obvious T cells responses to immunoreactions in the intratumoral tissues, there were fewer T cell intraepithelial attack immunoreactions than intratumoral infiltration immunoreactions, and the CD8+T response to immunoreactions was more intense than those CD4+T. These findings suggest that the response of T cells to immunoreactions is unbalanced in PDAC progression. We observed low T lymphocytes intratumoral infiltration and no T cell intraepithelial attack (T Ilow/Ano) in approximately half of the CD4+T cells and almost one-third of the CD8+T cells. This observation suggests that a subset of PDAC had a lower level of T cell response to immunoreactions, which was consistent with findings from previous studies [13]. Analysis of the interrelationships between the spatial distribution of CD4+ and CD8+T in PDAC tissues and clinicopathological variables showed that high intratumoral infiltration of both CD4+T and CD8+T was positively related to T stage progression. This finding suggested that the T cell response to immunoreactions was continuously stimulated and boosted in PDAC growth. More importantly, CD8+T intraepithelial attack was an independent favorable factor for prognosis, and its role was more prominent in the early stage of PDAC.
Previous studies reported conflicting results regar-ding whether the T cell response to immunoreactions plays an anti-tumor role in tumorigenesis [14-16]. Some researchers reported controversial relationship between the subgroup of TILs and prognosis in human cancer [17]. Wang proposed that CD4+T cells play a key role in the host anti-tumor immune response [18], while Fukunaga showed that the combination of CD8+ and CD4+T intratumoral infiltration rather than either of CD4+ or CD8+T alone was an independent prognostic indicator in PDAC tissues [19], and recently it was proved that regulatory CD4+T subpopulation cells (CD25+/FOXP3+) have immunosuppressive effects in pancreatic cancer and inhibit CD8+T intraepithelial attack [20]. With regard to the spatial distribution of TIL infiltration in PDAC tissues, one recent study showed that a high level of stromal CD45+T is a favorable prognostic indicator for overall survival of PDAC patients [21]. However, a study of Vulvar Squamous Cell Carcinoma showed that tumor-infiltrating CD4+ and CD8+T cells were not associated with patients’ survival [22]. In PDAC, a study shown that the high CD8+T infiltration in peritumoral normal tissues and low CD4+T infiltration in tumor tissues are favorable prognostic factors, authors believe high peritumoral CD8+T cells could be more suppress tumor growth and then prolong survival [12], while the other study showed that the high numbers of CD8+ TIL in the stromal but not in the intraepithelial were associated with improved OS in PDAC [6]. It seems inconsistent with our results. Our data indicated that CD4+T Ihigh and CD8+T Ihigh in tumor tissue were not significant prognostic factors, while the CD8+T Ayes pattern, although present in only a few cases, was an independent favorable prognostic factor for PDAC. Similar results were not found with CD4+T Ayes. In the detailed analysis, we divided the cases into four groups based on combinations of CD8+T intratumoral infiltration and CD8+T intraepithelial attack, and found that the survival times of the T IlowAyes group of both CD4+T and CD8+T cells was longer than the survival times of the group without infiltration (T Ilow/Ano). These results differ somewhat from the results of previous studies [19]. We present a new view that CD8+T Ayes played a major role in anti-tumor immunity in the tumor tissues, while the CD8+T Ihigh in the stroma around the tumor cell nests may not play an anti-tumor role. With regard to CD4+T cells, we hypothesized that CD4+T has a complex assistant action to inhibit or promote tumors under different circumstances, consistent with Fukunaga’s report [19] that there were subgroups of CD4+T other than Treg cells (non-regulatory CD4+ helper T cells) playing an active role in anti-tumor immunity [23].
We determined that only direct contact of T cells, especially CD8+T cells, with tumor cellswould kill tumor cells. In the present study, we adopted a new variable, lymphocyte intraepithelial attack, to estimate the immune microenvironment in tumor tissues. Although previous researchers generally hypothesized that CD8+T cells played a role in attacking tumor cells, these studies only examined the number of infiltrating lymphocytes to determine the strength of the CD8+T response to immunoreactions, and did not explore lymphocyte attack. We found that CD8+T cells were mostly distributed in the tumor stroma, instead of in the intraepithelial as an attack state in PDAC tissues. This state of lymphocyte intraepithelial attack was significantly different in PDAC tissues, suggesting that the variable of lymphocyte intraepithelial attack may be a more accurate index for the strength of anti-tumor CD8+T response immunoreactions.
The data indicated that CD8+T intraepithelial attack was an independent favorable factor in PDAC progression, and supported the significance of the new variable, lymphocyte intraepithelial attack. However, we showed that the survival time of patients in the CD8+T Ihigh/Ano group was shorter than the survival time of patients in the CD8+T Ilow/Ano group. This finding suggests that there are strong negative immune regulatory factors with more intense immuno-suppressive functions, or other immunosuppressive factors preventing CD8+T from approaching and attacking tumor cells [12] and thereby promoting tumor metastasis, in the CD8+T Ihigh/Ano PDAC tissues [24]. It was reported that the inflammatory response induced by the CD8+T outside the tumor test results in tumor tissue edema and rich blood supply that, without T cell intraepithelial attacks on tumor cells, created favorable conditions for the immune escape and metastasis of tumor cells [15, 25]. Mantovani argued that the inflammation in tumor tissue activated many molecular signals, recruited lymphocytes into the tumor microenvironment, and influenced the biological effects of immune cells through dramatic changes in gene expression, which tended to benefit tumor growth, promote remodeling of tumor tissue, and inhibit antitumor immune responses [26].
There is the question of why many PDAC tissues showed high T cell intratumoral infiltration without intraepithelial attack. The proposed mechanisms involved in negative immune regulatory factors in the T cell-inflamed tumor microenvironment include: 1) PD-L1 up-regulation and subsequent inhibition of T cells attacking tumor cells, 2) IDO up-regulation, 3) recruitment of T cells, and 4) antigenic immunogenicity reduction. In the non-inflamed tumor microenvironment, the following may reflect the mechanism: 1) a lack of innate immune cell recruitment, 2) oncogenic pathway exclude T cells, and 3) a lack of effector T-cell recruit-ment due to loss of effector chemokine production [27].
The NEJM [28] reported that monoclonal antibody drugs, such as PD-1 and PD-L1, blocked immunological checkpoints in non-small cell lung cancer, malignant melanoma and ovarian cancer, while their efficacy for pancreatic cancer was poor. Studies of melanoma and breast cancer have shown that without an immune-infiltrating microenvironment, similar to the state of the T Ilow/Ano presented here, immunotherapy has little efficacy in PDAC tissue and may accelerate the progression of disease in some patients [29, 30]. Although adoptive T cell therapy can increase the number of cytotoxic T lymphocytes in the body, most cytotoxic T lymphocytes do not attack the tumor cells, perhaps giving rise to and increasing the Ihigh/Ano state. Our results advance a theory that increasing the number of intraepithelial attack CD8+T cells in tumor nest may be a new immunotherapeutic approach to PDAC treatment, and may provide a novel pathway to find the new mechanism for tumor immunosuppression in the Ihigh/Ano microenvironment.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No. 81472279 and 81272663 to Jun Gao; No. 81372482 to Kaixuan Wang). The authors also would like to thank Shanghai Outdo Biotech Company.
Abbreviations
- PDAC
Pancreatic Ductal Adenocarcinoma
- TILs
Tumor Infiltrating Lymphocytes
- Treg
Regulatory T cells
- T
T lymphocytes
- CD4+T
CD4+T lymphocytes
- CD8+T
CD8+T lymphocytes
- TMA
Tissue Microarray chips
- OS
Overall Survival
- I
Intratumoral T Infiltration
- A
Intraepithelial T Attack
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of Changhai Hospital, Second Military Medical University, China.
HUMAN AND ANIMAL RIGHTS
All the procedures involving human subjects were compliant with the ethical guidelines of the 1975 Declaration of Helsinki. No animals were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Informed consent was signed by all patients or their families.
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher's website along with the published article.
REFERENCES
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2014;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Saif M.W. Pancreatic neoplasm in 2011: an update. JOP. 2011;12(4):316–321. [PubMed] [Google Scholar]
- 3.Ino Y., Yamazaki-Itoh R., Shimada K., et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu H., Hang J.J., Han T., Zhuo M., Jiao F., Wang L.W. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016;37(7):8657–8664. doi: 10.1007/s13277-015-4741-z. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka N., Yamazaki-Itoh R., Ino Y., et al. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140(1):310–321. doi: 10.1053/j.gastro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Lohneis P., Sinn M., Bischoff S., et al. Cytotoxic tumour-infiltrating T lymphocytes influence outcome in resected pancreatic ductal adenocarcinoma. Eur. J. Cancer. 2017;83:290–301. doi: 10.1016/j.ejca.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Carstens J.L., de Sampaio P.C., Yang D., et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 2017;8:15095. doi: 10.1038/ncomms15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher K., Haensch W., Röefzaad C., Schlag P.M. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 9.Huang Y., Wang F.M., Wang T., et al. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion. 2012;86:329–337. doi: 10.1159/000342801. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q., Lou W., Di W., Wu X. Prognostic value of tumor PD-L1 expression combined with CD8(+) tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int. Immunopharmacol. 2017;52:7–14. doi: 10.1016/j.intimp.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J., Wen H., Ju X., Bi R., Zuo W., Wu X. Clinical Significance of Programmed Death Ligand-1 and Intra-Tumoral CD8+ T Lymphocytes in Ovarian Carcinosarcoma. PLoS One. 2017;12:e0170879. doi: 10.1371/journal.pone.0170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Zhao G., Wu W., et al. Low intratumoral regulatory T cells and high peritumoral CD8(+) T cells relate to long-term survival in patients with pancreatic ductal adenocarcinoma after pancreatectomy. Cancer Immunol. Immunother. 2016;65(1):73–82. doi: 10.1007/s00262-015-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski T.F., Woo S.R., Zha Y., et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 2013;25(2):268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Melstrom L.G., Salazar M.D., Diamond D.J. The pancreatic cancer microenvironment: A true double agent. J. Surg. Oncol. 2017;116(1):7–15. doi: 10.1002/jso.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans A., Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front. Physiol. 2012;3:270. doi: 10.3389/fphys.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka N., Onozato K., Kosuge T., Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 17.Yu P., Fu Y.X. Tumor-infiltrating T lymphocytes: friends or foes. Lab. Invest. 2006;86(3):231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 18.Wang R.F. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22(5):269–276. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga A., Miyamoto M., Cho Y., et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Chellappa S., Hugenschmidt H., Hagness M., et al. Regulatory T cells that co-express RORγt and FOXP3 are pro-inflammatory and immunosuppressive and expand in human pancreatic cancer. OncoImmunology. 2016;5(4):e1102828. doi: 10.1080/2162402X.2015.1102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lianyuan T., Dianrong X., Chunhui Y., Zhaolai M., Bin J. The predictive value and role of stromal tumor-infiltrating lymphocytes in pancreatic ductal adenocarcinoma (PDAC). Cancer Biol. Ther. 2018;•••:1–10. doi: 10.1080/15384047.2017.1416932. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sznurkowski J.J., Zawrocki A., Emerich J., Biernat W. Prognostic significance of CD4+ and CD8+ T cell infiltration within cancer cell nests in vulvar squamous cell carcinoma. Int. J. Gynecol. Cancer. 2011;21(4):717–721. doi: 10.1097/IGC.0b013e3182131f36. [DOI] [PubMed] [Google Scholar]
- 23.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 24.Sarvaiya P.J., Guo D., Ulasov I., Gabikian P., Lesniak M.S. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4(12):2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogar P., Basso D., Fadi E., et al. Pancreatic cancer alters human CD4+ T lymphocyte function: a piece in the immune evasion puzzle. Pancreas. 2011;40(7):1131–1137. doi: 10.1097/MPA.0b013e31822077b8. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 27.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int. Immunol. 2016;28(8):383–391. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer J.R., Tykodi S.S., Chow L.Q., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aroldi F., Zaniboni A. Immunotherapy for pancreatic cancer: present and future. Immunotherapy. 2017;9(7):607–616. doi: 10.2217/imt-2016-0142. [DOI] [PubMed] [Google Scholar]
- 30.Skelton R.A., Javed A., Zheng L., He J. Overcoming the resistance of pancreatic cancer to immune checkpoint inhibitors. J. Surg. Oncol. 2017;116(1):55–62. doi: 10.1002/jso.24642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher's website along with the published article.