Abstract
Background:
Strategies to prevent anaemia in preterm infants include drawing fewer blood samples, the use of recombinant human erythropoietin and iron supplementation. Although iron sulfate is the most commonly used pharmaceutical formulation for iron supplementation, there are few studies comparing different iron salts in infants.
Objective:
This is a study of retrospective data comparison of two groups of preterm infants receiving erythropoietin to evaluate the efficacy of iron bisglycinate chelate to iron sulfate.
Subjects and
Methods:
Three-hundred infants of gestational age ≤32 weeks were enrolled: 225 were supplemented with iron sulfate (3 mg/kg/day) and 75 were supplemented with iron bisglycinate che-late (0.75 mg/kg/day). The effect on erythropoiesis was assessed with a general linear model that es-timates the response variables (values for Haemoglobin, Haematocrit, absolute values and percentage Reticulocytes, Reticulocyte Haemoglobin content) based on treatment, time, birth weight, and gesta-tional age.
Results:
Supplementation with iron bisglycinate chelate at a dose of 0.75 mg/kg/day demonstrated an efficacy comparable to iron sulfate at a dose of 3 mg/kg/day in both populations of preterm infants. The two cohorts had similar erythropoietic response, without significant differences.
Conclusions:
The higher bioavailability of iron bisglycinate chelate resulted in a lower load of ele-mental iron, a quarter of the dose, and achieved equivalent efficacy compared to iron sulfate. Iron bis-glycinate chelate may appear to be an alternative to iron sulfate in the prevention and treatment of pre-term newborn anaemia
Keywords: Preterm newborn anaemia, iron sulfate, bisglycinate chelate, treatment, haemoglobin, Iron deficiency
1. Introduction
More than 65% of preterm newborns develop Iron Deficiency (ID), earlier than full-term newborns [1]. Preterm newborns have poor iron stores at birth, as 80% of the iron is acquired by the foetus in the third trimester of pregnancy. The frequent blood sample drawings associated with an inadequate intake of iron and rapid postnatal growth contribute to developing ID and subsequent Iron Deficiency Anaemia (IDA) [2-4]. Furthermore, while nadir haemoglobin values
(Hb) in full-term infants are reached between the 6th and 8th week of life, in preterm infants it appears around the 4th week of life, dropping to values as low as 7-8 g/dL [5]. To prevent iron shortage, delayed clamping of the umbilical cord may be useful [6]; limiting the number of blood samples, by using micro methods and having available laboratory areas dedicated to newborn tests is fundamental.
Prophylactic iron supplementation has been universally demonstrated to be essential for prevention of the onset of ID and IDA in preterm infants. Although the need for iron treatment is indisputable, there is still no clear agreement on the kind of iron compound and preparation, the dosage/kg of elemental iron, the starting time of the supplementation with respect to efficacy, safety, tolerability, and cost [7-10]. Among iron preparations, iron sulfate remains the standard treatment in the prevention of anaemia [11, 12]. Studies in children and adults suggest that iron bisglycinate chelate could be a viable alternative to iron sulfate in terms of efficacy and tolerability. Iron bisglycinate chelate has a ferrous iron molecule bonded to two molecules of glycine, forming two heterocyclic rings. The amino acid chelating coating is digested only in the intestine, thereby optimising the absorption. As iron sulfate has not been available in our hospital since August 2013, we started adminstering iron bisglycinate for prophylaxis instead. To determine whether the use of iron bisglycinate is a viable alternative for preterm infants, we report the complete blood count values from this retrospective cohort study of preterm infants receiving iron prophylaxis, using ferrous sulfate (Fe-sol) to ferrous bisglycinate chelate (Fe-bis).
2. Material and methods
This is a study of retrospective data comparison of two groups of preterm infants. The Fe-sol cohort was developed from April 2006 to July 2013. The Fe-bis cohort was developed between August 2013 and December 2014. Both cohorts included all babies ≤32 weeks and >26 weeks of gestational age (GA) at birth admitted to our Neonatal Intensive Care Unit at the University Hospital Città della Salute e della Scienza of Turin (Italy). Exclusion criteria were infants born with any malformations, expired before receiving treatment, alloimmunization and treatment with intravenous (IV) iron saccharate (Venofer - Vifor France). Infants receiving both iron formulations during the transition period when they were switched from one product to another were also excluded. We used iron sulfate (Fer-in-sol drops - Mead Johnson Nutrition - USA) until August 2013; thereafter, we used iron bisglycinate chelate in drops (Tecnofer drops for children - Laboratori Baldacci - Pisa - Italy). Patient data were collected retrospectively through medical records. Specific approval by local ethical commitee was not required as observational study performed during diagnostic follow up (prot n. 24316/C28.1; website: www.cittadellasalute.to.it). Since there were no problems recruiting controls it was decided to match 1: 3 to increase the power of work.
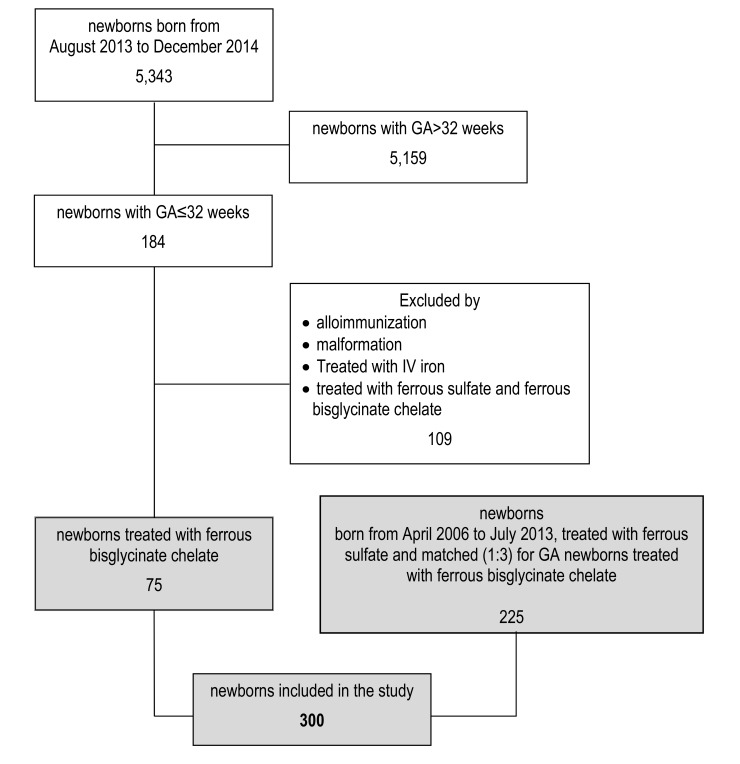
Recruitment of infants is shown in (Fig. 1). Non-invasive monitoring techniques, micro methods for performance of the laboratory tests to reduce the loss of iatrogenic blood and restrictive transfusion practices were used in all infants according to internal guidelines. Our nutritional policy includes parenteral nutrition, breast milk fortified with protein and mineral supplements in accordance with the usual specifications (FM 85 - Nestlé and PROTIFAR - Nutricia - Netherlands), and, in the case of hypogalactia/agalactia, babies were fed with enriched formula for preterm infants (Prenidina Ospedale - Nestlé). In addition, all infants received multivitamin supplements with 100 mcg/day of folic acid. Nevertheless, we can state that from 2006 to 2014 care protocols have not been changed as there are no differences in nutrition between the two groups.
Fig. (1).
Selection of newborns included in the study.
Iron Intake. As in usual local clinical practice, iron supplementation was started when the Reticulocyte Haemoglobin content (CHr) values reached 30-32 pg. Prophylaxis was carried out according to the indications of the pharmaceutical companies manufacturing the two products: iron sulfate 3drops/kg/day (equivalent to 3 mg/kg/day of elemental iron), iron bisglycinate chelate 3 drops/kg/day (equivalent to 0.75 mg/kg/day of elemental iron). When the CHr values were less than 30 pg, the iron dose was increased by 1 drop/kg/day.
Clinical and Laboratory Investigations: The Complete Blood Count tests were performed weekly from birth to discharge, according to routine medical assistance. The first blood sample was often collected through umbilical venous catheter, while subsequent samples from heel capillary blood collections. The 0.5 ml blood sample was collected in microtubes with EDTA (S-Monovette - Sarstedt - Germany). The samples obtained were processed with ADVIA 2120 innovation Hematology System - Siemens-Germany analyser.For each examination, Hb (g/dL), Haematocrit (HCT) (%), absolute Reticulocyte count (Ret. tot.) (109litre value) and percentage Reticulocyte value (Ret. perc.) (%) were assessed as indicators of haematopoiesis [14-16] and the CHr (pg) as an indicator of iron status [7, 13, 17, 18]. The start date of the iron supplementation, and the dosage at the beginning and at discharge (expressed in drops/kg) were collected. The number of infants who had blood transfusions was identified. We refer to the blood transfusion protocol of the Italian Society of Neonatology [19]. Data relating to breastfeeding at discharge (maternal or formula) were also collected.
Side effects: We monitored for side effects potentially related to the use of iron including necrotizing enterocolitis and retinopathy of the prematurity.
Statistical Analysis. Birth weight was expressed as a z-score, using the INeS charts as reference in order to correct for sex, GA, and parity (firstborn/laterborn) [20]. At first descriptive analyses were done by computing the Hb, HCT, Ret. tot. or perc., and CHr raw means and their confidence intervals (95%), by cohort (Fe-bis and Fe-sol) and sample order (regardless of the GA at which the sample was taken). A more detailed analysis was carried out by estimating the treatment effect on the blood profile with a general linear model in which the response variable (Hb, HCT, Ret. tot. and perc., and CHr value) was estimated as a function of: cohort, time (square root of age at sample-taking), class of birth weight (2 classes: z-score greater than or less than zero), GA class (2 classes: ≤29 and ≥30 completed weeks). The different interactions between the variables and the subjects were also included in the model as covariates.
3. Results
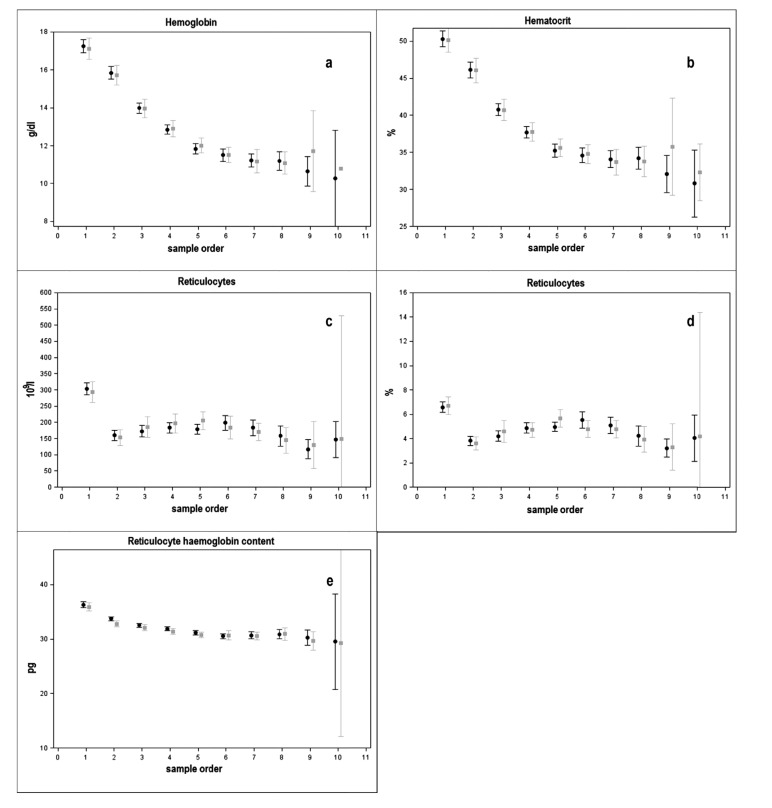
Characteristics of the two cohorts are listed in Table 1. Fig. (2) reports haematological values observed in each consequent blood sample for both cohorts. A progressive reduction of mean Hb and HCT was observed until the 6th week of age in both cohorts after which values stabilised respectively at 11 g/dL and 35% (approximately at the 7th sample) (Figs. 2a and b). The ret. tot. and perc. (Figs. 2c and d) were reduced during the first week, but their mean values increased between the second and the third week. The CHr mean values showed an initial reduction in the first two weeks of age, followed by stable values thereafter in both cohorts (Fig. 2e).
Table 1.
Characteristics of the newborns included in the study by group.
| Fe-sol (N=225) | Fe-bis (N=75) | |
|---|---|---|
| Males, n(%) | 111 (49%) | 39 (52%) |
| GA (wks), median (range) | 30 (26-32) | 30 (26-32) |
| Birth Weight (g), mean±ES | 1315±20.4 | 1334±37.8 |
| Birth weight (z-score*), mean±ES | -0,20±0.06 | -0,14±0.12 |
| Newborns Blood transfused, n (%) | 10 (4) | 2 (3) |
| Exclusive Breastfed at discharge, n (%) | 46 (20) | 20 (27) |
| Age at first examination(days), median (range) | 1(0-4) | 1 (0-8) |
| Age at start of iron supplementation (days), median (range) | 15 (3-65) | 16 (4-61) |
| Age at discharge, median (range) | 41 (12-137) | 38 (17-107) |
| Age at start of therapy with rHuEPO, median (range) | 12 (1-63) | 11 (5-33) |
| Age at the end of therapy with rHuEPO, median (range) | 43 (18-102) | 37 (17-106) |
| Hemochrome, n (range) | 5 (2-10) | 5 (2-10) |
| Dose at start of therapy (mg/Kg/die), mean±ES | 2.75±0.043 | 0.72±0.018 |
Fig. (2).
Row mean and their IC (95%) of hemoglobin (a), hematocrit (b), reticulocytes (c), reticulocytes % (d), reticulocyte hemoglobin content (e) by number of test (#test) and group (Fe-bis: grey, Fe-sol: black).
The general linear model explains about 80% of the observed variability of Hb and HCT values (R-square: 0.78 and 0.75 respectively), about 70% of the CHr values (R-Square: 0.69), and less than 35% of the ret. tot. and perc. values (R-square: 0.34 and 0.33 respectively). All the five hematopoietic variables analysed are significantly influenced by the age at sample-taking and by the class of birth weight. On the other hand, the two cohorts (Fe-sol, Fe-bis) did not show significant differences in the observation period for all the considered variables. No patient died during the treatment period.
4. Discussion
ID and IDA in the infants have a negative impact on long-term behavioural and neurocognitive development. They can also be associated with acoustic nerve fibres and hippocampus alterations. The etiopathogenetic origin of the neurological damage can be traced back to the essential role of iron in the neuronal energy metabolism, in the functionality of the neurotransmitters, and in myelination [1, 21, 22]. It has been shown that iron supplementation in premature infants allows higher levels of Hb and greater iron deposits to be attained, and a lower risk of developing ID and IDA [7, 23, 24]. Thus, prophylactic iron supplementation must be practiced in the majority of preterm infants to prevent the onset of ID and IDA [7]. On the other hand, in full-term infants with appropriate birth weight, iron stores present at birth will support an adequate haematopoiesis until the 6th month of age, so iron supplementation is usually not necessary. In preterm infants with early iron supplementation, iron stores at 2-3 weeks of age is three times greater compared to iron stores of preterm infants with later supplementation. These data suggest that early compared to late iron supplementation has better iron stores, but the iron stores are still lower if compared to the iron stores of full-term infants [25-28]. International guidelines suggest that a minimum of 2-4 mg/kg of oral elemental iron per day is needed to prevent ID [8-10]. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition recommends starting iron supplementation at 2-6 weeks of age (2-4 weeks in infants with very low birth weight) and must be continued after discharge until at least 6-12 months of age, depending on the toddler diet. Iron sulfate is the most common oral supplement. To our knowledge, there are no studies that have established the superiority of one iron compound compared to others for iron supplementation in childhood and infants [29-31]. Due to its high bioavailability, the iron bisglycinate chelate may be a viable alternative to iron sulfate in terms of efficacy and tolerability [32-37]. Pineda performed a clinical trial on children aged 6-36 months and observed the bioavailability of iron bisglycinate chelate at 90.9%, whereas that of iron sulfate at only 26.7% [33]. Moreover Bovell, in a study on adolescents, demonstrated that iron bisglycinate chelate when added to food was absorbed four times better than iron sulfate, maintaining its efficacy on haematopoiesis [34].. In 2014, Duque assessed a cohort of children at risk of IDA receiving iron sulfate or bisglycinate chelate iron at the same dosage: only the bisglycinate chelate iron cohort showed significantly higher ferritin concentration [36]. The same results emerged from other recent studies performed on adults [35, 37-39]. To our knowledge, only one clinical trial was conducted on preterm infants comparing iron sulfate to polysaccharide iron demonstrating that the two compounds had the same efficacy on Hb, serum iron and ferritin values, but the polysaccharide iron had a better tolerability [40].
In our study, the supplementation was started early at 15 days of age in both cohorts, as recommended by the most recent literature [25-28]. The percentages of infants receiving transfusions in the two cohorts were comparable. The assessment of the incidence of necrotizing enterocolitis and retinopathy of prematurity in the two populations was not among the primary objectives of the study, but evaluated as possible side effects; however, data on all infants admitted to our centre showed no difference between the two cohorts. Further, the rates of necrotizing enterocolitis and retinopathy of prematurity in both cohorts were inferior compared to the rates reported by the Vermont Oxford Network [41]. Treatment with two compounds did not show significant differences; in particular, the values of the haemopoietic variables were comparable. A progressive reduction of Hb and HCT was observed after 6 weeks of age in both cohorts, as expected in both preterm and term newborn. The ret. tot. and perc. were reduced during the first week, but their mean values increased between the second and the third week when treatment with rHuEPO and iron supplementation was initiated. Consequently, after the 6th week of age we observed a stabilisation of the Hb and HCT values, which is a sign of an adequate iron supplementation. The CHr mean values showed an initial reduction indicating a relative depletion of iron stores, followed by stable parameters in both cohorts. It is noteworthy that in our population no infant had a CHr value less than 29 pg, which is the threshold value established as an early marker of ID [42, 43]. This data should be interpreted both as a sign of adequate iron supplementation and an optimal adjustment of the iron dose according to the CHr value modification for the weight increase.
As expected, all the five hematopoietic variables analysed were significantly influenced by the age of infant at the time of the sample-taking and by the class of birth weight. No significant differences were observed between the two treatments.
In our patients, treatment with iron bisglycinate chelate demonstrated an equivalent haematopoietic response compared to iron sulfate when administered approximately at quarter of the dose: 0.75 mg/kg/day compared to 3 mg/kg/day. This observation suggests that iron bisglycinate chelate is better absorbed than iron sulfate, maintaining equal efficacy even in premature infants. Data from previous studies mainly conducted in children and adults were consistent with our findings, but our study was carried out exclusively on preterm infants and had larger cohorts compared to previous studies. The efficacy at lower doses may also ensure better tolerability. The administered dose at the beginning of the supplementation period did not require an increase over time, at least until discharge.
Limitations of this study are the potential bias inherent in non-randomised studies, and the possibility that there were changes in management practices (e.g. lipids composition of parenteral nutrition, light source for phototherapy) over the duration of the study which may have affected the cohorts differently as they were not contemporaneous. Nevertheless, our main care protocols underwent minor changes from 2006 to 2014 as well as our nutrition policy and the methods of blood collection and analysis of the samples. Moreover, even if there were no GA-related inclusion criteria, in our population we observed only preterm infants with GA>26 weeks, as premature infants of lower GA received iron supplementation more frequently intravenously especially in the early stages of supplementation.
Variable number of blood samples were recorded depending on both the GA at birth and the infants’ clinical conditions that sometimes warranted additional blood counts as well as increased hospital stay. Only a small number of infants had up to 10 samples drawn, which led to an increase of imprecision in the data mean estimation and resulted in a wide Confidence Interval (95%).
conclusion
In conclusion, iron bisglycinate chelate treatment at a dose of 0.75 mg/kg/day showed equal efficacy compared to iron sulfate treatment at a dose of 3 mg/kg/day in the haematopoietic response in preterm infants of GA≤32 weeks. This reduced dose may have avoided iron overload and potential adverse effects. Iron bisglycinate chelate may be considered a valid alternative to iron sulfate in the prevention and treatment of preterm newborn anaemia. It would be appropriate to conduct randomised controlled clinical trials to compare other iron compounds to iron bisglycinate chelate in preterm infants. Further studies should monitor infants for a longer period of time beyond the hospital stay in order to assess treatment responses over time. Similar studies may be useful to evaluate the efficacy of bisglycinate-chelate in other NICU settings where no routine use of EPO is practiced.
Acknowledgements
RB and PS wrote the study proposal and collected the data. RM performed all the analysis on blood samples and wrote the study proposal. ES analysed the data. TB collected the data and wrote the first draft of the manuscript. EAC wrote the subsequent versions of the manuscript. EB and AC advised on the study proposal and supervised the data analysis. All authors contributed to and approved the final manuscript.
List of abbreviations
- CHr
Reticulocyte Haemoglobin Content
- GA
Gestational Age
- Hb
Haemoglobin
- HCT
Hematocrit
- ID
Iron Deficiency
- IDA
Iron Deficiency Anaemia
- IV
Intravenous
- Ret. perc.
Reticulocyte Percent
- Ret. tot.
Reticulocyte Total Count
- rHuEPO
Recombinant Human Erythropoietin
Ethics Approval and Consent to Participate
Patient data were collected retrospectively through medical records. Specific approval by local ethical commitee was not required as observational study performed during diagnostic follow up.
Human and Animal Rights
No Animals/Humans were used for studies that are base of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Rao R., Georgieff M.K. Iron Therapy for Preterm Infants. Clin. Perinatol. 2009;36(1):27–42. doi: 10.1016/j.clp.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin J.C., Strauss R.G., Kulhavy J.C., et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics. 2000;106(2):E19. doi: 10.1542/peds.106.2.e19. [DOI] [PubMed] [Google Scholar]
- 3.Rao R., Georgieff M.K. Perinatal aspects of iron metabolism. Acta Paediatr. Suppl. 2002;91(438):124–129. doi: 10.1111/j.1651-2227.2002.tb02917.x. [DOI] [PubMed] [Google Scholar]
- 4.Bateman S.T., Lacroix J., Boven K., et al. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am. J. Respir. Crit. Care Med. 2008;178(1):26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 5.Strauss R.G. Anaemia of prematurity: Pathophysiology and treatment. Blood Rev. 2010;24(6):221–225. doi: 10.1016/j.blre.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raju T.N.K., Singhal N. Optimal timing for clamping the umbilical cord after birth. Clin. Perinatol. 2012;39(4):886–900. doi: 10.1016/j.clp.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills R.J., Davies M.W. Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database Syst. Rev. 2012;3(3):CD005095. doi: 10.1002/14651858.CD005095.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker R.D., Greer F.R., The Committee On Nutrition Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0-3 Years of Age). Pediatrics. 2010;126(5):1040–1050. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 9.Agostoni C., Buonocore G., Carnielli V.P., Curtis M., Darmaun D., Decsi T. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra T.R., Zlotkin Z.H., Boland M.P., Issenman R.M., Rousseau-Harsany E., Van Aerde J.E.E. Nutrient needs and feeding of premature infants. CMAJ. 1995;152(11):1765–1785. [PMC free article] [PubMed] [Google Scholar]
- 11.Cembranel F., Dallazen C., González-Chica D.A. Effectiveness of ferrous sulfate supplementation in the prevention of anemia in children: a systematic literature review and meta-analysis. Cad. Saude Publica. 2013;29(9):1731–1751. doi: 10.1590/0102-311x00152312. [DOI] [PubMed] [Google Scholar]
- 12.Cancelo-Hidalgo M.J., Castelo-Branco C., Palacios S., et al. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013;29(4):291–303. doi: 10.1185/03007995.2012.761599. [DOI] [PubMed] [Google Scholar]
- 13.David O., Bagna R., Borgione S., Bertino E., Fabris C. Use of reticulocyte parameters to monitor very low birth weight newborns receiving recombinant human erythropoietin. Ital J Pediatr PACINIEDITORE. 2002;28(5):372–376. [Google Scholar]
- 14.Forestier F., Daffos F., Galacteros F., Bardakjian J., Rainaut M., Beuzard Y. Hematological values of 163 normal fetuses between 18 and 30 weeks of gestation. Pediatr. Res. 1986;20(4):342–346. doi: 10.1203/00006450-198604000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Thomas C., Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin. Chem. 2002;48(7):1066–1076. [PubMed] [Google Scholar]
- 16.Christensen R.D., Henry E., Andres R.L., Bennett S.T. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology. 2011;99(4):289–294. doi: 10.1159/000320148. [DOI] [PubMed] [Google Scholar]
- 17.Brugnara C., Laufer M.R., Friedman A.J., Bridges K., Platt O. Reticulocyte hemoglobin content (CHr): Early indicator of iron deficiency and response to therapy. Blood. 1994;83(10):3100–3101. [PubMed] [Google Scholar]
- 18.Fishbane S., Galgano C., Langley R.C., Canfield W., Maesaka J.K. Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney Int. 1997;52(1):217–222. doi: 10.1038/ki.1997.323. [DOI] [PubMed] [Google Scholar]
- 19.Girelli G., Antoncecchi S., Casadei A.M., et al. Recommendations for transfusion therapy in neonatology. Blood Transfus. 2015;13(3):484–497. doi: 10.2450/2015.0113-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertino E., Spada E., Occhi L., et al. Neonatal anthropometric charts: The Italian neonatal study compared with other European studies. J. Pediatr. Gastroenterol. Nutr. 2010;51(3):353–361. doi: 10.1097/MPG.0b013e3181da213e. [DOI] [PubMed] [Google Scholar]
- 21.Lozoff B., Georgieff M.K. Iron Deficiency and Brain Development. Semin. Pediatr. Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Domellöf M., Braegger C., Campoy C., et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014;58(1):119–129. doi: 10.1097/MPG.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 23.Berglund S., Westrup B., Domellöf M. Iron supplements reduce the risk of iron deficiency anemia in marginally low birth weight infants. Pediatrics. 2010;126(4):e874–e883. doi: 10.1542/peds.2009-3624. [DOI] [PubMed] [Google Scholar]
- 24.Long H., Yi J.M., Hu P.L., et al. Benefits of Iron supplementation for low birth weight infants: A systematic review. BMC Pediatr. 2012;12(1):99. doi: 10.1186/1471-2431-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franz A.R., Mihatsch W.A., Sander S., Kron M., Pohlandt F. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics. 2000;106(4):700–706. doi: 10.1542/peds.106.4.700. [DOI] [PubMed] [Google Scholar]
- 26.Arnon S., Shiff Y., Litmanovitz I., et al. The efficacy and safety of early supplementation of iron polymaltose complex in preterm infants. Am. J. Perinatol. 2007;24(2):95–99. doi: 10.1055/s-2007-970179. [DOI] [PubMed] [Google Scholar]
- 27.Steinmacher J., Pohlandt F., Bode H., Sander S., Kron M., Franz A.R. Randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams: Neurocognitive development at 5.3 years’ corrected age. Pediatrics. 2007;120:538–546. doi: 10.1542/peds.2007-0495. [DOI] [PubMed] [Google Scholar]
- 28.HongXing J RongShan W, ShuJun C, AiPing W, XiYong L. Early and late iron supplementation for low birth weight infants: A meta-analysis. Ital. J. Pediatr. 2015;41:16. doi: 10.1186/s13052-015-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaber L., Rigler S., Taya A., et al. Iron polymaltose versus ferrous gluconate in the prevention of iron deficiency anemia of infancy. J. Pediatr. Hematol. Oncol. 2010;32(8):585–588. doi: 10.1097/MPH.0b013e3181ec0f2c. [DOI] [PubMed] [Google Scholar]
- 30.Amaral D., Galimberti G., Cuesta S., Pinto J., Ferrario C., Graciela E. Comparative evaluation of efficacy and tolerance of iron polymaltose complex and ferrous sulphate for treatment of iron deficiency anemia in infants. Rev. Fac. Cien. Med. Univ. Nac. Cordoba. 2012;69(2):97–101. [PubMed] [Google Scholar]
- 31.Haliotis F.A., Papanastasiou D.A. Comparative study of tolerability and efficacy of iron protein succinylate versus iron hydroxide polymaltose complex in the treatment of iron deficiency in children. Int. J. Clin. Pharmacol. Ther. 1998;36(6):320–325. [PubMed] [Google Scholar]
- 32.Pineda O., Ashmead H.D., Perez J.M., Lemus C.P. Effectiveness of iron amino acid chelate on the treatment of iron deficiency anemia in adolescents. J. Appl. Nutr. 1994;46(1-2):2–13. [Google Scholar]
- 33.Pineda O., Ashmead H.D. Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate. Nutrition. 2001;17(5):381–384. doi: 10.1016/s0899-9007(01)00519-6. [DOI] [PubMed] [Google Scholar]
- 34.Bovell-Benjamin A.C., Viteri F.E., Allen L.H. Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. Am. J. Clin. Nutr. 2000;71(6):1563–1569. doi: 10.1093/ajcn/71.6.1563. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari P., Nicolini A., Manca M.L., et al. Treatment of mild non-chemotherapy-induced iron deficiency anemia in cancer patients: Comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomed. Pharmacother. 2012;66(6):414–418. doi: 10.1016/j.biopha.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Duque X., Martinez H., Vilchis-Gil J., et al. Effect of supplementation with ferrous sulfate or iron bis-glycinate chelate on ferritin concentration in Mexican schoolchildren: A randomized controlled trial. Nutr. J. 2014;13:71. doi: 10.1186/1475-2891-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milman N., Jønsson L., Dyre P., et al. Ferrous bisglycinate 25 mg iron is as effective as ferrous sulfate 50 mg iron in the prophylaxis of iron deficiency and anemia during pregnancy in a randomized trial. J. Perinat. Med. 2014;42(2):197–206. doi: 10.1515/jpm-2013-0153. [DOI] [PubMed] [Google Scholar]
- 38.Falco P. Bisglycinated chelated iron in sideropenic anaemia treatment of celiac pazients with refractoriness to the others iron terapies and improvement of laboratoristic haemoglobinic parameters. Haematologica. 2013;98:224. [Google Scholar]
- 39.Iannello S., Spanti C., LaRosa R. Efficacia e tollerabilità del ferro bisglicinato chelato (TECNOFER) nel trattamento dell’anemia sideropenica lieve-moderata in pazienti donatori di sangue nel trattamento dell’anemia sideropenica. J. Health Sci. 2013;11(7):3–7. [Google Scholar]
- 40.Naude S., Clijsen S., Naulaers G., Daniels H., Vanhole C., Devlieger H. Iron supplementation in preterm infants: A study comparing the effect and tolerance of a Fe2+ and a nonionic FeIII compound. J. Clin. Pharmacol. 2000;40(12 Pt 2):1447–1451. [PubMed] [Google Scholar]
- 41.Vermont Oxford Network 2012.
- 42.Lorenz L., Arand J., Buchner K., Wacker-Gussmann A., Peter A., Poets C.F., Franz A.R. Reticulocyte haemoglobin content as a marker of iron deficiency. Arch. Dis. Child. Fetal Neonatal Ed. 2015;•••:F198–F202. doi: 10.1136/archdischild-2014-306076. [DOI] [PubMed] [Google Scholar]
- 43.Lönnerdal B., Georgieff M.K., Hernell O. Developmental Physiology of Iron Absorption, Homeostasis, and Metabolism in the Healthy Term Infant. J. Pediatr. 2015;167(4):S8–S14. doi: 10.1016/j.jpeds.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]