Abstract
The diagnosis and management of gastro-esophageal reflux (GER) and GER disease (GERD) in infants and children remains a challenge. Published guidelines and position papers, along with Embase, MEDLINE, and the Cochrane Database were reviewed and summarized with the intent to propose a practical approach and management of GER and GERD for healthcare providers and to standardize and improve the quality of care for infants and children. For this purpose, 2 algorithms were developed, 1 for infants <12 months of age and the other for older children. None of the signs and symptoms of GER and GERD are specific and there is no gold standard diagnostic test or tool. Nutritional management is recommended as a first-line approach in infants, while in children, a therapeutic trial with antacid medication is advised for early management. The practical recommendations from this review are intended to optimize the management of GER in infants and older children and reduce the number of investigations and inappropriate use of medication.
Keywords: Gastro-esophageal reflux, Gastroesophageal reflux disease, Esophagitis, Endoscopy, Impedance, Proton pump inhibitors, pH
INTRODUCTION
Joint committees of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) published guidelines on gastro-esophageal reflux (GER) and GER disease (GERD) in 2009 and 2018 [1,2]. This review is an update and a personal view of these guidelines.
A systematic literature search was performed from June 2015 (end date of the search for the NASPGHAN-ESPGHAN guidelines) up to September 2019, including Embase, MEDLINE, PubMed, and the Cochrane Database.
DEFINITION OF GER AND GERD
GER is the passage of gastric contents into the esophagus with or without regurgitation and/or vomiting. GER becomes GERD when reflux causes troublesome symptoms and/or complications (Table 1) [3]. GERD is obvious in the presence of regurgitation, heartburn, reflux esophagitis, or esophageal stenosis in older children, and highly suspected in cases of hematemesis and failure to thrive in a vomiting child. However, none of the symptoms and signs of GERD are specific.
Table 1. Symptoms and signs associated with GERD in infants and children.
| Symptoms | Signs |
|---|---|
| Heartburn/chest pain | Esophageal mucosal complications (esophagitis, esophageal stricture, Barrett's esophagus) |
| Epigastric pain | Recurrent/chronic desaturations |
| Regurgitation/vomiting | Recurrent aspiration pneumonia |
| Recurrent inconsolable crying/irritability | Laryngitis |
| Hematemesis | Recurrent otitis media |
| Feeding refusal | Abnormal posturing/Sandifer syndrome |
| Odyno/dysphagia, hoarseness | Failure to thrive/weight loss |
| Recurrent/chronic dry cough, wheezing/ALTE/BRUE | Dental erosions |
| Disturbed sleep | - |
| Seizure episodes | - |
GERD: gastro-esophageal reflux disease, ALTE: apparent life-threatening event, BRUE: brief resolved unexplained event.
Severe esophageal mucosal complications and hematemesis occur, fortunately, in only a minority of children. In the majority of cases, symptoms are mild to moderate, mainly decreasing the daily quality of life or nocturnal sleeping. However, defining the “troublesome” character of the symptoms is difficult and may differ among children, their ability to describe them, their caregivers and healthcare professionals. As a consequence, the definition of GERD mostly depends on the subjective interpretation of symptoms as they affect quality of life. Moreover, the spectrum of possible GERD symptoms in infants and children varies widely by age and is non-specific [1,4]. This may lead to both over- and under-diagnosis and unneeded treatment. In infants especially, it is often difficult to determine what is troublesome, what is pathologic, and what is physiologic. Many infants with GERD do cry excessively, but infants who cry excessively but do not regurgitate or vomit only seldomly suffer from GERD. Furthermore, the degree of parental anxiousness is the driving factor for diagnosis and management. The absence of a gold standard diagnostic tool hampers the diagnosis of GERD in infants and children further. However, GERD is a prominent phenomenon in children with underlying medical conditions, such as esophageal atresia, neurologic impairment and pulmonary problems, including cystic fibrosis. According to the guidelines of the NASPGHAN-ESPGHAN, refractory GERD is GERD which does not or insufficiently responds to optimal treatment after 8 weeks.
Clinical history and physical examination are important to identify alarm symptoms and signs and differentiate GERD from other disorders. Regurgitation is defined as the passage of refluxed contents into the pharynx, mouth, or out from the mouth [1,2]. Other terms, such as spitting-up, posseting, and spilling are considered equivalent to regurgitation. Vomiting is a coordinated autonomic and voluntary motor response causing the forceful expulsion of gastric contents through the mouth. Rumination is the effortless regurgitation of recently-ingested food into the mouth with subsequent mastication and re-swallowing. Rumination syndrome is not further discussed and is a distinct clinical entity in which rumination follows in minutes after ingestion of a meal, does not occur during sleep, and does not respond to standard treatment for GER. Rumination involves repetitive contractions of the abdominal wall muscles, diaphragm, and tongue [5,6].
Regurgitation and episodes of vomiting are frequent in infants. When repetitive regurgitation or vomiting is already present during the first 1 to 2 weeks of life, infections, anatomical anomalies, and metabolic disorders should be excluded. When the onset of regurgitation is after the age of 6 months, as well as when symptoms persist beyond the age of 12 months, other diagnoses than infant GER or GERD should be considered. Special attention should be given to the dietary history, since cow's milk protein allergy may perfectly mimic the symptoms of GERD.
Reflux hypersensitivity occurs in patients with esophageal symptoms (heartburn or chest pain) who lack endoscopic and/or multiple intraluminal impedance (MII) and/or pH metric evidence of GERD, but do have clinical evidence of a temporal association between reflux and symptoms [1,2]. Functional heartburn occurs in patients with esophageal symptoms (heartburn or chest pain) who also lack the objective evidence of reflux and who do not have evidence that symptoms are triggered by reflux episodes. Non-erosive reflux disease (NERD) describes patients with esophageal symptoms who lack evidence of esophagitis on endoscopy but do have an abnormal acid burden according to evidence provided by MII-pH and/or pH metric results of acid reflux that may or may not trigger symptoms. NERD might be the most frequent presentation of GERD.
SYMTOMS AND SIGNS
Symptoms and signs are listed in Tables 1 and 2. Excessive body weight is associated with an increased prevalence of GERD.
Table 2. ‘Red flag’ symptoms and signs that may be associated with disorders other than GERD.
| Symptoms | Signs |
|---|---|
| Regurgitation started <2 weeks of life or >6 months or persistent after 18 months of life | Abnormal (general or abdominal, neurological, respiratory) physical examination |
| Bilious, nocturnal or persistent vomiting | Abdominal distension |
| Chronic or bloody diarrhea | Fever |
| Hematemesis | Failure to thrive/weight loss |
| Dysuria | Lethargy or excessive irritability |
| Seizures | Abnormal muscle tone |
| Dysphagia | Bulging fontanel or excessive increase of head circumference or micro/macrocephaly |
| Recurrent pneumonia | Abnormal psychomotor development |
GERD: gastro-esophageal reflux disease.
Distressed infants are a special group of patients. Many infants with overt GERD are distressed and cry a lot because not only esophagitis but also esophageal dilatation caused by reflux (regurgitated milk), may cause discomfort and pain. Infants with GERD often present inconsolable crying and overt regurgitation or vomiting. However, crying may be caused by many conditions other than GERD and is not always the referral symptom in infants with GERD. Occult GERD, GERD in infants who do not regurgitate or vomit, is quite rare. The role of occult GERD in children presenting with chronic respiratory symptoms needs further evaluation. The length of the esophagus in an infant is about 10 cm and can only contain about 5 ml of liquid. In other words, it is difficult to understand how a minimal volume of reflux that restricts itself to a couple of centimeters can cause so much distress and pain.
DIAGNOSTIC INVESTIGATIONS
Barium contrast, ultrasound, and scintigraphy
These techniques may investigate reflux for a very limited time and mainly during the postprandial period. One or more reflux episodes can be detected in up to 50% of children undergoing radiologic imaging, regardless of symptoms. An upper gastrointestinal (GI) barium contrast study is mainly helpful to detect GI malformations and it can be useful in the diagnosis of hiatal hernia, malrotation, pyloric stenosis, duodenal web, duodenal stenosis, antral web, esophageal narrowing, Schatzki's ring, achalasia, esophageal stricture, and esophageal extrinsic compression. Persistent symptoms after anti-reflux surgery are another indication [7,8]. Ultrasonography is not indicated for GERD diagnosis as the results are clearly investigator-dependent. The sensitivity of ultrasound in the 15 minutes post-prandial is about 95% but the specificity is only 11% in comparison to pH-metry [9,10]. The correlation between esophageal wall thickness and esophagitis is also poor. Regarding scintigraphy, sensitivity and specificity are only moderate, at 69% and 78%, respectively [11]. Besides demonstrating tracer that refluxes into the esophagus, scintigraphy evaluates gastric emptying and may also show pulmonary aspiration [12].
Esophagogastroduodenoscopy with biopsies
Microscopic esophagitis is defined as the presence of eosinophils, papillary lengthening, and/or basal cell hyperplasia. Erosive esophagitis is defined as visible breaks in the esophageal mucosa. There is no specific symptom of esophagitis. The sensitivity of erosive esophagitis in diagnosing GERD was reported to range from 15% up to 71% and the sensitivity of microscopic esophagitis in diagnosing GERD was between 83% and 88%. The authors found that histologic esophagitis had a negative predictive value (NPV) of 62% and 73% [13,14]. If the endoscopic appearance of the mucosa in control patients was also considered, the NPV of upper endoscopy decreased to only 33% [15]. These findings indicate that a biopsy without the hallmarks of esophagitis or the absence of macroscopic lesions does not rule out the presence of GERD. In all 3 studies, if mentioned, histology and macroscopic appearance were normal in the control group, which automatically led to a reported specificity and NPV of 100% [13,14,15]. Thus, there is insufficient evidence to support the use of endoscopy with or without biopsy for the diagnosis of GERD in infants and children. However, endoscopy of the upper GI tract is useful to evaluate the mucosa in the presence of alarm symptoms or signs, such as hematemesis, dysphagia, or failure to thrive or anemia; to detect complications of GERD, such as erosive esophagitis, strictures, and Barrett's esophagus; or to diagnose conditions that might mimic GERD, such as eosinophilic esophagitis. GERD may exist despite the normal endoscopic appearance of the esophageal mucosa and the absence of histological abnormalities. The correlation between esophagitis and acid exposure time on pH-monitoring is quite poor, particularly in infants. The area under the curve is a pH-metry parameter that considers the acidity of the reflux episodes and was shown to correlate with esophagitis but has lost appeal in recent years and is no longer reported in commercially-available pH-metry reports [16].
Biomarkers
Biomarkers, such as salivary pepsin, have not been shown to be useful to diagnose GERD. Pepsin can be found in the mouth of almost one-third of control patients [17]. The sensitivity and specificity of pepsin in bronchoalveolar lavage (BAL) fluid to predict pathologic reflux by pH-metry and MII or endoscopy was 57% and 65%, respectively [18]. The positive predictive value (PPV) of pepsin for pathologic reflux by pH-metry, MII, or endoscopy was 50% and the NPV was 71%. The sensitivity, specificity, PPV, and NPV for the presence of pepsin in the BAL of patients with confirmed pulmonary aspiration were 80%, 100%, 100%, and 93% [19]. Pepsin was reported to be present in the BAL of 84% of patients with respiratory disease and reflux symptoms. However, 87% of children with respiratory disease but no reflux symptoms were pepsin-negative [20]. Moreover, no relation was found between salivary pepsin positivity, extra-esophageal symptoms, quality of life scores, or inflammation on bronchoscopy or esophagogastroduodenoscopy [21]. Moreover, the presence of pepsin has been shown to be between 50% and 100% in children depending on the number of positive samples considered [22]. Middle ear fluid analysis showed no association between pepsin and reflux symptoms [23]. Bile salts, pepsin, and lipid-filled macrophages in BAL fluids have been studied to demonstrate gastric aspiration secondary to reflux as a cause of chronic respiratory disease but were not useful [18,21,24,25].
When pulmonary aspiration is obvious, the biomarkers are positive. Also, when GERD is obvious because of the presence of classic symptoms, such as vomiting, these biomarkers are positive. However, they are not helpful in other patient groups and cannot be recommended for children with chronic respiratory diseases without GERD symptoms. The reason for this might simply be that chronic respiratory disease caused by GERD is seldom in the absence of GER symptoms in neurologically-normal children.
Manometry/motility studies
Manometry or motility studies do, of course, not measure reflux, but may be useful by demonstrating the etiology of GERD. High-resolution esophageal manometry may be helpful to diagnose rumination syndrome [26,27]. High-resolution manometry can also highlight esophageal motility disorders in those who present with symptoms similar to GERD.
Proton pump inhibitor (PPI) trials
A PPI diagnostic test is based on the hypothesis that symptoms responding to PPI administration suggest that they are (acid) GERD-induced. Since none of the trials in infants showed a better symptom reduction than placebo, regardless of the duration of the trial, the administration of PPIs cannot be recommended for infants as a diagnostic test [28]. The best improvement of symptoms in children occurs during the first 2 to 4 weeks [29,30,31,32]. According to data in adults with typical symptoms, 1 week of PPI is sufficient to observe a significant response. There are insufficient data to recommend a PPI trial in patients with extra-esophageal symptoms, possibly related to GERD.
The pH-metry and MII recording
Continuous esophageal pH monitoring was developed in the 1990s and for a long time was regarded as the best technique to measure reflux since it was the only technique available to measure GER outside the postprandial period. More recently, wireless pH recording has been proposed as an alternative to pH probe monitoring. The capsule is clipped to the esophageal mucosa and is supposed to drop off after 48 hours, although up to 5 days of recording has been reported. Pediatric studies have shown that the wireless pH recording results were comparable to the pH probe studies in patients who had both techniques performed simultaneously [33]. Complications of the device occurred in 0% to 15% of the patients and included esophageal tears, chest pain, and early detachment [34,35]. Outside the USA, this capsule is hardly used, although it certainly might be helpful in patients with behavioral disorders. Of course, impedance cannot be recorded with this device. Oropharyngeal monitoring is not recommended as most studies failed to show that the technique was appropriate.
The development of MII in combination with pH monitoring has challenged pH monitoring. Indeed, MII in combination with pH-metry allows the detection of both acid and non-acid reflux. Since MII is expensive, the technique is only available in a limited number of centers. The analysis of an MII is time-consuming and requires experience. The determination of a normal value range is difficult to obtain since it is not ethically acceptable to perform such a technique in healthy asymptomatic infants and children. Reference values in different age groups have been recently proposed and cut-off values for symptom association indices have also been proposed. However, data for true asymptomatic, presumably healthy children are not available.
The indications to perform pH-MII are: 1) to measure the efficacy of acid suppression medication; 2) to differentiate NERD, hypersensitive esophagus, and functional heartburn in patients with normal endoscopies and histology; 3) to correlate persistent troublesome symptoms with acid and non-acid GER events; and 4) to establish the role of acid and non-acid reflux in the etiology of esophagitis and other signs and symptoms suggestive of GERD.
Non-pharmacological treatment
Patient education, specific methods to prevent or treat symptoms, and patient empowerment have been shown to decrease parental and patient anxiety. Management should always start with parental support.
Despite the possible benefits of positioning in the management of GER, only supine positions can be recommended for infants because the risk of sudden infant death syndrome (SIDS) is associated with all other sleeping positions. Supine sleeping is universally recommended by the National Health Service and the American Academy of Pediatrics (AAP) as the safest position to prevent the risk of SIDS. Because elevating the head of an infant's crib while the infant is supine may result in the infant rolling to the foot of the crib into a position that may comprise respiration, elevating the head of the crib is not recommended by the AAP [36]. An anti-regurgitation (AR) bed was developed and is available in some European countries. However, limited data hamper a global recommendation.
There is no evidence that reduced feeding volume, more frequent feedings, or extensively hydrolyzed or amino-acid based formula is effective for the treatment of infants presenting with troublesome GER symptoms. However, there is a consensus that overfeeding is a risk factor for GER and regurgitation. Although the overall quality of studies is low to very low, there is a consensus that thickened formula reduces regurgitation. There is no evidence to suggest that 1 thickening agent is more effective than another [37]. The impact of thickened formula on non-regurgitation symptoms is not clear.
Safety concerns regarding rice cereals were raised because of high levels of inorganic arsenic, which may cause neurotoxicity and long-term cancer risk. Despite an Food and Drug Administration warning, rice cereal does have some advantages over other cereals, including its ability to dissolve easily, its low cost, and it has been used for a long time. Bean gum has the advantage of adding no calories. It is important to realize that breast milk cannot be used to thicken with cereal because of the amylases present. Troublesome regurgitation and GERD are (almost) never a good reason to stop breastfeeding. Commercially-prepared thickened formula is preferred over adding thickeners to formula because the effect of the thickener on the composition of the formula has been taken into account in commercially-prepared formula. Most of the infant formula companies have developed AR and Comfort formula. While the first is positioned to reduce regurgitation in the "happy spitter", the second is positioned in the management of the infant presenting with regurgitation and distress. These comfort formulas are thickened, often the proteins are partially hydrolyzed, and lactose is reduced. In some infants, the differential diagnosis between troublesome GER-symptoms, GERD, and cow's milk protein allergy may be hard to make. Extensively hydrolyzed formula reduces GER-symptoms in cases where the symptoms are due to an allergy or delayed gastric emptying. A thickened extensive hydrolysate will be effective, independent of the cause of the symptoms, but does not make a precise diagnosis.
Limited data suggest that Lactobacillus reuteri DSM 17938 given as drops daily may be effective in reducing episodes of regurgitation, and may prevent future episodes [2].
Obese children are at increased risk of developing GER symptoms [38,39]. There is insufficient evidence to recommend lifestyle modifications, such as avoidance of alcohol and tobacco, massage therapy, complementary therapy (hypnotherapy, homeopathy, acupuncture, and herbal medicine), and specific dietary modifications for the reduction of GERD symptoms.
PHARMACOLOGICAL TREATMENT
Anti-acid medication
Antacids and alginates neutralize acid and contain sodium/potassium bicarbonate, or aluminum, magnesium, or calcium salts. Alginates are reported to reduce reflux symptoms and the number of episodes of regurgitation and vomiting [40,41]. Alginates were also shown to reduce the number of reflux episodes measured by pH-MII [42]. The same study confirmed the reduction of symptoms [42]. Some studies failed to show efficacy of alginate. This may be due to the design of those studies, alternating a feeding without and with alginate [42]. The National Institute for Health and Care Excellence guidelines recommend alginates as an alternative treatment to feed thickening agents in breastfed infants or as a trial in infants whose symptoms persist despite conservative measures [43]. On-demand and short-term administration of alginate have no significant adverse effects. Aluminum-containing antacids should not be used in infants and children with renal dysfunction.
Over the last decade, the efficacy of different PPIs, including lansoprazole, esomeprazole, rabeprazole, pantoprazole, and omeprazole, has been evaluated although no study compared different PPIs [44]. All studies in infants failed to show that PPIs were any better than placebos to decrease crying, fussiness, cough, arching back, regurgitation, and vomiting. PPI adverse effects received recently a lot of attention when about 25% of patients developed small intestinal bowel bacterial overgrowth [45]. An increase in upper and lower respiratory tract infections, GI infections, and eczema were reported. PPIs are a risk factor for Clostridium difficile infections [46,47,48]. Since histamine 2 receptor antagonists (H2RAs), such as ranitidine are less effective in reducing gastric acidity than PPIs, PPIs are the first choice. However, certainly, when PPIs are not available, H2RAs can still be used to treat acid-related diseases. The choice of administering PPIs versus H2RAs should also consider the ease of administration and medication cost since reliable evidence regarding its efficacy is limited. There is no evidence for the superiority of any PPI or H2RAs in comparison with a drug from the same class. Lansoprazole at 7.5 or 15 mg twice daily for 2 weeks improved symptoms, defined as a decrease in Revised Infant Gastroesophageal Reflux Questionnaire scores, more than an extensively hydrolyzed formula [49].
Prokinetics
Baclofen was reported to reduce transient lower esophageal sphincter (LES) relaxations, reflux episodes, and to accelerate gastric emptying, but there have been no randomized trials for GERD in children [50]. Baclofen may be used for the management of GERD, but not as a first-choice drug because of the reported side effects, including dyspeptic symptoms, drowsiness, dizziness, fatigue, and a lowered threshold for seizures [51].
There is no evidence that domperidone or metoclopramide reduces visible regurgitation or vomiting in comparison with placebo but they cause more adverse effects [52,53]. The most common adverse effects are extrapyramidal symptoms (9%), diarrhea (6%), and sedation (6%) [54]. Prolongation of the corrected QT interval is the most important adverse effect of domperidone [55]. Domperidone is not available in the United States. Cisapride, a mixed serotonergic agent that facilitates the release of acetylcholine at synapses in the myenteric plexus, increased the risk of sudden death, resulting in limited access programs [1,2].
There is no evidence for the efficiency of bethanechol, which has a high incidence of side effects [56,57]. Erythromycin and azithromycin, motilin agonists, may be of benefit in patients with gastroparesis [1,2]. However, these drugs have not been shown to reduce GER [58].
SURGICAL TREATMENT
Anti-reflux surgery is usually proposed after other options have failed [1]. Fundoplication decreases GER because it increases LES baseline pressure, decreases the number of transient LES relaxations, and the nadir pressure during swallow-induced relaxation, increasing the length of the intra-abdominal esophagus, accentuating the angle of His and reducing a hiatal hernia, if present. Laparoscopic surgery has replaced open Nissen fundoplication [59]. Robot-assisted Nissen fundoplication does not afford substantial advantage [60]. Anti-reflux surgery may be indicated in children with confirmed GERD who have failed or are non-adherent to optimal medical therapy or who have life-threatening presentations of GERD.
Total esophagogastric disconnection (Bianchi procedure) is an alternative surgical procedure in resistant GERD cases. There is no evidence to recommend total esophagogastric disconnection in infants and children with GERD refractory to pharmacological treatment.
Despite the efficacy of fundoplication for the treatment of GERD refractory to medical treatment, there has been an interest in developing less invasive and equally effective, endoscopic treatments for GERD. There is no evidence to support routine radiofrequency ablation (Stretta procedure) in infants and children with GERD. However, the technique may be of benefit in selected cases, and if performed by experienced specialists. The same can be said about endoluminal endoscopic gastroplication. Today, this technique cannot be used in infants and toddlers because of the size of the equipment.
PRACTICAL APPROACH
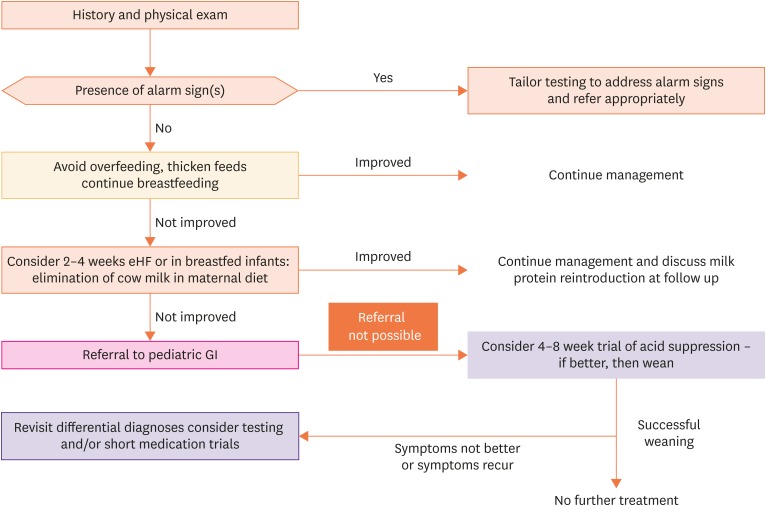
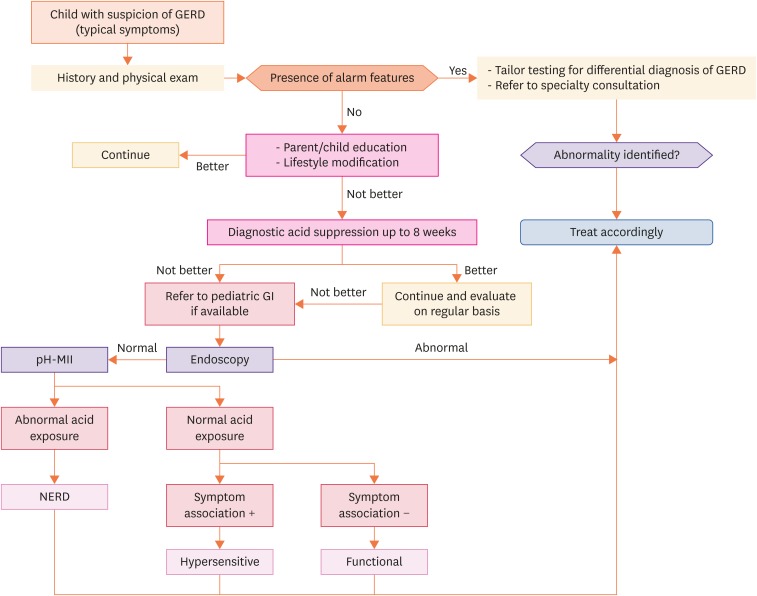
Two algorithms summarize the recommended practical approach, which is different in infants and young children (Fig. 1) and older children (Fig. 2). However, there is no evidence-based recommended practical approach because of insufficient evidence-based information. Moreover, both diagnostic and therapeutic possibilities differ from region to region. Last but not least, the organization of healthcare systems will also determine management.
Fig. 1. Infant with suspicion of GERD.
GERD: gastro-esophageal reflux disease, GI: gastrointestinal.
Fig. 2. Older children with suspicion of GERD.
GERD: gastro-esophageal reflux disease, NERD: Non-erosive reflux disease.
Infants
As always, optimal diagnosis and management start with taking a complete history and performing a complete physical examination. The healthcare provider is supposed to know the “red flags” or “alarm signs” and the most frequent differential diagnoses that may present with similar histories, symptoms, and signs. One example is the differential diagnosis in infants between GER, GERD, and cow's milk protein allergy.
In infants with overt regurgitation, in the absence of alarm symptoms, explaining the reasons why infants regurgitate (proportional large feeding volume, liquid feeding, supine position) and the natural evolution of the symptoms (spontaneous improvement between six and 18 months in the vast majority), to the parents is recommended. In general, regurgitation does not start before the age of 1 week or after the age of 6 months. Regurgitation and reflux are almost never reasons to stop breastfeeding. Frequency and the volume of feedings should be checked and adapted according to the age because overfeeding with large volumes occurs frequently in this age group. A trial with a thickened AR formula will decrease regurgitation and, thus, decrease parental anxiousness. Commercially-prepared thickened formula is preferable to formula thickened at home because the effect of the thickener on the composition of the formula is taken into account in commercial formula preparation. Some thickeners, such as bean gum may hamper iron absorption therefore, this micronutrient content is increased in the AR-formula. If the AR-formula does not successfully treat the symptoms after 1 to 2 weeks, an extensive hydrolysate trial for 2 to 4 weeks is recommended, certainly in cases where the infant presents with other symptoms indicative of atopic diseases, such as atopic dermatitis. Regarding all nutritional interventions, there is a consensus that a lack of improvement after 2 weeks is very unlikely to bring symptom relief afterward. In case of sufficient improvement, the AR-formula can be continued up to the age of 6 months, or until 12 months of age if symptoms relapse with a non-thickened formula. If an extensive hydrolysate was started, management of cow's milk protein allergy should be followed, with a challenge after 2 to 4 weeks, and continuation of the extensively hydrolyzed formula up to the age of 12 months or at least for 6 months, whichever is reached first. Many infant formula companies commercialized “comfort formula.” This type of formula is very often thickened, has a reduced lactose content, and contains a partially hydrolyzed protein. The efficacy of many of these formulas is poorly documented. In case the infant is also distressed, a trial with alginate for 2 to 4 weeks is recommended. Positional treatment is not recommended because the positions that decrease GER are positions with increased risk for SIDS. Although there are some data, evidence for the routine use of prebiotics, probiotics, massage, or herbal medications to treat GERD is too insufficient for their recommendation.
If nutritional management also remains unsuccessful, referral to a pediatric gastroenterologist is advised. In the case where referral is not possible, a 2 to 4 weeks trial of antacid medication (PPI and H2RA) is acceptable, although as stated, it is not recommended without an objective diagnosis of GERD. However, if the trial of antacid medication also fails, an appropriate referral is recommended. In the absence of alarm symptoms, diagnostic investigations are not recommended at the primary healthcare level.
Older children
The older the child, the closer the recommended approach is to the management in adults. Of course, at any age, the recommended approach starts with a complete history and physical examination. The healthcare provider should be aware of the alarm signs and most relevant differential diagnoses and act accordingly (Tables 2 and 3). In the absence of red flags, parent and child education and lifestyle modifications are the recommended first approaches. If there is insufficient improvement, a diagnostic trial with antacid medication for 2 to 4 weeks is recommended in children that are old enough to express themselves in a reliable way (>8–12 years). Again, if there is no improvement starts after 1 month, it is very unlikely that improvement will occur later. If there is sufficient improvement, the antacid medication should be continued and the necessity of continuation should be checked regularly, e.g., every 3 to 6 months. In the case of insufficient improvement, the patient should be referred for a diagnostic upper GI tract endoscopy with biopsies. In the case of abnormalities, appropriate management should be started. If the endoscopy and histology are normal, a pH-MII recording is recommended. If the result is abnormal, a diagnosis of NERD is established and management should be determined accordingly. If the result of the pH-MII is normal, but there is a positive symptom association, the patient suffers from a hypersensitive esophagus. In the case where there is also a negative symptom association, the conclusion is that the patient suffers from a functional GI disorder. Appropriate management is recommended for each of these situations.
Table 3. Differential diagnosis of GERD.
| GI | General/Extra-GI |
|---|---|
| Gastroenteritis | Infections |
| Obstructive abdomen | Respiratory/ENT Malformations |
| GI malformations | Allergy |
| Eosinophilic esophagitis/gastroenteropathy | Metabolic disorders |
| Gastritis/duodenitis/peptic ulcer | Neurologic/neuromuscular disease |
| Dysmotility disorders | Liver/renal disorder |
| Functional GI disorders | Pancreatitis |
| Pyloric stenosis, volvulus | Anorexia/self-induced vomiting |
| Cyclic vomiting | Inflammatory bowel disease |
| Superior mesenteric artery syndrome | Rumination syndrome |
| Foreign body | Cardiac disease |
GERD: gastro-esophageal reflux disease, ENT: ear-nose-throat, GI: gastrointestinal.
PROGNOSIS OF GERD
An onset of GERD symptoms under the age of 5, the presence of esophagitis, and the use of acid-suppression at the time of initial diagnosis may result in a less favorable outcome (Table 4). Firm conclusions, however, are limited by the poor quality of the studies. No evidence of an association between gender, ethnicity, family history of GERD, number of visits to the primary care physician, body weight at onset of GERD, the presence of respiratory symptoms at the onset of GERD, and the occurrence of GERD symptoms and complications at follow-up has been found. This topic is a priority for further research.
Table 4. Summary of the factors significantly related to the persistence of GERD.
| Prognostic factor | Determinant | Results |
|---|---|---|
| Age of onset GERD | <5 years | Increased rate |
| Initial diagnosis | GERD | Increased rate |
| Treatment at diagnosis | No or only antacids | Reduced rate |
| - | PPI or H2RAs+PPI | Increased rate |
GERD: gastro-esophageal reflux disease, PPI: proton pump inhibitor, H2RA: histamine 2 receptor antagonist.
CONCLUSION
The diagnosis and management of GER and GERD have remained challenges for years. Reviews and guidelines attempt to recommend the best approach according to existing evidence and consensus by key-opinion leaders. However, in many cases, the clinician must make management decisions based on diagnostic investigations with no certainty regarding outcome and, thus, results are often inconclusive.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 2.Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516–554. doi: 10.1097/MPG.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta SK, Hassall E, Chiu YL, Amer F, Heyman MB. Presenting symptoms of nonerosive and erosive esophagitis in pediatric patients. Dig Dis Sci. 2006;51:858–863. doi: 10.1007/s10620-006-9095-3. [DOI] [PubMed] [Google Scholar]
- 4.Martigne L, Delaage PH, Thomas-Delecourt F, Bonnelye G, Barthélémy P, Gottrand F. Prevalence and management of gastroesophageal reflux disease in children and adolescents: a nationwide cross-sectional observational study. Eur J Pediatr. 2012;171:1767–1773. doi: 10.1007/s00431-012-1807-4. [DOI] [PubMed] [Google Scholar]
- 5.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2006;130:1519–1526. doi: 10.1053/j.gastro.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 7.Dalla Vecchia LK, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, 3rd, Engum SA. Reoperation after Nissen fundoplication in children with gastroesophageal reflux: experience with 130 patients. Ann Surg. 1997;226:315–321. doi: 10.1097/00000658-199709000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider A, Gottrand F, Sfeir R, Duhamel A, Bonnevalle M, Guimber D, et al. Postoperative lower esophageal dilation in children following the performance of Nissen fundoplication. Eur J Pediatr Surg. 2012;22:399–403. doi: 10.1055/s-0032-1315807. [DOI] [PubMed] [Google Scholar]
- 9.Westra SJ, Wolf BH, Staalman CR. Ultrasound diagnosis of gastroesophageal reflux and hiatal hernia in infants and young children. J Clin Ultrasound. 1990;18:477–485. doi: 10.1002/jcu.1870180605. [DOI] [PubMed] [Google Scholar]
- 10.Jang HS, Lee JS, Lim GY, Choi BG, Choi GH, Park SH. Correlation of color Doppler sonographic findings with pH measurements in gastroesophageal reflux in children. J Clin Ultrasound. 2001;29:212–217. doi: 10.1002/jcu.1022. [DOI] [PubMed] [Google Scholar]
- 11.Patra S, Singh V, Chandra J, Kumar P, Tripathi M. Diagnostic modalities for gastro-esophageal reflux in infantile wheezers. J Trop Pediatr. 2011;57:99–103. doi: 10.1093/tropej/fmq056. [DOI] [PubMed] [Google Scholar]
- 12.Ravelli AM, Panarotto MB, Verdoni L, Consolati V, Bolognini S. Pulmonary aspiration shown by scintigraphy in gastroesophageal reflux-related respiratory disease. Chest. 2006;130:1520–1526. doi: 10.1378/chest.130.5.1520. [DOI] [PubMed] [Google Scholar]
- 13.Ravelli AM, Villanacci V, Ruzzenenti N, Grigolato P, Tobanelli P, Klersy C, et al. Dilated intercellular spaces: a major morphological feature of esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:510–515. doi: 10.1097/01.mpg.0000215312.78664.b9. [DOI] [PubMed] [Google Scholar]
- 14.Cucchiara S, Minella R, D'Armiento F, Franco M, Lervolino C, Campanozzi A, et al. Histologic grading of reflux oesophagitis and its relationship with intra-oesophageal and intragastric pH variables. Eur J Gastroenterol Hepatol. 1993;5:621–626. [Google Scholar]
- 15.Arasu TS, Wyllie R, Fitzgerald JF, Franken EA, Siddiqui AR, Lehman GA, et al. Gastroesophageal reflux in infants and children comparative accuracy of diagnostic methods. J Pediatr. 1980;96:798–803. doi: 10.1016/s0022-3476(80)80545-2. [DOI] [PubMed] [Google Scholar]
- 16.Vandenplas Y, Franckx-Goossens A, Pipeleers-Marichal M, Derde MP, Sacré-Smits L. Area under pH 4: advantages of a new parameter in the interpretation of esophageal pH monitoring data in infants. J Pediatr Gastroenterol Nutr. 1989;9:34–39. [PubMed] [Google Scholar]
- 17.Farhath S, He Z, Saslow J, Soundar S, Amendolia B, Bhat V, et al. Detection of pepsin in mouth swab: correlation with clinical gastroesophageal reflux in preterm infants. J Matern Fetal Neonatal Med. 2013;26:819–824. doi: 10.3109/14767058.2013.764408. [DOI] [PubMed] [Google Scholar]
- 18.Rosen R, Johnston N, Hart K, Khatwa U, Nurko S. The presence of pepsin in the lung and its relationship to pathologic gastro-esophageal reflux. Neurogastroenterol Motil. 2012;24:129–133. e84–125. doi: 10.1111/j.1365-2982.2011.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell S, McMaster C, Gibson D, Shields MD, McCallion WA. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastro-oesophageal reflux-related pulmonary aspiration. J Pediatr Surg. 2006;41:289–293. doi: 10.1016/j.jpedsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan U, Mitchell JD, Messina I, Day AS, Bohane TD. Assay of tracheal pepsin as a marker of reflux aspiration. J Pediatr Gastroenterol Nutr. 2002;35:303–308. doi: 10.1097/00005176-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Dy F, Amirault J, Mitchell PD, Rosen R. Salivary pepsin lacks sensitivity as a diagnostic tool to evaluate extraesophageal reflux disease. J Pediatr. 2016;177:53–58. doi: 10.1016/j.jpeds.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortunato JE, D'Agostino RB, Jr, Lively MO. Pepsin in saliva as a biomarker for oropharyngeal reflux compared with 24-hour esophageal impedance/pH monitoring in pediatric patients. Neurogastroenterol Motil. 2017;29:e12936. doi: 10.1111/nmo.12936. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly RC, He Z, Bloedon E, Papsin B, Lundy L, Bolling L, et al. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118(Suppl 116):1–9. doi: 10.1097/MLG.0b013e31817924a3. [DOI] [PubMed] [Google Scholar]
- 24.Kelly EA, Parakininkas DE, Werlin SL, Southern JF, Johnston N, Kerschner JE. Prevalence of pediatric aspiration-associated extraesophageal reflux disease. JAMA Otolaryngol Head Neck Surg. 2013;139:996–1001. doi: 10.1001/jamaoto.2013.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen R, Fritz J, Nurko A, Simon D, Nurko S. Lipid-laden macrophage index is not an indicator of gastroesophageal reflux-related respiratory disease in children. Pediatrics. 2008;121:e879–e884. doi: 10.1542/peds.2007-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tucker E, Knowles K, Wright J, Fox MR. Rumination variations: aetiology and classification of abnormal behavioural responses to digestive symptoms based on high-resolution manometry studies. Aliment Pharmacol Ther. 2013;37:263–274. doi: 10.1111/apt.12148. [DOI] [PubMed] [Google Scholar]
- 27.Kessing BF, Bredenoord AJ, Smout AJ. Objective manometric criteria for the rumination syndrome. Am J Gastroenterol. 2014;109:52–59. doi: 10.1038/ajg.2013.428. [DOI] [PubMed] [Google Scholar]
- 28.van der Pol RJ, Smits MJ, van Wijk MP, Omari TI, Tabbers MM, Benninga MA. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935. doi: 10.1542/peds.2010-2719. [DOI] [PubMed] [Google Scholar]
- 29.Haddad I, Kierkus J, Tron E, Ulmer A, Hu P, Sloan S, et al. Efficacy and safety of rabeprazole in children (1–11 years) with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2013;57:798–807. doi: 10.1097/MPG.0b013e3182a4e718. [DOI] [PubMed] [Google Scholar]
- 30.Fiedorek S, Tolia V, Gold BD, Huang B, Stolle J, Lee C, et al. Efficacy and safety of lansoprazole in adolescents with symptomatic erosive and non-erosive gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2005;40:319–327. doi: 10.1097/01.mpg.0000155369.54464.41. [DOI] [PubMed] [Google Scholar]
- 31.Baker R, Tsou VM, Tung J, Baker SS, Li H, Wang W, et al. Clinical results from a randomized, double-blind, dose-ranging study of pantoprazole in children aged 1 through 5 years with symptomatic histologic or erosive esophagitis. Clin Pediatr (Phila) 2010;49:852–865. doi: 10.1177/0009922810369253. [DOI] [PubMed] [Google Scholar]
- 32.Tolia V, Ferry G, Gunasekaran T, Huang B, Keith R, Book L. Efficacy of lansoprazole in the treatment of gastroesophageal reflux disease in children. J Pediatr Gastroenterol Nutr. 2002;35(Suppl 4):S308–S318. doi: 10.1097/00005176-200211004-00003. [DOI] [PubMed] [Google Scholar]
- 33.Croffie JM, Fitzgerald JF, Molleston JP, Gupta SK, Corkins MR, Pfefferkorn MD, et al. Accuracy and tolerability of the Bravo catheter-free pH capsule in patients between the ages of 4 and 18 years. J Pediatr Gastroenterol Nutr. 2007;45:559–563. doi: 10.1097/MPG.0b013e3180dc9349. [DOI] [PubMed] [Google Scholar]
- 34.Rao NM, Campbell DI, Rao P. Two years' experience of using the Bravo wireless oesophageal pH monitoring system at a single UK tertiary centre. Acta Paediatr. 2017;106:312–315. doi: 10.1111/apa.13667. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera J, Davis M, Horn D, Pfefferkorn M, Croffie JM. Esophageal pH monitoring with the BRAVO capsule: experience in a single tertiary medical center. J Pediatr Gastroenterol Nutr. 2011;53:404–408. doi: 10.1097/MPG.0b013e3182203caa. [DOI] [PubMed] [Google Scholar]
- 36.Moon RY Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030–1039. doi: 10.1542/peds.2011-2284. [DOI] [PubMed] [Google Scholar]
- 37.Salvatore S, Savino F, Singendonk M, Tabbers M, Benninga MA, Staiano A, et al. Thickened infant formula: what to know. Nutrition. 2018;49:51–56. doi: 10.1016/j.nut.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Koebnick C, Getahun D, Smith N, Porter AH, Der-Sarkissian JK, Jacobsen SJ. Extreme childhood obesity is associated with increased risk for gastroesophageal reflux disease in a large population-based study. Int J Pediatr Obes. 2011;6:e257–63. doi: 10.3109/17477166.2010.491118. [DOI] [PubMed] [Google Scholar]
- 39.Pashankar DS, Corbin Z, Shah SK, Caprio S. Increased prevalence of gastroesophageal reflux symptoms in obese children evaluated in an academic medical center. J Clin Gastroenterol. 2009;43:410–413. doi: 10.1097/MCG.0b013e3181705ce9. [DOI] [PubMed] [Google Scholar]
- 40.Ummarino D, Miele E, Martinelli M, Scarpato E, Crocetto F, Sciorio E, et al. Effect of magnesium alginate plus simethicone on gastroesophageal reflux in infants. J Pediatr Gastroenterol Nutr. 2015;60:230–235. doi: 10.1097/MPG.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 41.Miller S. Comparison of the efficacy and safety of a new aluminium-free paediatric alginate preparation and placebo in infants with recurrent gastro-oesophageal reflux. Curr Med Res Opin. 1999;15:160–168. doi: 10.1185/03007999909114087. [DOI] [PubMed] [Google Scholar]
- 42.Salvatore S, Ripepi A, Huysentruyt K, van de Maele K, Nosetti L, Agosti M, et al. The effect of alginate in gastroesophageal reflux in infants. Paediatr Drugs. 2018;20:575–583. doi: 10.1007/s40272-018-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies I, Burman-Roy S, Murphy MS Guideline Development Group. Gastro-oesophageal reflux disease in children: NICE guidance. BMJ. 2015;350:g7703. doi: 10.1136/bmj.g7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154:514–520.e4. doi: 10.1016/j.jpeds.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 45.Yadlapati R, Kahrilas PJ. The “dangers” of chronic proton pump inhibitor use. J Allergy Clin Immunol. 2018;141:79–81. doi: 10.1016/j.jaci.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 47.Rosen R, Amirault J, Liu H, Mitchell P, Hu L, Khatwa U, et al. Changes in gastric and lung microflora with acid suppression: acid suppression and bacterial growth. JAMA Pediatr. 2014;168:932–937. doi: 10.1001/jamapediatrics.2014.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trikha A, Baillargeon JG, Kuo YF, Tan A, Pierson K, Sharma G, et al. Development of food allergies in patients with gastroesophageal reflux disease treated with gastric acid suppressive medications. Pediatr Allergy Immunol. 2013;24:582–588. doi: 10.1111/pai.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khoshoo V, Dhume P. Clinical response to 2 dosing regimens of lansoprazole in infants with gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2008;46:352–354. doi: 10.1097/MPG.0b013e31815667d7. [DOI] [PubMed] [Google Scholar]
- 50.Omari TI, Benninga MA, Sansom L, Butler RN, Dent J, Davidson GP. Effect of baclofen on esophagogastric motility and gastroesophageal reflux in children with gastroesophageal reflux disease: a randomized controlled trial. J Pediatr. 2006;149:468–474. doi: 10.1016/j.jpeds.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 51.Li S, Shi S, Chen F, Lin J. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2014;2014:307805. doi: 10.1155/2014/307805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Loore I, Van Ravensteyn H, Ameryckx L. Domperidone drops in the symptomatic treatment of chronic paediatric vomiting and regurgitation. A comparison with metoclopramide. Postgrad Med J. 1979;55(Suppl 1):40–42. [PubMed] [Google Scholar]
- 53.Carroccio A, Iacono G, Montalto G, Cavataio F, Soresi M, Notarbartolo A. Domperidone plus magnesium hydroxide and aluminum hydroxide: a valid therapy in children with gastroesophageal reflux. A double-blind randomized study versus placebo. Scand J Gastroenterol. 1994;29:300–304. doi: 10.3109/00365529409094839. [DOI] [PubMed] [Google Scholar]
- 54.Lau Moon Lin M, Robinson PD, Flank J, Sung L, Dupuis LL. The Safety of metoclopramide in children: a systematic review and meta-analysis. Drug Saf. 2016;39:675–687. doi: 10.1007/s40264-016-0418-9. [DOI] [PubMed] [Google Scholar]
- 55.Morris AD, Chen J, Lau E, Poh J. Domperidone-associated QT interval prolongation in non-oncologic pediatric patients: a review of the literature. Can J Hosp Pharm. 2016;69:224–230. doi: 10.4212/cjhp.v69i3.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levi P, Marmo F, Saluzzo C, Dell'Olio D, Ansaldi N, Giuliani L, et al. Bethanechol versus antiacids in the treatment of gastroesophageal reflux. Helv Paediatr Acta. 1985;40:349–359. [PubMed] [Google Scholar]
- 57.Euler AR. Use of bethanechol for the treatment of gastroesophageal reflux. J Pediatr. 1980;96:321–324. doi: 10.1016/s0022-3476(80)80839-0. [DOI] [PubMed] [Google Scholar]
- 58.Rohof WO, Bennink RJ, de Ruigh AA, Hirsch DP, Zwinderman AH, Boeckxstaens GE. Effect of azithromycin on acid reflux, hiatus hernia and proximal acid pocket in the postprandial period. Gut. 2012;61:1670–1677. doi: 10.1136/gutjnl-2011-300926. [DOI] [PubMed] [Google Scholar]
- 59.Rothenberg SS. Two decades of experience with laparoscopic nissen fundoplication in infants and children: a critical evaluation of indications, technique, and results. J Laparoendosc Adv Surg Tech A. 2013;23:791–794. doi: 10.1089/lap.2013.0299. [DOI] [PubMed] [Google Scholar]
- 60.Hambraeus M, Arnbjörnsson E, Anderberg M. A literature review of the outcomes after robot-assisted laparoscopic and conventional laparoscopic Nissen fundoplication for gastro-esophageal reflux disease in children. Int J Med Robot. 2013;9:428–432. doi: 10.1002/rcs.1517. [DOI] [PubMed] [Google Scholar]