Abstract
Varicocele, defined as enlarged varicose veins in the scrotum, is the most common identifiable cause of male infertility. There are significant correlations between oxidative stress and varicocele-related infertility due to testicular hyperthermia, which can result in low sperm function. In addition, recent excessive oxidative stress can affect sperm telomere length and integrity of sperm DNA. Therefore, we assessed sperm telomere length as a potential marker of paternal genome integrity and leukocyte telomere length as an internal control (real-time PCR), along with sperm chromatin status (TUNEL and chromomycin A3 assay), and lipid peroxidation (Bodipy probe) in 18 infertile men with grade II or III varicocele, and 20 fertile men. Means of sperm parameters, sperm and leukocyte telomere length were significantly lower, while means of sperm DNA fragmentation, protamine deficiency, and lipid peroxidation were significantly higher in infertile men with varicocele compared to fertile men. Therefore, shortened telomere length in sperm and leukocytes is likely associated with increased oxidative stress related to the state of varicocele, which also accounts for increase in sperm DNA fragmentation. Thus, assessment of leukocyte telomere length could be taken as an indicator of antioxidant capacity in an individual, which also affects sperm function.
Introduction
The term “varicocele” was initially suggested by the British surgeon T.B. Curling in 1843 to define venous dilatation of plexus pampiniform1. This phenomenon is one of the most important cause of male infertility in 35% of men with primary and 80% of men with secondary infertility, compared to 15% of general adult male population2. Increased testicular temperature, related to altered blood flow in the pampiniform plexus, results in testicular dysfunction with increased testicular oxidative stress appearing to be the main mediator3,4.
At the level of semen, testicular dysfunction manifests as decreased sperm concentration, motility and morphology while at the cellular level, it is associated with higher DNA fragmentation, apoptosis and reduced chromatin integrity compared to the fertile population5–12. In addition to these adverse effects, many cellular enzymatic processes are affected due to altered enzyme kinetic properties at increased temperature13–16.
Chromosomal stability or genomic integrity is highly dependent on telomere length at the ends of each chromosome. Telomeres are characterized as heterochromatic structures with noncoding hexanucleotide TTAGGG repeats that are maintained by telomerases. The protective function of telomeres is mediated through the nucleoprotein complex “shelterin” that binds to these hexameric repeats at the ends of each chromosome. Shelterin protects the chromosome from being attacked by exonucleases and prevents end-to-end fusion between chromosomes by inhibiting DNA damage response being activated as recognition of a double-strand break, and overall prevents DNA degradation, recombination, and DNA end fusions17,18.
The average telomere length was estimated between 5 to 10 kb in human somatic cells and 10–20 kb in germ cells19. Unlike somatic cells, in germ, stem and cancerous cells, the length of the telomere is preserved through cell divisions20. This difference has been mainly related to reduction or absence of telomerase activity in somatic cells compared to the germ, stem and cancerous cells. Reduced telomere length in germ-line cells is associated with aberrant meiotic synapsis, recombination, chromosomal segregation, chromosomal disjunction, gamete aneuploidy, apoptosis and developmental arrest post fertilization21. Indeed, in the human embryo, critically short telomere lengths are associated with increased rate of cytoplasmic fragmentation, reduced blastocyst formation and, thereby infertility22.
The telomere length is a complex trait that is determined by various factors including age, oxidative stress, age of the father at the time of conception, infection and social status like smoking18. Considering the destructive effects of oxidative stress on telomere length and the fact that in varicocele state, oxidative stress increases23,24, we aimed to compare sperm telomere length as a potential marker of paternal genome integrity, along with sperm chromatin status, and lipid peroxidation between infertile men with varicocele and fertile individuals. In addition, leukocyte telomere length was assessed as an internal control. The result of current study clearly shows that the varicocele state, in addition to its effects on the sperm DNA integrity, sperm lipid peroxidation level, protamine content and sperm parameters, might also affect telomere length.
Results
Comparison of age and semen parameters between fertile and infertile men with varicocele groups
Semen characteristics, male age and paternal age at conception (PAC) were compared between fertile individuals and infertile men with varicocele (Table 1). Paternal age was significantly higher in fertile individuals compared to infertile men with varicocele but no significant difference was found in PAC between the two groups (p > 0.05). Despite this result, considering the effect of age as confounding factor on sperm telomere length by some researchers, therefore, we evaluated the effect of age as a possible confounding factor on telomere length by regression model in infertile men with varicocele and fertile men. This result showed that age did not significantly affect the study parameters in this manuscript. Therefore, the significant values presented in this manuscript are based on data adjusted for age. The mean value of semen parameters including sperm count, concentration and motility were significantly lower in infertile men with varicocele compared to the fertile individuals, while the percentage of abnormal morphology was significantly higher in infertile men with varicocele (p < 0.05). Semen volume showed no significant difference between the two groups (p > 0.05).
Table 1.
Comparison of male age and sperm parameters between infertile men with varicocele and fertile individuals.
| Parameters | Varicocele | Fertile | p-value |
|---|---|---|---|
| Male age (year) | 28.50 ± 5.52 | 41.38 ± 3.62 | <0.001 |
| Paternal age at conception (year) | 28.39 ± 7.47 | 30.30 ± 7.04 | 0.47 |
| Sperm concentration (106/ml) | 69.83 ± 12.53 | 135.1 ± 23.68 | 0.01 |
| Sperm count (106/ejaculate) | 209 ± 36.6 | 423.46 ± 70.56 | 0.007 |
| Sperm motility (%) | 43.75 ± 4.07 | 62.03 ± 2.58 | 0.001 |
| Abnormal sperm morphology (%) | 97.66 ± 0.2 | 96.88 ± 0.2 | 0.04 |
| Semen volume (ml) | 2.98 ± 0.3 | 3.36 ± 0.38 | 0.427 |
Comparison of sperm lipid peroxidation and chromatin status between fertile and infertile men with varicocele groups
Comparison of percentage sperm lipid peroxidation (34.83 ± 3.4 vs. 15.54 ± 1.47; p < 0.001), protamine deficiency (55.89 ± 2.63 vs. 28.4 ± 2.33; p < 0.001) and DNA fragmentation (12.43 ± 1.23 vs. 6.52 ± 1.17; p = 0.003) between infertile men with varicocele and fertile individuals showed that the aforementioned parameters, as negative markers of fertility, were significantly higher in individuals with varicocele (Fig. 1).
Figure 1.
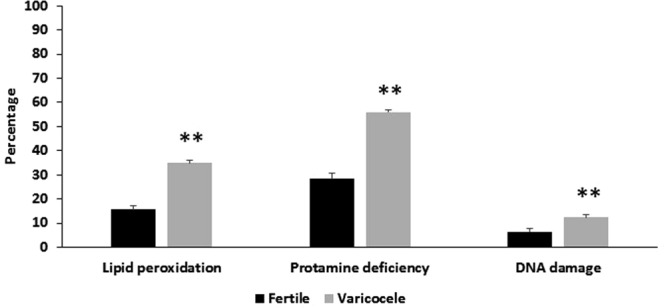
Comparison of mean percentage of sperm lipid peroxidation, protamine deficiency, and DNA damage between infertile men with varicocele and fertile individuals. *Shows P < 0.05 and **shows P < 0.001.
Comparison of sperm telomere length (STL), leucocyte telomere length (LTL) between fertile and infertile men with varicocele groups
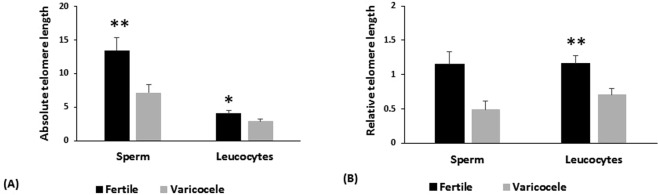
In this study, telomere length in sperm and leucocyte were evaluated by two methods; relative and absolute (Fig. 2). Means of absolute (7.17 ± 1.18 vs. 13.44 ± 1.87; p = 0.006) and relative (0.48 ± 0.12 vs. 1.14 ± 0.18; p = 0.004) telomere length in sperm were significantly lower in infertile men with varicocele compared to fertile individuals. Similar to telomere length in sperm, means of relative (0.71 ± 0.08 vs. 1.16 ± 0.11; p = 0.003) and absolute (2.92 ± 0.3 vs. 4.06 ± 0.44; p = 0.03) telomere length in leucocytes in infertile men with varicocele were also significantly lower than fertile individuals.
Figure 2.
Comparison of (A) absolute and (B) relative telomere length in sperm and leucocyte between fertile individuals and infertile men with varicocele. *Shows P < 0.05 and **shows P < 0.01.
Correlation between, male age, sperm parameters, sperm and leucocyte telomere length, sperm lipid peroxidation, and chromatin status
In this study, a positive significant correlation was observed between absolute sperm telomere length and absolute leucocyte telomere length (r = 0.396; p = 0.03) and negative significant correlations were observed between absolute sperm telomere length with percentage of DNA fragmentation (r = −0.401; p = 0.03), protamine deficiency (r = −0.438; p = 0.02) and sperm lipid peroxidation (r = −0.39; p = 0.04). Similarly, absolute leucocyte telomere length showed significant negative correlation with percentage of DNA fragmentation (r = −0.542; p < 0.001) and protamine deficiency (r = −0.401; p = 0.03). In addition, leukocyte telomere length significantly correlated with sperm concentration (r = 0.435, p = 0.01) and sperm count (r = 0.473; p < 0.001). A positive significant correlation was observed between absolute sperm telomere length and percentage of sperm motility (r = 0.376; p = 0.04).
Percentage of DNA fragmentation and protamine deficiency both showed negative significant correlations with sperm concentration and sperm count (p < 0.001). In addition, a very strong significant correlation was observed between the percentage of DNA fragmentation and protamine deficiency (r = 0.701; p < 0.001). Percentage of sperm lipid peroxidation also significantly negatively correlated with sperm count (r = −0.371; p = 0.04) and positively correlated with protamine deficiency (r = 0.552; p < 0.001) (Table 2). It is important to note that, when we assessed the correlation between aforementioned parameters with relative sperm and leucocyte telomere length, we obtained similar results as with absolute values. Therefore, to make the results concise, we presented only the correlations with absolute telomere length.
Table 2.
Shows the correlation among sperm parameters absolute sperm telomere length (STL), absolute leucocyte telomere length (LTL), sperm lipid peroxidation, protamine deficiency and DNA fragmentation.
| Parameters | STL | LTL | DNA fragmentation | Protamine deficiency | Lipid peroxidation |
|---|---|---|---|---|---|
| Absolute sperm telomere length (STL) | 1 | 0.396* | −0.401* | −0.438* | −0.390* |
| Absolute leucocyte telomere length (LTL) | 0.396* | 1 | −0.542** | −0.401* | −0.213 |
| Sperm concentration (106/ml) | 0.248 | 0.435* | −0.552** | −0.646** | −0.332 |
| Sperm count (106/ejaculate) | 0.359 | 0.473** | −0.544** | −0.729** | −0.371* |
| Sperm motility (%) | 0.376* | 0.175 | −0.261 | −0.327 | −0.331 |
| Abnormal sperm morphology (%) | −0.298 | −0.245 | 0.254 | 0.339 | 0.322 |
| DNA fragmentation (%) | −0.401* | −0.542** | 1 | 0.701** | 0.260 |
| Protamine deficiency (%) | −0.438* | −0.401* | 0.701** | 1 | 0.552** |
**P < 0.001 and *P < 0.05.
Discussion
Telomere length plays an important role in the maintenance of chromosomal stability and genomic integrity25. Therefore, researchers working in different fields have assessed the role of telomere length in aging-associated diseases, cancer and tumorigenesis, stem cell, coronary artery, cardiovascular disease and reproduction. In the latter field, they have assessed telomere length in oocyte, embryo, and sperm18,20,26. Some researchers believe that telomere length in human spermatozoa is unrelated to sperm parameters and DNA integrity. In this regard, Turner and Hartshorne stated that “our results lend weight to the idea that sperm telomere length is not crucial for male fertility, because telomere length resetting occurs in the embryo after fertilization. We propose that the oocyte probably modifies sperm telomere DNA, by a recombination based mechanism, before de novo telomerase transcription increases telomere length towards the blastocyst stage”25,27–30. In contrast, numerous studies suggest that sperm count, concentration, motility, and morphology show significant association with telomere length25,31. This disagreement could be related to confounding factors affecting both telomere length and infertility including age, oxidative stress, socioeconomic status, body mass index and genetic background18. Therefore, in this study, for the first time, the mean telomere lengths in infertile men with varicocele, which commonly presents with high oxidative stress, were compared to fertile individuals24,32.
In accordance with the literature, the results of this study also reveal that semen parameters of infertile men with varicocele showed significantly lower quality compared to fertile individuals, despite the fact that infertile men with varicocele were younger than the fertile individuals5,9,10,33. In addition, assessment of three important sperm functional parameters in these individuals revealed that degree of lipid peroxidation, protamine deficiency, and DNA damage were significantly higher in infertile men with varicocele. These observations are consistent with previous reports in the literature7,23,34–36. In this study, we assessed lipid peroxidation as the main marker of oxidative stress using Bodipy probe in infertile men with varicocele. We show that a high level of lipid peroxidation correlates with shortened sperm telomere length (Table 2).
Assessment of sperm and leucocyte telomere length by both absolute and relative methods show that for both cell types, the telomere length was significantly lower in infertile men with varicocele than fertile individuals. Comparison paternal age at conception revealed no difference between the two groups; however, mean age in fertile group was significantly higher than infertile men with varicocele. We initially thought that longer telomere length in fertile individuals is likely to be related to age, but assessment of telomere length in leucocyte revealed that in infertile men with varicocele, their leucocyte telomere length is also shorter than fertile men, despite their younger age, indicating that reduced telomere length in these individuals is likely to be independent of age and parental age at conception and is more likely to be an inherent problem in these individuals. Whether this inherent problem is a causative or associative factor with state of varicocele remains to be determined. Indeed, it has been shown that oxidative stress results in reduction of leucocyte telomere length. Antioxidant therapy can potentially decrease telomere attrition37.
Oxidative stress has been shown to affect the telomere length18. Indeed, we showed that both lipid peroxidation and DNA fragmentation, related to oxidative stress, are higher in these individuals. Increased oxidative stress at testicular level is a well-established fact in testes of infertile men with varicocele and may account for reduced telomere length in individuals with varicocele, but whether such an effect at somatic level could account for decreased telomere length in leucocytes remains to be determined38,39. The observed correlation between sperm and leucocyte telomere length may extend the support of this hypothesis. Therefore, the imbalance between ROS production and antioxidant capacity due to high percentage of abnormal sperm morphology, and number of leucocytes in infertile men with varicocele can induce oxidative stress and may account for decrease of both sperm and leucocyte telomere length15,32. Another possibility that may account for shortening of telomere length in sperm of infertile men with varicocele could be reduction of telomerase activity in testis. Indeed, concentration and activity of many testicular enzymes in infertile men with varicocele have reduced, possibly due to testicular hyperthermia, including: Topoisomerase I, phospholipase C zeta, acrosin activity, and apurinic/apyrimidinic endonuclease11,13,40,41.
It was also interesting to note that we observed significant negative correlations between telomere lengths of sperm and leukocytes as well as between DNA fragmentation and protamine deficiency. Unlike sperm telomere length, significant correlations were observed between sperm count and concentration with leukocyte telomere length. These results indicate that reduced telomere length renders sperm prone to loss of chromatin integrity, assessed as protamine deficiency or DNA fragmentation, or vice versa. This interaction may underlie the role of reduced antioxidant capacity at both testicular and somatic levels, which may render sperm prone to chromatin immaturity, increased DNA fragmentation, and reduced telomere length. Since we did not measure the antioxidant capacity at testicular and somatic levels, this hypothesis requires future verification. But our result reveals the potential of leukocyte telomere length as a biomarker for infertility.
In addition, significant correlations were observed between sperm motility (positive) and lipid peroxidation (negative) with sperm telomere length but not with leukocyte telomere length. These results indicate the importance of assessment of ROS at testicular level, which may be different from plasma levels. These observations are consistent with an in vitro study that showed that exposure of transcriptionally and translationally silent spermatozoa to H2O2 reduced telomere length, possibly directly, independent of reduced telomerase activity. This argument was justified by observation of oxidation of three guanine repeats in telomere repetitive sequences to 8-oxo-guanine42. In this study, the correlations observed among DNA fragmentation, lipid peroxidation and protamine deficiency are consistent with literature, suggesting that protamine deficiency and sperm immaturity render sperm prone to both DNA fragmentation and lipid peroxidation due to relaxation of chromatin and retention of extra cytoplasm, respectively23,33.
In conclusion, the result of this study shows that mean sperm telomere length in infertile men with varicocele is shorter than in fertile individuals and this is likely associated with increased oxidative stress correlated with the state of varicocele and which also accounts for increase sperm DNA fragmentation in these individuals. Further studies are needed to confirm these correlations. Furthermore, significant correlations were observed between leukocyte telomere length and semen parameters, sperm DNA fragmentation, and protamine deficiency. Therefore, assessment of leukocyte telomere length could be taken as indicator of antioxidant capacity in an individual that also reflects its effect on sperm function. Therefore, antioxidant therapy alone and/or along with varicocelectomy can improve fertility potential in infertile men with varicocele, which is in agreement with literature.
Materials and Methods
Participants
Following approval of the study by Royan Ethical Committee, semen samples and blood were collected from 20 fertile individuals referred for family balancing and 18 men with varicocele (II & III grade) referred to Isfahan Fertility and Infertility Center. Informed written consent was obtained from each participant. Infertile men with varicocele that (1) had other infertility related diseases, such as genital infection, hypogonadism, Klinefelter’s syndrome, testicular size discrepancy, anatomical disorders, abnormal hormonal profile, grade I varicocele, recurrent varicocele, azoospermia, previous history of scrotal trauma or surgery, and/or (2) were exposed to occupational agents and factors degrading male fertility, such as pesticides, solvents, heat, radiation, excessive alcohol, drug consumption, and/or (3) had other factors affecting seminal ROS level such as leukocytospermia, age >40 years, were excluded from this study. The diagnosis of varicocele was made by clinical examination and confirmed by color Doppler analysis. Clinical varicocele severity was graded according to the criteria of Dubin and Amelar RD43.
Sperm preparation
Standard semen analysis was carried out according to World Health Organization protocol and assessments were carried out by one trained individual44. An aliquot of semen sample was used for assessment of sperm motility, concentration, and morphology by using computer-aided sperm analysis (CASA) and the remaining sample was used for assessment of protamine deficiency, DNA fragmentation, sperm lipid peroxidation, and sperm telomere lengths by Chromomycin A3 staining, TUNEL assay, Bodipy probe, and real-time PCR, respectively.
Evaluation of sperm protamine deficiency using CMA3 staining
Chromomycin A3 (CMA3) staining was carried out according to Nasr-Esfahani et al.45. Briefly, semen samples were washed with PBS and fixed in Carnoy’s solution [methanol: glacial acetic acid 3:1 (Merck, Germany)]. Then, two smears were prepared for each sample and stained with CMA3 solution [0.25 mg/ml in McIlvaine buffer (7 ml citric acid 0.1 M, 32.9 ml Na2HPO4 .7H2O, 0.2 M, pH 7.0, containing 10 Mm MgCl2)] for 20 min. Next step, slides were washed and mounted. Status of sperm protamine content for each sample was evaluated using an Olympus fluorescence microscope (BX51, Tokyo, Japan) with the appropriate filters (460–470 nm), and 500 sperm cells were counted. Percentage of spermatozoa with bright yellow staining, as protamine deficient spermatozoa, was reported for each sample45.
Evaluation of sperm DNA fragmentation using TUNEL assay
DNA fragmentation was carried out according to Tavalaee et al. using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay46. Briefly, semen samples were washed with PBS and fixed in 4% methanol-free formaldehyde on slides for 30 min. Then slides were washed and permeabilized with 0.2% Triton X-100 in PBS for 5 min. Next, a commercial detection kit from Promega company was used for the detection of DNA fragmentation (Apoptosis Detection System Fluorescein, Promega, Mannheim, Germany), according to the manufacturer’s instructions. 500 spermatozoa were randomly selected and evaluated using an Olympus fluorescence microscope (BX51; Tokyo, Japan) with the appropriate filters (460–470 nm) at 100× magnification. The TUNEL negative spermatozoa fluoresced red (spermatozoon without fragmented DNA), whereas the TUNEL-positive spermatozoa fluoresced bright green (spermatozoon with fragmented DNA).
Assessment of sperm lipid peroxidation using the Bodipy probe
In this study, level of sperm lipid peroxidation was assessed according to the protocol by Aitken et al.47. Briefly, Bodipy C11 loading BODIPYw 581/591 C11 (D3861, Molecular Probes) was added to 2 × 106 spermatozoa at a final concentration of 5 mM. Then, tubes were incubated for 30 min at 37 °C and samples were then washed twice with PBS buffer. Percentage of lipid peroxidation in sperm or Bodipy positive spermatozoa was assessed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
DNA extraction and telomere length assay by quantitative real-time PCR
Genomic DNA was extracted from the remaining washed sperm and peripheral blood leukocytes using QIAamp DNA Mini Kit (QIAGEN, Milan, Italy) according to manufacturer’s recommendations. Sperm telomere length (STL) and leucocyte telomere length (LTL) were determined by real-time polymerase chain reaction (qRT-PCR) as previously described by Cawthon. Each sample was run in triplicate48. Telomere length was expressed as relative and/or absolute value. The relative telomere length was calculated by the telomere to single-copy gene (T/S) ratio, and expressed as 2(−ΔΔCt). For assessment of absolute telomere length according to modified method by NathanJ O’Callaghan et al.49, two standard curves were created by serial dilutions of known amounts of oligomer standards (36B4 and telomere) in each reaction, and then data were expressed as kbp.
Statistical analysis
All of the statistical analyses were carried out using the Statistical Program for Social Sciences (SPSS Inc., Version 11.0, Chicago, IL, USA). Data were expressed as standard error of the mean (means ± SEM), except age, which was reported as the standard deviation of means (means ± SDM). The Kolmogorov–Smirnov Z test was used to assess the normal data distribution. Independent sample t-test was used for comparison of variations between fertile and infertile men with varicocele groups. Pearson correlation was carried out to analyze the association between different parameters. p < 0.05 was considered statistically significant.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Royan Ethical Committee (Project NO: 96000088).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgements
This study was supported by Royan Institute. We express our gratitude to staff members of Isfahan Fertility and Infertility Center for their full support. This study was supported by Iran National Science Foundation (INSF) and Royan Institute.
Author Contributions
M.H.N.-E. Conception, design, data analysis, interpretation, manuscript writing and final approval of manuscript. M.T. Conception, design, collection and/or assembly of data, data analysis, interpretation, manuscript writing and final approval of manuscript. T.I. Analysis of quantitative real-time PCR and molecular exams. N.B. Supervisor of student. S.T. Semen analysis, prepared samples, and collected data. H.A. Urologist and management of infertile patients with varicocele for this study. Z.Z. & R.A.L. Manuscript writing and final approval of manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nöske HD, Weidner W. Varicocele-a historical perspective. World. J. Urol. 1999;17:151–157. doi: 10.1007/s003450050123. [DOI] [PubMed] [Google Scholar]
- 2.The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics World Health Organization. Fertil. Steril. 1992;57:1289–1293. doi: 10.1016/S0015-0282(16)55089-4. [DOI] [PubMed] [Google Scholar]
- 3.Miyaoka R, Esteves SC. A critical appraisal on the role of varicocele in male infertility. Adv. Urol. 2012;2012:597495. doi: 10.1155/2012/597495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Saleh RA. Role of oxidants in male infertility: rationale significance, and treatment. Urol. Clin. North. Am. 2002;29:817–827. doi: 10.1016/S0094-0143(02)00081-2. [DOI] [PubMed] [Google Scholar]
- 5.Nasr-Esfahani MH, Abasi H, Razavi S, Ashrafi S, Tavalaee M. Varicocelectomy: semen parameters and protamine deficiency. Int. J. Androl. 2009;32:115–122. doi: 10.1111/j.1365-2605.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 6.Azadi L, et al. Zaditen (Ketotifen), as mast cell blocker, improves sperm quality, chromatin integrity and pregnancy rate after varicocelectomy. Int. J. Androl. 2011;34:446–452. doi: 10.1111/j.1365-2605.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 7.Tavalaee M, et al. Semen parameters and chromatin packaging in microsurgical varicocelectomy patients. Int. J. Fertil. Steril. 2012;6:165–174. [PMC free article] [PubMed] [Google Scholar]
- 8.Samplaski MK, Lo KC, Grober ED, Zini A, Jarvi KA. Varicocelectomy to “upgrade” semen quality to allow couples to use less invasive forms of assisted reproductive technology. Fertil. Steril. 2017;108:609–612. doi: 10.1016/j.fertnstert.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Nasr-Esfahani MH, Tavalaee M. Origin and role of DNA damage in varicocele. Int. J. Fertil. Steril. 2012;6:141–146. [PMC free article] [PubMed] [Google Scholar]
- 10.Barekat F, et al. A Preliminary Study: N-acetyl-L-cysteine Improves Semen Quality following Varicocelectomy. Int. J. Fertil. Steril. 2016;10:120–126. doi: 10.22074/ijfs.2016.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janghorban-Laricheh E, et al. An association between sperm PLCζ levels and varicocele? J. Assist. Reprod. Genet. 2016;33:1649–1655. doi: 10.1007/s10815-016-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni K, Spiess AN, Schuppe HC, Steger K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: a systematic review and meta-analysis. Andrology. 2016;4:789–799. doi: 10.1111/andr.12216. [DOI] [PubMed] [Google Scholar]
- 13.Navaeian-Kalat E, et al. High total acrosin activity in varicocele individuals. Andrologia. 2012;1:634–641. doi: 10.1111/j.1439-0272.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 14.Durairajanayagam D, Agarwal A, Ong C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online. 2015;30:14–27. doi: 10.1016/j.rbmo.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Micheli L, et al. Evaluation of enzymatic and non-enzymatic antioxidants in seminal plasma of men with genitourinary infections, varicocele and idiopathic infertility. Andrology. 2016;4:456–464. doi: 10.1111/andr.12181. [DOI] [PubMed] [Google Scholar]
- 16.Mostafa T, Rashed L, Taymour M. Seminal cyclooxygenase relationship with oxidative stress in infertile oligoasthenoteratozoospermic men with varicocele. Andrologia. 2016;48:137–142. doi: 10.1111/and.12423. [DOI] [PubMed] [Google Scholar]
- 17.Longhese MP, Anbalagan S, Martina M, Bonetti D. The role of shelterin in maintaining telomere integrity. Front. Biosci. (Landmark Ed) 2012;1:1715–1728. doi: 10.2741/4014. [DOI] [PubMed] [Google Scholar]
- 18.Thilagavathi J, Venkatesh S, Dada R. Telomere length in reproduction. Andrologia. 2013;45:289–304. doi: 10.1111/and.12008. [DOI] [PubMed] [Google Scholar]
- 19.Samassekou O, Gadji M, Drouin R, Yan J. Sizing the ends: normal length of human telomeres. Ann. Anat. 2010;192:284–291. doi: 10.1016/j.aanat.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Campbell PJ. Telomeres and cancer: from crisis to stability to crisis to stability. Cell. 2012;148:633–635. doi: 10.1016/j.cell.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS. Genet. 2011;7:e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keefe DL, Parry JP. New approaches to assisted reproductive technologies. Semin. Reprod. Med. 2005;23:301–308. doi: 10.1055/s-2005-923387. [DOI] [PubMed] [Google Scholar]
- 23.Bahreinian M, et al. DNA hypomethylation predisposes sperm to DNA damage in individuals with varicocele. Syst. Biol. Reprod. Med. 2015;61:179–186. doi: 10.3109/19396368.2015.1020116. [DOI] [PubMed] [Google Scholar]
- 24.Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian. J. Androl. 2016;18:186–193. doi: 10.4103/1008-682X.170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cariati F, et al. Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality. Reprod. Biomed. Online. 2016;33:404–411. doi: 10.1016/j.rbmo.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, et al. Sperm telomere length is positively associated with the quality of early embryonic development. Hum. Reprod. 2015;30:1876–1881. doi: 10.1093/humrep/dev144. [DOI] [PubMed] [Google Scholar]
- 27.Turner, S. & Hartshorne, G. M. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol Hum Reprod. 2013 Aug; 1 9(8):510-8. 10.1093/molehr/gat021. Epub 2013 Mar 20. [DOI] [PubMed]
- 28.Liu L, Blasco M, Trimarchi J, Keefe D. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev. Biol. 2002;249:74–84. doi: 10.1006/dbio.2002.0735. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez S, et al. Critically short telomeres are associated with sperm DNA fragmentation. Fertil. Steril. 2005;84:843–845. doi: 10.1016/j.fertnstert.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Liu JP, Li H. Telomerase in the ovary. Reproduction. 2010;140:215–222. doi: 10.1530/REP-10-0008. [DOI] [PubMed] [Google Scholar]
- 31.Rocca MS, et al. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum. Reprod. 2016;31:1158–1163. doi: 10.1093/humrep/dew061. [DOI] [PubMed] [Google Scholar]
- 32.Garg H, Kumar R. An update on the role of medical treatment including antioxidant therapy in varicocele. Asian. J. Androl. 2016;18:222–8. doi: 10.4103/1008-682X.171657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni K, et al. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology. 2016;4:816–824. doi: 10.1111/andr.12210. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinpour E, et al. Sperm ubiquitination and DNA fragmentation in men with occupational exposure and varicocele. Andrologia. 2014;46:423–429. doi: 10.1111/and.12098. [DOI] [PubMed] [Google Scholar]
- 35.Esteves SC, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int. Urol. Nephrol. 2015;47:1471–1477. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 36.Cortés-Gutiérrez EI, et al. DNA damage in spermatozoa from infertile men with varicocele evaluated by sperm chromatin dispersion and DBD-FISH. Arch. Gynecol. Obstet. 2016;293:189–196. doi: 10.1007/s00404-015-3822-y. [DOI] [PubMed] [Google Scholar]
- 37.García-Calzón S, et al. GENOI members Dietary total antioxidant capacity is associated with leukocyte telomere lengthin a children and adolescent population. Clin. Nutr. 2015;34:694–699. doi: 10.1016/j.clnu.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Romeo C, Santoro G. Free radicals in adolescent varicocele testis. Oxid. Med. Cell. Longev. 2014;2014:912878. doi: 10.1155/2014/912878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mostafa T, Rashed LA, Zeidan AS, Hosni A. Glutathione-S-transferase-oxidative stress relationship in the internal spermatic vein blood of infertile men with varicocele. Andrologia. 2015;47:47–51. doi: 10.1111/and.12234. [DOI] [PubMed] [Google Scholar]
- 40.Fujisawa M, Yoshida S, Matsumoto O, Kojima K, Kamidono S. Decrease of topoisomerase I activity in the testes of infertile men with varicocele. Arch. Androl. 1988;21:45–50. doi: 10.3109/01485018808986732. [DOI] [PubMed] [Google Scholar]
- 41.Yoon SP. Apurinic/apyrimidinic endonuclease immunoreactivity in germ cells of experimental varicocele-induced rat testes. Acta. Histochem. 2013;115:887–892. doi: 10.1016/j.acthis.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Lafuente R. Sperm telomere length in motile sperm selection techniques:A qFISH approach. Andrologia. 2017;50:1–7. doi: 10.1111/and.12840. [DOI] [PubMed] [Google Scholar]
- 43.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil. Steril. 1970;21:606–609. doi: 10.1016/S0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 44.WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn. WHO Press, Geneva, Switzerland (2010).
- 45.Nasr-Esfahani MH, Razavi S, Mardani M. Relation between different human sperm nuclear maturity tests and in vitro fertilization. J. Assist. Reprod. Genet. 2001;18:219–225. doi: 10.1023/A:1009412130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavalaee M, Kiani-Esfahani A, Nasr-Esfahani MH. Relationship between phospholipase C-zeta, semen parameters, and chromatin status. Syst. Biol. Reprod. Med. 2017;63:259–268. doi: 10.1080/19396368.2017.1298006. [DOI] [PubMed] [Google Scholar]
- 47.Aitken RJ, Wingate JK, De Iuliis GN, McLaughlin EA. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol. Hum. Reprod. 2007;13:203–211. doi: 10.1093/molehr/gal119. [DOI] [PubMed] [Google Scholar]
- 48.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic. Acids. Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol. Proced. Online. 2011;31:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]